Abstract
Objective
Angiopoietins are postulated diagnostic biomarkers in children and adults with severe sepsis and septic shock. The diagnostic value of angiopoietins in children less than 5 years old has not been established, nor has their effect on permeability in the capillary microvasculature. We aim to determine if levels of angiopoietin-1 or -2 (angpt-1, -2) are diagnostic for severe sepsis/shock in young children and whether they affect the permeability of cultured human dermal microvascular endothelial cells (HDMEC).
Design
Prospective observational study of children< 5 years old. Patients were classified as non-systemic inflammatory response syndrome (SIRS), SIRS/sepsis and severe sepsis/septic shock.
Setting
Tertiary care pediatric hospitals.
Patients
Critically ill children.
Interventions
None
Measurements
Plasma angpt-1 and -2 levels were measured with ELISA. Expression of angpt-2 in endothelial cells was assessed with quantitative polymerase chain reaction. Permeability changes in cultured HDMECs were assessed with trans-endothelial electrical resistance (TEER) measurements.
Results
Angpt-1 levels were significantly higher in younger children compared to levels found in previous study of older children across disease severity (all p<0.001). Angpt-2 was significantly higher in this cohort with severe sepsis/septic shock compared to children without SIRS and SIRS/sepsis (all p<0.003). Angpt-2/1 ratio was also elevated in children with severe sepsis/septic shock but an order of magnitude less than older children (p<0.02, p=0.002). Angpt-1 and -2 did not affect basal HDMEC permeability or modulate leak in isolation or in the presence of tumor necrosis factor (TNF).
Conclusions
Angpt-2 levels and the angpt-2/1 ratio are appropriate diagnostic biomarkers of severe sepsis/septic shock in children less than 5 years old. Neither angpt-1 nor -2 affects basal HDMEC permeability alone or modulates TNF induced capillary leak.
Keywords: Angiopoietin-2, children biomarkers, capillary leak, trans-endothelial electrical resistance
Introduction
Sepsis is a significant cause of morbidity and mortality in children with higher mortality rates described in the very young(1). Since its timely identification and treatment continues to challenge pediatric critical care physicians, much effort has been devoted to isolating biomarkers to aid with diagnosis and eventually suggest therapies. The angiopoietins are a family of vascular growth factors that help to regulate endothelial growth and homeostasis(2). Two members of the angiopoietin family, angiopoietin-1 and -2 (angpt-1, angpt-2) have been postulated as diagnostic biomarkers in older children with severe sepsis and septic shock, but not yet in younger children (3–5). Moreover, the role of these angiopoietins in the important and complex pathophysiologic mechanisms of endothelial dysfunction in sepsis remains unclear(6).
The vascular endothelium’s function is essential to the control of fluid, solute, and macromolecule exchange between blood and tissue(6). Angpt-1 and angpt-2 influence endothelial permeability via activation and antagonism of the tyrosine kinase receptor, TIE-2(2). Angpt-1 acts as a TIE-2 receptor agonist, thereby promoting vessel stabilization through the synthesis of cadherins(7), inhibition of nuclear factor kappa-B (NFκB) migration to the nucleus (8) and the physiologic effects of endotoxin administration (9). Conversely, angpt-2 acts as a TIE-2 receptor antagonist in most circumstances and destabilizes blood vessels by increasing the phosphorylation of the myosin light chain complex (7) and promotes in vivo leak (7, 10, 11). It may also sensitize mouse venular endothelial cells to tumor necrosis factor (TNF) (12).
Furthermore, the effects of the perturbation of circulating angiopoietin levels in severe sepsis and septic shock on microvascular endothelial cells is less established. Many of the above studies investigated mouse models without regard to the specific vascular segment affected. Leak in the venular segment, characterized by disruption of adherins junctions, is physiologic and does not produce systemic effects; whereas capillary leak, characterized by disruption of claudin-5 dependent tight junctions, is pathologic and frequently leads to systemic consequences(13). The vascular segment most affected by angiopoietin axis perturbation in vivo is not known.
Several groups, including our own, demonstrated elevated peripheral blood levels of angpt-2 in critically ill patients with sepsis (7, 14–17), multiple trauma (18, 19), acute lung injury (7, 20, 21) and following cardiopulmonary bypass (22) when compared to healthy controls. More importantly, increased circulating blood angpt-2 levels appeared to be associated with adverse outcomes (14, 18–20, 22). Despite these data, it is not known whether the youngest pediatric patients differ from older children or adults. In our previous cohort, the overall median age was 11 years, with the severe sepsis/septic shock group’s median age of 13 years(4). An especially vulnerable population of children are under 5 years of age. These children have higher mortality rates than older children with severe sepsis or septic shock globally and may require special consideration(23, 24).
To investigate the diagnostic value of angpt-1 and -2 in younger children and infants, we will perform in vitro and in vivo studies. The temporal kinetics of these proteins in children less than 5 years of age throughout their first 4 days of a septic illness will be examined. We will compare these findings to our previously published results from an older cohort. We hypothesize that the angpt-1 and -2 temporal kinetics will be similar to those found in older children. To elucidate the effects of angiopoietins on capillary permeability, we will measure their effects on capillary endothelial cells in vitro, both in isolation and with tumor necrosis factor (TNF). We further hypothesize that angpt-1 will promote capillary barrier stability while angpt-2 would undermine it and that angpt-1 will mitigate and angpt-2 augment TNF-induced leak in human capillary endothelial cells.
Materials and Methods
This prospective observational study was approved through the Pediatric Protocol Review Committee and the Human Investigation Committee at the Yale University School of Medicine. All patients aged less than 5 years of age were evaluated for eligibility. Informed consent was obtained from parents of eligible patients within the first 24 hours of admission to the pediatric intensive care unit (PICU) between September 2013 until July 2016. Exclusion criteria included patients weighing less than 4 kg, hematocrits less than 25%, transfers from outside institutions greater than 24-hours after admission, patients receiving or having received corticosteroids within the previous 7-days, patients with a diagnosis of cancer, receiving renal replacement therapy, extracorporeal membrane oxygenation or plasma exchange, and those with known limitations of care. Blood samples were obtained in conjunction with routine labs obtained for clinical purposes by the PICU team. Therefore, patients for whom blood draws were not anticipated for other reasons or those whose predicted PICU admission was less than a 48-hour PICU were not approached for enrollment.
Enrolled patients were grouped within the first 24-hours of PICU admission into non-systemic inflammatory response syndrome (non-SIRS), SIRS/sepsis, and severe sepsis/septic shock according to the 2005 pediatric sepsis and organ dysfunction definitions (25). Demographics, comorbidities, severity of illness scores (i.e. pediatric index of mortality (PIM)-2 (26)) and indicators of endothelial leak (i.e. net fluid balance divided by patient weight) and outcome data including ICU length of stay (LOS) and mortality were collected. Net fluid balance was calculated by adding the total fluid given in 24 hours, including fluid administered outside of the PICU if documented, minus 24-hour urine output.
Blood Samples
Blood samples were collected in conjunction with clinical lab draws once per day for a maximum 4 samples. A 2-mL whole blood sample was collected in a sodium citrate tube and placed immediately in a refrigerator prior to centrifugation. Samples were then centrifuged at 4000 rpm for 10 minutes at 4°C to separate the cellular components from plasma. The plasma was then frozen at −80°C in 1.5mL Eppendorf tubes (Eppendorf, Hauppauge, NY) until analysis. Angpt-1 and angpt-2 levels were measured by commercially available sandwich enzyme-linked immunoassays (ELISA) using the manufacturer’s protocol (Human Angiopoietin-1 and Angiopoietin-2 Quantikine ELISA Kit; R&D Systems, Minneapolis, MN).
Human cell isolation and culture
Human dermal microvascular cells (HDMECs) were isolated under protocols approved by the Yale Institutional Review Board from de-identified tissue from Yale-New Haven Hospital, New Haven, CT, as previously described(27). Briefly, fresh skin was sectioned, dermatomed and incubated in dispase (Collaborative Biomedical Products) for 30 minutes at 37°C. The dermis was scraped into RPMI 1640 media (Life Technologies), filtered through a 70-pM nylon mesh and thenwas washed and plated in EGM2-MV (Lonza) onto tissue culture plastic (Becton Dickinson) pre-coated with human plasma fibronectin (EMD Millipore). Cells were cultured at 37°C in a humidified 5% CO atmosphere and positively selected for CD31 with magnetic beads (Miltenyi) per the manufacturer’s protocol. The selected cells were cultured and serially passaged in EMG2-MV (Lonza) onto 0.1% gelatin-coated (Sigma) tissue culture ware (Falcon). HDMECs were only serially passaged for an additional 5 passages. Commercially available human pulmonary microvascular cells (HPMECs, Lonza) and human umbilical venous endothelial cells (HUVECs, Yale Vascular Biology Core) were utilized and passaged as described above at identical passage numbers. We utilized HUVECs and HPMECs because they are commonly used for in vitro models of human vasculature. Both HPMECs and HDMECs, unlike the more commonly utilized HUVECs, were used to investigate tight junctions that replicate the capillary segment(28).
Trans-endothelial electrical resistance measurements (TEER)
TEER of HDMEC monolayers was assessed by electrical cell-substrate impedance (ECIS; 8 or 96 well arrays, catalog numbers # 96W20idf or #8W10E+, respectively, both with polyethylene terephthalate; from Applied BioPhysics) stimulated with 10 ng/mL TNF (Life Technologies), 100 ng/mL Angpt-1 and -2 (R&D Biosystems) or phosphate buffered saline (Sigma) as described(27). TEER is recorded in Ohms’ and reported as normalized TEER values (where the value of 1.0 represents the basal TEER measurement immediately before adding cytokine). Data were recorded by an ECIS Z-theta instrument controlled by a Dell personal computer equipped with ECIS software (Applied BioPhysics).
siRNA Inhibition of gene expression and polymerase chain reaction measurements
To block basal angpt-2 production using siRNA knock-down, the HDMECs are plated on gelatin-coated (Sigma) 6-well plates (Flacon, Corning) at 50% confluence were transfected with Lipofectamine RNAiMAX (Invitrogen) complexed to either of three different siRNA sequences that specifically targeted to ANGPT2 per the manufacturers protocol. The siRNA sequences used were commercially available (ThermoFisher s1359, s1260 and s1361 and Dharmacon-001210-02-05 for non-targeting control). All targeting siRNAs gave qualitatively similar results in preliminary ELISA experiments (not shown) and the most effective of the three, s1360, was used in all subsequent experiments. Briefly, siRNA complexes of Lipofectamine (Invitrogen) at 50 μg/ml and siRNA at 100 nM were prepared in Opti-MEM Reduced Serum Medium (Invitrogen), applied to cultures at a final concentration of 20 nM and incubated overnight. Cells were re-transfected 24 hours later then seeded into gelatin-coated ECIS 8-chamber arrays (Applied BioPhysics, catalog #8W10E+) at 100,000 cells per well or passaged for RNA or protein analysis. To isolate RNA, cells were washed with PBS and processed using the RNeasy Mini kit (Qiagen), according to the manufacturer’s protocol. The high-capacity cDNA reverse transcription kit (Applied Biosystems) was used to synthesize cDNA from RNA. All quantitative real-time RT-PCR (qRT-PCR) reactions were assembled with TaqMan gene expression master mix and predeveloped TaqMan gene expression probes (ThermoFisher, Hs02786624_g1 and Hs00169867_m1). Reactions were analyzed on a CFX96 real-time system using CFX Manager software (BioRad Laboratories). Data are presented as expression relative to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Western blot analysis
For immunoblot analyses to confirm angpt-2 knock down and TIE2 phosphorylation, HDMECs were cultured as described in C12 or C24 plastic wells (Flacon) were prepared for electrophoresis with SDS-PAGE gels (Bio-Rad) and transferred onto PVDF filters (Millipore), and immunoblotted as previously described(27). Antibodies for TIE2 and phospho-TIE2 (Cell Signaling Technologies, 4224S and 4221S) were used. Immunoblot images display bands of interest and controls from the same contiguous immunoblots with background levels minimized by adjusting all pixels equally with ImageJ 1.60i software (National Institute of Health).
Statistical Analysis
Based on means obtained from our previous study(4), using repeated measures 2-way ANOVA with 4 time points and an average autocorrelation of 0.20, we estimate that a sample size of 6 patients per group (18 total) will have 80% power at alpha of 2.5% is needed to detect a statistically significant difference over time in the mean levels of angpt-1, angpt-2, and their ratio between patients with severe sepsis/septic shock and either patients with non-SIRS or SIRS/sepsis diagnosis. Plasma samples were batch analyzed with the investigators blinded to all demographic and clinical data except group category. All angpt-1 samples and the non-SIRS and SIRS/sepsis Angpt-2 samples were diluted 15-fold, while the severe sepsis/septic shock angpt-2 samples were diluted 75-fold.
Due to the non-normal distribution of the sample outcome data, the plasma angpt-1 and angpt-2 levels were expressed as medians with interquartile ranges, and were natural log-transformed for subsequent analyses. Group comparisons of continuous variables were done using the Kruskal-Wallis test, followed with pair-wise comparisons using the Mann-Whitney U test. Categorical variables were compared using the Chi-square test, followed with pair-wise comparisons using the Fisher’s Exact test. The mean levels of outcomes of interest over time were compared among the three groups of patients using the covariance pattern modeling, which is similar to the repeated measures analysis of variance, but allows the use of different forms of covariance in the response variable over time. We used unstructured correlation over time. The fixed effects of group, time, and group by time interaction were examined in the models. Least square means and 95% confidence intervals (95%CI) were obtained at each time point for the three groups and plotted. Statistical significance for the between-group comparisons was established at alpha of 2.5% because we were primarily interested in the difference between severe sepsis/septic shock patients and either non-SIRS patients or SIRS/sepsis patients. We also used a sample of 45 children from our previously published study[4] to investigate whether the levels of angpt-1, angpt-2, and their ratio over time were different between children under 5 years of age and older children. Analyses were performed using SAS 9.4
Results
Patient Information
We enrolled 17 patients from 3 different children’s hospitals between September 2013 and July 2017. The median age was 2 years (IQR 0.25–3). Each patient had blood samples obtained on the day of admission and each subsequent day for up to four days. Five patients in the non-SIRS group, 2 in the SIRS/sepsis group and 2 in the severe sepsis/shock group had less than 4 blood samples drawn due to low hematocrits or lack of routine laboratory testing. Plasma samples were obtained from critically ill children in each of the following categories: Non-systemic inflammatory response syndrome (non-SIRS) (n=5), SIRS/sepsis (n=6), and severe sepsis/shock (n=6). There was equal distribution of gender, age, weight and comorbid conditions across the three groups (Table 1). Admission PIM2 scores increased significantly as the severity of illness increased representing the early-phase organ dysfunction associated with sepsis, severe sepsis and shock (Table 1). There were significantly higher rates of invasive ventilation, while differences in PICU and hospital length of stay in the SIRS/Sepsis and severe sepsis/septic shock groups compared to non-SIRS patients did not reach statistical significance (Table 2). One patient with septic shock died (Table 2).
Table 1.
Admission Demographics
| Variable | Non-SIRS n=5 | SIRS/Sepsis n=6 | Severe Sepsis/Shock n=6 | P-value |
|---|---|---|---|---|
| Gender, male | 5 (100%) | 2 (33%) | 4 (67%) | 0.071 |
| Age, years | 0.42 (0.17–0.75) | 2 (0.58–2.75) | 3 (3–3.75) | 0.182 |
| Weight, kg | 7.6 (4.7–8.2) | 13 (6.38–13.83) | 15.5 (8.25–16) | 0.422 |
| Comorbidity | 1 (20%) | 3 (50%) | 3 (50%) | 0.521 |
| PIM2, % | 0.9 (0.8–2.5) | 1.75 (0.95–3.6) | 30.75 (2.5–64.55) | 0.063 |
| Net Ins/Outs, mL | 179 (82–916) | 156 (−129–452) | 1310 (310–1826) | 0.212 |
Patient demographic information upon admission to the PICU. Data presented as medians with (interquartile ranges) or numbers with (%); kg=kilograms; PIM2=Pediatric Index of Mortality.
Chi-square test, followed by pair-wise Fisher’s Exact test;
Kruskal-Wallis test, followed by pair-wise Mann-Whitney test;
Linear Trend Analysis
Table 2.
Patient Outcomes
| Variable | Non-SIRS n=5 | SIRS/Sepsis n=6 | Severe Sepsis/Shock n=6 | P-value |
|---|---|---|---|---|
| Invasive Ventilation | 1 (20%) | 1 (17%) | 5 (83%) | 0.031 |
| PICU LOS, days | 4 (2–8) | 8 (5–11.75) | 6.5 (5.25–7) | 0.702 |
| Hospital LOS, days | 5 (4–18) | 14.5 (11.75–20.25) | 10 (7.5–11.75) | 0.482 |
| Mortality | 0 (0%) | 0 (0%) | 1 (17%) | --- |
Patient outcome data are presented as medians with (interquartile ranges) or numbers with (%). Invasive ventilation was through an endotracheal tube. LOS=length of stay
Chi-square test, followed by pair-wise Fisher’s Exact test;
Kruskal-Wallis test, follow by pair-wise Mann-Whitney test
Temporal Kinetics of Angpt-1, Angpt-2, and Angpt2/1 Ratio
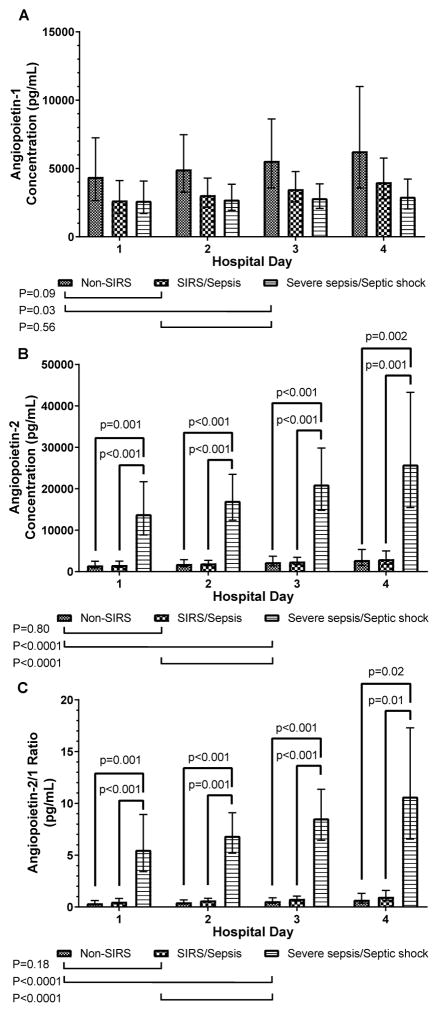
Angpt-1 levels were elevated across all hospital days between in the non-SIRS group compared to the severe sepsis/shock group (p=0.03), while a similar trend was observed for the SIRS/sepsis group vs. the severe sepsis/septic shock group (p=0.09, Figure 1A). Angpt-2 levels were significantly higher across all hospital days in the severe sepsis/shock group compared to non-SIRS and SIRS/sepsis groups (p<0.0001), with significant differences within each hospital day (Figure 1B). Due to the agonist/antagonist interaction with the TIE-2 receptor, we also determined the Angpt-2/1 ratio for each time point. The angpt-2/1 ratio was significantly elevated across all hospital days in the severe sepsis/shock group compared to the other two groups, with significant differences within each hospital day (p<0.001, Figure 1C).
Figure 1.
Angiopoietin levels and angpt-2/1 ratios from admission to hospital day 4.
Angiopoietin levels in children less than 5 years old admitted to the pediatric intensive care unit are compared. (A) Angpt-1 levels over the first 4 hospital days comparing groups within each day and over time. (B) Angpt-2 levels over the first 4 hospital days comparing groups within each day and over time. (C) Angpt-2/1 ratio over the first 4 hospital days comparing groups within each day and over time.
Comparison between the older and younger cohorts
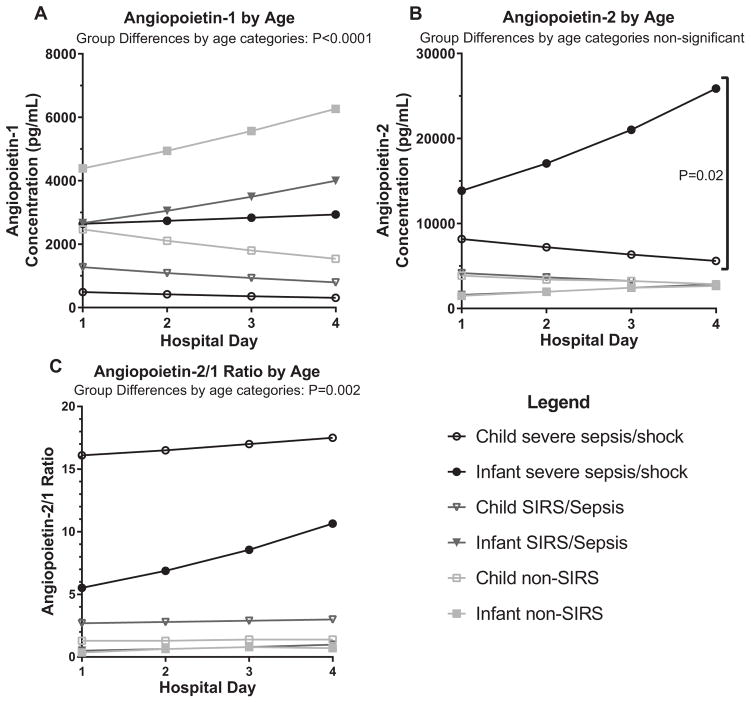
The current cohort differed from our previously reported cohort (overall median age 11 years, IQR 5.5–15(4)) in several ways (Figure 2A–C). Younger children had significantly higher levels of angpt-1 (p<0.0001) regardless of diagnoses. Additionally, significant differences were observed for angpt-2 between diagnoses, with the younger children with severe sepsis/shock having higher levels (p=0.02). Furthermore, the elevated angpt-1 levels in younger children resulted in significantly lower angpt-2/1 ratios (p=0.002) on most days, with a trajectory that continued to rise at day 4 compared to a peak at day 2 in the older cohort.
Figure 2.
Angiopoietin level comparisons between younger and older children cohorts
Angiopoietin levels are compared between the current infant cohort (median 0.42–3 years) and the previously reported child cohort (median 9.5–13 years) over the first 4 days of pediatric ICU admission. The infant groups are represented by closed shapes whereas the child groups are represented by closed shapes. (A) Angpt-1 levels by age. (B) Angpt-2 levels by age. (C) Angpt-2/1 ratio by age.
Expression, secretion and knock-down of angpt-2 in cultured cells
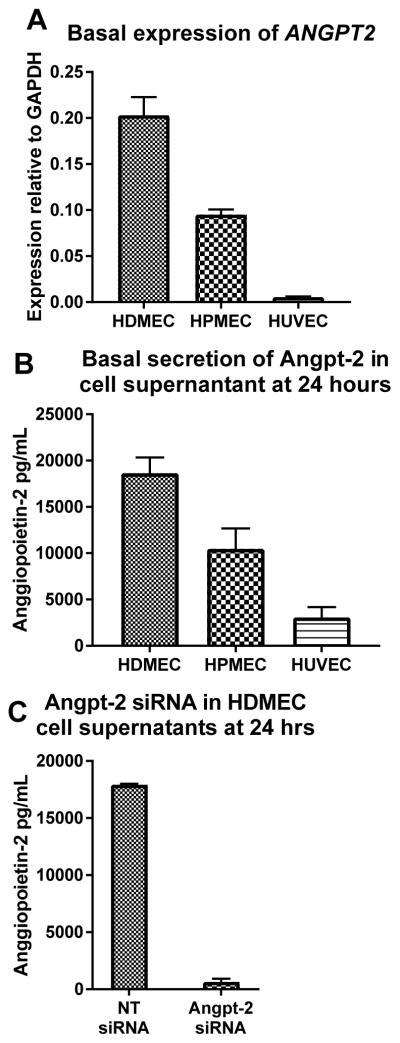
In standard culture conditions, HDMECs (mean: 20.3% of GAPDH expression, standard deviation: 1.9) express significantly more ANGPT2 than HPMECs (mean: 9.5%, SD: 0.6), which in turn express more than HUVECs (mean: 6%, SD: 0.1, all p<0.01, Figure 3A). Similarly, HDMEC secrete more Angpt-2 (mean: 18,643 pg/mL SD: 1,692) than HPMEC (mean: 10,476 pg/mL, SD: 2,204) and HUVECs (mean: 3,061 pg/mL, SD: 1,109, Figure 3B) after 24 hours. HDMECs were used for permeability experiments because, unlike HUVECs, they have been shown to replicate the capillary segment of the microvasculature(28), and Angpt-2 is suspected to originate from and act on the capillary micro-vessels. We used short-interfering (si)-RNA to knock down Angpt-2 secretion in HDMECs for all experiments with good effect (NT siRNA mean: 17,921 pg/mL, SD: 88.2, ANGPT2 siRNA mean 638 pg/mL, SD: 284, p=0.0001, Figure 3C).
Figure 3.
Expression and secretion of angpt-2 in cultured cells
Cultured endothelial cell angiopoietin-2 expression. (A) ANGPT2 expression relative to the house-keeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) comparing human dermal microvascular endothelial cells (HDMEC), human pulmonary microvascular endothelial cells (HPMEC) and human umbilical venous endothelial cells (HUVEC). (B) Angiopoietin-2 secretion across the three compared cell lines. (C) Angiopoietin-2 secretion in HDMEC after siRNA knock-down.
Analysis of angiopoietin effect on TEER and TIE2 activation
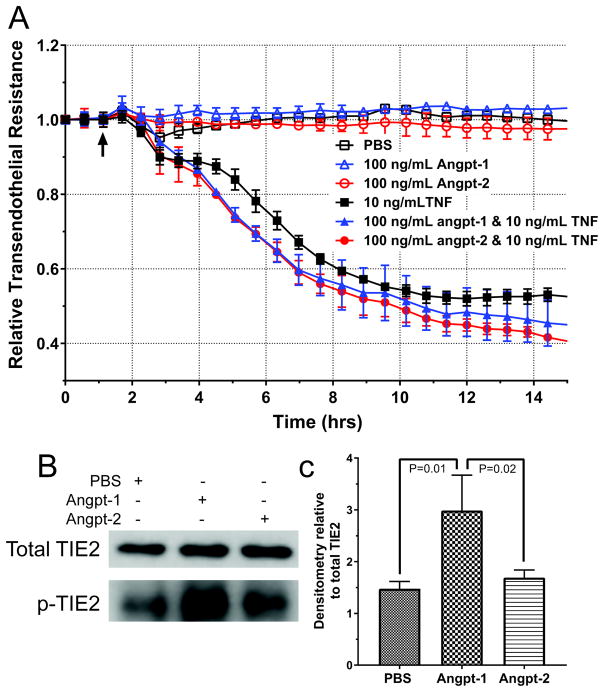
HDMECs with ANGPT2 knocked-down with siRNA demonstrate stable barriers around 4,000Ω, consistent with unmodified and non-targeting siRNA treated HDMECs. Surprisingly, the HDMECs barrier did not change when treated with 100 ng/mL of angpt-1 or -2 (Figure 4A, open shapes). Similarly, HDMEC pretreated with angpt-1 or -2 did not display any differences in TNF-induced permeability changes compared to untreated HDMEC (Figure 4A, closed shapes). Stimulation of HDMECs with angpt-1, but not angpt-2 resulted in increased phosphorylation of the TIE2 receptor (Figure 4B, quantification 4C). Dose response of HDMEC treated with angpt-1 or -2 and variable doses of TNF demonstrated with similar effect (Supplemental Figure 1). Importantly, basal levels of phosphorylated-TIE2 were observed in PBS and angpt-2 treated HDMECs indicating there may be significant levels of TIE2 signaling in culture and a significant difference from truly quiesced endothelium in vivo.
Figure 4.
Permeability changes in human dermal microvascular cells (HDMEC)
Trans-endothelial electrical resistance (TEER) of HDMEC monolayers does not change following treatment with 100ng/mL of angiopoietin-1 or -2 (angpt-1, -2) or control phosphate-buffered saline (PBS) (A, open shapes). TEER changes of HDMEC monolayers following cotreatment with angiopoietins and tumor necrosis factor (TNF) are not significantly different (A, solid shapes). Addition of 100 ng/mL of angpt-1 but not angpt-2 to HDMEC increases levels of phosphorylated TIE2 (B, quantification, C).
Discussion
We have demonstrated that children less than 5 years old admitted to the PICU with severe sepsis and shock have elevated plasma levels of angpt-2 and the angpt-2/1 ratio when compared to children of similar age with or without SIRS or sepsis. This result is consistent with the response in older children and adults. However, unlike studies in older children, we showed a significant elevation in angpt-1 levels, with consequently lower angpt-2/1 ratios, across all diagnosis groups, despite higher and more variable angpt-2 levels. We have also shown that angpt-1 and -2 do not appear to influence vascular permeability in the capillary segment without the presence of a known capillary modulator, TNF.
Children under 5 years of age have consistently shown higher severe sepsis prevalence and mortality than older patients(1, 24). Developing a clearer understanding of pathophysiology and focusing on earlier recognition in this age group may improve outcomes. Taken together, our data indicate that the angiopoietin axis is more perturbed in younger children with severe sepsis and shock than has been established in older children. Elevated and more variable angpt-1 levels in this age group is a novel observation of unknown significance and etiology. Possible explanations include increased numbers or activity of pericytes (known to secrete angpt-1), or a higher relative percentage of angiogenic endothelium(29). The significantly higher and continually rising (thought hospital day 4) angpt-2 levels in younger children compared to older children has not been previously described. We speculate that this may be the result of a more active vascular system in growing infants compared to adolescents as is seen in the bone growth plate of neonates(30).
Angpt-2 is widely thought to represent endothelial activation or injury in the setting of critical illness, however its effects on the capillary microvascular are not known. Multiple reports implicate angpt-2 in the diagnosis(31) and mechanism(32) of acute lung injury in vivo, however, none of these studies have identified the vascular segment most affected. To clarify this in our study, we used trans-endothelial electrical resistance (TEER) to assess monolayer permeability because this technique provides real-time, non-invasive, label free and quantitative measure of permeability and has greater sensitivity than tagged-protein trans-well experiments(28, 33). Previous TEER studies of HDMECs have shown that they replicate the capillary microvascular by forming high resistance barriers which are dependent on tight junctions, characterized by claudin-5, occludin and junctional adhesion molecule proteins(28). We found angpt-1 had no effect on the TEER of HDMEC despite increased TIE2 activation, while angpt-2 had no effect on TEER in the absence of TIE2 activation. These data cast doubt on the presumed effects of angiopoietins on permeability in the capillary segment, and may suggest that angiopoietins predominately alter permeability in the venular segment, as seen in early sepsis or following cardiopulmonary bypass(22). Moreover, the angiopoietins did not modulate the permeability-altering effect of TNF, a potent activator of the NF-κB signaling cascade(28).
The elevated angpt-2 levels and the angpt-2/1 ratios described in pediatric and adult cohorts of severe sepsis and septic shock may play a role in the necessary physiologic venular changes necessary for leukocyte trafficking to extravascular sites of infection(10). This physiologic increase in venular permeability, and subsequent edema, appears to be the result of a down regulation of integrin and cadherin proteins typically found on these segments of the vascular system(34) and may peak at about day 2–3 in survivors(3, 7). Children who have disease progression or organ failure may subsequently develop capillary leak irrespective of the actions of the angiopoietins on the endothelium. More research is needed to identify triggers that might shift from physiologic venular to pathologic capillary leak. Novel pharmacological therapies targeting these later triggers may allow for reductions in pediatric severe sepsis and septic shock mortality.
There are several limitations to our study. First, sample size calculations based on previous studies were performed using means and again with ½ means. The sample size totals remained small, ranging from 6 total patients to 15 total patients. Though we believe the statistics are valid, clinical conclusions may be difficult to make with such a small sample size. Enrollment occurred over a 3-year period due to three primary reasons: (1) few patients in the non-SIRS, SIRS and sepsis groups had clinical laboratory tests drawn every day, (2) many patients were receiving steroids (i.e. asthma treatment) or had received steroids (i.e. within bypass fluid), or (3) anticipated PICU length of stay was less than 48 hours. Despite this, significant differences were observed between groups and timepoints, indicating sufficient power. Additionally, treatment standards and guidelines remained similar over this 3-year period. Regarding our limited in vitro studies, there was basal phosphorylation of TIE2 in HDMECs, which may produce constitutively active downstream signaling (however phosphorylated TIE2 phosphorylation increased with angpt-1). HDMEC are isolated from skin and may not replicate the permeability responses of capillary endothelium in other organs, even though cutaneous edema is frequently seen in clinically significant vascular leak. Likewise, essential functions of capillaries may not be replicated in culture of human dermal microvascular cells. Future studies will focus on adapting in vivo models to explore the effects of angiopoietins on organ specific capillary segments.
Conclusions
This study further establishes angpt-2 as a diagnostic biomarker of severe sepsis and septic shock in children less than 5 years old, although elevated angpt-1 levels may make the angpt-2/1 ratio less diagnostic. Additionally, angpt-1 and -2 do not appear to mitigate or exacerbate basal or TNF-mediated permeability changes in the capillary vascular segment, although additional investigation is required.
Supplementary Material
TNF dose titration on human dermal microvascular cells (HDMEC)
Trans-endothelial electrical resistance (TEER) of HDMEC treated with either 100 ng/mL of angiopoietin (angpt)-1 or -2 and variable doses of TNF. The addition of angpt-1 or -2 does not modulate the effects of lower dose TNF on HDMEC permeability.
Acknowledgments
We would like to acknowledge the patients and families who are the inspiration for this work as well as the PICU physicians and nursing staff. Dr. Pierce received support from the National Institutes of Health (NIH, T32HD068201) and the Department of Pediatrics with Dr. Giuliano. Dr. Shabanova received support from CTSA (UL1 RR024139) from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS) and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
This work was conducted at Yale University with sample collection at Yale New Haven Children’s Hospital, Maria Fareri Children’s Hospital and Baystate Children’s Hospital.
This work was supported by NIH R01HL36003 and T32HD068201, NCRR and NCATS UL1 RR024139 as well as funds from the Departments of Immunobiology and Pediatrics.
Abbreviations
- Angpt-1
Angiopoietin-1
- Angpt-2
Angiopoietin-2
- NFκB
nuclear factor kappa-B
- TNF
tumor necrosis factor
- PICU
pediatric intensive care unit
- SIRS
systemic inflammatory response syndrome
- PIM2
pediatric index of mortality-2
- HDMEC
human dermal microvascular endothelial cells
- HPMEC
human pulmonary microvascular endothelial cells
- HUVEC
human umbilical vein endothelial cells
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- TEER
trans-endothelial electrical resistance
- ECIS
electrical cell-substrate impedance sensing
Footnotes
No reprints will be requested
References
- 1.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, Singhi SC, Erickson S, Roy JA, Bush JL, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parikh SM. Targeting Tie2 and the host vascular response in sepsis. Science Translational Medicine. 2016;8(335):1–3. doi: 10.1126/scitranslmed.aaf5537. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano JS, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS. Admission Angiopoietin Levels in Children with Septic Shock. Shock; PAP. 2007 [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano JS, Jr, Tran K, Li FY, Shabanova V, Tala JA, Bhandari V. The temporal kinetics of circulating angiopoietin levels in children with sepsis. Pediatr Crit Care Med. 2014;15(1):e1–8. doi: 10.1097/PCC.0b013e3182a553bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Bhandari V, Giuliano JS, Jr, CS OH, Shattuck MD, Kirby M. Angiopoietin-1, angiopoietin-2 and bicarbonate as diagnostic biomarkers in children with severe sepsis. PLoS One. 2014;9(9):e108461. doi: 10.1371/journal.pone.0108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce RW, Giuliano JS, Pober JS. Endothelial cell function and dysfunction in critically ill children. Pediatrics. 2017;140(1):e20170355. doi: 10.1542/peds.2017-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes DP, Marron MB, Brindle NP. The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-kappaB inhibitor ABIN-2. Circ Res. 2003;92(6):630–636. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 9.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation. 2005;111(1):97–105. doi: 10.1161/01.CIR.0000151287.08202.8E. [DOI] [PubMed] [Google Scholar]
- 10.Sturn DH, Feistritzer C, Mosheimer BA, Djanani A, Bijuklic K, Patsch JR, Wiedermann CJ. Angiopoietin affects neutrophil migration. Microcirculation. 2005;12(5):393–403. doi: 10.1080/10739680590960296. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari V, Elias JA. The role of angiopoietin 2 in hyperoxia-induced acute lung injury. Cell Cycle. 2007;6(9):1049–1052. doi: 10.4161/cc.6.9.4229. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 13.Redl H, Dinges HP, Buurman WA, Linden CJvd, Pober JS, Cotran RS, Schlag G. Expression of endothelial leukocyte adhesion molecule-1 in spetic but not traumatic/hypovolemic shock in the baboon. American Journal of Pathology. 1991;139(2):461–466. [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliano JS, Jr, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS. Admission angiopoietin levels in children with septic shock. Shock. 2007;28(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- 15.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35(1):199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 16.Siner JM, Bhandari V, Engle KM, Elias JA, Siegel MD. Elevated Serum Angiopoietin 2 Levels Are Associated with Increased Mortality in Sepsis. Shock. 2008 doi: 10.1097/SHK.0b013e318188bd06. [DOI] [PubMed] [Google Scholar]
- 17.Kumpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12(6):R147. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, Christiaans SC, Bir ND, Pittet JF. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247(2):320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 19.Giamarellos-Bourboulis EJ, Kanellakopoulou K, Pelekanou A, Tsaganos T, Kotzampassi K. Kinetics of angiopoietin-2 in serum of multi-trauma patients: correlation with patient severity. Cytokine. 2008;44(2):310–313. doi: 10.1016/j.cyto.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, Sukhatme VP. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29(6):656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63(10):903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 22.Giuliano JS, Jr, Lahni PM, Bigham MT, Manning PB, Nelson DP, Wong HR, Wheeler DS. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med. 2008;34(10):1851–1857. doi: 10.1007/s00134-008-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Liddell CA, Coates MM, Mooney MD, Levitz CE, Schumacher AE, Apfel H, Iannarone M, Phillips B, Lofgren KT, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9947):957–979. doi: 10.1016/S0140-6736(14)60497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 26.Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 27.Kluger MS, Clark PR, Tellides G, Gerke V, Pober JS. Claudin-5 controls intercellular barriers of human dermal microvascular but not human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(3):489–500. doi: 10.1161/ATVBAHA.112.300893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark PR, Kim RK, Pober JS, Kluger MS. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-kappaB-dependent phases. PLoS One. 2015;10(3):e0120075. doi: 10.1371/journal.pone.0120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avolio E, Rodriguez-Arabaolaza I, Spencer HL, Riu F, Mangialardi G, Slater SC, Rowlinson J, Alvino VV, Idowu OO, Soyombo S, et al. Expansion and characterization of neonatal cardiac pericytes provides a novel cellular option for tissue engineering in congenital heart disease. J Am Heart Assoc. 2015;4(6):e002043. doi: 10.1161/JAHA.115.002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horner A, Bord S, Kelsail A, Coleman N, Compston J. Tie2 Ligands Angiopoietin-1 and Angiopoietin-2 Are Coexpressed With Vascular Endothelial Cell Growth Factor in Growing Human Bone. Bone. 2001;28(1):65–71. doi: 10.1016/s8756-3282(00)00422-1. [DOI] [PubMed] [Google Scholar]
- 31.Zinter MS, Spicer A, Orwoll BO, Alkhouli M, Dvorak CC, Calfee CS, Matthay MA, Sapru A. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol. 2016;310(3):L224–231. doi: 10.1152/ajplung.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One. 2013;8(8):e70459. doi: 10.1371/journal.pone.0070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bischoff I, Hornburger MC, Mayer BA, Beyerle A, Wegener J, Furst R. Pitfalls in assessing microvascular endothelial barrier function: impedance-based devices versus the classic macromolecular tracer assay. Sci Rep; 2016;6:23671. doi: 10.1038/srep23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Felcht M, Kruse K, Kretschmer S, Deppermann C, Biesdorf A, Rohr K, Benest AV, Fiedler U, Augustin HG. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J Biol Chem. 2010;285(31):23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TNF dose titration on human dermal microvascular cells (HDMEC)
Trans-endothelial electrical resistance (TEER) of HDMEC treated with either 100 ng/mL of angiopoietin (angpt)-1 or -2 and variable doses of TNF. The addition of angpt-1 or -2 does not modulate the effects of lower dose TNF on HDMEC permeability.