Abstract
Background
The present study tested the hypothesis that network segregation, a graph theoretic measure of functional organization of the brain, is correlated with treatment response in patients with major depressive disorder (MDD) undergoing repetitive transcranial magnetic stimulation (rTMS).
Methods
Network segregation, calculated from resting state functional magnetic resonance imaging scans, was measured in 32 patients with MDD who entered a sham-controlled, double-blinded, randomized trial of rTMS to the left dorsolateral prefrontal cortex, and a cohort of 20 healthy controls (HCs). Half of the MDD patients received sham treatment in the blinded phase, followed by active rTMS in the open-label phase. The analyses focused on segregation of the following networks: default mode (DMN), salience (SN), fronto-parietal (FPN), cingulo-opercular (CON), and memory retrieval (MRN).
Results
There was no differential change in network segregation comparing sham to active treatment. However, in the combined group of patients who completed active rTMS treatment (in the blinded plus open-label phases), higher baseline segregation of SN significantly predicted more symptom improvement after rTMS. Compared to HCs at baseline, MDD patients showed decreased segregation in DMN, and trend-level decreases in SN and MRN.
Conclusion
The results highlight the importance of network segregation in MDD, particularly in the SN, where more normal baseline segregation of SN may predict better treatment response to rTMS in depression.
Keywords: Repetitive transcranial magnetic stimulation, Depression, Segregation, Salience network, Default mode network
Highlights
-
•
We examined network segregation in a cohort of MDD patients receiving rTMS treatment.
-
•
More normal segregation of SN predicted better response of depression to rTMS.
-
•
Patients with MDD had decreased network segregation in DMN.
1. Introduction
Studies using resting-state functional magnetic resonance imaging (fMRI) have revealed that spontaneous activation of the brain is organized into spatially segregated functional networks (Damoiseaux et al., 2006; Power et al., 2011). Recently, a graph theoretic measure of brain network organization, known as network (system) segregation, has been used to measure the balance of connections between areas within a network and outside a network (Chan et al., 2014; Cohen and D'Esposito, 2016; Gallen et al., 2016). The proportion of connections within versus between networks is a prerequisite for the balance between functional specialization and global integration (through sparse between-network connectivity) across a network (Wig, 2017). Network segregation reliably captures age-related, decreased specialization in brain function (Chan et al., 2014), and it correlates with variability in long-term memory independent of age (Chan et al., 2014). In older adults, better segregation of brain networks predicts greater improvements after cognitive training (Gallen et al., 2016). Taken together, these results suggest that network segregation may be important for psychiatric disorders.
Alterations in functional networks have been identified in major depressive disorder (MDD), including the default mode network (DMN), central executive network (CEN), and the salience network (SN) (Hamilton et al., 2012; Kaiser et al., 2015). These alterations are thought to contribute to the core cognitive and affective dysfunctions in MDD, leading to aberrations in self-referential thinking, autobiographical memory retrieval, affective interpretative bias, and cognitive control (Hamilton et al., 2012; Kaiser et al., 2015; Kim, 2012; Zhu et al., 2012). An important question is how network segregation may predict or change with treatment interventions.
Accordingly, we examined network segregation in a cohort of MDD patients receiving repetitive transcranial magnetic stimulation (rTMS) for their depression. rTMS is effective treatment for MDD, with effect sizes of meta-analytic studies ranging from 0.39–0.55 (Bakker et al., 2015; Connolly et al., 2012; Slotema et al., 2010); however, the antidepressant mechanisms of rTMS are largely unknown (Connolly et al., 2012; Slotema et al., 2010). Functional connectivity has been examined, and studies have shown that functional connectivity within and between brain networks tracked and predicted treatment response. rTMS treatment has been associated with alterations in connectivity in insula (a key node in SN) (Philip et al., 2018), dorsolateral prefrontal cortex (DLPFC, a key node in CEN) (Fox et al., 2012; Philip et al., 2018; Taylor et al., 2018), anterior cingulate cortex (ACC) within meso-cortico-limbic network (Tik et al., 2017), affective network (Taylor et al., 2018), between subgenual ACC (sgACC) and DMN (Liston et al., 2014; Philip et al., 2018; Taylor et al., 2018), and between DLPFC and DMN (Liston et al., 2014). Functional connectivity in DLPFC (Liston et al., 2014), SN (Ge et al., 2017), DMN (Ge et al., 2017), as well as between DMN and insula (Taylor et al., 2018) and between sgACC and DMN (Liston et al., 2014; Philip et al., 2018) have been found to predict rTMS treatment response to depression. These findings support the utility of functional connectivity measures to understand rTMS mechanisms. However, using seed-based functional connectivity (Fox et al., 2012; Liston et al., 2014; Philip et al., 2018; Taylor et al., 2018) or independent component analysis (ICA) methods (Ge et al., 2017; Tik et al., 2017), these studies did not address relationships between networks, as can be done with a graph-theoretic network measure such as segregation.
There are other graph-theoretic network measures, such as clustering coefficient, small worldness, local/global efficiency, modularity, degree, efficiency, and betweenness-centrality, to characterize the topological architectures of brain networks at different levels (Gong and He, 2015). Previous studies of measures on depression have yielded negative and inconsistent results (Gong and He, 2015). To our knowledge, no prior work has explored network segregation in MDD, and this approach has never previously been used to capture treatment response to a given therapy in psychiatric disorders. Given the reported associations of segregation with aging, cognition and response to cognitive training (Chan et al., 2014; Gallen et al., 2016), we thought it was important to test for a relationship between network segregation and the response to rTMS.
The study involved a cohort of patients who received rTMS in a controlled study. Half of the patients received rTMS during a blinded phase, while the other half first received sham rTMS, and then went on to receive active rTMS. We also compared baseline segregation in MDD patients with a cohort of healthy controls (HCs). To define networks for analysis, we used a 264-node parcellation, derived from a meta-analysis of task-based and resting-state fMRI studies (Power et al., 2011). From this parcellation, we focused on 5 networks identified as relevant for depression: the DMN, SN, two task control networks (fronto-parietal network, FPN; cingulo-opercular network, CON), and the memory retrieval network (MRN) (Dietsche et al., 2014; Hamilton et al., 2012; Kaiser et al., 2015; Wu et al., 2016). The overall question was whether or not network segregation identified a functional attribute relevant for MDD and the treatment of MDD.
2. Materials and methods
2.1. Subjects
Forty outpatient subjects with MDD, diagnosed with DSM-IV criteria using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998), were enrolled and randomized in the protocol between October 2013 and October 2015. They were recruited through advertisements in the community and referrals from clinicians at the University of Michigan Department of Psychiatry. Subjects were between 22 and 65 years of age, had failed at least one antidepressant medication trial, had at least moderate depressive severity (Montgomery-Asberg Depression Rating Scale [MADRS] ≥ 18) (Montgomery and Asberg, 1979) and ≤ 5 years in the current episode. The Antidepressant Treatment History Form (ATHF) (Sackeim, 2001) assessed treatment resistance. The same subjects were reported in a seed-based connectivity analysis (Taylor et al., 2018), where readers can find more details about the clinical trial.
Twenty HCs recruited from community advertisements for a separate protocol were selected to approximately match the age and gender distribution of the MDD patients. They were between 21 and 58 years of age. None of them had a lifetime history of Axis I psychiatric disorders as assessed with Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995), first-degree relatives with psychosis or other serious mental illness (requiring antipsychotic medication, hospitalization or ECT), and none were taking any medication affecting brain function.
The study protocol was approved by the University of Michigan Institutional Review Board and all subjects gave written consent before their participation.
2.2. Design and rTMS protocol
The protocol was a sham-controlled, randomized (1,1), double-blinded study. After initial screening and assessments, subjects entered a blinded treatment phase, beginning with the first MRI session (scan 1). In this blinded phase, subjects received 20 sessions of rTMS therapy or sham treatment, 5 days per week. The stimulation site for each participant was defined as the local maxima of left DLPFC activation during an n-back task (2-task – 0-back), which was targeted by the individualized neuronavigation (Brainsight Frameless system, Rogue Research, Montreal CA) (see Supplementary materials). rTMS treatments were delivered at 10 Hz frequency at 120% of motor threshold and 3000 pulses/session to the left DLPFC. At the end of the blinded treatment, the second MRI (scan 2) occurred, followed by open-label treatment: either 5 taper sessions over 2 weeks for those in the active arm during the blinded phase, or 20 sessions plus 5 taper sessions for those receiving sham stimulation in the blinded phase. The primary measurement for depression symptom changes over time was the MADRS, obtained weekly for the 20 sessions of rTMS treatment (baseline defined as week 0) and at the end of the last taper session. Categorical treatment response was defined as a 50% improvement from baseline MADRS, and remission was defined as MADRS <10.
2.3. Functional MRI acquisition and preprocessing
Image data were acquired by a 3 T GE 750 Discovery scanner (General Electric Healthcare, Chicago, IL). Blood oxygenation level dependent (BOLD) functional images were acquired with a T2*-weighted, reverse spiral acquisition (gradient recalled echo, TR = 2000 ms, TE = 30 ms, FA = 90°, field of view = 22 cm, 43 slices (40 slices for HCs), 3.0 mm thick/0 mm skip, equivalent to 64 × 64 voxel grid – yielding approximately isotropic voxels). Two hundred and forty acquisitions were acquired in the resting state with eyes open and fixated on a large ‘plus’ sign projected on a monitor. Four initial volumes were discarded to allow for equilibration of scanner signal. A high resolution T1 scan (3D SPGR) was obtained for anatomic normalization, as well as a T1 spin echo acquisition in the same prescription as the T2*BOLD scan.
All image data were preprocessed using the following standard steps: slice-time correction; realignment; warping of functional images to the MNI152 template (Statistical Parametric Mapping SPM8 package, Wellcome Institute of Cognitive Neurology, London), spatial smoothing (full-width at half maximum = 8 mm); linear detrending; regressing out of nuisance covariates (six head motion parameters, white matter signal, cerebrospinal fluid signal); and temporal band-pass filtering (0.01 Hz - 0.1 Hz). To assess and manage movement, we calculated frame-wise displacement (FD) (Power et al., 2012) from the translation and rotation parameters from the realignment file. A ‘scrubbing routine’ was used to censor any frame with FD > 0.5 mm from the following analyses, yielding a scrub ratio for each subject. Deleted frames of 60% or less was used as a cutoff for analyzable subjects as it has been demonstrated that as many as 60% of frames can be removed and still yield analyzable results (Fair et al., 2012; Power et al., 2012).
2.4. Segregation calculation
After preprocessing, for each 4D data set, time courses were extracted from 264, 10 mm diameter spheres (Power et al., 2011). From these time series, a 264 × 264 crosscorrelation matrix of Pearson rvalues was obtained and Z-transformed. Using the classification by Power and colleagues, each of the 264 nodes was assigned to one of 13 networks. For each network, segregation was defined as the relative strength of within-network connectivity compared to between-network connectivity. Specifically, it was calculated as follows (Chan et al., 2014):
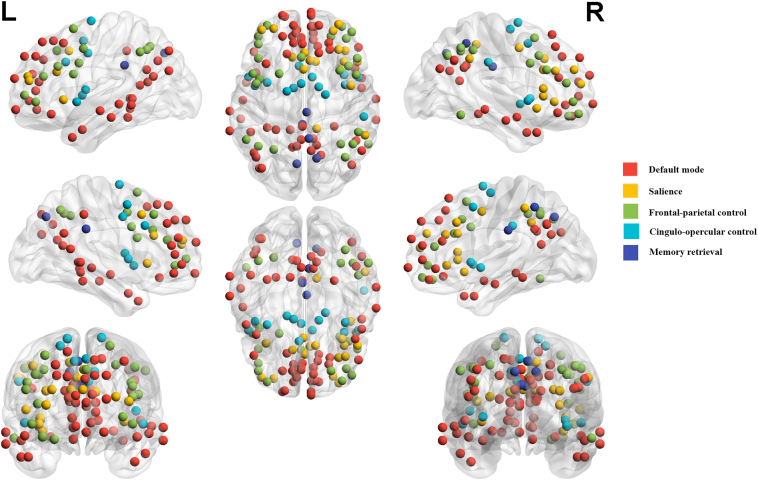
where is the mean connectivity strength of edges between all pairs of nodes within the same network and is the mean connectivity between nodes of one network to all nodes in other networks. Because of the uncertain meaning of negative correlations, negative z-values were excluded from the network analysis (Chai et al., 2012; Chan et al., 2014; Murphy et al., 2009). Also, diagonal values in the matrix were excluded from analysis. For each subject, 13 network segregation scores were computed, but we focused on the 5 networks of interest (DMN, SN, FPN, CON, and MRN) as specified above in the Introduction (Fig. 1).
Fig. 1.
The 5 networks-of-interest defined by Power et al. (2011).
2.5. Statistical analysis
To investigate the primary effect of rTMS on network segregation during the blinded treatment phase in MDD, separate repeated-measures analyses of covariance (ANCOVAs) were conducted on segregation of each of the 5 networks, with time (scan 1, scan 2) as within-subject factor, group (active, sham) as between-subjects factor, and age, FD and baseline MADRS score as covariates.
To test if baseline network segregation predicts symptom change following rTMS (blinded or open-label), hierarchical regression analyses were performed on the percentage MADRS change after 25 treatments. In this part of the analysis, patients in the active treatment arm were combined with patients who, after completing the sham arm, went on to receive active, open-label stimulation to maximize the power of the sample by including all patients who received rTMS. The predictors were baseline segregation scores, which came from scan 1 for the active arm patients and from scan 2 for the sham arm patients. We ran 6 regression models in total. In all of the regression models, age, FD and baseline MADRS scores were entered as predictors in step 1. To evaluate which networks together significantly predict rTMS treatment response above and beyond the predictors included in step 1, regression model 1 included segregation of all 5 networks as predictors in step 2, and we used stepwise method to determine which predictors to retain in the final model. To evaluate the predictive value of segregation of each of the 5 networks, we entered segregation of one of the 5 networks as the only predictor in step 2 for each of the other 5 regression models. Cook's distance(Cook, 1977) was used to identify possible outliers in the regression model, regression models were re-run without the datapoints with large Cook's distance, and robust regression was also performed to reduce the possible outlier effects (see details in Supplementary materials).
Since the results of the regression analyses suggested that higher segregation predicted better treatment response, we sought to clarify whether high or low segregation was normative. Hence, baseline network segregation scores in MDD patients were compared to the HCs using separate ANCOVAs, with age and movement as co-variates. For all the above analyses, alpha was set to p < .01, correcting for the 5 network comparisons. See supplementary materials for additional details.
3. Results
As previously reported (Taylor et al., 2018), of the 40 enrolled subjects, 34 completed scan 1 and 32 completed scan 1 and scan 2. Patients of both the sham and active treatment groups improved (in terms of MADRS score) after 20 treatments in the blinded phase, and there was no significant differential effect of stimulation (Supplementary Material Fig. S1). Of the patients who received sham rTMS, 2 subjects withdrew before entering open-label active treatment, and 3 subjects entered open-label treatment with remitted depression (MADRS score ≤ 8); these 5 subjects were thus excluded from the regression analyses of network segregation predictors of rTMS response. In the combined group (n = 27) of patients with active treatment and those who started with sham treatment and went on to receive active treatment, 48% exhibited a treatment response and 33% exhibited remission after 25 treatments. Demographics of the participants are summarized in Table 1.
Table 1.
Demographical and clinical variables among MDD patients and HCs.
| Blinded phase |
Active arm (N = 16) |
Sham arm (N = 16) |
t/χ2 | p | ||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| Age | 46.88 | 10.78 | 44.13 | 11.11 | 0.71 | 0.48 |
| Gender (female) | 11 (69%) | – | 10 (63%) | – | 0.14 | 0.71 |
| Medicated (%) | 16 (100) | – | 14 (87.5) | – | 2.13 | 0.14 |
| SES | 2.63 | 0.88 | 2.38 | 0.72 | 0.88 | 0.39 |
| ATHF-life | 5.06 | 3.17 | 5.56 | 2.13 | 0.52 | 0.60 |
| ATHF-current | 2.56 | 1.75 | 2.94 | 1.77 | 0.60 | 0.55 |
| MADRS-baseline | 25.44 | 5.73 | 21.94 | 3.13 | 2.14 | 0.04 |
| GAF-baseline | 52.63 | 4.67 | 55.56 | 4.27 | 1.86 | 0.07 |
| Response rate | 7 (44%) | 5 (31%) | 0.53 | 0.46 | ||
| Remission rate | 4 (25%) | 5 (31%) | 0.15 | 0.69 | ||
| Blinded + open-label phase |
MDD (N = 27) |
HCs (N = 20) |
t/χ2 | p | ||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| Age | 45.30 | 11.44 | 33.40 | 13.29 | 3.29 | 0.002 |
| Gender | 19 (70%) | – | 11 (55%) | – | 1.18 | 0.28 |
| Medicated (%) | 25 (92.6) | – | ||||
| Response | 13 (48%) | |||||
| Remission | 9 (33%) | |||||
Abbreviations: MDD, major depressive disorder; HCs, healthy control; SES: Socio-Economic Status; ATHF: Antidepressant Treatment History Form, total number of medication trials and augmentation strategies; MADRS: Montgomery-Asberg Depression Rating Scale; GAF: Global Assessment of Function.
3.1. Segregation changes over time in MDD
In the ANCOVAs comparing segregation changes over time in active arm vs. sham arm MDD patients, there were no significant effects of group or group × time interaction in any of the 5 networks (Supplementary Materials Fig. S2).
3.2. Baseline segregation prediction of symptom change following rTMS
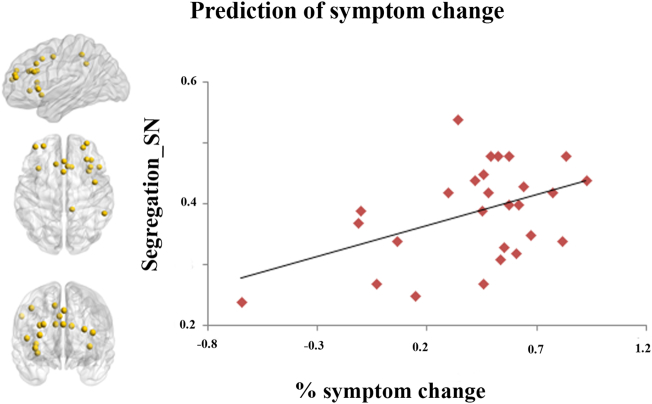
After controlling for age, FD and baseline MADRS score, segregation of the SN was the only significant predictor of symptom change among the 5 networks of interest (p = .006), either when these network predictors were considered simultaneously (Table 2, Model 1) or separately (Table 2, Models 2–6) in the regression models. The positive beta value suggested that more segregation of the SN predicted better response to rTMS treatment (Table 2, Fig. 2). No segregation metrics predicted response to real versus sham or overall stimulation at the end of the blinded phase (Supplementary materials, Table S3).
Table 2.
Baseline segregation of networks predicting symptom change in MDD patients (N = 27).
| Predictors | Variable statistics |
Model statistics |
||||
|---|---|---|---|---|---|---|
| β (s.e.) | t | p | ΔR2 | F | p | |
| Step 1 (for all models) | 0.11 | 0.93 | 0.443 | |||
| Age | −0.01 (0.01) | −1.20 | 0.242 | |||
| FD | −1.29 (1.21) | −1.07 | 0.296 | |||
| MADRS baseline | 0.01 (0.01) | 0.82 | 0.421 | |||
| Step 2 | ||||||
| Model 1a | ||||||
| Segregation_DMN | – | – | – | 0.27 | 3.30 | 0.029 |
| Segregation_SN | 2.44 (0.79) | 3.07 | 0.006 | |||
| Segregation_FPN | – | – | – | |||
| Segregation_MRN | – | – | – | |||
| Segregation_CON | – | – | – | |||
| Model 2 | ||||||
| Segregation_DMN | −0.99 (0.92) | −1.09 | 0.289 | 0.05 | 0.99 | 0.430 |
| Model 3 | ||||||
| Segregation_SN | 2.44 (0.79) | 3.07 | 0.006 | 0.27 | 3.03 | 0.029 |
| Model 4 | ||||||
| Segregation_FPN | −0.03 (0.97) | −0.03 | 0.979 | < 0.01 | 0.67 | 0.622 |
| Model 5 | ||||||
| Segregation_MRN | −0.22 (0.72) | −0.30 | 0.766 | < 0.01 | 0.69 | 0.606 |
| Model 6 | ||||||
| Segregation_CON | 0.69 (1.17) | 0.59 | 0.564 | 0.01 | 0.76 | 0.561 |
Abbreviations: MDD, major depression disorder; FD: frame displacement; DMN, default mode network; SN, salience network; FPN, frontal-parietal control network; CO, cingulo-opercular control network; MRN, memory retrieval network.
Predictors included segregation of DMN, SN, FPN, MRN, and CON. Using stepwise method, only segregation of SN was significant and retained in the final model.
Fig. 2.
Segregation of salience network (SN) predicted the treatment response of rTMS to depression (β = 2. 44, s.e. = 0.79, t = 3,07, p = .006).
3.3. Baseline segregation in MDD versus HC
The MDD and HC groups were similar in gender distribution, but they differed in age and movement (Table 1, Fig. S3). After controlling for the effects of age and movement, the ANCOVA results showed that MDD patients exhibited significantly less segregation in DMN (p = .005), and trend-level decreases in SN (p = .041) and MRN (p = .034), as compared to HCs (Fig. 3). Additional analyses confirmed that age and movement could not account for group differences (see supplementary materials, Table S1, S2 and Fig. S4).
Fig. 3.
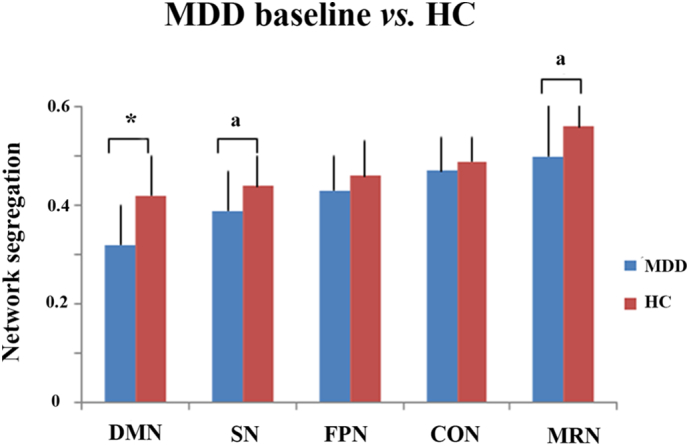
Segregation differences between MDD patients at baseline and HCs. MDD patients had significant decreases in segregation of DMN (F[1,43] = 8.74, p = .005, Eta2 = 0.169), and trend decrease in segregation of SN (F[1,43] = 4.42, p = .041, Eta2 = 0.093) and MRN (F[1,43] = 4.79, p = .034, Eta2 = 0.100) as compared to HCs. There were no significant differences between MDD and HCs in segregation of FPN (F[1,43] = 4.42, p = .889) and CON (F[1,43] = 0.06, p = .807). SN, salience network; DMN, default mode network; MRN, memory retrieval network; FPN, fronto-parietal control network, CO, cingulo-opercular control network; MDD, major depressive disorder; HCs, healthy controls. auncorrected p < .05, *uncorrected p < .01 (p < .05 when corrected for 5 multiple comparisons).
4. Discussion
The present study examined network segregation changes with rTMS therapy and network segregation as a predictor of response to rTMS. Although we did not find a significant effect of rTMS stimulation, relative to sham stimulation, on network segregation, we did find that segregation of SN predicted symptom improvement after rTMS. We also found that, compared to HCs, patients with MDD had significantly poorer segregation of DMN and a trend toward poorer segregation of SN and MRN. These findings have implications for future efforts to enhance treatment efficiency of rTMS as well as for our understanding of the pathophysiology of depression.
4.1. Response prediction in segregation of SN
An important finding of this study was that segregation of SN predicted symptom improvement following rTMS administered to the left DLPFC in MDD. In contrast to seed-based approaches or ICA, segregation measures network organization and interaction, a critical property indexing the balance between integration (connections between networks) and functional specialization (connections within a network). The segregation measure is relatively new, but as we discuss in the Introduction, recent findings suggest that it is functionally important in aging (Chan et al., 2014). The present study extends this line of work to depression, where MDD patients had marginally decreased segregation of SN compared with HCs at the baseline, suggesting that more segregation represents a more normative state.
The SN is a large-scale paralimbic-limbic system with key nodes including the anterior insula (AI), dorsal ACC (dACC), and amygdala. These nodes have rich connectivity with other brain areas (Averbeck and Seo, 2008; Craig, 2009; Menon and Uddin, 2010), permitting the SN to detect, integrate and process internal and external salient information (Menon and Uddin, 2010). Recent literature has demonstrated a critical role of SN in controlling switching between DMN (task-negative network) and task positive networks (Goulden et al., 2014; Menon, 2011). Dysfunction of SN has been found to play an important role in the pathopsychological mechanism of MDD, e.g. associated with the negative bias in patients with MDD (Downar et al., 2016; Hamilton et al., 2012; Hamilton et al., 2016). In addition, the dysfunction of SN, specifically the right AI, was significantly associated with altered dynamic communications between DMN and CEN in MDD patients, which may be related to the depressive biases toward internal and self-related thoughts at the expense of engaging with the external world (Kaiser et al., 2015; Manoliu et al., 2013). Based on these findings, our results may then provide support for the hypothesis that more normal segregation of SN confers greater treatment responses in MDD by fostering adaptive negative information processing and appropriate allocation of resources between a task-negative system and a task-positive system. Our findings are in line with results from several previous studies using ICA, multi-voxel pattern analysis, and seed-based connectivity analyses, showing that connectivity of many nodes within SN as well as between SN and other brain regions tracked MDD symptom improvement after rTMS (Ge et al., 2017; Philip et al., 2018). However, our results extend these findings by providing new insight about the potential of network segregation to predict treatment response to rTMS.
What remains unclear is whether the predictor, i.e., segregation of SN, is specific for response to DLPFC rTMS or a general biomarker of response across different treatment approaches. Previous studies have revealed that activity and connectivity within SN also tracked response to pharmacotherapy and psychotherapy in MDD (Fu et al., 2008; He et al., 2016; Langenecker et al., 2007; Sikora et al., 2016), and a recent large-scale meta-analysis revealed that the SN was a common core substrate affected across most psychiatric disorders (Goodkind et al., 2015). These findings may raise the possibility that SN function reflects a more general treatment response predictor, not specific to rTMS or even to MDD. There was no interaction between group and segregation of SN at the end of blinded phase, which would be consistent with a nonspecific response, but we did not find that SN segregation predicted overall response at the end of the blinded phase, which is not consistent with a non-specific response. However, it may have been the case that 20 sessions of stimulation did not cause enough symptom change to show a relationship with the network, as studies show that many patients respond to TMS after 20 sessions (Yip et al., 2017). The question of the specificity of SN segregation to treatment response remains a topic for future research.
4.2. Decreased segregation of DMN in depression
Our analysis also revealed significantly decreased segregation of DMN in patients with MDD. DMN consists of a set of brain regions that deactivate reliably during task performance, with key nodes anchored in the medial prefrontal cortex and posterior cingulate cortex (Raichle and Snyder, 2007). It has been associated with self-referential processing and emotion regulation as well as consciousness and memory processing (Andrews-Hanna et al., 2010; Kim, 2012; Mulders et al., 2015). From a clinical perspective, the decreased segregation of DMN may be involved in MDD via ruminative, self-referential thinking, as well as disruptions in emotion regulation. Seed-based methods and ICA analysis have consistently shown hyperconnectivity within the anterior part of DMN and between anterior DMN and SN (Mulders et al., 2015). In addition to DMN, we also detected a trend of less segregation of MRN in MDD patients as compared with HCs. In the parcellation by Power et al. (2011), the MRN consists of parts of the posterior cingulate, posterior medial and lateral parietal cortex, regions with strong activation in memory retrieval, and frequently included in the DMN in other parcellations (Allen et al., 2011; Yeo et al., 2011). Thus, less segregated MRN might contribute to the bias of retrieving negative life events in MDD patients. Altered connectivity within posterior DMN, between posterior DMN and anterior DMN, as well as between DMN and FPN were also frequently reported; however, the direction of alterations has been inconsistent (Kaiser et al., 2015; Mulders et al., 2015). The measurement of segregation provides an alternative perspective on the role of DMN in MDD by summarizing the information of within- and between-network connectivity, and showing that not only is regional connectivity disrupted in MDD, but more global functional organization of the network is abnormal.
4.3. Limitations
As described in the introduction, previous studies have revealed that overall network segregation decreases with age (Chan et al., 2014). While the relationship with age speaks to the significance of network segregation as an important marker of functional organization, it also presents a potential confound for our analysis, as the MDD patients and HC subjects were not well-matched on age. However, we found no significant correlations between age and the 5 networks-of-interest in either group, and no significant group by age interactions (Supplementary materials Table S1, Fig. S4), indicating that the main results of the present study, i.e., the treatment response prediction of SN as well as group difference in DMN, were not likely driven by age differences, although we cannot fully rule out the possibility because of our small sample size. It should be noted that previous studies relating age to decreased segregation typically examined only overall network segregation (i.e., mean segregation of all networks), not segregation of individual networks (Chan et al., 2014). To verify their findings, we did the same analysis (correlation between mean network segregation and age). As shown in Table S1, we did find the relationship in HCs but not in MDD. Our failure to find this relationship with age in the MDD patients may reflect a type 2 error, or it may reflect an altered relationship between aging and segregation in MDD.
Another limitation was that most subjects (30 out of 32; 25 out of 27) were on antidepressant medication. Previous studies have found medication effects on network connectivity properties in MDD (Frodl et al., 2011; Fu et al., 2015; Tadayonnejad et al., 2016), but no prior work has examined network segregation. Thus, our findings of differences in segregation between MDD patients and healthy controls should be treated with some caution as possible confounding effects of medication could not be addressed. Future studies with unmedicated MDD subjects will be required. It is also the case that we cannot rule out the possibility that medication may have moderated symptom improvement observed over the course of rTMS therapy. However, we note that practically, the presence of medications may make our results more generalizable, since most patients in regular clinical rTMS are also taking antidepressant medications (Carpenter et al., 2012; Taylor et al., 2017).
Several other limitations should also be noted. First, though the depression scores changed in the expected direction with an effect size (active vs. sham: 0.53) very similar to those reported in the literature (0.39–0.5) (Bakker et al., 2015; Connolly et al., 2012; Slotema et al., 2010), a significant clinical effect of active rTMS compared to sham treatment was not found. This fact must limit any conclusions about the apparent lack of an effect of rTMS on network segregations. Second, the relatively small sample in the present study, 32 MDD patients and 20 HCs has limited statistical power. Thus, findings must be regarded with caution, and future replication is needed. Third, movement may be another confounding factor of the results, although it is unlikely to account for our findings (Supplementary materials, Tables S1 and S2). Fourth, we failed to detect a prediction effect of segregation metrics to sham versus active stimulation or to overall stimulation, which limits conclusions about the specificity of the predictive value of SN segregation.
5. Conclusion
In conclusion, this preliminary study demonstrated that network segregation of SN predicted treatment response to rTMS effect in depression. Patients with MDD showed some degree of decreased segregation, particularly in DMN, suggesting that abnormal network segregation may be an important pathophysiological facet of MDD. These findings may inform the utility of functional network organization patterns in predicting treatment response and in understanding the psychopathology of depression.
Funding
This study was supported by NIMH R21MH098174, NIH Clinical Sciences Translational Award UL1TR000433, and NIMH K23MH108823. Research support was also provided by Neuronetics, which did not have a role in the design of the study.
Role of the sponsors: The sponsors provided funding only, except for the Clinical Sciences Translational Award, which assisted in the set-up of the clinical database.
Conflict of interest
The authors report the following disclosures relevant to the content of this article: Dr. Taylor has received research support from Neuronetics and St. Jude Medical (now Abbott). Dr. Maixner has received research support from St. Jude Medical (now Abbott). All other authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge the assistance of Kaitlin Cassady and Holly Gagnon for providing the script for segregation calculation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101719.
Appendix A. Supplementary data
Supplementary material
References
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A.M., Caprihan A., Turner J.A., Eichele T., Adelsheim S., Bryan A.D., Bustillo J., Clark V.P., Feldstein Ewing S.W., Filbey F., Ford C.C., Hutchison K., Jung R.E., Kiehl K.A., Kodituwakku P., Komesu Y.M., Mayer A.R., Pearlson G.D., Phillips J.P., Sadek J.R., Stevens M., Teuscher U., Thoma R.J., Calhoun V.D. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck B.B., Seo M. The statistical neuroanatomy of frontal networks in the macaque. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker N., Shahab S., Giacobbe P., Blumberger D.M., Daskalakis Z.J., Kennedy S.H., Downar J. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain. Stimulation. 2015;8:208–215. doi: 10.1016/j.brs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Carpenter L.L., Janicak P.G., Aaronson S.T., Boyadjis T., Brock D.G., Cook I.A., Dunner D.L., Lanocha K., Solvason H.B., Demitrack M.A. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress. Anxiety. 2012;29:587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castanon A.N., Ongur D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.Y., Park D.C., Savalia N.K., Petersen S.E., Wig G.S. Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R., D'Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K.R., Helmer A., Cristancho M.A., Cristancho P., O'Reardon J.P. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J. Clin. Psychiatry. 2012;73:e567–e573. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- Cook R.D. Detection of influential observations in linear regression. Technometrics. 1977;19:15–18. [Google Scholar]
- Craig A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietsche B., Backes H., Stratmann M., Konrad C., Kircher T., Krug A. Altered neural function during episodic memory encoding and retrieval in major depression. Hum. Brain Mapp. 2014;35:4293–4302. doi: 10.1002/hbm.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J., Blumberger D.M., Daskalakis Z.J. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn. Sci. 2016;20:107–120. doi: 10.1016/j.tics.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Nigg J.T., Iyer S., Bathula D., Mills K.L., Dosenbach N.U., Schlaggar B.L., Mennes M., Gutman D., Bangaru S., Buitelaar J.K., Dickstein D.P., Di Martino A., Kennedy D.N., Kelly C., Luna B., Schweitzer J.B., Velanova K., Wang Y.F., Mostofsky S., Castellanos F.X., Milham M.P. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. New York State Psychiatric Institute; New York: 1995. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Fox M.D., Buckner R.L., White M.P., Greicius M.D., Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Scheuerecker J., Schoepf V., Linn J., Koutsouleris N., Bokde A.L., Hampel H., Moller H.J., Bruckmann H., Wiesmann M., Meisenzahl E. Different effects of mirtazapine and venlafaxine on brain activation: an open randomized controlled fMRI study. J. Clin. Psychiatry. 2011;72:448–457. doi: 10.4088/JCP.09m05393blu. [DOI] [PubMed] [Google Scholar]
- Fu C.H., Williams S.C., Cleare A.J., Scott J., Mitterschiffthaler M.T., Walsh N.D., Donaldson C., Suckling J., Andrew C., Steiner H., Murray R.M. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Fu C.H., Costafreda S.G., Sankar A., Adams T.M., Rasenick M.M., Liu P., Donati R., Maglanoc L.A., Horton P., Marangell L.B. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. 2015;15:1–11. doi: 10.1186/s12888-015-0457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallen C.L., Baniqued P.L., Chapman S.B., Aslan S., Keebler M., Didehbani N., D'Esposito M. Modular brain network organization predicts response to cognitive training in older adults. PLoS One. 2016;11 doi: 10.1371/journal.pone.0169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R., Blumberger D.M., Downar J., Daskalakis Z.J., Dipinto A.A., Tham J.C.W., Lam R., Vila-Rodriguez F. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: a pilot study. J. Affect. Disord. 2017;218:75–81. doi: 10.1016/j.jad.2017.04.060. [DOI] [PubMed] [Google Scholar]
- Gong Q.Y., He Y. Depression, neuroimaging and connectomics: a selective overview. Biol. Psychiatry. 2015;77:223–235. doi: 10.1016/j.biopsych.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N., Khusnulina A., Davis N.J., Bracewell R.M., Bokde A.L., McNulty J.P., Mullins P.G. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Glover G.H., Bagarinao E., Chang C., Mackey S., Sacchet M.D., Gotlib I.H. Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Res. Neuroimaging. 2016;249:91–96. doi: 10.1016/j.pscychresns.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Cui Q., Zheng J., Duan X., Pang Y., Gao Q., Han S., Long Z., Wang Y., Li J., Wang X., Zhao J., Chen H. Frequency-specific alterations in functional connectivity in treatment-resistant and -sensitive major depressive disorder. J. Psychiatr. Res. 2016;82:30–39. doi: 10.1016/j.jpsychires.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. A dual-subsystem model of the brain's default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. NeuroImage. 2012;61:966–977. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Langenecker S.A., Kennedy S.E., Guidotti L.M., Briceno E.M., Own L.S., Hooven T., Young E.A., Akil H., Noll D.C., Zubieta J.K. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol. Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B., Voss H.U., Casey B.J., Etkin A., Dubin M.J. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthoffer D., Zimmer C., Forstl H., Bauml J., Riedl V., Wohlschlager A.M., Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N.S., Barredo J., van 't Wout-Frank M., Tyrka A.R., Price L.H., Carpenter L.L. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry. 2018;83:263–272. doi: 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. (discussion 1097-1089) [DOI] [PubMed] [Google Scholar]
- Sackeim H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry. 2001;62(Suppl. 16):10–17. [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. (quiz 34–57) [PubMed] [Google Scholar]
- Sikora M., Heffernan J., Avery E.T., Mickey B.J., Zubieta J.K., Pecina M. Salience network functional connectivity predicts placebo effects in major depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:68–76. doi: 10.1016/j.bpsc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotema C.W., Blom J.D., Hoek H.W., Sommer I.E. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Clin. Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R., Ajilore O., Mickey B.J., Crane N.A., Hsu D.T., Kumar A., Zubieta J.K., Langenecker S.A. Pharmacological modulation of pulvinar resting-state regional oscillations and network dynamics in major depression. Psychiatry Res. Neuroimaging. 2016;252:10–18. doi: 10.1016/j.pscychresns.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Bhati M.T., Dubin M.J., Hawkins J.M., Lisanby S.H., Morales O., Reti I.M., Sampson S., Short E.B., Spino C., Watcharotone K., Wright J. A naturalistic, multi-site study of repetitive transcranial magnetic stimulation therapy for depression. J. Affect. Disord. 2017;208:284–290. doi: 10.1016/j.jad.2016.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Ho S.S., Abagis T., Angstadt M., Maixner D.F., Welsh R.C., Hernandez-Garcia L. Changes in brain connectivity during a sham-controlled, transcranial magnetic stimulation trial for depression. J. Affect. Disord. 2018;232:143–151. doi: 10.1016/j.jad.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tik M., Hoffmann A., Sladky R., Tomova L., Hummer A., Navarro de Lara L., Bukowski H., Pripfl J., Biswal B., Lamm C., Windischberger C. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. NeuroImage. 2017;162:289–296. doi: 10.1016/j.neuroimage.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Wig G.S. Segregated Systems of Human Brain Networks. Trends Cogn. Sci. 2017;21:981–996. doi: 10.1016/j.tics.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Wu X., Lin P., Yang J., Song H., Yang R., Yang J. Dysfunction of the cingulo-opercular network in first-episode medication-naive patients with major depressive disorder. J. Affect. Disord. 2016;200:275–283. doi: 10.1016/j.jad.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zollei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip A.G., George M.S., Tendler A., Roth Y., Zangen A., Carpenter L.L. 61% of unmedicated treatment resistant depression patients who did not respond to acute TMS treatment responded after four weeks of twice weekly deep TMS in the Brainsway pivotal trial. Brain. Stimulation. 2017;10:847–849. doi: 10.1016/j.brs.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., Liao J., Zhong M., Wang W., Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material