Summary
Alteration in the frequency of monocyte subsets is a hallmark of tuberculosis–diabetes co‐morbidity (TB‐DM). To study this association, we examined the plasma levels of sCD14, sCD163, C‐reactive protein (CRP) and soluble tissue factor (sTF) in individuals with TB‐DM, TB or diabetes mellitus (DM), and in healthy controls (HC). Circulating levels of sCD14, sCD163 and sTF were significantly increased in TB‐DM and DM compared with TB and HC; however, CRP was significantly increased in TB‐DM and TB compared with DM and HC. During longitudinal follow up, sCD14, CRP and sTF levels remained significantly increased in TB‐DM compared with TB from baseline (pre‐treatment), during treatment (2nd month) and at the completion (6th month) of anti‐TB treatment (ATT), whereas sCD163 was significantly higher in TB‐DM compared with TB only at baseline. Moreover, the levels of sCD14 and sCD163 were significantly higher in TB‐DM individuals with bilateral and cavitary disease and exhibited a significant positive relationship with bacterial burden. Levels of sCD14, sCD163 and CRP exhibited a positive relationship with HbA1c levels. Within the TB‐DM group, those with known diabetes before incident TB (KDM) exhibited significantly higher levels of sCD14 and sCD163 compared with individuals with newly diagnosed DM with TB (NDM). Finally, KDM individuals on metformin treatment exhibited significantly lower levels of sCD14, sCD163 and CRP compared with those on non‐metformin‐containing regimens. Our data demonstrate that systemic monocyte activation marker levels reflect baseline disease severity and extent in TB‐DM, differentiate KDM from NDM and are modulated by ATT and metformin therapy.
Keywords: diabetes mellitus, monocytes, tuberculosis
Abbreviations
- ATT
anti‐TB treatment
- CRP
C‐reactive protein
- DM
diabetes mellitus
- HbA1c
glycated hemoglobin
- HC
healthy controls
- KDM
diabetic before incident TB
- NDM
newly diagnosed with DM
- sTF
soluble tissue factor
- TB
tuberculosis
Introduction
Tuberculosis (TB) and diabetes mellitus (DM) are two common diseases that independently have immense public health significance globally. Their association and consequences are well‐established,1, 2, 3 but some aspects need further research. It is not completely understood why patients with DM, predominantly those with poor glycemic control, are at highest risk of TB, although alterations have been found in both innate and adaptive immune responses.4, 5 Moreover, individuals with diabetes who have smear‐positive pulmonary TB have a higher possibility of failure during smear conversion after 2 months of therapy than patients without DM.6 On the one hand, DM constitutes a risk factor for active TB and might impact the disease presentation and response to treatment;1, 7 while on the other hand, TB may stimulate glucose intolerance and exacerbate dysglycemia in patients with DM.8, 9 The exact mechanisms underlying this interaction are still relatively vague and are in need of comprehensive evaluation.
Monocytes play a vital role in TB given their rapid migration to the lung upon initial Mycobacterium tuberculosis infection, where they differentiate into macrophages and dendritic cells for antigen presentation and secretion of cytokines.10 Monocytes are important to the innate immune system and play a crucial role in several inflammatory conditions associated with chronic infection, including TB.11 Plasma levels of soluble (s) CD14 have been established as a strong indicator of monocyte activation.12 A soluble form of CD163 was present in normal plasma13 and a heightened plasma concentration of sCD163 was seen in diseases linked to macrophage activity, including acute and chronic inflammation.14 Expression of soluble tissue factor (sTF) by cells of the monocyte/macrophage lineage was also shown to be involved in the development and progression of local and systemic inflammatory reactions.15, 16 C‐reactive protein (CRP) is an established marker of acute inflammation and its serum concentration correlates with the severity of systemic inflammation.17 Soluble markers derived from monocyte activation such as sCD14, sCD163, CRP and sTF in individuals have been broadly studied in the context of HIV infection.17, 18, 19, 20, 21 However, a detailed examination of the association of monocyte activation markers with tuberculosis–diabetes co‐morbidity (TB‐DM) or TB, and their relationship to disease pathology or bacterial burden, is presently unknown. We postulated that one potential mechanism for the increased severity of TB in individuals with diabetes could be excessive monocyte activation that promotes tissue injury and adverse TB disease outcomes. As macrophages and monocytes play a pivotal role in the progression of TB, the aim of this study was to elucidate the association of the systemic levels of monocyte activation markers, sCD14, sCD163, CRP and sTF in individuals with TB‐DM and those with TB and compare them with individuals with no TB but DM alone (DM) or healthy controls (HC).
Materials and methods
Ethics statement
This study was approved by the Ethics Committees of the Prof. M. Viswanathan Diabetes Research Center and the National Institute for Research in Tuberculosis. Informed written consent was obtained from all participants.
Study population
Plasma samples were collected from 44 individuals with active pulmonary TB with DM (TB‐DM), 44 individuals with active pulmonary TB (TB), 44 individuals with diabetes mellitus (DM), and 30 healthy control individuals with no TB or diabetes (HC) recruited in Chennai, India. All the enrolled DM and HC participants were negative for Quantiferon TB gold assay with no clinical symptoms of TB and normal chest X‐rays. The diagnosis of pulmonary TB was based on smear and culture positivity for M. tuberculosis. Chest X‐rays were used to define cavitary disease (TB‐DM, n = 13 and TB, n = 10) and non‐cavitary disease (TB‐DM, n = 31 and TB, n = 34) as well as unilateral (TB‐DM, n = 24 and TB, n = 25) versus bilateral (TB‐DM, n = 20 and TB, n = 19) lung involvement. Smear grades were used to determine bacterial burdens and were classified as 1+ (TB‐DM, n = 14 and TB, n = 19), 2+ (TB‐DM, n = 19 and TB, n = 14) and 3+ (TB‐DM, n = 11 and TB, n = 11). At the time of enrolment, all individuals with active TB had no record of prior TB disease or anti‐TB treatment (ATT). Glycemic status (DM or normoglycemia) was diagnosed on the basis of oral glucose tolerance test and/or glycated hemoglobin (HbA1c) levels (for those known to have diabetes), according to the WHO criteria. Among the 44 TB‐DM individuals, 22 were known diabetics (KDM) and 22 were newly diagnosed diabetics (NDM). Among the KDM individuals, 11 were on metformin‐containing anti‐diabetic medication and 11 were not. This was at the discretion of the treating physician and not related to extent or severity of diabetic disease. All the enrolled DM and HC individuals were negative for the Quantiferon TB gold assay with no clinical symptoms of TB and with normal chest X‐rays. The study groups were similar with regard to age and gender and the baseline characteristics of the study participants are shown in Table 1. Standard ATT was administered to TB‐DM and TB individuals using the directly observed treatment, short course strategy. At 2 and 6 months following ATT initiation, fresh plasma samples were obtained from TB‐DM and TB individuals. All TB‐DM and TB individuals were culture negative at the end of ATT.
Table 1.
Demographics of the study groups and biochemical parameters in TB‐DM, TB non‐DM, DM and HC
| Study demographics | TB‐DM | TB | DM | HC | |
|---|---|---|---|---|---|
| KDM | NDM | ||||
| No. of participants recruited | 22 | 22 | 44 | 44 | 30 |
| Gender (male/female) | 16/6 | 18/4 | 27/17 | 30/14 | 15/15 |
| Median age, years (range) | 52 (25–70) | 42 (29–70) | 39 (24–67) | 44 (33–68) | 34 (23–55) |
| Smear grade: 0/1+/2+/3+ | 0/4/10/8 | 0/10/9/3 | 0/19/14/11 | NA | NA |
| Cavitary disease (Y/N) | 9/13 | 4/18 | 10/34 | NA | NA |
| Lung lesions (unilateral/bilateral) | 13/9 | 11/11 | 25/19 | NA | NA |
| Fasting blood glucose, mg/dl | 166 (120–417) | 139 (111–375) | 93 (73–103) | 144 (95–405) | 75 (70–109) |
| Post‐prandial glucose, mg/dl | 350 (217–550) | 311 (202–505) | 110 (68–137) | 341 (210–543) | 98 (72–139) |
| Glycated hemoglobin level, % | 12·3 (8–15·6) | 10 (7·3–13·9) | 5·6 (5·0–5·8) | 10 (6·8–12·6) | 5·5 (5·0–5·9) |
DM, diabetes mellitus; HC, healthy controls; KDM, known DM with incident TB; NDM, newly diagnosed DM; TB, tuberculosis; TB‐DM, tuberculosis and diabetes mellitus co‐morbidity.
Values represent the geometric mean (and the 95% confidence intervals) except for age where the median (and the range) are depicted.
ELISA
Circulating levels of sCD14, sCD163 and CRP were measured using the Duoset ELISA Development System (R&D Systems, Minneapolis, MN). Quantikine ELISA kit (R&D Systems) was used for measuring sTF. The lowest detection limits were as follows: sCD14, 62·5 pg/ml; sCD163, 8·75 pg/ml; CRP, 0·781 pg/ml; sTF, 7·81 pg/ml.
Statistical analysis
Geometric means were used for measurements of central tendency. Statistically significant differences between the four groups were analyzed using the Kruskal–Wallis test with Dunn's correction for multiple comparisons. The Mann–Whitney U‐test was used to compare monocyte activation marker concentrations between pulmonary TB patients with and without DM, unilateral or bilateral lung lesions and cavitary or non‐cavitary disease. Linear trend post‐test was used to compare monocyte marker concentrations with smear grades (reflecting bacterial burdens) and Spearman rank correlation was used to compare monocyte marker concentrations with HbA1c levels. Analyses were performed using graphpad prism Version 7 (GraphPad, San Diego, CA, USA).
Results
Study population characteristics
The baseline characteristics including demographic and biochemical features of the study population are shown in Tables 1 and 2. No significant differences were observed in age, sex, smear or culture grades at baseline between the TB‐DM and TB groups (Table 1).
Table 2.
Demographics of the clinical data in TB‐DM
| Study demographics | TB‐DM | ||
|---|---|---|---|
| KDM | NDM | ||
| Metformin | Non‐metformin | ||
| No. of participants recruited | 11 | 11 | 22 |
| Smear grade: 0/1+/2+/3+ | 0/3/4/4 | 0/1/6/4 | 0/10/9/3 |
| Cavitary disease (Y/N) | 4/7 | 5/6 | 4/18 |
| Lung lesions (Unilateral/Bilateral) | 7/4 | 6/5 | 11/11 |
| Glycated hemoglobin level, % | 11 (7·7–15·6) | 10·2 (7·9–14·3) | 10 (7·3–13·9) |
KDM, known DM with incident TB; NDM, newly diagnosed DM; TB‐DM, tuberculosis and diabetes mellitus co‐morbidity.
Values represent the geometric mean (and the 95% confidence intervals).
Heightened levels of circulating monocyte activation markers in TB‐DM
To determine the systemic levels of circulating monocyte activation markers in TB‐DM and TB, we measured the circulating levels of sCD14, sCD163, CRP and sTF in TB‐DM, TB, DM and HC individuals (Fig. 1). As shown, the levels of sCD14 (median 3253 pg/ml in TB‐DM versus 2520 pg/ml in TB, 2778 pg/ml in DM and 2441 pg/ml in HC) were significantly higher in TB‐DM compared with TB and HC individuals and levels in DM were significantly increased compared with HC. Also, the levels of sCD163 (median 294·2 pg/ml in TB‐DM versus 241·4 pg/ml in TB, 334·1 pg/ml in DM and 132·7 pg/ml in HC) and sTF (median 10·8 pg/ml in TB‐DM versus 8·8 pg/ml in TB, 16·2 pg/ml in DM and 8·5 pg/ml in HC) were significantly higher in TB‐DM and DM compared with TB and HC individuals. However, CRP (median 91·8 pg/ml in TB‐DM versus 84·3 pg/ml in TB, 51 pg/ml in DM and 51·2 pg/ml in HC) was significantly increased in TB‐DM and TB compared with DM and HC. Hence, TB‐DM is associated with elevated systemic levels of circulating monocyte activation markers.
Figure 1.
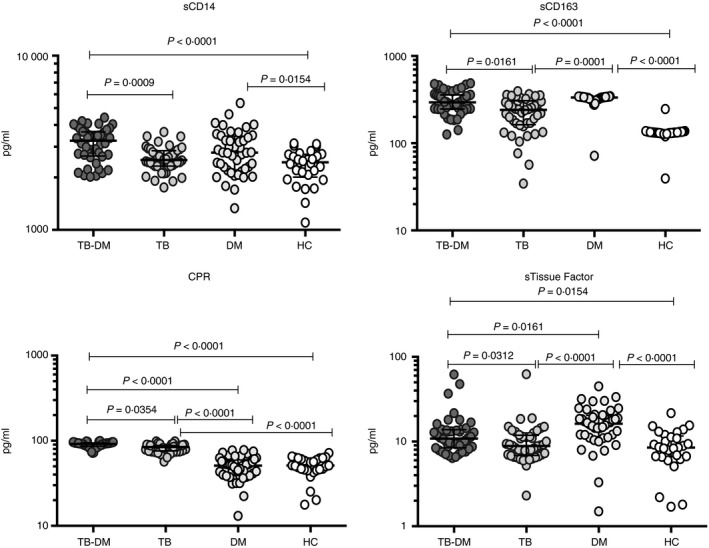
Elevated circulating levels of monocyte activation markers in tuberculosis–diabetes mellitus co‐morbidity (TB‐DM) and DM individuals. Plasma levels of sCD14, sCD163, C‐reactive protein (CRP) and soluble tissue factor (sTF) were measured in TB‐DM (n = 44), TB (n = 44) and DM (n = 44) individuals, and in healthy controls (HC) (n = 30) at baseline. Data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Kruskal–Wallis test with Dunn's post hoc for multiple comparisons.
Heightened circulating levels of monocyte activation markers in TB patients with diabetes compared with TB patients without diabetes during TB treatment
To determine whether the monocyte activation markers were altered by TB treatment, we measured the circulating levels of sCD14, sCD163, CRP and sTF in TB‐DM and TB at baseline (pre‐treatment), during treatment (2nd month) and at the completion of ATT (6th month). As shown Fig. 2, sCD14 (median 3253 pg/ml in TB‐DM versus 2520 pg/ml in TB); sCD163 (median 294·2 pg/ml in TB‐DM versus 241·4 pg/ml in TB); CRP (median 91·8 pg/ml in TB‐DM versus 84·3 pg/ml in TB) and sTF (median 10·8 pg/ml in TB‐DM versus 8·8 pg/ml in TB) levels were significantly higher in TB‐DM compared with TB pre‐treatment. Similarly, sCD14 (median 4029 pg/ml in TB‐DM versus 2197 pg/ml in TB); CRP (median 83 pg/ml in TB‐DM versus 73·6 pg/ml in TB) and sTF (median 11·2 pg/ml in TB‐DM versus 7·2 pg/ml in TB) levels were also significantly higher at 2 months of treatment. Finally, sCD14 (median 3732 pg/ml in TB‐DM versus 2746 pg/ml in TB); CRP (median 63·6 pg/ml in TB‐DM versus 49·9 pg/ml in TB) and sTF (median 12·7 pg/ml in TB‐DM versus 9·3 pg/ml in TB) levels were also significantly higher after 6 months of treatment.
Figure 2.
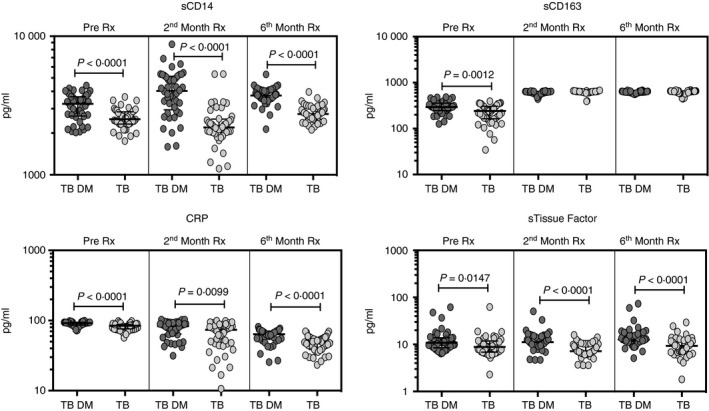
Tuberculosis–diabetes mellitus co‐morbidity (TB‐DM) is associated with increased frequencies of monocyte activation markers pre‐treatment and following treatment. Circulating levels of monocyte activation markers in TB‐DM (n = 44) and TB (n = 44) individuals at pre‐treatment and at 2 and 6 months following treatment are shown. Data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney test.
Circulating monocyte activation markers as markers of disease severity and bacterial burdens in TB‐DM
To determine the association between the systemic levels of circulating monocyte markers and disease severity in TB‐DM, we measured the circulating levels of sCD14, sCD163, CRP and sTF in TB‐DM and TB individuals with unilateral versus bilateral disease and cavitary versus non‐cavitary disease based on chest X‐ray. As shown in Fig. 3(a), the circulating levels of sCD14 (median 3474 pg/ml in bilateral versus 2888 pg/ml in unilateral disease) and sCD163 (median 342·1 pg/ml in bilateral versus 276·3 pg/ml in unilateral disease) were significantly higher in TB‐DM individuals with bilateral disease compared with those with unilateral disease. Similarly, as shown in Fig. 3(b), the circulating levels of sCD14 (median 3838 pg/ml in cavitary versus 2901 pg/ml in non‐cavitary disease) and sCD163 (median 359·6 pg/ml in bilateral versus 276·7 pg/ml in unilateral disease) were significantly higher in TB‐DM individuals with cavitary disease compared with those without. In contrast, as shown in the Supplementary material (Fig. S1A,B), no significant differences were observed in TB individuals between unilateral and bilateral disease and between cavitary and non‐cavitary disease. To determine the association of circulating monocyte markers and bacterial burdens, we performed a correlation of the circulating levels of sCD14, sCD163, CRP and sTF in TB‐DM and TB individuals with smear grades. As shown in Fig. 3(c), both sCD14 and sCD163 exhibited a significant positive correlation with smear grades in TB‐DM individuals, indicating a positive association of these factors with bacterial burdens. In contrast, as shown in Fig. S1(C), no significant correlation with smear grades was observed in individuals with TB. Hence, both disease severity and bacterial burden in TB‐DM are associated with elevated systemic levels of circulating monocyte‐associated activation markers.
Figure 3.
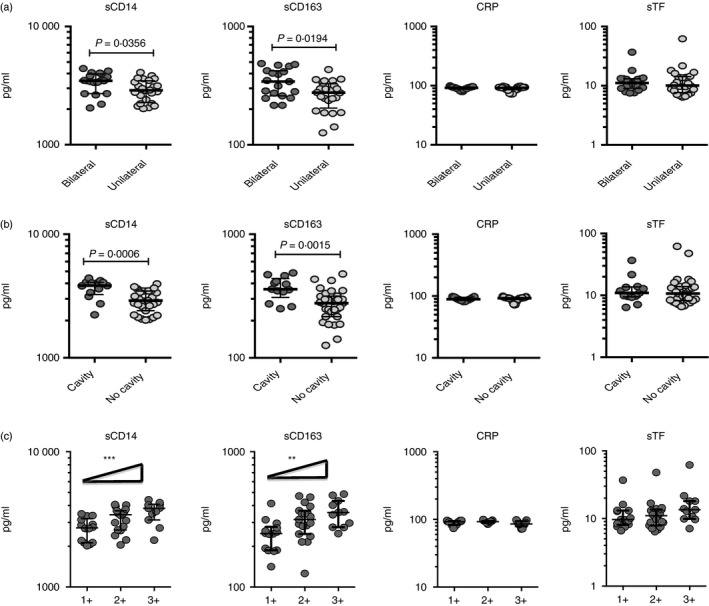
Elevated circulating levels of sCD14 and sCD163 in bilateral and cavitary disease and also marker of bacterial burden in individuals with tuberculosis–diabetes mellitus co‐morbidity (TB‐DM). (a) Plasma levels of sCD14, sCD163, C‐reactive protein (CRP) and soluble tissue factor (sTF) were measured in TB‐DM individuals with bilateral versus unilateral disease. (b) Plasma levels of sCD14, sCD163, CRP and sTF were measured in TB‐DM individuals with cavitary versus non‐cavitary disease. (c) Relationship between the plasma levels of sCD14, sCD163, CRP and sTF and smear grades as estimated by sputum smears was examined in TB‐DM individuals. Data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney test with Holm's correction for multiple comparisons. For bacterial burden correlation, P values were calculated using the linear trend post‐test.
Circulating monocyte activation markers exhibit a positive relationship with HbA1c in TB‐DM, are increased in KDM individuals, and decreased by metformin treatment
To determine the association between systemic levels of circulating monocyte markers and glycemic control in TB‐DM, we examined the relationship between the circulating levels of sCD14, sCD163, CRP and sTF in all TB individuals with and without DM with baseline HbA1c levels (Fig. 4a). As shown, the systemic levels of sCD14, sCD163 and CRP exhibited a significant positive relationship with HbA1c levels in TB individuals with and without DM, indicating a significant association of these factors with poor glycemic control. To determine whether monocyte marker levels differ between KDM (n = 22) and NDM (n = 22) in TB‐DM individuals, we first measured HbA1c levels in KDM and NDM and observed that HbA1c % was significantly higher in KDM compared with NDM (geometric mean 11·4% in KDM versus 8·9% in NDM, P = 0·0028). Second, we measured the baseline levels of sCD14, sCD163, CRP and sTF in KDM and NDM individuals. As shown in Fig. 4(b), systemic levels of sCD14 (median 3487 pg/ml in KDM versus 2836 pg/ml in NDM) and sCD163 (median 340·3 pg/ml in KDM versus 266·4 pg/ml in NDM) were significantly higher in KDM compared with NDM individuals. Hence, KDM is associated with elevated systemic levels of circulating monocyte markers at baseline. As the anti‐diabetic drug metformin is associated with protection against mortality in TB‐DM, we wanted to examine the circulating levels of sCD14, sCD163, CRP and sTF in KDM individuals on metformin treatment (n = 11) compared with those on non‐metformin regimens (n = 11). No significant differences were observed in HbA1c levels between KDM individuals on metformin compared with KDM individuals not on metformin. As shown in Fig. 4(c), systemic levels of sCD14 (median 2874 pg/ml in metformin versus 4035 pg/ml in non‐metformin), sCD163 (median 292 pg/ml in metformin versus 349·6 pg/ml in non‐metformin) and CRP (median 86·3 pg/ml in metformin versus 93·2 pg/ml in non‐metformin) were significantly diminished in KDM individuals on metformin compared with KDM individuals not on metformin. Hence, metformin therapy in KDM individuals is associated with diminished systemic levels of monocyte‐associated activation markers.
Figure 4.
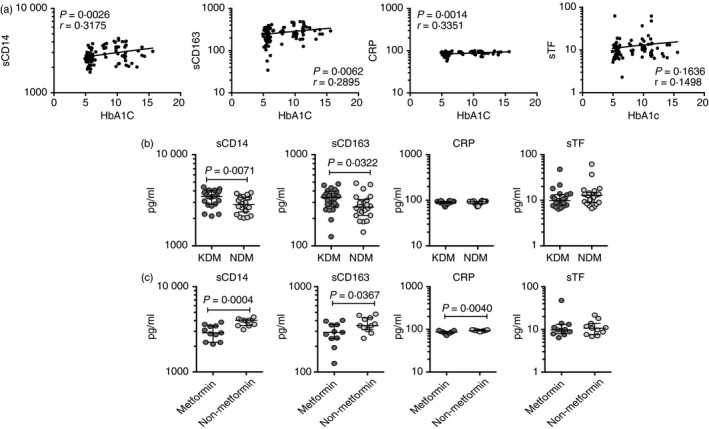
Significant correlation between circulating levels of monocyte activation markers and glycemic parameters and elevated circulating levels of monocyte activation markers in individuals with known diabetes mellitus (DM) before incident tuberculosis (TB) (i.e. KDM individuals). (a) Relationship between the plasma levels of sCD14, sCD163, C‐reactive protein (CRP) and soluble tissue factor (sTF) and HbA1c levels was examined in all TB individuals with and without DM at baseline. (b) Plasma levels of sCD14, sCD163, CRP and sTF were measured in individuals with tuberculosis–diabetes mellitus co‐morbidity (TB‐DM) with known diabetes (KDM) versus newly diagnosed diabetes (NDM) (c) Plasma levels of sCD14, sCD163, CRP and sTF were measured in KDM individuals on metformin treatment versus no metformin treatment. Data are represented as scatter plots with each circle representing a single individual. For HbA1c correlations, P values were calculated using the Spearman rank correlation. For KDM, P values were calculated using the Mann–Whitney test with Holm's correction for multiple comparisons.
Discussion
Diabetes mellitus is one of the most important risk factors in the worsening of TB and triples the risk of developing active TB disease.1 Now, approximately 15% of global TB cases can be attributed to DM co‐morbidity.22 Clinically, DM increases TB severity and worsens TB treatment outcomes,7 while equally TB hampers glycemic control.1 Monocytes and macrophages play an important role in TB immunopathogenesis.23 Pathogens, including M. tuberculosis, can influence macrophage differentiation and activation. Monocytes/macrophages have key roles in TB pathogenesis, as hosts for productive intracellular replication of M. tuberculosis,24 as mediators of tissue injury at sites of infection,25 and as effectors that contribute to an effective immune response. In the current study, we found that plasma biomarkers of monocyte activation are present in higher levels in individuals with TB‐DM compared with TB alone. Whether the increased monocyte activation reflects a causal mechanism of TB susceptibility in DM, or is a marker of increased TB disease severity and risk for tissue injury, remains to be determined. In this regard, we found that levels of sCD14 were higher in DM than HC individuals without active TB disease (Fig. 1). This suggests the potential that dysregulated monocyte activation in DM promotes TB susceptibility.
CD14 is a component of the innate immune system that functions as a co‐receptor for bacterial lipopolysaccharide and exists in membrane‐bound and soluble forms.26 Monocytes are an important source of sCD14 and upon cell activation release sCD14 into the circulation.27 It has been previously reported that sCD14 serum levels were significantly increased in individuals with pulmonary TB alone and pulmonary TB and HIV co‐infection compared with healthy controls.20 Earlier studies have also demonstrated that sCD14 levels were higher in HIV/TB with high (≥ 350 cells/μl) CD4 T‐cell counts compared with low CD4 T‐cell counts. In addition, sCD14 levels remained elevated in HIV/TB individuals with lower CD4 T‐cell counts despite treatment of TB.28 Finally, studies have also reported that sCD14 is elevated in the serum of patients with diabetes compared with those without.29, 30 In agreement with these reports, our study revealed that TB‐DM and DM individuals exhibited significantly higher systemic levels of sCD14 compared with TB and HC. In addition, before, during and after ATT, sCD14 levels remained elevated in TB‐DM compared with TB. Our data also revealed a novel association of sCD14 levels with the severity of TB disease (as estimated by bilateral and cavitary disease) and with estimated bacterial burden. Of additional interest was the finding that sCD14 levels correlated positively with HbA1c, indicating an association with poor glycemic control that to our knowledge has not previously been reported. It is of interest that sCD14 and sCD163 levels were not significantly different between TBDM and DM. Our interpretation of these data is that both TB and DM independently affect the monocyte markers. However, the effect of DM is powerful enough to override any significant differences that TB per se might cause. Apart from TB infection, it has also been previously reported that sCD14 expression was also significantly higher in other bacterial infections, such as pneumonia, and in cystic fibrosis and asthma compared with controls.31
CD163 is a scavenger receptor for the hemoglobin–haptoglobin complex. It is expressed exclusively on activated cells of the macrophage/monocyte lineage, especially on M2 phenotype cells.32, 33 Published studies have demonstrated that both proliferation and inflammatory activation of macrophages result in increased sCD163 levels.34, 35 Previous studies have shown increased serum concentrations of sCD163 in patients with active pulmonary TB compared with control participants.36 Other studies have also reported that during chronic HIV infection CD14+ CD16+ monocytes were correlated with high levels of sCD16337, 38 and published studies have also reported that TB patients with immune reconstitution inflammatory syndrome (TB‐IRIS) exhibited increased levels of sCD163 compared with non‐IRIS patients.11 Although sCD163 expression is induced by monocyte/macrophages in mycobacterial infection and HIV infections, little is known about the role of sCD163 in TB‐DM. Our data reveal that sCD163 levels are present at significantly enhanced levels in TB‐DM and DM compared with the other groups. TB‐DM is also characterized by elevated sCD163 levels at pre‐treatment, 2 and 6 months of ATT in comparison with TB individuals. Our study also reveals that sCD163 correlates with severity of disease and/or bacterial burdens in TB‐DM and that sCD163 exhibits a positive relationship with HbA1c levels. Hence, sCD163 appears to be associated with pathology and bacterial burdens in TB‐DM and could serve as a biomarker for severity of disease and therapeutic responses following treatment.
C‐Reactive protein is an acute‐phase reactant synthesized by hepatocytes under the influence of interleukin‐1 arising at sites of infection and inflammation39 and it is also used as a biomarker for the detection of inflammation in various active infections.40, 41 CRP has been shown to be useful in the clinical evaluation of respiratory tract infections in adults.42, 43 We previously reported that pulmonary TB displayed heightened serum CRP levels compared with latent TB and extrapulmonary TB.44 Previously published data also indicate that circulating CRP levels were significantly increased in active TB disease compared with controls.45, 46 During M. tuberculosis infection, CRP levels are increased in TB patients starting ATT and decreased during the first 2 months of treatment, and these elevated CRP levels reflected higher AFB smear grades and correlated with disease severity.44, 46 Similar to earlier findings, our data recapitulate those results and show that CRP levels are present at significantly enhanced levels in TB‐DM and TB compared with the other groups. In addition, as reported earlier in active TB, our findings also reveal that at baseline (pre‐treatment), CRP levels were increased and diminished following the 2nd month of ATT in the TB‐DM group. Finally, we found that CRP levels exhibited a positive correlation with HbA1c levels. This observation leads us to speculate that M. tuberculosis‐driven inflammatory tissue damage may initiate strong induction of CRP production.
Activated monocytes in the circulation and tissue macrophages are a major source of tissue factor (TF).47 Increased expression of TF by cells of the monocyte/macrophage lineage to the development and progression of local and systemic inflammatory reactions in many diseases.15, 22 Previously, it has been reported that M. tuberculosis induces TF expression in macrophages and M. tuberculosis signaling pathways that stimulate TF require the cooperation of multiple receptors and co‐factors including Toll‐like receptors.21 Our results demonstrate that sTF in plasma is significantly elevated in TB‐DM and DM compared with the other groups. In addition, before, during and after ATT, sTF levels remained elevated in TB‐DM compared with TB. However, sTF did not exhibit any correlation with severity of disease and/or bacterial burdens or with HbA1c levels. Hence, sTF levels appear to be associated with active TB with or without DM, although not directly associated with either severity of disease or bacterial burden.
A previously published study from our group reported that there was a bimodal distribution of baseline HbA1c between KDM and NDM individuals in our study cohort, with significantly higher baseline HbA1c in the KDM group.48 Our present study adds to this apparent heterogeneity in the presentation of TB‐DM co‐morbidity. We found significantly higher levels of sCD14 and sCD163 in KDM compared with NDM groups, suggesting increased severity of TB disease in KDM individuals. Metformin is the recommended first‐line anti‐hyperglycemic drug for the treatment of type 2 diabetes.49 Recently published studies revealed a potential role of metformin in improving the effective treatment of TB and indicated that metformin is a promising candidate adjunctive therapy for individuals with TB.49 A previously published study reports that metformin improves the chronic low‐grade inflammatory state and also alters the macrophage polarization both in vivo and in vitro.50 Our current findings provide additional evidence of a host‐directed therapeutic effect for metformin based on decreased systemic levels of sCD14, sCD163 and CRP. Also, as the non‐metformin groups of TB‐DM individuals were only on insulin or sulfonyl‐urea, it would not result in any confounding effect. Also, the duration of diabetes was not significantly different between the two groups.
In summary, our study provides additional evidence for an adverse effect of poorly controlled type 2 DM on TB disease severity as reflected by increased monocyte activation. The data also add to the growing body of evidence indicating heightened immune activation in this immune‐metabolic nexus. Longitudinal examination of monocyte markers demonstrated the evolution of these markers over the course of TB treatment and also provided evidence of unresolved inflammation at treatment completion in the TB‐DM group. We identified a positive correlation of monocyte activation with HbA1c, suggesting that chronic hyperglycemia drives this complication of DM as is the case for microvascular and macrovascular complications. Finally, we describe an anti‐inflammatory effect of metformin in TB that might relate to its reported disease‐modifying effects in TB.
Funding
This project has been funded in whole or in part with Federal funds from the Government of India's (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by CRDF Global (grant USB1‐31149‐XX‐13) and partly by SERB National Post Doctoral Fellowship. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, or CRDF Global. This work was also funded in part by the Division of Intramural Research, NIAID, NIH.
Author contributions
SB, HK and PKN designed the study; PKN, KM, YB and AN conducted experiments; PKN and KM acquired data; PKN, KM and SS analyzed data; VV, HK and SB contributed reagents and also revised subsequent drafts of the manuscript; VV, BSS and MN are responsible for the enrolment of participants and also contributed to acquisition and interpretation of clinical data; SB and PKN wrote the manuscript. All authors read and approved the final manuscript.
Disclosures
The authors declare that they have no competing interests.
Supporting information
Figure S1. No significant alterations in levels of monocyte activation markers in lung lesions, cavitary disease and bacterial burden in individuals with tuberculosis. (A) The plasma levels of sCD14, sCD163, C‐reactive protein (CRP) and soluble tissue factor (sTF) were measured in TB individuals with bilateral versus unilateral disease. (B) The plasma levels of sCD14, sCD163, CRP and sTF were measured in individuals with TB with cavitary versus non‐cavitary disease. (C) The relationship between the plasma levels of sCD14, sCD163, CRP and sTF and smear grades as estimated by sputum smears was examined in individuals with TB. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney test with Holm's correction for multiple comparisons. For bacterial burden correlation, P values were calculated using the linear trend post‐test.
Acknowledgements
The first author (Nathella Pavan Kumar) immensely thanks the Science and Engineering Research Board (SERB), Department of Science and Technology (DST) for providing the National Post Doctoral Fellowship (NPDF). We thank the staff of Department of Clinical Research and the Department of Bacteriology, NIRT for valuable assistance with bacterial cultures and radiology and the staff of MVDRC, RNTCP, especially Dr. Jayagopal Lavanya, and the Chennai corporation, especially Dr. Senthilnathan, for valuable assistance in recruiting the patients for this study. Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium.
Contributor Information
Nathella P. Kumar, Email: pavankumarn@nirt.res.in.
Subash Babu, Email: sbabu@mail.nih.gov.
References
- 1. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Restrepo BI, Camerlin AJ, Rahbar MH, Wang W, Restrepo MA, Zarate I et al Cross‐sectional assessment reveals high diabetes prevalence among newly diagnosed tuberculosis cases. Bull World Health Organ 2011; 89:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lonnroth K et al Bi‐directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health 2010; 15:1300–14. [DOI] [PubMed] [Google Scholar]
- 4. Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C et al Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn 2007; 3:228–45. [DOI] [PubMed] [Google Scholar]
- 5. Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015; 144:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faurholt‐Jepsen D, Range N, PrayGod G, Jeremiah K, Faurholt‐Jepsen M, Aabye MG et al Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health 2013; 18:822–9. [DOI] [PubMed] [Google Scholar]
- 7. Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K et al The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009; 9:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher‐Hoch SP, Mathews CE, McCormick JB. Obesity, diabetes and pneumonia: the menacing interface of non‐communicable and infectious diseases. Trop Med Int Health 2013; 18:1510–9. [DOI] [PubMed] [Google Scholar]
- 10. Stew SS, Martinez PJ, Schlesinger LS, Restrepo BI. Differential expression of monocyte surface markers among TB patients with diabetes co‐morbidity. Tuberculosis 2013; 93(Suppl):S78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrade BB, Singh A, Narendran G, Schechter ME, Nayak K, Subramanian S et al Mycobacterial antigen driven activation of CD14+ + CD16− monocytes is a predictor of tuberculosis‐associated immune reconstitution inflammatory syndrome. PLoS Pathog 2014; 10:e1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su GL, Simmons RL, Wang SC. Lipopolysaccharide binding protein participation in cellular activation by LPS. Crit Rev Immunol 1995; 15:201–14. [DOI] [PubMed] [Google Scholar]
- 13. Moller HJ, Peterslund NA, Graversen JH, Moestrup SK. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 2002; 99:378–80. [DOI] [PubMed] [Google Scholar]
- 14. Moller HJ. Soluble CD163. Scand J Clin Lab Invest 2012; 72:1–13. [DOI] [PubMed] [Google Scholar]
- 15. Drake TA, Cheng J, Chang A, Taylor FB Jr. Expression of tissue factor, thrombomodulin, and E‐selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol 1993; 142:1458–70. [PMC free article] [PubMed] [Google Scholar]
- 16. Vervloet MG, Thijs LG, Hack CE. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost 1998; 24:33–44. [DOI] [PubMed] [Google Scholar]
- 17. Pepys MB, Baltz ML. Acute phase proteins with special reference to C‐reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 1983; 34:141–212. [DOI] [PubMed] [Google Scholar]
- 18. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE et al Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker JV, Huppler Hullsiek K, Bradford RL, Prosser R, Tracy RP, Key NS. Circulating levels of tissue factor microparticle procoagulant activity are reduced with antiretroviral therapy and are associated with persistent inflammation and coagulation activation among HIV‐positive patients. J Acquir Immune Defic Syndr 2013; 63:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawn SD, Labeta MO, Arias M, Acheampong JW, Griffin GE. Elevated serum concentrations of soluble CD14 in HIV− and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti‐tuberculosis treatment. Clin Exp Immunol 2000; 120:483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kothari H, Rao LV, Vankayalapati R, Pendurthi UR. Mycobacterium tuberculosis infection and tissue factor expression in macrophages. PLoS One 2012; 7:e45700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng C, Hu M, Gao F. Diabetes and pulmonary tuberculosis: a global overview with special focus on the situation in Asian countries with high TB‐DM burden. Glob Health Action. 2017; 10:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol 2013; 35:563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J 2009; 50:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elkington PT, Ugarte‐Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J 2011; 38:456–64. [DOI] [PubMed] [Google Scholar]
- 26. Viriyakosol S, Mathison JC, Tobias PS, Kirkland TN. Structure–function analysis of CD14 as a soluble receptor for lipopolysaccharide. J Biol Chem 2000; 275:3144–9. [DOI] [PubMed] [Google Scholar]
- 27. Bufler P, Stiegler G, Schuchmann M, Hess S, Kruger C, Stelter F et al Soluble lipopolysaccharide receptor (CD14) is released via two different mechanisms from human monocytes and CD14 transfectants. Eur J Immunol 1995; 25:604–10. [DOI] [PubMed] [Google Scholar]
- 28. Toossi Z, Funderburg NT, Sirdeshmuk S, Whalen CC, Nanteza MW, Johnson DF et al Systemic immune activation and microbial translocation in dual HIV/tuberculosis‐infected subjects. J Infect Dis 2013; 207:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandez‐Real JM, Broch M, Richart C, Vendrell J, Lopez‐Bermejo A, Ricart W. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab 2003; 88:1780–4. [DOI] [PubMed] [Google Scholar]
- 30. Fernandez‐Real JM, Lopez‐Bermejo A, Castro A, Broch M, Penarroja G, Vendrell J et al Opposite relationship between circulating soluble CD14 concentration and endothelial function in diabetic and nondiabetic subjects. Thromb Haemost 2005; 94:615–9. [DOI] [PubMed] [Google Scholar]
- 31. Marcos V, Latzin P, Hector A, Sonanini S, Hoffmann F, Lacher M et al Expression, regulation and clinical significance of soluble and membrane CD14 receptors in pediatric inflammatory lung diseases. Respir Res 2010; 11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK et al Identification of the haemoglobin scavenger receptor. Nature 2001; 409:198–201. [DOI] [PubMed] [Google Scholar]
- 33. Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013; 13:621–34. [DOI] [PubMed] [Google Scholar]
- 34. Baeten D, Moller HJ, Delanghe J, Veys EM, Moestrup SK, De Keyser F. Association of CD163+ macrophages and local production of soluble CD163 with decreased lymphocyte activation in spondylarthropathy synovitis. Arthritis Rheum 2004; 50:1611–23. [DOI] [PubMed] [Google Scholar]
- 35. Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA et al Endotoxin induces rapid metalloproteinase‐mediated shedding followed by up‐regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol 2002; 72:711–7. [PubMed] [Google Scholar]
- 36. Suzuki Y, Shirai M, Asada K, Miwa S, Karayama M, Nakamura Y et al Utility of macrophage‐activated marker CD163 for diagnosis and prognosis in pulmonary tuberculosis. Ann Am Thorac Soc 2017; 14:57–64. [DOI] [PubMed] [Google Scholar]
- 37. Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K et al Monocyte‐activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis 2014; 210:1396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen P, Su B, Zhang T, Zhu X, Xia W, Fu Y et al Perturbations of monocyte subsets and their association with T Helper cell differentiation in acute and chronic HIV‐1‐infected patients. Front Immunol 2017; 8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peltola H, Laipio ML, Siimes MA. Quantitative C‐reactive protein (CRP) determined by an immunoturbidimetric method in rapid differential diagnosis of acute bacterial and viral diseases of children. Acta Paediatr Scand 1984; 73:273–4. [DOI] [PubMed] [Google Scholar]
- 40. Sproston NR, Ashworth JJ. Role of C‐Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018; 9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013; 50:23‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD et al Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 2010; 375:1920–37. [DOI] [PubMed] [Google Scholar]
- 43. Bjerrum L, Gahrn‐Hansen B, Munck AP. C‐reactive protein measurement in general practice may lead to lower antibiotic prescribing for sinusitis. Br J Gen Pract 2004; 54:659–62. [PMC free article] [PubMed] [Google Scholar]
- 44. Andrade BB, Pavan Kumar N, Mayer‐Barber KD, Barber DL, Sridhar R, Rekha VV et al Plasma heme oxygenase‐1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One 2013; 8:e62618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sharma V, Mandavdhare HS, Lamoria S, Singh H, Kumar A. Serial C‐reactive protein measurements in patients treated for suspected abdominal tuberculosis. Dig Liver Dis 2018; 50:559–62. [DOI] [PubMed] [Google Scholar]
- 46. Miranda P, Gil‐Santana L, Oliveira MG, Mesquita ED, Silva E, Rauwerdink A et al Sustained elevated levels of C‐reactive protein and ferritin in pulmonary tuberculosis patients remaining culture positive upon treatment initiation. PLoS One 2017; 12:e0175278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osterud B. Tissue factor/TFPI and blood cells. Thromb Res 2012; 129:274–8. [DOI] [PubMed] [Google Scholar]
- 48. Kornfeld H, West K, Kane K, Kumpatla S, Zacharias RR, Martinez‐Balzano C et al High Prevalence and Heterogeneity of Diabetes in Patients With TB in South India: A Report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study. Chest 2016; 149:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B et al Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014; 6:263ra159. [DOI] [PubMed] [Google Scholar]
- 50. Jing Y, Wu F, Li D, Yang L, Li Q, Li R. Metformin improves obesity‐associated inflammation by altering macrophages polarization. Mol Cell Endocrinol 2018; 461:256–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. No significant alterations in levels of monocyte activation markers in lung lesions, cavitary disease and bacterial burden in individuals with tuberculosis. (A) The plasma levels of sCD14, sCD163, C‐reactive protein (CRP) and soluble tissue factor (sTF) were measured in TB individuals with bilateral versus unilateral disease. (B) The plasma levels of sCD14, sCD163, CRP and sTF were measured in individuals with TB with cavitary versus non‐cavitary disease. (C) The relationship between the plasma levels of sCD14, sCD163, CRP and sTF and smear grades as estimated by sputum smears was examined in individuals with TB. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann–Whitney test with Holm's correction for multiple comparisons. For bacterial burden correlation, P values were calculated using the linear trend post‐test.


