Abstract
Preeclampsia results in increased susceptibility to hypertension and chronic kidney disease postpartum; however, the mechanisms responsible for disease progression in these women remain unknown. The purpose of this study was to test the hypothesis that two mechanisms contribute to the link between the maternal syndrome of preeclampsia and the increased postpartum risk of cardiovascular and renal disease: (a) increased T cells in the kidney and (b) a decreased nitric oxide: endothelin-1 ratio. Dahl S rats (a previously characterized model of preeclampsia superimposed on chronic hypertension) who experienced two pregnancies and virgin littermate controls were studied at six months of age. Mean arterial pressure was measured via telemetry, and renal injury was assessed through both histological analysis and measurement of urinary markers including nephrin, podocalyxin, and kidney injury marker 1. Contributing mechanisms were assessed through flow cytometric analysis of renal T cells, quantification of plasma tumor necrosis factor-α and interleukin-10, and quantification of urinary concentrations of nitric oxide metabolites and endothelin-1. Although prior pregnancy did not exacerbate the hypertension at six months, this group showed greater renal injury compared to virgin littermates. Flow cytometric analyses revealed an increase in renal T cells following pregnancy, and cytokine analysis revealed a systemic pro-inflammatory shift. Finally, the nitric oxide: endothelin-1 ratio was reduced. These results demonstrate that the link between the maternal syndrome of superimposed preeclampsia and postpartum risk of chronic kidney disease could involve both immune system activation and dysregulation of the nitric oxide: endothelin-1 balance.
Keywords: kidney disease, T cells, postpartum, women’s health, nitric oxide, endothelin
Introduction
Preeclampsia is a leading cause of maternal morbidity and mortality worldwide and affects up to 10% of pregnancies each year in the United States [1]. Preeclampsia is defined as new-onset hypertension plus one of a subset of signs of end-organ damage, such as proteinuria, during the second half of pregnancy. In 2011, the AHA formally recognized preeclampsia as a risk factor for cardiovascular disease [2]. Studies have shown that experiencing a preeclamptic pregnancy results in a 2–12 fold increased risk of stroke, heart attack, hypertension, chronic kidney disease, and end stage renal disease in the 5–15 years postpartum [3]. Animal models have also shown that experimentally induced preeclampsia leads to activation of pathways associated with cardiovascular disease and adverse vascular remodeling in response to injury beyond that of control animals exposed to the same injury. These findings suggest that preeclamptic pregnancies may be related to long-standing changes in the circulating maternal proteome and long-term adverse cardiovascular effects [4,5]. Although evidence supports an association between preeclampsia and long-term renal disease, it remains unclear whether preeclampsia itself is a risk factor or merely unmasks a preexisting risk in this patient population. Further studies to elucidate the mechanisms behind long-term changes in postpartum maternal health are needed to guide the development of follow-up recommendations [2].
Immune activation is well documented in preeclampsia [6,7]. While a degree of immune activation is present in normal pregnancy, this response is exacerbated in women with preeclampsia [8]. An imbalance in the CD4+ T cell subpopulations supports a maladaptive maternal response to pregnancy that contributes to preeclampsia [9–12]. Increased levels of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17), and interleukin-6 (IL-6) as well as decreased levels of regulatory cytokines such as interleukin-10 (IL-10) and interleukin-4 (IL-4) are present in the circulation and in the placenta during preeclampsia [6,7,13,14]. This shift toward systemic inflammation has been shown to increase vascular permeability and oxidative stress in the placenta, which could similarly contribute to postpartum renal injury [7]. Previous studies have shown that activation of the immune system can result in accumulation of memory T cells in the kidney, which then contributes to long-term sensitization to hypertensive stimuli and end-organ damage [15]. Though much of the previously published data on the immune system in hypertension has been produced in male animals, more recent studies and comprehensive reviews are increasingly noting a similar role for immune cells in hypertensive kidney injury of females as well [16].
There is clinical evidence that the altered immune state does not end at delivery in preeclamptic patients. Kvehaugen et al. [17] demonstrated a continued inflammatory state in women with a history of preeclampsia up to 5–8 years postpartum including increased markers of systemic inflammation (c-reactive protein, calprotectin) and antiangiogenic factors (soluble fms-like tyrosine kinase 1 or sFlt-1). Further studies are needed to determine whether the imbalance between pro-inflammatory and regulatory cytokines persists in the postpartum period of animal models and determine its role in mediating later-life cardiovascular disease risk. Therefore, immune activation during pregnancy could link the maternal syndrome of preeclampsia to the increased postpartum risk of cardiovascular disease.
Normal pregnancy is characterized by vasorelaxation, decreased blood pressure (BP), and increased renal blood flow (RBF). While the mechanisms behind this adaptation have not been fully elucidated, increased endogenous nitric oxide (NO) production is shown to play a key role [18]. Urinary excretion of NOx, the stable oxidation products of NO, increases during pregnancy even after correction for increased dietary NO intake via increased food consumption [19,20]. Blockade of NO synthesis abolishes the decrease in blood pressure normally seen in pregnancy [21], and NO activity has been shown to be severely reduced in preeclampsia [22]. Systemic inhibition of NO synthase produces a preeclamptic phenotype used in relevant animal studies [23]. Upregulation of NO in normal pregnancy also plays a role in mediating a response to vasoconstrictor substances, such as endothelin-1 (ET-1) to maintain the necessary low resistance, high flow vasculature [24]. ET-1 is increased early in the course of preeclampsia, and its rise in plasma concentration has been demonstrated to correlate with blood pressure, proteinuria and plasma sFlt-1 concentration [25]. Regulatory mechanisms of the two substances interact to maintain vascular tone, such that an increase in ET-1 should lead to a compensatory increase in NO production [26] followed by the action of NO to control circulating ET-1 by multiple mechanisms [27–30]. While previous reports support the role of dysregulation of the NO: ET-1 balance in the pathogenesis of preeclampsia, the importance of NO and ET-1 signaling in the progression of postpartum CKD has not been investigated.
Our laboratory and others have previously identified the Dahl salt sensitive (S) rat as a spontaneous model of preeclampsia superimposed on chronic hypertension [31,32]. Therefore, the first goal of this project was to determine the impact of preeclamptic pregnancy on the background of chronic hypertension on the progression of postpartum renal injury in the Dahl S rat. The second goal was to address the hypothesis that two mechanisms contribute to the link between the maternal syndrome of preeclampsia and the increased postpartum risk of cardiovascular and renal disease: (a) increased T cells in the kidney and (b) a decreased NO: ET-1 ratio.
Methods
The authors declare that all supporting data are available within the article, and data are available from the corresponding author upon reasonable request.
Animals
Dahl salt-sensitive S (SS/jr) rats were obtained from the colony maintained by Dr. Michael Garrett at the University of Mississippi Medical Center. Female rats who had experienced two pregnancies at 12 and 17 weeks of age and virgin littermate controls were studied at six months of age, following a seven-week recovery from the second pregnancy. All pregnant animals displayed the previously published phenotype of superimposed preeclampsia [31]. These groups will hereafter be referred to as “virgin” (n=8) and “prior pregnancy” (n=11). Pups were removed within 48 hours of birth to remove any confounding effects of lactation. All rats were fed normal chow (TD7034, 0.3% NaCl, Harlan Teklad, Madison, WI) and water ad libitum on a 12-hour light/dark cycle. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were monitored by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Mean Arterial Blood Pressure Measurements
Rats were implanted with telemetry devices (Data Sciences, Inc., St. Paul, MN) via the femoral artery for continual blood pressure monitoring at ~6 months of age as previously described [33]. After a 10-day recovery period, interval mean arterial pressure measurements were obtained for 14 days.
Urinary Measurements
Rats were placed in metabolic cages for 24-hour urine collection prior to tissue harvest. Urinary protein excretion was determined by Bradford Assay (Bio-Rad Laboratories, Hercules, CA). Proteinuria was defined as >20 mg protein/24 hours. Urinary excretion rates of endothelin-1 (no dilution, Quantiglo, R&D Systems, Minneapolis, MN), KIM-1 (1:2 dilution, Quantikine R&D Systems, Minneapolis, MN), nephrin (1:10 dilution), and podocalyxin (1:2 dilution, Exocell, Philadelphia, PA) were quantified via commercially available ELISA assays. During testing, urine sample dilutions were adjusted as needed to achieve linear fit for each assay. Urinary excretion of NOx was measured via Cayman Chemical nitrate/nitrite assay (1:50 dilution, Ann Arbor, MI).
Tissue Collection
At six months of age, rats were euthanized while on isoflurane anesthesia (Piramal Healthcare, Boston, MA). A terminal blood sample was obtained from the abdominal aorta, and the organs were subsequently perfused blood-free with saline. The kidneys were removed and retained for analyte measurement.
Plasma Measurements
Tumor necrosis factor (TNF)-α, interleukin (IL)-10 and endothelin-1 were measured in plasma via commercially available assays (ELISA, R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Plasma creatinine was measured by the picric acid method adapted for microtiter plates[34].
Renal Histological Analysis
Kidney sections were fixed in 10% formalin, paraffin embedded, sectioned, and stained with Masson’s trichrome stain. Images were captured using a Nikon 55i microscope with DS-Fi1 5-Meg Color C digital camera (Nikon, Melville, NY) and analyzed using Nis-Elements image-analysis software (version 3.03, Nikon Instruments, Melville, NY). Glomerular injury was assessed on a scale from 0 (normal) to 4 (severe) using previously described criteria [35] for 25 glomeruli per section per rat by 2 blinded examiners. All scores for a given animal were averaged to provide one animal’s final result. Tubulointerstitial injury was determined by evaluation of the same slides to quantify the percent fibrosis (blue staining) compared with the background in 10 randomly selected cortex images from each rat, five each from two sections. This was calculated by Nis-Elements software which compared the number of blue pixels to total pixels in each image, multiplied by 100. The mean of the ten images per animal was calculated to provide that animal’s final result.
Analysis of Renal T cells
Single cell suspensions from the kidney cortex were prepared in 5 mL RPMI (Roswell Park Memorial Institute) media containing 200 U/mL DNase and 10 mg/mL collagenase IV using the Gentle MACS Octo Dissociator (Milltenyi Biotec, Bergisch Gladbach, Germany) with a user-defined protocol for rodent kidney. The resulting suspension was filtered through a 70 μm cell strainer and washed with 1× PBS containing 2% FCS and 2 mmol/L EDTA. The single cell suspension was centrifuged at 300g for 10 minutes. The resulting cell pellet was then resuspended in 1X PBS, 2% FCS, 0.9% sodium azide at a concentration of 2 × 107 cells/mL. 1X106 cells were aliquoted into a flow cytometry tube and incubated with 0.5 μg of anti-rat CD32 (Rat FcR block, clone D34–485, BD Biosciences) and then stained with either isotype control antibodies or ant-rat CD3-PE (clone G4.18, BD Biosciences, anti-rat CD4-FITC (clone OX-35, BD Biosciences), and anti-rat CD8a-APC (clone OX-8, BD Biosciences) diluted 1:100 in 1X PBS, 2% FCS, 0.9% sodium azide. Cells were incubated on ice for 30 minutes and protected from light. Samples were analyzed on a Gallios (Becton Dickinson, Franklin Lakes, NJ) flow cytometer, and a total of100,000 events were acquired for each sample. Data were analyzed using Kaluza software (Beckman Coulter, Indianapolis, IN).
Statistical Analysis
All data are presented as mean ± SE. Statistical analyses were performed by Student’s t-test (between the virgin and prior pregnancy groups) using Sigma Plot 12 (Systat Software, Inc., San Jose, CA). Means were considered significantly different if p<0.05.
Results
Mean arterial pressure (MAP) is not different between virgin and prior pregnancy groups.
The Dahl S rat strain is a model of chronic hypertension that worsens with age, and the blood pressure observed in these rats is consistent with previous reports of significant hypertension that develops with age in this strain. Preeclampsia often results in a degree of postpartum hypertension and increases the risk for long-term chronic hypertension [36,37]. However, comparison of mean arterial pressure between Dahl S rats after two preeclamptic pregnancies and virgin littermates showed no significant difference (prior pregnancy: 184.6 mmHg ± 6.6; virgin: 185.4 mmHg ± 6.9, n=8/group).
Glomerular and tubular injury is exacerbated in prior pregnancy animals compared to virgin animals.
In the absence of long-term BP differences, renal injury may progress due to secondary mechanisms including intermittent blood pressure increases and immune cell infiltration. Despite similar BP, prior pregnancy animals had greater renal injury compared to virgin animals. Urinary protein excretion was significantly increased in prior pregnancy animals (Fig. 1), indicating renal injury. Histological examination of glomeruli showed a significant difference in the degree of injury using a visual grading scale for glomerulosclerosis as previously described [38] (Fig. 2). To further examine and quantify damage to the glomeruli, urinary excretion rates of nephrin and podocalyxin were measured by ELISA. These analyses showed significant increases in urinary excretion of both analytes in prior pregnancy compared to virgin animals (Fig. 3A-3B). To compare tubular damage, 10x images of Masson’s trichrome stained slides from renal cortex and medulla were used to quantify blue-colored pixels. Analysis demonstrated a significant increase in tubulointerstitial fibrosis in prior pregnancy animals as compared with virgin animals (Fig. 4A–C). KIM-1 excretion was quantified as an indicator of proximal tubule damage and was also significantly increased in prior pregnancy rats (Fig. 4D). No significant differences in plasma creatinine levels were observed between the groups (data not shown).
Figure 1.
Renal injury is exacerbated in prior pregnancy animals compared to virgin animals. Urinary protein excretion was significantly increased in prior pregnancy animals (195.13 mg/day ± 44.77) compared to virgin animals (96.34 mg/day ± 20.26). * p<0.05 vs virgin. n=7–10/group
Figure 2.
Glomerular injury is exacerbated in prior pregnancy animals compared to virgin animals. Semi-quantitative histological analysis with glomerulosclerosis score showed increased glomerular injury in prior pregnancy animals. (A) Average glomerulosclerosis score on a 0–4 scale revealed increased objective estimation of glomerular injury (prior pregnancy: 3.16 ± 0.24; virgin: 2.34 ± 0.37, *p<0.05 vs virgin). (B) Histological image of virgin glomerulus. (C) Histological image of glomerulus after two pregnancies. n=4/group
Figure 3.
Urinary markers of glomerular injury showed increases in prior pregnancy animals compared to virgin animals. (A) Urinary nephrin excretion was significantly increased (prior pregnancy: 3.11 ug/day ± 0.92; virgin: 0.62 ug/day ± 0.37). (B) Urinary podocalyxin excretion was also significantly increased (prior pregnancy: 21.04 ug/day ± 7.57; virgin: 4.86 ug/day ± 1.02). * p<0.05 vs virgin. n=7–11/group
Figure 4.
Tubular injury is exacerbated in prior pregnancy animals compared to virgin animals. Tubulointerstitial injury profile after prior preeclamptic pregnancy in Dahl S rats: (A) Quantitative analysis of tubulointerstitial fibrosis showed increased fibrosis in prior pregnancy animals compared to virgin animals (prior pregnancy: 8.33% ± 1.08; virgin: 4.88% ± 1.19). (B) Histological image of virgin tubulointerstitium. (C) Histological image of tubulointerstitium after two pregnancies. (D) Kidney Injury Marker 1 (KIM-1) increase reflects increased renal injury after two pregnancies (prior pregnancy: 16.77 ng/day ± 3.90; virgin: 8.17 ng/day ± 1.45). * p<0.05 vs virgin. n=7–11/group
CD4+ T cells are increased in prior pregnancy animals compared to virgin animals.
One possible mechanism of kidney injury is immune cell infiltration [39]. Since our animals displayed no long-term blood pressure difference from virgin controls, we focused on potential immune cell contributions to kidney injury. Flow cytometric analyses revealed a significant increase in CD3+ T cells (Fig. 5B) and specifically CD3+/CD4+ T cells (Fig. 5C) in the kidney in prior pregnancy animals compared to virgin animals.
Figure 5.
CD4+ T cells are increased in prior pregnancy animals compared to virgin animals. (A) Representative plots of total T cells (CD3+), CD3+/CD4+ T cells and CD3+/CD8+ T Cells isolated from the kidney cortex of virgin and prior pregnancy animals. (B) Total T cells are significantly increased in the cortex of prior pregnancy animals (prior pregnancy: 21.26 ± 1.48; virgin: 12.90 ± 2.92). (C) Effector T cells are significantly increased in the cortex of prior pregnancy animals (prior pregnancy: 15.49 ± 1.56; virgin: 9.13 ± 1.95). * p<0.05 vs virgin. n=6–9/group
Pro- and anti-inflammatory cytokines are imbalanced in prior pregnancy animals compared to virgin animals.
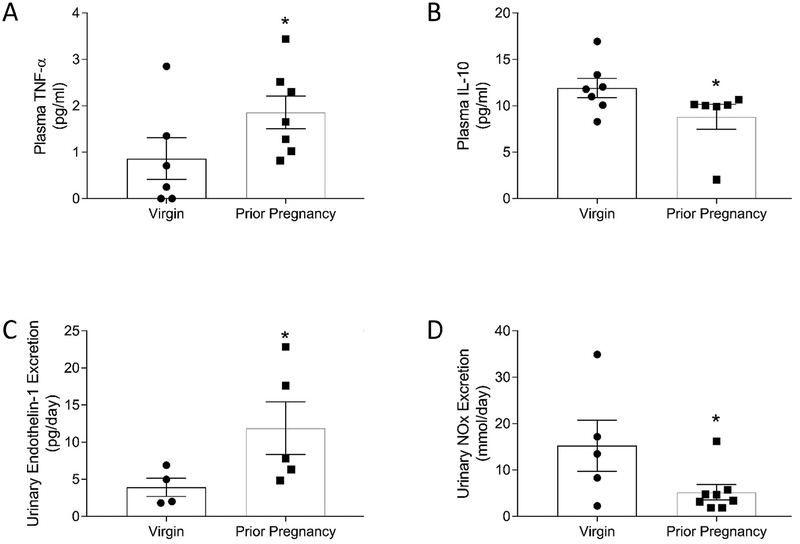
To further explore the systemic long-term immune changes following pregnancy plasma levels of TNF-α and IL-10 were measured. A significantly greater concentration of the pro-inflammatory cytokine TNF-α (Fig. 6A) and a lower level of the anti-inflammatory cytokine interleukin-10 (Fig. 6B) were observed, suggesting a postpartum imbalance between pro- and anti-inflammatory states at six months of age.
Figure 6.
There is dysregulation of the balance between pro- and anti-inflammatory cytokines and between nitric oxide (NO) and endothelin-1 (ET-1) in prior pregnancy animals compared to virgin animals. Plasma concentrations of tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) and urinary excretion rates of ET-1 and NO metabolites (NOx) in 6 month old virgin female Dahl S rats and rats following 2 pregnancies. Levels of the inflammatory cytokine TNF-α were greater (A) (prior pregnancy: 1.46 pg/mL ± 0.30; virgin: 0.77 pg/mL ± 0.32) while levels of the anti-inflammatory cytokine IL-10 were less (B) (prior pregnancy: 8.81 pg/mL ± 1.36; virgin: 11.93 ± 1.03) following pregnancy compared to age-matched littermates. (C) Urinary ET-1 was increased after prior pregnancy compared to virgin animals (prior pregnancy: 9.22 pg/day ± 2.99; virgin: 3.93 pg/day ± 1.23). (D) Urinary NOx (nitric oxide metabolite) excretion was significantly decreased in Dahl S rats after prior pregnancy (prior pregnancy: 5.22 mmol/day ± 1.64; virgin: 13.02 mmol/day ± 5.02). * p<0.05 vs virgin. n=4–7/group
Prior pregnancy rats have greater urinary excretion of ET-1 and lower NO metabolite excretion compared to virgin littermates.
Another potential mechanism contributing to the exacerbated renal injury of the prior pregnancy animals is dysregulation of the nitric oxide (NO)/endothelin-1 (ET-1) balance. Urinary excretion of ET-1 was significantly increased in prior pregnancy animals (Fig. 6C), but no difference in plasma concentration between these animals and virgin animals was observed (prior pregnancy: 0.51 pg/mL ± 0.07; virgin: 0.51 pg/mL ± 0.09, n=8–11/group, p=0.49), indicating greater renal production of ET-1 in prior pregnancy rats [40]. Prior pregnancy animals also showed a significant decrease in urinary excretion of NO metabolites (Fig. 6D).
Discussion
The salient findings from this study are that there is accelerated renal injury at six months of age in animals who have experienced two preeclamptic pregnancies compared to virgin controls in the context of chronic hypertension that occurs independently of blood pressure differences between the groups. We observed exacerbated glomerular and tubulointerstitial injury, supported by histological analysis and urinary measurements, in Dahl S rats following 2 pregnancies as compared to age-matched virgin littermates. We documented an increased percentage of T cells in renal tissue samples from prior pregnancy rats which is also reflected by an imbalance between pro- and anti-inflammatory cytokines in the systemic circulation. Finally, we established the presence of an imbalance between ET-1 and NO production in these animals with a decrease in in NO and an increase in ET-1 production.
Our data support the hypothesis that preeclampsia superimposed on chronic hypertension is an independent risk factor for later cardiovascular and renal disease due to the contribution of several mechanisms that are not yet well-defined. Preeclampsia and cardiovascular disease (CVD) share many risk factors and there has been controversy about whether preeclampsia itself is a risk factor or merely unmasks a pre-existing risk in this patient population. Higher pre-pregnancy blood pressure or pre-existing hypertension correlates with increased odds ratio (OR) of preeclampsia and with poorer postpartum outcomes compared to history of preeclampsia alone [41]. However, the risk for preeclampsia cannot be fully explained by pre-existing cardiovascular risk, and several studies have demonstrated that the risk persists even after adjusting for typical cardiovascular risk factors [36,37]. Van Rijn et al. described an association of preeclampsia with later cardiovascular risk but also supported the involvement of traditional risk factors which are exacerbated by preeclampsia to enhance postpartum disease progression [42]. The fact remains that women who have experienced preeclampsia are predisposed both to repeated episodes of preeclampsia in future pregnancies and to other CVD events in the postpartum period [43]. Therefore, the first goal of this study was to determine if preeclamptic pregnancy would accelerate the progression of renal disease in the Dahl S rat, a well-established model of hypertension and CKD that also spontaneously develops a superimposed preeclamptic phenotype during pregnancy [31].
As expected, we observed hypertension and renal injury in virgin female Dahl S rats at 6 months of age consistent with age-dependent development reported for this strain[44]. We did not observe any effect of prior pregnancy on long-term control of blood pressure in this strain; however, the physiologic changes we observed may affect BP in the context of a “second hit” such as dietary insults (high salt or high fat), increased angiotensin II sensitivity, or menopause and loss of protective ovarian hormones. Future studies will assess sensitivity to these stressors in this model. Even in the absence of any difference in MAP, our results demonstrate a detrimental renal phenotype after two preeclamptic pregnancies that differentiate these animals from their virgin littermate counterparts. This is in contrast to a similar study performed following three successive pregnancies in the spontaneously hypertensive rat (SHR), a model of genetic hypertension that does not display a preeclamptic phenotype during pregnancy. Work by Chris Baylis demonstrated that there are no differences in renal function between virgin animals and those having experienced three prior pregnancies at 45 weeks of age. In addition, neither virgin or prior pregnancy SHR developed proteinuria despite an age-dependent increase in arterial pressure, which also did not differ between groups [45]. Therefore, the degree of renal injury observed in these Dahl S postpartum rats must be due to additional mechanisms other than the isolated pregnancy-related increase in blood pressure.
An increase in renal T cells, and specifically in CD4+ T cells, supports evidence by Arriaga, et al. [14] that there may exist an imbalance between subpopulations of CD4+ T cells favoring pro-inflammatory cytokine-secreting cells over more beneficial, tolerance-producing cells during preeclamptic pregnancy. Evidence suggests that these cells remain in the kidney and other organs subject to hypertensive damage and retain a “memory” of the initial insult which sensitizes individuals to exacerbated end-organ damage on secondary insults [15]. For example, Xue et al. have demonstrated the ability of a subpressor dose of angiotensin II (Ang II) to sensitize the hypertensive response to subsequent doses of Ang II [46]. Continued secretion of pro-inflammatory cytokines such as TNF-α from these memory cells and a lack of protective cytokines such as IL-10 from regulatory immune cells promote immune cell infiltration and remodeling of the vasculature throughout the body. This detrimental cytokine profile results in continuous renal damage and increasing risk for cardiovascular and renal disease [7]. Future investigations will focus on more specific classification of the infiltrating T cell populations in the kidney of postpartum rats and investigate the effects of manipulating these cell populations on long-term renal health.
Cytokines may also play a role in stimulating production of endothelium-derived contracting factors such as ET-1, exacerbating the NO/ET-1 imbalance [47]. Chronic infusion of the pro-inflammatory cytokine IL-1β significantly increases plasma levels of ET-1 through increased gene expression and possibly also through increased endothelin converting enzyme (ECE) activity [48]. Conversely, neutralization of TNF-α is shown to reduce arterial blood pressure, renal inflammation and fibrosis, as well as reducing ET-1 concentration and improving NO release [49]. Thus, infiltration of immune cells, which is shown to be increased during and after preeclamptic pregnancy [50], and concurrent secretion of pro-inflammatory cytokines such as TNF-α contribute to endothelial dysfunction of cardiovascular and chronic kidney diseases.
ET-1 has been implicated in many facets of cardiovascular disease, including hypertension, atherosclerosis, and chronic kidney disease (CKD), and blockade of ET receptors increases renal blood flow (RBF) and preserves glomerular filtration rate (GFR) in animal models of CKD. Renal ET-1 mRNA expression and urinary excretion of ET-1 correlate with extent of kidney damage and proteinuria, and upregulation of ET pathways induces tubulointerstitial damage independent of any BP change [51]. Previous studies have demonstrated that urinary ET-1 levels reflect renal production of ET-1 and that increased ET-1 excretion is a marker of renal injury [40,52]. Within normal renal physiology, an elevation of ET-1 is usually accompanied shortly thereafter by increased production of NO, followed shortly by increased excretion of NO metabolites. In this manner, ET-1 and NO share a synergistic relationship to maintain endothelial health and appropriate renal blood flow [53]. However, in spite of elevated urinary excretion of ET-1, postpartum Dahl S rats demonstrated a decreased NOx excretion. This suggests that there may be an imbalance between NO and ET-1 production which may contribute to renovascular and tubulointerstitial damage in these animals. Future studies will more closely examine the imbalance in this relationship, determine the origin of increased ET-1 and decreased NO production, and determine the efficacy of ET receptor blockade on the progression of CKD following preeclampsia.
Based on these results, we suggest a link between the maternal syndrome of superimposed preeclampsia and the increased postpartum risk of chronic kidney disease through increased T cell infiltration in the kidney and decreased NO: ET-1 ratio. Further studies are necessary to investigate the potential role of endothelin blockade in the progression of CKD and to further explore the subpopulations of T cells in the kidney postpartum. Furthermore, it will be important to determine if blood pressure control during preeclamptic pregnancy can reduce the risk of future CKD, and studies are ongoing to determine the efficacy of antihypertensive therapies during pregnancy in this model to slow the progression of renal injury.
Perspectives
Preeclampsia is a widespread and serious complication of pregnancy which bestows a large healthcare burden on women and their families worldwide [1,43]. Limited therapeutic interventions exist, in part due to lack of knowledge concerning the etiology and effects of preeclampsia, before, during, and after gestation. Our laboratory has previously characterized the Dahl S rat as a spontaneous model of superimposed preeclampsia [31], which is a key step towards uncovering the etiology of and proposing novel therapeutic targets for preeclampsia. This project provides a necessary foundation for such work by detailing the profile of postpartum renal injury present after preeclampsia in the setting of chronic hypertension and identifying potential mechanisms involved. Defining postpartum cardiovascular and renal risk factors will allow us to improve recommendations for intrapartum and postpartum treatment regimens to improve the health of preeclamptic mothers.
Supplementary Material
Novelty and Significance.
What is new?
This paper proposes and provides evidence for a role of the NO: ET-1 balance and infiltration of T cells as mechanisms for kidney injury after multiple preeclamptic pregnancies.
What is relevant?
A woman who has had preeclampsia once is more likely to have preeclampsia with subsequent pregnancies, yet the effect of multiple preeclamptic pregnancies on the resultant deficits in kidney function has not been investigated. Additionally, though postpartum effects of preeclampsia have been studied previously, the relevant mechanisms for the changes seen have not yet been elucidated.
Summary
In summary, this paper combines a more longitudinal and translational view of postpartum renal health, which may more closely approximate that of a woman with more than one child born of a preeclamptic pregnancy, with supporting evidence for the relevant and underlying mechanisms.
Acknowledgments
We thank Ashley Johnson, Divya Patel, Jennifer Mooney, and Joshua Jefferson for expert technical assistance.
Sources of Funding
Research reported in this publication was supported by the National Institute of Health under award numbers R01HL134711 (J.M. Sasser), P20GM104357 (J.M. Sasser, pilot project), F30DK118864 (H.R. Turbeville), F32HL137393 (E.B. Taylor), R01HL136684 (M.J. Ryan) and R01HL137673 (M.R. Garrett). The content is solely the responsibility of us and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Dean Franklin Young Investigator Award from Data Sciences International/American Physiological Society (J.M. Sasser).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open [Internet]. 2011;1(1):e000101 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22021762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy, American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Washington, DC: American College of Obstetricians and Gynecologists; 2013. x, 89 pages. [DOI] [PubMed] [Google Scholar]
- 3.Paauw ND, Luijken K, Franx A, Verhaar MC, Lely AT. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci [Internet]. 2016;130(4):239–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26769659 [DOI] [PubMed] [Google Scholar]
- 4.Pruthi D, Khankin EV, Blanton RM, Aronovitz M, Burke SD, McCurley A, et al. Exposure to experimental preeclampsia in mice enhances the vascular response to future injury. Hypertens (Dallas, Tex 1979). 2015. April;65(4):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bytautiene E, Bulayeva N, Bhat G, Li L, Rosenblatt KP, Saade GR. Long-term alterations in maternal plasma proteome after sFlt1-induced preeclampsia in mice. Am J Obstet Gynecol. 2013. May;208(5):388.e1–388.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaMarca B, Cornelius D, Wallace K. Elucidating Immune Mechanisms Causing Hypertension During Pregnancy. Physiology [Internet]. 2013. July 1;28(4):225–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23817797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, et al. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol - Regul Integr Comp Physiol [Internet]. 2016/04/22. 2016. July 1;311(1):R1–9. Available from: http://ajpregu.physiology.org/lookup/doi/10.1152/ajpregu.00052.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and Long-Term Changes in Plasma Inflammatory Markers Associated With Preeclampsia. Hypertension [Internet]. 2004. November 1;44(5):708 LP–714. Available from: http://hyper.ahajournals.org/content/44/5/708.abstract [DOI] [PubMed] [Google Scholar]
- 9.Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens pregnancy. 2009;28(3):300–11. [DOI] [PubMed] [Google Scholar]
- 10.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009. December;183(11):7023–30. [DOI] [PubMed] [Google Scholar]
- 11.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, et al. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015. October;309(8):R884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargent IL, Borzychowski AM, Redman CWG. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2007;14 Spec No:111–7. [DOI] [PubMed] [Google Scholar]
- 13.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999. September;163(6):3491–5. [PubMed] [Google Scholar]
- 14.Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The Predominant Th1 Cytokine Profile in Maternal Plasma of Preeclamptic Women Is Not Reflected in the Choriodecidual and Fetal Compartments. J Soc Gynecol Investig [Internet]. 2005. July 28;12(5):335–42. Available from: http://journals.sagepub.com/doi/10.1016/j.jsgi.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Itani HA, Harrison DG. Memories that last in hypertension. Am J Physiol Ren Physiol [Internet]. 2015;308(11):F1197–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25834073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillis EE, Sullivan JC. Sex Differences in Hypertension: Recent Advances. Vol. 68, Hypertension (Dallas, Tex. : 1979). 2016. p. 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial Function and Circulating Biomarkers Are Disturbed in Women and Children After Preeclampsia. Hypertension [Internet]. 2011. July 1;58(1):63–9. Available from: http://hyper.ahajournals.org/cgi/doi/10.1161/HYPERTENSIONAHA.111.172387 [DOI] [PubMed] [Google Scholar]
- 18.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest. 1995. July;96(1):482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng A, Engels K, Baylis C. Impact of nitric oxide deficiency on blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int. 1996. October;50(4):1132–8. [DOI] [PubMed] [Google Scholar]
- 20.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, et al. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J Off Publ Fed Am Soc Exp Biol. 1993. April;7(6):566–71. [PubMed] [Google Scholar]
- 21.Wight E, Kung CF, Moreau P, Takase H, Luscher TF. Chronic blockade of nitric oxide-synthase and endothelin receptors during pregnancy in the rat: effect on pregnancy outcome. J Soc Gynecol Investig. 1998;5(3):132–9. [DOI] [PubMed] [Google Scholar]
- 22.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol. 2005. December;1(2):98–114; quiz 120. [DOI] [PubMed] [Google Scholar]
- 23.Sasser JM, Murphy SR, Granger JP. Emerging drugs for preeclampsia--the endothelium as a target Vol. 20, Expert opinion on emerging drugs. England; 2015. p. 527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mildenberger E, Biesel B, Siegel G, Versmold HT. Nitric oxide and endothelin in oxygen-dependent regulation of vascular tone of human umbilical vein. Am J Physiol Heart Circ Physiol. 2003. October;285(4):H1730–7. [DOI] [PubMed] [Google Scholar]
- 25.Verdonk K, Saleh L, Lankhorst S, Smilde JEI, van Ingen MM, Garrelds IM, et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertens (Dallas, Tex 1979). 2015. June;65(6):1316–23. [DOI] [PubMed] [Google Scholar]
- 26.Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertens (Dallas, Tex 1979). 2000. June;35(6):1237–41. [DOI] [PubMed] [Google Scholar]
- 27.Raoch V, Rodriguez-Pascual F, Lopez-Martinez V, Medrano-Andres D, Rodriguez-Puyol M, Lamas S, et al. Nitric oxide decreases the expression of endothelin-converting enzyme-1 through mRNA destabilization. Arterioscler Thromb Vasc Biol. 2011. November;31(11):2577–85. [DOI] [PubMed] [Google Scholar]
- 28.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004. May;286(5):L984–91. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg FC, Wolf K, Sandner P, Lorenz C, Riegger GA, Pfeifer M. The NO donor molsidomine reduces endothelin-1 gene expression in chronic hypoxic rat lungs. Am J Physiol Lung Cell Mol Physiol. 2001. February;280(2):L258–63. [DOI] [PubMed] [Google Scholar]
- 30.Ohkita M, Takaoka M, Sugii M, Shiota Y, Nojiri R, Matsumura Y. The role of nuclear factor-kappa B in the regulation of endothelin-1 production by nitric oxide. Eur J Pharmacol. 2003. July;472(3):159–64. [DOI] [PubMed] [Google Scholar]
- 31.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015. July;309(1):R62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takushima S, Nishi Y, Nonoshita A, Mifune H, Hirata R, Tanaka E, et al. Changes in the nitric oxide-soluble guanylate cyclase system and natriuretic peptide receptor system in placentas of pregnant Dahl salt-sensitive rats. J Obstet Gynaecol Res. 2015. April;41(4):540–50. [DOI] [PubMed] [Google Scholar]
- 33.Sasser JM, Baylis C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am J Physiol Regul Integr Comp Physiol. 2010. February;298(2):R433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allcock GH, Venema RC, Pollock DM. ETA receptor blockade attenuates the hypertension but not renal dysfunction in DOCA-salt rats. Am J Physiol. 1998. July;275(1 Pt 2):R245–52. [DOI] [PubMed] [Google Scholar]
- 35.Sasser JM, Molnar M, Baylis C. Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N(omega)-nitro-L-arginine methyl ester hypertensive rats. Hypertens (Dallas, Tex 1979). 2011. August;58(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garovic VD, Bailey KR, Boerwinkle E, Hunt SC, Weder AB, Curb D, et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. Vol. 28, Journal of hypertension. 2010. p. 826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald SD, Yusuf S, Walsh MW, Lonn E, Teo K, Anand SS, et al. Increased cardiovascular risk after pre-eclampsia in women with dysglycaemia. Diabet Med. 2013. January;30(1):e1–7. [DOI] [PubMed] [Google Scholar]
- 38.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984. August;26(2):137–43. [DOI] [PubMed] [Google Scholar]
- 39.Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease--what have we learned in 10 years? Semin Dial. 2010;23(5):498–509. [DOI] [PubMed] [Google Scholar]
- 40.Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertens (Dallas, Tex 1979). 1992. July;20(1):89–95. [DOI] [PubMed] [Google Scholar]
- 41.Scantlebury DC, Hayes SN. How does preeclampsia predispose to future cardiovascular disease? Curr Hypertens Rep. 2014. September;16(9):472. [DOI] [PubMed] [Google Scholar]
- 42.van Rijn BB, Nijdam M-E, Bruinse HW, Roest M, Uiterwaal CS, Grobbee DE, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol. 2013. May;121(5):1040–8. [DOI] [PubMed] [Google Scholar]
- 43.Enkhmaa D, Wall D, Mehta PK, Stuart JJ, Rich-Edwards JW, Merz CNB, et al. Preeclampsia and Vascular Function: A Window to Future Cardiovascular Disease Risk. J Womens Health (Larchmt). 2016. March;25(3):284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pai AV, West CA, Arlindo de Souza AM, Cheng X, West DAJ, Ji H, et al. Salt-sensitive (Rapp) Rats from Envigo Spontaneously Develop Accelerated Hypertension Independent of Ovariectomy on a Low Sodium Diet. Am J Physiol Regul Integr Comp Physiol. 2018. July; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Immediate Baylis C. and long-term effects of pregnancy on glomerular function in the SHR. Am J Physiol. 1989. December;257(6 Pt 2):F1140–5. [DOI] [PubMed] [Google Scholar]
- 46.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertens (Dallas, Tex 1979). 2012. February;59(2):459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Possomato-Vieira JS, Khalil RA. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv Pharmacol. 2016;77:361–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, et al. Interleukin-1β, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Vol. 295, American Journal of Physiology - Renal Physiology. 2008. p. F446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Therrien FJ, Agharazii M, Lebel M, Larivière R. Neutralization of Tumor Necrosis Factor-Alpha Reduces Renal Fibrosis and Hypertension in Rats with Renal Failure. Am J Nephrol. 2012;36(2):151–61. [DOI] [PubMed] [Google Scholar]
- 50.Taylor EB, Sasser JM. Natural killer cells and T lymphocytes in pregnancy and pre-eclampsia Vol. 131, Clinical science (London, England : 1979). England; 2017. p. 2911–7. [DOI] [PubMed] [Google Scholar]
- 51.Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006. April;17(4):943–55. [DOI] [PubMed] [Google Scholar]
- 52.Ohta K, Hirata Y, Shichiri M, Kanno K, Emori T, Tomita K, et al. Urinary excretion of endothelin-1 in normal subjects and patients with renal disease. Kidney Int. 1991. February;39(2):307–11. [DOI] [PubMed] [Google Scholar]
- 53.Zeng Y, Li M, Chen Y, Wang S. Homocysteine, endothelin-1 and nitric oxide in patients with hypertensive disorders complicating pregnancy. Vol. 8, International Journal of Clinical and Experimental Pathology. 2015. p. 15275–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.