Abstract
Vaccination is a biological process that administrates antigenic materials to stimulate an individual’s immune system to develop immunity to a specific pathogen. It is the most effective tool to prevent illness and death from infectious diseases or diseases leading to cancers. Because many recombinant and synthetic antigens are poorly immunogenic, adjuvant is essentially added to vaccine formula that can potentiate the immune responses, offer better protection against pathogens and reduce the amount of antigens needed for protective immunity. To date, there are nearly 100 different types of adjuvants associated with about 400 vaccines that are either commercially available or under development. Among these adjuvants, many of them are particulates and nano-scale in nature. Nanoparticles represent a wide range of materials with novel physicochemical properties that exhibit immunostimulatory effects. However, the mechanistic understandings on how their physicochemical properties affect immunopotentiation remain elusive. In this article, we aim to review current development status of nanomaterial-based vaccine adjuvants, and further discuss their acting mechanisms, understanding of which will benefit the rational design of effective vaccine adjuvants with improved immunogenicity for prevention of infectious disease as well as therapeutic cancer treatment.
Engineered nanomaterials as vaccine adjuvants are capable of potentiating the immune responses through different mechanisms.
1. Introduction
Vaccination remains one of the most effective tools to stimulate protective immune responses against infectious disease.1–3 Since Edward Jenner’s use of cowpox materials to provide protection against smallpox in 1796, vaccination has saved millions of lives. It has completely eliminated smallpox, the near-complete eradiation of poliomyelitis, and a significant decrease in the incidence of diseases including diphtheria, tetanus, pertussis, measle, hepatitis A, hepatitis B, etc.4 In addition, vaccination can prevent diseases that lead to cancers. The hepatitis B vaccine is 95% effective in infection prevention and the development of chronic disease and liver cancer.5 Gardasil, a human papillomavirus (HPV) vaccine, can protect against two types of HPV that cause 70% of cervical cancer, 70% of vaginal cancer and 50% of vulvar cancer.6 Successful vaccines contain not only protective antigens, but also adjuvants that trigger innate and adaptive immune activations for optimal and long lasting immunogenicity.7 Statistics by Vaxjo, a web- based central database and analysis system that stores vaccine adjuvants and their usages in vaccine development, shows that 93 vaccine adjuvants have been used in 379 vaccines against 78 pathogens, cancers and allergies.8 Among these, only very few vaccine adjuvants are licensed for use in humans. For example, the Food and Drug Administration (FDA) of the United States approves four adjuvants including aluminum salts, AS03, AS04, and MF59 (Table 1).4, 9 In Europe, besides these four adjuvants, virosomes have been licensed and safely used since 1994.10
Table 1.
Approved vaccine adjuvants in human and their active components.
| Adjuvants | Components | Disease | Stage | Reference |
|---|---|---|---|---|
| Aluminum salts | Aluminum hydroxide, aluminum phosphate, aluminum potassium sulfate, aluminum hydroxyphosphate sulfate | Hepatitis A, hepatitis B, DTaP-HepB- IPV, human papillomavirus etc. | Licensed (US, EU) | 4, 9 |
| AS03* | Squalene, DL-α-tocopherol, polysorbate 80 | Influenza | Licensed (US, EU) | 4, 9 |
| AS04 | Aluminum-absorbed TLR4 agonist | Hepatitis B, human papilloma virus | Licensed (US, EU) | 4, 9 |
| MF59 | Oil-in-water emulsion of squalene oil | Influenza | Licensed (US, EU) | 4, 9 |
| Virosome | Viral envelop | Influenza and Hepatitis A | Licensed (EU) | 10 |
*AS03 is included in the H5N1 influenza vaccine, however, is not commercially available.
Most of the vaccine adjuvants currently being used or under development are nano-range particulates.11 Compared to traditional materials, nanomaterials can protect the antigen from the surrounding biological milieu, increase their half-life, minimize the systemic toxicity, promote the delivery of immunomodulatory and immunostimulatory substances to antigen presenting cells, or trigger the activation of antigen-specific T cells.12 Although particulates are widely used as vaccine adjuvants, the mechanisms of these nano-particulate adjuvants in simulating innate and adaptive immunity are poorly understood.11 In this review, we aim to introduce current status of the development of nanomaterial-based vaccine adjuvant and to bridge the concept of materials science and immunology. The understanding of immuno-stimulating mechanisms will provide knowledge to design more effective engineered prophylactic and therapeutic vaccine for the prevention and treatment of infectious disease and cancer.
2. Nanoparticles as Adjuvants
Nanomaterials refer to particulate materials having a length scale in the range of 1 nm to 100 nm in at least one dimension.13 Broader definition raises the range up to 1 μm, based on their similar physicochemical properties to nanoscale particles. They have unique physicochemical properties including size, shape, surface chemistry, roughness and surface coatings that are distinctive from bulk materials, and have been widely used in biomedical applications including drug/gene delivery, vaccines, imaging, and medical devices.14–21 Among these, nanomaterials is known to generate or enhance immunological responses originate from the interactions at the nano-bio interface.13, 22, 23 One of the most important advantages of ENMs is that it is possible to control their properties through engineered design, allowing the selection of the most effective adjuvant formulations using in vitro and in vivo approaches in a systemic fashion. For example, studies have demonstrated that by controlling the physicochemical properties of nanomaterials including the ability to generate ROS, aspect ratio,24, 25 dispersion state,26 size27–29 and surface functionalization,28–30 it is possible to modulate the immune activation and further enhance immune responses to antigens.22–31
3. Engineered Nanomaterials (ENMs) as Vaccine Adjuvants
Nanomaterials are demonstrated to provide adjuvant activity by enhancing the delivery of antigens to the immune system or by potentiating innate and/or adaptive immune responses.13 Research groups have developed various ENM-based adjuvants to provoke long-lasting immune responses (Table 2). We will discuss the major groups of nanomaterial-based adjuvants and ways to improve the adjuvant effects.
Table 2.
Nanomaterial-based vaccine adjuvants.
| Nanomaterials | Physicochemical Properties in Study | Model Antigens | Reference |
|---|---|---|---|
| Aluminum hydroxide | Size | Bacillus anthracis protective antigen | 32 |
| Aluminum oxyhydroxide | Shape, crystallinity, hydroxyl content | OVA | 31 |
| Gold | Shape, size | West Nile virus envelope protein | 33 |
| Silver | N/A | OVA | 34 |
| Mesoporous silica | 3-D rod and surface chemistry | OVA, CpG-ODN | 35, 36 |
| PLGA | N/A | Staphylococcal enterotoxin B toxoid | 37 |
| γ-PGA | N/A | HIV-1 | 38 |
| Chitosan | N/A | OVA, Hybrid-1 | 39, 40 |
| PLGA-DMAEMA-co- PAA-co-BMA | Blend ratio | OVA | 41 |
| PEI | N/A | Influenza hemagglutinin, HSV-2 glycoprotein D | 7 |
| DDA liposome | N/A | BBG2Na, a recombinant fusion protein produced in E. coli | 42 |
3.1. Metal- and Metal Oxide-Based Vaccine Adjuvants
3.1.1. Aluminum-based Vaccine Adjuvants
Aluminum-based vaccine adjuvants have been in routine use in human vaccination against various diseases including DTaP (Diphtheria, Tetanus, acellular Pertussis), Human Papillomavirus, Pneumococcal, Hepatitis A and Hepatitis B with safe record for over eighty years (Table 3).43 Depending on the commercial sources, they are composed of aluminum hydroxide (Alhydrogel), aluminum phosphate (Adju-Phos), aluminum potassium sulfate (Alum), aluminum hydroxyphosphate sulfate (AHSA) or a mixture of aluminum and magnesium hydroxides (Imject Alum)43–45 These commercially available aluminum salts have different physicochemical properties.46 For example, aluminum hydroxides are needle-like particles with diameters of 2 nm, while aluminum phosphates are plate-like particles with primary size of 50 nm. However, their names do not correctly describe their adjuvant structures. X-ray diffraction analysis and infrared spectroscopy have identified aluminum hydroxide adjuvant as crystalline aluminum oxyhydroxide, AlOOH; and aluminum phosphate is determined as amorphous aluminum hydroxyphosphate, Al(0H)x(P04)y.46 In addition, the key physicochemical properties that determine the nano-bio interaction and the stimulation of immune responses still remain unknown.
Table 3.
| Vaccine | Trade name and manufacture | Age of usage | Type of aluminum salts |
|---|---|---|---|
| Anthrax | Biothrax, Emergent BioSolutions, Inc. | 18−65 (y) | Aluminum hydroxide |
| Td | Decavac, Sanoft Pastern Limited | >7(y) | Aluminum potassium sulfate |
| Td | Tenivac, Sanoft Pasteur Limited | >7(y) | Aluminum phosphate |
| DTaP | Daptacel, Sanofi Pasteur Limited | 6 (w)-6 (y) | Aluminum phosphate |
| Tdap | Adacel, Sanofi Pasteur Limited | 10−64 (y) | Aluminum phosphate |
| Tdap | Boostrix, GlaxoSmithKline Biologicals | >10 (y) | Aluminum hydroxide |
| DTaP-IPV | Kinrix, GlaxoSmithKline Biologicals | 4−6 (y) | Aluminum hydroxide |
| DTaP-HepB-IPV | Pediarix, GlaxoSmithKline Biologicals | 4−6 (y) | Aluminum hydroxide |
| DTaP-IPV/Hib | Pentacel, Sanofi Pasteur Limited | 6 (w)-4 (y) | Aluminum phosphate |
| Haemophilus influenzae | PedvaxHIB, Merck & Co., Inc | 2−71 (m) | Aluminum hydroxyphosphate sulfate |
| Haemophilus influenzae/Hepatitis B | Comvax, Merck & Co., Inc | 2−15 (m) | Aluminum hydroxyphosphate sulfate |
| Hepatitis A | Havrix, Glaxo SmithKline Biologicals | >12 (m) | Aluminum hydroxide |
| Hepatitis B | Engerix-B, GlaxoSmithKline Biologicals | All age | Aluminum hydroxide |
| Hepatitis B | Recombivax HB, Merck & Co., Inc | All age | Aluminum hydroxyphosphate sulfate |
| Hepatitis A / Hepatitis B | Twinrix, GlaxoSmithKline Biologicals | >18 (y) | Aluminum hydroxide, Aluminum phosphate |
| Human Papillomavirus (HPV) | Cervarix, GlaxoSmithKline Biologicals | 9−25 (y) | Aluminum hydroxide |
| Human Papillomavirus (HPV) | Gardasil, Merck & Co., Inc Ixiaro Intercell | 9−25 (y) | Aluminum hydroxyphosphate sulfate |
| Japanese Encephalitis | Biomedical Ltd. PCV13-Prevnar 13, | >2 (m) | Aluminum hydroxide |
| Pneumococcal | Wyeth Pharmaceuticals, Inc. | >65 (y) | Aluminum phosphate |
AP: acellular pertussis; D: diphtheria; Hib: Haemophilus influenzae type b; IPV: inactivated poliovirus; T: tetanus; w: weeks; m: months; y: years.
Size effects of aluminum salts were studied by Li et al. using ovalbumin and Bacillus anthracis protective antigen protein as model antigens.32 Aluminum hydroxide nanoparticles with mean diameters of 112 nm and 9 μm were prepared. It is demonstrated that aluminum hydroxide nanoparticles (~112 nm) exhibited more potent antigen-specific antibody response than that of the micro-sized (~9 μm) particles. The stronger adjuvant activity of nano-sized particles was correlated with their ability to more effectively facilitate the uptake of the antigens.
Effects of surface coating was studied by Wang et al. who prepared phospholipid bilayer-coated aluminum nanoparticles (50 nm in size) through chemisorption, and compared their adjuvant effects with naked particles. The coated adjuvant was more readily taken up by antigen-presenting cells and could induce robust antigen-specific humoral (antibody production) and cellular (cytokine production, e.g., IFN-γ in splenocyte supernatants) immunoresponses with less local inflammation.48
Recently, in a study by us, we elucidated the role of shape and aspect ratio in aluminum oxyhydroxide-induced adjuvant effects (Figure 1).31 A comprehensive library of γ-phase aluminum oxyhydroxide nanoparticles (γ-ΑlΟΟΗ, boehmite) with variation in shape, crystal structure, and surface hydroxyl groups was established using a hydrothermal method. It is demonstrated that shape, crystallinity and hydroxyl content of AlOOH nanoparticles played important roles in the induction of immune responses in vitro and in vivo. AlOOH nanorods induced NLRP3 inflammasome activation and IL-1β production that was dependent on their hydroxyl contents and reactive oxygen species (ROS) generation ability in both THP-1 cells and bone marrow-derived dendritic cells (BMDCs) in vitro. AlOOH nanorods also induced the maturation of BMDCs. By using ovalbumin (OVA) as a model antigen, it is shown that vaccination with AlOOH nanorods exhibited higher antibody production including OVA specific IgG1 and IgE in mice compared to commercial Imject Alum. Adoptive DC transfer was also used to demonstrate that ex vivo boosting of APC activity predicts the ability of AlOOH nanorods to exert an adjuvant effect in intact animals. This study shows that the intrinsic properties of AlOOH nanorods play an important role in inducing NLRP3 inflammasome activation that correlates well with the in vivo immune-potentiating effects, which suggests a potential mechanism involved in aluminum-based adjuvants. More research is needed on understanding the relevant mechanisms of other types of aluminum-based adjuvants, which may activate similar pathways or through other mechanisms.
Figure 1.
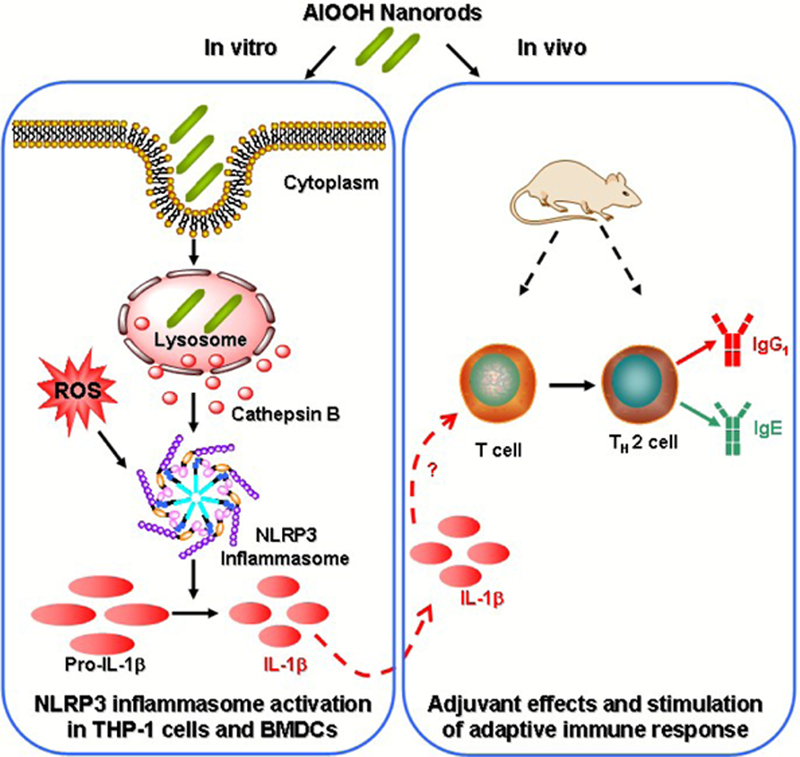
Engineered aluminum oxyhydroxide (A100H) nanorods as vaccine adjuvants. The shape, crystallinity and hydroxyl content play an important role in NLRP3 inflammasome activation and boosting of antigen-specific immune responses.
3.1.2. Gold Nanoparticles
Gold nanoparticles (AuNPs) with controlled physicochemical properties are the subject of intensive studies and applications in biology and medicine.33, 49, 50 Niikura et al. investigated the effect of shape and size of gold nanoparticle coated by West Nile virus envelope protein (WNVE) on their immunological responses in vitro and in vivo.33 It is showed that the Au rods were most efficiently internalized into cells and induced the secretion of the inflammasome-related cytokines including IL-1β and IL-18. While in vivo study showed that Sphere40 (40 nm) was more effective than other shapes (cube and rod) or smaller sphere (20 nm) in antibody production, possibly caused by the production of inflammatory cytokines by Sphere40. However, the detailed mechanisms and generality of the dominant factor underlying the effects of size and shape of Au NPs on immune responses need further investigation. Additionally, although the gold nanoparticles are generally considered safe, they are not biodegradable and repeated use will lead to bioaccumulation, which may lead to long term effects that are yet to be determined.
3.1.3. Silver Nanoparticles
Due to their unique antimicrobial properties, silver nanoparticles are widely used in commercial products and more than 30% of nanomaterial-based consumer products contain nano-sliver.51 Beyond these widely accepted commercial applications, Xu et al. evaluated the adjuvant effect of silver (Ag) nanoparticles (spherical, 141 nm) and showed that AgNPs could induce the increase of OVA specific IgG1/IgG2a ratio and IgE, indicating the elicitation of Th2-biased immune responses. Further mechanistic study showed that the adjuvant effect of AgNPs was mainly ascribed to the recruitment and activation of local leukocytes, especially macrophages.34 However, the weakness of AgNPs is the dissolution of the particles that may lead to complete degradation.51 In addition, AgNPs have been shown to be toxic to mammalian cells and they can also induce acute inflammation in animal lungs and systemic inflammation, so the potential toxicity could outweigh their beneficial adjuvant effects.51, 52
3.2.4. 3D Mesoporous Silica Rod
Mesoporous silica nanoparticles have been experiencing an outstanding growth in recent years because of their biocompatibility and unique properties.53,54 Recently, Kim et al. has demonstrated that injected high-aspect-ratio mesoporous silica rods (MSRs) could spontaneously assemble in vivo to form macroporous structures that provide a three dimensional cellular microenvironment for host immune cells.35 In mice, substantial numbers of dendritic cells are recruited to the pores between the scaffold rods. The recruitment of dendritic cells and their subsequent homing to lymph nodes can be modulated by sustained release of inflammatory signals and adjuvants from the scaffold. Moreover, injection of an MSR-based vaccine formulation enhances systemic helper T cells, serum antibody and cytotoxic T-cell levels compared to bolus controls. At the site of the injection, the MSRs are biodegradable within a few months. These findings suggest that plantable MSRs may serve as a multifunctional vaccine platform to modulate host immune cell function and provoke adaptive immune responses. A follow-up study by Li et al. showed that functionalization of MSR scaffold could change their immunogenic potential in vivo. MSR scaffold with poly(ethylene-glycol) (PEG) would reduce its immunogenicity and thus decrease immune cell infiltration. In contrast, modifying the MSR scaffold with PEG-Arg-Gly-Asp (PEG-RGD) would enhance immune cell adhesion and infiltration.36
In summary, metal and metal oxides represent a group of materials with tunable physicochemical properties that exhibit superior adjuvant potentials. However, there are some potential problems need to be considered during engineered design. First, the biopersistence of the materials, e.g., solubility, clearance by macrophages and dendritic cells, and the site of injection determine material’s adjuvancy as well as adverse inflammatory reaction. Thus, material safety is always a major concern, e.g., silver nanoparticles. The injection of metal- or metal oxide-based adjuvants could induce local inflammatory reaction, which can be associated with clinical symptoms of pain, swelling, and redness at the injection site, although these localized reactions are usually mild and of short duration.44
3.2. Polymeric Nanoparticles
Polymers represent a type of materials with facile synthesis, superior biocompatibility and biodegradability. Various polymeric nanoparticles have been developed for biomedical application including gene and drug deliveries.55–61 Synthetic polymers have the advantage of sustaining the release of the encapsulated therapeutic agent over a period of days to several weeks.37
3.2.1. Poly(lactic-co-glycolic acid)
Among various polymeric nanomaterials, poly(lactic-co-glycolic acid) (PLGA) is a copolymer that has been approved by the Food and Drug Administration (FDA) and widely used in biomedical applications.55 Desai et al. has demonstrated that PLGA nanoparticles containing encapsulated staphylococcal enterotoxin B toxoid showed strong adjuvant properties.37 In this study, biodegradable nanospheres in the range of 100–150 nm were formulated using PLGA (50:50). Immunization of animals with equal doses of toxoid, either using nanospheres or alum induced a comparable systemic immune response (IgG, IgM and IgA titers). The systemic immune response of animals injected with nanoparticles was comparable to that obtained following injection of alum. However, it reached a maximum at 7 weeks post-immunization, and then gradually declined with time. A booster dose of toxoid at 19 weeks induced a similar secondary immune response that was higher than the primary immune response.37 In addition, Cruz et al. determined the size effect of PLGA on delivering antigen to human dendritic cells in vitro.62 It is demonstrated that encapsulation of antigen resulted in almost 38% degradation for both NPs and micron-sized particles (MPs) 6 days after particle ingestion by DCs, compared to 94% when nonencapsulated and soluble antigen was used. In contrast to the MPs, which were taken up rather nonspecifically, the NPs effectively targeted human DCs. Consequently, targeted delivery improved antigen presentation of NPs and induced antigen-dependent T cell responses at 10–100 fold lower concentrations than non-targeted NPs. However, the potential shortcoming for PLGA as adjuvant is their short half-life because they are often rapidly degraded. On the one hand, this could be beneficial because it is relatively safe due to their biodegradability; on the other hand, the immune boosting effects are shorter without long term protection, which may require more boosting injections. Future work is needed to design PLGA-based adjuvants that have optimal balance between the two aspects.
3.2.2. Poly (γ-glutamic acid)
Poly(γ-glutamic acid) (γ-PGA) is a capsular exopolymer produced by bacteria. γ-PGA nanoparticles can be degraded by γ-glutamyl transpeptidase that is widely distributed in the human body. Nanoparticles composed of amphiphilic γ-PGA and hydrophobic amino acids can immobilize proteins, peptides, and chemicals onto their surfaces and/or encapsulate these substances into the particles.38 Wang et al. synthesized nanoparticles composed of g-PGA-graft-PAE by a precipitation and dialysis method. They demonstrated that γ-PGA nanoparticles are effective adjuvants that can support the induction of both HIV-1-specific humoral and cellular immune responses, which are needed for an effective anti-AIDS vaccine.38 Further mechanistic study showed that the production of inflammatory cytokines from macrophages and maturation of dendritic cells were impaired in MyD88 knockout and TLR4-deficient mice compared with their wild-types, when the cells were stimulated with γ-PGA NPs. The immunization of these KO mice with antigen-carrying γ-PGA NPs also results in diminished induction of antigen-specific cellular immune responses, suggesting the involvement of TLR4 and MyD88 signaling pathways.11 Similar to PLGA, γ-PGA nanoparticles may also be subjected to rapid clearance that makes it difficult to induce long term immune responses.
3.2.3. Chitosan
Chitosan is a type of naturally occurring polysaccharide. Due to its well recognized biocompatibility, low toxicity and degradability by human enzymes, it has been used as delivery vehicles for various biomolecules including peptides, proteins, antigens, genes and oligonucleotides.63, 64 Due to its superior properties, chitosan is also explored as vaccine adjuvant for enhanced immune responses, especially the Th1 response. Mori et al. found that when combined with TLR9 agonist CpG, the CpG-chitosan complex could induce NLRP3-dependent antigen specific Th1 and Thl7 responses.39 Study by Carroll et al. showed that when the chitosan was complexed with Hybrid-1, a fusion protein based on immunodominant antigens from Mycobacterium tuberculosis, it mediated enhanced antigen specific Th1 and IgG2C responses that were dependent on both enzyme cyclic-di- GMP-AMP synthase (cGAS) and stimulator of IFN genes (STING).40 Further mechanistic study demonstrated that the chitosan cationic polymer can actually engage the cGAS-STING DNA sensing pathway for the enhancement of cellular immunity.40 However, limited human studies are performed using chitosan thus far, and there are no chitosan-related products currently available on the market.64
3.2.4. Polyethyleneimine
Polyethyleneimine (PEI) is a type of cationic polymer that has been used as gene delivery reagents in vitro65 and DNA vaccine delivery in vivo.66 It is demonstrated that PEI have potent mucosal adjuvant activity for viral subunit glycoprotein antigens. A single intranasal administration of influenza hemagglutinin or herpes simplex virus type-2 (HSV-2) glycoprotein D with PEI elicited robust antibody-mediated protection from an otherwise lethal infection, and was superior to existing experimental mucosal adjuvants.7 It is found that linear PEI forms had similar potency as a mucosal adjuvant, cholera toxin subunit B (CTB), whereas branched PEI forms of 750 kD and 25 kD gave titers of antigen-specific mucosal IgA more than tenfold higher than those elicited by CTB. Detailed mechanistic study showed that PEI formed nanoscale complexes with antigen, which were taken up by antigen-presenting cells, promoted dendritic cell trafficking to draining lymph nodes and induced non-proinflammatory cytokine responses. PEI adjuvanticity required release of host double stranded DNA that triggered Irf3-dependent signaling pathway. Potential weakness of PEI is that high molecular PEI is toxic to cells and the mechanism involves plasma membrane damage and/or lysosomal damage by the proton-sponge effects.67, 68 Another weakness is that PEI with high molecular weight may bind to DNA tightly that could not be released to the cytosol, thus diminishing the effectiveness of DNA vaccines.67 So PEIs with optimal molecular weight that are safe and effective should be examined for adjuvant purposes.
3.2.5. pH-responsive polymer
pH-responsive, endosomolytic polymer nanoparticles were originally designed for small interfering RNA delivery.69 There is advantage to use these particles as adjuvant because after cellular uptake (DC), the endosomolytic property allows the rapid release of digested antigen oligopeptides for presentation outside the cells to boost immune responses. Recently, based on this principle, Wilson et al. developed micellar nanoparticles that were assembled from amphiphilic diblock copolymers. The particles were composed of an ampholytic core-forming block and a redesigned polycationic corona block doped with thiol-reactive pyridyl disulfide groups to enable dual-delivery of antigens and immunostimulatory CpG oligodeoxynucleotide (CpG ODN) adjuvants.70 Conjugation of OVA to nanoparticles significantly enhanced antigen cross-presentation in vitro relative to free OVA or unconjugated physical mixture of the parent compounds. Subcutaneous vaccination of mice with OVA-nanoparticle conjugates elicited a significantly higher CD8+ T cell response compared to mice vaccinated with free OVA or a physical mixture of the two components. Significantly, immunization with OVA- nanoparticle conjugates complexed with CpG ODN (dual-delivery) enhanced CD8+ T cell responses 7-, 18-, and 8-fold relative to immunization with conjugates, OVA administered with free CpG, or a formulation containing free OVA and CpG complexed to micelles, respectively. Similarly, dual-delivery carriers significantly increased Th1 responses and elicited a balanced IgG1/IgG2c antibody response. The pH responsiveness is an advantageous design that could be integrated into other adjuvant platforms. Recently, Tran et al. developed composition tunable polymer blend particles by mixing PLGA and a pH-responsive polymer (DMAEMA-co-PAA-co-BMA). It was demonstrated that polymer blend particles are able to deliver antigens into both class I and II antigen presentation pathways in vitro.41 By using a mouse and OVA as a model antigen, it is demonstrated that a significantly higher and sustained level of CD4+ and CD8+ T cell responses as well as comparable IgG production are elicited with polymer blend particles than PLGA particles and commercial vaccine adjuvant, Alum.41 Similar to PLGA, the stability or degradation rate of these polymers should be optimized to allow time to mount and maintain more effective immune responses.
All together, polymeric materials as vaccine adjuvants are capable of enhancing the vaccine potential against infectious diseases as well as cancers. The adjuvant activities of polymers depends on their solubility, molecular weight, degree of branching and conformation of the polymer.71 However, polymeric materials also present potential concerns as other materials do. While benefiting from their unique physicochemical properties, it is difficult to make a prediction on an empirical basis which adjuvant will work most effectively with particular antigens.72 Although most polymers exhibit good biocompatibility, safety is a major concern for cationic polymers. For example, PEI showed superior adjuvanticity,7 however, high molecular PEI could cause plasma membrane damage and/or lysosomal damage and lead to cytotoxicity.67, 68
3.3. Liposome
Liposomes are a type of material with spherical lipid bilayers ranging from 50 nm to 1000 nm in diameter. It serves as delivery vehicles for biomedical applications, including drug delivery, cancer treatment and vaccine.73 As vaccine adjuvants, various liposome formulations have been investigated, including DOTAP, DC-Chol, DDA, and DOTIM.42, 74 Although liposome itself has low immunostimulatory effects, it could increase the number of antigen presenting cells to the administration site75 and further enhance the cellular uptake of antigens.76 Commonly, various types of liposome is combined with other immunostimulating ligands, e.g., dsRNA, MPLA, CpG DNA, to generate more specialized and directed immune responses against specific diseases.74 The weakness for liposomes is their stability, and recent studies on liposomes with solid support such as mesoporous nanoparticles showed substantially prolonged stability that may be used for development of vaccine adjuvants.77
4. Immune Activation Mechanisms by ENMs
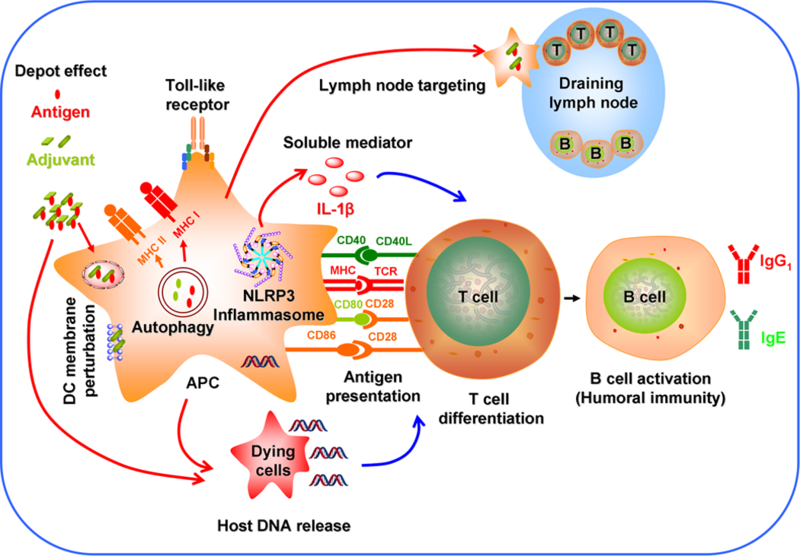
It is known that the properties of engineered nanomaterials (ENMs) play a major role in influencing the immune system shown as above.78 Thus, understanding the molecular mechanisms of immune activation is critical in rational design of ENMs for optimal and long lasting immuno-potentiating effects. Although nanomaterials themselves do not have specific immunostimulatory effect, they play an important role in directing immunity towards either a bacterial or viral defense pathway.79 Currently, the immunostimulatory activity of nanomaterials as adjuvants has been attributed to the following mechanisms:13, 80–82(1) depot effect that promotes the persistence, stability, and gradual release of antigens;83, 84 (2) activation of NLRP3 inflammasome;22, 31 (3) perturbation of dendritic cell membrane;85 (4) autophagic regulation; (5) delivery of antigens to the draining lymph nodes;86 (6) Toll-like receptor (TLR)-dependent signal transduction;11, 87 (7) repetitive antigen display in which the spatial organization of the antigens on the particle surface facilitates B cell receptor co-aggregation, triggering and activation;88 (8) T cell differentiation;89, 90 (9) antigen presentation in which exogenously acquired-antigens are processed into MHC class I or MHC class II pathways;41, 91 (10) host DNA release;92 and (11) release of soluble mediators such as cytokines, chemokines and immunomodulatory molecules that regulate the immune response (Figure 2).13
Figure 2.
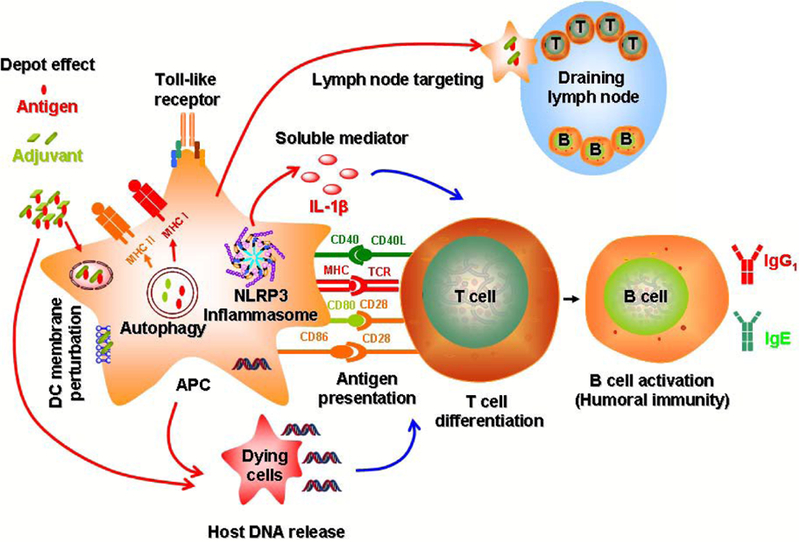
Mechanisms of immune system activation by engineered nanomaterials include (1) depot effect; (2) activation of NLRP3 inflammasome; (3) perturbation of dendritic cell membrane; (4) autophagic regulation; (5) delivery of antigens to the draining lymph nodes; (6) Toll-like receptor (TLR)-dependent signal transduction; (7) B cell activation; (8) T cell differentiation; (9) antigen presentation; (10) host DNA release; and (11) release of soluble mediators.
4.1. Depot effect
Adjuvants can act as depot for antigens, presenting the antigen over a long period of time and maximizing the immune response before the clearance of the antigen.83, 84 In 1931, Glenny et al. suggested that precipitation of antigens with aluminum salts reduced the rate of antigen elimination from the injection site.84 In the experiment, the injection sites were collected from guinea pigs 3 days after injection of alum-precipitated or soluble diphtheria toxoid. Then the injection sites were macerated and injected in naive guinea pigs. The recipients of the material from the aluminum-precipitated diphtheria toxoid injected animal developed immune response, but not other guinea pigs. Additionally, it is demonstrated that antigens adsorbed to cationic liposomes compared to neutral liposome -adsorbed antigens have better retention at the injection site.83 The longer retention time resulted in increased antigen presentation by antigen presenting cells, IFN-γ and IL-17 production, and higher differentiated population of antigen specific T-cells. The cationic liposomes are retained for a longer period of time than neutral liposomes at the site of injection.83 However, the depot effect was challenged by Holt et al. who showed that excision of the injection site after 7 or more days post injection of the aluminum- precipitated diphtheria toxoid, did not interfere with the development of a humoral immune response to diphtheria toxoid.93
4.2. NLRP3 inflammasome activation
The NLRP3 inflammasome is an intracellular protein complex that is assembled and activated upon various stimuli in macrophages and DCs.22 Recently, it was shown that aluminum salts (Alum) could activate the NLRP3 inflammasome and IL-1β production in macrophages, which could explain its ability to induce local inflammation, recruitment of APCs, enhanced antigen uptake, dendritic cell maturation, and stimulation of T-cell activation and T-cell differentiation.22, 89, 94–100 It is noted that long aspect ratio (LAR) ENMs (e.g., nanowires and carbon nanotubes) trigger activation of the inflammasome secondary to shape-dependent and oxidative stress effects at lysosomal level.24, 26, 101 Using NLRP3 knockout mice, Eisenbarth et al. showed that Alum failed to boost OVA- specific antibody responses in NLRP3, ASC, and caspase-1 knockout mice.89 Similarly, Kool et al. showed that the collection of Alum-induced inflammatory cells in the peritoneal cavity is decreased in NLRP3 deficient mice, supporting the role of the NLRP3 inflammasome in the induction of adjuvant effects.102 Sun et al. shows excellent correlation between NLRP3 activation at cellular level and the generation of in vivo adjuvant effects.31 Although it is generally agreed that Alum is capable of inducing NLRP3 inflammasome activation at the cellular level, there is some disagreement about the necessity of this pro-inflammatory response pathway in generating adjuvant effects in vivo.89, 102–105 Franchi et al. showed that the NLRP3 inflammasome was not required for the induction of an antigen-specific antibody response during immunization with Alum.103 Moreover, Kool et al. in another study demonstrated that while NLRP3 deficient mice were partially defective at priming antigen-specific T cells, these animals mounted a normal OVA-specific IgG1 response.104 Due to the existing conflicting reports, further research is needed to clarify the role of NLRP3 inflammasome in initiation of immunity by adjuvants in vivo.
4.3. Perturbation of dendritic cell membrane
Flach et al. reported, independent of inflammasome and membrane proteins, Alum could bind to dendritic cell (DC) membrane lipids with substantial force.85 Although Alum does not have a specific receptor on the DC surface, it can directly engages lipids in the plasma membrane of DCs, leading to lipid sorting similar to monosodium urate (MSU) crystals that involves the aggregation of immunoreceptor signaling motif (ITAM)- containing receptors, and subsequent spleen tyrosine kinase (Syk)- and phosphoinositide 3-kinase (PI3K)-mediated phagocytic responses.85 However, Alum does not enter the cells, it instead delivers the soluble antigen across the plasma membrane. DCs engaged by Alum develop a strong affinity for CD4+ T cells. In contrast, another independent study by Mold et al. identified intracellular aluminum adjuvant in THP-1 cells,106 consistent with the study by Sun et al.31 The discrepancy among these studies, especially on the cellular uptake, may come from the type of cell lines chosen, thus further detailed studies are needed to clarify the role of DC membranes in immunostimulation.
4.4. Autophagic regulation
Autophagic regulation plays a role in the maintenance of cellular homeostasis under normal conditions and during cellular stress.107, 108 In innate immunity, autophagy is responsible for delivering antigens endosomal toll-like receptors109 and clearance of damaged mitochondria and ROS.110 It has been identified as a pathway to deliver cytoplasmic and nuclear antigens to MHC class II molecules for presentation to CD4+ T cells.107 It has also been implicated in MHC class I cross-presentation of tumor antigen and the activation of CD8+ T cells.107 Recent study by Li et al. indicates that alpha- alumina (α-Al2O3) nanoparticles are capable of delivering tumor antigens to the autophagosome-related cross-presentation pathway in dendritic cells. As a result, immunization of mice with a-A1203 nanoparticles conjugated to either a model tumor antigen or autophagosomes derived from tumor cells could lead tumor regression.111 In addition, autophagy also play a role in NLRP3 inflammasome activation,108 which would impact the adjuvant effects by nanoparticles.
4.5. Lymph node targeting
Lymph nodes are major sites of B cells, T cells and other immune cells. They are important for the proper function of the immune system, acting as filters for foreign particles and cancer cells.112–114 Reddy et al. showed that nanoparticles can be used as a vaccine platform by targeting lymph node-residing dendritic cells via interstitial flow and activating these cells.86 Following intradermal injection, interstitial flow could transport 25 nm nanoparticles efficiently into lymphatic capillaries and their draining lymph nodes, targeting nearly half of the lymph node-residing dendritic cells, whereas 100 nm nanoparticles were only 10% as efficient. Hanson et al. demonstrated that Cyclic dinucleotides encapsulated within PEGylated lipid nanoparticles have the potential to target lymph nodes and further promote both strong antigen-specific T cell priming and high antibody titers.115 Additionally, Liu et al. demonstrated that administration of structurally optimized CpG-DNA/peptide vaccines in mice resulted in marked increases in lymph node accumulation, T-cell priming and enhanced anti-tumor efficacy.116 Thus, lymph nodes targeting should be considered as a factor in adjuvant design.
4.6. Toll-like receptor (TLR) signaling
Toll-like receptors (TLRs) are a class of proteins that play a critical role in the innate immune system. They are single, membrane-spanning, non-catalytic receptors expressed on macrophages and dendritic cells that recognize structurally conserved molecules derived from microbes.117 Stimulation of various TLRs could induce distinct patterns of gene expression that leads to the activation of innate immunity and instructs the development of antigen-specific acquired immunity.117 Thus far, thirteen different TLRs have been identified in humans and mice. Uto et al. described that biodegradable nanoparticles (NPs) elaborated with poly(γ-glutamic acid) (γ-PGA) are able to induce potent innate and adaptive immune responses through TLR4 signaling pathway.11 The production of inflammatory cytokines from macrophages and the maturation of dendritic cells were impaired in TLR4-deficient mice compared with their wild-types, when the cells were stimulated with γ-PGA NPs. Chen et al. demonstrated that the size of nanoparticles that carries the CpG oligodeoxynucleotides (ODN) plays an important role in TLR9 activation.118 The size of materials affects their ability to regulate endosomal pH, thus the TLR9-mediated differential cytokine productions that regulate both innate and adaptive immunity.118 In short, selection of optimal TLR agonists and combine that with nanoparticles could enhance the specific adjuvant effects.
4.7. B cell activation
B cells are a type of lymphocyte in the development of humoral immunity. The primary functions of B cells are to synthesize antibodies in response to antigens, to perform the role of antigen-presenting cells and to develop memory B cells after activation by antigen interaction. B cells could also release cytokines that are used for regulating immune functions.119 Study shows that antigen binding to the B cell receptor (BCR) could induce receptor clustering, cell spreading, and the formation of signaling microclusters, triggering B cell activation.120 Temchura et al. demonstrated that calcium phosphate nanoparticles could be preferentially bound and internalized by antigen-specific B- cells.121 Co-cultivation of antigen-specific B-cells with the Hen Egg Lysozyme functionalized calcium phosphate nanoparticles also increases surface expression of B- cell activation markers. Further, functionalized nanoparticles are able to effectively cross-link B-cell receptors at the surface of antigen-matched B-cells and were 100-fold more efficient in the activation of B-cells than soluble antigens.121
4.8. T cell differentiation
T cells are another type of lymphocyte that plays an important role in cell-mediated immunity. The protective effects of vaccine depend on both the quantity and quality of memory T cells.122 Studies have demonstrated that the most effective activators of T cells are mature dendritic cells.122 The T cell-dendritic cell interaction usually requires three signals.123 The first signal is provided by processed antigenic peptides bound to MHC molecules recognized by the T cell receptors (TCRs), and the second signal by the binding of costimulatory molecules to their ligands on the T cells as well as the third signal that is provided by cytokines and instructs the differentiation of T cells into Th1 or Th2 effector cells. Sokolovska et al. found that aluminum-containing adjuvants activate DCs and influence their ability to direct Th1 and Th2 responses.90 It is demonstrated that aluminum adjuvants could directly enhance the antigen presentation to T cells, the expression of costimulatory molecule CD86 and the production of IL-1β and IL-18 by dendritic cells.90 These results demonstrate that aluminum-containing adjuvants are not just delivery vehicles for antigens, but directly activate DCs to effectively initiate immune responses and influence the ability of DCs to direct Th1 and Th2 responses.
4.9. Antigen presentation
Dendritic cells (DC) are highly specialized antigen presenting cells that could take up exogenous material from the extracellular environment for presentation in the context of MHC molecules, including MHC I and MHC II. Among these, MHC II molecules are primarily express by professional antigen presenting cells, e.g., dendritic cells, macrophages and B cells.124 Cytosolic endogenous proteins can be presented by MHC class II molecules. McKee et al. showed that aluminum salts could introduce host DNA into the cytoplasm of dendritic cells, where it engages receptors that promote MHC class II presentation and better dendritic cell-T cell interactions.92 In addition to presentation of antigenic epitopes on MHC class II molecules to CD4+ T cells, DCs can also shuttle antigen to the MHC class I processing pathway for CD8+ T cell activation, a process termed cross-presentation.125 This enables DCs that have engulfed tumor antigen to activate antigen-specific CD8+ T cells capable of tumor cell killing. Cross presentation present antigens without using the endogenous proteasomal processing pathway used by the MHC II molecule processing. Schnurr et al. showed that antigen formulation determines antigen processing.91 It is demonstrated that ISCOMATRIX adjuvant (a particulate adjuvant comprising saponin, cholesterol and phospholipid) induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II.
4.10. Host DNA release
Either non-self nucleic acids from invading microbes or self nucleic acids left by incomplete clearance during cell damage have been shown to evoke innate immune responses.126, 127 Those nucleic acids can engage intracellular DNA sensors, including toll-like receptor 9 (TLR9),128, 129 DNA-dependent activator of interferon-regulatory factors (DAI)130, 131 and AIM2 inflammasome.132, 133 The engagement of DNA sensors initiates a cascade of signaling pathways, resulting in secretions of proinflammatory and inflammatory cytokines. Sun et al. showed that DNA delivered by hydroxylapatite biominerals activated DAI and AIM2 inflammasomes and mediated production of pro- inflammatory cytokines.134 In addition, after injection, host DNA could rapidly coat injected adjuvants. McKee et al. found that Alum could act as an adjuvant by introducing host DNA into the cytoplasm of antigen-bearing dendritic cells, where it engages receptors that promote MHC class II presentation and stronger DC-T cell interactions.92 On the same note, DNase coinjection could reduce CD4+ T cell priming.
4.11. Soluble mediators
Soluble mediators such as cytokine, chemokine and immunomodulatory molecules could influence innate and adaptive immune function and T cell polarization.135 For example, IL-1β is a pro-inflammatory cytokine that has potentiating effects on function of many innate and specific immunocompetent cells and may mediate inflammatory diseases by initiating immune and inflammatory responses.136–139 Ben-Sasson et al. showed that IL-1β could cause a marked increase in the degree of expansion of naive and memory CD4+ T cells in response to antigen challenge, it also increases the proportion of cytokine- producing transgenic CD4+ T cells, especially IL-17- and IL-4-producing cells, strikingly increases serum IgE levels and serum IgG1 levels.140 This study indicates that IL-1β signaling in T cells markedly induces robust and durable primary and secondary CD4+ T cell responses. Another example is IL-10. IL-10 is a cytokine secreted by Treg cells that are associated with immune suppression, and it is considered as a “cytokines synthesis inhibitor factor”.141 It has been shown that DC-based vaccines that induce suppression of IL-10-producting Treg exhibited enhanced efficacy,142 and inhibition of IL-10 could enhance the magnitude of CD4+ T cell immunity and protection following vaccination.143
In summary, various mechanisms are involved in the immunostimulatory activity of nanoscale materials. An effective adjuvant will likely engage multiple pathways and there is no one-size-fits-all model can be derived. Understanding of the detailed mechanisms of these processes could significantly advance the progress of nanomaterial-based immunotherapy for both infectious diseases and cancers.
5. Challenges in Development of ENM-based Adjuvants
The development of nanomaterial-based vaccine adjuvants is faced with several challenges. Though ENM-based adjuvants have been shown to be effective in potentiating immune responses, the physicochemical properties that make them attractive could also potentially generate toxicity, so safety is a major concern.144 In addition, the exact molecular and immunological mechanisms involved in the adjuvant potentiation remain to be elucidated, and some experimental results are even contradictory, e.g., the involvement of NLRP3 inflammasomes and perturbation of dendritic cell membrane in Alum-induced adjuvant effects. Furthermore, the ENM formulation that works for one antigen may not work for another because of the differential immune potentiating mechanisms. Thus, further work needs to be done to design safer and effective ENM- based adjuvants for specific antigens.
In addition, cancers are still one of the leading causes of morbidity and mortality worldwide. In 2012, there are 14 million new cases and 8.2 million cancer-related deaths, and the new cases are predicted to rise by 70% over the next two decades.145 Thus, effective preventative and therapeutic treatment are highly in demand. Beyond traditional treatments including surgery, chemotherapy, and radiation therapy, vaccination for cancer prevention and treatment have been drawing more and more attentions. Firstly, vaccination can prevent diseases that lead to cancers. For example, the hepatitis B vaccine is 95% effective in preventing the development of chronic disease and liver cancer;5 Gardasil, a human papillomavirus (HPV) vaccine, can protect against two types of HPV that cause 70% of cervical cancer, 70% of vaginal cancer and 50% of vulvar cancer.6 Furthermore, vaccination can be used for cancer treatment. Thus far, the US FDA has approved immunotherapies including sipuleucel-T (Provenge) for metastatic prostate cancer, talimogene laherparepvec (T-VEC) for metastatic melanoma,146 and checkpoint inhibitors targeting PD-1 (Tecentriq) and CTLA-4 (Yervoy) for bladder cancer and metastatic melanoma, respectively.147, 148 Among these immunotherapies, cytokines and growth factors, e.g., interleukin 2 (IL-2), and granulocyte-macrophage colony-stimulating factor (GM-CSF) are commonly used as adjuvants in vaccines to augment the immune response.146 Currently, with the advancement of nanotechnology, nanomaterials with immunostimulatory properties are either served as adjuvants or carrier to deliver adjuvants or checkpoint inhibitors to augment the immune responses targeting cancer cells.111, 149, 150 Although such studies are in pre-mature stage, many promising nanomaterials are under pre-clinical and clinical investigations.151, 152 As we’re gaining more understanding on the acting mechanisms of nanomaterial-based adjuvants, therapeutic cancer vaccines with nano scale adjuvants could be developed to benefit the cancer patients.
6. Conclusions and Perspectives
The usage of engineered nanomaterials that possess unique physicochemical properties in adjuvants enables researchers to potentially achieve improved protection against infectious diseases. Currently, there are many nanomaterial-based adjuvants available or in development that have been shown to be effective to potentiate immune responses induced by a variety of antigens. However, there are some challenges on safety and understanding of the molecular mechanisms, which need to be addressed. Through a systemic approach including engineered design to control the properties and further exploration on the mechanisms of action using in vitro and in vivo approaches will answer these challenges to develop better ENM based adjuvants.
A new trend on adjuvant design is also emerging that could be beneficial for the development of ENM-based adjuvants. Recent studies show that when a combination of nanomaterial adjuvants and additional specific immunostimulatory substances, e.g., TLR agonists, they can specifically activate Th1 and/or Th2 responses. For example, triggering of TLR4, 7 could stimulate the production of cytokines/chemokines (TNF-α, IL-2, IL-12 and IL-6) and type I interferons (IFNs) that promote the immune system to activate Th1 responses to eliminate exogenous influenza viruses.153 Clinical trials suggest that TLR ligands can be safe and effective as vaccine adjuvants, and there are vaccines already licensed in the US and Europe containing such ligands.154 Combinational adjuvant, such as AS04, is able to elicit multiple protective immune responses. Thus, combining nanomaterial-based vaccine adjuvants with these novel TLR ligands that modulate the innate immunity could generate synergistic effects to significantly benefit development of more effective vaccine adjuvants. At the same time, this may reduce the amount of antigen and/or the number of immunizations needed. These efforts will facilitate the development of safe and more effective nanomaterial-based adjuvants to combat infectious diseases and cancer. Additionally, ENM-based adjuvants could be helpful for the development of therapeutic vaccines for cancers as well as diagnostic purpose for autoimmune diseases, which are under intense research and investigation.155–158
Supplementary Material
Acknowledgements:
This work was primarily supported by the US National Institute of Environmental Health Sciences Grants, R01 ESO16746, R01 ES022698, and U01 ES027237, and leveraged support from the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number DBI 0830117 and 1266377. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily represent the views of the National Science Foundation, the Environmental Protection Agency or the National Institute of Health.
References
- 1.Pulendran and R Ahmed, Cell, 2006, 124, 849–863. [DOI] [PubMed] [Google Scholar]
- 2.Ogra PL, Faden H and Welliver RC, Clin. Microbiol. Rev, 2001, 14, 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitney CG, Farley ΜM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A and Active Bacterial Core S, N. Engl. J. Med, 2003, 348, 1737–1746. [DOI] [PubMed] [Google Scholar]
- 4.Rappuoli R, Mandl CW, Black S and De Gregorio E, Nat. Rev. Immunol, 2011, 11, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The WHO, http://www.who.int/mediacentre/factsheets/fs204/enA/, (accessed July, 2016).
- 6.Merck Sharp & Dohme Corp., http://www.gardasil.comA/, (accessed July, 2016).
- 7.Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho L-P, Lambe T, Puthia M, Svanborg C, Scherer EM, Krashias G, Williams A, Blattman JN, Greenberg PD, Flavell RA, Moghaddam AE, Sheppard NC and Sattentau QJ, Nat. Biotechnol, 2012, 30, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayers S, Ulysse G, Xiang Z and He Y, J. Biomed. Biotechnol, 2012, 831486, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peek LJ, Middaugh CR and Berkland C, Adv Drug Deliv Rev, 2008, 60, 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moser C, Muller M, Kaeser MD, Weydemann U and Amacker M, Expert Rev Vaccines, 2013, 12, 779–791. [DOI] [PubMed] [Google Scholar]
- 11.Uto T, Akagi T, Yoshinaga K, Toyama M, Akashi M and Baba M, Biomaterials, 2011, 32, 5206–5212. [DOI] [PubMed] [Google Scholar]
- 12.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y and Santamaria P, ACS Nano, 2015, 9, 16–30. [DOI] [PubMed] [Google Scholar]
- 13.Smith DM, Simon JK and Baker JR Jr, Nat. Rev. Immunol, 2013, 13, 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikoba U, Peng H, Li H, Miller C, Yu C and Wang Q, Nanoscale, 2015, 7, 4291–4305. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Wang S, Wang Y, Wang X, Wang Q and Chen M, Biotechnol. Adv, 2014, 32, 1301–1316. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Li M, Sun S, Li B, Du D, Sun J, Cao F, Li H, Jia F, Wang T, Chang N, Yu H, Wang Q and Peng H, Biomaterials, 2014, 35, 3697–3707. [DOI] [PubMed] [Google Scholar]
- 17.Ding F, Deng H, Du Y, Shi X and Wang Q, Nanoscale, 2014, 6, 9477–9493. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TX, Huang L, Gauthier M, Yang G and Wang Q, Nanomedicine, 2016, 11, 1169–1185. [DOI] [PubMed] [Google Scholar]
- 19.Li ΜH, Yu H, Wang TF, Chang ND, Zhang JQ, Du D, Liu MF, Sun SL, Wang R, Tao HQ, Geng SL, Shen ZY, Wang Q and Peng HS, Journal of Materials Chemistry B, 2014, 2, 1619–1625. [DOI] [PubMed] [Google Scholar]
- 20.Lipomi DJ, Vosgueritchian M, Tee BCK, Hellstrom SL, Lee JA, Fox CH and Bao Z, Nature Nanotechnology, 2011, 6, 788–792. [DOI] [PubMed] [Google Scholar]
- 21.Gao XH, Cui YY, Levenson RM, Chung LWK and Nie SM, Nat. Biotechnol, 2004, 22, 969–976. [DOI] [PubMed] [Google Scholar]
- 22.Sun B, Wang X, Ji Z, Li R and Xia T, Small, 2013, 9, 1595–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nel AE, Maedler L, Velegol D, Xia T, Hoek EΜV, Somasundaran P, Klaessig F, Castranova V and Thompson M, Nat. Mater, 2009, 8, 543–557. [DOI] [PubMed] [Google Scholar]
- 24.Ji Z, Wang X, Zhang H, Lin S, Meng H, Sun B, George S, Xia T, Nel A and Zink J, ACS Nano, 2012, 6, 5366–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, Eisenbarth SC and Flavell RA, Proc. Natl. Acad. Sci. U. S. A, 2011, 108, 14867–14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Xia T, Duch M, Ji Z, Zhang H, Li R, Sun B, Lin S, Meng H, Liao Y-P, Wang M, Song T-B, Yang Y, Hersam M and Nel A, Nano Lett, 2012, 12, 3050–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Petrilli V, Tschopp J, O’Neill LAJ and Lavelle EC, Proc. Natl. Acad. Sci. U. S. A, 2009, 106, 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishige T, Yoshioka Y, Inakura H, Tanabe A, Yao X, Narimatsu S, Monobe Y, Imazawa T, Tsunoda S.-i., Tsutsumi Y, Mukai Y, Okada N and Nakagawa S, Biomaterials, 2010, 31, 6833–6842. [DOI] [PubMed] [Google Scholar]
- 29.Yang E-J, Kim S, Kim JS and Choi I-H, Biomaterials, 2012, 33, 6858–6867. [DOI] [PubMed] [Google Scholar]
- 30.Lunov O, Syrovets T, Loos C, Nienhaus GU, Mailӓnder V, Landfester K, Rouis M and Simmet T, Acs Nano, 2011, 5, 9648–9657. [DOI] [PubMed] [Google Scholar]
- 31.Sun B, Ji Z, Liao Y-P, Wang M, Wang X, Dong J, Chang CH, Li R, Zhang H, Nel AE and Xia T, ACS Nano, 2013, 7, 10834–10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Aldayel AM and Cui Z, J. Controlled Release, 2014, 173, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, Ijiro K and Sawa H, Acs Nano, 2013, 7, 3926–3938. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Tang H, Liu JH, Wang H and Liu Y, Toxicol. Lett, 2013, 219, 42–48. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G and Mooney DJ, Nat Biotech, 2015, 33, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li WA, Lu BY, Gu L, Choi Y, Kim J and Mooney DJ, Biomaterials, 2016, 83, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai ΜP, Hilfinger JM, Amidon GL, Levy RJ and Labhasetwar V, J. Microencapsul, 2000, 17, 215–225. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Uto T, Akagi T, Akashi M and Baba M, J. Med. Virol, 2008, 80, 11–19. [DOI] [PubMed] [Google Scholar]
- 39.Mori A, Oleszycka E, Sharp FA, Coleman M, Ozasa Y, Singh M, O’Hagan DT, Tajber L, Corrigan ΟI, McNeela EA and Lavelle EC, Eur. J. Immunol, 2012, 42, 2709–2719. [DOI] [PubMed] [Google Scholar]
- 40.Carroll EC, Jin L, Mori A, Muñoz-Wolf N, Oleszycka E, Hannah BT Moran S Mansou Craig P. McEntee E Lambe Else M. Agger P Andersen C Cunningham P Hertzog Katherine A. Fitzgerald A Bowie G and Lavelle Ed C., Immunity, 2016, 44, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran KK, Zhan X and Shen H, Advanced Healthcare Materials, 2014, 3, 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klinguer-Hamour C, Libon C, Plotnicky-Gilquin H, Bussat M-C, Revy L, Nguyen T, Bonnefoy J-Y, Corvaia N and Beck A, Vaccine, 2002, 20, 2743–2751. [DOI] [PubMed] [Google Scholar]
- 43.Baylor NW, Egan W and Richman P, Vaccine, 2002, 20, S18–S23. [DOI] [PubMed] [Google Scholar]
- 44.HogenEsch H, Front. Immunol, 2013, 3, 1–13. [Google Scholar]
- 45.Sun B, Ji Z and Xia T, Aluminum-Based Nano-adjuvants, Springer Science+Business Media Dordrecht, 2014. [Google Scholar]
- 46.Hem SL and HogenEsch H, Expert Rev. Vaccines, 2007, 6, 685–698. [DOI] [PubMed] [Google Scholar]
- 47.The CDC, http://www.cdc.gov/vaccines/vac-gen/additives.htm. (accessed July, 2016).
- 48.Wang T, Zhen Y, Ma X, Wei B and Wang N, ACS Applied Materials & Interfaces, 2015, DOI: 10.1021/acsami.5b00348. [DOI] [PubMed] [Google Scholar]
- 49.Boisselier E and Astruc D, Chemical Society Reviews, 2009, 38, 1759–1782. [DOI] [PubMed] [Google Scholar]
- 50.Dykman L and Khlebtsov N, Chemical Society Reviews, 2012, 41, 2256–2282. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Ji Z, Chang CH, Zhang H, Wang M, Liao Y-P, Lin S, Meng H, Li R, Sun B, Winkle LV, Pinkerton KE, Zink JI, Xia T and Nel AE, Small, 2014, 10, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.George S, Lin S, Jo Z, Thomas CR, Li L, Mecklenburg M, Meng H, Wang X, Zhang H, Xia T, Hohman JN, Lin S, Zink J, Weiss PS and Nel AE, Acs Nano, 2012, 6, 3745–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallet-Regi M, Balas F and Arcos D, Angewandte Chemie-InternationalEdition, 2007, 46, 7548–7558. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann F, Cornelius M, Morell J and Froba M, Angew. Chem. Int. Ed. Engl, 2006, 45, 3216–3251. [DOI] [PubMed] [Google Scholar]
- 55.Langer R and Tirrell DA, Nature, 2004, 428, 487–492. [DOI] [PubMed] [Google Scholar]
- 56.Kataoka K, Harada A and Nagasaki Y, Adv. Drug Del. Rev, 2001, 47, 113–131. [DOI] [PubMed] [Google Scholar]
- 57.Moghimi SM, Hunter AC and Murray JC, Pharmacol. Rev, 2001, 53, 283–318. [PubMed] [Google Scholar]
- 58.Jain RA, Biomaterials, 2000, 21, 2475–2490. [DOI] [PubMed] [Google Scholar]
- 59.Vila A, Sanchez A, Tobio M, Calvo P and Alonso MJ, J. Controlled Release, 2002, 78, 15–24. [DOI] [PubMed] [Google Scholar]
- 60.Peng H, Liu X, Wang G, Li M, Bratlie KM, Cochran E and Wang Q, Journal of Materials Chemistry B, 2015, 3, 6856–6870. [DOI] [PubMed] [Google Scholar]
- 61.Wang Q, Cheng H, Peng H, Zhou H, Li PY and Langer R, Adv. Drug Del. Rev, 2015, 91, 125–140. [DOI] [PubMed] [Google Scholar]
- 62.Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, Torensma R and Figdor CG, J. Controlled Release, 2010, 144, 118–126. [DOI] [PubMed] [Google Scholar]
- 63.Bhattarai N, Gunn J and Zhang M, Adv. Drug Del. Rev, 2010, 62, 83–99. [DOI] [PubMed] [Google Scholar]
- 64.Bemkop-Schniirch A and Dünnhaupt S, Eur. J. Pharm. Biopharm, 2012, 81, 463–469. [DOI] [PubMed] [Google Scholar]
- 65.Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M and Wagner E, J. Gene Med, 2001, 3, 362–372. [DOI] [PubMed] [Google Scholar]
- 66.Torrieri-Dramard L, Lambrecht B, Ferreira HL, Van den Berg T, Klatzmann D and Bellier B, Mol. Ther, 2011, 19, 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI and Nel AE, Acs Nano, 2009, 3, 3273–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Xia T, Meng H, Xue M, George S, Ji Z, Wang X, Liu R, Wang M, France B, Rallo R, Damoiseaux R, Cohen Y, Bradley KA, Zink JI and Nel AE, Acs Nano, 2011, 5, 2756–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Convertine AJ, Benoit DS, Duvall CL, Hoffman AS and Stayton PS, J Control Release, 2009, 133, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A and Stayton PS, ACS Nano, 2013, 7, 3912–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shakya AK and Nandakumar KS, Journal of the Royal Society Interface, 2013, 10, 20120536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohan T, Verma P and Rao DN, The Indian Journal of Medical Research, 2013, 138, 779–795. [PMC free article] [PubMed] [Google Scholar]
- 73.Banerjee R, Biomater J. Appl, 2001, 16, 3–21. [DOI] [PubMed] [Google Scholar]
- 74.Christensen D, Korsholm KS, Andersen P and Agger EM, Expert Rev. Vaccines, 2011, 10, 513–521. [DOI] [PubMed] [Google Scholar]
- 75.Cremel M, Hamzeh-Cognasse H, Genin C and Delezay O, Vaccine, 2006, 24, 5744–5754. [DOI] [PubMed] [Google Scholar]
- 76.Smith Korsholm K, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C and Andersen P, Immunology, 2007, 121, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X, Situ A, Kang Y, Villabroza KR, Liao Y, Chang CH, Donahue T, Nel AE and Meng H, ACS Nano, 2016, 10, 2702–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Archibald DD and Mann S, Nature, 1993, 364, 430–433. [Google Scholar]
- 79.Leleux J and Roy K, Advanced Healthcare Materials, 2013, 2, 72–94. [DOI] [PubMed] [Google Scholar]
- 80.Silva JM, Videira M, Gaspar R, Preat V and Florindo HF, J. Controlled Release, 2013, 168, 179–199. [DOI] [PubMed] [Google Scholar]
- 81.Kanapathipillai M, Brock A and Ingber DE, Adv. Drug Del. Rev, 2014, 79–80, 107–118. [DOI] [PubMed] [Google Scholar]
- 82.Yue H and Ma G, Vaccine, 2015, 33, 5927–5936. [DOI] [PubMed] [Google Scholar]
- 83.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P and Perrie Y, J. Controlled Release, 2010, 145, 102–108. [DOI] [PubMed] [Google Scholar]
- 84.Glenny AT, Buttle GAH and Stevens MF, J. Pathol. Bacteriol, 1931, 34, 267–275. [Google Scholar]
- 85.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ΜE, Vilaysane A, Mucsi AD, Fong Y, Prenner E, Ling CC, Tschopp J, Muruve DA, Amrein MW and Shi Y, Nat. Med, 2011, 17, 479–487. [DOI] [PubMed] [Google Scholar]
- 86.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA and Hubbell JA, Nat Biotech, 2007, 25, 1159–1164. [DOI] [PubMed] [Google Scholar]
- 87.Ishii KJ and Akira S, J. Clin. Immunol, 2007, 27, 363–371. [DOI] [PubMed] [Google Scholar]
- 88.Kaba SA, McCoy ΜE, Doll TAPF, Brando C, Guo Q, Dasgupta D, Yang Y, Mittelholzer C, Spaccapelo R, Crisanti A, Burkhard P and Lanar DE, PLoS ONE, 2012, 7, e48304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisenbarth SC, Colegio OR, O’Connor W Jr., Sutterwala FS and Flavell RA, Nature, 2008, 453, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sokolovska A, Hem SL and HogenEsch H, Vaccine, 2007, 25, 4575–4585. [DOI] [PubMed] [Google Scholar]
- 91.Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, Cebon J, Maraskovsky E and Endres S, J. Immunol, 2009, 182, 1253–1259. [DOI] [PubMed] [Google Scholar]
- 92.McKee AS, Burchill MA, Munks MW, Jin L, Kappler JW, Friedman RS, Jacobelli J and Marrack P, Proc. Natl. Acad. Sci. U. S. A, 2013, 110, E1122–E1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holt L, Developments in Diphtheria Prophylaxis, London: Heinemann, 1950. [Google Scholar]
- 94.Martinon F, Mayor A and Tschopp J, in Annu. Rev. Immunol, 2009, vol. 27, pp. 229–265. [DOI] [PubMed] [Google Scholar]
- 95.Schroder K and Tschopp J, Cell, 2010, 140, 821–832. [DOI] [PubMed] [Google Scholar]
- 96.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ and Ting JPY, Immunity, 2009, 30, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose- Girma M, Lee WP, Weinrauch Y, Monack DM and Dixit VM, Nature, 2006, 440, 228–232. [DOI] [PubMed] [Google Scholar]
- 98.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA and Sutterwala FS, Proc. Natl. Acad. Sci. U. S. A, 2008, 105, 9035–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ and Golenbock DT, Nat. Immunol, 2008, 9, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KHG, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC and O’Neill LAJ, Nat. Immunol, 2010, 11, 897–U1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamilton RF Jr., Wu N, Porter D, Buford M, Wolfarth M and Holian A,Part. Fibre. Toxicol, 2009, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN and Tschopp J, J. Immunol, 2008, 181, 3755–3759. [DOI] [PubMed] [Google Scholar]
- 103.Franchi L and Nunez G, Eur. J. Immunol, 2008, 38, 2085–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN and Tschopp J, J. Immunol, 2008, 181, 3755–3759. [DOI] [PubMed] [Google Scholar]
- 105.McKee AS, Munks MW, MacLeod ΜKL, Fleenor CJ, Van Rooijen N, Kappler JW and Marrack P, J. Immunol, 2009, 183, 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mold M, Eriksson H, Siesjo P, Darabi A, Shardlow E and Exley C, Sci. Rep, 2014, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crotzer VL and Blum JS, J. Immunol, 2009, 182, 3335–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li R, Ji Z, Qin H, Kang X, Sun B, Wang M, Chang CH, Wang X, Zhang H, Zou H, Nel AE and Xia T, ACS Nano, 2014, 8, 10280–10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yordy B, Tal MC, Hayashi K, Arojo O and Iwasaki A, Int. Immunol, 2013, 25, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filomeni G, De Zio D and Cecconi F, Cell Death Differ, 2015, 22, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li H, Li Y, Jiao J and Hu H-M, Nature Nanotechnology, 2011, 6, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farokhzad OC and Langer R, ACS Nano, 2009, 3, 16–20. [DOI] [PubMed] [Google Scholar]
- 113.Bachmann MF and Jennings GT, Nat. Rev. Immunol, 2010, 10, 787–796. [DOI] [PubMed] [Google Scholar]
- 114.Dobrovolskaia MA, Aggarwal P, Hall JB and McNeil SE, Mol Pharm, 2008, 5, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanson MC, Crespo ΜP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo ΜB, Mueller S and Irvine DJ, J Clin Invest, 2015, 125, 2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C and Irvine DJ, Nature, 2014, 507, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Akira S and Takeda K, Nature Reviews Immunology, 2004, 4, 499–511. [DOI] [PubMed] [Google Scholar]
- 118.Chen HC, Sun B, Iran KK and Shen H, Biomaterials, 2011, 32, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Banchereau J and Steinman RM, Nature, 1998, 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 120.Ketchum C, Miller H, Song W and Upadhyaya A, Biophys. J, 2014, 106, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Temchura VV, Kozlova D, Sokolova V, Uberla K and Epple M, Biomaterials, 2014, 35, 6098–6105. [DOI] [PubMed] [Google Scholar]
- 122.Kaech SM, Wherry EJ and Ahmed R, Nat. Rev. Immunol, 2002, 2, 251–262. [DOI] [PubMed] [Google Scholar]
- 123.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YT, Pulendran B and Palucka K, Annu. Rev. Immunol, 2000, 18, 767–811. [DOI] [PubMed] [Google Scholar]
- 124.Neefjes J, Jongsma MLM, Paul P and Bakke O, Nat. Rev. Immunol, 2011, 11, 823–836. [DOI] [PubMed] [Google Scholar]
- 125.Villadangos JA and Schnorrer P, Nat. Rev. Immunol, 2007, 7, 543–555. [DOI] [PubMed] [Google Scholar]
- 126.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O and Akira S, Nature Immunology, 2006, 7, 40–48. [DOI] [PubMed] [Google Scholar]
- 127.Yoshida H, Okabe Y, Kawane K, Fukuyama H and Nagata S, Nature Immunology, 2005, 6, 49–56. [DOI] [PubMed] [Google Scholar]
- 128.Ishii KJ and Akira S, Trends in Immunology, 2006, 27, 525–532. [DOI] [PubMed] [Google Scholar]
- 129.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K and Akira S, Nature, 2000, 408, 740–745. [DOI] [PubMed] [Google Scholar]
- 130.Takaoka A, Wang Z, Choi ΜK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y and Taniguchi T, Nature, 2007, 448, 501–505. [DOI] [PubMed] [Google Scholar]
- 131.Wang ZC, Choi ΜK, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K and Taniguchi T, Proceedings of the National Academy of Sciences of the United States of America, 2008, 105, 5477–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hornung V and Latz E, Nature Reviews Immunology, 2010, 10, 123–130. [DOI] [PubMed] [Google Scholar]
- 133.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL and Superti-Furga G, Nat. Immunol, 2009, 10, 266–272. [DOI] [PubMed] [Google Scholar]
- 134.Sun B and Shen H, Biomaterials, 2015, 54, 106–115. [DOI] [PubMed] [Google Scholar]
- 135.Pettengill MA, van Haren SD and Levy O, Front Immunol, 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dinarello CA, Annu. Rev. Immunol, 2009, 27, 519–550. [DOI] [PubMed] [Google Scholar]
- 137.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins ΜK and Mescher MF, J. Immunol, 1999, 162, 3256–3262. [PubMed] [Google Scholar]
- 138.Sutton C, Brereton C, Keogh B, Mills KHG and Lavelle EC, J. Exp. Med, 2006, 203, 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Awasthi S and Cox RA, BioTechniques, 2003, 35, 600–604. [DOI] [PubMed] [Google Scholar]
- 140.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA and Paul WE, Proc. Natl. Acad. Sci. U. S. A, 2009, 106, 7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Redford PS, Murray PJ and O’Garra A, Mucosal Immunol, 2011, 4, 261–270. [DOI] [PubMed] [Google Scholar]
- 142.Savelkoul HFJ, Ferro VA, Strioga ΜM and Schijns VEJC, Vaccines, 2015, 3, 148–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brooks DG, Walsh KB, Elsaesser H and Oldstone ΜB, Proc. Natl. Acad. Sci. U. S. A, 2010, 107, 3018–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van der Laan JW, Gould S and Tanir JY, Vaccine, 2015, 33, 1507–1514. [DOI] [PubMed] [Google Scholar]
- 145.The WOH, http://www.who.int/mediacentre/factsheets/fs297/enA (accessed July, 2016).
- 146.The National Cancer Institute, http://www.cancer.gov/about-cancer/causes- prevention/vaccines-fact-sheet#q8. (accessed July, 2016).
- 147.The FDA, http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm501762.htm, (accessed July, 2016).
- 148.The FDA, http://www.fda.gOv/NewsEvents/Newsroom/PressAnnouncements/ucmll93237.htm, (accessed July, 2016).
- 149.Fan Y and Moon JJ, Vaccines, 2015, 3, 662–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheung AS and Mooney DJ, Nano Today, 2015, 10, 511–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao L, Seth A, Wibowo N, Zhao C-X, Mitter N, Yu C and Middelberg APJ, Vaccine, 2014, 32, 327–337. [DOI] [PubMed] [Google Scholar]
- 152.Jia F, Liu X, Li L, Mallapragada S, Narasimhan B and Wang Q,JControl Release, 2013, 172, 1020–1034. [DOI] [PubMed] [Google Scholar]
- 153.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya ΗI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R and Pulendran B, Nature, 2011, 470, 543–U136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Steinhagen F, Kinjo T, Bode C and Klinman DM, Vaccine, 2011, 29, 3341–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maughan CN, Preston SG and Williams GR, J. Pharm. Pharmacol, 2015, 67, 426–449. [DOI] [PubMed] [Google Scholar]
- 156.Ozao-Choy J, Lee DJ and Fanes ΜB, Surg. Clin. North Am, 2014, 94, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Orange JS, Ballow M, Stiehm ER, Balias ZK, Chinen J, De La Morena M, Kumararatne D, Harville TO, Hesterberg P, Koleilat M, McGhee S, Perez EE, Raasch J, Scherzer R, Schroeder H, Seroogy C, Huissoon A, Sorensen RU and Katial R, J. Allergy Clin. Immunol, 2012, 130, S1–S24. [DOI] [PubMed] [Google Scholar]
- 158.Petros RA and DeSimone JM, Nat. Rev. DrugDiscov, 2010, 9, 615–627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


