Abstract
Aim
The aim of this narrative review was to explore the potential contributions of CAM to reduce antibiotic use.
Methods
We searched PubMed, Embase, and Cochrane Database of Systematic Reviews with a specific, limited set of search terms and collected input from a group of expert CAM researchers to answer the question: What is known about the contribution of CAM health and health promotion concepts, infection prevention, and infection treatment strategies to reduce antibiotic use? Results. The worldview-related CAM health concepts enable health promotion oriented infection prevention and treatment aimed at strengthening or supporting the self-regulating ability of the human organism to cope with diseases. There is some evidence that the CAM concepts of health (promotion) are in agreement with current conceptualization of health and that doctors who practice both CAM and conventional medicine prescribe less antibiotics, although selection bias of the presented studies cannot be ruled out. There is some evidence that prevention and some treatment strategies are effective and safe. Many CAM treatment strategies are promising but overall lack high quality evidence.
Conclusions
CAM prevention and treatment strategies may contribute to reducing antibiotic use, but more rigorous research is necessary to provide high quality evidence of (cost-)effectiveness.
1. Introduction
Resistance to antibiotics is a complex and growing, international public health problem [1, 2]. Worldwide strategies to control antimicrobial resistance (AMR) and its major consequences (increased mortality, economic impact) are being developed [3, 4]. Currently these strategies appear to be insufficient, as, for example, demonstrated by the unchanged average European consumption rates of antibiotics during the years 2011–2014 [4], although in the UK in 2015 for the first time fewer antibiotics were being prescribed by GPs and clinicians across all healthcare settings than in 2014 [5].
Among others, finding alternatives for antibiotics [2, 6], alone or as part of a delayed prescription approach, may provide a good strategy to optimize appropriate use of antibiotics, meeting both doctors' and patients' needs [7–9]. Alternative nonantibiotic strategies (for symptom relief and/or fighting bacteria) that are currently being studied are, among others, phage therapy, antibodies, immune stimulation, lysins, probiotics, and peptides [2].
At the moment, formal policies advising on the need for alternative strategies to antibiotics do not include the study and/or application of complementary and alternative (CAM) therapies for symptom relief and/or treatment of infections and CAM preventive strategies to reduce the use of antibiotics, although observational studies in Europe have shown that CAM practices and hospitals may have lower antibiotic prescription rates compared to conventional practices [10], due to additional strategies regarding prevention and treatment of infections [11]. In this article we use the term CAM, although elsewhere terms as traditional and complementary medicine [12] or complementary and integrative medicine [13] are used.
Given the mismatch between the urgent need for nonantibiotic strategies and the lack of use of CAM strategies embedded in current conventional policies and clinical practice, we performed a narrative review to determine what is known about the contribution of CAM to help reduce antibiotic use.
2. Material and Methods
2.1. Research Questions
What are the worldview differences between CAM and conventional medicine, relevant for prevention and treatment of infections and the AMR problem?
What are the hypothesized CAM contributions to reduce antibiotic use?
-
Is there evidence
- that supports the proposition that CAM prevention and treatment strategies can lead to the prescription and consumption of fewer antibiotics?
- that CAM prevention and treatment strategies are effective and safe?
2.2. Design
We chose to perform a narrative review based on (1) searches in three databases with a specific, limited set of search terms and (2) input from CAM (research) experts, in order to get a first broad overview of the domain of (possible) contributions of CAM to reduce antibiotic use. Based on the results of this broad narrative review, more methodologically rigorous scoping reviews and/or systematic reviews on subareas of this scientific field can and must be performed.
2.3. Identification of Relevant Studies
Searches were performed in PubMed, Embase, and the Cochrane Database of Systematic Reviews (from onset to June 2017).
Search terms used for PubMed and Embase were “health concept”, “prevention AND infection”, “lifestyle AND infection”, “treatment AND infection”, “antibiotic prescription”, “antibiotic consumption”, each in combination with each of the following search terms for CAM: “complementary medicine”, “alternative medicine”, “herbal”, “ayurveda OR ayurvedic”, “homeopathy OR homeopathic”, “TCM OR traditional Chinese medicine”, “anthroposophy OR anthroposophic” (for example, “health concept” AND “complementary medicine”; “health concept” AND “alternative medicine”, “health concept” AND “herbal”, etcetera).
Search keywords used for the Cochrane Database of Systematic Reviews were “alternative medicine AND prevention”, “complementary medicine AND prevention”, “complementary medicine AND infection”, “herbal AND infection”, “ayurveda OR ayurvedic”, “homeopathy OR homeopathic”, “TCM OR traditional Chinese medicine”, “TCM OR traditional Chinese medicine AND infection”, “anthroposophy OR anthroposophic”.
2.4. Study Selection
The inclusion criteria used in the narrative review were as follows:
-
Main research domain:
- CAM or IM (mandatory)
-
Research topics (one research topic is mandatory):
- Worldview
- Health (promotion) concept
- Antibiotic prescription
- Antibiotic consumption
- Prevention of infection
- Treatment of infection
The exclusion criteria in this study were as follows:
Only conventional prevention or treatment strategies for infections
CAM/IM for noninfection indications
Study collections were exported to Excel and duplicates were removed. Selection of relevant papers was carried out in two stages and both stages were performed independently by two reviewers. In the first stage, both reviewers read the titles and the abstracts to select potentially relevant papers according to the inclusion criteria. Disagreements were resolved through discussion and consensus with the other review author. In the second stage, the full texts of the included articles were evaluated.
2.5. Input from CAM Experts
An international group of CAM clinical and/or research experts was invited for one or two workshops (25/26 January 2017 and 16/17 February 2017) in Frankfurt (Germany), in which, among other things, the scope of the review, barriers and facilitators of the integration of CAM strategies in conventional medicine, and future research activities on the CAM contribution to reduce antibiotic use were discussed. This group and some other CAM experts were also invited to give their input to draft versions of the article.
2.6. Analyses
The aim of the qualitative analyses of this narrative review is to map the relevant themes and to provide a first broad overview of the studied domain. As a result, the review does not provide an exact, narrow focused overview of the state of science of each of the subareas (concepts, prevention, treatment per indication) as is done in a scoping review, it does not include all relevant articles if this is not necessary for the mapping purpose, and it does not judge the methodological quality of the scientific evidence of studies on CAM prevention and treatments for specific indications (e.g., assessment of the methodological quality of RCTs with GRADE or of systematic reviews with the AMSTAR 2 checklist), as is done in a systematic review.
3. Results
3.1. Search Results
See Figure 1.
Figure 1.
3.2. Worldview Aspects of CAM and Conventional Medicine Relevant for Prevention and Treatment of Infections and the AMR Problem
Worldviews are frameworks of meaning and meaning-making that shape how individuals perceive particular issues and their possible solutions and that influence the willingness of individuals to participate in these worldview-related solutions [14]. Medical systems are based on specific, often implicitly handled, worldviews that shape concepts of health, disease, and treatment that in their turn underlie preventive, diagnostic, and treatment strategies applied in clinical practice (Figure 2). A good understanding of the similarities and differences between conventional medicine and CAM worldviews and related concepts, and infection prevention and treatment strategies is expected to contribute to integrate the best of both worlds [15] and is therefore here shortly described.
Figure 2.
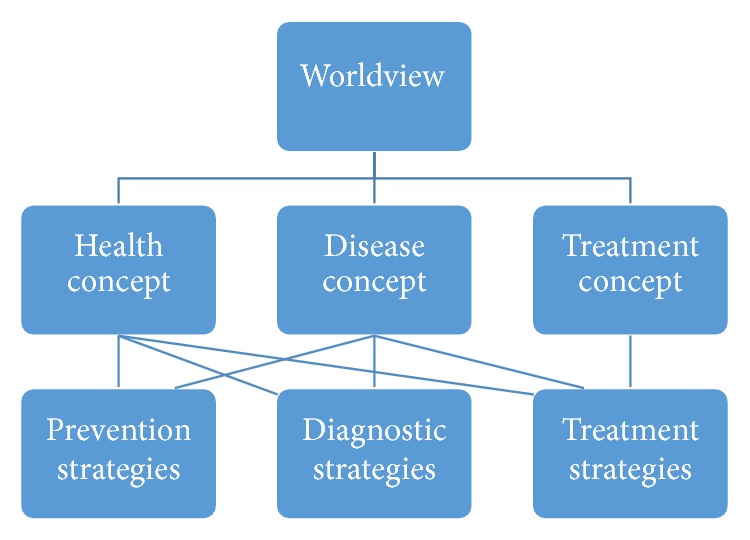
Worldviews, concepts, and clinical practice.
The main worldview in conventional medicine is the biomedical model. Treatment within this model is mainly oriented at “fighting the disease” both in prevention and treatment, in order to regain the default situation of health [16].
CAM systems (e.g., anthroposophic medicine, ayurveda, homeopathy, traditional Chinese medicine, naturopathy) are whole medical systems, complete systems of theory and practice that have evolved independently over time in different cultures and apart from conventional medicine or western medicine [15, 17, 18]. In daily clinical practice, based on the nonatomistic holistic worldview and related health and disease concepts, CAM stimulates a health promotion oriented lifestyle (prevention) and treats patients with the aim of strengthening or supporting the self-healing or self-regulating ability of the human organism [19] to cope with diseases [20–26].
The differences in worldview and related concepts of health and disease are also expressed in the differences in the main prevention and treatment strategies for infections. Conventional medicine historically was and is (more) focused on fighting disease, more or less implicitly regarding health as the absence of disease. CAM historically focused (more) on health promotion strategies.
The main conventional preventive strategies are vaccinations, hygiene, improving nutrition, and isolation measures [3]. Their aim is respectively to produce immunity and to prohibit contact with microorganisms. The main CAM preventive strategies are lifestyle changes/interventions and medical measures/interventions that strengthen resilience [27]. Their aim is to improve the physiological ability to self-manage and adapt to infections.
The main conventional treatment strategies are antimicrobial treatments that kill or reduce the growth of microbes and reduce disease-related symptoms, like discomfort of fever and pain. The main treatment strategies of CAM are the medicinal and nonpharmaceutical treatments that support the organism to overcome the infection by itself by means of strengthening the self-regulating abilities of the organism (“changing the host's capacities”).
Both in conventional medicine and in CAM, there are currently developments that are aimed at integrating the best of both worlds of fighting disease and health promotion approaches [15, 22].
3.3. What Is the Evidence That Supports the Hypothesized CAM Contributions?
3.3.1. Health and Health Promotion Concept
The key elements of the CAM concepts of health are the following: (1) health is the result of a self-regulating inner activity, and (2) health is aimed at restoring wholeness of the organism and balance within or between the functions of body, soul, and spirit [24, 65]. In agreement with the health concept, health promotion can be logically defined as the process of enabling individuals, groups, or societies to increase control over, and to improve, their physiological, psychosocial, and spiritual (meaning in life) health [66]. Health promotion thus aims to improve the development and quality of the self-regulating abilities on these levels and aims to restore balance between opposite functions.
In 1948, the World Health Organization (WHO) defined health as ‘a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity'. In 2011, Huber et al. [67] redefined health as ‘the ability to self-manage and adapt'. This concept is in line with current other concepts of health (e.g., resilience, salutogenesis), emphasizing the role of self-regulating abilities in the physiological, psychosocial, and ‘meaning in life' level as internal resources of the human being to remain or become (more) healthy [22, 68].
One of the mechanisms of acquiring health by self-regulation is the active balancing of opposite functions in the organism, which is increasingly described in the literature on, for example, apoptosis [69] (programmed cell death as an opposite function to ongoing cell division in organisms), wound healing [70], and chronobiology [71]. Imbalances of one of the two opposite functions is related to disease states [72].
In conclusion, we state that there is some evidence that CAM health and health promotion concepts are internally consistent and increasingly in agreement with current health conceptualization in conventional medicine. Their degree of agreement with empirical facts is described in the next paragraphs on health promotion oriented prevention and treatment strategies.
3.3.2. Less Prescription and Consumption of Antibiotics
Several, mostly observational, studies (Table 1) support the hypothesis that practices of doctors who practice both CAM and conventional medicine compared to their conventional colleagues have lower antibiotic prescription rates (measured as past use, antibiotics use ever, in the first 12 months of life and after 12 months of life, consumption, prescription rates) and their patient groups have lower antibiotic consumption rates, although in these studies selection bias (e.g., patients that do not want antibiotics may choose more often a CAM doctor) cannot be ruled out.
Table 1.
Studies on prescription and consumption rates of antibiotics in CAM practices and in families with an alternative lifestyle.
| Study type | Results | Study characteristics |
|---|---|---|
| Cross-sectional study comparing children from anthroposophic families and children with a non-anthroposophic lifestyle [28] | Past use of antibiotics: (i) anthroposophic children: 52% (ii) non-anthroposophic children: 90% (odds ratio (OR): 0.62, 95% CI: 0.43 - 0.91) |
N = 295 anthroposophic children and 380 non-anthroposophic children, age 5-13 years. Sweden |
|
| ||
| Cross-sectional study comparing children from anthroposophic families and children with a non-anthroposophic lifestyle [29] | Antibiotics use ever, in the first 12 months of life and after 12 months of life: (i) all significantly lower in children with an anthroposophic lifestyle (p < 0.001) |
N = 6.630 children, age 5-13 years (4.606 from Steiner schools and 2.024 from reference schools) in 5 European countries (Austria, 11%; Germany, 39%; The Netherlands, 22%; Sweden, 9%; Switzerland, 20%) |
|
| ||
| KOALA Birth Cohort Study comparing families with an alternative and a non-alternative lifestyle [30] | Families with an alternative lifestyle: (i) antibiotic use was less frequent (13.8% vs. 24.1%) (p-value not presented) |
N= 2.343 conventional children and 491 alternative lifestyle children. The Netherlands |
|
| ||
| Observational study on prescribing practices of anthroposophic medicine (AM) doctors in the treatment of upper respiratory tract infections [31] | Prescription rate for antibiotics (6.3%) was well below the German average | 21.818 prescriptions for 12.081 patients (73.7% children) with 19.050 cases of URTI were analysed. Antibiotics were given in 6.3% of cases (minimum: common cold 1.9%, maximum: tonsillitis 24.3%). Germany |
|
| ||
| Prospective, non-randomised comparison of outcomes in patients self-selected to anthroposophic or conventional therapy under real-world conditions [10] | 5.5% of the patients in the AM group and 33.6% in the conventional group received antibiotics (p < 0.0001) | 29 primary care practices (Austria, Germany, Netherlands, UK and USA). N= 1.016 outpatients, age ≥ 1 month, consulting an anthroposophic (N = 715) or conventional physician (N = 301) with a chief complaint of acute (≤ 7 days) sore throat, ear pain, sinus pain, runny nose or cough |
|
| ||
| Prospective, non-randomised comparison of outcomes in patients self-selected to anthroposophic or conventional therapy under real-world conditions [32] | 5.5% of the patients in the AM group and 25.6% in the conventional group received antibiotics (p < 0.001) | N = 529 children <18 years from Europe (Austria, Germany, Netherlands, and UK) or USA with acute respiratory or ear infections |
|
| ||
| Observational study on the treatment of patients with upper respiratory tract infections: homeopathic GPs vs. conventional GPs [33] | Significantly lower consumption of antibiotics (OR = 0.43, 95% CI: 0.27-0.68) in the homeopathic patients group | N = 518 adults and children with URTI (79.3% rhinopharyngitis). France |
|
| ||
| Randomized trial, children 6 months to 11 years old, diagnosed with AOM and managed with a delayed antibiotic approach, randomized to standard therapy alone or standard therapy plus a homeopathic ear drop preparation [34] | Significantly less antibiotic use in the homeopathic group (26.9% vs. 41.2%) (p-value not presented) | N = 456 patient visits were compared: 281 received homeopathy, 175 received conventional medicine. Germany, Switzerland, Austria, USA |
|
| ||
| Observational study among parents of children [35] | Use of homeopathic products not associated with decreased antibiotic consumption (adjusted OR = 1.02, 95% CI: 0.84 - 1.24). | N = 9.723 parents of children, age: 3–4.5 years. United Kingdom |
3.3.3. Effects and Safety of Prevention Strategies
The main CAM prevention strategies are lifestyle changes/interventions and medical measures/interventions that strengthen and/or support the physiological ability of the organism to self-manage and adapt to infections. Prevention CAM strategies are aimed at (1) reducing stress, insomnia, depression, and anxiety (that are all associated with higher susceptibility to infections), (2) promoting healthy diets and physical exercise (reducing both the risk of infectious diseases), (3) supporting the fever reaction of the organism to infections (to enable the organism to overcome the infection by itself), and (4) preventing infections with natural products.
Chronic Stress, Insomnia, Depression, and Anxiety Associated with Higher Susceptibility to Acute Infectious Illness. Chronic stress suppresses or dysregulates innate and adaptive immune responses by altering the Type 1–Type 2 cytokine balance, inducing low-grade chronic inflammation, and suppressing numbers, trafficking, and function of immunoprotective cells. Chronic stress can suppress protective immune responses and/or exacerbate pathological immune responses [73, 74]. Higher reported stress levels [75, 76], short sleep duration (< 6 or 7 hours/night) and poor sleep continuity [77, 78], depression [79], and anxiety [80] are all associated with higher susceptibility to acute infectious illness (e.g., common cold, pneumonia).
Chronobiology and General Physiological Recovery. Chronobiology research has demonstrated that rhythms are present in the whole human organism and all its cells and that they are responsible for the ordered balancing in time between, for example, degenerative and regenerative physiological processes, performance and recovery, sympathetic and parasympathetic activity, work and relaxation, and wakefulness and sleep [71, 81]. Biorhythms are important for recovery in several physiological functions. The speed of recovery differs between biorhythms in the range from very quick recovery (membrane recovery in milliseconds), local tissue recovery in minutes, moderately quick recovery of fatigue by sleeping in 24 hours, until longer recovery periods (vegetative recovery/self-healing (weeks) and trophic/plastic adaptation and growth (months)) [82].
Preventive CAM Strategies. Several preventive CAM strategies aim to reduce stress, insomnia, depression, and anxiety that are linked to an increased susceptibility to infections [77–80]. CAM prevention includes often promotion of a rhythmic lifestyle [71, 82] in order to support general physiological recovery. Meditation programs can reduce the negative dimensions of psychological stress [83–87]. Mindfulness is currently recommended as a useful method for improving mental health and reducing symptoms of stress, anxiety, and depression [88, 89]. Regular sauna visits, both in children and adults, reduce the frequency and severity of influenza infections, and the incidence of the common cold [90]. In athletes (intermediate trackers compared to untrained) the immune system is more stimulated with an increased number of white blood cells, lymphocyte, neutrophil, and basophil counts after the sauna session. The main working mechanisms of becoming more resilient (among others to infections) after sauna are optimization of temperature and circulation regulation of skin and mucous membranes, vegetative stabilization with decrease of sympathetic tone (stress reduction), stimulation of nonspecific resistance parameters, and strengthening the antioxidative protection potential and thus the defense against free radicals [91]. Two RCTs demonstrated that balneotherapy is beneficial for stress and fatigue reduction in comparison with music or no therapy group [92] and reduces distress by reducing the health risk posed by distress (by 26%), by increasing the health resources (by 11%), and by reducing probability of general health risk (by 18%) [93]. Based on a systematic review of 23 articles [94] it was concluded among others that the administration of various forms of therapeutic massage exerted a reduced risk of neonatal sepsis and reduced neonatal stress in very preterm neonates, based on increased vagal activity, increased gastric activity, and increased serum insulin levels. A review of 14 studies on the effects of massage on older people in residential care settings concluded that older people perceive positive effects of massage on factors such as pain, sleep, emotional status, and psychosocial health [95]. Tai-chi is associated with improvements in psychological well-being including reduced stress, anxiety, depression, and mood disturbance and increased self-esteem [96]. Steiner or Waldorf school education is associated with lower cortisol levels of children [97–99] and better adjustment to higher education (less anxiety and depression symptoms, greater life satisfaction and academic achievement) compared to children from conventional schools [100]. In Steiner or Waldorf schools, knowledge of biorhythms is applied in the design of the curriculum [101].
Changes of diets are related to rapid changes of the human gut microbiome [102] that is related to the human health and disease status. Currently probiotics, prebiotics, and polyphenols are among the most well established dietary strategies available for modulating either the composition or metabolic/immunological activity of the human gut microbiota [103]. Several “normal” diet ingredients are able to positively influence the immune system [104]. A systematic review and meta-analysis with 14 included studies demonstrated that, overall, flavonoid supplementation decreased URTI incidence by 33% (95% CI: 31%-36%) compared with control, with no apparent adverse effects [105]. In a mice model, it was demonstrated that the gut microbiota plays a protective role in the host defense against pneumococcal pneumonia [106]. Polyphenols appear to protect athletes from virus infections following rigorous exercise [107].
A systematic review of 28 articles demonstrated that exercise has considerable effects on markers of cellular aspects of the immune system [108]. Current theories regard exercise as a powerful stimulus of immune function [109]. Regular exercise has been shown to improve neutrophil microbicidal functions which reduce the risk of infectious disease, and may be related to improved vaccine responses [110].
Fever induction is the result of a fine interplay between the innate immune system and the neuronal circuitry within the central and peripheral nervous systems. It results in the increase of metabolic rate and the enhancement of immune-protective mechanisms (both innate and adaptive) during infection [111]. A small rise in body temperature inhibits bacterial and viral replication (creation of a thermal restriction zone) [111, 112], while at the same time accelerating the immune response (increasing the mobility of polymorphonuclear cells, increasing phagocytosis and T-helper cell adherence, and prevention of lymphocytes cell reduction (CD4 T cells and B cells activity)), and attenuating the immune response/protection against the collateral damage (increased heat shock protein causing a decrease of NF-κB, reduced TNFα, and reduced IFNγ) [113]. In men, early acute respiratory distress syndrome (ARDS), an elevated peak temperature in the first 24 hours in ICU in critically ill patients with an infection [114], is associated with improved survival rates (adjusted OR: 0.56, 95% CI: 0.48–0.66). It is now evident that antipyretic treatment (paracetamol, aspirin, or ibuprofen) does not prevent seizures [115]. A recent review and meta-analysis demonstrated that antipyretic treatment does not prolong the fever or illness, but may alter inflammatory processes, especially in the early phase during which the immune response develops. It may lead to a reduction of the initial adaptive response. Antipyretic treatment has been shown to increase the spread of infection and prolong influenza, chicken pox, and common colds at the population level and may increase both the rate and duration of viral shedding, further increasing the pathogen's transmission rate; this effect has been shown experimentally for influenza in ferrets. A higher transmission rate in general will lead to larger epidemics and hence to greater morbidity and mortality [116].
Finally, prevention of infections is achieved by use of natural products, for example, prevention of wound and gastrointestinal infections by apitherapy and respiratory tract infections by probiotics [53, 117, 118].
3.3.4. Effects of Treatment Strategies
Evidence of the effects of CAM medicinal treatment strategies comes from 12 Cochrane reviews (Table 2), 16 non-Cochrane reviews (Table 3), 15 clinical studies (Section 3.3.4 (2)), and 20 studies on traditional use and in vitro studies (Section 3.3.4 (3)). Systematic reviews were categorized per indication (respiratory tract infections (Cochrane reviews (CRs): 7, Non-Cochrane reviews (NCRs): 13); urinary tract infections (CRs: 2, NCRs: 1); and other infections (CRs: 3, NCRs: 0). In addition the results of two NCRs on antibiotic-associated diarrhoea were described. Clinical studies were categorized per indication: acute respiratory and ear infections (observational studies: 2), otitis media (RCT:1, observational study: 1), infected wounds and MRSA (RCTs: 4), and other infections (RCTs: 7, observational studies: 2).
Table 2.
Cochrane reviews of CAM treatments of infections.
| Treatment and indication | Main conclusions | Study characteristics |
|---|---|---|
| Respiratory tract infections (RTIs) | ||
|
| ||
| Immunostimulants (IS) (including herbal treatments) for preventing respiratory tract infection in children [36] | IS reduce the incidence of acute RTIs by 40% on average in susceptible children Further RCTs are required |
Thirty-five placebo-controlled trials (N = 4.060). The use of IS was shown to reduce ARTIs measured as the total numbers of ARTIs (MD -1.24; 95% CI: -1.54 to -0.94) and the difference in ARTI rates (MD -38.84%; 95% CI: -46.37% to -31.31%). |
|
| ||
| Oral Astragalus (Huang qi) for the prevention of frequent acute respiratory tract infections in children [37] | Insufficient evidence of the effectiveness and safety | No studies met the inclusion criteria |
|
| ||
| Garlic for prevention of the common cold [38] | There is insufficient clinical trial evidence Further RCTs are required |
Only one trial met the inclusion criteria. N= 146 participants. Interventions: either a garlic supplement (with 180 mg of allicin content) or a placebo (once daily) for 12 weeks. Results: 24 occurrences of the common cold in the garlic intervention group compared with 65 in the placebo group (p < 0.001), resulting in fewer days of illness in the garlic group compared with the placebo group (111 versus 366). The number of days to recovery from an occurrence of the common cold was similar in both groups (4.63 versus 5.63). |
|
| ||
| Echinacea for the common cold [39] | There is possibly a weak benefit from some Echinacea products | Twenty-four double-blind trials with 4.631 participants including a total of 33 comparisons of Echinacea preparations and placebo met the inclusion criteria. None of the 12 prevention comparisons reporting the number of patients with at least one cold episode found a statistically significant difference. However a post hoc pooling of their results suggests a relative risk reduction of 10% to 20%. Of the six treatment trials reporting data on the duration of colds, only two showed a significant effect of Echinacea over placebo. |
|
| ||
| Pelargonium sidoides for acute rhinosinusitis, the common cold and acute bronchitis [40] |
P. sidoides may be effective in alleviating symptoms of acute rhinosinusitis and the common cold in adults, but doubt exists. It may be effective in relieving symptoms in acute bronchitis in adults and children, and sinusitis in adults The overall quality of the evidence was considered low for main outcomes in acute bronchitis in children and adults, and very low for acute sinusitis and the common cold |
Of 10 eligible studies, eight were included in the analyses; two were of insufficient quality. Three trials (746 patients, low quality of evidence) of efficacy in acute bronchitis in adults showed effectiveness for most outcomes in the liquid preparation but not for tablets. Three other trials (819 children, low quality of evidence) showed similar results for acute bronchitis in children. One study in patients with sinusitis (n = 103 adults, very low quality of evidence) showed significant treatment effects (complete resolution at day 21; RR 0.43, 95% CI: 0.30-0.62). One study in the common cold demonstrated efficacy after 10 days, but not five days (very low quality of evidence). |
|
| ||
| Chinese herbals for sore throat [41] | Some Chinese herbal medicines appeared efficacious Due to methodological weaknesses no final conclusions could be drawn |
12 studies involving 1.954 participants. Ten studies were identified as being of methodologically poor quality and two studies as being of medium quality. No meta-analyses. Six formulations were shown to be superior to the control in improving recovery: Ertong Qingyan Jiere Koufuye was more effective than Fufang Shuanghua Koufuye for acute pharyngitis (odds ratio (OR) 2.52; 95% CI: 1.11-5.74); Yanhouling mixture was more effective than gentamicin atomised inhalation for acute pharyngitis (OR 5.39; 95% CI: 2.69-10.81); Qinganlan Liyan Hanpian was more effective than Fufang Caoshanhu Hanpian for acute pharyngitis (OR 2.25; 95% CI: 1.08-4.67); sore throat capsules were more effective than antibiotics (intravenous cefalexin) for acute pharyngitis or acute tonsillitis (OR 2.36; 95% CI: 1.01-5.51); compound dandelion soup was more effective than sodium penicillin for acute purulent tonsillitis (OR 5.06; 95% CI: 1.70-15.05); and eliminating heat by nourishing yin and relieving sore-throat methods combined with Dikuiluqan Hanpian were more effective than Dikuiluqan Hanpian alone for children with chronic pharyngitis (OR 2.63; 95% CI: 1.02-6.79). Another six formulations were shown to be equally efficacious as the control. |
|
| ||
| Chinese medicinal herbs for acute bronchitis [42] | There is insufficient quality data | None of 74 studies involving 6.877 participants met the inclusion criteria. |
|
| ||
| Urinary tract infections (UTIs) | ||
|
| ||
| Chinese herbal medicine (CHM) for recurrent urinary tract infections [43] | CHM as an independent intervention or in conjunction with antibiotics may be beneficial for treating recurrent UTIs during the acute phase of infection and may reduce the recurrent UTI incidence for at least six months post-treatment Better quality evidence is needed |
Seven RCTs involved a total of 542 women; of these, five recruited post-menopausal women (aged from 56 to 70 years) (422 women). All studies were assessed to be at high risk of bias. Analysis of three studies involving 282 women that looked at CHM versus antibiotics suggested that CHM had a higher rate of effectiveness for acute UTI (RR 1.21, 95% CI: 1.11–1.33) and reduced recurrent UTI rates (RR 0.28, 95% CI: 0.09-0.82). Analysis of two studies involving 120 women that compared CHM plus antibiotics versus antibiotics alone found the combined intervention had a higher rate of effectiveness for acute UTI (RR 1.24, 95% CI: 1.04-1.47) and resulted in lower rates of recurrent infection six months after the study (RR 0.53, 95% CI: 0.35-0.80). One study comparing different CHM treatments found Er Xian Tang was more effective in treating acute infection in post-menopausal women than San Jin Pian (80 women: RR 1.28, 95% CI: 1.03-1.57). Analysis showed that active CHM treatments specifically formulated for recurrent UTI were more effective in reducing infection incidence than generic CHM treatments that were more commonly used for acute UTI (RR 0.40, 95% CI: 0.21-0.77). |
|
| ||
| Probiotics for preventing urinary tract infections in adults and children [44] | There is insufficient quality data | Nine studies involved 735 people. Four studies compared probiotic with placebo, two compared probiotic with no treatment, two compared probiotics with antibiotics in patients with UTI, and one study compared probiotic with placebo in healthy women. All studies aimed to measure differences in rates of recurrent UTI. Overall, there was a high risk of bias in the included studies. No significant reduction in the risk of recurrent symptomatic bacterial UTI was found between patients treated with probiotics and placebo (6 studies, 352 participants: RR 0.82, 95% CI: 0.60-1.12; I2 = 23%). No significant reduction in the risk of recurrent symptomatic bacterial UTI was found between probiotic and antibiotic treated patients (1 study, 223 participants: RR 1.12, 95% CI: 0.95-1.33). |
|
| ||
| Other infections | ||
|
| ||
| Chinese medicinal herbs for preventing infection in nephrotic syndrome [45] | A compound of Chinese medicinal herbs—Tiaojining—may have positive effects on prevention of nosocomial or unspecified infection with no obvious serious adverse events in children with nephrotic syndrome Better quality evidence is needed |
Twelve studies conducted in China, including 762 children with nephrotic syndrome were identified. No studies were identified in adults. All studies compared one kind of prophylactic pharmacotherapy (intravenous immunoglobulin (IVIG), thymosin, oral transfer factor, mannan peptide tablet, Bacillus Calmette-Guerin (BCG) vaccine injection, polyvalent bacterial vaccine (Lantigen B) and two kinds of Chinese medicinal herbs: a compound of Chinese medicinal herbs (Tiaojining) and Huang qi (astragalus) granules) plus baseline treatment with baseline treatment alone. No RCTs were identified comparing antibiotics, non-pharmacological prophylaxis, or pneumococcal vaccination. Four studies showed a significantly beneficial effect of IVIG on preventing nosocomial or unspecified infection in children with nephrotic syndrome (RR 0.47, 95% CI: 0.31-0.73). Thymosin (RR 0.50, 95% CI: 0.26-0.97), oral transfer factor (RR 0.51, 95% CI: 0.35-0.73), BCG vaccine injection (RR 0.68, 95% CI: 0.48-0.95), Huang qi granules (RR 0.62, 95% CI: 0.47-0.83) and Tiaojining (RR 0.59, 95% CI: 0.43-0.81) were also effective in reducing the risk of infection in children with nephrotic syndrome. However mannan peptide tablet (RR 0.46, 95% CI: 0.21-1.01) and polyvalent bacterial vaccine (RR 0.24, 95% CI: 0.06-1.00) were not superior to baseline treatment in reducing the risk of infection for nephrotic children. |
|
| ||
| Honey for infected post-operative wounds [46] | Honey appeared to heal infected post-operative wounds more quickly than antiseptics and gauze | One trial (N = 50) on infected post-operative wounds. Honey healed infected post-operative wounds more quickly than antiseptic washes followed by gauze and was associated with fewer adverse events (moderate quality evidence, RR of healing: 1.69, 95% CI: 1.10-2.61). |
|
| ||
| Chinese herbal medicines for skin and soft-tissue infections [47] | No RCTs that met the inclusion criteria > No conclusion | |
Table 3.
Non-Cochrane reviews with some evidence of effectiveness of CAM treatments of infections.
| Treatment and indication | Main conclusions | Study characteristics |
|---|---|---|
| Respiratory tract infections (RTIs) | ||
|
| ||
| Andrographis paniculata for symptomatic relief of acute respiratory tract infections in adults and children [48] |
A. paniculata appears beneficial and safe for relieving ARTI symptoms and shortening time to symptom resolution. However, these findings should be interpreted cautiously owing to poor study quality and heterogeneity. Well-designed trials evaluating the effectiveness and potential to reduce antibiotic use of A. paniculata are warranted |
33 RCTs with a total of 7.175 patients were included. Most trials evaluating A. paniculata (as a monotherapy and as a herbal mixture) provided commercially but seldom reported manufacturing or quality control details. A. paniculata improved cough (n = 596, standardised mean difference SMD: -0.39, 95% CI: -0.67 to -0.10) and sore throat (n = 314, SMD: -1.13, 95% CI: -1.37 to -0.89) when compared with placebo. A. paniculata (alone or plus usual care) has a statistically significant effect in improving overall symptoms of ARTIs when compared to placebo, usual care, and other herbal therapies. Evidence also suggested that A. paniculata (alone or plus usual care) shortened the duration of cough, sore throat, and sick leave/time to resolution when compared with usual care. The methodological quality of included trials was overall poor. |
|
| ||
| Pelargonium sidoides preparation (EPS 7630) for acute bronchitis, acute rhinosinusitis and acute tonsillopharyngitis [49] | Superiority of EPS 7630 to placebo in reducing both symptom severity and time until complete recovery for all indications investigated | 13 trials with a total of 3.392 participants were included, 10 of which could be entered into meta-analyses of efficacy (AB: 6/8 trials; ARS: 2/2 trials; ATP: 2/3 trials). In ARS, all trials included adults only, whereas studies in ATP had been conducted with children only. EPS 7630 was superior to placebo in reducing both symptom severity and time until complete recovery for all indications investigated. Significant advantages for the herbal drug were also observed for time until the onset of a meaningful treatment effect, global therapy outcome, and days off work, school, or kindergarten. In AB, efficacy could also be shown for both subsets defined by age. |
|
| ||
| Pelargonium sidoides for acute rhinosinusitis [50] | Positive evidence | Seven trials on P. sidoides (EPs 7630, Umckaloabo®), Myrtol (GeloMyrtol® forte), BNO 1016 (Sinupret® extract), BNO 101 (Sinupret®), Cyclamen europaeum (Nasodren®), and Esberitox® were included. Risk of bias was heterogeneous. EPs 7630 appeared to be useful in the treatment of ARS. Myrtol showed benefits against a placebo compound, and BNO 1016 and BNO 101 might be helpful; however, there was little evidence for the effectiveness of Cyclamen europaeum and Esberitox® (p-values not presented). |
|
| ||
|
Echinacea and Pelargonium sidoides for treatment of RTIs in children [51] |
Because of conflicting evidence in the included studies, no concrete conclusion on effects of Echinacea could be drawn so far. In the case of P. sidoides, there is moderate evidence for efficacy and safety in the treatment of RTIs in children | Eleven trials with 2.181 participants were included. No clear evidence for Echinacea (4 trials) or an herbal compound preparation (1 trial) in preventing RTI symptoms was found. Meta-analysis revealed evidence for efficacy (responder rates: RR: 2.56; 95% CI: 1.54 – 4.26; p < .01) and safety (patients with adverse events: RR: 1.06, 95% CI: 0.42 – 2.66; p = .9) of P. sidoides in treating RTI symptoms compared with placebo (6 trials). |
|
| ||
| Probiotics for prevention of upper respiratory tract infections (URTIs) in children [52] | Probiotics decrease the incidence of URTIs | 23 trials with a total of 6.269 children (age: 0 -18). None of the trials showed a high risk of bias. The quality of the evidence of outcomes was moderate. Probiotic consumption significantly decreased the number of subjects having at least 1 RTI episode (17 RCTs, 4.513 children, RR: 0.89, 95% CI: 0.82–0.96, p= 0.004). Children supplemented with probiotics had fewer numbers of days of RTIs per person compared with children who had taken a placebo (6 RCTs, 2.067 children, MD: −0.16, 95% CI: −0.29 to 0.02, p = 0.03) and had fewer numbers of absence days from day care/school (8 RCTs, 1.499 children, MD: −0.94, 95% CI: −1.72 to −0.15, p = 0.02). However, there was no statistically significant difference of illness episode duration between probiotic intervention group and placebo group (9 RCTs, 2.817 children, MD: −0.60, 95% CI: −1.49 to 0.30, p = 0.19). |
|
| ||
| Probiotics for prevention of URTIs in immunocompetent children [53] | Modest effect both in diminishing the incidence of URTIs and the severity of the infection symptoms | 14 RCTs applied to a pediatric population with high-quality methodology. At least one beneficial effect of prophylactic probiotic was observed in the majority of RCTs. The long-term administration of probiotics appeared to have a good safety profile in childhood and none of the studies reported any serious adverse events related to the probiotic strain. Probiotics in immunocompetent children have a modest effect in diminishing both the incidence of URTIs (number of subjects having at least 1 respiratory symptom episode (RR: 0.89, 95% CI: 0.82 – 0.96, p = 0.004); children supplemented with probiotics had fewer number of days of RTIs per person compared with children who had taken a placebo (weighted MD: 0.16, 95% CI: 0.29-0.02, p = 0.03)) and the severity of the infection symptoms. |
|
| ||
|
Sanren Decoction (made of almonds, Amomum cardamomum, barley, talc, tetrapanax papyrifera, folia bambosae, Magnolia officinalis, Pinellia ternate) for URTIs [54] |
Higher cure rate and effectiveness rate than control group High quality evidence is required |
Seven studies with 571 URTI patients. The cure rate (OR = 3.51, 95% CI: 2.19-5.15, p < 0.001) and effectiveness rate (OR = 3.91, 95% CI: 2.58-5.90, p < 0.001) of Sanren Decoction's treatment on URTI were significantly higher than those of control group. |
|
| ||
| Shuanghuanglian injection for URTIs [55] | Better effect than common antibiotics on helping relieve some symptoms and decrease the course of acute upper respiratory tract infections High quality evidence is required |
Eight trials with 857 participants. SHL injection showed significant effect on reducing the time to resolution of fever (3 trials, 297 patients; MD: 0.82 day, 95% CI: 0.6-1.04, p < 0.00001) and the resolution time of cough (2 trials, 209 patients; MD: 0.9 day, 95% CI: 0.58-1.23, p < 0.00001), when compared with ribavirin and/or lincomycin. SHL injections had significant effect on reducing the resolution time of sore throat (1 trial, 79 patients; MD: 1.39 day, 95% CI: 0.88-1.9) and nasal congestion and discharge (1 trial, 130 patients; MD: 0.74 day, 95% CI: 0.11-1.37) (p-values not presented). |
|
| ||
| Homeopathy for URTIs [56, 57] | Positive results | 29 studies of different designs (17 RCTs) with 5.062 patients on the domain ‘Upper Respiratory Tract Infection/Allergy' (URTI/A) showed an overall positive result in favour of homeopathy. 6 out of 7 of the controlled studies demonstrated at least equivalence with conventional medical interventions and 8 out of 16 placebo controlled studies significance in favour of homeopathy. This positive trend was maintained in the evaluation of subgroups. |
|
| ||
| Individualized homeopathy for children with URTI, tonsillitis and acute sinusitis [58] | Homeopathy is a more or at least not inferior cost-effective method than placebo or conventional and antibiotic treatments | Six clinical trials (N= not presented). A significant difference in the median total symptom score in patients receiving homeopathy compared to the recipients of placebo in control groups (p = 0.026). Homeopathic strategies yielded significantly better results compared to antibiotic strategies in terms of medical efficacy (p ≤ 0.001). |
|
| ||
| Herbal medicine for cough [59] | Strong evidence for Andrographis paniculata and ivy/primrose/thyme-based preparations Moderate evidence for Pelargonium sidoides |
34 RCTs (N = 7.083) on P. sidoides (11 RCTs), Echinacea (8 RCTs), A. paniculata (6 RCTs), ivy/primrose/thyme (4 RCTs), essential oils (4 RCTs) and bakumondoto (1 RCT) were included. Controls were mainly placebo. Most studies had a low risk of bias. The meta-analysis revealed strong evidence for A. paniculata (SMD = -1.00, 95% CI: -1.85 to -0.15; p < 0.001) and ivy/primrose/thyme (RR = 1.40, 95% CI: 1.23-1.60; p < 0.001) in treating cough; moderate evidence for P. sidoides (RR = 4.60; 95% CI: 2.89-7.31; p < 0.001), and limited evidence for Echinacea (SMD = -0.68; 95% CI: -1.32 to -0.04; p = 0.04). |
|
| ||
| Chinese herbal medicine for postinfectious cough [60] | Improvement of core symptoms of postinfectious cough Enhancement of quality of life |
12 RCTs with moderate-to-high levels of evidence. Methodological quality was considered high in three trials, while in the other nine studies the unclear risk of bias was in the majority. Findings suggested that, compared with western conventional medicine or placebo, Chinese herbal medicine could effectively improve core symptoms of postinfectious cough, act better and have earlier antitussive effect, and enhance patients' quality of life. No serious adverse event was reported. |
|
| ||
| Chinese medicine for respiratory diseases [61] | Chinese medicine was more effective than anti-viral medicine | Six economic evaluations and cost studies were included, of which 4 studies' quality was low, 1 was high and 1 was medium. All studies adequately documented effectiveness of interventions. However, the costs of interventions were not well reported in 2 studies. 2 studies inadequately conducted sensitivity analysis and discounting. The diseases of 6 studies included bronchitis (2 studies), upper respiratory tract infection, herpangina, hand-foot-and-mouth disease and viral pneumonia. The studies results showed that cost-effectiveness of Xiyanping injection is poorer than Tanreqing injection and has more adverse reaction in 2 studies, and it is poorer than Yanhuning injection, but with less adverse reaction in 2 studies. Xiyanping injection is better than anti-viral medicine in 2 studies. 1 study indicated that Xiyanping is more cost-effective by atomized than intravenous drip. |
|
| ||
| Urinary tract infections (UTIs) | ||
|
| ||
| Cranberry for UTIs [62] | Evidence supporting clinical efficacy of cranberry product UTI prophylaxis exists in the following populations: women with rUTI, women with rUTI over 49 years old, children, rUTI, post-gynecological surgery patients, patients carrying a double-J ureteral stent, high-UTI-risk long-term care facility (LTCF) patients, prostatic adenocarcinoma patients treated with radiotherapy, and renal transplant patients with rUTI. An absence of clinical efficacy for cranberry product UTI prophylaxis exists in populations of women with rUTI (other studies), elderly males and females, neuropathic bladder/spinal injury patients, pregnant women, children (other studies), radiotherapy patients, low-UTI-risk LTCF patients, and MS patients with neurogenic bladder. |
22 relevant articles: three SRs, two SRs with MAs, eight RCTs, five NRSs, and four guidelines with relevant recommendations. |
|
| ||
| Antibiotic-associated diarrhoea | ||
|
| ||
| Probiotics for antibiotic-associated diarrhoea (AAD) [63] | Reduction of AAD | A total of 82 RCTs met inclusion criteria. The majority used Lactobacillus-based interventions alone or in combination with other genera; strains were poorly documented. The pooled relative risk in a DerSimonian-Laird random-effects meta-analysis of 63 RCTs, which included 11.811 participants, indicated a statistically significant association of probiotic administration with reduction in AAD (relative risk: 0.58; 95% CI: 0.50-0.68; p < .001; I(2), 54%; [risk difference: -0.07; 95% CI, -0.10 to -0.05], [number needed to treat: 13; 95% CI: 10.3-19.1]) in trials reporting on the number of patients with AAD. This result was relatively insensitive to numerous subgroup analyses. However, there exists significant heterogeneity in pooled results and the evidence is insufficient to determine whether this association varies systematically by population, antibiotic characteristic, or probiotic preparation. |
|
| ||
| Probiotics for prevention of AAD [64] | Preventive effects on AAD in adults (18–64 years) but not the elderly (> 65 years) | 30 RCTs met the predefined inclusion criteria and were included in the meta-analysis. There was considerable heterogeneity among the trials (p < .001); thus, subgroup analyses were performed. The meta-analysis resulted in a pooled relative risk (RR) of AAD of 0.69 (95% CI: 0.62-0.76) in a fixed effects model and 0.58 (95% CI: 0.48-0.71) in a random effects model, as compared with placebo. The positive association between intake of probiotic and reduced risk of AAD was observed in adults (RR: 0.47; 95% CI: 0.4-0.56). In contrast, in elderly patients, there was no positive effect (RR: 0.94; 95% CI: 0.76-1.15) of probiotic use and AAD. |
The Cochrane and non-Cochrane reviews demonstrate that some CAM treatment strategies for respiratory infections (both children and adults) are promising and that some have been shown to be effective in systematic reviews. CAM treatment strategies for other infections such as urinary tract infections (adult women) and skin infections are promising, but more rigorous research is necessary to provide high quality evidence.
(1) Other Non-Cochrane Reviews. See Table 3.
(2) Individual Clinical Studies. There are many other CAM treatments for infections which have been studied in a clinical trial, but for which no systematic review has yet been completed.
Acute Respiratory and Ear Infections. An international, multicenter, cohort study, comparing homeopathic and conventional treatment of acute respiratory and ear complaints in a primary care setting with 1.577 patients (857 received homeopathic and 720 conventional treatment), demonstrated that homeopathic treatment was not inferior to conventional treatment. More statistically significant favorable results for homeopathy were as follows: onset of improvement within the first 7 days after treatment was significantly faster upon homeopathic treatment both in children and adults, and adverse drug reactions occurred more frequently in adults of the conventional group than in the homeopathic group [119]. A prospective observational study comparing anthroposophic (AM) and conventional treatment of children with acute respiratory or ear infections under routine primary care conditions demonstrated that AM treatment was associated with much lower use of antibiotics (5% vs. 26%, during the four-week follow-up) and also much lower use of analgesics/antipyretics (3% vs. 26%) and was safe. AM patients demonstrated somewhat quicker symptom resolution and higher caregiver satisfaction [32].
Otitis Media. There is some evidence that Juzen-taiho-to, a Kampo or traditional Japanese herbal medicine, is effectively preventing recurrent acute otitis media (AOM) in children [120]. A prospective nonrandomized, comparative study underlined these results in the treatment of children with chronic otitis media with effusion. The frequency of antibiotic use was significantly less with the integrative concept using integrative-anthroposophic treatment (17.9% vs. 82.9%) [121].
Infected Wounds and MRSA. Tea Tree Oil (TTO) is an essential oil derived mostly from the leaves and terminal branchlets of the Australian native plant Melaleuca alternifolia from the Myrtaceae family [122]. Although no Cochrane systematic review has been conducted yet with regard to effects of TTO on MRSA infections, there is low quality evidence that 10% TTO soap significantly reduces MRSA quantity in infected wounds. TTO has been studied as an alternative treatment option for MRSA infections [123–126]. In a RCT a 10% TTO cream and 5% TTO body wash were more effective in clearing MRSA on skin compared with 4% chlorhexidine gluconate soap and 1% silver sulfadiazine cream [124].
Other Infections. There is some evidence that CAM use in patients with cancer is associated with a reduction in hospitalizations and requirements for antibiotics [127]. Trials of complementary interventions (vitamin A, probiotics, cranberry, nasturtium, and horseradish) for prevention of recurrent urinary tract infection in children generally gave favorable results but were not conclusive [128]. Herbal therapies appeared to be at least as effective as rifaximin for resolution of small intestine bacterial overgrowth and as effective as triple antibiotic therapy for SIBO rescue therapy for rifaximin nonresponders [129]. Long-term vaginal administration of Lactobacillus rhamnosus appears to be a useful complementary approach in the management of bacterial vaginosis [130]. A RCT, comparing the effects of vaginal cream with thyme and garlic and Metronidazole vaginal gel on treatment of bacterial vaginosis, demonstrated that both treatments were equally effective [131].
(3) Traditional Use and/or In Vitro Studies. Many CAM treatments have not been studied (at all or not for specific indications) in clinical studies with humans. They have only a status of long traditional use and/or have demonstrated antimicrobial effects in vitro. We describe here a very small selection of examples without any claim on completeness in selecting the examples. The importance of describing them here is that, given the urgent need for alternative, nonantibiotic treatments, this group of CAM treatments might be a source of development of new nonantibiotic alternatives. Positive experience during long periods of traditional use and/or positive results of in vitro studies provide a reason to study these remedies in clinical studies with patients.
Anemarrhena asphodeloides had been used in China, Japan, and Korea for thousands of years and demonstrates both antimicrobial and antiviral activities in vitro [132, 133]. Asparagus racemosus might be an alternative to antibiotics during UTI infections [134, 135]. Diodia scandens and Phyllanthus amarus might be effective against MRSA [136], extract 220D-F2 from the root of Rubus ulmifolius can be used to inhibit S. aureus biofilm formation to a degree that can be correlated with increased antibiotic susceptibility without toxic effects on normal mammalian cells [137], Woodfordia fruticosa appears to be effective against Pseudomonas pseudoalcaligenes and Gram-negative bacteria [138, 139], a review identified 255 (70% of 365) plant species from a wide range of families that have shown antimycobacterial activity [140], and protocatechuic acid (PCA, 3,4-dihydroxybenzoic acid) is a phenolic compound found in many food plants that demonstrates antimicrobial activities and also exerts synergistic interaction with some antibiotics against resistant pathogens [141]. Asparagus racemosus demonstrated positive effects in vitro against several UTI Gram-negative and Gram-positive pathogens [135]. Apitherapy is used in many traditional medical systems, among others, for the prevention of respiratory infections [142]. Natural honey demonstrated antiacanthamoebic properties [143], antibacterial effects [144], and antibiofilm effects against S. aureus [145] in wound healing. Melissa officinalis L. demonstrates both antibacterial and antifungal effects [146]. Several studies describe a list of promising CAM treatments for several infections, based on clinical experience and/or in vitro studies [147–150].
Andrographis paniculata has already been shown in clinical trials to be effective for respiratory infections (see above). In addition to this it may be effective against other infections. It is currently used in ayurveda, homeopathy [151], and TCM [152]. In vitro studies demonstrated dose-dependent antibacterial effects of A. paniculata: against E. coli, Klebsiella, Staphylococcus and Pseudomonas [153], Salmonella, Shigella, Gram A Streptococci, S. aureus, MRSA, Pseudomonas aeruginosa [154], Salmonella typhimurium, E. coli, Shigella sonnei, Streptococcus pneumonia, Streptococcus pyogenes, Legionella pneumophila, and Bordetella pertussis [155]. However in one study no activity against E. coli or Klebsiella pneumoniae was detected [154].
Pelargonium sidoides has already been tested in clinical trials for respiratory infections (see above) but may also be helpful in the treatment of other infections. Its traditional use was for the treatment of diarrhoea. Mechanistic evidence from a review in 2014 [156] concluded the following: ‘experimental results from in vitro studies indicate that bioactive phytochemical constituents of Pelargonium sidoides may not possess a direct antimicrobial effect, but instead act by interfering with microbial binding to host cell receptors, inhibition of key enzymes and the production of antimicrobial effector molecules such as nitric oxide and interferons (IFNs) by the host cells.' However, other in vitro studies did demonstrate growth inhibition of Escherichia coli, Shigella sonnei, Staphylococcus aureus OK2a and S. aureus ATCC6538, Salmonella typhi, S. typhimurium, Shigella flexneri, and Staphylococcus aureus OK2b [157].
Several studies also demonstrate the potential of treatment with natural products of viral infections, for example, licorice for several viral infections [158].
(4) Nonpharmaceutical Interventions. There is some evidence that acupuncture is effective in pain reduction of acute sore throat [159, 160]. Blue light demonstrates bactericidal effects in vitro and in vivo [161–163]. Based on the expertise of anthroposophic nurses, there is some practice-based evidence that external applications with essential oils may have effects on symptom relief [164].
3.3.5. Safety
General side effects of antibiotics use are antibiotic-associated diarrhoea (AAD) that happens in 5-39% of patients that are prescribed antibiotics [63] and candidiasis, obesity (associated with childhood use of antibiotics before 2 years) [165–169], allergies (in 5-10% of all patients [170] and in 10-30% of hospital patients [171]), increase of irritable bowel syndrome (IBS), and irritable bowel disease (IBD) symptoms [172]. In the treatment of AOM for every 14 children treated with antibiotics, one child experienced an adverse event (such as vomiting, diarrhoea, or rash) that would not have occurred if antibiotics had been withheld [173].
According to a systematic review of results reported in RCTs with 111 studies of a single herb and 133 of multiple herbs with a total of 15,441 participants, herbal treatment may be considered as safe: ‘There were 480 cases (3.1%) of adverse events (344 for single, 136 for multiple herb studies; p < 0.01). A total of 259 cases reported blood test abnormalities, including five cases of abnormality in hepatic functional enzymes. The most frequently reported adverse event was digestive symptoms (44.3%), followed by nervous system symptoms (17.3%) and behaviors such as loss of appetite (16.3%).' [174] However, a few herbal treatments have been associated with severe adverse events [175–177]. In reaction to these problems, pharmacovigilance systems have been established in the main producing countries of Chinese herbals [178] and Ayurvedic herbals [179], and quality standards for these medicinal products (MPs) have been improved [180, 181]. Other concerns are the inadequate knowledge of their mode of action, potential adverse reactions, contraindications, and interactions with existing orthodox pharmaceuticals and functional foods [182, 183].
Adverse reactions to homeopathic and anthroposophic MPs are infrequent and usually of mild to moderate severity, and anaphylactic reactions occur but are very rare [15, 57, 184].
4. Discussion
Given the mismatch between the urgent need for nonantibiotic strategies and the lack of use of CAM strategies embedded in current conventional policies and clinical practice, we performed a narrative review, based on searches in three databases (PubMed, Embase, and the Cochrane Database of Systematic Reviews) with a specific, limited set of search terms and input from CAM (research and clinical) experts, to explore and map what is known about the contribution of CAM health and health promotion concepts, infection prevention, and infection treatment strategies to reduce antibiotic use. This review has found significant evidence to support the safety and effectiveness of a range of CAM treatments for respiratory infections, based on many systematic reviews. It is now important to assess how this information can be used to recommend alternatives to antibiotics and so avoid unnecessary use of antibiotics in clinical practice. For other types of infection (such as urinary infections and skin infections) there are some promising clinical trials, but more research is needed before recommendations can be made.
4.1. Strengths and Limitations
The main strength of this review is the broad overview on this domain: the differences between conventional medicine and CAM regarding worldview, health (promotion) concepts, related prevention and treatment strategies for infections, and supporting evidence. This broad scope may contribute to providing a more transparent view on the differences between conventional medicine and CAM and on the possible contribution of CAM strategies. The promising results may also provide a broader interest in conventional medicine to study the contribution of CAM to the reduction of antibiotic use, and provide interest in the professional integration of the best of both worlds of CAM and conventional medicine in prevention and treatment strategies of infections, in line with, for example, the current ‘Traditional Medicine Strategy: 2014-2023' of the World Health Organization (WHO) [12].
The review has also several limitations. The first one is that it is a narrative review that is aimed at exploring relevant themes and at providing a first broad overview of the studied domain. The review thus does not provide an exact, narrow focused overview of the state of science of each of the subareas (concepts, prevention, treatment per indication) as is done in a scoping review, and it does not judge the methodological quality of the scientific evidence of studies on CAM prevention and treatments for specific indications, as is done in a systematic review. A second limitation is that the number of databases searched was limited to three (PubMed, Embase, Cochrane Database of reviews), that a limited number of search terms was used, that the acquired additional records were not collected in a systemized way, and that input was given by a selected group of CAM research and/or clinical experts, which might have led to a selection regarding the content provided as input. A third limitation is that the quality of the studies was often low. For example, many of the studies on prescription of antibiotics (may have) used self-selected samples. It is therefore not obvious that it would be possible to reduce use of antibiotics simply by transferring patients from conventional to integrative doctors, because the reason may be that patients self-selecting to go to CAM doctors are less likely to demand antibiotics.
4.2. Medical and Methodological Barriers and Facilitators of the Implementation of CAM Strategies into Conventional Medicine
Besides, in many cases, the absence of high quality evidence, the implementation of CAM prevention and treatment strategies into conventional medicine is hindered by several other medical and methodological barriers.
Whereas CAM modalities were tolerated in clinical practice in many western countries until the end of the 20th century, they are increasingly becoming scientifically criticized [15, 185]. According to many scientists, CAM treatments are justified with prescientific or unscientific paradigms that are not in agreement with currently accepted medical theories. Therefore, on theoretical grounds, it is their and others' opinion that CAM must not be integrated with conventional medicine [186–188]. A second group of barriers concerns the quality of CAM prevention and treatment strategies. The image of CAM, in conventional science and medicine, is often that there is no high quality evidence of specific effects of CAM strategies for conventional indications as tested in clinical studies and analyzed in systematic reviews and meta-analyses [186]. In addition, there are concerns regarding the safety and drug interactions of CAM treatments [189–191], because some medicinal products have been associated with repeated, severe adverse reactions [192, 193]. Other concerns are environmental contaminations (e.g., air pollution, soil contaminations), cultivation practices (e.g., pesticides, fungicides, microorganisms, endotoxins), manufacturing procedures (e.g., microorganisms, endotoxins), and inappropriate use [194, 195].
Another group of barriers concerns implementation of CAM strategies. First, whereas some conventional guidelines are still based on clinical expertise and/or lower level of evidence (e.g., in pediatrics and surgery), input for guidelines from CAM based on clinical expertise and/or lower level of evidence is not easily accepted, based on the described negative image of CAM. Secondly there are regulatory barriers. In essence, although European regulatory systems are available for homeopathy and herbal medicines, they currently do not match the specific features of whole medical system products with regard to assessment of quality [196], effectiveness and safety, and handling of multicomponent products [15]. Thirdly, the available evidence and knowledge on prevention and treatment strategies are not easily accessible for the target populations (e.g., doctors, pharmacists, patients) [197]. Fourthly, although many patients already use CAM MPs [198, 199] and shared-decision making is promoted in clinical practice, many doctors do not want to prescribe CAM alternatives for antibiotics, due to patient pressure to prescribe antibiotics [200–202], fear of ineffectiveness of CAM treatments [203], lack of knowledge on CAM in general [204], insufficient information on effectiveness and safety, (assumed) insufficient regulation of herbal practitioners, concerns about herbal quality control and potential herb–drug interactions [205, 206], and a lack of communication between doctors and patients about this topic [204, 207].
An important methodological barrier for CAM is that methodologies that are currently used to acquire high quality evidence (RCTs, systematic reviews, and meta-analyses of RCTs) often do not match CAM, so-called whole medical system, interventions. The current golden standard of EBM, the double-blind, placebo-controlled RCT, is often not applicable to test efficacy and effectiveness of a CAM intervention due to its complexity [208, 209]. Furthermore there are significant regulatory barriers to conducting clinical trials of complex or individualized mixtures of herbal medicines. CAM treatments for conventional indications are often individualized based on additional CAM diagnoses, are aimed at restoring balances rather than symptom reduction, often contain different treatments as part of a complex intervention (multimodal), and are system effects and health promotion oriented. As a result of this mismatch between demanded methodologies and CAM interventions, there is a lack of RCTs. And the available RCTs with protocolled interventions might lead to false-negative results (meaning that in reality the treatment has (larger) beneficial effects but these are not captured in the research study), because of the lack of individualization. Therefore, CAM researchers argue that there is a need for additional methods, e.g., pragmatic studies, observational studies, a mix of qualitative and quantitative studies, and n=1 studies, in order to meet the complexity of CAM interventions [210]. In addition, a “reversed research strategy” for assessing CAM has been suggested, starting with studies of the context, paradigms, philosophical understanding, and utilization, then subsequently the safety status of the whole system, comparative effectiveness of the whole system, specific efficacy of components, and finally the underlying biological mechanisms [78, 107]. A second barrier is that there is a lack of structural funding of research on CAM prevention and treatment strategies in many countries (although in China the research on TCM is increasingly structurally funded), with a sponsorship bias as a result [211]. Most of CAM is not patentable and not profitable, has little lobby, and is complicated and multifactorial, and therefore research is underfunded.
On the other hand, there are also several facilitators for the integration of CAM prevention and treatment strategies for infections. First of all, the position of the patient in healthcare is increasingly important (as expressed in developments like, for example, shared-decision making, patient reported outcomes, and experiences) and CAM is increasingly used and demanded by patients worldwide [43, 207, 212]. Secondly, the increasing burden of the global AMR problem opens opportunities for CAM alternative prevention and treatment approaches as expressed in a NHS funded study on CAM treatment of UTI [213] and a EU funded European research network for CAM researchers on infections [214]. Thirdly, the CAM concept of health promotion is increasingly in agreement with conceptualization of health in conventional medicine [66, 67, 215]. CAM prevention and treatment health promotion strategies are thus not, as often assumed, justified with a prescientific or unscientific paradigm, but are based on theories that are increasingly accepted in conventional medicine. Fourthly, there is a growing scientific interest in and knowledge of systems biology/systems and personalized approaches in conventional science and medicine, which makes it easier to accept the CAM systems approaches [22]. Fifthly, there are positive examples of the integration of CAM strategies, especially in low-income countries [216], that can result in more confidence in CAM. Sixthly, there is an increasing interest in the delayed prescription strategy, which fits with the CAM treatment strategy; during the delayed prescription period patients can use CAM treatments. Reduced antibiotics prescription for uncomplicated infections, without additional CAM treatment, is already relatively safe with only a very slight increase in the incidence of complicated infections [217, 218]. In addition, suggesting actions parents could take to reduce their child's symptoms (providing parents positive treatment recommendations, such as CAM treatments) is associated with decreased risk of antibiotic prescribing [219]. Last but not least, there are some good examples of positive results from CAM prevention and treatment strategies of infections with high quality evidence from systematic reviews/meta-analyses [51, 59, 63].
4.3. Future Research and Other Nonresearch Perspectives
The narrative review provides concrete leads for future research and other activities. First, in general this narrative review must be followed by scoping reviews and/or systematic reviews, examining each of the subareas (e.g., prescription rates of antibiotics) separately, providing a more complete overview of the subarea (scoping review) and a better judgment of the quality of the scientific evidence (systematic review).
Although this first narrative review on CAM approaches to reduce antibiotic use, based on a systematic search strategy, is limited and not yet a systematic review of the literature, it can help to structure directions and types of further research. The following topics seem to be important for future research in the domains that were studied here (health and health promotion concepts, antibiotic prescription and consumption, safety and effects of prevention and treatment strategies). Regarding the CAM health (promotion) concepts, more studies validating the health concept and exploring and testing the health promotion working mechanisms (in general and specific regarding infections) are necessary. With regard to the comparison of prescription and consumption rates of antibiotics in CAM practices and conventional practices, more studies are needed in other integrative primary and secondary care facilities, studies on specific indications, and better studies controlling for selection bias. With regard to the safety and effects of CAM prevention and treatment strategies, more research is needed regarding the effects and safety of CAM lifestyle/prevention strategies on the development of resilience to infections; nosocomial infectious diseases and resistance rates, hygiene management in different types of hospitals (CAM vs. conventional); and the identification, collection, and/or systematization of CAM expert knowledge based on traditional use.
Regarding treatment, further research building on current high levels of evidence could focus on use of CAM as alternatives to antibiotics for respiratory infections. This research and respective methodologies could build on the existing systematic reviews. Decision aids and guidelines need to be developed, piloted, and evaluated, to guide clinicians and patients in their choice of CAM therapies. Economic analyses will also be important to guide policy development in this area.
For other disease areas, there is as yet insufficient evidence for the development of guidelines or decision aids, so more research is needed to build the evidence base with this goal in mind. There is interesting preliminary evidence from systematic reviews on use of CAM for treatment of urinary tract infections and prevention of recurrent UTIs. Further rigorous research is needed in order to find the optimal CAM treatments for these conditions, as alternatives for antibiotics. Another important area is the treatment of antibiotic-resistant skin infections, such as wounds infected with MRSA. There are some preliminary clinical trials, but no systematic reviews in this area. Many antibiotics are prescribed for skin infections, especially acne, yet our search did not identify any RCTs of CAM treatments for acne. Systematic reviews and more rigorous clinical trials are needed to find the most effective CAM approaches for these conditions.
In many countries worldwide a wide range of CAM treatments are used daily but not well observed by the scientific community. Such research and knowledge gaps as well as low levels of evidence can derive from low research capacity and /or methodological challenges. They may be relevant for further research and for public health policy. AMR public health policy will become more interested in learning about the potential contribution of CAM to reduce prescription of and demand for antibiotics. Preliminary research may serve as a starting point with respect to some of these fields of current CAM practice, to highlight the most promising therapies, for example, through observational studies such as retrospective treatment-outcome studies [220].
Finally, according to the group of CAM experts, other issues, supporting the study of the CAM contribution and the integration of the contribution of CAM into conventional medicine, should be addressed in future activities: adequate research infrastructure should be developed and optimized (e.g., (academic) institutes, methodologies, funding) to test CAM multicomponent and/or multimodal treatments of infections; adequate regulatory infrastructures should be developed to regulate multicomponent and/or multimodal CAM treatments of infections; adequate information on the CAM contributions needs to be provided for different stakeholder groups (e.g., doctors, pharmacists, patients), through the development of best practices, decision support tools, training, communication, and implementation strategies including proposals for the integration of CAM/IM contributions in guidelines. There need to be more studies of patients' demands, decision criteria, and awareness of side effects of antibiotics and both patients' and doctors' perceptions on antibiotics and the AMR problem. There is a need for cross-country analyses of socioeconomic factors, insurance policies and regulation with regard to antibiotic prescription, effectiveness of public health policies, and implementation of guidelines as well as CAM prevention and treatment strategies.
5. Conclusions
There is some evidence that CAM prevention and treatment strategies can lead to the prescription and consumption of fewer antibiotics.
There is some, most often low quality, evidence that CAM prevention and treatment strategies are safe and effective (reduction of incidence of recurrent infections, overall symptoms and specific symptoms of infections, symptom severity, and time to recovery/sick leave).
Some of the CAM treatment strategies for respiratory infections are promising and some have been shown to be effective in systematic reviews. Guidelines and decision aids are needed for patients and clinicians.
CAM treatment strategies for other infections such as urinary tract infections and skin infections are promising, but more rigorous research is necessary to provide high quality evidence before guidelines can be developed.
The worldview differences between CAM and conventional medicine, relevant to prevention and treatment of infections and the AMR problem, are the differences between the biomedical model and the whole medical system model.
The worldview-related CAM health concepts enable health promotion oriented prevention and treatment of infections aimed at strengthening or supporting the self-regulating ability of the human organism to cope with diseases.
-
The hypothesized CAM contributions to the reduction of antibiotic use are
- prevention strategies aimed at reducing stress, insomnia, depression, and anxiety (all associated with increased susceptibility to acute infectious illness), promoting healthy diets and physical exercise (both reducing the risk of infectious diseases), supporting the fever reaction of the organism (to overcome the infections by itself), and preventing infections with natural products;
- treatment strategies with natural medicinal products.
Abbreviations
- 95% CI:
95% confidence interval
- AAD:
Antibiotic-associated diarrhoea
- AB:
Acute bronchitis
- AM:
Anthroposophic medicine
- AMR:
Antimicrobial resistance
- AOM:
Acute otitis media
- A. paniculata:
Andrographis paniculata
- ARDS:
Acute respiratory distress syndrome
- ARS:
Acute rhinosinusitis
- ARTI:
Acute respiratory tract infection
- ATP:
Acute tonsillopharyngitis
- BCG:
Bacillus Calmette-Guerin
- CAM:
Complementary and Alternative Medicine
- CD4:
Cluster of differentiation 4
- CHM:
Chinese herbal medicine
- E. coli:
Escherichia coli
- EBM:
Evidence-based medicine
- EU:
European Union
- GP:
General practitioner
- IBD:
Irritable bowel disease
- IBS:
Irritable bowel syndrome
- ICU:
Intensive care unit
- IFNγ:
Interferon gamma
- IM:
Integrative Medicine
- IVIG:
Intravenous immunoglobulin
- IS:
Immunostimulant
- LTC:
Long-term care
- MA:
Meta-analysis
- MAP:
Mitogen-activated protein
- MBC:
Minimal bactericidal concentration
- MD:
Mean difference
- MIC:
Minimal inhibitory concentration
- MPs:
Medicinal products
- MRSA:
Multiresistant Staphylococcus aureus
- MS:
Multiple sclerosis
- N:
Number
- NF-κB:
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NHS:
National Health Service
- NRS:
Nonrandomized controlled study
- OR:
Odds ratio
- PCA:
Protocatechuic acid
- P. sidoides:
Pelargonium sidoides
- RCT:
Randomized controlled trial
- RR:
Relative risk
- RTI:
Respiratory tract infection
- rUTI:
Recurrent urinary tract infection
- S. aureus:
Staphylococcus aureus
- SIBO:
Small Intestinal Bacterial Overgrowth
- SMD:
Standardized mean difference
- S. typhimurium:
Salmonella typhimurium
- TNFα:
Tumor necrosis factor alpha
- TTO:
Tea tree oil
- UK:
United Kingdom
- URTI:
Upper respiratory tract infection
- UTI:
Urinary tract infection
- USA:
United States of America
- WHO:
World Health Organization.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have the following possible conflicts of interest. However, these conflicts of interest did not actually influence the design, analyses, and the reporting of this study in the current article. Erik W. Baars is a member of the European Scientific Cooperative on Anthroposophic Medicinal Products (ESCAMP), Freiburg, Germany, and a professor of anthroposophic medicine at the University of Applied Sciences Leiden, The Netherlands. In the past, he received research funding from CAM pharmaceutical industries. Eefje Belt-van Zoen has no conflicts of interest to declare. Thomas Breitkreuz is Chairman of the Commission C for Anthroposophic Medicinal Products (AMPs) at the Federal Institute for Drugs and Medical Devices, in Bonn, Germany. He is President of the Federation of Anthroposophic Medical Associations (IVAA), member of the executive board of the Physicians' Association for Anthroposophic Medicine in Germany (GAÄD), and member of the European Scientific Cooperative on Anthroposophic Medicinal Products (ESCAMP). David Martin has lectured at events organized or sponsored by CAM pharmaceutical companies out of conviction and has thereby always declined any remuneration. He also teaches in several institutions for integrative and anthroposophic medicine and holds a chair for medical theory, integrative and anthroposophic medicine, none of which involve conflicts of interest. Harald Matthes is Head of the working group Integrative and Anthroposophic Medicine and Managing Director of the Havelhöhe Research Institute. He is a board member of the Tumor Center of Nonprofit and Confessional Hospitals in Berlin, a board member of the Hufelandgesellschaft (umbrella organization of the medical societies for integrative and complementary medicine), a member of the Medical Commission of the German Hospital Association (DKG), a member of the Pharmacovigilance Committee (§67) at the BfArM, and President of the German Academy of Homeopathy and Naturopathy (DAHN e.V.), and he holds a chair in Integrative and Anthroposophic Medicine at the Charite University in Berlin. Tido von Schoen-Angerer has no conflicts of interest to declare. Georg Soldner is member of the Commission C for Anthroposophic Medicinal Products at the Federal Institute for Drugs and Medical Devices in Bonn, Germany. He is also Vice-Leader of the medical section of the Goetheanum in Dornach, Switzerland, a board member of “Medizinisches Seminar Bad Boll”, and a postgraduate training initiative in anthroposophic medicine. Jan Vagedes declares that there are no conflicts of interest regarding the publication of this paper. Herman van Wietmarschen is a member of the European Scientific Cooperative on Anthroposophic Medicinal Products (ESCAMP). He is also a board member of the Academy of Integrative Medicine in the Netherlands. Olga Patijn declares that there are no conflicts of interest regarding the publication of this paper. Merlin Willcox is a General Medical Practitioner and Academic Clinical Lecturer in the Department of Primary Care and Population Sciences at the University of Southampton. Paschen von Flotow is Head of the Sustainable Business Institute (SBI). His academic research on socioeconomic issues of sustainable development such as AMR has been funded by various public and private funding institutions, including CAM pharmaceutical industry. He was member of the board of the German chapter of the European Network for Business Ethics (EBEN) until June 2018. Michael Teut is a member of the Commission D for Homeopathic Medicinal Products at the Federal Institute for Drugs and Medical Devices, in Bonn, Germany. He received funding for research on homeopathy from Karl und Veronica Carstens-Stiftung, Homöopathie-Stiftung des Deutschen Zentralvereins homöopathischer Ärzte, omoeon e.V., and Max Tiedemann Stiftung. He also received travel expenses for participation in homeopathic congresses or network-meetings from Deutscher Zentralvereins homöopathischer Ärzte and Robert Bosch Stiftung. Klaus von Ammon has no conflicts of interest to declare. Madan Thangavelu declares that there are no conflicts of interest regarding the publication of this paper. Ursula Wolf is Managing Director at the Institute of Complementary Medicine at the University of Bern. She is President Elect of the International Society for Complementary Medicine Research ISCMR. Josef Hummelsberger is the member of the directorate board of the SMS (International Society of Chinese Medicine (Germany) and Berufsverband Akupunktur (Germany). Ton Nicolai is the coordinator and spokesman of EUROCAM, a foundation uniting European organizations of patients and health professionals (doctors, veterinarians, and other practitioners) using Complementary and Alternative Medicine. Philippe Hartemann is Distinguished Professor of Public Health in the School of Medicine of Nancy, University of Lorraine, member of numerous scientific boards in Environmental Health, without any conflicts of interest in the field of this article. Henrik Szőke is Acting Head of the CAM Department of the University of Pécs in Hungary, is Founder and Leader of AntroMedicArt and AMEMA associations, and is member of the board of MAOT (Hungarian Association of Acupuncture Doctors) society. Michael McIntyre is Chair of the European Herbal and Traditional Medicine Practitioners Association for which he received an emolument. Esther T. van der Werf is member of the scientific advisory board of the Portland Centre Integrative Medicine (UK). In the past, she received research funding from CAM pharmaceutical industries. Roman Huber is Head of the Center for Complementary Medicine, University of Freiburg, Germany. He declares no conflicts of interest.
Authors' Contributions
Erik W. Baars and Eefje Belt-van Zoen performed the searches. Erik W. Baars wrote the draft versions of the article. All (co-)authors (Erik W. Baars, Eefje Belt-van Zoen, Thomas Breitkreuz, David Martin, Harald Matthes, Tido von Schoen-Angerer, Georg Soldner, Jan Vagedes, Herman van Wietmarschen, Olga Patijn, Merlin Willcox, Paschen von Flotow, Michael Teut, Klaus von Ammon, Madan Thangavelu, Ursula Wolf, Josef Hummelsberger, Ton Nicolai, Philippe Hartemann, Henrik Szőke, Michael McIntyre, Esther T. van der Werf, and Roman Huber) made substantial contributions to conception and design, or analysis and interpretation of data; have been involved in drafting the manuscript or revising it critically for important intellectual content; and have given final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.EMA. Antimicrobial resistance. 2017, http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000439.jsp.
- 2.O'Neill J. Tackling drug-resistant infections globally: final report and recommendations. London, UK: Wellcome Trust & HM Government; 2016. [Google Scholar]
- 3.World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. [DOI] [PubMed] [Google Scholar]
- 4.Smith E., Lichten C. A., Taylor J., et al. Evaluation of the EC Action Plan Against the Rising Threats from Antimicrobial Resistance. 2016. [Google Scholar]
- 5.Hopkins S., Johnson A. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) London, UK: Public Health England; 2016. [Google Scholar]
- 6.National Institute for Health and Care Excellence. Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. NG15. 2015. [Google Scholar]
- 7.Rodrigues A. T., Roque F., Falcão A., Figueiras A., Herdeiro M. T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. International Journal of Antimicrobial Agents. 2013;41(3):203–212. doi: 10.1016/j.ijantimicag.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Akkerman A. E., M. M. Kuyvenhoven, van der Wouden J. C., Verheij T. J. M. Prescribing antibiotics for respiratory tract infections by GPs: management and prescriber characteristics. British Journal of General Practice. 2005;55(511):114–118. [PMC free article] [PubMed] [Google Scholar]
- 9.McKay R., Mah A., Law M. R., McGrail K., Patrick D. M. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrobial Agents and Chemotherapy. 2016;60(7):4106–4118. doi: 10.1128/AAC.00209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamre H. J., Fischer M., Heger M., et al. Anthroposophic vs. conventional therapy of acute respiratory and ear infections: a prospective outcomes study. Wiener Klinische Wochenschrift. 2005;117(7-8):256–268. doi: 10.1007/s00508-005-0344-9. [DOI] [PubMed] [Google Scholar]
- 11.Kok E. T., Jong M. C., Gravendeel B., Van Leeuwen W. B., Baars E. W. Resistance to antibiotics and antifungal medicinal products: can complementary and alternative medicine help solve the problem in common infection diseases? the introduction of a dutch research consortium. Evidence-Based Complementary and Alternative Medicine. 2015;2015:6. doi: 10.1155/2015/521584.521584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO Traditional Medicine Strategy 2014–2023. 2013. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 13.McClafferty H., Dodds S., Brooks A., et al. Pediatric Integrative Medicine in Residency (PIMR): Description of a New Online Educational Curriculum. Children. 2015;2(1):98–107. doi: 10.3390/children2010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt A. H.-d. Exploring worldviews and their relationships to sustainable lifestyles: Towards a new conceptual and methodological approach. Ecological Economics. 2012;84:74–83. doi: 10.1016/j.ecolecon.2012.09.009. [DOI] [Google Scholar]
- 15.Baars E. W., Hamre H. J. Whole medical systems versus the system of conventional biomedicine: a critical, narrative review of similarities, differences, and factors that promote the integration process. Evidence-Based Complementary and Alternative Medicine. 2017;2017:13. doi: 10.1155/2017/4904930.4904930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcum J. A. An Introductory Philosophy of Medicine: Humanizing Modern Medicine. Vol. 99. Springer Science & Business Media; 2008. [Google Scholar]
- 17.Schroen Y., Wietmarschen H., van Wang M. East is East and West is West, and never the twain shall meet? Science. 2014;346(6216):S10–S12. [Google Scholar]
- 18.Koithan M., Bell I. R., Niemeyer K., Pincus D. A complex systems science perspective for whole systems of complementary and alternative medicine research. Forschende Komplementärmedizin. 2012;19(1):7–14. doi: 10.1159/000335181. [DOI] [PubMed] [Google Scholar]
- 19.Bie G. v. d., Scheffers T., Tellingen C. v. The Healing Process. Organ of Repair. Bolk’s Companion; 2008. [Google Scholar]
- 20.Goldman A. W., Burmeister Y., Cesnulevicius K., et al. Bioregulatory systems medicine: an innovative approach to integrating the science of molecular networks, inflammation, and systems biology with the patient's autoregulatory capacity? Frontiers in Physiology. 2015;6(225) doi: 10.3389/fphys.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kröz M., Reif M., Pranga D., et al. The questionnaire on autonomic regulation: a useful concept for integrative medicine? Journal of Integrative Medicine. 2016;14(5):315–321. doi: 10.1016/S2095-4964(16)60264-9. [DOI] [PubMed] [Google Scholar]
- 22.Baars E. W. Evidence-based curative health promotion: a systems biology-orientated treatment of seasonal allergic rhinitis with Citrus/Cydonia comp [Doctoral thesis] Wageningen University; 2011. [Google Scholar]
- 23.Leung K. F., Liu F. B., Zhao L., Fang J. Q., Chan K., Lin L. Z. Development and validation of the Chinese Quality of Life Instrument. Health and Quality of Life Outcomes. 2005;3:p. 26. doi: 10.1186/1477-7525-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Tang Y.-L. On the concept of health in traditional Chinese medicine and its characteristics and advantages. Zhonghua yi shi za zhi (Beijing, China : 1980) 2010;40(1):13–14. [PubMed] [Google Scholar]
- 25.Bhopal R. S. Bhye Bhaddi: A food and health concept of Punjabi Asians. Social Science & Medicine. 1986;23(7):687–688. doi: 10.1016/0277-9536(86)90116-4. [DOI] [PubMed] [Google Scholar]
- 26.Jagtenberg T., Evans S., Grant A., Howden I., Lewis M., Singer J. Evidence-based medicine and naturopathy. The Journal of Alternative and Complementary Medicine. 2006;12(3):323–328. doi: 10.1089/acm.2006.12.323. [DOI] [PubMed] [Google Scholar]
- 27.Heckenbach K., Tabali M., Matthes H. Wie häufig werden Kinder bei anthroposophischen Ärzten geimpft? DMW - Deutsche Medizinische Wochenschrift. 2012;137(S 03) doi: 10.1055/s-0032-1323291. [DOI] [Google Scholar]
- 28.Alm J. S., Swartz J., Lilja G., Scheynius A., Pershagen G. Atopy in children of families with an anthroposophic lifestyle. The Lancet. 1999;353(9163):1485–1488. doi: 10.1016/S0140-6736(98)09344-1. [DOI] [PubMed] [Google Scholar]
- 29.Flöistrup H., Swartz J., Bergström A., et al. Allergic disease and sensitization in Steiner school children. The Journal of Allergy and Clinical Immunology. 2006;117(1):59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Kummeling I., Thijs C., Penders J., et al. Etiology of atopy in infancy: The KOALA Birth Cohort Study. Pediatric Allergy and Immunology. 2005;16(8):679–684. doi: 10.1111/j.1399-3038.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 31.Jeschke E., Lüke C., Ostermann T., Tabali M., Hübner J., Matthes H. Prescribing practices in the treatment of upper respiratory tract infections in anthroposophic medicine. Forschende Komplementärmedizin. 2007;14(4):207–215. doi: 10.1159/000104171. [DOI] [PubMed] [Google Scholar]
- 32.Hamre H. J., Glockmann A., Schwarz R., et al. Antibiotic use in children with acute respiratory or ear infections: prospective observational comparison of anthroposophic and conventional treatment under routine primary care conditions. Evidence-Based Complementary and Alternative Medicine. 2014;2014:17. doi: 10.1155/2014/243801.243801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimaldi-Bensouda L., Bégaud B., Rossignol M., et al. Management of Upper Respiratory Tract Infections by Different Medical Practices, Including Homeopathy, and Consumption of Antibiotics in Primary Care: The EPI3 Cohort Study in France 2007–2008. PLoS ONE. 2014;9(3):p. e89990. doi: 10.1371/journal.pone.0089990. [DOI] [Google Scholar]
- 34.Riley D., Fischer M., Singh B., Haidvogl M., Heger M. Homeopathy and conventional medicine: An outcomes study comparing effectiveness in a primary care setting. The Journal of Alternative and Complementary Medicine. 2001;7(2):149–159. doi: 10.1089/107555301750164226. [DOI] [PubMed] [Google Scholar]
- 35.Wye L., Hay A. D., Northstone K., Bishop J., Headley J., Thompson E. Complementary or alternative? The use of homeopathic products and antibiotics amongst pre-school children. BMC Family Practice. 2008;9(1):1–8. doi: 10.1186/1471-2296-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del-Rio-Navarro B. E., Espinosa-Rosales F. J., Flenady V., Sienra-Monge J. J. Cochrane Review: Immunostimulants for preventing respiratory tract infection in children. Evidence-Based Child Health: A Cochrane Review Journal. 2012;7(2):629–717. doi: 10.1002/ebch.1833. [DOI] [PubMed] [Google Scholar]
- 37.Su G., Chen X., Liu Z., et al. Oral Astragalus (Huang qi) for preventing frequent episodes of acute respiratory tract infection in children. Cochrane Database of Systematic Reviews. 2016;12 doi: 10.1002/14651858.CD011958.CD011958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lissiman E., Bhasale A. L., Cohen M. Garlic for the common cold. Cochrane Database of Systematic Reviews. 2014;11 doi: 10.1002/14651858.CD006206.pub4.CD006206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karsch-Völk M., Barrett B., Kiefer D., Bauer R., Ardjomand-Woelkart K., Linde K. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2014;2 doi: 10.1002/14651858.CD000530.pub3.CD000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmer A., Günther J., Motschall E., Rücker G., Antes G., Kern W. V. Pelargonium sidoides extract for treating acute respiratory tract infections. Cochrane Database of Systematic Reviews. 2013;10 doi: 10.1002/14651858.CD006323.pub3.CD006323 [DOI] [PubMed] [Google Scholar]
- 41.Huang Y., Wu T., Zeng L., Li S. Chinese medicinal herbs for sore throat. Cochrane Database of Systematic Reviews. 2012;3 doi: 10.1002/14651858.CD004877.pub3.CD004877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang L., Li K., Wu T. Chinese medicinal herbs for acute bronchitis. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD004560.pub4.CD004560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flower A. W. L., Lewith G., Liu J., Li Q. Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database of Systematic Reviews. 2015;6 doi: 10.1002/14651858.cd010446.pub2.CD010446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwenger E. M., Tejani A. M., Loewen P. S. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database of Systematic Reviews. 2015;12 doi: 10.1002/14651858.CD008772.pub2.CD008772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H. M., Tang J. L., Sha Z. H., Cao L., Li Y. P. Interventions for preventing infection in nephrotic syndrome. Cochrane Database of Systematic Reviews. 2004;2 doi: 10.1002/14651858.CD003964.pub2.CD003964 [DOI] [PubMed] [Google Scholar]
- 46.Jull A. B., Cullum N., Dumville J. C., Westby M. J., Deshpande S., Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews. 2015;3 doi: 10.1002/14651858.CD005083.pub4.CD005083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y. F., Que H. F., Wang Y., Cui X. J. Chinese herbal medicines for treating skin and soft-tissue infections. Cochrane Database of Systematic Reviews. 2014;(7) doi: 10.1002/14651858.CD010619.pub2.CD010619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu X.-Y., Wu R.-H., Logue M., et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE. 2017;12(8) doi: 10.1371/journal.pone.0181780.e0181780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthys H., Malek F., Kamin W. EPs® 7630 in Acute Respiratory Tract Infections–a Systematic Review and Meta-Analysis of Randomised Clinical Trials. Forsch Komplementärmed. 2014;21:57–58. [Google Scholar]
- 50.Koch A. K., Klose P., Lauche R., et al. A Systematic Review of Phytotherapy for Acute Rhinosinusitis. Complementary Medicine Research. 2016;23(3):165–169. doi: 10.1159/000447467. [DOI] [PubMed] [Google Scholar]
- 51.Anheyer D., Cramer H., Lauche R., Saha F. J., Dobos G. Herbal Medicine in Children With Respiratory Tract Infection: Systematic Review and Meta-Analysis. Academic Pediatrics. 2018;18(1):8–19. doi: 10.1016/j.acap.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Li X., Ge T., et al. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95(31) doi: 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozen M., Sandal G. K., Dinleyici E. C. Probiotics for the prevention of pediatric upper respiratory tract infections: A systematic review. Expert Opinion on Biological Therapy. 2015;15(1):9–20. doi: 10.1517/14712598.2015.980233. [DOI] [PubMed] [Google Scholar]
- 54.Mingming Q. i., Jian M. a., Xiaojin C. Randomized and controlled trials of Sanren Decoction’s treatment on upper respiratory infections: a meta-analysis. International Journal of Clinical and Experimental Medicine. 2016;9(7):12954–12958. [Google Scholar]
- 55.Zhang H., Chen Q., Zhou W., et al. Chinese medicine injection shuanghuanglian for treatment of acute upper respiratory tract infection: a systematic review of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/987326.987326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergemann S. M., Bornhöft G., Bloch D., Vogt-Frank C., Righetti M., Thurneysen A. Homeopathy in Healthcare–Effectiveness, Appropriateness, Safety, Costs. Springer; 2011. Clinical studies on the effectiveness of homeopathy for URTI/A (Upper Respiratory Tract Infections and Allergic Reactions) pp. 127–157. [Google Scholar]
- 57.Bornhöft G., Wolf U., Ammon K. v., et al. Effectiveness, Safety and Cost-Effectiveness of Homeopathy in General Practice – Summarized Health Technology Assessment. Complementary Medicine Research. 2006;13(2):19–29. doi: 10.1159/000093586. [DOI] [PubMed] [Google Scholar]
- 58.Mostafaei M., Ahmadabad M. S., Rafiee H. Effects of homeopathy on the treatment of infections in children. Avicenna Journal of Phytomedicine. 2015;5 [Google Scholar]
- 59.Wagner L., Cramer H., Klose P., et al. Herbal Medicine for Cough: a Systematic Review and Meta-Analysis. Complementary Medicine Research. 2015;22(6):359–368. doi: 10.1159/000442111. [DOI] [PubMed] [Google Scholar]
- 60.Liu W., Jiang H.-L., Mao B. Chinese herbal medicine for postinfectious cough: a systematic review of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine. 2013;2013:14. doi: 10.1155/2013/906765.906765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Huang S., Zhu W. Cost-Effectiveness of the Treatment of Respiratory Diseases of Xiyanping Injection : A Systematic Review. Value in Health. 2014;17(7):p. A779. doi: 10.1016/j.jval.2014.08.365. [DOI] [PubMed] [Google Scholar]
- 62.Health C. A. f. D. a. T. i. Cranberry Products or Topical Estrogen-Based Therapy for the Prevention of Urinary Tract Infections: A Review of Clinical Effectiveness and Guidelines. 2016. [PubMed] [Google Scholar]
- 63.Hempel S., Newberry S. J., Maher A. R. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. The Journal of the American Medical Association. 2012;307(18):1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 64.Jafarnejad S., Shab-Bidar S., Speakman J. R., Parastui K., Daneshi-Maskooni M., Djafarian K. Probiotics Reduce the Risk of Antibiotic-Associated Diarrhea in Adults (18-64 Years) but Not the Elderly (> 65 Years): A Meta-Analysis. Nutrition in Clinical Practice: Official Publication of The American Society for Parenteral and Enteral Nutrition. 2016;31(4):502–513. doi: 10.1177/0884533616639399. [DOI] [PubMed] [Google Scholar]
- 65.Huber R., Michalsen A. Checkliste Komplementärmedizin. Georg Thieme Verlag; 2014. [Google Scholar]
- 66.Eriksson M., Lindström B. Antonovsky's sense of coherence scale and its relation with quality of life: a systematic review. Journal of Epidemiology and Community Health. 2007;61(11):938–944. doi: 10.1136/jech.2006.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huber M., Knottnerus J. A., Green L., et al. How should we define health? British Medical Journal. 2011;343(7817) doi: 10.1136/bmj.d4163.d4163 [DOI] [Google Scholar]
- 68.Mittelmark M. B., Sagy S., Eriksson M., et al. The Handbook of Salutogenesis. Springer International Publishing; 2017. [DOI] [PubMed] [Google Scholar]
- 69.Reutelingsperger C. P. Celdood, een nieuw perspectief op leven. 2010, https://cris.maastrichtuniversity.nl/portal/en/publications/celdood-een-nieuw-perspectief-op-leven(386db645-4d58-4ad0-8409-e2ccbf14e833).html.
- 70.Menke N. B., Ward K. R., Witten T. M., Bonchev D. G., Diegelmann R. F. Impaired wound healing. Clinics in Dermatology. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Hildebrandt G., Moser M., Lehofer M. Chronobiologie und Chronomedizin. Stuttgart, Germany: Hippokrates; 1998. [Google Scholar]
- 72.Colombo J., Arora R., DePace N. L., Vinik A. I. Clinical Autonomic Dysfunction. Cham: Springer International Publishing; 2015. [DOI] [Google Scholar]
- 73.Dhabhar F. S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunologic Research. 2014;58(2-3):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 74.Marsland A. L., Bachen E. A., Cohen S., Rabin B., Manuck S. B. Stress, immune reactivity and susceptibility to infectious disease. Physiology & Behavior. 2002;77(4-5):711–716. doi: 10.1016/S0031-9384(02)00923-X. [DOI] [PubMed] [Google Scholar]
- 75.Cohen S., Tyrrell D. A. J., Smith A. P. Psychological stress and susceptibility to the common cold. The New England Journal of Medicine. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 76.Cohen S., Williamson G. M. Stress and infectious disease in humans. Psychological Bulletin. 1991;109(1):5–24. doi: 10.1037/0033-2909.109.1.5. [DOI] [PubMed] [Google Scholar]
- 77.Prather A. A., Janicki-Deverts D., Hall M. H., Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel S. R., Malhotra A., Gao X., Hu F. B., Neuman M. I., Fawzi W. W. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35(1):97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiecolt-Glaser J. K., Glaser R. Depression and immune function central pathways to morbidity and mortality. Journal of Psychosomatic Research. 2002;53(4):873–876. doi: 10.1016/S0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 80.Campisi J., May J., Burch K., et al. Anxiety-inducing Facebook behavior is associated with higher rates of upper respiratory infection in college-aged users. Computers in Human Behavior. 2017;76:211–217. doi: 10.1016/j.chb.2017.07.022. [DOI] [Google Scholar]
- 81.Moser M., Frühwirth M., Penter R., Winker R. Why life oscillates - From a topographical towards a functional chronobiology. Cancer Causes & Control. 2006;17(4):591–599. doi: 10.1007/s10552-006-0015-9. [DOI] [PubMed] [Google Scholar]
- 82.Heusser P. Physiologische Grundlagen der Gesundheitsförderung und das anthroposophisch-medizinische Konzept. 2002. [Google Scholar]
- 83.Goyal M., Singh S., Sibinga E. M. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma M. Yoga as an alternative and complementary approach for stress management a systematic review. Journal of evidence-based complementary & alternative medicine. 2013 doi: 10.1177/2156587213503344.2156587213503344 [DOI] [PubMed] [Google Scholar]
- 85.Abbott R. A., Whear R., Rodgers L. R., et al. Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. Journal of Psychosomatic Research. 2014;76(5):341–351. doi: 10.1016/j.jpsychores.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Riley K. E., Park C. L. How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry. Health Psychology Review. 2015;9(3):379–396. doi: 10.1080/17437199.2014.981778. [DOI] [PubMed] [Google Scholar]
- 87.Pascoe M. C., Bauer I. E. A systematic review of randomised control trials on the effects of yoga on stress measures and mood. Journal of Psychiatric Research. 2015;68:270–282. doi: 10.1016/j.jpsychires.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 88.Fjorback L. O., Arendt M., Ornbol E., Fink P., Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy—a systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica. 2011;124(2):102–119. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- 89.Gu J., Strauss C., Bond R., Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clinical Psychology Review. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Ernst E., Pecho E., Wirz P., Saradeth T. Regular sauna bathing and the incidence of common colds. Annals of Medicine. 1990;22(4):225–227. doi: 10.3109/07853899009148930. [DOI] [PubMed] [Google Scholar]
- 91.Brenke R. Das Potenzial der Sauna im Rahmen der Prävention - eine Übersicht neuerer Erkenntnisse. Complementary Medicine Research. 2015;22(5):320–325. doi: 10.1159/000441402. [DOI] [PubMed] [Google Scholar]
- 92.Rapolienė L., Razbadauskas A., Sąlyga J., Martinkėnas A. Stress and Fatigue Management Using Balneotherapy in a Short-Time Randomized Controlled Trial. Evidence-Based Complementary and Alternative Medicine. 2016;2016:10. doi: 10.1155/2016/9631684.9631684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rapolienė L., Razbadauskas A., Jurgelėnas A. The Reduction of Distress Using Therapeutic Geothermal Water Procedures in a Randomized Controlled Clinical Trial. Advances in Preventive Medicine. 2015;2015:10. doi: 10.1155/2015/749417.749417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Álvarez M. J., Fernández D., Gómez-Salgado J., Rodríguez-González D., Rosón M., Lapeña S. The effects of massage therapy in hospitalized preterm neonates: A systematic review. International Journal of Nursing Studies. 2017;69:119–136. doi: 10.1016/j.ijnurstu.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 95.McFeeters S., Pront L., Cuthbertson L., King L. Massage, a complementary therapy effectively promoting the health and well-being of older people in residential care settings: a review of the literature. International Journal of Older People Nursing. 2016;11(4):266–283. doi: 10.1111/opn.12115. [DOI] [PubMed] [Google Scholar]
- 96.Wang C., Bannuru R., Ramel J., Kupelnick B., Scott T., Schmid C. H. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complementary and Alternative Medicine. 2010;10(1):p. 23. doi: 10.1186/1472-6882-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stenius F., Swartz J., Lindblad F., et al. Low salivary cortisol levels in infants of families with an anthroposophic lifestyle. Psychoneuroendocrinology. 2010;35(10):1431–1437. doi: 10.1016/j.psyneuen.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Swartz J., Lindblad F., Arinell H., Theorell T., Alm J. Anthroposophic lifestyle and salivary cortisol are associated with a lower risk of sensitization during childhood. Pediatric Allergy and Immunology. 2015;26(2):153–160. doi: 10.1111/pai.12342. [DOI] [PubMed] [Google Scholar]
- 99.Swartz J., Stenius F., Alm J., Theorell T., Lindblad F. Lifestyle and salivary cortisol at the age of 12 and 24 months. Acta Paediatrica. 2012;101(9):979–984. doi: 10.1111/j.1651-2227.2012.02732.x. [DOI] [PubMed] [Google Scholar]
- 100.Shankland R., Genolini C., França L. R., Guelfi J.-D., Ionescu S. Student adjustment to higher education: The role of alternative educational pathways in coping with the demands of student life. Higher Education. 2010;59(3):353–366. doi: 10.1007/s10734-009-9252-7. [DOI] [Google Scholar]
- 101.Education T. F. o. W. Learning through rhythm. 2017, https://www.freunde-waldorf.de/en/the-friends/publications/catalogue-waldorf-education/learning-through-rhythm/
- 102.David L. A., Maurice C. F., Carmody R. N., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marchesi J. R., Adams D. H., Fava F., et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bermon S., Castell L. M., Calder P. C., et al. Consensus statement immunonutrition and exercise. Exercise Immunology Review. 2017;23:8–50. [PubMed] [Google Scholar]
- 105.Somerville V. S., Braakhuis A. J., Hopkins W. G. Effect of flavonoids on upper respiratory tract infections and immune function: A systematic review and meta-analysis. Advances in Nutrition. 2016;7(3):488–497. doi: 10.3945/an.115.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schuijt T. J., Lankelma J. M., Scicluna B. P., et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmed M., Henson D. A., Sanderson M. C., Nieman D. C., Gillitt N. D., Lila M. A. The protective effects of a polyphenol-enriched protein powder on exercise-induced susceptibility to virus infection. Phytotherapy Research. 2014;28(12):1829–1836. doi: 10.1002/ptr.5208. [DOI] [PubMed] [Google Scholar]
- 108.Dinh H. C., Beyer I., Mets T., et al. Effects of Physical Exercise on Markers of Cellular Immunosenescence: A Systematic Review. Calcified Tissue International. 2017:1–23. doi: 10.1007/s00223-016-0212-9. [DOI] [PubMed] [Google Scholar]
- 109.Turner J. E. Is immunosenescence influenced by our lifetime “dose” of exercise? Biogerontology. 2016;17(3):581–602. doi: 10.1007/s10522-016-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartlett D. B., Huffman K. M. The Ageing Immune System and Health. Springer; 2017. Lifestyle interventions to improve immunesenescence; pp. 161–176. [Google Scholar]
- 111.Evans S. S., Repasky E. A., Fisher D. T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nature Reviews Immunology. 2015;15(6):335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Casadevall A., Hogan D. A. Thermal Restriction as an Antimicrobial Function of Fever. PLoS Pathogens. 2016;12(5):p. e1005577. doi: 10.1371/journal.ppat.1005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Launey Y., Nesseler N., Mallédant Y., Seguin P. Clinical review: fever in septic ICU patients—friend or foe? Critical Care. 2011;15(3, article 222):p. 1. doi: 10.1186/cc10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Young P. J., Saxena M., Beasley R., et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Medicine. 2012;38(3):437–444. doi: 10.1007/s00134-012-2478-3. [DOI] [PubMed] [Google Scholar]
- 115.Smith J. A is for aphorisms: Feed a fever, starve a cold? Or could it be starve a fever, feed a cold? Australian Family Physician. 2015;44(1):77–78. [PubMed] [Google Scholar]
- 116.Earn D. J., Andrews P. W., Bolker B. M. Population-level effects of suppressing fever. Proceedings of the Royal Society B Biological Science. 2014;281(1778) doi: 10.1098/rspb.2013.2570.20132570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eteraf-Oskouei T., Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iranian Journal of Basic Medical Sciences. 2013;16(6):p. 732. [PMC free article] [PubMed] [Google Scholar]
- 118.Lenoir-Wijnkoop I., Gerlier L., Roy D., Reid G. The clinical and economic impact of probiotics consumption on respiratory tract infections: Projections for Canada. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0166232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haidvogl M., Riley D. S., Heger M. Homeopathic and conventional treatment for acute respiratory and ear complaints: a comparative study on outcome in the primary care setting. BMC Complementary and Alternative Medicine. 2007;7:p. 7. doi: 10.1186/1472-6882-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito M., Maruyama Y., Kitamura K., et al. Randomized controlled trial of juzen-taiho-to in children with recurrent acute otitis media. Auris Nasus Larynx. 2017;44(4):390–397. doi: 10.1016/j.anl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Szőke H., Maródi M., Sallay Z., Székely B., Sterner M.-G., Hegyi G. Integrative versus Conventional Therapy of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children: A Prospective Observational Study. Forschende Komplementärmedizin. 2016;23(4):231–239. doi: 10.1159/000448440. [DOI] [PubMed] [Google Scholar]
- 122.Carson C. F., Riley T. V. Safety, efficacy and provenance of tea tree (Melaleuca alternifolia) oil. Contact Dermatitis. 2001;45(2):65–67. doi: 10.1034/j.1600-0536.2001.045002065.x. [DOI] [PubMed] [Google Scholar]
- 123.Caelli M., Porteous J., Carson C. F., Heller R., Riley T. V. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. The Journal of Hospital Infection. 2000;46(3):236–237. doi: 10.1053/jhin.2000.0830. [DOI] [PubMed] [Google Scholar]
- 124.Dryden M., Dailly S., Crouch M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. The Journal of Hospital Infection. 2004;56(4):283–286. doi: 10.1016/j.jhin.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 125.Blackwood B., Thompson G., Mcmullan R., et al. Tea tree oil (5%) body wash versus standard care (johnson's baby softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. Journal of Antimicrobial Chemotherapy. 2013;68(5):1193–1199. doi: 10.1093/jac/dks501. [DOI] [PubMed] [Google Scholar]
- 126.Lee R. L. P., Leung P. H. M., Wong T. K. S. A randomized controlled trial of topical tea tree preparation for MRSA colonized wounds. International Journal of Nursing Sciences. 2014;1(1):7–14. doi: 10.1016/j.ijnss.2014.01.001. [DOI] [Google Scholar]
- 127.Ching T. H., BscPharm J. C. Clinical outcomes for cancer patients using complementary and alternative medicine. Alternative Therapies in Health and Medicine. 2012;18(1):p. 12. [PubMed] [Google Scholar]
- 128.Williams G., Craig J. C. Prevention of recurrent urinary tract infection in children. Current Opinion in Infectious Diseases. 2009;22(1):72–76. doi: 10.1097/QCO.0b013e328320a885. [DOI] [PubMed] [Google Scholar]
- 129.Chedid V., Dhalla S., Clarke J. O., et al. Herbal Therapy is Equivalent to Rifaximin for the Treatment of Small Intestinal Bacterial Overgrowth. Global Advances in Health and Medicine. 2014;3(3):16–24. doi: 10.7453/gahmj.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marcone V., Calzolari E., Bertini M. Effectiveness of vaginal administration of Lactobacillus rhamnosus following conventional metronidazole therapy: How to lower the rate of bacterial vaginosis recurrences. New Microbiologica. 2008;31(3):429–433. [PubMed] [Google Scholar]
- 131.Asadi M., Forouhari S., Jahromi B. N., Zarei A., Sayadi M., Rad S. K. Comparison of the effects of Mycocin vaginal cream and Metronidazole vaginal gel on treatment of bacterial vaginosis: A randomized clinical trial. International Journal of Medical Research & Health Sciences. 2016;5(8):250–256. [Google Scholar]
- 132.Bae G., Yu J.-R., Lee J., Chang J., Seo E.-K. Identification of Nyasol and structurally related compounds as the active principles from Anemarrhena asphodeloides against respiratory syncytial virus (RSV) Chemistry & Biodiversity. 2007;4(9):2231–2235. doi: 10.1002/cbdv.200790181. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y., Dan Y., Yang D. The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2014;153(1):42–60. doi: 10.1016/j.jep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 134.Alok S., Jain S. K., Verma A., Kumar M., Mahor A., Sabharwal M. Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): A review. Asian Pacific Journal of Tropical Disease. 2013;3(3):242–251. doi: 10.1016/S2222-1808(13)60049-3. [DOI] [Google Scholar]
- 135.Chouhan D. A., Pande P. S. Studies onantibacterial potential of asparagus racemosus extract against bacteria causing UTI. International Journal of Pharmaceutical Research. 2014;6(2):p. 101. [Google Scholar]
- 136.Ojo S. K. S., Esumeh F. I., Uyoh A. A. Antimicrobial effect of diodia scandens and phyllanthus amarus on multi-drug resistance pattern of Staphylococci from clinical sources of surgical unit of a tertiary hospital. American Journal of Drug Discovery and Development. 2013;3(4):271–278. doi: 10.3923/ajdd.2013.271.278. [DOI] [Google Scholar]
- 137.Quave C. L., Estévez-Carmona M., Compadre C. M., et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0028737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Das P. K., Goswami S., Chinniah A., et al. Woodfordia fruticosa: Traditional uses and recent findings. Journal of Ethnopharmacology. 2007;110(2):189–199. doi: 10.1016/j.jep.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 139.Kumar D., Sharma M., Sorout A., Saroha K., Verma S. Woodfordia fruticosa Kurz.: A Review on its Botany, Chemistry and Biological activities. Journal of Pharmacognosy and Phytochemistry. 2016;5(3):p. 293. [Google Scholar]
- 140.Gautam R., Saklani A., Jachak S. M. Indian medicinal plants as a source of antimycobacterial agents. Journal of Ethnopharmacology. 2007;110(2):200–234. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 141.Semaming Y., Pannengpetch P., Chattipakorn S. C., Chattipakorn N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evidence-Based Complementary and Alternative Medicine. 2015;2015:11. doi: 10.1155/2015/593902.593902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuksel S., Akyol S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. Journal of Intercultural Ethnopharmacology. 2016;5(3):308–311. doi: 10.5455/jice.20160331064836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yousuf F. A., Mehmood M. H., Malik A., Siddiqui R., Khan N. A. Antiacanthamoebic properties of natural and marketed honey in Pakistan. Asian Pacific Journal of Tropical Biomedicine. 2016;6(11):967–972. doi: 10.1016/j.apjtb.2016.05.010. [DOI] [Google Scholar]
- 144.Malone M., Tsai G. Wound healing with Apitherapy: A Review of the Effects of Honey. Journal of Apitherapy. 2016;1(1):29–32. doi: 10.5455/ja.20160620031837. [DOI] [Google Scholar]
- 145.Zamora L. G., Beukelman C. J., Van Den Berg A. J. J., et al. An insight into the antibiofilm properties of Costa Rican stingless bee honeys. Journal of Wound Care. 2017;26(4):168–177. doi: 10.12968/jowc.2017.26.4.168. [DOI] [PubMed] [Google Scholar]
- 146.Miraj S., Azizi N., Kiani S. A review of chemical components and pharmacological effects of Melissa officinalis L. Der Pharmacia Lettre. 2016;8(6):229–237. [Google Scholar]
- 147.Dubreuil J. D. Antibacterial and antidiarrheal activities of plant products against Enterotoxinogenic Escherichia coli. Toxins. 2013;5(11):2009–2041. doi: 10.3390/toxins5112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sharma A., Flores-Vallejo R. D. C., Cardoso-Taketa A., Villarreal M. L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. Journal of Ethnopharmacology. 2017;208:264–329. doi: 10.1016/j.jep.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 149.Komiazyk M., Palczewska M., Sitkiewicz I., Groves P. Use of plant extracts to control and treat AB5 enterotoxin-related diarrhea. Polish Journal of Microbiology. 2014;63(1):3–14. [PubMed] [Google Scholar]
- 150.Rath S., Padhy R. N. Antibacterial efficacy of five medicinal plants against multidrug-resistant enteropathogenic bacteria infecting under-5 hospitalized children. Journal of Integrative Medicine. 2015;13(1):45–57. doi: 10.1016/S2095-4964(15)60154-6. [DOI] [PubMed] [Google Scholar]
- 151.Joshi V. Some plants used in ayurvedic and homoeopathic medicine. Journal of Pharmacognosy and Phytochemistry. 2013;2(1) [Google Scholar]
- 152.Ji L.-L., Wang Z., Dong F., Zhang W.-B., Wang Z.-T. Andrograpanin, a compound isolated from anti-inflammatory traditional Chinese medicine Andrographis paniculata, enhances chemokine SDF-1α-induced leukocytes chemotaxis. Journal of Cellular Biochemistry. 2005;95(5):970–978. doi: 10.1002/jcb.20464. [DOI] [PubMed] [Google Scholar]
- 153.Agrawal R., Prachi P., Agrawal N. Chemopeventive effects of andographis panniculata extract in vivo and in vitro models. Med One. 2016;1(4):p. 2. [Google Scholar]
- 154.Akbar S. Andrographis paniculata: a review of pharmacological activities and clinical effects. Alternative Medicine Review. 2011;16(1):66–77. [PubMed] [Google Scholar]
- 155.Jayakumar T., Hsieh C.-Y., Lee J.-J., Sheu J.-R. Experimental and clinical pharmacology of andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evidence-Based Complementary and Alternative Medicine. 2013;2013:16. doi: 10.1155/2013/846740.846740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moyo M., Van Staden J. Medicinal properties and conservation of Pelargonium sidoides DC. Journal of Ethnopharmacology. 2014;152(2):243–255. doi: 10.1016/j.jep.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saheed S., Tom A. A. Antimicrobial and Antidiarrheal Activities of Pelargonium luridum (Andrews) Sweet Root Extracts. Pharmacologia. 2016;7(4):202–210. doi: 10.5567/pharmacologia.2016.202.210. [DOI] [Google Scholar]
- 158.Wang L., Yang R., Yuan B., Liu Y., Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharmaceutica Sinica B (APSB) 2015;5(4):310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han Y. j., Lee S. H., Lee J. Y. Recent Clinical Research on Effect of Acupuncture on Sore Throat. The Journal of Pediatrics of Korean Medicine. 2016;30(2):47–55. doi: 10.7778/jpkm.2016.30.2.047. [DOI] [Google Scholar]
- 160.Moss D. A., Crawford P. Ear acupuncture for acute sore throat: A randomized controlled trial. Journal of the American Board of Family Medicine. 2015;28(6):697–705. doi: 10.3122/jabfm.2015.06.150014. [DOI] [PubMed] [Google Scholar]
- 161.Adair T. L., Drum B. E. RNA-Seq reveals changes in the Staphylococcus aureus transcriptome following blue light illumination. Genomics Data. 2016;9:4–6. doi: 10.1016/j.gdata.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dai T., Gupta A., Murray C. K., Vrahas M. S., Tegos G. P., Hamblin M. R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates. 2012;15(4):233–236. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Y., Wu X., Chen J., et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and in Vivo Studies. The Journal of Infectious Diseases. 2016;213(9):1380–1387. doi: 10.1093/infdis/jiw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baars E., Hoekman J. De wetenschappelijke stand van zaken van de uitwendige therapie. Leiden, Netherlands: Hogeschool Leiden; 2012. [Google Scholar]
- 165.Mikkelsen K. H., Allin K. H., Knop F. K. Effect of antibiotics on gut microbiota, glucose metabolism and body weight regulation: a review of the literature. Diabetes, Obesity and Metabolism. 2016;18(5):444–453. doi: 10.1111/dom.12637. [DOI] [PubMed] [Google Scholar]
- 166.Turta O., Rautava S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC Medicine. 2016;14(1, article no. 57) doi: 10.1186/s12916-016-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Scott F. I., Horton D. B., Mamtani R., et al. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology. 2016;151(1):120–129.e5. doi: 10.1053/j.gastro.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paolella G., Vajro P. Childhood obesity, breastfeeding, intestinal microbiota, and early exposure to antibiotics: what is the link? JAMA Pediatrics. 2016;170(8):735–737. doi: 10.1001/jamapediatrics.2016.0964. [DOI] [PubMed] [Google Scholar]
- 169.Scheepers L. E. J. M., Penders J., Mbakwa C. A., Thijs C., Mommers M., Arts I. C. W. The intestinal microbiota composition and weight development in children: The KOALA Birth Cohort Study. International Journal of Obesity. 2015;39(1):16–25. doi: 10.1038/ijo.2014.178. [DOI] [PubMed] [Google Scholar]
- 170.Romano A., Warrington R. Antibiotic Allergy. Immunology and Allergy Clinics of North America. 2014;34(3):489–506. doi: 10.1016/j.iac.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 171.Trubiano J. A., Beekmann S. E., Worth L. J., et al. Open Forum Infectious Diseases. Oxford University Press; 2016. Improving antimicrobial stewardship by antibiotic allergy delabeling: Evaluation of knowledge, attitude, and practices throughout the emerging infections network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chey W. D., Kurlander J., Eswaran S. Irritable bowel syndrome: a clinical review. The Journal of the American Medical Association. 2015;313(9):949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 173.Venekamp R. P., Sanders S. L., Glasziou P. P., Del Mar C. B., Rovers M. M. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews. 2015;6 doi: 10.1002/14651858.CD000219.pub4.CD000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee J. Y., Jun S. A., Hong S. S., Ahn Y. C., Lee D. S., Son C. G. Systematic Review of Adverse Effects from Herbal Drugs Reported in Randomized Controlled Trials. Phytotherapy Research. 2016:1412–1419. doi: 10.1002/ptr.5647. [DOI] [PubMed] [Google Scholar]
- 175.Prakash S., Hernandez G. T., Dujaili I., Bhalla V. Lead poisoning from an Ayurvedic herbal medicine in a patient with chronic kidney disease. Nature Reviews Nephrology. 2009;5(5):297–300. doi: 10.1038/nrneph.2009.41. [DOI] [PubMed] [Google Scholar]
- 176.Genuis S. J., Schwalfenberg G., Siy A.-K. J., Rodushkin I. Toxic element contamination of natural health products and pharmaceutical preparations. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049676.e49676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu M., Fang M., Hu Y., Wang X. Four types of traditional Chinese medicine inducing epileptic seizures. Seizure. 2012;21(5):311–315. doi: 10.1016/j.seizure.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 178.Zhang L., Yan J. B., Liu X. M., et al. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: current status and future perspective. Journal of Ethnopharmacology. 2012;140(3):519–525. doi: 10.1016/j.jep.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 179.Baghel M. The national pharmacovigilance program for Ayurveda, Siddha and Unani drugs: Current status. International Journal for Ayurveda Research. 2010;1(4):p. 197. doi: 10.4103/0974-7788.76779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gao H. M., Wang Z. M., Li Y. J., Qian Z. Z. Overview of the quality standard research of traditional Chinese. Frontiers of Medicine. 2011;5(2):195–202. doi: 10.1007/s11684-011-0134-x. [DOI] [PubMed] [Google Scholar]
- 181.Gupta P., Daswani P., Birdi T. Approaches in fostering quality parameters for medicinal botanicals in the Indian context. Indian Journal of Pharmacology. 2014;46(4):363–371. doi: 10.4103/0253-7613.135946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Neurology. 2013;4 doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Byard R. W., Musgrave I., Maker G., Bunce M. What risks do herbal products pose to the australian community? Medical Journal of Australia. 2017;206(2):86–90. doi: 10.5694/mja16.00614. [DOI] [PubMed] [Google Scholar]
- 184.Hamre H. J., Glockmann A., Heckenbach K., Matthes H. Use and Safety of Anthroposophic Medicinal Products: An Analysis of 44,662 Patients from the EvaMed Pharmacovigilance Network. Drugs - Real World Outcomes. 2017;4(4):199–213. doi: 10.1007/s40801-017-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Atwood 4th. K. C. Naturopathy, pseudoscience, and medicine: myths and fallacies vs truth. MedGenMed : Medscape general medicine. 2004;6(1):p. 33. [PMC free article] [PubMed] [Google Scholar]
- 186.Anlauf M., Hein L., Hense H.-W., et al. Complementary and alternative drug therapy versus science-oriented medicine. GMS German Medical Science. 2015;13:1–47. doi: 10.3205/000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perry R., Watson L. K., Terry R., Onakpoya I., Ernst E. British general practitioners' attitudes towards and usage of homeopathy: A systematic review of surveys. Focus on Alternative and Complementary Therapies. 2013;18(2):51–63. doi: 10.1111/fct.12018. [DOI] [Google Scholar]
- 188.Righetti M. Homöopathieforschung: Problematik und Ergebnisse zur Wirksamkeit - mit Resultaten aus dem Programm Evaluation Komplementärmedizin PEK. Schweizerische Zeitschrift für Ganzheitsmedizin. 2007;19(2):104–108. doi: 10.1159/000283649. [DOI] [Google Scholar]
- 189.Sammons H. M., Gubarev M. I., Krepkova L. V., et al. Herbal medicines: challenges in the modern world. Part 2. European Union and Russia. Expert Review of Clinical Pharmacology. 2016;9(8):1117–1127. doi: 10.1080/17512433.2016.1189326. [DOI] [PubMed] [Google Scholar]
- 190.Enioutina E. Y., Salis E. R., Job K. M., Gubarev M. I., Krepkova L. V., Sherwin C. M. T. Herbal Medicines: challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expert Review of Clinical Pharmacology. 2017;10(3):327–338. doi: 10.1080/17512433.2017.1268917. [DOI] [PubMed] [Google Scholar]
- 191.Brooks K. M., George J. M., Kumar P. Drug interactions in HIV treatment: complementary & alternative medicines and over-the-counter products. Expert Review of Clinical Pharmacology. 2017;10(1):59–79. doi: 10.1080/17512433.2017.1246180. [DOI] [PubMed] [Google Scholar]
- 192.Teschke R., Zhang L., Long H., et al. Traditional Chinese Medicine and herbal hepatotoxicity: a tabular compilation of reported cases. Annals of Hepatology. 2015;14(1):7–19. doi: 10.1016/S0415-6412(16)30121-7. [DOI] [PubMed] [Google Scholar]
- 193.Wu F., Wang T. Risk assessment of upper tract urothelial carcinoma related to aristolochic acid. Cancer Epidemiology, Biomarkers & Prevention. 2013;22(5):812–820. doi: 10.1158/1055-9965.EPI-12-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lai J.-N., Tang J.-L., Wang J.-D. Observational Studies on Evaluating the Safety and Adverse Effects of Traditional Chinese Medicine. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/697893.697893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu S.-H., Chuang W.-C., Lam W., Jiang Z., Cheng Y.-C. Safety surveillance of Traditional Chinese Medicine: current and future. Drug Safety. 2015;38(2):117–128. doi: 10.1007/s40264-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Job K. M., Kiang T. K. L., Constance J. E., Sherwin C. M. T., Enioutina E. Y. Herbal medicines: challenges in the modern world. Part 4. Canada and United States. Expert Review of Clinical Pharmacology. 2016;9(12):1597–1609. doi: 10.1080/17512433.2016.1238762. [DOI] [PubMed] [Google Scholar]
- 197.Fcahs S. V. M. M. F. Complementary and alternative medicine: a survey of its use in children with chronic respiratory illness. Canadian Journal of Respiratory Therapy. 2014;50(1):p. 27. [PMC free article] [PubMed] [Google Scholar]
- 198.Zuzak T. J., Zuzak-Siegrist I., Simões-Wüst A. P., Rist L., Staubli G. Use of complementary and alternative medicine by patients presenting to a paediatric Emergency Department. European Journal of Pediatrics. 2009;168(4):431–437. doi: 10.1007/s00431-008-0765-3. [DOI] [PubMed] [Google Scholar]
- 199.Italia S., Wolfenstetter S. B., Teuner C. M. Patterns of Complementary and Alternative Medicine (CAM) use in children: a systematic review. European Journal of Pediatrics. 2014;173(11):1413–1428. doi: 10.1007/s00431-014-2300-z. [DOI] [PubMed] [Google Scholar]
- 200.Little P., Dorward M., Warner G., Stephens K., Senior J., Moore M. Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: Nested observational study. British Medical Journal. 2004;328(7437):444–446. doi: 10.1136/bmj.38013.644086.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kautz-Freimuth S., Redaèlli M., Samel C., Civello D., Altin S. V., Stock S. Parental views on acute otitis media (AOM) and its therapy in children - results of an exploratory survey in German childcare facilities. BMC Pediatrics. 2015;15(1) doi: 10.1186/s12887-015-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Broides A., Bereza O., Lavi-Givon N., Fruchtman Y., Gazala E., Leibovitz E. Parental acceptability of the watchful waiting approach in pediatric acute otitis media. World Journal of Clinical Pediatrics. 2016;5(2):p. 198. doi: 10.5409/wjcp.v5.i2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marchisio P., Bianchini S., Galeone C., et al. Use of complementary and alternative medicine in children with recurrent acute otitis media in Italy. International Journal of Immunopathology and Pharmacology. 2011;24(2):441–449. doi: 10.1177/039463201102400217. [DOI] [PubMed] [Google Scholar]
- 204.Jansons L. L., Lynch R. L., LeBlanc A., Tilburt J. C. Shared decision making in complementary and alternative medicine therapies. Pediatric Annals. 2012;41(12):522–527. doi: 10.3928/00904481-20121126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Flower A., Winters D., Bishop F. L., Lewith G. The challenges of treating women with recurrent urinary tract infections in primary care: a qualitative study of GPs' experiences of conventional management and their attitudes towards possible herbal options. Primary Health Care Research & Development. 2015;16(6):597–606. doi: 10.1017/S1463423615000201. [DOI] [PubMed] [Google Scholar]
- 206.King A. R., Russett F. S., Generali J. A., Grauer D. W. Evaluation and implications of natural product use in preoperative patients: A retrospective review. BMC Complementary and Alternative Medicine. 2009;9:p. 38. doi: 10.1186/1472-6882-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de Silva J. E. S., Souza C. A. S., Silva T. B., et al. Use of herbal medicines by elderly patients: A systematic review. Archives of Gerontology and Geriatrics. 2014;59(2):227–233. doi: 10.1016/j.archger.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 208.Kienle G. S., Hamre H. J., Kiene H., et al. Methodological aspects of integrative and person-oriented health care evaluation. Complementary Medicine Research. 2017;24(1):23–28. doi: 10.1159/000460511. [DOI] [PubMed] [Google Scholar]
- 209.Julliard K. N., Citkovitz C., McDaniel D. Towards a Model for Planning Clinical Research in Oriental Medicine. Explore: The Journal of Science and Healing. 2007;3(2):118–128. doi: 10.1016/j.explore.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 210.Fischer H. F., Junne F., Witt C. Key issues in clinical and epidemiological research in complementary and alternative medicine–a systematic literature review. Forschende Komplementärmedizin/Research in Complementary Medicine. 2012;19(2):51–60. doi: 10.1159/000343126. [DOI] [PubMed] [Google Scholar]
- 211.Lexchin J. Sponsorship bias in clinical research. International Journal of Risk and Safety in Medicine. 2012;24(4):233–242. doi: 10.3233/JRS-2012-0574. [DOI] [PubMed] [Google Scholar]
- 212.Flower A., Bishop F. L., Lewith G. How women manage recurrent urinary tract infections: An analysis of postings on a popular web forum. BMC Family Practice. 2014;15(1):p. 162. doi: 10.1186/1471-2296-15-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Trill J., Simpson C., Webley F., et al. Uva-ursi extract and ibuprofen as alternative treatments of adult female urinary tract infection (ATAFUTI): Study protocol for a randomised controlled trial. Trials. 2017;18(1):p. 421. doi: 10.1186/s13063-017-2145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.JPIAMR. The fourth JPIAMR call: “AMR Networks/Working Groups”. 2017, http://www.jpiamr.eu/fourth-joint-callresult/
- 215.Ali A., Katz D. L. Disease Prevention and Health Promotion: How Integrative Medicine Fits. American Journal of Preventive Medicine. 2015;49(5):S230–S240. doi: 10.1016/j.amepre.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Manyi-Loh C. E., Clarke A. M., Mkwetshana N. F., Ndip R. N. Treatment of Helicobacter pylori infections: mitigating factors and prospective natural remedies. African Journal of Biotechnology. 2010;9(14):2032–2042. [Google Scholar]
- 217.Gulliford M. C., Moore M. V., Little P., et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. British Medical Journal. 2016;354 doi: 10.1136/bmj.i3410.i3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kronenberg A., Bütikofer L., Odutayo A., et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ. 2017;359:p. j4784. doi: 10.1136/bmj.j4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mangione-Smith R., Zhou C., Robinson J. D., Taylor J. A., Elliott M. N., Heritage J. Communication practices and antibiotic use for acute respiratory tract infections in children. Annals of Family Medicine. 2015;13(3):221–227. doi: 10.1370/afm.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Willcox M. L., Graz B., Falquet J., Diakite C., Giani S., Diallo D. A "reverse pharmacology" approach for developing an anti-malarial phytomedicine. Malaria Journal. 2011;10(1):p. S8. doi: 10.1186/1475-2875-10-s1-s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.