Abstract
The Hofmann-Löffler-Freytag (HLF) reaction is a prototypical example of radical-based remote functionalization of unactivated C(sp3)–H bond. While 1,5-hydrogen atom transfer (1,5-HAT) of the amidyl radical is thermodynamically favorable and is well-established, the method for the subsequent functionalization of the translocated carbon radical is still limited. We report herein two catalytic remote C(sp3)–H functionalization protocols. Cu(MeCN)4PF6-catalyzed reaction of 2-alkyl benzohydrazides 3 with TMSN3 in the presence of MeCO2OtBu affords the γ-azido amides 4, while CuCl-catalyzed reaction of 3 with Togni’s reagent provides 2-(β-trifluoromethylvinyl)benzamides 5 via an oxidative δ-trifluoromethylation of the alkyl group. Mechanistic studies suggest that the γ-azidation of benzohydrazides 3 goes through 1,5-HAT followed by a Cu-mediated azido transfer cascade, while the oxidative δ-trifluoromethylation of 3 proceeds via, after 1,5-HAT process, a radical-polar crossover mechanism.
Functionalization of carbon radicals formed via 1,5-hydrogen atom transfer of amidyl radicals is an underdeveloped protocol for remote C–H functionalization. Here, the authors report a Cu-catalyzed γ-azidation and oxidative δ-trifluoromethylation of benzohydrazides for the synthesis of more functionalized amides.
Introduction
The Hofmann–Löffler–Freytag (HLF) reaction1,2 that converts the N-haloamines to pyrrolidines constitutes a prototypical example of radical-based remote C(sp3)–H bond functionalization process3. The reaction, discovered in 1883, is a complex domino process involving the generation of the aminium radical followed by regioselective 1,5-hydrogen atom transfer (HAT), halogenation, and cyclization4–8. The instability of the N-haloamines, as well as the generally harsh conditions required for the generation and subsequent hydrogen atom abstraction of aminium radicals, limited nevertheless the full exploitation of its synthetic potential. A major breakthrough addressing this issue came from Suárez’s group. In a series of seminal papers, they reported that amides/sulfonamides can be converted to the corresponding amidyl and sulfonamidyl radicals with iodine/lead tetraacetate9,10 or iodine/phenyliodine diacetate11 as oxidants, and these electron-withdrawing group-attached nitrogen-centered radicals readily undergo intramolecular HAT under mild neutral conditions. Recently, Müniz et al.12–15 showed that the amidyl radical can be generated directly from the secondary amide under mild catalytic oxidative conditions, while the group of Knowles16 and Rovis17,18 demonstrated independently that photoredox conditions were very effective for the same purpose. Complementary to this work, significant efforts have also been made recently in identifying tailored precursors for the generation of the N-centered radicals19–25. The development of these easily available and stable amidyl radical precursors in conjunction with the recent advent of metal and visible-light photoredox catalysts allowed the execution of the HLF reaction under much milder conditions, therefore providing opportunities to expand the repertoire of the remote C(sp3)–H functionalization process. Indeed, besides the cyclization to N-heterocycles9–11,26–32, halogenation22,23,33–36 and C–S bond formation21 that are inherent to the classic radical chain mechanism of the HLF reaction, C–C bond formation via radical addition16–18,37–39, azidation/cyanation19,20,22, acetoxylation40, thiolation/alkynylation20,22, and arylation24,25 have been developed very recently.
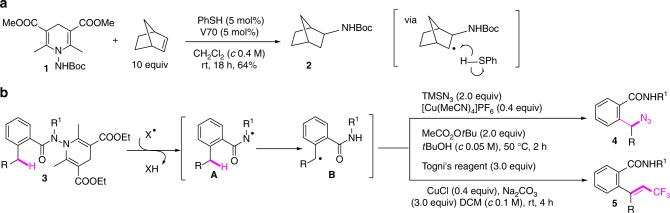
Studer et al. demonstrated that amidyl radicals can be generated from N-aminated dihydropyridines 1 and be used in the hydroamination of olefins (Fig. 1a)41,42. Mechanistically, the process is initiated by the polarity-matched abstraction of the Hantzsch ester’s C-4 hydrogen by the electrophilic thiophenol radical to afford, after fragmentation, the amidyl radical and pyridine. The addition of the former to the double bond followed by hydrogen transfer from thiophenol to the resulting radical adduct afforded the product 2 with concurrent regeneration of the thiophenol radical to propagate the chain. Inspired by these results and in connection with our interest in remote C(sp3)–H functionalization processes24,43,44, we hypothesized that if the amidyl radical A was generated by action of an electrophilic radical species (X•) whose reduced form X–H was reluctant to transfer its hydrogen to the relayed C-centered radical B, then it would be possible to functionalize B by other reactive species to afford remote functionalized amides. We assumed that electrophilic trifluoromethyl and tert-butoxy radicals could satisfy these criteria as CF3–H and tBuO–H would not be able to propagate the chain reaction due to their high bond dissociation energies (CF3–H: 107.4 kcal/mol, tBuO–H: 106.3 kcal/mol). We report herein the Cu-catalyzed γ-azidation and oxidative δ-trifluoromethylation of benzohydrazides 3 for the synthesis of amides 4 and 5, respectively (Fig. 1b). Conversion of ethyl to trifluoromethylvinyl group is, to the best of our knowledge, unknown.
Fig. 1.
Amidyl radicals and HLF reaction. a Studer’s work on the use of N-aminated dihydropyridines as precursors of N-centered radicals: hydroamination of alkenes; b benzohydrazides in HLF reaction: functionalization of remote C(sp3)–H bond. V70 = 2,2’-azobis(2,4-dimethyl-4-methoxy valeronitrile); TMSN3 = trimethylsilyl azide; MeCO2OtBu = tert-butyl peroxyacetate
Results
Cu-catalyzed γ-C(sp3)–H azidation of benzohydrazides
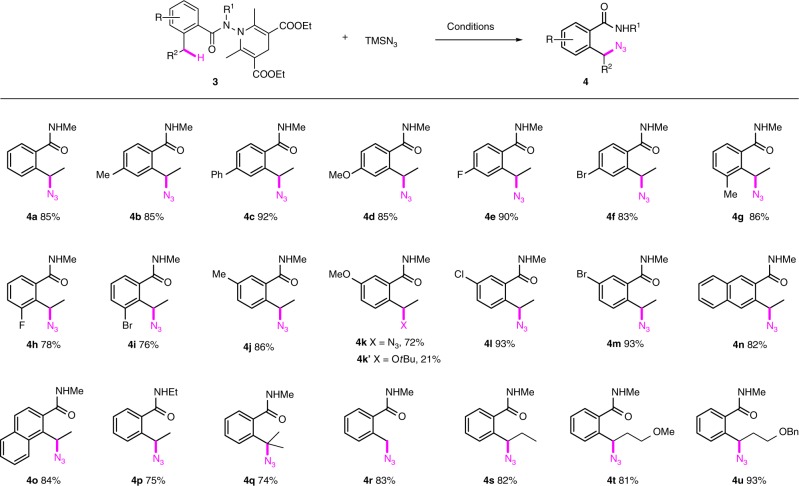
While azidation of alkenes are well developed45–48, direct azidation of C(sp3)–H bond remains challenging49. Indeed, the γ-azidation of amides was unknown at the outset of this work. However, Studer et al. have very recently reported such a transformation using TfN3 as an azide donor19. We focused on the use of stable and readily available TMSN3 as an azide source and the benzohydrazides 341,42 as precursors of amidyl radicals. In line with our working hypothesis and knowing that peroxide is compatible with the Cu-catalyzed carboazidation process50,51, the reaction of 3a (R2 = Me, R = R1 = H) with TMSN3 was investigated in the presence of copper salts and peroxides. After systematic screening of the copper sources [CuCl, CuBr, CuI, CuOTf·benzene, Cu(MeCN)4PF6, Cu(OAc)2, Cu(OTf)2, CuSO4, Cu(acac)2, Cu(ClO4)2], the peroxides (tBuOOH, tBuOOtBu, cumene hydroperoxide, PhCO2OtBu, MeCO2OtBu), and the solvents (tBuOH, DCE, 1,4-dioxane, MeCN, CF3CH2OH), the optimum conditions found consisted of heating a tBuOH solution of 3a (c 0.05 M) and TMSN3 (2.0 equiv) at 50 °C in the presence of Cu(MeCN)4PF6 (0.4 equiv) and MeCO2OtBu (2.0 equiv, 50 wt. % in odorless mineral spirits from Sigma). Under these conditions, the azido amide 4a was isolated in 85% yield (Fig. 2). A small amount of 2-(1-(tert-butoxy)ethyl)-N-methylbenzamide (see Supplementary Information) was also isolated from the reaction mixture.
Fig. 2.
γ-Azidation of 2-alkyl benzohydrazides. 3 (0.1 mmol), TMSN3 (0.2 mmol), Cu(MeCN)4PF6 (0.04 mmol), MeCO2OtBu (0.2 mmol), tBuOH (2.0 mL, c 0.05 M), 50 °C, 2 h
The scope of this azidation protocol was next examined (Fig. 2). A range of 2-alkyl substituted benzohydrazides were converted to the corresponding 2-(1-azidoalkyl)benzamides in good-to-excellent yields. Halides (F, Cl, Br) at different position of the aromatic ring was compatible with the reaction conditions affording the azido derivatives suitable for further functionalization. The primary, secondary, and tertiary benzylic carbons were all successfully functionalized to afford the corresponding azides in good to excellent yields. In the case of 3k (R = 4-MeO), the desired azido compound 4k (72%) was isolated together with a significant amount of 4k’ (X = OtBu, 21%) probably due to the presence of a strong electron-donating para-methoxy group.
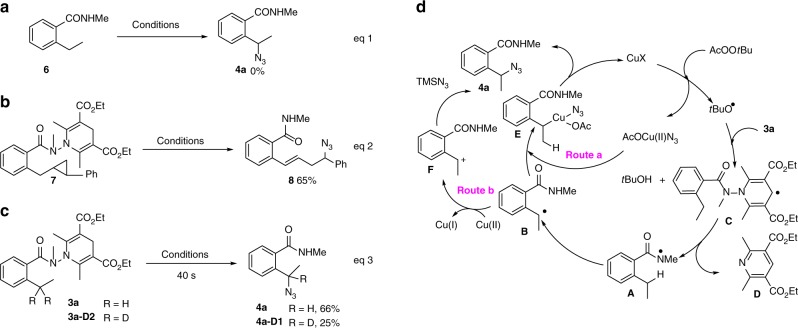
A series of control experiments were carried out to gain insight on the reaction mechanism. Treatment of N-methyl-2-ethylbenzamide (6) under standard conditions failed to produce even a trace amount of azido amide 4a indicating that 6 was not an intermediate of the reaction (Fig. 3, a). In line with the radical mechanism, azidation of 3a was completely inhibited in the presence of TEMPO or BHT, and a radical clock experiment involving cyclopropane-containing substrate 7 afforded azide 8 in 65% yield (Fig. 3, b). A side-by-side kinetic experiment using 3a and 3a-D2 provided an intermolecular KIE value of 2.6 (Fig. 3, c) suggesting that the 1,5-HAT, an off-catalytic cycle process, might be a rate-limiting step in the present domino azidation process.
Fig. 3.
Mechanistic studies on the γ-azidation of 2-alkyl benzohydrazides. a Control experiment; b radical clock experiment; c KIE experiment; d possible reaction pathway; conditions: 6, 7 or 3a/3a-D2 (0.1 mmol), TMSN3 (0.2 mmol), Cu(MeCN)4PF6 (0.04 mmol), MeCO2OtBu (0.2 mmol), tBuOH (2.0 mL, c 0.05 M), 50 °C, 2 h
On the basis of these results, a possible reaction pathway for the γ-azidation of benzohydrazide 3a is proposed (Fig. 3, d). Reduction of peroxide by Cu(I)X salt would produce AcOCuX salt and tert-butoxy radical, which would abstract the C-4 hydrogen from dihydropyridine to give tBuOH and dihydropyridine radical C. Fragmentation of the latter would lead to the amidyl radical A and pyridine D. Subsequent 1,5-HAT of A would generate the benzyl radical B which, upon radical rebound with AcOCu(II)N3 (Fig. 3, d, route a), would afford Cu(III) species E. Reductive elimination would then deliver the γ-azido amide 4a with the concurrent release of Cu(I) salt. The formation of 2-(1-(tert-butoxy)ethyl)-N-methylbenzamide could be accounted for by the presence of minor amount of tBuOCuX species in the reaction mixture52.
An alternative pathway would involve the oxidation of radical B to benzyl cation F that could then be trapped by TMSN3 to provide 4a (Fig. 3, d, route b). To verify this possibility, the reaction of 3o (R = 5-OMe, cf Fig. 2) with TMSN3 was carried out in a mixture of solvent (tBuOH/MeOH = 1:1) under otherwise identical conditions. Azide 4o was still isolated in 65% yield together with a small amount of 2-(1-(tert-butoxy)ethyl)-N-methylbenzamide (6%). Methoxylated product was not observed. This result together with the fact that isoindolinone53–55 was not formed under our conditions are in accord with the Cu-mediated redox azido transfer mechanism50,51.
Cu-catalyzed oxidative δ-C(sp3)-trifluoromethylation
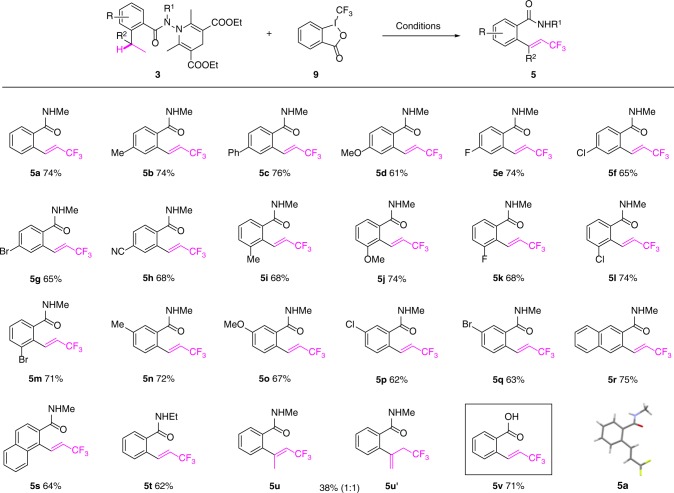
Trifluoromethylation of 2-alkyl benzohydrazides was next examined. The reaction of 3a with Togni’s reagent 946–48 in the presence of metal salts was chosen as a benchmark reaction. Instead of obtaining the N-methyl-2-(1,1,1-trifluoropropan-2-yl)benzamide, our initial experiments allowed us to isolate unexpectedly the compound 5a whose structure was confirmed by X-ray crystallographic analysis (Fig. 4). Since the reaction converting formally an ethyl group to a trifluoromethylvinyl group was unknown56, conditions were further optimized toward its formation by varying the catalysts (Cu(I), Cu(II), Fe(II), Fe(III) salts), the solvents (DCM, DCE, toluene, 1,4-dioxane), and the additives (NaHCO3, Na2CO3, K2CO3, NaH2PO4, etc.) (see Supplementary Information). The optimum conditions found consisted of stirring a solution of 3a and Togni’s reagent 9 (3.0 equiv.) in DCM (c 0.10 M) in the presence of CuCl (0.4 equiv.) and Na2CO3 (3.0 equiv.) at room temperature. Under these conditions, 5a was isolated in 74% yield.
Fig. 4.
Oxidative δ-trifluoromethylation of 2-alkyl benzohydrazides. 3 (0.1 mmol), Togni’s reagent 9 (0.3 mmol), CuCl (0.04 mmol), DCM (1.0 mL), Na2CO3 (0.3 mmol), rt, 4 h
The generality of this oxidative γ-trifluoromethylation of 2-ethyl benzohydrazides was next examined (Fig. 4). The presence of the electron-donating (Me, OMe) and -withdrawing groups (CN) and halides (F, Cl, Br, I) at different positions of the aromatic ring were well tolerated to afford the corresponding 2-(β-trifluoromethylvinyl)benzamides (5a–5q) in good yields. In the case of 3 m (R = 3-Br), the corresponding isoindolinone resulting from the intramolecular C–N bond formation was isolated in 5% yield. N-methyl 3-ethyl and N-methyl 1-ethyl-2-naphthohydrazides were similarly transformed to the corresponding β-trifluoromethylvinyl substituted naphthoamides 5r and 5 s. The N,2-diethyl benzohydrazide was chemoselectively converted to N-ethyl-2-(β-trifluoromethylvinyl)benzamide 5t without event. Finally, 2-isopropyl benzohydrazide was converted to a mixture of two isomers 5 u/5 u’ (1:1) in a moderate yield. The methyl amide 5a was readily hydrolyzed to the corresponding carboxylic acid 5 v (HOAc–H2O, 20% H2SO4), providing therefore a versatile functional group for further functionalization.
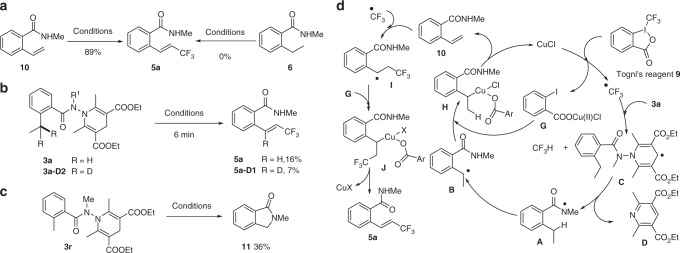
Addition of TEMPO to the reaction of 3a with 9 inhibited completely the process and only 2,2,6,6-tetramethyl-1-(trifluoromethoxy)piperidine was formed. Reducing the amount of Togni’s reagent 9 to 1.5 equiv. under otherwise identical conditions decreased the yield of 5a (42% yield) with concurrent formation of N-methyl-2-vinyl benzamide (10) and N-methyl-2-ethylbenzamide (6) in yields of 12 and 4%, respectively (Fig. 5, a). Resubmitting 10 to the standard conditions afforded 5a in 89% yield57, while 6 remained unchanged under standard conditions. A side-by-side experiment using 3a and 3a-D2 provided an intermolecular KIE (kH/kD) value of 2.3 indicating that the 1,5-HAT step could be a rate-limiting step in our domino process (Fig. 5, b). Finally, treatment of 3r under standard conditions furnished 11 in 36% yield. This result indicated that the 1,5-HAT of the in situ generated amidyl radical occurred. However, in contrast to the azidation process, the transfer of CF3 to the resulting translocated benzyl radical was not fast enough to compete with the intramolecular C–N bond formation (Fig. 5, c).
Fig. 5.
Mechanistic studies on the oxidative δ-trifluoromethylation of 2-alkyl benzohydrazides. a Control experiment; b KIE experiment; c isoindolinone formation; d possible reaction pathway; conditions: 10 or 6 or 3a/3a-D2 (0.1 mmol), Togni’s reagent 9 (0.3 mmol), CuCl (0.04 mmol), DCM (1.0 mL, c 0.1 M), Na2CO3 (0.3 mmol), rt, 4 h
On the basis of the results of these control experiments, a possible reaction pathway is proposed (Fig. 5, d). Single-electron transfer between Togni’s reagent 9 and CuCl would produce the CF3 radical and Cu(II) salt G. The abstraction of the C-4 hydrogen of the Hantzsch ester 3a by CF3• generated radical C which underwent fragmentation to provide the amidyl radical A and pyridine D. The 1,5-HAT of A furnished the then translocated benzyl radical B, which upon radical rebound with Cu(II) salt G, would afford H. Reductive elimination leading to the formation of C–Cl and C–OCOAr bonds may not be kinetic competent, the alternative β-hydride elimination took place to afford 2-vinyl benzamide (10). Subsequent addition of CF3 radical to the newly generated double bond would generate the benzyl radical I which, upon radical rebound and β-hydride elimination, would afford the observed product 5a with concurrent generation of the Cu(I) species. Following this reaction pathway, at least two equivalents of Togni’s reagent 9 are needed to complete this domino process, which is in accordance with the result of our control experiment.
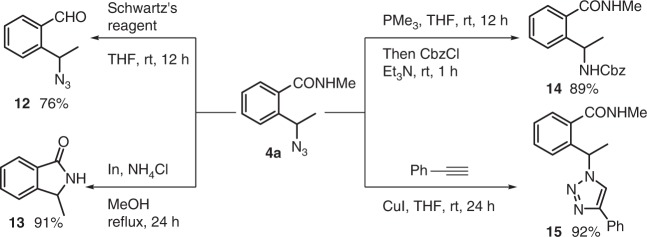
Post-transformations of azido amide 4a were carried out to illustrate its synthetic potential (Fig. 6). Chemoselective reduction of the amide function in 4a with Schwartz’s reagent delivered azido aldehyde 12 in 76% yield. Heating to reflux a MeOH solution of 4a with indium in the presence of NH4Cl afforded the isoindolinone 13 in 91% yield via probably a reduction/transamidation sequence. On the other hand, Staudinger reduction of azide followed by in situ acylation with CbzCl furnished carbamate 14, while the click reaction of 4a with phenylacetylene provided the triazole 15 in 92% yield.
Fig. 6.
Transformation of azido amide 4a. Schwartz’s reagent = bis(cyclopentadienyl)zirconium(IV) chloride hydride, CbzCl = benzyl chloroformate
Discussion
The classic Hofmann–Löffler–Freytag (HLF) reaction converts the protonated N-chloroamines to pyrrolidines under thermal or photochemical conditions. In this transformation, the carbon-centered radical, resulting from the 1,5-HAT of the in situ generated aminium radical, abstracts a chlorine atom from the ammonium salt forming therefore a radical chain process. While many different transformations could in principle be envisaged for the functionalization of the translocated C-centered radical, only very few reaction types have actually been successfully combined with the 1,5-HAT process.
In this paper, we presented two copper-catalyzed remote C(sp3)–H functionalization processes in which the metal-catalyzed cross-coupling reactions were successfully merged with the radical 1,5-HAT process. The salient feature of these transformations was that the copper salts served not only as a reductant to generate the amidyl radicals from benzohydrazides 3, but also catalyzed the functionalization of the translocated carbon radical. Specifically, Cu(MeCN)4PF6-catalyzed reaction of 2-alkyl benzohydrazides 3 with TMSN3 in the presence of MeCO2OtBu affords the γ-azido amides 4, while CuCl-catalyzed reaction of 3 with Togni’s reagent provides 2-(β-trifluoromethylvinyl)benzamides 5 via an oxidative δ-trifluoromethylation of the alkyl group. Mechanistic studies suggest that the γ-azidation of benzohydrazides 3 goes through 1,5-HAT followed by a Cu-mediated azido transfer cascade, while the oxidative δ-trifluoromethylation of 3 proceeds via, after 1,5-HAT process, a radical-polar crossover mechanism.
In view of the availability and reliability of a wide range of metal-catalyzed cross-coupling reactions, we believe that their combination with the 1,5-HAT process will open a new avenue for the development of powerful synthetic tools58.
Methods
Cu-catalyzed γ-C(sp3)–H azidation of benzohydrazides
A screw cap tube was charged with Cu(CH3CN)4PF6 (14.9 mg, 0.04 mmol, 0.4 equiv.), substrate 3 (0.1 mmol, 1.0 equiv.), TMSN3 (26.5 μL, 0.2 mmol, 2.0 equiv.), and tBuOH (2.0 mL). The mixture was stirred at 50 °C for 2 min, then MeCO2OtBu (32.2 μL, 0.2 mmol, 2.0 equiv.) was added to the above mixture. After being stirred for 2 h at 50 °C under N2 atmosphere, the reaction mixture was quenched with water, extracted with EtOAc. The organic extracts were washed with brine, dried over Na2SO4. The solvent was removed under reduced pressure. The residue was purified by flash column chromatography (SiO2, eluent: petroleum ether/EtOAc = 2/1) to give 4.
Cu-catalyzed oxidative δ-C(sp3)–H trifluoromethylation
To a screw cap tube charged with CuCl (4.0 mg, 0.04 mmol, 0.4 equiv.) and Na2CO3 (31.8 mg, 0.3 mmol, 3.0 equiv.) was added a solution of Togni’s reagent 9 (94.8 mg, 0.3 mmol, 3.0 equiv.) in DCM (0.5 mL) under argon atmosphere. After stirring for 2 min, substrate 3 (0.1 mmol, 1.0 equiv.) in DCM (0.5 mL) was added to the above mixture. After being stirred for 4 h at room temperature under argon atmosphere, the reaction mixture was quenched with water, extracted with EtOAc. The organic extracts were washed with Na2CO3 solution and brine, dried over Na2SO4. The solvent was removed under reduced pressure. The residue was purified by flash column chromatography (SiO2, eluent: petroleum ether/EtOAc = 2/1) to give 5.
Supplementary information
Acknowledgements
We thank EPFL (Switzerland), the Swiss National Science Foundation (SNSF 20020_155973; SNSF 20021_178846) for financial support.
Author contributions
X.B., Q.W. and J.Z. conceived and designed the experiments. X.B. carried out the experiments. X.B., Q.W. and J.Z. interpreted the results and co-wrote the paper.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and the Supplementary Information, as well as from the authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1866223. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/Search?Ccdc=1866223&Author=Bao%20Xu&Access = referee
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Sangit Kumar, Wei Yu and the other anonymous reviewer for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-08741-w.
References
- 1.Hofman AW. Ueber die Einwirkung des Broms in Alkalischer Lösung auf die Amine. Ber. Dtsch. Chem. Ges. 1883;16:558–560. doi: 10.1002/cber.188301601120. [DOI] [Google Scholar]
- 2.Löffler K, Freytag C. Über das ω‐Oxy‐α‐propyl‐piperidin und eine neue Synthese des Piperolidins (δ‐Coniceins) Ber. Dtsch. Chem. Ges. 1909;42:3427–3431. doi: 10.1002/cber.19090420377. [DOI] [Google Scholar]
- 3.Chu JCK, Rovis T. Complementary strategies for directed C(sp3)−H functionalization: a comparison of transition‐metal‐catalyzed activation, hydrogen atom transfer, and carbene/nitrene transfer. Angew. Chem. Int. Ed. 2018;57:62–101. doi: 10.1002/anie.201703743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zard SZ. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 2008;37:1603–1618. doi: 10.1039/b613443m. [DOI] [PubMed] [Google Scholar]
- 5.Chiba S, Chen H. sp3 C–H oxidation by remote H-Radical shift with oxygen- and nitrogen-radicals: a recent update. Org. Biomol. Chem. 2014;12:4051–4060. doi: 10.1039/C4OB00469H. [DOI] [PubMed] [Google Scholar]
- 6.Jeffrey JL, Sarpong R. Intramolecular C(sp3)–H amination. Chem. Sci. 2013;4:4092–4106. doi: 10.1039/c3sc51420j. [DOI] [Google Scholar]
- 7.Chen JR, Hu XQ, Lu LQ, Xiao WJ. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016;45:2044–2056. doi: 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- 8.Stateman LM, Nakafuku KM, Nagib DA. Remote C-H functionalization via selective hydrogen atom transfer. Synthesis. 2018;50:1569–1586. doi: 10.1055/s-0036-1591930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández, R., Rivera, A., Salazar, J. A. & Suárez, E. Nitroamine radicals as intermediates in the functionalization of non-activated carbon atoms. J. Chem. Soc. Chem. Commun. 958–959 (1980).
- 10.Betancor C, Concepción JI, Hernández R, Salazar JA, Suárez E. Intramolecular functionalization of phosphoramidate radicals. synthesis of 1,4-epimine compounds. J. Org. Chem. 1983;48:4430–4432. doi: 10.1021/jo00171a066. [DOI] [Google Scholar]
- 11.De Armas P, et al. Synthesis of 1,4-epimine compounds. iodosobenzene diacetate, an efficient reagent for neutral nitrogen radical generation. Tetrahedron Lett. 1985;26:2493–2496. doi: 10.1016/S0040-4039(00)94862-7. [DOI] [Google Scholar]
- 12.Martínez C, Muñiz K. An iodine-catalyzed Hofmann–Löffler reaction. Angew. Chem. Int. Ed. 2015;54:8287–8291. doi: 10.1002/anie.201501122. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Muñiz K. Selective piperidine synthesis exploiting iodine-catalyzed Csp3-H amination under visible light. ACS Catal. 2017;7:4122–4125. doi: 10.1021/acscatal.7b00928. [DOI] [Google Scholar]
- 14.Becker P, Duhamel T, Stein CJ, Reiher M, Muñiz K. Cooperative light-activated iodine and photoredox catalysis for the amination of Csp3-H bonds. Angew. Chem. Int. Ed. 2017;56:8004–8008. doi: 10.1002/anie.201703611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker P, Duhamel T, Martínez C, Muñiz K. Designing homogeneous bromine redox catalysis for selective aliphatic C-H bond functionalization. Angew. Chem. Int. Ed. 2018;57:5166–5170. doi: 10.1002/anie.201800375. [DOI] [PubMed] [Google Scholar]
- 16.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu, J. C. K. & Rovis T. Amide-directed photoredox-catalysed C.–C. Bond formation at unactivated sp3 C–H bonds. Nature539, 272–275 (2016). [DOI] [PMC free article] [PubMed]
- 18.Chen DF, Chu JCK, Rovis T. Directed γ-C(sp3)−H alkylation of carboxylic acid derivatives through visible light photoredox catalysis. J. Am. Chem. Soc. 2017;139:14897–14900. doi: 10.1021/jacs.7b09306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia Y, Wang L, Studer A. Site-selective remote radical C-H functionalization of unactivated C-H bonds in amides using sulfone reagents. Angew. Chem. Int. Ed. 2018;57:12940–12944. doi: 10.1002/anie.201807455. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Xia Y, Bergander K, Studer A. Remote site-specific radical alkynylation of unactivated C−H bonds. Org. Lett. 2018;20:5817–5820. doi: 10.1021/acs.orglett.8b02514. [DOI] [PubMed] [Google Scholar]
- 21.Na CG, Alexanian EJ. A general approach to site-specific, intramolecular C-H functionalization using dithiocarbamates. Angew. Chem. Int. Ed. 2018;57:13106–13109. doi: 10.1002/anie.201806963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morcillo SP, et al. Photoinduced remote functionalization of amides and amines using electrophilic nitrogen radicals. Angew. Chem. Int. Ed. 2018;57:12945–12949. doi: 10.1002/anie.201807941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groendyke, B. J., AbuSalim, D. I. & Cook, S. P. Iron-catalyzed. & fluoroamide-directed, C. −H. fluorination. J. Am. Chem. Soc. 138, 12771–12774 (2016). [DOI] [PubMed]
- 24.Li Z, Wang Q, Zhu J. Copper-catalyzed arylation of remote C(sp3)-H bonds in carboxamides and sulfonamides. Angew. Chem. Int. Ed. 2018;57:13288–13292. doi: 10.1002/anie.201807623. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Z., Stateman, L. M. & Nagib, D. A. δ C-H (Hetero)arylation via Cu-catalyzed radical relay. Chem. Sci. 10, 1207–1211 (2019). [DOI] [PMC free article] [PubMed]
- 26.Katohgi M, Togo H, Yamaguchi K, Yokoyama M. New synthetic method to 1,2-benzisothiazoline-3-one-1,1-dioxides and 1,2-benzisothiazoline-3-one-1-oxides from N-Alkyl(o-methyl)arenesulfonamides. Tetrahedron. 1999;55:14885–14900. doi: 10.1016/S0040-4020(99)00974-6. [DOI] [Google Scholar]
- 27.Fan R, Pu D, Wen F, Wu J. δ and α-sp3 C-H bond oxidation of sulfonamides with PhI(OAc)2/I2 under metal-free conditions. J. Org. Chem. 2007;72:8994–8997. doi: 10.1021/jo7016982. [DOI] [PubMed] [Google Scholar]
- 28.Yang M, et al. Silver-catalysed direct amination of unactivated C–H bonds of functionalized molecules. Nat. Commun. 2014;5:4707. doi: 10.1038/ncomms5707. [DOI] [PubMed] [Google Scholar]
- 29.Paz NR, et al. Chemoselective intramolecular functionalization of methyl groups in nonconstrained molecules promoted by N-iodosulfonamides. Org. Lett. 2015;17:2370–2373. doi: 10.1021/acs.orglett.5b00866. [DOI] [PubMed] [Google Scholar]
- 30.Richers J, Heilmann M, Drees M, Tiefenbacher K. Synthesis of lactones via C−H functionalization of nonactivated C(sp3)-H bonds. Org. Lett. 2016;18:6472–6475. doi: 10.1021/acs.orglett.6b03371. [DOI] [PubMed] [Google Scholar]
- 31.Qin Q, Yu S. Visible-light-promoted remote C(sp3)-H amidation and chlorination. Org. Lett. 2015;17:1894–1897. doi: 10.1021/acs.orglett.5b00582. [DOI] [PubMed] [Google Scholar]
- 32.Wappes EA, Fosu SC, Chopko TC, Nagib DA. Triiodide-mediated δ-amination of secondary C-H bonds. Angew. Chem. Int. Ed. 2016;55:9974–9978. doi: 10.1002/anie.201604704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy LR, Reddy BVS, Corey EJ. Efficient method for selective introduction of substituents as C(5) of isoleucine and other α-amino acids. Org. Lett. 2006;8:2819–2821. doi: 10.1021/ol060952v. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Myers MC, Yu JQ. Copper-catalyzed bromination of C(sp3)-H bonds distal to functional groups. Angew. Chem. Int. Ed. 2017;56:306–309. doi: 10.1002/anie.201608210. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Mei TS, Yu JQ. “γ,δ,ε-C(sp3)–H functionalization through directed radical H-abstraction. J. Am. Chem. Soc. 2015;137:5871–5874. doi: 10.1021/jacs.5b02065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Short MA, Blackburn JM, Roizen JL. Sulfamate esters guide selective radical‐mediated chlorination of aliphatic C-H bonds. Angew. Chem. Int. Ed. 2018;57:296–299. doi: 10.1002/anie.201710322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu W, Genoux A, Li Z, Nevado C. γ-Functionalizations of amines through visible-light-mediated, redox-neutral C-C bond cleavage. Angew. Chem. Int. Ed. 2017;56:10521–10524. doi: 10.1002/anie.201704068. [DOI] [PubMed] [Google Scholar]
- 38.Yuan W, Zhou Z, Gong L, Meggers E. Asymmetric alkylation of remote C(sp3)-H bonds by combining proton-coupled electron transfer with chiral Lewis acid catalysis. Chem. Commun. 2017;53:8964–8967. doi: 10.1039/C7CC04941B. [DOI] [PubMed] [Google Scholar]
- 39.Friese FW, Mück-Lichtenfeld C, Studer A. Remote C−H functionalization using radical translocating arylating groups. Nat. Commun. 2018;9:2808. doi: 10.1038/s41467-018-05193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou T, Luo FX, Yang MY, Shi ZJ. Silver-catalyzed long-distance aryl migration from carbon center to nitrogen center. J. Am. Chem. Soc. 2015;137:14586–14589. doi: 10.1021/jacs.5b10267. [DOI] [PubMed] [Google Scholar]
- 41.Guin J, Fröhlich R, Studer A. Thiol-catalyzed stereoselective transfer hydroamination of olefins with N-aminated dihydropyridines. Angew. Chem. Int. Ed. 2008;47:779–782. doi: 10.1002/anie.200703902. [DOI] [PubMed] [Google Scholar]
- 42.Chou CM, Guin J, Mück-Lichtenfeld C, Grimme S, Studer A. Radical-transfer hydroamination of olefins with N-aminated dihydropyridines. Chem. Asian J. 2011;6:1197–1209. doi: 10.1002/asia.201000881. [DOI] [PubMed] [Google Scholar]
- 43.Piou T, Neuville L, Zhu J. Activation of a C(sp3)-H bond by a transient σ-alkylpalladium(ii) complex: synthesis of spirooxindoles through a palladium-catalyzed domino carbopalladation/C(sp3)-C(sp3) bond-forming process. Angew. Chem. Int. Ed. 2012;51:11561–11565. doi: 10.1002/anie.201206267. [DOI] [PubMed] [Google Scholar]
- 44.Piou T, Bunescu A, Wang Q, Neuville L, Zhu J. Palladium-catalyzed through-space C(sp3)-H and C(sp2)H bond activation by 1,4-palladium migration: efficient synthesis of [3,4]-fused oxindoles. Angew. Chem. Int. Ed. 2013;52:12385–12389. doi: 10.1002/anie.201306532. [DOI] [PubMed] [Google Scholar]
- 45.Wu K, Liang Y, Jiao N. Azidation in the difunctionalization of olefins. Molecules. 2016;21:352. doi: 10.3390/molecules21030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Studer AA. “Renaissance” in radical trifluoromethylation. Angew. Chem. Int. Ed. 2012;51:8950–8958. doi: 10.1002/anie.201202624. [DOI] [PubMed] [Google Scholar]
- 47.Merino E, Nevado C. Addition of CF3 across unsaturated moieties: a powerful functionalization tool. Chem. Soc. Rev. 2014;43:6598–6608. doi: 10.1039/C4CS00025K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charpentier J, Früh N, Togni A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem. Rev. 2015;115:650–682. doi: 10.1021/cr500223h. [DOI] [PubMed] [Google Scholar]
- 49.Huang X, Groves JT. Taming azide radicals for catalytic C−H azidation. ACS Catal. 2016;6:751–759. doi: 10.1021/acscatal.5b02474. [DOI] [Google Scholar]
- 50.Bunescu A, Ha TM, Wang Q, Zhu J. Copper-catalyzed three-component carboazidation of alkenes with acetonitrile and sodium azide. Angew. Chem. Int. Ed. 2017;56:10555–10558. doi: 10.1002/anie.201705353. [DOI] [PubMed] [Google Scholar]
- 51.Bao X, Yokoe T, Ha TM, Wang Q, Zhu J. Copper-catalyzed methylative difunctionalization of alkenes. Nat. Commun. 2018;9:3725. doi: 10.1038/s41467-018-06246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochi JK, Bemis A. Catalytic reactions of peroxides: direct initiation by Cuprous Species. Tetrahedron. 1968;24:5099–5113. doi: 10.1016/S0040-4020(01)88421-0. [DOI] [Google Scholar]
- 53.Verma A, et al. Transition metal free intramolecular selective oxidative C(sp3)-N coupling: synthesis of N-Aryl-isoindolinones from 2-alkylbenzamides. Chem. Commun. 2015;51:1371–1374. doi: 10.1039/C4CC08717H. [DOI] [PubMed] [Google Scholar]
- 54.Nouawa-Kumada K, Kadokawa J, Kameyama T, Kondo Y. Copper-catalyzed sp3 C-H aminative cyclization of 2-alkyl-N-arylbenzamides: an approach for the synthesis of N-Aryl-isoindolinones. Org. Lett. 2015;17:4479–4481. doi: 10.1021/acs.orglett.5b02235. [DOI] [PubMed] [Google Scholar]
- 55.Bunescu A, Wang Q, Zhu J. Copper-mediated/catalyzed oxyalkylation of alkenes with alkylnitriles. Chem. Eur. J. 2014;20:14633–14636. doi: 10.1002/chem.201405102. [DOI] [PubMed] [Google Scholar]
- 56.Yu P, Zheng SC, Yang NY, Tan B, Liu XY. Phosphine‐catalyzed remote β‐C-H functionalization of amines triggered by trifluoromethylation of alkenes: one‐pot synthesis of bistrifluoromethylated enamides and oxazoles. Angew. Chem. Int. Ed. 2015;54:4041–4045. doi: 10.1002/anie.201412310. [DOI] [PubMed] [Google Scholar]
- 57.Feng C, Loh TP. Direct directing-group-assisted copper-catalyzed olefinic trifluoromethylation of electron-deficient alkenes. Angew. Chem. Int. Ed. 2013;52:12414–12417. doi: 10.1002/anie.201307245. [DOI] [PubMed] [Google Scholar]
- 58.Bao, X., Wang, Q. & Zhu, J. Dual photoredox/copper catalysis for the remote C(sp3)-H functionalization of alcohols and alkyl halides via N-alkoxypyridinium salts. Angew. Chem. Int. Ed. 58, 2139–2143 (2019). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and the Supplementary Information, as well as from the authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1866223. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/Search?Ccdc=1866223&Author=Bao%20Xu&Access = referee