Abstract
Increased epilepsy prevalence is reported in onchocerciasis (OC) endemic areas and is associated with the occurrence of distinct syndromes such as nodding disease and Nakalanga syndrome. o date, a causal relationship between OC and epilepsy is still a matter of controversy. We conducted a case-control study of participants with epilepsy and age-and gender-matched presumably healthy controls to elucidate the relationships between OC and epilepsy and explore the role of inflammation and growth factors in an OC endemic area in the Democratic Republic of Congo (DRC). Eighty-two participants with epilepsy (mean age±SD: 23.2±8.7 years) and 27 controls (mean age±SD:22.3±12.0 years) underwent snip skin biopsies to infection status. Serum concentrations of cytokines, were measured using a Luminex Multiplex Assay determine Onchocerca volvulus chemokines, and growth factors kit. Children < 19 years of age underwent neurocognitive assessments using the Kaufman Assessment Battery for Children, 2nd edition (KABC-II). Overall, epilepsy was associated with OC (OR=4.51, z=3.11, p=0.0019), and children with OC were more likely to be severely stunted (OR=11.67, z=2.62, p=0.0087). The relationship between epilepsy and OC was no longer significant (z=1.27, p=0.20) when stunting was included as a correcting covariate. Epilepsy was associated with poor KABC-II test scores, high serum levels of IL-17, and low levels of IL-1RA, IL-8, and EGF. KABC-II testing scores correlated with serum levels of IL-10, MCP-1 and HGF. Familial history of epilepsy occurred frequently. Future studies should consider cytokines and/or growth factors when assessing susceptibility to epilepsy in OC endemic areas. Additional investigations, preferentially in low-prevalence OC areas, may provide further insights into the concept, risk, and burden of river epilepsy.
Keywords: Cognition, Epilepsy, Growth Factors, Inflammation, Onchocerciasis, Stunting
1. INTRODUCTION
Onchocerciasis (OC), commonly known as river blindness, is a parasitic disease caused by the filarial nematode Onchocerca volvulus (OV). The parasite is transmitted through the bites of blackflies that breed near fast flowing streams or rivers. Microfilariae transmitted through the blackfly bite die and induce an inflammatory response, which can cause itching and corneal damage. If untreated, the corneal damage can ultimately lead to blindness. While dermatologic forms are commonly seen, ocular forms and putatively neurologic complicated forms are being reported with increasing frequency in children as young as 5 years old in select areas of sub-Saharan Africa (Robert Colebunders et al., 2017; Dowell et al., 2013; Levick et al., 2017). This begets the possible existence of a third neurologic complicated form, termed epilepsy syndromes and/or neurodevelopmental deficits (ENDD) (Pion et al., 2009), which may have serious global health implications, since millions of people are infected with OV, almost exclusively in the developing world. This threat is compounded for the several hundred-million plus inhabitants of sub-Saharan Africa, a subcontinent torn apart by armed conflicts, poor infrastructure, and socio-ecological collapse (Fonkwo, 2008). The pathogenesis of ENDD, however, is not known (Marin et al., 2006) and the terminology “river blindness epilepsy” may well fit the description of the yet to be elucidated OC-associated NDD (Pion et al., 2009). Proposed risk factors for OC-associated ENDD include: 1) migration of OV to the central nervous system in heavily infected subjects (Duke, Vincelette, & Moore, 1976), 2) co-infections with Loa loa (R. Colebunders et al., 2016) or Wolbachia, an OV-endosymbiotic bacteria (Higazi et al., 2005; Idro et al., 2016), 3) OV strain genetic variations (Higazi et al., 2005), 4) immunological responses (Soboslay et al., 1991), 5) treatment with ivermectin in the presence of loaisis (Twum-Danso, 2003), and 6) unknown toxic exposures and/or malnutrition (Foltz et al., 2013).
Recent reports indicated an increase in the prevalence of ENDD, including a distinct type of fatal epilepsy known as “nodding syndrome” (NS), in OC endemic areas of southern Sudan (Spencer et al., 2013), Uganda (Kaiser et al., 1996), and possibly the Democratic Republic of Congo (DRC) (Levick et al., 2017). Other studies defined another NS-like entity as Nakalanga syndrome (NKS). While recent reports have presented logical, evidence-based criterion for NS and NKS (Foger et al., 2017), this lack of agreement indicates a baseline characterization of epilepsy in OC endemic areas is still needed. Findings from such studies are essential to determine biomarkers, attributable risks, and diagnostic algorithms to fully define distinct types of epilepsy syndromes linked to OC, e.g., NS and NKS (vide supra). This paper summarizes findings from a case-control study aimed to elucidate the relationships between OC and epilepsy, provide a baseline characterization of epilepsy, and explore the role of inflammation and growth factors in ENDD in an OC endemic area of the DRC.
2. METHODS
2.1. Study Population
The study was performed in September 2014 in the Kifuma catchment area (KCA) of the Masa Health Care Zone (MHZ), District of Kasangulu, Bas-Congo Province, DRC. The MHZ (~ 1000 sq. miles) serves a population of roughly 100,000 inhabitants. The KCA consists of 33 villages with ~ 6000 inhabitants, the majority of whom are from the Lemfu ethnic group. Participants were recruited from clinics near three villages: Kifuma, Kalama, and Lalu Sefu (Figure 1). A routine 2014 survey from the DRC Ministry of Health reported an overall prevalence of 59.5 (54.3 – 64.5)% stunting in the study population. Almost half of the households in the study area were in a situation of food insecurity as determined by the Standardized Monitoring and Assessment of Relief and Transitions (SMART) assessment (http://http://smartmethodology.org/about-smart/)(DRC Ministry of Health). Kasangulu is an OC endemic area in which mass distribution of ivermectin was carried out ~ 2 years prior to the current study (DRC Ministry of Health).
Figure 1. Study Area.
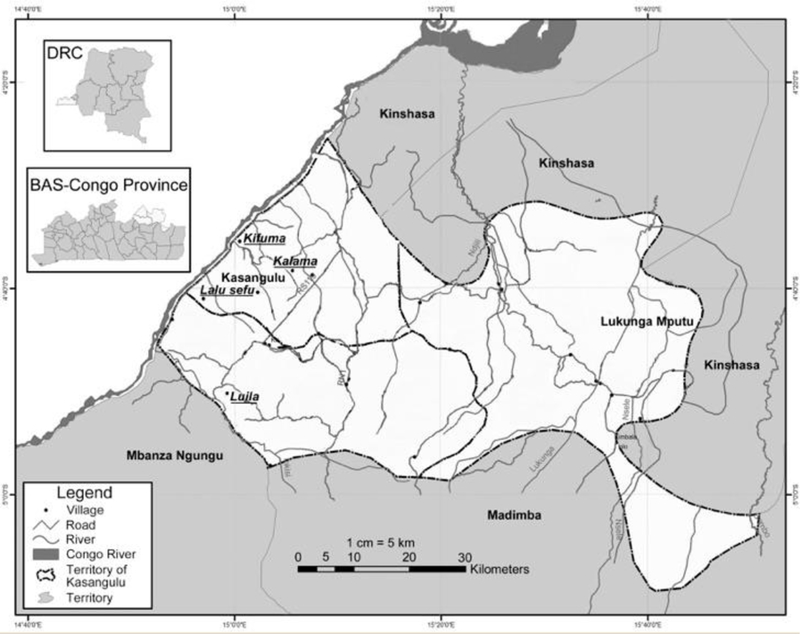
Map of the DRC showing the Kifuma catchment area, including the three villages from which participants were recruited: Kifuma, Kalama, and Lalu Sefu. All are situated along the Congo River, south of Kinshasa, the DRC capital (Source: DRC Ministry of Health).
2.2. Study Design
We implemented a case-control design which enrolled participants with epilepsy and age-and gender-matched presumably healthy controls. Epilepsy status was determined according to the International League Against Epilepsy (Fisher et al., 2014). Each case was required to have at least two unprovoked seizures occurring >24 hours apart within 10 years of study enrollment. Epilepsy status was confirmed by an expert neurologist (author KJK). Participants with no epilepsy were matched as controls by village, age, and gender. All study participants were recruited through an active search of health records from the local rural clinics, public announcements using a megaphone, church announcements, and meetings with community leaders. We initially planned to enroll only children < 19 years of age. However, because of limited access to care in the study area, a large number of adult family members asked for participation and were therefore enrolled. To confirm OV infection, up to 6 skin snip biopsies were taken from iliac crest, scapular areas and lower calves of participants (two from each site) for microscopic examination following Giemsa staining. A ~ 5ml blood specimen was collected from each subject for biochemical analyses (vide infra).
2.3. Specific Protocols for Biochemical Analyses and Neurocognitive Testing
2.3.1. Measurements of Serum Cytokines, Chemokines, and Growth Factors
Blood was collected through venipuncture in Vacutainer tubes with no anticoagulants and kept at room temperature for approximately 2 hours. The blood was then centrifuged at 15,000 rpm for 15 min, and resulting serum was removed and aliquoted into cryotubes then flash-frozen in liquid nitrogen. Serum was then shipped to Kinshasa and stored at −80°C until shipment on dry ice to Oregon Health & Science University for biochemical analyses. Fifty μL serum was used to measure concentrations of the following cytokines, chemokines, and growth factors: EGF, Eotaxin, FGF, G-CSF, GM-CSF, HGF, IFN-α, IFN-γ, IL-1ra, IL-1β, IL-2, IL-2r, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p40/p70), IL-13, IL-15, IL-17, IP-10, MCP-1, MIG, MIP-1α, MIP-1β, RANTES, TNF-α, and VEGF using a human magnetic 30-plex Luminex cytokine kit (Thermo Fisher Scientific). Growth factors were included in the analysis as putative markers of neurological damage associated with chronic undernutrition, mental and physical disability, and stunting; all potential features of epilepsy syndromes encountered in OC endemic areas. Samples were run in duplicate, and values were discarded when the coefficient of variation was greater than 10%. The two concentrations measured were averaged and used for statistical analyses.
2.3.2. Neurocognitive Assessments
Neurocognitive assessments were performed only on children less than 19 years old, using the Kaufman Assessment Battery for Children, 2nd edition (KABC-II), previously validated and used in the DRC (Bangirana et al., 2009). The KABC-II testing battery has 13 subtests to assess 4 foundational domains of cognition: (a) learning: storing and efficiently retrieving newly learned or previously learned information; (b) sequential processing: taking in and holding information and then using it within a few seconds; (c) simultaneous processing: perceiving, storing, manipulating and thinking with visual patterns; and (d) planning: solving novel problems by using reasoning abilities such as induction and deduction. Mental Processing Index was used as composite score of overall cognitive performance.
2.4. Statistical Analyses
All statistical analysis was carried out using R, 3.2 and R, 3.3. Logistic regression was used to assess the influences of the demographic variables of interest on the odds of participants to present with epilepsy, OC, or both (comorbidity). Odds ratios were calculated for a predefined battery of demographic covariates and evaluated to determine which were significantly associated with greater likelihood of presenting with one of the disease conditions. Covariates included gender, being a youth under the age of 19, and nutritional status (stunting). Based on the World Health Organization Height-for-age z-score (HAZWHO) criteria, children were considered either mildly stunted (z-score between −2 and −3) or severely stunted (z-score less than −3). Collinearity of the independent variables was examined by combining covariates into models using multiple regression along with assessments of variance inflation. Cytokine, chemokine, and growth factor levels, as well as KABC -II subdomain scores were compared (disease groups vs. healthy) using one-way ANOVA followed by post hoc analysis using a Bonferroni’s corrected t test. Mixed-effect models were used to assess whether epilepsy status had an effect on the relationship between levels of cytokines, chemokines, or growth factors and KABC-II subdomain scores. Serum cytokine levels were natural log transformed prior to statistical analysis. All associations were considered significant at p<0.05.
Ethics Statement
Community consent was obtained from the village leaders. Since most recruited participants were unable to read, informed consent to participate in the study was verbally obtained from each subject, and from each parent or guardian of any recruited child < 19 years old. Child assent was verbally obtained following parent or guardian informed consent. Ethical approval was obtained from the OHSU Institutional Review Board and the ethics committee of the DRC Ministry of Health.
3. RESULTS
3.1. Participation Rates and Participant Demographic Characteristics
A total of 164 consenting participants were initially enrolled in the study. Fifty-five (33.5%) were excluded from analysis for several reasons, such as insufficient samples to perform laboratory analyses and withdrawal from the study. Several of the presumably healthy children withdrew their participation fearing stigma from being enrolled in a study that specifically targeted children with epilepsy. Of the 164 initially recruited participants, 109 (66.5%) (51 females and 58 males, mean age±SD: 23.2±9.5 years) completed relevant procedures and were included in the current analysis (Figure 2).
Figure 2. Study Participation Rates.
A large proportion of controls (20 out of 39 excluded from analysis) withdrew due to fears of stigma for being enrolled in a study that targeted children with epilepsy.
Of the above mentioned 109 participants, 82 (75.2%) (39 females and 43 males, mean age±SD: 23.2±8.7 years) met the criteria for epilepsy and 27 (24.8%) were included as controls with no epilepsy (12 females and 15 males, mean age±SD: 22.3±12.0 years). Forty-two participants (38.5%) were children < 19 years old (27 with epilepsy and 15 with no epilepsy; 18 females and 24 males; overall mean age±SD: 14.5±2.6 years) while 67 (61.5%) were ≥ 19 years old (55 with epilepsy and 12 with no epilepsy; 33 females and 34 males; mean age±SD: 28.2±8.6 years).
3.2. Clinical and Biological Findings
3.2.1. Onset of Epilepsy and Types of Seizures
The mean (SD) age at the onset of epilepsy was 11.1 (3.3), 14.5 (5.2), or 13.5 (4.9) years for children < 19 years old, adults ≥ 19 years old, and all participants, respectively. The majority (80.5%) of participants with epilepsy reported generalized tonic-clonic seizures (with tiredness frequently reported as a triggering factor). Absence and complex seizures were also commonly reported (Table 1).astmedicalhistoriesweredifficultto obtain due widespread illiteracy and poorly maintained medical records.
Table 1.
Clinical Features of Seizures.
| Types of Seizures | Children (n=27) |
Adults (n=55) |
Overall (n=82) |
|---|---|---|---|
| Tonic Clonic | 20 | 46 | 66 |
| Absence | 6 | 9 | 15 |
| Myoclonic | 0 | 2 | 2 |
| Tonic | 4 | 4 | 8 |
| Atonic | 1 | 4 | 5 |
| Partial | 1 | 0 | 1 |
| Complex | 5 | 17 | 22 |
| Family History of Epilepsy in First Degree Relative |
11 | 31 | 42 |
| Family History of Epilepsy in Second Degree Relative |
12 | 27 | 39 |
| Association with OV infection (Giemsa Positive) |
21 | 47 | 68 |
| Association with mild stunting | 10 | Not Applicable | 10 |
| Associated with severe stunting | 18 | Not Applicable | 18 |
A large percentage of participants (75.2%) tested positive for OV. OV was found in 68/82 (83%) subjects with epilepsy and 14/27 (52%) control participants. Similar trends were seen in those < 19 years of age, as 21/27 (78%) children with epilepsy and 7/15 (47%) children with no epilepsy tested positive for OV.
3.2.2. Association Models
Univariate analyses showed no association between epilepsy and gender (p = 0.78) or historyoftreatmentwithivermectin(p = 0.66). Family history of epilepsy was reported in a large number of participants (52% for first degree relative, 48% for second degree relative). However, only epilepsy in second degree relatives was significantly more common in cases compared to controls (p = 0.01, Fischer’s exact test). Overall, epilepsy was associated with OC (OR=4.51, z=3.11, p=0.0019). Adults were more likely to have epilepsy (OR=2.54, z=2.06, p=0.039) or both OC and epilepsy (OR=2.35, z=2.09, p=0.036) than children. he above relationship between epilepsy and OC did not change after adjusting for age and gender. No association was found between epilepsy and stunting (mild stunting: z=0.91, p=0.37; severe stunting: z=0.86, p=0.39). However, severe stunting was significantly associated with OC (OR=11.67, z=2.62, p=0.0087) (Figure 3). Evaluation of collinearity determined that when stunting was included as a correcting covariate, the relationship between epilepsy and OC was no longer significant (z=1.27, p=0.20), and the relationship between severe stunting and OC was greatly attenuated (z=2.52, p=0.012). Assessment of variance inflation found a 39% increase in the model variance of OC due to the inclusion of stunting as a covariate, implying moderate confounding between the two variables. Contrasting rates with respect to stunting status was not feasible due to small sample size.
Figure 3. Forest plots of the relationships between disease status and sex, age, stunting, and having a second disease.
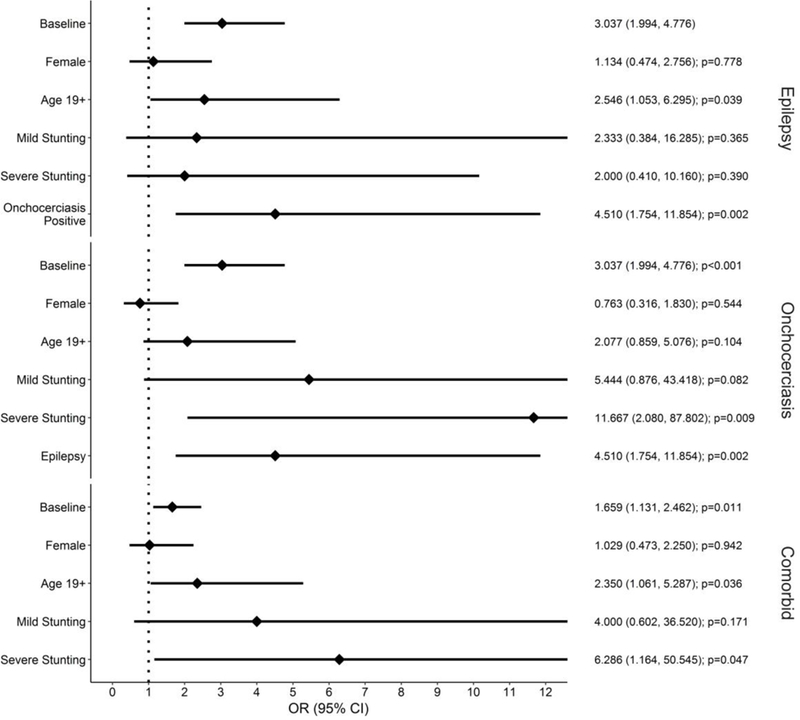
Odds ratios (on the right) and 95% confidence intervals (in parenthesis), along with p-values are shown for a number of covariates based on their associations with epilepsy, OC status, or comorbidity (epilepsy and OC).
3.2.3. Systemic Inflammation and Growth Factors in Epilepsy and Onchocerciasis
Overall, participants with epilepsy only had higher serum levels of IL-17 [mean±SD in healthy controls (n = 13): 1.54±0.54 pg/ml vs. 2.30±0.98 pg/ml in those with epilepsy (n 12), p<0.05] but lower levels of IL-1RA (mean±SD in controls: 6.14±0.67 pg/ml vs. 5.52±0.83 pg/ml in those with epilepsy, p<0.05), IL-8 (mean±SD in controls: 3.92±1.07 pg/ml vs. 3.07±0.79 pg/ml in those with epilepsy, p<0.05), and EGF (mean±SD in controls: 3.61±1.19 pg/ml vs. 2.94± 1.26 pg/ml in those with epilepsy, p<0.05) (Figure 4).Inchildrenwhounderwentcognitive assessments, the only significant difference between groups was an increase in IL-17 in children with epilepsy only compared to controls [mean±SD in controls (n = 8): 1.75±0.47 pg/ml vs. 3.11±0.89 pg/ml in those with epilepsy (n = 5), p<0.05] (Figure 4B).
Figure 4. Serum concentrations of cytokines, chemokines, and growth factors.

A) All participants. n =13 for healthy group, n = 12 for epilepsy only group, n = 14 for OC only group, n = 65 for OC and epilepsy group. B) Only children (< 19 years old). n = 8 for healthy group, n = 5 for epilepsy only group, n = 7 for OC only group, n = 18 for OC and epilepsy group. OC = onchocerciasis. * = p<.05, ** = p<.01, compared to healthy controls. Bars denote mean±SD.
3.3. Neurocognition Performance in Children with Epilepsy and/or Onchocerciasis
Children with epilepsy and no OC had significantly lower scores than healthy children in the sequential processing (Healthy: 74±17 vs. Epilepsy: 52±7, p = 0.0022), simultaneous processing (Healthy: 73±19 vs. Epilepsy: 39±5, p = 0.0040), learning (Healthy: 78.72±19.38 vs. Epilepsy: 54.25±7.49, p = 0.0018), and planning (Healthy: 88.66±13.38 vs. Epilepsy: 65.31±3.28, p = 0.0006) neurocognition domains, as well as for the Mental Processing Index (Healthy: 71.44±15.29 vs. Epilepsy: 44.56±4.58, p = 0.0002). Children with epilepsy and OC also had significantly lower scores than healthy children in the sequential processing (Healthy: 74.21±16.74 vs. Epilepsy and OC: 52.21±7.015, p = 0.0001), simultaneous processing (Healthy: 73.21±19.08 vs. Epilepsy and OC: 46.15±15.29, p = 0.0035), learning (Healthy: 78.72±19.38 vs. Epilepsy and OC: 54.75±6.34, p<0.001), and planning (Healthy: 88.66±13.38 vs. Epilepsy and OC: 70.73±6.39, p<0.001), and the Mental Processing Index (Healthy: 71.44±15.29 vs. Epilepsy and OC: 47.73±6.06, p<0.0001). While children with OC and no epilepsy had lower scores than healthy children in all domains, only Mental Processing Index was significant (Healthy: 71.44±15.29 vs. OC: 57.19±6.06, p = 0.020) (Figure 5).
Figure 5. Neurocognitive evaluations.

KABC-II neuropsychological testing scores assessing different cognition subdomains, as well as Mental Processing Index, a composite score for the overall cognitive performance. OC = onchocerciasis. * = p<0.05, = p<0.01, *** = p<0.001 compared to healthy controls. n= 6 for healthy group, n = 5 for epilepsy only group, n = 7 for OC only group, n = 24 for OC and epilepsy group.
Based on our findings that cognitive dysfunction and altered systemic inflammation occur in epilepsy rather than OC, we sought to determine whether there was any relationship between serum biomarkers of interest (vide supra) and cognitive performance in children with and without epilepsy, regardless of OV status. Interestingly, we found that while there were no relationships between the aforementioned markers and the KABC-II subdomain scores in children with epilepsy, there were significant relationships between KABC-II subdomain scores and IL-10, MCP-1, and HGF in children without epilepsy. Furthermore, a disease effect on these relationships was noticeable for at least one KABC-II subdomain score (Figure 6).
Figure 6. Correlations between serum levels of cytokines, growth factors, and neurocognition testing scores.
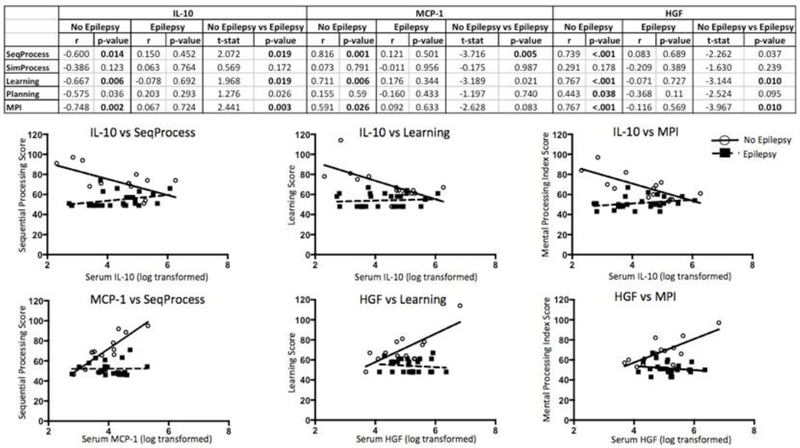
Correlations between serum cytokine concentration and KABC-II subdomain scores. Top: table with correlation coefficients (r) for the relationships between serum levels of cytokines, chemokines, and growth factors and KABC-II subdomain testing scores. T-stats are given for the differences in relationships between serum cytokine, chemokine, or growth factor levels and KABC-II subdomain scores for children with epilepsy and children without epilepsy.Only cyto kines where at least one t-stat was significant are shown. Bottom: graphs show differential relationships between serum biomarker levels and KABC-II subdomain testing scores for children with and without epilepsy when t-stat is significant (p<0.05). SeqProcess = Sequential Processing, SimProcess = Simultaneous Processing, MPI = Mental Processing Index. -values less than 0.05 are bolded. n = 13 for No Epilepsy group, n=23 for Epilepsy group.
4. Discussion
We conducted a case control study to define the baseline characteristics of epilepsy in an OC endemic area of the DRC. Our findings provide insights into the relationships between epilepsy, deficits in foundational domains of child cognition, and OC -all in context of poor nutrition. The causal relationships between OC and epilepsy have yet to be established, despite several studies suggesting that distinct epileptic entities, termed nodding syndrome (NS) and Nakalanga syndromes (NKS) are pathophysiologically linked to OC (R. Colebunders et al., 2016; Robert Colebunders et al., 2017). While we intentionally did not investigate these syndromes in our study, we still found their key clinical features, such as epileptic seizures at the onset at puberty, stunting, and poor cognition, in our study participants.
We showed that stunting was a key feature of OC but not epilepsy. Conversely, cognitive dysfunction was associated with epilepsy but not OC. Our findings are consistent with studies showing that chronic undernutrition is common in poverty-stricken OC endemic areas of the developing world (Kanan & Swar, 2016; Kismul, Acharya, Mapatano, & Hatløy, 2018). In addition, it is conceivable that children with untreated epilepsy, such as those in our study area, would likely present with neurocognitive deficits and, possibly, poor neurodevelopmental trajectories (van Rijckevorsel, 2006). As such, an immediate public health response to improve access to care, including means of cognitive rehabilitation, is warranted in our study area.
The associations between OC and epilepsy in our participants are not indicative of any causal relationships. They may be a result of a convenient sampling procedure – i.e., active recruitment of participants with self-reported epileptic seizures from a known OC-endemic area. Nevertheless, our study findings are consistent with those from several previous studies that also reported similar associations, including cognitive impairment and/or stunting in children with OC and epilepsy (Robert Colebunders et al., 2017; Newell, Vyungimana, & Bradley, 1997; Ovuga, Kipp, Mungherera, & Kasoro, 1992). Familial history of epilepsy, which was significant in second-degree relatives, is in agreement with a previously suggested heritability component (R. Colebunders et al., 2015). Further insights into the possible causal relationships between OC and epilepsy should be pursued through carefully conducted epidemiological studies, preferentially in areas with low OC endemicity. In these studies, care should be taken to optimize representation of various types of epileptic seizures (e.g., myoclonic, atonic seizures) following protocols that minimize bias inself-reportedevents.hallengesforsuch rigorous approaches are compounded by lack of standardized and validated protocols to conduct epilepsy research in Africa (Muzumdar, 2016). We found no association between ivermectin treatment and epilepsy occurrence. Interpreting this finding does require caution, since records of mass distribution were not readily available and information on whether subjects had even taken ivermectin or not was self-reported, thus less reliable and subject to recall bias.
We found that participants with epilepsy but no OC had increased serum levels of IL-17, but decreased levels IL-1RA, EGF, and IL-8 in comparison to presumably healthy participants. IL-17 was the only cytokine that was significantly different when children were analyzed separately. This cytokine is implicated in neutrophil activation (Liu et al., 2016) and elevated levels in the serum and cerebrospinal fluid were reported in epileptic individuals (Mao et al., 2013). Interestingly, levels of IL-8, which is also associated with neutrophil function (Pieper, Pieloch, & Galla, 2013), were significantly lower in participants with epilepsy only, suggesting possible cross-talk between IL-17-and IL-8-mediated signaling. This interpretation must be done with caution as our findings still need to be reproduced and validated against other markers of inflammation using appropriate design and sample size. Our finding that IL-1RA is decreased in individuals with epilepsy is consistent with a previous study suggesting it may play a role in epileptogenesis (Youn et al., 2012). Whether the differential levels of select cytokines or chemokines reflect an immune response to hidden infections commonly seen in sub-Saharan Africa should also be considered.
Analyses performed within study groups revealed significant correlations between markers of inflammation or select growth factors and cognitive performance scores. Interestingly, all of these relationships occurred in the non-epilepsy group. We found a strong negative correlation between serum IL-10 and cognitive performance scores that held true across multiple subdomains of cognition. IL-10 is an anti-inflammatory cytokine that is important in ameliorating chronic inflammation and preventing autoimmunity. However, excessive or mistimed IL-10 induction can prevent clearance of infection, and is associated with increased pathology during parasitic infection (Couper, Blount, & Riley, 2008). Alternatively, we found that levels of MCP-1 and HGF positively correlated with select KABC-II scores. MCP-1 is an important chemokine for various populations of immune cells, including monocytes, granulocytes, and subsets of lymphocytes. It is considered one of the key chemokines for recruiting immune cells from the periphery to brain (Mahad & Ransohoff, 2003), and is important in infections prevalent in sub-Saharan Africa that are known to affect cognition, such as trypanosomiasis (Amin et al., 2009) and cerebral malaria (Dunst, Kamena, & Matuschewski, 2017). The fact that we found a positive relationship between MCP-1 (a pro-inflammatory cytokine) and cognition, plus a negative relationship between IL-10 (an anti-inflammatory cytokine) and cognition in presumably healthy children, but not those with disease, indicate that cross-talk and balance between pro-and anti-inflammatory mechanisms may play a role in cognition. Furthermore, the fact that these relationships were lost in children with epilepsy suggests a disease effect. Studies show that OV has powerful immunomodulatory capabilities (Greene, Gbakima, Albiez, & Taylor, 1985), which may prevent the immune system from mounting a response necessary to clear the infection (Lechner et al., 2012). These interesting results, along with previous studies, underscore the importance of additional studies to elucidate the relationships between inflammation, growth, and cognition in children of the developing world. Early detection of brain damage and intervention are of paramount importance for the prevention of disease progression and further neurological insults
Limitations to the present study are mainly those inherent to the convenient sampling approach, self-reporting of epileptic seizures, and small sample size. Our participants were almost entirely recruited from rural areas, where inhabitant shad limited access to healthcare and many basic services. Therefore, many of the participants, including those presumably healthy, may have had unreported or undiagnosed medical issues that could have confounded results. ample size in some groups, especially the “healthy” (no OC and no epilepsy) was small (due to participant dropout, as discussed in the Methods section), which presents a challenge for establishing a true “healthy” reference. However, the purpose of this study was not to simply compare children with epilepsy to healthy children, but rather to elucidate the relationships between epilepsy and OC while providing a baseline characterization of epilepsy in an OC endemic area. In spite of the small number of “healthy” participants, We were still able to achieve statistical significance in several measures, even after stringent adjustments. he fact that we found a significant relationship between OC status and epilepsy status in both adults and children shows a great need for studies investigating the effects and relationships of these two diseases throughout an individual’s life. Nutritional status should always be considered as a potential confounder. For many people in Sub-Saharan Africa, OC and epilepsy are lifelong diseases, and therefore studies should not only focus on children, but rather the progression of these diseases as one ages. Due to our desire to limit the complexity of this study, we did not account for other infections such as Loa loa, Taenia solium, and Toxoplasma gondii that could influence both epilepsy status and serum cytokine levels. OV infection could also be ascertained using molecular biology techniques including polymerase chain reactions to increase sensitivity. In spite of these limitations, this study provides a thorough baseline characterization of inhabitants of a previously understudied area within the DRC. As a result of this work, future studies can be performed to conduct more complex analyses, including additional covariates such as additional infections beyond OC.
In summary, we defined baseline characteristicsofepilepsyinaselectOC endemic area in the DRC, providing new information to better understand the association between epilepsy and OC in the context of poor nutrition. erum cytokines and growth factors may help differentiate different epilepsy-associated syndromes. This baseline information will be helpful in refining the definition and evaluating the attributable risks and biomarkers of epilepsy in OC endemic areas, as well as developing diagnostic algorithms and cognition rehabilitation paradigms for those with cognitive deficits.
HIGHLIGHTS.
Epilepsy, but not onchocerciasis (OC), is associated with cognition deficits.
OC, but not epilepsy, is associated with severe stunting in OC endemic areas.
Future studies should consider the immunomodulatory effects of OC on epilepsy risk.
Acknowledgements and Funding
This work was funded by the NIH Fogarty International Center Grant #R21TW01000402 (DDT). We are grateful to all the study participants including the Kifuma community leaders, Dr. Kambaja B., Dr. Musasa H., Dr. Bumoko G, Dr. Sombo T., and nurse F. Kutona.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin N, Rottenberg ME, Thomsen AR, Mumba D, Fenger C, Kristensson K,… Masocha W (2009). Expression and Role of CXCL10 during the Encephalitic Stage of Experimental and Clinical African Trypanosomiasis. J Infect Dis, 200(10), 1556–1565. 10.1086/644597 [DOI] [PubMed] [Google Scholar]
- Bangirana P, Seggane M, Allebeck P, Giordani B, John CC, Opoka OR, … Boivin MJ (2009). A preliminary examination of the construct validity of the KABC-II in Ugandan children with a history of cerebral malaria. Afr Health Sci, 9(3), 186–192. [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, Mandro M, Mokili JL, Mucinya G, Mambandu G, Pfarr K, … Laudisoit A (2016). Risk factors for epilepsy in Bas-Uele Province, Democratic Republic of the Congo: a case-control study. Int J Infect Dis, 49, 1–8. 10.1016/j.ijid.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, Post R, O’Neill S, Haesaert G, Opar B, Lakwo T, … Hendy A (2015). Nodding syndrome since2012:recentprogress,challengesand recommendations for future research. Trop Med Int Health, 20(2), 194–200. [DOI] [PubMed] [Google Scholar]
- Colebunders R, Njamnshi AK, van Oijen M, Mukendi D, Kashama JM, Mandro M, … Idro R (2017). Onchocerciasis-associated epilepsy: From recent epidemiological and clinical findings to policy implications. Epilepsia Open, 2(2), 145–152. 10.1002/epi4.12054 doi:10.1111/tmi.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper KN, Blount DG, & Riley EM (2008). IL-10: The Master Regulator of Immunity to Infection. The Journal of Immunology, 180(9), 5771–5777. 10.4049/jimmunol.180.9.5771 [DOI] [PubMed] [Google Scholar]
- Dowell SF, Sejvar JJ, Riek L, Vandemaele KAH, Lamunu M, Kuesel AC, … Mbonye AK (2013). Nodding Syndrome. Emerg Infect Dis, 19(9), 1374–1373. 10.3201/eid1909.130401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke BO, Vincelette J, & Moore PJ (1976). Microfilariae in the cerebrospinal fluid, and neurological complications, during treatment of onchocerciasis with diethylcarbamazine. Tropenmed Parasitol, 27(2), 123–132. [PubMed] [Google Scholar]
- Dunst J, Kamena F, & Matuschewski K (2017). Cytokines and Chemokines in Cerebral Malaria Pathogenesis. Frontiers in Cellular and Infection Microbiology, 7, 324 10.3389/fcimb.2017.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou,., Bogacz,., Cross JH, Elger CE, … Wiebe S (2014). ILAE official report: a practical clinical definition of epilepsy. Epilepsia, 55(4), 475–482. 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- Foger K,Gora-Stahlberg G,Sejvar J, Ovuga E, Jilek-Aall L, Schmutzhard E, …Winkler AS (2017). Nakalanga Syndrome: Clinical Characteristics, Potential Causes, and Its Relationship with Recently Described Nodding Syndrome. PLoS Negl Trop Dis, 11(2), e0005201 10.1371/journal.pntd.0005201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz JL, Makumbi I, Sejvar JJ, Malimbo M, Ndyomugyenyi R, Atai-Omoruto AD, … Lwamafa DKW (2013). An Epidemiologic Investigation of Potential Risk Factors for Nodding Syndrome in Kitgum District, Uganda. PLoS One, 8(6), e66419 10.1371/journal.pone.0066419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonkwo PN (2008). Pricing infectious disease: The economic and health implications of infectious diseases. EMBO Reports, 9(Suppl 1), S13–S17. 10.1038/embor.2008.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene BM, Gbakima AA, Albiez EJ, & Taylor HR (1985). Humoral and cellular immune responses to Onchocerca volvulus infection in humans. Rev Infect Dis, 7(6), 789–795. [DOI] [PubMed] [Google Scholar]
- Higazi B, Filiano A, Katholi CR, Dadzie Y, Remme JH, & Unnasch TR (2005). Wolbachia endosymbiont levels in severe and mild strains of Onchocerca volvulus. Molecular and Biochemical Parasitology, 141(1), 109–112. 10.1016/j.molbiopara.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Idro R, Opar B, Wamala J, Abbo C, Onzivua S, Mwaka DA, … Aceng JR (2016). Is nodding syndrome an Onchocerca volvulus-induced neuroinflammatory disorder? Uganda’s story of research in understanding the disease. International Journal of Infectious Diseases, 45(Supplement C), 112–117. 10.1016/j.ijid.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Kaiser C, Kipp W, Asaba G, Mugisa C, Kabagambe G, Rating D, & Leichsenring M (1996). The prevalence of epilepsy follows the distribution of onchocerciasis in a west Ugandan focus. Bull World Health Organ, 74(4), 361–367. [PMC free article] [PubMed] [Google Scholar]
- Kanan SOH, & Swar MO (2016). Prevalence and outcome of severe malnutrition in children less than five-year-oldin Omdurman Paediatric Hospital, Sudan. Sudanese Journal of Paediatrics, 16(1), 23–30. [PMC free article] [PubMed] [Google Scholar]
- Kismul H, Acharya P, Mapatano MA, & Hatløy A (2018). Determinants of childhood stunting in the Democratic Republic of Congo: further analysis of Demographic and Health Survey 2013– 14. BMC Public Health, 18, 74 10.1186/s12889-017-4621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner CJ, Gantin RG, Seeger T, Sarnecka A, Portillo J, Schulz-Key H, … Soboslay PT (2012). Chemokines and cytokines in patients with an occult Onchocerca volvulus infection. Microbes Infect, 14(5), 438–446. 10.1016/j.micinf.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Levick B, Laudisoit A, Tepage F, Ensoy-Musoro C, Mandro M, Bonareri Osoro C, … Colebunders R (2017). High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLOS Neglected Tropical Diseases, 11(7), e0005732 10.1371/journal.pntd.0005732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Lauridsen HM, Amezquita R, Pierce W, Jane-Wit D, Fang C, … Pober JS (2016). IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J Immunol, 197(6), 2400–2408. 10.4049/jimmunol.1600138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DJ,&Ransohoff RM(2003). The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Semin Immunol, 15(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Mao L-Y, Ding J, Peng W-F, Ma Y, Zhang Y-H, Fan W, & Wang X (2013). Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia, 54(9), e142–e145. 10.1111/epi.12337 [DOI] [PubMed] [Google Scholar]
- Marin B, Boussinesq M, Druet-Cabanac M, Kamgno J, Bouteille B, & Preux P-M (2006). Onchocerciasis-related epilepsy? Prospects at a time of uncertainty. Trends in Parasitology, 22(1), 17–20. 10.1016/j.pt.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Muzumdar D (2016). Epilepsy surgery in the developing world: Facts and challenges. International Journal of Surgery, 36(Part B), 403–404. 10.1016/j.ijsu.2016.11.131 [DOI] [PubMed] [Google Scholar]
- Newell D, Vyungimana F, & Bradley JE (1997). Epilepsy, retarded growth and onchocerciasis, in two areas of different endemicity of onchocerciasis in Burundi. Trans R Soc Trop Med Hyg, 91(5), 525–527. [DOI] [PubMed] [Google Scholar]
- Ovuga E, Kipp W, Mungherera M, & Kasoro S (1992). Epilepsy and retarded growth in a hyperendemic focus of onchocerciasis in rural western Uganda. East Afr Med J, 69(10), 554–556. [PubMed] [Google Scholar]
- Pieper C, Pieloch P, & Galla HJ (2013). Pericytes support neutrophil transmigration via interleukin-8 across a porcine co-culture model of the blood-brain barrier. Brain Res, 1524, 1–11. 10.1016/j.brainres.2013.05.047 [DOI] [PubMed] [Google Scholar]
- Pion SDS, Kaiser C, Boutros-Toni F, Cournil A, Taylor MM, Meredith SEO, … Boussinesq M (2009). Epilepsy in Onchocerciasis Endemic Areas: Systematic Review and Meta-analysis of Population-Based Surveys. PLOS Neglected Tropical Diseases, 3(6), e461. 10.1371/journal.pntd.0000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboslay PT, Dreweck CM, Taylor HR, Brotman B, Wenk P, & Greene BM (1991). Experimental onchocerciasis in chimpanzees. Cell-mediated immune responses, and production and effects of IL-1 and IL-2 with Onchocerca volvulus infection. J Immunol, 147(1), 346–353. [PubMed] [Google Scholar]
- Spencer PS, Vandemaele K, Richer M, Palmer VS, Chungong S, Anker M, … Tumwine JK (2013). Nodding syndrome in Mundri county, South Sudan: environmental, nutritional and infectious factors. Afr Health Sci, 13(2), 183–204. 10.4314/ahs.v13i2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twum-Danso NAY (2003). Loa loa encephalopathy temporally related to ivermectin administration reported from onchocerciasis mass treatment programs from 1989 to 2001: implications for the future. Filaria Journal, 2(Suppl 1), S7–S7. 10.1186/1475-2883-2-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijckevorsel K (2006). Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure, 15(4), 227–234. 10.1016/j.seizure.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Youn YA, Kim SJ, Sung IK, Chung Y, Kim YH, & Lee IG (2012). Serial examination of serum IL-8, IL −10 and IL-1Ra levels is significant in neonatal seizures induced by hypoxic -ischaemic encephalopathy. Scand J Immunol, 76(3), 286–293. 10.1111/j.1365-3083.2012.02710.x [DOI] [PubMed] [Google Scholar]



