Abstract
Emerging studies indicate that striatal cholinergic interneurons play an important role in synaptic plasticity and motor control under normal physiological conditions, while their disruption may lead to movement disorders. Here we discuss the involvement of the cholinergic system in motor dysfunction, with a focus on the role of the nicotinic cholinergic system in Parkinson’s disease and drug-induced dyskinesias. Evidence for a role for the striatal nicotinic cholinergic system stems from studies showing that administration of nicotine or nicotinic receptor drugs protects against nigrostriatal degeneration and decreases L-dopa-induced dyskinesias. In addition, nicotinic receptor drugs may ameliorate tardive dyskinesia, Tourette’s syndrome and ataxia, although further study is required to understand their full potential in the treatment of these disorders. A role for the striatal muscarinic cholinergic system in movement disorders stems from studies showing that muscarinic receptor drugs acutely improve Parkinson’s disease motor symptoms, and may reduce dyskinesias and dystonia. Selective stimulation or lesioning of striatal cholinergic interneurons suggests they are primary players in this regulation, although multiple central nervous systems appear to be involved.
Implications
Accumulating data from preclinical studies and clinical trials suggest that drugs targeting CNS cholinergic systems may be useful for symptomatic treatment of movement disorders. Nicotinic cholinergic drugs, including nicotine and selective nAChR receptor agonists, reduce L-dopa-induced dyskinesias, as well as antipsychotic-induced tardive dyskinesia, and may be useful in Tourette’s syndrome and ataxia. Subtype selective muscarinic cholinergic drugs may also provide effective therapies for Parkinson’s disease, dyskinesias and dystonia. Continued studies/trials will help address this important issue.
Overview
Extensive studies over nearly half a century provide overwhelming evidence for a role of the basal ganglia in the control of voluntary movement and the pathophysiology of movement disorders.1–3 In this regard, the basal ganglia do not work in isolation but function in concert with the substantia nigra, cortex, thalamus, raphe nuclei, brain stem nuclei, and other regions (Figure 1). A basal ganglia region central in this regulation is the striatum, with extensive work suggesting a significant involvement of the striatal cholinergic system.4–7 This idea stems from numerous studies showing that lesions of the striatum disrupt movement while drugs that modulate the cholinergic system can improve motor disabilities in preclinical studies and/or clinical trials.8–12
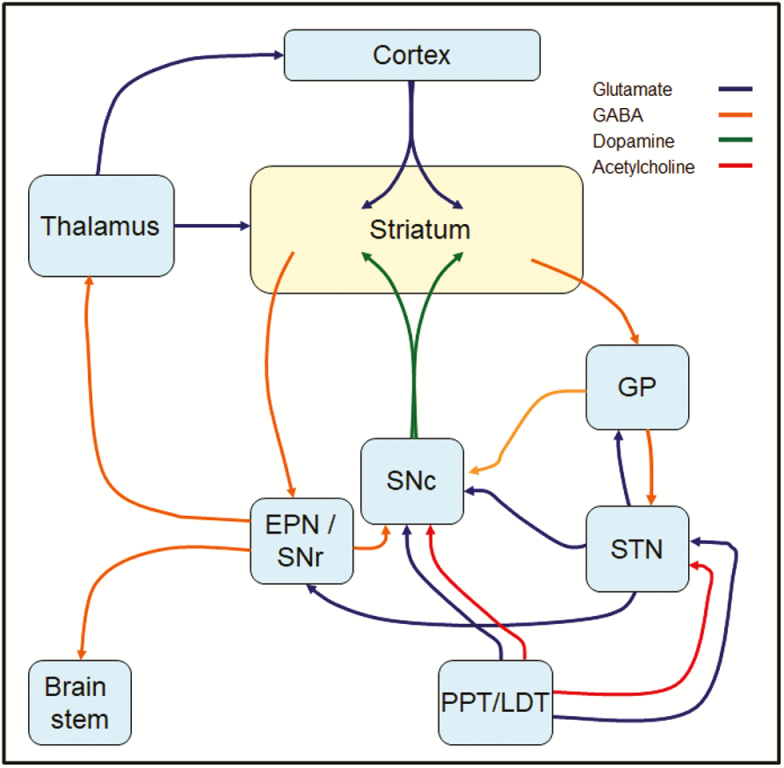
Figure 1.
Direct and indirect pathway circuitry within the basal ganglia. Dopaminergic projections from the substantia nigra pars compacta (SNc) and cortical glutamatergic afferents synapse onto the medium spiny neurons (MSNs) of the striatum. These neurons are classically subdivided into the “direct” or “indirect” pathways based on their expression of D1 or D2 dopamine receptors, respectively. Direct pathway D1 MSNs project directly to the enteropeduncular nucleus (EPN; internal segment of the globus pallidus in primates) or the substantia nigra pars reticulata (SNr), and thence to the brain stem or thalamus/cortex, respectively. Indirect pathway D2 MSNs project to the globus pallidus (GP; external segment of the globus pallidus in primates) en route to the EPN and SNr via the SNc or the subthalamic nucleus (STN). Depicted are also the cholinergic projections from the pedunculopontine tegmental (PPT) and laterodorsal tegmental (LDT) nuclei to the striatum, STN and SNc, which in addition to cholinergic interneurons regulate basal ganglia function.
The objective of this article is to present emerging data that reinforces the assumption of a critical role for the striatal cholinergic system in movement disorders, with a focus on the nicotinic cholinergic system. We first briefly review the anatomy of striatal neuronal circuits and summarize evidence for a role of cholinergic interneurons in movement dysfunction. These combined studies form the basis for understanding the beneficial role of nicotinic, as well as muscarinic receptor drugs in improving various types of motor disabilities.
Cholinergic Interneurons and Striatal Circuitry
Striatal circuitry consists of various intrinsic neuron subtypes, as well as an extensive array of excitatory and inhibitory connections from the substantia nigra, cortex, thalamus, raphe nuclei, locus coeruleus, and other regions (Figures 1 and 2). These inputs synapse onto striatal neurons that may be of several subtypes. These include GABAergic medium spiny neurons (MSNs) that form the greater majority (95%) of striatal neurons, as well as smaller populations of several types of striatal interneurons that constitute the remaining 5% of neurons.5,13–18
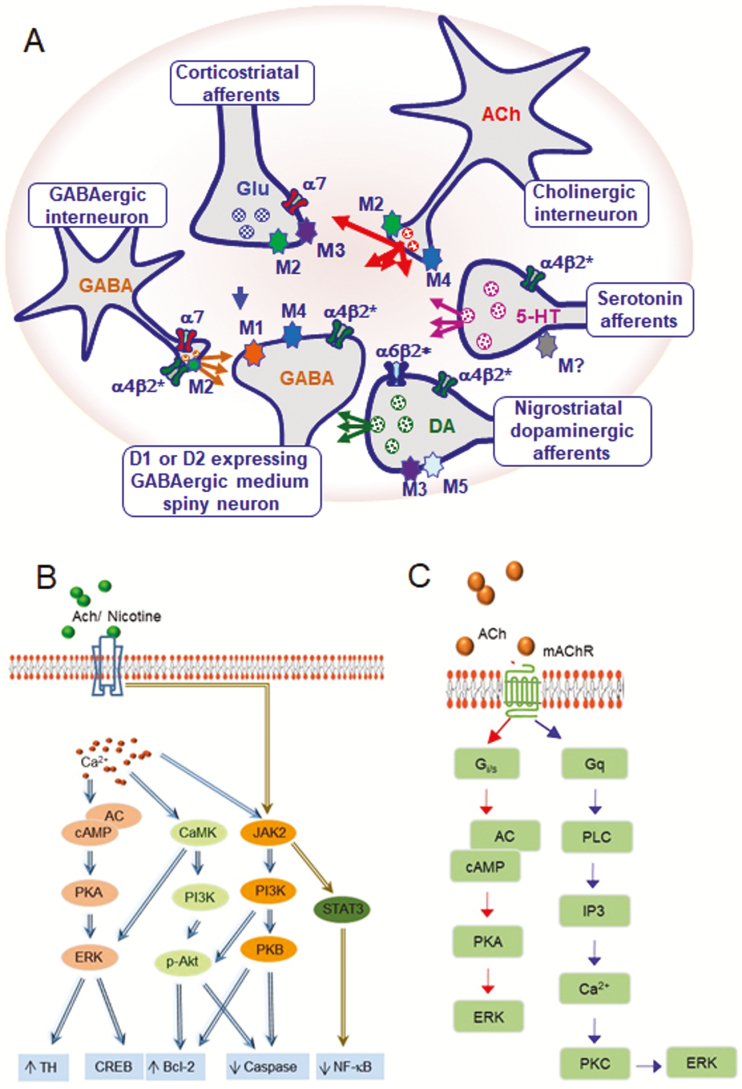
Figure 2.
Cholinergic signaling via nAChRs and muscarinic acetylcholine receptors (mAChRs) regulates striatal function. (A) Diagrammatic representation of the primary striatal neurotransmitter systems. Cholinergic interneurons are the primary source of striatal acetylcholine (ACh) and regulate its function via pre-and post-synaptic nAChRs and muscarinic receptors. Acetylcholine regulates the activity of direct and indirect GABAergic medium spiny neurons (MSNs) by acting at α4β2* nAChRs, as well as M1 and/or M4 muscarinic receptors. In addition, acetylcholine modulates striatal dopamine (DA) release via an interaction at α6β2* and α4β2* nAChRs along with M2 and/or M4 muscarinic receptors on nigrostriatal dopaminergic and serotonergic (5-HT) terminals, which further regulates the output of direct and indirect pathway MSNs. Likewise, acetylcholine can modulate GABAergic interneuron activity via α7 and α4β2* nAChRs, as well as M2 muscarinic receptors. Acetylcholine can further control striatal function via α7 nAChRs and M2 and M3 muscarinic receptors located on the excitatory glutamatergic (GLU) inputs arising from the cortex. (B) Molecular signaling modulated by nAChRs. Stimulation of nAChRs increases intracellular Ca2+ which promotes activation of PKA and CAMKII to initiate ERK1/2 cascade activity. nAChR signaling can also occur via Ca2+ -independent mechanisms thru the JAK2/STAT3 pathway. (C) Molecular signaling via mAChRs. These receptors are coupled to G proteins. M2 and M4 receptors couple preferentially to Gi/0, whereas M1, M3 and M5 receptors mainly couple to Gq/11. Upon stimulation, M2 and M4 receptors inhibit adenylyl cyclase (AC) activity, leading to a decrease in intracellular cAMP levels and PKA activity to ultimately regulate ERK1/2 activity. M1, M3, and M5 receptors activate PKC by means of upstream PLC activation and increase in IP3 and Ca2+ levels. PKC activity leads to the activation of the MAP kinase cascade and ERK1/2.
MSNs are medium sized projection neurons with wide-ranging dendritic trees densely covered with spines that extensively arborize and synapse with striatal interneurons and numerous incoming neurons.5,13–18 The primary afferents to MSNs are glutamatergic corticostriatal, glutamatergic thalamostriatal, and dopaminergic nigrostriatal neurons (Figures 1 and 2). Additional afferents include serotonergic raphestriatal, noradrenergic locus coeruleus, and cholinergic pedunculopontine projections. MSNs innervate a variety of basal ganglia structures, including the globus pallidus and substantia nigra.5,13–18 There appear to be two functionally distinct subpopulations of MSNs that are responsible for different aspects of motor control, which act in a somewhat opposing fashion. These include the D1 dopamine receptor expressing direct pathway MSNs that project to and disinhibit the inhibitory output neurons of the globus pallidus internus and substantia nigra pars reticulata (Figure 1); this pathway is thought to be the driving factor for movement facilitation under normal physiological conditions. By contrast, indirect pathway MSNs express D2 dopamine receptors and project to the globus pallidus externus to disinhibit the subthalamic nucleus and promote the tonic inhibitory output of the globus pallidus internus/substantia nigra reticulate (Figure 1). The indirect pathway is thought to be inhibited during movement and active during lack of movement. Overall motor function involves a complex balance between the direct and indirect pathways to allow for the fine control of motivation and action. Degeneration of the dopaminergic nigrostriatal pathway as occurs in Parkinson’s disease results in an imbalance in the functions mediated by the direct and indirect pathway leading to the resultant bradykinesia and other movement abnormalities observed in this disorder.
In addition to the GABAergic MSN projection neurons, the striatum also contains several subtypes of medium size striatal GABAergic interneurons with distinct physiological, chemical, and morphological properties.5,13–18 These include interneurons that selectively express calcium-binding proteins such as parvalbumin or calretinin, as well as various neuropeptides and enzymes, including neuropeptide Y, somatostatin, and nitric oxide synthase. Some examples of interneuron subtypes include GABAergic parvalbumin-immunoreactive fast-spiking interneurons, GABAergic low-threshold spiking interneurons, GABAergic calretinin-immunoreactive interneurons, and tyrosine hydroxylase-immunoreactive interneurons. For a discussion on the role of these different interneuron subtypes in striatal function, the reader is referred to some recent excellent reviews.5,13–18 The involvement of these select neuronal populations in neurological disease is an area just beginning to be understood.
Besides medium size interneurons, there exists a small population of large aspiny cholinergic interneurons in the striatum.5,13–18 Although they are sparse in number (~2% of the neuronal population), they have very widespread dendritic and axonal fields that extensively arborize throughout the striatum to synapse with the GABAergic projection neurons and interneurons described above. In addition, they extensively overlap with nigrostriatal dopaminergic terminals, corticostriatal glutamatergic afferents, serotonergic terminals from the raphe, and other inputs to the striatum. These cholinergic interneurons are tonically active and fire action potentials at a slow rate to result in a continuous pulsatile release of acetylcholine under basal conditions. Acetylcholine release from cholinergic interneurons is controlled by numerous neurotransmitter systems as recently reviewed by Lim and coworkers.16 This includes both intrinsic and extrinsic GABAergic inputs, with data indicating that GABA can directly modulate acetylcholine release through stimulation of GABAA receptors on striatal cholinergic neurons.19,20 Groups I and II metabotropic glutamate receptors located on the axon terminals of striatal cholinergic interneurons also modulate acetylcholine release,21,22 as do ionotropic glutamate receptors.16 Dopamine directly regulates striatal cholinergic transmission via D1/D5 and D2 dopamine receptors.23–27 Evidence for this stems from a variety of studies including pharmacological work showing that selective D1 receptor agonists increased acetylcholine release, while both D1 receptor antagonists and D2 receptor agonists reduced acetylcholine release.23,24 Thus, D1 and D2 receptors have opposing roles in the control of striatal acetylcholine release to allow for the fine tuning of dopamine receptor-mediated regulation of locomotor activity.11
Histamine also influences acetylcholine release from striatal interneurons via an action at H1, H2, and H3 receptors.28,29 In addition, serotonin released from raphe-striatal neurons is able to inhibit the release of acetylcholine from striatal interneurons through a variety of serotonin receptor subtypes.30,31 This multimodal regulation of acetylcholine release by numerous neurotransmitters allows for extensive fine tuning of striatal function. The acetylcholine released from cholinergic interneurons subsequently acts at cholinergic receptors located on neuronal terminals and/or cell bodies of dopaminergic, GABAergic, glutamatergic, and serotonergic neurons, as well as on the cholinergic neurons themselves (Figure 2). Acetylcholine then exerts its effects by acting at both nicotinic and muscarinic receptors as described in detail below.
Nicotinic Acetylcholine Receptor Signaling in Striatum
Neuronal nicotinic acetylcholine receptors (nAChRs) are members of the Cys-loop gene super family of ligand-gated ion channels.11,32,33 They consist of complexes of five subunits around a central hydrophilic pore or channel. Twelve distinct neuronal nAChR subunits have been identified to date and fall into two major subclasses, including α (α2 to α10) and β (β2 to β4) subunits. The α subunits bear the distinction of possessing an acetylcholine recognition site, whereas the β subunits do not, although they influence the properties of acetylcholine binding to the α subunit.11,32,33 nAChRs have been classified into two main types that may be heteromeric or homomeric. The results of mRNA expression work, nAChR subunit knockout experiments, nAChR subunit selective antibody testing and nAChR subtype selective drug studies indicate that the main heteromeric receptor subtypes in the striatum are α4β2* and α6β2* nAChRs, with the asterick indicating the presence of other subunits in the receptor complex. These may be the α5 and β3 subunits to yield α4α5β2, and α4α6β2β3 receptors.34 The primary homomeric receptor present in the striatum is the α7 subtype, which is composed of five identical α subunits.
nAChR subtypes are differentially distributed throughout the brain and may be localized on presynaptic nerve terminals or postsynaptically on neuronal cell bodies11 (Figure 2). In the striatum, the greater majority of nAChRs are expressed on incoming afferent terminals arising from the substantia nigra, cortex, raphe nuclei, and other regions. In addition, nAChRs may be located on GABAergic and cholinergic interneurons within the striatum, although their numbers are sparse as suggested from the results of in situ hybridization studies that identified little, if any, nAChR subunit mRNA in this region.35
Functionally, the different nAChR subtypes mediate fast excitatory transmission in response to acetylcholine or nAChR agonists, when exposed to rapidly changing concentrations of agonist. However, volume transmission of acetylcholine is known and the pharmacokinetics of agonist delivery to the CNS may result in relatively slow changes in agonist concentrations and therefore slower kinetics of nAChR function. In addition, the different nAChR subtypes have diverse functional and pharmacological properties, and may thus mediate unique and varied cellular activities.11,32,33
Activation of presynaptic nAChRs enhances permeability to small monovalent and divalent cations such as Na+, K+, and Ca2+ to facilitate release of various striatal neurotransmitters into the synaptic cleft. nAChR-evoked striatal dopamine release is one of the best studied. Cholinergic interneurons extensively overlap with nigrostriatal dopaminergic terminals expressing α4β2* and α6β2* nAChRs (α4β2, α4α5β2, α6β2β3, α4α6β2β3) to modulate dopamine release.34,36,37 Striatal α7 nAChRs located on corticostriatal glutamatergic efferents also indirectly regulate dopamine release by modulating striatal glutamate release.38 Additionally, acetylcholine released from cholinergic interneurons can also stimulate α4β2* nAChRs to induce GABA release from GABAergic interneurons.39 nAChR stimulation may also elicit 5-HT release from striatal raphe nucleus afferents.40 Thus, acetylcholine released from striatal cholinergic interneurons can act at distinct nAChR subtypes on different neurotransmitter terminals to result in an intricate regulation of striatal function. This, in turn, has the potential to allow for a complex control of movement under physiological conditions, and to result in varied movement deficits under pathological conditions (Table 1).
Table 1.
Involvement of CNS cholinergic systems in movement disorders
| System | Movement disorder | Cholinergic system implicated | Cholinergic receptors involved | Reference |
|---|---|---|---|---|
| Nicotinic cholinergic | Parkinson’s disease | Striatal cholinergic interneurons | α4β2*, α6β2*, α7 | 41–45 |
| L-dopa-induced dyskinesias | Striatal cholinergic interneurons | α4β2*, α6β2*, α7 | 46–56 | |
| Tardive dyskinesia | Striatal cholinergic interneurons | β2* | 10 | |
| Tourette’s syndrome | Striatal cholinergic interneurons | β2* | 57,58 | |
| Ataxia | Cerebellar cholinergic system | β2*, α7 | 59,60 | |
| Gait disturbances in Parkinson’s disease | Pedunculopontine system | Not known | ||
| Muscarinic cholinergic | Parkinson’s disease | Striatal cholinergic interneurons | M1, M4 | 4,61 |
| L-dopa-induced dyskinesias | Striatal cholinergic interneurons | M4, other | 12,62,63 | |
| Dystonia | Striatal cholinergic interneurons | M1, M2, M4 | 64–66 |
Role of the Nicotinic Cholinergic System in Movement
Parkinson’s Disease
Parkinson’s disease is the second most common neurodegenerative disease with an incidence of 1% over the age of 60. It is characterized by movement disabilities including tremor, rigidity, bradykinesia/hypokinesia, and postural instability as well as numerous other deficits in cognition, affect, sleep, and autonomic nervous system function.9,67–69 Parkinson’s disease is associated with a generalized loss of neuronal systems throughout the brain, with the most prominent feature being a degeneration of nigrostriatal dopaminergic neurons. This results in a decline in dopamine release and a reduced stimulation of D1 and D2 receptors on MSNs of the direct pathway and indirect pathway, respectively. This ensuing dysregulation of dopamine function leads to an overall decline in movement facilitation mediated by the direct pathway and increase in the inhibitory influence of the direct pathway to result in the bradykinesia, rigidity, freezing, and other motor deficits observed in Parkinson’s disease. This idea is supported by extensive work showing that dopamine replacement with the dopaminergic precursor 3,4-dihydroxyphenylalanine (L-dopa) and/or treatment with dopaminergic receptor agonists dramatically improve the motor symptoms.9,67–69
However, variable and incomplete responses to dopaminergic therapy suggest the involvement of other neurotransmitter systems, with a prominent role for the cholinergic one.5,7,8,70,71 Indeed, muscarinic cholinergic antagonists were the first drugs used to treat Parkinson’s disease, as is discussed in a later section. In addition, studies have been done to evaluate the effect of nAChR drugs on acute motor symptoms in Parkinson’s disease. Although there was improvement in about half the trials, no change or a worsening was found in the others.72–82 Possible explanations for these variable results include differences in the duration of nicotine treatment and/or in the nicotine dosing regimen, the relatively small study sizes, the clinical tests used and the stage of Parkinson’s disease. Of particular note, however, is that the improvement in motor symptoms were associated with the open-label studies, while no effect or a worsening was observed in the double-blinded trials. These clinical data are consistent with results in parkinsonian animal models that generally also found no acute improvement in motor symptoms with nAChR drugs.83,84
Although nicotine and nAChR drugs may not improve acute motor symptoms in Parkinson’s disease, there is an extensive literature suggesting that nAChR drugs may protect against nigrostriatal damage. This idea initially stemmed from the results of epidemiological studies which consistently found a negative association between Parkinson’s disease and tobacco use. This reduced incidence of Parkinson’s disease with tobacco use appeared to be due to a true biological effect of smoking based on several lines of evidence, as follows85–89: (1) the effect was dose and time dependent, with the decline in Parkinson’s disease incidence greater with more years of smoking and more cigarettes smoked; (2) the reduced risk was lost with smoking cessation; (3) there was a decreased incidence of Parkinson’s disease with other forms of tobacco; (4) twin studies showed that Parkinson’s disease develops less in the twin that smoked;90 and (5) lastly, the decreased risk of Parkinson’s disease in smokers was not due to a selective mortality.85–89
Although there are many chemical components in cigarette smoke, a role for nicotine in the apparent protective effect was suggested from studies showing that nicotine and nAChR drugs reduced neuronal damage in culture systems.91,92 More importantly, extensive studies in toxin-induced parkinsonian models, including MPTP-treated monkeys, MPTP-treated mice, and 6-hydroxydopamine-treated rats and mice, also demonstrated that nicotine and nAChR agonist administration protected against nigrostriatal dopaminergic damage.91 Studies with selective nAChR drugs and α4, α6, α7, or β2 nicotinic receptor subunit knockout mice suggested a role for both β2* and α7 nAChR subtypes.41–45 These preclinical studies formed the basis for an ongoing Michael J. Fox Foundation funded clinical trial, to investigate the ability of the nicotine patch to protect against Parkinson’s disease (ClinicalTrials.gov Identifier NCT01560754).
L-Dopa-Induced Dyskinesias
L-dopa-induced dyskinesias (LIDs) are abnormal involuntary movements that occur as a side effect of L-dopa therapy, the gold standard treatment for Parkinson’s disease motor symptoms.93–96 They generally only arise after months or more commonly years of L-dopa treatment; however, they occur in most patients to some degree and may become debilitating.97 At present, therapeutic options are limited. Amantadine has historically been the only drug approach; however, data on efficacy were limited.94–96 Recent placebo-controlled trials utilizing a novel sustained-release formulation amantadine support efficacy, although side effects were common and may limit generalizability of use.98,99 Deep brain stimulation has proved very effective but involves surgery with its related drawbacks.100–102 Additional approaches for treatment would thus be a great asset.
As might be expected, the nigrostriatal dopaminergic system is key in the development of LIDs.103–105 Administration of L-dopa is thought to lead to unregulated dopamine release and excessive activity of striatal MSN projection neurons of the D1 direct pathway. D2-mediated activity of the indirect pathway also becomes overactive, with a resultant decline in activity of this pathway to lead to the overall enhanced motor activity characteristic of dyskinesias.93,96 In addition, numerous other CNS neurotransmitters have been implicated including the serotonergic, glutamatergic, opioid, GABAergic, noradrenergic, histaminergic, and various peptidergic systems.93,94,96,106–108 More recent work also indicates a role for striatal cholinergic interneurons and the nicotinic cholinergic system. Experimental studies show that ablation of striatal cholinergic interneurons in mice before initiation of L-dopa treatment markedly reduced LIDs without compromising the therapeutic efficacy of L-dopa.109 Additionally, long duration optical stimulation of cholinergic interneurons decreased LIDs, again without affecting parkinsonism.62 These combined studies provide direct evidence for the involvement of cholinergic interneurons in selectively regulating LIDs.
A role for the nicotinic system stems from extensive preclinical work showing that administration of nicotine to parkinsonian monkeys, mice, and rats decreased LIDs 50–60% (Table 2).46,83,84 Long term molecular mechanisms appeared to be involved since the reduction in LIDs required several weeks to develop, and was maintained for several weeks after nicotine discontinuation. Importantly, all modes of nicotine administration tested reduced LIDs,83,110 with no tolerance in the ability of nicotine to reduce dyskinesias.111 These latter properties indicate that nicotine treatment may be useful in the clinic. Evidence that the effect of nicotine is mediated via nAChRs stem from studies with α4, α6, α7, and β2 nAChR null mutant mice, and work which showed that β2* and α7 subtype selective nAChR agonists reduced LIDs in both monkeys and rodents (Table 2).46–56
Table 2.
nAChR drugs decrease LIDs in parkinsonian rats, mice, or monkeys
| Receptor subtype | Drug | Decline in LIDs | References |
|---|---|---|---|
| Nonselective agonist | Nicotine | ~35–60% | 62,83,84,110–112 |
| Varenicline | ~10–50% | 48,49 | |
| β2* selective agonist | ABT-089 | ~50% | 51 |
| ABT-894 | ~60% | 51 | |
| AZD1446 | ~30% | 113 | |
| Sazetidine | ~23% | 50 | |
| TC2696 | ~30% | 50 | |
| TC-8831 | ~25–50% | 47,48,50 | |
| TC-10600 | ~30% | 50 | |
| β2* nonselective antagonist | Mecamylamine | 62,110 | |
| α7 selective agonist | ABT-107 | ~60% | 54 |
| ABT-126 | ~60% | 55 | |
| AQ051 | ~60% | 56 |
It should be noted that nicotine or nAChR agonist administration had no acute symptomatic effects on parkinsonism in either rodents or nonhuman primates. Moreover, optical stimulation of cholinergic interneurons or cholinergic interneuron ablation did not affect parkinsonism.62,109 These data indicate that cholinergic interneurons selectively regulate LIDs.
A mechanism that explains the decline in LIDs with nAChR agonists and the longer duration optical stimulation of cholinergic interneurons is receptor desensitization. Notably, the extent of the decline in LIDs with longer duration optical stimulation was 50–60%, which is similar to that observed with nicotine and nAChR agonist treatment.62 Numerous studies have shown that chronic exposure nicotine and nAChR agonists result in nAChR desensitization and a consequent functional receptor blockade.114,115 The longer duration optical stimulation would be expected to enhance extracellular acetylcholine levels and consequently desensitize nAChRs. This receptor desensitization may subsequently lead to further molecular changes to mediate overall functional changes. Possible mechanisms may include those previously implicated in nicotine-mediated neuroprotection. Nicotine modulates neurotoxicity by enhancing phosphatidylinositol 3-kinase and altering levels of phosphorylated AKT, as well as Src, B-cell lymphoma (Bcl) 2, and Bcl-x.116,117 The mitogen-activated protein kinase/extracellular signal-regulated kinases pathway and the JAK2/STAT3 pathway have also been implicated in nAChR-mediated neuroprotection,118–121 as well as other downstream mechanisms including alterations in phospholipase C,118 nerve growth factor,122 proinflammatory cytokines,123 caspases, and reactive oxygen species124Figure 2).
In addition to numerous preclinical studies, a small clinical trial has been conducted to evaluate the potential of nicotine to reduce LIDs in Parkinson’s disease patients. Oral nicotine (designated NP002) administration to 50 patients for several months significantly reduced a variety of outcome measures related to LIDs (www.prnewswire.com/news-releases/neuraltus-pharmaceuticals-reports-clinical-results-from-phase-12-np002-study-in-the-treatment-of-dyskinesias-resulting-from-levodopa-therapy-for-parkinsons-disease-111255279.html).
Overall, these results demonstrate that striatal cholinergic interneurons play a critical role in LIDs. Moreover, the finding that nicotine and nAChR drugs targeting β2* and α7 nAChRs reduce LIDs in parkinsonian animal models and in a small clinical trial suggest that nAChR drugs may be useful therapeutically.
Tardive Dyskinesia
Antipsychotics are key in treating schizophrenia and bipolar disorder, and are also used off-label for depression, sleep disorders, autism, attention deficit hyperactivity disorder, tic disorders, obsessive compulsive disorder, and post-traumatic stress disorder.125–129 They exert their beneficial effect by blocking D2 dopamine receptors and reducing excess dopaminergic activity in brain regions linked to neurological disorders. However, they also affect dopaminergic systems associated with motor control, and induce side effects including tardive dyskinesia.130 These are potentially irreversible late onset repetitive abnormal involuntary movements primarily of the face and limbs.126,131–134 They occur in up to 30% of treated patients and may be debilitating and socially stigmatizing. The second-generation antipsychotics cause less tardive dyskinesia; however, it still develops at an annual incidence of 4%.126,135–138 Acquired sensitivity or dysregulation of nigrostriatal dopamine signaling has been hypothesized to underlie the development of tardive dyskinesia. This idea is based on studies showing that selective vesicular monoamine transporter 2 inhibitors (VMAT2I), such as tetrabenazine, act by reducing dopamine release in the synaptic cleft. Tetrabenazine efficacy is often limited by side effects, though recently valbenazine and deutetrabenazine have been shown efficacious and well tolerated in clinical trials.139,140 These agents collectively reduce but do not resolve tardive movements, therefore additional or adjunctive therapeutic options are needed. However, this has proved difficult most likely due to our incomplete understanding of the cellular and molecular mechanisms that underlie tardive dyskinesia.
One possible new approach may involve the use of nAChR drugs. Pre-clinical studies in rodents point to a beneficial effect of nicotine against tardive dyskinesia. Chronic nicotine dosing of haloperidol-treated rats or mice reduced vacuous chewing movements (VCMs),10,141 an analog of tardive dyskinesia in rodents. Both oral or minipump nicotine treatment attenuated VCMs ~50%.141 This decrease appeared to be due to an interaction at nAChRs, since varenicline, an agonist that acts at several nAChR subtypes also reduced haloperidol-induced VCMs.10 Unexpectedly, the general nAChR agonist varenicline reduced VCMs to a greater extent (90%) than nicotine (50%),10 possibly due to an interaction at 5-HT3 receptors.142–145 Optogenetic studies also showed that stimulation of striatal cholinergic interneurons or striatal D2 MSNs reduced haloperidol-induced VCMs ~50% via an interaction at nAChRs.62
The animal studies above suggest that nAChR drugs may reduce tardive dyskinesia in humans. Schizophrenic patients are well known to consume several packs of cigarettes per day,146,147 and thus would consume nicotine in this manner. Unfortunately, data in humans are unclear as to whether smoking improves tardive dyskinesia. This most likely relates to inconsistencies among studies in neuroleptic dosing, cigarette consumption, length of time of antipsychotic medication and smoking, association with alcohol consumption, differential psychiatric morbidities, and other variables.148–151 One query that arises is why smoking schizophrenics exhibit tardive dyskinesia at all if nicotine attenuates their occurrence. However, it should be noted that nicotine administration maximally reduced abnormal movements only up to 50% in the animal studies.141,152 Thus, tardive dyskinesia may be less pronounced in schizophrenic patients that smoke. A carefully controlled, double-blind clinical trial is essential to address the question whether smoking reduces tardive dyskinesia in the clinic.
In summary, preclinical studies provide direct evidence for a role of both striatal cholinergic interneurons and D2 MSNs in tardive dyskinesia. Moreover, work with nAChR drugs in rodents indicate that such agents may be useful to reduce antipsychotic-induced tardive dyskinesia. Further clinical trials will help understand the potential of nAChR drugs to reduce tardive dyskinesia in humans.
Tourette’s Syndrome
Tourette’s syndrome is a relatively common disorder (1% incidence) that arises in childhood and is characterized by motor and vocal tics, and common comorbid symptoms of obsession, compulsion, impulsivity, distractibility, and hyperactivity.153–155 Antidopaminergic therapy is one of the most effective symptomatic treatments with dopamine receptor blockers improving the motor and vocal tics.153–156 However, it is only partially effective and there are unacceptable side effects. Other medications are also used, again with only partial success.153,155,156 Thus alternative approaches are essential.
Although pathophysiology of the dopaminergic system is a major problem in Tourette’s syndrome, the motor symptoms are also linked to a dysfunction of the striatal cholinergic system.157,158 Preclinical evidence for this idea stems from studies showing that targeted ablation of 50% of striatal cholinergic interneurons in mice led to tic-like stereotypies and a loss of coordination.158 Clinically, there is a down regulation of striatal interneuron transcripts and a decreased number of cholinergic interneurons in Tourette’s syndrome brains.159
Because of this link between the striatal dopaminergic and cholinergic systems, the nAChR agonist nicotine was tested in Tourette’s syndrome. Initial open label trials with the nicotine gum or patch showed a decrease in tics and improved attention in haloperidol-treated Tourette’s patients.160–164 In addition, the nicotine patch reduced symptoms in haloperidol-treated patients in a double-blind placebo-controlled trial.165 The acetylcholinesterase inhibitor donepezil also significantly reduced tics in an open-label study.166 The observation that the beneficial response in Tourette’s was of longer duration with the nicotine patch than gum,167 suggested that nAChR desensitization or blockade may be involved.167,168 This possibility led to two trials with the nAChR blocker mecamylamine. There was improvement in relieving the motor and vocal tics, as well as some behavioral measures, in a retrospective open-label study of 24 patients. Mecamylamine was also somewhat effective in a double-blind placebo-controlled study in haloperidol-treated patients.57,58
These clinical trial data, coupled with the experimental animal studies, indicate an involvement of the striatal nicotinic cholinergic system in Tourette’s syndrome and suggest that nAChR drugs have potential as an adjunct to antipsychotic therapy.
Ataxia
Ataxia is a motor disorder characterized by poor coordination of voluntary muscle movements. It is associated with various genetic abnormalities that result in mitochondrial and other cellular deficits, which lead to spinocerebellar, Friedreich’s, Fragile X associated, and other forms of ataxia.
Currently, there is an absence of therapeutic options for ataxia169; however, drugs that enhance CNS cholinergic activity appear useful. The centrally acting acetylcholinesterase inhibitor physostigmine, which increases brain acetylcholine levels, improved spinocerebellar degeneration and various inherited ataxias in open label and double-blind randomized trials, possibly via an interaction with the nicotinic cholinergic system.170–172 On the other hand, physostigmine was not effective against autosomal dominant cerebellar ataxia and idiopathic cerebellar ataxia.173 In earlier work, the acetylcholine precursor choline improved Friedreich’s ataxia, idiopathic cerebellar degeneration, multiple sclerosis-linked ataxia, and ataxias associated with sporadic cerebellar degeneration and atypical spinocerebellar degeneration.174–177
Trials with more selective agents have also been done. In small case reports, the nAChR agonist varenicline improved ataxia and imbalance in one individual with Fragile X tremor/ataxia syndrome, enhanced proprioception in two patients with Friedreich’s ataxia and ameliorated gait, balance, and depth perception in a patient with spinocerebellar ataxia.178–180 A pilot double-blind, placebo-controlled, randomized trial with 20 patients with spinocerebellar ataxia showed that 2 months of varenicline treatment improved axial symptoms and rapid alternating movements.181 However, in a different study minimal benefit was observed in patients with other forms of ataxia.182
Studies in animal models of ataxia have been done to understand the receptor subtype and location of the nAChRs involved. Acute intracerebellar administration of nicotine or an α4β2* nAChR agonist reduced ethanol-induced ataxia.183 This improvement did not occur with intracerebellar administration of an α4β2* nAChR antagonist providing evidence for a role for cerebellar α4β2* nAChRs.184 This idea is supported by other studies showing that nicotine and the β2* selective nAChR agonist varenicline reduced ataxia in rats with a lesion of the olivocerebellar pathway.59 α7 nAChR drugs administered into the cerebellum also attenuated ethanol-induced ataxia, providing evidence for a role of cerebellar α7 nAChRs.60
Thus, there also appears to be dysfunction of the cholinergic system in ataxia. This appears more closely linked to aberrant nicotinic cholinergic signaling in the cerebellum (Table 1) than striatum and involves both α4β2* and α7 nAChRs. Drugs targeting these receptors may therefore be useful for the treatment of certain forms of ataxia.
Muscarinic Acetylcholine Receptor Signaling in Striatum
In addition to nAChRs, the striatum also densely expresses muscarinic acetylcholine receptors, including the M1 through M5 subtypes.16,185,186 These G-protein coupled receptors (Figure 2) serve a longer term modulatory role (over 100’s msec) in contrast to the ionotropic nAChRs that typically mediate transmission on a more rapid time scale (msec). It has long been known that striatal muscarinic cholinergic receptors are critical in motor function as evidence by the observation that muscarinic antagonists reduce motor symptoms in Parkinson’s disease.8,187
Muscarinic receptors are distributed throughout the striatum with M1 and M4 receptors expressed on GABAergic MSNs4,188,189 (Figure 2). The M3 and M5 appear to be on nigrostriatal nerve terminals where they play a key role in dopamine release.190,191 M2 and M4 muscarinic receptors present on striatal cholinergic terminals serve an autoinhibitory role, with M4 muscarinic antagonists inhibiting striatal acetylcholine release while M2 antagonists increase release.189,192–194 This released acetylcholine may subsequently regulate striatal dopamine release195 and consequently modulate motor control.
Various intracellular signaling pathways may mediate these functional changes (Figure 2). M2 and M4 receptors couple preferentially to Gi/0, whereas M1, M3, and M5 receptors mainly couple to Gq/11. Upon activation, M2 and M4 receptors inhibit adenylyl cyclase (AC) activity which leads to a decrease in intracellular cAMP levels and PKA activity that subsequently regulates ERK1/2 activity. M1, M3, and M5 receptors activate PKC by means of upstream PLC activation and increase in IP3 and Ca2+ levels. PKC activity leads to the activation of the MAP kinase cascade and ERK1/2.
Role of the Muscarinic Cholinergic System in Movement
Parkinson’s Disease
As mentioned, muscarinic receptor blockers were the first drugs used to provide acute relief of Parkinson’s disease motor symptoms and drugs such as trihexyphenidyl, benztropine, and others are still sometimes used in a secondary role, particularly for tremor. The rationale for their use derived from work suggesting that normal motor function appeared to be a balance between dopaminergic and muscarinic cholinergic signaling in the striatum, which is disrupted in Parkinson’s disease.11 Anticholinergic drugs appear to correct the disequilibrium that develops between striatal dopaminergic inputs and the intrinsic cholinergic innervation.11 The efficacy of anticholinergics is attributed to a decrease in the over activity of cholinergic interneurons and the hyperactivity of corticostriatal glutamate neurotransmission that arises with nigrostriatal damage. In addition, studies in parkinsonian animal models indicate that this improvement may be due to a blockade of postsynaptic M1 and M4 receptors on MSNs to alleviate lesion-induced motor deficits.4,61 Evidence that cholinergic interneurons are key players stems from studies showing that optogenetic activation and inhibition of these neurons modulates motor deficits in parkinsonian mouse models.196,197 Although muscarinic cholinergic drugs were initially useful in the treatment of Parkinson’s disease, they are now less used because of side effects, including cognitive impairment, confusion, constipation, dry mouth, urinary issues, and others.
In contrast to the benefit of muscarinic receptor blockers on motor function in Parkinson’s disease, acetylcholinesterase inhibitors that increase acetylcholine’s action at both muscarinic receptors and nAChRs yielded no significant improvement in Parkinson’s disease motor symptoms.71 They may, however, reduce gait disturbances and the risk of falls in a subgroup of patients with Parkinson’s disease.198–201 With respect to mechanisms, recent preclinical studies in mice lacking the vesicular acetylcholine transporter from mesopontine nuclei suggest that cholinergic neurons in the pedunculopontine nucleus are critical for gait (Table 1) and may be the target for cholinesterase inhibitors.202,203
In summary, antimuscarinic drugs may still be used for Parkinson’s disease treatment in combination with other antiparkinsonian drugs.187 A drawback is their side effect profile which is less favorable than other antiparkinsonian medications as neuropsychiatric and cognitive adverse events may develop.187 Selective muscarinic subtype antagonists may prove more promising as potential targets for the symptomatic treatment of parkinsonian-like motor symptoms. Additionally, acetylcholinesterase inhibitors may be useful for gait disturbances.
L-Dopa-Induced Dyskinesias
Extensive work points to a role of the striatal nicotinic cholinergic system in LIDs, as detailed in a previous section. In addition, muscarinic cholinergic receptors may be involved with the muscarinic receptor antagonist dicyclomine reducing LIDs in a mouse parkinsonian model.63 An M4 muscarinic positive allosteric modulator also decreased LIDs in mouse and nonhuman primate parkinsonian models via long term depression of corticostriatal glutamatergic synapses, suggesting that M4 muscarinic receptors may selectively be involved.12 Striatal cholinergic interneurons most likely play a role, as short duration optogenetic stimulation of these neurons induces LIDs that are blocked by the muscarinic antagonist atropine.62 However, atropine also blocked the stimulation-induced decrease in LIDs that arises with long duration optogenetic stimulation62; this observation suggests that nonspecific muscarinic receptor drugs such as atropine may not reduce LIDs clinically. Possibly subtype selective drugs may prove useful in the treatment of LIDs.
Tardive Dyskinesia
Less work has been done to understand the involvement of the muscarinic system in tardive dyskinesia. However, because of the close interrelationship between the dopaminergic and cholinergic system in the basal ganglia, a variety of cholinergic agents have been tested in clinical trials. These drugs generally failed to show a clear benefit for the treatment of tardive dyskinesia and, in addition, resulted in side effects including cognitive problems, dry mouth, urinary disturbances, constipation and others.204 Some studies have also suggested that muscarinic blockers cause a worsening of tardive dyskinesia205 and would therefore not be useful. Possibly the development of subtype selective muscarinic receptor drugs would prove of benefit.
Dystonia
Dystonia is a movement disorder characterized by twisted posturing due to abnormal muscle contraction. The finding that anticholinergic therapy is often beneficial in dystonia patients suggested an involvement of the cholinergic system in its pathophysiology.206 Evidence from animal models of dystonia indicate a role for the basal ganglia,18 with a crucial involvement of the striatal cholinergic system. Elevated extracellular striatal acetylcholine was identified in a knock-in mouse model of human DYT1 dystonia (TorAE/+ mice), suggestive of a striatal hypercholinergic state.64 The mutation in TorAE mice and consequent enhanced extracellular acetylcholine levels may lead to an imbalance in acetylcholine-dopamine interactions. A selective M1 antagonist and M2/M4 muscarinic antagonist specifically targeted to muscarinic receptors expressed by cholinergic interneurons improved the dystonic behavior.64–66 These data directly indicate a role for striatal cholinergic interneurons and the muscarinic system in dystonia.
Summary
Accumulating data from preclinical studies and clinical trials suggest that drugs targeting CNS cholinergic systems may be useful for symptomatic treatment of various movement disorders. In particular, extensive studies in multiple animal models show that nicotinic cholinergic drugs reduce L-dopa-induced dyskinesias, as well as antipsychotic-induced tardive dyskinesia. Both the general nAChR agonist nicotine and selective nAChR agonists effectively improved movement. In addition, there is some evidence that nicotine and other general nAChR agonist may be useful in Tourette’s syndrome and ataxia, although the data is less compelling possibly because the nAChR drugs tested to date stimulate all nAChR subtypes, whereas only select subtypes may be affected in these latter diseases. Studies/trials with subtype selective drugs would help address this issue. Muscarinic cholinergic drugs, particularly subtype selective agonists and/or antagonist, also have the potential to provide effective therapies for Parkinson’s disease, dyskinesias, and dystonia; continued studies/trials with subtype selective drugs are necessary to understand their full potential.
It should be noted that the best therapeutic strategy for any disease is to reduce or halt disease progression. In this regard, extensive studies have shown that nicotine and nAChR drugs reduce neurodegeneration in animal models of Parkinson’s disease. A Michael J. Fox funded trial with Parkinson’s disease patients is currently in progress to address this important question in the clinic.
Funding
This work was supported by grant NS R56NS095965 from the National Institutes of Health.
Declaration of Interests
None declared.
References
- 1. Obeso JA, Marin C, Rodriguez-Oroz C, et al. . The basal ganglia in Parkinson’s disease: Current concepts and unexplained observations. Ann Neurol. 2008;64 (Suppl 2):S30–S46. [DOI] [PubMed] [Google Scholar]
- 2. Barroso-Chinea P, Bezard E. Basal Ganglia circuits underlying the pathophysiology of levodopa-induced dyskinesia. Front Neuroanat. 2010;4. doi: 10.3389/fnana.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Féger J. Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. Mov Disord. 2015;30(9):1155–1170. [DOI] [PubMed] [Google Scholar]
- 4. Ztaou S, Maurice N, Camon J, et al. . Involvement of striatal cholinergic interneurons and M1 and M4 muscarinic receptors in motor symptoms of Parkinson’s disease. J Neurosci. 2016;36(35):9161–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanimura A, Pancani T, Lim SAO, et al. . Striatal cholinergic interneurons and Parkinson’s disease. Eur J Neurosci. 2017. July 5. doi: 10.1111/ejn.13638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tozzi A, de Iure A, Di Filippo M, et al. . The distinct role of medium spiny neurons and cholinergic interneurons in the D₂/A₂A receptor interaction in the striatum: Implications for Parkinson’s disease. J Neurosci. 2011;31(5):1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pisani A, Bonsi P, Centonze D, Gubellini P, Bernardi G, Calabresi P. Targeting striatal cholinergic interneurons in Parkinson’s disease: Focus on metabotropic glutamate receptors. Neuropharmacology. 2003;45(1):45–56. [DOI] [PubMed] [Google Scholar]
- 8. Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117(2):232–243. [DOI] [PubMed] [Google Scholar]
- 9. Vijverman AC, Fox SH. New treatments for the motor symptoms of Parkinson’s disease. Expert Rev Clin Pharmacol. 2014;7(6):761–777. [DOI] [PubMed] [Google Scholar]
- 10. Quik M, Zhang D, Perez XA, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quik M, Wonnacott S. α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev. 2011;63(4):938–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen W, Plotkin JL, Francardo V, et al. . M4 muscarinic receptor signaling ameliorates striatal plasticity deficits in models of L-DOPA-induced dyskinesia. Neuron. 2015;88(4):762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzales KK, Smith Y. Cholinergic interneurons in the dorsal and ventral striatum: Anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci. 2015;1349:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deffains M, Bergman H. Striatal cholinergic interneurons and cortico-striatal synaptic plasticity in health and disease. Mov Disord. 2015;30(8):1014–1025. [DOI] [PubMed] [Google Scholar]
- 15. Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53(4):590–605. [DOI] [PubMed] [Google Scholar]
- 16. Lim SA, Kang UJ, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci. 2014;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonsi P, Cuomo D, Martella G, et al. . Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eskow Jaunarajs KL, Bonsi P, Chesselet MF, Standaert DG, Pisani A. Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia. Prog Neurobiol. 2015;127–128:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ikarashi Y, Yuzurihara M, Takahashi A, Hirohisa Ishimaru, Shiobara T, Maruyama Y. Modulation of acetylcholine release via GABAA and GABAB receptors in rat striatum. Brain Res. 1999;816(1):238–240. [DOI] [PubMed] [Google Scholar]
- 20. DeBoer P, Westerink BH. GABAergic modulation of striatal cholinergic interneurons: An in vivo microdialysis study. J Neurochem. 1994;62(1):70–75. [DOI] [PubMed] [Google Scholar]
- 21. Marti M, Paganini F, Stocchi S, Bianchi C, Beani L, Morari M. Presynaptic group I and II metabotropic glutamate receptors oppositely modulate striatal acetylcholine release. Eur J Neurosci. 2001;14(7):1181–1184. [DOI] [PubMed] [Google Scholar]
- 22. Pisani A, Bonsi P, Catania MV, et al. . Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J Neurosci. 2002;22(14):6176–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acquas E, Di Chiara G. Role of dopamine D1 receptors in the control of striatal acetylcholine release by endogenous dopamine. Neurol Sci. 2001;22(1):41–42. [DOI] [PubMed] [Google Scholar]
- 24. Damsma G, Tham CS, Robertson GS, Fibiger HC. Dopamine D1 receptor stimulation increases striatal acetylcholine release in the rat. Eur J Pharmacol. 1990;186(2–3):335–338. [DOI] [PubMed] [Google Scholar]
- 25. Stoof JC, De Boer T, Sminia P, Mulder AH. Stimulation of D2-dopamine receptors in rat neostriatum inhibits the release of acetylcholine and dopamine but does not affect the release of gamma-aminobutyric acid, glutamate or serotonin. Eur J Pharmacol. 1982;84(3–4):211–214. [DOI] [PubMed] [Google Scholar]
- 26. Clos MV, García-Sanz A, Vivas NM, Badia A. D2 dopamine receptors and modulation of spontaneous acetylcholine (ACh) release from rat striatal synaptosomes. Br J Pharmacol. 1997;122(2):286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drukarch B, Schepens E, Stoof JC. Muscarinic receptor activation attenuates D2 dopamine receptor mediated inhibition of acetylcholine release in rat striatum: Indications for a common signal transduction pathway. Neuroscience. 1990;37(1):1–9. [DOI] [PubMed] [Google Scholar]
- 28. Prast H, Tran MH, Fischer H, et al. . Histaminergic neurons modulate acetylcholine release in the ventral striatum: Role of H3 histamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360(5):558–564. [DOI] [PubMed] [Google Scholar]
- 29. Bell MI, Richardson PJ, Lee K. Histamine depolarizes cholinergic interneurones in the rat striatum via a H(1)-receptor mediated action. Br J Pharmacol. 2000;131(6):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonsi P, Cuomo D, Ding J, et al. . Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: Implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology. 2007;32(8):1840–1854. [DOI] [PubMed] [Google Scholar]
- 31. Vizi ES, Hársing LG Jr, Zsilla G. Evidence of the modulatory role of serotonin in acetylcholine release from striatal interneurons. Brain Res. 1981;212(1):89–99. [DOI] [PubMed] [Google Scholar]
- 32. Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol. 2015;124:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96(Pt B):302–311. [DOI] [PubMed] [Google Scholar]
- 34. Gotti C, Guiducci S, Tedesco V, et al. . Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30(15):5311–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marks MJ, Pauly JR, Gross SD, et al. . Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12(7):2765–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drenan RM, Grady SR, Steele AD, et al. . Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 2010;30(29):9877–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem Pharmacol. 2009;78(7):744–755. [DOI] [PubMed] [Google Scholar]
- 38. Kaiser S, Wonnacott S. Alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58(2):312–318. [DOI] [PubMed] [Google Scholar]
- 39. Grilli M, Zappettini S, Zoli M, Marchi M. Presynaptic nicotinic and D(2) receptors functionally interact on dopaminergic nerve endings of rat and mouse nucleus accumbens. J Neurochem. 2009;108(6):1507–1514. [DOI] [PubMed] [Google Scholar]
- 40. Takahashi H, Takada Y, Nagai N, Urano T, Takada A. Nicotine increases stress-induced serotonin release by stimulating nicotinic acetylcholine receptor in rat striatum. Synapse. 1998;28(3):212–219. [DOI] [PubMed] [Google Scholar]
- 41. Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132(8):1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeyarasasingam G, Tompkins L, Quik M. Stimulation of non-alpha7 nicotinic receptors partially protects dopaminergic neurons from 1-methyl-4-phenylpyridinium-induced toxicity in culture. Neuroscience. 2002;109(2):275–285. [DOI] [PubMed] [Google Scholar]
- 43. Park HJ, Lee PH, Ahn YW, et al. . Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26(1):79–89. [DOI] [PubMed] [Google Scholar]
- 44. Bordia T, McGregor M, Papke RL, Decker MW, McIntosh JM, Quik M. The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions. Exp Neurol. 2015;263:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stuckenholz V, Bacher M, Balzer-Geldsetzer M, et al. . The α7 nAChR agonist PNU-282987 reduces inflammation and MPTP-induced nigral dopaminergic cell loss in mice. J Parkinsons Dis. 2013;3(2):161–172. [DOI] [PubMed] [Google Scholar]
- 46. Huang LZ, Grady SR, Quik M. Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors. J Pharmacol Exp Ther. 2011;338(3):932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnston TH, Huot P, Fox SH, et al. . TC-8831, a nicotinic acetylcholine receptor agonist, reduces L-DOPA-induced dyskinesia in the MPTP macaque. Neuropharmacology. 2013;73:337–347. [DOI] [PubMed] [Google Scholar]
- 48. Zhang D, Mallela A, Sohn D, et al. . Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson’s disease. J Pharmacol Exp Ther. 2013;347(1):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M. Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats. Neuropharmacology. 2011;60(6):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quik M, Campos C, Bordia T, et al. . α4β2 Nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology. 2013;71:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang D, Bordia T, McGregor M, McIntosh JM, Decker MW, Quik M. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2014;29(4):508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quik M, Park KM, Hrachova M, et al. . Role for α6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice. Neuropharmacology. 2012;63(3):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quik M, Campos C, Grady S. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias; studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol. 2013;86(8):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang D, McGregor M, Decker MW, Quik M. The α7 nicotinic receptor agonist ABT-107 decreases L-Dopa-induced dyskinesias in parkinsonian monkeys. J Pharmacol Exp Ther. 2014;351(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang D, McGregor M, Bordia T, et al. . α7 nicotinic receptor agonists reduce levodopa-induced dyskinesias with severe nigrostriatal damage. Mov Disord. 2015;30(14):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Di Paolo T, Grégoire L, Feuerbach D, Elbast W, Weiss M, Gomez-Mancilla B. AQW051, a novel and selective nicotinic acetylcholine receptor α7 partial agonist, reduces l-Dopa-induced dyskinesias and extends the duration of l-Dopa effects in parkinsonian monkeys. Parkinsonism Relat Disord. 2014;20(11):1119–1123. [DOI] [PubMed] [Google Scholar]
- 57. Silver AA, Shytle RD, Sanberg PR. Mecamylamine in Tourette’s syndrome: a two-year retrospective case study. J Child Adolesc Psychopharmacol. 2000;10(2):59–68. [DOI] [PubMed] [Google Scholar]
- 58. Silver AA, Shytle RD, Sheehan KH, Sheehan DV, Ramos A, Sanberg PR. Multicenter, double-blind, placebo-controlled study of mecamylamine monotherapy for Tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 2001;40(9):1103–1110. [DOI] [PubMed] [Google Scholar]
- 59. Wecker L, Engberg ME, Philpot RM, et al. . Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia. Neuropharmacology. 2013;73:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taslim N, Saeed Dar M. The role of nicotinic acetylcholine receptor (nAChR) α7 subtype in the functional interaction between nicotine and ethanol in mouse cerebellum. Alcohol Clin Exp Res. 2011;35(3):540–549. [DOI] [PubMed] [Google Scholar]
- 61. Jackson MJ, Swart T, Pearce RK, Jenner P. Cholinergic manipulation of motor disability and L-DOPA-induced dyskinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated common marmosets. J Neural Transm (Vienna). 2014;121(2):163–169. [DOI] [PubMed] [Google Scholar]
- 62. Bordia T, Perez XA, Heiss J, Zhang D, Quik M. Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias. Neurobiol Dis. 2016;91:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci USA. 2011;108:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Scarduzio M, Zimmerman CN, Jaunarajs KL, Wang Q, Standaert DG, McMahon LL. Strength of cholinergic tone dictates the polarity of dopamine D2 receptor modulation of striatal cholinergic interneuron excitability in DYT1 dystonia. Exp Neurol. 2017;295:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martella G, Maltese M, Nisticò R, et al. . Regional specificity of synaptic plasticity deficits in a knock-in mouse model of DYT1 dystonia. Neurobiol Dis. 2014;65:124–132. [DOI] [PubMed] [Google Scholar]
- 66. Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87(3):306–314. [DOI] [PubMed] [Google Scholar]
- 67. Pahwa R, Lyons KE. Treatment of early Parkinson’s disease. Curr Opin Neurol. 2014;27(4):442–449. [DOI] [PubMed] [Google Scholar]
- 68. Jenner P. Treatment of the later stages of Parkinson’s disease - pharmacological approaches now and in the future. Transl Neurodegener. 2015;4 June 25:3. doi: 10.1016/j.nbd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. LeWitt PA, Fahn S. Levodopa therapy for Parkinson disease: a look backward and forward. Neurology. 2016;86 (14 Suppl 1):S3–S12. [DOI] [PubMed] [Google Scholar]
- 70. Perez-Lloret S, Peralta MC, Barrantes FJ. Pharmacotherapies for Parkinson’s disease symptoms related to cholinergic degeneration. Expert Opin Pharmacother. 2016;17(18):2405–2415. [DOI] [PubMed] [Google Scholar]
- 71. Pagano G, Rengo G, Pasqualetti G, et al. . Cholinesterase inhibitors for Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(7):767–773. [DOI] [PubMed] [Google Scholar]
- 72. Marshall J, Schnieden H. Effect of adrenaline, noradrenaline, atropine, and nicotine on some types of human tremor. J Neurol Neurosurg Psychiatry. 1966;29(3):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci. 1993;117(1–2):28–32. [DOI] [PubMed] [Google Scholar]
- 74. Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000;43(1–3):274–282. [PubMed] [Google Scholar]
- 75. Mitsuoka T, Kaseda Y, Yamashita H, et al. . Effects of nicotine chewing gum on UPDRS score and P300 in early-onset parkinsonism. Hiroshima J Med Sci. 2002;51(1):33–39. [PubMed] [Google Scholar]
- 76. Villafane G, Cesaro P, Rialland A, et al. . Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur J Neurol. 2007;14(12):1313–1316. [DOI] [PubMed] [Google Scholar]
- 77. Hanagasi HA, Lees A, Johnson JO, Singleton A, Emre M. Smoking-responsive juvenile-onset Parkinsonism. Mov Disord. 2007;22(1):115–119. [DOI] [PubMed] [Google Scholar]
- 78. Clemens P, Baron JA, Coffey D, Reeves A. The short-term effect of nicotine chewing gum in patients with Parkinson’s disease. Psychopharmacology (Berl). 1995;117(2):253–256. [DOI] [PubMed] [Google Scholar]
- 79. Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology. 2001;57(6):1032–1035. [DOI] [PubMed] [Google Scholar]
- 80. Shoulson I. Randomized placebo-controlled study of the nicotinic agonist SIB-1508Y in Parkinson disease. Neurology. 2006;66(3):408–410. [DOI] [PubMed] [Google Scholar]
- 81. Ebersbach G, Stöck M, Müller J, Wenning G, Wissel J, Poewe W. Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord. 1999;14(6):1011–1013. [DOI] [PubMed] [Google Scholar]
- 82. Villafane G, Thiriez C, Audureau E, et al. . High-dose transdermal nicotine in Parkinson’s disease patients: a randomized, open-label, blinded-endpoint evaluation phase 2 study. Eur J Neurol. 2018;25(1):120–127. [DOI] [PubMed] [Google Scholar]
- 83. Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327(1):239–247. [DOI] [PubMed] [Google Scholar]
- 84. Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol. 2007;62(6):588–596. [DOI] [PubMed] [Google Scholar]
- 85. Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. . Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72(6):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26 (Suppl 1):S1–S58. [DOI] [PubMed] [Google Scholar]
- 87. Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010;25 (Suppl 1):S58–S62. [DOI] [PubMed] [Google Scholar]
- 88. Searles Nielsen S, Gallagher LG, Lundin JI, et al. . Environmental tobacco smoke and Parkinson’s disease. Mov Disord. 2012;27(2):293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Elbaz A, Moisan F. Update in the epidemiology of Parkinson’s disease. Curr Opin Neurol. 2008;21(4):454–460. [DOI] [PubMed] [Google Scholar]
- 90. Tanner CM, Goldman SM, Aston DA, et al. . Smoking and Parkinson’s disease in twins. Neurology. 2002;58(4):581–588. [DOI] [PubMed] [Google Scholar]
- 91. Quik M, O’Neill M, Perez XA. Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci. 2007;28(5):229–235. [DOI] [PubMed] [Google Scholar]
- 92. Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bastide MF, Meissner WG, Picconi B, et al. . Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol. 2015;132:96–168. [DOI] [PubMed] [Google Scholar]
- 94. Rascol O, Perez-Lloret S, Ferreira JJ. New treatments for levodopa-induced motor complications. Mov Disord. 2015;30(11):1451–1460. [DOI] [PubMed] [Google Scholar]
- 95. Iravani MM, McCreary AC, Jenner P. Striatal plasticity in Parkinson’s disease and L-dopa induced dyskinesia. Parkinsonism Relat Disord. 2012;18 (Suppl 1):S123–S125. [DOI] [PubMed] [Google Scholar]
- 96. Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65(1):171–222. [DOI] [PubMed] [Google Scholar]
- 97. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–458. [DOI] [PubMed] [Google Scholar]
- 98. Pahwa R, Tanner CM, Hauser RA, et al. . Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED Study). Mov Disord. 2015;30(6):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Oertel W, Eggert K, Pahwa R, et al. . Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord. 2017;32(12):1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Larson PS. Deep brain stimulation for movement disorders. Neurotherapeutics. 2014;11(3):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Perestelo-Pérez L, Rivero-Santana A, Pérez-Ramos J, Serrano-Pérez P, Panetta J, Hilarion P. Deep brain stimulation in Parkinson’s disease: meta-analysis of randomized controlled trials. J Neurol. 2014;261(11):2051–2060. [DOI] [PubMed] [Google Scholar]
- 102. Aviles-Olmos I, Kefalopoulou Z, Tripoliti E, et al. . Long-term outcome of subthalamic nucleus deep brain stimulation for Parkinson’s disease using an MRI-guided and MRI-verified approach. J Neurol Neurosurg Psychiatry. 2014;85(12):1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Feyder M, Bonito-Oliva A, Fisone G. L-DOPA-induced dyskinesia and abnormal signaling in striatal medium spiny neurons: focus on dopamine D1 receptor-mediated transmissioN. Front Behav Neurosci. 2011;5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aubert I, Guigoni C, Håkansson K, et al. . Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57(1):17–26. [DOI] [PubMed] [Google Scholar]
- 105. Suárez LM, Solís O, Caramés JM, et al. . L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry. 2014;75(9):711–722. [DOI] [PubMed] [Google Scholar]
- 106. Calabresi P, Di Filippo M, Ghiglieri V, Picconi B. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov Disord. 2008;23 (Suppl 3):S570–S579. [DOI] [PubMed] [Google Scholar]
- 107. Sgambato-Faure V, Cenci MA. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson’s disease. Prog Neurobiol. 2012;96(1):69–86. [DOI] [PubMed] [Google Scholar]
- 108. Al Dakheel A, Beaulieu-Boire I, Fox SH. Emerging drugs for levodopa-induced dyskinesia. Expert Opin Emerg Drugs. 2014;19(3):415–429. [DOI] [PubMed] [Google Scholar]
- 109. Won L, Ding Y, Singh P, Kang UJ. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. J Neurosci. 2014;34(8):3090–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333(3):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Quik M, Mallela A, Ly J, Zhang D. Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2013;28(10):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. Nicotine-mediated improvement in L-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis. 2013;50:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mather J, Burdette, Cebers, et al. Potential of AZD1446, a novel nicotinic agonist, for the treatment of L-DOPA-induced dyskinesia in Parkinson’s disease. Society for Neuroscience Abstr. 2014;43:137. [Google Scholar]
- 114. Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84(4):329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Buccafusco JJ, Beach JW, Terry AV Jr. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328(2):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kihara T, Shimohama S, Sawada H, et al. . Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276(17):13541–13546. [DOI] [PubMed] [Google Scholar]
- 117. Shimohama S, Kihara T. Nicotinic receptor-mediated protection against beta-amyloid neurotoxicity. Biol Psychiatry. 2001;49(3):233–239. [DOI] [PubMed] [Google Scholar]
- 118. Ren K, Puig V, Papke RL, Itoh Y, Hughes JA, Meyer EM. Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J Neurochem. 2005;94(4):926–933. [DOI] [PubMed] [Google Scholar]
- 119. Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: Cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20(12):2093–2101. [DOI] [PubMed] [Google Scholar]
- 120. Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J Biol Chem. 2002;277(47):44920–44924. [DOI] [PubMed] [Google Scholar]
- 121. Toborek M, Son KW, Pudelko A, King-Pospisil K, Wylegala E, Malecki A. ERK ½ signaling pathway is involved in nicotine-mediated neuroprotection in spinal cord neurons. J Cell Biochem. 2007;100(2):279–292. [DOI] [PubMed] [Google Scholar]
- 122. Ren K, King MA, Liu J, et al. . The alpha7 nicotinic receptor agonist 4OH-GTS-21 protects axotomized septohippocampal cholinergic neurons in wild type but not amyloid-overexpressing transgenic mice. Neuroscience. 2007;148(1):230–237. [DOI] [PubMed] [Google Scholar]
- 123. Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem Int. 2010;56(1):135–142. [DOI] [PubMed] [Google Scholar]
- 124. Parada E, Egea J, Romero A, del Barrio L, García AG, López MG. Poststress treatment with PNU282987 can rescue SH-SY5Y cells undergoing apoptosis via α7 nicotinic receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic Biol Med. 2010;49(11):1815–1821. [DOI] [PubMed] [Google Scholar]
- 125. Gershanik OS, Gómez Arévalo GJ. Typical and atypical neuroleptics. Handb Clin Neurol. 2011;100:579–599. [DOI] [PubMed] [Google Scholar]
- 126. Tarsy D, Lungu C, Baldessarini RJ. Epidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugs. Handb Clin Neurol. 2011;100:601–616. [DOI] [PubMed] [Google Scholar]
- 127. Zupancic ML. Role of atypical antipsychotics in rapid cycling bipolar disorder: A review of the literature. Ann Clin Psychiatry. 2011;23(2):141–149. [PubMed] [Google Scholar]
- 128. Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. J Manag Care Pharm. 2012;18(5 Suppl B):S1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Khouzam HR. Identification and management of tardive dyskinesia: A case series and literature review. Postgrad Med. 2015;127(7):726–737. [DOI] [PubMed] [Google Scholar]
- 130. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses. 2010;4(1):56–73. [DOI] [PubMed] [Google Scholar]
- 132. Turrone P, Remington G, Kapur S, Nobrega JN. The relationship between dopamine D2 receptor occupancy and the vacuous chewing movement syndrome in rats. Psychopharmacology (Berl). 2003;165(2):166–171. [DOI] [PubMed] [Google Scholar]
- 133. Lockwood JT, Remington G. Emerging drugs for antipsychotic-induced tardive dyskinesia: Investigational drugs in Phase II and Phase III clinical trials. Expert Opin Emerg Drugs. 2015;20(3):407–421. [DOI] [PubMed] [Google Scholar]
- 134. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: A systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414–425. [DOI] [PubMed] [Google Scholar]
- 135. Peluso MJ, Lewis SW, Barnes TR, Jones PB. Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. Br J Psychiatry. 2012;200(5):387–392. [DOI] [PubMed] [Google Scholar]
- 136. Woods SW, Morgenstern H, Saksa JR, et al. . Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: A prospective cohort study. J Clin Psychiatry. 2010;71(4):463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21(2):151–156. [DOI] [PubMed] [Google Scholar]
- 138. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: Therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Anderson SM, Brunzell DH. Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS One. 2012;7(11):e48665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fernandez HH, Factor SA, Hauser RA, et al. . Randomized controlled trial of deutetrabenazine for tardive dyskinesia: The ARM-TD study. Neurology. 2017;88(21):2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bordia T, McIntosh JM, Quik M. Nicotine reduces antipsychotic-induced orofacial dyskinesia in rats. J Pharmacol Exp Ther. 2012;340(3):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J Pharmacol Exp Ther. 2011;339(1):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Creed MC, Hamani C, Bridgman A, Fletcher PJ, Nobrega JN. Contribution of decreased serotonin release to the antidyskinetic effects of deep brain stimulation in a rodent model of tardive dyskinesia: Comparison of the subthalamic and entopeduncular nuclei. J Neurosci. 2012;32(28):9574–9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Creed-Carson M, Oraha A, Nobrega JN. Effects of 5-HT(2A) and 5-HT(2C) receptor antagonists on acute and chronic dyskinetic effects induced by haloperidol in rats. Behav Brain Res. 2011;219(2):273–279. [DOI] [PubMed] [Google Scholar]
- 145. Naidu PS, Kulkarni SK. Effect of 5-HT1A and 5-HT2A/2C receptor modulation on neuroleptic-induced vacuous chewing movements. Eur J Pharmacol. 2001;428(1):81–86. [DOI] [PubMed] [Google Scholar]
- 146. Aubin HJ, Rollema H, Svensson TH, Winterer G. Smoking, quitting, and psychiatric disease: A review. Neurosci Biobehav Rev. 2012;36(1):271–284. [DOI] [PubMed] [Google Scholar]
- 147. Mobascher A, Winterer G. The molecular and cellular neurobiology of nicotine abuse in schizophrenia. Pharmacopsychiatry. 2008;41 (Suppl 1):S51–S59. [DOI] [PubMed] [Google Scholar]
- 148. Nilsson A, Waller L, Rosengren A, Adlerberth A, Wilhelmsen L. Cigarette smoking is associated with abnormal involuntary movements in the general male population–a study of men born in 1933. Biol Psychiatry. 1997;41(6):717–723. [DOI] [PubMed] [Google Scholar]
- 149. Yassa R, Lal S, Korpassy A, Ally J. Nicotine exposure and tardive dyskinesia. Biol Psychiatry. 1987;22(1):67–72. [DOI] [PubMed] [Google Scholar]
- 150. Menza MA, Grossman N, Van Horn M, Cody R, Forman N. Smoking and movement disorders in psychiatric patients. Biol Psychiatry. 1991;30(2):109–115. [DOI] [PubMed] [Google Scholar]
- 151. Zhang XY, Yu YQ, Sun S, et al. . Smoking and tardive dyskinesia in male patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1765–1769. [DOI] [PubMed] [Google Scholar]
- 152. Bordia T, Zhang D, Perez XA, Quik M. Striatal cholinergic interneurons and D2 receptor-expressing GABAergic medium spiny neurons regulate tardive dyskinesia. Exp Neurol. 2016;286:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Münchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology. 2013;68:143–149. [DOI] [PubMed] [Google Scholar]
- 154. Thomas R, Cavanna AE. The pharmacology of Tourette syndrome. J Neural Transm (Vienna). 2013;120(4):689–694. [DOI] [PubMed] [Google Scholar]
- 155. Termine C, Selvini C, Rossi G, Balottin U. Emerging treatment strategies in Tourette syndrome: What’s in the pipeline?Int Rev Neurobiol. 2013;112:445–480. [DOI] [PubMed] [Google Scholar]
- 156. Silay YS, Jankovic J. Emerging drugs in Tourette syndrome. Expert Opin Emerg Drugs. 2005;10(2):365–380. [DOI] [PubMed] [Google Scholar]
- 157. Udvardi PT, Nespoli E, Rizzo F, Hengerer B, Ludolph AG. Nondopaminergic neurotransmission in the pathophysiology of Tourette syndrome. Int Rev Neurobiol. 2013;112:95–130. [DOI] [PubMed] [Google Scholar]
- 158. Xu M, Kobets A, Du JC, et al. . Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci USA. 2015;112(3):893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lennington JB, Coppola G, Kataoka-Sasaki Y, et al. . Transcriptome analysis of the human striatum in Tourette syndrome. Biol Psychiatry. 2016;79(5):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sanberg PR, McConville BJ, Fogelson HM, et al. . Nicotine potentiates the effects of haloperidol in animals and in patients with Tourette syndrome. Biomed Pharmacother. 1989;43(1):19–23. [DOI] [PubMed] [Google Scholar]
- 161. McConville BJ, Fogelson MH, Norman AB, et al. . Nicotine potentiation of haloperidol in reducing tic frequency in Tourette’s disorder. Am J Psychiatry. 1991;148(6):793–794. [DOI] [PubMed] [Google Scholar]
- 162. Dursun SM, Reveley MA, Bird R, Stirton F. Longlasting improvement of Tourette’s syndrome with transdermal nicotine. Lancet. 1994;344(8936):1577. [DOI] [PubMed] [Google Scholar]
- 163. Silver AA, Shytle RD, Philipp MK, Sanberg PR. Case study: Long-term potentiation of neuroleptics with transdermal nicotine in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35(12):1631–1636. [DOI] [PubMed] [Google Scholar]
- 164. Dursun SM, Kutcher S. Smoking, nicotine and psychiatric disorders: Evidence for therapeutic role, controversies and implications for future research. Med Hypotheses. 1999;52(2):101–109. [DOI] [PubMed] [Google Scholar]
- 165. Silver AA, Shytle RD, Philipp MK, Wilkinson BJ, McConville B, Sanberg PR. Transdermal nicotine and haloperidol in Tourette’s disorder: A double-blind placebo-controlled study. J Clin Psychiatry. 2001;62(9):707–714. [DOI] [PubMed] [Google Scholar]
- 166. Cubo E, Fernández Jaén A, Moreno C, Anaya B, González M, Kompoliti K. Donepezil use in children and adolescents with tics and attention-deficit/hyperactivity disorder: An 18-week, single-center, dose-escalating, prospective, open-label study. Clin Ther. 2008;30(1):182–189. [DOI] [PubMed] [Google Scholar]
- 167. Sanberg PR, Vindrola-Padros C, Shytle RD. Translating laboratory discovery to the clinic: From nicotine and mecamylamine to Tourette’s, depression, and beyond. Physiol Behav. 2012;107(5):801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shytle RD, Silver AA, Sanberg PR. Comorbid bipolar disorder in Tourette’s syndrome responds to the nicotinic receptor antagonist mecamylamine (Inversine). Biol Psychiatry. 2000;48(10):1028–1031. [DOI] [PubMed] [Google Scholar]
- 169. Trujillo-Martín MM, Serrano-Aguilar P, Monton-Alvarez F, Carrillo-Fumero R. Effectiveness and safety of treatments for degenerative ataxias: A systematic review. Mov Disord. 2009;24(8):1111–1124. [DOI] [PubMed] [Google Scholar]
- 170. Kark RA, Budelli MM, Wachsner R. Double-blind, triple-crossover trial of low doses of oral physostigmine in inherited ataxias. Neurology. 1981;31(3):288–292. [DOI] [PubMed] [Google Scholar]
- 171. Kark RA, Blass JP, Spence MA. Physostigmine in familial ataxias. Neurology. 1977;27(1):70–72. [DOI] [PubMed] [Google Scholar]
- 172. Rodriguez-Budelli MM, Kark RA, Blass JP, Spence MA. Action of physostigmine on inherited ataxias. Adv Neurol. 1978;21:195–202. [PubMed] [Google Scholar]
- 173. Wessel K, Langenberger K, Nitschke MF, Kömpf D. Double-blind crossover study with physostigmine in patients with degenerative cerebellar diseases. Arch Neurol. 1997;54(4):397–400. [DOI] [PubMed] [Google Scholar]
- 174. Livingstone IR, Mastaglia FL, Pennington RJ, Skilbeck C. Choline chloride in the treatment of cerebellar and spinocerebellar ataxia. J Neurol Sci. 1981;50(2):161–174. [DOI] [PubMed] [Google Scholar]
- 175. Blattel RA. Use of choline in the treatment of ataxia associated with multiple sclerosis. Can Med Assoc J. 1979;121(12):1568. [PMC free article] [PubMed] [Google Scholar]
- 176. Legg NJ. Oral choline in cerebellar ataxia. Br Med J. 1978;2(6149):1403–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Philcox DV, Kies B. Choline in hereditary ataxia. Br Med J. 1979;2(6190):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zesiewicz TA, Sullivan KL. Treatment of ataxia and imbalance with varenicline (chantix): Report of 2 patients with spinocerebellar ataxia (types 3 and 14). Clin Neuropharmacol. 2008;31(6):363–365. [DOI] [PubMed] [Google Scholar]
- 179. Zesiewicz TA, Sullivan KL, Freeman A, Juncos JL. Treatment of imbalance with varenicline Chantix®: Report of a patient with fragile X tremor/ataxia syndrome. Acta Neurol Scand. 2009;119(2):135–138. [DOI] [PubMed] [Google Scholar]
- 180. Zesiewicz TA, Sullivan KL, Gooch CL, Lynch DR. Subjective improvement in proprioception in 2 patients with atypical Friedreich ataxia treated with varenicline (Chantix). J Clin Neuromuscul Dis. 2009;10(4):191–193. [DOI] [PubMed] [Google Scholar]
- 181. Zesiewicz TA, Greenstein PE, Sullivan KL, et al. . A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78(8):545–550. [DOI] [PubMed] [Google Scholar]
- 182. Connolly BS, Prashanth LK, Shah BB, Marras C, Lang AE. A randomized trial of varenicline (chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;79(22):2218. [DOI] [PubMed] [Google Scholar]
- 183. Al-Rejaie S, Dar MS. Behavioral interaction between nicotine and ethanol: Possible modulation by mouse cerebellar glutamate. Alcohol Clin Exp Res. 2006;30(7):1223–1233. [DOI] [PubMed] [Google Scholar]
- 184. Taslim N, Al-Rejaie S, Saeed Dar M. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: A functional interaction. Neuroscience. 2008;157(1):204–213. [DOI] [PubMed] [Google Scholar]
- 185. Brann MR, Ellis J, Jørgensen H, Hill-Eubanks D, Jones SV. Muscarinic acetylcholine receptor subtypes: Localization and structure/function. Prog Brain Res. 1993;98:121–127. [DOI] [PubMed] [Google Scholar]
- 186. Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012;(208):223–241. [DOI] [PubMed] [Google Scholar]
- 187. Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lv X, Dickerson JW, Rook JM, Lindsley CW, Conn PJ, Xiang Z. M1muscarinic activation induces long-lasting increase in intrinsic excitability of striatal projection neurons. Neuropharmacology. 2017;118:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: Light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14(5 Pt 2):3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22(15):6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Petkova-Kirova P, Giovannini MG, Kalfin R, Rakovska A. Modulation of acetylcholine release by cholecystokinin in striatum: Receptor specificity; role of dopaminergic neuronal activity. Brain Res Bull. 2012;89(5-6):177–184. [DOI] [PubMed] [Google Scholar]
- 192. Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22(5):1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bonsi P, Martella G, Cuomo D, et al. . Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci. 2008;28(24):6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lachowicz JE, Duffy RA, Ruperto V, et al. . Facilitation of acetylcholine release and improvement in cognition by a selective M2 muscarinic antagonist, SCH 72788. Life Sci. 2001;68(22–23):2585–2592. [DOI] [PubMed] [Google Scholar]
- 195. Threlfell S, Clements MA, Khodai T, et al. . Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30(9):3398–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kravitz AV, Freeze BS, Parker PR, et al. . Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Maurice N, Liberge M, Jaouen F, et al. . Striatal cholinergic interneurons control motor behavior and basal ganglia function in experimental parkinsonism. Cell Rep. 2015;13(4):657–666. [DOI] [PubMed] [Google Scholar]
- 198. Bohnen NI, Albin RL, Müller ML, Chou K. Advances in therapeutic options for gait and balance in Parkinson’s disease. US Neurol. 2011;7(2):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26(14):2496–2503. [DOI] [PubMed] [Google Scholar]
- 200. Young MF, Wecker L. Regulation of gait and balance: The underappreciated role of neuronal nicotinic receptor agonists. Curr Pharm Des. 2016;22(14):1998–2003. [DOI] [PubMed] [Google Scholar]
- 201. Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75(14):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Janickova H, Rosborough K, Al-Onaizi M, et al. . Deletion of the vesicular acetylcholine transporter from pedunculopontine/laterodorsal tegmental neurons modifies gait. J Neurochem. 2017;140(5):787–798. [DOI] [PubMed] [Google Scholar]
- 203. Falkenburger B. ExPPNing how acetylcholine improves gait in Parkinson’s disease: An editorial highlight for ‘deletion of the vesicular acetylcholine transporter from pedunculopontine/laterodorsal tegmental neurons modifies gait’. J Neurochem. 2017;140(5):688–691. [DOI] [PubMed] [Google Scholar]
- 204. Jankelowitz SK. Treatment of neurolept-induced tardive dyskinesia. Neuropsychiatr Dis Treat. 2013;9:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ogino S, Miyamoto S, Miyake N, Yamaguchi N. Benefits and limits of anticholinergic use in schizophrenia: Focusing on its effect on cognitive function. Psychiatry Clin Neurosci. 2014;68(1):37–49. [DOI] [PubMed] [Google Scholar]
- 206. Jankovic J. Medical treatment of dystonia. Mov Disord. 2013;28(7):1001–1012. [DOI] [PubMed] [Google Scholar]