Abstract
High levels of uric acid are associated with greater risk of stress-related cardiovascular illnesses that occur disproportionately among African Americans. Whether hyperuricemia affects biological response to acute stress remains largely unknown, suggesting a need to clarify this potential connection. The current study examined how salivary uric acid (sUA) is associated with resting and reactive blood pressure – two robust predictors of hypertension and related cardiovascular disease and disparity. Healthy African Americans (N=107; 32% male; M age=31.74 years), completed the Trier Social Stress Test to induce social-evaluative stress. Systolic and diastolic blood pressure readings were recorded before, during, and after the task to assess resting and reactive change in blood pressure. Participants also provided a saliva sample at baseline that was assayed for sUA. At rest, and controlling for age, sUA was modestly associated with higher systolic (r = .201, p = .044), but not diastolic (r = .100, p = .319) blood pressure. In response to the stressor task, and once again controlling for age, sUA was also associated with higher total activation of both systolic (r = .219, p = .025) and diastolic blood pressure (r = .198, p < .044). A subsequent moderation analysis showed that associations between sUA and BP measures were significant for females, but not for males. Findings suggest that uric acid may be implicated in hypertension and cardiovascular health disparities through associations with elevated blood pressure responses to acute social stress, and that low levels of uric acid might be protective, particularly for females.
Keywords: Uric Acid, Trier Social Stress Test, Cardiovascular Illness, Blood Pressure, Health Disparities, Stress Reactivity
1. Introduction
In the United States, cardiovascular disease (CVD) disproportionately burdens African Americans (American Heart Association, 2013). In addition to socioeconomic variables such as education and poverty and decreased access to preventive care, psychosocial variables, such as discrimination, are viewed as key determinants of CVD disparities (Adler, 2013; Ladwig et al., 2014). One particularly important precursor to CVD and disparity is psychobiological stress (Dimsdale, 2008). In tandem, African Americans have higher rates of many stress-related CVD risk factors (Blankstein et al., 2011; Flegal, Carroll, Ogden, & Curtin, 2010). This includes hypertension – an especially potent CVD precursor (Mouton, Hayden, & Southerland, 2017). African Americans are likewise over-burdened by prehypertension (PHT) – a modifiable risk factor for hypertension and CVD that carries a considerable potential for intervention (Wang & Wang, 2004). Given these connections and prospects, there is a critical need for further knowledge of the ways in which cognitive and biological stress processes contribute to hypertension, and vice versa.
One crucial, yet perhaps still underappreciated way to enrich current understanding of CVD and disparity is to examine physiological responses that occur within the context of exposure to acute stress (Phillips & Hughes, 2011). The causal role of deregulated stress reactivity in many illnesses is increasingly well-substantiated (Lovallo, 2015), and this especially includes CVD (Lovallo, 2011). Support for links to CVD is provided by the cardiovascular reactivity hypothesis, which holds that reactivity to stressors, if prolonged or exaggerated, can promote hypertension and other physical changes that eventuate in CVD (Obrist, 2012). Meta-analytic evidence confirms that reactivity responses are clinically significant – heightened event phase reactivity and slow stressor event recovery, above and beyond the effects of resting BP, are prospectively linked to CVD, including both hypertension and atherosclerosis (Chida & Steptoe, 2010; Panaite, Salomon, Jin, & Rottenberg, 2015). Thus, an important next step in CVD prevention may be to identify ways to promote adaptive stress reactivity responses (Lovallo, 2011), particularly among at-risk individuals.
In the present study, we advance current understanding of the links between stress, hypertension and CVD by considering connections between uric acid (UA) and reactive blood pressure responses to social evaluative stress. UA is produced primarily in the liver during the breakdown of purine nucleotides (El Ridi & Tallima, 2017). Systemic elevations in UA may be related to dietary behaviors, such as the consumption of high purine foods and fructose, as well as high-fat diets (Dornas 2015; Kanbay 2016). Impairments in renal function may also contribute to high UA levels (Kushiyama 2014; Sah and Qing, 2015). Systemically, UA has several physiologic functions; it is a powerful antioxidant and a mediator and amplifier of the type 2 inflammatory response. Of current interest, UA is especially implicated in hypertension via its capacity to activate the renin-angiotensin system – high levels of uric acid induce growth factors, hormones, and cytokines that activate signal transduction pathways that express inflammation, and that eventuate in increased arterial pressure and hypertension (El Ridi & Tallima, 2017). With these various mechanisms of action, UA has been implicated in the pathophysiology of several diseases, with high levels of UA (hyperuricemia) linked with chronic kidney disease, metabolic syndrome, insulin-resistance, obesity, and Type-2 diabetes (Feig, 2014; Feig, Kang, & Johnson, 2008). Of current interest, hyperuricemia is also strongly associated with hypertension and stroke (for reviews, Feig, 2014; Feig et al., 2008). Whether UA plays a causal role in CVD continues to be debated, though there is growing support for the notion that UA may be an independent risk factor (Feig et al., 2008; Kawai et al., 2012), and that UA may also play a causal role in CVD disparities (Johnson, Titte, Cade, Rideout, & Oliver, 2005). Of relevance to stress, UA has been implicated in mood disorders, such as depression and anxiety disorders (Cheffer et al., 2018), and in regulating the psychobiological response to stress (Goodman et al., 2016).
Although UA has been well linked to many long-term measures of CVD risk, including chronic hypertension, connections between UA and acute stress reactivity are less well understood. Little is yet known about whether UA plays a role in physiological responses that occur in reaction to social evaluative stress. This includes the reactive responses of systolic and diastolic blood pressure (BP), which are likely directly implicated in linking UA to hypertension. Systolic BP, peak pressure in the arteries during the cardiac cycle, is considered to be more strongly linked to CVD risk than diastolic BP, or the lowest pressure at the resting phase of the cardiac cycle (Gerin, Goyal, Mostofsky, & Shimbo, 2008; Kannel, 2000). However, although systolic BP may generally carry greater risk information than diastolic BP, diastolic BP is considered to be a stronger predictor among younger individuals (<40 years; Perry et al. 2000). BP reactivity may play a mechanistic role in hypertension through relaying UA activation of the renin-angiotensin system and suppression of endothelial nitric oxide elaboration, which leads to vasoconstriction. In turn, continual suppression and constriction may eventuate in atherosclerosis, and in long-term, sodium-sensitive hypertension (Feig, 2014). Although there is support for its potential clinical relevance via stress reactivity, and a viable physiological mechanism that implicates hypertension, our review of the literature uncovered only one study that has examined links between UA and BP reactivity in response to acute social stress. Namely, Mrug and colleagues (2017) showed that higher UA excretion, as measured by 12 hour urine collection, predicted greater BP reactivity to acute psychosocial stress, as well as higher resting BP 18 months later, in a predominantly African American sample of urban adolescents. Links between UA and BP reactivity among African American adults remain unknown – better understanding this association appears critical given that incidence of chronic hypertension and CVD both progress with age.
In further connecting UA to the stress reactivity literature, another need involves non-invasive measurement of UA. At present, UA is typically measured using venous blood or urine. However, venipuncture is a relatively invasive procedure that may not be well suited to stress reactivity studies, particularly those conducted with underserved populations, as the invasive process of venipuncture may introduce a stressor that confounds efforts to evaluate acute stress responses (Girgis, Shea, & Husband, 1988). In parallel, although providing a useful measure of sustained UA output, urine collection may be ill-suited to studying rapid change in UA, and standardized collection of urine may not be feasible in all stress reactivity study contexts. One alternative modality that is gaining momentum as a route for noninvasively assessing UA is measurement in oral fluids. Emerging studies confirm a modest-to-strong positive association between circulating and salivary levels of UA (Cheng, Xia, Peng, & Zhou, 2013; Nunes, Brenzikofer, & Macedo, 2011; Soukup et al., 2012; Xia et al., 2012). Indeed, measurement of salivary uric acid (sUA) appears to be both stable and indicative of trait-like individual differences (Riis et al., 2018). Salivary UA levels are also associated with established measures of cardiovascular risk, including BP (Soukup et al., 2012). Taken together, emerging studies illuminate that oral fluid may be ideally suited to evaluating the role of UA in stress reactivity. However, this potential application of sUA has not yet been evaluated.
In the present study, we sought to further consider how biological response to acute social stress might be implicated in CVD disparities. Specifically, we examined whether sUA would be associated with resting BP, and also reactive BP responses to social-evaluative stress. Increasingly, evidence suggests that social stress plays a central role in racial health disparities e.g., Chae, et al., 2014), and that social stress predicts racial health disparities above and beyond socioeconomic stress (for review, Adler & Rehkopf, 2008). We focused on BP given known connections to UA, and also because BP is a robust predictor of hypertension and related CVD and disparity. Moreover, numerous studies have shown that BP is highly reactive to acute stress. A community sample of healthy African Americans completed the Trier Social Stress Test (Kirschbaum, Pirke, & Hellhammer, 1993) to induce social-evaluative stress. Systolic and diastolic blood pressure readings were recorded before, during, and after the task to assess baseline and reactive change in BP. Participants also provided a saliva sample at baseline that was assayed for sUA. Based on the links between UA and cardiovascular risk factors including BP, coupled with positive associations between serum and salivary UA levels, we expected that sUA would be positively associated with both resting and reactive BP responses. With an eye towards literature that has suggested possible sex differences (Fang & Alderman, 2000; Tuttle, Short, & Johnson, 2001), we also conducted a moderation analysis to explore whether links between sUA and BP differed between males and females. Previous studies have shown that men generally have higher UA levels compared to women, but that heightened UA may be a greater CVD risk factor for women compared to men (Baker et al., 2005; Feig, Kang, & Johnson, 2008; Martinez, Ruelas, & Granger, 2017). These latter findings suggest that we may expect to see a stronger association between for sUA and BP for women compared to men.
2. Method
This study was performed in adjunct to alternate considerations of this data (Lucas et al., 2016), after obtaining the subsequently described measurement of sUA. Procedures for recruiting participants and implementing the stressor task are therefore identical to a previous description.
2.1. Participants
Participants were recruited from the Detroit metropolitan community via advertisements and completed a brief online prescreen survey to determine eligibility. Eligibility criteria included being 18 years of age or older and African American, and not taking an interfering medication or having a pre-existing medical or psychiatric condition that would preclude undertaking a minor stress induction. A sample of 118 participants enrolled and completed all study procedures approved by the Institutional Review Board. The present sample was limited to the 104 participants (33 male, 71 female) who had complete data for sUA and BP measures. Data were excluded for 3 participants whose sUA levels were below the acceptable threshold (see Supporting Information for further information). Participants’ age ranged from 17 to 60 years (M = 31.41, SD = 13.84). Table 1 provides additional demographic information. All participants provided informed consent, received modest financial compensation for participating in a single 3-hour laboratory session, and were fully debriefed following study completion.
Table 1.
Sample Characteristics (N = 104).
| Demographic Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 33 (31.7%) |
| Female | 71 (68.3%) |
| Age | |
| 17–20 | 26 (25.0%) |
| 21–30 | 39 (37.5%) |
| 31–40 | 12 (11.5%) |
| 41–50 | 7 (6.7%) |
| 51–60 | 19 (18.3%) |
| Missing | 1 (1.0%) |
| Income | |
| Less than $15,000 | 42 (40.4%) |
| $15,000-$24,999 | 16 (15.4%) |
| $25,000-$34,999 | 12 (11.5%) |
| $35,000-$49,999 | 11 (10.6%) |
| $50,000-$74,999 | 12 (11.5%) |
| $75,000-$99,999 | 7 (7.7%) |
| $100,000 and above | 3 (2.9%) |
| Education | |
| Less than High School | 1 (1.0%) |
| High School/GED | 51 (49.0%) |
| Some College or Trade School | 27 (26.0%) |
| College Graduate | 17 (16.3%) |
| Professional/Advanced Degree | 8 (7.7%) |
2.2. Procedures
2.2.1. Task procedure.
The Trier Social Stress Test (TSST) was used to induce mild psychosocial stress and associated physiological responses (Kirschbaum, Pirke, & Hellhammer, 1993). All sessions were scheduled for late morning or early afternoon. Participants were first given 10 minutes to acclimate, and the remaining TSST protocol was then presented, which included a task description phase, a 10-minute speech preparation period, and a 10-minute performance (5-minute speech and 5-minute arithmetic task) given in front of a 2-person panel, consisting of one male and one female. Participants were given a 1-hour recovery period following task performance.
2.2. Measures
2.2.1. Saliva collection and preparation.
Participants provided at least 2 ml whole saliva by passive drool at baseline, following the 10-minute acclimation period. Two minutes were allotted to saliva collection, and additional time was allotted if participants failed to produce 2 ml. Collection time and volume were recorded. After collection, samples were stored at −80°C until shipped frozen overnight for laboratory analysis. Participants were asked to refrain from consuming food, caffeine, citric drinks and dairy, and to avoid exercise or brushing teeth in the 30 minutes prior to saliva collection, and to report adherence to these guidelines. Participants also self-reported oral health by answering four yes-no questions: ‘did you brush your teeth today?’ (Yes = 63, No=41) ‘did your gums bleed today? (Yes=11, No=93), ‘do you have any mouth bruises?’ (Yes = 4, No = 100) and ‘have you had any recent dental work?’ (Yes = 1, No = 103). Oral health variables were subsequently probed for potential associations with sUA (See Supporting Information).
2.2.2. Salivary uric acid determination.
Saliva samples were collected in accordance with guidelines set forth by previous research (Granger, Hibel, Fortunato, & Kapelewski, 2009; Granger et al., 2012; Riis et al., 2018). Prior research has indicated that the serum to saliva correlation of UA is robust (r = .69; Riis et al., 2018). Saliva samples were assayed in duplicate for sUA using a commercially available enzymatic reaction kit specifically designed for use with saliva and following the manufacturer’s instructions (Catalog #1–3802, Salimetrics, Carlsbad, CA). Samples were frozen, thawed, and centrifuged at 1500 g for 15 minutes prior to assay in order to remove mucins. The sUA test kit enables detection of UA in saliva through production of a red chromogen after brief incubation, which is measured at a wavelength of 515 nm. The amount of UA present in saliva is directly proportional to the increase in wavelength absorbance. The test volume was 10 μl and the lower limit of detection (LLD) was 0.07 mg/dL. The average of duplicate tests was used in subsequently described statistical analyses. On average, the intraassay CV was less than 5% and the inter-assay CV less than 10%.
2.2.3. Blood pressure measurement.
Resting and reactive blood pressure were measured using a Dinamap 8100 (Critikon, Tampa, FL). The Dinamap 8100 is a fully portable, non-invasive blood pressure device that measures systolic and diastolic pressure, as well as pulse rate and mean arterial pressure, using the oscillometric technique. This instrument has been used and evaluated in numerous studies and has achieved acceptable or better standards in a vast majority according to accuracy criteria established by the British Hypertensive Society (BHS) and the Association for the Advancement of Medical Instrumentation (Jin, Donaghue, Fairchild, Chan, & Silink, 2001). Blood pressure readings were taken following the protocol established by the BHS (Gerin, Goyal, Mostofsky, & Shimbo, 2008). The blood pressure cuff was applied to participants’ non-dominant arm, and the lower edge of the cuff was placed 2 cm above the elbow crease, with the marked arrow placed over the brachial artery. An appropriate cuff size was selected using measurement of the mid-upper arm circumference, and the cuff was wrapped sufficiently tight to allow two fingers to be inserted at the top and bottom.
Blood pressure readings were collected from each participant at six time points that corresponded to resting, reactive, and recovery periods (Lucas et al., 2016). Readings were collected in triplicate at one minute intervals for all six time points. The initial (resting) blood pressure measurement occasion was taken following the 10-minute acclimation period. The second and third occasions occurred immediately before and after the TSST performance. Occasions 4 through 6 took place during the recovery period –15, 30, and 60 minutes after task completion. Prior to taking measurements at all occasions, participants were comfortably seated, with their feet flat to the floor, and the arm was raised to heart level and supported. Participants were also instructed to relax and not to speak during blood pressure measurements. An average systolic and diastolic reading was calculated at each timepoint using all three readings. Table 2 presents systolic and diastolic blood pressure readings across all six timepoints, as well as bivariate correlations and coefficients of variation.
Table 2.
Systolic and Diastolic Blood Pressure Across Stress Reactivity Timepoints.
| M (SD) | 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|---|
| 1. T1 Systolic BP | 120.54 (16.62) | 3.625 | |||||
| 2. T2 Systolic BP | 128.93 (17.67) | .88** | 3.510 | ||||
| 3. T3 Systolic BP | 129.94 (16.70) | .82* | .93** | 4.053 | |||
| 4. T4 Systolic BP | 121.50 (16.35) | .85** | .85** | .86**** | 4.742 | ||
| 5. T5 Systolic BP | 121.03 (16.16) | .87** | .86** | .87** | .89** | 3.899 | |
| 6. T6 Systolic BP | 121.43 (16.52) | .86** | .84** | .85** | .87** | .92** | 4.127 |
| 1. T1 Diastolic BP | 66.47 (11.84) | 5.944 | |||||
| 2. T2 Diastolic BP | 72.15 (11.73) | .87** | 5.904 | ||||
| 3. T3 Diastolic BP | 73.38 (12.42) | .85** | .90** | 5.682 | |||
| 4. T4 Diastolic BP | 69.82 (12.33) | .90** | .85** | .86** | 5.680 | ||
| 5. T5 Diastolic BP | 69.20 (11.99) | .87** | .85** | .84** | .92** | 6.293 | |
| 6. T6 Diastolic BP | 69.18 (12.90) | .80** | .79** | .80** | .85** | .88** | 5.460 |
Coefficients of variation presented on diagonal.
p < .05.
p < .01.
2.4. Statistical analysis
We considered associations between sUA and resting systolic and diastolic BP using readings collected at the first (resting) timepoint. To assess associations between sUA and overall BP reactivity, total activation measures were calculated for systolic and diastolic BP. These summations were calculated across the six measurements using a well-established area under the curve (AUC) method of integration and mathematical formulas developed specifically for use in biological reactivity paradigms (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). This approach uses a trapezoidal method to summarize changes across time for each individual participant, and we calculated total activation (AUCg) to represent total reactivity.
We conducted two-step hierarchical multiple regression analyses to assess the effect of sUA on baseline (time 1 systolic and diastolic) and total activation (AUCg systolic and diastolic) BP measures. Significance was assessed using R2 change and individual regression weights of predictors newly entered at each step. Age was entered as a covariate on the first step of each regression. This study also included two minor variations to the traditional TSST protocol, which were fully crossed and simultaneously implemented ten minutes prior to the fourth salivary collection timepoint (i.e. recovery phase). A substantive consideration of these manipulations is provided elsewhere (Lucas et al., 2016; Lucas, Pierce, et al., 2017). For present purposes, the two experimental variations were also entered as covariates on the first step for AUCg regression models. For all regression models, sUA was entered on the second step.
Finally, we conducted moderation analyses using the PROCESS macro for SPSS (Hayes, 2013) to determine whether sex moderated the association between sUA and each indicator of BP. Models with baseline BP specified as the dependent variable statistically controlled for age, and models with AUCg BP specified as the dependent variable controlled for age and the two experimental manipulations. Significance was determined through 95% bias-corrected bootstrapped confidence intervals based on 5,000 bootstrapped samples. Confidence intervals that do not contain zero are statistically significant at p < .05.
Prior to analysis, sUA and BP measures were assessed for univariate normality, and both measures showed significant positive skew. Thus, the subsequently presented statistical analyses were also conducted using variable transformations to correct for skew (see Supporting Information).
3. Results
3.1. Preliminary analyses
Zero order and partial correlations were first computed to evaluate the associations between sUA and BP measures, with and without controlling for effects of age, which was positively associated with sUA (r = .220, p = .025), baseline BP (rsystolic = .495, p < .001; rdiastolic = .524, p < .001) and reactive BP (rsystolic = .462, p < .001; rdiastolic = .527, p < .001). As seen in Table 3, zero order correlations indicated that sUA was significantly positively associated with baseline systolic BP (p < .001), baseline diastolic BP (p = .039), reactive systolic BP (p < .001), and reactive diastolic BP (p = .001). Associations with baseline systolic BP (p = .044), reactive systolic BP (p = .008), and reactive diastolic BP (p = .014) remained significant once age was controlled, whereas baseline diastolic BP did not (p = .319). As shown in Table 4, sUA also remained significantly associated with both reactive systolic BP (ΔR2 = .05; p = .012) and diastolic BP (ΔR2 = .05; p = .010) after accounting for age as well as TSST protocol variations entered on Step 1.
Table 3.
Correlations between salivary uric acid and blood pressure (N = 104)
| M (SD) | Zero-order correlations | Partials correlations controlling for age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 1. | 2. | 3. | 4. | 5. | ||
| 1. sUA | 2.67 (1.88) | - | - | ||||||||
| 2. Systolic BP | 120.88 (16.55) | .34** | - | .20* | - | ||||||
| 3. Diastolic BP | 66.77 (11.94) | .20* | .71** | - | .10 | .61** | - | ||||
| 4. AUCg Systolic PB | 17711.36 (3015.41) | .37** | .84** | .62** | - | .26** | .78** | .50** | - | ||
| 5. AUCg Diastolic BP | 10023.64 (1921.52) | .31** | .71** | .86** | .84** | - | .24* | .62** | .80** | .80** | - |
p < .05.
p < .01.
Table 4.
Hierarchical regression results for sUA predicting BP
| Baseline Systolic BP | Baseline Diastolic BP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p | F | R2 | Δ R2 | β | p | F | R2 | Δ R2 | |
| Step 1 | 33.71** | .25 | - | 39.91** | .28 | - | ||||
| Age | .50 | < .01** | .53 | < .01** | ||||||
| Step 2 | 19.55** | .28 | .03* | 20.39** | .29 | .01 | ||||
| Age | .46 | < .01** | .52 | < .01** | ||||||
| sUA | .18 | .04* | .08 | .34 | ||||||
| AUCg Systolic BP | AUCg Diastolic BP | |||||||||
| β | p | F | R2 | Δ R2 | β | p | F | R2 | Δ R2 | |
| Step 1 | 9.75** | .23 | - | 13.22** | .29 | - | ||||
| Age | .45 | < .01** | .52 | < .01** | ||||||
| Proc. | −.13 | .16 | −.03 | .71 | ||||||
| Dist. | −.004 | .97 | .07 | .40 | ||||||
| Step 2 | 9.37** | .28 | .05* | 12.22** | .34 | .05* | ||||
| Age | .40 | < .01** | .47 | < .01** | ||||||
| Proc. | −.10 | .28 | −.003 | .97 | ||||||
| Dist. | .03 | .74 | .11 | .22 | ||||||
| sUA | .23 | .01* | .23 | .01* | ||||||
Note. p < .05.
p < .01.
sUA = salivary uric acid. Proc = procedural fairness manipulation. Dist. = distributive justice manipulation.
3.2. Moderation by Sex.
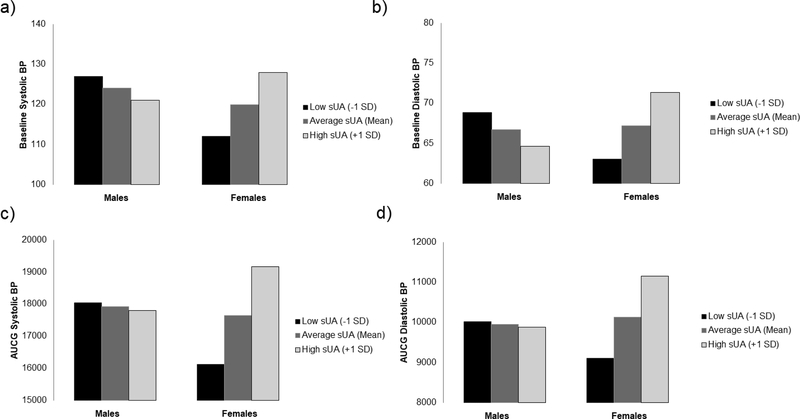
Next, we tested whether sex moderated the associations between sUA and BP indicators. Results revealed a significant interaction for baseline systolic BP, B = 3.08, SE = .74, p < .001, 95% CI [1.62, 5.55]. As shown in Figure 1A, sUA was significantly positively associated with baseline systolic BP for females (effect = 4.49, SE = 1.04, p < .001; 95% CI [2.42, 5.67]), but was unrelated to baseline systolic BP for males (effect = −1.68, SE = 1.06, p = .118, 95% CI [3.79, 0.43]). In tandem, Figure 1A shows there was little difference between males and females when sUA was average (mean) or high (+1 SD), whereas females had considerably lower BP when sUA levels were low (−1 SD; Cohen, Cohen, West, & Aiken, 2003). Results for baseline diastolic BP were consistent with baseline systolic BP, as the interaction was again significant, B = 1.77, SE = .56, p = .002, 95% CI [.66, 2.88]. As seen in Figure 1B, sUA and baseline diastolic BP were also positively associated for females (effect = 2.35, SE = 0.79, p = .004; 95% CI [0.77, 3.92]), but unrelated for males (effect = −1.20, SE = 0.81, p = .143; 95% CI [−2.80, 0.41]).
Figure 1.
Moderating effect of sex on association between sUA and BP
Moderation analyses were repeated with reactive systolic and diastolic BP. Consistent with the baseline BP models, the interaction was significant for reactive systolic BP, B = 469.83, SE = 146.61, p = .002, 95% CI [178.77, 760.89], and reactive diastolic BP, B = 308.39, SE = 90.18, p < .001, 95% CI [129.37, 487.41]. As seen in Figure 1C, sUA and reactive systolic BP were positively associated for females (effect = 866.82, SE = 211.62, p < .001; 95% CI [446.71, 1286.94]), but were unrelated for males (effect = −72.84, SE = 202.41, p = .720; 95% CI [474.68, 329.00]). As seen in 1D, sUA and reactive diastolic BP were also positively associated for females (effect = 579.52, SE = 130.16, p < .001; 95% CI [321.12, 837.92]), but were unrelated for males (effect = −37.26, SE = 124.50, p = .765; 95% CI [−284.42, 209.90]).
4. Discussion
This study adds to a nascent, but growing literature that is exploring how UA connects to CVD disparity. Prior research has established that high UA is associated with greater CVD risk (Fieg et al., 2008), and more recent research highlights that UA may be implicated especially through hypertension (Loeffler, Navas-Acien, Brady, Miller, & Fadrowski, 2012; Viazzi et al., 2013). Adding to this literature, our study is among the first to demonstrate that UA may play a role in CVD among African Americans through connections to increased BP reactivity that occurs in response to social evaluative threat, as UA appears to be positively associated with acute BP responses (see also, Mrug et al., 2017). This hitherto underappreciated connection may be important, as accumulating evidence underscores that stress reactivity may eventuate in hypertension, and thus contribute to extant CVD (Panaite et al., 2015). Moreover, hypertension is a strong CVD risk factor that is especially prevalent among African Americans (Wang & Wang, 2004), such that understanding drivers of BP reactivity to acute stress may be vital to addressing CVD disparities.
This study also suggests that UA may be differentially associated with BP between males and females. Across resting and reactive systolic and diastolic BP measures, UA and BP were positively associated for females, but not for males, who showed a very modest but not statistically significant negative association (see also Supporting Information). One possible interpretation is that low levels of UA may be protective against hypertension through promoting adaptive stress responses, but only for women. Along these lines, available literature supports that UA may be especially strongly linked to CVD in women. Notably, prospective epidemiological research has shown that, although high UA is associated with greater CVD mortality in general, this association is more pronounced in women than in men (Fang & Alderman, 2000). Moreover, UA has been linked to angiographic evidence of CVD especially in women (Tuttle, Short, & Johnson, 2001). Another important consideration is that both our own and others observed sex differences in the links between UA and CVD might be driven in part by dietary health behavior, as some research suggests that hyperuricemia may be more strongly linked to diet in men than women (Gao et al., 2007). Thus, one important direction for future research is to disentangle the extent to which associations between UA and CVD risk factors or events are attributable to dietary behavior, and whether diet explains sex differences in links to CVD. Although our findings corroborate that diet is a possible direction for future research, we note that the current sex-moderator findings should be interpreted cautiously, given a relatively modest sample size.
In illuminating connections to stress reactivity and potential sex differences, the present study may also carry preliminary implications for advancing efforts to reduce CVD disparities through formal intervention. Given that the current sample was only comprised of African American participants, we are unable to make comparisons across racial groups. Existing research on cardiovascular risk has consistently provided evidence for racial disparities; thus, understanding the specific cardiovascular risk factors among African Americans may provide insight into the processes that result in such disparities. One prospect that is ripe for future study is to evaluate whether dietary and other UA-lowering interventions reduce CVD and disparities through affecting UA-directed stress reactivity responses. Increasingly, evidence suggests that UA is an independent risk factor for CVD (Kawai et al., 2012), such that dietary interventions that reduce consumption of fructose and/or purine-rich foods might reduce CVD and disparity. The present study builds on this potential in suggesting that effects of dietary intervention might be observable through changes in acute stress responses, including BP responses that carry implications for hypertension and extant CVD disparities among African Americans. Given the evidence that acute and chronic stressors have the potential to modify BP and other physiological indicators of CVD risk, it is important to continue to explore intervention opportunities that target stress reduction and adaptive coping. Indeed, a systematic review and meta-analysis concluded that stress reduction programs, such as meditation, are associated with significant reductions in BP (Rainforth et al., 2007). Specifically targeting stressors that are more common among African Americans (e.g., discrimination) could potentially reduce some CVD disparities through this mechanism. We also note that emerging evidence points towards linkages between stress reactivity and the gut microbiome, which is largely reflective of diet (Luna & Foster, 2015). Moreover, evidence suggests that BP may play a role in Alzheimer’s disease and dementias (Snyder et al., 2015), which could suggest implications for UA-targeted interventions in treating or preventing age-related cognitive illnesses. However, prospects of dietary and other interventions that seek to reduce UA must be balanced against the potential for high levels of UA to also relay beneficial anti-oxidant health effects. For example, high UA has been associated with a decreased risk of Parkinson’s disease (de Lau, Koudstaal, Hofman, & Breteler, 2005), and the role of UA in preventing age-related cognitive diseases in general continues to be debated (Latourte, Bardin, & Richette, 2018).
In tandem to furthering knowledge of its potential role in CVD disparities, this study also demonstrates the potential utility of measuring UA in saliva. UA has been well linked to CVD disease and disparity through measurements taken via whole blood and urine. However, these collection modalities are more onerous than oral fluid collection, and are therefore potentially less conducive to exploring stress reactivity. Thus, one contribution of this research is to demonstrate the potential utility of measuring UA in saliva, which may better lend itself to both field and laboratory-based reactivity evaluations. One intriguing possibility to investigate in future research that was not presently considered is to evaluate the temporal patterns and reactivity of UA itself. Indeed, salivary measurement may prove highly practical in considering whether UA also carries a reactivity profile, and in subsequently evaluating the role of UA in the multisystem stress response, which is increasingly gaining salience (e.g., Laurent, Lucas, Pierce, Goetz, & Granger, 2016; Lucas, Wegner, et al., 2017).
Several limitations suggest both a cautious interpretation and other future directions. First, only African Americans were studied. This group has experienced pronounced disparities in a number of cardiovascular illnesses, due in considerable part to disparities in CVD risk factors that include hypertension. Thus, focusing on African Americans was sensible for initially considering links between sUA and BP. Nonetheless, future research must consider whether sUA is linked to BP in other groups that may also disproportionately experience CVD. Second, compared to large-scale epidemiological and individual difference studies, this study is characterized by a relatively small sample size. Although our sample size compares favorably to many stress reactivity studies, findings should nonetheless be interpreted cautiously, particularly for associations between sUA and baseline BP measures. Related, there were more younger than older individuals in our sample, and more females than males. To this point, diastolic BP may be more predictive of CVD risk among younger (<40 years) individuals (Perry et al., 2000), highlighting the importance of considering both systolic and diastolic BP in the current study. Future studies that recruit broader age ranges and more males may reveal additional strength or nuance in the associations between CVD risk factors and sUA, especially to in that the CVD risk increases with age. Larger studies could also consider a broader range of covariates that might influence these associations, as well as cohort effects. Further, past research has shown that BMI is positively associated with sUA and BP; however, we only had self-reported BMI for a subset of the sample, and are therefore unable to draw meaningful conclusions about its role in this context (see Supporting Information). Third, interpretation of links between sUA and BP is based on correlational data. Although some evidence suggests elevations in UA precede changes in BP (Feig, 2014), we cannot definitively suggest a causal relationship. One intriguing direction for future research may be to evaluate links to stress reactivity using pharmacological administration paradigms. For example, stress reactivity paradigms that use xanthine oxidase inhibitors (XOI’s) to artificially lower UA levels could be used to further assess a causal role in stress reactivity responses. Indeed, to the extent that UA-lowering drugs are widely available and routinely administered, such a paradigm may be both useful and practical. Finally, this study only examined BP as an indicator of CVD risk. Although BP is an especially potent CVD risk factor, particularly among African Americans, there are numerous other risk factors that could also be associated with sUA in ways that are clinically significant (Feig et al., 2008). Related, although highly correlated with serum UA, clinically meaningful values of sUA have not yet been established, such that the current research cannot speak to whether participants displayed high, normal, or low levels of UA. Limitations notwithstanding, this study provides a needed advance in showing that UA is associated with stress reactivity in ways that may carry implications for hypertension and CVD. Future research may further elucidate how these connections contribute to CVD disparities, including among African Americans.
Supplementary Material
Highlights.
Salivary uric acid predicts resting and reactive blood pressure responses to stress.
Effects of salivary uric acid on blood pressure were observed for women.
Considering stress effects of uric acid can advance understanding of racial health disparities.
Acknowledgments
This research was supported by Award Number R21HL097191 from the National Heart, Lung, and Blood Institute awarded to the first author. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC and these relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and University of California at Irvine. We thank Mercedes Hendrickson, Nathan Weidner, Lenwood Hayman, Edyta Debowska, Kaitlyn Simmonds, Kevin Wynne, Stefan Goetz, Rhiana Wegner, and the Clinical Research Center at Wayne State University for assistance with data collection. Finally, we appreciate biotechnical support with salivary assays provided by Carla Slike, Becky Zavacky, and Jessica Acevedo.
Footnotes
Conflict of Interest
In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC and these relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and University of California at Irvine. We wish to confirm that there are no other known potential conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We further confirm that any aspect of the work covered in this manuscript that has involved human participants has been conducted with the ethical approval of all relevant bodies. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NE (2013). Health Disparities Taking on the Challenge. Perspectives on Psychological Science, 8, 679–681. [DOI] [PubMed] [Google Scholar]
- Adler NE, & Rehkopf DH (2008). US disparities in health: descriptions, causes, and mechanisms. Annu. Rev. Public Health, 29, 235–252. [DOI] [PubMed] [Google Scholar]
- Association, A. H. (2013). African Americans and cardivascular disaeases.
- Baker JF, Krishnan E, Chen L, & Schumacher HR (2005). Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? The American Journal of Medicine, 118, 816–826. [DOI] [PubMed] [Google Scholar]
- Blankstein R, Budoff MJ, Shaw LJ, Goff DC, Polak JF, Lima J, . . . Nasir K (2011). Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology, 58, 364–374. [DOI] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, & Epel ES (2014). Discrimination, racial bias, and telomere length in African-American men. American Journal of Preventive Medicine, 46(2), 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheffer A, Castillo A, Corrêa-Velloso J, Gonçalves M, Naaldijk Y, Nascimento I, . . . Ulrich H (2018). Purinergic system in psychiatric diseases. Molecular psychiatry, 23, 94. [DOI] [PubMed] [Google Scholar]
- Cheng P, Xia Y, Peng C, & Zhou Z (2013). Evaluation of dialysis in patients with end-stage renal disease by salivary urea, creatinine and uric acid. Zhong nan da xue xue bao. Yi xue ban= Journal of Central South University. Medical sciences, 38, 1260–1263. [DOI] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2010). Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. Hypertension, 55, 1026–1032. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ: Erlbaum. [Google Scholar]
- de Lau LM, Koudstaal PJ, Hofman A, & Breteler MM (2005). Serum uric acid levels and the risk of Parkinson disease. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 58, 797–800. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE (2008). Psychological stress and cardiovascular disease. Journal of the American College of Cardiology, 51, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ridi R, & Tallima H (2017). Physiological functions and pathogenic potential of uric acid: A review. Journal of advanced research, 8, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, & Alderman MH (2000). Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971–1992. Jama, 283, 2404–2410. [DOI] [PubMed] [Google Scholar]
- Feig DI (2014). Serum uric acid and the risk of hypertension and chronic kidney disease. Current opinion in rheumatology, 26, 176–185. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang D-H, & Johnson RJ (2008). Uric acid and cardiovascular risk. New England Journal of Medicine, 359, 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, & Curtin LR (2010). Prevalence and trends in obesity among US adults, 1999–2008. Jama, 303, 235–241. [DOI] [PubMed] [Google Scholar]
- Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, & Ascherio A (2007). Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension, 50, 306–312. [DOI] [PubMed] [Google Scholar]
- Gerin W, Goyal TM, Mostofsky E, & Shimbo D (2008). The measurement of blood pressure in cardiovascular research. Handbook of physiological research methods in health psychology, 115–131. [Google Scholar]
- Girgis A, Shea J, & Husband A (1988). Immune and psychological responses to acute venipuncture stress. Medical Science Research. [Google Scholar]
- Goodman AM, Wheelock MD, Harnett NG, Mrug S, Granger DA, & Knight DC (2016). The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience, 339, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, & Out D (2012). Focus on methodology: Salivary bioscience and research on adolescence: An integrated perspective. Journal of Adolescence, 35, 1081–1095. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34, 1437–1448. [DOI] [PubMed] [Google Scholar]
- Hayes A (2013). PROCESS SPSS Macro [Computer software and manual]. Google Scholar. [Google Scholar]
- Jin R, Donaghue K, Fairchild J, Chan A, & Silink M (2001). Comparison of Dinamap 8100 with sphygmomanometer blood pressure measurement in a prepubertal diabetes cohort. Journal of paediatrics and child health, 37, 545–549. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Titte S, Cade JR, Rideout BA, & Oliver WJ (2005). Uric acid, evolution and primitive cultures. Paper presented at the Seminars in nephrology. [DOI] [PubMed] [Google Scholar]
- Kannel WB (2000). Elevated systolic blood pressure as a cardiovascular risk factor. The American Journal of Cargiology, 85, 251–255. [DOI] [PubMed] [Google Scholar]
- Kawai T, Ohishi M, Takeya Y, Onishi M, Ito N, Yamamoto K, . . . Rakugi H (2012). Serum uric acid is an independent risk factor for cardiovascular disease and mortality in hypertensive patients. Hypertension Research, 35), 1087. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Ladwig K-H, Lederbogen F, Albus C, Angermann C, Borggrefe M, Fischer D, . . . Jünger J (2014). Position paper on the importance of psychosocial factors in cardiology: update 2013. GMS German Medical Science, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latourte A, Bardin T, & Richette P (2018). Uric acid and cognitive decline: a double-edge sword? Current opinion in rheumatology, 30, 183–187. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Lucas T, Pierce J, Goetz S, & Granger DA (2016). Coordination of cortisol response to social evaluative threat with autonomic and inflammatory responses is moderated by stress appraisals and affect. Biological psychology, 118, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler LF, Navas-Acien A, Brady TM, Miller ER, & Fadrowski JJ (2012). Uric acid level and elevated blood pressure in US adolescents. Hypertension, 59, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR (2011). Do low levels of stress reactivity signal poor states of health? Biological psychology, 86, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR (2015). Can exaggerated stress reactivity and prolonged recovery predict negative health outcomes? The case of cardiovascular disease. Psychosomatic Medicine, 77, 212214. [DOI] [PubMed] [Google Scholar]
- Lucas T, Lumley MA, Flack JM, Wegner R, Pierce J, & Goetz S (2016). A preliminary experimental examination of worldview verification, perceived racism, and stress reactivity in African Americans. Health Psychology, 35, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Pierce J, Lumley MA, Granger DA, Lin J, & Epel ES (2017). Telomere length and procedural justice predict stress reactivity responses to unfair outcomes in African Americans. Psychoneuroendocrinology, 86, 104–109. [DOI] [PubMed] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, & Granger DA (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosomatic Medicine, 79, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna RA, & Foster JA (2015). Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Current opinion in biotechnology, 32, 35–41. [DOI] [PubMed] [Google Scholar]
- Martinez AD, Ruelas L, & Granger DA (2017). Association between body mass index and salivary uric acid among Mexican origin infants, youth and adults: Gender and developmental differences. Developmental Psychobiology, 59, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton CP, Hayden M, & Southerland JH (2017). Cardiovascular health disparities in underserved populations. Primary Care: Clinics in Office Practice, 44, e37–e71. [DOI] [PubMed] [Google Scholar]
- Mrug S, Mrug M, Morris AM, Reynolds N, Patel A, Hill DC, & Feig DI (2017). Uric Acid Excretion Predicts Increased Blood Pressure Among American Adolescents of African Descent. The American journal of the medical sciences, 353, 336–341. [DOI] [PubMed] [Google Scholar]
- Nunes LAS, Brenzikofer R, & Macedo DV (2011). Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clinical biochemistry, 44, 1440–1444. [DOI] [PubMed] [Google Scholar]
- Obrist PA (2012). Cardiovascular psychophysiology: A perspective: Springer Science & Business Media. [Google Scholar]
- Panaite V, Salomon K, Jin A, & Rottenberg J (2015). Cardiovascular recovery from psychological and physiological challenge and risk for adverse cardiovascular outcomes and all-cause mortality. Psychosomatic Medicine, 77, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry HM, Miller JP, Baty JD, Carmody SE, & Sambhi MP (2000). Pretreatment blood pressure as a predictor of 21-year mortality. American Journal of Hypertension, 13, 734–733. [DOI] [PubMed] [Google Scholar]
- Phillips AC, & Hughes BM (2011). Introductory paper: Cardiovascular reactivity at a crossroads: where are we now? Biological psychology, 86, 95–97. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Rainforth MV, Schneider RH, Nidich SI, Gaylord-King C, Salerno JW, & Anderson JW (2007). Stress reduction programs in patients with elevated blood pressure: a systematic review and meta-analysis. Current Hypertension Reports, 9, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Bryce CI, Matin MJ, Stebbins JL, Kornienko O, Huisstede L. v., & Granger DA (2018). The validity, stability, and utility of measuring uric acid in saliva. Biomarkers in medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, . . . Schaffer CB (2015). Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s & Dementia, 11, 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup M, Biesiada I, Henderson A, Idowu B, Rodeback D, Ridpath L, . . . Bridges KG (2012). Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetology & Metabolic Syndrome, 4, 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle KR, Short RA, & Johnson RJ (2001). Sex differences in uric acid and risk factors for coronary artery disease. The American Journal of Cardiology, 87, 1411–1414. [DOI] [PubMed] [Google Scholar]
- Viazzi F, Antolini L, Giussani M, Brambilla P, Galbiati S, Mastriani S, . . . Genovesi S (2013). Serum uric acid and blood pressure in children at cardiovascular risk. Pediatrics, peds. 2013–0047. [DOI] [PubMed] [Google Scholar]
- Wang Y, & Wang QJ (2004). The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Archives of Internal Medicine, 164, 2126–2134. [DOI] [PubMed] [Google Scholar]
- Xia Y, Peng C, Zhou Z, Cheng P, Sun L, Peng Y, & Xiao P (2012). Clinical significance of saliva urea, creatinine, and uric acid levels in patients with chronic kidney disease. Journal of Central South University: Medical Sciences, 37(11), 1171–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.