Abstract
Objective:
We examined the relationship between urine tenofovir (TFV) levels measured with a novel immunoassay, which permits point-of-care (POC) testing, with HIV seroconversion and objective adherence metrics in a large PrEP demonstration project.
Design:
Secondary analysis of stored specimens from an open-label PrEP cohort study
Methods:
We examined the association between undetectable urine TFV levels and HIV seroconversion in iPrEx-OLE using generalized estimating equations. We examined rank correlations between levels of TFV and emtricitabine (FTC) in urine, dried blood spots (DBS), and hair and determined the sensitivity and specificity of undetectable urine TFV for predicting dosing cut-offs in DBS.
Results:
The median urinary TFV level was 15,000 ng/ml in those who remained HIV-negative (n=105; IQR:1,000–45,000); 5,500 in those who eventually seroconverted (n=11; IQR:1,000–12,500); and all were undetectable at seroconversion (n=9; p<0.001). Decreasing strata of urine TFV levels were associated with future HIV seroconversion (p=0.03). An undetectable urine TFV was 100% sensitive and 81% specific when compared to an undetectable DBS TFV-DP level and 69% sensitive, but 94% specific, when compared to low adherence by DBS (<2 doses/week).
Conclusions:
Urine TFV detection by a novel antibody-based assay was associated with protection from HIV acquisition among individuals on PrEP. Urine TFV levels were correlated with hair and DBS levels and undetectable urine TFV was 100% sensitive in detecting non-adherence. By implementing the immunoassay into a POC strip test, PrEP non-adherence could be detected in real-time, allowing rapid intervention.
Keywords: Immunoassay, PrEP, Point-of-care, Urine, HIV infection, Antiretroviral adherence, Tenofovir
INTRODUCTION
The accurate interpretation of major tenofovir (TFV) disoproxil fumarate (TDF)/emtricitabine (FTC)-based pre-exposure prophylaxis (PrEP) trials relied on the use of pharmacologic metrics of adherence, rather than self-report [1–3]. Pharmacologic metrics assess TFV/FTC drug levels (or their metabolites) in biomatrices such as plasma or dried blood spots (DBS). For instance, in the iPrEx Open-Label Extension (iPrEx-OLE) study, HIV-incidence decreased from 4.7 infections per 100 person-years in those with no detectable TFV-diphosphate (TFV-DP) in DBS to 0 with high estimated adherence (≥4 tablets/week) [1]. Objective adherence metrics have therefore been incorporated into PrEP implementation programs to aid interpretation of effectiveness [4–7].
Although PrEP adherence has been higher in demonstration projects than clinical trials after PrEP’s efficacy was known, adherence to PrEP over time, or PrEP persistence, has been a challenge among at-risk populations [4, 8, 9]. In the United States (U.S.) PrEP Demo Project, one-fifth of participants had DBS drug levels consistent with sub-optimal adherence by study end (<4 tablets/week) [4]. A demonstration project among young U.S. men who have sex with men (MSM; ages 18–22) found sub-optimal adherence in 76% of individuals by study end [6].
Investigators have attempted to use PrEP drug levels in plasma, urine, and DBS to target adherence interventions to individuals with the highest need [7, 10–12]. However, the turn-around time of the current available methods to analyze drug levels in any biomatrix using liquid chromatography tandem-mass spectrometry (LC-MS/MS) may limit the impact [11, 13]. Spectrometry-based methods for PrEP drug levels will also be difficult to implement clinically due to expense and the need for trained personnel [13, 14]. Antibody-based measurement of TFV is attractive because it can be developed into a rapid strip test available at the point-of-care (POC), similar to a urine pregnancy test, allowing immediate adherence intervention [13, 14].
We developed a novel antibody-based immunoassay for TFV that evaluates recent PrEP adherence (within 96 hours) and have previously shown that urine TFV levels by the immunoassay are highly correlated with those quantified via LC-MS/MS in healthy volunteers [14]. In a sub-study of iPrEx-OLE, we examine for the first time the association between urine TFV levels measured by the immunoassay and HIV seroconversion events in individuals taking PrEP, and compare urine tenofovir (TFV) levels by the immunoassay to drug level measurement in DBS and hair.
METHODS:
Participants Who Qualified for the iPrEx-OLE Urine Adherence Substudy
The iPrEx-Open Label Extension (OLE) provided PrEP to 1,085 MSM and 140 transwomen [1]. Urine was collected every 12 weeks and DBS was prepared 4 and 8 weeks after PrEP initiation, and then every 12 weeks. DBS assays for TFV-DP and FTC-triphosphate (FTC-TP) were analyzed at all visits for participants who seroconverted in iPrEx-OLE and in a random subset of those who remained HIV-negative [1]. Hair samples for TFV and FTC were collected every 12 weeks for all who provided opt-in consent and analyzed among seroconverters and a random subset of those who remained HIV-negative[15]. Participants who qualified for the correlation analysis required sample availability from all three biomatrices (urine, DBS, hair) at one or more visits over the duration of iPrEx-OLE (median 72 weeks). Additional urine samples from seroconverters (n=10) were included in the specific analysis looking at the association between urine TFV levels and seroconversion. All individuals in the study provided informed consent, including for sample storage and further testing, the institutional review board from each study site approved the study.
Laboratory Procedures
The development of the antibody specific for TFV and the performance characteristics of the resultant immunoassay were recently described [14]. Any TFV level below the lower limit of quantification (LLOQ) was considered negative (<1000ng/ml). The upper limit of quantification for the immunoassay is 50,000 ng/ml. Using previously described and validated LC-MS/MS-based methods, TFV-DP and emtricitabine triphosphate (FTC-TP) concentration were measured in DBS [16], and FTC and TFV concentrations were measured in hair [17].
Statistical Analysis
For the seroconversion analysis, urine TFV concentrations via the immunoassay were compared using Kruskal Wallis’ test among individuals 1) at the seroconversion visit; 2) prior to the seroconversion visit; and 3) those who remained HIV-negative. We analyzed receiver operating curves (ROC) to identify two urine TFV cut-points compared to the outcome of future HIV seroconversion. Mixed-effects logistic regression examined the association between the cut-points and HIV seroconversion only in the samples collected prior to the seroconversion visit. Spearman correlation coefficients and scatterplots were examined to assess the relationship between TFV urine concentrations via the immunoassay and both TFV-DP and FTC-TP levels in DBS and TFV and FTC levels in hair for participants with samples from all three biomatrices. The sensitivity and specificity of the urine assay at an undetectable urine TFV level (<1000 ng/ml) was compared to two levels of inadequate adherence defined by TFV-DP concentrations in DBS: (1) the limit of quantification (<3.5 fmol/punch) and (2) very low adherence (<350 fmol/punch, estimated average weekly adherence of <2 tablets/week) [1, 18]. We selected the lower limit of detection for the urine assay (1000ng/ml) as the optimal single cut-off based on analysis of ROC curves and prior data examining LC-MS/MS-based methods to quantify TFV levels in urine [10].
RESULTS:
Urine, DBS, and hair samples were available from 125 individuals across 162 person-visits. The median age of the 125 participants was 30 years (interquartile range (IQR): 24–40). Overall, 5% were transwomen; 14% were Black and 44% Latino. Overall, 34% of participants who remained HIV negative had urine tenofovir levels indicating poor recent adherence compared to 60% of individuals prior to seroconversion, and 100% at the time of seroconversion. Of the 20 individuals who HIV seroconverted in this substudy, samples were available at 14 person-visits from 11 individuals prior to seroconversion and from 9 participants at the actual visit where seroconversion was documented. The median time between sample collection and HIV seroconversion in these 20 participants was 15 weeks (intraquartile range (IQR): 0–36 weeks).
Urine TFV levels among HIV seroconverters
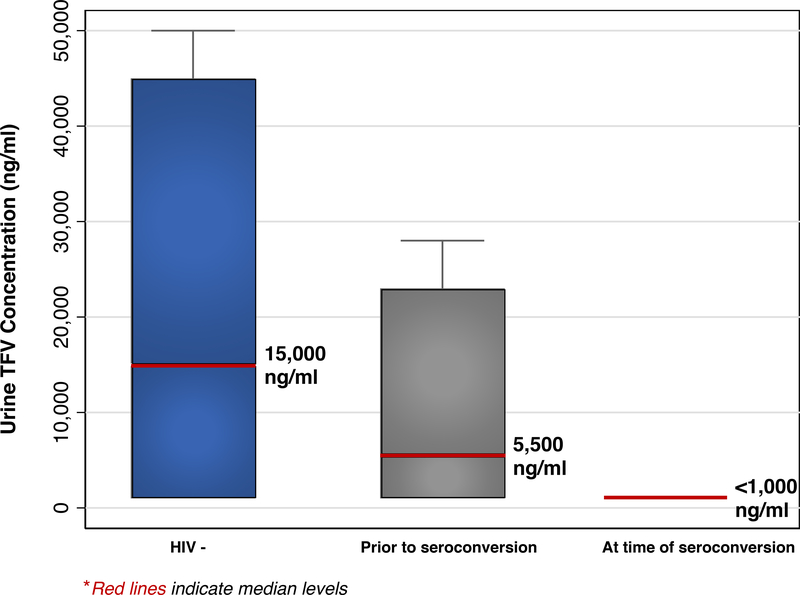
The median urinary TFV level by the immunoassay was 15,000 ng/ml (IQR: BLQ-45,000) in those who remained HIV-negative (n=105); 5,500 ng/ml (IQR: BLQ-23,000) in those who eventually seroconverted (p<0.001; n=11; median of 36 weeks prior to seroconversion); and undetectable (<1000 ng/mL) in all 9 individuals at the seroconversion visit (p<0.001) (Figure 1). The optimal urine cut-points for future HIV seroconversion based on ROC analysis were 1,000 and 25,000 ng/ml (Supplemental Figure 1). Decreasing strata of urine TFV levels were associated with future HIV seroconversion (p=0.03). A low/undetectable vs. high (≤1000 vs. >25,000 ng/ml) urine TFV was associated with future HIV seroconversion (odds ratio (OR) 13.7; 95% confidence interval (CI): 1.25–1197), while the p-value when comparing an intermediate and high level was 0.11 (OR 6.3; 95%CI 0.23–474).
Figure 1:
Box plot showing ELISA-immunoassay urine TFV levels among iPrEx OLE participants who did not acquire HIV; those prior to seroconversion (median 36 weeks); and those at the time of seroconversion
Association of TFV urine levels with DBS and hair concentrations
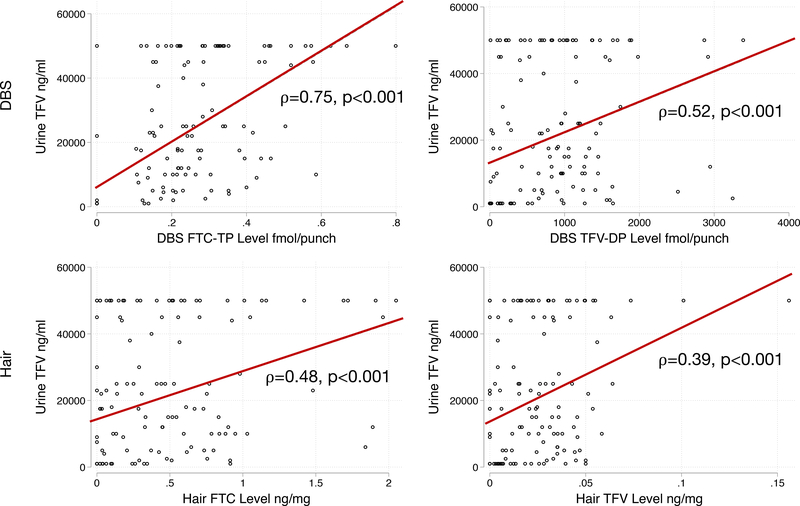
Across 152 person-visits in the correlation analysis, urine TFV levels correlated with hair concentrations of TFV (Rho (ρ)=0.39, p<0.001) and FTC (ρ=0.48, p<0.001), as well as with DBS concentrations of TFV-DP (ρ=0.52, p<0.001) and FTC-TP (ρ=0.75, p<0.001) (Figure 2). An undetectable urine TFV level was 100% sensitive and 81% specific when compared to an undetectable TFV-DP concentration in DBS. When comparing an undetectable urine TFV level to very low adherence estimated by DBS TFV-DP level (<2 tablets/week), the urine test was 69% sensitive and 94% specific (Supplemental Figure 2).
Figure 2:
Correlation of Urine Tenofovir (TFV) with Dried Blood Spot (DBS) Emtricitabine Triphosphate (FTC-TP) and Tenofovir Diphosphate (TFV-DP) and Hair FTC and TFV
Discussion:
We demonstrate for the first time that urine TFV levels by a novel immunoassay are associated with future HIV acquisition in a diverse sample of MSM and transwomen enrolled in a PrEP demonstration project. Urine TFV levels correlated with hair and DBS metrics, established methodologies essential to interpretation of later-stage PrEP trials [1, 3, 5, 6]. An undetectable urine tenfovir level with the immunoassay was highly sensitive in detecting non-adherence and was highly specific in detecting low adherence via DBS metrics.
TFV levels in urine, like in plasma, are a short-term measure of adherence, detecting TFV ingestion 4 days prior to measurement [10]. PrEP drug/metabolite levels in hair and DBS, respectively, provide estimates of adherence over certain established time frames (shortest to longest time frames: DBS FTC-TP< DBS TFV-DP< hair FTC< hair TFV levels) [19, 20]. The correlation coefficients of TFV levels in urine (shortest-term metric) and PrEP metrics in DBS/hair follow a monotonic pattern according to duration of exposure represented by each established metric (Figure 2). Although DBS and hair levels better assess drug-taking over longer time periods, they require specialized personnel and LC-MS/MS equipment, resulting in high cost and long turn-around times [13, 14]. Antibody-based assays, such as the one described here, can be developed into point-of-care lateral flow immunoassays (LFAs). LFAs require no special equipment or training to implement, permitting real-time adherence feedback at the clinic or even at home, with a projected cost of approximately <$2 U.S. dollars [14].
A limitation of this study is the lack of case-cohort sampling among the specimens, prohibiting examination of HIV incidence. We were only able to retrospectively test the urine samples by the immunoassay that were stored in iPrEx-OLE. Moreover, urine was not available on all seroconverters in iPrEx-OLE for this analysis. There were 28 seroconverters on PrEP in the overall cohort and we had data on 20 (71%) [1]. The immunoassay may be limited in assessing adherence in the context of intermittent PrEP if previous exposures occurred more than 4 days prior. Future studies should measure TFV levels by the immunoassay prospectively, as well as a long-term adherence measurement in DBS or hair, to more accurately determine short and long-term patterns of adherence in diverse populations. Finally, the LFA for POC TFV urine level monitoring is still under development (anticipated data January 2019) using cut-offs for high, moderate, and low levels of adherence provided by a directly-observed (DOT) study [21].
A real-time test showing low TFV levels will allow immediate feedback and could trigger established PrEP adherence interventions. Further qualitative research is needed to understand how to deliver results in a motivating, non-judgmental manner. Prior studies noted that adherence feedback using laboratory-based assays is valuable to patients, and discussion of sub-optimal drug level results can improve subsequent adherence [7, 11, 12]. Furthermore, feedback delivered in a patient-centered manner, emphasizing protection from HIV rather than adherence, increases acceptance [11, 12]. A low-cost, POC adherence metric could allow clinics to focus their resources on individuals who need the most support [7], an important consideration as PrEP delivery moves towards automated and home-based delivery to meet demand and patient preference [7, 22, 23].
In conclusion, we apply a novel immunoassay which quantifies TFV levels in urine to a cohort study for the first time and show that TFV levels are associated with both future HIV acquisition and other established pharmacologic metrics of adherence. The antibody-based assay is expected to be available as a low-cost, POC adherence metric within the year. Studies evaluating the POC adherence metric’s potential to motivate and support PrEP persistence in diverse populations are needed.
Supplementary Material
Acknowledgements:
Work supported by NIAID/NIH 2R01AI098472 (PI: M.G.), R01AI143340 (PI: M.G.), R01AI118575 (PI: R.M.G.), and 5T32AI060530 (recipient: M.A.S.). S.P.B. and R.M.G. have lead studies in which Gilead Sciences donated study drug. The other authors have no conflicts of interest to declare.
Conflicts of Interest and Source of Funding: S.P.B. and R.M.G. have lead studies in which Gilead Sciences donated study drug. The other authors have no conflicts of interest to declare. Work supported by NIAID/NIH 2R01AI098472 (PI: M.G.), R01AI143340 (PI: M.G.), R01AI118575 (PI: R.M.G.), and 5T32AI060530 (recipient: M.A.S.).
References:
- 1.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis 2018; 66(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med 2016; 176(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinsztejn B, Hoagland B, Moreira RI, Kallas EG, Madruga JV, Goulart S, et al. Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV 2018; 5(3):e136–e145. [DOI] [PubMed] [Google Scholar]
- 6.Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, et al. An HIV Preexposure Prophylaxis Demonstration Project and Safety Study for Young MSM. J Acquir Immune Defic Syndr 2017; 74(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, Bushman L, et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr 2017; 76(5):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan PA, Mena L, Patel R, Oldenburg CE, Beauchamps L, Perez-Brumer AG, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc 2016; 19(1):20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusie LK, Orengo C, Burrell D, Ramachandran A, Houlberg M, Keglovitz K, et al. PrEP Initiation and Retention in care over five years, 2012–2017: Are quarterly visits too much? Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalley-Chareczko L, Clark D, Conyngham C, Zuppa A, Moorthy G, Mounzer K, et al. Delivery of TDF/FTC for Pre-exposure Prophylaxis to Prevent HIV-1 Acquisition in Young Adult Men Who Have Sex With Men and Transgender Women of Color Using a Urine Adherence Assay. J Acquir Immune Defic Syndr 2018; 79(2):173–178. [DOI] [PubMed] [Google Scholar]
- 11.Celum CL, Delany-Moretlwe S, Hosek S, Dye BJ, Bekker LG, Mgodi N, et al. Risk Behavior, Perception and Reasons for PrEP among Young African Women in HPTN 082 [Abstract 1049]. In: Conference on Retroviruses and Opportunistic Infections Boston; 2018. [Google Scholar]
- 12.vanderStraten A, Katz A, Balan I, Reddy K, Etima J, Woeber K, et al. A qualitative evaluation of women’s experience receiving drug feedback in MTN-025/HOPE - an HIV prevention open-label trial of the dapivirine vaginal ring [Abstract THPEC334]. In: AIDS. Amsterdam; 2018. [Google Scholar]
- 13.Anderson PL. What Can Urine Tell Us About Medication Adherence? EClinicalMedicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi M, Bacchetti P, Rodrigues WC, Spinelli MA, Koss CA, Drain PK, et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinicalMedicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016; 3(11):e521–e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014; 9(1):e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2018; 62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH, et al. Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing. Antimicrob Agents Chemother 2016; 60(11):6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P, et al. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis 2015; 212(9):1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cressey TR, Siriprakaisil O, Klinbuayaem V, Quame-Amaglo J, Kubiak RW, Sukrakanchana PO, et al. A randomized clinical pharmacokinetic trial of Tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis 2017; 17(1):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegler AJ, Mayer KH, Liu AY, Patel RR, Ahlschlager LM, Kraft CS, et al. Developing and assessing the feasibility of a home-based PrEP monitoring and support program. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stekler JD, McMahan V, Ballinger L, Viquez L, Swanson F, Stockton J, et al. HIV Pre-exposure Prophylaxis Prescribing Through Telehealth. J Acquir Immune Defic Syndr 2018; 77(5):e40–e42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.