Abstract
The complement system significantly contributes to the development of inflammatory and neuropathic pain, but the underlying mechanisms are poorly understood. Recently, we identified the signaling pathway responsible for thermal hypersensitivity induced by the complement system component C5a. Here, we examine the mechanisms of another important action of C5a, induction of mechanical hypersensitivity. We found that intraplantar injection of C5a produced a dose-dependent mechanical sensitization, and that this effect was blocked by chemogenetic ablation of macrophages in both male and female mice. Knockout (KO) of TRPV1 or pretreatment with the TRPV1 antagonists, AMG9810 or 5’-iodoresiniferatoxin (5’-IRTX), significantly reduced C5a-induced mechanical sensitization. Notably, local administration of 5’-IRTX 90 minutes after C5a injection resulted in a slow, but complete, reversal of mechanical sensitization, indicating that TRPV1 activity was required for maintaining C5a-induced mechanical hypersensitivity. This slow reversal suggests that neurogenic inflammation and neuropeptide release may be involved. Indeed, pretreatment with a calcitonin gene related peptide (CGRP) receptor antagonist (but not an antagonist of the neurokinin 1 receptor), prevented C5a-induced mechanical sensitization. Furthermore, intraplantar injection of CGRP produced significant mechanical sensitization in both wild-type (WT) and TRPV1 KO mice. Taken together, these findings suggest that C5a produces mechanical sensitization by initiating macrophage-to-sensory-neuron signaling cascade that involves activation of TRPV1 and CGRP receptor as critical steps in this process.
Summary
The complement system component C5a produces mechanical hypersensitivity by initiating macrophage-to-nociceptor signaling cascade that involves activation of TRPV1 and CGRP receptor as critical steps in this process.
1. Introduction
The complement system is a central arm of innate immunity and includes >30 soluble and membrane-bound proteins that collectively act as a first line of defense against infection and tissue damage-associated conditions. Activated components of the complement system participate in host defenses through a range of mechanisms including recruitment and activation of immune cells, opsonization of pathogens or necrotic cells and killing of cells [24; 52; 75]. Recent studies found that complement activity modulates pain sensitivity in experimental models of inflammatory pain including osteoarthritis [78], rheumatoid arthritis [22; 44], ankylosing spondylitis [82], post-surgical pain [11; 27; 37] and more generic models of inflammatory pain [44; 69]. Additionally, meta-analysis of microarray data revealed that genes from the complement system are among those most frequently and strongly altered after induction of neuropathic or inflammatory pain [32], with several studies specifically highlighting upregulation of complement activity after peripheral nerve injury [17; 23; 32; 34; 67; 73]. However, which signaling pathways trigger pain hypersensitivity as a result of complement activity is not well understood.
The complement component C5a is a highly potent pro-inflammatory and pronociceptive product of complement system activation that is rapidly generated in response to injury or infection. This 74 amino acid polypeptide acts primarily through a canonical G-protein coupled receptor, C5aR1 (also called C5aR or CD88) [2; 52]. Numerous studies have shown that the C5a/C5aR1 levels are elevated in various pain states. For example in humans, C5a/C5aR1 was found to be elevated in patients with rheumatoid arthritis [22; 28; 78] and acute pancreatitis [54]. Similarly, in murine models of postsurgical, neuropathic, and inflammatory pain, C5a and C5aR1 are significantly upregulated [11; 27; 57; 63]. Furthermore, direct administration of C5a elicits mechanical and thermal hypersensitivity in rodents [26; 27; 35; 44; 63; 69]. Consistent with the sensitizing action of C5a, both genetic deletion and pharmacologic antagonism of C5aR1 produce analgesia in murine models of inflammatory, arthritic, post-surgical and neuropathic pain [11; 23; 27; 37; 44; 63].
In spite of a growing body of literature supporting a role of C5a as an important modulator of pain processing, the mechanisms underlying C5a-induced sensitization are still unclear, particularly those that apply to mechanical hypersensitivity. In this study, we examine the cellular and molecular mechanisms underlying C5a-induced mechanical sensitization. By utilizing chemogenetic depletion, we identify macrophages as key intercellular mediators between complement activity and primary afferent neuron sensitization. Furthermore, we find that the activity of TRPV1 is essential for both the development and maintenance of C5a-induced mechanical hypersensitivity in mice. Finally, our data indicate that activation of the CGRP receptor is also required for the development of C5a-evoked mechanical hypersensitivity.
2. Methods
2.1. Animals
All experiments involving mice and the procedures used therein were approved by the University of Iowa Institutional Animal Care and Use Committee and were carried out in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of mice used and their suffering. Mice (6–10 weeks of age) were housed with food and water ad libitum under a 12-hr light/dark cycle. C57BL/6J, TRPV1 KO (Jackson Labs #003770; C57BL/6J), TRPA1 KO (Jackson Labs #006401; mixed B6;129) and MAFIA (Jackson Labs #005070; C57BL/6J) mice were obtained from The Jackson Laboratory (Farmington, CT).
2.2. Chemogenetic depletion of macrophages
For designer drug-inducible macrophage depletion experiments we used transgenic macrophage Fas-induced apoptosis (MAFIA) mice [3]. In these mice, a drug-inducible suicide gene is expressed under the control of the macrophage/monocyte-specific colony-stimulating factor (CSF) 1 receptor (c-fms) promoter; its activation by the dimerizing compound AP20187 (Clontech Cat #635069) triggers Fas-induced apoptosis in macrophages and dendritic cells [3]. MAFIA mice do not seem to be depleted of microglia following systemic administration of AP20187, most likely due to its poor penetration through the blood brain barrier [3; 77]. Male or female MAFIA mice were administered either 2 mg/kg of AP20187 dissolved in vehicle (10% PEG-400 and 1.7% Tween-80 in PBS) or vehicle alone daily via intraperitoneal injection for 5 days as previously described [63]. Behavioral testing was performed 6 days after the first injection of AP20187 or vehicle by an investigator blinded to treatment conditions.
2.3. Drug administration
Pharmacological agents were injected into the plantar surface of the hindpaw using a 33-gauge needle coupled to a Hamilton syringe. Injections included: 500 ng recombinant mouse C5a in 10 µL PBS (unless otherwise stated), 5 µg of CGRP in 10 µL of PBS, or 10 ng NGF in 10 µL PBS. NGF neutralizing antibody (1 µg in 10 µL PBS) or IgG (control; 1 µg in 10 µL PBS) was administered into the plantar skin of the hindpaw 30 min prior to injection of C5a. For delivery of the TRPV1 antagonist, 67 ng of AMG9810 was mixed with 500 ng of C5a, and this mixture was injected in a total volume of 10 µL PBS. 5’-IRTX (10 µL of 50 nM) was injected into the plantar surface of the hindpaw either 30 min before or 90 min after C5a was injected. The neurokinin 1 (NK1) receptor antagonist SR140333 was delivered at either 1 nmol subcutaneously (SC) or 1 mg/kg intraperitoneally (i.p.), 30 min prior to C5a injection. The CGRP receptor antagonist BIBN4096 was delivered i.p. at 10 mg/kg, 30 min prior to C5a injection.
2.4. Testing of evoked pain behavior
Mechanical and thermal sensitivity of mouse hindpaws were measured using the von Frey filament threshold calculation and Hargreaves test, respectively, by an investigator blinded to treatment conditions as previously described [26; 38; 43; 58]. Mice were acclimatized to the behavioral testing chambers for 2 h per day beginning 3 days before testing. Mice were placed inside a clear acrylic box (100 X 100 X 150 mm) on an elevated wire mesh platform (holes in mesh are ~5X5mm). Mechanical sensitivity was assayed by calculating the 50% response threshold to 5 presentations each of 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4 and 2 g von Frey filaments (Stoelting, Wood Dale, IL). Filaments were presented in order of the lowest strength (0.04 g) to the highest strength (2.0 g).
Thermal sensitivity was measured using an IITC Plantar Analgesia Meter (IITC Life Sciences; CA) with the glass table-top heated to a thermo-neutral temperature (30°C). Nociceptive thermal sensitivity was measured by focusing a beam of light on the plantar surface of the hindpaw to generate heat. The time required for the stimulus to elicit withdrawal of the hindpaw (paw withdrawal latency) was recorded using the programmable digital timer of the IITC Plantar Analgesia Meter. Baseline latency was determined shortly (15–30 min) before drug administration, by averaging the results of three tests separated by a 5-min interval.
2.5. Testing of spontaneous pain behavior
Mice were acclimatized to the test Hargreaves chambers for 2 hr per day beginning 3 days before testing. Spontaneous pain behaviors were measured by video recording mice at baseline (prior to injection) in the Hargreaves chamber. Then, capsaicin (1.6 µg in 10 µL) was injected into the plantar surface of the hindpaw, and the mice were placed back in the chamber, and continuously recorded for 20 min. After the experiment, the video was replayed for a blinded observer and the time spent flinching or leaking each hindpaw was recorded and collated for 5-min blocks of time. Thirty min prior to capsaicin injection WT animals were pre-treated by intraplantar injection of either 10 µL of 50 nM 5’-IRTX or 10 µL of PBS. TRPV1 KO animals were naïve prior to capsaicin injection.
2.6. Reagents
Recombinant mouse C5a, CGRP receptor antagonist BIBN4096 (Olcegepant), NK1 receptor antagonist SR140333, and TRPV1 antagonists AMG9810 and 5’-IRTX, were purchased from R&D Systems/Tocris. Recombinant rat calcitonin gene related peptide (α-CGRP; Cat# C0292) and capsaicin (cat# M2028) were purchased from Sigma. NGF was purchased from AbD Serotec. NGF neutralizing antibody and IgG control were purchased from Exalpha Biologicals (Cat# L148M and 0G11, respectively; Shirley, MA). AP20187 (B/B homodimerizer Cat #635069) was from Takara/Clontech.
2.7. Statistical analysis
All data are expressed as mean ± SEM. The data were analyzed using two-way repeated measures ANOVA with Holm-Sidak post hoc test (time course with comparison of 2 or more groups to all other groups at the same time point), or two-way repeated measures ANOVA with Dunnett post hoc test (time course with comparison to respective baseline values). A value of p<0.05 was considered statistically significant in all cases. Statistical analyses were performed using the GraphPad Prism 7 software.
3. Results
3.1. C5a produces dose-dependent mechanical hypersensitivity
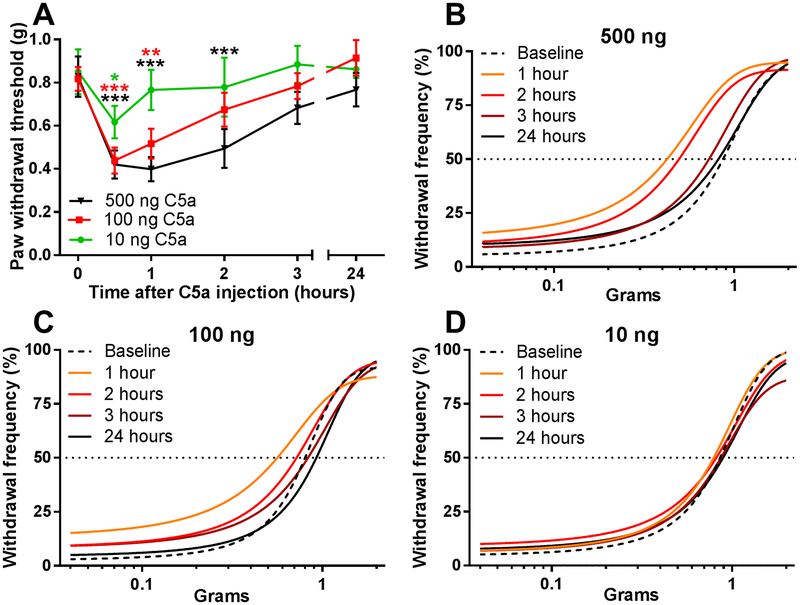
Previous reports have described a wide range of C5a doses for inducing thermal and mechanical hypersensitivity in rodents. As amounts used for intraplantar injection ranged from 10–40 ng/hindpaw at the low end [26; 69] to 200–500 ng/hindpaw at the high end [26; 27; 44], we first characterized the dependence of mechanical sensitivity on C5a dose in WT mice (Fig. 1). Administration of 10, 100 and 500 ng C5a into the hindpaw produced increasingly stronger and longer lasting mechanical hypersensitivity, which returned to baseline levels 24 hours after the injection (Fig. 1A). Examination of behavioral responses to individual filaments using a non-linear regression fit of the paw withdrawal frequency (Fig. 1B-D) revealed a distinct leftward shift in the 50% paw withdrawal frequency that depended on the C5a dose and the post-injection time at which the measurements were taken. Based on these data and the previous reports [26; 27; 44], 500 ng was chosen as the standard dose for all subsequent experiments.
Figure 1. C5a produces dose-dependent mechanical hypersensitivity.
(A) Paw withdrawal thresholds in WT (C57BL/6J) mice, as measured before (time=0 hr) and after injection of 10 ng (green circles; n=4), 100 ng (red squares; n=4), or 500 ng (black triangle; n=5) of C5a (in 10 µL of saline) into the plantar surface of the hindpaw. Significant main effect of time: F5,50=18.31; p<0.001 (two-way repeated-measures ANOVA). *p<0.05, **p<0.01, ***p<0.001, relative to baseline (Dunnett post hoc test). Color of each asterisk denotes significance of change relative to baseline for the corresponding test group. (B-D) Dose response curves were calculated based on the experiments in (A) using non-linear, four parameter regression model for 500 ng (B), 100 ng (C), and 10 ng (D) C5a injection. Horizontal dotted black line indicates 50% withdrawal frequency.
3.2. Macrophages are required for C5a-induced mechanical hypersensitivity in both male and female mice
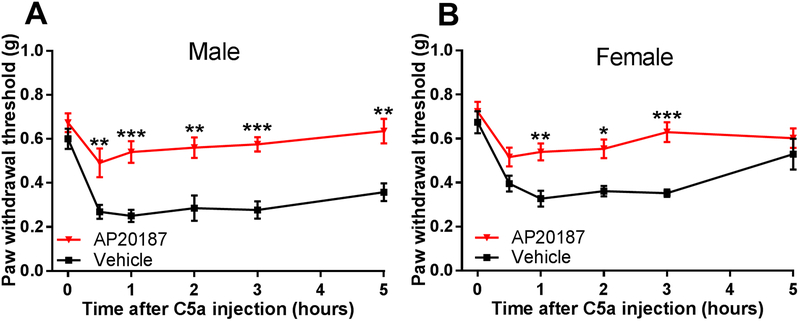
In the skin, C5aR1 is predominantly expressed in macrophages, and its activation induces robust Ca2+ mobilization in these cells [63]. We therefore examined the role of macrophages in C5a-induced mechanical sensitization by utilizing the chemogenetic mouse model of macrophage depletion known as macrophage FAS-induced apoptosis (MAFIA). The transgenic MAFIA mice express a drug-inducible suicide gene under the control of the macrophage/monocyte-specific colony-stimulating factor (CSF) 1 receptor (c-fms) promoter [3]. Systemic administration of the designer drug AP20187 for 5 days, which significantly depletes macrophage populations in mouse hindpaws [63], resulted in a significant reduction of C5a-induced mechanical hypersensitivity (Fig. 2). In vehicle-treated MAFIA mice, intraplantar injection of C5a induced a strong and long-lasting mechanical hypersensitivity, which was similar to the effects observed in WT mice (Fig. 1A). Given that different immune cells can mediate pain hypersensitivity in male and female mice [66], we also examined the effects of C5a administration and macrophage depletion in female mice. As in male mice, intraplantar injection of C5a produced significant mechanical hypersensitivity in female mice, and this response was markedly attenuated by macrophage depletion (Fig. 2B). Thus, macrophages are required for the induction of C5a-induced mechanical sensitization in both male and female mice.
Figure 2. Macrophages are required for C5a-induced mechanical sensitization.
(A and B) Paw withdrawal thresholds as measured before (time=0 hr) and after injection of C5a (500 ng) into the hindpaw plantar surface of male (A) or female (B) MAFIA mice treated with vehicle (black) or AP20187 (red; macrophage-depleted) for 5 days prior to behavioral testing as described in the Methods. Males: AP20187 n=8, vehicle n=5. Females: AP20187 n=6, vehicle n=5. Significant main effect of time: F5,55=7.46, p<0.0001 for males and F5,55=11.46, p<0.0001 for females; significant main effect of AP20187 treatment: F1,11=54.47, p<0.0001 for males and F1,9=28.36, p<0.001 for females (two-way repeated-measures ANOVA). *p<0.05, **p<0.01, ***p<0.001, for AP20187 relative to vehicle-injected mice (Holm-Sidak post hoc test). In addition, there was no significant difference in the response to C5a between vehicle(AP20187)-treated male and female mice (two-way repeated-measures ANOVA with Holm-Sidak post hoc test).
3.3. TRPV1 is required for the initiation and maintenance of C5a-induced mechanical hypersensitivity
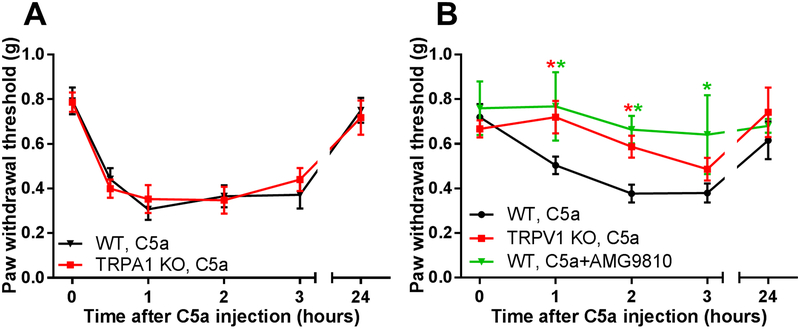
In order to identify an ion channel or receptor capable of mediating the pronociceptive effects of C5a, we tested a potential involvement of TRPA1, which has been implicated in multiple forms of mechanical nociception [30; 31; 33; 48]. We found that TRPA1 KO mice did not differ from WT mice in terms of their response to C5a administration (Fig. 3A), suggesting that TRPA1 does not contribute to C5a-induced mechanical sensitization.
Figure 3. C5a-induced mechanical sensitization requires TRPV1, but not TRPA1.
(A) Hindpaw mechanical sensitivity was measured before and after intraplantar injection of C5a (500 ng), in WT (black; n=6) and TRPA1 KO (red; n=7) mice. There was no statistically significant difference in the response of C5a relative to WT mice (two-way repeated measures ANOVA with Holm-Sidak post hoc test). Significant main effect of time: F5,66=25.46, p<0.001. (B) Mechanical sensitization to 500 ng C5a was determined for 3 treatment groups: (1) WT mice injected with C5a alone (black circles; n=10), (2) WT mice co-injected with C5a and 67 ng of the TRPV1 antagonist AMG9810 (green triangles; n=6), and (3) TRPV1 KO mice injected with C5a (red squares; n=9). Paw withdrawal thresholds were measured before (time=0 hr) and at multiple times after C5a/drug injection. Significant main effect of time: F4,104=4.61, p<0.001; Significant main effect of treatment/genotype: F2,104=7.779, p<0.001 (two-way ANOVA test). *p<0.05, is relative to “WT, C5a” (Holm-Sidak post hoc test). Green and red asterisks correspond to “WT, C5a+AMG” and “TRPV1KO, C5a” mouse groups, respectively.
Recently, we reported that another TRP channel, TRPV1, mediates C5a-induced thermal hypersensitivity [63]. In addition, several studies showed that TRPV1 can contribute to mechanical sensitization [14; 43; 45; 76]. Therefore, we tested TRPV1 for a role in C5a-mediated mechanical sensitization, and found that the effect of C5a was significantly diminished in TRPV1 KO mice (Fig. 3B). Consistent with this genetic approach, pre-treatment with the TRPV1 antagonists, AMG9810 (Fig. 3B) or 5’-IRTX (Fig. 4A), significantly decreased C5a-induced mechanical hypersensitivity.
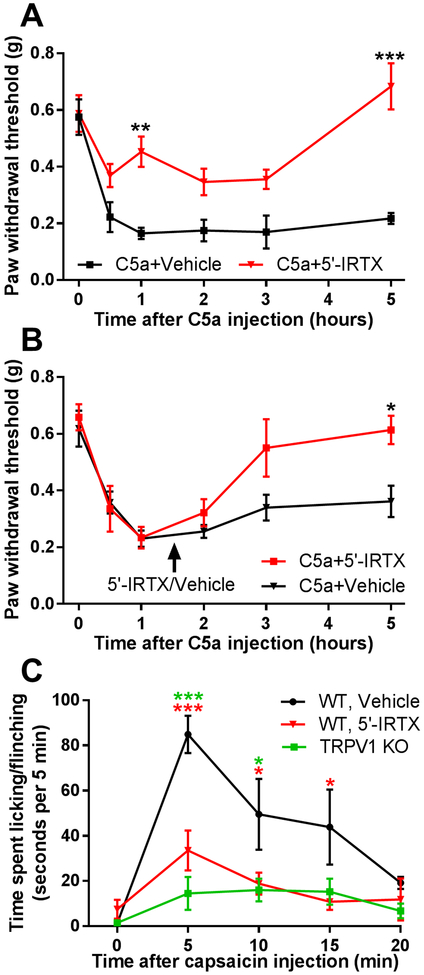
Figure 4. TRPV1 is required for both initiation and maintenance of C5a-induced mechanical hypersensitivity.
(A) Pre-treatment with the TRPV1 antagonist 5’-IRTX (10 µL of 50 nM) reduced mechanical hypersensitivity elicited by C5a (500 ng). 5’-IRTX (red, n=5) or vehicle (black, n=4) were injected into the plantar surface of the hindpaw 30 min prior to C5a administration. There was a significant interaction between the effects of time and 5’-IRTX treatment: F5,35=4.596, p=0.0025. Significant main effect of time: F5,35=13.11, p<0.001; Significant main effect of 5’-IRTX treatment: F1,7=32.55, p<0.001 (two-way repeated-measures ANOVA). **p<0.01, ***p<0.001, relative to vehicle-treated mice (Holm-Sidak post hoc test). (B) Delayed (90 min post C5a; arrow) treatment with 5’-IRTX (10 µL of 50 nM; red, n=5), but not vehicle (black, n=5), slowly reversed mechanical hypersensitivity induced by C5a (500 ng). There was a significant interaction between the effects of time and 5’-IRTX treatment: F5,40=2.637, p=0.0375; Significant main effect of time: F5,40=18.17, p<0.001 (two-way repeated-measures ANOVA). *p<0.05, relative to sham (Holm-Sidak post hoc test). (C) Pre-treatment with 5’-IRTX (10 µL of 50 nM) blocked spontaneous pain behaviors elicited by intraplantar injection of capsaicin (1.6 µg in 10 µL). WT mice were pre-treated with either 5’-IRTX (red, n=6) or vehicle (black, n=6) by subcutaneous injection into the plantar surface of the hindpaw 30 min prior to capsaicin administration. TRPV1 KO mice (green, n=4) were naïve prior to capsaicin injection. Spontaneous pain behaviors were quantified as cumulative time spent licking and flinching. Baseline (time=0 min) was obtained 5 min prior to capsaicin injection. There was a significant interaction between the effects of time and treatment: F8,52=3.065, P=0.0067; Significant main effect of time: F4,52=9.795, p<0.001; Significant main effect of 5’-IRTX treatment/genotype: F2,13=13.29, p<0.001 (two-way repeated-measures ANOVA). *p<0.05, ***p<0.001, “WT, 5’-IRTX” (red asterisks) or “TRPV1 KO” (green asterisks) relative to “WT, Vehicle” (Holm-Sidak post hoc test).
To address whether TRPV1 contributes only to the initiation of mechanical hypersensitivity or is also required for its maintenance, we blocked TRPV1 activity after C5a-induced mechanical hypersensitivity had fully developed. Local administration of 5’-IRTX 90 min after C5a injection produced a slow (2–3 hrs) but complete reversal of C5a-induced mechanical hypersensitivity (Fig. 4B). The slow time course of the 5’-IRTX effect suggests that TRPV1 functions as a regulator of mechanical sensitivity rather than a direct sensor of mechanical stimulus in the context of the C5a effect. In the latter case, the reversal of mechanical hypersensitivity would have been expected to occur much faster. Indeed, 30 min of pretreatment with 5’-IRTX was sufficient to prevent the development of spontaneous nocifensive behavior in response to intraplantar administration of the TRPV1 agonist capsaicin (Fig. 4C). Overall, these results demonstrate that TRPV1 is required for both the initiation and maintenance of C5a-induced mechanical hypersensitivity. They also suggest that C5a-dependent TRPV1 activation triggers additional signaling events, e.g. release of neuroactive peptides and neurogenic inflammation that ultimately produce prolonged sensitization of mechanosensitive afferent fibers.
3.4. CGRP receptor is required for C5a-induced mechanical sensitization
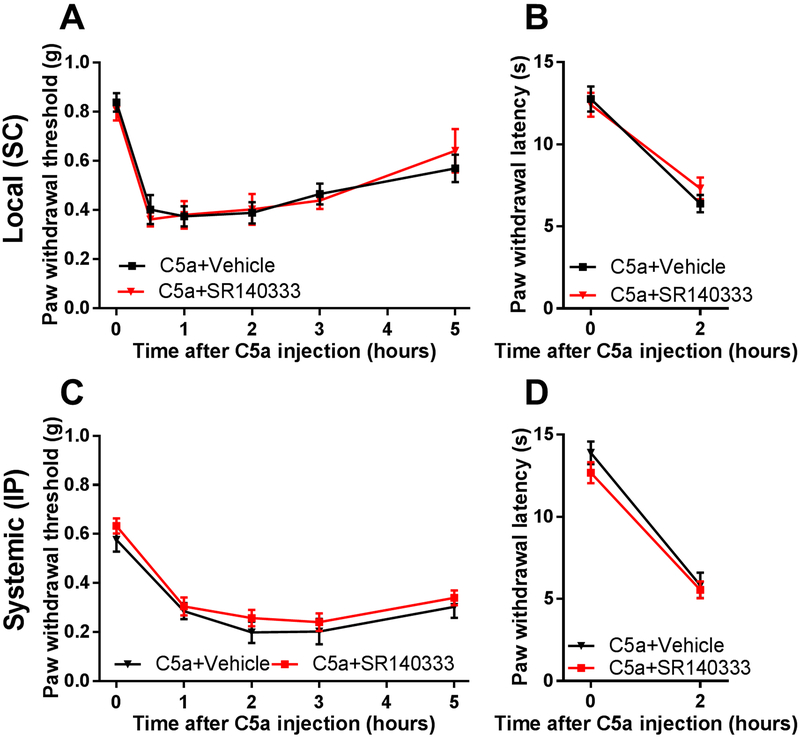
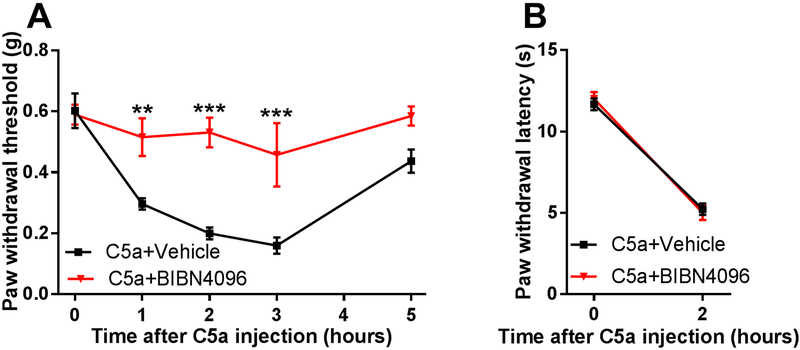
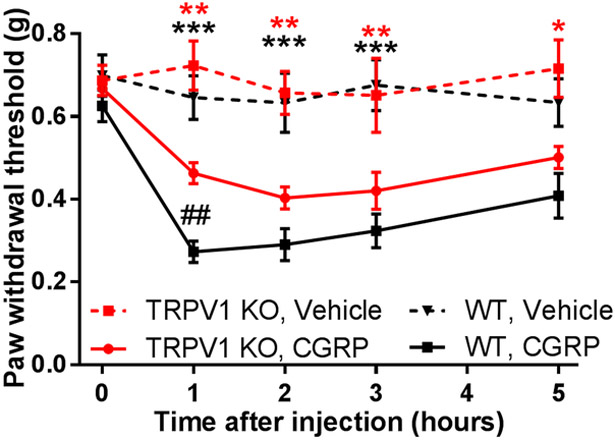
A large subset of TRPV1-positive neurons express the neuroactive peptides, substance P (SP) and CGRP [5; 71], that could be released upon TRPV1 activation to produce neurogenic inflammation and mechanical sensitization [19; 49; 51]. Therefore, we examined the role of these neuropeptides and their receptors in C5a-induced mechanical hypersensitivity. First, we tested the effect of pharmacologic blockade of the SP receptor, neurokinin 1 receptor (NK1R), using its selective antagonist SR140333 [16; 53]. Neither local (Fig. 5A, B) nor systemic administration (Fig. 5C, D) of SR140333 significantly affected mechanical (Fig. 5A, C) or thermal (Fig. 5B, D) hypersensitivity induced by C5a. In contrast, pretreatment with the selective CGRP receptor antagonist BIBN4096 [55] completely prevented C5a-induced mechanical sensitization (Fig. 6A), although it had no effect on C5a-induced thermal hypersensitivity (Fig. 6B). Thus, CGRP, but not SP, appeared to play a significant role in mediating C5a-induced mechanical sensitization. This conclusion was further supported by testing the effects of intraplantar administration of CGRP. This treatment resulted in prominent mechanical hypersensitivity (Fig. 7) not only in WT but also in TRPV1 KO mice, consistent with the idea that CGRP acts downstream of TRPV1 in the signaling cascade that mediates C5a-induced mechanical sensitization.
Figure 5. NK1 receptor is not required for C5a-induced mechanical or thermal hypersensitivity.
(A and B) Pre-treatment with the NK1 receptor antagonist SR140333 via subcutaneous administration (SC; 1 nmol; n=7) 30 min prior to C5a injection (500 ng) did not significantly affect C5a-induced mechanical (A) or thermal (B) hypersensitivity relative to vehicle-treated mice (n=6) (two-way repeated-measures ANOVA with Holm-Sidak post hoc test). Significant main effect of time: F5,55=24.8, p<0.0001 for mechanical sensitivity and F1,11=95.12, p<0.0001 for thermal sensitivity. (C and D), Pre-treatment with the NK1 receptor antagonist, SR140333 via intraperitoneal administration (IP; 1 mg/kg; n=6) 30 min prior to C5a injection (500 ng) did not significantly affect C5a-induced mechanical (C) or thermal (D) hypersensitivity relative to vehicle-treated mice (n=5) (two-way repeated-measures ANOVA with Holm-Sidak post hoc test). Significant main effect of time: F4,32=43.73, p<0.0001 for mechanical sensitivity and F1,9=110, p<0.0001 for thermal sensitivity.
Figure 6. CGRP receptor is required for C5a-induced mechanical, but not thermal, hypersensitivity.
Pre-treatment with the CGRP receptor antagonist BIBN4096 (10 mg/kg IP; n=8) 30 min prior to C5a injection (500 ng) significantly reduced C5a-induced mechanical (A) but not thermal (B) hypersensitivity, compared to vehicle pre-treated animals (n=8). (A) For mechanical sensitivity, there was a significant interaction between the effects of time and treatment: F4,56=4.74, P=0.0023. Significant main effect of time: F4,56=13.38, p<0.0001; Significant main effect of BIBN4096 treatment: F1,14=20.42, p<0.001 (two-way repeated-measures ANOVA). **p<0.01, ***p<0.001, relative to vehicle (Holm-Sidak post hoc test). (B) BIBN4096 pretreatment did not significantly affect C5a-induced thermal hypersensitivity (two-way repeated-measures ANOVA with Holm-Sidak post hoc test). Significant main effect of time: F1,14=206.7, p<0.0001.
Figure 7. CGRP administration induces mechanical hypersensitivity.
Intraplantar injection of CGRP (5 µg in 10 µL) in WT (solid black; n=7) and TRPV1 KO mice (solid red; n=11) produced significant mechanical sensitization relative to vehicle-injected WT (dashed black; n=11) and TRPV1 KO (dashed red; n=4). Baseline corresponds to 0 hr time point. There was a significant interaction between the effects of time and treatment: F12,84=2.561, P=0.0061. Significant main effect of time: F4,84=10.2, p<0.001; Significant main effect of treatment/genotype: F3,21=23.76, p<0.001 (two-way repeated-measures ANOVA). *p<0.05, **p<0.01, ***p<0.001, CGRP relative to vehicle injection (Holm-Sidak. post hoc test). Red and black asterisks correspond to TRPV1 KO and WT, respectively. ## p<0.01 is “WT, CGRP” relative to “TRPV1 KO, CGRP” (two-way repeated-measures ANOVA with Holm-Sidak post hoc test, main effects listed above).
3.5. NGF contributes to C5a-induced mechanical sensitization.
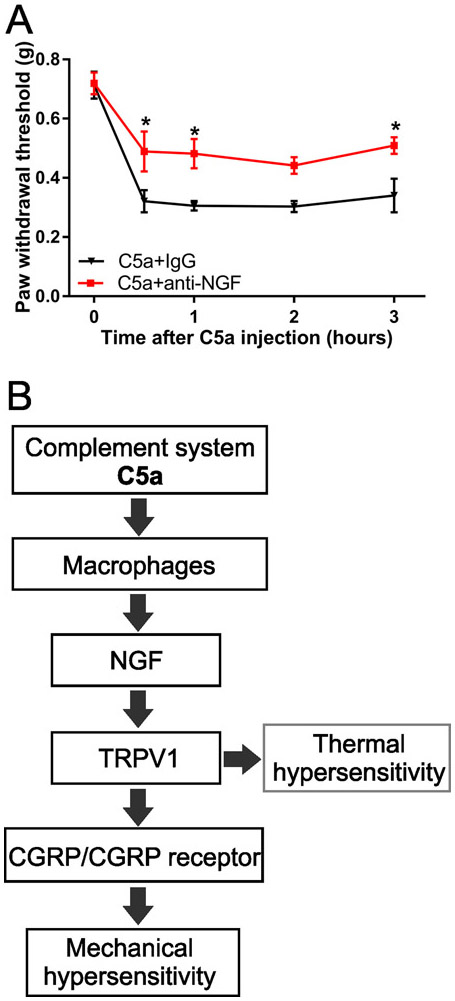
Having shown that C5a produces mechanical sensitization that depends on macrophages and TRPV1, we next sought to identify the factor(s) that could signal from C5a-stimulated macrophages to TRPV1-expressing nociceptors. Recently we found that nerve growth factor (NGF) can play such a role in mediating C5a-induced thermal hypersensitivity [63]. Given that NGF is also expressed and released by macrophages [20; 40; 47] and that it sensitizes TRPV1 via TrkA receptor activation [10; 39; 83; 84], we tested whether NGF plays a role in C5a-induced mechanical sensitization. Specifically, we utilized an NGF-neutralizing antibody (anti-NGF) that was validated by others [8; 9; 79] and used previously by our group [63]. Pretreatment with anti-NGF, but not a control IgG, 30 min prior to C5a administration significantly reduced the effect of C5a on paw withdrawal threshold (Fig. 8A), suggesting that NGF contributes to C5a-induced mechanical sensitization.
Figure 8. C5a-induced mechanical sensitization depends on NGF.
(A) Mechanical hypersensitivity induced by C5a (500 ng, intraplantar) was reduced by pretreatment with an NGF neutralizing antibody (1 mg; 30 min prior to C5a injection; red; n=6), but not by control IgG (1 mg; 30 min prior to C5a injection; black; n=6). Significant main effect of time: F4,40=35.08, p<0.001; Significant main effect of anti-NGF treatment: F1,10=11.04, p=0.0077 (two-way repeated-measures ANOVA). *p<0.05, relative to IgG treatment (Holm-Sidak post hoc test). (B) Proposed model describes the pronociceptive signaling of complement C5a in the periphery by integrating conclusions from the current study focused on mechanical hypersensitivity with those from our previous work focused on thermal hypersensitivity [63].
4. Discussion
The results presented here define a novel pathway through which C5a produces mechanical hypersensitivity. From the cellular perspective, we show that C5a acts through macrophages to initiate an intercellular signaling cascade that sensitizes primary afferents to mechanical stimuli. At the molecular level, we demonstrate that this macrophage-to-sensory-neuron signaling critically depends on TRPV1 and CGRP receptor activation in eliciting strong and prolonged mechanical hypersensitivity (Fig. 8B).
4.1. Macrophages in C5a signaling and pain sensitization
Recognition of the modulatory role of the immune system in nociception has led to a rapid growth of knowledge surrounding neuro-immune interactions in pain. We have examined one aspect of neuro-immune modulation and determined how activation of the complement cascade produces mechanical hypersensitivity. Although the hyperalgesic activity of the complement cascade has been highlighted in numerous reports over the last decade [23; 32; 37; 44], the cellular mechanisms linking the complement system to mechanical hypersensitivity had not been determined. Here, we show that C5a-induced mechanical sensitization is dependent on macrophages (Fig. 2). Recent studies suggested that differential activation of immune cell populations in male and female mice is responsible for sexual dimorphism observed in some chronic pain conditions [66]. To this end, male and female mice did not differ significantly with respect to the effects of C5a and macrophage depletion (Fig. 2), indicating that the particular complement-mediated pain pathway described in our work is similar in both sexes.
Our findings revealing the key role of macrophages in mediating the pronociceptive effects of C5a appear to disagree with a previous report suggesting that C5a acted through neutrophils [69]. However, that conclusion was based on experiments using the cytotoxic agent vinblastine that in addition to depleting neutrophils, also eliminates most myeloid cells including monocytes and macrophages [7; 42]. The specificity of the MAFIA model for macrophage/monocyte-derived cells versus other myeloid cells such as neutrophils [3] argues that macrophages are the primary responders to C5a in the skin. This does not rule out that in a chronic disease state such as rheumatoid arthritis, surgical pain, or neuropathic pain, other immune cells such as neutrophils may still contribute to C5a signaling. Indeed, neutrophils are known to be C5a-sensitive [59; 65] and important for wound/disease responses and healing [29].
Overall, identification of macrophages as the primary first responders to complement activation in skin adds to a rapidly expanding spectrum of macrophage functions in the pathogenesis of chronic inflammatory and neuropathic pain states [36; 50; 60-62; 80].
4.2. The role of TRPV1 and NGF in C5a-induced mechanical sensitization
We demonstrated that TRPV1, and not TRPA1, is an essential mediator of C5a-induced mechanical sensitization using both genetic and pharmacologic approaches (Fig. 3, 4). We also found that NGF plays a significant role in C5a-induced mechanical sensitization (Fig. 8A), consistent with the proposed role of NGF as a critical signaling mediator in macrophage-to-nociceptor communication (Fig. 8B) [63]. NGF acts via the TrkA receptor to facilitate membrane trafficking and function of TRPV1 through multiple signaling pathways [10; 83; 84]. This could potentially promote the release of CGRP and other neuropeptides [56], causing paracrine sensitization of mechanosensitive fibers, similar to capsaicin-induced neurogenic inflammation that also results in mechanical sensitization [14; 15; 45]. The proposed involvement of neuropeptide release and neurogenic inflammation downstream of TRPV1 activation is consistent with the slow reversal of C5a-induced mechanical hypersensitivity by administering the TRPV1 antagonist 5’-IRTX (Fig. 4B). Notably, in human subjects intradermal C5a injection produced a significant wheal-and-flare reaction, which was prevented by local pre-treatment with lidocaine [81]; the latter suggests that C5a-induced inflammation has a neurogenic component, consistent with our findings.
Although the NGF-neutralizing antibody significantly reduced C5a-induced mechanical hypersensitivity in our experiments, this inhibition was incomplete (Fig. 8A). This is in contrast to the full inhibition of C5a-induced thermal hypersensitivity by the same antibody [63]. This also differs from the nearly complete blockade of C5a-induced mechanical sensitization achieved by macrophage depletion (Fig. 2), TRPV1 KO (Fig. 3, 4) or CGRP receptor antagonism (Fig. 6). It is possible that besides NGF, additional inflammatory factors mediate the sensitizing effects of C5a downstream of macrophages. This is consistent with our previous report that a large number of cytokines and pro-inflammatory modulators are rapidly mobilized in the skin in response to injection of C5a [63], and that many of these mediators (e.g., TNF-α, IL1-β and IL-6) can produce mechanical sensitization [1; 41; 69]. Future studies will be necessary to determine whether there are additional factors mobilized by C5a that significantly contribute to macrophage-to-sensory-neuron signaling and the resulting mechanical hypersensitivity.
4.3. CGRP in C5a-induced mechanical sensitization
We found that the CGRP, but not NK1, receptors are essential for C5a-induced mechanical sensitization (Fig. 5, 6). Moreover, intraplantar administration of CGRP mimicked the behavioral effects of C5a. Importantly, the latter action did not require TRPV1 (Fig. 7), suggesting that CGRP acts downstream of TRPV1 in the pronociceptive C5a signaling pathway described here. The most likely explanation for our findings is that TRPV1-dependent mobilization of CGRP from peptidergic afferent fibers, and the resulting activation of CGRP receptors, are essential signaling events in C5a-induced mechanical sensitization (Fig. 8B). CGRP is an important mediator of pronociceptive signaling both in the peripheral and central nervous systems. TRPV1-dependent release of CGRP in the periphery is well documented both in rodents and humans [4; 18; 64; 72], and this process plays an important role in the development of neurogenic inflammation and peripheral sensitization [25; 51]. Peripherally released CGRP can activate receptors expressed in vascular smooth muscles to produce vasodilation, and in macrophages to regulate chemotaxis, phagocytosis and cytokine secretion [56; 68; 74]. Interestingly, macrophages can accumulate around CGRP-positive fibers in the setting of inflammatory pathology [70], and CGRP release from peptidergic fibers during inflammation activates macrophages, further enhancing neurogenic inflammation [21]. CGRP receptors are also found in DRG neurons suggesting that CGRP can act directly at primary sensory fibers to change their excitability [12]. Indeed, CGRP was shown to enhance tetrodotoxin-resistant voltage-gated Na+ currents in DRG neurons via protein kinases A- and C-dependent mechanisms [46]. Thus, CGRP can contribute to C5a-induced mechanical sensitization through both nerve fibers and non-neuronal cells in the skin.
Whether the proposed TRPV1- and CGRP-dependent mechanical sensitization involves the same or distinct populations of nociceptors is unclear. Based on our results and published literature, it is likely that C5a-induced mechanical sensitization involves at least two serially activated populations of fibers, first, a peptidergic-TRPV1/CGRP population, and second, a TRPV1-negative mechanically-sensitive population. This idea is consistent with the markedly delayed reversal of mechanical sensitization produced by the TRPV1 antagonist 5’-IRTX (Fig. 4B), which was substantially slower than the time required for TRPV1 inhibition per se (Fig. 4C). In addition, this model is in agreement with the finding that distinct subsets of sensory fibers mediate noxious thermal (TRPV1-positive) and mechanical (Mrgprd-positive) behavioral responses [6]. Finally, optogenetic silencing of CGRPα-expressing neurons in naive animals significantly reduced acute thermal sensitivity, but did not affect response to mechanical stimulation [13], further supporting the idea that distinct subsets of primary sensory fibers are involved in the development of C5a-induced mechanical hypersensitivity.
In conclusion, we identified critical signaling components that mediate complement C5a-induced mechanical sensitization in the periphery (Fig. 8B). Our work highlights the complexity of neuro-immune interaction in the development of pain hypersensitivity and defines C5a/C5aR1, NGF, TRPV1 and CGRP receptors as key elements of this interaction that could be therapeutically targeted for alleviating pain driven by hyperactivation of the complement system.
Acknowledgments
This work was supported by National Institutes of Health grants NS096246 and DK116624, and Iowa Neuroscience Institute Research Program of Excellence grant. C.A.W. was supported by a predoctoral fellowship through NIH T32 Grant NS045549, by an individual predoctoral fellowship through Pharmaceutical Research & Manufacturers of America Foundation and by a postdoctoral fellowship through NIH T32 Grant DK112751.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
REFERENCES
- [1].Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 2007;449(7162):607–610. [DOI] [PubMed] [Google Scholar]
- [2].Brennan FH, Anderson AJ, Taylor SM, Woodruff TM, Ruitenberg MJ. Complement activation in the injured central nervous system: another dual-edged sword? J Neuroinflammation 2012;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. Journal of leukocyte biology 2004;75(4):612–623. [DOI] [PubMed] [Google Scholar]
- [4].Calcott G, White JP, Nagy I. Xenon fails to inhibit capsaicin-evoked CGRP release by nociceptors in culture. Neurosci Lett 2011;499(2):124–126. [DOI] [PubMed] [Google Scholar]
- [5].Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 2011;31(28):10119–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A 2009;106(22):9075–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen MG, Schooley JC. Effects of ionizing radiation and vinblastine on the proliferation of peritoneal macrophage precursors in the mouse. Radiat Res 1970;41(3):623–636. [PubMed] [Google Scholar]
- [8].Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. Journal of neuropathology and experimental neurology 2009;68(11):1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng HT, Dauch JR, Oh SS, Hayes JM, Hong Y, Feldman EL. p38 mediates mechanical allodynia in a mouse model of type 2 diabetes. Mol Pain 2010;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001;411(6840):957–962. [DOI] [PubMed] [Google Scholar]
- [11].Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology 2006;104(6):1274–1282. [DOI] [PubMed] [Google Scholar]
- [12].Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 2005;490(3):239–255. [DOI] [PubMed] [Google Scholar]
- [13].Cowie AM, Moehring F, O’Hara C, Stucky CL. Optogenetic Inhibition of CGRPα Sensory Neurons Reveals Their Distinct Roles in Neuropathic and Incisional Pain. J Neurosci 2018;38(25):5807–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Culp WJ, Ochoa J, Cline M, Dotson R. Heat and mechanical hyperalgesia induced by capsaicin. Cross modality threshold modulation in human C nociceptors. Brain 1989;112 ( Pt 5):1317–1331. [DOI] [PubMed] [Google Scholar]
- [15].Davis AJ, Perkins MN. Substance P and capsaicin-induced mechanical hyperalgesia in the rat knee joint; the involvement of bradykinin B1 and B2 receptors. Br J Pharmacol 1996;118(8):2206–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Denadai-Souza A, Camargo Lde L, Ribela MT, Keeble JE, Costa SK, Muscara MN. Participation of peripheral tachykinin NK1 receptors in the carrageenan-induced inflammation of the rat temporomandibular joint. Eur J Pain 2009;13(8):812–819. [DOI] [PubMed] [Google Scholar]
- [17].Doolen S, Cook J, Riedl M, Kitto K, Kohsaka S, Honda CN, Fairbanks CA, Taylor BK, Vulchanova L. Complement 3a receptor in dorsal horn microglia mediates pronociceptive neuropeptide signaling. Glia 2017;65(12):1976–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Franco-Cereceda A, Henke H, Lundberg JM, Petermann JB, Hokfelt T, Fischer JA. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides 1987;8(2):399–410. [DOI] [PubMed] [Google Scholar]
- [19].Gamse R, Holzer P, Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol 1980;68(2):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Garaci E, Caroleo MC, Aloe L, Aquaro S, Piacentini M, Costa N, Amendola A, Micera A, Calio R, Perno CF, Levi-Montalcini R. Nerve growth factor is an autocrine factor essential for the survival of macrophages infected with HIV. Proc Natl Acad Sci U S A 1999;96(24):14013–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Glowka TR, Steinebach A, Stein K, Schwandt T, Lysson M, Holzmann B, Tsujikawa K, de Jonge WJ, Kalff JC, Wehner S. The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterol Motil 2015;27(7):1038–1049. [DOI] [PubMed] [Google Scholar]
- [22].Grant EP, Picarella D, Burwell T, Delaney T, Croci A, Avitahl N, Humbles AA, Gutierrez-Ramos JC, Briskin M, Gerard C, Coyle AJ. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med 2002;196(11):1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci 2007;27(32):8699–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol 2014;32:433–459. [DOI] [PubMed] [Google Scholar]
- [25].Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017;158(4):543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jang JH, Clark JD, Li X, Yorek MS, Usachev YM, Brennan TJ. Nociceptive sensitization by complement C5a and C3a in mouse. Pain 2010;148:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jang JH, Liang D, Kido K, Sun Y, Clark DJ, Brennan TJ. Increased local concentration of complement C5a contributes to incisional pain in mice. J Neuroinflammation 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jose PJ, Moss IK, Maini RN, Williams TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis 1990;49(10):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- [30].Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006;50(2):277–289. [DOI] [PubMed] [Google Scholar]
- [31].Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 2009;29(15):4808–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].LaCroix-Fralish ML, Austin JS, Zheng FY, Levitin DJ, Mogil JS. Patterns of pain: meta-analysis of microarray studies of pain. Pain 2011;152(8):1888–1898. [DOI] [PubMed] [Google Scholar]
- [33].Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One 2012;7(8):e43597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain 2008;137(1):182–201. [DOI] [PubMed] [Google Scholar]
- [35].Levine JD, Gooding J, Donatoni P, Borden L, Goetzl EJ. The role of the polymorphonuclear leukocyte in hyperalgesia. J Neurosci 1985;5(11):3025–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li Z, Wei H, Piirainen S, Chen Z, Kalso E, Pertovaara A, Tian L. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav Immun 2016;58:107–117. [DOI] [PubMed] [Google Scholar]
- [37].Liang DY, Li X, Shi X, Sun Y, Sahbaie P, Li WW, Clark JD. The complement component C5a receptor mediates pain and inflammation in a postsurgical pain model. Pain 2012;153(2):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loo L, Shepherd AJ, Mickle AD, Lorca RA, Shutov LP, Usachev YM, Mohapatra DP. The C-type natriuretic peptide induces thermal hyperalgesia through a noncanonical Gbetagamma-dependent modulation of TRPV1 channel. J Neurosci 2012;32(35):11942–11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 2011;31(29):10516–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mallat M, Houlgatte R, Brachet P, Prochiantz A. Lipopolysaccharide-stimulated rat brain macrophages release NGF in vitro. Dev Biol 1989;133(1):309–311. [DOI] [PubMed] [Google Scholar]
- [41].Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain 2010;151(2):345–355. [DOI] [PubMed] [Google Scholar]
- [42].Martin F, Olsson NO, Jeannin JF. Effects of four agents that modify microtubules and microfilaments (vinblastine, colchicine, lidocaine and cytochalasin B) on macrophage-mediated cytotoxicity to tumor cells. Cancer Immunol Immunother 1981;1981:113–119. [Google Scholar]
- [43].Mickle AD, Shepherd AJ, Loo L, Mohapatra DP. Induction of thermal and mechanical hypersensitivity by parathyroid hormone-related peptide through upregulation of TRPV1 function and trafficking. Pain 2015;156(9):1620–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moriconi A, Cunha TM, Souza GR, Lopes AH, Cunha FQ, Carneiro VL, Pinto LG, Brandolini L, Aramini A, Bizzarri C, Bianchini G, Beccari AR, Fanton M, Bruno A, Costantino G, Bertini R, Galliera E, Locati M, Ferreira SH, Teixeira MM, Allegretti M. Targeting the minor pocket of C5aR for the rational design of an oral allosteric inhibitor for inflammatory and neuropathic pain relief. Proc Natl Acad Sci U S A 2014;111(47):16937–16942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain 1997;71(2):179–186. [DOI] [PubMed] [Google Scholar]
- [46].Natura G, von Banchet GS, Schaible HG. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. Pain 2005;116(3):194–204. [DOI] [PubMed] [Google Scholar]
- [47].Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, Yonenobu K, Yoshikawa H, Noguchi K. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain 2002;99(1–2):121–132. [DOI] [PubMed] [Google Scholar]
- [48].Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain 2007;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch 2005;451(1):151–159. [DOI] [PubMed] [Google Scholar]
- [50].Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010;16(11):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 2002;302(3):839–845. [DOI] [PubMed] [Google Scholar]
- [52].Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. Journal of Immunology 2013;190(8):3831–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rittner HL, Lux C, Labuz D, Mousa SA, Schafer M, Stein C, Brack A. Neurokinin-1 receptor antagonists inhibit the recruitment of opioid-containing leukocytes and impair peripheral antinociception. Anesthesiology 2007;107(6):1009–1017. [DOI] [PubMed] [Google Scholar]
- [54].Roxvall L, Bengtson A, Heideman M. Anaphylatoxin generation in acute pancreatitis. J Surg Res 1989;47(2):138–143. [DOI] [PubMed] [Google Scholar]
- [55].Rudolf K, Eberlein W, Engel W, Pieper H, Entzeroth M, Hallermayer G, Doods H. Development of human calcitonin gene-related peptide (CGRP) receptor antagonists. 1. Potent and selective small molecule CGRP antagonists. 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]car bonyl]-D-tyrosyl]-l-lysyl]-4-(4-pyridinyl)piperazine: the first CGRP antagonist for clinical trials in acute migraine. J Med Chem 2005;48(19):5921–5931. [DOI] [PubMed] [Google Scholar]
- [56].Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94(4):1099–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sahbaie P, Li X, Shi X, Clark JD. Roles of Gr-1+ leukocytes in postincisional nociceptive sensitization and inflammation. Anesthesiology 2012;117(3):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008;28(19):4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 2009;20(2):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shepherd AJ, Copits BA, Mickle AD, Karlsson P, Kadunganattil S, Haroutounian S, Tadinada SM, de Kloet AD, Valtcheva MV, McIlvried LA, Sheahan TD, Jain S, Ray PR, Usachev YM, Dussor G, Krause EG, Price TJ, Gereau RWt, Mohapatra DP. Angiotensin II Triggers Peripheral Macrophage-to-Sensory Neuron Redox Crosstalk to Elicit Pain. J Neurosci 2018;38(32):7032–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shepherd AJ, Mickle AD, Golden JP, Mack MR, Halabi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RWt, Mohapatra DP. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 2018;115(34):E8057–E8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11(11):762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, Woodruff TM, Clark JD, Usachev YM. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. J Neurosci 2016;36(18):5055–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depre M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974). Br J Clin Pharmacol 2010;69(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sogkas G, Vogtle T, Rau E, Gewecke B, Stegner D, Schmidt RE, Nieswandt B, Gessner JE. Orai1 controls C5a-induced neutrophil recruitment in inflammation. Eur J Immunol 2015;45(7):2143–2153. [DOI] [PubMed] [Google Scholar]
- [66].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18(8):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Strong JA, Xie W, Coyle DE, Zhang JM. Microarray analysis of rat sensory ganglia after local inflammation implicates novel cytokines in pain. PLoS One 2012;7(7):e40779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tang Y, Feng Y, Wang X. Calcitonin gene-related peptide potentiates LPS-induced IL-6 release from mouse peritoneal macrophages. J Neuroimmunol 1998;84(2):207–212. [DOI] [PubMed] [Google Scholar]
- [69].Ting E, Guerrero AT, Cunha TM, Verri WA Jr., Taylor SM, Woodruff TM, Cunha FQ, Ferreira SH. Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol 2008;153(5):1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Toriya Y, Hashiguchi I, Maeda K. Immunohistochemical examination of the distribution of macrophages and CGRP-immunoreactive nerve fibers in induced rat periapical lesions. Endod Dent Traumatol 1997;13(1):6–12. [DOI] [PubMed] [Google Scholar]
- [71].Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18(1):145–153. [DOI] [PubMed] [Google Scholar]
- [72].van Oosterhout WP, Schoonman GG, Garrelds IM, Danser AH, Chan KY, Terwindt GM, Ferrari MD, MaassenVanDenBrink A. A human capsaicin model to quantitatively assess salivary CGRP secretion. Cephalalgia 2015;35(8):675–682. [DOI] [PubMed] [Google Scholar]
- [73].Vega-Avelaira D, Geranton SM, Fitzgerald M. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Mol Pain 2009;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vignery A, Wang F, Ganz MB. Macrophages express functional receptors for calcitonin-gene-related peptide. J Cell Physiol 1991;149(2):301–306. [DOI] [PubMed] [Google Scholar]
- [75].Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov 2010;9(1):43–56. [DOI] [PubMed] [Google Scholar]
- [76].Walder RY, Radhakrishnan R, Loo L, Rasmussen LA, Mohapatra DP, Wilson SP, Sluka KA. TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. Pain 2012;153(8):1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang NK, Lai CC, Liu CH, Yeh LK, Chou CL, Kong J, Nagasaki T, Tsang SH, Chien CL. Origin of fundus hyperautofluorescent spots and their role in retinal degeneration in a mouse model of Goldmann-Favre syndrome. Disease models & mechanisms 2013;6(5):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, Crish JF, Bebek G, Ritter SY, Lindstrom TM, Hwang I, Wong HH, Punzi L, Encarnacion A, Shamloo M, Goodman SB, Wyss-Coray T, Goldring SR, Banda NK, Thurman JM, Gobezie R, Crow MK, Holers VM, Lee DM, Robinson WH. Identification of a central role for complement in osteoarthritis. Nat Med 2011;17(12):1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, Louis JC. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther 2007;322(1):282–287. [DOI] [PubMed] [Google Scholar]
- [80].Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience 2006;142(3):809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yancey KB, Hammer CH, Harvath L, Renfer L, Frank MM, Lawley TJ. Studies of human C5a as a mediator of inflammation in normal human skin. J Clin Invest 1985;75(2):486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yang C, Ding P, Wang Q, Zhang L, Zhang X, Zhao J, Xu E, Wang N, Chen J, Yang G, Hu W, Zhou X. Inhibition of Complement Retards Ankylosing Spondylitis Progression. Sci Rep 2016;6:34643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005;24(24):4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci 2007;34(4):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]