Abstract
Background
Epidemiological studies found that genetic factors are among the causes of schizophrenia, exclusively the genes involved in the dopamine system. Prior to this, the role of dopamine receptor D2 (DRD2) gene promoter polymorphisms and schizophrenia has been studied extensively, but there are still some uncertainties about these associations. The present study is focusing on the association between the DRD2 gene promoter region polymorphisms and schizophrenia in the northern Chinese Han population.
Methods
We sequenced 2,111‐bp fragment of DRD2 gene promoter region in 306 schizophrenic patients and 324 healthy controls to find association between DRD2 and schizophrenia. SPSS version 18 0.0 was used to calculate odds ratios (OR), 95% confidence intervals (CIs).The Hardy–Weinberg equilibrium test and the confirmation of haplotypes were calculated using Haploview version 4.1. The association of schizophrenic risk of DRD2 genotypes, alleles, and haplotypes between case and control groups was calculated using the chi‐squared test. PS program was used to calculate the Power analysis.
Results
The genotype frequencies of rs7116768 (p = 0.025) and rs1799732 (p = 0.042) were associated meagerly. After Bonferroni correction, there was no association found between DRD2 gene promoter region with schizophrenia risk in the northern Chinese Han population.
Conclusions
In this study, we did not find any significant difference between schizophrenia and the polymorphisms of DRD2 gene promoter region. A more forceful conclusion remains to be verified by further confirmatory experiments.
Keywords: association, dopamine, promoter region, schizophrenia
1. INTRODUCTION
Schizophrenia is a multifactorial common psychiatric disorder (Casey, Rodriguez, Northcott, Vickar, & Shihabuddin, 2011), in which both environmental and genetic factors play an important role. In Schizophrenia degree of inheritance, involvement may be more than 80% (Sullivan, Kendler, & Neale, 2003). Different hypotheses were created to explain the pathogenesis of schizophrenia and propose that these interact to funnel through one final common pathway of presynaptic striatal hyperdopaminergia. (Howes & Kapur, 2009). Dopamine receptors are proteins that bind to dopamine specifically. Mutations in the genes may lead to the changes of their expression or their ability to bind to dopamine which initiates the process of schizophrenia. The dopamine receptor D2 (DRD2) gene is located in 11q22~23 extends over 270 kb and contains eight exons and seven introns. Long use of classic antipsychotic medications, which may preferentially antagonize dopamine receptors. Previous studies which includes the autopsy results of patients with schizophrenia (Camps, Cortes, Gueye, Probst, & Palacios, 1989; Hess, Bracha, Kleinman, & Creese, 1987), findings from PET studies (Breier et al., 1997; Laruelle et al., 1996, 1997), and pharmacological evidence (Seeman, 2002; Worrel, Marken, Beckman, & Ruehter, 2000) suggested a close relationship for DRD2 gene and schizophrenia.
Up till now, most studies related to the association between the promoter region in DRD2 gene and schizophrenia mainly focused on rs1799732. Arinami, Gao, Hamaguchi, and Toru (1997) suggested that the del C allele in schizophrenic patients can reduce the level of the expression of DRD2 gene encoding area by 20%~40%. Subsequently, the following studies did not find a consistent conclusion in different geographical areas, such as England (Breen et al., 1999), Spain (Doehring, Kirchhof, & Lotsch, 2009), and in Japan (Hori, Ohmori, Shinkai, Kojima, & Nakamura, 2001). Ikeda et al. (2008) reported that rs1799978 is associated with the treatment and drug reaction against schizophrenia. The C allele of rs12364283 is associated with enhanced transcription and increased density of D2 receptors (Bertolino et al., 2009; Zhang et al., 2007). However, to fully explore the association of DRD2 gene and schizophrenia, more and more studies are required for better understanding.
In the current study, we try to explore whether the polymorphisms in the promoter region of DRD2 gene was involved in schizophrenia among the northern Chinese Han population.
2. MATERIALS AND METHODS
2.1. Study subjects
In this study, 306 schizophrenic patients and 324 healthy subjects were recruited. The schizophrenia group (n = 306) which came from the Third People's Hospital of Liaoning Province comprised of 154 males and 152 females, and the control group (n = 324) included 157 males and 167 females. The control samples were provided by China Medical University, and the individuals with any mental illness and other serious diseases were excluded. The mean age of patients was 44.6 ± 7.3 (mean ± standard deviation) years, and the mean age of healthy subjects was 45.3 ± 15.9 years. The mean onset age of case group was 25.68 ± 5.12. The disease duration was 5–23 years. The patients included were all paranoid schizophrenia. All patients were assessed for age at first hospitalization, first degree relatives with a history of mental illness, alcohol or drug abuse, antipsychotic reactions to schizophrenia, suicide attempts, and anticholinergic medication. Only patients who fully meet the diagnostic and statistical manual of mental disorders (fourth edition) were included in this study, which were diagnosed by expert professionals. Each of the subjects signed a written informed consent form before participating in this study. Sample collection and analysis have been approved by the Ethics Committee of China Medical University.
2.2. DNA extraction
Peripheral blood samples collected from each subject were stored in the EDTA tube. Genomic DNA was extracted applying phenol–chloroform method (Kramvis, Bukofzer, & Kew, 1996).
2.3. Segment selection and primer designing
In order to detect polymorphisms which have more possibility to impact the expression of DRD2 gene, we chose a 2,111‐bp fragment upstream of the 5′ untranslated region (5'UTR) in DRD2 gene. The fragment is closest to the coding region, and the polymorphisms within the fragment are more likely to affect gene expression. Sequencing primers were designed using the Primer Premier 5 Design Program (www.premierbiosoft.com). The sense and antisense primers used by PCRs were 5′‐CAACCATATCTGTAATGGCTGATCC‐3′ and 5′‐CTTCTAAGTGGCGAGGAGGCTAC‐3′, respectively.
2.4. Polymerase chain reaction amplification
Polymerase chain reactions were performed on TaKaRa PCR Thermal Cycler Dice™ (TP650) system (Japan) in a 20 µl reaction volume contains 4 µl 5× PrimeSTAR buffer, 2 µl dNTPs, 1.4 µl DMSO, 3 µl 15× each primer, 0.3 µl of PrimeStar HS polymerase 0.75 U (TaKaRa, Japan), and 3 µl of genomic DNA. The PCR condition was predenaturation at 98°C for 5 min, denaturation temperature of 98°C for 10 s, annealing temperature of 60.5°C for 5 s, extension temperature of 72°C for 2 min, and the number of cycles was 30.
2.5. Sequencing and alignment
The PCR products of around 2000 bp size were sent to the Taihe Biotechnology Co. (Beijing, China) Because of the technical limitations, it was not possible to sequence 2000 bp so we sequenced it in several segments. Primer details are in Table 1.
Table 1.
Sequencing primers of DRD2 gene
| Primer | Sequence 5′−3′ |
|---|---|
| A | CAACCATATCTGTAATGGCTGATCC |
| B | GGCGGTCGAGGGTTGCGTTCC |
| C | AGACCTGAAGTCAGAAAACG |
| D | GGAGTGGCCGCACAAACTTCTGGTC |
After successful sequencing, we aligned the sequenced sequences with the reference sequences which had been reported in the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/gene/) to identify polymorphisms.
2.6. Statistical analysis
Odds ratios (OR) and 95% confidence intervals (CIs) were calculated by SPSS 18.0 software (IBM, Armonk, NY, USA). The Hardy–Weinberg equilibrium test and the haplotype verification were evaluated using Haploview 4.1 software (Broad Institute, Cambridge, MA, USA). The chi‐square test was used to assess the associations between alleles, genotypes, haplotypes, and risk of schizophrenia, respectively. Bonferroni correction was used in multiple independent tests (p < 0.0125 was statistically significant). PS program (Dupont & Plummer, 1998) was used to calculate the Power analysis.
3. RESULTS
3.1. Genotype analysis
We detected six SNPs (rs7116768, rs1047479195, rs1799732, rs1799978, rs12364283, and rs80202441) through the analysis of sequencing results. Allele frequencies and genotype frequencies of the detected SNP loci are listed in Table 2.
Table 2.
Genotype and allele frequencies of DRD2 SNPs in control subjects and schizophrenia patients
| SNP | Case | Control | p‐Value | OR | 95% CI | Power | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Rs7116768 | 0.025 | |||||||
| G/G | 257 | 83.99 | 275 | 84.88 | ||||
| G/C | 33 | 10.78 | 44 | 13.58 | ||||
| C/C | 16 | 5.23 | 5 | 1.54 | ||||
| G/G + G/C | 290 | 94.77 | 319 | 98.46 | 0.013 | 0.284 | 0.103–0.785 | 0.729 |
| C allele | 65 | 10.5 | 54 | 8.3 | 0.178 | 1.307 | 0.895–1.910 | 0.283 |
| Rs1047479195 | 0.403 | |||||||
| C/C | 284 | 92.81 | 306 | 94.44 | ||||
| C/A | 20 | 6.54 | 14 | 4.32 | ||||
| A/A | 2 | 0.65 | 4 | 1.23 | ||||
| A/A + C/A | 22 | 7.19 | 18 | 5.55 | 0.418 | 1.317 | 0.692–2.506 | 0.135 |
| A allele | 24 | 3.9 | 22 | 3.4 | 0.654 | 1.161 | 0.644–2.094 | 0.079 |
| Rs1799732 | 0.042 | |||||||
| Ins/Ins | 257 | 83.99 | 275 | 84.88 | ||||
| Ins/Del | 34 | 11.11 | 44 | 13.58 | ||||
| Del/Del | 15 | 4.90 | 5 | 1.54 | ||||
| Ins/Ins + Ins/Del | 291 | 95.1 | 319 | 98.46 | 0.021 | 0.304 | 0.109–0.847 | 0.669 |
| Del allele | 64 | 10.5 | 54 | 8.3 | 0.209 | 1.285 | 0.878–1.879 | 0.253 |
| Rs1799978 | 0.120 | |||||||
| A/A | 209 | 68.30 | 208 | 64.20 | ||||
| G/A | 83 | 27.12 | 87 | 26.85 | ||||
| G/G | 14 | 4.58 | 28 | 8.64 | ||||
| A/A + G/A | 292 | 95.42 | 296 | 91.36 | 0.054 | 1.973 | 1.018–3.823 | 0.534 |
| G allele | 111 | 18.14 | 143 | 22.92 | 0.092 | 0.782 | 0.593–1.032 | 0.423 |
The SNPs with minor allele frequency <0.01 were excluded. The p‐value was calculated by 2 × 3 and 2 × 2 chi‐square test, in which the codominant model, the recessive model, and the allele model were corrected by Bonferroni's correction and the p < 0.05/4was statistically significant. The statistical power is considered to be enough to detect any significant difference when power >0.8. The false discovery rate <0.05.
Among six SNPs, four SNPs (rs12364283, rs1799732, rs7116768, and rs8020241) were in HWE in control groups (p > 0.05). The frequencies of rs12364283 and rs80202441 were too low to carry out a statistical analysis (the minor allele frequencies were 0.003 and 0.006, respectively). Rs1799978 and rs1047479195 were not associated with schizophrenia (p‐value was 0.120 and 0.403, respectively). However, the genotype frequencies of rs7116768 and rs1799732 had a relevance to the occurrence of schizophrenia (p‐value was 0.025 and 0.042, respectively). However, when a Bonferroni correction was applied to mitigate against the so‐called “multiple comparison problem” (where for a significant p‐value of 0.5, 5% of tests are likely to be significant by chance), no significances were found. There was no significant difference in the allele frequencies of each site between the case group and the control group.
Studies have shown that gender differences affect the correlation between candidate genes and schizophrenia (Hoenicka et al., 2010). We evaluated the association of the detected SNPs with schizophrenia risk using gender as a classification criterion conducting by chi‐square test, as shown in Tables 3 and 4. We found that in the male population, the C allele (p = 0.015) and C/C genotype frequency (p = 0.037) of rs7116768 may be associated with schizophrenia susceptibility. However, when a Bonferroni correction was applied to mitigate against the so‐called “multiple comparison problem” (where for a significant p‐value of 0.5, 5% of tests are likely to be significant by chance), no significances were found. We do not have enough statistical power to detect any significant difference in the overall sample size and separately for men and women by the standards of 0.8.
Table 3.
Genotype and allele frequencies of DRD2 SNPs in control male subjects and schizophrenia male patients
| SNP | Case | Control | p‐Value | OR | 95% CI | Power | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Rs7116768 | 0.037 | |||||||
| G/G | 131 | 85.06 | 132 | 84.08 | ||||
| G/C | 14 | 9.10 | 23 | 14.65 | ||||
| C/C | 9 | 5.84 | 2 | 1.27 | ||||
| G/G + G/C | 145 | 94.16 | 155 | 98.73 | 0.034 | 0.208 | 0.044–0.978 | 0.586 |
| C allele | 32 | 10.39 | 54 | 8.60 | 0.015 | 1.791 | 1.121–2.863 | 0.625 |
| Rs1047479195 | 0.576 | |||||||
| C/C | 143 | 92.86 | 150 | 95.54 | ||||
| C/A | 9 | 5.84 | 5 | 3.18 | ||||
| A/A | 2 | 1.30 | 2 | 1.27 | ||||
| A/A + C/A | 11 | 7.14 | 7 | 4.46 | 0.341 | 1.648 | 0.622–4.370 | 0.180 |
| A allele | 13 | 4.22 | 9 | 2.87 | 0.392 | 0.670 | 0.282–1.590 | 0.117 |
| Rs1799732 | 0.075 | |||||||
| Ins/Ins | 131 | 85.06 | 132 | 84.08 | ||||
| Ins/Del | 15 | 9.74 | 23 | 14.65 | ||||
| Del/Del | 8 | 5.19 | 2 | 1.27 | ||||
| Ins/Ins + Ins/Del | 146 | 94.81 | 155 | 98.73 | 0.059 | 0.235 | 0.049–1.127 | 0.500 |
| Del allele | 64 | 10.06 | 54 | 8.60 | 0.262 | 0.792 | 0.530–1.184 | 0.121 |
| Rs1799978 | 0.120 | |||||||
| A/A | 109 | 70.78 | 104 | 66.24 | ||||
| G/A | 36 | 23.38 | 39 | 24.84 | ||||
| G/G | 9 | 5.84 | 14 | 8.92 | ||||
| A/A + G/A | 145 | 94.16 | 143 | 92.86 | 0.387 | 1.577 | 0.662–3.760 | 0.153 |
| G allele | 54 | 17.53 | 67 | 21.34 | 0.265 | 1.276 | 0.856–1.901 | 0.251 |
The SNPs with minor allele frequency <0.01 were excluded. The p‐value was calculated by 2 × 3 and 2 × 2 chi‐square test, in which the codominant model, the recessive model, and the allele model were corrected by Bonferroni's correction and the p < 0.05/4was statistically significant. The statistical power is considered to be enough when power >0.8. The false discovery rate <0.05.
Table 4.
Genotype and allele frequencies of DRD2 SNPs in control female subjects and schizophrenia female patients
| SNP | Case | Control | p‐Value | OR | 95% CI | Power | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Rs7116768 | 0.373 | |||||||
| G/G | 126 | 82.89 | 143 | 85.63 | ||||
| G/C | 19 | 12.50 | 21 | 12.57 | ||||
| C/C | 7 | 4.61 | 3 | 1.80 | ||||
| G/G + G/C | 145 | 95.39 | 164 | 98.20 | 0.202 | 0.379 | 0.096–1.492 | 0.305 |
| C allele | 33 | 10.86 | 27 | 8.08 | 0.277 | 0.722 | 0.423–1.232 | 0.178 |
| Rs1047479195 | 0.485 | |||||||
| C/C | 141 | 92.86 | 156 | 93.41 | ||||
| C/A | 11 | 5.84 | 9 | 5.39 | ||||
| A/A | 0 | 1.30 | 2 | 1.20 | ||||
| A/A + C/A | 11 | 7.24 | 11 | 6.59 | 0.829 | 0.904 | 0.380–2.149 | 0.055 |
| A allele | 11 | 3.62 | 13 | 3.89 | 1.000 | 1.079 | 0.476–2.445 | 0.054 |
| Rs1799732 | 0.373 | |||||||
| Ins/Ins | 126 | 82.89 | 143 | 85.63 | ||||
| Ins/Del | 19 | 12.5 | 21 | 12.57 | ||||
| Del/Del | 7 | 4.61 | 3 | 1.80 | ||||
| Ins/Ins +Ins/Del | 145 | 95.39 | 164 | 98.20 | 0.202 | 0.379 | 0.096–1.492 | 0.374 |
| Del allele | 33 | 10.86 | 27 | 8.08 | 0.277 | 0.722 | 0.423–1.232 | 0.178 |
| Rs1799978 | 0.710 | |||||||
| A/A | 100 | 65.79 | 105 | 62.87 | ||||
| G/A | 43 | 28.29 | 48 | 28.74 | ||||
| G/G | 9 | 5.92 | 14 | 8.38 | ||||
| A/A + G/A | 143 | 94.08 | 153 | 91.62 | 0.517 | 1.454 | 0.610–3.463 | 0.133 |
| G allele | 61 | 20.07 | 76 | 22.75 | 0.441 | 1.173 | 0.803–1.716 | 0.139 |
The SNPs with minor allele frequency <0.01 were excluded. The p‐value was calculated by 2 × 3 and 2 × 2 chi‐square test, in which the codominant model, the recessive model, and the allele model were corrected by Bonferroni's correction and the p < 0.05/4was statistically significant. The statistical power is considered to be enough to detect any significant difference when power >0.8. The false discovery rate <0.05.
3.2. Linkage disequilibrium and haplotypes
We employed Haploview 4.2 program to assess the Linkage disequilibrium (LD) block and haplotypes of the four SNPs. Among rs7116768, rs1799732, rs1047479195, and rs1799978, an LD block was made in Figure 1 (rs7199732 and rs7116768 D′ = 1.0, r 2 = 1.0; rs1799978 and rs7116768 D′ = 1.0, r 2 = 0.026; rs7199978 and rs7116768 D′ = 0.934, r 2 = 0.108; rs1799978 and rs1047479195 D′ = 1.0, r 2 = 0.026).
Figure 1.
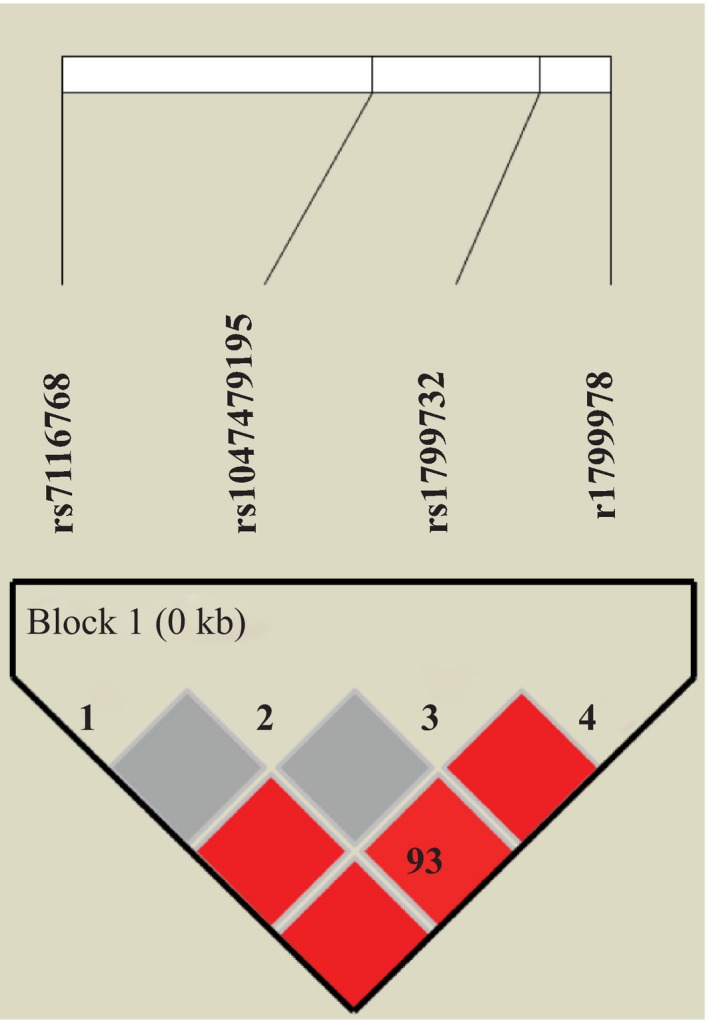
Linkage disequilibrium block composed by rs7116768, rs1799732, rs1047479195, and rs1799978. The number is the value of multiallelic D′, which represents the level of recombination between the two blocks
The relationship of haplotype distribution with schizophrenia was also analyzed. We found no association for both haplotype and the risk of schizophrenia (shown in Table 5).
Table 5.
Haplotype analysis of DRD2 SNPs in control subjects and schizophrenia patients
| Haplotype | SNP | Case | Control | p‐Value | OR | CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs7116768 | Rs1047479195 | Rs1799732 | Rs1799978 | N | % | N | % | ||||
| 1 | G | C | insC | A | 216 | 70.6 | 225 | 69.4 | 0.794 | 1.056 | 0.751–1.485 |
| 2 | G | C | insC | G | 46 | 14.9 | 61 | 18.8 | 0.243 | 0.763 | 0.502–1.160 |
| 3 | C | C | delC | A | 32 | 10.5 | 27 | 8.3 | 0.412 | 1.285 | 0.750–2.200 |
Haplotype with frequency <0.05 was excluded.
4. DISCUSSION
In the present study, we found both of the rs7116768 and rs7199732 have a statistical difference between schizophrenic patients and control group. Nevertheless, after the Bonferroni correction, both of the observed associations with schizophrenia tended to disappear. Similarly, we did not find any association between DRD2 promoter polymorphisms and schizophrenia in the gender groups, which are consistent with the study of Xiao et al. (2013). SNP is a bimodal genetic marker for low heterozygosity. In order to improve the heterozygosity and to use the genetic information more effectively, we performed haplotype analysis. And no statistical difference was found in all of the four haplotypes between schizophrenia patients and control subjects.
Previous studies mainly focused on the relationship between DRD2 gene and schizophrenia were largely limited to coding regions (Liu et al., 2014; Liu, Liu, An, Zhang, & Wang, 2012; Yang et al., 2016) and showed that the DRD2 gene coding region polymorphisms of rs1076560, rs6277, and so on were associated with schizophrenia in the Chinese Han population (Fan et al., 2010; Zheng, Shen, & Xu, 2012). As for the promoter region of DRD2 gene, previous studies were mainly focused on rs1799732 (shown in Table 6). Our results are also lining with previous studies which mostly were negative studies (Parsons et al., 2007; Stober et al., 1998; Tallerico, Ulpian, & Liu, 1999), except for several positive ones. As for the studies with positive findings, China (Peng, Wang, Cheng, Zhang, & Jiang, 2005) and Brazil (Cordeiro, Siqueira‐Roberto, Zung, & Vallada, 2009) showed that del C allele was a protective factor while Scotland (Breen et al., 1999) had an adverse conclusion.
Table 6.
Previous studies on the association with rs1799732 and the etiology of schizophrenia
| Author (Year) | Region | SNP | Sample size (case/control) | p‐Value |
|---|---|---|---|---|
| Breen et al. (1999) | Scotland | rs1799732 | 439/437 | 0.02 |
| Peng et al. (2005) | China | rs1799732 | 120/100 | <0.05 |
| Cordeiro et al. (2009) | Brazil | rs1799732 | 229/733 | 0.001 |
| Xiao et al. (2013) | China | rs1799732 | 120/100 | <0.05 |
| Dubertret et al. (2010) | France | rs1799732 | 108 trios | 0.991 |
| Rohrmeier et al. (2003) | Germany | rs1799732 | 190 trios | 0.696 |
| Himei et al. (2002) | Japan | rs1799732 | 190/103 | 0.520 |
| Behravan et al. (2008) | Iran | rs1799732 | 38/63 | 0.94 |
| Parsons et al. (2007) | Spain | rs1799732 | 119/165 | >0.05 |
These inconsistent results may be due to the use of case–control study in these studies. The method is used widely because of its convenience and precision positioning. However, the approach is vulnerable to the effect of racial stratification, sample population, and sample content. Sample population issues may also explain the inconsistencies between our results and some meta‐analyses conducted in Asia (He et al., 2016; Wang et al., 2016) and even in China (Zhao et al., 2016). China is a vast country with a great geographical distance between north and south. In previous studies, most of the samples were from southern China, such as Wuhan (Peng et al., 2005; Xiao et al., 2013). We cannot exclude the influence of population structure differences on the results, so these conclusions cannot fully represent the situation of Han population in north China. This study can provide references for the association between DRD2 promoter region and schizophrenia in the Han population in north China. The case–control study based on blood relationship is more referable because it excludes the influence factors of the population admixture (Kazeem & Farrall, 2005) such as French (Dubertret et al., 2010) and Germanic (Rohrmeier et al., 2003) family studies, they also end up at negative findings. Himei et al. (2002) found no association for several DRD2 gene polymorphisms with schizophrenia and a severe association of positive symptoms of patients with the −141 C Del allele by studying the association with each polymorphism and PANSS score. All of these evidences implied that rs1799732 may not be directly related to the occurrence of schizophrenia, but through its del C allele affects the symptoms of schizophrenia patients.
Related to rs7116768, there was no reported study involved the association with this SNP locus and schizophrenia. Our study found that rs7116768 was slightly associated with schizophrenia in the northern Chinese Han population. Rs7116768 locates in 5'UTR in DRD2 gene, and the base sequence of this region is transcribed for RNA without being translated into amino acids. The transcriptional products are removed during the modification of mature mRNA. This SNP site may be in a target region of transcription factors and cis‐regulatory elements. It may influence the expression level of DRD2 gene by regulating the expression and stability of mRNA, and then change the activity of dopamine neurotransmitters to lead the onset of schizophrenia. The number of samples of this study is not sufficient for significantly reflecting the association between this polymorphic site and the risk of schizophrenia. Usage of Bonferroni correction in this study may increase the false‐negative rate. However, to fully explore the association with rs7116768 and schizophrenia, much more data are needed.
5. CONCLUDING REMARKS
In this study, we found no association for DRD2 gene promoter region with schizophrenia risk in the northern Chinese Han population. But we cannot definitively exclude the possible association between DRD2 gene promoter region and schizophrenia risk. Additional studies of rs7116768 and other DRD2 SNPs will be required on a large data set. We hope our study data can provide a reference for future research.
CONFLICT OF INTEREST
The authors report no conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
X.Z. wrote the initial manuscript. X.Z., A.A., Y.L., Y.L., and J.Y. conducted the experiment. X.Z., J.Y., J.X., J.X., M.D., A.A., and B.W. analyzed the results. A.A modified the manuscript. All authors reviewed the manuscript.
ETHICAL APPROVAL
All participants gave their informed consent in writing after the study aims and procedures were carefully explained to them in their own language. The study was approved by the ethical review board of the China Medical University, Shenyang Liaoning Province, People's Republic of China and in accordance with the standards of the Declaration of Helsinki.
ACKNOWLEDGMENT
This study was supported by National Natural Science Foundation of China (No. 81601653).
Zhang X, Ding M, Adnan A, et al. No association between polymorphisms in the promoter region of dopamine receptor D2 gene and schizophrenia in the northern Chinese Han population: A case–control study. Brain Behav. 2019;9:e01193 10.1002/brb3.1193
Contributor Information
Jun Yao, Email: yaojun198717@163.com.
Bao‐jie Wang, Email: baojiewangcmu@gmail.com.
REFERENCES
- Arinami, T. , Gao, M. , Hamaguchi, H. , & Toru, M. (1997). A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Human Molecular Genetics, 6(4), 577–582. 10.1093/hmg/6.4.577 [DOI] [PubMed] [Google Scholar]
- Bertolino, A. , Fazio, L. , Caforio, G. , Blasi, G. , Rampino, A. , Romano, R. , … Sadee, W. (2009). Functional variants of the dopamine receptor D2 gene modulate prefronto‐striatal phenotypes in schizophrenia. Brain, 132(Pt 2), 417–425. 10.1093/brain/awn248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behravan, J. , Hemayatkar, M. , Toufani, T. , & Abdollahian, E. (2008). Linkage and Association of DRD2 Gene Taq I Polymorphism with Schizophrenia in an Iranian Population. Archives of Iranian Medicine, 11(3), 252–256. https://doi.org/08113/AIM.004 [PubMed] [Google Scholar]
- Breen, G. , Brown, J. , Maude, S. , Fox, H. , Collier, D. , Li, T. , … StClair, D. (1999). 141 C del/ins polymorphism of the dopamine receptor 2 gene is associated with schizophrenia in a British population. American Journal of Medical Genetics, 88(4), 407–410. [DOI] [PubMed] [Google Scholar]
- Breier, A. , Su, T. P. , Saunders, R. , Carson, R. E. , Kolachana, B. S. , de Bartolomeis, A. , … Pickar, D. (1997). Schizophrenia is associated with elevated amphetamine‐induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences U S A, 94(6), 2569–2574. 10.1073/pnas.94.6.2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps, M. , Cortes, R. , Gueye, B. , Probst, A. , & Palacios, J. M. (1989). Dopamine receptors in human brain: Autoradiographic distribution of D2 sites. Neuroscience, 28(2), 275–290. 10.1016/0306-4522(89)90179-6 [DOI] [PubMed] [Google Scholar]
- Casey, D. A. , Rodriguez, M. , Northcott, C. , Vickar, G. , & Shihabuddin, L. (2011). Schizophrenia: Medical illness, mortality, and aging. International Journal of Psychiatry in Medicine, 41(3), 245–251. 10.2190/PM.41.3.c [DOI] [PubMed] [Google Scholar]
- Cordeiro, Q. , Siqueira‐Roberto, J. , Zung, S. , & Vallada, H. (2009). Association between the DRD2‐141C Insertion/Deletion polymorphism and schizophrenia. Arquivos De Neuro‐Psiquiatria, 67(2A), 191–194. 10.1590/S0004-282X2009000200004 [DOI] [PubMed] [Google Scholar]
- Doehring, A. , Kirchhof, A. , & Lotsch, J. (2009). Genetic diagnostics of functional variants of the human dopamine D2 receptor gene. Psychiatric Genetics, 19(5), 259–268. 10.1097/YPG.0b013e32832d0941 [DOI] [PubMed] [Google Scholar]
- Dubertret, C. , Bardel, C. , Ramoz, N. , Martin, P. M. , Deybach, J. C. , Ades, J. , … Gouya, L. (2010). A genetic schizophrenia‐susceptibility region located between the ANKK1 and DRD2 genes. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 34(3), 492–499. 10.1016/j.pnpbp.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Dupont, W. D. , & Plummer, W. D. Jr (1998). Power and sample size calculations for studies involving linear regression. Controlled Clinical Trials, 19(6), 589–601. 10.1016/S0197-2456(98)00037-3 [DOI] [PubMed] [Google Scholar]
- Fan, H. , Zhang, F. , Xu, Y. , Huang, X. , Sun, G. , Song, Y. , … Liu, P. (2010). An association study of DRD2 gene polymorphisms with schizophrenia in a Chinese Han population. Neuroscience Letters, 477(2), 53–56. 10.1016/j.neulet.2009.11.017 [DOI] [PubMed] [Google Scholar]
- He, H. , Wu, H. , Yang, L. , Gao, F. , Fan, Y. , Feng, J. , & Ma, X. (2016). Associations between dopamine D2 receptor gene polymorphisms and schizophrenia risk: A PRISMA compliant meta‐analysis. Neuropsychiatric Disease and Treatment, 12, 3129–3144. 10.2147/NDT.S118614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, E. J. , Bracha, H. S. , Kleinman, J. E. , & Creese, I. (1987). Dopamine receptor subtype imbalance in schizophrenia. Life Sciences, 40(15), 1487–1497. 10.1016/0024-3205(87)90381-X [DOI] [PubMed] [Google Scholar]
- Himei, A. , Koh, J. , Sakai, J. , Inada, Y. , Akabame, K. , & Yoneda, H. (2002). The influence on the schizophrenic symptoms by the DRD2Ser/Cys311 and ‐141C Ins/Del polymorphisms. Psychiatry and Clinical Neurosciences, 56(1), 97–102. 10.1046/j.1440-1819.2002.00935.x [DOI] [PubMed] [Google Scholar]
- Hoenicka, J. , Garrido, E. , Ponce, G. , Rodriguez‐Jimenez, R. , Martinez, I. , Rubio, G. , … Palomo, T. (2010). Sexually dimorphic interaction between the DRD1 and COMT genes in schizophrenia. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics., 153B(4), 948–954. 10.1002/ajmg.b.31065 [DOI] [PubMed] [Google Scholar]
- Hori, H. , Ohmori, O. , Shinkai, T. , Kojima, H. , & Nakamura, J. (2001). Association analysis between two functional dopamine D2 receptor gene polymorphisms and schizophrenia. American Journal of Medical Genetics, 105(2), 176–178. 10.1002/ajmg.1196 [DOI] [PubMed] [Google Scholar]
- Howes, O. D. , & Kapur, S. (2009). The dopamine hypothesis of schizophrenia: Version III–the final common pathway. Schizophrenia Bulletin, 35(3), 549–562. 10.1093/schbul/sbp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M. , Yamanouchi, Y. , Kinoshita, Y. , Kitajima, T. , Yoshimura, R. , Hashimoto, S. , … Iwata, N. (2008). Variants of dopamine and serotonin candidate genes as predictors of response to risperidone treatment in first‐episode schizophrenia. Pharmacogenomics, 9(10), 1437–1443. 10.2217/14622416.9.10.1437 [DOI] [PubMed] [Google Scholar]
- Kazeem, G. R. , & Farrall, M. (2005). Integrating case‐control and TDT studies. Annals of Human Genetics, 69(Pt 3), 329–335. 10.1046/j.1529-8817.2005.00156.x [DOI] [PubMed] [Google Scholar]
- Kramvis, A. , Bukofzer, S. , & Kew, M. C. (1996). Comparison of hepatitis B virus DNA extractions from serum by the QIAamp blood kit, GeneReleaser, and the phenol‐chloroform method. Journal of Clinical Microbiology, 34(11), 2731–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle, M. , Abi‐Dargham, A. , van Dyck, C. H. , Gil, R. , D'Souza, C. D. , Erdos, J. , … Innis, R. B. (1996). Single photon emission computerized tomography imaging of amphetamine‐induced dopamine release in drug‐free schizophrenic subjects. Proceedings of the National Academy of Sciences U S A, 93(17), 9235–9240. 10.1073/pnas.93.17.9235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle, M. , D'Souza, C. D. , Baldwin, R. M. , Abi‐Dargham, A. , Kanes, S. J. , Fingado, C. L. , … Innis, R. B. (1997). Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology, 17(3), 162–174. 10.1016/S0893-133X(97)00043-2 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Fan, D. , Ding, N. , Hu, Y. , Cai, G. , Wang, L. , … Pan, F. (2014). The relationship between DRD2 gene polymorphisms (C957T and C939T) and schizophrenia: A meta‐analysis. Neuroscience Letters, 583, 43–48. 10.1016/j.neulet.2014.09.024 [DOI] [PubMed] [Google Scholar]
- Liu, Z. W. , Liu, J. L. , An, Y. , Zhang, L. , & Wang, Y. M. (2012). Association between Ser311Cys polymorphism in the dopamine D2 receptor gene and schizophrenia risk: A meta‐analysis in Asian populations. Genetics and Molecular Research, 11(1), 261–270. 10.4238/2012.February.8.1 [DOI] [PubMed] [Google Scholar]
- Parsons, M. J. , Mata, I. , Beperet, M. , Iribarren‐Iriso, F. , Arroyo, B. , Sainz, R. , … Kerwin, R. (2007). A dopamine D2 receptor gene‐related polymorphism is associated with schizophrenia in a Spanish population isolate. Psychiatric Genetics, 17(3), 159–163. 10.1097/YPG.0b013e328017f8a4 [DOI] [PubMed] [Google Scholar]
- Peng, D. H. , Wang, G. H. , Cheng, Z. L. , Zhang, D. X. , & Jiang, K. D. (2005). Association of schizophrenia with a promoter polymorphism in the dopamine D2 receptor gene. Zhonghua Yi Xue Yi Chuan Xue Za Zhi, 22(1), 94–95. [PubMed] [Google Scholar]
- Rohrmeier, T. , Putzhammer, A. , Sartor, H. , Knapp, M. , Albus, M. , Borrmann‐Hassenbach, M. , … Eichhammer, P. (2003). No association of 141C‐ins/del polymorphism in the D2 dopamine receptor gene in schizophrenia. Psychiatrische Praxis, 30(Suppl 2), S212–215. [PubMed] [Google Scholar]
- Seeman, P. (2002). Atypical antipsychotics: Mechanism of action. Canadian Journal of Psychiatry, 47(1), 27–38. 10.1177/070674370204700106 [DOI] [PubMed] [Google Scholar]
- Stober, G. , Jatzke, S. , Heils, A. , Jungkunz, G. , Knapp, M. , Mossner, R. , … Lesch, K. P. (1998). Insertion/deletion variant (‐141C Ins/Del) in the 5' regulatory region of the dopamine D2 receptor gene: Lack of association with schizophrenia and bipolar affective disorder. Short Communication. Journal of Neural Transmission, 105(1), 101–109. 10.1007/s007020050041 [DOI] [PubMed] [Google Scholar]
- Sullivan, P. F. , Kendler, K. S. , & Neale, M. C. (2003). Schizophrenia as a complex trait: Evidence from a meta‐analysis of twin studies. Archives of General Psychiatry, 60(12), 1187–1192. 10.1001/archpsyc.60.12.1187 [DOI] [PubMed] [Google Scholar]
- Tallerico, T. , Ulpian, C. , & Liu, I. S. (1999). Dopamine D2 receptor promoter polymorphism: No association with schizophrenia. Psychiatry Research, 85(2), 215–219. 10.1016/S0165-1781(98)00125-5 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Liu, L. , Xin, L. , Fan, D. , Ding, N. , Hu, Y. , … Pan, F. (2016). The ‐141C Ins/Del and Taq1A polymorphism in the dopamine D2 receptor gene may confer susceptibility to schizophrenia in Asian populations. Journal of Clinical Neuroscience, 30, 1–7. 10.1016/j.jocn.2015.10.052 [DOI] [PubMed] [Google Scholar]
- Worrel, J. A. , Marken, P. A. , Beckman, S. E. , & Ruehter, V. L. (2000). Atypical antipsychotic agents: A critical review. American Journal of Health System Pharmacy, 57(3), 238–255. [DOI] [PubMed] [Google Scholar]
- Xiao, L. , Shen, T. , Peng, D. H. , Shu, C. , Jiang, K. D. , & Wang, G. H. (2013). Functional ‐141C Ins/Del polymorphism in the dopamine D2 receptor gene promoter and schizophrenia in a Chinese Han population. Journal of International Medical Research, 41(4), 1171–1178. 10.1177/0300060513483415 [DOI] [PubMed] [Google Scholar]
- Yang, B. , Niu, W. , Chen, S. , Xu, F. , Li, X. , Wu, X. , … He, G. (2016). Association study of dopamine receptor genes polymorphisms with the risk of schizophrenia in the Han Chinese population. Psychiatry Research, 245, 361–364. 10.1016/j.psychres.2016.08.052 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Bertolino, A. , Fazio, L. , Blasi, G. , Rampino, A. , Romano, R. , … Sadee, W. (2007). Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proceedings of the National Academy of Sciences U S A, 104(51), 20552–20557. 10.1073/pnas.0707106104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Huang, Y. , Chen, K. , Li, D. , Han, C. , & Kan, Q. (2016). 141C insertion/deletion polymorphism of the dopamine D2 receptor gene is associated with schizophrenia in Chinese Han population: Evidence from an ethnic group‐specific meta‐analysis. Asia‐Pacific Psychiatry: Official Journal of the Pacific Rim College of Psychiatrists, 8(3), 189–198. 10.1111/appy.12206 [DOI] [PubMed] [Google Scholar]
- Zheng, C. , Shen, Y. , & Xu, Q. (2012). Rs1076560, a functional variant of the dopamine D2 receptor gene, confers risk of schizophrenia in Han Chinese. Neuroscience Letters, 518(1), 41–44. 10.1016/j.neulet.2012.04.052 [DOI] [PubMed] [Google Scholar]


