Abstract
The cause of insulin resistance in obesity and type 2 diabetes mellitus (T2DM) is not limited to impaired insulin signalling but also involves the complex interplay of multiple metabolic pathways. The analysis of large data sets generated by metabolomics and lipidomics has shed new light on the roles of metabolites such as lipids, amino acids and bile acids in modulating insulin sensitivity. Metabolites can regulate insulin sensitivity directly by modulating components of the insulin signalling pathway, such as insulin receptor substrates (IRSs) and AKT, and indirectly by altering the flux of substrates through multiple metabolic pathways, including lipogenesis, lipid oxidation, protein synthesis and degradation and hepatic gluconeogenesis. Moreover, the post-translational modification of proteins by metabolites and lipids, including acetylation and palmitoylation, can alter protein function. Furthermore, the role of the microbiota in regulating substrate metabolism and insulin sensitivity is unfolding. In this Review, we discuss the emerging roles of metabolites in the pathogenesis of insulin resistance and T2DM. A comprehensive understanding of the metabolic adaptations involved in insulin resistance may enable the identification of novel targets for improving insulin sensitivity and preventing, and treating, T2DM.
Nutrient metabolism is fundamental for the survival, growth and development of all organisms and it requires the coordinated regulation of many metabolic pathways. Insulin regulates the metabolism of carbohydrates, lipids and proteins through the canonical insulin signalling cascade, which involves the insulin receptor (IR), insulin receptor substrate (IRS) proteins, PI3K and AKT1,2. Insulin resistance — defined as impaired signal transduction and biological actions in response to insulin stimulation — is a fundamental mechanism causing type 2 diabetes mellitus (T2DM). When insulin secretion can no longer compensate for insulin resistance, T2DM occurs. Despite increasing knowledge of the insulin signalling cascade, few insulin-sensitizing agents are available to treat T2DM in the clinic. Directly targeting the insulin signalling pathway to improve insulin sensitivity in patients with T2DM is difficult, and there are concerns that activating PI3K and AKT may increase the risk of, or exacerbate, cancer3.
In the post-genomic era, the integration of functional genomics, transcriptomics, proteomics, metabolomics and lipidomics has broadened our understanding of the molecular mechanisms underlying insulin resistance and T2DM. The technology for analysing metabolomics and lipidomic data sets is rapidly evolving, and it has enabled us to identify novel metabolites that regulate insulin sensitivity and/or novel roles for known metabolites in this process.
In this Review, we provide an overview of the current landscape of metabolite families, in particular, of lipids and related metabolites, which have emerged from omics studies as being linked to insulin sensitivity. Lipids have diverse roles as signalling molecules, metabolic substrates and cellular membrane components. Lipids can also modify proteins that influence insulin sensitivity. Chain length and the degree of desaturation of the fatty acid moieties in lipid molecules increase the complexity of assigning biological roles to lipid classes. Moreover, whether lipids are synthesized endogenously or obtained through diet influences their accumulation and/or metabolism and subsequent biological roles. Thus, it is not surprising that controversy exists regarding the causative role of, and mechanisms for the effects of, specific lipid classes on the development of insulin resistance. However, new high-resolution metabolomic techniques enable the identification of lipid subclasses and novel families of lipids that regulate insulin sensitivity.
In addition to lipids, we also provide updates on amino acids and other metabolites such as ketones. We primarily focus on data from preclinical studies in animal models in which the mechanisms of insulin sensitivity have been investigated. However, we relate these data to human studies and highlight discrepancies between preclinical and human data. It is still unknown whether many of the metabolite alterations discussed play a causative role in, or are markers of, insulin resistance. Also, when such alterations are known to be causative, the underlying mechanism is often unknown. Finally, we discuss how targeting the pathways that regulate metabolites involved in insulin resistance may lead to novel strategies for treating T2DM.
Lipids signal to regulate metabolism
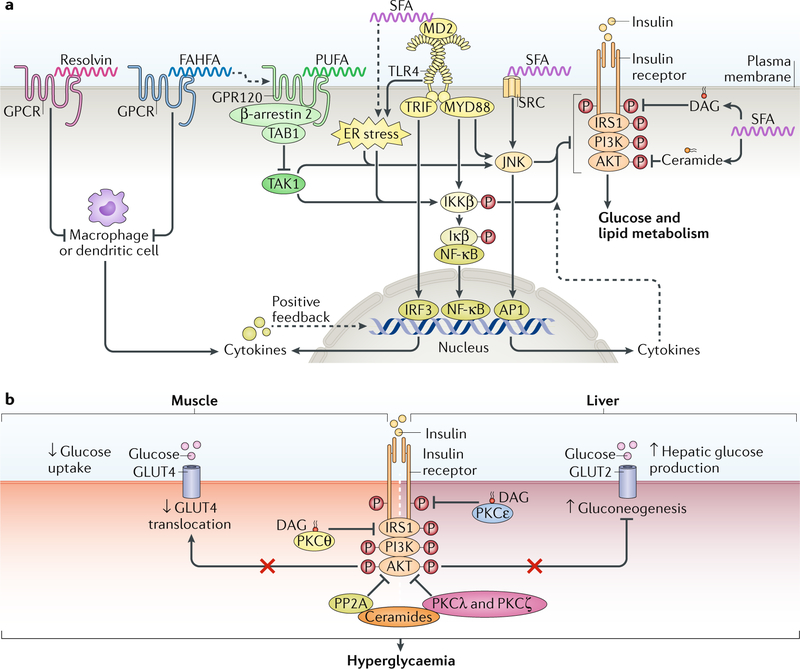
Our understanding of lipids as signalling molecules can be traced back more than seven decades, when fatty acid-derived eicosanoids such as prostaglandins, thromboxanes and leukotrienes were found to signal through G protein-coupled receptors (GPCRs) to exert a broad spectrum of biological functions4,5. Since then, other signalling lipids (such as fatty acids, diacylglycerol (DAG), sphingolipids and fatty acid esters of hydroxy fatty acids) that regulate intracellular pathways to impact insulin action and metabolism have been identified (FIG. 1).
Fig. 1 |. Lipids as signalling molecules that regulate metabolism.
Several signalling lipids, including fatty acids, fatty acid esters of hydroxy fatty acids (FAHFAs), diacylglycerol (DAG) and ceramides, regulate intracellular pathways to influence insulin resistance. a | Fatty acids regulate insulin sensitivity and inflammation. Saturated fatty acids (SFAs) activate Toll-like receptor 4 (TLR4), possibly via its co-receptor, myeloid differentiation protein 2 (MD2), which, through the adaptor proteins TIR-domain-containing adaptor-inducing interferon-β (TRIF) and myeloid differentiation primary response protein MYD88, increases the activity of pro-inflammatory transcription factors. TRIF activation promotes the nuclear translocation of interferon regulatory factor 3 (IRF3) to increase the expression of cytokines. MYD88 activation increases phosphorylation of inhibitor of nuclear factor-κB (NF-κB) kinase (IKKβ), which further phosphorylates inhibitor of NF-κB (IκB), leading to nuclear translocation of NF-κB to increase pro-inflammatory cytokine expression. MYD88 also activates Jun N-terminal kinase (JNK) to increase the activity of transcription factor activator protein 1 (AP1), thereby altering the expression of cytokines. Pro-inflammatory cytokines, through their receptors (not shown), further activate these pro-inflammatory transcription factors to establish a positive feedback loop for sustained inflammation. SFA-activated TLR4 and cytokine production impair insulin signalling through IKKβ and JNK activation, but the direct targets of IKKβ and JNK in the insulin signalling pathway remain to be identified. SFA-mediated activation of endoplasmic reticulum (ER) stress, the SRC–JNK pathway and the incorporation of SFAs into diacylglycerol (DAG) and ceramides also impair insulin signalling by inhibiting phosphorylation of the insulin receptor, insulin receptor substrate 1 (IRS1) or AKT. Polyunsaturated fatty acids (PUFAs) exert anti-inflammatory effects by activating G protein-coupled receptor 120 (GPR120), which recruits β-arrestin 2 and sequesters TAK1 binding protein 1 (TAB1) to inhibit the TAK1-mediated activation of JNK and IKKβ. PUFA, FAHFAs and resolvins may exert anti-inflammatory effects by activating G protein-coupled receptors (GPCRs) in antigen-presenting cells such as macrophages to inhibit cytokine production. The dashed lines indicate indirect effects. b | DAG and ceramide may induce insulin resistance. DAG accumulates ectopically in insulin-resistant muscle and liver. In muscle, DAG-activated protein kinase Cθ (PKCθ) promotes the phosphorylation of IRS1 on Ser1101 in mice, impairing IRS1 phosphorylation on tyrosine and attenuating insulin signalling. In liver, DAG-activated PKCε promotes phosphorylation of IR on Thr1160 to suppress insulin signalling. As a result of DAG increase, glucose uptake via insulin responsive glucose transporter 4 (GLUT4) in muscle is reduced, and glucose output via GLUT2 from liver is increased; these changes induce hyperglycaemia. Ceramides contribute to hyperglycaemia by activating protein phosphatase 2 A (PP2A), which dephosphorylates AKT, and stimulating PKCλ and PKCζ, which prevent AKT from associating with the membrane, thereby inhibiting AKT activity.
Saturated fatty acids.
Saturated fatty acids (SFAs) (see Supplementary Box 1) are detrimental to insulin sensitivity in animals and cell culture6–9; SFAs impair insulin signalling by activating pro-inflammatory signalling pathways and/or by providing substrates for the synthesis of potentially detrimental lipids such as DAG and ceramides (FIG. 1a) (see below). However, human studies evaluating the role of SFAs on insulin resistance and diabetes present conflicting data. Intralipid infusion or a high-SFA diet (80% calories from fat with ~63% from SFAs) for 24 hours induces insulin resistance10–12. By contrast, chronic consumption of dietary SFAs is not associated with incident diabetes. However, replacing SFAs with unsaturated fats in diets may have some beneficial effects for insulin sensitivity and is likely to reduce the risk of T2DM13,14.
Multiple mechanisms contribute to SFA-induced insulin resistance in animals and cultured cells. SFAs activate Toll-like receptors (TLRs), especially TLR4, which is expressed on immune cells, in white adipose tissue (WAT) and in liver, to stimulate the downstream pro-inflammatory processes6–9,15. Pharmacological inhibition, or whole-body or WAT-specific deletion, of TLR4 in mice prevents insulin resistance caused by lipid infusion9,15,16. Moreover, mice with a liver-specific knockout of the gene encoding TLR4 are protected from high-fat diet (HFD)-induced glucose intolerance, insulin resistance and hepatic steatosis17. Therefore, TLR4 is necessary for SFA-induced insulin resistance even though structure studies and binding assays indicate that SFAs are not direct ligands of TLR4 (REFS18,19). One study suggested that palmitic acid, referred to as C16:0 (where C16 indicates the number of carbon atoms and 0 indicates the number of double bonds), directly binds to the TLR4 co-receptor myeloid differentiation protein 2 (MD2; also known as Ly96))20 and that the knockdown or inhibition of MD2 prevented the pro-inflammatory response to palmitic acid20. Further studies are needed to determine whether the SFA–MD2 interaction is important for SFA-induced insulin resistance.
TLR4 activation initiates intracellular signalling pathways involving the adaptor proteins myeloid differentiation primary response protein MYD88 and TIR-domain-containing adaptor-inducing interferon-β (TRIF; also known as TICAM1), which activate pro-inflammatory pathways to increase the production of pro-inflammatory cytokines; these pro-inflammatory cytokines induce feedforward signalling cascades to exacerbate pro-inflammatory effects (FIG. 1a). TLR4-mediated activation of inhibitor of nuclear factor-κB (NF-κB) kinase subunit-β (IKKβ) and Jun N-terminal kinase (JNK) pathways, likely via MYD88, is thought to directly mediate SFA-induced insulin resistance. Indeed, IKKβ and JNK knockout mice are protected from HFD-induced insulin resistance21,22, but the mechanism remains to be elucidated. Although IKKβ-mediated and JNK-mediated phosphorylation of IRS1 on Ser307 in mice (Ser312 in humans) impairs insulin signalling in vitro23,24, the relevance of this phosphorylation in SFA-induced insulin resistance is questionable, as IRS1-S307A knock-in mice (that is, mice expressing IRS1 that cannot be phosphorylated at Ser307) are insulin resistant and glucose intolerant25,26.
SFAs induce endoplasmic reticulum (ER) stress in metabolic organs and immune cells, which can activate NF-κB signalling and the inflammatory cascade to exacerbate insulin resistance27,28. Whole-body knockout of TLR4 in mice attenuates HFD-induced ER stress in muscle, liver and adipose tissue, indicating that TLR4 is necessary for SFA-induced ER stress29. However, in cultured myotubes, the TLR4-specific inhibitor TAK-242 attenuated palmitate-induced inflammatory cytokine expression but not ER stress, suggesting that TLR4 signalling is not the predominant mediator of SFA-induced ER stress under some conditions30. SFAs may also induce ER stress by altering the composition of intracellular phospholipids. For example, phosphatidylcholines (PCs) with incorporated SFAs integrate into the ER membrane, impair its fluidity and trigger ER stress in multiple cell types, including macrophages31,32. Furthermore, thioesterase superfamily member 2 (THEM2; also known as ACOT13), a long-chain fatty acyl-CoA thioesterase that incorporates SFAs into PCs, was found to reduce the fluidity of the ER membrane, resulting in increased hepatic ER stress, insulin resistance and gluconeogenesis33.
SFA-induced insulin resistance may also occur when SFAs alter the membrane distribution of proto-oncogene tyrosine-protein kinase SRC, leading to its activation and the induction of JNK signalling; JNK signalling inhibits AKT in the insulin cascade34. In addition, palmitic acid may trigger the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome pathway to reduce insulin sensitivity by inactivating 5′-AMP-activated protein kinase (AMPK), which results in defective autophagy and the generation of mitochondrial reactive oxygen species (ROS)35. SFAs are also substrates for ceramides, DAGs and phospholipids, which also may impair insulin action (see below), further highlighting that SFAs can impair insulin sensitivity via multiple pathways.
Although animal and cell culture studies have provided important insights into the mechanisms of SFA-induced insulin resistance, how these mechanisms translate to dietary SFA intake in humans, which is not associated with insulin resistance, is unclear13,14. The composition of the SFAs and of other nutrients in diets may mitigate the association between SFA consumption and insulin resistance. Indeed, multiple studies show that even-chain SFAs, especially palmitic acid (16:0) and stearic acid (18:0), are associated with insulin resistance, whereas odd-chain SFAs may be associated with a lower incidence of T2DM36–40. Future studies focusing on the roles of different species of SFAs in insulin resistance may solve some controversies in human studies and provide information for dietary guidelines.
Polyunsaturated fatty acids.
In contrast to SFAs, polyunsaturated fatty acids (PUFAs), in particular, the essential long-chain omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Supplementary Box 1), are anti-inflammatory and have been associated with improved insulin sensitivity in animal studies41–43. However, the effects of PUFAs in humans are controversial, and most studies suggest that omega-3 PUFAs have no beneficial effects on cardiovascular outcomes or glucose levels41,44.
Omega-3 PUFAs exert anti-inflammatory effects by activating the GPCR GPR120 (also known as FFAR4), which recruits the adaptor protein β-arrestin 2; β-arrestin 2 sequesters TAK1-binding protein 1 (TAB1), resulting in the inhibition of TGFβ-activated kinase 1 (TAK1; also known as MAP3K7) to prevent JNK and IKKβ activity (FIG. 1a). DHA-enriched diets decrease inflammatory macrophage infiltration and inflammation in the WAT of wild-type, but not GPR120-knockout, mice42. Several cell-based studies also suggest that GPR120 mediates the anti-inflammatory effects of PUFAs45,46. However, whether GPR120 promotes the insulin-sensitizing effects of omega-3 PUFAs is less clear47,48.
Using untargeted lipidomic approaches, the omega-3 PUFA metabolites specialized pro-resolving mediators (SPMs) were found to be present during the resolution phase of acute inflammation49. SPMs are structurally classified as resolvins, protectins and maresins, and resolvins are further classified as E series (RvE1–RvE3) or D series (RvD1–RvD5) depending on whether they are generated from EPA or DHA, respectively. DHA is also a precursor for protectins (such as protectin D1 (PD1)) and their isomers, protectin DX (PDX), maresin 1 (MaR1) and MaR2. The production of some SPMs, and particularly of RvD1, RvD2 and PD1, is impaired in the WAT of obese mice and humans50,51. Treating obese mice with RvD1 and RvE1 improves insulin sensitivity and attenuates WAT and hepatic inflammation52,53. The reduced inflammation is at least partially the result of macrophage polarization towards an M2 (that is, alternatively activated) and anti-inflammatory phenotype54. Several GPCRs mediate the anti-inflammatory effects of SPMs, although the signalling pathways have not been fully elucidated55–57 (FIG. 1a). For example, the GPCRs N-formyl peptide receptor 2 (FPR2) and GPR32 are necessary for the RvD1-mediated inhibition of TAK1 (REF.56). RvE1 interacts with the GPCR CHEMR23 (also known as CMKLR1) to enhance macrophage phagocytosis through the PI3K–AKT–ribosomal protein S6 kinase pathway58. RvE1 treatment also induces the expression of adiponectin, glucose transporter 4 (GLUT4; also known as SLC2A4) and IRS1 in WAT and improves insulin sensitivity in ob/ob mice52, but the role of CHEMR23 in causing these effects is unknown. Further studies are needed to determine the physiological roles of these SPM receptors in regulating insulin sensitivity and glucose homeostasis.
Monounsaturated fatty acids.
Monounsaturated fatty acids (MUFAs) are enriched in plant-based oils including avocado, olive and peanut oils. The most common dietary MUFA is oleic acid (C18:1 n-9; where n-9 indicates that the single double bond is between carbons 9 and 10), followed by palmitoleic acid (POA, also known as palmitoleate, C16:1 n-7) and vaccenic acid (C18:1 n-6) (see Supplementary Box 1). The intake of MUFAs, especially those in olive oil, may be associated with reduced cardiovascular mortality and improved insulin sensitivity13,59,60. Untargeted plasma metabolomics data obtained using liquid chromatography followed by mass spectrometry and combined with Mendelian randomization analysis identified a causative role for reduced serum levels of oleic acid and POA in insulin resistance61. Oleic acid reverses palmitate and tumour necrosis factor (TNF)-induced impairments in AKT phosphorylation by inhibiting JNK and NF-κB activation in cells62. Furthermore, feeding mice an oleic acid-rich HFD reduced the secretion of interleukin-1β (IL-1β) from adipose tissue and improved insulin sensitivity63. Oleic acid is also a substrate of oleoylethanola-mide, which is reported to act as an endogenous ligand of peroxisome proliferator-activated receptor-α (PPARα) to regulate food intake and body weight64,65.
Palmitoleate is a common constituent of animal fats and plant seed oils, but it can also be synthesized through de novo lipogenesis. Palmitoleate produced from adipocytes may regulate systemic insulin sensitivity66. Indeed, palmitoleate treatment reverses HFD-induced pro-inflammatory macrophage polarization, potentially by activating AMPK and GPR120 but not PPARα in mice42,67,68. However, human studies of the relationship between palmitoleate and metabolic syndrome are conflicting61,69,70. Thus, most PUFAs and MUFAs have anti-inflammatory effects in cells and mice, but dietary PUFAs and MUFAs have limited or no beneficial effects on insulin sensitivity in humans. The PUFA and MUFA doses used in mice are too high and not suitable for human use. Nevertheless, understanding which signalling pathways these lipids regulate may yield novel anti-inflammatory and insulin-sensitizing targets.
Branched fatty acid esters of hydroxy fatty acids.
Branched fatty acid esters of hydroxy fatty acids (FAHFAs) are structurally novel lipids that are synthesized in humans, animals and plants and that have beneficial metabolic and anti-inflammatory effects71. An in silico analysis predicted that >1,000 FAHFAs exist in nature72, and >20 FAHFA families have been identified71–73. These families are differentiated by their acyl-chain composition; each family contains isomers that differ in the position of the ester bond between acyl chains. FAHFAs containing DHA are present at low levels in serum and WAT in mice and humans, and these levels are increased by long-term treatment with omega-3 fatty acid73.
Palmitic acid esters of hydroxy stearic acid (PAHSAs) have been studied most extensively. PAHSAs are present at highest levels in white and brown adipose tissue compared with other tissues in mice; adipose tissue has eight PAHSA isomers, other tissues have at least three PAHSA isomers, and mouse and human serum have six71. PAHSA levels are reduced in the serum and subcutaneous WAT of insulin-resistant mice and humans, and serum PAHSA levels correlate with insulin sensitivity in humans71. A single oral dose of 5-PAHSA or 9-PAHSA in mice fed chow that are glucose intolerant from ageing or in insulin-resistant mice on a HFD improves glucose tolerance. In aged mice on a chow diet, PAHSAs augment glucose-stimulated insulin and glucagon-like peptide 1 (GLP1) secretion in vivo but these secretory effects are not seen in HFD-fed mice. GLP1 can decrease blood sugar levels by enhancing insulin secretion. It also reduces appetite, resulting in weight loss in some people. GLP1 analogues are currently being used to treat T2DM and have been shown to have cardioprotective effects. Chronic PAHSA treatment improves insulin sensitivity and glucose tolerance in mice on both diets74. In vitro, PAHSAs directly enhance the secretion of GLP1 from entero-endocrine cells and glucose-stimulated insulin secretion (GSIS) from human pancreatic islets. They also enhance insulin-stimulated glucose transport and GLUT4 translocation in adipocytes. PAHSAs are anti-inflammatory. They decrease HFD-induced adipose inflammation in obese, insulin-resistant mice and attenuate lipopolysaccharide (LPS)-induced dendritic cell activation and cytokine production in vitro71 (FIG. 1a). The anti-inflammatory effects are further demonstrated by their ability to reduce the severity of colitis in a mouse model75.
PAHSAs activate the long form of GPR120 albeit at high concentrations. GPR120 is necessary for PAHSAs to enhance insulin-stimulated glucose transport and GLUT4 translocation to the plasma membrane in adipocytes71. PAHSAs are also selective agonists for GPCR 40 (GPR40; also known as FFAR1), as demonstrated by the fact that they increase Ca2+ flux through this receptor but not cAMP generation. PAHSA-mediated GPR40 activation promotes glucose homeostasis, as blocking GPR40 reverses the improvements in glucose tolerance and insulin sensitivity observed in PAHSA-treated chow- and HFD-fed mice and directly inhibits PAHSA-mediated augmentation of GSIS in human islets74. Although PAHSAs do not activate the GLP1 receptor, it is necessary for them to enhance glucose tolerance74, perhaps because PAHSAs stimulate the secretion of GLP1 from entero-endocrine cells. As GPR120 and GPR40 do not mediate all PAHSA effects, PAHSAs are likely to activate other GPCRs, the identification of which could drive the discovery of new treatments for T2DM and immune-mediated diseases. The naturally evolved relative affinities that PAHSAs have for multiple GPCRs may make them more effective at treating these disorders than synthetic agonists.
FAHFA biosynthesis is stimulated by increased glucose uptake into adipocytes, which activates the lipogenic and glycolytic transcription factor carbohydrate-responsive element-binding protein (ChREBP)71. Full-length FAHFAs are synthesized from labelled FAHFA precursors in mice71, but the biosynthetic enzymes responsible for this are not yet known. However, several FAHFA-degrading enzymes have been identified76. A gain-of-function mutation in carboxyl ester lipase (CEL), a major FAHFA hydrolase in pancreas77, is thought to cause type 8 maturity-onset diabetes of the young78. As this mutant has greater hydrolytic activity against FAHFAs than wild-type CEL77, patients expressing it may undergo increased FAHFA hydrolysis in the pancreas. This could result in lower FAHFA levels, which might impair GSIS and thereby result in diabetes.
Interestingly, R-form stereoisomers of 9-PAHSA are more abundant than S-form stereoisomers in WAT79. Cells favour the production of R-9-PAHSA, and CEL selectively hydrolyses S-9-PAHSA79. These observations highlight the role of stereochemistry in the production and degradation of PAHSAs and may facilitate the identification of the enzymes responsible for PAHSA biosynthesis79. In summary, FAHFAs are anti-diabetic and anti-inflammatory lipids that are synthesized in mammalian tissues. Levels of at least one family member of FAHFAs, PAHSAs, are reduced in insulin-resistant people. Understanding the pathways that regulate PAHSA biosynthesis and degradation could inform approaches to restore PAHSA levels and prevent and treat T2DM and inflammatory diseases.
Diacylglycerol.
Ectopic lipid accumulation in muscle and liver is associated with insulin resistance in animals and humans80. However, whether lipid deposition causes insulin resistance, or vice versa, and which lipids in muscle and liver might cause insulin resistance, are unclear. The increased triglyceride (TAG) levels that are associated with obesity do not appear to cause insulin resistance81,82. DAG, an immediate precursor of TAG (BOX 1), is elevated in muscle and liver in obesity and T2DM and has been linked to insulin resistance in some but not all studies, by activating protein kinase C (PKC) isoforms, which modify the phosphorylation of insulin signalling molecules (see below) (FIG. 1b). The PKC family of serine/threonine-protein kinases contains conventional PKCs (cPKCs: PKCα, PKCβI, PKCβII and PKCγ), which require DAG and Ca2+ for activation, novel PKCs (nPKCs: PKCδ, PKCε, PKCη and PKCθ), which require DAG for activation and atypical PKCs (aPKCs: PKCζ and PKCλ), which do not require DAG or Ca2+ for enzymatic activity.
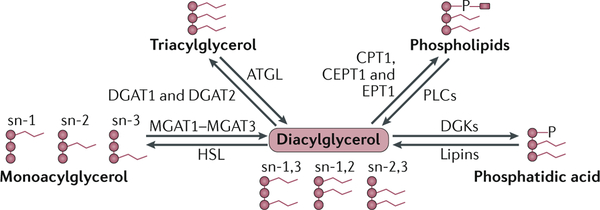
Box 1 |. Diacylglycerol synthesis.
Diacylglycerol (DAG) levels are dynamically regulated by multiple pathways (see the figure). First, DAG can be synthesized from monoacylglycerol catalysed by monoacylglycerol acyltransferases (MGAT1–MGAT3), which esterify monoacylglycerol at stereospecific numbering 1 (sn)-1, sn-2 or sn-3 of glycerol to form sn-1,2, sn-1,3 or sn-2,3 DAG. second, DAG can be generated by the hydrolysis of triacylglycerol (triglycerides) and membrane phospholipids by adipose triglyceride lipase (ATGL) and phospholipases (PLCs), respectively. third, dephosphorylation mediated by lipins (lipin 1 (LPIN1) and LPIN3) of phosphatidic acid (PA) that is embedded in the endoplasmic reticulum membrane also generates DAGs. Each of these reactions can be reversed to reduce DAG levels. specifically, hormone-sensitive lipase (HSL) hydrolyses DAGs to generate monoacylglycerols. Diacylglycerol acyltransferase 1 (DGAT1) and DGAT2 esterify DAG to generate TAG. DAGs can be substrates for the synthesis of phospholipids, which is catalysed by cholinephosphotransferase 1 (CPT1), choline/ethanolaminephosphotransferase 1 (CEPT1) and ethanolaminephosphotransferase 1 (EPT1). Finally, DAGs can be phosphorylated by DAG kinase (DGK) to generate PA (see the figure). All these pathways have been studied in the context of insulin resistance, with inconclusive results. Knockdown of MGAT1 in liver improves glucose tolerance and hepatic insulin signalling in high-fat-diet-fed and ob/ob mice but also unexpectedly increases DAG levels310. However, the systemic deletion of Mgat1 in ob/ob mice does not alter insulin sensitivity311. The adenovirus-mediated knockdown of LPIN1 and LPIN2 reduces hepatic DAGs and the activation of protein kinase Cε (PKCε) and improves insulin sensitivity312,313. However, liver-specific knockout of LPIN1 does not affect insulin sensitivity88,314. DGKδ haploinsufficiency increases the level of DAG in muscles and causes insulin resistance315, but DGKθ overexpression in primary hepatocytes causes insulin resistance despite decreased levels of DAG316. Finally, and paradoxically, the overexpression of DGAT2 in liver increases the level of DAGs, whereas DGAT2 knockdown reduces the accumulation of hepatic lipids and improves insulin sensitivity317–319. The discrepancies in data derived from these models highlight the complex regulation of cellular DAG levels; altering one regulatory pathway may trigger compensatory changes in other pathways. In addition, different DAG species may have different effects on PKC activity94. However, as most studies reporting that an increase in DAG-induced PKC activation causes insulin resistance do not identify the DAG species involved, it is unclear whether specific DAG species or DAG levels are important.
An increase in muscle and hepatic DAGs in obesity and T2DM leads to the activation of the nPKCs PKCθ and PKCε81,82 (FIG. 1b). In both mice and humans, lipid infusion increases the accumulation of DAG in muscle, enhances the activity of PKCθ and causes insulin resistance83,84. Mice lacking PKCθ are protected from insulin resistance in muscle during lipid infusion85. Mechanistically, PKCθ promotes the phosphorylation of IRS1 at Ser1101, which prevents it from activating AKT83,86. In liver, increased DAG enhances the activity of PKCε, which leads to the phosphorylation of IR at Thr1160 to impair insulin signalling87,88. Mice that express a version of IR that cannot be phosphorylated at Thr1160 (Thr1160Ala) are protected from HFD-induced hepatic insulin resistance87. Knocking down PKCε in the liver of rats fed a HFD reverses lipid-induced defects in insulin signalling and hepatic insulin resistance89. Therefore, the ectopic accumulation of DAG in muscle and liver may modulate insulin sensitivity by activating nPKCs.
In insulin-resistant states such as obesity and T2DM, insulin fails to suppress hepatic gluconeogenesis, while insulin-stimulated lipogenesis remains intact88,90. The ‘selective’ hepatic insulin resistance occurs downstream of IR activation. It is unclear how DAG-mediated PKCε activation and impairment of insulin signalling at the IR level fit with the mechanism of selective hepatic insulin resistance. In addition, animal models lacking or overexpressing genes that regulate DAG metabolism do not always support a causative role of DAG in insulin resistance88 (BOX 1). Indeed, the genetic deletion of several lipolysis-related genes causes hepatic steatosis and elevated DAG levels but not insulin resistance91,92. Total levels of DAG are also higher in muscles of endurance-trained athletes, and these athletes have a higher sensitivity to insulin93. The accumulation of DAG in membranes versus lipid droplets has different effects on PKC activation80, and DAG species with different acyl chains activate PKC with varying potencies94, further complicating the role of DAG in insulin resistance. Future studies should investigate specific DAG species and their activation in individual cellular compartments to determine their role in insulin sensitivity.
Sphingolipids and ceramides.
Sphingolipids are structurally similar to glycerolipids but have a serine (rather than glycerol) backbone to which acyl chains are attached. Sphingolipids may act as structural components of membranes and as signalling molecules95. Ceramides, which contain a long-chain amino alcohol sphingosine and a fatty acid, are the best-studied sphingolipids in relation to insulin resistance (BOX 2). Although elevated ceramide levels are usually associated with insulin resistance, not all ceramide species are metabolically detrimental, and the chain length and saturation of fatty acids in ceramides affect their biological functions96.
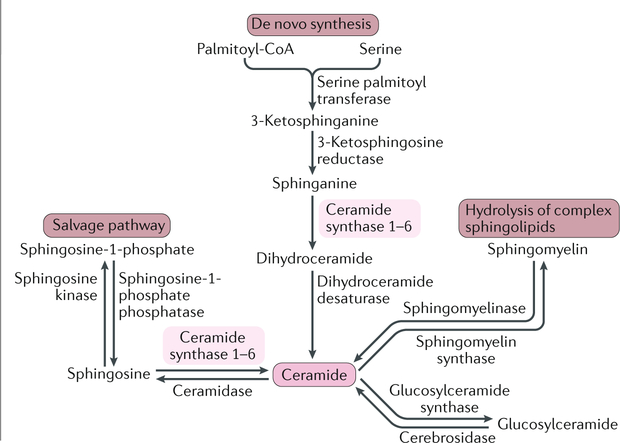
Box 2 |. Sphingolipid metabolism.
Ceramides can be generated through de novo synthesis, the salvage pathway or the hydrolysis of complex sphingolipids320 (see the figure). De novo synthesis occurs in the endoplasmic reticulum, where palmitoyl-Coa and serine are condensed by serine palmitoyltransferase to form 3-ketosphinganine. 3-Ketosphinganine is then reduced to sphinganine by 3-ketosphingosine reductase, which is further acylated through a family of six ceramide synthase (CERS) enzymes (CERS1–CERS6) and desaturated by dihydroceramide desaturase to form ceramides. Each CERS isoform preferentially generates ceramides with specific acyl-chain lengths. For instance, CERS1, CERS5 and CERS6 generate C18 ceramides and C16 ceramides, respectively, and CERS2 selectively produces ceramides containing very-long-chain fatty acids (C22–C26 ceramides). In the salvage pathway, the sphingosine backbone of ceramides is broken down and reused for ceramide synthesis. Ceramides are degraded by ceramidases, which deacylate ceramides to generate sphingosine. Sphingosine is then phosphorylated by sphingosine kinase 1 (SPK1) or SPK2 to sphingosine-1-phosphate (S1P). S1P binds to S1P receptors (S1PR1–S1PR5), which are G protein-coupled receptors. S1P may induce insulin resistance in liver and muscle321,322, although adiponectin-stimulated S1P is associated with improved hepatic insulin sensitivity97. Finally, ceramides may be released from the hydrolysis of complex sphingolipids such as sphingomyelin (via the action of sphingomyelinase) and glycosphingolipids such as glucosylceramide (via the action of glucosylceramide synthase (GCS).
Insulin resistance may lead to elevated ceramide levels by causing inflammation, reducing adiponectin levels and increasing the availability of ceramide precursors97,98. Elevated ceramides may in turn further impair insulin sensitivity99. Inhibition of de novo ceramide synthesis decreases intramyocellular ceramide levels and improves insulin sensitivity in obese mice100. Furthermore, the genetic deletion or pharmacological inhibition of sphingomyelinase, which generates ceramides from sphingomyelin, protects against diet-induced hyperglycaemia and insulin resistance101. Mechanistically, ceramides interfere with insulin signalling (FIG. 1b) by allosterically activating protein phosphatase 2A (PP2A), which dephosphorylates AKT at Thr308 and Ser473, the phosphorylation of which is critical for insulin-stimulated AKT activation102,103. Ceramides also directly activate the aPKCs PKCλ and PKCζ, which prevents AKT from binding to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to block insulin signalling104–106.
The association between ceramides and insulin resistance has not been observed in all studies107. Mass spectrometry has enabled the detection of ceramide species with different length fatty acid chains and different degrees of saturation108, and data suggest that specific ceramide species rather than the total ceramide levels influence insulin resistance96,108. In humans, the levels of muscle C18:0, C16:0 and C18:1 ceramide species, but not total ceramides, inversely correlated with insulin sensitivity109,110. Ceramide species containing C16–C22 long-chain fatty acids (LCFAs) and >C22 very-long-chain fatty acids (VLCFAs) were increased and decreased, respectively, in the liver of mice on a HFD111. Consistent with this, overexpression in hepatocytes of ceramide synthase 2 (CERS2), which catalyses the synthesis of VLCFA ceramides, enhanced insulin signalling despite elevating the total level of ceramide111. CERS2 haploinsufficiency was compensated for by an increase in CERS6 expression, which resulted in the increased production of C16:0 ceramide in liver and predisposed mice to steatohepatitis and insulin resistance112. Furthermore, hepatic overexpression of CERS6 impaired insulin-stimulated AKT phosphorylation112. Conversely, CERS6 knockout reduced C16:0 ceramide in WAT and liver and ameliorated HFD-induced obesity and insulin resistance113. Therefore, specific ceramide species may play different roles in regulating insulin sensitivity.
Lipids as metabolic substrates
Over half a century ago, Randle and colleagues recognized that fatty acids and glucose compete for oxidation; in isolated heart and skeletal muscle, utilization of one of these nutrients directly inhibited the use of the other without hormonal mediation114. This substrate competition forms the basis of the concept of ‘metabolic flexibility’, which refers to metabolic substrate shift during physiological changes such as fasting and feeding, although hormones are involved at the whole-body level115. Kelley and colleagues used the term ‘metabolic inflexibility’ to describe the impairment in substrate shift between fatty acid and carbohydrate utilization in obesity and T2DM116,117.
Lipids play a central role in metabolic inflexibility and the associated insulin resistance. For example, excessive dietary lipid intake and enhanced lipogenesis may cause ectopic lipids to accumulate in metabolic organs. Furthermore, adipose lipolysis is increased in obesity and T2DM, increasing the release of glycerol and fatty acids118–120 (FIG. 2). Inflammatory cytokines such as TNF and IL-6 enhance lipolysis by increasing the expression of lipolytic enzymes121. IL-6-neutralizing antibodies reduce lipolysis and improve insulin sensitivity in HFD-fed rats119. Adipocyte-secreted retinol-binding protein 4 (RBP4) increases the production of pro-inflammatory cytokines from macrophages, which promotes adipocyte lipolysis122,123. Furthermore, insulin suppresses adipose lipolysis by inhibiting the rate-limiting enzyme hormone-sensitive lipase (HSL)124. Therefore, insulin resistance itself enhances lipolysis118. All these events increase the uptake and deposition of lipids and alter metabolic flux in muscle and liver.
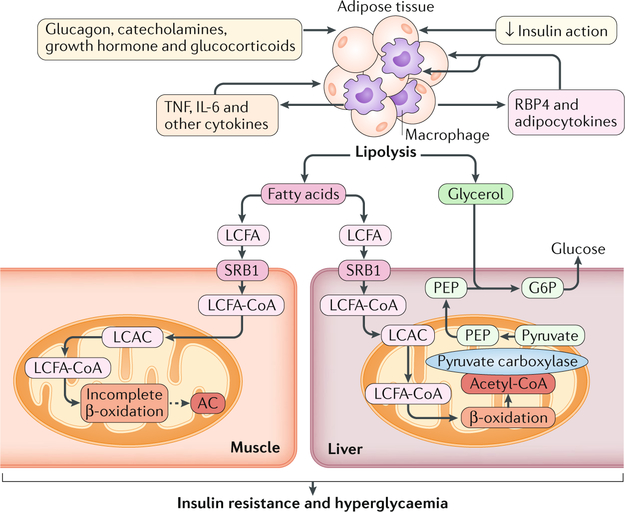
Fig. 2 |. Alterations in lipid metabolism are associated with insulin-resistant states.
Obesity and type 2 diabetes mellitus are associated with increased lipolysis in adipose tissue owing to the action of, or resistance to, multiple hormones and to the increased production of cytokines (such as tumour necrosis factor (TNF) and interleukin-6 (IL-6)) by adipose tissue macrophages. The release of TNF and IL-6 from macrophages is potentiated by the secretion, from adipocytes, of adipocytokines such as retinol-binding protein 4 (RBP4). Fatty acids including long-chain fatty acids (LCFAs) that are released by lipolysis are taken up by muscle and liver via the fatty acid transporter scavenger receptor class B member 1 (SRB1). In muscle, LCFA thioesters (LCFA-CoAs) are imported into mitochondria for β-oxidation via the carnitine shuttle, in which LCFA-CoAs are converted into long-chain acylcarnitines (LCACs). Incomplete β-oxidation in insulin-resistant states causes accumulation of acylcarnitines (ACs) (shown as a dashed line) of varying lengths, which are associated with insulin resistance and hyperglycaemia. In the liver, LCFAs are imported into the mitochondria and are oxidized to generate acetyl-CoA, which activates pyruvate carboxylase, leading to increased production of phosphoenolpyruvate (PEP) from pyruvate. Glycerol generated from lipolysis, in addition to PEP, is converted into glucose-6-phosphate (G6P), resulting in increased glucose production. Overall, the increased flux of metabolic substrates into liver causes insulin resistance and hyperglycaemia. Although an increase in AC muscle content correlates with insulin resistance, a causative effect has not been established in vivo.
Increased fatty acid influx can impair mitochondrial function in muscle. Under physiological conditions, fatty acids are activated to acyl-CoAs, which are converted into acylcarnitines so that acyl-CoAs can be transported across the mitochondrial membrane for oxidation. Acylcarnitines are sensitive biomarkers of shifts in nutrient delivery to, and flux through, mitochondrial metabolic pathways. In obesity and T2DM, impaired mitochondrial function in muscle and increased fatty acid influx causes incomplete β-oxidation, leading to the accumulation of acyl-CoAs and their acylcarnitine counterparts125,126 (FIG. 2). In cultured muscle cells, acylcarnitines impair insulin signalling and/or glucose uptake and induce oxidative stress and mitochondrial dysfunction125,127. In mice, inhibition of muscle acetylcarnitine export causes the accumulation of C6–C20-chain acylcarnitines and glucose intolerance128. Pharmacological inhibition of l-carnitine transport decreases the level of acylcarnitines in plasma and tissue and improves insulin sensitivity in obese mice129. These results from cell culture and mouse studies suggest that acylcarnitine accumulation from increased lipid influx and deposition in muscle mechanistically link metabolic inflexibility to insulin resistance. However, human studies on the roles of acylcarnitines and l-carnitine supplementation are lacking130. Note that it is possible that acylcarnitines with different carbon lengths may have different effects on insulin resistance.
Lipids as metabolic substrates may also regulate insulin sensitivity by modulating hepatic glucose production (FIG. 2). The glycerol released from lipolysis can serve as a direct substrate for hepatic gluconeogenesis131. Fatty acids released by lipolysis are delivered to liver and metabolized to acetyl-CoA119, which enhances the activity of pyruvate carboxylase to push pyruvate into the gluconeogenic pathway119. Therefore, by acting as metabolic substrates, lipids may influence glucose homeostasis by regulating muscle insulin signalling and hepatic gluconeogenesis.
Phospholipids and insulin sensitivity
Phospholipids are major components of cellular membranes, and they comprise two fatty acids at stereospecific numbering (sn)-1 and sn-2 positions and an alcohol-modified phosphate group at the sn-3 position. Phosphatidylcholine (PC) is the most abundant phospholipid, accounting for 40–50% of total cellular phospholipids; phosphatidylethanolamine (PE), which is localized in mitochondrial inner membranes, is the second most abundant phospholipid. PC and PE synthesis is controlled by different enzymes, but PE can be enzymatically converted into PC (BOX 3). Specific PC species rather than total PC concentrations regulate insulin sensitivity.
Box 3 |. Synthesis of phosphatidylcholine and phosphatidylethanolamine.
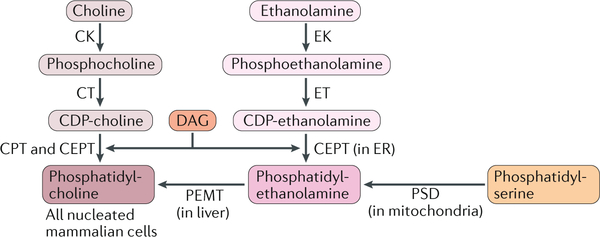
In the majority of cells, phosphatidylcholine (PC) is synthesized via the cytidine 5-diphosphate (CDP)-choline pathway, in which choline is phosphorylated to phosphocholine by choline kinase (CK) and then converted into CDP-choline by CTP:phosphocholine cytidylyltransferase (CT; encoded by PCYT1) (see the figure). subsequently, CDP-choline is combined with diacylglycerol (DAG) by two enzymes that are integrated into the endoplasmic reticulum (ER): CDP-choline:1,2-diacylglycerol cholinephosphotransferase (CPT) and CDP-choline:1,2-diacylglycerol choline/ethanolamine phosphotransferase (CEPT). The CDP-choline pathway is present in all nucleated mammalian cells. However, in the liver, up to 30% of PC is generated by the conversion of phosphatidylethanolamine (PE) into PC by phosphatidylethanolamine N-methyltransferase (PEMT) (see the figure).
PE can be synthesized by two major pathways: the CDP-ethanolamine pathway in the ER and the phosphatidylserine decarboxylase (PSD) pathway in mitochondria. the CDP-ethanolamine pathway is similar to that of PC synthesis. Phosphoethanolamine is converted into CDP-ethanolamine by CTP:phosphoethanolamine cytidylyltransferase (encoded by PCYT2) and then added to DAG by CEPT to form PE. the PSD pathway occurs exclusively in mitochondria, where phosphatidylserine (PS) is decarboxylated by PSD to form PE. The synthesis of PS, which is controlled by two PS synthases, is the rate-limiting step for PE synthesis in the PSD pathway. EK, ethanolamine kinase.
In the 1930s, Charles Best, who co-discovered insulin, reported that the PC precursor choline reduces fat deposition in rat liver132, providing evidence that choline and PC may regulate lipid metabolism. Choline-deficient diets are now commonly used to induce hepatic steatosis in mice133,134. Furthermore, decreasing the levels of hepatic PC and PE by knocking down enzymes involved in their synthesis (BOX 3) exacerbates HFD-induced non-alcoholic steatohepatitis (NASH) without, surprisingly, causing insulin resistance135,136. These data suggest that total levels of PC and PE do not regulate insulin sensitivity.
Instead, specific phospholipids may regulate insulin sensitivity. Indeed, PC(C12:0/C12:0) (with C12:0 fatty acids at n-1 and n-2 positions) activates the orphan nuclear receptor liver receptor homologue 1 (LRH1; also known as NR5A2) to increase bile acid synthesis, resulting in improved hepatic steatosis, insulin sensitivity and glucose homeostasis in mice137. PPARδ-activated hepatic PC(18:0/18:1) promotes fatty acid uptake and metabolism in muscle in a PPARα-dependent manner, and treating obese mice with PC(18:0/18:1) improves glucose tolerance and insulin sensitivity138. Phospholipids can be regulated by ‘remodelling’ (also called the Lands cycle), which changes the fatty acids at positions sn-1 and sn-2. Lysophosphatidylcholine acyltransferase-3 (LPCAT3) preferentially synthesizes unsaturated fatty acid-containing PCs and is induced by liver X receptors (LXRs) and PPARδ in liver139,140. Hepatic knockdown of LPCAT3 induces ER stress and inflammation139, while its overexpression improves insulin sensitivity and glucose tolerance in wild-type and ob/ob mice139,141. Future studies identifying the intracellular signals generated by specific phospholipid species will help clarify their role in insulin sensitivity.
Lipids and metabolites modify proteins
Post-translational modifications (PTMs) of proteins increase functional diversity of the proteome without altering amino acid sequence. PTMs regulate protein activity, localization and interaction with other cellular components. Although protein phosphorylation and protein dephosphorylation are common PTMs that extensively regulate protein function, lipids and other non-lipid metabolites may serve as substrates for additional PTMs, among which acetyl-CoA-mediated protein acetylation has been extensively studied.
Acetyl-CoA and protein acetylation.
Acetyl-CoA links nutrient metabolism to many biological functions, including the PTM of proteins at lysine residues by acetylation. Acetylation, which regulates protein function, is a reversible process that is mediated by lysine acetyltransferases (KATs) and deacetylases (DACs)142,143 (Supplementary Box 2). In yeast, stem cells and cancer cells, the activity of KATs and DACs is regulated by the availability of acetyl-CoA, with high acetyl-CoA levels increasing protein acetylation. However, acetyl-CoA does not modify proteins ‘nonspecifically’ because the KATs have varying Kd for acetyl-CoA144–148. Unfortunately, elucidating whether acetyl-CoA levels regulate protein acetylation in metabolic tissues and organs is challenging. In vivo intracellular acetyl-CoA levels are regulated by the availability of multiple substrates (glucose, lipids and amino acids), metabolic flux (as determined by the tricarboxylic acid (TCA) cycle, de novo lipogenesis, ketogenesis and steroid synthesis)149 and inter-organ crosstalk (for example, the elevation of acetyl-CoA in liver mediated by adipose lipolysis)119. Acetyl-CoA dynamics may explain the discrepancies in tissue acetyl-CoA levels recorded in obese mice on a HFD with studies reporting elevated119 or reduced149 hepatic acetyl-CoA levels. Measuring subcellular acetyl-CoA levels is also technically challenging. Therefore, a major focus has been on studying the regulation of KATs and DACs by hormones and environmental cues and how this affects the function of target proteins.
KATs and DACs may regulate insulin sensitivity by modulating the acetylation of proteins in the insulin signalling cascade (BOX 4; FIG. 3a) and of other proteins involved in glucose and lipid metabolism (FIG. 3b). The histone acetyltransferase GCN5 (also known as KAT2A) may play a dual role in regulating hepatic gluconeogenesis. In the fed state, GCN5 acetylates PPARγ co-activator-1α (PGC-1α) to inhibit its activity, resulting in the reduced expression of gluconeogenic enzymes and reduced glucose production150,151. In fasted and obese diabetic states, GCN5 is phosphorylated by protein kinase A (PKA), which increases its acetyltransferase activity for histones and attenuates that for PGC-1α. The increased histone acetylation recruits transcription factors such as forkhead box protein O1 (FOXO1) and hepatocyte nuclear factor 4α (HNF-4α)) to gluconeogenic genes to promote gluconeogenesis152. Hepatic gluconeogenesis is also regulated by p300, which acetylates and stabilizes CREB-regulated transcription co-activator 2 (CRTC2), a transcriptional cofactor of cAMP-responsive element-binding protein (CREB)153. This mechanism appears to be important for the activation of early gluconeogenic genes because CRTC2 is deacetylated by sirtuin 1 (SIRT1) and degraded later during fasting153. p300 also acetylates glucokinase (GCK) regulatory protein (GKRP), which increases its stability, sequesters GCK in the nucleus and inhibits the participation of GCK in glycolysis154. Finally, in the liver of obese mice, p300 promotes hyper-acetylation of the bile acid receptor farnesoid X-activated receptor (FXR) to impair its activity155,156. FXR deficiency causes ectopic lipid deposition in liver and muscle, leading to insulin resistance157. Thus, protein acetylation regulates glucose and lipid metabolism.
Box 4 |. Acetylation of insulin signalling proteins.
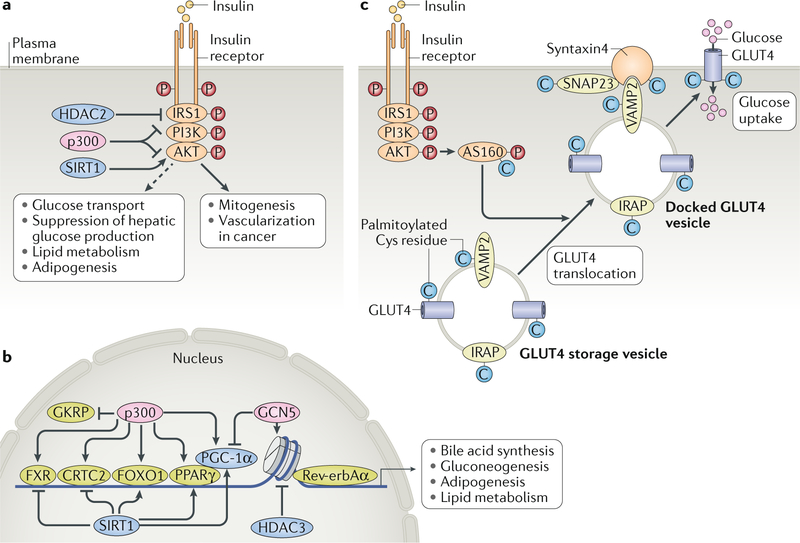
Acetylation of insulin receptor substrate (IRS) proteins by the histone acetyltransferase p300 and their deacetylation by histone deacetylase 2 (HDAC2) are both associated with impaired insulin signalling323,324. Mass spectrometry identified 15 acetylated lysine residues in IRS1 and IRS2 in mouse liver324. Serial mutagenesis revealed that acetylation of Lys1017, Lys1080 and Lys1131 in IRS1 and of Lys1173 and Lys1264 in IRS2 may be critical for insulin signalling324. Additional acetylation sites (Lys52 and Lys61) in human IRS1 were identified by non-targeted proteomics, but their relevance to insulin signalling is unclear325,326. p300 and the p300/CBP-associated factor (P/CAF) also acetylate AKT in the pleckstrin homology (PH) domain at Lys14 and Lys20, thereby blocking AKT binding to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) at the plasma membrane327. Conversely, deacetylation of these lysine residues by sirtuin 1 (SIRT1, which is NaD+ dependent), promotes AKT–PIP3 binding to activate aKt327. In addition, p300 and SIRT1 regulate AKT activation by modulating the acetylation of rapamycin-insensitive companion of mtOR (RICTOR), an essential scaffold protein within mTOR complex 2 (MTORC2). RICTOR acetylation increases mTORC2 activity and promotes AKT phosphorylation at Ser473, which is critical for insulin-stimulated AKT activation328. Finally, SIRT7 suppresses AKT phosphorylation at Ser473 via deacetylating and activating FK506-binding protein, a scaffold protein for the AKT-specific phosphatase PH domain leucine-rich repeat-containing protein phosphatase (PHLPP), in cancer cells329. However, the significance of acetylation of proteins involved in insulin signalling in systemic insulin resistance is unclear. For instance, although SIRT1 deacetylates and activates AKT in cultured cells327, transgenic SIRT1 overexpression in skeletal muscle does not increase AKT phosphorylation or insulin sensitivity330.
Fig. 3 |. Lipids and metabolites modify proteins to regulate metabolism.
Protein acetylation regulates insulin signalling and glucose metabolism. a | Histone acetyltransferase p300, histone deacetylase 2 (HDAC2) and sirtuin 1 (SIRT1) modulate acetylation of insulin receptor substrate 1 (IRS1) and/or AKT in cancers, causing mitogenesis and vascularization, but their effects on activation of insulin-stimulated metabolic pathways remain to be determined (shown as a dashed line). b | In the nucleus, these acetyltransferases and deacetylases also modulate the activity of transcription factors, including farnesoid X-activated receptor (FXR), CREB-regulated transcription co-activator 2 (CRTC2), forkhead box protein O1 (FOXO1) and peroxisome proliferator-activated receptor-γ (PPARγ), and of PPARγ co-activator 1α (PGC-1α)) to regulate bile acid synthesis, gluconeogenesis, adipogenesis and lipid metabolism. Moreover, the modulation of histone acetylation by histone deacetylase 3 (HDAC3), which interacts with Rev-erbAα, regulates the circadian rhythm of lipid and glucose metabolism. p300 also acetylates glucokinase (GCK) regulatory protein (GKRP), which sequesters GCK in the nucleus to inhibit its participation in glycolysis. c | Protein palmitoylation regulates glucose transport in adipocytes. In adipocytes, glucose transporter 4 (GLUT4), components of the GLUT4 storage vesicle (namely, vesicle-associated membrane protein 2 (VAMP2) and insulin-responsive aminopeptidase (IRAP)) and GLUT4 trafficking proteins (including synaptosomal-associated protein 23 (SNAP23), syntaxin 4 and AKT substrate of 160 kDa (AS160)) are palmitoylated at cysteine residues, which regulates their activity. Decreased palmitoylation is associated with impaired insulin-stimulated GLUT4 translocation.
p300-mediated protein acetylation can be reversed by sirtuins, especially SIRT1, which is associated with increased insulin sensitivity158. Global SIRT1 overexpression improves insulin sensitivity, glucose tolerance and hepatic steatosis159,160, and SIRT1 heterozygous knockout mice have hepatic steatosis and disrupted energy homeostasis161,162. Studies from global and tissue-specific SIRT1 transgenic and knockout mice indicate that SIRT1 in WAT and liver, but not muscle, regulates insulin sensitivity and glucose metabolism161–166. SIRT1 may promote WAT remodelling by deacetylating PPARγ to attain a more thermogenic brown adipose tissue (BAT)-like phenotype, thereby improving insulin sensitivity and glucose metabolism167. Although multiple studies indicate that SIRT1 activation in liver increases insulin sensitivity161,168,169, understanding the molecular mechanisms involved is complicated by the fact that SIRT1 deacetylates and activates PGC-1α and FOXO1, which promote hepatic gluconeogenesis151,170–172. SIRT1-mediated deacetylation of FXR and CRTC2 may also offset its effects on PGC-1α and FOXO1 under certain conditions155–157.
SIRT3, SIRT4 and SIRT5, which are localized in mitochondria, also regulate lipid and glucose metabolism. SIRT3 is dominant, and it regulates global mitochondrial protein acetylation173. Mitochondrial proteins are hyperacetylated in SIRT3 knockout mice and, although this is associated with reduced ATP production in metabolic organs such as liver, heart and kidney, it does not produce a metabolic phenotype in mice on a chow diet174,175. However, on a HFD, SIRT3 knockout mice are prone to obesity, insulin resistance and hepatic steatosis173,176. Interestingly, muscle and liver-specific SIRT3 knockout mice show no metabolic abnormalities, even on a HFD177, which is consistent with growing evidence showing that impaired mitochondrial function is associated with, but may not cause, insulin resistance178–180. SIRT7 deletion has been reported to both prevent and promote hepatic steatosis181–183. These often contrasting metabolic effects seen in different sirtuin studies reflect the complexity of studying how protein acetylation regulates glucose and lipid metabolism; different diets may alter the tissue levels of acetyl-CoA and NAD+, which are critical for p300 and sirtuin activity184. In addition, protein acetylation is temporally and spatially regulated, as observed in GCN5, p300 and SIRT1-modulated gluconeogenesis152,153.
Histone deacetylase 3 (HDAC3) regulates hepatic lipid metabolism via its interaction with Rev-erbAα (also known as NR1D1), a nuclear receptor that regulates circadian rhythm (FIG. 3b). During the fed period, when the animal is active, low Rev-erbAα levels reduce the association of HDAC3 with the liver genome, resulting in lipid synthesis and accumulation. During the inactive and fasting period, increased Rev-erbAα levels and HDAC3 activity suppress lipid accumulation185. Liver-specific HDAC3 deletion causes continuous lipid synthesis, leading to severe hepatosteatosis186, yet these mice are more sensitive to insulin, perhaps owing to the limited availability of gluconeogenic substrates186. By contrast, mice with a muscle-specific deletion of HDAC3 are insulin resistant187 yet have greater endurance and resistance to muscle fatigue than wild-type mice, as they activate anaplerotic pathways that are driven by AMP deaminase 3 and the catabolism of branched-chain amino acids (BCAAs)187. Furthermore, loss of HDAC3 in heart and/or skeletal muscle results in lipid accumulation and cardiac hypertrophy188,189. Insulin also promotes the HDAC3-mediated deacetylation and activation of phosphoglycerate kinase 1 to increase glycolytic ATP production and regulate redox state190. The relevance of this pathway in insulin resistance remains to be investigated.
Acetyl-CoA can also be used to acetylate glucosamine-6-phosphate to synthesize uridine-diphosphate-GlcNAc, which is the substrate for protein O-GlcNAcylation — that is, the attachment of O-linked N-acetylglucosamine (O-GlcNAc) moieties to proteins. The role of protein O-GlcNAcylation in regulating insulin signalling and lipid and glucose metabolism was recently reviewed191. Overall, protein acetylation is a critical regulator of insulin sensitivity and metabolism, and this is a complex and evolving field of research.
In addition to acetylation, lysine residues in proteins can be post-translationally modified by succinylation, malonylation and glutarylation, all of which involve the addition of acyl moieties192,193. Proteomics has identified thousands of proteins that are modified by these metabolites, although the functional consequences of these modifications are largely unknown. The substrates for these modifications, such as succinyl-CoA, malonyl-CoA and glutaryl-CoA, are intermediates of multiple metabolic pathways, including the TCA cycle, lipogenesis, fatty acid oxidation and amino acid metabolism. Future studies will clarify whether these PTMs regulate or reflect insulin sensitivity.
Palmitate and protein palmitoylation.
LCFAs such as palmitic acid or myristic acid can also modify proteins. Palmitoylation of proteins at cysteine residues is established by a family of 24 Asp–His–His–Cys motif-containing acyltransferases (DHHCs)194 and removed by palmitoyl protein thioesterases27,195. N-myristoylation is mediated by peptide N-myristoyltransferase 1, which irreversibly attaches myristate to a conserved acceptor glycine either co-translationally or post-translationally196. Hundreds of proteins can be acylated, but few acylated proteins are known to be involved in insulin signalling and/or action. A recent non-targeted proteomic analysis revealed that GLUT4, proteins in the GLUT4 storage vesicle (including vesicle-associated membrane protein 2 (VAMP2) and insulin-responsive aminopeptidase (IRAP)) and other proteins involved in GLUT4 trafficking (including synaptosomal-associated protein 23 (SNAP23), syntaxin 4 and AKT substrate of 160 kDa (AS160; also known as TBC1D4)) are palmitoylated in adipocytes197 (FIG. 3c). Palmitoylation of GLUT4 at Cys223, which is mediated by zinc-finger DHHC domain-containing protein 7 (DHHC7; also known as ZDHHC7), is critical for insulin-stimulated GLUT4 translocation198,199. GLUT4 translocation does not occur in the adipocytes of DHHC7 knockout mice, which develop insulin resistance and glucose intolerance198. Although DHHC7 has substrates in addition to GLUT4, this study demonstrates that protein palmitoylation has metabolic consequences. Interestingly, the induction of de novo lipogenesis by insulin in endothelial cells seems to be critical for the palmitoylation of endothelial nitric oxide synthase (eNOS; also known as NOS3), and disruption of this pathway, as seen in insulin resistance, leads to endothelial dysfunction and inflammation200. Furthermore, HFD-induced insulin resistance and the accumulation of palmitic acid in the hippocampus increase the expression of DHHC3 (also known as ZDHHC3) in a FOXO3-dependent manner, leading to palmitoylation of the AMPA glutamate receptor subunit GluA1, which reduces its activity and impairs cognitive function201. Additional studies are required to characterize the mechanisms by which protein palmitoylation influences insulin action, metabolic homeostasis and cognitive function.
Short-chain fatty acids in metabolism
Acetate, propionate and butyrate represent 90% of short-chain fatty acids (SCFAs), which are fermentation products of dietary complex carbohydrates such as fibres that are released by the gut microbiota202,216. Obesity and insulin resistance in humans and rodents are associated with alterations in gut microbial composition, with the enrichment of certain species of bacteria such as the butyrate-producing Firmicutes. and propionate-producing Bacteroidetes being different between healthy, lean and obese, insulin-resistant states202,203. Microbiota transplantation from obese mice or humans into lean, germ-free mice alters the levels of SCFAs and increases adiposity202,204. Conversely, faecal transplants from lean, healthy human donors into obese individuals improve insulin sensitivity and increase the abundance of certain butyrate-producing bacteria205. Studies in small cohorts of humans also suggest that SCFAs (particularly propionate) lower glucose levels206,207. Thus, an altered microbiota and consequently altered SCFA production may affect insulin sensitivity and energy metabolism in patients with obesity and T2DM.
Most but not all studies show that SCFA administration reduces body weight and/or adiposity in rodents and humans by suppressing appetite and increasing energy expenditure and oxidative metabolism208–213. SCFAs act through GPR41, GPR43 and GPR109 (REFS214–218). SCFAs, particularly acetate, can cross the blood–brain barrier in rodents and humans211,219, so their anorexigenic effects may be caused by GPCR activation in the brain. SCFAs also suppress appetite by stimulating the secretion of leptin from WAT220,221 and of peptide YY (PYY) and GLP1 from the gut208,222–224. In addition, SCFAs activate intestinal gluconeogenesis, which has systemic insulin-sensitizing effects and promotes satiety and increased energy expenditure via the periportal neural system209.
SCFAs alter peripheral metabolism by suppressing lipolysis and increasing oxidative metabolism in WAT, liver and skeletal muscle in an AMPK-dependent and/or PPARα-dependent manner225,226. Furthermore, SCFAs increase the number, differentiation and activity of colonic regulatory T cells227,228 and suppress adipose inflammation in mice220,229. In humans, SCFAs suppress the pro-inflammatory effects of LPS in several immune cell types230,231, and acetate reduces the levels of circulating TNF in obese women222. However, the metabolic effects of SCFAs may be species-specific, as SCFAs promote adipogenesis in murine and porcine, but not human, adipocytes in a GPR43-dependent manner232–234. Furthermore, acetate increases GSIS from mouse, but not human, islets, which may also be a result of species-specific differences in GPR43 signalling214,235. In conclusion, SCFAs have promising metabolic effects in rodents. However, long-term SCFA intervention studies have marginal benefits in humans216, so their relevance to insulin sensitivity in humans is unclear.
Amino acids regulate insulin resistance
The association of elevated serum amino acid levels with obesity and insulin resistance was observed more than four decades ago236. The advancement in metabolomics analysis and mass spectrometry-based isotope labelling techniques has renewed interest in this area, especially towards understanding the relationship between BCAAs and insulin resistance.
Branched-chain amino acids.
Elevated circulating levels of the BCAAs leucine, isoleucine and valine has been associated with obesity, insulin resistance, T2DM, fatty liver, coronary artery disease and other complications of metabolic syndrome237–241. Elevated BCAA levels may also serve as a biomarker for the development of metabolic syndrome242. Bariatric surgery and pharmacological or dietary interventions causing weight loss and metabolic improvements lower the level of circulating BCAAs237,243–247. Several mechanisms have been implicated in raising BCAA levels in insulin resistance and/or obesity (FIG. 4). The expression level of BCAA catabolic enzymes in WAT is reduced in animal models and humans with obesity and/or insulin resistance246,248,249. This reduction in BCAA catabolism in WAT may be due to increased WAT inflammation and ER stress250. Transplanting WAT from normal mice reduces the elevated BCAA levels in mice with systemic genetic deletion of a rate-controlling BCAA catabolic enzyme249,251. How liver and muscle contribute to elevated BCAA levels in the states of insulin resistance and obesity is less clear as the expression of BCAA catabolic enzymes has been reported to be increased, decreased or unchanged under these conditions246,249,252–254. Increased enrichment of BCAA-producing bacteria in the gut microbiota255 and disruption of hypothalamic insulin signalling that results in decreased hepatic BCAA catabolism252 has been reported to also contribute to elevated BCAAs in obesity and insulin resistance.
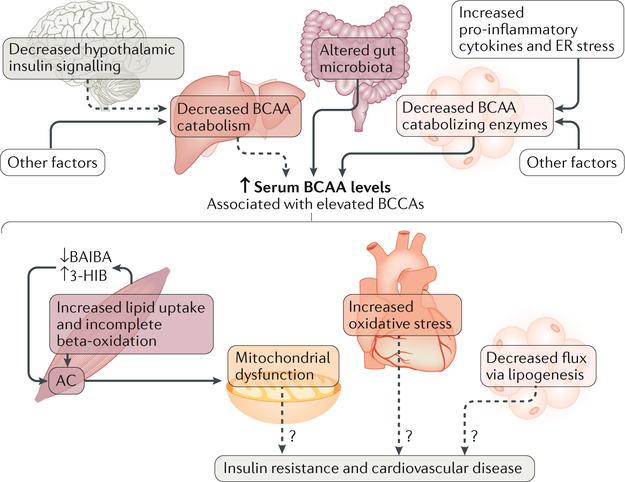
Fig. 4 |. Increased levels of branched-chain amino acids are associated with insulin resistance.
Several factors may increase serum levels of branched-chain amino acids (BCAAs) in states of insulin resistance and obesity, including increased BCAA production by gut microbiota, decreased expression of BCAA catabolizing enzymes in white adipose tissue (WAT) resulting from increased WAT inflammation and endoplasmic reticulum (ER) stress, and possibly decreased catabolism of hepatic BCAAs, which is linked to impaired hypothalamic insulin signalling. Although elevated serum levels of BCAAs are associated with insulin resistance and cardiovascular disease, whether this is causative needs further investigation. BCAAs increase incomplete lipid oxidation in muscle, resulting in the accumulation of acylcarnitines and mitochondrial dysfunction. Altered valine catabolism in muscle increases the production of 3-hydroxyisobutyrate (3-HIB) and decreases the secretion of β-aminoisobutyric acid (BAIBA), which results in increased lipid oxidation and the accumulation of acylcarnitines. Elevated cardiac BCAAs may cause oxidative stress in heart. In adipose tissue, impaired BCAA catabolism may reduce substrate flux into lipogenesis. All these factors may contribute to metabolic dysfunction in insulin resistance, type 2 diabetes mellitus and cardiovascular disease. Dashed lines indicate possible effects that have not been conclusively validated.
It is unclear whether the elevated levels of BCAAs associated with obesity and/or insulin resistance cause insulin resistance. Mendelian randomization in a large human genetic study suggests that elevated levels of BCAAs cause insulin resistance and T2DM256. However, animal and human studies supplementing with or restricting the levels of BCAAs report conflicting effe cts241,254,257–260. The rodent studies are confounded by several variables including species (mouse versus rat), the type of BCAA supplemented (leucine seems to be permissive for insulin signalling and induces satiety257,258) and the route of BCAA administration. Data suggesting that BCAAs promote mitochondrial dysfunction are stronger. BCAAs are tightly linked to lipid metabolism (FIG. 4), and BCAA overload increases acylcarnitine accumulation in muscle241, which may exacerbate mitochondrial dysfunction126. The conjugation of acyl-CoA to glycine and their export from skeletal muscle can limit acylcarnitine accumulation in the muscle. Indeed, obese and insulin-resistant Zucker fatty rats have lower muscle glycine and urine acetyl-glycine levels than their lean counterparts, and BCAA restriction restores these parameters and is associated with improved metabolic homeostasis254. Serum 3-hydroxyisobutyrate (3-HIB), a valine metabolite secreted by muscle, is elevated in obesity and may stimulate fatty acid uptake and promote lipid accumulation in muscle261. By contrast, the valine metabolite β-aminoisobutyric acid (BAIBA), which is also secreted by muscle (in a PGC-1α-dependent manner), increases thermogenesis and the oxidative capacity of liver and adipose tissue via PPARα. Unlike 3-HIB levels, BAIBA levels are associated with reduced cardiometabolic risks and are increased by exercise in humans262. In addition, BCAA catabolism is impaired in heart failure, resulting in the accumulation of BCAA metabolites that may cause oxidative stress and impair mitochondrial function263,264. BCAAs also contribute to lipogenic substrates acetyl-CoA and propionyl-CoA in adipocytes. The impaired catabolism of BCAAs in adipocytes in obesity may result in less substrate and therefore reduced de novo lipogenesis, which is associated with insulin resistance265. Furthermore, monomethyl branched-chain fatty acids (mmBCFAs), which are synthesized from BCAAs, are decreased in WAT of individuals with obesity and insulin resistance and increased after gastric bypass surgery266. However, the biological functions of mmBCFAs are unknown.
In short, BCAA catabolism activates pathways that cause mitochondrial dysfunction, but whether mitochondrial dysfunction causes insulin resistance is unclear. Therefore, further studies are needed to verify whether elevated levels of circulating BCAAs in obesity, insulin resistance and T2DM cause insulin resistance.
Methionine and circulating aromatic amino acids.
Methionine is an essential amino acid in humans; the methionine derivative S-adenosyl-methionine is a methyl donor for the methylation of DNA, histones and other proteins. Methionine restriction rapidly reduces adiposity and improves insulin sensitivity and fatty liver in mice267–270. In humans, restriction of methionine intake with a 58% decline in serum methionine causes weight loss despite being coupled with increased energy intake271. It is not clear how methionine restriction increases energy expenditure while also increasing food intake267–270. Levels of fibroblast growth factor 21 (FGF21) are increased tenfold in plasma and liver within the first 12 hours of methionine restriction, and this increase is sustained over several weeks268. However, the induction of FGF21 may be necessary for the effects of methionine restriction on energy expenditure but not weight loss, suggesting that other factors are involved in regulating methionine restriction-induced leanness270. Methionine restriction also induces adiponectin secretion, although adiponectin is dispensable for methionine restriction-mediated insulin sensitivity272. Methionine restriction-mediated insulin sensitivity is partially cell autonomous, as methionine depletion from cell culture media increases insulin-stimulated AKT phosphorylation in HepG2 cells268. Methionine restriction also alters histone and DNA methylation in liver and WAT273, but whether these epigenetic modifications influence methionine restriction-mediated energy expenditure and insulin sensitivity is unknown.
Metabolomics studies show that the circulating aromatic amino acids (AAAs) phenylalanine and tryptophan are elevated in individuals with obesity and insulin resistance242,247. Importantly, AAAs may predict whether individuals will develop T2DM274,275. Metabolic improvements as a result of gastric bypass surgery are associated with lower AAAs276. To date, the relationship between AAAs and insulin resistance is only correlational. Further studies are needed to determine whether elevated AAAs cause insulin resistance and/or obesity.
Other metabolites
In addition to lipids and amino acids, other metabolites, including ketones, bile acids and nucleotides, may also be involved in regulating insulin sensitivity.
Ketone bodies, a ketogenic diet and insulin sensitivity.
Ketone bodies (that is, acetone, acetoacetate and β-hydroxybutyrate (β-OHB)) are alternative metabolic substrates during periods of nutrient deprivation. Ketones are both derived from acetyl-CoA and metabolized to acetyl-CoA, which interfaces with metabolic pathways such as fatty acid oxidation, BCAA metabolism and glycolysis. During periods of nutrient deprivation, ketones are generated in the liver and delivered to metabolically active organs such as the brain and heart for energy production. Ketogenesis is enhanced during fasting when the expression and activity of the rate-limiting ketogenic enzyme 3-hydroxy-3-methylglutaryl CoA synthase (HMGCS2) are induced and TCA cycle intermediates are preferentially used for gluconeogenesis. HMGCS2 knockdown in liver blocks ketogenesis, resulting in the accumulation of acetyl-CoA; the accumulation of acetyl-CoA activates mitochondrial pyruvate carboxylase to drive pyruvate towards gluconeogenesis119. Therefore, mice with hepatic HMGCS2 knockdown are hyperglycaemic on a chow diet, and they develop severe fatty liver and NASH owing to the increased flux of acetyl-CoA into de novo lipogenesis on a HFD277. HMGCS2 is also expressed in extrahepatic organs such as kidney, muscle, heart and adipose tissue278, but the physiological relevance is unclear.
Ketone bodies are also signalling molecules. β-OHB binds to GPR109A and inhibits WAT lipolysis279, which may serve as a negative feedback mechanism given that lipolysis provides ketogenic substrates. β-OHB also antagonizes GPR41 signalling in the sympathetic nervous system, which lowers sympathetic tone280 and may be a physiological adaptation to starvation. In addition, β-OHB inhibits HDAC1 and HDAC2 activity, which increases histone acetylation at promoters of FOXO3 and metallothionein 2 (REF.281) — factors that help protect against oxidative stress. Finally, β-OHB may inhibit the NLRP3 inflammasome282,283. Although WAT lipolysis, the oxidative stress response and inflammation contribute to insulin sensitivity284,285, it is unclear whether ketone bodies such as β-OHB influence lipid and glucose metabolism through these pathways.
Ketogenic diets, which generally have >70% calories from fats and <10% calories from carbohydrates, increase energy expenditure, reduce obesity and enhance insulin sensitivity in rodents and humans286,287. Although the beneficial effects of ketogenic diets in mice require an increase in FGF21 levels, ketogenic diets reduce circulating levels of FGF21 in humans288,289, indicating that the FGF21 pathway is not important in humans on a ketogenic diet. Beta-adrenergic receptors are required for a ketogenic diet-induced increase in energy expenditure in mice because mice lacking all three beta-adrenergic receptors do not increase energy expenditure and gain weight on a ketogenic diet, despite elevated FGF21 levels290. Although ketogenic diets are effective for weight loss, it may be hard for individuals to follow these diets.
Therefore, elucidating the mechanisms for ketogenic diet-induced weight loss, including alterations of metabolites, may provide novel targets for obesity treatment.
Bile acids.
Bile acids (Supplementary Box 3) are signalling molecules that regulate glucose and lipid metabolism through FXR and the Takeda G-protein coupled receptor 5 (TGR5; also known as GPBAR1). Bile acid-activated FXR reduces the activity of transcription factors hepatocyte nuclear factor 4α (HNF4α) and LRH1 to inhibit the synthesis of liver bile acid, forming a negative feedback loop. Furthermore, FXR activation in intestine induces the expression of the intestinal hormone FGF15 in mice (FGF19 in humans), which inhibits cholesterol 7α-hydroxylase (CYP7A1; the rate-limiting enzyme for bile acid synthesis) in liver by activating FGF receptor 4 (FGFR4)–β-klotho signalling291. In addition to negatively modulating bile acid production, FXR activation regulates lipid and glucose metabolism and therefore insulin sensitivity. Specifically, FXR knockout mice are insulin resistant and glucose intolerant owing to the deposition of ectopic lipids in liver and muscle157. FXR also inhibits the hepatic lipogenic factor sterol regulatory element-binding protein 1 (SREBP1) and promotes PPARα-induced β-oxidation292.
TGR5, which is expressed ubiquitously, increases intracellular cAMP signalling293,294. Dietary supplementation of cholic acid in mice prevents and reverses HFD-induced weight gain, potentially by increasing TGR5-dependent energy expenditure in muscle and BAT295,296. This could result from the conversion of tetraiodothyronine (T4) into the more potent triiodothyronine (T3) (REFS295,296), which activates genes via thyroid hormone receptors to promote energy metabolism. The cholic acid-derived secondary bile acid deoxycholic acid (DCA) is more potent in activating, and has a higher specificity for, TGR5 than cholic acid. Disruption of FGFR4–β-klotho signalling by knocking out β-klotho results in higher levels of DCA, which activates TGR5 and stimulates BAT thermogenesis297. The β-klotho knockout mice are therefore resistant to diet-induced obesity298. TGR5 is also expressed on certain intestinal entero-endocrine cells (L cells) and epithelial cells, from which it facilitates GLP1 release. Interestingly, TGR5 also increases GLP1 production from pancreatic β-cells by increasing the activity of prohormone convertase 1 (PCSK1), which cleaves proglucagon to generate GLP1 (REF.299). Bile acids or selective agonists of TGR5 improve liver and pancreatic function and enhance glucose tolerance in obese mice300. The biological functions of TGR5 may also explain many of the beneficial metabolic effects of gastric bypass surgery, as weight loss, improved fatty liver and reduced insulin resistance are lost in TGR5 knockout mice subjected to vertical sleeve gastrectomy301. In short, bile acid-mediated FXR and TGR5 pathways appear to be attractive targets for treating obesity and diabetes.
Nucleotides.
More than 30 years ago, Weber and colleagues described the role of insulin in regulating the synthesis of nucleotides302. Although the roles of purines in cellular energetics and signalling are well defined303, an interest in the pyrimidine nucleoside uridine in metabolism has emerged. Fasting increases the levels of circulating uridine by inducing the production of uridine from adipose tissue, which lowers core body temperature and metabolic rate; refeeding reduces plasma uridine levels, as uridine synthesis in adipose tissue is decreased and biliary clearance of uridine is increased304. In humans, plasma uridine levels are increased in individuals with T2DM305 and hypertension306 and correlate with insulin resistance. In agreement, short-term or chronic uridine supplementation in mice results in glucose intolerance and reduced insulin signalling307. However, uridine supplementation also ameliorates hepatic steatosis and has cardioprotective effects308,309. Thus, the full spectrum of effects of uridine in cardiometabolic disease needs further study.
Perspective
Recent technological and bioinformatics advances have enabled the measurement of thousands of metabolites in biological samples. Signatures of amino acid, fatty acid and bile acid subtypes are being identified in association with altered metabolic states, especially obesity and T2DM. This presents several challenges, including that of determining which metabolite signatures cause the metabolic aberrations in question and which are consequences of them. Also, how these changes in metabolite levels are initiated, and in which tissues, needs to be determined. Studies also need to address how dietary nutrients alter cellular metabolites and the cellular pathways that these regulate, whether the effects of gut-derived metabolites are local or peripheral and the mechanisms involved. Additionally, it will be key to determine which observations in animal models are relevant to humans. Deepening our mechanistic understanding of how metabolites act as signalling molecules, and engage specific receptors to regulate hormone secretion, immune responses, insulin action and neuronal function, will be important in addressing these issues. In addition, it will be important to identify the functional consequences of the metabolite or lipid modification of protein structure and cell membrane dynamics. The sheer number of these metabolites and the interplay between their metabolic pathways, coupled with their ability to alter inter-tissue communication, make this a compelling frontier for understanding metabolic disease and inventing novel approaches to treat T2DM.
Supplementary Material
Toll-like receptors.
(TLRs). A family of pattern-recognition receptors with a role in innate immunity. For example, TLR4 is activated upon dimerization with the co-receptor myeloid differentiation protein 2 (MD2); signalling via TLR4–MD2 results in the transcription of pro-inflammatory genes.
Mendelian randomization.
A novel epidemiological study design that uses genetic variants to investigate the causal relationship of a biomarker to the risk of having a phenotype or disease.
Stereoisomers.
Two molecules that have the same molecular formula but differ in the orientation of atoms. Enantiomers are stereoisomers that are mirror images of each other; an enantiomer is labelled ‘R’ in its right-handed configuration and ‘S’ in its left-handed configuration.
Stereospecific numbering (sn).
The sn position is often used to define the configuration of glycerol-containing metabolites. When the OH group on the second carbon (sn-2) of glycerol is oriented to the left, the top (first) carbon is at the sn-1 position and the bottom (third) carbon is at the sn-3 position.
Non-alcoholic steatohepatitis (NASH).
Hepatitis caused by excessive fat deposition in the liver that is not related to heavy alcohol use.
Lands cycle.
Cycles to remodel phospholipids by first de-acylating and then re-acylating them, thereby altering the fatty acid moiety to generate mature phospholipids.
Anaplerotic pathways.
Pathways that replenish tricarboxylic acid cycle intermediates, which can then be used for energy production or for gluconeogenesis in the liver.
Periportal neural system.
The nervous system that innervates the liver.
L cells.
A subset of entero-endocrine cells that secrete gut peptides such as glucagon-like peptide 1 (GLP1), incretins, etc.
Acknowledgements
The authors thank A. Santoro for comments on the manuscript. Q.Y. is supported by US National Institutes of Health (NIH) grant R01 DK100385; B.B.K. is supported by NIH R01 DK43051, R01 DK106210, R01 DK107405 and a grant from the JPB Foundation; A.V. is supported by NIH R01 DK106210.
Footnotes
Competing interests
B.B.K. is an inventor on patents related to the fatty acid esters of hydroxy fatty acids. The other authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haeusler RA., McGraw TE. & Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol 19, 31–44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi CM, Emanuelli B & Kahn CR Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol 7, 85–96 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Martini M, De Santis MC, Braccini L, Gulluni F & Hirsch E PI3K/AKT signaling pathway and cancer: an updated review. Ann. Med 46, 372–383 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Dennis EA & Norris PC Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol 15, 511–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wymann MP & Schneiter R Lipid signalling in disease. Nat. Rev. Mol. Cell Biol 9, 162–176 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Chavez JA & Summers SA Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys 419, 101–109 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Davis JE, Gabler NK, Walker-Daniels J & Spurlock ME The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Horm. Metab. Res 41, 523–530 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Holland WL et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest 121, 1858–1870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest 116, 3015–3025 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koska J et al. A human model of dietary saturated fatty acid induced insulin resistance. Metabolism 65, 1621–1628 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Stephens FB et al. Lipid-induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Diabetes 64, 1615–1620 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Boesch C, Kuk JL & Arslanian S Effects of an overnight intravenous lipid infusion on intramyocellular lipid content and insulin sensitivity in African-American versus Caucasian adolescents. Metabolism 62, 417–423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamura F et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med 13, e1002087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riserus U, Willett WC & Hu FB Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res 48, 44–51 (2009).References 13 (meta-analysis) and 14 (review) report that SFA consumption is not associated with an increased risk of incident diabetes but that replacing dietary carbohydrates and SFAs with PUFAs is linked to improved glycaemia and insulin sensitivity.
- 15.Tao C et al. Short-term versus long-term effects of adipocyte toll-like receptor 4 activation on insulin resistance in male mice. Endocrinology 158, 1260–1270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y et al. TAK-242, a Toll-like receptor 4 antagonist, protects against aldosterone-induced cardiac and renal injury. PLoS ONE 10, e0142456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia L et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun 5, 3878 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park BS et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Schaeffler A et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 126, 233–245 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y et al. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat. Commun 8, 13997 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirosumi J et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Yuan M et al. Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293, 1673–1677 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Gao Z et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem 277, 48115–48121 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Rui L et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Invest 107, 181–189 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copps KD et al. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab 11, 84–92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copps KD & White MF Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 (2012).References 25 and 26 show that, contrary to the results of cell-based experiments relating Ser307 phosphorylation of IRS1 to impaired insulin signalling, Ser307 in mice is a positive regulatory site that maintains proximal insulin signalling.
- 27.Frakes AE & Dillin A The UPR(ER): sensor and coordinator of organismal homeostasis. Mol. Cell 66, 761–771 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS & Davis RJ Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol 8, a006072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierre N et al. Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS ONE 8, e65061 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry BD et al. Palmitate-induced ER stress and inhibition of protein synthesis in cultured myotubes does not require Toll-like receptor 4. PLoS ONE 13, e0191313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robblee MM et al. Saturated fatty acids engage an IRE1alpha-dependent pathway to activate the NLRP3 inflammasome in myeloid cells. Cell Rep 14, 2611–2623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leamy AK, Egnatchik RA & Young JD Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res 52, 165–174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ersoy BA, Maner-Smith KM, Li Y, Alpertunga I & Cohen DE Thioesterase-mediated control of cellular calcium homeostasis enables hepatic ER stress. J. Clin. Invest 128, 141–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holzer RG et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147, 173–184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen H et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol 12, 408–415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corpeleijn E et al. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia 49, 2392–2401 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Forouhi NG et al. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med 13, e1002094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurotani K et al. High levels of stearic acid, palmitoleic acid, and dihomo-gamma-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr. Res 32, 669–675.e3 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Pfeuffer M & Jaudszus A Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv. Nutr 7, 730–734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weitkunat K et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am. J. Clin. Nutr 105, 1544–1551 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Lalia AZ & Lanza IR Insulin-sensitizing effects of omega-3 fatty acids: lost in translation? Nutrients 8, E329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh DY et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storlien LH et al. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237, 885–888 (1987). [DOI] [PubMed] [Google Scholar]
- 44.Bosch J et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med 367, 309–318 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Li X, Yu Y & Funk CD Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 27, 4987–4997 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Liu Y et al. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology 143, 81–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjursell M et al. The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS ONE 9, e114942 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paerregaard SI et al. FFAR4 (GPR120) signaling is not required for anti-inflammatory and insulin-sensitizing effects of omega-3 fatty acids. Mediators Inflamm 2016, 1536047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serhan CN et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med 192, 1197–1204 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claria J, Nguyen BT, Madenci AL, Ozaki CK & Serhan CN Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol 304, C1141–C1149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuhofer A et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 62, 1945–1956 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez-Periz A et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J 23, 1946–1957 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellmann J, Tang Y, Kosuri M, Bhatnagar A & Spite M Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 25, 2399–2407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titos E et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol 187, 5408–5418 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Chiang N, Dalli J, Colas RA & Serhan CN Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med 212, 1203–1217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsiao HM et al. Resolvin D1 attenuates polyinosinic-polycytidylic acid-induced inflammatory signaling in human airway epithelial cells via TAK1. J. Immunol 193, 4980–4987 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serhan CN Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohira T et al. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem 285, 3451–3461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian F, Korat AA, Malik V & Hu FB Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 39, 1448–1457 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwingshackl L & Hoffmann G Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis 13, 154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nowak C et al. Effect of insulin resistance on monounsaturated fatty acid levels: a multi-cohort non-targeted metabolomics and Mendelian randomization study. PLoS Genet 12, e1006379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perdomo L et al. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol 14, 75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finucane OM et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes 64, 2116–2128 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez de Fonseca F et al. An anorexic lipid mediator regulated by feeding. Nature 414, 209–212 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Schwartz GJ et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab 8, 281–288 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao H et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan KL et al. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J. Biol. Chem 290, 16979–16988 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Souza CO et al. Palmitoleic acid (n-7) attenuates the immunometabolic disturbances caused by a high-fat diet independently of PPARalpha. Mediators Inflamm 2014, 582197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mozaffarian D et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am. J. Clin. Nutr 92, 1350–1358 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nestel P, Clifton P & Noakes M Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J. Lipid Res 35, 656–662 (1994). [PubMed] [Google Scholar]
- 71.Yore MM et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014).This study reports the discovery of a novel class of lipids, branched FAHFAs, and shows that FAHFAs have anti-diabetic and anti-inflammatory effects.
- 72.Ma Y et al. An in silico MS/MS library for automatic annotation of novel FAHFA lipids. J. Cheminform 7, 53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuda O et al. Docosahexaenoic acid-derived fatty acid esters of hydroxy fatty acids (FAHFAs) with anti-inflammatory properties. Diabetes 65, 2580–2590 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Syed I et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab 27, 419–427.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J. et al. Branched fatty acid esters of hydroxy fatty acids (FAHFAs) protect against colitis by regulating gut innate and adaptive immune responses. J. Biol. Chem 291, 22207–22217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parsons WH et al. AIG1 and ADTRP are atypical integral membrane hydrolases that degrade bioactive FAHFAs. Nat. Chem. Biol 12, 367–372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolar MJ et al. Branched fatty acid esters of hydroxy fatty acids are preferred substrates of the MODY8 protein carboxyl ester lipase. Biochemistry 55, 4636–4641 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raeder H et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat. Genet 38, 54–62 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Nelson AT et al. Stereochemistry of endogenous palmitic acid ester of 9-hydroxystearic acid and relevance of absolute configuration to regulation. J. Am. Chem. Soc 139, 4943–4947 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shulman GI Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med 371, 1131–1141 (2014).This is a comprehensive review on the roles of ectopic lipids, especially DAGs, in insulin resistance, dyslipidaemia and cardiometabolic disease.
- 81.Erion DM & Shulman GI Diacylglycerol-mediated insulin resistance. Nat. Med 16, 400–402 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samuel VT, Petersen KF & Shulman GI Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szendroedi J et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111, 9597–9602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu C et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem 277, 50230–50236 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Kim JK et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J. Clin. Invest 114, 823–827 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y et al. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J. Biol. Chem 279, 45304–45307 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Petersen MC et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest 126, 4361–4371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finck BN & Hall AM Does diacylglycerol accumulation in fatty liver disease cause hepatic insulin resistance? Biomed. Res. Int 2015, 104132 (2015).This review article discusses the controversies on the roles of DAGs in insulin resistance.
- 89.Samuel VT et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest 117, 739–745 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferris HA & Kahn CR Unraveling the paradox of selective insulin resistance in the liver: the brain-liver connection. Diabetes 65, 1481–1483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown JM et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res 51, 3306–3315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turpin SM et al. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 54, 146–156 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Amati F et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60, 2588–2597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kishimoto A, Takai Y, Mori T, Kikkawa U & Nishizuka Y Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J. Biol. Chem 255, 2273–2276 (1980). [PubMed] [Google Scholar]
- 95.Hannun YA & Obeid LM Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol 19, 175–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park JW, Park WJ & Futerman AH Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 1841, 671–681 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Holland WL et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17, 55–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peraldi P, Hotamisligil GS, Buurman WA, White MF & Spiegelman BM Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem 271, 13018–13022 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Chavez JA & Summers SA A ceramide-centric view of insulin resistance. Cell Metab 15, 585–594 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Ussher JR et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deevska GM et al. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem 284, 8359–8368 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blouin CM et al. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59, 600–610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chalfant CE et al. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J. Biol. Chem 274, 20313–20317 (1999). [DOI] [PubMed] [Google Scholar]
- 104.Bourbon NA, Sandirasegarane L & Kester M Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem 277, 3286–3292 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Hajduch E et al. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J 410, 369–379 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Powell DJ, Hajduch E, Kular G & Hundal HS Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/ Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol 23, 7794–7808 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petersen MC & Shulman GI Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci 38, 649–665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merrill AH Jr Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev 111, 6387–6422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bergman BC et al. Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia 59, 785–798 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Chung JO, Koutsari C, Blachnio-Zabielska AU, Hames KC & Jensen MD Intramyocellular ceramides: subcellular concentrations and fractional de novo synthesis in postabsorptive humans. Diabetes 66, 2082–2091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montgomery MK et al. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: a beneficial role for very long-chain sphingolipid species. Biochim. Biophys. Acta 1861, 1828–1839 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Raichur S et al. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 20, 687–695 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Turpin SM et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20, 678–686 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Randle PJ, Garland PB, Hales CN & Newsholme EA The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963). [DOI] [PubMed] [Google Scholar]
- 115.Goodpaster BH & Sparks LM Metabolic flexibility in health and disease. Cell Metab 25, 1027–1036 (2017).This comprehensive review discusses the mechanisms for insulin resistance induced by metabolic inflexibility in muscle and adipose tissue.
- 116.Kelley DE, Goodpaster B, Wing RR & Simoneau JA Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol 277, E1130–E1141 (1999). [DOI] [PubMed] [Google Scholar]
- 117.Kelley DE & Mandarino LJ Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49, 677–683 (2000).References 116 and 117 show the evidence for and describe the concept of metabolic inflexibility in the development of insulin resistance.
- 118.Guilherme A, Virbasius JV, Puri V & Czech MP Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol 9, 367–377 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perry RJ et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).This study shows that adipose inflammation-stimulated lipolysis increases the influx of acetyl-CoA into the liver, which activates pyruvate carboxylase and promotes hepatic gluconeogenesis, leading to hyperglycaemia. This occurs in insulin-resistant states such as obesity and T2DM.
- 120.Zhang HH, Halbleib M, Ahmad F, Manganiello VC & Greenberg AS Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 51, 2929–2935 (2002). [DOI] [PubMed] [Google Scholar]
- 121.Grant RW & Stephens JM Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am. J. Physiol. Endocrinol. Metab 309, E205–E213 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Lee SA, Yuen JJ, Jiang H, Kahn BB & Blaner WS Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology 64, 1534–1546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Q et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Morigny P, Houssier M, Mouisel E & Langin D Adipocyte lipolysis and insulin resistance. Biochimie 125, 259–266 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Aguer C et al. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J 29, 336–345 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koves TR et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7, 45–56 (2008).This paper provides evidence of increased incomplete β-oxidation of lipids in states of insulin resistance and obesity, resulting in the accumulation of acylcarnitines.
- 127.Muoio DM & Neufer PD Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15, 595–605 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muoio DM et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 15, 764–777 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liepinsh E et al. Decreased acylcarnitine content improves insulin sensitivity in experimental mice models of insulin resistance. Pharmacol. Res 113, 788–795 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Bene J, Hadzsiev K & Melegh B Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes 8, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nurjhan N, Consoli A & Gerich J Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J. Clin. Invest 89, 169–175 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Best CH & Ridout JH The effects of cholesterol and choline on liver fat. J. Physiol 86, 343–352 (1936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Z & Vance DE Phosphatidylcholine and choline homeostasis. J. Lipid Res 49, 1187–1194 (2008). [DOI] [PubMed] [Google Scholar]
- 134.Raubenheimer PJ, Nyirenda MJ & Walker BR A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 55, 2015–2020 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Meikle PJ & Summers SA Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol 13, 79–91 (2017). [DOI] [PubMed] [Google Scholar]
- 136.van der Veen JN et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta 1859, 1558–1572 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Lee JM et al. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature 474, 506–510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu S et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502, 550–554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rong X et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab 18, 685–697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singh AB & Liu J Identification of hepatic lysophosphatidylcholine acyltransferase 3 as a novel target gene regulated by peroxisome proliferator-activated receptor delta. J. Biol. Chem 292, 884–897 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cash JG & Hui DY Liver-specific overexpression of LPCAT3 reduces postprandial hyperglycemia and improves lipoprotein metabolic profile in mice. Nutr. Diabetes 6, e206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drazic A, Myklebust LM, Ree R & Arnesen T The world of protein acetylation. Biochim. Biophys. Acta 1864, 1372–1401 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Menzies KJ, Zhang H, Katsyuba E & Auwerx J Protein acetylation in metabolism — metabolites and cofactors. Nat. Rev. Endocrinol 12, 43–60 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Moussaieff A et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21, 392–402 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Lee JV et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab 20, 306–319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Donohoe DR et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cai L, Sutter BM, Li B & Tu BP Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 42, 426–437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wellen KE et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carrer A et al. Impact of a high-fat diet on tissue acyl-coA and histone acetylation levels. J. Biol. Chem 292, 3312–3322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lerin C et al. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab 3, 429–438 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Rodgers JT et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434, 113–118 (2005). [DOI] [PubMed] [Google Scholar]
- 152.Sakai M et al. The GCN5-CITED2-PKA signalling module controls hepatic glucose metabolism through a cAMP-induced substrate switch. Nat. Commun 7, 13147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park JM, Kim TH, Jo SH, Kim MY & Ahn YH Acetylation of glucokinase regulatory protein decreases glucose metabolism by suppressing glucokinase activity. Sci. Rep 5, 17395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fang S et al. The p300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. J. Biol. Chem 283, 35086–35095 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kemper JK et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab 10, 392–404 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma K, Saha PK, Chan L & Moore DD Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest 116, 1102–1109 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Houtkooper RH, Pirinen E & Auwerx J Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol 13, 225–238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Banks AS et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8, 333–341 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M & Tschop MH Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA 105, 9793–9798 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Purushotham A, Xu Q & Li X Systemic SIRT1 insufficiency results in disruption of energy homeostasis and steroid hormone metabolism upon high-fat-diet feeding. FASEB J 26, 656–667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu F et al. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/− mice: a role of lipid mobilization and inflammation. Endocrinology 151, 2504–2514 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chalkiadaki A & Guarente L High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 16, 180–188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gillum MP et al. SirT1 regulates adipose tissue inflammation. Diabetes 60, 3235–3245 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.White AT et al. Skeletal muscle-specific overexpression of SIRT1 does not enhance whole-body energy expenditure or insulin sensitivity in young mice. Diabetologia 56, 1629–1637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White AT et al. High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am. J. Physiol. Endocrinol. Metab 307, E764–772 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qiang L et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 150, 620–632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25, 1664–1679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang RH et al. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Invest 121, 4477–4490 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Daitoku H et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl Acad. Sci. USA 101, 10042–10047 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matsuzaki H et al. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl Acad. Sci. USA 102, 11278–11283 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rodgers JT & Puigserver P Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl Acad. Sci. USA 104, 12861–12866 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hirschey MD et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 44, 177–190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ahn BH et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl Acad. Sci. USA 105, 14447–14452 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lombard DB et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol 27, 8807–8814 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lantier L et al. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes 64, 3081–3092 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fernandez-Marcos PJ et al. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci. Rep 2, 425 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hancock CR et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl Acad. Sci. USA 105, 7815–7820 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Holloszy JO “Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes 62, 1036–1040 (2013).References 178 and 179 provide evidence that impaired mitochondrial function may not be a causative factor for insulin resistance.
- 180.Holloway GP, Bonen A & Spriet LL Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am. J. Clin. Nutr 89, 455S–462S (2009). [DOI] [PubMed] [Google Scholar]
- 181.Ryu D et al. A SIRT7-dependent acetylation switch of GABPbeta1 controls mitochondrial function. Cell Metab 20, 856–869 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Shin J et al. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep 5, 654–665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoshizawa T et al. SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway. Cell Metab 19, 712–721 (2014). [DOI] [PubMed] [Google Scholar]
- 184.Canto C, Menzies KJ & Auwerx J NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22, 31–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Feng D et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun Z et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med 18, 934–942 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hong S et al. Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat. Med 23, 223–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Montgomery RL et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J. Clin. Invest 118, 3588–3597 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun Z et al. Diet-induced lethality due to deletion of the Hdac3 gene in heart and skeletal muscle. J. Biol. Chem 286, 33301–33309 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang S et al. Insulin and mTOR pathway regulate HDAC3-mediated deacetylation and activation of PGK1. PLoS Biol 13, e1002243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang X & Qian K Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol 18, 452–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hirschey MD & Zhao Y Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol. Cell Proteomics 14, 2308–2315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Choudhary C, Weinert BT, Nishida Y, Verdin E & Mann M The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol 15, 536–550 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Resh MD Fatty acylation of proteins: the long and the short of it. Prog. Lipid Res 63, 120–131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guan X & Fierke CA Understanding protein palmitoylation: biological significance and enzymology. Sci. China Chem 54, 1888–1897 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yalovsky S, Rodr Guez-Concepcion M & Gruissem W Lipid modifications of proteins-slipping in and out of membranes. Trends Plant Sci 4, 439–445 (1999). [DOI] [PubMed] [Google Scholar]
- 197.Ren W, Jhala US & Du K Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte 2, 17–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Du K, Murakami S, Sun Y, Kilpatrick CL & Luscher B DHHC7 palmitoylates glucose transporter 4 (Glut4) and regulates Glut4 membrane translocation. J. Biol. Chem 292, 2979–2991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ren W, Sun Y & Du K Glut4 palmitoylation at Cys223 plays a critical role in Glut4 membrane trafficking. Biochem. Biophys. Res. Commun 460, 709–714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wei X et al. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J. Biol. Chem 286, 2933–2945 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Spinelli M et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun 8, 2009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [DOI] [PubMed] [Google Scholar]
- 203.Schwiertz A et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18, 190–195 (2010). [DOI] [PubMed] [Google Scholar]
- 204.Ridaura VK et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).References 202 and 204 provide evidence of altered gut microbiota in obesity and insulin resistance and show that this increases the propensity to develop obesity and insulin resistance.
- 205.Vrieze A et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 e917 (2012). [DOI] [PubMed] [Google Scholar]
- 206.Todesco T, Rao AV, Bosello O & Jenkins DJ Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr 54, 860–865 (1991). [DOI] [PubMed] [Google Scholar]
- 207.Venter CS, Vorster HH & Cummings JH Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol 85, 549–553 (1990). [PubMed] [Google Scholar]
- 208.Chambers ES et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.De Vadder F et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014). [DOI] [PubMed] [Google Scholar]
- 210.den Besten G et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes 64, 2398–2408 (2015). [DOI] [PubMed] [Google Scholar]
- 211.Frost G et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun 5, 3611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gao Z et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Perry RJ et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ang Z & Ding JL GPR41 and GPR43 in obesity and inflammation — protective or causative? Front. Immunol 7, 28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Brown AJ et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem 278, 11312–11319 (2003). [DOI] [PubMed] [Google Scholar]
- 216.Canfora EE, Jocken JW & Blaak EE Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol 11, 577–591 (2015). [DOI] [PubMed] [Google Scholar]
- 217.Karaki S et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol 39, 135–142 (2008). [DOI] [PubMed] [Google Scholar]
- 218.Thangaraju M et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69, 2826–2832 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jiang L et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J. Clin. Invest 123, 1605–1614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Al-Lahham SH et al. Regulation of adipokine production in human adipose tissue by propionic acid. Eur. J. Clin. Invest 40, 401–407 (2010). [DOI] [PubMed] [Google Scholar]
- 221.Xiong Y. et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl Acad. Sci. USA 101, 1045–1050 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Freeland KR & Wolever TM Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br. J. Nutr 103, 460–466 (2010). [DOI] [PubMed] [Google Scholar]
- 223.Psichas A et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. (Lond.) 39, 424–429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tolhurst G et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Aberdein N, Schweizer M & Ball D Sodium acetate decreases phosphorylation of hormone sensitive lipase in isoproterenol-stimulated 3T3-L1 mature adipocytes. Adipocyte 3, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ge H et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 (2008). [DOI] [PubMed] [Google Scholar]
- 227.Arpaia N et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Maslowski KM et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Al-Lahham S et al. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur. J. Clin. Invest 42, 357–364 (2012). [DOI] [PubMed] [Google Scholar]
- 230.Liu T et al. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-kappaB pathway in RAW264.7 cells. Inflammation 35, 1676–1684 (2012). [DOI] [PubMed] [Google Scholar]
- 231.Cox MA et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol 15, 5549–5557 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li G, Yao W & Jiang H Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J. Nutr 144, 1887–1895 (2014). [DOI] [PubMed] [Google Scholar]
- 233.Dewulf EM et al. Evaluation of the relationship between GPR43 and adiposity in human. Nutr. Metab 10, 11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hong YH et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005). [DOI] [PubMed] [Google Scholar]
- 235.Priyadarshini M et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol. Endocrinol 29, 1055–1066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Felig P, Marliss E & Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med 281, 811–816 (1969). [DOI] [PubMed] [Google Scholar]
- 237.Cheng S et al. Adipose tissue dysfunction and altered systemic amino acid metabolism are associated with non-alcoholic fatty liver disease. PLoS ONE 10, e0138889 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Iwasa M et al. Elevation of branched-chain amino acid levels in diabetes and NAFL and changes with antidiabetic drug treatment. Obes. Res. Clin. Pract 9, 293–297 (2015). [DOI] [PubMed] [Google Scholar]
- 239.Bhattacharya S et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 232, 191–196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shah SH et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet 3, 207–214 (2010). [DOI] [PubMed] [Google Scholar]
- 241.Newgard CB et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9, 311–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wurtz P et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36, 648–655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Newgard CB Metabolomics and metabolic diseases: where do we stand? Cell Metab 25, 43–56 (2017).This comprehensive review discusses the emerging roles of metabolites, especially BCAAs, in insulin resistance.
- 244.Shah SH & Newgard CB Integrated metabolomics and genomics: systems approaches to biomarkers and mechanisms of cardiovascular disease. Circ. Cardiovasc. Genet 8, 410–419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Newgard CB Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15, 606–614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.She P et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab 293, E1552–E1563 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang TJ et al. Metabolite profiles and the risk of developing diabetes. Nat. Med 17, 448–453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lackey DE et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab 304, E1175–E1187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Herman MA, She P, Peroni OD, Lynch CJ & Kahn BB Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem 285, 11348–11356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Burrill JS et al. Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes. Mol. Endocrinol 29, 411–420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zimmerman HA, Olson KC, Chen G & Lynch CJ Adipose transplant for inborn errors of branched chain amino acid metabolism in mice. Mol. Genet. Metab 109, 345–353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shin AC et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab 20, 898–909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lefort N et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 59, 2444–2452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.White PJ et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab 5, 538–551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pedersen HK et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016). [DOI] [PubMed] [Google Scholar]
- 256.Lotta LA et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med 13, e1002179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Smith GI et al. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 64, 1555–1563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Macotela Y et al. Dietary leucine — an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 6, e21187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zeanandin G et al. Differential effect of long-term leucine supplementation on skeletal muscle and adipose tissue in old rats: an insulin signaling pathway approach. Age (Dordr) 34, 371–387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xiao F et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 63, 841–850 (2014). [DOI] [PubMed] [Google Scholar]
- 261.Jang C et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med 22, 421–426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Roberts LD et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19, 96–108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sun H et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133, 2038–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Li T et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab 25, 374–385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Green CR et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol 12, 15–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Su X et al. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: effects of obesity and weight loss. Obesity (Silver Spring) 23, 329–334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Malloy VL et al. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5, 305–314 (2006). [DOI] [PubMed] [Google Scholar]
- 268.Stone KP, Wanders D, Orgeron M, Cortez CC & Gettys TW Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 63, 3721–3733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wanders D et al. UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J 29, 2603–2615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wanders D et al. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes 66, 858–867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Epner DE, Morrow S, Wilcox M & Houghton JL Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr. Cancer 42, 158–166 (2002). [DOI] [PubMed] [Google Scholar]
- 272.Stone KP et al. Compromised responses to dietary methionine restriction in adipose tissue but not liver of ob/ob mice. Obesity (Silver Spring) 23, 1836–1844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mentch SJ et al. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab 22, 861–873 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chen T et al. Tryptophan predicts the risk for future type 2 diabetes. PLoS ONE 11, e0162192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Shah SH et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55, 321–330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Laferrere B et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl Med 3, 80re82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cotter DG et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J. Clin. Invest 124, 5175–5190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Puchalska P & Crawford PA Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25, 262–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Taggart AK et al. D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem 280, 26649–26652 (2005). [DOI] [PubMed] [Google Scholar]
- 280.Kimura I et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl Acad. Sci. USA 108, 8030–8035 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shimazu T et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Goldberg EL et al. beta-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep 18, 2077–2087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Youm YH et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med 21, 263–269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC & Crispim D Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 74, 1–9 (2017). [DOI] [PubMed] [Google Scholar]
- 285.Houstis N, Rosen ED & Lander ES Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 (2006). [DOI] [PubMed] [Google Scholar]
- 286.Fisher FM & Maratos-Flier E Understanding the physiology of FGF21. Annu. Rev. Physiol 78, 223–241 (2016). [DOI] [PubMed] [Google Scholar]
- 287.Foster GD et al. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med 348, 2082–2090 (2003). [DOI] [PubMed] [Google Scholar]
- 288.Chavez AO et al. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 32, 1542–1546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fazeli PK et al. FGF21 and the late adaptive response to starvation in humans. J. Clin. Invest 125, 4601–4611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Douris N et al. Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Mol. Metab 6, 854–862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chavez-Talavera O, Tailleux A, Lefebvre P & Staels B Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694. e3 (2017). [DOI] [PubMed] [Google Scholar]
- 292.Preidis GA, Kim KH & Moore DD Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J. Clin. Invest 127, 1193–1201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kawamata Y et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem 278, 9435–9440 (2003). [DOI] [PubMed] [Google Scholar]
- 294.Maruyama T et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun 298, 714–719 (2002). [DOI] [PubMed] [Google Scholar]
- 295.Broeders EP et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22, 418–426 (2015). [DOI] [PubMed] [Google Scholar]
- 296.Watanabe M et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006). [DOI] [PubMed] [Google Scholar]
- 297.Somm E et al. beta-Klotho deficiency protects against obesity through a crosstalk between liver, microbiota, and brown adipose tissue. JCI Insight 2, 91809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fujisaka S et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J. Clin. Invest 126, 4430–4443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kumar DP et al. Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet alpha cells to promote glucose homeostasis. J. Biol. Chem 291, 6626–6640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Thomas C et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10, 167–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ding L et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology 64, 760–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Weber G et al. Regulation of purine and pyrimidine metabolism by insulin and by resistance to tiazofurin. Adv. Enzyme Regul 23, 81–99 (1985). [DOI] [PubMed] [Google Scholar]
- 303.Pelley JW (ed.) Purine, Pyrimidine, and Single Carbon Metabolism, (Elsevier, 2012). [Google Scholar]
- 304.Deng Y et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 355, eaaf5375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yamamoto T et al. Relationship between plasma uridine and insulin resistance in patients with non-insulin-dependent diabetes mellitus. Nucleosides Nucleotides Nucleic Acids 29, 504–508 (2010). [DOI] [PubMed] [Google Scholar]
- 306.Hamada T et al. Plasma levels of uridine correlate with blood pressure and indicators of myogenic purine degradation and insulin resistance in hypertensive patients. Circ. J 71, 354–356 (2007). [DOI] [PubMed] [Google Scholar]
- 307.Urasaki Y, Pizzorno G & Le TT Chronic uridine administration induces fatty liver and pre-diabetic conditions in mice. PLoS ONE 11, e0146994 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Krylova IB, Bulion VV, Selina EN, Mironova GD & Sapronov NS Effect of uridine on energy metabolism, LPO, and antioxidant system in the myocardium under conditions of acute coronary insufficiency. Bull. Exp. Biol. Med 153, 644–646 (2012). [DOI] [PubMed] [Google Scholar]
- 309.Le TT et al. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J. Lipid Res 54, 1044–1057 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hall AM et al. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes 63, 2284–2296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Agarwal AK et al. Mogat1 deletion does not ameliorate hepatic steatosis in lipodystrophic (Agpat2−/−) or obese (ob/ob) mice. J. Lipid Res 57, 616–630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ryu D et al. Endoplasmic reticulum stress promotes LIPIN2-dependent hepatic insulin resistance. Diabetes 60, 1072–1081 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ryu D et al. TORC2 regulates hepatic insulin signaling via a mammalian phosphatidic acid phosphatase, LIPIN1. Cell Metab 9, 240–251 (2009). [DOI] [PubMed] [Google Scholar]
- 314.Schweitzer GG et al. Liver-specific loss of lipin-1-mediated phosphatidic acid phosphatase activity does not mitigate intrahepatic TG accumulation in mice. J. Lipid Res 56, 848–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chibalin AV et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 132, 375–386 (2008). [DOI] [PubMed] [Google Scholar]
- 316.Zhang C et al. Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol. J. Biol. Chem 290, 3519–3528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jornayvaz FR et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc. Natl Acad. Sci. USA 108, 5748–5752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Monetti M et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6, 69–78 (2007). [DOI] [PubMed] [Google Scholar]
- 319.Choi CS et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem 282, 22678–22688 (2007). [DOI] [PubMed] [Google Scholar]
- 320.Aburasayn H, Al Batran R & Ussher JR Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab 311, E423–435 (2016). [DOI] [PubMed] [Google Scholar]
- 321.Fayyaz S et al. Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia 57, 373–382 (2014). [DOI] [PubMed] [Google Scholar]
- 322.Hu W, Bielawski J, Samad F, Merrill AH Jr & Cowart LA Palmitate increases sphingosine-1-phosphate in C2C12 myotubes via upregulation of sphingosine kinase message and activity. J. Lipid Res 50, 1852–1862 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kaiser C & James SR Acetylation of insulin receptor substrate-1 is permissive for tyrosine phosphorylation. BMC Biol 2, 23 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Cao J et al. Endotoxemia-mediated activation of acetyltransferase P300 impairs insulin signaling in obesity. Nat. Commun 8, 131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.LaBarge S, Migdal C & Schenk S Is acetylation a metabolic rheostat that regulates skeletal muscle insulin action? Mol. Cells 38, 297–303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhao S et al. Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sundaresan NR et al. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci. Signal 4, ra46 (2011). [DOI] [PubMed] [Google Scholar]
- 328.Glidden EJ et al. Multiple site acetylation of Rictor stimulates mammalian target of rapamycin complex 2 (mTORC2)-dependent phosphorylation of Akt protein. J. Biol. Chem 287, 581–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Yu J et al. Regulation of serine-threonine kinase Akt activation by NAD+-dependent deacetylase SIRT7. Cell Rep 18, 1229–1240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Boutant M & Canto C SIRT1 metabolic actions: integrating recent advances from mouse models. Mol. Metab 3, 5–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.