Abstract
Background and objectives
Several major risk factors for cancer involve vascular oversupply of energy to affected tissues. These include obesity, diabetes and chronic inflammation. Here, we propose a potential mechanistic explanation for the association between energy oversupply and cancer risk, which we call the metabolic cancer suppression hypothesis: We hypothesize that oncogenesis is normally suppressed by organismal physiology that regulates and strictly limits normal energy supply to somatic cells, and that this protection is removed by abnormal oversupply of energy.
Methodology
We evaluate this hypothesis using a computational model of somatic cell evolution to simulate experimental manipulation of the vascular energy supply to a tissue. The model simulates the evolutionary dynamics of somatic cells during oncogenesis.
Results
In our simulation experiment, we found that under plausible biological assumptions, elevated energy supply to a tissue led to the evolution of elevated energy uptake by somatic cells, leading to the rapid evolution of both defining traits of cancer cells: hyperproliferation, and tissue invasion.
Conclusions and implications
Our results support the hypothesis of metabolic cancer suppression, suggesting that vascular oversupply of energetic resources to somatic cells removes normal energetic limitations on cell proliferation, and that this accelerates cellular evolution toward cancer. Various predictions of this hypothesis are amenable to empirical testing, and have promising implications for translational research toward clinical cancer prevention.
Keywords: cancer, cell metabolism, cell energetics, oncogenesis, cancer prevention
BACKGROUND AND OBJECTIVES
It is generally accepted that cancer develops through somatic mutation and clonal selection, but selection is more difficult to observe. As a result, quantitative measures of selection in cancer evolution have lagged, and the relative importance of selection has been debated [1]. Relevant to this debate, it has recently been shown that the somatic ‘driver’ mutations most strongly associated with cancers, and most positively selected in cancers, are also common in normal somatic tissues [2, 3], where they do not reach such high frequency, suggesting that they are not strongly selected in these normal tissues, despite being under strong selection in cancers [4]. This suggests that the difference between normal cells versus those involved in oncogenesis resides not only in what mutations arise, but also in what mutations are positively selected. This in turn suggests that a critical influence on the oncogenesis process lies in the distinctive selective microenvironment of those tissues that give rise to cancer.
The idea has been suggested previously that oncogenic microenvironments may somehow select for the malignant properties of cancer cells, but usually without detailing specific and testable hypotheses. One specific suggestion is that on all biological scales, disturbed resource-rich environments select for ‘profiteering phenotypes’, such as those of cancer cells [5]. This suggestion is plausible based on the described parallels between cancer evolution and classical ecology. However, no causal mechanism was suggested for the effect on cancer risk, and no explicit connection was drawn to cancer epidemiology. We therefore undertake here to formalize this hypothesis in a computational model to study its dynamics and to evaluate how well it conforms with the principles of clonal evolution by natural selection, and with recognized patterns in cancer epidemiology.
Multiple cancer risk factors may share the same underlying mechanism
Several major risk factors for cancer involve vascular oversupply of energy to affected tissues (Supplementary Table S1). Although each of these four factors is strongly associated with cancer risk, causal mechanisms have generally not been demonstrated.
Here, we propose the metabolic cancer suppression hypothesis as a potential mechanistic explanation for the association between cancer risk and vascular oversupply of energy to tissues, which is present in each of the four conditions listed in Supplementary Table S1. The hypothesis is that dysregulated oversupply of energetic resources to somatic cells abrogates the normal metabolic cancer suppression created through organismal limitation of tissue energy supply, and thereby accelerates cellular evolution toward cancer.
MICROENVIRONMENTAL ECOLOGY SHAPES THE SOMATIC EVOLUTION OF CANCER
As principles from ecology and evolutionary biology are increasingly applied to cancer biology, e.g. [5] this perspective emphasizes the importance of the cell microenvironment, which provides the selective pressures that drive somatic cell evolution during oncogenesis. Here, we apply this evolutionary ecology perspective to cancer etiology and prevention. Using an in silico evolutionary model of oncogenesis, we formalize and evaluate the hypothesis that cellular access to excess energy is a direct causal factor in oncogenesis. We suggest that cancer is normally suppressed by organismal physiology that regulates and strictly limits normal energy supply to somatic cells, and thereby limits their proliferation. We call this the metabolic cancer suppression hypothesis.
Because it is difficult to directly observe the interactions among mutations, microenvironments, cell fitness, and the resulting evolutionary dynamics, computational models play an important role in understanding mechanisms of somatic cell evolution in oncogenesis [6, 7]. A previous in silico evolutionary model showed that even when the rate and types of mutation were held constant, somatic cell evolution in the spatially structured environments of tissues depended on the conditions of cell micro-environments, as did cancer outcomes [8].
ENERGY DISTRIBUTION AND ALLOCATION ARE TIGHTLY CONTROLLED IN NORMAL MAMMALIAN TISSUES
Distribution of energy resources through the mammalian vascular system is optimized for the whole organism, through a distribution system that equitably meets the energetic needs of all somatic cells [9, 10]. A previous in silico model showed that greater cell access to energy can increase selection for mutations supporting hyperproliferation and cell motility [8]. This suggested the possibility that energy excess might be the common microenvironmental mechanism underlying multiple cancer risk factors that apparently involve supply of energy resources to affected tissues (Supplementary Table S1).
MULTIPLE CANCER RISK FACTORS APPARENTLY INVOLVE CELLULAR ACCESS TO EXCESS ENERGY
Caloric restriction, or ‘energy restriction’ dietary regimens, which restrict energy intake to minimal survival levels, have strong cancer preventive effects in animal models, with experimental results suggesting the relation between energy supply and cancer risk is dose-dependent and robust. Indeed, ‘calorie restriction is arguably the most potent, broadly acting dietary regimen for suppressing the carcinogenesis process’ [11].
In most discussions of mechanisms of cancer risk, diet and the other factors listed in Table 1 have been considered separately from each other and were often discussed primarily in terms of distinct signaling or molecular pathways. Many possible molecular mechanisms have been considered to explain the anticancer effects of reduced energy supply, including the intervening effects of various hormones, growth factors and adipose-derived factors [12]. We agree that these regulatory pathways are important. However, no single pathway is clearly dominant, and they all function to regulate energy allocation and usage in response to changing energy availability. We suggest that all these regulatory pathways function to balance the same underlying biophysical constraints: that energy availability is often biologically limiting, and that cell proliferation is energetically expensive. This energetic cost is manifested in the fact that for unicellular organisms in very energetically restricted conditions, no cell proliferation occurs, and metabolism is constrained to only the most essential survival functions [13]. Within multicellular organisms, the same constraint on cells is observed, but energy availability in a somatic cell’s microenvironment is determined by the organism’s vascular network. When freed from this organismal constraint by growth in culture, mammalian cells increase their energy consumption by several orders of magnitude [9]. Under the normal constraint of limited vascular energy delivery, the rapid cell proliferation characteristic of cancer poses a profound metabolic challenge that requires substantial reorganization of cell metabolism [14]. Under starvation conditions, normal cells reduce energy expenditure and divert it from growth to maintenance. In cancer cells, this response is blocked by oncogene expression, leaving cancer cells more prone than normal cells to death under starvation conditions [15].
Table 1.
Four major cancer risk factors that may reflect the same underlying energetic mechanisma
| Risk factor | Local or systemic? | Proposed mechanism of risk |
|---|---|---|
| Dietary caloric intake | Systemic | Hyperglycemia |
| Hyperglycemia | Systemic | Cell hyperproliferation |
| Obesity | Systemic | History of hyperglycemia |
| Chronic inflammation | Both | History of energy oversupply |
The right-most column summarizes the proximal causal mechanisms we propose in this article.
We propose that the ultimate mechanism by which dietary caloric restriction reduces cancer is that reduced energy availability to cells suppresses cell proliferation, which, in turn, reduces the potential for cell evolution toward cancer. Moreover, we propose that this mechanism is not unique to the risk factor of dietary intake, but that it also applies to the other risk factors listed in Table 1. If energetic excess in the diet leads to energetic excess in the cell microenvironment, that link can only be through increased energy distribution via the vascular distribution network, suggesting a role for hyperglycemia in this causal chain.
Indeed, the risk of several types of cancer is significantly associated with diabetes and hyperglycemia [16], and this association appears to be causal, with diabetes and hyperglycemia increasing cancer mortality across multiple cancer types. There is strong and growing evidence that degree of hyperglycemia influences cancer risk [17]. Obesity appears to arise from, but not contribute to, this causal chain, as hyperglycemia is associated with cancer risk for several organ sites independently of obesity [18].
Despite lack of evidence for a direct causal relationship, obesity and overweight are associated with increased death rates from many different cancer types at multiple organ sites [19]. Because of the strength of this association, it was tempting to assume that obesity directly causes cancer, e.g. [20]. However, the available evidence does not support that assumption. Although obesity is generally associated with cancer risk, this association does not hold for those people described as the ‘metabolically healthy obese’ [21]. Our hypothesis predicts that the most direct cancer risk factor associated with obesity is a history of chronic hyperglycemia. Indeed, experimental evidence from an animal model indicates that their association arises because obesity and cancer both arise from the same upstream causal factors, including a history of chronic positive energy balance and chronic hyperglycemia [22].
In contrast to the clearly energy-related risk factors in Table 1, it may be less obvious how chronic inflammation fits in with our hypothesis. Chronic inflammation is associated with many types of cancer [23], and anti-inflammatory medication has shown promise in cancer prevention [24]. We suggest that chronic inflammation does indeed cause cancer risk by increasing energy delivery to tissues and cells. Inflammation is a complex condition involving several physiological processes, but discussions of its role in oncogenesis often focus solely on immunological aspects [23]. However, prior to its later immunological manifestations, the first part of the inflammation response is vascular. The characteristic redness, heat and swelling of inflammation result from vasodilation—enlargement of blood vessels in the affected tissue. Even a slight vessel dilation can cause a large increase in resource delivery (Fluid delivery through a cylindrical vessel is proportional to the fourth power of vessel diameter, generating a steep increase with vasodilation, and the effect may be stronger in microvasculature, where blood cells must deform to squeeze through microscopic vessels.). Clinical data on humans confirm that vasodilation increases oxygen delivery to tissues [25]. For the same physical reasons, vasodilation presumably increases delivery of oxidizable energy substrates as well, providing more energy and potentially supporting increased cell proliferation. Some chemical agents that cause vasodilation do also increase local cell proliferation in animal models (e.g. [26]).
Normal physiology includes regulated short-term vasodilation with various functions, including temporary inflammation supporting immune response and healing. In contrast, chronic inflammation, with its chronic vasodilation, is a pathologically dysregulated state [27]. Chronic local inflammation of specific organs is associated with cancers of those organs [28]. Chronic systemic low-grade inflammation, also termed ‘metaflammation’, may reportedly account for as much as 90% of all human cancer [29] or even as much as 95% [30].
METHODOLOGY
To formalize and evaluate the hypothesis that energetic excess exacerbates cancer risk, we used an agent- or individual-based model, which is a computer simulation in which each individual cell is tracked explicitly, along with its properties [31, 32].
Like any useful model, ours was highly simplified and therefore unrealistic in many details. Building a fully realistic and complete representation of the system under study is not the goal of effective modeling [31, 32]. Rather than trying to represent every aspect of oncogenesis, our model was designed to represent one specific question about this complex process: Might normal physiological constraints on cell energetics plausibly play a role in suppressing cancer? This question served as a filter to exclude all those elements of real biology that were not essential to formalizing our question.
In computer software, we built a stochastic agent-based model representing a population of somatic cells in a 2D grid space representing a small monolayer of somatic tissue cells embedded with a vascular capillary bed. The model architecture followed that of previous modeling work [8], and rested on similar assumptions. The model was implemented using the NetLogo 6.0 modeling platform [33, 34]. The model algorithm is briefly summarized below, and is described in more detail in a Supplementary Appendix. The full model source code (for NetLogo 6.0) is also available on request.
Brief model description
The model was a stochastic agent-based computational model with discrete time steps, and discrete 2D space, representing a tissue layer for in silico experiments.
The central assumptions of the model were the following:
Cancer arises through the evolution of somatic cells due to cell-heritable somatic changes (genetic or epigenetic mutations) affecting cell traits that influence Darwinian cell fitness (rates of survival and proliferation).
Somatic cell evolution is limited by the rate of cell division, which provides limiting opportunities for both mutational change and Darwinian selection among cells.
Cell division is energetically expensive and can be limited by energy scarcity in the cell microenvironment [9, 13].
Normal vascular physiology is regulated to provide tissues with sufficient energetic resources for cell survival and function, but not for cell division, except under temporary special conditions wherein cell proliferation is needed by the multicellular organism. Thus, individual somatic cells do not normally control their own energy supply.
The agent-based computer model consisted of collections of three types of software ‘agents’ representing three key elements of solid tissues. Those three agent types were as follows:
‘Capillaries’, the terminal delivery units of the vascular system, delivered energetic resources to tissues at a rate that was normally regulated by the multicellular organism for overall organismal benefit.
‘Microenvironments’: Each capillary was surrounded by a tissue microenvironment containing a level of energetic resources that reflected both the rate of resource delivery by the capillary, and the combined rate of resource uptake by the multiple cells residing within the microenvironment.
‘Cells’ took up energetic resources from their microenvironment, at a rate determined both by the cell’s internally determined resource demand, and by resource availability in its microenvironment. When combined cell demand exceeded microenvironmental availability, cells competed for energetic resources based on their heritable resource demand. Cells used energy first for survival and basic functioning; then, used any excess for biosynthesis and cell division. Cells that did not meet their energetic requirement for survival died, while those acquiring sufficient internal energy reserves divided, with trait inheritance subject to stochastic mutation.
SIMULATION EXPERIMENT
We used the model described above to perform one core experiment: To test the effect of energy availability on oncogenesis, we held constant all other parameters (Supplementary Table S1), and systematically varied the rate of vascular energy delivery. Because the model was stochastic, for each setting of energy delivery rate, we performed 1000 simulation runs with different random number seeds and combined the results for statistical analysis.
Simulation termination and scoring of outcomes
We interpreted as cancer cells, any cells in which accumulated mutations had increased resource uptake (supporting hyper-proliferation), and had also increased motility, relative to unmutated cells. We interpreted a simulation run as having a cancer outcome when cancer cells became predominant (>50%) in our simulated piece of tissue, and some cancer cells had also escaped the bounds of the simulated experimental tissue piece by moving out into surrounding normal tissue (representing tissue invasion and malignancy). Each simulation run continued until it reached a cancer outcome, at which point the run was automatically terminated and the elapsed time was automatically recorded for analysis.
RESULTS
Stability of normal tissue
Under default conditions, representing normal tissue, vascular energetic supply was just enough to meet the combined basal power requirements (BPRs) [13] of all cells in each capillary microenvironment. This resulted in a stable tissue, in which somatic cells were very slow to evolve any changes. Because the potential resource uptake of normal cells slightly exceeded BPR (Supplementary Table S1), normal cells occasionally did accumulate sufficient energy reserves to divide and proliferate even under the control condition of normal resource delivery, but this occurred only when resource availability increased locally, due to the stochastic death of other cells in the microenvironment. Through this cell turnover from background mortality, some cell lineages did eventually acquire mutations, and did slowly evolve first increased resource demand and consumption (leading to hyperproliferation), and later, cell motility (leading to malignant tissue invasion).
Normal tissue was not easily destabilized by mutations. From the earliest time steps of a model run, mutations arose that increased cells’ demand for energy resources, and thus increased their growth and proliferation relative to normal cells, but the resulting neoplastic clones were self-limiting without motility, which evolved later. A high-consumption clone could undergo only a few rounds of cell division before the resource demand of the growing clone exceeded the delivery rate of its local capillary, so that resource limitation prevented further proliferation. Non-invasive neoplasms were therefore self-limiting to the microscopic scale, except through slow growth limited by resource diffusion between microenvironments, which allowed few mitotic opportunities for further mutation and evolution. This situation persisted until cell motility evolved within the neoplastic clone, followed by malignant invasion of surrounding capillary neighborhoods and ultimately a cancer outcome.
Experimental simulation results
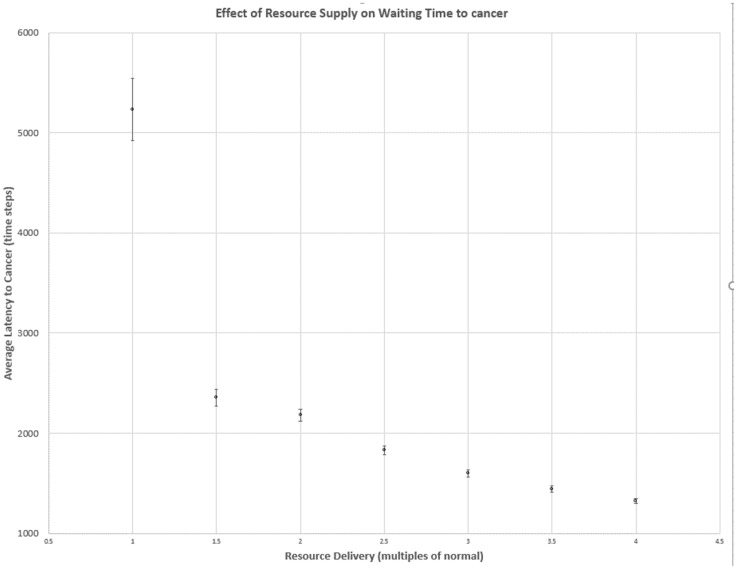
Tissue stability was maximized, and cancer was maximally delayed, under the normal minimal energy supply, providing cells with only their BPR (Fig. 1). Any lower supply of energy caused cell loss and tissue wasting. In contrast, under conditions of energy oversupply, mutated cell clones rapidly evolved first increased energy demand, allowing hyperproliferation, then increased motility. Under conditions of resource oversupply, this process culminated in cancer through the same evolutionary route as under normal energy supply, but much more rapidly. Any increase in energy delivery above the basal maintenance level resulted in significantly reduced waiting time to cancer (Fig. 1); (t-test of the smallest delivery increase vs normal rate, P < 0.01). Further increases caused diminishing, but still significant, reductions of waiting time (Jonckheere-Terpstra trend test: P < 0.0002).
Figure 1.
Effect of increased vascular delivery of energetic resource. Circle markers show average over 1000 simulation runs, and error bars show 95% CIs
SENSITIVITY ANALYSIS
Some parameters in our computational model were set to default values that we could not rigorously justify on biological grounds, because the relevant measurements have not been published. Therefore, we carried out a sensitivity analysis on all nine default parameter values (Supplementary Table S1), to determine whether our key result was robust. Our only goal was to evaluate how deviations from the default value of each parameter affected the relationship between resource delivery rate and waiting time to cancer. Hence, we focused on two-way interactions between fixed parameter values and the (variable) resource delivery rate in their effect on cancer outcome. Using the one-factor-at-a-time approach [35, 36], we varied each of the default parameter values (Supplementary Table S2) separately, using both a 2-fold increase and a two-fold decrease for each. In each such variant, our key result held: each experimental value of increased energy delivery above the normal (BPR) level significantly reduced waiting time to cancer (t-tests, P < 0.05 for each parameter setting).
CONCLUSIONS
Based on our simulation results, we conclude that the metabolic cancer suppression hypothesis reflects sound reasoning about somatic cell evolution toward cancer and is a plausible candidate mechanism for the cancer risk factors listed in Table 1.
Limitations and caveats
Because our model was intentionally simplified to represent a focused question, rather than all aspects of oncogenesis, its omission of many factors known to be important in oncogenesis is not meant to imply that they are unimportant.
Simulation studies alone cannot establish causality in the real world. Their main contribution is to formalize hypotheses that may then become plausible enough and important enough, to merit empirical testing. Toward that goal, we attempted to evaluate whether the metabolic cancer suppression hypothesis is logically coherent internally, and is consistent with important empirical patterns in cancer epidemiology. The scientific method is most effective when it exploits multiple competing hypotheses [37]. But here, we propose and evaluate just one hypothesis. The publication bias for such studies is probably large, as negative evaluations are unlikely to be published. Therefore, we suggest that a critical reader should focus on whether our assumptions are valid, and whether their logical implications were correctly formalized in the computational model.
Cancer risk in the framework of somatic evolution
Some early ideas about mechanisms of cancer risk came from observations that chemical exposures can act either as cancer initiators, by inducing somatic mutation, or as promoters, by inducing cell proliferation [38]. Our current results help to integrate those classical ideas into the modern framework of somatic cell evolution, building on, e.g. [39–41]. In our simulated experiment, excess energy availability acted as a cancer promoter, accelerating oncogenesis by increasing cell proliferation. Increased cell proliferation was oncogenic because it modified selective pressures, creating positive selection for oncogenic cell traits that in normal tissue were weakly, or even negatively, selected.
Our simulation results are consistent with empirical observations of cancer risk that is normally low but increasing with age, and also with the metabolic risk exposures we modeled. Our observation that positive selection for cell motility only occurred in cell lineages that had already evolved abnormally high resource demand and cell proliferation replicated earlier simulation results [8].
A possible common mechanism behind superficially distinct cancer risk factors
Throughout biology, Darwinian evolution is typically shaped by resource ecology [42], so it is unsurprising to find the same relationship in the somatic Darwinian evolution that underlies oncogenesis. The unique feature of somatic cell evolution is that the resource ecology of somatic cells is controlled by the multicellular organism, including its systems for internal resource allocation. Indeed, the comparative study of multicellular organisms has shown that equitable internal resource allocation is one of the universal foundations of multicellularity, and that, throughout all multicellular life, ‘disruption of, or manipulation of, resource transport systems are central characteristics of cancer’ [43]. Our results support and explain that observation.
To seek mechanistic understanding of diverse cancer risk factors, it is customary to investigate the unique molecular pathways involved in each. In parallel, however, this field can gain other useful insights by searching for commonalities and general principles suggested by relevant evolutionary and ecological theory. If our current hypothesis is substantiated by empirical tests, this mechanistic insight may help in designing effective health interventions by targeting general physiological processes rather than specific molecular aberrations, which are more sporadic and heterogeneous, and are also common outside of oncogenesis.
Testable predictions and translational opportunities
One key empirical prediction from our hypothesis and results is that any pathological condition that abnormally increases delivery of energetic resources to tissues is expected to increase cancer risk in the affected tissues. The translational corollary for cancer prevention is that such risk factors can be ameliorated by maintaining or restoring the normally restricted vascular energy delivery. Although we have highlighted four important causes of oversupply of energetic resources, other causes likely exist, and each constitutes opportunities for empirical tests of the hypothesis, and for translational application. For example, our hypothesis predicts that chronic exposure to any agent with a vasodilating effect may increase cancer risk. Alcohol drinking may be the most important such candidate. It is an established risk factor for several malignancies, based on statistical association [44, 45], but no clear mechanism has yet been established. It is known, however, that alcohol drinking is a powerful vasodilator, and is reported to be, ‘even more effective in this respect than the normal vasodilator drugs’ [46].
Our hypothesis suggests likely mechanisms of several other cancer risk factors beyond those in Table 1. Just as our mechanistic hypothesis can explain the generalization that obesity is often, but not always, a cancer risk factor, it can also explain the generalization that chronic inflammation is often, but not always, carcinogenic. Our more mechanistically specific formulation is that inflammation is oncogenic particularly when it involves vasodilation. This would exclude as risk factors some forms of chronic inflammation such as psoriasis, which suppresses vasodilation [47]. One translational corollary would be a prediction that those anti-inflammatory agents most effective in cancer prevention will be those that are most effective at ameliorating chronic vasodilation.
Our hypothesis can also address open mechanistic questions about how physical activity protects against cancer risk. Higher physical activity is associated with reduced risk of many cancer types. Several physiologic and biochemical mechanisms have been hypothesized to link physical activity to cancer risk, but the mechanistic connection remains unclear [48]. Our hypothesis predicts that the effect of physical activity most relevant to cancer prevention may be reduction of chronic hyperglycemia. Some empirical evidence is consistent with this prediction. In a study tracking how various clinical biomarkers respond to levels of physical activity, one of the largest effects was seen for blood glucose [49]. If our prediction is met, blood glucose monitoring may become useful for tracking the anti-cancer effectiveness of exercise regimens through this surrogate endpoint that can provide faster response and higher sensitivity than long-term monitoring of cancer outcomes. This is a readily testable prediction.
More generally, future progress in preventing cancer through lifestyle management may depend on providing better support and motivation to follow existing guidelines that have proven effective [50]. According to the present hypothesis, tracking chronic hyperglycemia and chronic vasodilation may hold promise for rapid quantitative feedback to reduce risk through guided lifestyle management.
Supplementary Material
ACKNOWLEDGEMENTS
At the NCI Division of Cancer Prevention, we thank Richard M. Fagerstrom for his input on the study design. For their exceptional administrative support, we also thank Lawrence Morris and Joy Osborne.
FUNDING
JWP is supported as an employee of the National Cancer Institute (NCI). The findings, opinions, and recommendations expressed here are those of the authors and not necessarily those of the NCI.
Conflict of interest: None declared.
REFERENCES
- 1. Fortunato A, Boddy A, Mallo D. et al. Natural selection in cancer biology: from molecular snowflakes to trait hallmarks. Cold Spring Harb Perspect Med 2017;7: Article Number: a029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martincorena I, Roshan A, Gerstung M. et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015;348:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abelson S, Collord G, Ng SWK. et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 2018;559:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martincorena I, Raine KM, Gerstung M. et al. Universal patterns of selection in cancer and somatic tissues. Cell 2017;171:1029–41.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ducasse H, Arnal A, Vittecoq M. et al. Cancer: an emergent property of disturbed resource-rich environments? Ecology meets personalized medicine. Evol Appl 2015;8:527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang L, Wang ZH, Sagotsky JA. et al. Multiscale agent-based cancer modeling. J Math Biol 2009;58:545–59. [DOI] [PubMed] [Google Scholar]
- 7. Pepper JW, Vydelingum NA, Dunn BK. et al. Agent-based models in cancer prevention research In: Kora ABs. (ed.). Advances in Computational Modeling Research: Nova Science Publishers, 2013, 1105–16. [Google Scholar]
- 8. Aktipis CA, Maley CC, Pepper JW.. Dispersal evolution in neoplasms: the role of disregulated metabolism in the evolution of cell motility. Cancer Prevent Res 2012;5:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. West GB, Woodruff WH, Brown JH.. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci USA 2002;99:2473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Savage VM, Herman AB, West GB. et al. Using fractal geometry and universal growth curves as diagnostics for comparing tumor vasculature and metabolic rate with healthy tissue and for predicting responses to drug therapies. Discrete Continuous Dyn Syst Ser B 2013;18:1077–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hursting SD, Smith SM, Lashinger LM. et al. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis 2010;31:83–9. [DOI] [PubMed] [Google Scholar]
- 12. Longo VD, Fontana L.. Calorie restriction and cancer prevention: metabolic and molecular mechanisms . Trends Pharmacol Sci 2010;31:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kempes CP, van Bodegom PM, Wolpert D. et al. Drivers of bacterial maintenance and minimal energy requirements. Front Microbiol 2017;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeBerardinis RJ, Lum JJ, Hatzivassiliou G. et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 15. Buono R, Longo VD.. Starvation, stress resistance, and cancer. Trends Endocrinol Metab 2018;29:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vigneri P, Frasca F, Sciacca L. et al. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–23. [DOI] [PubMed] [Google Scholar]
- 17. Chowdhury TA. Diabetes and cancer. QJM 2010;103:905–15. [DOI] [PubMed] [Google Scholar]
- 18. Stattin P, Bjor O, Ferrari P. et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007;30:561–7. [DOI] [PubMed] [Google Scholar]
- 19. Calle EE, Rodriguez C, Walker-Thurmond K. et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 20. Hursting SD, Digiovanni J, Dannenberg AJ. et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prevent Res 2012;5:1260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bluher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol 2014;171:R209–19. [DOI] [PubMed] [Google Scholar]
- 22. Hursting SD, Nunez NP, Varticovski L. et al. The obesity-cancer link: lessons learned from a fatless mouse. Cancer Res 2007;67:2391–3. [DOI] [PubMed] [Google Scholar]
- 23. Colotta F, Allavena P, Sica A. et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–81. [DOI] [PubMed] [Google Scholar]
- 24. Kostadinov RL, Kuhner MK, Li X. et al. NSAIDs modulate clonal evolution in barrett's esophagus. PLoS Genet 2013;9:e1003553.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bihari D, Smithies M, Gimson A. et al. The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N Engl J Med 1987;317:397–403. [DOI] [PubMed] [Google Scholar]
- 26. Jothi DJ, Dhanraj M, Solaiappan S. et al. Brugia malayi asparaginyl - tRNA synthetase stimulates endothelial cell proliferation, vasodilation and angiogenesis. Plos One 2016;11:e0146132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marelli G, Sica A, Vannucci L. et al. Inflammation as target in cancer therapy. Curr Opin Pharmacol 2017;35:57–65. [DOI] [PubMed] [Google Scholar]
- 28. Peek RM, Mohla S, DuBois RN.. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a national cancer institute–sponsored meeting. Cancer Res 2005;65:8583–6. [DOI] [PubMed] [Google Scholar]
- 29. Sethi G, Shanmugam MK, Ramachandran L. et al. Multifaceted link between cancer and inflammation. Biosci Rep 2012;32:1–15. [DOI] [PubMed] [Google Scholar]
- 30. Aggarwal BB. Inflammation, a silent killer in cancer is not so silent! Curr Opin Pharmacol 2009;9:347–50. [DOI] [PubMed] [Google Scholar]
- 31. Railsback SF, Grimm V.. Agent-Based and Individual-Based Modeling: A Practical Introduction. Princeton University Press, 2012. [Google Scholar]
- 32. Otto SP, Day T.. A Biologist’s Guide to Mathematical Modeling in Ecology and Evolution. Princeton: Princeton University Press, 2007. [Google Scholar]
- 33. Wilensky U. NetLogo (software). Evanston, IL: Center for Connected Learning and Computer-Based Modeling, 1999. [Google Scholar]
- 34. Wilensky U, Rand W.. An Introduction to Agent-Based Modeling: Modeling Natural, Social, and Engineered Complex Systems with NETLogo. London: MIT Press, 2015. [Google Scholar]
- 35. Czitrom V. One-factor-at-a-time versus designed experiments. Am Stat 1999;53:126–31. [Google Scholar]
- 36. Cacuci DG. Sensitivity & Uncertainty Analysis, Volume 1: Theory. Chapman & Hall/CRC, 2003. [Google Scholar]
- 37. Platt JR. Strong inference - certain systematic methods of scientific thinking may produce much more rapid progress than others. Science 1964;146:347–53. [DOI] [PubMed] [Google Scholar]
- 38. Vincent TL, Gatenby RA.. An evolutionary model for initiation, promotion, and progression in carcinogenesis. Int J Oncol 2008;32:729–37. [PubMed] [Google Scholar]
- 39. Swanton C, Bardelli A, Polyak K. et al. Cancer Evolution. Cold Springs Harbor, NY: Cold Springs Harbor Laboratories Press, 2017, 304 [Google Scholar]
- 40. Merlo LM, Pepper JW, Reid BJ. et al. Cancer as an evolutionary and ecological process. Nat Rev Cancer 2006;6:924–35. [DOI] [PubMed] [Google Scholar]
- 41. Pepper JW, Findlay CS, Kassen R. et al. Cancer research meets evolutionary biology. Evol Appl 2009;2:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Langevelde F, Prins HHT.. Introduction to Resource Ecology In: Prins HHT, Van Langevelde Fs (eds).Resource Ecology: Spatial and Temporal Dynamics of Foraging. Dordrecht, The: Netherlands: Springer, 2008, 1–6 [Google Scholar]
- 43. Aktipis CA, Boddy AM, Jansen G. et al. Cancer across the tree of life: cooperation and cheating in multicellularity. Philos Trans R Soc Lond B Biol Sci 2015;370: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LoConte NK, Brewster AM, Kaur JS. et al. Alcohol and cancer: a statement of the american society of clinical oncology. J Clin Oncol 2017; JCO.2017.76.1155. [DOI] [PubMed] [Google Scholar]
- 45. Xi B, Veeranki SP, Zhao M. et al. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in US adults. J Am Coll Cardiol 2017;70:913–22. [DOI] [PubMed] [Google Scholar]
- 46. Gillespie JA. Vasodilator properties of alcohol. Br Med J 1967;2:274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alba BK, Greaney JL, Ferguson SB. et al. Endothelial function is impaired in the cutaneous microcirculation of adults with psoriasis through reductions in nitric oxide-dependent vasodilation. Am J Physiol Heart Circ Physiol 2017; doi: 10.1152/ajpheart.00446.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moore SC, Lee I, Weiderpass E. et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016;176:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luke A, Dugas LR, Durazo-Arvizu RA. et al. Assessing physical activity and its relationship to cardiovascular risk factors: NHANES 2003-2006. BMC Public Health 2011;11:387.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kohler LN, Garcia DO, Harris RB. et al. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev 2016;25:1018–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.