Abstract
Social stress in the form of conflict between romantic partners is a salient correlate of substance use disorders (SUD), and also plays an integral role in SUD treatment outcomes. Neuroimaging has advanced the study of social stress on SUD etiology, course, and treatment. However, no neuroimaging paradigms have yet been developed to examine neural responses to conflict among romantic couples. In order to fill this gap in the literature, the goal of this exploratory study was to examine the preliminary feasibility of a novel relationship conflict fMRI paradigm. We compared the effects of an auditory relationship conflict versus a neutral cue on functional connectivity in corticolimbic brain regions, and the associations between neural activities and self-report ratings of relationship adjustment, substance use problems, and intimate partner violence. We also explored sex differences in neural correlates of relationship conflict versus neutral cues. Participants demonstrated increased functional connectivity between the amygdala and the prefrontal cortex during the relationship conflict cue compared to the neutral cue. Intimate partner violence was associated with functional connectivity. Sex differences emerged in neural responses to the relationship conflict cue compared to the neutral cue. Collectively, the findings demonstrate preliminary validity of this novel neuroimaging paradigm for couples.
Keywords: Couples, Addiction, Neuroimaging, Social stress, Intimate partner violence
1. Introduction
Substance use disorders (SUD) are a tremendous public health problem (Whiteford et al., 2013). Couple conflict occurs in both distressed and healthy relationships, and constructive conflict is central to adaptive relationship functioning (Lavner et al., 2016; Williamson et al., 2015). However, a large and complex literature demonstrates that social stress, particularly in the form of relationship conflict among romantic couples, is a salient correlate of SUD. The consensus in this literature is that SUD shares a mutually causal association with relationship conflict such that SUD has detrimental effects on relationship conflict, and relationship conflict precipitates excessive substance use (Breslau et al., 2011; Leonard and Eiden, 2007; Testa and Derrick, 2013).
For example, laboratory-based spousal interactions are negatively impacted by alcohol administration (Testa et al., 2014) and acute intoxication is a well-established causal factor in the incidence of relationship conflict in the form of intimate partner violence (IPV) (Devries et al., 2014; Margolin et al., 2013; Mattson et al., 2010; Quigley et al., 2013; Testa and Derrick, 2013; Testa et al., 2003). SUD is both an acute and longitudinal predictor of relationship conflict (Cranford et al., 2011; Field and Caetano, 2003), and married couples in which one partner meets diagnostic criteria for SUD are nearly twice as likely to divorce compared to those without SUD (Breslau et al., 2011).
Neuroimaging has proven integral to advancing our understanding of the etiological underpinnings of SUD, and has informed the development of novel interventions to treat SUD. Valid neuroimaging paradigms, such as alcohol and drug cue paradigms, have proven crucial to the identification of targets for pharmacological treatments, examination of prognostic indicators of pharmacological and behavioral treatment response, and characterization and clarification of treatment outcomes (Cabrera et al., 2016; Goldstein and Volkow, 2011; Gu et al., 2010; Jasinska et al., 2014; Schacht et al., 2013; Sinha, 2007; Volkow and Baler, 2013). Toward that end, neuroimaging studies employing a vast array of paradigms have advanced the literature examining mechanisms by which social stress and substance use behaviors are linked (Sinha, 2012; Sinha and Li, 2007). The literature in this area continues to advance at a fast pace and with a high level of precision with regard to the roles that specific brain regions play in risk, resilience, and recovery/relapse to SUD. Specifically, much attention has focused on the role of impaired prefrontal cortex (PFC) regulation of limbic brain regions such as the amygdala (AMY)(Koob and Volkow, 2016). The AMY plays a crucial role in threat perception, fear conditioning, emotional salience, and heightened memory for emotional events (Pitman et al., 2012; Wassum and Izquierdo, 2015). The PFC is responsible for executive functioning and disrupting habitual or compulsive behaviors that are not adaptive (Pitman et al., 2012). Dysregulation of the PFC-AMY circuitry (i.e., lack of “top down” control) likely makes it difficult to modulate maladaptive cognitions such as craving-related thoughts or behaviors like impulsive substance use (Goldstein and Volkow, 2011; Koob and Volkow, 2016). Using validated paradigms such as facial recognition and the Trier Social Stress Task, individuals with SUD demonstrate lower PFC-AMY functional connectivity in response to stress and emotion cues compared to healthy controls (Crunelle et al., 2015; Kaag et al., 2018; O’Daly et al., 2012; Wade et al., 2017). Lower corticolimbic connectivity is also associated with a significantly shorter time to relapse (Beck et al., 2012; McHugh et al., 2014). However, other studies have reported greater PFC-AMY functional connectivity in substance users in some experimental conditions. For example, in one study (O’Daly et al., 2012), individuals who had experienced single detoxifications, multiple detoxification, and social drinkers were compared on their responses to implicit versus explicit fearful faces recognition task. Findings indicate that increased functional connectivity occurred between corticolimbic brain regions during the implicit task. In another study, Seo et al. (2011) examined functional connectivity in response to stress and alcohol cues among socially drinking men and women. Increased connectivity in different brain regions was observed by sex and in response to stress versus alcohol cues. Some studies have examined neural correlates of general anger and aggression, including functional connectivity of the PFC with other brain regions. These studies have been conducted among individuals with criminal convictions, traumatic brain injuries, normative populations and individuals with intermittent explosive disorder and schizophrenia (see Rosell and Siever, 2015, for review). To our knowledge, only one study to date has examined neural correlates of aggression associated with substance use. Denson et al. (2018) found that healthy young men intoxicated by alcohol demonstrated decreased activity in the prefrontal cortex, caudate, and ventral striatum while activity in the hippocampus was heightened compared to participants in the placebo condition. In this study, aggressive behavior was positively associated with activation in the medial and dorsolateral prefrontal cortex compared to participants in the placebo condition.
No studies to date have examined neural correlates of relationship conflict among normative couples or couples with SUD. A significant barrier to advancing this area of the literature is that neuroimaging procedures have not been developed or adapted for use among couples, despite the tremendous progress that has been made to validate and refine neuroimaging paradigms for individuals. The development of a neuroimaging paradigm for couples is essential to conduct studies aimed at clarifying neural processes underlying the bidirectional association between relationship functioning and SUD. Understanding the neural response to relationship conflict may support SUD treatment development given the importance of relationship health in successful treatment outcomes.
Relationship conflict is known to impede effective treatment for SUD, while adaptive relationship functioning is known to facilitate SUD treatment outcomes (Cranford et al., 2011; Rodriguez et al., 2013; Testa et al., 2014). Specifically, adaptive relationship functioning positively influences SUD treatment initiation, outcome, and maintenance of treatment gains (Grosso et al., 2013; Lebow et al., 2012; Meis et al., 2013; O’Farrell and Clements, 2012). Recent studies suggest that women in SUD treatment with higher relationship satisfaction experience fewer cravings (Owens et al., 2013). Individuals who have supportive partners have greater motivation for SUD treatment and drink less during treatment for alcohol use disorder (Grosso et al., 2013; McCrady et al., 2002). Some studies have indicated that partner involvement in treatment for alcohol use disorders may help prevent relapse (Nattala et al., 2010; Walitzer and Dermen, 2004). While the effects of maladaptive relationship functioning on substance use behaviors and treatment have been examined less thoroughly, literature has demonstrated that maladaptive relationship functioning is associated with greater substance use (Cranford et al., 2011; Quigley et al., 2013; Rodriguez et al., 2013; Testa et al., 2014). One study found a daily temporal association between relationship conflict in the form of psychological IPV victimization and substance use among men (Testa and Derrick, 2013), and another study observed a longitudinal association between poor relationship functioning and alcohol use disorder development (Whisman et al., 2006). Additional research shows that relationship conflict may have a causal effect on relapse (Maisto et al., 1995; Mattson et al., 2010). Maladaptive relationship functioning might play a particularly salient role in SUD among women. Relationship distress been shown to be a primary reason for women’s SUD treatment seeking and is associated with increased risk for SUD relapse (Green et al., 2008; Lemke et al., 2007).
The well-documented efficacy of couples therapies to treat SUD further underscores the importance of the role of relationship functioning in SUD and recovery. Recent studies indicate that couples SUD treatment outperforms individual SUD treatment (McCrady et al., 2016; McHugh et al., 2010; Powers et al., 2008; Schumm et al., 2014). Notably, adaptive changes in relationship functioning are a significant mechanism of change in treatment outcomes (Magill et al., 2015; McCrady et al., 2016; McCrady et al., 2002; Schumm et al., 2014). Thus, continuing to refine and improve methods to enhance couples treatment development research in the area of SUD is critical.
The current study is the first to explore the feasibility of a novel neuroimaging paradigm in order to examine neural correlates of relationship conflict. To accomplish this goal, we adapted an auditory cue paradigm developed by Sinha and Tuit (2012) which is commonly employed in the context of individual neuroimaging research, especially among individuals with SUD (Sinha et al., 2009; Sinha et al., 2006; Sinha and Li, 2007). In its original form, personalized stress paradigms are created for each individual participant relevant to any topic they recently found stressful (i.e., being fired from a job, being pulled over by police, being reprimanded by a work superior). This auditory cue paradigm has been used most commonly to elicit stress in order to examine the effects of stress on substance craving, vulnerability to SUD relapse, and to identify the neural underpinnings that link emotional distress with substance use behaviors. Couple conflict may differ in that it is co-created by both members of the couple and is a shared experience, although each partner might have different emotional, behavioral, and neurobiological responses to this unique stressor. The present adaptation was designed to measure each partner’s unique response to the shared experience. Couple conflict is known to elicit emotional and physiological stress responses that predict relationship and health outcomes (Ditzen et al., 2007; Heffner et al., 2006; Kiecolt-Glaser et al., 2003). However, it is not yet known whether relationship conflict among distressed, substance-misusing couples shares neural underpinnings with SUD or responses to other stressors that have previously been examined.
Areas that have commonly been identified as relevant to the link between social stress and substance use behaviors include striatallimbic-prefrontal regions such as the AMY, anterior cingulate cortex, caudate, putamen, thalamus, and striatum (Sinha, 2008). These regions have been established as relevant through the use of neuroimaging studies comparing neural activation under stress and substance cues as compared to neutral cues and through the comparison of individuals with substance use disorders and healthy individuals. It is hypothesized that these regions link stress with addiction through their involvement in emotional processing and regulation and the ability to regulate impulsive behavior (Sinha, 2012; Wilcox et al., 2016). It is hypothesized that in addition to the causal link established between acute stress states and vulnerability to substance use and relapse, individuals with chronic excessive substance use might incur maladaptive changes to this neural circuitry which, over time, inhibits one’s ability to adaptively manage stress (Sinha, 2001).
We examined the effects of relationship conflict cues versus neutral cues on (1) corticolimbic functional activation and connectivity, and (2) associations between corticolimbic functional connectivity during the conflict cue and self-report ratings of relationship adjustment, substance use problems, and IPV victimization and perpetration. Although this study is preliminary, sex differences in the neurobiological link between stress reactivity and SUD have been observed (Fox and Sinha, 2009; Li et al., 2005; Potenza et al., 2012). In addition, the role of sex is a critical focus of the current clinical and preclinical literature examining both behavioral and neurobiological mechanisms of substance use disorder development, change in substance use behavior, treatment engagement, and treatment outcome (Bazargan-Hejazi et al., 2016; Becker and Koob, 2016; Greenfield et al., 2007; Holmes et al., 2016; Sugarman et al., 2014). These differences appear to emerge as early as during adolescence (Johnson et al., 2015; Kuhn, 2015). Thus, a secondary goal of this study was to explore sex differences in neural correlates of relationship conflict versus neutral cues in order to inform the design future studies aimed at refining this paradigm.
As a result of previous literature demonstrating increased functional connectivity in corticolimbic brain regions in response to stress versus neutral cues (O’Daly et al., 2012; Seo et al., 2011), we hypothesized that participants would demonstrate greater corticolimbic reactivity and functional connectivity for conflict cues as compared to the neutral cues, as an index of an elevated stress response. The amygdala served as the limbic region of interest given its role in stress reactivity. The PFC region of interest was the lateral orbitofrontal (OFC) cortex given its role in conscious regulation of amygdala responses (Wilcox et al., 2016). We also hypothesized that increased AMY-OFC connectivity in response to relationship conflict cues would be associated with poorer relationship adjustment, greater quantity/frequency of substance use, and greater severity of alcohol and drug problems and IPV victimization and perpetration. Finally, we hypothesized that females would show greater corticolimbic reactivity and AMY-OFC connectivity than males in response to relationship conflict cues as reported in some prior studies examining stress versus drug cues (Potenza et al., 2012; Seo et al., 2011).
2. Method
2.1. Participants
All procedures were IRB approved and participants provided written informed consent prior to completing any study procedures. Participants were recruited using advertisements placed in the community, local treatment clinics, and on the internet. Participation was open to couples aged 18–65 of any sex and sexual orientation who were able to comprehend English and function at an intellectual level sufficient to provide informed consent and accurately complete assessment instruments (as determined by a Mini Mental Status Exam score ≥ 26). Eleven couples enrolled in the study. One couple withdrew prior to the neuroimaging scan, resulting in a total sample of 10 couples (N = 20 total participants; 9 males, 11 females). The current sample included one same-sex female couple. In addition, at least one partner within each dyad was required to have engaged in hazardous drinking (i.e., 4 or more drinks for women, 6 or more for men) during the past 60 days or meet DSM-IV diagnostic criteria for current substance use disorder and relationship distress as indicated by a score of ≤ 13 on the DAS-4 (Sabourin et al., 2005). Exclusion criteria included (1) meeting DSM-IV criteria for a history of or current psychotic or bipolar affective disorder, (2) current suicidal or homicidal ideation and intent, (3) pregnancy for women, and (4) past 6 months severe and unilateral IPV with current partner. Additional exclusion criteria were any contraindications for MRI scanning (i.e., metal in or on the body, orthodontics, and history of concussion or head injury, claustrophobia). Sample demographic and clinical characteristics are presented in Table 1.
Table 1.
Demographic and clinical characteristics (N=20).
| Characteristics | Total SampleN=20 |
Menn=9 | Womenn=11 | p | |
|---|---|---|---|---|---|
| Range | Mean (SD) | ||||
| Demographics | |||||
| Age (years) | 22–58 | 39.45 ± 10.27 | 38.89 ± 10.97 | 39.91 ± 10.18 | 0.73 |
| Income | $0–$52,000 | $20,268 ± $17,848 | $14,511 ± $9,424 | $18,140 ± $16,897 | 0.61 |
| Education (years) | 10–18 | 12.25 ± 1.85 | 11.50 ± 0.93 | 12.85 ± 2.21 | 0.94 |
| Employment, n (%) | 0.65 | ||||
| Unemployed | 10 (50.0%) | 4 (44.4%) | 6 (54.5%) | ||
| Part-time | 3 (15.0%) | 1 (11.1%) | 2 (18.2%) | ||
| Full-time | 4 (20.0%) | 2 (22.2%) | 2 (18.2%) | ||
| Student | 1 (5.0%) | 1 (11.1%) | 0 (0.0%) | ||
| Disabled | 1 (5.0%) | 1 (11.1%) | 0 (0.0%) | ||
| Did not report | 1 (5.0%) | 0 (0.0%) | 1 (9.1%) | ||
| Race, n (%) | 0.45 | ||||
| Black/African American | 16 (80.0%) | 8 (88.9%) | 8 (72.7%) | ||
| White/Caucasian | 3 (15.0%) | 1 (11.1%) | 2 (18.2%) | ||
| More than one race | 1 (5.0%) | 0 (0.0%) | 1 (9.1%) | ||
| Relationship Status, n (%) | 0.58 | ||||
| Married | 5 (25.0%) | 3 (33.3%) | 2 (18.2%) | ||
| Cohabitating | 8 (40.0%) | 4 (44.4%) | 5 (45.5%) | ||
| Dating | 7 (35.5%) | 2 (22.2%) | 3 (27.3%) | ||
| Did not report | 1 (5.0%) | 0 (0.0%) | 1 (9.1%) | ||
| Relationship length (months) | 2–27 | 7.05 ± 7.72 | 7.56 ± 7.97 | 6.60 ± 7.89 | 0.33 |
| Clinical characteristics | |||||
| Substance use disorder, n (%) | |||||
| Alcohol abuse | 12 (60.0%) | 7 (77.8%) | 5 (45.5%) | 0.07 | |
| Alcohol dependence | 7 (35.0%) | 3 (33.3%) | 4 (36.4%) | 0.64 | |
| Drug abuse | 15 (75.0%) | 8 (88.9%) | 7 (63.6%) | 0.26 | |
| Drug dependence | 14 (70.0%) | 7 (77.8%) | 7 (63.6%) | 0.61 | |
| Alcohol problems (AUDIT) | 0–29 | 10.80 ± 9.30 | 13.44 ± 8.43 | 8.64 ± 9.80 | 0.02 |
| Drug problems (DAST) | 0–8 | 3.60 ± 2.54 | 4.00 ± 2.92 | 3.27 ± 2.28 | 0.49 |
| TLFB TDU alcohol | 0–30 | 12.00 ± 11.03 | 17.40 ± 12.00 | 8.64 ± 9.44 | 0.02 |
| TLFB TDU marijuana | 0–30 | 15.85 ± 14.56 | 17.20 ± 14.88 | 13.18 ± 14.81 | 0.69 |
| TLFB TDU cocaine | 0–25 | 5.60 ± 8.25 | 6.80 ± 9.80 | 4.36 ± 6.44 | 0.53 |
| TLFB TDU stimulants | 0–12 | 0.60 ± 2.68 | 0.00 ± 0.00 | 1.09 ± 3.62 | 0.33 |
| TLFB TDU opiates | 0–8 | 0.40 ± 1.79 | 0.00 ± 0.00 | 0.72 ± 2.41 | 0.33 |
| Dyadic adjustment | 5–20 | 11.37 ± 2.48 | 11.56 ± 3.43 | 11.20 ± 1.32 | 0.87 |
| Psych IPV victimization | 0–137 | 31.22 ± 33.70 | 25.56 ± 43.39 | 35.91 ± 23.91 | 0.21 |
| Phys IPV victimization | 0–48 | 3.25 ± 10.66 | 0.22 ± 0.67 | 5.73 ± 14.16 | 0.26 |
| Sex IPV victimization | 0–79 | 5.18 ± 17.39 | 10.50 ± 25.42 | 0.75 ± 1.29 | 0.19 |
| Psych IPV perpetration | 2–124 | 31.86 ± 34.04 | 21.80 ± 29.82 | 40.25 ± 36.28 | 0.21 |
| Phys IPV perpetration | 0–92 | 9.64 ± 22.43 | 9.20 ± 29.09 | 10.00 ± 16.37 | 0.93 |
| Sex IPV perpetration | 0–103 | 7.41 ± 22.63 | 15.40 ± 32.55 | 0.75 ± 2.05 | 0.13 |
Note. Current (past 6 months) substance use disorder reported. Relationship status and length are reported only at the group level. IPV = intimate partner violence. IPV victimization and perpetration data represent severity scores from the Revised Conflict Tactics Scale (CTS-2). Psych = Psychological. Phys = Physical. Sex = Sexual. TLFB = Time Line Follow Back. TDU = Total days using. Group differences in continuous characteristics were assessed using t-tests or chi-square tests where appropriate.
The most frequent topic of conflict was disagreements about finances/money, followed by conflict over substance use and trust. All of the participants in this study (N = 20, 100%) reported at least one instance of IPV perpetration during the past six months. Eighteen participants (90.0%) who participated in this study reported at least one instance of IPV victimization. Both participants who reported no IPV victimization were men.
2.2. Procedure
This study was carried out in accordance with the Declaration of Helsinki. All study procedures were IRB-approved. Potential participants were screened by telephone and those meeting preliminary eligibility criteria scheduled for an in-person appointment. Procedures took place in one study visit. All participants completed informed consent and baseline assessment procedures in a private room separate from their partner. Women completed a urine pregnancy test. If negative, both partners completed breathalyzer tests and urine drug screens. Both partners within each couple completed all study procedures.
2.3. Measures
2.3.1. Diagnostic evaluation
The Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) was used to assess current and history of DSM-IV psychiatric diagnoses, including substance abuse and dependence. Intellectual capacity to participate was assessed using the Mini Mental Status Exam (Folstein et al., 1975).
2.3.2. Substance use
The Time Line Followback (TLFB; Sobell and Sobell, 1992) is a calendar-assisted, semi-structured interview which was used to assess quantity and frequency of alcohol and both illicit and prescription drugs (e.g., prescription opioids, benzodiazepines, and psychostimulants). Participants report the total number of days substances are used and the amount of substance used (e.g., standard drink units for alcohol, number of joints for marijuana) during the 60 days prior to participating in the study.
Alcohol and drug use problem severity was assessed using the 10-item self-report Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001). Items are rated on a scale from 0 (never) to 4 (more than 4 times per week) and summed to obtain a total score. Drug use problems were assessed with the 10-item version of the Drug Abuse Screening Test (DAST; Skinner, 1982). Each item was rated 0 (no) or 1 (yes) and summed to produce a total score.
2.3.3. Relationship adjustment
The brief version of the Dyadic Adjustment Scale (DAS-4; Sabourin et al., 2005) is a self-report questionnaire used to assess couple functioning. Item scores were summed to obtain a total score ranging from 1 to 21. Higher scores reflect higher levels of relationship adjustment, and scores below 13 reflect relationship distress.
2.3.4. Intimate partner violence
IPV victimization and perpetration was measured using the 78-item Revised Conflict Tactics Scale (CTS-2; Straus et al., 2003). Response categories that comprised a range of values were recoded (Straus et al., 2003) (i.e., 3–5 times (recoded to 4), 6–10 times (recoded to 8), 11–20 times (recoded to 15), and more than 20 times (recoded to 25)). Severity scores for each participant were calculated for victimization and perpetration subscales by summing the respective responses for the psychological, physical, and sexual IPV subscales. The sexual IPV subscale scores excluded items 15 and 16. Analyses were conducted by examining the maximum victimization and perpetration score within each couple in order to minimize reporting discrepancies between partners.
2.4. Auditory cue development
Each individual participant created an auditory neutral cue recording pertaining to their normal morning routine. Consistent with the manualized procedures developed by Sinha and Tuit (2012), a script of this recording was developed and translated into a 3-min audio recording. In order to develop relationship conflict cues, each partner identified a topic of relationship difficulty and couples completed an audio-recorded conflict task. A coin flip determined the topic of the discussion (i.e. disagreements about finances/household spending; infidelity/suspected infidelity; substance use behaviors) and the couple was asked to work towards a resolution or agreement on that topic. This procedure is extensively used in observational studies examining relationship conflict (Hahlweg et al., 2000; Hellmuth and McNulty, 2008; Miller et al., 2013). The recorded conflict discussion was subsequently translated into an audio recording which served as the relationship conflict cue. Both partners within each couple listened to the identical conflict cue during the neuroimaging scan.
2.5. Functional neuroimaging procedures
A relationship conflict cue block was created consisting of the recorded conflict discussion divided into two three-min segments each. Similarly, a neutral block was created in which participants heard a script describing their typical morning routine at home divided into two segments of 3-min each. During the relationship conflict cue, participants saw a visual display that said “allow” and were instructed to allow themselves to experience any thoughts or emotions related to the cue. Order of presentation of the relationship conflict versus neutral cues was counterbalanced between participants. Three-minute blocks of the relationship conflict cue alternated with three-minute blocks of the neutral cue with no inter-stimulus interval or silent period. A standard fixation point was presented for 30 s before the start of the cue. Presentation of the cues used E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA) Audio Stimulus Presentation Software (http://www.pstnet.com).
Neuroimaging data were acquired on a Siemens Trio 3T scanner using a 32-channel head coil. Functional images were obtained using an echoplanar imaging (EPI) sequence (36 slices, TR=2200 ms, TE=35 ms, FOV=192 mm, slice thickness =3 mm). A high-resolution T1-weighted MPRAGE anatomical image was also obtained for each participant (TR=1900 ms, TE=2.26 ms, voxel dimensions 1.0 × 1.0 × 1.0 mm, 192 slices).
2.6. Data analytic plan
Post-acquisition preprocessing and statistical analysis of all of imaging data were performed using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB’s Software Library). Pre-processing consisted of skull stripping using the Brain Extraction Tool (BET), slice timing correction, high-pass temporal filtering with a cutoff of 100 s, MCFLIRT motion correction, and spatial smoothing (using a Gaussian kernel of FWHM 5 mm). FLIRT (FMRIB’s Linear Image Registration Tool) was used to transform the images to standard space (Montreal Neurological Institute; MNI).
The primary analysis was a functional connectivity analysis using a Psychophysiological interaction (PPI) seed-based approach (Friston et al., 1997). Mean BOLD time series were extracted from four seed regions. Bilateral amygdala regions were selected as seed regions based on their role in prior studies of stress reactivity and SUD (Banks et al. 2007; Koob and Volkow, 2016). The left and right amygdala seed regions-of-interest (seed ROIs) were defined by the amygdala regions in the Harvard–Oxford probabilistic structural atlas. Additionally, the lateral OFC, a region previous studies have found to play an important role in emotion regulation and modulation of habitual behavioral responses (Hartikainen et al., 2012; Hooker et al., 2010; Wilcox et al., 2014; Wilcox et al., 2016), was selected as the prefrontal seed region due to its recruitment in the experimental cues. OFC ROIs were defined as 10-mm spheres centered on MNI coordinates of −38, 22, −12 for the left OFC and 38, 22, −12 for the right OFC.
A whole-brain PPI analysis was conducted to characterize differences in functional connectivity between the relationship conflict cue and the neutral cue using the four seed regions (bilateral amygdala, bilateral OFC). The first level PPI analysis for each subject included two psychological variables to represent the relationship conflict and neutral cues and one physiological variable (the time series in one of the seed regions described above), with two interaction terms representing the interaction of the physiological regressor and the conflict condition (PPIC) or the physiological regressor and the neutral condition (PPIN). A separate PPI analysis was conducted for each of the four seed regions. Head motion parameters were added as regressors of no interest. Group-level analyses were carried out using FLAME 1 (FMRIB’s Local Analysis of Mixed Effects) to generate z statistical images contrasting males and females. Contrasts were specified as Males > Females, Males < Females, Males mean, Females mean, and Group mean. For clusters showing significant between-group differences in activation, the most probable anatomical label from the Harvard-Oxford Cortical and Subcortical Structural Atlas packaged in FSL was used with MNI coordinates (x, y, and z).
To examine associations between functional connectivity for the conflict cue and relevant clinical measures, we extracted parameter estimates converted to percent signal change (using featquery) in the following connections of interest: left amygdala seed-left frontal operculum (LAMG-LFO), right amygdala seed-left frontal operculum (RAMG-LFO), left amygdala seed-left inferior frontal gyrus (LAMG-LIFG), right amygdala seed-left inferior frontal gyrus (RAMG-LIFG), left OFC seed-right amygdala (LOFC-RAMG) and right OFC seed-right amygdala (ROFC-RAMG). We then used Spearman-rank correlations to determine whether AMY-PFC connectivity in the relationship conflict cue was modulated by self-report measurement of alcohol (AUDIT) and drug (DAST) problems, relationship adjustment (DAS-4), or IPV (CTS-2). If the correlation was significant for the conflict cue, we tested whether it was also significant for the neutral cue to determine the specificity of the association to processing conflict. These correlations were intended to establish effect sizes to help formulate hypotheses for future studies; therefore, there was no correction for multiple tests.
General linear model (GLM)-based analyses were also conducted to identify (a) the full network of regions involved in listening to relationship conflict, (b) to isolate sex differences in activation of this network and (c) to ensure that the OFC seed region was implicated in relationship conflict cue processing. Motion parameters were included as covariates of no interest along with predictors for each of the two experimental conditions (conflict cue, neutral cue). The primary contrast of interest was Conflict > Neutral cues. Group-level analyses were performed by combining data from all participants in a mixed effect GLM (using FLAME1) to identify regions where males and females exhibited differential brain responses to the two experimental cues. In addition to examining the group mean, four group contrasts were specified: Males > Females, Males < Females, Males mean, and Females mean. For each significant cluster of activation (cluster corrected Z = 2.3 P < 0.05), the most probable anatomical label from the Harvard–Oxford cortical and subcortical structural Atlas packaged in FSL was used with MNI coordinates (x, y, and z).
3. Results
3.1. PPI analysis with the bilateral amygdala seeds
None of the contrasts revealed significant connectivity with the right or left amygdala seed regions in the group as a whole. However, IPV perpetration correlated positively with RAMG-LIFG connectivity during the neutral condition only (rho = 0.461 (p = 0.04). IPV perpetration was not associated with LAMG-LIFG connectivity during either cue. IPV victimization correlated positively with RAMG-LIFG connectivity during conflict (rho = 0.487 (p = 0.02) but also for the neutral condition (rho = 0.57, p < 0.001). IPV victimization was not associated with LAMG-LIFG connectivity during either cue. Correlations by sex were not significant for any of the correlations with clinical measures.
Sex differences emerged for the PPIC condition. During the relationship conflict cue (PPIC), females showed greater functional connectivity than males between the right amygdala and the left inferior frontal cortex (opercular portion) and mid-cingulate (see Table 2). This activation extended into other regions, including the left caudate and the triangular portion of the inferior frontal gyrus (Fig. 1a). Males did not show any significant connectivity with the two amygdala seeds.
Table 2.
Results of PPI analysis.
| Seed region | Contrast | Group comparison | Region/Peak | MNI coordinates |
Z-score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Right AMY | PPIC | F > M | L Mid-Cingulate / | −3 | 14 | 27 | 3.46 |
| R Anterior Cingulate | 14 | 8 | 34 | ||||
| L Inf. Frontal Opercular/ | −47 | 18 | 6 | 3.47 | |||
| L Precentral | −52 | 4 | 10 | ||||
| Right OFC | PPIC > PPIN | M > F | R Hippocampus/Thalamus | 22 | −20 | −7 | 3.46 |
| R Hippocampus | 28 | −32 | −8 | ||||
| White matter / | −14 | −42 | 23 | 3.42 | |||
| Cerebrospinal Fluid | −14 | 32 | 20 | ||||
| PPIC | Group mean | R Frontal Pole/ | 3 | 65 | 2 | 3.83 | |
| R Frontal Pole | −6 | 68 | 0 | ||||
| Left OFC | PPIC > PPIN | F > M | R Superior Temporal / | 44 | −10 | −11 | 3.92 |
| R Middle Temporal | 60 | −6 | −18 | ||||
| Female mean | R Insula / | 40 | 1 | −15 | 4.02 | ||
| R Middle temporal | 60 | −6 | −18 | 60 | |||
Note. R = Right. L = Left. F = Female. M = Male. PPIC = Psychophysiological Interaction Parameter for the conflict condition. PPIN = Psychophysiological Interaction Parameter for the neutral condition. Inf = Inferior.
Fig. 1.
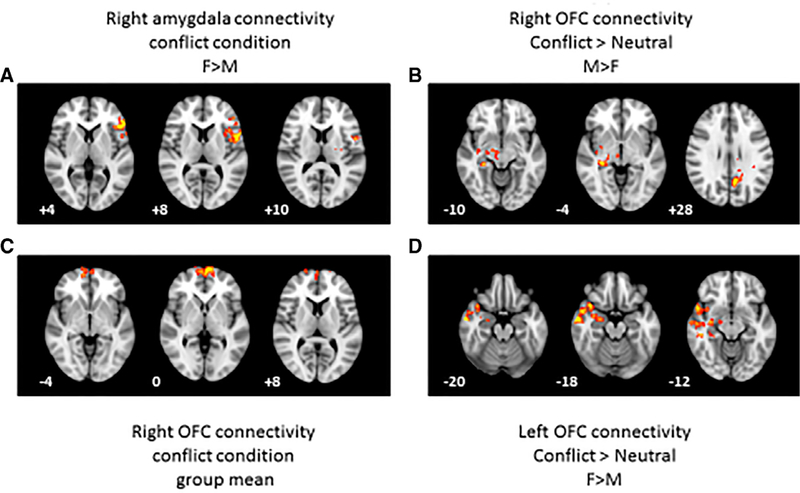
Results from the voxel-wise psychophysiological interaction (PPI) analysis. (A) Regions that were more strongly functionally connected to the right amygdala seed region in the conflict condition for females vs. males. (B) Regions that were more strongly functionally connected to the right orbitofrontal cortex (OFC) in the conflict versus neutral contrast for males vs. females. (C) Regions that were more strongly functionally connected to the right orbitofrontal cortex (OFC) in the conflict condition for the group mean. (D) Regions that were strongly functionally connected to the left OFC seed region in the conflict vs. neutral contrast for females vs. males. All activation was significant at a cluster corrected threshold of 2.33, Z < 0.05. The left hemisphere is shown on the right of each image. z-coordinates in Montreal Neurological Institute space are shown for each slice.
3.2. PPI analysis with the bilateral orbitofrontal cortex seeds
The group as a whole showed right OFC-right frontal pole connectivity for the conflict condition alone (PPIC; see Fig. 1b). During the relationship conflict cue as compared to the neutral cue (PPIC > PPIN), the right orbitofrontal cortex (OFC) was more strongly functionally connected to the right amygdala / hippocampus in males (see Table 2; Fig. 1c). A second cluster of activation included several other brain regions, such as the posterior cingulate; however, the average locus of activation across these diverse regions fell in the cerebrospinal fluid (CSF) and white matter. There were no regions that were more strongly functionally connected in females as compared to males for this contrast.
During the conflict cue compared to the neutral cue (PPIC > PPIN) in direct comparison to males, females showed greater left OFC functional connectivity with the right middle temporal gyrus (Table 2; Fig. 1d), and this activation also extended into the right insula, right amygdala and temporal pole. Males did not show any regions with greater left OFC functional connectivity than females during the relationship conflict cue. Correlations with clinical measures were not significant for any of the contrasts using the OFC seeds.
3.3. General linear model results
The exploratory contrasts (Males > Females or Females > Males) did not reveal any regions differentially activated by sex for the relationship conflict cue compared to the neutral cue. However, there was extensive activation for the other contrasts, as shown in Table 3. Relationship conflict recruited an extended network of regions including the frontal pole, lateral temporal cortex, the caudate nucleus and precuneus.
Table 3.
Results of general linear model analysis.
| Contrast | Group comparison | Region | MNI coordinates |
Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| C>N | Male mean | R Frontal Pole | 6 | 48 | 46 | 3.5 |
| R Caudate | 16 | 26 | 0 | 3.15 | ||
| C>N | Female mean | L Frontal Pole | −2 | 58 | 20 | 3.42 |
| C>N | Group mean | L Frontal Pole | −4 | 58 | 22 | 3.57 |
| R Caudate | 14 | 26 | 0 | 3.33 | ||
| L Caudate | −8 | 6 | 4 | 3.30 | ||
| R Superior Temporal | 60 | −20 | −2 | 3.14 | ||
| L Precuneus | −10 | −56 | 32 | 3.21 | ||
| L Superior Temporal | −52 | −30 | −2 | 2.92 | ||
| Conflict | Male mean | R Middle Temporal | 58 | −24 | −6 | 4.12 |
| L Superior Temporal | −58 | −24 | 0 | 4.14 | ||
| L Inferior Frontal Opercula | −44 | 14 | 24 | 3.72 | ||
| L Precuneus | −8 | −52 | 34 | 3.46 | ||
| Conflict | Female mean | R Inferior Frontal Opercula | 50 | 18 | 2 | 4.06 |
| R Superior Temporal | 56 | −24 | 4 | 4.06 | ||
| R Anterior Cingulate | 0 | 34 | 16 | 3.69 | ||
| Conflict | Group mean | L Superior Temporal | −60 | −22 | −2 | 4.25 |
| R Superior Temporal | 50 | −24 | 2 | 4.26 | ||
| L Frontal Pole | −6 | 56 | 20 | 3.82 | ||
| R Precuneus | 10 | −52 | 34 | 3.58 | ||
Note. C = Conflict. N = Neutral. L = Left. R = Right.
4. Discussion
Converging literature indicates that social stress in the form of relationship conflict is a salient correlate of SUD. The extent to which a couple is functioning adaptively versus maladaptively plays an important role in SUD etiology, course, and treatment. Neuroimaging studies have been successfully employed to identify neural correlates of SUD, with a particular focus on the neural underpinnings of the link between social stress and SUD. However, no studies to date have examined the neural correlates of relationship conflict among couples. This remaining limitation in the literature is due primarily to the absence of a paradigm designed to accomplish this goal. This study addressed this gap in the literature by examining the preliminary feasibility of a novel paradigm for assessing neural processes during relationship conflict among couples with substance misuse. The development of a neuroimaging protocol for couples is essential for clarifying the neural processes contributing to the bidirectional association between relationship functioning and SUD and, subsequently, improving the methods employed to conduct SUD treatment development research. Although preliminary, results from this study provide a platform for future research to refine this approach and to examine the validity of the paradigm in an adequately powered study.
The GLM findings indicated that the relationship conflict cue elicited a robust neural response, engaging a large network of brain regions including the frontal pole, precuneus, lateral temporal cortex, the caudate and thalamus. The functional connectivity hypotheses were partially supported by the PPI analysis in that participants showed greater functional connectivity between the hippocampus and the right OFC during the relationship conflict cue as compared to the neutral cue. This pattern of findings offers initial support for validity of the task in that the relationship conflict cue elicited a neural response consistent with those observed in other stressor paradigms in SUD populations (Sinha et al., 2009; Sinha and Li, 2007). As such, the MRI-adapted version of the relationship conflict cue could potentially be used to examine neural processes associated with relationship conflict and SUD. This finding should inform hypotheses examined in future adequately powered studies.
The paradigm also revealed potential sex differences in functional connectivity for the relationship conflict cue. Similar to other work examining socially-oriented stressors such as social threat and emotion recognition (Etkin et al., 2011; Filkowski et al., 2017; Tobia et al., 2017), women showed greater functional connectivity between the amygdala and several regions of the prefrontal cortex during the conflict cue, as compared to males. Three previous studies have found dysregulated PFC-AMY connectivity among women with IPV victimization (Fonzo et al., 2010; Satterthwaite et al., 2016; Simmons et al., 2008); however, participants in both of these studies were recruited specifically due to having a diagnosis of posttraumatic stress disorder resulting from IPV victimization. In addition, previous literature demonstrates extensive sex differences in neural correlates of addiction, with a specific focus on the role of stress in addiction (Becker and Hu, 2008; Fattore, 2015; Milivojevic et al., 2017). For example, Potenza et al. (2012) reported greater corticostriatal-limbic reactivity in female cocaine users in response to stress cues compared to males. This suggests that women experiencing relationship conflict and substance misuse in their relationship may recruit a more extensive network of prefrontal brain regions when processing relationship conflict. Women not only had greater connectivity with paralimbic regions (right amygdala-anterior cingulate connectivity and left OFC-insula connectivity) during the relationship conflict cue, but they also showed increased connectivity with regions implicated in cognitive processing (right amygdala - left IFG and left OFC and temporal regions). In contrast, men were more likely to recruit a single limbic circuit involving the right hippocampus and right OFC) in response to the relationship conflict cue. Seo et al. (2011) similarly reported that fMRI response to stress cues was isolated to limbic and paralimbic regions in male social drinkers (amygdala, hippocampus, cingulate and insula) but fMRI response to stress cues engaged lateral prefrontal regions (superior and middle frontal cortex) in female social drinkers. Similar to Seo et al. (2011), we speculate that the prefrontal and temporal lobe regions are involved in cognitive processing. However, the examinations of sex differences in this study are exploratory and these preliminary findings should be interpreted with caution. These exploratory analyses were examined as a result of previous literature suggesting differences in functional connectivity among men and women with substance misuse, and other literature suggesting neurobiological differences in the association between social stress and substance use disorders between men and women. In the absence of larger sample powered to rigorously test sex differences, the value of these preliminary findings is that they suggest that future studies examining this paradigm, particularly among populations with substance misuse, should be designed with the intention to examine possible sex differences in brain responses to the paradigm.
In the present context, these left-hemisphere regions have been associated with processing language. The inferior frontal gyrus is consistent with Broca’s area, a major locus for production of speech. The left temporal cortex has been associated with processing the meaning of speech and semantic content. Given the verbal nature of the relationship conflict cues, engagement of left-hemisphere language regions for this task is not surprising. However, the present findings further suggest that women demonstrate greater co-activation of these regions with the amygdala in stressful contexts.
Although more research is needed to refine this procedure, replicate the findings, and conduct a rigorous examination of the validity of the procedure, the results suggest that the neural patterns identified in response to the relationship conflict cue could reveal sex-specific social stress responses. Because examinations of sex differences in addiction have focused primarily on hormonal, neurochemical, and structural/functional differences with some attention to behavioral correlates such as emotion regulation (Cahill, 2006; Fox and Sinha, 2009; Milivojevic et al., 2017), it is critical for future studies to thoroughly examine behavioral, relational, and personality characteristics that might also explain or be associated with the neurobiological sex differences often observed in populations with SUD. Such patterns may be important for determining if the sex differences revealed in this study can be replicated and if so, clarifying why theses sex differences emerged in our examination of the connection between relationship functioning and SUD.
With regard to self-report clinical measures, IPV victimization and perpetration were associated with increased functional connectivity between the right amygdala and left prefrontal cortex during the conflict or neutral cues. Whereas IPV perpetration was only associated with this connectivity during the neutral cue, IPV victimization was associated with this connectivity during both the neutral and conflict cues. This suggests that IPV victimization and perpetration may be an especially important set of variables to examine when conducting examinations of functional connectivity as it may contribute to individual differences in neural patterns and their connections with behavior. While numerous studies have examined neural correlates of stress tasks, only three have done so in a sample specifically recruited due to IPV exposure (Fonzo et al., 2010; Satterthwaite et al., 2016; Simmons et al., 2008). These studies did not examine neural correlates of relationship conflict or IPV specifically. Because no other clinical measures were associated with functional connectivity, and because IPV is highly prevalent and salient in SUD populations (Afifi et al., 2009; Chermack et al., 2008; Leonard and Homish, 2008), our preliminary findings suggest that IPV is an important consideration in the refinement of this procedure.
Another important consideration is that IPV is heterogenous with regard to frequency, severity, and directionality (Henning et al., 2006; Langhinrichsen-Rohling et al., 2012). The neurocognitive effects of head trauma are also associated with IPV (Kwako et al., 2011). Future studies should explicitly examine this factor as it relates to neural correlates of couple functioning and IPV. Future studies would also benefit from applying this procedure among couples with co-occurring SUD and IPV and adapting the procedure to examine IPV-specific cues. Examining sex differences in response to IPV-specific cues is also an important future direction. Sex plays a prominent role in the link between social stress and addiction (Bobzean et al., 2014), and sex and gender roles are a longstanding and prominent topic of debate in the IPV field. For example, debate exists regarding motivations for use of IPV, the extent and nature of negative sequelae of IPV, and the impact of societal gender roles in the occurrence and maintenance of IPV between men and women in both heterosexual and same-sex couples.
In light of the expansion of couple interventions to other areas of mental and physical health intervention research (e.g., depression, cancer, and smoking) (Barbato and D’Avanzo, 2008; LaChance et al., 2015; Scott et al., 2004), results from this study may contribute to the refinement and adaptation of these procedures for use in other populations. While this study is the first to provide evidence to support the use of a relationship conflict paradigm that can be used during MRI scanning, additional work is needed to determine the full utility of the paradigm and to advance theory connecting relationship functioning with SUD.
The goal of the current study was to establish the utility and feasibility of a relationship conflict fMRI paradigm. Given this goal, some of the analyses were considered exploratory and did not correct for multiple comparisons, as in evaluating correlations between fMRI connectivity and clinical measures. Larger samples are needed in future work to increase statistical power and allow for more nuanced analyses that consider important covariates and moderators. The small sample size of the present study also limited our ability to account for non-independence of data within couples, which is a critical next step for future studies on this topic. Relatedly, only two couples in this sample were substance-discordant (one as opposed to both partners reported SUD), which prevented us from examining possible neural factors associated with this pattern within couples. For instance, research suggests that concordant versus discordant substance use behavior contributes significantly to SUD, treatment, IPV, and relationship outcomes among couples (Crane et al., 2016; Homish and Leonard, 2007; Leonard et al., 2014), and both directionality and severity of IPV are likely to play a role in partners’ responses to relationship conflict cues in SUD populations (Langhinrichsen-Rohling et al., 2012; Straus, 2008). Because relationship conflict and SUD have a bidirectional association and share common psychosocial underpinnings such as impulsivity, poor coping, and poor emotion regulation skills, future studies can improve on the current design by including a more comprehensive clinical battery.
There are several other limitations to consider. The present study did not employ a healthy control group including non-distressed, non-substance misusing couples, which is an important next step for future studies. While a validated interview instrument was employed to assess SUD diagnostic criteria, measurement can be improved in future studies by employing more nuanced structured clinical interviews to assess DSM-5 (rather than DSM-IV) diagnostic criteria for SUD. The study is limited by a lack of pre-post procedure ratings of subjective reactivity to the conflict discussion and the fMRI scan, as well as a lack of observational coding of specific partner behaviors during the conflict cue. Assessing participants’ objective conflict behaviors and self-reported subjective responses to the procedure will allow future studies to more accurately characterize partners’ experience of the procedures and to examine the extent to which the conflict content was meaningful to each participant. Importantly, these data will help characterize similarities and discrepancies between partners’ experiences of a co-created conflict, which is a common theme in couple research and treatment. Short-term longitudinal studies are also needed to examine the directionality of effects between relationship functioning and substance use chronicity, severity, and concordance within dyads. Couples in this study also varied in terms of the length of their relationship and overall, relationship length reported by participants was brief. Results may have differed among more established couples. Future studies should examine additional relationship factors such as commitment level when assessing neural correlates of dyadic conflict. Future studies would also benefit from exploring neural responses to substance use-specific conflict in order to map more clearly onto content commonly applied in couples therapies to treat SUD. Given the exploratory nature of the current study, we did not examine physiological reactivity to the cues employed here. Future research would benefit from including measures such as heart rate and cortisol reactivity, which have been employed in previous studies of stress reactivity.
In summary, the findings provide modest, preliminary support of the validity of the relationship conflict paradigm tested in this study. The findings suggest that a future study that is adequately designed with appropriate control groups and powered to test the validity of the paradigm is warranted. Findings also suggest that future studies to refine and adapt the procedure are warranted to advance the SUD treatment development field. This procedure could serve as an assessment tool to shed light on specific neural pathways through which relationship conflict increases risk of SUD, and vice versa. Understanding these neural pathways will inform theory relating to SUD and its relational underpinnings, and inform the development of future interventions that incorporate brain imaging as part of the initial assessments and symptom tracking.
Acknowledgements
This manuscript is the result of work supported, in part, by the National Institute on Child Health and Human Development and the Office of Research on Women’s Health (K12HD055885), the National Institute on Alcohol Abuse and Alcoholism (K23AA023845), the National Center for Advancing Translational Sciences (UL1TR001450) and the National Institute on Drug Abuse (K02 DA039229).
Footnotes
Declaration of interest
The authors have no conflicts of interest to disclose.
References
- Afifi TO, MacMillan H, Cox BJ, Asmundson GJG, Stein MB, Sareen J, 2009. Mental health correlates of intimate partner violence in marital relationships in a nationally representative sample of males and females. J. Interpers. Violence 24, 1398–1417. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, 2001. The Alcohol Use Disorders Identification Test World Health Organization, Geneva, Switzerland. [Google Scholar]
- Barbato A, D’Avanzo B, 2008. Efficacy of couple therapy as a treatment for depression: a meta-analysis. Psychiatr. Q 79, 121–132. [DOI] [PubMed] [Google Scholar]
- Bazargan-Hejazi S, De Lucia V, Pan D, Mojtahedzadeh M, Rahmani E, Jabori S, Bazargan M, 2016. Gender comparison in referrals and treatment completion to residential and outpatient alcohol treatment. Subst. Abus. Res. Treat 10, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Wüstenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A, 2012. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch. Gen. Psychiatry 69, 842–852. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M, 2008. Sex differences in drug abuse. Front. Neuroendocrinol 29, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex differences in animal models: focus on addiction. Pharmacol. Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean S, DeNobrega AK, Perrotti LI, 2014. Sex differences in the neurobiology of drug addiction. Exp. Neurol 259, 64–74. [DOI] [PubMed] [Google Scholar]
- Breslau J, Miller E, Jin R, Sampson NA, Alonso J, Andrade LH, Bromet EJ, De Girolamo G, Demyttenaere K, Fayyad J, 2011. A multinational study of mental disorders, marriage, and divorce. Acta Psychiatr. Scand 124, 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, Wang GJ, 2016. Neuroimaging the effectiveness of substance use disorder treatments. J. Neuroimmune Pharmacol 11, 408–433. [DOI] [PubMed] [Google Scholar]
- Cahill L, 2006. Why sex matters for neuroscience. Nat. Rev. Neurosci 7, 477–484. [DOI] [PubMed] [Google Scholar]
- Chermack ST, Murray RL, Walton MA, Booth BA, Wryobeck J, Blow FC, 2008. Partner aggression among men and women in substance use disorder treatment: correlates of psychological and physical aggression and injury. Drug Alcohol Depend 98, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane CA, Testa M, Schlauch RC, Leonard KE, 2016. The couple that smokes together: dyadic marijuana use and relationship functioning during conflict. Psychol. Addict. Behav 30, 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Floyd FJ, Schulenberg JE, Zucker RA, 2011. Husbands’ and wives’ alcohol use disorders and marital interactions as longitudinal predictors of marital adjustment. J. Abnorm. Psychol 120, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Kaag AM, van den Munkhof HE, Reneman L, Homberg JR, Sabbe B, van den Brink W, van Wingen G, 2015. Dysfunctional amygdala activation and connectivity with the prefrontal cortex in current cocaine users. Hum. Brain Mapp 36, 4222–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Blundell KA, Schofield TP, Schira MM, Krämer UM, 2018. The neural correlates of alcohol-related aggression. Cognit. Affect. Behav. Neurosci 18, 203–215. [DOI] [PubMed] [Google Scholar]
- Devries KM, Child JC, Bacchus LJ, Mak J, Falder G, Graham K, Watts C, Heise L, 2014. Intimate partner violence victimization and alcohol consumption in women: a systematic review and meta‐analysis. Addiction 109, 379–391. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M, 2007. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology 32, 565–574. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate cortex and medial prefrontal cortex. Trends Cognit. Sci 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, 2015. Reward processing and drug addiction: does sex matter? Front. Neurosci 9, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CA, Caetano R, 2003. Longitudinal model predicting partner violence among white, black, and Hispanic couples in the United States. Alcohol. Clin. Exp. Res 27, 1451–1458. [DOI] [PubMed] [Google Scholar]
- Filkowski MM, Olsen RM, Duda B, Wanger TJ, Sabatinelli D, 2017. Sex differences in emotional perception: meta analysis of divergent activation. NeuroImage 147, 925–933. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. ``Mini-mental state’’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB, 2010. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry 68, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R, 2009. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv. Rev. Psychiatry 17, 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ, 1997. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–229. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KE, Pugh LA, McCrady BS, Epstein EE, 2008. Unique aspects of female-primary alcoholic relationships. Addict. Disord. Treat 7, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM, 2007. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend 86, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JA, Epstein EE, McCrady BS, Gaba A, Cook S, Backer-Fulghum LM, Graff FS, 2013. Women’s motivators for seeking treatment for alcohol use disorders. Addict. Behav 38, 2236–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y, 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage 53, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlweg K, Kaiser A, Christensen A, Fehm‐Wolfsdorf G, Groth T, 2000. Self‐report and observational assessment of couples’ conflict: the concordance between the communication patterns questionnaire and the KPI observation system. J. Marriage Fam 62, 61–67. [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT, 2012. Orbitofrontal cortex biases attention to emotional events. J. Clin. Exp. Neuropsychol 34, 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner KL, Loving TJ, Kiecolt-Glaser JK, Himawan LK, Glaser R, Malarkey WB, 2006. Older spouses’ cortisol responses to marital conflict: associations with demand/withdraw communication patterns. J. Behav. Med 29, 317. [DOI] [PubMed] [Google Scholar]
- Hellmuth JC, McNulty JK, 2008. Neuroticism, marital violence, and the moderating role of stress and behavioral skills. J. Personal. Soc. Psychol 95, 166–180. [DOI] [PubMed] [Google Scholar]
- Henning K, Renauer B, Holdford R, 2006. Victim or offender? Heterogeneity among women arrested for intimate partner violence. J. Fam. Violence 21, 351–368. [Google Scholar]
- Holmes AJ, Hollinshead MO, Roffman JL, Smoller JW, Buckner RL, 2016. Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. J. Neurosci 36, 4038–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homish GG, Leonard KE, 2007. The drinking partnership and marital satisfaction: the longitudinal influence of discrepant drinking. J. Consult. Clin. Psychol 75, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Gyurak A, Verosky SC, Miyakawa A, Ayduk Ö, 2010. Neural activity to a partner’s facial expression predicts self-regulation after conflict. Biol. Psychiatry 67, 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y, 2014. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM, Fairman B, Gilreath T, Xuan Z, Rothman EF, Parnham T, Furr-Holden C, 2015. Past 15-year trends in adolescent marijuana use: differences by race/ethnicity and sex. Drug Alcohol Depend 155, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaag AM, Reneman L, Homberg JR, Van Den Brink W, van Wingen GA, 2018. Enhanced amygdala-striatal functional connectivity during the processing of cocaine cues in male cocaine users with a history of childhood trauma. Front. Psychiatry 9, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB, 2003. Love, marriage, and divorce: newlyweds’ stress hormones foreshadow relationship changes. J. Consult. Clin. Psychol 71, 176–188. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, 2015. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol. Ther 153, 55–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Glass N, Campbell J, Melvin KC, Barr T, Gill JM, 2011. Traumatic brain injury in intimate partner violence: a critical review of outcomes and mechanisms. Trauma Violence Abus 12, 115–126. [DOI] [PubMed] [Google Scholar]
- LaChance H, Cioe PA, Tooley E, Colby SM, O’Farrell TJ, Kahler CW, 2015. Behavioral couples therapy for smoking cessation: a pilot randomized clinical trial. Psychol. Addict. Behav 29, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhinrichsen-Rohling J, Selwyn C, Rohling ML, 2012. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse 3, 199–230. [Google Scholar]
- Lavner JA, Karney BR, Bradbury TN, 2016. Does couples’ communication predict marital satisfaction, or does marital satisfaction predict communication? J. Marriage Fam 78, 680–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow JL, Chambers AL, Christensen A, Johnson SM, 2012. Research on the treatment of couple distress. J. Marital Fam. Ther 38, 145–168. [DOI] [PubMed] [Google Scholar]
- Lemke S, Brennan PL, Schutte KK, Moos RH, 2007. Upward pressures on drinking: exposure and reactivity in adulthood. J. Stud. Alcohol Drugs 68, 437–445. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Eiden RD, 2007. Marital and family processes in the context of alcohol use and alcohol disorders. Ann. Rev. Clin. Psychol 3, 285–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE, Homish GG, 2008. Predictors of heavy drinking and drinking problems over the first 4 years of marriage. Psychol. Addict. Behav 22, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE, Smith PH, Homish GG, 2014. Concordant and discordant alcohol, tobacco, and marijuana use as predictors of marital dissolution. Psychol. Addict. Behav 28, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Kosten TR, Sinha R, 2005. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol. Psychiatry 57, 487–494. [DOI] [PubMed] [Google Scholar]
- Magill M, Kiluk BD, McCrady BS, Tonigan JS, Longabaugh R, 2015. Active ingredients of treatment and client mechanisms of change in behavioral treatments for alcohol use disorders: progress 10 years later. Alcoholi. Clin. Exp. Res 39, 1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, McKay JR, O’Farrell TJ, 1995. Relapse precipitants and behavioral marital therapy. Addict. Behav 20, 383–393. [DOI] [PubMed] [Google Scholar]
- Margolin G, Ramos MC, Baucom BR, Bennett DC, Guran EL, 2013. Substance use, aggression perpetration, and victimization: temporal co-occurrence in college males and females. J. Interpers. Violence 28, 2849–2872. [DOI] [PubMed] [Google Scholar]
- Mattson RE, O’Farrell TJ, Monson CM, Panuzio J, Taft CT, 2010. Female perpetrated dyadic psychological aggression predicts relapse in a treatment sample of men with substance use disorders. J. Fam. Violence 25, 33–42. [Google Scholar]
- McCrady BS, Epstein EE, Hallgren KA, Cook S, Jensen NK, 2016. Women with alcohol dependence: a randomized trial of couple versus individual plus couple therapy. Psychol. Addict. Behav 30, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Hayaki J, Epstein EE, Hirsch LS, 2002. Testing hypothesized predictors of change in conjoint behavioral alcoholism treatment for men. Alcoholi. Clin. Exp. Res 26, 463–470. [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Stein EA, Adinoff B, 2014. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front. Psychiatry 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Hearon BA, Otto MW, 2010. Cognitive-behavioral therapy for substance use disorders. Psychiatr. Clin. N. Am 33, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis LA, Griffin JM, Greer N, Jensen AC, MacDonald R, Carlyle M, Rutks I, Wilt TJ, 2013. Couple and family involvement in adult mental health treatment: a systematic review. Clin. Psychol. Rev 33, 275–286. [DOI] [PubMed] [Google Scholar]
- Milivojevic V, Fox HC, Sinha R, 2017. Sex differences and the role of sex hormones in stress reactivity, emotion regulation and craving in drug and alcohol dependence. Alcohol 60, 217–218. [Google Scholar]
- Miller MW, Wolf EJ, Reardon AF, Harrington KM, Ryabchenko K, Castillo DT, Freund R, Heyman RE, 2013. PTSD and conflict behavior between veterans and their intimate partners. J. Anxiety Disord 27, 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattala P, Leung K, Nagarajaiah, Murthy P, 2010. Family member involvement in relapse prevention improves alcohol dependence outcomes: a prospective study at an addiction treatment facility in India. J. Stud. Alcohol Drugs 71, 581–587. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SC, Stephens DN, Duka T, 2012. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology 37, 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell TJ, Clements K, 2012. Review of outcome research on marital and family therapy in treatment for alcoholism. J. Marital Fam. Ther 38, 122–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MD, Hallgren KA, Ladd BO, Rynes K, McCrady BS, Epstein E, 2013. Associations between relationship satisfaction and drinking urges for women in alcohol behavioral couples and individual therapy. Alcoholi. Treat. Q 31, 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I, 2012. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci 13, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R, 2012. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am. J. Psychiatry 169, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Vedel E, Emmelkamp PG, 2008. Behavioral couples therapy (BCT) for alcohol and drug use disorders: a meta-analysis. Clin. Psychol. Rev 28, 952–962. [DOI] [PubMed] [Google Scholar]
- Quigley BM, Crane CA, Testa M, 2013. Dyadic alcohol use as a moderator of the relationship between partner conflict and subsequent day relationship functioning: a daily diary analysis. Alcoholi. Clin. Exp. Res 37, 24A [Google Scholar]
- Rodriguez LM, Overup CS, Neighbors C, 2013. Perceptions of partners’ problematic alcohol use affect relationship outcomes beyond partner self-reported drinking: Alcohol use in committed romantic relationships. Psychol. Addict. Behav 27, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell DR, Siever LJ, 2015. The neurobiology of aggression and violence. CNS Spectr 20, 254–279. [DOI] [PubMed] [Google Scholar]
- Sabourin S, Valois P, Lussier Y, 2005. Development and validation of a brief version of the dyadic adjustment scale with a nonparametric item analysis model. Psychol. Assess 17, 15–27. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Cook PA, Bruce SE, Conway C, Mikkelsen E, Satchell E, Vandekar SN, Durbin T, Shinohara RT, Sheline YI, 2016. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol. Psychiatry 21, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta‐analysis and systematic review. Addict. Biol 18, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm JA, O’Farrell TJ, Kahler CW, Murphy MM, Muchowski P, 2014. A randomized clinical trial of behavioral couples therapy versus individually based treatment for women with alcohol dependence. J. Consult. Clin. Psychol 82, 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JL, Halford WK, Ward BG, 2004. United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. J. Consult. Clin. Psychol 72, 1122. [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R, 2011. Sex differences in neural responses to stress and alcohol context cues. Hum. Brain Mapp 32, 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB, 2008. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol. Psychiatry 64, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2001. How does stress increase risk of drug abuse and relapse? Psychopharmacology 158, 343–359. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2007. The role of stress in addiction relapse. Curr. Psychiatry Rep 9, 388–395. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2012. How does stress lead to risk of alcohol relapse? Alcohol Res. Curr. Rev 34, 432. [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM, 2009. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34, 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ, 2006. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry 63, 324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CR, 2007. Imaging stress and cue-induced drug and alcohol craving: association to relapse and clinical implications. Drug Alcohol Rev 26, 25–31. [DOI] [PubMed] [Google Scholar]
- Sinha R, Tuit K, 2012. Imagery Script Development Procedures Manual CreateSpace, Charleston, SC. [Google Scholar]
- Skinner HA, 1982. The drug abuse screening test. Addict. Behav 7, 363–371. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption Humana Press, Totowa, NJ. [Google Scholar]
- Straus MA, 2008. Dominance and symmetry in partner violence by male and female university students in 32 nations. Child. Youth Serv. Rev 30, 252–275. [Google Scholar]
- Straus MA, Hamby SL, Warren WL, 2003. The Conflict Tactics Scales Handbook: Revised Conflict Tactics Scale (CTS2) and CTS: Parent-Child Version (CTSPC) Western Psychological Services, Los Angeles, CA, USA. [Google Scholar]
- Sugarman DE, Kaufman JS, Trucco EM, Brown JC, Greenfield SF, 2014. Predictors of drinking and functional outcomes for men and women following inpatient alcohol treatment. Am. J. Addict 23, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Crane CA, Quigley BM, Levitt A, Leonard KE, 2014. Effects of administered alcohol on intimate partner interactions in a conflict resolution paradigm. J. Stud. Alcohol Drugs 75, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Derrick JL, 2013. A daily process examination of the temporal association between alcohol use and verbal and physical aggression in community couples. Psychol. Addict. Behav 28, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Livingston JA, Leonard KE, 2003. Women’s substance use and experiences of intimate partner violence: a longitudinal investigation among a community sample. Addict. Behav 28, 1649–1664. [DOI] [PubMed] [Google Scholar]
- Tobia MJ, Hayashi K, Ballard G, Gotlib IH, Waugh CE, 2017. Dynamic functional connectivity and individual differences in emotions during social stress. Hum. Brain Mapp 38, 6185–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, 2013. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry 70, 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade NE, Padula CB, Anthenelli RM, Nelson E, Eliassen J, Lisdahl KM, 2017. Blunted amygdala functional connectivity during a stress task in alcohol dependent individuals: a pilot study. Neurobiol. Stress 7, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitzer KS, Dermen KH, 2004. Alcohol-focused spouse involvement and behavioral couples therapy: evaluation of enhancements to drinking reduction treatment for male problem drinkers. J. Consult. Clin. Psychol 72, 944–955. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A, 2015. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev 57, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Uebelacker LA, Bruce ML, 2006. Longitudinal association between marital dissatisfaction and alcohol use disorders in a community sample. J. Fam. Psychol 20, 164. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CL, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA, 2014. Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev. Neurosci 25, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Pommy JM, Adinoff B, 2016. Neural circuitry of impaired emotion regulation in substance use disorders. Am. J. Psychiatry 173, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson HC, Rogge RD, Cobb RJ, Johnson MD, Lawrence E, Bradbury TN, 2015. Risk moderates the outcome of relationship education: a randomized controlled trial. J. Consult. Clin. Psychol 83, 617–629. [DOI] [PubMed] [Google Scholar]


