Abstract
Background
Migraine is a complex genetic disorder that is brought about by multiple genetic and environmental factors. We aimed to assess whether migraine frequency is associated with genetic susceptibility.
Methods
We investigated in 2829 migraine patients (14% males) whether ‘migraine frequency’ (measured as the number of migraine days per month) was related to ‘genetic load’ (measured as the number of parents affected with migraine) using a validated web-based questionnaire. In addition, we investigated associations with age-at-onset, migraine subtype, use of acute headache medication, and comorbid depression.
Results
We found an association between the number of migraine days per month and family history of migraine for males (p = 0.03), but not for females (p = 0.97). This association was confirmed in a linear regression analysis. Also, a lower age-at-onset (p < 0.001), having migraine with aura (p = 0.03), and a high number of medication days (p = 0.006) were associated with a stronger family history of migraine, whereas lifetime depression (p = 0.13) was not.
Discussion
Migraine frequency, as measured by the number of migraine days per month, seems associated with a genetic predisposition only in males. A stronger family history of migraine was also associated with a lower age-at-onset, a higher number of medication days, and migraine with aura. Our findings suggest that specific clinical features of migraine seem more determined by genetic factors.
Keywords: Genetics, migraine, chronic migraine, migraine attack frequency, medication overuse, depression
Introduction
Over the past decade, genome-wide association studies (GWAS) have made an important contribution to the identification of genetic components involved in migraine (1). These studies reinforced the concept that migraine is a complex, genetic disorder with multiple genetic variants, each with a small effect size, and environmental factors together conferring migraine susceptibility. It remains, however, an enigma to what extent (endo)phenotypes and individual features of migraine, for example, age-at-onset or migraine type, are determined by genetic susceptibility and whether this applies to the same extent in females and males. Epidemiological studies showed that an early onset of disease was associated with an increased relative risk of migraine in first-degree relatives (2,3), an observation commonly made when a disease is genetic (4,5). Epidemiological studies also showed that migraine with aura appeared to cluster in families more profoundly than migraine without aura, pointing towards a possibly higher genetic susceptibility in the former migraine subtype (6,7). It seems paradoxical that (robust) associations with genetic loci were discovered only for migraine without aura in GWAS (8), although this may simply reflect that migraine with aura may instead be determined by rarer medium- and high-risk alleles that are not captured in a GWAS.
Assessing the genetic susceptibility of (endo)phenotypes and features of migraine can be of help for diagnosing patients, understanding pathology, and ultimately to find targets for treatment. One clinical feature that is particularly of interest to study is the number of monthly migraine days, not least because the individual and societal burden is much higher when patients suffer from many days of migraine per month – to the point that the disorder is termed chronic migraine (9). Here we investigated whether a higher number of migraine days per month is associated with a higher genetic susceptibility. For this, we used familial occurrence of migraine in parents as a measure of genetic load. As results from GWAS in migraine suggest that genetic effects may be larger in male patients, possibly explained by the major influence of environmental/hormonal factors on migraine prevalence in females, we specifically investigated whether this association was influenced by sex (10). In addition, we evaluated previously reported associations of age-at-onset and migraine type with genetic load. Finally, we investigated whether there was an association of the number of migraine days and genetic load with medication use and lifetime depression, as both are factors known to convert episodic migraine into a chronic disorder (11–13).
Materials and methods
Patients
For this retrospective study we used data from the LUMINA (Leiden University Migraine Neuro-Analysis programme) study population (see Supplemental material). Continuously, subjects (≥18 years of age) are recruited into LUMINA via a validated self-reporting, web-based questionnaire (14). Subjects applied to participate in headache research via the project’s website and reside throughout the Netherlands. All participants that meet the screening criteria have been asked to fill out an extensive migraine questionnaire, based on International Classification of Headache Disorders (ICHD-3, beta version) criteria (15). This questionnaire has been validated by semi-structured telephone interviews in 1038 participants in a previous study, which showed a specificity for migraine of 0.95 (14). For the current study, we included all participants with a diagnosis of migraine with aura or migraine without aura. The questionnaire includes several illustrations depicting auras and for all aura subtypes we asked for presence of several known features (e.g. scintillations, visual field defects, zigzag lines, pins-and-needles, numbness). Participants with migraine with aura were always classified as migraine with aura, regardless of whether they also suffered from attacks of migraine without aura. We excluded all participants (n = 132) who responded ‘yes’ to the question “whether a relative already participates in the LUMINA population” to limit chances that multiple subjects from the same family would be included. The study was approved by the Medical Ethics Committee of Leiden University Medical Centre and all participants provided written informed consent.
Measurements
Besides questions to reliably diagnose migraine, the web-based questionnaire includes questions about general demographics (age, sex), number of migraine days per month, age-at-onset of migraine, family history of migraine, use of acute headache medication (current use of prophylactic medication was, unfortunately, not included in the questionnaire), and lifetime depression. All questions for this study were answered on a single occasion by each participant.
To assess migraine frequency, participants were asked to fill in the average number of migraine days per month over the preceding three months. In our analyses, we included migraine days per month as a continuous measure, as we aimed to investigate a general correlation between frequency and genetic load. Age-at-onset was determined by asking the participants’ age when they suffered their first migraine attack (so not the age at diagnosis). Participants were asked to indicate whether their mother and father had a (lifetime) migraine diagnosis; a question that could also be answered with ‘unknown’. We used the number of parents affected with migraine as the measure of genetic load (with both parents affected reflecting the highest genetic load). Siblings were not included in the analyses because proportions of siblings with migraine cannot be compared reliably with varying total numbers of siblings for each participant. Moreover, siblings considered unaffected might still develop migraine later in life, thereby possibly underestimating genetic load.
To determine the level of acute medication use, we asked participants how many days per month, over the past three months, they had used acute migraine medication, and, specifically, on how many days they used: a) triptans; and b) simple analgesics; or c) ergotamines for migraine. Opioids were not included as these are generally not prescribed for headache in the Netherlands.
Lifetime depression was measured as a dichotomous variable as described previously (16). To this end, we used validated cut-off scores for the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D) and the Centre for Epidemiologic Studies Depression scale (CES-D), in combination with a previously used and published algorithm for depression and an additional question on depression diagnoses in the past (HADS-D ≥ 8, or CES-D ≥ 16, or use of antidepressants as an indication of depression, or having been diagnosed with depression in the past) (17).
Data analysis and statistics
All data were self-reported through web-based questionnaires and although field restrictions limited type errors and other mistakes, some erroneous data may have occurred (for example, reporting more than 31 migraine days per month). Therefore, for each analysis, we excluded subjects for which data obviously was erroneous, missing but needed for the analysis, or indicated as ‘unknown’.
We compared the number of migraine days per month, age-at-onset of migraine, migraine subtype (i.e. migraine with or without aura), number of medication days per month, and presence of lifetime depression between participants with either no, one or both parents affected with migraine, and specifically considered differences between male and female migraine patients. Because of the distribution of the data, we calculated median values and interquartile range (IQR) and performed non-parametric Jonckheere-Terpstra tests (a test that is comparable to the Kruskal-Wallis test but more suitable for ordinal data) for continuous variables and Pearson’s Chi Square tests for categorical variables. In addition, we performed a linear regression model to assess the association between migraine frequency and family history of migraine, adjusted for variables that may influence this association, with a focus on sex. All statistics were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Results with p < 0.05 were considered statistically significant.
Results
Association of the number of migraine days per month and family history of migraine
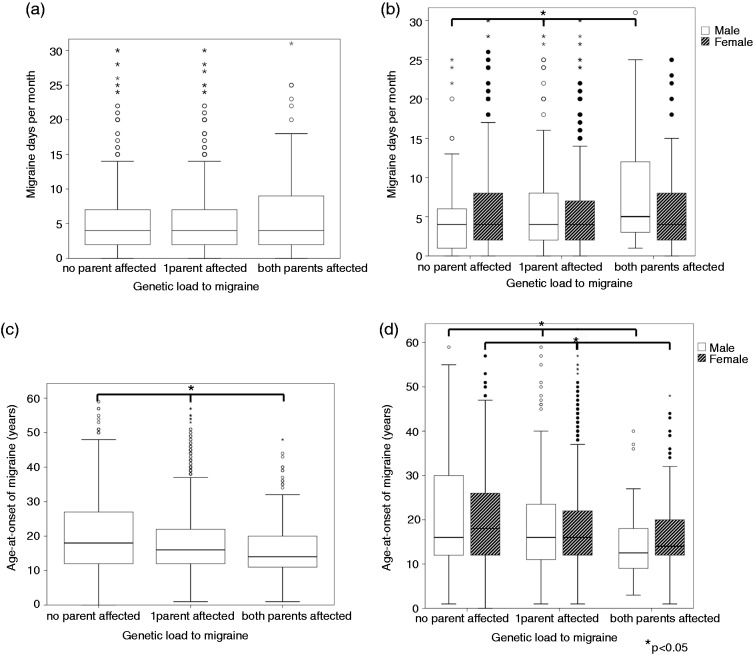
Of 2829 LUMINA participants, migraine status of both parents (n = 5658) and the number of migraine days was available. The majority of cases were female (n = 2442; 86%). Migraine patients more often suffered from migraine without aura (63%) than migraine with aura (37%) (Table 1). When considering all migraine patients, there was no difference in the number of migraine days per month among the groups with no, one or both parents affected with migraine (p = 0.41), which indicates that there was no association between genetic load and the number of migraine days per se (Table 2, Figure 1(a)). Next, we performed the analysis separately for male and female patients. The number of migraine days did not differ between male (median (IQR) = 4 (2–7) days) and female (median (IQR) = 4 (2–8) days) migraine patients (p = 0.27). However, in males, the median (IQR) number of migraine days per month was 4 (1–6), 4 (2–8) and 5 (3–13) with no, one, or both affected parents, respectively (p = 0.03) (Figure 1(b)), so migraine frequency significantly increased with an increasing number of affected parents. Notably, no such relation was observed for female patients (p = 0.97). Of note, migraine frequency might be influenced by the subject’s age, but we did not find a relationship between age and number of migraine days per month (Pearson correlation coefficient 0.05, p = 0.006).
Table 1.
Descriptive statistics of study participants with distribution of sex, migraine subtype, and lifetime depression across groups with an increasing genetic load (reflected by the proportion of affected parents).
| Total group of migraine patients | Subgroups by parents’ migraine status |
Pearson χ2 Test* | |||
|---|---|---|---|---|---|
| No parent affected | One parent affected | Both parents affected | |||
| Age, median (IQR) (years) | 41.9 (32.3–50.0) | 41.7 (31.9–50.0) | 42.2 (33.1–50.2) | 39.5 (29.5–48.2) | |
| Sex | |||||
| Female, n (%) | 2442 (86%) | 885 (36%) | 1418 (58%) | 139 (6%) | χ2(2) = 0.67 |
| Male, n (%) | 387 (14%) | 137 (35%) | 224 (58%) | 26 (7%) | p = 0.72 |
| Migraine subtype | |||||
| MO, n (%) | 1787 (63%) | 674 (38%) | 1019 (57%) | 94 (5%) | χ2(2) = 6.99 |
| MA, n (%) | 1042 (37%) | 348 (33%) | 623 (60%) | 71 (7%) | p = 0.03 |
| Lifetime depression | |||||
| Yes, n (%) | 991 (43%) | 343 (35%) | 581 (59%) | 67 (7%) | χ2(2) = 4.16 |
| No, n (%) | 1292 (57%) | 465 (36%) | 765 (59%) | 62 (5%) | p = 0.13 |
Pearson χ2 test comparing distribution of presented variables over the categories of: 0 = no parents affected; 1 = one parent affected; 2 = both parents affected with migraine.
IQR: interquartile range; MO: migraine without aura; MA: migraine with aura.
Table 2.
Number of migraine days per month, age-at-onset of migraine, and number of days with medication compared across groups with an increasing genetic load (reflected by the proportion of affected parents).
| Total group of migraine patients | Subgroups by parents’ migraine status |
Jonckheere- Terpstra test* | |||
|---|---|---|---|---|---|
| No parent affected | One parent Affected | Both parents affected | |||
| Number of migraine days/ month, median (IQR) | 4.0 (2.0–8.0) | 4.0 (2.0–7.0) | 4.0 (2.0–7.3) | 4.0 (2.0–9.0) | p = 0.41 |
| Age-at-onset of migraine, median (IQR) (years) | 16.0 (12.0–24.0) | 18.0 (12.0–27.0) | 16.0 (12.0–22.0) | 14.0 (11.0–20.0) | p < 0.001 |
| Number of days of acute headache medication use, median (IQR) (days/ month) | |||||
| Combined total | 5.0 (2.0–10.0) | 4.0 (2.0–9.0) | 5.0 (2.0–10.0) | 6.0 (2.0–12.0) | p = 0.006 |
| Simple analgesics | 4.0 (1.0–8.0) | 3.0 (1.0–7.0) | 4.0 (2.0–8.0) | 5.0 (1.0–10.0) | p < 0.001 |
| Triptans | 2.0 (0.0–6.0) | 2.0 (0.0–6.0) | 2.0 (0.0–6.0) | 2.0 (0.0–6.0) | p = 0.82 |
Jonckheere-Terpstra test comparing distribution of presented variables over the categories of: 0 = no parents affected; 1 = one parent affected; 2 = both parents affected with migraine.
IQR: interquartile range.
Figure 1.
Box-plots showing the distribution of migraine days per month and age-of-onset in the three categories of increasing genetic load. Box-plots (a) and (c) show the distribution of migraine days per month (a) and age-at-onset of migraine (c) in the three categories of no, one or both parents affected with migraine. Box-plots (b) and (d) show the same distributions for male and female LUMINA participants separately. (a), (c): Circles (white and black) represent outliers; small asterisks represent extreme outliers. (b), (d): White bars: Male participants; patterned bars: Female participants. Horizontal bars with large asterisks: Statistical comparison among the three categories of no, one of both parents affected with p-value < 0.05.
In a linear regression model adjusted for age, sex, lifetime depression diagnosis, acute medication use, migraine subtype, and age-at-onset of migraine we did not find an association between migraine frequency and family history of migraine either (p = 0.14) (Table 3). All variables except sex (p = 0.81) had a significant (p < 0.05) effect on migraine frequency. To further investigate the influence of sex on the effect of family history of migraine, we ran a second linear regression model including an interaction term for sex and family history of migraine. Adding the interaction term in model 2 resulted in a dramatic change towards (borderline) significance of the separate effects of family history and sex on migraine frequency compared to model 1, supporting that the effect of family history on migraine frequency depends on sex. Looking at specific parameter estimates, the effect was stronger (β = −1.77; p = 0.15) for the comparison between zero and both parents affected (n = 165) with migraine compared to the comparison between zero and one parent affected (n = 1642) with migraine (β = −1.43; p = 0.02). The negative interaction value indicates that the lower the value of sex (male) the more positive the effect of family history on migraine frequency.
Table 3.
Linear regression associations between migraine frequency (migraine days per month) and family history of migraine.
| β | 95% CI | p-value | |
|---|---|---|---|
| Model 1 | |||
| Family history of migraine | 0.14 | ||
| (0 vs. 1 affected parent, | −0.16 | −0.57; 0.26 | 0.46 |
| 0 vs. 2 affected parents) | 0.69 | −0.20; 1.58 | 0.13 |
| Age (years) | 0.03 | 0.01; 0.05 | 0.001 |
| Sex (male vs. female) | 0.07 | −0.51; 0.65 | 0.81 |
| Lifetime depression | 0.57 | 0.17; 0.97 | 0.005 |
| Acute medication use | 0.31 | 0.28; 0.34 | <0.001 |
| Migraine subtype (MA vs. MO) | 1.05 | 0.64; 1.45 | <0.001 |
| Age-at-onset of migraine (years) | −0.03 | −0.05; 0.01 | 0.01 |
| Model 2 | |||
| Family history of migraine | 0.09 | ||
| (0 vs. 1 affected parent, | 1.08 | −0.05; 2.21 | 0.06 |
| 0 vs. 2 affected parents) | 2.20 | −0.02; 4.42 | 0.05 |
| Age (years) | 0.03 | 0.01; 0.05 | 0.001 |
| Sex (male vs. female) | 1.02 | 0.06; 1.99 | 0.04 |
| Lifetime depression | 0.56 | 0.16; 0.95 | 0.006 |
| Acute medication use | 0.31 | 0.28; 0.34 | <0.001 |
| Migraine subtype (MA vs. MO) | 1.04 | 0.64; 1.45 | <0.001 |
| Age-at-onset of migraine (years) | −0.03 | −0.05; −0.01 | 0.009 |
| Family history * sex | 0.05 | ||
| (0 vs. 1 affected parent, male vs. female, | −1.43 | −2.64; −0.21 | 0.02 |
| 0 vs. 2 affected parents, male vs. female) | −1.77 | −4.19; 0.64 | 0.15 |
Note: Data are unstandardized regression coefficients (β) with 95% confidence intervals (CI) and p-values. Model 1 was adjusted for age, sex, lifetime depression diagnosis, acute medication use, migraine subtype (MO: migraine without aura; MA: migraine with aura), and age-at-onset of migraine. Model 2 was additively adjusted for the interaction term of family history of migraine and sex.
Other associations of migraine characteristics and family history of migraine
A positive association was found between the number of days per month on which acute headache medication was used and family history (p = 0.006) (Table 2). Looking more into detail at the different types of pain medication, this effect was exclusively explained by the use of simple analgesics (p < 0.001). Ergotamines were used by only 49 migraine patients, with a median (IQR) of 0 (0–0) days per month in the total group of migraine patients and in all three subgroups with either no, one or both parents affected. As may be expected, the number of migraine days per month was higher in the group with comorbid lifetime depression (n = 2283; with comorbid depression median (IQR) 4 (2–8) days and without comorbid depression median (IQR) 3 (2–6) days; p < 0.001), but there was no association of presence of lifetime depression with genetic susceptibility to migraine (so an increasing number of affected parents with migraine) (p = 0.13) (Table 1).
Finally, in our cohort, a) migraine with aura was associated with a stronger family history than migraine without aura (p = 0.03) (Table 1) and (b), a lower age-at-onset of migraine was associated with a stronger family history (p < 0.001) (Table 2).
Discussion
In this large study, we show that migraine frequency, as measured by the number of migraine days per month, is associated with a genetic load, measured by a higher number of parents with migraine, but only in males. Furthermore, a stronger family history of migraine is associated with a lower age-at-onset, a higher number of acute headache medication days, and migraine with aura.
Our study’s main strength is the large number of participants (n = 2829), allowing us to detect small effects, in contrast to a previous small (n = 344) negative study in a paediatric migraine population (3). In addition, our migraine questionnaire has been validated and the algorithm used can deliver reliable diagnoses of both migraine with or without aura (14). Notably, we were able to demonstrate that having (more) parents with migraine was associated with a lower age-at-onset of migraine, so these features seem to be influenced by genetic susceptibility (2). In addition, we were able to demonstrate a higher proportion of cases with migraine with aura when one or both parents also have migraine, in congruence with a previous report (6). These findings support that our measure of affected parents can be used as a measure of genetic susceptibility (“load”). Only a small proportion of participants reported that both their parents had migraine (5–7%), which likely created a lack of statistical power in the linear regression analysis. However, we feel that having even one affected parent is a clear indication of possibly inherited genetic factors that may make an individual more susceptible to developing migraine attacks, making the category of one affected parent just as relevant in our analysis.
Although the prevalence of migraine has consistently been shown to be higher in women (18–21), genetic susceptibility was reported to be similar in men and women (22–24) or even higher in men (10), suggesting that, in females, other causes than genetic factors play a dominant role (e.g. female sex hormones). Despite average migraine frequencies in men and women being described as similar (25), women are at an increased risk of transitioning from episodic to chronic migraine (26). This phenomenon could be explained by either a greater exposure to or a greater susceptibility to environmental (e.g. sex hormonal) factors in women, resulting in an increase in migraine days more easily than in men. Although we found a significant effect in males with respect to an increase in the number of migraine days when one or two parents also had migraine, it should be noted that the median migraine days in male migraine patients only changed from four to five days per month with increasing genetic load, indicating that the effect is minimal. Of note, only 14% of participants were male and we do not know how our results would be if more males had participated. One of the key factors responsible for an increase in migraine days is medication overuse (16). We found that the frequency of using simple analgesics increased with an increase of genetic load. Thus, migraine frequency in relation to genetic load may be influenced by this overuse of acute headache medication, but with our study it is impossible to determine what is cause or consequence. Copying medication use habits from first-degree relatives may be of great importance as well. Furthermore, we were not able to include the current use of prophylactic treatment in our analyses, which may be a confounding factor.
Between participants with and without a diagnosis of lifetime depression, migraine days differed in our investigated subgroup of the LUMINA population, in congruence with an earlier study (16). As genetic factors are also thought to play a role in the development of depression (27), chances to develop a high frequency of migraine attacks may also be influenced indirectly by genetic factors relevant to depression. Regardless, we could not detect an increase in prevalence of lifetime depression with increasing genetic load to migraine, which suggests that besides possible shared genetic factors influencing migraine and depression (28), depression-specific factors may be responsible for the development of depression in migraine patients.
In our cohort, we confirmed the association between a lower age-at-onset of migraine and an increased genetic load (2). Associations between a lower age-at-onset and increased genetic load were also found in other complex diseases (2,4,5). It may be interesting to investigate whether migraine patients with an early age-at-onset carry a higher number of genetic variants that are known to increase the susceptibility to migraine from GWAS but, given the current relatively low number of 38 variants (29), such an investigation will be more fruitful when hunderds of variants have been found. We also confirmed the association of genetic load and having migraine with aura (2,6), but except for a single variant (30) current GWAS have not been able to discover robust associations for this migraine subtype. Migraine frequency was not included as an endophenotype in genome-wide association studies but no association with migraine frequency, nor with the migraine with aura subtype, was found in a recent genetic risk score analysis (31). In addition, in a Danish study, a few SNPs identified from migraine GWAS showed nominal associations with many lifetime attacks and prolonged migraine attacks, but none associated with early onset of migraine (32).
We considered the risk of bias in our study. First, we chose not to compare our results of genetic load and migraine frequency to results from population-based studies, as we expected both values to be higher in LUMINA due to an increased motivation of subjects to participate in migraine research when multiple relatives are affected or when migraine is severe. By comparing family history and migraine days within the LUMINA population, these selection biases were probably avoided. Second, our estimation of genetic load is based on self-reported migraine family history. Previous studies showed an underestimation of migraine family history obtained through relatives (33,34). However, as LUMINA participants apply themselves to participate via our website, we feel that they are likely more occupied with their migraine complaints and history than migraine patients from the general population. As a result, they will probably be more aware of their relatives’ headache complaints. Moreover, we feel the issue of bias is less important for our study as we did not intend to report absolute quantifications of familial occurrence of migraine, but merely investigated associations between genetic load and migraine characteristics. Third, age-at-onset and migraine frequency were assessed retrospectively, which could cause bias if the ability to remember attacks differs among groups. We do not expect the family history to be of such an influence on the ability to assess migraine frequency over the past three months. However, as parents who suffer from migraine themselves are likely able to recognise migraine in their children, leading to an earlier age at diagnosis, we decided to ask for age at first migraine attack and not age at diagnosis. We also feel that a first migraine attack often is considered a severe event, which is likely to be remembered. We thus expect the error range in reported age-at-onset to be small. For all investigated retrospective parameters, the possibly errors in reported values are also, to a certain extent, compensated by the large size of our population, allowing us to still detect significant effects.
In conclusion, migraine frequency, as measured by the number of migraine days per month, is associated with genetic load in males but not females. Furthermore, higher genetic load is associated with lower age-at-onset, higher number of acute medication days, and migraine with aura. These clinical characteristics could be targets for future epidemiologic and genetic migraine research.
Supplemental Material
Supplemental material for Linking migraine frequency with family history of migraine by Nadine Pelzer, Mark A Louter, Erik W van Zwet, Dale R Nyholt, Michel D Ferrari, Arn MJM van den Maagdenberg, Joost Haan and Gisela M Terwindt in Cephalalgia
Article highlights
Migraine frequency, as measured by the number of migraine days per month, appears associated with a genetic predisposition in males, but not females.
A stronger family history of migraine was associated with a lower age-at-onset, a higher number of medication days, and migraine with aura.
Author contributions
NP undertook acquisition and analysis of clinical data, and drafted/revised the manuscript; MAL undertook acquisition and analysis of clinical data, and revised the manuscript; EWvZ and DRN undertook statistical analysis of clinical data, and revised the manuscript; MDF, AMJMvdM, JH and GMT revised the manuscript and supervised study.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N Pelzer, MA Louter, EW van Zwet, DR Nyholt and J Haan report no disclosures. MD Ferrari reports grants and consultancy or industry support from Medtronic and independent support from the European Community, NWO, NIH and the Dutch Heart Foundation. AMJM van den Maagdenberg reports independent support from NWO and the European Community. GM Terwindt reports independent support from NWO, European Community, the Dutch Heart Foundation, and the Dutch Brain Foundation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants of the Netherlands Organization for Scientific Research (NWO) [VIDI 91711319 to GMT] and the European Community (EC) [FP7-EUROHEADPAIN - no. 602633 to AMJMvdM, MDF and GMT]. The funding agencies had no role in the design or conduct of the study.
References
- 1.Nyholt DR, van den Maagdenberg AM. Genome-wide association studies in migraine: Current state and route to follow. Curr Opin Neurol 2016; 29: 302–308. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Bigal ME, Kolodner K, et al. Familial risk of migraine: Variation by proband age-at-onset and headache severity. Neurology 2006; 66: 344–348. [DOI] [PubMed] [Google Scholar]
- 3.Eidlitz-Markus T, Haimi-Cohen Y, Zeharia A. Association of age-at-onset of migraine with family history of migraine in children attending a pediatric headache clinic: A retrospective cohort study. Cephalalgia 2015; 35: 722–727. [DOI] [PubMed] [Google Scholar]
- 4.Brant SR, Picco MF, Achkar JP, et al. Defining complex contributions of NOD2/CARD15 gene mutations, age-at-onset, and tobacco use on Crohn’s disease phenotypes. Inflamm Bowel Dis 2003; 9: 281–289. [DOI] [PubMed] [Google Scholar]
- 5.Escott-Price V, Nalls MA, Morris HR, et al. Polygenic risk of Parkinson disease is correlated with disease age-at-onset. Ann Neurol 2015; 77: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell MB, Iselius L, Olesen J. Migraine without aura and migraine with aura are inherited disorders. Cephalalgia 1996; 16: 305–309. [DOI] [PubMed] [Google Scholar]
- 7.Cologno D, De PA, Manzoni GC. Familial occurrence of migraine with aura in a population-based study. Headache 2003; 43: 231–234. [DOI] [PubMed] [Google Scholar]
- 8.Anttila V, Winsvold BS, Gormley P, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet 2013; 45: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep 2012; 16: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyholt DR, Anttila V, Winsvold BS, et al. Concordance of genetic risk across migraine subgroups: Impact on current and future genetic association studies. Cephalalgia 2015; 35: 489–499. [DOI] [PubMed] [Google Scholar]
- 11.Fuh JL, Wang SJ, Lu SR, et al. Does medication overuse headache represent a behavior of dependence? Pain 2005; 119: 49–55. [DOI] [PubMed] [Google Scholar]
- 12.Vieira DS, Naffah-Mazacoratti MG, Zukerman E, et al. Cerebrospinal fluid GABA levels in chronic migraine with and without depression. Brain Res 2006; 1090: 197–201. [DOI] [PubMed] [Google Scholar]
- 13.Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache 2006; 46: 1334–1343. [DOI] [PubMed] [Google Scholar]
- 14.van Oosterhout WP, Weller CM, Stam AH, et al. Validation of the web-based LUMINA questionnaire for recruiting large cohorts of migraineurs. Cephalalgia 2011; 31: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 15.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 16.Louter MA, Bosker JE, van Oosterhout WP, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain 2013; 136: 3489–3496. [DOI] [PubMed] [Google Scholar]
- 17.Louter MA, Wardenaar KJ, Veen G, et al. Allodynia is associated with a higher prevalence of depression in migraine patients. Cephalalgia 2014; 34: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 18.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: The GEM study. Neurology 1999; 53: 537–542. [DOI] [PubMed] [Google Scholar]
- 19.Henry P, Auray JP, Gaudin AF, et al. Prevalence and clinical characteristics of migraine in France. Neurology 2002; 59: 232–237. [DOI] [PubMed] [Google Scholar]
- 20.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 21.Steiner TJ, Scher AI, Stewart WF, et al. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia 2003; 23: 519–527. [DOI] [PubMed] [Google Scholar]
- 22.Gervil M, Ulrich V, Kaprio J, et al. The relative role of genetic and environmental factors in migraine without aura. Neurology 1999; 53: 995–999. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich V, Gervil M, Kyvik KO, et al. The inheritance of migraine with aura estimated by means of structural equation modelling. J Med Genet 1999; 36: 225–227. [PMC free article] [PubMed] [Google Scholar]
- 24.Low NC, Cui L, Merikangas KR. Sex differences in the transmission of migraine. Cephalalgia 2007; 27: 935–942. [DOI] [PubMed] [Google Scholar]
- 25.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache 2001; 41: 646–657. [DOI] [PubMed] [Google Scholar]
- 26.Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008; 48: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 27.Hyde CL, Nagle MW, Tian C, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet 2016; 48: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stam AH, de Vries B, Janssens AC, et al. Shared genetic factors in migraine and depression: Evidence from a genetic isolate. Neurology 2010; 74: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gormley P, Anttila V, Winsvold BS, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 2016; 48: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anttila V, Stefansson H, Kallela M, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet 2010; 42: 869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisanu C, Preisig M, Castelao E, et al. A genetic risk score is differentially associated with migraine with and without aura. Hum Genet 2017; 136: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esserlind AL, Christensen AF, Steinberg S, et al. The association between candidate migraine susceptibility loci and severe migraine phenotype in a clinical sample. Cephalalgia 2016; 36: 615–623. [DOI] [PubMed] [Google Scholar]
- 33.Ottman R, Hong S, Lipton RB. Validity of familiy history data on severe headache and migraine. Neurology 1993; 43: 1954–1960. [DOI] [PubMed] [Google Scholar]
- 34.Russell MB, Fenger K, Olesen J. The family history of migraine. Direct versus indirect information. Cephalalgia 1996; 16: 156–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Linking migraine frequency with family history of migraine by Nadine Pelzer, Mark A Louter, Erik W van Zwet, Dale R Nyholt, Michel D Ferrari, Arn MJM van den Maagdenberg, Joost Haan and Gisela M Terwindt in Cephalalgia