Abstract
Angiogenesis plays an essential role in the progression of rheumatoid arthritis (RA). RGD peptide shows high affinity and selectivity for integrin αvβ3, which is one of the most extensively examined target of angiogenesis. Nimesulide could improve the anti-rheumatic profile of methotrexate. But the clinical application was limited due to water-insolubility of both methotrexate and nimesulide and lacking targeting ability. Therefore, this study aimed to design a targeted drug delivery system of micelles mediated by RGD plus the passive targeting of micelles to solve the application problems of methotrexate and nimesulide (M/N), and thus enhance their combined therapeutic effect on RA.
Methods: RGD was conjugated with NHS-PEG-PLA to form RGD-PEG-PLA for the preparation of RGD-modified drug-loaded micelles (R-M/N-PMs). The size and zeta potential of micelles were measured by dynamic light scattering. Morphology was detected by transmission electron microscopy. The inhibition effect of R-M/N-PMs on angiogenesis was assessed by the chick chorioallantoic membrane assay. The real-time fluorescence imaging analysis was conducted to examine the in vivo distribution of the fluorescence-labeled R-M/N-PMs. Rats arthritis model induced by Freund's adjuvant was used to evaluate the in vivo anti-inflammatory efficacy of R-M/N-PMs.
Results: The in vitro study indicated successful development of R-M/N-PMs. R-M/N-PMs could markedly suppress the angiogenesis of chick embryos. The fluorescence-labeled R-M/N-PMs mainly accumulated in arthritic joints. RGD enhanced the targeting ability of micelles and thus promoted retention of micelles in arthritic joints. Moreover, R-M/N-PMs significantly alleviated the joint swelling while reducing bone erosion and serum levels of inflammatory cytokines. It helped to recover the bone microstructure of arthritic rats.
Conclusion: Our results confirmed that the targeted delivery of the combination of a low dose of methotrexate and nimesulide mediated by RGD-modified polymeric micelles could enhance the therapeutic effect on rheumatoid arthritis. These findings provide a promising potential for the clinical therapy of rheumatoid arthritis.
Keywords: RGD, Polymeric micelles, Methotrexate, Nimesulide, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA), a chronic autoimmune disease, is characterized by infiltration of inflammatory mononuclear cells, excessive synovial hyperplasia, pannus formation over the joint surface and progressive joint destruction 1. At present, there is no cure for RA because of its complex etiology and multifactorial pathogenesis 2, 3. Current strategies for the treatment of RA include disease-modifying anti-rheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GC), and novel biologics like TNFα-blocking agents 4, 5 and B-cell targeted therapy 6. However, because of the high cost of novel biologics and the severe side effects of glucocorticoids, the conventional DMARDs are still widely used in clinical therapy 7. According to the guidelines of the American College of Rheumatology, newly diagnosed RA patients receive NSAIDs for reducing acute pain and suppressing inflammation in combination with DMARDs for relieving disease activity and preventing joint damage 8. Methotrexate (MTX), which is a chemotherapy drug and an immunosuppressant, is one of the most commonly used DMARDs for the treatment of RA 9-11 . However, MTX was reported to cause significant systemic toxicity, especially for highly proliferative cells in the gastrointestinal mucosa and bone marrow. Nimesulide (NIM), which belongs to the NSAID class of drugs and is a COX-2 inhibitor, is widely used for symptomatic alleviation of RA 12. The combined application of MTX and NIM for RA was suggested to increase therapeutic efficacy and minimize the side effects of MTX 13. However, some disadvantages like poor water-solubility of both MTX and NIM and poor pharmacokinetics and narrow safety dose of MTX limit their application in the treatment of various diseases. Moreover, both MTX and NIM lack tissue specificity, which leads to serious side effects including reproductive toxicity, gastrointestinal lesions, and cardiovascular complications. Therefore, nanotherapeutics and the targeted delivery strategies for improving the target tissue accumulation of drugs have attracted considerable attention in recent years for the treatment of RA 14-16.
It has been reported that the polymeric micelles, as nano-sized drug carriers, show various advantages such as prolonging the circulation time in blood, improving the solubility of hydrophobic drugs, controlling the drug release pattern, and protecting the cargos from degradation in vivo 17, 18. Therefore, polymeric micelles are considered ideal carriers for specific drugs with low solubility and side effects such as MTX and NIM. More importantly, polymeric micelles may be passively targeted to sites of inflammation according to the ELVIS (Extravasation through Leaky Vasculature and Inflammatory cell-mediated Sequestration) mechanism 19.
Inflammation and angiogenesis are two crucial factors in the initiation and persistence of arthritic disease and targeting both features may yield effective therapeutic strategies for successfully treating RA 20. RGD peptide is known to have an affinity for αvβ3 integrin that is over-expressed on angiogenic endothelial cells 21. We hypothesized that RGD-mediated polymeric micelles loaded with low-dose methotrexate and nimesulide, would drive both passive and active targeting, and enhance the combined therapeutic effect of both drugs on rheumatoid arthritis. We, therefore, conjugated RGD onto an amphiphilic copolymer of NHS-PEG3400-PLA2000, one of the biocompatible and biodegradable materials approved by FDA 22, 23, to form RGD-PEG3400-PLA2000. Subsequently, we prepared RGD-modified drug-loaded micelles (R-MTX-PMs, R-NIM-PMs) by the filming-rehydration method in which the combination of R-MTX-PMs and R-NIM-PMs was designated as R-M/N-PMs. We assessed the toxicity of micelles by the in vitro hemolysis test and detected the inhibitory effect of R-M/N-PMs on angiogenesis using the chick chorioallantoic membrane assay. Furthermore, we conducted the real-time fluorescence imaging analysis to examine the in vivo distribution of the fluorescence-labeled R-M/N-PMs and performed in vivo studies in a rat model with adjuvant-induced arthritis to assess the anti-inflammatory efficacy of R-M/N-PMs.
Materials and Methods
Materials
Methotrexate was supplied by the National Institutes for Food and Drug Control (Beijing, China). Nimesulide was obtained from Tokyo Chemical Industry Corporation (Tokyo, Japan; purity >98%). mPEG3400-PLA2000 and NHS-PEG3400-PLA2000 polymer were purchased from Xi'an Ruixi Biotechnology Company (Xi'an, China). RGD tri-peptide was obtained from Nanjing Peptide Biotech Company (Nanjing, China; purity >95%). Methanol and acetonitrile (HPLC grade) were purchased from Kelong Chemical Reagent Factory (Chengdu, China). Complete Freund's adjuvant (CFA) was acquired from Chondrex (Washington DC, USA). ELISA kits were from Shanghai Qiaodu Biotechnology Company (Shanghai, China).
Cell lines and animals
The murine macrophage cell line Raw264.7 and human umbilical vein endothelial cell line (HUVEC) were purchased from the Shanghai Cell Institute, China Academy of Sciences, and preserved in our laboratory. Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibico Laboratories (Grand Island, NY, USA). 3-(4,5 dimethylthiozol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma (USA). Paraformaldehyde was provided by Jinshan Chemical Company (Chengdu, China). Both Raw264.7 and HUVEC cells were cultured in DMEM containing 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C with 5% CO2.
Male Sprague-Dawley rats (160 ± 20 g) were supplied by the Experimental Animal Center of Southwest Medical University (Lu zhou, China). The rats were maintained under standardized conditions. All animal tests were approved by the Institutional Animal Care and Ethics Committee of Southwest Medical University (permit No. 2017050009).
Preparation of RGD-modified micelles loaded with MTX and NIM
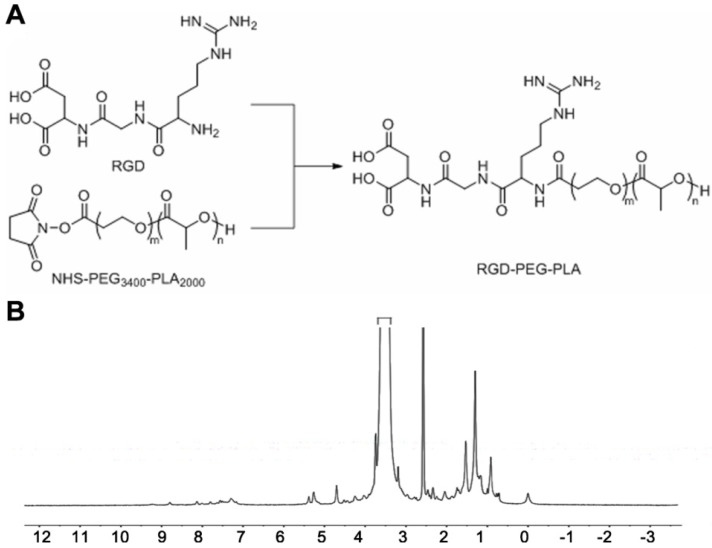
To prepare the RGD-modified micelles loaded with MTX and NIM, we first synthesized the copolymer RGD-PEG3400-PLA2000 as shown in Figure 1A, using NHS-PEG3400-PLA2000 as a crosslinker as described previously 22. Briefly, 130 mg of NHS-PEG3400-PLA2000 was dissolved in anhydrous N, N-dimethyl formamide (DMF) and mixed with 25 μL of anhydrous triethylamine (TEA). Subsequently, 10 mg of RGD was added to the mixture, stirred at room temperature for 24 h and then dialyzed (MWCO 3,500 Da) against deionized water for 48 h to remove the unconjugated RGD. The solution was immediately lyophilized after dialysis and subjected to 1H NMR (400 MHz, DMSO-d6) detection to confirm the conjugation of RGD with PEG3400-PLA2000.
Figure 1.
Synthesis and 1H NMR spectrum of RGD-PEG3400-PLA2000. (A) NHS-PEG3400-PLA2000 was reacted with RGD in anhydrous N, N-dimethyl formamide (DMF) containing triethylamine (TEA), with 1: 1.2: 1.2 molar ratio of NHS-PEG3400-PLA2000, RGD, and TEA. The mixture was stirred at room temperature for 24 h. (B) 1H NMR (DMSO-d6) spectrum was used to identify the synthesized polymers.
Polymeric micelles were prepared by the filming-rehydration method according to the published literature with minor modification 22, 23. In brief, the preformed RGD-PEG3400-PLA2000 copolymer (40 mg) and MTX/NIM (4 mg) were dissolved in 2 mL DMF. The mixture was dried under reduced pressure at 50 °C until a dry thin-film formed. To remove any residual DMF, it was maintained in a vacuum drying chamber for overnight at room temperature. Then, 2 mL saline was added and kept in an incubator at 37 °C with slow shaking for 1 h. Subsequently, the solution was ultrasonicated for 15 min at 25 °C. Finally, the micelles solution was centrifuged at 15,000 × g for 10 min to remove the unloaded drugs. The blank micelles (PMs) and RGD-free micelles were also prepared according to the described procedure.
Characterization of micelles
The size and zeta potential of micelles including PMs, MTX-PMs, NIM-PMs, R-MTX-PMs and R-NIM-PMs were measured by the dynamic light scattering (DLS) method using a Malvern Zetasizer Nano ZS90 (Malvern Instruments, U.K.). Morphology of micelles was observed by transmission electron microscopy (TEM). Critical micelle concentration (CMC) was measured by a fluorescence technique using pyrene as the fluorescence probe as described previously 24. Encapsulation efficiency (EE) and drug loading (DL) of micelles were measured using high-performance liquid chromatography (HPLC). Measurements were performed on an Agilent ZORBAX Eclipse XDB-C18 column (5 μm, 150 × 4.6 mm). For the detection of MTX, the flow phase was prepared with a mixture of sodium dihydrogen phosphate (10 mmoL/L) and methanol (26:74, v/v). The detection wavelength was 302 nm, which corresponds to the maximum absorption of MTX. In case of NIM, the flow phase was the mixture of 0.1% phosphoric acid (pH 7.0) and acetonitrile (60:40, v/v) and the detector was set to monitor the signal at 295 nm. All measurements were conducted at 25 °C with a flow rate of 1.0 mL/min and an injection volume of 20 μL. The selectivity, linearity, precision, and recovery of methods were fully validated. Encapsulation efficiency and drug loading were determined using the following formulae:
| EE (%) = (Weight of the drug encapsulated in micelles) / (Weight of the total drug) × 100% |
| DL (%) = (Weight of the drug encapsulated in micelles) / (Weight of the total micelles) × 100% |
In vitro release of MTX and NIM from micelles
In vitro release of micelles including MTX-PMs, NIM-PMs, R-MTX-PMs, and R-NIM-PMs were investigated using the dialysis method with PBS with 1% Tween-80 as the release medium. Briefly, three batches of the preformed micelles were added into dialysis bags with a molecular weight cut-off of 3.5 kDa (Millipore) and placed in 50 mL of release medium. The whole device was placed in a water bath at 37 °C for 96 h. At the pre-designed time points of 0.5, 1, 2, 4, 6, 8, 12, 24, 36, 48, 72 and 96 h, 2.0 mL of the solution from the release medium was taken and replaced with fresh medium. The sample was brought up to 5 mL with methanol and filtered through a 0.22-μm membrane (Millipore). The concentrations of MTX or NIM in various micelles were determined by the HPLC method described above.
Toxicity assay of micelles by in vitro hemolysis
Rat red blood cells (RBCs) were collected to evaluate the hemolytic potential of MTX/NIM-loading micelles. RBCs were centrifuged 5 times at 2,000 × g for 8 min and suspended in 10 mL of saline. 500 μL of different concentrations (5.65, 56.5, 565 μg/mL) of MTX-PMs, NIM-PMs, M/N-PMs (MTX-PMs + MIN-PMs), and R-M/N-PMs (R-MTX-PMs + R-MIN-PMs) were incubated with 500 μL of RBCs suspension (2%) with normal saline and cell lysis solution (RIPA) as negative and positive controls, respectively 25. After incubation at 37 °C for 3 h, the samples were centrifuged at 2,500 g for 10 min. 150 μL of the supernatants were collected and placed into 96-well plates to analyze the hemoglobin content by microplate reader at 540 nm. The hemolysis rate (%) was calculated according to the following formula:
| Hemolysis rate (%) = (Asample - Anegative control) / (Apositive control - Anegative control) ×100%. |
Chick chorioallantoic membrane assay
The chick chorioallantoic membrane (CAM) assay was conducted to analyze the anti-angiogenic activity of micelles formulation 21, 26, 27. Chicken eggs were fertilized and incubated at 37 °C with a relative humidity of 60%. On day 6, all eggs were swabbed with ethanol solution (75%). A small hole was made at the end of the chamber with a hypodermic needle. The second hole was made in the center of the egg which was directly located over the avascular portion of the embryonic membrane. By using a negative pressure through the first hole to separate CAM from the shell, a false air chamber was created. After an additional incubation for 48 h, a 1.0-cm-diameter window was made with an embroidery needle and a tweezer, and the shell membrane was peeled away. Sterile filter papers (about 6 mm2), pretreated with saline as control and PMs, MTX-PMs, NIM-PMs, M/N-PMs, R-MTX-PMs, R-NIM-PMs, R-M/N-PMs, were placed on the vessels surface and air-dried under sterile conditions. After incubating for 24 h, the area around the filter paper was peeled off and photographed using a Canon digital camera.
Construction of the arthritis model induced by CFA
We used CFA to induce arthritis in the rats. Briefly, 0.12 mL of CFA that contained 10 mg/mL of heat-killed mycobacteria was subcutaneously injected at the base of the rat tail 11, 28 using saline injection as a control. During disease progression, all rats could freely access sterile food and water. The arthritis progression was monitored daily.
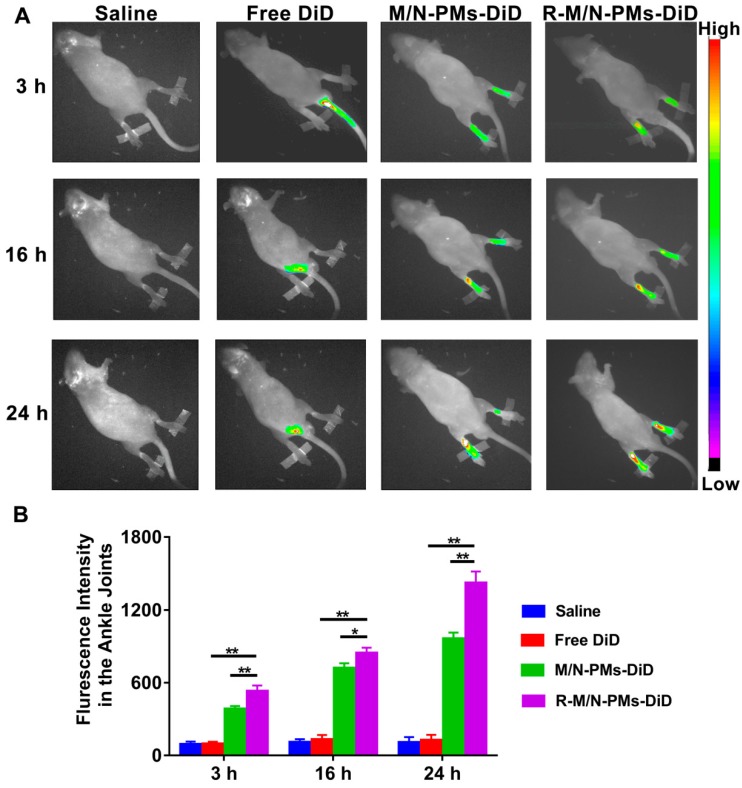
Distribution of R-M/N-PMs in arthritic rats
Following the intravenous injection in SD rats with arthritis, the distribution of micelles was investigated by the in vivo small animal imaging system (Bruker, Fx Pro/FX, USA). DiD (KeyGEN BioTECH), a hydrophobic infrared fluorescent dye, was used as the tracer (5 μg DiD per rat) in free form or encapsulated into polymeric micelles 14. Arthritic rats with whole body hair removed by depilation cream were randomly divided into four groups (n=3) of saline, DiD, M/N-PMs-DiD, and R-M/N-PMs-DiD. Each rat received an intravenous injection of 0.2 mL of different micelle solutions, in which the MTX and NIM dose was 0.6 mg/kg and 3.0 mg/kg, respectively, as determined by our preliminary experiments and according to a published report 13. The rats were anesthetized at the designated time points (3 h, 16 h, and 24 h) and visualized in imaging systems, in which the filters for excitation and emission were set at 644 nm and 665 nm, respectively, to measure fluorescence intensity of DiD. The images of each rat used the same intensity scale with the same range of minimum and maximum values. The fluorescence intensity was quantified using NIH Image-Pro Plus 6.0.
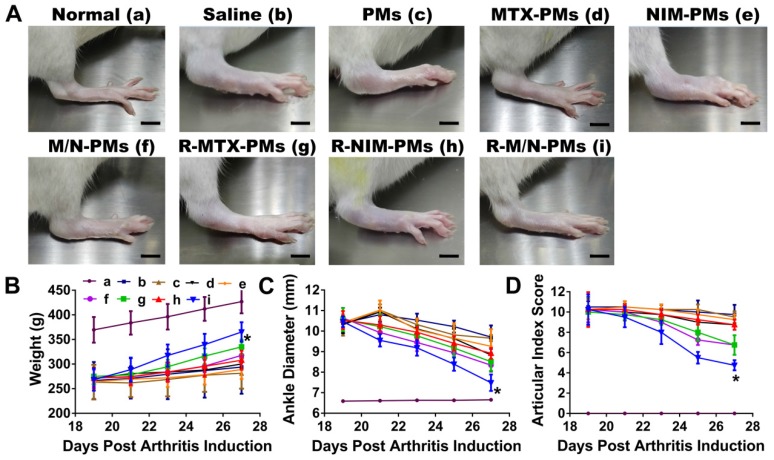
Measurement of weight, ankle diameter, and articular index score in arthritic rats
After injection with CFA to induce arthritis, the diet, mental state, fur color, walking gait, and joint swelling of the rats were observed every day. The weight of each rat was measured every other day during the disease progression from day 0. On day 19 post-induction, arthritis in rats was at the peak level, showing severe swelling and erythema in limbs, especially the hind limbs. Arthritic rats were randomly assigned to eight groups (n = 5) and injected intravenously with 0.2 mL of saline, PMs, MTX-PMs, NIM-PMs, M/N-PMs, R-MTX-PMs, R-NIM-PMs and R-M/N-PMs on days 19, 21, 23, and 25 after arthritis induction, in which the MTX dose was 0.6 mg/kg and the NIM dose was 3.0 mg/kg. The normal healthy rats were injected with equal volume of saline as control. The ankle diameter from medial to lateral of each rat was detected every other day using a digital caliper. The articular index (AI) score in each limb of all animals was assessed from day 19 to day 27 post-arthritis inductions following a previously described method with minor modifications 29. In brief, the AI scoring was made according to a numeric system with a score ranging from 0 to 4, in which the absence of swelling or erythema was considered to be 0; a mild swelling and/or erythema was 1; a moderate edema and signs involving the tarsals was 2; a visible edema with limited use of the joint and signs extending to the metatarsals was 3; and an excessive edema with joint rigidity and severe signs involving the entire paw was 4. The final arthritis score of each rat was the sum of the two hind limb scores.
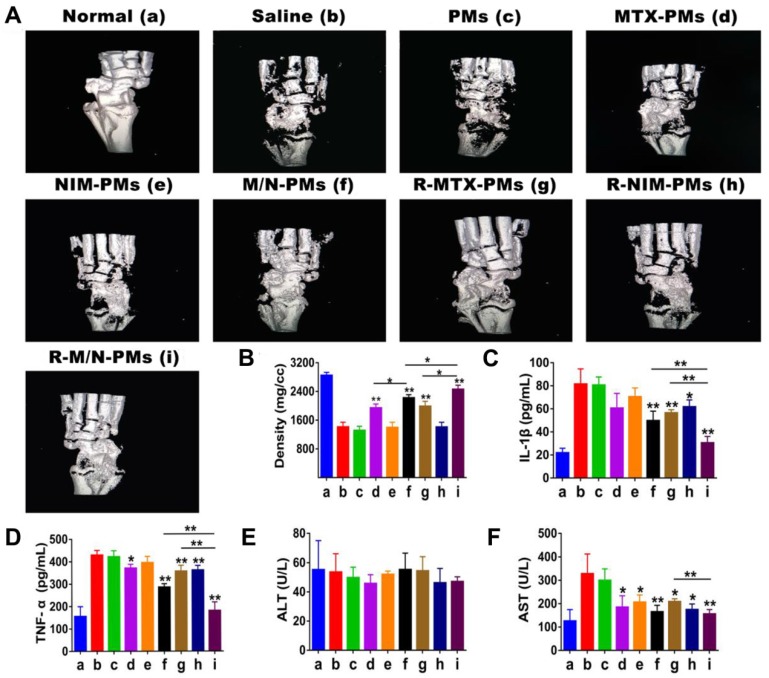
Micro-computed tomography analysis of ankle joints
On day 27 after induction, rats of each group (n = 5) were euthanized and the left ankle joints were fixed by 10% neutral buffered formalin for 48 h to evaluate the bone density of ankle joints using a high-resolution Micro-computed tomography (Micro-CT, SIEMENS healthcare, Berlin and Munich Germany) 11, 30. Parameters of Micro-CT scanning were set as voltage 55 kV, current 189 μA, exposure time 230 ms, resolution 6.2 μm and aluminum filter 0.5 mm. The three-dimensional (3D) reconstruction was performed for visualization and data analysis.
Analysis of serum markers and assessment of the immune organs index
On day 27 after induction, the blood, spleens and thymuses were collected from each group. Expression levels of the pro-inflammatory cytokines, TNF-α and IL-1β, in serum were measured using ELISA kits according to the standard protocol. Serum levels of aspartate aminotransferase (AST) and alanine transaminase (ALT) were measured using an automatic biochemical analyzer.
Spleens and thymuses collected from rats were accurately weighed to calculate the spleen and thymus index according to the following formula:
| Spleen index (%) = (Spleen weight) / (Body weight) × 100% |
| Thymus Index (%) = (Thymus weight) / (Body weight) × 100%. |
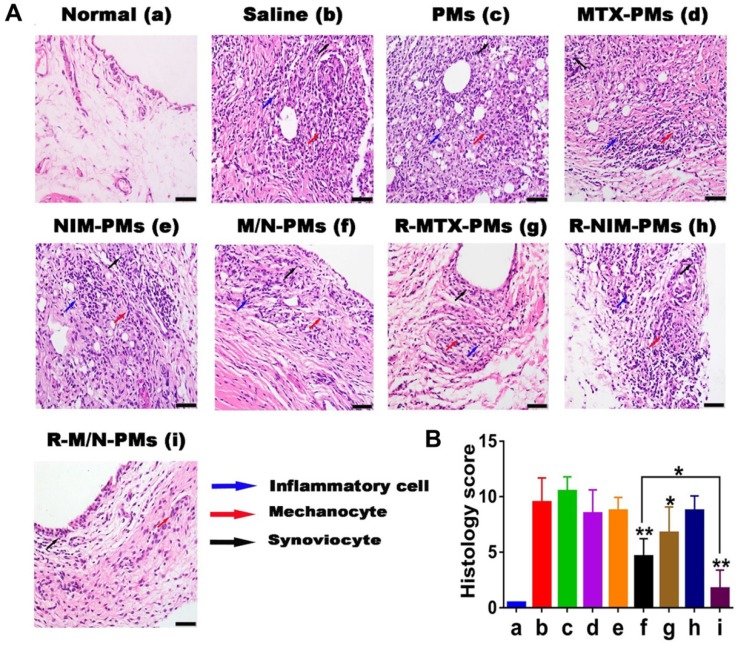
Joint tissue histological evaluation
The ankle joints were decalcified in a neutral calcium EDTA decalcifying solution (14%) for 4-6 days until the joint tissue became soft with barbless resistance after complete decalcification. The decalcified ankle joints were bisected longitudinally on the median axis and paraffin-embedded by standard histological methods. Tissue sections (5 μm) were processed and H&E stained for histological evaluation by a pathologist, who was blinded to the treatments.
Pathological changes were evaluated with modification of the previously described semi-quantitative scoring system for experimental arthritis in rats 31 as follows: synovial cell proliferation (0-2 points), pannus formation (0-3 points), mononuclear cell infiltration (0-3 points), infiltration of neutrophils in soft tissues around the joints (0-3 points), cell infiltration and distal tibia bone destruction (0-3 points), and chondrocyte infiltration (0-2 points). The H&E score of an ankle joint is the sum of histopathological features scores.
Statistical analysis
Results were scored as the mean ± standard deviation (SD) and analyzed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Statistical comparisons were made to assess the difference between various groups using one-way analysis of variance (ANOVA). A P value less than 0.05 was considered statistically significant.
Results
Synthesis and structure identification of RGD-PEG3400-PLA2000
1H NMR was utilized to determine the formation of RGD-PEG3400-PLA2000. As shown in Figure 1B, the peak at 5.21 ppm corresponds to the tertiary PLA proton (m, -CH), and that at 3.62 ppm represents the protons of the repeating units in the PEG chain (m, OCH2-CH2O). The peak of 1.51 ppm is for the pend methyl group of PLA chain (m, -CH3). The chemical shift at 8.32 ppm is assigned to the H protons in RGD blocks.
Characterization of particle size, zeta potential, and morphology of micelles
Based on DLS detection, the micelles showed small size and narrow size distribution (Table 1). The particle size of blank micelles (PMs) was 29.70 ± 0.58 nm with a PDI of 0.17 ± 0.04. After drug loading, it increased to 32.30 ± 0.86 nm and 59.70 ± 6.10 nm for MTX-PMs and NIM-PMs, respectively, and the particle sizes of R-MTX-PMs and R-NIM-PMs were 34.30 ± 0.61 nm and 60.20 ± 3.21 nm, respectively. Thus, there was no significant difference in the particle sizes between MTX-PMs vs. R-MTX-PMs and NIM-PMs vs. R-NIM-PMs (P > 0.05). The PDI data showed the uniformity of all particles and the zeta potential data indicated that all micelles had a neutral charge.
Table 1.
Characteristics of micelles: size distribution, zeta potential, drug loading, and encapsulation efficiency.
| Micelles | Particle Size (nm) |
PDI | Zeta Potential (mV) | Drug Loading |
Encapsulation Efficiency |
|---|---|---|---|---|---|
| PMs | 29.70 ± 0.58 | 0.171 ± 0.04 | 0.00 ± 1.53 | - | - |
| MTX-PMs | 32.30 ± 0.86 | 0.236 ± 0.10 | 1.57 ± 0.06 | 4.97 ± 0.86 | 84.60 ± 9.51 |
| R-MTX- PMs | 34.30 ± 0.61 | 0.238 ± 0.21 | 1.60 ± 0.06 | 5.03 ± 0.67 | 85.36 ± 6.56 |
| NIM-PMs | 59.70 ± 6.10 | 0.321 ± 0.04 | 1.57 ± 2.14 | 5.67 ± 0.25 | 89.06 ± 8.58 |
| R-NIM- PMs | 60.20 ± 3.21 | 0.319 ± 0.07 | 1.60 ± 1.98 | 5.78 ± 0.32 | 90.78 ± 7.85 |
Abbreviation: PDI, polydispersity index. Results are expressed as mean ± SD from three independent experiments.
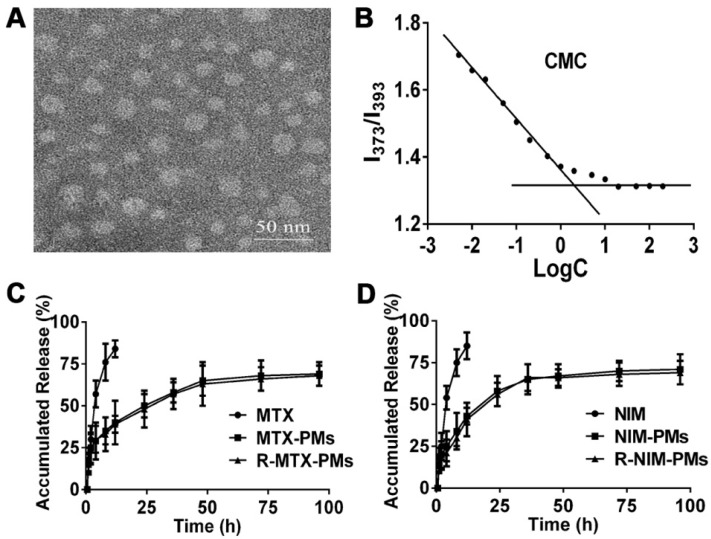
MTX and NIM were incorporated into the PEG3400-PLA2000 micelles to form R-MTX-PMs and R-NIM-PMs with an encapsulation efficiency of 85.36 ± 6.56% and 90.78 ± 7.85% and drug loading yield of 5.03 ± 0.67% and 5.78 ± 0.32%, respectively. TEM demonstrated micelles with a uniform spherical shape and an average size of about 30 nm (Figure 2A). This result was consistent with that obtained from the dynamic light scattering.
Figure 2.
Characterization of micelles. (A) Morphology of blank micelles detected by transmission electron microscopy (TEM), bar =50 nm. (B) Critical micelle concentration (CMC) of polymeric micelles. In vitro release profile of (C) MTX and (D) NIM in PBS containing 1% Tween-80. Results are presented as mean ± SD (n = 3).
CMC was estimated to be 2.51×10-3 mg/mL through the fluorescence method taking pyrene as a probe (Figure 2B). The in vitro release profile of MTX-PMs, NIM-PMs, R-MTX-PMs and R-NIM-PMs are shown in Figure 2C-D. All micelles formulations exhibited a sustained release profile compared to free MTX and NIM, in which both free drugs were released rapidly with up to about 85% cumulative release over the first 24 h. In contrast, only about 60% of MTX and 65% of NIM were released from drug-loaded micelles after 96 h. These results suggested that the micelle formulations of MTX-PMs, NIM-PMs, R-MTX-PMs, and R-NIM-PMs showed the advantage of controlled release at 37 °C in neutral medium.
Analysis of hemolytic activity of micelles
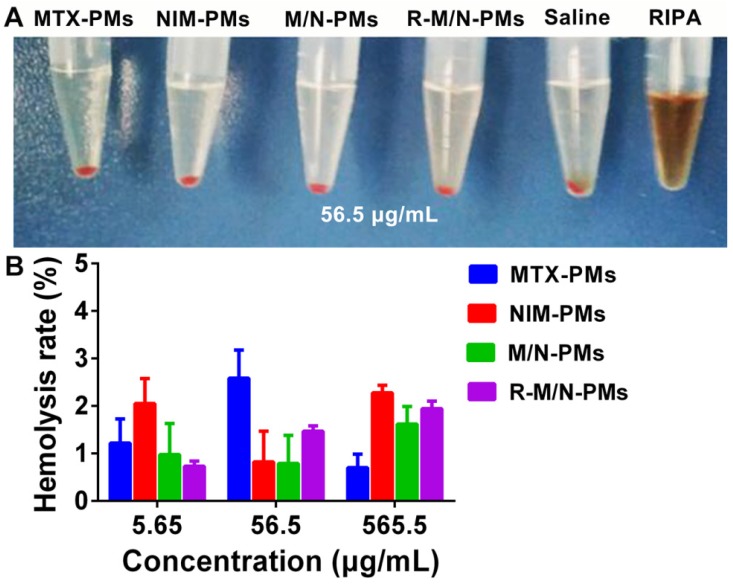
Since the micelles are intended for the in vivo study, we first evaluated their biocompatibility by using the in vitro hemolysis test. As shown in Figure 3A, the positive control of RIPA induced massive hemolysis, while the micelle samples including MTX-PMs, NIM-PMs, M/N-PMs, and R-M/N-PMs induced erythrocyte lysis at a level similar to the negative control. Figure 3B shows that even at a concentration of up to 565 μg/mL, no obvious hemolytic activity was observed by any of the micelle samples with an average hemolysis rate of less than 3%. Therefore, these results demonstrated good biocompatibility of all four micelle formulations, MTX-PMs, NIM-PMs, M/N-PMs, and R-M/N-PMs.
Figure 3.
Hemolytic activity of micelles. (A) Red blood cells from healthy SD rats were incubated at 37 °C with different micelle formulations of MTX-PMs, NIM-PMs, M/N-PMs, and R-M/N-PMs with saline as negative and RIPA as positive controls. (B) Hemolytic activity of increasing concentrations of various micelle formulations
Effect of micelles on angiogenesis
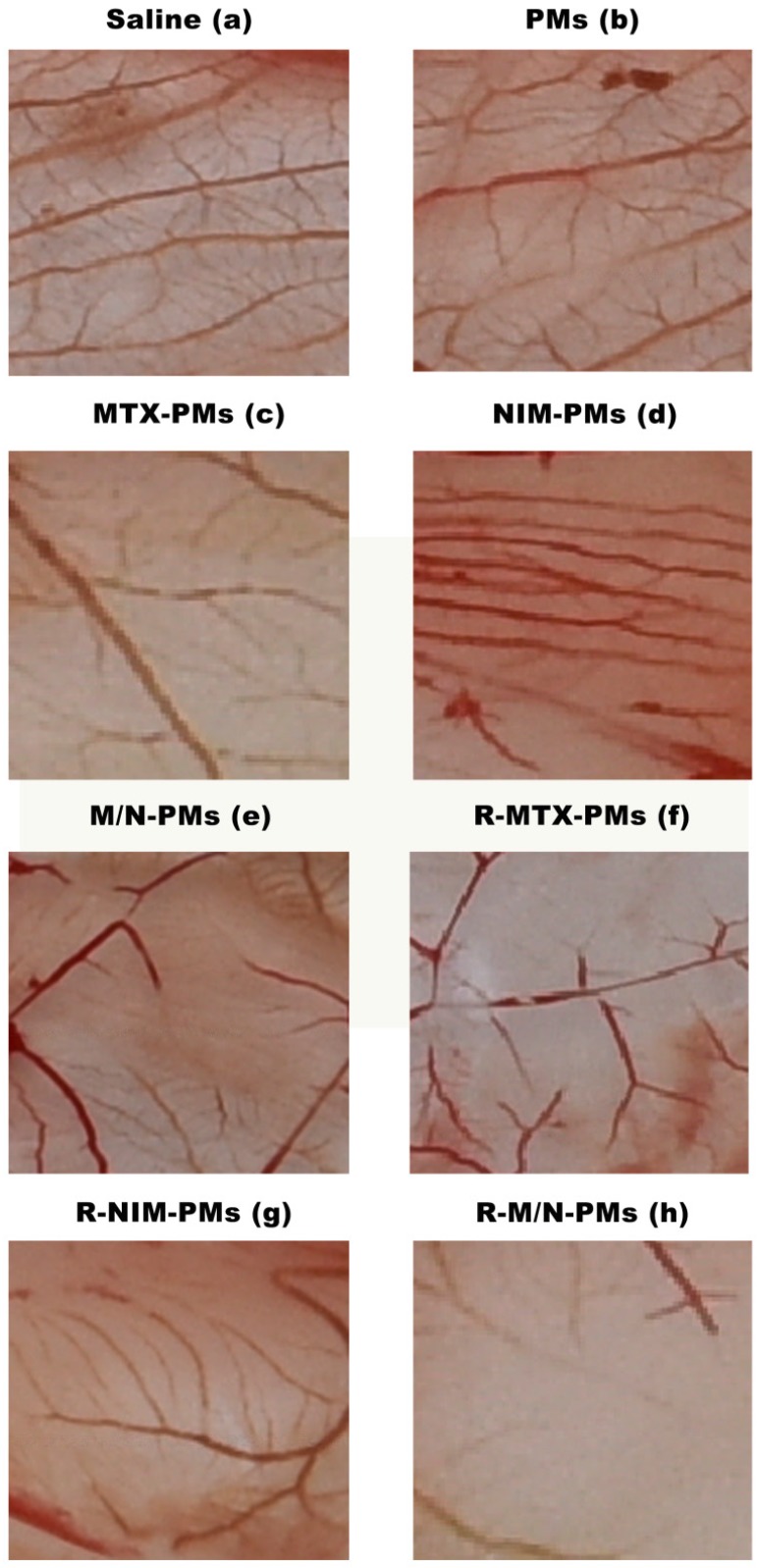
To verify whether the micelle formulations could suppress angiogenesis, we used the CAM in vivo angiogenesis model in this study. As shown in Figure 4, compared to the saline group, the newly formed blood vessel branch points were decreased in the groups treated with MTX-PMs, NIM-PMs, M/N-PMs, R-MTX-PMs, R-NIM-PMs, and R-M/ N-PMs, whereas in the PMs group they remained at the normal level. Among all micelle formulations, R-M/N-PMs showed the most potent inhibitory effect on angiogenesis of chick embryos in the CAM assay.
Figure 4.
Inhibitory effects of micelle formulations on neovascularization in chick embryos. The CAM assay was performed as described in M&M section. Compared to the Saline control and the group treated with PMs, all other formulations showed a decrease in blood vessel branch points with the most significant decrease with R-M/N-PMS.
Retention of R-M/N-PMs in arthritic joints
Real-time fluorescence imaging analysis revealed that systemically administered M/N-PMs- DiD or R-M/N-PMs-DiD mainly accumulated in arthritic joints (Figure 5A). The fluorescence signal was negligible in the limbs of arthritic rats receiving free DiD, but intense fluorescence in arthritic joints was detected as early as at 3 h post-injection and lasted for more than 24 h. R-M/N-PMs-DiD showed stronger fluorescence signal in arthritic joints compared with M/N-PMs-DiD. To further investigate the biodistribution of R-M/N-PMs, the semi-quantitative analysis of fluorescence signal was carried out (Figure 5B). The graph illustrated a similar pattern as fluorescence images. These results suggested that R-M/N-PMs-DiD persisted longer in circulation and accumulated selectively in arthritic joints and that RGD enhanced the targeting ability of micelles and promoted their retention in arthritic joints. This is consistent with the result from the tissue biodistribution detection shown in Figure S4.
Figure 5.
In vivo distributions of micelles in arthritic rats (A) and average fluorescence intensity detected from ankle joints (B). The arthritic rats received an intravenous injection of free DiD, M/N-PMs-DiD, R-M/N-PMs-DiD, and saline as control. Data were presented as mean ± SD (n = 3; *P < 0.05, **P < 0.01).
Sustained amelioration of joint inflammation by R-M/N-PMs in arthritic rats
Mild swelling and erythema were observed on day 15 after CFA injection. On day 19, arthritis was fully developed when severe swelling and erythema appeared in the hind limbs and moderate symptoms in the front paws. Various micelle formulations were injected via the tail vein and then the ankle joint diameter and the joint index score were measured 5 times every other day. As shown in Figure 6A, arthritis progression appeared significantly different between different treatment groups after the 5th drug administration on day 27, which was visible by macroscopic examination.
Figure 6.
In vivo therapeutic effects of micelles in rats with adjuvant-induced arthritis. On day 19 post-induction, the rats with established arthritis were given different micelle formulations every other day for 4 times. (A). Representative photographs of hind legs were taken on the 27th day (B), weight, (C) ankle diameter, and (D) articular index scores show the strongest effect of the R-M/N-PMs micelles. Results are shown as mean ± SD (n=5). Symbols P represented statistical significance with *P < 0.050. The bar is 75 mm.
In terms of the weight changes, compared to the saline control group, there was a continuous and pronounced increase from day 19 to 27 post-arthritis induction in the R-M/N-PMs group (p <0.01) (Figure 6B). Both saline and PMs groups showed a slight weight increase in rats whereas a moderate increase was observed in other groups.
The drug-loaded micelle formulations including MTX-PMs, NIM-PMs, M/N-PMs, R-MTX-PMs, R-NIM-PMs, and R-M/N-PMs resulted in a greater decrease in both ankle swelling (Figure 6C) and AI scores (Figure 6D) from day 19 to 27 post-arthritis induction, showing slower disease progression than saline and PMs. The ankle diameter of the R-M/N-PMs group appeared to be significantly lower (P < 0.05) than the other micelle-treated groups and saline group; but was significantly higher (P < 0.05) than the normal healthy control. No significant differences were observed between PMs and saline groups (P >0.05).
Impact of micelles on the bone microstructure and serum biochemical markers
Figure 7A shows the three-dimensional reconstruction images of the bone microstructure obtained from micro-CT. The quantitative analysis of bone mineral density (BMD) is presented in Figure 7B. From these images and BMD data, it is evident that severe bone damage occurred in the saline and PMs groups with extensive erosion of the entire tibia. Compared to the saline group, MTX-PMs- and R- MTX-PMs-treated animals demonstrated a moderate erosion of the ankle bone (P < 0.05); M/N-PMs and R-M/N-PMs significantly helped to recover bone microstructure with minor erosion (P <0.01).
Figure 7.
Impact of micelles on the bone microstructure and serum biomarkers. (A) Significant restoration of the bone microarchitecture analyzed by micro CT on day 27 after induction and (B) Quantitative analysis of bone mineral density. The maximum effect was observed in both parameters by R-M/N-PMs. Serum biomarkers of (C) IL-1β and (D) TNF-α analyzed by ELISA; that of (E) ALT and (F) AST measured using an automatic biochemical analyzer. Results are shown as mean ± SD (n=4). Symbols P represented statistical significance with *P < 0.05 and **P < 0.01.
It has been reported that IL-1β and TNF-α are the most important pro-inflammatory cytokines involved in the progression of joint synovial damage in RA 32, 33. Following arthritis induction, both IL-1β and TNF-α increased significantly compared to the healthy controls (Figure 7C-D). The increased levels of IL-1β and TNF-α were maintained after treatment with saline and PMs, whereas treatment with NIM-PMs resulted in a slight decrease and with MTX-PMs, R-MTX-PMs and R-NIM-PMs led to a moderate decrease in both cytokines (P < 0.05). However, both M/N-PMs and R-M/N-PMs significantly decreased the expression levels of IL-1β and TNF-α (P <0.01), with R-M/N-PMs leading to a greater decrease.
In the normal controls, the serum levels of ALT were similar to those in animals treated with saline and micelle formulations (Figure 7E). But arthritis progression increased the AST level (Figure 7F) that was maintained after treatment with saline or PMs. Compared to saline or PMs group, treatment with other drug-loaded micelles resulted in a pronounced reduction of AST (P < 0.05, P <0.01). This result indicated that the targeted delivery of R-M/N-PMs likely help to reduce the liver toxicity in the arthritic rats.
RA is an immune disease, so an index of the immune organs could reflect the disease progression to a certain extent. As shown in Table 2, both spleen and thymus indices of arthritic rats treated with saline and PMs were significantly higher than that of the healthy controls. Compared to the saline group, M/N-PMs and R-M/N-PMs remarkably reduced the indices of spleen and thymus (P <0.01) reaching almost the normal levels.
Table 2.
Spleen and thymus index of rats post-treatment with various micelle formulations
| Groups | Dosage (mg/kg) | Spleen index (%) | Thymus index (%) |
|---|---|---|---|
| Normal | - | 0.198 ± 0.013 | 0.134 ± 0.023 |
| Saline | - | 0.293 ± 0.028 | 0.169 ± 0.018 |
| PMs | 3.0 | 0.283 ± 0.019 | 0.163 ± 0.017 |
| MTX-PMs | 0.6 | 0.234 ± 0.025 | 0.150 ± 0.023 |
| NIM-PMs | 3.0 | 0.250 ± 0.027 | 0.152 ± 0.025 |
| M/N-PMs | 0.6MTX + 3.0NIM | 0.211± 0.019** | 0.142 ± 0.017** |
| R-MTX-PMs | 0.6 | 0.216 ± 0.031* | 0.151 ± 0.029 |
| R-NIM-PMs | 3.0 | 0.218 ± 0.033* | 0.154 ± 0.012 |
| R-M/N-PMs | 0.6MTX + 3.0NIM | 0.210 ± 0.016** | 0.140 ± 0.014** |
Results are presented as mean ± S.D. (n=5). Symbols represent statistical significance compared with the control group (*P < 0.05, **P < 0.01).
Histological analysis of ankle joints
Representative images from the tissue histopathology analyses of the ankle joints isolated at the end point of the in vivo experiment are shown in Figure 8A. Compared to healthy control animals, histological analysis revealed marked periosteal expansion, inflammatory cell infiltration, pannus formation, and distal tibia bone destruction in saline- and PMs-treated groups. However, the treatment groups of M/N-PMs and R-M/N-PMs displayed markedly reduced joint damage and cellular infiltration with bone and cartilage morphology maintained at a level similar to that of the healthy rats. The sum of the score from each animal was recorded and is shown in Figure 8B. A statistically significant difference was found between saline vs. other groups, M/N-PMs (P < 0.01), R-MTX-PMs (P < 0.05), and R-M/N-PMs (P < 0.01) as well as M/N-PMs vs. R-M/N-PMs (P < 0.05) groups. No significant difference was found between saline and PMs.
Figure 8.
Histopathology analysis of the ankle joint. (A) H&E staining. The inflammatory cells, melanocytes, and synoviocyte are marked by bold blue, red, and black arrows, respectively. The bar is 100 μm. (B) Histopathology scores. Results are shown as mean ± SD (n=5). Symbols P represented statistical significance with *P < 0.05 and **P < 0.01.
Discussion
Angiogenesis plays an important role in the progression of RA, which is considered an angiogenesis-dependent disease 34. We conducted this study to evaluate whether the combination of methotrexate and nimesulide mediated by RGD-modified polymeric micelles, R-M/N-PMs, could target and inhibit angiogenesis and thus enhance the therapeutic effect of drugs on rheumatoid arthritis. We successfully synthesized RGD-functionalized polymers, RGD-PEG3400-PLA2000, to formulate RGD-modified drug-loaded micelles by the thin-film hydration method. R-M/N-PMs not only significantly inhibited the viability of the inflammatory RAW264.7 cells but also markedly suppressed angiogenesis of chick embryos in the CAM assay. We further conducted the real-time fluorescence imaging analyses using the in vivo small animal imaging system and observed that systemically administered R-M/N-PMs labeled by DiD mainly distributed to arthritic joints and that RGD enhanced the targeting ability of micelles and thus promoted the retention of micelles in arthritic joints. Most importantly, the in vivo study of the arthritic rats demonstrated that R-M/N-PMs reduced the joint swelling, immune organs index, bone erosion, and serum levels of inflammatory cytokines and thus enhanced the therapeutic efficacy for rheumatoid arthritis.
The particle size of the polymeric micelles was between 25 nm and 65 nm suitable for tail vein injection in rats. However, the zeta potential indicated that the micelles had a neutral charge. Zeta potential and CMC of micelles are the two important characteristics that determine the stability of micelles. In general, a high absolute value of zeta potential contributes to the better dynamic stability of the micelles 35. However, the zeta potential value of the polymeric micelles prepared in this study indicated that they might be unstable, but the CMC determination suggested reasonable stability. We, therefore, conducted the stability experiment and found that the micelle solution, when stored at 4 °C, showed no changes for 30 days with an average drug-leakage rate of about 1% (data not shown). This stability may be due to the high content of PEG on the micelle surface providing a repulsive force to reduce the surface tension between particles and thus help maintain the stability. This observation is consistent with a previous published report 36, 37.
The RGD peptide is a specific ligand for αvβ3-integrin expressed on endothelial cells at sites of inflammation 38. In our study, the in vitro cell viability assay indicated that RGD-containing micelles significantly enhanced the inhibition effect on cell-growth of LPS-stimulated Raw264.7 cells compared to the RGD-free micelles demonstrating the significance of RGD in the therapy of inflammation-related diseases. In the in vivo assay of arthritic rat model, both R-M/N-PMs and M/N-PMs, when intravenously injected, mainly distributed to the arthritic joints. This may be due to the passive targeting of micelles to the inflammation sites through the ELVIS mechanism (Extravasation through Leaky Vasculature and Inflammatory cell-mediated Sequestration) in which, after systemic administration, both R-M/N-PMs and M/N-PMs could pass through the leaky vasculature of inflammatory lesions and internalize into the inflammatory infiltrates and locally activated resident cells 39. However, at 24 h post-injection, the R-M/N-PMs group maintained stronger fluorescence signal than the M/N-PMs group because of the high affinity and selectivity of RGD with integrin αvβ3 thus promoting the retention of micelles in arthritic joints.
The CAM assay is often used to study tumor angiogenesis 40. Since the angiogenesis in rheumatoid arthritis is similar to that in tumors 41, we used this assay to investigate whether the micelle formulations could inhibit angiogenesis. Methotrexate is a cytotoxic drug and therefore MTX-PMs showed a stronger inhibitory effect on neovascularization than NIM-PMs with nimesulide, an anti-inflammatory drug. In the R-M/N-PMs group, the combination of methotrexate and nimesulide together with the RGD's specificity for the integrin receptor over-expressed in neovascular endothelial cells resulted in the most significant inhibition effect among all the micelle formulations.
In vivo toxicity problems of nano-drug delivery systems, such as liver toxicity, blood compatibility, immunogenicity and myelo-suppression, have been one of the main factors limiting their clinical application. As shown in the supplementary file (Figure S4), the major organs for nano-sized micelles to reach in the healthy are liver and spleen, which is consistent with the other report 42. But in the arthritic rats, micelles mainly accumulated on ankle joints. This may be one of the reasons that the targeted delivery of R-M/N-PMs was found to help reduce systemic toxicity in the arthritic rats, which suggested by the result from the blood levels of ALT and AST.
It has been reported that the use of NSAIDs partially alleviates the symptoms of rheumatoid arthritis but cannot prevent its long-term disease progression 43. Therefore, nimesulide did not lead to a significant difference between the R-NIM-PMs group and the saline group in the histopathology score of the ankle joint. However, Al-Abd et al. reported that nimesulide improved the anti-rheumatic profile of methotrexate in the collagen-induced arthritic mice model 13. In our present study, we found that the combination therapy of methotrexate and nimesulide mediated by RGD-modified polymeric micelles enhanced the therapeutic efficacy in rheumatoid arthritis. However, in our study, we formulated methotrexate and nimesulide separately into the polymeric micelles. The rats with arthritis were given methotrexate (0.6 mg/kg) and nimesulide (3.0 mg/kg) as micelle formulations by intravenous injection every other day, in which not only the dose of both methotrexate and nimesulide was significantly decreased but also the simple administration method of 'same time and same injection' provided a more convenient procedure for the potential clinical application.
In summary, the combination therapy of methotrexate and nimesulide mediated by RGD-modified polymeric micelles showed promising results in the rat model of rheumatoid arthritis. However, rheumatoid arthritis is a chronic autoimmune disease and the animal models of rheumatoid arthritis can be affected by multiple factors, such as animal strains, feeding environment, and concentration of Mycobacterium tuberculosis. Although the animal model of rheumatoid arthritis used in this study is very similar to the human rheumatoid arthritis in terms of its pathological features, it lacks the chronic course of the human disease. Therefore, in the future, it is necessary to study other animal models that more closely mimic the human disease to obtain more useful information for the clinical therapy of rheumatoid arthritis.
Conclusion
We successfully developed a novel targeted drug delivery system, RGD-modified polymeric micelles loaded with low-doses of methotrexate and nimesulide in a fixed dose combination, which significantly enhanced the therapeutic effect on rheumatoid arthritis. Our strategy holds great promise for future clinical applications to alleviate a common debilitating human ailment.
Supplementary Material
Supplementary methods and figures.
Acknowledgments
All authors are thankful for getting help and supports from the following research platforms of the Key Laboratory of Medical Electrophysiology of Ministry of Education, Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease, the Drug Discovery Research Center, the Department of Medicinal Chemistry, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan 646000, China.
Funding
This work was supported by the General Program of Science and Technology Agency of Sichuan Province (2017JY0160, 2018RZ0120); the Collaborative Fund of Luzhou Government and Southwest Medical University (2016LZXNYD-J06 and 2017LZXNYD-T07); the Science and Technology Project of the Health Planning Committee of Sichuan (18PJ547); the Key Science and Technology Project of Luzhou Government (2018-SYF-19); the Key Fund, the Youth Fund and the Transformation Project of Science and Technology Achievements of Southwest Medical University (2018-ZRZD-018, 2017-ZRQN-073, 2018002), the Opening Project of the Key Laboratory of Drug Targeting and Drug Delivery System of the Ministry of Education (Sichuan University), and the Collaborative Project of Luzhou TCM Hospital and Southwest Medical University (2017-LH004).
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine transaminase
- AI
articular index
- CFA
complete Freund's adjuvant
- CMC
critical micelle concentration
- CAM
chick chorioallantoic membrane
- DMEM
Dulbecco's modified eagle's medium
- DMSO
dimethyl sulfoxide
- DMF
N,N-dimethyl formamide
- DLS
dynamic light scattering
- DL
drug loading
- EE
encapsulation efficiency
- FBS
fetal bovine serum
- HPLC
high-performance liquid chromatography
- H&E
hematoxylin-eosin staining
- HUVEC
human umbilical vein endothelial cell line
- LPS
lipopolysaccharide
- MTX
methotrexate
- Micro-CT
micro-computed tomography
- MTT
3-(4,5 dimethylthiozol-2-yl)-2,5-diphenyl-tetrazolium bromide
- NIM
nimesulide
- RBCs
red blood cells
- RA
rheumatoid arthritis
- RIPA
ristocetin-induced platelet agglutination
- SD
standard deviation
- TEM
transmission electron microscopy
- TEA
anhydrous triethylamine.
References
- 1.Hua S, Dias TH. Hypoxia-inducible factor (HIF) as a target for novel therapies in rheumatoid arthritis. Front pharmacol. 2016;7:184. doi: 10.3389/fphar.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateen S, Zafar A, Moin S, Khan AQ, Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta. 2016;455:161–71. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Mankia K, Emery P. Preclinical rheumatoid arthritis: progress toward prevention. Arthritis Rheumatol. 2016;68:779–88. doi: 10.1002/art.39603. [DOI] [PubMed] [Google Scholar]
- 4.Caramaschi P, Bambara LM, Pieropan S, Tinazzi I, Volpe A, Biasi D. Anti-TNFalpha blockers, autoantibodies and autoimmune diseases. Joint Bone Spine. 2009;76:333–42. doi: 10.1016/j.jbspin.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Fehér J, Lengyel G. Effectiveness and safety of biological therapy with adalimumab. Orv Hetil. 2009;150:1215–22. doi: 10.1556/OH.2009.28681. [DOI] [PubMed] [Google Scholar]
- 6.Bracewell C, Isaacs JD, Emery P, Ng WF. Atacicept, a novel B cell-targeting biological therapy for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2009;9:909–19. doi: 10.1517/14712590903033919. [DOI] [PubMed] [Google Scholar]
- 7.Martinez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding) Clin Exp Rheumatol. 2009;27:678–84. [PubMed] [Google Scholar]
- 8.Ding H, Gao G, Zhang L, Shen G, Sun W, Gu Z. et al. The protective effects of curculigoside A on adjuvant-induced arthritis by inhibiting NF-small ka, CyrillicB/NLRP3 activation in rats. Int Immunopharmacol. 2016;30:43–9. doi: 10.1016/j.intimp.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Ding J, Feng X, Chang F, Wang Y, Gao Z. et al. Scavenger receptor-mediated targeted treatment of collagen-induced arthritis by dextran sulfate-methotrexate prodrug. Theranostics. 2017;7:97–105. doi: 10.7150/thno.16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Chang F, Ding J, Wang J, Gao Z, Zhuang X. et al. Scavenger receptor-targeted dextran sulfate-methotrexate prodrug for treatment of collagen-induced arthritis. J Control Release. 2017;259:e98. doi: 10.7150/thno.16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi R, Majoros I, Misra AC, Koch AE, Campbell P, Marotte H. et al. Folate receptor-targeted dendrimer-methotrexate conjugate for inflammatory arthritis. J Biomed Nanotechnol. 2015;11:1431–41. doi: 10.1166/jbn.2015.2077. [DOI] [PubMed] [Google Scholar]
- 12.Suleyman H, Cadirci E, Albayrak A, Halici Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr Med Chem. 2008;15:278–83. doi: 10.2174/092986708783497247. [DOI] [PubMed] [Google Scholar]
- 13.Al-Abd AM, Inglis JJ, Nofal SM, Khalifa AE, Williams RO, El-Eraky WI. et al. Nimesulide improves the disease modifying anti-rheumatic profile of methotrexate in mice with collagen-induced arthritis. Eur J Pharmacol. 2010;644:245–50. doi: 10.1016/j.ejphar.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Feng X, Ding J, Chang F, Chen X. Nanotherapeutics relieve rheumatoid arthritis. J Control Release. 2017;252:108–24. doi: 10.1016/j.jconrel.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Ding J, Zhang Y, Chang F, Wang J, Gao Z. et al. Activated macrophage-targeted dextran-methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. J Mater Chem B. 2016;4:2102–13. doi: 10.1039/c5tb02479j. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Chang F, Ding J, Gao Z, Zhuang X, Chen X. Treatment of collagen-induced arthritis by activated macrophage-targeted dextran-methotrexate/folate conjugate. Nanomedicine. 2018;14:1815–6. [Google Scholar]
- 17.Feng XR, Ding JX, Gref R, Chen XS. Poly(β-cyclodextrin)-mediated polylactide-cholesterol stereocomplex micelles for controlled drug delivery. Chinese J Polym Sci. 2017;35:693–9. [Google Scholar]
- 18.Shen K, Li D, Guan J, Ding J, Wang Z, Gu J. et al. Targeted sustained delivery of antineoplastic agent with multicomponent polylactide stereocomplex micelle. Nanomedicine. 2017;13:1279–88. doi: 10.1016/j.nano.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Yuan F, Quan LD, Cui L, Goldring SR, Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev. 2012;64:1205–19. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari M, Onuoha SC, Pitzalis C. Going with the flow: harnessing the power of the vasculature for targeted therapy in rheumatoid arthritis. Drug Discov Today. 2016;21:172–9. doi: 10.1016/j.drudis.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Wang X, Zhang Y, Yang S, Wang J, Zhang X. et al. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J Drug Target. 2009;17:459–67. doi: 10.1080/10611860902974085. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Xia H, Wang J, Li Y. High intensity focused ultrasound-responsive release behavior of PLA-b-PEG copolymer micelles. J Control Release. 2009;139:31–9. doi: 10.1016/j.jconrel.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X. et al. Paclitaxel-loaded pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J pharm. 2009;376:176–85. doi: 10.1016/j.ijpharm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Basu Ray G, Chakraborty I, Moulik SP. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J Colloid Interf Sci. 2006;294:248–54. doi: 10.1016/j.jcis.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Jain A, Agarwal A, Majumder S, Lariya N, Khaya A, Agrawal H. et al. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Control Release. 2010;148:359–67. doi: 10.1016/j.jconrel.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Park BC, Park SY, Lee JS, Mousa SA, Kim JT, Kwak MK. et al. The anti-angiogenic effects of 1-furan-2-yl-3-pyridin-2-yl-propenone are mediated through the suppression of both VEGF production and VEGF-induced signaling. Vasc Pharmacol. 2009;50:123–31. doi: 10.1016/j.vph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Kim BS, Park H, Ko SH, Lee WK, Kwon HJ. The sphingosine-1-phosphate derivative NHOBTD inhibits angiogenesis both in vitro and in vivo. Biochem Bioph Res Co. 2011;413:189–93. doi: 10.1016/j.bbrc.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Jiang J, Chen W, Jiang H, Zhang Z, Sun X. Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J Control Release. 2016;230:64–72. doi: 10.1016/j.jconrel.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Cui M, Ding L, Xia L, Lu J, Shen H. Interleukin-34 aggravates the severity of arthritis in collagen-induced arthritis mice by inducing interleukin-17 production. J Interferon Cytokine Res. 2018;38:221–5. doi: 10.1089/jir.2017.0095. [DOI] [PubMed] [Google Scholar]
- 30.Quan L, Zhang Y, Crielaard BJ, Dusad A, Lele SM, Rijcken CJF. et al. Nanomedicines for inflammatory arthritis: head-to-head comparison of glucocorticoid-containing polymers, micelles, and liposomes. ACS nano. 2014;8:458–66. doi: 10.1021/nn4048205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan LD, Purdue PE, Liu XM, Boska MD, Lele SM, Thiele GM. et al. Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy. Arthritis Res Ther. 2010;12:R170. doi: 10.1186/ar3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PC, Williams RO. Combination cytokine blockade: the way forward in therapy for rheumatoid arthritis? Arthritis Rheumatol. 2015;67:14–6. doi: 10.1002/art.38893. [DOI] [PubMed] [Google Scholar]
- 33.Reum Son A, Kim DY, Hun Park S, Yong Jang J, Kim K, Ju Kim B. et al. Direct chemotherapeutic dual drug delivery through intra-articular injection for synergistic enhancement of rheumatoid arthritis treatment. Sci Rep. 2015;5:14713. doi: 10.1038/srep14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gayetskyy S, Museyko O, Kasser J, Hess A, Schett G, Engelke K. Characterization and quantification of angiogenesis in rheumatoid arthritis in a mouse model using μCT. BMC Musculoskelet Disord. 2014;15:298. doi: 10.1186/1471-2474-15-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Liu M, Du S. Optimization of madecassoside liposomes using response surface methodology and evaluation of its stability. Int J Pharm. 2014;473:280–5. doi: 10.1016/j.ijpharm.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Bae Y, Alani AW, Rockich NC, Lai TS, Kwon GS. Mixed pH-sensitive polymeric micelles for combination drug delivery. Pharm Res. 2010;27:2421–32. doi: 10.1007/s11095-010-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Xu W, Li S, Qiu H, Li Z, Wang C. et al. Polylactide-cholesterol stereocomplex micelle encapsulating chemotherapeutic agent for improved antitumor efficacy and safety. J Biomed Nanotechnol. 2018;14:2102–13. doi: 10.1166/jbn.2018.2624. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Niu G, Wu H, Chen X. Clinical application of radiolabeled RGD peptides for PET imaging of integrin alphavbeta3. Theranostics. 2016;6:78–92. doi: 10.7150/thno.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan L, Zhang Y, Dusad A, Ren K, Purdue PE, Goldring SR. et al. The evaluation of the therapeutic efficacy and side effects of a macromolecular dexamethasone prodrug in the collagen-induced arthritis mouse model. Pharm Res. 2016;33:186–93. doi: 10.1007/s11095-015-1776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Scanlon CS, Banerjee R, Russo N, Inglehart RC, Willis AL. et al. The histone methyltransferase EZH2 mediates tumor progression on the chick chorioallantoic membrane assay, a novel model of head and neck squamous cell carcinoma. Transl Oncol. 2013;6:273–81. doi: 10.1593/tlo.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lupia E, Montrucchio G, Battaglia E, Modena V, Camussi G. Role of tumor necrosis factor-alpha and platelet-activating factor in neoangiogenesis induced by synovial fluids of patients with rheumatoid arthritis. Eur J Immunol. 1996;26:1690–4. doi: 10.1002/eji.1830260804. [DOI] [PubMed] [Google Scholar]
- 42.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinblatt ME. Rheumatoid arthritis: treat now, not later! Ann Intern Med. 1996;124:773–4. doi: 10.7326/0003-4819-124-8-199604150-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and figures.