Abstract
Nanotechnology has the potential to make a beneficial impact on several agricultural, forestry, and environmental challenges, such as urbanization, energy constraints, and sustainable use of resources. However, new environmental and human health hazards may emerge from nano-enhanced applications. This raises concerns for agricultural workers who may become primarily exposed to such xenobiotics during their job tasks. The aim of this review is to discuss promising solutions that nanotechnology may provide in agricultural activities, with a specific focus on critical aspects, challenging issues, and research needs for occupational risk assessment and management in this emerging field. Eco-toxicological aspects were not the focus of the review. Nano-fertilizers, (nano-sized nutrients, nano-coated fertilizers, or engineered metal-oxide or carbon-based nanomaterials per se), and nano-pesticides, (nano-formulations of traditional active ingredients or inorganic nanomaterials), may provide a targeted/controlled release of agrochemicals, aimed to obtain their fullest biological efficacy without overdosage. Nano-sensors and nano-remediation methods may detect and remove environmental contaminants. However, limited knowledge concerning nanomaterial biosafety, adverse effects, fate, and acquired biological reactivity once dispersed into the environment, requires further scientific efforts to assess possible nano-agricultural risks. In this perspective, toxicological research should be aimed to define nanomaterial hazards and levels of exposure along the life-cycle of nano-enabled products, and to assess those physico-chemical features affecting nanomaterial toxicity, possible interactions with agro-system co-formulants, and stressors. Overall, this review highlights the importance to define adequate risk management strategies for workers, occupational safety practices and policies, as well as to develop a responsible regulatory consensus on nanotechnology in agriculture.
Keywords: Nanotechnology, Nanotoxicology, Nano-enabled agrochemicals, Nano-enhanced environmental remediation, Nanocellulose, Occupational risk assessment and management
Definitions
Summary of the principle nano-agriculture related definitions employed in the manuscript.
| Nanomaterial | A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm - 100 nm | European Commission, 2011 |
| Nanofertilizer | Nanomaterials which can supply one or more nutrients to the plants and enhance their growth and yields | Liu and Lal, 2015 |
| Nanomaterial-enhanced fertilizer |
Nanomaterials, that, when augmented with conventional fertilizers can improve their performance, without directly providing crops with nutrients | Liu and Lal, 2015 |
| Nanopesticide | Any pesticide formulation that involves either very small particles of a pesticide active ingredient or other small engineered structures with useful pesticidal properties | Bergeson, 2010 |
| Nano-coating | Nano-coatings or surface coatings of nanomaterials, on fertilizer or pesticide active ingredients, can hold the material more strongly due to higher surface tension than the conventional surfaces and thus help in controlled release | Ghormade et al., 2011 |
| Nano-enabled formulation |
Nano-enabled formulation encompasses those emulsions made of smaller micelles formed with smaller amount of surfactants, or microcapsules with a well-defined nano-pore network | Kah, 2015 |
| Nanospheres | Aggregate in which the active compound is homogeneously distributed into the polymeric matrix | Perlatti et al., 2012 |
| Nanocapsule | Aggregate in which the active compound is concentrated near the center core, lined by the polymer matrix | |
| Nanogel | Hydrophilic, generally cross-linked, polymers which can absorb high volumes of water | |
| Nano-sensor | Nanoscale devices capable of detecting and responding to physico-chemical and biological stimuli. Nanomaterials may provide larger surface area for the immobilization of the target-recognition elements or confer the sensor their own optical-physical and electrochemical properties | Omanović-Mikličanin and Maksimović, 2016 |
1. Introduction
Nanotechnology has the potential to make an impact on several agricultural and environmental challenges, such as urbanization, energy and resource constraints, sustainable use of resources, run- off and accumulation of pesticides and fertilizers (Chen and Yada, 2011; Ditta, 2012; Parisi et al., 2015). With a constantly growing population the demand for higher agricultural yields and more effective strategies to optimize agricultural practices are needed, therefore the use of nanoscale materials in agricultural science is increasing (Gogos etal., 2012). Nanotechnology, in fact, may play substantial impact on sustainable agriculture and precision farming development. This ultimately aims to maximize agriculture output (i.e., crop yields), while minimizing input (i.e., fertilizers, pesticides, and herbicides) through monitoring environmental variables and applying targeted action (Fraceto et al., 2016; Servin et al., 2015).
In this field, growing interest has been focused on nano-enhanced solutions due to their potential to improve seed germination, growth and plant protection through the controlled release of agrochemicals, with the consequent reduction in the amounts of chemical products applied and the minimization of nutrient losses in fertilization. Additionally, nanotechnology in agriculture may provide innovative solutions to protect and remediate water and soils, thus boosting global food production and quality in an eco- friendly manner (Biswal et al., 2012; Ditta, 2012; Khot et al., 2012; Prasad et al., 2014; Sekhon, 2014; Sonkaria et al., 2012).
All of the leading producers of agricultural chemicals are actively researching nanotechnology for use in agriculture (De Rosa et al., 2010). Some companies, over the last decade, have already deposited patents comprising a wide range of protocols for the production and application of nanopesticide formulations (Peters et al., 2016). However, the most recent European Food Safety Authority “Inventory of nanotechnology applications in the agricultural, feed and food sector” (Peters et al., 2014) pointed out that only few encapsulated formulations for nanopesticides are already available on the market. However, other nano-agrochemicals applications primarily including nano-encapsulates, nanocomposites, silica and silver (Ag) nanomaterials, as well as nanoclays are still in a developmental stage for both pesticidal and biocide purposes, and may be expected to emerge in the market in the future (Peters et al., 2014).
Nevertheless, emerging uses of nanotechnology in agriculture and many other sectors of the global economy continue to raise questions and express concern over possible human and environmental health implications (Kah, 2015; Scott and Chen, 2013). The deliberate introduction of nano-sized materials within agricultural activities, in fact, could result in unintended health outcomes (Gogos et al., 2012; Kah et al., 2013). In this scenario, environmental and human exposure due to nanomaterial residues in soil and crops are expected to increase with exposure routes including possible bioaccumulation in the environment and food chain. Therefore, the anticipated innovative and improved activities of nano-enhanced applications may result in both new benefits and new hazards to human and environmental health. In this perspective, the purpose of achieving sustainable agriculture overlaps the need for the development of a “green nanotechnology”, a conceptual approach to balance the benefits provided by nano-products in solving environmental challenges with the assessment and management of environmental, health, and safety risks potentially posed by nanoscale materials. Additionally, potential risks derived from nanomaterial exposure should be assessed using an appropriately tailored life-cycle perspective. This means taking into account all the phases in which nano-solutions may be found, from the application into the field, potential incorporation into food supply, to the disposal or re-use of the products together with possible influences exerted by peculiar agro-system conditions that may all affect nanomaterial hazardous properties and risk characterization.
From an occupational health perspective, this seems an even more urgent issue to assess, since earliest exposures to nanomaterials may occur for agricultural workers who may become highly and chronically in contact with these still not-fully explored xenobiotics, while performing their routine job tasks. In this context, the increasing interest in the use of nanotechnology in agriculture raises questions on potential risks for occupational exposure to these materials as well as to how specifically assess, communicate and manage these risks for regulatory purposes (Kookana et al., 2014). The potential great variety of nano-substances employed, in fact, are still not fully understood toxicologically once dispersed into physico-chemically changing environments, and their unique modes of employment/application in the agro-fields, do not only require a “nano-focused” attention, but more specific “nano-agricultural” oriented strategies for occupational risk assessment and management processes.
In this review, some potential applications of nanoscale science, engineering and nanotechnology for agriculture, specifically aimed at improving and protecting agronomic yields and crop production as well as to detect and remediate environmental pollutants, have been addressed with attention focused on emerging occupational risks in this field. Additionally, toxicological research priorities, aimed to obtain information concerning nanomaterial hazardous behaviors, exposure evaluation, dose-response relationships and environmental fate have been identified. These may be all important to improve future knowledge concerning possible human and, more specifically, occupational health implications of nano-innovations and to define suitable approaches for nano-risk assessment, considering also the potential adaptation of existing occupational risk assessment models and procedures for use with agricultural nanotechnology. This review aims to highlight some critical issues that should be taken into consideration when attempting to define adequate occupational risk management strategies, safety practices and policies. Overall, these intriguing topics should be addressed to develop an ethical and responsible regulatory consensus on nanotechnology in agriculture.
2. Nanotechnology enabled agrochemicals
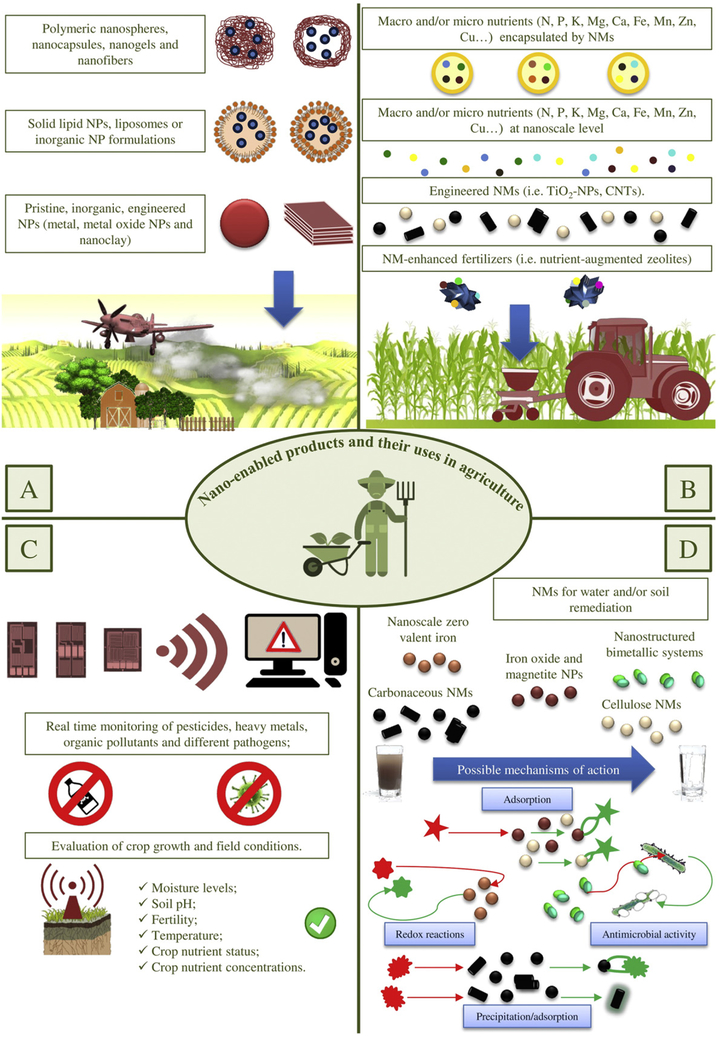
Agrochemicals play a key role in agriculture production. However, when traditionally applied, they may be decomposed or removed by climatic factors, i.e., wind, sunlight, and rain (Liu et al., 2008). A significant proportion of agrochemicals do not reach their target species and, therefore, periodic application is required. Multiple applications of agrochemicals not only increase the cost, but also leads to undesirable side effects to plants, environment, and to the health of persons exposed through the food chain (Kumari and Yadav, 2014). Nanotechnology, by virtue of nanomaterial related properties, has the potential for agro-biotechnological applications to face these challenging issues (Table 1 and Fig. 1).
Table 1.
Nanotechnology in agriculture: applications, opportunities and practical challenges.
| NANOTECHNOLOGY in AGRICULTURE | |||
|---|---|---|---|
| Applications | Opportunities | Practical challenges | |
| Nano-enabled agrochemicals |
Nanofertilizers ✓ Macronutrient nanofertilizers; ✓ Micronutrient nanofertilizers; ✓ Nutrient-loaded nanofertilizers, i.e. nutrient augmented zeolites; ✓ Plant growth enhancers, i.e. TiO 2-NPs, CNTs. |
✓ Targeted and controlled nutrient release; ✓ Increased nutrient availability and plant uptakeefficiency; ✓ Increase denzymatic activity; ✓ Reduced adverse impact of conventional compounds. |
✓ Nanomateriai phytotoxicity; ✓ Variability and reactivity of nanomateriais in the environment; ✓ Possible adverse effects for exposed workers under real application conditions. |
|
Nanopesticides ✓ Nano-emulsions, -dispersions, -spheres,- capsules, and -gels of traditional pesticides; ✓ Solid lipid NPs, coated liposomes, or inorganic NPs associated with active ingredients; ✓ Engineereed NPs, i.e. Ag-and TiO 2-NPs. |
✓ Greater pesticide solubility, mobility and durability; ✓ Reduced amount of ingredients via targeted/controlled release; ✓ Reduced resistance and damage to nontarget organisms. |
✓ Biosafety of nanopesticides; ✓ Toxicological profile; interactions with co-formulants; environmental fate; ✓ Long term effects on the environment and chronically exposed workers under real application conditions. |
|
| Detection and remediation of environmental pollution |
Sensingdevices ✓ Nanosensorsto monitor a varietyof pesticides, pathogens as well as to assess crop-growth, field conditions, and early biological aiterations. |
✓ Improved detection limits for real time monitoring ot toxicants; ✓ Precise farming support: reduction of inputs, sustainable use of resources. |
✓ Fabrication and validation of sensitive instruments; ✓ Environmental and health consequences of nanomateriai relesed from devices. |
|
Water and soilremedlation ✓ Zero valentiron-NPs, metal oxide-NPs, i.e. TiO2, Fe3O4, bimetallic-NPs, carbon basednanomaterials have been explored for degradation of chlorinated, halogenated compounds and heavymetals. |
✓ Destruction and transformation of toxic contaminants with high removal effectiveness; ✓ Reduced clean-up time; ✓ Cost and energy-efficient processes. |
✓ Environmental and health effects of nanomateriais and released products; ✓ No knowledge on nanomateriai largescale application, regeneration, reutilization. |
|
| Nanoscale agricultural products |
Water remediation and crop coating ✓ Cellulose nanocrystals or nanofibers derived from plant based resources are promising adsorbents for water remediation; ✓ Nano-cellulose may stabilize reactive NPs or improve membrane properties; ✓ A cellulose nanofiber-based coating for reducing cherry rain-cracking has been recently developed. |
✓ Good adsorbent properties due to nanocellulose high surface area, mechanical properties, biocompatibility, easily functionalizable surface, sustainable sourceas, biodegradability; ✓ Improved membrane strength, selectivity, permeability, hydrophilicity, biofouling resistance. |
✓ In vitro studies demonstrated some cytotoxic and genotoxic effects of cellulose nanomateriais; ✓ In vivo exposure via the respiratory tract induced lung inflammatory and oxidative stress reactions; ✓ Concerns regarding nanocellulose high aspect ratio, stiffness, bio-durability. |
Ag-NPs, silver nanoparticles; CNTs, carbon nanotubes; Fe3O4, magnetite; NPs, nanoparticles; TiO2-NPs, titanium dioxide nanoparticles.
Fig. 1.
Nano-enabled products and related uses in agriculture.A: Nano-pesticide formulations (silver, copper, aluminum, mesoporous silica and titanium dioxide nanoparticles (TiO2-NPs); B: Nano-fertilizers (macro- and micro-nutrients at nanoscale level or encapsulated by nanomaterials (NMs) such as multi walled carbon nanotubes (CNTs) and TiO2-NPs; C: Nanosensors (noble metal NPs, metal oxide NPs and metal-nanocluster including gold, silver, platinum and copper, quantum dots, graphene and CNTs); D: Nanoproducts for water and/or soil remediation (silica, silver, copper, aluminum, zero valent iron, palladium, nano-structured bimetallic systems, cellulose based and carbonaceous NMs).
2.1. Nanofertilizers
In the context of sustainable agriculture, employing nanotechnology in development and application of new types of fertilizers is regarded as one of the promising approaches to significantly increase plant growth and agronomic yields to meet incoming challenges of food availability and environmental protection (Liu and Lal, 2015). Substituting nanofertilizers for traditional methods of fertilizer applications is a way to release nutrients into the soil gradually and in a targeted/controlled manner. This may prevent eutrophication and run-off causing water and soil pollution (Sekhon, 2014; Wilson et al., 2008).
Nutrients can be encapsulated by nanomaterials, coated with a thin protective nanoscale polymeric film, or delivered as nano-emulsions or nanoparticles (NPs) (De Rosa et al., 2010). Nanofertilizers can supply one or more nutrients to the plants and enhance their growth, or can improve the performance of conventional fertilizers (Liu and Lal, 2015). For instance, nanocoatings on fertilizer particles can hold the material more strongly on the plant due to the higher surface tension (Ghormade et al., 2011; Yang et al., 2012). Nanomaterial-enhanced fertilizers may increase plant-uptake efficiency of nutrients and/or reduce the adverse impacts of conventional fertilizer application (Liu and Lal, 2015). Nutrient-augmented-zeolites are an important example for this group (Malekian et al., 2011; Perrin et al., 1998; Zwingmann et al., 2011). Nano-porous properties of zeolites confer a high specific surface area and a high selection toward plant macronutrients, which may be slowly released for specific plant uptake, thus reducing nutrient loss and environmental risks and improving their efficacy. However, future research should focus on the potential of developing other types of nutrient augmented nanomaterials with a safer and more effective profile.
Plant-photosynthesis efficacy and enzyme activity could be enhanced by other nanomaterials, such as titanium dioxide (TiO2)-NPs (Gao et al., 2008; Song et al., 2012a,b; Yang et al., 2007) and carbon nanotubes (CNTs) (Khodakovskaya et al., 2013; Lahiani et al., 2013; Villagarcia et al., 2012), which were reported to increase plant germination and growth, despite the fact that these particles did not contain any essential nutrients. Increased growth rate could reflect the photo-sterilization and photo- generation of “active oxygen like superoxide and hydroxide anions” induced by TiO2-NPs that can increase the seed stress resistance and promote capsule penetration for intake of water and oxygen needed for fast germination. Comparably, multi walled-CNTs can penetrate seeds and increase the germination rate by enhancing the seed water uptake and utilization efficiency of the plants (Khodakovskaya et al., 2009, 2013). Although beneficial, the possible phytotoxicity, bioaccumulation and bioavailability of a variety of nanomaterials to different plant species need to be thoroughly understood under “environmentally realistic scenarios” (Khot et al., 2012). To define the beneficial effects as well as the possible toxicological profiles of nanomaterials for fertilizing purposes, scientific research should understand how nano-agrochemicals may interfere with important plant-microbial relationships which are all critical for soil fertility and agricultural productivity.
2.2. Nanopesticides
Nanotechnology is also receiving increasing interest in the pesticide sector with the development of a range of plant protection products that are termed “nanopesticides” (Gogos et al., 2012; Kah et al., 2013; Khot et al., 2012; Pérez-de-Luque and Rubiales, 2009). The term “nanopesticide” is used to describe any pesticide formulation that “involve either very small particles of a pesticide active ingredient or other small engineered structures with useful pesticidal properties” (Bergeson, 2010; Kookana et al., 2014). Nano-formulations for pesticidal purposes may offer benefits due to their greater solubility, mobility and durability; the opportunity to reduce the amount of active ingredients used; the possibility to employ products releasing less harmful chemicals to non-target organisms thus reducing the development of resistance; as well as the ability to provide ingredient protection against premature degradation (Kah et al., 2013; Kah and Hofmann, 2014; Sasson et al., 2007).
Nanopesticides cannot be considered as a single entity. The solubility of poorly water soluble pesticides can be increased by means of nano-emulsions and nano-dispersions, which can enhance the bioavailability of the active ingredient while avoiding a number of adjuvants which may be toxic for non-target organisms (Anjali et al., 2010; Elek et al., 2010; Suresh Kumar et al., 2013; Yang et al., 2009). Polymeric nano-spheres and nano-capsules, together with nanogels and nanofibers, have been developed as formulations primarily aimed at slow and controlled release profile of the active ingredients serving as protective reservoirs and carriers (Anton et al., 2008; Bhagat et al., 2013; Brunel et al., 2013; Kah et al., 2013; Kah and Hofmann, 2014; Xiang et al., 2013). More complex nano-formulations for the delivery of pesticides, such as solid lipid NPs, coated liposomes or formulations involving inorganic NPs associated with organic active ingredients (i.e., mesoporous silica or calcium carbonate as carriers for slow release and TiO2-NPs to photocatalyze the organic ingredients after release to reduce residues on plants and in soils) have been investigated (Ao et al., 2013; Bang et al., 2009; Kang et al., 2012; Nguyen et al., 2012a, 2012b; Qian et al., 2011; Song et al., 2012a, 2012b). However, future research, at all levels, from the nanoscale to in field settings, should be pursued to understand nano-metal particle toxicity, environmental fate and suitable applications.
Finally, in some cases, pristine, inorganic, engineered NPs, not intentionally produced for pesticidal purposes, such as metal, metal oxide, and nanoclay-NPs, or a solubilized form of the NPs, may “drive” this biological effect. Ag has long been known for its antimicrobial properties and several in vitro studies have demonstrated that Ag-NPs may significantly inhibit the growth of plant pathogens in a dose-dependent manner (Chun et al., 2010; Jo et al., 2009; Jung et al., 2010; Kim et al., 2009, 2012; Min et al., 2009). Silicon, TiO2-, alumina- and copper- NPs have also been suggested as potential candidates for controlling a range of agricultural pests, enhancing plant tolerance of various abiotic and biotic stresses, and improving the performance of plant growth compared to their coarser bulk materials (Gogos et al., 2012; Kah and Hofmann, 2014; Kim et al., 2012; Mondal and Mani, 2012; Norman and Chen, 2011; Paretet al., 2013a, 2013b).
Although possible benefits of nano-pesticide formulations have been suggested, concerns regarding their agricultural application have emerged particularly on the biosafety of such products and their long-term effects on the environment and humans, and specifically for chronically exposed workers. Importantly, the application of approved pesticide formulations, not intentionally released in the environment in the nanometric size should be carefully considered. Synthetic amorphous silicon dioxide as a nanomaterial in the form of stable aggregated particles of > 1 μm size has been recently approved as an insecticide in Europe (ECHA, 2014). However, although the exposure to nanoscale primary particles was not expected during the specific intended biocidal use of the chemical, the hazards and risks related to the individual NPs of silicon dioxide, maybe derived from environmental dissolution processes, could not be definitively ruled out. In this scenario, more substantial information appears necessary to eventually update this position and understand possible unexpected exposure and adverse effects. Comparable considerations may be done for copper as fungicide for agricultural crops (US-EPA, 2009), whose possible nanoscale “biotransformation” once released into the environment, may provide the chemical a peculiar biocidal and toxicological behavior. Therefore, scientific research should be aimed to understand the complex interplay between environmental conditions and applied chemicals or nano- enabled chemicals in order to define how primary physico-chemical particle properties and thus biological reactivity of the substances may change in response to different agrosystem stressors, as well how this response may vary according to the pristine or environmentally acquired features of the substances.
The improved solubility and bioavailability of nanopesticides may affect their environmental fate, as well as their toxicokinetic and dynamic behavior once adsorbed by organisms. Therefore, a robust toxicological assessment of the potential risks associated with the use of nanopesticides, both as nano-formulations of traditional active ingredients or nanomaterials that exhibit pesticidal activity, should be performed.
3. Nanotechnology for detection and remediation of environmental pollutants
Sensitive detection and efficient removal of an increasing number of persistent and emerging environmental pollutants are major challenges in our industrialized world (Liu et al., 2011a, 2011b). Sensors, diagnostic, and remediation devices for on-site application may allow close monitoring of environmental conditions, therefore increasing plant growth and protection as well as agricultural productivity, while reducing the use of agrochemicals in a precision farming perspective (Ghormade et al., 2011). Nanomaterials are promising materials in overall strategies to detect and remediate environmental contaminants (Baruah and Dutta, 2009; Zhang and Fang, 2010) (Fig. 1).
3.1. Environmental monitoring of toxicants and pathogens
Environmental security is one of the fundamental requirements of public well–being (Wanekaya et al., 2008). However, it still remains a major global challenge. There is a need to develop techniques that can detect and monitor environmental pollutants in different biological matrices, both in a sensitive and selective manner, to enable effective remediation. This seems an even more challenging issue in the agricultural sector where the accurate monitoring of pollutant concentrations has become imperative both for the protection of ecological systems, food supplies and human health.
In this scenario, nanotechnology may be developed and deployed for real time monitoring of a wide variety of fertilizers, herbicide, pesticide, insecticide, heavy metals, organic pollutants and pathogens (Chen and Yada, 2011; Kumar et al., 2015). The increased application of pesticides in various agricultural activities has made it necessary to explore the unique chemical and physical properties of nanomaterials to develop innovative sensors for pesticide residue detection. These “nanosensors,” intended as analytical devices having at least one sensing dimension no > 100 nm, fabricated for monitoring physico-chemical properties in places otherwise difficult to reach, may offer greater sensitivity, low detection limits, selectivity, fast detection rates, and more portability than conventional detection techniques (Fraceto et al., 2016; Liu et al., 2008). Nanomaterials may also offer improved detection limits for bacterial, viral and fungal pathogen determination in plants (Baac et al., 2006; Boonham et al., 2008; Yao et al., 2009). Moreover, nanosensors may also offer the opportunity to assess crop growth and field conditions including moisture levels, soil pH, fertility and temperature, crop nutrient status and concentrations, as well as early biological alterations induced by stressing stimuli in plants in order to support sustainable agriculture and enhanced productivity (Rai et al., 2012; Wang et al., 2010). The use of a network of sensors, global positioning, and information systems through an agricultural area could measure and report on a number of different environmental, crop and pest variables. These reports would support choices for fertilization strategies, reduction of inputs, and better management of time and environmental resources.
The development of nanosensor systems requires investigation to address nanomaterial sensitivity to common toxicant residues, pathogens and environmental parameters, the need for a multi-residue identification in the real agricultural scenario, the fabrication and validation of suitable detection instruments as well as issues related to nanomaterial exposure to the surrounding environment. A thorough understanding of such aspects and their implications is necessary before widely introducing nanomaterials in such a complex agricultural production system and should also be supported by increasing parallel regulatory frameworks.
3.2. Nanotechnology for water and soil remediation
Environmental pollution is one of the greatest problems that the world is facing today (Das et al., 2015). Soils and groundwater may be contaminated by toxic pollutants from either natural or anthropogenic sources at concentrations capable of posing great risk to human health or the environment (Thomè et al., 2015). Nanotechnology has been viewed as potentially providing sustainable solutions to these global challenges related to the protection of soils and water. Nanotechnology based techniques can contribute to new cost-effective methods for the removal of soil pollutants such as heavy metals/metalloids, dyes and organic pollutants (Gupta et al., 2013; Hua et al., 2012; Li et al., 2016; Park et al., 2013; Sánchez et al., 2011; Singh et al., 2013; Sivakumar et al., 2012; Udom et al., 2013; Wang et al., 2013; Xu et al., 2012; Zhao et al., 2011), as well as for water and wastewater treatment (Feng et al., 2013; Ng et al., 2013; Ruiz-Hitzky et al., 2013; Shukla et al., 2013). Nano-remediation methods involve the application of reactive nanomaterials for transformation and detoxification of pollutants. Nanomaterials have highly desired properties and flexibility for both in situ applications, to directly treat the environmental matrices in the subsurface, as well as in ex situ uses, when matrices must be removed from the site before treatment (Reddy and Lee, 2013). NPs may be able to access very small spaces in the subsurface and remain suspended in groundwater, achieving a wider distribution compared to larger, macro-sized particles (Li et al., 2016). Nano-remediation has the potential to reduce the overall costs of cleaning up large-scale contaminated sites, and also to reduce clean-up time, eliminate the need for treatment and disposal of contaminated soil, and reduce some contaminant concentrations to near zero-all in situ.
Many different nanoscale materials have been explored for environmental remediation, such as metal, metal-oxide and bimetallic NPs, carbon nanotubes and fibers. Of these, nanoscale zero-valent iron (ZVI-NPs) has been widely investigated, mainly due to its low toxicity and low cost in production (Fu et al., 2014; Tosco et al., 2014; Yan et al., 2013). ZVI-NPs are electron-donor molecules that have the potential to participate in the degradation of chlorinated compounds and the reduction of heavy metals through redox reactions (O′Carroll et al., 2013). For instance, ZVI-NPs have been researched for the removal of heavy metals such as cadmium from aqueous solutions (Boparai et al., 2011), and chromium [Cr(VI)] from soil polluted with tannery wastes (Singh et al., 2012a), and from wastewater (Fu et al., 2013; Lv et al., 2011). The possibility to eliminate nutrients such as nitrogen from activated sludge (Wu et al., 2013) and phosphorus from aqueous solutions (Liu et al., 2013) through ZVI-NPs has been also evaluated. However, challenging issues, such as the rapid aggregation and settling of ZVI-NPs and their possible reactions with a number of natural environmental constituents currently prevent their widespread commercial application (O′Carroll et al., 2013).
Some metal oxide-NPs have been used for the removal of several heavy metals and organic compounds as well. Iron oxides have been widely used in the environmental field as potential adsorbents due to their redox cycle, ion exchange, high affinity for contaminants (Braunschweig et al., 2013; Lee et al., 2013), and magnetic properties (Zhang et al., 2011, 2013). Magnetite (Fe3O4), another member of the iron oxides, has been used for pollutant adsorption (Adeleye et al., 2016). After contaminant removal, magnetic oxides can be easily recovered from aqueous media, making the cleaning process more cost- effective. Nanostructured bimetallic systems—i.e., palladium/Fe, Ag/Fe, and Ni/Fe—eventually stabilized with carboxy-methyl cellulose, polymers, and surfactants have been developed to overcome NP agglomeration. Therefore, these systems have been studied to effectively remove heavy metals, dyes, and halogenated compounds and to kill bacteria (Li et al., 2016; Singh etal., 2012b; Zhou et al., 2011). Porous Ti silicate and Al nanocomposite (Al2O3/TiO2) can be used to remove heavy metals, particularly lead (Pb2+) and Cd2+ (Das et al., 2015). However, although efficient in removing pollutants, the field application of bimetallic NPs should be viewed with caution given their potential to generate reactive intermediates and final toxic products (Li et al., 2011; Yuan et al., 2012). Carbonaceous nanomaterials and their composites exhibited good adsorption capacities for different classes of halogenated organics and polychlorinated biphenyls (Bikshapathi et al., 2011; Ma et al., 2011; Peng et al., 2003; Velzeboer et al., 2014; Wang et al., 2014a,b; Zhou et al., 2011) as well as organic compounds, polycyclic aromatic hydrocarbons, volatile organic compounds, herbicides, industrial dyes, and heavy metals in water (Farghali et al., 2013; Liu et al., 2011a,b; Hou et al., 2013; Hüffer et al., 2012; Moradi et al., 2011; Wang et al., 2014a, Wang et al., 2014b).
Even though potential beneficial effects on the destruction and transformation of toxic contaminants have been suggested for nanomaterials, there is a still lack of information about their regeneration and reusability, large-scale application, and efficiency in wastewaters and contaminated soils. Additionally, little is known about the nanomaterial life-cycle i.e., from their introduction into the environment, through their “active working phase,” up to the disposal of pollutant-loaded nanomaterials—about the possible release of metal ions and nanomaterial impact on different ecosystems. All these issues should be addressed by toxicological research on both the in-lab and in-field scale. This appears essential to define those chemical reactions and physical mechanisms that determine the fate of nanomaterials into the environments and efficacy of remediation, as well as those factors that influence their ability for decontamination. It is important to develop and validate toxicological models able to predict remediation at a wide range of field sites as well as possible environmental and human health toxicity derived from the deliberate injection of NPs into soils and groundwater for agrochemical delivery or remediation purposes (Thomé et al., 2015). These issues seem even more important to be addressed, considering preliminary studies reporting adverse effects of different nanomaterials, including those employed as nano-fertilizers and pesticides, as well as for water and soil remediation, in several in vitro and in vivo models (Iavicoli et al., 2011, 2012, 2013). Overall, proper evaluation—particularly full-scale, ecosystem- wide studies—of agricultural employment of nanomaterials and nano-remediation should provide useful information for preventing potential adverse occupational exposure (Karn et al., 2009, 2011).
4. Nanoscale agricultural products
Some of the products of agriculture and agroforestry can be nanoscale. Cellulose nanomaterials from a variety of plant-based resources can be derived as either nanofibers (i.e., from brown cotton and curaua) or nanocrystals (i.e., from trees). Nanocellulose is an attractive environmental option due to the abundant and renewable parent sources, potential for biodegradability of final products, and overall decreased carbon footprint of the process. All of these characteristics add up to decreased cost to produce and use nanocellulose over time.
In the agricultural setting, nanocellulose has been introduced into the field of protective coatings, for seeds, plants, and foodstuffs. Nanocellulose composite coatings are valued for their mechanical and barrier properties, biodegradability, and crop safety applications. They are used as edible coatings/films for crop harvesting and storage, as well as to protect perishable plants or plant parts, and other objects. Such nanocellulose-based coatings/films are effective to protect fresh and processed agricultural products (US Patent Application Publication, 2016). Jung et al. (2016) have developed and validated a cellulose nanofiber-based hydrophobic coating (InnofreshTM) for reducing rain-induced cherry cracking in preliminary field validation trials.
Cellulose nanomaterials are a promising alternative adsorbent for water remediation due to their high surface area-to-volume ratio, remarkable mechanical properties, biocompatibility, and sustainable source, as well as inherent environmental inertness (Carpenter et al., 2015). Cellulose nanomaterial's easily functionalizable surface allows also for the incorporation of chemical moieties that may improve adsorption capacity of pollutants, including metal ions and organic contaminants (Hokkanen et al., 2016; Korhonen et al., 2011; Yu et al., 2013). Cellulose nanomaterials may find passive application as scaffolds or particle stabilizers for reactive NPs (Snyder et al., 2013) or to improve membrane tensile strength, surface hydrophilicity, permeability, selectivity, and resistance to biofouling (Lalia et al., 2013; Qu et al., 2010). Moreover, innovative nutrient delivery systems based on porous nanoscale cellulose could reduce nitrogen loss by promoting enhanced plant nitrogen uptake (Yu et al., 2013). A novel cellulose acetate-coated compound fertilizer with controlled-release and water-retention has been prepared to serve as a suitable moisture-holding additive in the soil for agricultural purposes (Wu and Liu, 2008).
Despite the increased interest in the development of nanocellulosic products, few studies fully address exposure levels and potential toxicity. Concerning this latter aspect, some in vitro investigations demonstrated that exposure to nanocrystalline and microfibrillated cellulose caused either no cell death or marginal cytotoxicity (Dong et al., 2012a,b; Ni et al., 2012; Vartiainen et al., 2011; Yang et al., 2013), while others reported a significant reduction in cellular viability (Clift et al., 2011; Pereira et al., 2013). However, very limited information is currently available referencing the potential risk associated with inhalation exposure to nanocellulose (Cullen et al., 2002; Dong et al. 2009; Lin and Dufresne, 2014; Tatrai et al., 1995; Warheit et al., 1998).
5. Critical issues of occupational risk in nano-agricultural field
5.1. Risk assessment
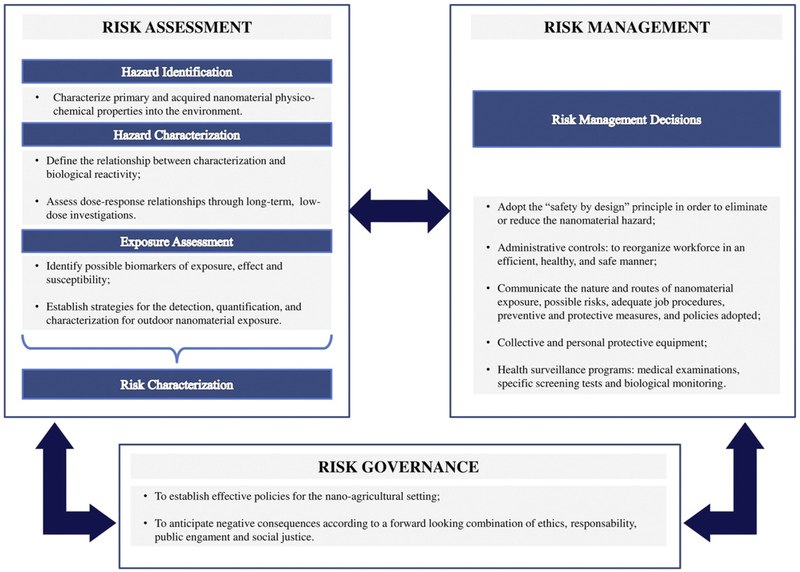
Risk assessment of agricultural chemical impact on human health is not an easy process because of the great variety of substances employed, mixtures used in the field, differences in exposure dose, and geographic as well as meteorological characteristics of the agricultural areas where agro- chemicals are applied (Bolognesi, 2003; Damalas and Eleftherohotinos, 2011; Pastor et al., 2003). Nano-specific risk assessment is a challenging issue since the assumptions used to assess the risks of conventional chemicals, together with test methodologies and modelling paradigms for behavior in the environment and possible human uptake, may not be appropriate for nano-enabled products (Damalas and Eleftherohotinos, 2011) (Fig. 2).
Fig. 2.
Main elements, actions and recommendations of the nano – agricultural risk assessment and management programs.
The hazard identification of nano-formulations needs to focus on the active ingredient concentration properties and the nano-component. A review of the body of literature on potential environmental and health hazards of NPs points out the challenge of interpretation for the purpose of hazard identification (Krug, 2014). If the nano-component simply protects the active ingredient from degradation, then the fate and behavior of the nano-component may be the same as in conventional pesticide formulation. In the case of pristine, inorganic, engineered NPs directly employed as biologically effective fertilizers, pesticides, or in the soil or water remediation, the hazardous behavior should be carefully viewed in a life-cycle perspective in the environment from the introduction into application fields up to the disposal of working residues (Shatkin and Kim, 2015).
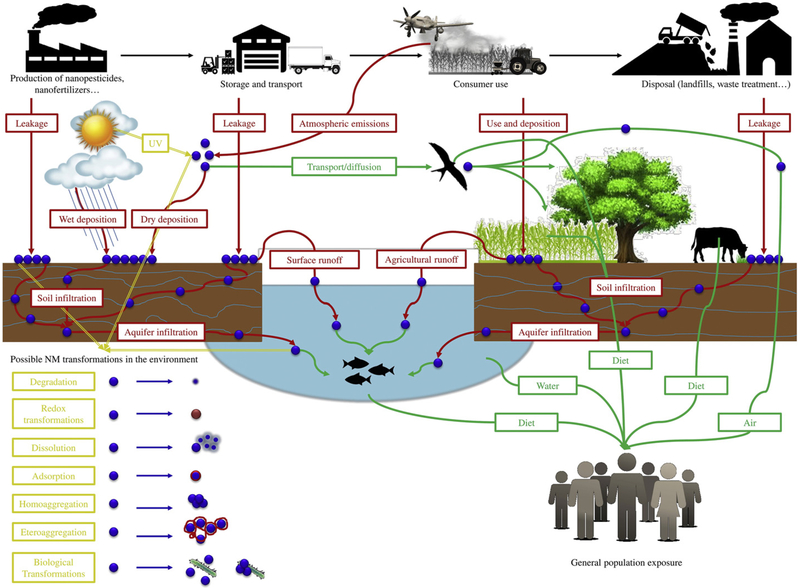
Intentional and enhanced input of nanomaterials into agricultural ecosystems poses a number of questions regarding the environmental fate and transportation of these materials into the environment that still have to be answered (Fig. 3).
Fig. 3.
Life-cycle of nano-enabled products used in agriculture.
This seems an even more urgent issue to face considering the large number of nano-formulations potentially employed in the agricultural practice as well as the uncertainties concerning possible interactions with variable environmental factors. These may include the still unknown influence of naturally occurring ultrafine particles on the fate of nano-agrochemicals; the uncertainties concerning the alterations caused by aging, soil, and water features; as well as those induced by the diverse work procedures adopted together with the difficulties in quantifying all these variables into an adequate risk assessment process. However, these aspects should be taken into careful consideration because they may affect the physico-chemical characterization of nanomaterials, changing their toxicological profile and thus occupational risks.
Therefore, assessing hazards posed by nanomaterials in the agricultural field may go beyond the standard strategies for assessing hazardous features from conventional chemicals or from the same nanomaterials applied in different settings. This may primarily require evaluation of a series of physico-chemical parameters, such as the nanomaterial composition, chemical form, morphology, surface area, functionalization, and dustiness (Evans et al., 2013). Moreover, number concentration and particle size distribution, together with the ratio of “free” and active ingredient bounded- nanomaterials that may all be important in determining the substance bioavailability, bioactivity, and potential toxicity should be assessed (Kookana et al., 2014). Additionally, agricultural specific dynamic- parameters such as time, bio-concentration factors, aggregation, and sedimentation rate may all be considered as indicators of the behavior of nano-sized agro-chemicals in the environment, providing ulterior information regarding their persistence, bio-accumulation potential, and hazardous profile (Kookana et al., 2014). Studies on the harmful properties of agricultural nanochemicals should consider the possible interactions between nanosized chemicals and the multiple stressors existing in the agro-ecosystems that may result in antagonistic, synergistic, and additive “mixture” of effects, modifying toxicities induced by the single substances and, potentially, susceptibilities to environmental and health adverse effects (Handy and Shaw, 2007; Kahru and Savolainen, 2010; Klaine et al., 2008, 2012; Perez et al., 2009; Scown et al., 2010).
In this context, the challenging issue to understand the environmental and human health and safety implications of nano-solutions intentionally developed and applied for agriculture is further complicated by the possible additional risks due to the “nano-emerging” contamination caused by biosolids. Biosolids containing nanosized metals derived from consumer products (i.e., Ag, TiO2, and ZnO-NPs) and employed as land-fertilizers, landfilling and incineration as other methods of biosolid disposal, together with irrigation with wastewater or contaminated surface water may all be considered as a possible alternative source of nanomaterials into the environment (Chen et al., 2015; Judy et al., 2015; OECD, 2012). The toxicological implications of such peculiar contaminations, since these are characterized by “aged” nanomaterials, perhaps transformed during waste treatment have not been fully explored. These may constitute an additional source of nanosized micronutrients or nanobiocidal substances and may interact with intentionally introduced nanomaterials, possibly modifying risks for human and ecosystem health. Moreover, little is known about the amount of biosolid-derived nanomaterials that may enter the food webs or cause direct or indirect toxicity to plants, microbial communities, or other soil organisms, in turn affecting the ecosystem that holds nanomaterials applied for agricultural purposes, maybe influencing their environmental behavior and efficacy. The complex interplay between environmental matrices—including resident plant and microbial species, chemical substances, and human beings—may provide variable profiles of risks for the exposed populations and requires concerted human and ecotoxicological efforts to find more meaningful strategies to define models able to predict possible adverse outcomes.
These general considerations are in line with the position expressed by the U.S. Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Scientific Advisory Panel and consulted by the U.S. Environmental Protection Agency (EPA), concerning the adequacy of current procedures for evaluation of the hazards and exposure associated with nanometal pesticides. The Panel concluded that there may be potential for pesticides containing nanoscale materials to pose different risks to humans and the environment than pesticides that do not contain nanomaterials. Models generally employed for non-nano pesticides may be not appropriate for those containing nanomaterials. In this case, additional information on metrics for dose and exposure (i.e., particle size, mean and distribution, surface area, number, and mass concentration) and on other parameters (e.g., shape, agglomeration, stability in application environments, surface chemistry, aspect ratio, and stiffness) is necessary to improve our understanding of the processes involved and to develop alternative approaches for hazard evaluation and characterization (FIFRA-SAP, 2009).
In this complex scenario, toxicological research should be viewed as the basis of hazard identification and characterization. Linking toxicology testing to hazard determination is not new to the global chemical industry. However, moving to the nanoscale has revealed new or heightened biological activity driven by size and physico-chemical properties that should be specifically explored. Importantly, better understanding will facilitate assessment of hazards for innovative nanomaterials in the agricultural field and thus the design of “safer” nanomaterials both for the environment and for worker health (Schulte et al., 2013a). Preliminary, short-term, toxicity testing has demonstrated the pro-oxidant and pro-inflammatory action of nanomaterials; their ability to induce adverse effects on animal respiratory, cardiovascular, and nervous systems; as well as their potential to act as endocrine disruptors (Iavicoli et al., 2013). Although this information can be used to anticipate hazards from a small number of nanomaterials and implement exposure control measures, there is ultimately a need for standardized approaches for toxicological evaluation and setting priorities for toxicity testing on long-term and low-dose investigations as those potentially experienced by workers (Schulte et al., 2014).
The measurement of exposures to nanomaterials is critical in assessing and managing risks in the nano- agricultural field. Exposure assessment includes issues relating to reliable detection, quantification, and characterization of nanoscale materials overcoming the limited “chemical speciation concept” widely used in the general analytical chemistry (Kah et al., 2013). To just identify hazardous components is not informative enough; there is also a need to know who is being exposed to nano-formulations, at what exposure concentrations, and how exposure may be affected by changes in job tasks. Workers who may receive the greatest exposure due to the nature of their work are those engaged in nanomaterial synthesis and incorporation into agricultural products; agricultural workers who mix, load, and transport nano-formulated pesticides and fertilizers; as well as licenced and trained ap-plicators. During nano-formulation handling and application, exposure is affected by product form, type of packaging, presence of toxic chemical adjuvant employed in the chemical formulation, together with weather conditions at the time of application, including air temperature, humidity, and windy conditions. However, irrespective of whether the occupation involves the direct use of nano-formulations, the presence of such chemicals in the working environment—including atmosphere, soil, sediments, or water—may constitute a potential bystander occupational exposure for farm workers who may be exposed to nano-chemicals, perhaps including residues building up over time, based on job tasks and environmental conditions (Damalas and Eleftherohotinos, 2011). Additionally, nano-contamination of water resources and presence of residues on food products, may represent another source of nanomaterial exposure not only for involved workers but also for the general population, whose entity is difficult to estimate (Fig. 3).
In this scenario, to “quantify the exposure concentrations” appears a complex endeavour, especially considering the difficulties in monitoring outdoor levels of nanoscale chemical substances and the limited information concerning appropriate exposure metrics to employ. Additionally, nano- formulations may consist of various forms of organic and/or inorganic ingredients that may also vary with time during storage and/or during/after application as a consequence of the physical, chemical, and biological processes occurring within the environment. Analytical techniques should be able to determine such changeable properties that may affect the behavior of the nano-formulations in terms of solubility, sorption, degradation, and availability in nonequilibrium environmental compartments where differentiating manufactured nanomaterials from those of background is also extremely difficult under realistic low-concentrations conditions. Other “external” factors affecting the stability of nanomaterials—such as possible interactions with containers/tubing, matrix effects, changes in pH, the effects of impurities, degradation of coatings, functionalization, and/or emulsifiers that ensure nanomaterial stability—may also need to be taken into account while assessing occupational exposure (Tiede et al., 2009).
Measuring outdoor pesticides and herbicides can be accomplished by the use of NIOSH Methods 5600–5602 for organophosphates, chlorinated and organonitrogen herbicides, which call for the use of collection of air samples using XAD-resin sorbent tubes (NIOSH, 1994). Due to their small size and propensity to penetrate to the deep lung, determining the potential for exposure to aerosolized nanomaterials are of a high priority. Evaluating exposure to the nanomaterial of interest may be challenging if it is a nanomaterial without a sampling method; however, there is some useful guidance available. Initial guidance was published in 2011 and relies on a general framework for conducting workplace characterization; understanding exposure potential to nanomaterials; accounting for background aerosols; constructing exposure groups; and selecting appropriate instrumentation for monitoring, providing appropriate choice of exposure limits, and describing criteria by which exposure management decisions should be made. NIOSH has provided recommended exposure limits (RELs) for nano TiO2 and carbon nanotubes and carbon nanofibers. The supporting documentation for these RELs are published in Current Intelligence Bulletins and include sampling information for the nanomaterials (NIOSH, 2011, 2013). For nanomaterials without an REL, NIOSH authors also published a technique that includes the use of real-time particle counters in addition to a pair of filter-based samples where one is analyzed for the element of interest (e.g., using standard analytical chemistry methods for trace metals) and the other is analyzed using electron microscopy for particle size, morphology, etc. (Eastlake et al., 2016; Methner et al., 2010; NIOSH, 2009). Sampling may need to be conducted on several days under various atmospheric conditions to obtain representative data.
Coupling the complexity in understanding the dynamic behavior of nanomaterials in the environment and the possibility of a rapid change in their physico-chemical properties, makes exposure evaluation a challenging issue. This currently prevents enough data to adequately support exposure modelling, particularly for those slow —/targetedrelease nano-formulations. Additionally, pesticide formulations, protective clothing and gear, application equipment, and personal hygiene practices, together with the amount sprayed and type of nozzle used should all be viewed as factors influencing real worker exposure. Moreover, risk assessment for general pesticide application tends to rely on models derived from personal measurements of dermal exposure. Biological monitoring may act as a fundamental complementary method to the environmental exposure assessment. The direct measurement of chemicals in biological fluids may be accurate predictors of internal dose already absorbed by agricultural workers. Unfortunately, in the case of engineered NPs no definite biomarkers of exposure have been proposed, particularly for this complex occupational exposure setting. Therefore, in vivo toxicokinetic studies should be performed to define possible indicators to be subsequently validated and applied in occupational biomonitoring field studies. Additionally, upon entering the body, translocation and distribution of the nano-chemicals across different organs and tissues may occur. Fate in tissues may be determined by the organism's potential to bio-transform molecules as a step toward elimination and by the potential of nanomaterials to interact with the relevant cellular receptors and be up-taken (Kookana et al., 2014). In this preliminary phase of knowledge, the definition of possible target organs of nanomaterial action and the development of biomarkers of early effect appear to be useful means to define the toxicological profile of such xenobiotics and eventually adopt adequate preventive and protective measures. Overall, quantifying internal exposure to nano-sized materials and early biological alterations induced may be important to understand the relationship between nanomaterial properties and toxicity and seem essential to support an adequate risk assessment process.
5.2. Risk management
The ultimate goal of risk assessment is to provide quantitative predictions of given risks, enabling their evidence-based management (Savolainen et al., 2010). However, vast uncertainty about hazards, exposures, and risks in the emerging nano-agricultural field make it imperative to adopt a dynamic precautionary management approach before all of the evidence is completed for this direct and intentional application of nanomaterials in the environment. This means that risk management strategies and guidance will be changing and continuously evaluated, improved, and verified as health and safety assessments and risk information become more substantial (Iavicoli et al., 2014a; Schulte et al., 2013b). To effectively address potential agricultural nanotechnology-related risks, a suitable management plan including a hierarchy of controls should be emphasized. This should be focused on the adoption of strategies aimed to eliminate or substitute exposures, followed by risk minimization through the application of administrative controls that can be applied at all stages of the life-cycle of nanomaterials up to the use of personal protective equipment (Fig. 2).
The most effective approach, at the top of the hierarchy of controls, is to eliminate or design out hazards from nanomaterials according to the long-standing principles of “prevention through design” and “safety by design” (Geraci et al., 2015; Schulte et al., 2008a, 2008b). To this aim, understanding connections between nanomaterial physico-chemical properties and biological reactions, seems important to guide the modifications of some nanomaterial features, thus profoundly reducing or mitigating their potential toxicity while maintaining agricultural/environmental remediation utility. Therefore, in this extremely variable and still not understood exposure scenario, sustainable agriculture may take advantage of the primary preventive measure to produce nanomaterials and nano-enabled products in ways that can minimize human and environmental harm. Well-established principles of green chemistry can be followed to design and produce innovative nanomaterials, thus avoiding dependence on processes that might result in environmental pollutants and health risks for occupationally exposed populations. Green engineering “embraces the concept that decisions to protect human health and the environment can have the greatest impact and cost effectiveness when applied early to the design and development phase of a process or product” (Bergeson, 2013). Green engineering considers the full life-cycle of a product, from the extraction of the materials through manufacturing, product use, and end of life. Green nanotechnology's focus on the full life-cycle can better prepare users to minimize generating new hazards through unintended consequences.
In vitro and in vivo models for screening the impact of characterization changes on nanomaterial bio-activity, as well as the dose-response relationships, should be clearly developed. Moreover, other aspects, such as the pH of a nanomaterial suspension as well as the oxidation state of the material, should be investigated as potential modifiable factors able to predict nanomaterial interactions in different biological environments. Using high-throughput screening and evaluation techniques has the potential to aid in more rapid identification of nanomaterial hazards and mechanisms of toxicity. They can also allow for the development of predictive models to design inherently safer products and greener nanotechnology in order to achieve meaningful worker protection.
In addition to developing “safer” nanomaterials, minimizing or eliminating exposures through proper containment are major means of control of materials in the occupational environment. Administrative controls should include the identification of potentially exposed workers and the definition of which tasks pose the highest risks for exposure in order to allocate and organize the workforce in the most efficient, healthy, and safe manner in terms of number and expertise. Training programs should be defined through which companies communicate to workers information sufficient to understand the nature and routes of potential nanomaterial workplace exposure, possible risks, adequate job procedures, preventive and protective measures, and policies adopted. In training and risk communication, it may be important to overcome the frequently insufficient or inadequate information on substances classified as nanomaterials as well as for chemical products containing manufactured nanomaterials. Industry may play a key role, for instance, by supplying the necessary data and product information and by sharing technical, scientific and policy expertise (Watson et al., 2011). Product labels and safety data sheets (SDS) should be reviewed for information concerning quantity, quality, traceability, proper application, as well as health and safety information regarding nanomaterials, either as active nano-ingredients or more complex nano-formulations, though the quality of such information may vary and may not present a complete documentation of the health and safety concerns (Eastlake et al., 2012). Risk communication should be viewed as an integral part of the risk management strategies providing suitable and easily accessible technical, health, and regulatory information to the occupational and general population. This is extremely important to ensure appropriate information is available regarding the benefits and challenges of nanotechnology in agriculture and environmental remediation, protecting public and occupational opinion from both unrealistic hopes and stigmatization of these innovative applications.
Engineering controls such as use of closed transfer systems, low drift nozzles, and carbon filters for the tractor cab should be used whenever possible. In addition, the use of personal protective equipment should be considered to protect outdoor workers, but not as a primary preventive measure. Respiratory and eye protection, together with gloves, aprons, and coats, are effective measures for preventing dermal exposure. Information on variables such as the quantity of nanomaterials being handled, their physical form and dispersibility, as well as the task duration may be important to assist the adoption of the most appropriate protective measures for given occupational processes (NIOSH, 2009). As each one of these variables increases, the chance of exposure becomes greater and does the need for more efficient exposure control measures.
Additionally, as a measure of secondary prevention, occupational health surveillance programs can be useful components of a nanomaterial risk management plan. They may include elements of hazard and medical surveillance (i.e., monitoring of health outcomes or biological changes) as well as medical surveillance of the effects at group and individual levels (Nasterlack et al., 2008; NIOSH, 2013; Schulte and Trout, 2011; Schulte et al., 2008c). The essential steps of these surveillance programs should be the initial, periodic, and post-incident medical examinations with specific medical screening tests; the worker training to recognize and report symptoms of exposure to a given hazard; and the employer actions in response to identification of potential hazards and risks to health (Trout and Schulte, 2010). Additionally, biological monitoring strategies aimed to detect internal doses of different xenobiotics, indicators of early biological effect, and biomarkers of susceptibility to nanomaterial insults, where applicable should be defined, validated, and eventually applied in occupational surveillance plans. Importantly, biological monitoring information may be an integral instrument for a comprehensive nanotechnology risk assessment and management process tailored to individual subjects or specific subpopulations involved in this innovative nanotechnology field (Iavicoli etal., 2014b, 2016; Schulte and Hauser, 2012). Toxicological research in in vitro and in vivo models may provide helpful data regarding the mechanisms of nanomaterial toxicity as well as information concerning the toxicokinetic and dynamic behavior of these chemicals once adsorbed into the organisms. These may be important to extrapolate possible sensitive and specific exposure and early effect biomarkers to be validated in occupational settings. Moreover, regarding susceptibility, scientific efforts should be focused on the determination of possible biomarkers indicative of an elevated sensitivity to nanomaterial effects, which should provide quantitative estimates of a population variability to be employed into an adequate occupational nanomaterial risk assessment as well as in the adoption of specific or implemented workplace preventive and protective measures (Iavicoli et al., 2016).
Epidemiological research may be useful to enhance the impact of occupational health surveillance through the periodic analysis of aggregated data in order to identify patterns of worker health that may be linked to job activities and practices (NIOSH, 2013; Schulte et al., 2009). Additionally, exposure registries may be useful in setting the stage for this kind of research. Registries can be created to enumerate and identify exposed individuals, to provide them with adequate information and guidance as well as with primary or secondary prevention measures concerning potential nanomaterial exposure risks (Schulte and Trout, 2011). Overall, aligning health and safety purposes with business goals can improve the profitability and sustainability of agricultural nanotechnology by protecting employee skill, experience, and knowledge while implementing societal beneficial aspects related to this emerging technology.
5.3. Risk governance
At the societal level, the rapid growth of nanotechnology requires regulatory changes that could shape application of nanomaterials in agriculture and therefore contribute to adequately assess and manage potential risks in a precautionary manner (Fig. 2). This relies on the concept that results and benefits of innovative technologies may be dependent in part on the regulatory systems and policy atmosphere (Watson et al., 2011). The US EPA uses its authority under the FIFRA to register pesticides and recently proposed to treat nanoscale versions of approved conventional substances as “new” for purposes of registration (US EPA, 2011). In Europe, nanoscale pesticide active ingredients and formulations are covered by the Plant Protection Products Regulation (EC 1107/2009 (EC, 2009). This regulates the authorization and use of pesticides in the European community and applies to products either alone or in mixtures, in whatever size, shape, or physical state. In addition to nano-formulations of traditional active ingredients, nanomaterials that exhibit pesticidal activity also need to be considered (e.g., nano-Ag). These materials would have to be assessed in the same way as any other active ingredient collecting data on the toxicity and environmental fate. The key question, however, remains whether, and to what extent, current procedures for hazard identification and characterization are able to deal with such nano-products and whether new/enhanced properties would be identified when following standard protocols.
Therefore, academic, industry, governmental bodies and stakeholders should provide concerted actions to obtain a general description of the nanomaterial characteristics and toxicological behavior, to define their current applications in the nano-agricultural field, as well as their potential intended uses. Efforts should be focused on establishing new, nanomaterial-specific policy or effective application of existing policies to nanomaterials, with a specific focus on the nano-agricultural setting.
Construction of relevant occupational safety practices and policies should be present from the beginning of an activity involving nanomaterials rather than implemented later as a reaction to the definition of unsafe conditions. Due to the safety concerns about some nanomaterials and the problem of inappropriate generalization due to the huge range of nanotechnological applications, it is necessary to address this gap in the regulation of such xenobiotics. It should be filled by using the findings of the ongoing projects in toxicity testing, decision-making on nanomaterial characterization and testing protocols, as well as exposure and precautionary management data.
An alternative nanotechnology governance approach based on a forward-looking combination of ideas in anticipatory ethics, future oriented responsibility, upstream public engagement, and deliberation and theories of justice may provide a useful support (Hester et al., 2015). This aims to build an intellectual and societal capacity to anticipate negative consequences before they arise in the hope that such an approach could be the antithesis of the retrospective imposition of responsibility and liability after the harm is done, which is the outcome of traditional regulatory and ethical approaches. It would at the same time be responsive to the real-time state of scientific knowledge and uncertainty and sufficiently flexible to be able to keep pace with and adapt to evolving scientific knowledge.
6. Concluding remarks
Nanotechnology is a useful tool in modern agriculture, and agri-food nanotechnology is anticipated to become a driving economic force in the near future to face emerging agricultural and environmental challenges principally related to the needs for an increased productivity, sustainability, and security of agriculturally produced foods (Parisi et al., 2015).
Some interesting critical aspects emerged from our review:
Beneficial expectations: nanotechnology promises the development of “high-tech” agricultural fields equipped with a range of intelligent nanotools that allow for the precise management and control of inputs. It may be helpful to implement delivery systems for agrochemicals, improve plant breeding, and create new nano-bio-industrial products for environmental pollutant detection and remediation. It promises to reduce the impact of modern agriculture on the environment and input costs, while improving quality and quantity of yields.
Emerging concerns: despite the promising potential of nanotechnology in the agri-food sector, there are still potential toxicological hazards and risks. Release of nanomaterials into the environment may occur when they are used as nanofertilizers, nanopesticides, as well as in applications for pollutant detection, cleanup, and water treatment. Nanomaterial human health, safety, and ecological implications are not well understood. Particularly, uncertainties remain concerning the possible health effects on workers who may be exposed for extended periods of time to a wide variety of nanomaterials at variable environmental concentrations.
Future research needs: scientific efforts should overcome the lack of toxicological and environmental effect information through greater research into the hazardous properties and biological reactivity of nanomaterials and determinant physico- chemical properties, employing experimental settings more realistically resembling exposure conditions experienced in the agricultural field. Human and ecotoxicological research should concertedly understand the complex interplay between agro-ecosystems, nanoscale substances, levels of exposure, and human beings. This may include defining the influences that the environmental bio-physico and chemical characteristics have on nanomaterial- acquired biological reactivity in a lifecycle perspective. All this information should be shared by the scientific community, industry, and governmental regulatory agencies in order to clearly define the hazardous profile of nanomaterials under variable exposure scenarios. This information can then be used to develop suitable risk assessment procedures and precautionary management measures including the identification of adequate environmental exposure limits and biological limit values as guidelines to assist in the control of possible health risks.
Overall, appreciation of these aspects may lead to the development of broad policy and international regulatory consensus incorporating ethical analysis, public engagement, and participation into decision making. These are central topics to successful nanotechnology application in terms of ecosystem solution and occupational health and safety management, in order to achieve a long-term sustainability of this emerging technology in the agricultural field.
Abbreviations:
- Ag
silver
- CNTs
carbon nanotubes
- Fe
iron
- Fe3O4
magnetite
- NPs
nanoparticles
- TiO2
titanium dioxide
- ZnO
zinc oxide
- ZVI-NPs
zero-valent iron nanoparticles
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of organizations to which they are affiliated.
Conflict of interest statement
The authors declare they have no conflict of interest.
References
- Adeleye AS, Conway JR, Garner K, Huang Y, Su Y, Keller AA, 2016. Engineered nanomaterials for water treatment and remediation: costs, benefits, and applicability. Chem. Eng. J 286:640–662. 10.1016/j.cej.2015.10.105. [DOI] [Google Scholar]
- Anjali CH, Sudheer Khan S, Margulis-Goshen K, Magdassi S, Mukherjee A, Chandrasekaran N, 2010. Formulation of water-dispersible nanopermethrin for larvicidal applications. Ecotoxicol. Environ. Saf 73:1932–1936. 10.1016/j.ecoenv.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Anton N, Benoit JP, Saulnier P, 2008. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J. Control. Release 128:185–199. 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Ao M, Zhu Y, He S, Li D, Li P, Li J, Cao Y, 2013. Preparation and characterization of 1-naphthylacetic acid-silica conjugated nanospheres for enhancement of controlled-release performance. Nanotechnology 24:035601 10.1088/0957-4484/24/3/035601. [DOI] [PubMed] [Google Scholar]
- Baac H, Hajós JP, Lee J, Kim D, Kim SJ, Shuler ML, 2006. Antibody-based surface plasmon resonance detection of intact viral pathogen. Biotechnol. Bioeng 94:815–819. 10.1002/bit.20882. [DOI] [PubMed] [Google Scholar]
- Bang SH, Yu YM, Hwang IC, Park HJ, 2009. Formation of size-controlled nano carrier systems by self-assembly. J. Microencapsul. 26:722–733. 10.3109/02652040902726994. [DOI] [PubMed] [Google Scholar]
- Baruah S, Dutta J, 2009. Nanotechnology applications in pollution sensing and degradation in agriculture: a review. Environ. Chem. Lett 7:191–204. 10.1007/s10311-009-0228-8. [DOI] [Google Scholar]
- Bergeson LL, 2010. Nanosilver: US EPA's pesticide office considers how best to proceed? Environ. Qual. Manag 19:79–85. 10.1002/tqem.20255. [DOI] [Google Scholar]
- Bergeson LL, 2013. Sustainable nanomaterials: emerging governance systems. ACS Sustain. Chem. Eng 1:724–730. 10.1021/sc4000863. [DOI] [Google Scholar]
- Bhagat D, Samanta SK, Bhattacharya S, 2013. Efficient management of fruit pests by pheromone nanogels. Sci. Rep 3:1294 10.1038/srep01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikshapathi M, Mathur GN, Sharma A, Verma N, 2011. Surfactant-enhanced multiscale carbon webs including nanofibers and Ni-nanoparticles for the removal of gaseous persistent organic pollutants. Ind. Eng. Chem. Res 51:2104–2112. 10.1021/ie200741e. [DOI] [Google Scholar]
- Biswal SK, Nayak AK, Parida UK, Nayak PL, 2012. Applications of nanotechnology in agriculture and food sciences. IJSID 2, 21–36. [Google Scholar]
- Bolognesi C, 2003. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat. Res 543:251–272. 10.1016/S1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Boonham N, Glover R, Tomlinson J, Mumford R, 2008. Exploiting generic platform technologies for the detection and identification of plant pathogens. Eur. J. Plant Pathol 121:355–363. 10.1007/s10658-008-9284-3. [DOI] [Google Scholar]
- Boparai HK, Joseph M, O'Carroll DM, 2011. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater 186:458–465. 10.1016/j.jhazmat.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Braunschweig J, Bosch J, Meckenstock RU, 2013. Iron oxide nanoparticles in geomicrobiology: from biogeochemistry to bioremediation. New Biotechnol. 30:793–802. 10.1016/j.nbt.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Brunel F, El Gueddari NE, Moerschbacher BM, 2013. Complexation of copper(II) with chitosan nanogels: toward control of microbial growth. Carbohydr. Polym. 92:1348–1356. 10.1016/j.carbpol.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Carpenter AW, de Lannoy CF, Wiesner MR, 2015. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol 49:5277–5287. 10.1021/es506351r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Unrine JM, Judy JD, Lewis RW, Guo J, McNear DH Jr., Tsyusko OV, 2015. Toxicogenomic responses of the model legume Medicago truncatula to aged Biosolids containing a mixture of nanomaterials (TiO2, Ag, and ZnO) from a pilot wastewater treatment plant. Environ. Sci. Technol. 49:8759–8768. 10.1021/acs.est.5b01211. [DOI] [PubMed] [Google Scholar]
- Chen H, Yada R, 2011. Nanotechnologies in agriculture: new tools for sustainable development. Trends Food Sci. Technol 22:585–594. 10.1016/j.tifs.2011.09.004. [DOI] [Google Scholar]
- Chun JP, Choi JS, Ahn YJ, 2010. Utilization of fruit bags coated with nano-silver for controlling black stain on fruit skin of ‘Niitaka’ pear (Pyrus pyrifolia). Hortic. Environ. Biotechnol 51, 245–248. [Google Scholar]
- Clift, Foster EJ, Vanhecke D, Studer P, Wick P, Gehr P, Rothen-Rutishauser B, Weder C, 2011. Investigation of the interaction of cellulose nanofibers derived from cotton with a sophisticated human lung cell coculture. Biomacromolecules 12:3666–3673. 10.1021/bm200865j. [DOI] [PubMed] [Google Scholar]
- Cullen RT, Miller BG, Jones AD, Davis JM, 2002. Toxicity of cellulos fibres. Ann. Occup. Hyg 46 (Suppl. 1):81–84. 10.1093/annhyg/mef628. [DOI] [Google Scholar]
- Damalas CA, Eleftherohorinos IG, 2011. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 8:1402–1419. 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sen B, Debnath N, 2015. Recent trends in nanomaterials applications in environmental monitoring and remediation. Environ. Sci. Pollut. Res. Int 22:18333–18344. 10.1007/s11356-015-5491-6. [DOI] [PubMed] [Google Scholar]
- De Rosa MC, Monreal C, Schnitzer M, Walsh R, Sultan Y, 2010. Nanotechnology in fertilizers. Nat. Nanotechnol 5:91 10.1038/nnano.2010.2. [DOI] [PubMed] [Google Scholar]
- Ditta A, 2012. How helpful is nanotechnology in agriculture? Adv. Nat. Sci. Nanosci. Nanotechnol. 3:033002 10.1088/2043-6262/3/3/033002. [DOI] [Google Scholar]
- Dong H, Strawhecker KE, Snyder JF, Orlicki JA, Reiner RS, Rudie AW, 2012b. Cellulose nanocrystals as a reinforcing material for electrospun poly(methyl methacrylate) fibers: formation, properties and nanomechanical characterization. Carbohydr. Polym 87:2488–2495. 10.1016/j.carbpol.2011.11.015. [DOI] [Google Scholar]
- Dong P, Hirani AA, Colacino KR, Lee YW, Roman M, 2012a. Cytotoxicity and cellular uptake of cellulose nanocrystalls. Nano Life 02 (03), 1241006. [Google Scholar]
- Eastlake A, Hodson L, Geraci CL, Crawford C, 2012. A critical evaluation of material safety data sheets (MSDS) for engineered nanomaterials. J. Chem. Health Saf 53:S108–S112. 10.1016/j.jchas.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastlake A, Beaucham C, Martinez K, Dahm M, Sparks C, Hodson L, Geraci C, 2016. Refinement of the nanoparticle mission assessment technique into the nanomaterial exposure assessment technique (NEAT 2.0). J. Occup. Environ. Health 13 (9). 10.1080/15459624.2016.1167278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA, 2014. Synthetic amorphous silicon dioxide (PT18) - Assessment Report Finalized in the Standing Committee on Biocidal Products at Its Meeting on 13 march 2014. Available at. http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/1378-18/1378-8_Assessment_Report.pdf (Accessed on 07 October 2016).
- Elek N, Hoffman R, Raviv U, Resh R, Ishaaya I, Magdassi S, 2010. Novaluron nanoparticles: formation and potential use in controlling agricultural insect pests. Colloids Surf. A Physicochem. Eng. Asp. 372:66–72. 10.1016/j.colsurfa.2010.09.034. [DOI] [Google Scholar]
- European Commission (EC), 2009. Regulation (EC) No. 1107/2009 of the European Parliament and of the council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/ 414/EeC. Available at. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (Accessed on 07 October 2016).
- European Commission, 2011. Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Available at. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011H0696&from=EN (Accessed on 05 May 2017).
- Evans DE, Turkevich LA, Roettgers CT, Deye GJ, Baron PA, 2013. Dustiness of fine and nanoscale powders. Ann. Occup. Hyg 57:261–277. 10.1093/annhyg/mes060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farghali A, Bahgat M, ElRouby W, Khedr M, 2013. Decoration of multi-walled carbon nanotubes (MWCNTs) with different ferrite nanoparticles and its use as an adsorbent. J. Nanostruct. Chem 3,1–12. [Google Scholar]
- Feng C, Khulbe KC, Matsuura T, Tabe S, Ismail AF, 2013. Preparation and characterization of electro-spun nanofiber membranes and their possible applications in water treatment. Sep. Purif. Technol 102:118–135. 10.1016/j.seppur.2012.09.037. [DOI] [Google Scholar]
- FIFRA-SAP, 2009. Evaluation of the hazard and exposure associated with nanosilver and other nanometal pesticide products. U.S. Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Scientific Advisory Panel (SAP). Available at. https://www.silverinstitute.org/site/wp-content/uploads/2014/04/EPA_SAP_SNWGpresentation_Nov2009.pdf (Accessed on 07 October 2016).
- Fraceto LF, Grillo R, deMedeiros GA, Scognamiglio V, Rea G, Bartolucci C, 2016. Nanotechnology in agriculture: which innovation potential does it have? Front. Environ. Sci 4:20 10.3389/fenvs.2016.00020. [DOI] [Google Scholar]
- Fu F, Dionysiou DD, Liu H, 2014. The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J. Hazard. Mater 267:194–205. 10.1016/j.jhazmat.2013.12.062. [DOI] [PubMed] [Google Scholar]
- Fu F, Han W, Tang B, Hu M, Cheng Z, 2013. Insights into environmental remediation of heavy metal and organic pollutants: simultaneous removal of hexavalent chromium and dye from wastewater by zero-valent iron with ligand-enhanced reactivity. Chem. Eng. J 232:534–540. 10.1016/j.cej.2013.08.014. [DOI] [Google Scholar]
- Gao F, Liu C, Qu C, Zheng L, Yang F, Su M, Hong F, 2008. Was improvement of spinach growth by nano-TiO(2) treatment related to the changes of Rubisco activase? Biometals 21:211–217. 10.1007/510534-007-9110-y. [DOI] [PubMed] [Google Scholar]
- Geraci CL, Heidel D, Sayes C, Hodson L, Schulte PA, Eastlake A, Brenner S, 2015. Perspectives on the design of safer nanomaterials and manufacturing processes. J. Nanopart. Res 17:366 10.1007/s/1051-015-3152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghormade V, Deshpande MV, Paknikar KM, 2011. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 29:792–803. 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Gogos A, Knauer K, Bucheli TD, 2012. Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J. Agric. Food Chem 60:9781–9792. 10.1021/jf302154y. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA, 2013. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interf. Sci. 193-194:24–34. 10.1016/j.cis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Handy RD, Shaw BJ, 2007. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 9:125–144. 10.1080/13698570701306807. [DOI] [Google Scholar]
- Hester K, Mullins M, Murphy F, Tofail SAM., 2015. Anticipatory ethics and governance (AEG): towards a future care orientation around nanotechnology. NanoEthics 9:123–136. 10.1007/s11569-015-0229-y. [DOI] [Google Scholar]
- Hokkanen S, Bhatnagar A, Sillanpää M, 2016. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 91:156–173. 10.1016/j.watres.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhu D, Wang X, Wang L, Zhang C, Chen W, 2013. Adsorption of phenanthrene, 2- naphthol, and 1-naphthylamine to colloidal oxidized multiwalled carbon nanotubes: effects of humic acid and surfactant modification. Environ. Toxicol. Chem 32:493–500. 10.1002/etc.2088. [DOI] [PubMed] [Google Scholar]
- Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q, 2012. Heavy metal removal from water/wastewater by nanosized metal oxides: a review. Hazard. Mater 211-212:317–331. 10.1016/j.jhazmat.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Hüffer T, Kah M, Hofmann T, Schmidt TC, 2012. How redox conditions and irradiation affect sorption of PAHs by dispersed fullerenes (NC60). Environ. Sci. Technol 47:6935–6942. 10.1021/es303620c. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Leso V, Bergamaschi A, 2013. The effects of nanomaterials as endocrine disruptors. Int. J. Mol. Sci 14:16732–16801. 10.3390/ijms140816732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I, Leso V, Bergamaschi A, 2012. Toxicological effects of titanium dioxide nanoparticles: a review of in vivo studies. J. Nanomater, 964381 (36pp.). 10.1155/2012/964381. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Leso V, Fontana L, Bergamaschi A, 2011. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci 15, 481–508. [PubMed] [Google Scholar]
- Iavicoli I, Leso V, Manno M, Schulte PA, 2014b. Biomarkers of nanomaterial exposure and effect: current status. J. Nanopart. Res 16:2302 10.1007/s11051-014-2302-9. [DOI] [Google Scholar]
- Iavicoli I, Leso V, Ricciardi W, Hodson LL, Hoover MD, 2014a. Opportunities and challenges of nanotechnology in the green economy. Environ. Health 7 (13):78 10.1186/1476-069X-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I, Leso V, Schulte PA, 2016. Biomarkers of susceptibility: state of the art and implications for occupational exposure to engineered nanomaterials. Toxicol. Appl. Pharmacol 299:112–124. 10.1016/j.taap.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YK, Kim BH, Jung G, 2009. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 93:1037–1043. 10.1094/Pdis-93-10-1037. [DOI] [PubMed] [Google Scholar]
- Judy JD, McNear DH Jr., Chen C, Lewis RW, Tsyusko OV, Bertsch PM, Rao W, Stegemeier J, Lowry GV, McGrath SP, Durenkamp M, Unrine JM, 2015. Nanomaterials in biosolids inhibit nodulation, shift microbial community composition, and result in increased metal uptake relative to bulk/dissolved metals. Environ. Sci. Technol 49:8751–8758. 10.1021/acs.est.5b01208. [DOI] [PubMed] [Google Scholar]
- Jung J, Deng Z, Simonson J, Bastias RM, Zhao Y, 2016. Development and preliminary field validation of water-resistant cellulose nanofiber based coatings with high surface adhesion and elasticity for reducing cherry rain-cracking. Sci. Hortic 200:161–169. 10.1016/j.scienta.2016.01.016. [DOI] [Google Scholar]
- Jung JH, Kim SW, Min JS, Kim YJ, Lamsal K, Kim KS, Lee YS, 2010. The effect of nano-silver liquid against the white rot of the green onion caused by Sclerotium cepivorum. Mycobiology 38:39–45. 10.4489/MYCO.2010.38.1.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kah M, 2015. Nanopesticides and nanofertilizers: emerging contaminants or opportunities for risk mitigation? Front. Chem 3:1–6. 10.3389/fchem.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kah M, Beulke S, Tiede K, Hofmann T, 2013. Nanopesticides: state of knowledge, environmental fate, and exposure modeling. Crit. Rev. Env. Sci. Technol 16:1823–1867. 10.1080/10643389.2012.671750. [DOI] [Google Scholar]
- Kah M, Hofmann T, 2014. Nanopesticide research: current trends and future priorities. Environ. Int 63:224–235. 10.1016/j.envint.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Kahru A, Savolainen K, 2010. Potential hazard of nanoparticles: from properties to biological and environmental effects. Toxicology 269:89–91. 10.1016/j.tox.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kang MA, Seo MJ, Hwang IC, Jang C, Park HJ, Yu YM, Youn YN, 2012. Insecticidal activity and feeding behavior of the green peach aphid, Myzus persicae, after treatment with nano types of pyrifluquinazon. J. Asia Pac. Entomol 15:533–541. 10.1016/j.aspen.2012.05.015. [DOI] [Google Scholar]
- Karn B, Kuiken T, Otto M, 2009. Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environ. Health Perspect 117:1813–1831. 10.1289/ehp.0900793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn B, Kuiken T, Otto M, 2011. Nanotechnology and in situ remediation: a review of the benefits and potential risks. Cien. Saude Colet 16, 165–178. [DOI] [PubMed] [Google Scholar]
- Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS, 2009. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3:3221–3227. 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- Khodakovskaya MV, Kim BS, Kim JN, Alimohammadi M, Dervishi E, Mustafa T, Cernigla CE, 2013. Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 9:115–123. 10.1002/smll.201201225. [DOI] [PubMed] [Google Scholar]
- Khot LA, Sankaran S, Maja JM, Ehsani R, Schuster EW, 2012. Applications of nanomaterials in agricultural production and crop protection: a review. Crop. Prot 35:64–70. 10.1016/j.cropro.2012.01.007. [DOI] [Google Scholar]
- Kim SW, Kim KS, Lamsal K, Kim YJ, Kim SB, Jung M, Sim SJ, Kim HS, Chang SJ, Kim JK, Lee YS, 2009. An in vitro study of the antifungal effect of silver nanoparticles on oakwilt pathogen Raffaelea sp. J. Microbiol. Biotechnol 19:760–764. 10.4014/jmb.0812.649. [DOI] [PubMed] [Google Scholar]
- Kim SW, Jung JH, Lamasal K, Kim YS, Min JS, Lee YS, 2012. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 40:53–58. 10.5941/MYCO.2012.40.1.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR, 2008. Nanomaterials in the environment, behavior, fate, bioavailability and effects. Environ. Toxicol. Chem 27:1825–1851. 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Koelmans AA, Horne N, Carley S, Handy RD, Kapustka L, Nowack B, von der Kammer F, 2012. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem 31:3–14. 10.1002/etc.733. [DOI] [PubMed] [Google Scholar]
- Kookana RS, Boxall AB, Reeves PT, Ashauer R, Beulke S, Chaudhry Q, Cornelis G, Fernandes TF, Gan J, Kah M, Lynch I, Ranville J, Sinclair C, Spurgeon D, Tiede K, Van den Brink PJ, 2014. Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J. Agric. Food Chem 62:4227–4240. 10.1021/jf500232f. [DOI] [PubMed] [Google Scholar]
- Korhonen JT, Kettunen M, Ras RHA, Ikkala O, 2011. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interfaces 3:1813–1816. 10.1021/am200475b. [DOI] [PubMed] [Google Scholar]
- Krug HF, 2014. Nanosafety research- are we on the right track? Angew. Chem. Int. Ed 3:12304–12319. 10.1002/anie.201403367. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kim KH, Deep A, 2015. Recent advancements in sensing techniques based on functional materials for organophosphate pesticides. Biosens. Bioelectron 70:469–481. 10.1016/j.bios.2015.03.066. [DOI] [PubMed] [Google Scholar]
- Kumari A, Yadav SK, 2014. Nanotechnology in agri-food sector. Crit. Rev. Food Sci. Nutr 8:975–984. 10.1080/10408398.2011.621095. [DOI] [PubMed] [Google Scholar]
- Lahiani MH, Dervishi E, Chen J, Nima Z, Gaume A, Bins AS, Khodakovskaya MV, 2013. Impact of carbon nanotube exposure to seeds of valuable crops. aCs Appl. Mater. Interfaces 5:7965–7973. 10.1021/am402052x. [DOI] [PubMed] [Google Scholar]
- Lalia BS, Guillen E, Arafat HA, 2013. Hashaikeh, nanocrystalline cellulose reinforced pvdf- hfp membranes for membrane distillation application. Desalination 332:134–141. 10.1016/j.desal.2013.10.030. [DOI] [Google Scholar]
- Lee HU, Lee SC, Lee YC, Vrtnik S, Kim C, Lee S, Lee YB, Nam B, Lee JW, Park SY, Lee SM, Lee J, 2013. Sea-urchin-like iron oxide nanostructures for water treatment. J. Hazard. Mater 262:130–136. 10.1016/j.jhazmat.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Li Q, Chen X, Zhuang J, Chen X, 2016. Decontaminating soil organic pollutants with manufactured nanoparticles. Environ. Sci. Pollut. Res. Int 23:11533–11548. 10.1007/s11356-016-6255-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Yuan S, Wan J, Long H, Tong M, 2011. A combination of electrokinetics and Pd/Fe PRB for the remediation of pentachlorophenol-contaminated soil. J. Contam. Hydrol 124:99–107. 10.1016/j.jconhyd.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Lin N, Dufresne A, 2014. Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J 59:302–325. 10.1016/j.eurpolymj.2014.07.025. [DOI] [Google Scholar]
- Liu F, Chung S, Oh G, Seo TS, 2011a. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces 4:922–927. 10.1021/am201590z. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen H, Zou X, Xie Q, Qing C, Chen D, Frost RL, 2013. Removal of phosphorus using NZVI derived from reducing natural goethite. Chem. Eng. J 234:80–87. 10.1016/j.cej.2013.08.061. [DOI] [Google Scholar]
- Liu R, Lal R, 2015. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ 514:131–139. 10.1016/j.scitotenv.2015.01.104. [DOI] [PubMed] [Google Scholar]
- Liu Y, Su G, Zhang B, Jiang G, Yan B, 2011b. Nanoparticle-based strategies for detection and remediation of environmental pollutants. Analyst 136:872–877. 10.1039/c0an00905a. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tong Z, Prud'homme RK, 2008. Stabilized polymeric nanoparticles for controlled and efficient release of bifenthrin. Pest Manag. Sci 64:808–812. 10.1002/ps.1566. [DOI] [PubMed] [Google Scholar]
- Lv X, Xu J, Jiang G, Xu X, 2011. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 85:1204–1209. 10.1016/j.chemosphere.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ma X, Anand D, Zhang X, Talapatram S, 2011. Adsorption and desorption of chlorinated compounds from pristine and thermally treated multiwalled carbon nanotubes. J. Phys. Chem. C 115:4552–4557. 10.1021/jp1117272. [DOI] [Google Scholar]
- Malekian R, Abedi-Koupai J, Eslamian SS, 2011. Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J. Hazard. Mater 185:970–976. 10.1016/j.jhazmat.2010.09.114. [DOI] [PubMed] [Google Scholar]
- Methner M, Hodson L, Geraci C, 2010. Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials – Part A. J. Occup. Environ. Hyg 7:163–176. 10.1080/15459620903508066. [DOI] [PubMed] [Google Scholar]
- Min JS, Kim KS, Kim SW, Jung JH, Lamsal K, Bin Kim S, Jung M, Lee YS, 2009. Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant Pathol. J 25, 376–380. [Google Scholar]
- Mondal KK, Mani C, 2012. Investigation of the antibacterial properties of nanocopper against Xanthomonas axonopodis pv. punicae, the incitant of pomegranate bacterial blight. Ann. Microbiol 62:889–893. 10.1007/s13213-011-0382-7. [DOI] [Google Scholar]
- Moradi O, Yari M, Moaveni P, Norouzi M, 2011. Removal of p-nitrophenol and naphthalene from petrochemical wastewater using SWCNTs and SWCNT-COOH surfaces, fullerenes. Nanotubes Carbon Nanostruct. 20:85–98. 10.1080/1536383X.2010.533309. [DOI] [Google Scholar]
- Nasterlack M, Zober A, Oberlinner C, 2008. Considerations on occupational medical surveillance in employees handling nanoparticles. Int. Arch. Occup. Environ. Health 81:721–726. 10.1007/s00420-007-0245-5. [DOI] [PubMed] [Google Scholar]
- Ng LY, Mohammad AW, Leo CP, Hilal N, 2013. Polymeric membranes incor-porated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308:15–33. 10.1016/j.desal.2010.11.033. [DOI] [Google Scholar]
- Nguyen HM, Hwang IC, Park JW, Park HJ, 2012a. Enhanced payload and photo-protection for pesticides using nanostructured lipid carrierswith corn oil as liquid lipid. J. Microencapsul 29:596–604. 10.3109/02652048.2012.668960. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Hwang IC, Park JW, Park HJ, 2012b. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest. Manag. Sci 68:1062–1068. 10.1002/ps.3268. [DOI] [PubMed] [Google Scholar]
- Ni H, Zeng S, Wu J, Cheng X, Luo T, Wang W, Zeng W, Chen Y, 2012. Cellulose nanowhiskers: preparation, characterization and cytotoxicity evaluation. Biomed. Mater. Eng 22:121–127. 10.3233/BME-2012-0697. [DOI] [PubMed] [Google Scholar]
- NIOSH, 2009. National Institute for Occupational Safety and Health: Approaches to Safe Nanotechnology Managing the Health and Safety Concerns Associated with Engineered Nanomaterials. Publication No. 2009-125. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute of Occupational Safety and Health, DHHS (NIOSH), Cincinnati, Ohio, USA: http://www.cdc.gov/niosh/docs/2009-125/pdfs/2009-125.pdf (Access on 07 October 2016). [Google Scholar]
- NIOSH, 1994. National Institute for Occupational Safety and Health: Manual of Analytical Methods (NMAM), Fourth Edition, 8/15/94. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute of Occupational Safety and Health, DHHS (NIOSH), Cincinnati, Ohio, USA. [Google Scholar]
- NIOSH, 2013. National Institute for Occupational Safety and Health: Occupational Exposure to Carbon Nanotubes and Nanofibers. Publication No. 2013-145. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute of Occupational Safety and Health, DHHS (NIOSH), Cincinnati, Ohio, USA. [Google Scholar]
- NIOSH, 2011. National Institute for Occupational Safety and Health: Occupational Exposure to Titanium Dioxide. Publication No. 2011-160. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute of Occupational Safety and Health, DHHS (NIOSH), Cincinnati, Ohio, USA. [Google Scholar]
- Norman DJ, Chen J, 2011. Effect of foliar application of titanium dioxide on bacterial blight of geranium and xanthomonas leaf spot of poinsettia. Hortscience 46,426–428. [Google Scholar]
- O'Carroll D, Sleep B, Krol M, Boparai H, Kocur C, 2013. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour 51:104–122. 10.1016/j.advwatres.2012.02.005. [DOI] [Google Scholar]
- OECD, Organisation for Economic Co-operation and Development, 2012. New and Emerging Water Pollutants arising from Agriculture New and Emerging Water Pollutants arising from Agriculture, COM/TAD/CA/ENV/EPOC(2010)17/FINAL.Available at. https://www.oecd.org/tad/sustainable-agriculture/49848768.pdf (Accessed on 07 October 2016).
- Omanović-Mikličanin E, Maksimovićx M, 2016. Nanosensors applications in agriculture and food industry. Bull. Chem. Technol. Bosnia and Herzegovina 47, 59–70. [Google Scholar]
- Paret ML, Palmateer AJ, Knox GW, 2013a. Evaluation of a light-activated nanoparticle formulation of titanium dioxide with zinc for management of bacterial leaf spot on rosa ‘Noare’. Hortscience 48, 189–192. [Google Scholar]
- Paret ML, Vallad GE, Averett DR, Jones JB, Olson SM, 2013b. Photocatalysis: effect of light activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology 103:228–236. 10.1094/phyto-08-12-0183-r. [DOI] [PubMed] [Google Scholar]
- Parisi C, Vigani M, Rdríguez-Cerezo E, 2015. Agricultural nanotechnologies: what are the current possibilities. Nano Today 10:124–127. 10.1016/j.nantod.2014.09.009. [DOI] [Google Scholar]
- Park H, Park Y, Kim W, Choi W, 2013. Surface modification of TiO2 photocat-alyst for environmental applications. J. Photochem. Photobiol. C 15:1–20. 10.1016/j.jphotochemrev.2012.10.001. [DOI] [Google Scholar]
- Pastor S, Creus A, Parrón T, Cebulska-Wasilewska A, Siffel C, Piperakis S, Marcos R, 2003. Biomonitoring of four European populations occupationally exposed to pesticides: use of micronuclei as biomarkers. Mutagenesis 18:249–258. 10.1093/mutage/18.3.249. [DOI] [PubMed] [Google Scholar]
- Peng X, Li Y, Luan Z, Di Z, Wang H, Tian B, Jia Z, 2003. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 376:154–158. 10.1016/S0009-2614(03)00960-6. [DOI] [Google Scholar]
- Pereira MM, Raposo NR, Brayner R, Teixeira EM, Oliveira V, Quintao CC, Camargo S, Mattoso LH, Brandao HM, 2013. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 24:075103 10.1088/0957-4484/24/7/075103. [DOI] [PubMed] [Google Scholar]
- Perez S, la Farre M, Barcelo D, 2009. Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment. Trends Anal. Chem 28:820–832. 10.1016/j.trac.2009.04.001. [DOI] [Google Scholar]
- Pérez-de-Luque A, Rubiales D, 2009. Nanotechnology for parasitic plant control. Pest. Manag. Sci 65:540–545. 10.1002/ps.1732. [DOI] [PubMed] [Google Scholar]
- Perlatti B, Luisa de Souza Bergo P, Fatima das Graças M, da Silva F, Batista Fernandes J, Rossi Forim M, 2012. Polymeric Nanoparticle-Based Insecticides: A Controlled Release Purpose for Agrochemicals. InTech Publishers; 10.5772/53355. [DOI] [Google Scholar]
- Perrin TS, Drost DT, Boettinger JL, Norton JM, 1998. Ammonium-loaded clinoptilolite: a slow-release nitrogen fertilizer for sweet corn. J. Plant. Nutr 21:515–530. 10.1080/01904169809365421. [DOI] [Google Scholar]
- Prasad R, Kumar V, Prasad KS, 2014. Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr. J. Biotechnol 13:705–713. 10.5897/ajbx2013.13554. [DOI] [Google Scholar]
- Peters RJB, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P, Marvin HJP, Mech A, Botelho Moniz F, Quiros Pesudo L, Rauscher H, Schoonjans R, Undas AK, Vettori MV, Weigel S, Aschberger K, 2016. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci. Technol 54:155–164. 10.1016/j.tifs.2016.06.008. [DOI] [Google Scholar]
- Peters RJB, Brandhoff P, Weigel S, Marvin H, Bouwmeester H, Aschberger K, Rauscher H, Amenta V, Arena M, Botelho Moniz F, Gottardo S, Mech A, 2014. Inventory of Nanotechnology applications in the agricultural, feed and food sector. EFSA supporting publication 2014:EN-621. Available at. http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2014.EN-621/pdf (Accessed on 05 May 2017). [Google Scholar]
- Qian K, Shi T, Tang T, Zhang S, Liu X, Cao Y, 2011. Preparation and characterization of nano-sized calcium carbonate as controlled release pesticide carrier for validamycin against Rhizoctonia solani. Microchim. Acta 173:51–57. 10.1007/s00604-010-0523-x. [DOI] [Google Scholar]
- Qu P, Tang H, Gao Y, Zhang L, Wang S, 2010. Polyethersulfone composite membrane blended with cellulose fibrils. BioRes. 5, 2323–2336. [Google Scholar]
- Rai V, Acharya S, Dey N, 2012. Implications of nanobiosensors in agriculture. J. Biomater. Nanobiotechnol 3:315–324. 10.4236/jbnb.2012.322039. [DOI] [Google Scholar]
- Reddy DH, Lee SM, 2013. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid. Interface Sci 201-202: 68–93. 10.1016/j.cis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Ruiz-Hitzky E, Darder M, Fernandes FM, Wicklein B, Alcântara ACS, Aranda P, 2013. Fibrous clays based bionanocomposites. Prog. Polym. Sci 38:1392–1414. 10.1016/j.progpolymsci.2013.05.004. [DOI] [Google Scholar]
- Sánchez A, Recillas S, Font X, Casals E, González E, Puntes V, 2011. Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment. Trends Anal. Chem 30:507–516. 10.1016/j.trac.2010.11.011. [DOI] [Google Scholar]
- Sasson Y, Levy-Ruso G, Toledano O, Ishaaya I, 2007. Nanosuspensions: emerging novel agrochemical formulations In: Ishaaya I, Nauen R, Horowitz AR (Eds.), Insecticides Design Using Advanced Technologies. Springer-Verlag, Netherlands, pp. 1–32. [Google Scholar]
- Savolainen K, Alenius H, Norppa H, Pylkkänen L, Tuomi T, Kasper G, 2010. Risk assessment of engineered nanomaterials and nanotechnologies - a review. Toxicology 269:92–104. 10.1016/j.tox.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Geraci CL, Hodson LL, Zumwalde RD, Kuempel ED, Murashov V, Martinez KF, Heidel DS, 2013b. Overview of risk management for engineered nanomaterials. J. Phys. Conf. Ser 429:012062 10.1088/1742-6596/429/1/012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA, Geraci CL, Murashov V, Kuempel ED, Zumwalde RD, Castranova V, Hoover MD, Hodson L, Martinez KF, 2014. Occupational safety and health criteria for responsible development of nanotechnology. J. Nanopart. Res 16:2153 10.1007/s11051-013-2153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA, Geraci C, Zumwalde R, Hoover M, Kuempel E, 2008a. Occupational risk management of engineered nanoparticles. J. Occup. Environ. Hyg 5:239–249. 10.1080/15459620801907840. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Hauser JE, 2012. The use of biomarkers in occupational research, practice, and policy. Toxicol. Lett. 213:91–99. 10.1016/j.toxlet.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Schulte PA, McKernan LT, Heidel DS, Okun AH, Dotson GS, Lentz TJ, Geraci CL, Heckel PE, Branche CM, 2013a. Occupational safety and health, green chemistry, and sustainability: a review of areas of convergence. Environ. Health 12:31. 10.1186/1476-069X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA, Rinehart R, Okun A, Geraci CL, Heidel DS, 2008b. National prevention through design (PtD) initiative. J. Safety Res 39:115–121. 10.1016/j.jsr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Schubauer-Berigan MK, Mayweather C, Geraci CL, Zumwalde R, McKernan JL, 2009. Issues in the development of epidemiologic studies of workers exposed to engineered nanoparticles. J. Occup. Environ. Med 51:323–335. 10.1097/JOM.0b013e3181990c2c. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Trout DB, 2011. Nanomaterials and worker health: medical surveillance, exposure registries, and epidemiologic research. J. Occup. Environ. Med 53 (6 Suppl):S3–S7. 10.1097/JOM.0b013e31821b1b28. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Trout D, Zumwalde RD, Kuempel E, Geraci CL, Castranova V, Mundt DJ, Mundt KA, Halperin WE, 2008c. Options for occupational health surveillance of workers potentially exposed to engineered nanoparticles: state of the science. J. Occup. Environ. Med 50:517–526. 10.1097/JOM.0b013e31816515f7. [DOI] [PubMed] [Google Scholar]
- Scott NR, Chen H, 2013. Nanoscale science and engineering for agriculture and food systems. Ind. Biotechnol 9,17–18. [Google Scholar]
- Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S, Lead JR, Stone V, Fernandes TF, Jepson M, van Aerle R, Tyler CR, 2010. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol. Sci 115:521–534. 10.1093/toxsci/kfq076. [DOI] [PubMed] [Google Scholar]
- Sekhon BS, 2014. Nanotechnology in agri-food production: an overview. Nanotechnol. Sci. Appl 7:31–53. 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin A, Elmer W, Mukherjee A, De la Torre-Roche R, Hamdi H, White JC, Bindraban P, Dimkpa C, 2015. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res 17:92 10.1007/s11051-015-2907-7. [DOI] [Google Scholar]
- Shatkin JA, Kim B, 2015. Cellulose nanomaterials: life cycle risk assessment and environmental health and safety roadmap. Environ. Sci. Nano 2:497–499. 10.1039/C5EN00059A [DOI] [Google Scholar]
- Shukla SK, Mishra AK, Arotiba OA, Mamba BB, 2013. Chitosan-based nano-materials: a state-of-the-art review. Int. J. Biol. Macromol 59:46–58. 10.1016/j.ijbiomac.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Singh R, Misra V, Mudiam MKR, Chauhan LKS, Singh RP, 2012b. Degradation of -HCH spiked soil using stabilized Pd/Fe0 bimetallic nanoparticles: pathways, kinetics and effect of reaction conditions. J. Hazard. Mater 237-238, 355–364. [DOI] [PubMed] [Google Scholar]
- Singh R, Misra V, Singh RP, 2012a. Removal of Cr(VI) by nanoscale zero- valent iron (nZVI) from soil contaminated with tannery wastes. Bull. Environ. Contam. Toxicol 88:210–214. 10.1007/s00128-011-0425-6. [DOI] [PubMed] [Google Scholar]
- Singh S, Mahalingam H, Singh PK, 2013. Polymer-supported titanium dioxidephotocatalysts for environmental remediation: a review. Appl. Catal. A 462-463:178–195. 10.1016/j.apcata.2013.04.039. [DOI] [Google Scholar]
- Sivakumar R, Thomas J, Yoon M, 2012. Polyoxometalate-based molecular/nanocomposites: advances in environmental remediation by photocatalysis and biomimetic approaches to solar energy conversion. J. Photochem. Photobiol. C 13, 277–298. [Google Scholar]
- Snyder A, Bo Z, Moon R, Rochet JC, Stanciu L, 2013. Reusable photocatalytic titanium dioxide-cellulose nanofiber films. J. Colloid Interf. Sci 399, 92–98. [DOI] [PubMed] [Google Scholar]
- Song G, Gao Y, Wu H, Hou W, Zhang C, Ma H, 2012b. Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ. Toxicol. Chem 31:2147–2152. 10.1002/etc.1933. [DOI] [PubMed] [Google Scholar]
- Song MR, Cui SM, Gao F, Liu YR, Fan CL, Lei TQ, Liu DC, 2012a. Dispersible silica nanoparticles as carrier for enhanced bioactivity of chlorfenapyr. J. Pestic. Sci 37:258–260. 10.1584/jpestics.D12-027. [DOI] [Google Scholar]
- Sonkaria S, Ahn SH, Khare V, 2012. Nanotechnology and its impact on food and nutrition: a review. Recent Pat. Food Nutr. Agric 4, 8–18. [DOI] [PubMed] [Google Scholar]
- Suresh Kumar RS, Shiny PJ, Anjali CH, Jerobin J, Goshen KM, Magdassi S, Mukherjee A, Chandrasekaran N, 2013. Distinctive effects of nano-sized permethrin in the environment. Environ. Sci. Pollut. Res. Int 20:2593–2602. 10.1007/s11356-012-1161-0. [DOI] [PubMed] [Google Scholar]
- Tatrai E, Adamis Z, Bohm U, Meretey K, Ungvary G, 1995. Role of cellulose in wood dust- induced fibrosing alveo-bronchiolitis in rat. J. Appl. Toxicol 15:45–48. 10.1002/jat.2550150110. [DOI] [PubMed] [Google Scholar]
- Thomé A, Reddy KR, Reginatto C, Cecchin I, 2015. Review of nanotechnology for soil and groundwater remediation: Brazilian perspectives. Water Air Soil Pollut. 226:121 10.1007/s11270-014-2243-z. [DOI] [Google Scholar]
- Tiede K, Hassellov M, Breitbarth E, Chaudhry Q, Boxall ABA, 2009. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 1216:503–509. 10.1016/j.chroma.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Tosco T, Papini MP, Viggi CC, Sethi R, 2014. Nanoscale zerovalent iron particles for groundwater remediation: a review. J. Clean. Prod. 77:10–21. 10.1016/j.jclepro.2013.12.026. [DOI] [Google Scholar]
- Trout DB, Schulte PA, 2010. Medical surveillance, exposure registries, and epidemiologic research for workers exposed to nanomaterials. Toxicology 269:128–135. 10.1016/j.tox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Udom I, Ram MK, Stefanakos EK, Hepp AF, Goswami DY, 2013. One dimensional-ZnO nanostructures: synthesis, properties and environmental applications. Mater. Sci. Semicond. Process 16:2070–2083. 10.1016/j.mssp.2013.06.017. [DOI] [Google Scholar]
- US-EPA, Environmental Protection Agency, 2009. Reregistration Eligibility Decision (RED) for Coppers.Available at. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-26_26-May-09.pdf (Accessed on 07 October 2016).
- US-EPA, Environmental Protection Agency, 2011. Pesticides; Policies Concerning Products Containing Nanoscale Materials; Opportunity for Public Comment.Available at. https://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14943.pdf (Accessed on 07 October 2016).
- US Patent Application Publication, 2016. Nano-cellulose edible coatings and uses thereof. Pub. No.: US 2016/0002483. Pub date: Jan. 7, 2016.Available at. http://www.freepatentsonline.com/20160002483.pdf (Accessed on 08 May 2017).
- Vartiainen J, Pöhler T, Sirola K, Pylkkänen L, Alenius H, Hokkinen J, Tapper U, Lahtinen P, Kapanen A, Putkisto K, Hiekkataipale P, Eronen P, Ruokolainen J, Laukkanen A, 2011. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 18, 775–786. [Google Scholar]
- Velzeboer I, Kwadijk CJAF, Koelmans AA, 2014. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol 48:4869–876. 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Villagarcia H, Dervishi E, de Silva K, Biris AS, Khodakovskaya MV, 2012. Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small 8:2328–2334. 10.1002/smll.201102661. [DOI] [PubMed] [Google Scholar]
- Wanekaya AK, Chen W, Mulchandani A, 2008. Recent biosensing developments in environmental security. J. Environ. Monit 10:703–712. 10.1039/b806830p. [DOI] [PubMed] [Google Scholar]
- Wang F, Haftka JJH, Sinnige TL, Hermens JLM, Chen W, 2014b. Adsorption of polar, nonpolar, and substituted aromatics to colloidal graphene oxide nanoparticles. Environ. Pollut 186:226–233. 10.1016/j.envpol.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen Z, Chen B, 2014a. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol 48:4817–4825. 10.1021/es405227u. [DOI] [PubMed] [Google Scholar]
- Wang S, Sun H, Ang HM, Tadé MO, 2013. Adsorptive remediation of environmen-tal pollutants using novel graphene-based nanomaterials. Chem. Eng. J 226:336–347. 10.1016/j.cej.2013.04.070. [DOI] [Google Scholar]
- Wang Z, Wei F, Liu SY, Xu Q, Huang JY, Dong XY, Yu JH, Yang Q, Zhao YD, Chen H, 2010. Electrocatalytic oxidation of phytohormone salicylic acid at copper nanoparticles - modified gold electrode and its detection in oilseed rape infected with fungal pathogen Sclerotinia sclerotiorum. Talanta 80:1277–1281. 10.1016/j.talanta.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Snajdr SI, Hartsky MA, Frame SR, 1998. Two-week inhalation study in rats with cellulose fibers. Int. Congr. Ser 1153:579–582. 10.1093/annhyg/mef642. [DOI] [Google Scholar]
- Watson SB, Gergely A, Janus ER, 2011. Where is “Agronanotechnology” heading in the United States and European union? Nat. Resour. Environ 26, 8–12. [Google Scholar]
- Wilson MA, Tran NH, Milev AS, Kannangara GSK, Volk H, Lu, 2008. Nanomaterials in soils. Geoderma 146:291–302. http://dx.doi.Org/10.1016/j.geoderma.2008.06.004. [Google Scholar]
- Wu D, Shen Y, Ding A, Mahmood Q, Liu S, Tu Q, 2013. Effects of nanoscale zerovalent iron particles on biological nitrogen and phosphorus removal and microorganisms in activated sludge. J. Hazard. Mater 262:649–655. 10.1016/j.jhazmat.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu M, 2008. Preparation and characterization of cellulose acetate-coated compound fertilizer with controlled-release and water-retention. Polym. Adv. Technol 19:785–792. 10.1002/pat.1034. [DOI] [Google Scholar]
- Xiang C, Taylor AG, Hinestroza JP, Frey MW, 2013. Controlled release of nonionic compounds from poly(lactic acid)/cellulose nanocrystal nanocomposite fibers. J. Appl. Polym. Sci 127:79–86. 10.1002/app.36943. [DOI] [Google Scholar]
- Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Lai C, Wei Z, Huang C, Xie GX, Liu ZF, 2012. Use of iron oxide nanomaterials in wastewater treatment: a review. Sci. Total. Environ. 424:1–10. 10.1016/j.scitotenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Yan W, Lien HL, Koel BE, Zhang WX, 2013. Iron nanoparticles for environmental clean-up: recent developments and future outlook. Environ. Sci 15:63–77. 10.1039/C2EM30691C. [DOI] [PubMed] [Google Scholar]
- Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P, 2007. The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol. Trace Elem. Res 119:77–88. 10.1007/s12011-007-0046-4. [DOI] [PubMed] [Google Scholar]
- Yang FL, Li XG, Zhu F, Lei CL, 2009. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem 57:10156–10162. 10.1021/jf9023118. [DOI] [PubMed] [Google Scholar]
- Yang X, Bakaic E, Hoare T, Cranston ED, 2013. Injectable polysaccharide hydrogels reinforced with cellulose nanocrystals: morphology, rheology, degradation, and cytotoxicity. Biomacromolecules 14:4447–4455. 10.1021/bm401364z. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hong-tao Z, Jian W, Meng X, Yang L, Yu-long Z, 2012. Preparation and properties of modified polyvinyl alcohol film for encapsulation of fertilizer. Acta Metall. Sin 18:1295–1301. 10.11674/zwyf.2012.11357. [DOI] [Google Scholar]
- Yao KS, Li SJ, Tzeng KC, Cheng TC, Chang CY, Chiu CY, Liao CY, Hsu JJ, Lin ZP, 2009. Fluorescence silica nanoprobe as a biomarker for rapid detection of plant pathogens. Adv. Mater. Res 79-82:513–516. 10.4028/www.scientific.net/AMR.79-82.513. [DOI] [Google Scholar]
- Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W, 2013. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 25:933–943. 10.1016/S1001-0742(12)60145-4. [DOI] [PubMed] [Google Scholar]
- Yuan S, Long H, Xie W, Liao P, Tong M, 2012. Electrokinetic transport of CMC-stabilized Pd/Fe nanoparticles for the remediation of PCP-contaminated soil. Geoderma 185–186:18–25. 10.1016/j.geoderma.2012.03.028. [DOI] [Google Scholar]
- Zhang F, Zhu Z, Dong Z, Cui Z, Wang H, Hu W, Zhao P, Wang P, Wei S, Li R, Ma J, 2011. Magnetically recoverable facile nanomaterials: synthesis, charac-terization and application in remediation of heavy metals. Microchem. J 98:328–333. 10.1016/j.microc.2011.03.005. [DOI] [Google Scholar]
- Zhang L, Fang M, 2010. Nanomaterials in pollution trace detection and environmental improvement. Nano Today 5:128–142. 10.1016/j.nantod.2010.03.002. [DOI] [Google Scholar]
- Zhang J, Zhai S, Li S, Xiao Z, Song Y, An Q, Tian G, 2013. Pb(II) removal of Fe3O4@ SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem. Eng. J 215–216:461–471. 10.1016/j.cej.2012.11.043. [DOI] [Google Scholar]
- Zhao X, Lv L, Pan B, Zhang W, Zhang S, Zhang Q, 2011. Polymer-supported nano-composites for environmental application: a review. Chem. Eng. J 170:381–394. 10.1016/j.cej.2011.02.071. [DOI] [Google Scholar]
- Zhou H, Han J, Baig SA, Xu X, 2011. Dechlorination of 2,4-dichlorophenoxyaceticacid by sodium carboxymethyl cellulose-stabilized Pd/Fe nanoparticles. J. Hazard. Mater 198:7–12. 10.1016/j.jhazmat.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Zwingmann N, Mackinnon IDR, Gilkes RJ, 2011. Use of a zeolite synthesised from alkali treated kaolin as a K fertiliser: glasshouse experiments on leaching and uptake of K by wheat plants in sandy soil. Appl. Clay Sci 53:684–690. 10.1016/j.clay.2011.06.00. [DOI] [Google Scholar]