Keywords: nerve regeneration, ischemic stroke, rehabilitation, constraint-induced movement therapy, nerve growth factors, functional recovery, neuronal plasticity, real time-polymerase chain reaction, western blot assay, rats, neural regeneration
Abstract
Constraint-induced movement therapy is an effective rehabilitative training technique used to improve the restoration of impaired upper extremity movement after stroke. However, whether constraint-induced movement therapy is more effective than conventional rehabilitation in acute or sub-acute stroke remains controversial. The aim of the present study was to identify the optimal time to start constraint-induced movement therapy after ischemic stroke and to explore the mechanisms by which constraint-induced movement therapy leads to post-stroke recovery. Sixty-four adult male Sprague-Dawley rats were randomly divided into four groups: sham-surgery group, cerebral ischemia/reperfusion group, early constraint-induced movement therapy group, and late constraint-induced movement therapy group. Rat models of left middle cerebral artery occlusion were established according to the Zea Longa line embolism method. Constraint-induced movement therapy was conducted starting on day 1 or day 14 in the early constraint-induced movement therapy and late constraint-induced movement therapy groups, respectively. To explore the effect of each intervention time on neuromotor function, behavioral function was assessed using a balance beam walking test before surgery and at 8 and 21 days after surgery. The expression levels of brain-derived neurotrophic factor, nerve growth factor and Nogo receptor were evaluated using real time-polymerase chain reaction and western blot assay to assess the effect of each intervention time. The results showed that the behavioral score was significantly lower in the early constraint-induced movement therapy group than in the cerebral ischemia/reperfusion and late constraint-induced movement therapy groups at 8 days. At 21 days, the scores had significantly decreased in the early constraint-induced movement therapy and late constraint-induced movement therapy groups. At 8 days, only mild pyknosis appeared in neurons of the ischemic penumbra in the early constraint-induced movement therapy group, which was distinctly better than in the cerebral ischemia/reperfusion group. At 21 days, only a few vacuolated cells were observed and no obvious inflammatory cells were visible in late constraint-induced movement therapy group, which was much better than at 8 days. The mRNA and protein expression levels of brain-derived neurotrophic factor and nerve growth factor were significantly higher, but expression levels of Nogo receptor were significantly lower in the early constraint-induced movement therapy group compared with the cerebral ischemia/reperfusion and late constraint-induced movement therapy groups at 8 days. The changes in expression levels at 21 days were larger but similar in both the early constraint-induced movement therapy and late constraint-induced movement therapy groups. Besides, the protein nerve growth factor level was higher in the late constraint-induced movement therapy group than in the early constraint-induced movement therapy group at 21 days. These results suggest that both early (1 day) and late (14 days) constraint-induced movement therapy induces molecular plasticity and facilitates functional recovery after ischemic stroke, as illustrated by the histology. The mechanism may be associated with downregulation of Nogo receptor expression and upregulation of brain-derived neurotrophic factor and nerve growth factor expression.
Chinese Library Classification No. R454; R364
Introduction
Stroke is a major cause of disability, primarily resulting in hemiplegia that has a devastating effect on a person’s capability to perform daily activities (Dobkin et al., 2005; Pang et al., 2006; Feigin et al., 2010). This occurs even when most patients show significant gains in motor function soon after stroke onset (Langhorne et al., 2011). Approximately 85% of survivors present with motor impairments in the upper extremity, and as many as 75% of them continue to have problems for the following 3–6 months (Kunkel et al., 1999; Mozaffarian et al., 2016). It is accepted that rehabilitation can maximize the recovery of functional disorders in stroke patients.
Constraint-induced movement therapy (CIMT), developed by Taub et al. (1993), is a neuro-rehabilitation approach characterized by restraint of the less affected upper limb and the enforced use of the affected arm. A series of studies has demonstrated that CIMT is an effective rehabilitative training technique to improve restoration of impaired upper extremity movement after a stroke (DeBow et al., 2003; Liepert et al., 2006; Gauthier et al., 2008; Langhorne et al., 2011). CIMT has produced more promising results in the rehabilitation of patients after stroke compared with traditional rehabilitation measures, especially in the chronic phase (> 6 months) after stroke (Wolf et al., 2006). However, whether CIMT has a higher efficacy than conventional rehabilitation in acute or sub-acute stroke phases remains controversial. The stage at which to start CIMT has become an important question.
Some scholars believe that, beginning CIMT at the acute stage after a stroke is harmful, causing exaggeration of the neural injury or local cortical hyperthermia (Humm et al., 1999; DeBow et al., 2004; Dromerick et al., 2009). However, other studies indicated that acute CIMT can promote functional recovery after ischemic stroke or spinal hemisection (Joo et al., 2012; Lang et al., 2013; Kwakkel et al., 2015). The risk versus benefit of CIMT conducted immediately after stroke has been inconclusive. Several previous studies addressed CIMT effects on cerebral ischemia (Zhang et al., 2015) and a few studies focused on CIMT effects after subcortical hemorrhage (Ishida et al., 2015; Liu et al., 2016).
Previous studies indicated that cerebral ischemia affects multiple factors, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and Nogo-receptor (NgR) expression levels, which play an important role in the recovery of stroke (Lee et al., 2008; Ochodnicky et al, 2011; Luo et al., 2017; Zhang et al., 2018). It is possible that early or late application of CIMT may have diverse effects in cerebral ischemia models.
The aim of the present study was to identify the optimal time to begin CIMT after a middle cerebral artery occlusion and to explore the mechanism by which CIMT leads to post-stroke recovery. This study compared the effects on behavior, histology, and the protein and gene expression of early and late CIMT on an ischemic brain injury rat model.
Materials and Methods
Animals
A total of 64 specific-pathogen-free male Sprague-Dawley rats weighing 250–280 g and aged 8 weeks old were provided by the Experimental Animal Center of Shandong University, China (Laboratory animal license number: 20130009). All rats were housed under controlled temperatures (23°C) in a 12-hour light/dark cycle with free access to food and water for 1 week before experiment. The study protocol was approved by the Animal Ethics Committee of Affiliated Hospital of Shandong Traditional Chinese Medicine University, China on February 23, 2017 (Approval number: 2017013).
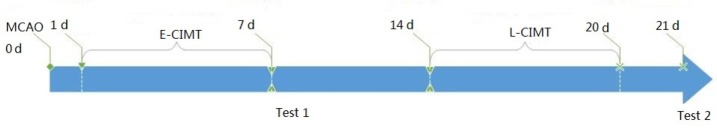
The rats were divided into four groups (n = 16): sham-surgery group, cerebral ischemia/reperfusion group (cerebral ischemia and non-treated), early CIMT group (E-CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following cerebral ischemia/reperfusion), and late CIMT group (L-CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke). The timeline of the experiments is shown in Figure 1. At each time point, 8 and 21 days after ischemic insult, 8 rats from each group were randomly selected for behavioral assessments, and then decapitated. The brains from four rats in each group were used for real time-polymerase chain reaction (RT-PCR) and western blot assay and four for histological analysis.
Figure 1.
Protocol of the present study.
Cerebral infarction model rats were made according to the Zea Longa line embolism method, followed by 7 days CIMT from day 1 or day 14. Behavioral assessment by neurological score and balance beam walking test was carried out after CIMT (test 1 at day 8, and test 2 at day 21). Hematoxylin-eosin staining, real-time polymerase chain reaction and western blot assay for Nogo-receptor, brain-derived neurotrophic factor and nerve growth factor were also performed after early and late CIMT. E-CIMT: Early CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following ischemic stroke; L-CIMT: late CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke; MCAO: middle cerebral artery occlusion; CIMT: constraint-induced movement therapy; d: day(s).
Animal model establishment
The ischemic rat model was established according to the Zea Longa line embolism method (Longa et al., 1989). Rats were intraperitoneally anesthetized with 10% chloral hydrate and fixed on the operating table. An incision was made in the middle of the neck and superficial fascia, causing blunt separation of the sternocleidomastoid muscle and the sternal hyoid muscle. The left common carotid artery, external carotid artery, and internal carotid artery were separated to avoid damage to the vagus nerve. The proximal end of the common carotid artery was ligated and the external carotid artery was temporarily clamped by an artery clamp. The far end of the common carotid artery was then threaded. At the upper part of common carotid artery bifurcation, a “V”-shaped incision was made into the middle cerebral artery, 17–20 mm from the bifurcation of external carotid artery and internal carotid artery. The surgical line was ligated and fixed. Each rat was housed singly in a cage with access to water and food. Rats with neurological scores of 1–3 were enrolled in the present study.
Neurological function assessment
Neurological scores of rats in each group were evaluated according to the Zea Longa criteria (Uluc et al., 2011; Li et al., 2018) at 8 and 21 days after surgery. A 4-point scale was adopted as follows: 0, no neurological deficit symptoms, normal activity; 1: inverted tails, unable to stretch contralateral forepaws; 2: turn to the opposite side when crawling; 3: the body inclined to the opposite side while walking; 4: cannot walk, lacks consciousness.
Balance beam walking test
The neuro-behavioral outcome was evaluated using the balance beam walking test at 8 and 21 days after surgery. The beam dimensions were 2.5 cm width and 150 cm length, at a height of 70 cm above a sawdust cushion. A box was placed at the end of the beam for rats to rest on between tests. Before the first test, rats were trained to walk on the beam from one end to the other and then stayed in the box after walking. A 5-point scale was adopted as follows (Zausinger et al., 2000): 0, the rat was able to balance and walk on the beam using its forelimbs symmetrically; 1, the rat was able to balance and walk on the beam using its unaffected limb preferentially; 2, the rat was able to balance and walk on the beam mostly relying on the unaffected limb; 3, once it started to move, the rat was not able to balance on the beam; 4, the rat fell off the beam immediately. The test was conducted three times for each rat by a blinded rater to the group division. The average scores were calculated for statistical analysis. The balance beam walking test was performed before surgery and at 8 and 21 days after surgery.
Histological analysis
At 8 and 21 days after surgery, the tissues extracted from the ipsilateral hemisphere were fixed in 10% neutral formaldehyde, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. A microimaging system (Olympus, Tokyo, Japan) was used to observe the pathological changes in the four groups at 200× magnification.
Real-time PCR
TRIzol reagent kit (Molecular Probes, Invitrogen, Eugene, OR, USA) was used to extract total RNA from the ipsilateral hemisphere according to the manufacturer’s protocol at 8 and 21 days after induction of ischemia. Real-time PCR was performed using Exicycler™ 96 quantitative PCR Amplifier (BIONEER, Daejeon, Korea). The primers for TGF-b1 and Smad3 were purchased from the Applied Biosystems, and the β-actin expression level was used as the internal reference. The primer sequences are listed in Table 1. The reaction condition was set at 95°C for 10 minutes, 40 cycles at 95°C for 10 seconds, 60°C for 20 seconds, and 72°C for 30 seconds. The expression levels of target genes were normalized to those of the housekeeping gene β-actin and were calculated using 2-ΔΔCT method (Bachman et al., 2013).
Table 1.
Oligonucleotide primers for real-time polymerase chain reaction
| Name | Sequence (5′–3′) | Product size (bp) |
|---|---|---|
| NgR | Forward: GTC CCT TCC AGA CCA ATC AGC | 170 |
| Reverse: GCC ATT GCC TGG TGG AGT GT | ||
| BDNF | Forward: CAG TAT TAG CGA GTG GGT CA | 213 |
| Reverse: GAT TGG GTA GTT CGG CAT T | ||
| NGF | Forward: GCG TAA TGT CCA TGT TGT TC | 123 |
| Reverse: GTT TAG TCC AGT GGG CTT CA | ||
| β-Actin | Forward: GGA GAT TAC TGC CCT GGC TCC TAG C | 155 |
| Reverse: GGC CGG ACT CAT CGT ACT CCT GCT T |
NgR: Nogo-receptor; BDNF: brain-derived neurotrophic factor; NGF: nerve growth factor.
Western blot assay
Four rats from each group were sacrificed on day 8 and day 21 after middle cerebral artery occlusion. Tissues extracted from the sensorimotor cortex of the ipsilateral hemisphere were selected for western blot assay. Under deep anesthesia, the brain tissue was rapidly removed, dissected and homogenized in a cell lysis buffer containing a complete protease inhibitor cocktail. The lysates were incubated on ice for 5 minutes and centrifuged at 12,000 r/min for 10 minutes at 4°C. Protein levels of the supernatant lysates were measured using Bio-Rad DC protein assay. Proteins were separated by electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gel and blotted for 1 hour at 15 V onto a polyvinylidene fluoride membrane. The membranes were incubated at 4°C overnight with the following primary antibodies: rabbit anti-mouse anti-NgR, rabbit anti-mouse anti-BDNF, and rabbit anti-mouse anti-NGF (Beyotime, China). All of the antibodies were used at 1:1000 final dilutions in 5% skimmed milk in Tris-buffered saline/0.5% Tween. Equal gel loading was confirmed using anti-actin (1:5000 in 5% skimmed milk in Tris-buffered saline/0.5% Tween). The membranes were washed with Tris-buffered saline/0.5% Tween and incubated with horseradish peroxidase-conjugated secondary antibody (1:20,000 in 5% skimmed milk in Tris-buffered saline/0.5% Tween) for 45 minutes. Immunoreactivity was visualized using the enhanced chemiluminescence method. Densitometry was performed using Digital Gel Image Processing System (Liuyi, Beijing, China).
Statistical analysis
Data are presented as the mean ± SEM. Statistical analysis was performed using SAS 9.3 software (SAS Institute, Cary, NC, USA). All data were analyzed using one-way analysis of variance followed by Student-Newman-Keuls post hoc test. A P < 0.05 was considered statistically significant.
Results
E-CIMT effect on neurological function in rats with ischemic brain injury
Neurological score
As shown in Table 2, the difference of neurological scores among the four groups was statistically significant at 8 days (P = 0.013), using analysis of variance. Further analysis showed that the score in E-CIMT was significantly lower than that of the cerebral ischemia/reperfusion group and the L-CIMT group (P < 0.05), but there was no statistical difference between the cerebral ischemia/reperfusion group and the L-CIMT group (P > 0.05). This would be expected since L-CIMT had not begun by the day 8 point.
Table 2.
Neurological scores at 8 and 21 days by Zea Longa criteria
| Group | 8 days | 21 days | t | P |
|---|---|---|---|---|
| Sham-surgery | 0 | 0 | 0.000 | 1.000 |
| Cerebral ischemia/reperfusion | 1.88±0.31 | 1.75±0.28 | 0.880 | 0.395 |
| E-CIMT | 1.38±0.22*# | 1.25±0.31* | 0.970 | 0.352 |
| L-CIMT | 2.00±0.18 | 1.63±0.21* | 3.780 | 0.002 |
| F | 5.320 | 3.580 | – | – |
| P | 0.013 | 0.043 | – | – |
Data are expressed as the mean ± SEM (n = 8; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). *P < 0.05, vs. cerebral ischemia/reperfusion group; #P < 0.05, vs. L-CIMT group. E-CIMT: Early CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following ischemic stroke; L-CIMT: late CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke; CIMT: constraint-induced movement therapy.
The score was significantly lower at 21 days than that at 8 days in the L-CIMT group (P < 0.001). The analysis of variance showed that there was statistically significant difference in neurological function scores among the four groups at 21 days (P = 0.043). The scores of the E-CIMT and the L-CIMT groups were significantly lower than that of the cerebral ischemia/reperfusion group (QE-CIMT vs. ischemia/reperfusion group = 3.14, P < 0.05; QL-CIMT vs. ischemia/reperfusion group = 3.77, P < 0.05), but there was no significant difference between the E-CIMT and the L-CIMT groups (P > 0.05).
When evaluating the scores resulting from the balance beam walking test in the cerebral ischemia/reperfusion group, scores for the E-CIMT group and L-CIMT group were all significantly higher than the sham-surgery group at 8 days (P < 0.05). However, the score was significantly lower in the E-CIMT group compared with the cerebral ischemia/reperfusion and L-CIMT groups (P < 0.05). The difference in the scores between the cerebral ischemia/reperfusion and L-CIMT groups was not statistically significant (P > 0.05).
At 21 days after L-CIMT intervention, the scores in the E-CIMT and L-CIMT groups were similar (P > 0.05) and both significantly lower than each at 8 days (P < 0.05) and were both lower than that in the cerebral ischemia/reperfusion group (P < 0.05). The score was significantly higher in the cerebral ischemia/reperfusion group than in the sham-surgery group (P < 0.05; Table 3).
Table 3.
Balance beam walking test scores at 8 and 21 days
| Group | 8 days | 21 days | t | P |
|---|---|---|---|---|
| Sham-surgery | 0 | 0 | 0.000 | 1.000 |
| Cerebral ischemia/reperfusion | 3.00±0.41 | 2.83±0.38 | 0.860 | 0.405 |
| E-CIMT | 2.33±0.22*# | 1.58±0.31*† | 5.580 | < 0.001 |
| L-CIMT | 2.91±0.38 | 1.33±0.21*† | 10.290 | < 0.001 |
| F | 8.79 | 40.43 | – | – |
| P | 0.001 | < 0.001 | – | – |
Data are expressed as the mean ± SEM (n = 8; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). *P < 0.05, vs. cerebral ischemia/reperfusion group; #P < 0.05, vs. L-CIMT group; †P < 0.05, vs. 8 days. E-CIMT: Early CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following ischemic stroke; L-CIMT: late CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke; CIMT: constraint-induced movement therapy.
E-CIMT effect on histological changes in the ipsilateral hemisphere of rats with ischemic brain injury
The results of hematoxylin-eosin staining in sham-surgery group are shown in Figure 2A. The neurons in the cortex of the sham-surgery group had no obvious lesions at 8 and 21 days. The nuclei were round, the nucleoli clear, and all the intracellular morphology was clearly visible. The intercellular space was also normal.
Figure 2.
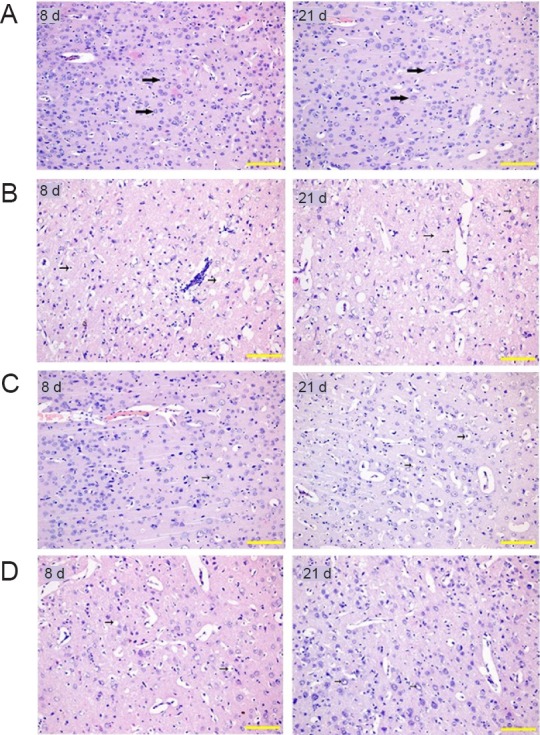
Effect of CIMT on histological changes in rats with ischemic brain injury (hematoxylin-eosin staining).
(A) Histological changes of sham-surgery group at 8 and 21 days: No obvious lesions at 8 and 21 days. The nucleus was rounded; the nucleolus was clear; the intercellular space was normal. (B) Histological changes of cerebral ischemia/reperfusion group at 8 and 21 days: At 8 days, obvious pyknosis, cytoplasmic vacuolization and vacuolar degeneration were observed. Neuronal number decreased and intercellular space increased. At 21 days, the neuropile became very rarefacted and glial cells increased in addition to the pathological changes at 8 days. (C) Histological changes of E-CIMT group at 8 and 21 days: At 8 days, the mild pyknosis of neurons seen in the ischemic penumbra in E-CIMT group was significantly less than in the cerebral ischemia/reperfusion group. By 21 days, the severity of the lesions showed improvement compared with that at 8 days, but much less than in the ischemia/reperfusion only group. (D) Histological changes of L-CIMT group at 8 and 21 days: At 8 days, the detrimental histological changes from both the L-CIMT and cerebral ischemia/reperfusion groups were more severe than in the E-CIMT group. At 21 days, the severity in the L-CIMT group was slightly less than in the E-CIMT group. Obvious pyknosis and few vacuolated cells were also observed. Original magnification: 200×. Scale bars: 20 μm. E-CIMT: Early CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following ischemic stroke; L-CIMT: late CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke; CIMT: constraint-induced movement therapy; d: days.
As exhibited in Figure 2B, obvious pyknosis, cytoplasmic vacuolization and vacuolar degeneration were apparent in the cerebral ischemia/reperfusion group at 8 days. The total neuronal number had also decreased and a corresponding increase in intercellular space in the ischemic penumbra. At 21 days, further pathological changes had occurred; the neuropil became very rarefacted and the number of glial cells increased in the cerebral ischemia/reperfusion group.
Figure 2C shows the result of histological changes in the E-CIMT group. At 8 days, only mild pyknosis appeared in neurons of the ischemic penumbra in the E-CIMT group, which was distinctly better than in the cerebral ischemia/reperfusion group. By 21 days, the severity of the lesions showed some further, slight improvement compared with that at 8 days. However, this was not as great as the improvement shown in the L-CIMT group at 21 days.
As expected, there was no significant difference between L-CIMT and cerebral ischemia/reperfusion groups at 8 days, and they both exhibited more severe damage than in the E-CIMT group (Figure 2D). At 21 days, there was much improvement in the L-CIMT group. Although there was some obvious pyknosis, only a few vacuolated cells were observed and no obvious inflammatory cells were visible.
E-CIMT effect on mRNA expression of BDNF, NGF and NgR in rats with ischemic brain injury
The mRNA expression levels of BDNF, NGF and NgR were calculated using real-time PCR. As shown in Figure 3, there was a significant difference in the expression levels of these transcripts among the four groups at 8 days (P < 0.05). Compared with the sham-surgery group, the expressions levels of BDNF, NGF and NgR were significantly increased in the cerebral ischemia/reperfusion, E-CIMT, and L-CIMT groups. At day 8, the mRNA expression levels of BDNF and NGF were significantly higher in the E-CIMT group than in the cerebral ischemia/reperfusion and L-CIMT groups (all P < 0.05). In addition, the E-CIMT group exhibited significantly lower mRNA expression of NgR compared with the cerebral ischemia/reperfusion and L-CIMT groups (P < 0.05).
Figure 3.
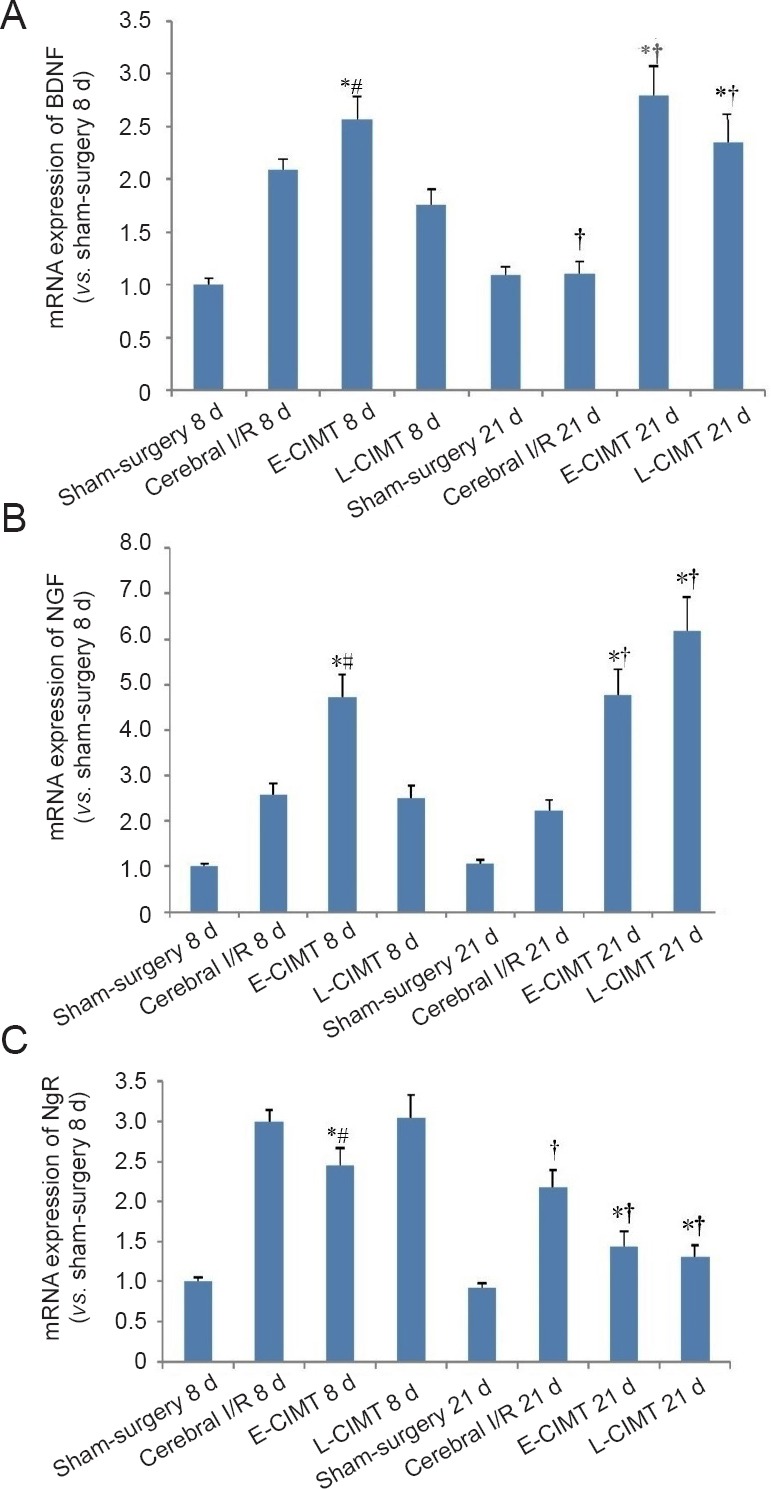
CIMT effect on mRNA expression levels of BDNF, NGF, and NgR in rats with ischemic brain injury.
(A) At 8 days, mRNA expression levels of BDNF were higher in the E-CIMT group than in the cerebral I/R and L-CIMT groups. The expression levels of BDNF had significantly increased at 21 days compared with those at 8 days in the L-CIMT group using 2-ΔΔCT method. The expression levels of BDNF were higher in the E-CIMT and L-CIMT groups than in the cerebral I/R group. (B) Results of mRNA expression of NGF were similar to those for BDNF. (C) At 8 days, the E-CIMT group exhibited remarkably lower mRNA expression of NgR compared with the cerebral I/R and L-CIMT groups. The mRNA expression levels of NgR had significantly decreased at 21 days compared with those at 8 days in the cerebral I/R, E-CIMT, and L-CIMT groups. The expression levels in E-CIMT and L-CIMT groups were lower than that in the cerebral I/R group. Data are expressed as the mean ± SEM (n = 8; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). *P < 0.05, vs. cerebral I/R group; #P < 0.05, vs. L-CIMT group; †P < 0.05, vs. 8 days. E-CIMT: Early CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following ischemic stroke; L-CIMT: late CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke. BDNF: Brain-derived neurotrophic factor; NGF: nerve growth factor; NgR: Nogo-receptor; CIMT: constraint-induced movement therapy; I/R: ischemia/reperfusion; d: days.
The mRNA expression levels of NgR were significantly lower at 21 days than those at 8 days in each of the cerebral ischemia/reperfusion, E-CIMT, and L-CIMT groups (P < 0.05). In the L-CIMT group, the expression levels of BDNF and NGF were significantly higher at 21 days than those at 8 days (P < 0.05). There were significant differences in the expression levels of BDNF, NGF and NgR among the four groups at 21 days (P < 0.05). The NgR expression levels in the E-CIMT and L-CIMT groups were significantly lower than in the cerebral ischemia/reperfusion group. However, no significant difference in NgR expression level was identified between the E-CIMT and L-CIMT groups (P > 0.05). The expression levels of NGF and BDNF were both higher in the E-CIMT and L-CIMT groups than in the cerebral ischemia/reperfusion group (P < 0.05).
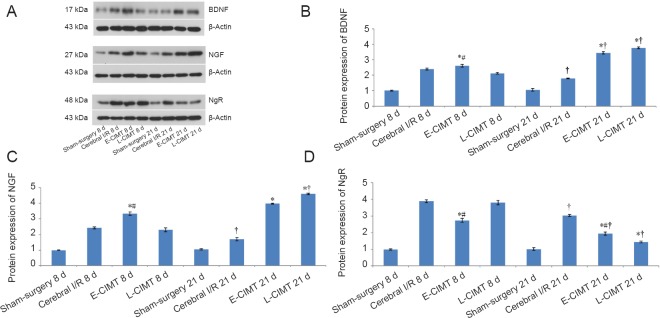
E-CIMT effect on protein expression of BDNF, NGF and NgR in rats with ischemic brain injury
The protein levels of BDNF, NGF and NgR were measured by western blot assay at 8 and 21 days. Figure 4 shows that at 8 days the BDNF, NGF and NgR levels were significantly higher in the cerebral ischemia/reperfusion, E-CIMT, and L-CIMT groups than in the sham-surgery group (P < 0.05). BDNF and NGF levels were significantly higher in the E-CIMT group than in the cerebral ischemia/reperfusion and L-CIMT groups (P < 0.05). The NgR level was significantly lower in the E-CIMT group than in the cerebral ischemia/reperfusion and L-CIMT groups (P < 0.05). No significant difference was detected between cerebral ischemia/reperfusion and L-CIMT groups (P > 0.05).
Figure 4.
CIMT effect on protein expression levels of BDNF, NGF and NgR in rats with ischemic brain injury.
(A) Protein expression levels of BDNF, NGF and NgR in different groups with β-actin as the internal control. (B) Western blot assay for BDNF protein expression at 8 and 21 days: BDNF expression was significantly higher in the E-CIMT group than in the cerebral I/R and L-CIMT groups at day 8. The relative expression of BDNF was increased at 21 days compared with that at 8 days in the E-CIMT and L-CIMT groups. BDNF levels in the E-CIMT and L-CIMT groups were significantly higher than in the cerebral I/R and sham-surgery groups (P < 0.05). (C) Protein expression of NGF at 8 and 21 days: The changes in protein expression of NGF were similar to those of BDNF. (D) Protein expression of NgR at 8 and 21 days: At 8 days, the NgR level in the E-CIMT group was significantly lower than in the cerebral I/R and L-CIMT groups. At day 21 NgR expression was significantly reduced compared with that at 8 days in the cerebral I/R, E-CIMT and L-CIMT groups. Expressions of NgR in the E-CIMT and L-CIMT groups were lower than in the cerebral I/R group. Data are expressed as the mean ± SEM (n = 8; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). *P < 0.05, vs. cerebral I/R group; #P < 0.05, vs. L-CIMT group; †P < 0.05, vs. 8 days. E-CIMT: Early CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 1 following ischemic stroke; L-CIMT: late CIMT, forced-limb use of the affected forelimb for 7 days, starting on day 14 following ischemic stroke. BDNF: Brain-derived neurotrophic factor; NGF: nerve growth factor; NgR: Nogo-receptor; CIMT: constraint-induced movement therapy; I/R: ischemia/reperfusion; d: days.
At 21 days, the relative expression levels of BDNF and NFG in the E-CIMT and L-CIMT groups had significantly increased compared with those in the cerebral ischemia/reperfusion group and sham-surgery groups (P < 0.05). The NgR expression levels in the cerebral ischemia/reperfusion, E-CIMT and the L-CIMT groups were significantly lower at 21 days than those at 8 days (P < 0.05). The NgR protein expression had fallen significantly lower in the E-CIMT and L-CIMT groups compared with the cerebral ischemia/reperfusion group (P < 0.05). There were no significant differences in NgR and BDNF expression levels between the E-CIMT and L-CIMT groups (P > 0.05). However, the NGF level was higher in the L-CIMT group than in the E-CIMT group at 21 days. The NGF level was significantly higher in the cerebral ischemia/reperfusion group than in the sham-surgery group (P < 0.05).
Discussion
In our study, exploring an appropriate time to give CIMT after stroke and its mechanism of action, we determined the effects of E-CIMT and L-CIMT on motor function in the affected upper limb through behavioral, histological, and molecular analyses. Our results found that both E-CIMT and L-CIMT induced significant functional improvement after ischemic stroke. Both E-CIMT and L-CIMT caused increased mRNA and protein expressions of neurotrophic factors BDNF and NGF, and decreased NgR expression.
As previously reported (Ishida et al., 2015; Kwakkel et al., 2016), our findings indicated that E-CIMT improved the motor function of impaired forelimbs in the rat models of ischemic stroke. The beneficial effects of E-CIMT have also been reported in animal models of intracerebral hemorrhage and other ischemic models (Maier et al., 2008; Ishida et al., 2011). Somewhat contradictory, E-CIMT has been shown to be detrimental to recovery because it causes neuronal damage and motor dysfunction via excitotoxicity and hyperthermia in ischemic models and after intracerebral hemorrhages (ICH) (Kozlowski et al., 1996; Liu et al., 2016). The reason for these contradictory results might lie in the different locations of the lesions. Interestingly, our results were not consistent with a previous study, which found that E-CIMT failed to enhance recovery after ischemic cortical stroke (Diederich et al., 2012). Our study demonstrated that L-CIMT also induces molecular and morphological changes, leading to functional recovery of the impaired limb, which was not consistent with the previous study using the ICH model. This suggests that recovery may depend on the pathology of stroke.
BDNF and NGF are both essential factors for neuronal remodeling and synaptic plasticity, playing an important role in the recovery of stroke (Ploughman et al., 2009; Livingston et al., 2014). Several studies reported that CIMT promoted the expressions of BDNF and NGF, which aid axonal growth and nerve recovery (Routtenberg et al., 2000; Shen et al., 2002). This is supported by our study. Furthermore, the Nogo-A protein has a key role in constraining axonal regeneration after the central nervous system is damaged (Schmandke et al., 2014; Sui et al., 2015; Lu et al., 2018), by retarding neurite outgrowth. Neuronal expression of Nogo-A spreads from the initial stroke lesion and becomes globally elevated by 28 days (Cheatwood et al., 2008). Treatment with antibodies against Nogo-A after injury improves functional recovery and neuroanatomical plasticity (Seymour et al., 2005; Tsai et al., 2011; Lindau et al., 2014; Podraza et al., 2017). Our results have shown that E-CIMT or L-CIMT enhanced the expression of BDNF and NGF, and decreased the level of NgR. These events suggest that they may be part of the mechanism by which CIMT achieves functional recovery.
There are important caveats to be noted in the present study. Our results indicate that the expression of neurotrophic growth-related factors increased after model establishment. However, the expression changes after ischemia/reperfusion are only short-term and did not translate into behavioral benefits. Besides, the results from this study indicated that CIMT applied at either early or late stages stimulates morphological and molecular changes, resulting in functional recovery of the affected limb. However, the expression levels of BDNF and NGF in the L-CIMT group were similar to or better than that in the E-CIMT group at 21 days. This indicates that there is no advantage in the early application of CIMT rather than later. These results provide new insights into the possible neural mechanisms of CIMT for limb function recovery.
This study has some limitations. First, to keep effects comparable between E-CIMT and L-CIMT groups, rats in both groups received 7 days of training, starting from day 1 and day 14, respectively. However, patients given E-CIMT usually received a longer training time in clinical practice. Second, our present findings only compared the short-term effects of E-CIMT and L-CIMT on ischemic rat model. The authors will explore the above issues in future studies.
In summary, both E-CIMT and L-CIMT induced molecular plasticity and improved functional recovery after ischemic stroke. The recovery effect of L-CIMT was slightly better than that of E-CIMT.
Additional file: Open peer review reports 1 (66.7KB, pdf) and 2 (98.3KB, pdf) .
Acknowledgments:
The authors would like to thank Jie Gao from Shandong Maternity and Child Hospital for his cooperation in the statistical analysis.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the Natural Science Foundation of Shandong Province of China, No. 2014ZRB14502 (to XHL). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The study protocol was approved by the Animal Ethics Committee of Affiliated Hospital of Shandong Traditional Chinese Medicine University, China on February 23, 2017 (Approval number: 2017013). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Jun-feng Zhang, Institute of Basic Medical Sciences, Xi’an Medical University, China; Midori A. Yenari, University of California, USA.
Funding: This study was supported by the Natural Science Foundation of Shandong Province of China, No. 2014ZRB14502 (to XHL).
P-Reviewers: Zhang JF, Yenari MA; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Azab M, Al-Jarrah M, Nazzal M, Maayah M, Sammour MA, Jamous M. Effectiveness of constraint-induced movement therapy (CIMT) as home-based therapy on Barthel Index in patients with chronic stroke. Top Stroke Rehabil. 2009;16:207–211. doi: 10.1310/tsr1603-207. [DOI] [PubMed] [Google Scholar]
- 2.Bachman J. Reverse-transcription PCR (RT-PCR) Methods Enzymol. 2013;530:67–74. doi: 10.1016/B978-0-12-420037-1.00002-6. [DOI] [PubMed] [Google Scholar]
- 3.Berretta A, Tzeng YC, Clarkson AN. Post-stroke recovery: the role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev Neurother. 2014;14:1335–1344. doi: 10.1586/14737175.2014.969242. [DOI] [PubMed] [Google Scholar]
- 4.Cheatwood JL, Emerick AJ, Schwab ME, Kartje GL. Nogo-A expression after focal ischemic stroke in the adult rat. Stroke. 2008;39:2091–2098. doi: 10.1161/STROKEAHA.107.507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S, Ma M, Ma Y, Wang Z, Xu G, Liu X. Combination therapy with intranasal NGF and electroacupuncture enhanced cell proliferation and survival in rats after stroke. Neurol Res. 2009;31:753–758. doi: 10.1179/174313209X382557. [DOI] [PubMed] [Google Scholar]
- 6.DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–1026. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 7.DeBow SB, McKenna JE, Kolb B, Colbourne F. Immediate constraint-induced movement therapy causes local hyperthermia that exacerbates cerebral cortical injury in rats. Can J Physiol Pharmacol. 2004;82:231–237. doi: 10.1139/y04-013. [DOI] [PubMed] [Google Scholar]
- 8.Diederich K, Quennet V, Bauer H, Müller HD, Wersching H, Schäbitz WR, Minnerup J, Sommer C. Successful regeneration after experimental stroke by granulocyte-colony stimulating factor is not further enhanced by constraint-induced movement therapy either in concurrent or in sequential combination therapy. Stroke. 2012;43:185–192. doi: 10.1161/STROKEAHA.111.622159. [DOI] [PubMed] [Google Scholar]
- 9.Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology. 2009;73:195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humm JL, Kozlowski DA, Bland ST, James DC, Schallert T. Use-dependent exaggeration of brain injury: is glutamate involved? Exp Neurol. 1999;157:349–358. doi: 10.1006/exnr.1999.7061. [DOI] [PubMed] [Google Scholar]
- 14.Ishida A, Tamakoshi K, Hamakawa M, Shimada H, Nakashima H, Masuda T, Hida H, Ishida K. Early onset of forced impaired fore limb use causes recovery of fore limb skilled motor function but no effect on gross sensory-motor function after capsular hemorrhage in rats. Behav Brain Res. 2011;225:126–134. doi: 10.1016/j.bbr.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Ishida A, Misumi S, Ueda Y, Shimizu Y, Cha-Gyun J, Tamakoshi K, Ishida K, Hida H. Early constraint-induced movement therapy promotes functional recovery and neuronal plasticity in a subcortical hemorrhage model rat. Behav Brain Res. 2015;284:158–166. doi: 10.1016/j.bbr.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Joo HW, Hyun JK, Kim TU, Chae SH, Lee YI, Lee SJ. Influence of constraint-induced movement therapy upon evoked potentials in rats with cerebral infarction. Eur J Neurosci. 2012;36:3691–3697. doi: 10.1111/ejn.12014. [DOI] [PubMed] [Google Scholar]
- 17.Kunkel A, Kopp B, Müller G, Villringer K, Villringer A, Taub E, Flor H. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–628. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]
- 18.Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14:224–234. doi: 10.1016/S1474-4422(14)70160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwakkel G, Winters C, van Wegen EE, Nijland RH, van Kuijk AA, Visser-Meily A, de Groot J, de Vlugt E, Arendzen JH, Geurts AC, Meskers CG EXPLICIT-Stroke Consortium. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: the explicit-stroke randomized clinical trial. Neurorehabil Neural Repair. 2016;30:804–816. doi: 10.1177/1545968315624784. [DOI] [PubMed] [Google Scholar]
- 20.Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang KC, Thompson PA, Wolf SL. The EXCITE Trial: reacquiring upper-extremity task performance with early versus late delivery of constraint therapy. Neurorehabil Neural Repair. 2013;27:654–663. doi: 10.1177/1545968313481281. [DOI] [PubMed] [Google Scholar]
- 22.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee TH, Yang JT, Ko YS, Kato H, Itoyama Y, Kogure K. Influence of ischemic preconditioning on levels of nerve growth factor, brain-derived neurotrophic factor and their high-affinity receptors in hippocampus following forebrain ischemia. Brain Res. 2008;1187:1–11. doi: 10.1016/j.brainres.2007.09.078. [DOI] [PubMed] [Google Scholar]
- 24.Li RQ, Wan MY, Shi J, Wang HL, Liu FL, Liu CM, Huang J, Liu RC, Ma L, Feng XD. Catgut implantation at acupoints increases the expression of glutamate aspartate transporter and glial glutamate transporter-1 in the brain of rats with spasticity after stroke. Neural Regen Res. 2018;13:1013–1018. doi: 10.4103/1673-5374.233444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liepert J, Haevernick K, Weiller C, Barzel A. The surround inhibition determines therapy-induced cortical reorganization. Neuroimage. 2006;32:1216–1220. doi: 10.1016/j.neuroimage.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Lindau NT, Bänninger BJ, Gullo M, Good NA, Bachmann LC, Starkey ML, Schwab ME. Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain. 2014;137:739–756. doi: 10.1093/brain/awt336. [DOI] [PubMed] [Google Scholar]
- 27.Liu YH, Zhao Y, Huang FZ, Chen YH, Wang HX, Bonney E, Liu BQ. Combination of early constraint-induced movement therapy and fasudil enhances motor recovery after ischemic stroke in rats. Int J Neurosci. 2016;126:168–173. doi: 10.3109/00207454.2014.998759. [DOI] [PubMed] [Google Scholar]
- 28.Livingston-Thomas JM, McGuire EP, Doucette TA, Tasker RA. Voluntary forced use of the impaired limb following stroke facilitates functional recovery in the rat. Behav Brain Res. 2014;261:210–219. doi: 10.1016/j.bbr.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Hsiang F, Chang JH, Yao XQ, Zhao H, Zou HY, Wang L, Zhang QX. Houshiheisan and its components promote axon regeneration after ischemic brain injury. Neural Regen Res. 2018;13:1195–1203. doi: 10.4103/1673-5374.235031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J, Zheng H, Zhang L, Zhang Q, Li L, Pei Z, Hu X. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci. 2017;18:E455. doi: 10.3390/ijms18020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochodnický P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30:1227–1241. doi: 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- 34.Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87:1–9. doi: 10.1016/j.apmr.2005.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 36.Podraza KM, Mehta Y, Husak VA, Lippmann E, O’Brien TE, Kartje GL, Tsai SY. Improved functional outcome after chronic stroke with delayed anti-Nogo-A therapy: a clinically relevant intention-to-treat analysis. J Cereb Blood Flow Metab. 2017;38:1327–1338. doi: 10.1177/0271678X17730994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rijntjes M, Hamzei F, Glauche V, Saur D, Weiller C. Activation changes in sensorimotor cortex during improvement due to CIMT in chronic stroke. Restor Neurol Neurosci. 2011;29:299–310. doi: 10.3233/RNN-2011-0600. [DOI] [PubMed] [Google Scholar]
- 38.Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic over expression of a brain growth protein. Proc Natl Acad Sci U S A. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmandke A, Schmandke A, Schwab ME. Nogo-A: multiple roles in CNS development, maintenance, and disease. Neuroscientist. 2014;20:372–386. doi: 10.1177/1073858413516800. [DOI] [PubMed] [Google Scholar]
- 40.Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O’Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 41.Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui YP, Zhang XX, Lu JL, Sui F. New insights into the roles of Nogo-A in CNS biology and diseases. Neurochem Res. 2015;40:1767–1785. doi: 10.1007/s11064-015-1671-5. [DOI] [PubMed] [Google Scholar]
- 43.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 44.Tsai SY, Papadopoulos CM, Schwab ME, Kartje GL. Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke. 2011;42:186–190. doi: 10.1161/STROKEAHA.110.590083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uluç K, Miranpuri A, Kujoth GC, Aktüre E, Başkaya MK. Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. J Vis Exp. 2011;48:1978. doi: 10.3791/1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 47.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, et al. Writing Group Members. Executive summary: heart disease and stroke statistics--2016 update: a report from the american heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 48.Zausinger S, Hungerhuber E, Baethmann A, Reulen H, Schmid-Elsaesser R. Neurological impairment in rats after transient middle cerebral artery occlusion: a comparative study under various treatment paradigms. Brain Res. 2000;863:94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, He Q, Li YY, Li C, Bai YL, Hu YS, Zhang F. Constraint-induced movement therapy promotes motor function recovery and downregulates phosphorylated extracellular regulated protein kinase expression in ischemic brain tissue of rats. Neural Regen Res. 2015;10:2004–2010. doi: 10.4103/1673-5374.172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang DL, Li D, Wang MJ. Mechanism and application of stem cell transplantation in the treatment of ischemic stroke. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:5393–5398. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.