Abstract
Objective:
Electroencephalogram burst-suppression during general anesthesia is associated with post-operative delirium (POD). Whether burst-suppression causes POD or merely reflects susceptibility to POD is unclear. We hypothesized decreased intraoperative alpha (8–12 Hz) and beta (13–33 Hz) power prior to the occurrence of burst-suppression in susceptible patients.
Methods:
We analyzed intraoperative electroencephalogram data of cardiac surgical patients undergoing cardiopulmonary bypass (CPB). We detected the incidence and duration of CPB burst-suppression with an automated burst-suppression detection algorithm. We analyzed EEG data with multi-taper spectral estimation methods. We assessed associations between patient characteristics and burst-suppression using Binomial and Zero-inflated Poisson Regression Models.
Results:
We found significantly decreased alpha and beta power (7.8–22.95 Hz) in the CPB burst-suppression cohort. The odds ratio for the association between point estimates for alpha and beta power (7.8–22.95 Hz) and the incidence of burst-suppression was 0.88 (95% CI: 0.79 to 0.98). The incidence rate ratio for the association between point estimates for power between the alpha and beta range and the duration of burst-suppression was 0.89 (95% CI: 0.84 to 0.93).
Conclusion:
Decreased intra-operative power within the alpha and beta range was associated with susceptibility to burst-suppression during CPB.
Significance:
This dynamic may be used to develop principled neurophysiological-based approaches to aid the preemptive identification and targeted care of POD vulnerable patients.
Keywords: General anesthesia, EEG oscillations, alpha and beta oscillations, Burst-suppression, Post-operative delirium
Introduction
Electroencephalogram (EEG) burst-suppression consists of quasi-periodic alternations between isoelectricity and brief bursts of electrical activity such as spikes, sharp waves, or slow waves (Young, 2000, Akeju et al. , 2017). It reflects a brain state of relative cortical quiescence that is not observed during normal behavioral states of wake or sleep(Young, 2000, Brown et al. , 2010, Akeju et al. , 2017). Instead, it is closely associated with cortical pathologies such as diffuse anoxic brain injury, hypothermia and Ohtahara syndrome(Young, 2000, Brown et al. , 2010, Akeju et al. , 2017). Burst-suppression is also fundamental to the practice of medicine. For example, refractory status epilepticus is routinely managed by titrating anesthetic drugs to burst suppression (Brown et al. , 2010). Intraoperative EEG burst-suppression during general anesthesia that is maintained with clinically relevant concentrations of anesthetics that potentiate the γ amino butyric acid A (GABAA) receptor has been associated with post-operative delirium (POD) (Soehle et al. , 2015, Fritz et al. , 2016, Fritz et al. , 2018).
POD is an acute brain dysfunction associated with increased morbidity and healthcare costs (Marcantonio, 2017, Palanca et al. , 2017). It is unclear whether intraoperative burst-suppression causes POD or merely reflects susceptibility to POD. This distinction is clinically relevant. For instance, if burst-suppression causes POD, EEG guided low-dose anesthetic protocols that reduce the incidence of intraoperative burst-suppression may decrease the burden of POD. Whereas, if burst-suppression merely reflects an underlying susceptibility to POD, protocols that identify and pre-emptively provide targeted care to patients with a high burden of intraoperative burst-suppression may decrease the burden of POD.
A history of POD has been strongly associated with a long-term cognitive decline (Saczynski et al. , 2012, Inouye et al. , 2016, Marcantonio, 2017). Further, patients with cognitive impairment on cognitive screening tests are more likely to be diagnosed with POD (Kalisvaart et al. , 2006, Robinson et al. , 2012, Saczynski et al. , 2012, Inouye et al. , 2016, Culley et al. , 2017). Therefore, we aimed to study whether patients that exhibited burst-suppression during cardiopulmonary bypass (CPB), a period with stable anesthetic management and physiologic manipulations, were neurophysiologically distinct from patients that did not exhibit burst-suppression. A neurophysiologic distinction would strongly suggest that patients that exhibit burst-suppression, at clinically relevant anesthetic doses, possess a neurobiological predisposition to burst-suppression. We a priori hypothesized decreased intraoperative alpha and beta oscillation power – EEG oscillations that reflect cortical pyramidal and interneuron cell integrity (McCarthy et al. , 2008, Ching et al. , 2010) – as this neurophysiological distinction.
METHODS
Ethics Statement
The Partners Human Research Committee approved this human research study.
Data Collection
We reviewed our database of EEG recordings obtained during general anesthesia and identified all patients that underwent CPB during cardiac surgery. All EEG data were collected using a four-channel frontal EEG device (Sedline, Masimo, Irvine, CA). We identified a total of 138 EEG recordings from this database. We excluded from analyses: 33 cases without CPB, 8 cases with deep hypothermic cardiac arrest, and 18 artifact-laden cases. Thus, data from 79 patients were analyzed. We extracted patient characteristics and surgical details from the Society of Thoracic Surgeon’s National Database. We reviewed the medical records to ensure that none of the patients had known neurological abnormalities. Isoflurane was the sole hypnotic agent that was administered for maintenance of general anesthesia.
We recorded EEG data using the Sedline Sedtrace electrode arrays placed on the forehead at Fp1, Fp2, F7, and F8, with the ground electrode at Fpz, and reference electrode approximately 1 cm above Fpz. Data were recorded with a pre-amplifier bandwidth of 0.5 to 92 Hz, a sampling rate of 250Hz, with 16-bit, 29 nV resolution. Electrode impedance was less than 5kΩ in each channel. We selected EEG data segments using information from the electronic medical record and spectral analysis of the EEG.
For each patient, we carefully selected 2 minute EEG segments that represented the maintenance phase of general anesthesia during surgery. The data were selected from a period at least 15 minutes after the initial induction bolus of an intravenous hypnotic and while the expired concentration of isoflurane was stable. We visually inspected the selected segments in both the time and spectral domains to ensure approximately stability and data quality. These data have not been previously reported in any previous publication.
Burst-suppression Detection
We used a previously validated algorithm to identify periods of EEG suppression (Chemali et al. , 2013, An et al. , 2015). This algorithm detects suppressions by comparing an estimate of the local signal variance with a threshold. Segments with below-threshold variance lasting at least 0.5 seconds were classified as suppressions and were assigned values of one. Other segments were assigned values of zero. We used this binary signal to compute the burst-suppression probability (BSP) using a Bayesian binary filter algorithm. The BSP represents the instantaneous probability that the EEG is in the suppressed state and increases from zero to one as the amount of suppression increases in the EEG.
Spectral Analysis
We computed multitaper spectral estimates using the Chronus toolbox with the following parameters: window length T = 2 seconds without overlap, time-bandwidth product TW = 3, number of tapers K = 5. We equally weighted the signals from Fp1, Fp2, F7 and F8 .
Statistical Analysis
Power analysis:
There was no a priori power analysis to guide our sample size in data collection. The data analyzed were based on availability and our previous experience in related research (Akeju et al. , 2014, Akeju et al. , 2015, Purdon et al. , 2015, Lee et al. , 2017).
EEG:
We implemented an empirical bootstrap approach to assess statistical significance for the difference in spectra at each frequency (i.e., 99% confidence interval of the median difference between groups). First, we resampled the spectral estimates for each non-overlapping window and obtained subject level median spectral estimate for the resampled data. Next, we obtained the median spectral estimates across subjects for each group and computed the difference between groups. We repeated this procedure 5,000 times and calculated the 99% confidence interval of the median difference at each frequency. We rejected the null hypothesis when the confidence interval of the median difference at each frequency exceeded the significance threshold over a contiguous frequency range ≥ 2W. Because We matched patients by age and gender prior to analyses.
Regression:
The 27 events (burst-suppression) in our dataset limit our ability to make principled inferences to 3 variables of interest using traditional regression methods. Therefore, we employed data-driven regression analyses. We constructed a Binomial Regression Model with adaptive elastic net penalty to assess associations between the presence (YES/NO) of burst-suppression and the following ten variables of interest: gender, age, alpha and beta (7.8–22.9 hz) power, depression, diabetes, sleep apnea, CPB perfusion time, CPB temperature nadir, hours in the ICU duration, and CPB isoflurane concentration. We also constructed a Zero-inflated Poisson Regression Model with adaptive elastic net penalty to assess associations between the duration of burst-suppression as a percentage CPB total time and the variables of interest listed above. For each patient, the median of the summed multitaper spectral estimates between 7.8 and 22.9 Hz for each spectral window multiplied by Δ frequency was computed as point estimates that were analyzed in our regression models. Regression models were constructed using JMP®, Pro 13 (SAS Institute Inc., Cary, NC).
Results
Electroencephalogram dynamics of patients that exhibited burst-suppression were distinct from patients that did not exhibit burst-suppression
Table 1 summarizes characteristics and co-administered medications of the cardiac surgical cohort. General anesthetic dosing between the groups during the EEG epochs analyzed (pre-CPB) was not significantly different (burst-suppression cohort, mean isoflurane expired concentration, 0.9 [SD, 0.2]; no suppression cohort (age and gender matched), mean isoflurane expired concentration, 0.8 [SD, 0.3]; P = 0.5135, Wilcoxon). We observed decreased alpha and beta oscillation power in the spectrogram of patients that subsequently exhibited burst-suppression (n = 27; mean age, 67.9 [SD,9.3]) compared to age and gender matched patients that did not exhibit burst-suppression during CPB (Fig 1A, 1B; n = 27; mean age, 69.1 [SD, 9.1]). To quantify these differences, we compared the spectra between both groups and found significant differences in power (Fig. 1C; no burst-suppression > burst-suppression, 7.8–22.95 Hz).
Table 1.
Patient Characteristics
| Burst suppression = 27 | No Burst suppression = 52 | |
|---|---|---|
| Female, n (%) | 9 (33.3) | 6 (11.5) |
| Age ± SD | 67.9 ± 9.3 | 65.3 ±8.7 |
| Weight (kg), mean ± SD | 76.4 ± 17.4 | 85.3 ±16.5 |
| Height (cm), mean ± SD | 170.1 ± 12.3 | 172.8 ±7.4 |
| Comorbidities | ||
| Diabetes, n (%) | 9 (33.3) | 17(32.7) |
| Sleep Apnea, n (%) | 4 (14.8) | 4 (7.7) |
| Depression, n (%) | 6 (22.2) | 1 (1.9) |
| Cross lamp time (min), mean ± SD | 85.3 ± 28.7 | 94.3 ± 62.5 |
| CPB Perfusion time (min), mean ± SD | 123.3 ± 36 | 114.2 ± 54.7 |
| CPB temp nadir (F), mean ± SD | 33.8 ± 2.3 | 34.0 ± 1.5 |
| Hours in ICU, mean ± SD | 62.3 ± 83.5 | 45.9 ± 37.2 |
| Surgery Type | ||
| Isolated CABG | 11 (40.7) | 25 (48.1) |
| MV Repair | 1 (3.7) | 2 (3.8) |
| AVR/CABG | 2 (7.4) | 5 (9.6) |
| Isolated MVR | 1 (3.7) | 2 (3.8) |
| AVR/MVR | 0 (0) | 2 (3.8) |
| Isolated AVR | 3 (11.1) | 2 (3.8) |
| MV Repair/CABG | 1 (3.7) | 2 (3.8) |
| Other | 8 (29.6) | 12 (23.1) |
| CPB Isoflurane, % ± SD | 0.9 ± 0.2 (n = 27) | 0.9 ± 0.3 (n = 52) |
| Propofol (mg), mean ± SD | 117.0 ± 50.6 (n = 20) | 91.1 ± 62.3 (n = 45) |
| Etomidate (mg), mean ± SD | 12.4 ± 4.3 (n = 5) | 15.3 ± 8.1 (n = 3) |
| Midazolam (mg), mean ± SD | 3.8 ± 1.4 (n = 27) | 4.1 ±1.4 (n = 51) |
| Muscle Relaxants | ||
| Rocuronium, n (%) | 19 (70.4) | 39 (75) |
| Cisatracuronium, n (%) | 6 (22.2) | 7 (25) |
| Opioids | ||
| Fentanyl (mcg), mean ± SD | 852.8 ± 351.3 (n = 27) | 857.7 ± 235.9 (n = 52) |
| Morphine (mg), mean ± SD | 17.5 ± 5 (n = 4) | 15.0 ± 5.8 (n = 4) |
| Hydromorphone (mg), mean ± SD | 1 ± 0 (n = 3) | 1.5 ± 0.5 (n = 6) |
AVR; Aortic Valve Replacement; CABG; Coronary Artery Bypass Graft, F; Fahrenheit, ICU; Intensive Care Unit, MV; mg, milligram; mcg, microgram; Mitral Valve, MVR; Mitral Valve Replacement, n; number.
Figure 1.
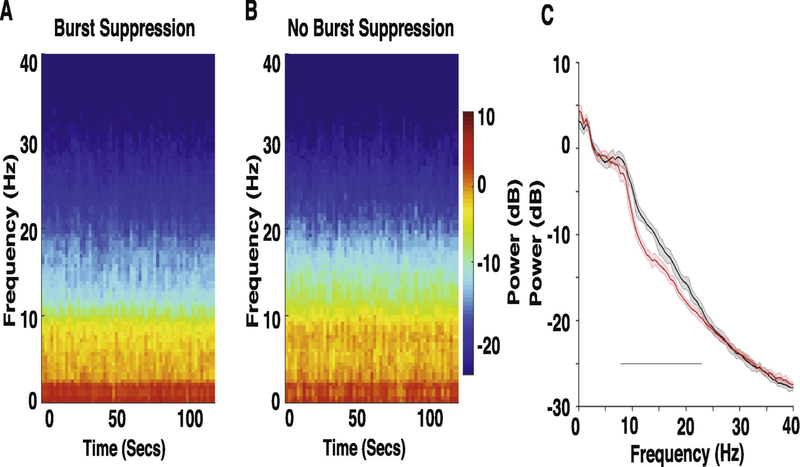
Spectral comparison of EEG obtained prior to the onset of CPB in the CPB Burst suppression versus CPB No Burst suppression cohorts. (A, B) Median frontal spectrograms of Burst suppression (n = 27) and No Burst suppression (n = 27) patient cohorts. (C) Overlay of median Burst suppression (red) and median No Burst suppression (black) frontal spectra. Bootstrapped median spectra are presented, and the shaded regions represent the 95% confidence interval for the uncertainty around each bootstrapped median spectrum. We observed differences in power between the spectra (No Burst suppression > Burst suppression: 7.8–22.95 Hz). Black line represents significantly different regions.
Electroencephalogram alpha and beta power point estimates were associated with the incidence and duration of burst-suppression
The odds ratio for the association between point estimates for alpha and beta power (7.8–22.95 Hz) and the incidence of burst-suppression was 0.88 (95% CI: 0.79 to 0.98). Gender, Age, Alpha and beta (7.8–22.95 Hz) power, and depression were the only variables that did not result in zero-valued coefficients in our model (Table 2).
Table 2.
Burst suppression Odds Ratios from Binomial Regression Model and % Duration of Burst suppression Incidence Rate Ratios from Zero Inflated Poisson Regression Model
| Variable | Binomial Regression Model for Developing Burst suppression During CPB | Zero Inflated Poisson Model for % Duration of Burst suppression |
|---|---|---|
| Odds Ratios (95% CI) | Incidence Rate Ratios (95% CI) | |
| Gender | ||
| Male/Female | 0.45 (0.14–1.43) | 1 |
| Female/Male | 2.21 (0.70–7.01) | 1 |
| Age | 1 | 1 |
| Alpha and beta Power | 0.88 (0.79–0.98) | 0.89 (0.84–0.93) |
| Depression | 0.18 (0.03–1.20) | 0.80 (0.51–1.25) |
| Diabetes | ||
| Y/N | 1 | 0.75 (0.46–1.21) |
| N/Y | 1 | 1.33 (0.83–2.14) |
| Sleep Apnea | ||
| Y/N | 1 | 1 |
| N/Y | 1 | 1 |
| CPB Perfusion time | 1 | 0.99 (0.98–1) |
| CPB temp nadir | 1 | 0.98 (0.88–1.08) |
| Hours in ICU | 1 | 1 |
| CPB Isoflurane expired concentration | 1 | 0.76 (0.24–2.44) |
CPB, Cardiopulmonary Bypass; CI, Confidence Interval; ICU, Intensive Care Unit
The incidence rate ratio for the association between point estimates for alpha and beta power and the duration of burst-suppression during CPB was 0.89 (95% CI: 0.84 to 0.93). Alpha and beta (7.8–22.95 Hz) power, Depression, Diabetes, CPB perfusion time, CPB temperature nadir, and CPB isoflurane expired concentration were the only variables that did not result in zero-valued coefficients in our model (Table 2).
DISCUSSION
In this investigation, we found that decreased EEG power between 7.8 to 22.95 Hz during stable isoflurane general anesthesia maintenance (after the induction of general anesthesia) was associated with the later incidence and duration of burst-suppression during CPB. Because decreased alpha and beta oscillation power preceded the onset of burst-suppression, our findings suggest that intraoperative EEG dynamics within the alpha and beta range may be further developed as EEG biomarkers for burst-suppression, and by proxy, POD vulnerability. We note that alpha and beta (7.8–22.9 Hz) power, and depression were the only variables that did not result in zero-valued coefficients in our data driven regression models.
EEG oscillations associated with anesthetic drugs that potentiate the GABAA receptor
Anesthetic drugs that potentiate the GABAA receptor induce frontal EEG beta oscillations at sedative doses and alpha oscillations at general anesthetic doses. These medications include but are not limited to zolpidem (Kalisvaart et al. , 2006, Monk et al. , 2008, Robinson et al. , 2012), midazolam (McCarthy et al. , 2008, Ching et al. , 2010), thiopental (Kiersey et al. , 1951), propofol (Chemali et al. , 2013, Akeju et al. , 2015, An et al. , 2015), and derivatives of ether anesthesia (desflurane, isoflurane, sevoflurane) (Akeju et al. , 2014, Akeju et al. , 2016, Pavone et al. , 2017). Models to explain EEG alpha and beta oscillatory dynamics suggest that an increase in GABAA decay-time and conductance causes cortical low threshold spiking (LTS) interneuron antisynchrony that patterns pyramidal cell spiking into a beta oscillation (13–33 Hz) (McCarthy et al. , 2008). Further increases in GABAA decay-time and conductance modulate pyramidal and thalamic relay cell spiking into a thalamocortical alpha oscillation (Ching et al. , 2010). Thus, EEG alpha and beta oscillation power may reflect the integrity of cortical, and possibly, cognitive circuits, and burst-suppression may more readily manifest in patients with impairments in these circuits.
EEG oscillations associated with GABAA receptor drugs and neurocognitive function
Giattino et al. recently demonstrated that intraoperative frontal alpha oscillation power was positively correlated with neurocognitive function (Giattino et al. , 2017). In other compelling studies, a thiopental challenge resulted in decreased frontal beta oscillation power in Alzheimer’s disease patients (Holschneider et al. , 1997, Holschneider et al. , 2000). This finding was positively correlated with cognitive function (Holschneider et al. , 2000). Thus, the EEG oscillations that are induced by anesthetic drugs may elicit neurophysiological biomarkers that are not readily discernable from EEG recordings obtained during the awake state. We note that healthy aging is associated with an age-dependent decrease in anesthesia-induced frontal alpha oscillation power (Akeju et al. , 2015, Purdon et al. , 2015, Lee et al. , 2017). However, similar to the accelerated decrease in the awake-occipital alpha oscillation power that is associated with neurodegeneration in patients with mild cognitive impairment and Alzheimer’s disease (Rossini et al. , 2007, Babiloni et al. , 2013, Babiloni et al. , 2015), significantly decreased anesthesia-induced frontal alpha and beta power may reflect sub-clinical neurodegenerative changes.
Intraoperative EEG burst-suppression, post-operative delirium and causality
Roach et al. studied differences in neurologic and neuropsychologic outcomes in cardiac surgical patients randomized to either “sufentanil only” or “sufentanil plus propofol-titrated-to-burst-suppression” anesthetic groups (Roach et al. , 1999). The authors found that the incidence and severity of neurologic and neuropsychologic dysfunction, depression, and anxiety were similar in both groups (Roach et al. , 1999). This finding suggests that anesthetic-induced burst-suppression in cognitively normal patients is unlikely to result in POD. Rather, burst-suppression may be more evident, at clinically relevant anesthetic doses, in patients that possess an underlying vulnerability to POD. Results from trials, such as the ENGAGES study (Wildes et al. , 2016), are expected to make clear the effect of principled low dose anesthetic protocols on POD and other clinically relevant outcomes.
Limitations and Future Directions
A key limitation of our study is the observational nature of the data that were analyzed. However, it is unlikely that variations in clinical management could account for the magnitude of the EEG changes in our analyses. Burst-suppression is typically regarded as a spatially homogenous phenomenon. However, anesthetic-drug induced burst-suppression may not be spatially homogenous (Lewis et al. , 2013, An et al. , 2015). Thus, because we recorded EEG signals from frontal channels, epochs of non-spatially homogenous burst-suppression from other scalp locations may have been missed. Future high-density EEG studies are therefore necessary to make clear whether burst-suppression recorded from frontal brain regions are more closely associated with POD compared to burst-suppression recorded from other brain regions.
Large randomized controlled studies such as the MINDDS trial (Shelton et al. , 2018) that couple structured delirium assessments to intra-operative EEG dynamics are necessary to enable causal inferences on the association between alpha and beta power, burst-suppression, and POD. Also, these studies may in more detail inform on whether patients with diagnoses such as sleep apnea and depression that have previously associated with POD should receive focused peri-operative care (Flink et al. , 2012, Roggenbach et al. , 2014, Mollon et al. , 2016, Nadler et al. , 2017). These studies may also make clear the extent to which susceptibility to POD as defined by significant deviations in EEG alpha and beta power “norms” may be modified by targeted peri-operative management. We note that even at equal anesthetic drug doses, EEG dynamics and biomarkers of brain vulnerability that are derived and validated in healthy patients may not be readily applicable to critically ill patients because systemic inflammation increases neuronal sensitivity to GABAA receptor potentiating drugs (Avramescu et al. , 2016). Thus, studies that are specific to critically ill patients are especially essential. Further, intra-operative EEG power dynamics and machine learning algorithms may be leveraged to benefit clinical diagnosis (i.e., perioperative stroke, abnormal brain aging/subclinical neurodegeneration) and to provide objective means to sub-categorize patients with neuropsychiatric diagnosis (i.e., depression, autism spectrum disorder).
Conclusions
Decreased intra-operative alpha and beta power is associated with susceptibility to burst-suppression during CPB. This dynamic may be used to develop principled neurophysiological-based approaches to aid the preemptive identification and targeted care of POD vulnerable patients. Further studies are necessary to define the cumulative distribution and objective cut points for alpha and beta power during general anesthesia.
Highlights.
Intraoperative electroencephalogram burst-suppression is associated with post-operative delirium.
Decreased alpha and beta power was evident in the EEG prior to the occurrence of burst-suppression.
Decreased EEG alpha and beta power predicted the later incidence and duration of burst-suppression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
OA has received speaker’s honoraria from Masimo Corporation, and is listed as an inventor on pending patents on EEG monitoring that are assigned to Massachusetts General Hospital, some of which are assigned to Masimo Corporation. OA has received institutionally distributed royalties for these licensed patents. All other authors declare that no competing interests exist.
REFERENCES
- Akeju O, Brown EN. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol 2017;44:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Hamilos AE, Song AH, Pavone KJ, Purdon PL, Brown EN. GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol 2016;127:2472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Pavone KJ, Thum JA, Firth PG, Westover MB, Puglia M, et al. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. Br J Anaesth 2015;115 Suppl 1:i66–i76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 2014;121:990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Jonnalagadda D, Moura V, Purdon PL, Brown EN, Westover MB. Spatial variation in automated burst suppression detection in pharmacologically induced coma. Conf Proc IEEE Eng Med Biol Soc 2015;2015:7430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramescu S, Wang DS, Lecker I, To WT, Penna A, Whissell PD, et al. Inflammation Increases Neuronal Sensitivity to General Anesthetics. Anesthesiology 2016;124:417–27. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Lizio R, Vecchio F, Baglieri A, Bernardini S, et al. Resting state cortical electroencephalographic rhythms are related to gray matter volume in subjects with mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp 2013;34:1427–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Boccardi M, Lizio R, Lopez S, Carducci F, et al. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2015;36:556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010;363:2638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemali J, Ching S, Purdon PL, Solt K, Brown EN. Burst suppression probability algorithms: state-space methods for tracking EEG burst suppression. J Neural Eng 2013;10:056017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A 2010;107:22665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley DJ, Flaherty D, Fahey MC, Rudolph JL, Javedan H, Huang CC, et al. Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology 2017;127:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flink BJ, Rivelli SK, Cox EA, White WD, Falcone G, Vail TP, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012;116:788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, et al. Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth Analg 2016;122:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BA, Maybrier HR, Avidan MS. Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth 2018;121:241–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giattino CM, Gardner JE, Sbahi FM, Roberts KC, Cooter M, Moretti E, et al. Intraoperative Frontal Alpha-Band Power Correlates with Preoperative Neurocognitive Function in Older Adults. Front Syst Neurosci 2017;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Leuchter AF. Attenuation of brain high frequency electrocortical response after thiopental in early stages of Alzheimer’s dementia. Psychopharmacology (Berl) 2000;149:6–11. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Leuchter AF, Uijtdehaage SH, Abrams M, Rosenberg-Thompson S. Loss of high-frequency brain electrical response to thiopental administration in Alzheimer’s-type dementia. Neuropsychopharmacology 1997;16:269–75. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 2016;12:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisvaart KJ, Vreeswijk R, de Jonghe JF, van der Ploeg T, van Gool WA, Eikelenboom P. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc 2006;54:817–22. [DOI] [PubMed] [Google Scholar]
- Kiersey DK, Bickford RG, Faulconer A Jr. Electro-encephalographic patterns produced by thiopental sodium during surgical operations; description and classification. Br J Anaesth 1951;23:141–52. [DOI] [PubMed] [Google Scholar]
- Lee JM, Akeju O, Terzakis K, Pavone KJ, Deng H, Houle TT, et al. A Prospective Study of Age-dependent Changes in Propofol-induced Electroencephalogram Oscillations in Children. Anesthesiology 2017;127:293–306. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Ching S, Weiner VS, Peterfreund RA, Eskandar EN, Cash SS, et al. Local cortical dynamics of burst suppression in the anaesthetized brain. Brain 2013;136:2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med 2017;377:1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci 2008;28:13488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016;98-B:818–24. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008;108:18–30. [DOI] [PubMed] [Google Scholar]
- Nadler JW, Evans JL, Fang E, Preud’Homme XA, Daughtry RL, Chapman JB, et al. A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia 2017;72:729–36. [DOI] [PubMed] [Google Scholar]
- Palanca BJA, Wildes TS, Ju YS, Ching S, Avidan MS. Electroencephalography and delirium in the postoperative period. Br J Anaesth 2017;119:294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone KJ, Su L, Gao L, Eromo E, Vazquez R, Rhee J, et al. Lack of Responsiveness during the Onset and Offset of Sevoflurane Anesthesia Is Associated with Decreased Awake-Alpha Oscillation Power. Front Syst Neurosci 2017;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Pavone KJ, Akeju O, Smith AC, Sampson AL, Lee J, et al. The Ageing Brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth 2015;115 Suppl 1:i46–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach GW, Newman MF, Murkin JM, Martzke J, Ruskin A, Li J, et al. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology 1999;90:1255–64. [DOI] [PubMed] [Google Scholar]
- Robinson TN, Wu DS, Pointer LF, Dunn CL, Moss M. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg 2012;215:12–7; discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbach J, Klamann M, von Haken R, Bruckner T, Karck M, Hofer S. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Crit Care 2014;18:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol 2007;83:375–400. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KT, Qu J, Bilotta F, Brown EN, Cudemus G, D’Alessandro DA, et al. Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018;8:e020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol 2015;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildes TS, Winter AC, Maybrier HR, Mickle AM, Lenze EJ, Stark S, et al. Protocol for the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) study: a pragmatic, randomised clinical trial. BMJ Open 2016;6:e011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GB. The EEG in coma. J Clin Neurophysiol 2000;17:473–85. [DOI] [PubMed] [Google Scholar]


