Abstract
Introduction
The prevalence of Clostridium difficile infection is rapidly increasing worldwide, but prevalence is difficult to estimate in developing countries where awareness, diagnostic resources, and surveillance protocols are limited. As diarrhea is the hallmark symptom, we conducted a systematic review and meta-analysis to determine the prevalence and incidence of C. difficile infection in patients in these regions who presented with diarrhea.
Methods
We conducted a systematic literature search of MEDLINE/PubMed, Scopus, and Latin-American and Caribbean Health Sciences Literature databases to identify and analyze data from recent studies providing prevalence or incidence rates of C. difficile-associated diarrhea in developing countries within four regions: Africa–Middle East, developing Asia, Latin America, and China. Our objectives were to determine the current prevalence and incidence density rates of first episodes of C. difficile-associated diarrhea in developing countries.
Results
Within the regions included in our analysis, prevalence of C. difficile infection in patients with diarrhea was 15% (95% CI 13–17%) (including community and hospitalized patients), with no significant difference across regions. The incidence of C. difficile infection in 17 studies including this information was 8.5 per 10,000 patient-days (95% CI 5.83–12.46). Prevalence was significantly higher in hospitalized patients versus community patients (p = 0.0227).
Conclusion
Our prevalence estimate of 15% is concerning; however, low awareness and inconsistent diagnostic and surveillance protocols suggest this is markedly underestimated. Enhanced awareness and management of C. difficile infection in patients with diarrhea, along with improvements in infection control and surveillance practices, should be implemented to reduce prevalence of C. difficile-associated diarrhea in developing countries.
Funding
Pfizer Inc.
Electronic supplementary material
The online version of this article (10.1007/s40121-019-0231-8) contains supplementary material, which is available to authorized users.
Keywords: Clostridium difficile infection, C. difficile-associated diarrhea, Developing countries, Nosocomial diarrhea
Introduction
The incidence of Clostridium difficile infection has greatly increased in recent years as new strains have emerged and antimicrobial resistance has increased (i.e., hypervirulent C. difficile ribotype 027 and ribotype 078) [1–7]. Precise global epidemiologic data are difficult to obtain, due to continually changing prevalence data and variations in surveillance practices. One recent US analysis estimated 453,000 C. difficile cases, 29,000 deaths, and a healthcare-related incidence of 92.8 per 100,000 persons [8]. European epidemiological data indicate wide variation in reported incidence of C difficile infection [9]. The survey of hospitalized patients in 97 institutions reported a weighted mean incidence of 4.1 per 10,000 patient-days, with individual country rates ranging from 0 to 36.3. In Latin America, Asia, and Africa, recent and comprehensive epidemiologic data on C. difficile infection are limited [1, 10].
Commonly associated with antibiotic use [4, 11–13], C. difficile was previously regarded primarily as a nosocomial infection [11, 14]; however, community-acquired C. difficile has now emerged as a significant public health threat [3, 8, 11, 14–16]. The epidemiology of community-acquired infection is not well understood [17]. While both nosocomial and community-acquired infection can be severe and even fatal, patients with community-acquired infection often do not have the risk factors (e.g., age ≥ 65, treatment with antibiotics, certain comorbidities) known to be associated with nosocomial infection [11, 13, 14, 17, 18].
Clostridium difficile-associated diarrhea (CDAD) is the hallmark symptom of clinical infection and can range in severity from mild diarrhea to fulminant colitis [12, 15]. C. difficile is recognized as a leading cause of healthcare-associated diarrhea [11, 19] and as the main contributing factor in gastroenteritis-associated hospitalizations and deaths [12, 20]. Mortality associated with CDAD is high, particularly in patients ≥ 65 years with comorbid conditions, severe disease, or hypervirulent strains [21].
In developing countries, surveillance data on C. difficile infection are not readily available, likely due to limitations in awareness, laboratory capacity and capabilities, and surveillance systems [22–24]. A recent review of the burden of C. difficile infection in developing countries noted that patients with diarrhea are not routinely tested for this pathogen, and, when tested, it is very often with enzyme immunoassay (EIA) rather than stool culture [25]. Clinical Practice Guidelines for C. difficile note that EIA is less sensitive than stool culture and is therefore an inferior alternative [11, 19]. As C. difficile has become resistant to many antimicrobials [1, 10], prevention of infection through the implentation of infection control and hospital epidemiology programs must be a priority [11].. With a better understanding of the epidemiologic trends related to CDAD, enhanced approaches to the prevention and control of C. difficile infection and associated diarrhea may be achieved. The primary objective of this systematic review and meta-analysis was to determine the current prevalence of CDAD first episodes (nosocomial or community-acquired) in developing countries. The secondary objective was determination of hospital incidence rates (cases per 10,000 patient-days) within the same case parameters.
Methods
Search Strategy and Selection Criteria
We conducted a systematic literature search of the MEDLINE/PubMed, Scopus, and Latin-American and Caribbean Health Sciences Literature (LILACS) databases for studies providing prevalence or incidence rates of CDAD in developing countries. While there is no uniformly adopted definition of “developing countries,” it is generally accepted that this classification includes low- to middle-income countries. For the purposes of our analysis, we included such countries from four different regions: Africa–Middle East (AfME), developing Asia (Asia), Latin America (LATAM), and China. The full list of countries/territories is in Table 1.
Table 1.
Developing countries included in this analysis
|
Africa–Middle East (AfME) Bahrain Egypt Iraq Kuwait Lebanon Oman Qatar Saudi Arabia South Africa Tunisia United Arab Emirates Iran Ghana Zimbabwe Developing Asia (ASIA) Hong Kong India Indonesia Malaysia Pakistan Philippines Singapore Taiwan Thailand |
Latin America (LATAM) Argentina Bolivia Brazil Caribbean Region Chile Colombia Costa Rica Ecuador El Salvador Guatemala Honduras Mexico Nicaragua Panama Paraguay Peru Uruguay Venezuela |
| China |
To quantify the current burden, our search was limited to publications from January 2000 to December 2017. No language restrictions were applied; however, we required that at least the abstract of a paper be available in English in order for it to be included in our full analysis. Details of our search strategy, including specific search terms and strings, are described in the Supplementary Materials.
To capture both nosocomial and community-acquired CDAD, the selected studies included cases in hospitalized patients and/or outpatients. We also considered factors such as whether the cases occurred during a known outbreak period, and age groups covered by the studies (adult, pediatric, or both). Clinical data were extracted from each study for analysis.
Review articles and other publications citing data from more than one study were not included; however, their citations were used to identify individual studies that had not already been identified in the literature search. Studies were included if they provided prevalence (proportion) data and/or estimated incidence density rates (cases per 10,000 patient-days). Studies were excluded if they included only recurrent or asymptomatic cases, if there was evidence of explicit selection bias (e.g., unclear denominator), or if they considered rates of admissions and/or discharges (unless they also considered patient-days).
The decision for inclusion of each study was made by Drs. Fernández and Correa, functioning as independent reviewers and screening the studies by title and abstract. Resolution of any differences was determined by Dr. Curcio.
Assessment of Study Quality
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model [26] in structuring our literature analysis. In the context of our meta-analysis, the main source of quality variation results from the biases introduced during the selection of patients (e.g., when the denominator is smaller than the sum of all symptomatic cases). Because the presence of this type of selection bias has been used as an exclusion criterion in our analysis, it is assumed that the studies we selected are relatively homogeneous in quality.
Statistical Analysis
For the primary analysis of prevalence of CDAD, studies that provided information on the proportion of occurrence were included. The secondary objective of incidence density rate (cases per 10,000 patient-days) was analyzed on the studies providing incidence data. A subanalysis was also carried out on the prevalence data, stratifying studies across the four specified geographic regions (Table 1). An overall effect-size analysis was also performed using the incidence density rate data.
The potential for publication bias was evaluated using a funnel plot as a graphical method to determine the trials’ effect estimates of effect size against the measure of precision for all included studies [27]. We also utilized the Egger linear regression test for funnel plot asymmetry, which may be indicative of bias in meta-analyses [28].
A meta-regression analysis, which is generally performed to identify relationships between dependent and independent variables across studies and/or subgroups [29], was conducted relevant to the route of C. difficile acquisition, geographic regions, occurrence during known outbreaks, and age groups. For those covariates demonstrating statistical significance in the meta-regression analysis, a subanalysis was conducted.
Because a proportion as an effect to be measured raises particular concerns, a funnel plot was used for the meta-analysis to stabilize variances across studies prior to pooling the data [30, 31]. In contrast, the logarithmic transformation of the variable was used to analyze the incidence density values [32]. The random effects model was utilized for the global effect size when significant heterogeneity across studies was shown.
For the statistical analysis of the values expressed as a proportion, the Metaprop package in R software (v.3.4.2) was used. For the analysis of the values expressed as incidence, the implementation of the transformation suggested by Stijnen et al. [32] was used from the Metafor package of the same R software.
Compliance with Ethics Compliance
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Literature Selection
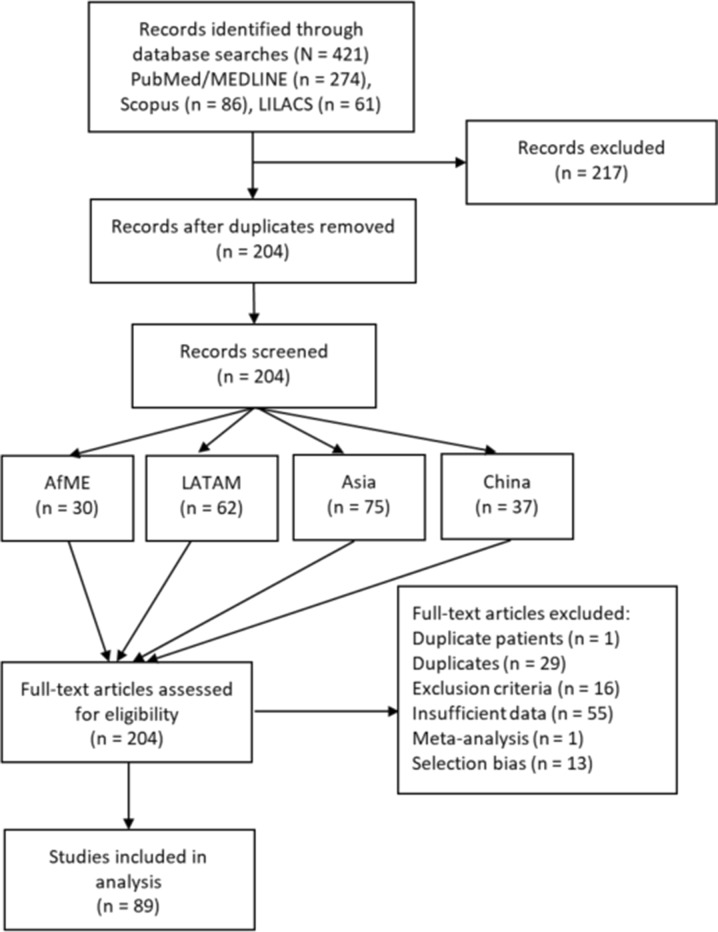
As shown in Fig. 1, the initial literature search in MEDLINE/PubMed, Scopus, and LILACS yielded 421 results, 51 of which were identified from a meta-analysis conducted by Borren and colleagues [33]. After removal of duplicate titles and abstracts, 204 full-text publications were screened for inclusion in the analysis, with the greatest number of articles pertaining to Asia (n = 75). Reviews of full-text articles resulted in another 115 exclusions, giving a total of 89 articles were selected for inclusion in our systematic review and meta-analysis.
Fig. 1.
Flowchart of articles considered for inclusion in analysis
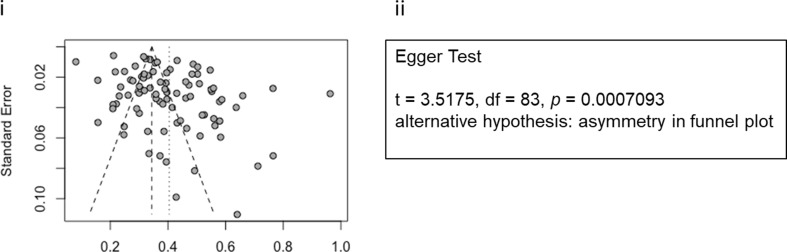
Publication Bias Analysis
The funnel plot and the Egger test for funnel plot asymmetry (p < 0.001) both demonstrated significant publication bias, indicating a high variability across the studies included in our analysis (Fig. 2).
Fig. 2.
Publication bias assessment demonstrates high variability across studies. (i) Funnel plot; lack of simmetry in points around center of the graph indicates publication bias. (ii) Egger test [28]; the very small p value offers evidence pointing in the same direction
Meta-Analysis
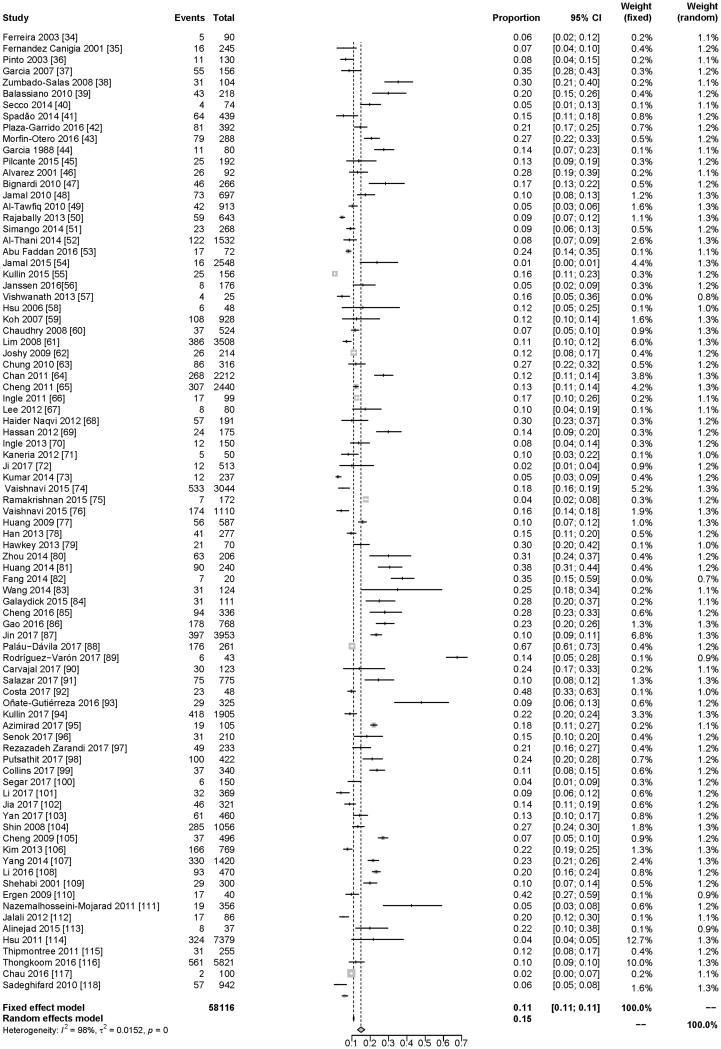
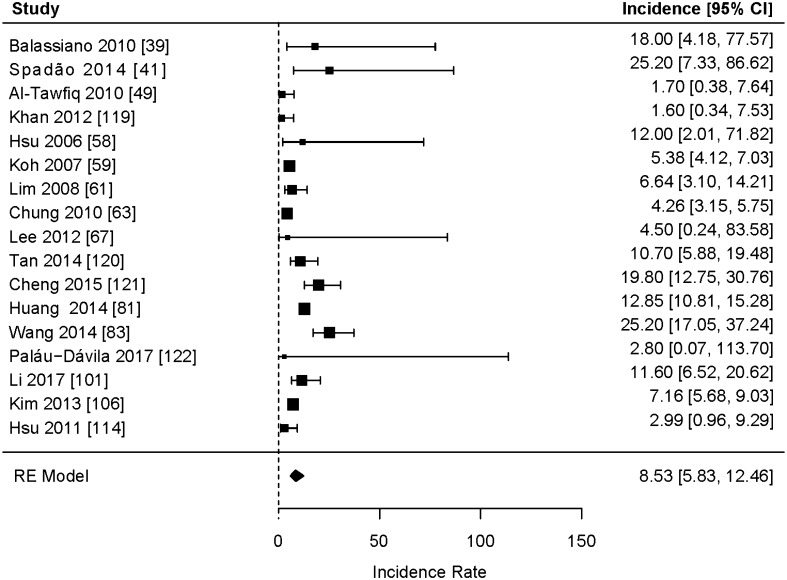
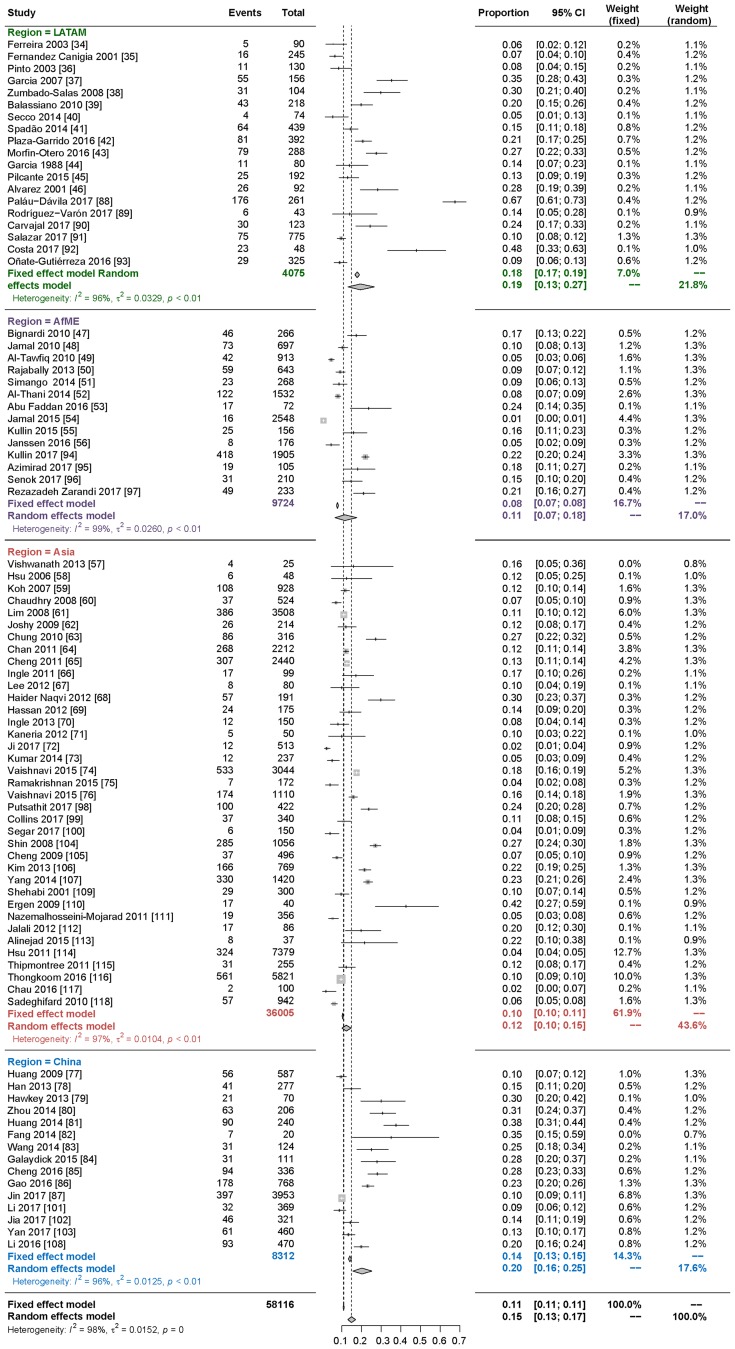
Based on the 85 studies in this meta-analysis that included prevalence data (Fig. 3) [34–118], among patients in developing countries with diarrhea, a first episode of C. difficile infection was determined to be the cause in 15% of cases (95% CI 13–17%) (including community and hospitalized patients). In the 17 studies that included incidence data (Fig. 4), the incidence density rate of C. difficile infection among patients with diarrhea was 8.5 per 10,000 patient-days (95% CI 5.83–12.46).
Fig. 3.
Meta-analysis of prevalence of CDAD based on the 85 studies including prevalence data
Fig. 4.
Meta-analysis of incidence of CDAD based on the 17 studies including incidence data
A stratified subanalysis of the prevalence studies by geographic regions (Fig. 5) showed that, among patients with diarrhea, a first episode of C. difficile infection was present in 19% in LATAM (95% CI 13–27%), 11% in AfME (95% CI 7–18%), 12% in Asia (95% CI 10–15%), and 20% in China (95% CI 16–25%). There were no statistically significant differences across regions.
Fig. 5.
Prevalence of CDAD stratified by region
Meta-Regression Analysis
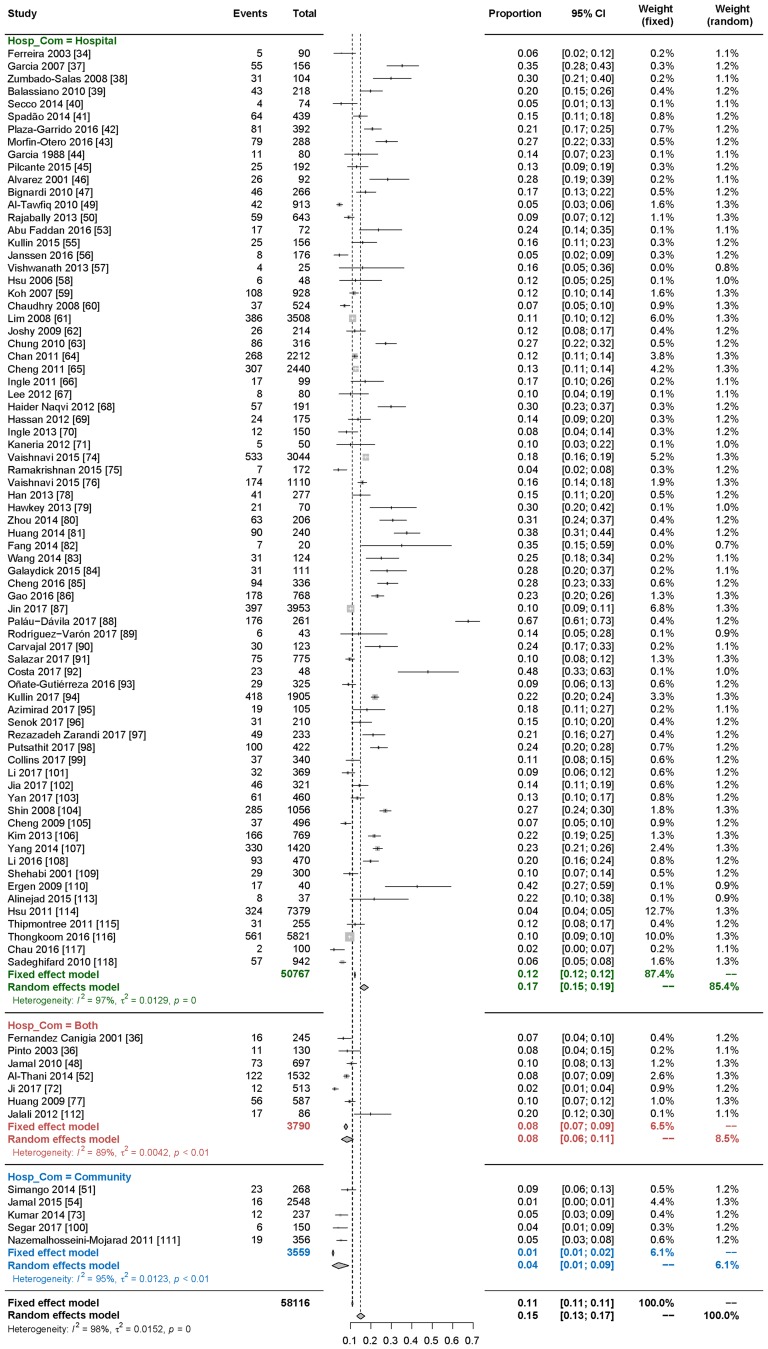
The only covariate demonstrating statistical significance in the meta-regression analysis was the route of CDAD acquisition (Table 2). In studies including only hospitalized patients, the values of the ratio were significantly higher than in studies including community-acquired cases or both nosocomial and community-acquired [p = 0.0227 (95% CI 0.0165–0.2196)]. Based on this finding, a subanalysis of prevalence by route of acquisition was conducted on studies including acquisition data. As shown in Fig. 6, among patients presenting with diarrhea, C. difficile infection was significantly more prevalent in studies involving hospitalized patients [17% (95% CI 15–19%)] versus studies including community patients [4% (95% CI 1–9%)] or both [8% (95% CI 6–11%)]. While we intended to examine CDAD prevalence and incidence by age, we found very few studies that clearly stratified by age groups. Our subgroup meta-analysis showed a prevalence of 12% for pediatric patients, and 16% prevalence for adults; however, the scarcity of pediatric data made it impossible to determine an age-related trend.
Table 2.
Summary of meta-regression analysis
| Variable | Value | Coefficient | 95% CI | p value |
|---|---|---|---|---|
| Route of acquisition (ref = Both) | Community | − 0.0600 | − 0.2088 to 0.0888 | 0.4290 |
| Hospital | 0.1181 | 0.0165 to 0.2196 | 0.0227 | |
| Outbreak (ref = No) | Yes | − 0.0265 | − 0.2860 to 0.2329 | 0.8413 |
| Age group (ref = Adult) | Both | − 0.0463 | − 0.1280 to 0.0355 | 0.2676 |
| Pediatric | − 0.0832 | − 0.2267 to 0.0603 | 0.2556 | |
| Unknown | − 0.2065 | − 0.4719 to 0.0589 | 0.1272 | |
| Region (ref = AfME) | Asia | − 0.0345 | − 0.1184 to 0.0494 | 0.4205 |
| China | 0.0518 | − 0.0508 to 0.1543 | 0.3226 | |
| LATAM | 0.0611 | − 0.0367 to 0.1589 | 0.2209 |
Coefficients, confidence intervals for coefficients and p values for variables in meta regression. Studies including exclusively hospital-acquired CDAD showed a significant positive value. The remaining variables had non-significant coefficients
Fig. 6.
Subanalysis of significant covariate: prevalence by mode of CDAD acquisition
Discussion
In this meta-analysis of CDAD in developing countries, we found 15% of diarrhea cases to be attributable to C. difficile infection (including community and hospitalized patients). The proportions were highest in LATAM and China, but there was no significant difference across regions. This study outcome was specific to determining the cause of diarrhea in patients presenting with this symptom. Due to inconsistencies in study designs, comparisons to other parts of the world are not always easily drawn, and lower prevalences of C. difficile infection in patients with diarrhea have been observed (range 7.4–12.7%). For example, in a prospective analysis of 4659 fecal samples from inpatients in the United Kingdom with suspected antibiotic-associated diarrhea, C. difficile was determined to be the causative pathogen in 12.7% of cultures [119]. A Berlin study that included analysis of 693 cultures from inpatients with antibiotic-associated diarrhea detected C. difficile in 11.4% of samples [120]. A smaller study conducted at a major hospital in Houston, Texas, USA, found C. difficile to be the causative pathogen in 7.4% of 81 inpatients experiencing diarrhea [121]. Based on these limited data, the prevalence of CDAD is higher in the developing countries included in our study.
Similarly, the incidence of C. difficile infection among patients with diarrhea in developing countries, which we determined to be 8.5 per 10,000 patient-days (95% CI 5.56–11.79), is not easily compared to incidence rates in more developed regions such as the US and Europe. However, this undoubtedly low incidence rate is approximately twice that reported in Europe. The hospital-based European survey noted above resulted in a weighted incidence of 4.1 per 10,000 patient-days, with considerable variation in individual country rates [9]. It was noted that factors such as diagnostic procedures and capabilities were inconsistent, making it difficult to compare epidemiologic trends across countries within this survey. US data from the 2011 survey referenced above indicate a mean crude incidence of 9.3 and 4.8 per 10,000 persons for healthcare-associated and community-acquired C. difficile infection, respectively [8]. A report from the US Centers for Disease Control and Prevention estimated the mean crude incidence for C. difficile infection in 2015 to be 14.9 per 10,000 persons (8.3 and 6.6 for healthcare-associated and community-acquired infection, respectively) [122]. While the US rate of 14.9 per 10,000 persons appears to be comparable to that of developing countries in our analysis, we must recognize that we would expect reported rates to be higher in developed regions where C. difficile infection is more proactively assessed and managed.
Although we found no statistically significant differences in prevalence across regions, due to numerous factors related to diagnosis and management, it may be reasonably assumed that prevalence might be underestimated. It has been observed that awareness of C. difficile as a cause of diarrhea is relatively low in developing regions such as Asia and Latin America [22, 123]. For example, a 2015 survey of physicians in Latin American countries reported low-to-moderate knowledge of C. difficile diagnosis and management [124]. Similarly, a survey of physicians in Taiwan found marked differences between internationally accepted treatment guidelines for C. difficile and actual clinical practice [125]. In addition, diagnostic capacity and capabilities in developing countries are generally suboptimal [22, 23, 124, 126]. Thus, CDAD is likely under-recognized and insufficiently managed in these regions.
Our meta-regression analysis found the route of acquisition to be the only prespecified variable to demonstrate a statistically significant difference, with the value of the ratio for CDAD being higher in hospitalized patients than in community patients. Our subanalysis corroborated this finding, showing the prevalence of CDAD to be significantly higher in studies including only hospitalized patients versus those including community patients or both. A number of factors may be contributing to this finding. The risk of hospital-acquired infections such as C. difficile is greater in developing countries, which lack resources for effective infection control programs [127, 128]. Hospitalized patients are more likely to have the recognized risk factors for C. difficile infection, namely advanced age, antibiotic use, and certain comorbidities. Prescribing rates for antibiotics, particularly those strongly associated with C. difficile infection (e.g., cephalosporins, fluoroquinolones), are higher in the developing countries than in other regions [129].
While the data on healthcare-acquired CDAD are salient, it is important to consider that community acquisition is a relatively new trend that until recently has not been a focus of epidemiologic research. Of the 85 studies included in our analysis that provided prevalence data, only 12 included cases of community-acquired CDAD (7 included both hospital-acquired and community-acquired cases). This under-surveillance is likely masking a higher rate of community-acquired disease in these developing countries. In areas where surveillance is more aggressive, proportions of community-acquired CDAD have been shown to be much higher. In the US, for example, community onset accounted for almost 50% of C. difficile infections in 2010 [2].
Study limitations include, as previously noted, the potential for significant publication bias related to high variability among the studies selected. Publication bias in meta-analyses is often attributable to the number of included studies being lower than the total number of studies conducted [27]. By closely adhering to the PRISMA model in selecting studies for inclusion [26], we feel we have minimized this concern. However, the definition of CDAD according to microbial testing may have varied across studies. Inconsistencies in study designs also make it challenging to compare prevalence in developing countries to that in more developed areas, such as the US and Europe. Along those lines, wide variation in diagnostic protocols and surveillance practices would suggest that prevalence and incidence of CDAD are underestimated. In addition, in most of the included studies in our meta-analysis, populations were poorly defined, which might make comparisons unclear.
We found no data related to CDAD in long-term care facilities, which would now be included under the umbrella of nosocomial acquisition. We found only 17 articles with data on incidence rates, which makes that aspect of the analysis underpowered.
Conclusion
Low awareness of CDAD in developing countries and inconsistent surveillance protocols likely cause marked underestimates of prevalence and incidence rates in developing countries. In this meta-analysis, we estimated prevalence of C. difficile infection to be 15% in patients with diarrhea; however, this should be considered the tip of the iceberg in light of limited diagnostic resources and protocols, as well as the low level of awareness, in developing countries. Hospital-acquired CDAD was found to be a greater concern than community-acquired disease, although the latter is undoubtedly trending upward. Heightened awareness of CDAD among healthcare providers; as well as enhancements in diagnostic capabilities, infection control, and surveillance protocols; should be implemented to better manage and prevent C. difficile infection and associated diarrhea in developing countries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship of this study and article processing charges were funded by Pfizer Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
The statistical analysis was conducted by Content Medicine in Buenos Aires, Argentina; funding was provided by Pfizer Inc. Ann L. Davis, MPH, CMPP, an employee of Pfizer Inc, provided writing support for the manuscript under the direction of the authors.
Disclosures
Daniel Curcio is an employee of Pfizer, Inc. and may hold stock in the company. Alejandro Cané is an employee of Pfizer, Inc. and may hold stock in the company. Francisco Andrés Fernández and Jorge Correa have nothing to disclose.
Compliance with Ethics Compliance
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7571009.
References
- 1.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23(3):529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Vital signs: preventing Clostridium difficile infections. Morb Mortal Wkly Rep. 2012;61(9):157–162. [PubMed] [Google Scholar]
- 3.To KB, Napolitano LM. Clostridium difficile infection: update on diagnosis, epidemiology, and treatment strategies. Surg Infect (Larchmt) 2014;15(5):490–502. doi: 10.1089/sur.2013.186. [DOI] [PubMed] [Google Scholar]
- 4.Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis. 2016;3(1):23–42. doi: 10.1177/2049936115622891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 6.O’Donoghue C, Kyne L. Update on Clostridium difficile infection. Curr Opin Gastroenterol. 2011;27(1):38–47. doi: 10.1097/MOG.0b013e3283411634. [DOI] [PubMed] [Google Scholar]
- 7.Smits WK. Hype or hypervirulence: a reflection on problematic C. difficile strains. Virulence. 2013;4(7):592–6. [DOI] [PMC free article] [PubMed]
- 8.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 10.Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;8(1):1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RL, Suda KJ, Evans CT. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev. 2017;3:CD004610. [DOI] [PMC free article] [PubMed]
- 13.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 14.Bloomfield LE, Riley TV. Epidemiology and risk factors for community-associated Clostridium difficile infection: a narrative review. Infect Dis Ther. 2016;5(3):231–251. doi: 10.1007/s40121-016-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pituch H. Clostridium difficile is no longer just a nosocomial infection or an infection of adults. Int J Antimicrob Agents. 2009;33(Suppl 1):S42–S45. doi: 10.1016/S0924-8579(09)70016-0. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed 23 Oct 2018.
- 17.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107(1):89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173(14):1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66(7):987–994. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 20.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55(2):216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 21.Bauer KA, Johnston JEW, Wenzler E, et al. Impact of the NAP-1 strain on disease severity, mortality, and recurrence of healthcare-associated Clostridium difficile infection. Anaerobe. 2017;48:1–6. doi: 10.1016/j.anaerobe.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JW, Xiao M, Kudinha T, et al. The role of glutamate dehydrogenase (GDH) testing assay in the diagnosis of Clostridium difficile infections: a high sensitive screening test and an essential step in the proposed laboratory diagnosis workflow for developing countries like China. PLoS ONE. 2015;10(12):e0144604. doi: 10.1371/journal.pone.0144604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins DA, Hawkey PM, Riley TV. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control. 2013;2(1):21. doi: 10.1186/2047-2994-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester JD, Cai LZ, Mbanje C, Rinderknecht TN, Wren SM. Clostridium difficile infection in low- and middle-human development index countries: a systematic review. Trop Med Int Health. 2017;22(10):1223–1232. doi: 10.1111/tmi.12937. [DOI] [PubMed] [Google Scholar]
- 25.Roldan GA, Cui AX, Pollock NR. Assessing the Burden of Clostridium difficile Infection in Low- and Middle-Income Countries. J Clin Microbiol. 2018;56(3):e01747-17. doi: 10.1128/JCM.01747-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed]
- 27.Jin ZC, Zhou XH, He J. Statistical methods for dealing with publication bias in meta-analysis. Stat Med. 2015;34(2):343–360. doi: 10.1002/sim.6342. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet. 1998;351(9096):123–127. doi: 10.1016/S0140-6736(97)08468-7. [DOI] [PubMed] [Google Scholar]
- 30.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–8. [DOI] [PubMed]
- 31.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 33.Borren NZ, Ghadermarzi S, Hutfless S, Ananthakrishnan AN. The emergence of Clostridium difficile infection in Asia: a systematic review and meta-analysis of incidence and impact. PLoS ONE. 2017;12(5):e0176797. doi: 10.1371/journal.pone.0176797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira CE, Nakano V, Durigon EL, Avila-Campos MJ. Prevalence of Clostridium spp. and Clostridium difficile in children with acute diarrhea in Sao Paulo city, Brazil. Mem Inst Oswaldo Cruz. 2003;98(4):451–4. [DOI] [PubMed]
- 35.Fernandez Canigia L, Nazar J, Arce M, Dadamio J, Smayevsky J, Bianchini H. Clostridium difficile diarrhea: frequency of detection in a medical center in Buenos Aires, Argentina. Rev Argent Microbiol. 2001;33(2):101–107. [PubMed] [Google Scholar]
- 36.Pinto LJ, Alcides AP, Ferreira EO, et al. Incidence and importance of Clostridium difficile in paediatric diarrhoea in Brazil. J Med Microbiol. 2003;52(Pt 12):1095–1099. doi: 10.1099/jmm.0.05308-0. [DOI] [PubMed] [Google Scholar]
- 37.Garcia C, Samalvides F, Vidal M, Gotuzzo E, Dupont HL. Epidemiology of Clostridium difficile-associated diarrhea in a Peruvian tertiary care hospital. Am J Trop Med Hyg. 2007;77(5):802–805. [PubMed] [Google Scholar]
- 38.Zumbado-Salas R, Gamboa-Coronado Mdel M, Rodriguez-Cavallini E, Chaves-Olarte E. Clostridium difficile in adult patients with nosocomial diarrhea in a Costa Rican hospital. Am J Trop Med Hyg. 2008;79(2):164–165. [PubMed] [Google Scholar]
- 39.Balassiano IT, Dos Santos-Filho J, de Oliveira MP, et al. An outbreak case of Clostridium difficile-associated diarrhea among elderly inpatients of an intensive care unit of a tertiary hospital in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis. 2010;68(4):449–455. doi: 10.1016/j.diagmicrobio.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Secco DA, Balassiano IT, Boente RF, et al. Clostridium difficile infection among immunocompromised patients in Rio de Janeiro, Brazil and detection of moxifloxacin resistance in a ribotype 014 strain. Anaerobe. 2014;28:85–89. doi: 10.1016/j.anaerobe.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Spadão F, Gerhardt J, Guimarães T, et al. Incidence of diarrhea by Clostridium difficile in hematologic patients and hematopoietic stem cell transplantation patients: risk factors for severe forms and death. Rev Inst Med Trop Sao Paulo. 2014;56(4):325–331. doi: 10.1590/S0036-46652014000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plaza-Garrido A, Barra-Carrasco J, Macias JH, et al. Predominance of Clostridium difficile ribotypes 012, 027 and 046 in a university hospital in Chile, 2012. Epidemiol Infect. 2016;144(5):976–979. doi: 10.1017/S0950268815002459. [DOI] [PubMed] [Google Scholar]
- 43.Morfin-Otero R, Garza-Gonzalez E, Aguirre-Diaz SA, et al. Clostridium difficile outbreak caused by NAP1/BI/027 strain and non-027 strains in a Mexican hospital. Braz J Infect Dis. 2016;20(1):8–13. doi: 10.1016/j.bjid.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia LB, de Uzeda M. Occurrence of Clostridium difficile in the feces of children of Rio de Janeiro, RJ, Brazil. Rev Inst Med Trop Sao Paulo. 1988;30(6):419–423. doi: 10.1590/s0036-46651988000600006. [DOI] [PubMed] [Google Scholar]
- 45.Pilcante J, Rojas P, Ernst D, et al. Clostridium difficile infection in Chilean patients submitted to hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2015;37(6):388–394. doi: 10.1016/j.bjhh.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez M, Gonzalez R, Briceno I, et al. Diagnosis of Clostridium difficile diarrhea: in search of a more efficient clinical focus. Rev Med Chil. 2001;129(6):620–625. [PubMed] [Google Scholar]
- 47.Bignardi GE, Settle C. Glutamate dehydrogenase as confirmatory test for Clostridium difficile toxin A/B-positive stools. J Hosp Infect. 2010;75(4):327–328. doi: 10.1016/j.jhin.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Jamal W, Rotimi VO, Brazier J, Duerden BI. Analysis of prevalence, risk factors and molecular epidemiology of Clostridium difficile infection in Kuwait over a 3-year period. Anaerobe. 2010;16(6):560–565. doi: 10.1016/j.anaerobe.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Al-Tawfiq JA, Abed MS. Clostridium difficile-associated disease among patients in Dhahran, Saudi Arabia. Travel Med Infect Dis. 2010;8(6):373–376. doi: 10.1016/j.tmaid.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Rajabally NM, Pentecost M, Pretorius G, Whitelaw A, Mendelson M, Watermeyer G. The Clostridium difficile problem: a South African tertiary institution’s prospective perspective. S Afr Med J. 2013;103(3):168–172. doi: 10.7196/samj.6012. [DOI] [PubMed] [Google Scholar]
- 51.Simango C, Uladi S. Detection of Clostridium difficile diarrhoea in Harare, Zimbabwe. Trans R Soc Trop Med Hyg. 2014;108(6):354–357. doi: 10.1093/trstmh/tru042. [DOI] [PubMed] [Google Scholar]
- 52.Al-Thani AA, Hamdi WS, Al-Ansari NA, Doiphode SH, Wilson GJ. Polymerase chain reaction ribotyping of Clostridium difficile isolates in Qatar: a hospital-based study. BMC Infect Dis. 2014;14:502. doi: 10.1186/1471-2334-14-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu Faddan NH, Aly SA, Abou Faddan HH. Nosocomial Clostridium difficile-associated diarrhoea in Assiut University Children’s Hospital, Egypt. Paediatr Int Child Health. 2016;36(1):39–44. doi: 10.1179/2046905514Y.0000000167. [DOI] [PubMed] [Google Scholar]
- 54.Jamal W, Pauline E, Rotimi V. A prospective study of community-associated Clostridium difficile infection in Kuwait: epidemiology and ribotypes. Anaerobe. 2015;35(Pt B):28–32. doi: 10.1016/j.anaerobe.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Kullin B, Meggersee R, D’Alton J, et al. Prevalence of gastrointestinal pathogenic bacteria in patients with diarrhoea attending Groote Schuur Hospital, Cape Town, South Africa. S Afr Med J. 2015;105(2):121–125. doi: 10.7196/samj.8654. [DOI] [PubMed] [Google Scholar]
- 56.Janssen I, Cooper P, Gunka K, et al. High prevalence of nontoxigenic Clostridium difficile isolated from hospitalized and non-hospitalized individuals in rural Ghana. Int J Med Microbiol. 2016;306(8):652–656. doi: 10.1016/j.ijmm.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Vishwanath S, Singhal A, D’Souza A, Mukhopadhyay C, Varma M, Bairy I. Clostridium difficile infection at a tertiary care hospital in south India. J Assoc Physicians India. 2013;61(11):804–806. [PubMed] [Google Scholar]
- 58.Hsu MS, Wang JT, Huang WK, Liu YC, Chang SC. Prevalence and clinical features of Clostridium difficile-associated diarrhea in a tertiary hospital in northern Taiwan. J Microbiol Immunol Infect. 2006;39(3):242–248. [PubMed] [Google Scholar]
- 59.Koh TH, Tan AL, Tan ML, Wang G, Song KP. Epidemiology of Clostridium difficile infection in a large teaching hospital in Singapore. Pathology. 2007;39(4):438–442. doi: 10.1080/00313020701444507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhry R, Joshy L, Kumar L, Dhawan B. Changing pattern of Clostridium difficile associated diarrhoea in a tertiary care hospital: a 5 year retrospective study. Indian J Med Res. 2008;127(4):377–382. [PubMed] [Google Scholar]
- 61.Lim PL, Barkham TM, Ling LM, Dimatatac F, Alfred T, Ang B. Increasing incidence of Clostridium difficile-associated disease, Singapore. Emerg Infect Dis. 2008;14(9):1487–1489. doi: 10.3201/eid1409.070043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshy L, Chaudhry R, Dhawan B. Detection and characterization of Clostridium difficile from patients with antibiotic-associated diarrhoea in a tertiary care hospital in North India. J Med Microbiol. 2009;58(Pt 12):1657–1659. doi: 10.1099/jmm.0.000125-0. [DOI] [PubMed] [Google Scholar]
- 63.Chung CH, Wu CJ, Lee HC, et al. Clostridium difficile infection at a medical center in southern Taiwan: incidence, clinical features and prognosis. J Microbiol Immunol Infect. 2010;43(2):119–125. doi: 10.1016/S1684-1182(10)60019-9. [DOI] [PubMed] [Google Scholar]
- 64.Chan M, Lim PL, Chow A, Win MK, Barkham TM. Surveillance for Clostridium difficile infection: ICD-9 coding has poor sensitivity compared to laboratory diagnosis in hospital patients, Singapore. PLoS ONE. 2011;6(1):e15603. doi: 10.1371/journal.pone.0015603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng VC, Yam WC, Lam OT, et al. Clostridium difficile isolates with increased sporulation: emergence of PCR ribotype 002 in Hong Kong. Eur J Clin Microbiol Infect Dis. 2011;30(11):1371–1381. doi: 10.1007/s10096-011-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ingle M, Deshmukh A, Desai D, et al. Prevalence and clinical course of Clostridium difficile infection in a tertiary-care hospital: a retrospective analysis. Indian J Gastroenterol. 2011;30(2):89–93. [Google Scholar]
- 67.Lee YC, Wang JT, Chen AC, Sheng WH, Chang SC, Chen YC. Changing incidence and clinical manifestations of Clostridium difficile-associated diarrhea detected by combination of glutamate dehydrogenase and toxin assay in Northern Taiwan. J Microbiol Immunol Infect. 2012;45(4):287–295. doi: 10.1016/j.jmii.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Haider Naqvi SA, Chaudhry FF. Clostridium difficile postantibiotic diarrhoea diagnosis. J Coll Physicians Surg Pak. 2012;22(10):640–643. doi: 10.2012/JCPSP.640643. [DOI] [PubMed] [Google Scholar]
- 69.Hassan SA, Othman N, Idris FM, et al. Prevalence of Clostridium difficile toxin in diarhoeal stool samples of patients from a tertiary hospital in North Eastern Penisular Malaysia. Med J Malaysia. 2012;67(4):402–405. [PubMed] [Google Scholar]
- 70.Ingle M, Deshmukh A, Desai D, et al. Clostridium difficile as a cause of acute diarrhea: a prospective study in a tertiary care center. Indian J Gastroenterol. 2013;32(3):179–183. doi: 10.1007/s12664-013-0303-8. [DOI] [PubMed] [Google Scholar]
- 71.Kaneria MV, Paul S. Incidence of Clostridium difficile associated diarrhoea in a tertiary care hospital. J Assoc Physicians India. 2012;60:26–28. [PubMed] [Google Scholar]
- 72.Ji DD, Huang IH, Lai CC, et al. Prevalence and characterization of enterotoxigenic Bacteroides fragilis and toxigenic Clostridium difficile in a Taipei emergency department. J Microbiol Immunol Infect. 2017;50(1):83–89. doi: 10.1016/j.jmii.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Kumar N, Ekka M, Raghunandan P, et al. Clostridium difficile infections in HIV-positive patients with diarrhoea. Natl Med J India. 2014;27(3):138–140. [PubMed] [Google Scholar]
- 74.Vaishnavi C, Singh M, Kapoor P, Kochhar R. Clinical and demographic profile of patients reporting for Clostridium difficile infection in a tertiary care hospital. Indian J Med Microbiol. 2015;33(2):326–327. doi: 10.4103/0255-0857.153570. [DOI] [PubMed] [Google Scholar]
- 75.Ramakrishnan N, Sriram K. Antibiotic overuse and Clostridium difficile infections: the Indian paradox and the possible role of dietary practices. Nutrition. 2015;31(7–8):1052–1053. doi: 10.1016/j.nut.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Vaishnavi C, Singh M, Mahmood S, Kochhar R. Prevalence and molecular types of Clostridium difficile isolates from faecal specimens of patients in a tertiary care centre. J Med Microbiol. 2015;64(11):1297–1304. doi: 10.1099/jmm.0.000169. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Wu S, Wang M, et al. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int J Antimicrob Agents. 2009;33(4):339–342. doi: 10.1016/j.ijantimicag.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 78.Han XH, Du CX, Zhang CL, et al. Clostridium difficile infection in hospitalized cancer patients in Beijing, China is facilitated by receipt of cancer chemotherapy. Anaerobe. 2013;24:82–84. doi: 10.1016/j.anaerobe.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Hawkey PM, Marriott C, Liu WE, et al. Molecular epidemiology of Clostridium difficile infection in a major chinese hospital: an underrecognized problem in Asia? J Clin Microbiol. 2013;51(10):3308–3313. doi: 10.1128/JCM.00587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou FF, Wu S, Klena JD, Huang HH. Clinical characteristics of Clostridium difficile infection in hospitalized patients with antibiotic-associated diarrhea in a university hospital in China. Eur J Clin Microbiol Infect Dis. 2014;33(10):1773–1779. doi: 10.1007/s10096-014-2132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang H, Wu S, Chen R, et al. Risk factors of Clostridium difficile infections among patients in a university hospital in Shanghai, China. Anaerobe. 2014;30:65–69. doi: 10.1016/j.anaerobe.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 82.Fang WJ, Jing DZ, Luo Y, et al. Clostridium difficile carriage in hospitalized cancer patients: a prospective investigation in eastern China. BMC Infect Dis. 2014;14:523. doi: 10.1186/1471-2334-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Cai L, Yu R, Huang W, Zong Z. ICU-Onset Clostridium difficile infection in a university hospital in China: a prospective cohort study. PLoS ONE. 2014;9(11):e111735. doi: 10.1371/journal.pone.0111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galaydick J, Xu Y, Sun L, et al. Seek and you shall find: prevalence of Clostridium difficile in Wuhan, China. Am J Infect Control. 2015;43(3):301–302. doi: 10.1016/j.ajic.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Cheng JW, Xiao M, Kudinha T, et al. The first two Clostridium difficile ribotype 027/ST1 isolates identified in Beijing, China—an emerging problem or a neglected threat? Sci Rep. 2016;6:18834. doi: 10.1038/srep18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao Q, Wu S, Huang H, et al. Toxin profiles, PCR ribotypes and resistance patterns of Clostridium difficile: a multicentre study in China, 2012–2013. Int J Antimicrob Agents. 2016;48(6):736–739. doi: 10.1016/j.ijantimicag.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 87.Jin D, Luo Y, Huang C, et al. Molecular Epidemiology of Clostridium difficile Infection in Hospitalized Patients in Eastern China. J Clin Microbiol. 2017;55(3):801–810. doi: 10.1128/JCM.01898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paláu-Dávila L, Garza-González E, Gutiérrez-Delgado EM, Camacho-Ortiz A. Predictors of severe outcomes in patients with Clostridium difficile infection from a Hispanic population. Indian J Gastroenterol. 2017;36(1):38–42. doi: 10.1007/s12664-016-0722-4. [DOI] [PubMed] [Google Scholar]
- 89.Rodríguez-Varón A, Muñoz OM, Pulido-Arenas J, Amado SB, Tobón-Trujillo M. Antibiotic-associated diarrhea: clinical characteristics and the presence of Clostridium difficile. Rev Gastroenterol Mex. 2017;82(2):129–33. [DOI] [PubMed]
- 90.Carvajal C, Pacheco C, Jaimes F. Clinical and demographic profile and risk factors for Clostridium difficile infection. Biomedica. 2017;37(1):53–61. doi: 10.7705/biomedica.v37i1.2915. [DOI] [PubMed] [Google Scholar]
- 91.Salazar CL, Reyes C, Atehortua S, et al. Molecular, microbiological and clinical characterization of Clostridium difficile isolates from tertiary care hospitals in Colombia. PLoS ONE. 2017;12(9):e0184689. doi: 10.1371/journal.pone.0184689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Costa CL, Mano de Carvalho CB, Gonzalez RH, et al. Molecular epidemiology of Clostridium difficile infection in a Brazilian cancer hospital. Anaerobe. 2017;48:232–6. [DOI] [PubMed]
- 93.Oñate-Gutiérreza JM, Villegasa MV, Correa A. Prevalencia y factores relacionados con la infección por Clostridium difficile en un centro hospitalario de alta complejidad en Cali (Colombia). Infection. 2016:9–14.
- 94.Kullin B, Wojno J, Abratt V, Reid SJ. Toxin A-negative toxin B-positive ribotype 017 Clostridium difficile is the dominant strain type in patients with diarrhoea attending tuberculosis hospitals in Cape Town, South Africa. Eur J Clin Microbiol Infect Dis. 2017;36(1):163–175. doi: 10.1007/s10096-016-2790-x. [DOI] [PubMed] [Google Scholar]
- 95.Azimirad M, Krutova M, Nyc O, et al. Molecular typing of Clostridium difficile isolates cultured from patient stool samples and gastroenterological medical devices in a single Iranian hospital. Anaerobe. 2017;47:125–128. doi: 10.1016/j.anaerobe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 96.Senok AC, Aldosari KM, Alowaisheq RA, et al. Detection of Clostridium difficile antigen and toxin in stool specimens: comparison of the C. difficile quik chek complete enzyme immunoassay and GeneXpert C. difficile polymerase chain reaction assay. Saudi J Gastroenterol. 2017;23(4):259–62. [DOI] [PMC free article] [PubMed]
- 97.Rezazadeh Zarandi E, Mansouri S, Nakhaee N, Sarafzadeh F, Iranmanesh Z, Moradi M. Frequency of antibiotic associated diarrhea caused by Clostridium difficile among hospitalized patients in intensive care unit, Kerman, Iran. Gastroenterol Hepatol Bed Bench. 2017;10(3):229–234. [PMC free article] [PubMed] [Google Scholar]
- 98.Putsathit P, Maneerattanaporn M, Piewngam P, Kiratisin P, Riley TV. Prevalence and molecular epidemiology of Clostridium difficile infection in Thailand. New Microb New Infect. 2017;15:27–32. doi: 10.1016/j.nmni.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collins DA, Gasem MH, Habibie TH, et al. Prevalence and molecular epidemiology of Clostridium difficile infection in Indonesia. New Microb New Infect. 2017;18:34–37. doi: 10.1016/j.nmni.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Segar L, Easow JM, Srirangaraj S, Hanifah M, Joseph NM, Seetha KS. Prevalence of Clostridium difficile infection among the patients attending a tertiary care teaching hospital. Indian J Pathol Microbiol. 2017;60(2):221–225. doi: 10.4103/0377-4929.208383. [DOI] [PubMed] [Google Scholar]
- 101.Li C, Duan J, Liu S, et al. Assessing the risk and disease burden of Clostridium difficile infection among patients with hospital-acquired pneumonia at a University Hospital in Central China. Infection. 2017;45(5):621–628. doi: 10.1007/s15010-017-1024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jia H, Yang H, Du P, et al. Analysis of toxin and multilocus sequence typing of Clostridium difficile strains isolated from China-Japan Friendship Hospital. Chin J Microbiol Immunol. 2017;37:297–302. [Google Scholar]
- 103.Yan J, Liang J, Lv T, et al. Epidemiology of Clostridium difficile in a County Level Hospital in China. Jundishapur J Microbiol. 2017;10(6):e14376. [Google Scholar]
- 104.Shin BM, Kuak EY, Yoo HM, et al. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000–2005. J Med Microbiol. 2008;57(Pt 6):697–701. doi: 10.1099/jmm.0.47771-0. [DOI] [PubMed] [Google Scholar]
- 105.Cheng VC, Yam WC, Chan JF, To KK, Ho PL, Yuen KY. Clostridium difficile ribotype 027 arrives in Hong Kong. Int J Antimicrob Agents. 2009;34(5):492–493. doi: 10.1016/j.ijantimicag.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Kang JO, Kim H, et al. Epidemiology of Clostridium difficile infections in a tertiary-care hospital in Korea. Clin Microb Infect. 2013;19(6):521–527. doi: 10.1111/j.1469-0691.2012.03910.x. [DOI] [PubMed] [Google Scholar]
- 107.Yang BK, Do BJ, Kim EJ, et al. The simple predictors of pseudomembranous colitis in patients with hospital-acquired diarrhea: a prospective observational study. Gut Liver. 2014;8(1):41–48. doi: 10.5009/gnl.2014.8.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Huang Y, Li Y, Nie Y. Clinical characteristics of Clostridium difficile-associated diarrhea among patients in a tertiary care center in China. Pak J Med Sci. 2016;32(3):736–741. doi: 10.12669/pjms.323.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shehabi AA, Abu-Ragheb HA, Allaham NA. Prevalence of Clostridium difficile-associated diarrhoea among hospitalized Jordanian patients. East Mediterr Health J. 2001;7(4–5):750–755. [PubMed] [Google Scholar]
- 110.Ergen EK, Akalin H, Yilmaz E, et al. Nosocomial diarrhea and Clostridium difficile associated diarrhea in a Turkish University Hospital. Med Mal Infect. 2009;39(6):382–387. doi: 10.1016/j.medmal.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 111.Nazemalhosseini-Mojarad E, Azimirad M, Razaghi M, et al. Frequency of Clostridium difficile among patients with gastrointestinal complaints. Gastroenterol Hepatol Bed Bench. 2011;4(4):210–213. [PMC free article] [PubMed] [Google Scholar]
- 112.Jalali M, Khorvash F, Warriner K, Weese JS. Clostridium difficile infection in an Iranian hospital. BMC Res Notes. 2012;5:159. doi: 10.1186/1756-0500-5-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alinejad F, Barati M, Satarzadeh Tabrisi M, Saberi M. Hospital acquired diarrhea in a burn center of Tehran. Iran J Microbiol. 2015;7(6):310–314. [PMC free article] [PubMed] [Google Scholar]
- 114.Hsu LY, Tan TY, Koh TH, et al. Decline in Clostridium difficile-associated disease rates in Singapore public hospitals, 2006 to 2008. BMC Res Notes. 2011;4:77. doi: 10.1186/1756-0500-4-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thipmontree W, Kiratisin P, Manatsathit S, Thamlikitkul V. Epidemiology of suspected Clostridium difficile-associated hospital-acquired diarrhea in hospitalized patients at Siriraj Hospital. J Med Assoc Thai. 2011;94(Suppl 1):S207–S216. [PubMed] [Google Scholar]
- 116.Thongkoom P, Kanchanahareutai S, Chantrakooptungkul S, Rahule S. Characteristics and Demographic Distributions of Toxigenic Clostridium difficile Strains in Rajavithi Hospital, 2009-2015. J Med Assoc Thai. 2016;99(Suppl 2):S195–S200. [PubMed] [Google Scholar]
- 117.Chau ML, Hartantyo SH, Yap M, et al. Diarrheagenic pathogens in adults attending a hospital in Singapore. BMC Infect Dis. 2016;16:32. doi: 10.1186/s12879-016-1354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sadeghifard N, Salari MH, Ghassemi MR, Eshraghi S, Amin Harati F. The incidence of nosocomial toxigenic Clostridium difficile associated diarrhea in Tehran tertiary medical centers. Acta Med Iran. 2010;48(5):320–325. [PubMed] [Google Scholar]
- 119.Asha NJ, Tompkins D, Wilcox MH. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J Clin Microbiol. 2006;44(8):2785–2791. doi: 10.1128/JCM.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heimesaat MM, Granzow K, Leidinger H, Liesenfeld O. Prevalence of Clostridium difficile toxins A and B and Clostridium perfringens enterotoxin A in stool samples of patients with antibiotic-associated diarrhea. Infection. 2005;33(5–6):340–344. doi: 10.1007/s15010-005-5067-3. [DOI] [PubMed] [Google Scholar]
- 121.Garey KW, Graham G, Gerard L, et al. Prevalence of diarrhea at a university hospital and association with modifiable risk factors. Ann Pharmacother. 2006;40(6):1030–1034. doi: 10.1345/aph.1H028. [DOI] [PubMed] [Google Scholar]
- 122.Centers for Disease Control and Prevention. 2015 Annual Report for the Emerging Infections Program for Clostridium difficile Infection. Updated 24 July 2017. https://www.cdc.gov/hai/eip/Annual-CDI-Report-2015.html. Accessed 1 Aug 2018.
- 123.Balassiano IT, Yates EA, Domingues RM, Ferreira EO. Clostridium difficile: a problem of concern in developed countries and still a mystery in Latin America. J Med Microbiol. 2012;61(Pt 2):169–179. doi: 10.1099/jmm.0.037077-0. [DOI] [PubMed] [Google Scholar]
- 124.Cane A, Curcio D, Quintana A. Physician knowledge of Clostridium difficile: a Latin American survey. Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases. April 9–12, 2016. Amsterdam, Netherlands.
- 125.Hung YP, Lee JC, Lin HJ, et al. Perceptions of Clostridium difficile infections among infection control professionals in Taiwan. J Microbiol Immunol Infect. 2017;50(4):521–526. doi: 10.1016/j.jmii.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Pittet D, Allegranzi B, Storr J, et al. Infection control as a major World Health Organization priority for developing countries. J Hosp Infect. 2008;68(4):285–292. doi: 10.1016/j.jhin.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 127.Pittet D, Allegranzi B, Storr J, Donaldson L. ‘Clean Care is Safer Care’: the Global Patient Safety Challenge 2005–2006. Int J Infect Dis. 2006;10(6):419–424. doi: 10.1016/j.ijid.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.