Abstract
Introduction
Major trauma is a leading cause of death and disability in young adults, especially from massive non-compressible torso haemorrhage. The standard technique to control distal haemorrhage and maximise central perfusion is resuscitative thoracotomy with aortic cross-clamping (RTACC). More recently, the minimally invasive technique of resuscitative endovascular balloon occlusion of the aorta (REBOA) has been developed to similarly limit distal haemorrhage without the morbidity of thoracotomy; cost–utility studies on this intervention, however, are still lacking. The aim of this study was to perform a one-year cost–utility analysis of REBOA as an intervention for patients with major traumatic non-compressible abdominal haemorrhage, compared to RTACC within the U.K.’s National Health Service.
Methods
A retrospective analysis of the outcomes following REBOA and RTACC was conducted based on the published literature of survival and complication rates after intervention. Utility was obtained from studies that used the EQ-5D index and from self-conducted surveys. Costs were calculated using 2016/2017 National Health Service tariff data and supplemented from further literature. A cost–utility analysis was then conducted.
Results
A total of 12 studies for REBOA and 20 studies for RTACC were included. The mean injury severity scores for RTACC and REBOA were 34 and 39, and mean probability of death was 9.7 and 54%, respectively. The incremental cost-effectiveness ratio of REBOA when compared to RTACC was £44,617.44 per quality-adjusted life year. The incremental cost-effectiveness ratio, by exceeding the National Institute for Health and Clinical Effectiveness’s willingness-to-pay threshold of £30,000/quality-adjusted life year, suggests that this intervention is not cost-effective in comparison to RTACC. However, REBOA yielded a 157% improvement in utility with a comparatively small cost increase of 31.5%.
Conclusion
Although REBOA has not been found to be cost-effective when compared to RTACC, ultimately, clinical experience and expertise should be the main factor in driving the decision over which intervention to prioritise in the emergency context.
Keywords: Cost–utility, cost-effectiveness, resuscitative endovascular balloon aortic occlusion, resuscitative thoracotomy with aortic cross-clamping, major haemorrhage, major trauma
Introduction
Trauma accounts for 12,500 deaths across England and Wales each year and is the leading cause of death in those aged 44 and under.1 There are an estimated 20,000 cases of major trauma in England annually and the burden is set to increase over the next 20 years, posing a major public health problem.1,2 Major trauma is defined as an injury severity score (ISS) greater than 15.1,3 Whilst extremity haemorrhage is amenable to direct control via compression or proximal tourniquet application, massive bleeding within the chest or abdomen is not and is referred to as non-compressible torso haemorrhage (NCTH). Without intervention, severe NCTH leads to exsanguinating cardiac arrest. Interventions are typically aimed at temporarily halting distal haemorrhage whilst maintaining central (cardiac and cerebral) perfusion followed by a definitive procedure at the site of bleeding.
First performed in 1900, resuscitative thoracotomy with aortic cross-clamping (RTACC) is the current standard and most commonly performed intervention following NCTH, despite being hugely invasive with significant associated complications.4,5 Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a less invasive method of aortic occlusion, first reported during the Korean War in 1954. It feeds an endovascular balloon into the aorta via the femoral artery; it can then be inflated to occlude distal flow in the aorta, limiting haemorrhage and maintaining central perfusion. In large animal models and several human studies, REBOA has demonstrated its potential in terms of minimal invasiveness, improved survival rate,6–9 increased blood pressure,7,10,11 and improved brain oxygenation and carotid arterial blood flow.11,12 Despite promising initial findings, data regarding complications are still lacking and will be needed before widespread implementation is likely to occur. In addition, the limited availability of economic evaluations of REBOA serves as another barrier to its adoption.
One of the methods by which the National Institute for Health and Clinical Excellence (NICE) ascertains the cost-effectiveness of health interventions is a cost–utility analysis.13 This allows the comparison of two interventions whose measurable outcomes, measured in quality-adjusted life years (QALY), and clinical pathways may differ but address the same problem. Utility, which is a component of QALYs, refers to the satisfaction derived from consuming a good. In health economics, it is essentially an indication of the preference and therefore, quality of life associated with a particular health state; a score of 1 being perfectly healthy and 0 being death.14 Thus, QALYs are a weighted measure of utility, taking into consideration the extra life years a patient might live at an adjusted quality of life. For example, a patient who undergoes a procedure that gives him another 10 years to live with a utility of 0.8 for the period would gain eight QALYs (0.8 × 10 years). A cost–utility analysis ultimately yields a single comparable measure termed the incremental cost-effectiveness ratio (ICER), which describes the cost per QALY gained by one intervention over the other; the ICER is then compared to the health system’s willingness to pay (WTP) in making decisions to fund health interventions.15 As an example, an ICER of £18,000 means two things: the procedure in question delivers gains in QALYs over its comparator; and each QALY gained over its comparator costs £18,000. Within the NHS, the WTP remains at £20,000–£30,000, hence if the ICER is less than the WTP, then an intervention is deemed cost-effective and would be recommended by NICE for NHS funding.
The aim of this study is to perform a cost–utility analysis of REBOA as an intervention for patients with major traumatic NCTH above the aortic bifurcation within the abdomen compared to RTACC, the current gold standard.3,16 In the current climate of financial constraints, this study may ultimately support emergency clinicians in deciding which intervention to prioritise in their departments in this subset of patients within the NHS setting.
Methodology
Search criteria
PUBMED, Google Scholar and OvidSP were initially searched using the terms: ‘REBOA (Resuscitative Endovascular Occlusion of the Aorta)’, ‘endovascular occlusion’, ‘Resuscitative Thoracotomy’, ‘Emergency Thoracotomy’, ‘Aortic Cross Clamping’, ‘Cost Analysis’, ‘Cost Utility’, ‘Major Trauma’, ‘Major Haemorrhage’, ‘Injury Severity’, ‘Survival’, ‘Complications’, ‘Side-Effects’, ‘Indications’. This search enabled the development of the decision trees and generation of a list of complications associated with both REBOA and RTACC. To generate the complications’ data pertaining to the survival rates and utility after initial intervention, PUBMED, Google Scholar and OvidSP were again searched. This time the key terms included the complications: ‘REBOA (Resuscitative Endovascular Occlusion of the Aorta)’, ‘Endovascular Occlusion’, ‘Resuscitative Thoracotomy’, ‘Emergency Thoracotomy’, ‘Aortic Cross Clamping’, ‘Intervention’, ‘Surgery’, ‘Laparoscopic Packing’, ‘Utility’, ‘Quality Of Life’ (QOL), ‘EQ-5D’, ‘Traumatic’, ‘Haemothorax’, ‘Limb Ischaemia’, ‘Necrosis’, ‘Amputation’, ‘Empyema’, ‘Reoperation’, ‘Infection’, ‘Sepsis’, ‘Pneumonia’, ‘Thrombosis’, ‘Deep Vein Thrombosis’, ‘Dialysis’, ‘Acute Kidney Injury (AKI)’, ‘Neurological Impairment’, ‘Intensive Care’, ‘Major trauma’, ‘Disability’ and ‘Mortality’. Studies were included if they reported on the QOL or mortality of the complication regardless of the cause. This was done due to the inability to obtain relevant studies reporting on the individual complication in a subgroup of patients with NCTH. Studies were excluded if the full text was inaccessible for free, if they did not report QOL in the EQ-5D format (for QoL data) or if they were not in English. The list of complications was taken from literature reporting the use of either RTACC or REBOA which reported a complication directly attributable to these interventions. Due to the limited data available regarding utility and QoL from complications directly associated with REBOA and RTACC, these values were preferentially taken from studies where patients had undergone major trauma and had these complications, although these were also scarce. Most of these values were obtained from studies reporting on non-trauma patients as these were the only data available. Where no data existed at all, the utility of the complication was estimated by consultation of medical professionals. Although this was the largest limitation of the study, small changes in utility data did not impact the overall findings, as demonstrated in the sensitivity analysis (Table S4).
Literature review
Published evidence on the use of either intervention is of low quality, with much less literature on the use of REBOA. Due to the nature of the setting in which they are used, randomised controlled trials are difficult to perform. There was a significant heterogeneity of patients included and we chose not to differentiate between blunt and penetrating trauma in our analysis, in an effort to approximate the setting as close to real-world clinical practice as possible as some studies did not discriminate outcomes based on the mechanism of injury. Pertaining to complications, this study only included those that were likely to lead to different levels of reimbursements for hospital trusts in the NHS and for which there was clear evidence of direct causation by either REBOA or RTACC.
The studies used in this analysis are listed in the online supplementary information and a breakdown of included study numbers can be seen in Figure 1. Only patients sustaining NCTH were included as this is the only indication for REBOA. A meta-analysis of survival rates and clinical outcomes was primarily used to collect data for RTACC outcomes, including survival rates, number of procedures performed and numbers of patients who survived with residual neurological impairment.17 Other studies with data regarding complications and neurological outcomes were added but not duplicated with any studies already included. There is one included cost–utility analysis of RTACC that was accessible in the literature but none for REBOA.18
Figure 1.
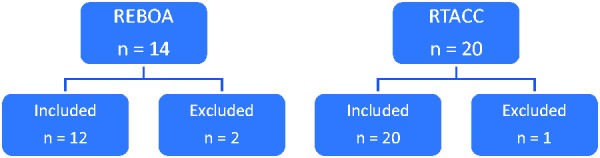
Inclusion and exclusion of studies for both techniques.
This analysis obtained information regarding REBOA predominantly from Morrison et al.’s19 systematic review and the DuBose et al.20 study whose patients had suffered traumatic NCTH. One study was excluded from this analysis as the abstract was not available, and an additional study was excluded as it did not represent a case of trauma.19,20 The exclusion criteria used meant that the full paper had to be available, each case had to be related to trauma where REBOA or RTACC was used before a further intervention occurred, could not be a case report and mentioned the outcomes of their patient populations. This meant that two studies were excluded from the REBOA arm and one from the RTACC arm.
Several studies stated the amount of blood products used; however, the majority did not and so this study calculated a weighted average of the units of red blood cells (RBCs) used as this had the highest volume of evidence. It was assumed that all patients were unconscious whilst the procedure was being performed, in which case intubation would be required.21,22 There are a wide range of survival rates reported for both techniques which may be due to varying sample sizes, severity of injury and variability of the procedures performed after the haemorrhage control intervention.23,24 Additionally, although REBOA is being increasingly performed in the U.K. and Japan, RTACC remains the standard in the US where a significant amount of data was collected.
Complication rates likewise varied greatly across the studies, with most simply reporting that they were observed without providing specific figures. The meta-analysis did not include complication rates and therefore, other studies were used. Similarly, RTACC studies reported a wide range of neurological outcomes, with impairment rates reported between 0 and 50%.25 No neurological impairment was reported in patients who underwent REBOA. There was insufficient data on the length of stay of patients in either the Intensive Care Unit (ICU) or the general ward, as well as the duration spent on a ventilator. Therefore, data from Campbell et al.26 were used in this analysis. The most applicable studies to determine the utility values of complications were chosen based on their reference to patients undergoing trauma, the ISS or patient population being similar to that of this study. Individual complication values pertaining utility and mortality were applied to instances of NCTH as no other data were available with stronger links to major trauma.
Decision tree
The decision tree for patients undergoing REBOA was designed by primarily using data from Morrison et al.19 and for RTACC from Rhee et al.17 Complications related to access were excluded as they did not have any important sequelae as were complications that were not directly attributed to the intervention, such as pericarditis and abdominal abscess following RTACC.22,27,28
The probability of each chance node was calculated using the weighted probability of the studies reporting the occurrence of the event, according to the number of patients involved. Where there were no data, the study was excluded from the calculations. Overall probabilities of each chance node add up to 1. Costs and utilities were added and attributed to the relevant nodes. Further information explaining the decision tree nodes can be found in the supplementary information’s Methodology section.
Discounting
Due to the nature of the injuries, according to Holbrook et al.,29 either patients make a near total recovery within the first year post-injury or they do not survive to discharge; for this reason, a one-year evaluation was conducted and as the majority of the costs were incurred within this year, no discounting was performed.
Financial costs
The costs of these interventions have been evaluated from a U.K. NHS perspective, not taking into consideration productivity losses or other indirect societal costs. The majority of the costs were obtained from the NHS reference costs or tariffs30 with several notable exceptions. The blood products which are ‘unbundled’ or additional expenses were calculated in addition to the core spell tariff using the NICE costing guidelines on blood transfusion.31 The amount of blood needed per procedure was obtained from the literature. In addition, the cost of the REBOA kit was added onto the core spell tariff as it is unlikely that such a novel treatment has been taken into consideration when setting the reference cost for treating major trauma. The cost of this kit was obtained directly from the retailer at its current retail price.32 Patients who experienced a neurological event were costed with a neurological event spell; however, those who were discharged with residual neurological impairment were given an additional cost of neurological rehabilitation for a year. This cost was obtained from the literature, based on a 2009 figure and inflated accordingly.33
All costs were in pounds (£) and did not require conversion. All costs were 2016/2017 values with the exception of the blood products, which were inflated to 2017 costs, and the neurological rehabilitation. The data used to inflate costs were based on the consumer price index from the Office for National Statistics.34 Discounting was not applied as all costs were incurred within a year.
Costs of REBOA and RTACC
Following a serious injury, patients were modelled to receive either REBOA or RTACC as emergency measures to stabilise the patient until they are able to receive definitive care. The cost of these interventions was based on a multiple trauma diagnoses core spell which was obtained from the NHS tariffs.30 The NHS discriminates trauma costs based on the ISS which was obtained from the literature, as well as the Therapeutic Intervention Score which was obtained through the modelling of a typical major trauma patient.35 This was done to select the most accurate tariff. Costs of the blood products which were obtained from the literature were added to the core spell tariff.
Following the REBOA or RTACC intervention, those patients who survived would then require a more definitive intervention (laparotomy or angioembolisation) for the abdominal bleeding regardless of which initial intervention was received. These costs were obtained using the NHS tariff then weighted against the likelihood of each patient undergoing the procedure to provide one cost which was named ‘definitive intervention’.30
Cost of complications
The most common complications that were reported in the literature were included as well as the less common complications but which had either a high cost or significantly lower quality of life such as leg amputation. The cost of these procedures and treatments were based on the tariff costs.30 Most of these costs were inpatient, except for patients who were discharged home requiring dialysis, and those with neurological impairment, whose costs were calculated as outpatient. The cost of treating neurological impairment per year was obtained from the literature.32 The cost of an outpatient dialysis session was obtained from the NHS tariffs and multiplied by the total number of expected sessions/year.
As the injury considered is critical multi-trauma, all complications were assumed to be major and associated with complexity and comorbidity as defined by the NHS in their tariff stratification. Finally, for those tariffs that stratified the costs according to age, the ‘19 and over’ category was chosen as the average age of patients who present with major trauma is greater than 30 in the literature. The tariffs which were most relevant and representative were selected for each condition in order to make the total cost as accurate as possible.
QALYs
Each end node QALY displayed in the decision trees represents the sum of all the utilities for each health state (quality of life scores (QoLs)) multiplied by the length of life (LOL) for that corresponding state. The QoLs have a value between 0 and 1, which represents death and perfect health, respectively. The LOL value represents the LOL (in years) spent in a particular health state. All LOL values were obtained from the literature. QoL scores were obtained from either the literature or direct assessment using EQ-5D questionnaires. The EQ-5D is a standardised numerical measure of health outcomes, used by NICE. The EQ-5D was the chosen measure of utility for this analysis as the questionnaire and resources are openly accessible and it allowed for the calculation of utility/QoL scores for complications where QoL values were not available in the literature based on self-conducted surveys.
In the context of NCTH, it has been assumed that irrespective of the intervention (RTACC or REBOA), patients are ventilated for the same amount of days on average (three days), spend 4.93 days in the ICU and 19.58 days in the ward. The QoL scores for each of these health states, therefore, have been assumed to be equal 0, 0.286 and 0.730, respectively. If a complication arises after intervention, the LOL for the number of days in the ward is reduced, such that the number of days in the complication state and number of days in the ward totals 19.58 days. The study follows the patient for a total of one year, thus the LOL of the final health state of the patient pathway totals 337.49 days. Discounting of QALYs was not done as this is a one-year evaluation.
ICER
The costs and QALYs of comparative treatments were calculated from the decision tree. The ICER was calculated using the equation
Results
Decision trees
The outcome of the decision tree designs for both intervention can be seen in Figures 2 and 3. Detailed analysis of how each value was derived can be found in the supplementary information (REBOA Probabilities and RTACC Probabilities section).
Figure 2.
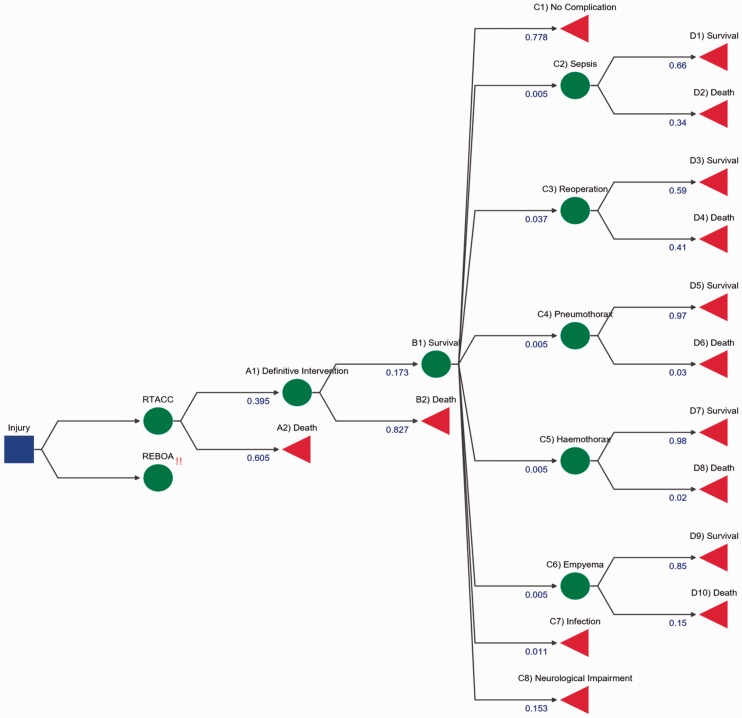
RTACC decision tree. The initial decision node is demonstrated by a blue box, where a decision for an intervention occurs, in this case RTACC. The chance nodes are represented by green circles whereby probability dictates which node is travelled down. Terminal nodes are represented by red triangles which highlight the end of the path and outcome of the journey. Each blue number under the node title is representative of weighted probabilities of a patient undertaking the route. For example, Death and Survive (B nodes) should add up to 1. Numbers on the diagram are rounded for display purposes and may not add up to 1.
Figure 3.
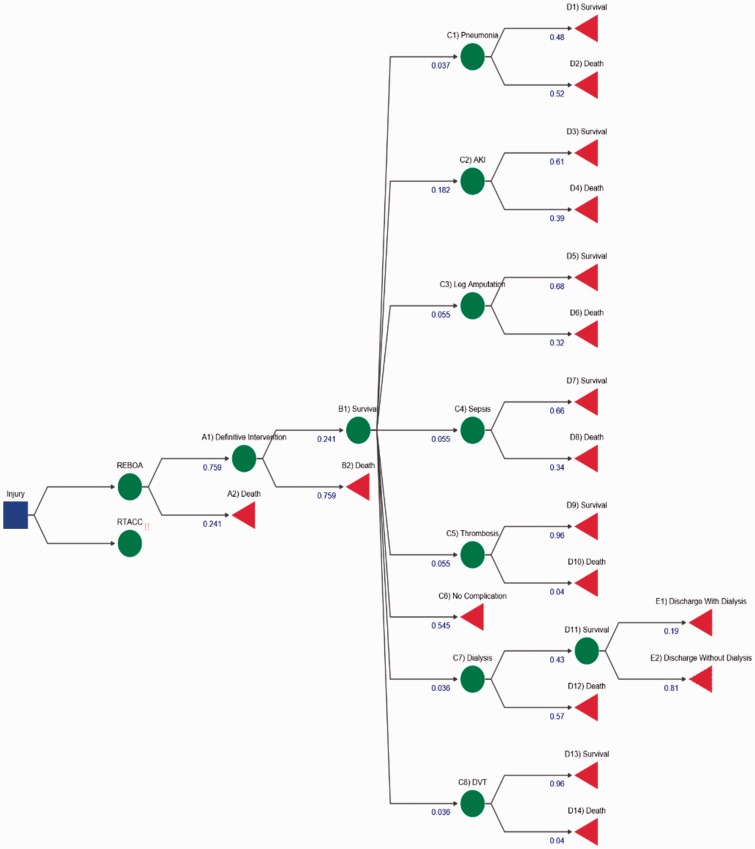
REBOA decision tree. All shapes, colours and numbers represent the same things as in Figure 2, but for the REBOA pathway.
ICER
The ICER of REBOA intervention in patients with NCTH with RTACC as comparator is
These values were derived from literature; the total utility is the sum of weighted utility of each branch, taking into consideration the probability of a patient undertaking an event and the outcome from that specific event. The total costs were calculated by taking the sum cost of each intervention or complication using the NHS tariffs for 2016/2017 and weighting them against the probability of use. This information was found from literature and detailed in the supplementary information (Methodology section). This ICER value means that for each QALY gained by using REBOA instead of RTACC, it will cost an extra £44,617.44. The positive cost and positive QALY difference denote that there is a trade-off. REBOA is more effective than RTACC but also costlier, thus acceptance of REBOA as a more effective intervention is dependent on the maximum WTP. In the U.K., the WTP per QALY gained is £20,000–£30,000 for general treatment, so REBOA would not be deemed more cost-effective for clinical use according to the NHS as it is higher than the threshold.
Sensitivity analysis
The sensitivity analysis was performed using alternative values available from published literature. The analysis used the minimum and maximum values for probability of survival from either REBOA or RTACC and the intervention (node B1/2). It also assessed the minimum and maximum cost of REBOA or RTACC, the cost in relation to the ISS, addition of blood products used as well as the proportion of patients undergoing angioembolisation or laparotomy and packing. Finally, the impact of variation in utility was assessed using the minimum and maximum values for the EQ-5D values. The values used can be found in the supplementary information (Table S4). This was performed to assess whether alternative data values from the literature yielded significant changes to cost-effectiveness outcomes.
Discussion
The main aim of this study was to assess the cost–utility of using REBOA compared to RTACC from an NHS perspective in the subset of critically ill patients who have NCTH. The ICER for REBOA in this study was £44,617.44 suggesting that, according to current NICE thresholds, this intervention would not be deemed cost-effective. Importantly, one of the main predictors of outcome, the ISS, was similar in both sets of patients, making these interventions comparable clinically.
The large ICER has several possible explanations. RTACC involves less costly equipment, whereas the REBOA kit itself costs close to £1000.33 The improvement in utility provided by REBOA is small at only 0.102 in absolute terms, despite a 157% relative improvement in utility. This means that although a small absolute increase in how well a patient is doing is observed, mainly due to the high mortality rates seen in this type of trauma, the study suggests that from a clinical point of view, REBOA provides considerable benefits, giving outcomes 157% higher than using RTACC. Therefore, a patient will have a much higher chance of surviving NCTH if REBOA is used instead of RTACC. The relative increase in costs is nonetheless less at 31.5%. The utility of RTACC may be overstated due to the fact that there was poor quality data based on studies that did not follow-up their patients nor include complications; furthermore, as there is more experience using RTACC, there were more patients than for REBOA, which may have led to a bias. Additionally, there was a greater amount of RBC units used in the REBOA group compared to RTACC. Finally, the probability of surviving to definitive intervention following REBOA was greater, leading to an accrual of costs to the system, which are not compensated by a sufficient improvement in the utility of these patients.
This is the first cost–utility analysis comparing these two interventions, although the rationale for looking at cost in such critically ill patients, who have very poor outcomes to begin with, might be disputed from a clinical point of view. In 2007, Brown concluded that RTACC costs $16,125 per QALY when compared to no intervention.18 When this is converted into pounds, at the March 2017 exchange rate of $1.23/£ and adjusted for inflation using the consumer price index, would equate to approximately £17,185.20.36 The Brown study differed from this analysis in several ways, notably in its setting, the USA, with greater healthcare costs overall; the utility at discharge of 1 and the comparator being no treatment.13 It is important to note that no market forces factors were taken into account when calculating costs because this analysis was from the perspective of the NHS and not from an individual hospital.
This study looked at how changes in the probabilities impacted the ICER. Of the survival data, the ICER was most sensitive to changes to the probability of death following REBOA. When the minimum reported probability of survival to definitive intervention and after definitive intervention were used, the change in ICER was 152.82 and 226.79%, respectively. In terms of cost, of the variables analysed, the one that impacted the ICER most was the number of RBC units used in the RTACC arm, with the maximal reported use yielding a 62.12% change in ICER. For the utilities, the focus was on assessing the impact of the self-conducted survey on the ICER. Virtually no change occurred, with the largest ICER fluctuation of 0.64% occurring with the lowest utility for ICU stay.
Of this analysis only, three ICER changes led to a value that was within NICE’s WTP threshold. These were maximum use of RBC in the RTACC arm, having no complications in the RTACC arm, and having a probability of survival to definitive intervention of 1 in the REBOA arm. Of all the variables examined in this sensitivity analysis, survival was the one that impacted the ICER the most. REBOA survival was the most impactful because those who survive to discharge have a considerably better outcome than those who survive RTACC. The upper limit of the NICE threshold is £30,000 per QALY, thus REBOA would not be recommended by NICE solely based on the NHS’ WTP.13
This study does have a number of limitations, many of them derived from the numerous assumptions that had to be made which are summarised in the online supplementary information (Table S5). These mostly arise from the poor quality of evidence available in the literature for both arms of this study. No study was able to randomise patients and was performed in units with variable experiences in using the techniques, thus reducing standardisation. Several gaps in the information available meant that probabilities had to be calculated from a small data set and explain why many assumptions were made. Importantly, several complications could not be attributed to the procedure itself and had to be excluded altogether. The analysis was limited to outcomes over one year and so costs are likely to be underestimated and benefits overestimated over the course of a lifetime. Evidence-based QoL was used where possible, and utility was based on Ringburg et al.’s paper.37 This report may also have included patients who did not sustain abdominal trauma as part of their multiple injuries as there were no studies in whom all patients had sustained abdominal trauma, with or without other types of trauma, and thus this utility may not be fully generalisable. As a result, the analysis included papers reporting the mortality and QoL of the complications in non-traumatic patients although, where possible, post-traumatic patients were included even though they did not have NCTH. This admittedly reduces the relevance of our data; however, the sensitivity analysis demonstrates that small changes in utility would not change the overall outcome (Table S4). There was no data on the utility of patients who were unconscious, intubated and ventilated; thus, it was assumed to be 0. Furthermore, there were little data available on the utility of several health states and so a self-conducted EQ-5D survey was performed on several experienced medical professionals in order to achieve a consensus view.
Conclusion
The objective of this report was to evaluate the cost–utility of a novel treatment for major trauma. Based on the findings of this study, according to NICE thresholds, REBOA has not been found to be cost-effective when compared to RTACC. Due to both the poor quality of studies used and the limited number of patients included in the REBOA arm of this analysis, we feel our results should be interpreted with caution. Importantly, the aim of this study was not to recommend which intervention should be used over the other, but provide information supporting clinical decision-making in a cost-constrained NHS setting. Ultimately, clinical experience and expertise should be the main factor in driving the decision over which intervention to prioritise. This study acts as a starting point in understanding which intervention is more cost-effective, although further research is needed for a more robust analysis of these interventions. Compared to RTACC, REBOA is a relatively new intervention, so greater experience with it may ultimately lead to better outcomes in the future and consequently a more favourable ICER.
Supplementary Material
Acknowledgements
We would like to acknowledge Marisa Miraldo of Imperial College Business School for her support pertaining this economic analysis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent
Not applicable.
Ethical approval
Not applicable.
Contributorship
All members of the team were involved with gathering data from relevant sources and conceiving the study. MSR gathered in-depth information on the REBOA arm and developed the decision tree graphics and CVZ gathered more specific data on the RTACC arm and analysed the contraindications. FA-H analysed the costs of each treatment. CT, MM and JG gathered detailed data for the QOLs and performed the calculations for them. MSR and CT performed the sensitivity analysis. MSR, CT, FA-H and CVZ edited the first draft, with MSR and CT working on the final drafts.
Provenance and peer review
Not commissioned, externally peer reviewed.
References
- 1.TARN. The trauma audit and research network, https://www.tarn.ac.uk/Home.aspx (2017, accessed 1 March 2017).
- 2.NAO. Major trauma care in England, https://www.nao.org.uk/wp-content/uploads/2010/02/0910213es.pdf (2010, accessed 1 March 2017).
- 3.Blyth A. Thoracic Trauma, British Medical Journal 2014; 348: bmj.g1137 (accessed 1 March 2017).
- 4.Brautigan MW, Tietz G. Emergency thoracotomy in an urban community hospital: initial cardiac rhythm as a new predictor of survival. Am J Emerg Med 1985; 3: 311–315. [DOI] [PubMed] [Google Scholar]
- 5.Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage a review with contemporary definitions and management strategies. Surg Clin North Am 2012; 92: 843–858. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JJ, Percival TJ, Markov NP, et al. Aortic balloon occlusion is effective in controlling pelvic hemorrhage. J Surg Res 2012; 177: 341–347. [DOI] [PubMed] [Google Scholar]
- 7.Avaro J-P, Mardelle V, Roch A, et al. Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma 2011; 71: 720–726. [DOI] [PubMed] [Google Scholar]
- 8.Martinelli T, Thony F, Decléty P, et al. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma 2010; 68: 942–948. [DOI] [PubMed] [Google Scholar]
- 9.Brenner ML, Moore LJ, DuBose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg 2013; 75: 506–511. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JJ, Ross JD, Houston R, et al. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of noncompressible torso hemorrhage. Shock 2014; 41: 130–137. [DOI] [PubMed] [Google Scholar]
- 11.Scott DJ, Eliason JL, Villamaria C, et al. A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg 2013; 75: 122–128. [DOI] [PubMed] [Google Scholar]
- 12.Markov NP, Percival TJ, Morrison JJ, et al. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery 2013; 153: 848–856. [DOI] [PubMed] [Google Scholar]
- 13.NICE. Judging the cost effectiveness of public health activities. Local government briefing LGB10, https://www.nice.org.uk/advice/lgb10/chapter/judging-the-cost-effectiveness-of-public-health-activities (2013, accessed 1 March 2017).
- 14.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull 2010; 96: 5–21. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Economic aspects of drug use (pharmacoeconomy). In: Introduction to Drug Utilization research. Oslo: World Health Organization, 2003, 26–28, http://apps.who.int/medicinedocs/pdf/s4876e/s4876e.pdf (accessed 1 March 2017).
- 16.NHS England. NHS standard contract for major trauma service (all ages), https://www.england.nhs.uk/wp-content/uploads/2014/04/d15-major-trauma-0414.pdf (2013, accessed 1 March 2017).
- 17.Rhee PM, Acosta J, Bridgeman A, et al. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000; 190: 288–298. [DOI] [PubMed] [Google Scholar]
- 18.Brown TB, Romanello M, Kilgore M. Cost-utility analysis of emergency department thoracotomy for trauma victims. J Trauma Acute Care Surg 2007; 62: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 19.Morrison JJ, Galgon RE, Jansen JO. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Acute Care Surg 2016; 80: 324–334. [DOI] [PubMed] [Google Scholar]
- 20.DuBose JJ, et al. The AAST prospective Aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg 2016; 81: 409–419. [DOI] [PubMed] [Google Scholar]
- 21.Abe T, Uchida M, Nagata I, et al. Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: a nationwide cohort study in Japan. Crit Care 2016; 20: 400–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz HP, Steinmetz B, Lieberman J, et al. Emergency thoracotomy: survival correlates with physiologic status. J Trauma Acute Care Surg 1992; 32: 780–788. [DOI] [PubMed] [Google Scholar]
- 23.Feliciano DV, Bitondo CG, Cruse PA, et al. Liberal use of emergency center thoracotomy. Am J Surg 1986; 152: 654–659. [DOI] [PubMed] [Google Scholar]
- 24.Branney S, Moore E, Feldhaus K, et al. Critical analysis of two decades of experience with postinjury emergency department thoracotomy in a regional trauma center. J Trauma Acute Care Surg 1998; 45: 87–94. [DOI] [PubMed] [Google Scholar]
- 25.Flynn T, Ward R and Miller P. Emergency room thoracotomy. Ann Emerg Med 1982; 11: 11413–11416. [DOI] [PubMed]
- 26.Campbell HE, Stokes EA, Bargo DN, et al. Quantifying the healthcare costs of treating severely bleeding major trauma patients: a national study for England. Crit Care 2015; 19: 276–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millikan JS, Moore EE. Outcome of resuscitative thoracotomy and descending aortic occlusion performed in the operating room. J Trauma Acute Care Surg 1984; 24: 387–392. [DOI] [PubMed] [Google Scholar]
- 28.Van Waes OJF, Van Riet PA, Van Lieshout EMM, et al. Immediate thoracotomy for penetrating injuries: ten years’ experience at a Dutch level I trauma center. Eur J Trauma Emerg Surg 2012; 38: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holbrook TL, Anderson JP, Sieber WJ, et al. Outcome after major trauma: 12-month and 18-month follow-up results from the Trauma recovery project. J Trauma Acute Care Surg 1999; 46: 765–773. [DOI] [PubMed] [Google Scholar]
- 30.NHS England. 2016/2017 National Prices and National Tariff Workbook, https://www.gov.uk/government/publications/nhs-national-tariff-payment-system-201617 (2017, accessed 1 March 2017).
- 31.NICE. Costing statement: Blood transfusion implementing the NICE guideline on blood transfusion (NG24), https://www.nice.org.uk/guidance/ng24/resources/costing-statement-2177158141 (2015, accessed 1 March 2017).
- 32.SP Services. REBOA Kit, https://www.spservices.co.uk/item/Brand_REBOAKit_1_0_5519_1.html (2017, accessed 5 March 2017).
- 33.Luengo-Fernandez R, Silver LE, Gutnikov SA, et al. Hospitalization resource use and costs before and after TIA and stroke: results from a population-based cohort study (OXVASC). Value Health 2013; 16: 280–287. [DOI] [PubMed] [Google Scholar]
- 34.OFN Statistics. Inflation and price indices, https://www.ons.gov.uk/economy/inflationandpriceindices (2017, accessed 5 March 2017).
- 35.Lefering R, Zart M, Neugebauer EAM. Retrospective evaluation of the simplified Therapeutic Intervention Scoring System (TISS-28) in a surgical intensive care unit. Intensive Care Med 2000; 26: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 36.Thisismoney.co.uk. Historic inflation calculator: how the value of money has changed since 1900, http://www.thisismoney.co.uk/money/bills/article-1633409/Historic-inflation-calculator-value-money-changed-1900.html (2017, accessed 1 March 2017).
- 37.Ringburg AN, Polinder S, van Ierland MCP, et al. Prevalence and prognostic factors of disability after major trauma. J Trauma Acute Care Surg 2011; 70: 916–922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.