Abstract
Testing negative for human papillomavirus (HPV) predicts long-term reassurance against invasive cervical cancer (ICC). To provide realistic estimates of effectiveness for new screening programs, we studied ICC risk after a 7-year repeated multimethod screening effort. In 1993–1994, 10,049 women aged 18–97 years were enrolled into a population-based cohort study of cervical HPV in Guanacaste, Costa Rica. Women were screened at different intervals according to enrollment results. Each visit (mean 3.2, 90% attendance) included split-sample conventional, automated, and liquid-based cytology, visual inspection, cervicography, and PCR-based HPV testing. Abnormal screening led to colposcopy and excisional treatment as appropriate during the study. Referral to colposcopy for HPV in the absence of other findings was introduced only at the last visit. Population-based Costa Rica Cancer Registry linkage identified cohort women diagnosed with ICC in the 18 years following cohort enrollment. The ICC cumulative risk was 0.4% (n = 38); 18 were diagnosed with ICC after study participation. Of these, 9 were missed at the screening step (negative screening or below the referral threshold, refused screening or colposcopy), 5 attended colposcopy but were not diagnosed as CIN2+, and 4 were treated for CIN2/3 but progressed to ICC nonetheless. Decreasing age-standardized ICC rates for the 1993–2011 period were observed in Guanacaste; cohort women showed additional 31% ICC incidence reduction with apparent downstaging of cancers that occurred. ICC risk following negative HPV testing in the optimal age range 30–50 years was extremely low. Real-life screening effectiveness following introduction is lower than the potential near-complete efficacy predicted by HPV natural history.
Keywords: cervical cancer screening, cervical cancer risk 18-years after enrollment into the Guanacaste cohort, screening frequency
Introduction
Cervical cancer is caused almost exclusively by persistent human papillomavirus (HPV) infection and is theoretically highly preventable through primary and secondary prevention. Accordingly, many countries have some cervical cancer prevention program.1–4 Nonetheless, invasive cervical cancer (ICC) still remains a serious public health problem; with an estimated 527,600 new cases and 265,700 deaths in 2012, it is the second most commonly diagnosed cancer and third leading cause of death among women in developing countries.5 Some projections estimate that an aging world population could lead to increased numbers of cases in the years ahead, unless prevention efforts are increased.6
The primary prevention goal is vaccination to avoid carcinogenic HPV infections that could lead to cervical precancer (more stringently defined as CIN3, less stringently as CIN2/ CIN3) and eventually to ICC. However, existing vaccines against carcinogenic HPV types, while highly efficacious at preventing new infections, do not treat infections detectable at the time of vaccination.7–9 Consequently, vaccination programs tend to target preadolescent girls, leaving several generations of older girls and women depending on secondary prevention through screening to prevent ICC. In less affluent countries, the introduction of HPV vaccination is still pending or has only recently started mainly through the Global Alliance for Vaccine Immunization10 (GAVI Alliance, GAVI homepage). Thus, secondary prevention of ICC through screening will likely be needed for decades to come.
Properly organized cytology-based screening programs have been in place for forty or more years in some regions achieving reductions up to 65% in ICC incidence.3 The goal is to diagnose cervical precancers and treatable early cancers, while avoiding overtreatment of the common HPV infections that would clear without treatment. However, in most low and some middle income countries, cytology-based programs have failed to make a major impact, due to the lack of infrastructure and funding constraints imposed by competing health needs.11–14
HPV DNA detection tests have proven to be considerably more sensitive than cytology screening for detecting cervical precancer.15 Compared with a negative cytology result, a negative HPV DNA test provides stronger and longer reassurance against developing cancer.16 Based on the natural history and average peak ages of HPV, precancers, and cancers, it has been proposed that a negative test, obtained at ages 30–50 years after the peak of HPV acquisition in late adolescence/early adulthood, and before the average age of cancer diagnosis, might predict very low risk of ICC.17,18
Specifically, in recent years, HPV-based screening programs have started in countries with only limited or moderate resources.19 It has been proposed,18 following the results of simulation models20,21 for countries or regions without established cervical cancer screening programs, that one or two rounds of screening using HPV testing among women 30–50, combined with treatment of screen-detected high-risk infections (HR-HPV) and related precursor lesions, could profoundly reduce population lifetime cervical cancer risk. The present study provides rare real-life population-based data regarding the long-term impact on ICC rates after the introduction into a previously poorly screened, high risk region of state-of-the-art-cervical screening, namely repeated screening using multiple methods which were considered either cutting edge at the time (e.g., liquid-based cytology) or experimental methods (e.g., HCT later supplanted by HC2® with a lower positivity threshold and more HPV types targetted).
Methods
We linked the Guanacaste Natural History Study Cohort, a 10,049 women cohort, to the Costa Rica population-based Cancer Registry data up to18 years after initiation of the project, to determine the impact on cervical cancer risk achieved with the multimodal intensive screening with close follow-up and excisional treatment provided during the study years.
Costa Rica is a small upper middle-income country and Guanacaste is a rural province located in the Northwest of the country. At the time that the Guanacaste Cohort was launched, cervical cancer incidence rates among Guanacaste women had been among the highest in the country, over 20 cases annually per 100,000 women, since registration began. The 5-year moving average age-standardized cervical cancer incidence rates of Costa Rica and Guanacaste from 1981 to 2012 are shown in Figure 1.
Figure 1.
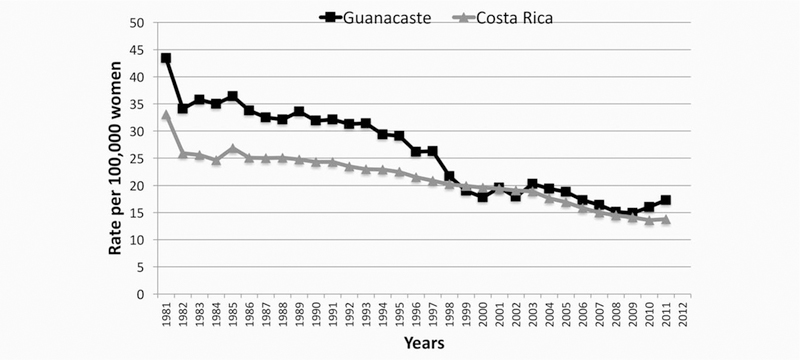
Cervical cancer standardized incidence rate, 5-year moving average, Guanacaste and Costa Rica 1981–2011.
Between June 1993 and December 1994, we began the population-based cohort study, aimed at understanding the natural history of HPV infection and cervical neoplasia, and the evaluation of new screening and management methods. A total of 10,049 women from randomly selected areas of Guanacaste who were 18 years or older at the time were recruited with a participation rate of 93.6%. The cohort represents approximately one-sixth of the adult women in the province. All women signed an informed consent at enrollment and the study protocol was reviewed annually by institutional review boards in Costa Rica and at National Cancer Institute in the United States. Details regarding this population-based cohort design, methods and follow-up have been described elsewhere.22–24
We mention some details of the project to demonstrate the intensive effort undertaken to bring state-of-the-art screening to Guanacaste. At enrollment women were screened with multiple cytology tests using a split sample (liquid-based and conventional, the latter also read using a now-defunct computer-assisted method called PapNet); two visual methods after application of acetic acid (direct visual examination by a nurse and an expert colposcopist reading of a magnified image of the cervix called Cervicography); and detection of HPV DNA with an early test (HCT) (Qiagen, Gaithersburg, MD) that targeted 11 carcinogenic types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, and 58) at a threshold of 10 pg/mL of DNA from exfoliated cervical cells preserved in specimen transport medium (STM; Qiagen). Previous to the study start and for the study duration, extensive training was conducted for all aspects of the study including conventional and liquid-based cytology methods; all cytologic results were reported using the Bethesda system. The study gynecologist was trained in colposcopy and LEEP techniques in the US. There was ongoing quality-control measurement of all study components. We exported both cytology and visual images for expert rereview in the US, and conducted PCR-based HPV retesting in an expert US laboratory (RDB) that had analytic sensitivity at least as good as commercial, FDA-approved assays still on the US market (like HC2; Qiagen).
At enrollment, an abnormal cytology or visual method (i.e., ASC-US or worse cytology reading, equivocal or suspicious of CIN2 or worse cervicographic image, suspicious for cancer by naked eye evaluation) led to referral of women to colposcopy and biopsy of the worst appearing lesion(s). During follow-up visits, women were sent for colposcopic evaluation if their test results showed persistent LSIL or ASC-US cytology, any HSIL cytology result, suspicion of cancer by naked eye examination, equivocal reading or suspicion of CIN2 or worse reading by cervigram). Women with prevalent CIN2 or worse histopathologic diagnoses were referred for treatment as appropriate and censored from the study (n = 142). Additionally women who had a hysterectomy previous to enrollment (n = 659) and those with an enrollment cytologic result of high-grade squamous intraepithelial lesions (HSIL) or other, even less common high grade screening result that did not lead to histological confirmation of CIN2+ (n = 151) were referred for continued care outside the study and censored from further follow-up within the study. There were three women who were excluded from further follow-up by mistake (see Fig. 2).
Figure 2.
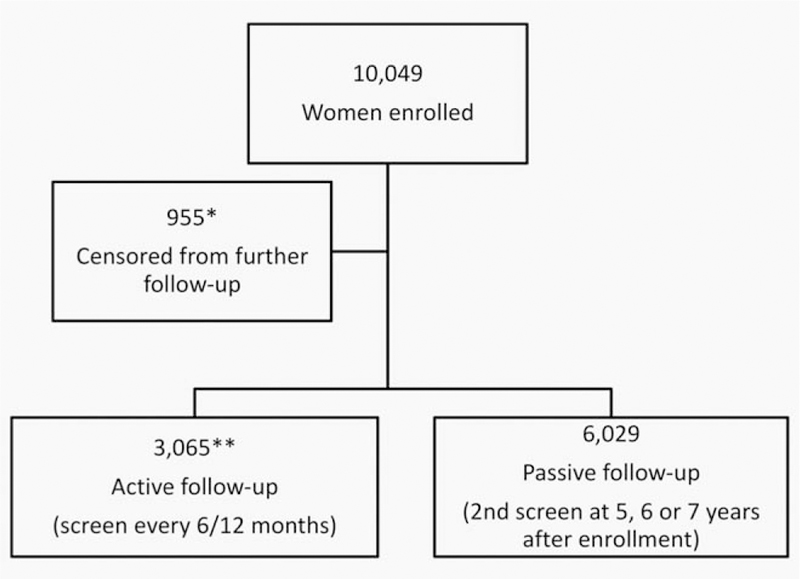
CONSORT diagram. *659: previously histerectomized; 142: histologic CIN 2 or worse diagnosis at enrollment; 151: highgrade screening result at enrollment without histologic confirmation; and 3: excluded by mistake. **410: virginal women under 27 years of age at enrollment; 2,115: minor abnormalities at enrollment, and 540: random control group.
Cohort follow-up was continued for 7 years, and women were screened at each visit as at the enrollment visit, with the exception of the semiautomated reading of the conventional cytology that was used only for the first year of the study. HPV testing using HCT was stopped after enrollment, and an MY09/11 L1 degenerate primer PCR system with AmpliTaq Gold polymerase (TaqGold; Perkin-Elmer-Cetus, Norwalk, CT) was used to test all specimens (but not used initially for clinical management). Dot-blot hybridization was used for HPV typing: probes were specific for types 2, 6, 11, 13, 16, 18, 26, 31–35, 39, 40, 42–45, 51–59, 61, 62, 64, 66–74, 81–85, and 89.19,25 To confirm the sensitivity of the main assay, approximately 2,000 initially negative specimens, including those with or without cytological abnormalities, were retested with additional PCR primers (i.e., GP5+ and FAP). Extremely few additional positive results arose from this retesting.26
The screening intervals varied for women according to an algorithm that classified them according to their crudely estimated risk of CIN2+ development based on their enrollment test results. Therefore, a group of 3,065 women called the “active sub-cohort” was screened every 6–12 months (Fig. 2). It included 2,115 women who at enrollment tested positive for HPV (HCT) or had minor abnormalities on any of the cytology or visual tests, or had a history of five or more sexual partners in their lifetime. We also screened actively 410 women who were 18–27 years old virgins at enrollment, and a random sample of 540 women who were sexually active and had negative enrollment screening test results as controls. During follow-up, women from the active cohort were shifted to a 6-month screening interval if they presented with an LSIL cytologic result or low-grade equivalent cervicographic finding. The “passive sub-cohort” consisted of 6,029 women who had negative enrollment tests and who were screened again at 5, 6, or 7 years after enrollment. At that time, if they presented with abnormal cytology results, they were called back for screening every six months until the completion of the study.
During follow-up, active and passive sub-cohort women found at screening to have high-grade cytology or equivalent cervigram assessment were referred for colposcopic evaluation and treatment as needed and censored from further follow-up in the study. Treatment with loop electrosurgical excision procedure (LEEP) was introduced shortly before the end of enrollment, after training in the US of the Costa Rican experienced gynecologist, replacing cold-knife cone as the preferred treatment procedure for precancerous lesions.
At the end of the study, women were also sent for a colposcopic evaluation if they showed evidence of persistent infection with a carcinogenic HPV type during the last 2 years of the study or were HPV 16 or HPV 18 positive at their last screening visit. For this final HPV infection evaluation, each woman’s complete history of HPV infections according to the MY09/MY11 PCR-based test results was reviewed.
We examined the long-term incidence of ICC using the Costa Rica National Tumor Registry, which was created by Executive Decree in 1976 with compulsory notification of cancer cases to the registry. In 1981, the registry reached countrywide coverage and since then it has maintained good completeness and validity indicators. Quality assurance processes like verification of registration completeness by reviewing all death certificates with a diagnosis of cancer (death clearance process), case identification verification and several edit checks for data completeness and validity were incorporated with time. The Costa Rica registry is one of few population-based cancer registries with nation-wide coverage. Its data have met the quality criteria needed for inclusion of the data in volumes V–X of the scientific publication Cancer Incidence in Five Continents published every 5 years by the International Agency for Research on Cancer and the International Association of Cancer Registries.27,28
The Cancer Registry dataset was complete up to 2011 at the time of linkage with the Guanacaste cohort dataset for a maximum of 18 years of cancer registration since cohort recruitment. All 10,049 cohort women were considered for this linkage. A comparison algorithm was developed using Visual Basic programming language (version 11.0, 2012) that provided an overall percent agreement between any two records based on similarities using the woman’s national personal identification number or cedula, date of birth and full name. For a better name comparison, three variables were generated after indexing the woman’s full name with the phonetic algorithms Soundex, Metaphone and DoubleMetaphone. Minor modifications were done to these algorithms to improve their performance addressing Spanish special characters. All invasive cancers (behavior code 3) with a location of “C53” (uterine cervix) were considered, regardless of histology type. A pair of records with a percent agreement of 90% or above was considered a match, while those in the 80–89% agreement range were hand reviewed to decide if there was a match (16 were determined as matches out of 23). No matches were found among pairs with an agreement of 70–79% (n = 20), therefore no further manual reviews were done.
We reasoned that the primary purpose of cervical screening is to detect and treat precursor lesions; therefore, as a measure of programmatic population benefit, we calculated the cumulative yield of CIN2/3 cases diagnosed during the cohort study. All these cases were diagnosed by a panel of Costa Rican and US experts.24 To indirectly determine the impact of the study effort regarding ICC risk in the 18 years after enrollment into the cohort, we computed the observed over expected ratio or standardized incidence ratio.29 The expected number of ICC cases among the cohort women was estimated using the 1993–2011 age-specific cervical cancer incidence rates provided by the cancer registry for the Guanacaste province.
We counted cancer outcomes detected during the 18 years subsequent to enrollment for all cohort women and the cancer cumulative proportion was stratified by time at diagnosis (i.e., during the study or after the study completion) for all age groups combined. The study charts and Cancer Registry documents (hospital and pathology laboratory reports) were reviewed for women with a cervical cancer diagnosed after study completion to determine what possible screening program step (i.e., screening, colposcopy, treatment, and adherence to follow-up) likely failed to prevent cervical cancer for that particular woman.
Results
Demographic characteristics at enrollment of the Guanacaste cohort women have been described elsewhere.22–24 In brief, the median age at enrollment was 38 ranging from 18 to 97 years old; the median age of first sexual intercourse was 18 years with 26.6% having started at age 17 or 18 years; 5.8% (95% CI 5.4–6.3%) reported not having had sexual intercourse and 69.2% reported 1 or 2 sexual partners in their lifetime. Women attended a mean of 3.2 screening visits overall during the study, with those in active follow up attending a median of 7 screening visits and those in the passive follow up group attending a median of 2. Overall 12.9% of sexually active women were positive for 1 of 13 HR HPV types by MY09/MY11 PCR at enrollment.
Eighteen cases of ICC were diagnosed in Guanacaste cohort women (out of 38 total in the cohort) after participation in the study was completed. The overall 18-year cumulative cervical cancer risk was 0.38% (95% CI 0.27–0.52%), 5 and 10 years after enrollment into the cohort the cumulative risks for cancer were 0.18% (95% CI 0.11–0.28%) and 0.32% (95% CI 0.22–0.45%), respectively (Fig. 3). Of the 20 cases diagnosed during the study, 11 women were found to have prevalent cancer at enrollment and 9 more were diagnosed with ICC during the study follow-up phase. During the study, 266 women had a CIN2/3 histologic diagnosis for a cumulative yield during the study of 2.6% (266/10,049).
Figure 3.
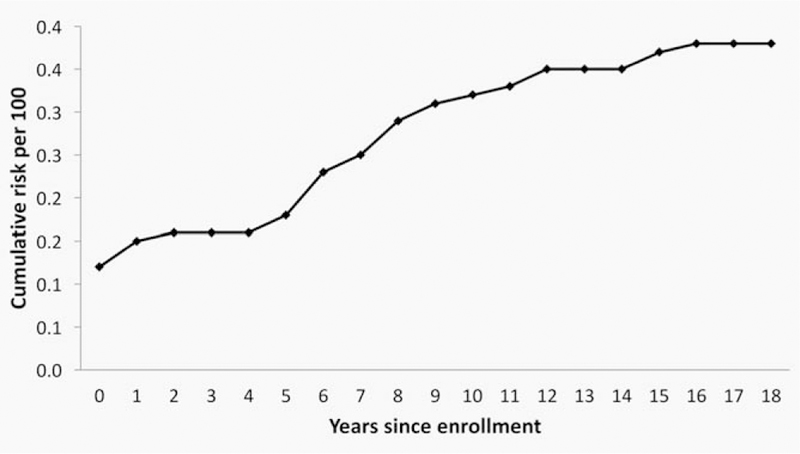
18-Year cumulative cervical cancer risk, Guanacaste cohort (×100).
To compare with the 38 cases of ICC observed between 1993 and 2011 among cohort women, the expected number of ICC cases was estimated to be 55 using the indirect standardization method and the Guanacaste province age-specific rates for the years 1993–2011, yielding an observed over expected ratio of 0.69. Therefore, in addition to the decreasing trend observed for all women in the Guanacaste province during the study period, the ICC risk was reduced by 31% for women who participated in the study when compared to the risk observed for all women in the Guanacaste province.
We did not have stage data for the ICC cases diagnosed in Guanacaste outside of the cohort study. However, downstaging is quite likely, as the Guanacaste cohort women diagnosed with ICC during the study (n = 20) had a median age at diagnosis of 41 years while 47 years was the median age for all women from the Guanacaste province diagnosed with ICC (n = 490) during the 1993–2011 period. Moreover, among the 38 cohort women diagnosed with ICC, those diagnosed after study completion were significantly older at the time of enrollment and at the time of diagnosis (56% were 70 years or older at diagnosis) than women diagnosed during the study (60% were younger than 50 years of age) (Kruskal–Wallis H test p < 0.01 with ties) (Fig. 4). Women diagnosed during the study who died lived 13 years on average after diagnosis with an average age of 61 years at death, while those diagnosed after the study ended died 4.2 years after diagnosis with an average age of 77 years.
Figure 4.
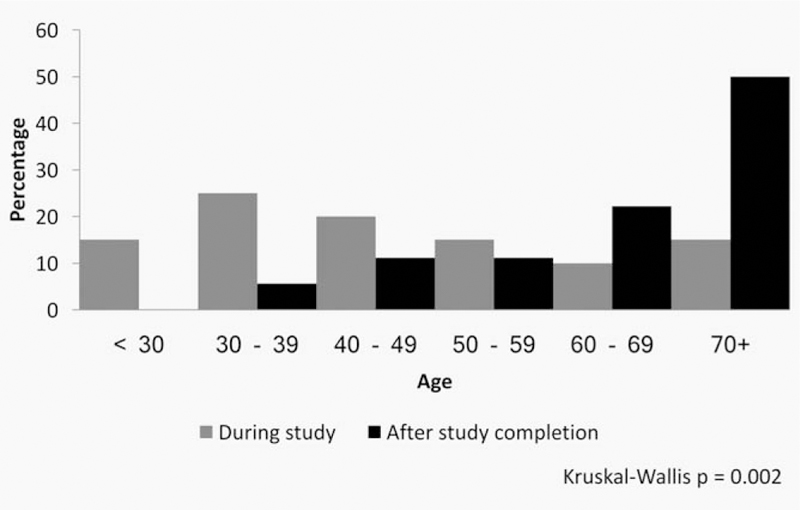
Age at cancer diagnosis by time of diagnosis.
We carefully reviewed the documentation available to determine what step of the study screening program failed to produce a cancer diagnosis for the 18 women diagnosed after completing their study follow up, that is, screening, colposcopy or treatment of precancerous lesions. Of the 18 cases, 9 were missed at the screening step, 5 were determined as screen positive and were referred to colposcopy but not diagnosed as CIN2 or worse, and 4 women were determined to be screen positive, attended the colposcopy clinic, were treated as having CIN2/3 but progressed nonetheless (Fig. 5).
Figure 5.
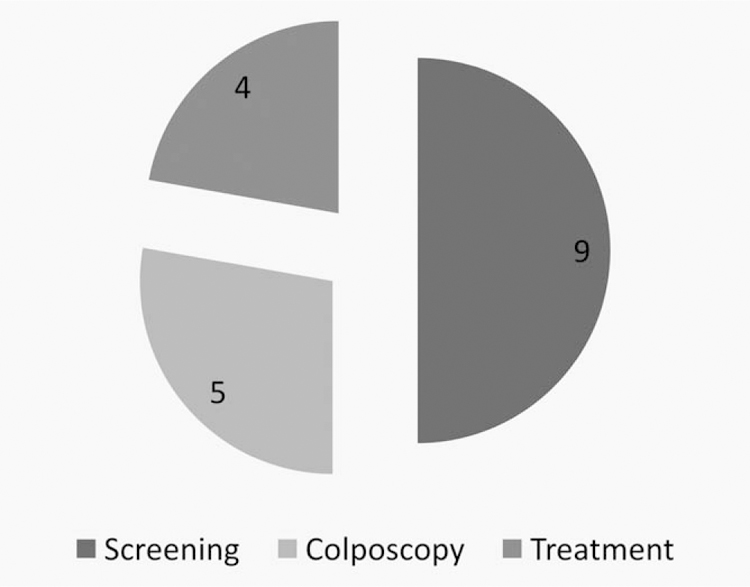
Cancer cases diagnosed after study end by program failure step.
Of note, the project included adult women of all ages. If hypothetically restricted to an ideal screening age range of 30–50 years old during the course of the study, the cumulative incidence of ICC was 0.25% (15/6105) and only 4 of these women were diagnosed after the end of the study participation. Two were HPV negative.
All four women who were treated for precancerous lesions detected during the study and had a cervical cancer reported to the cancer registry at a later time were initially treated within the first year of the LEEP technique introduction into the project (data not shown). Older age and having passed menopause at time of enrollment screening were the main characteristics of the five women with subsequent cancer classified as colposcopy clinic failures (screened positive, were referred to colposcopy but were not diagnosed as CIN2/3).
For nine women, we judged that the screening step failed (see Table 1). Six out of these women tested completely negative during the study including the PCR test (cases 1, 2, 3, 4, 5, and 6). Of note, women listed as cases 1, 2, 3, and 5 were around or past menopausal age at the time of enrollment.
Table 1.
Screening failure cases
| Date |
Cytology |
HPV test |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age at enrollment |
Cancer diagnosis |
Study screening visit |
Conventional | Conventional automatized reading |
Liquid-based | Cervigram | Visual inspection Naked-eye |
HCT | MY09/11 PCR test |
| 1 | 81 | August 2001 | July 93 | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| August 94 | Reactive changes | Negative | Negative | Probably normal | Negative | ND | Negative | |||
| 2 | 48 | November 99 | July 93 | Reactive changes | Negative | Negative | Negative | Negative | Negative | Negative |
| October 99 | Reactive changes | ND | Negative | Negative | Negative | ND | Negative | |||
| 3 | 52 | April 2009 | September 93 | Reactive changes | Negative | Negative | Negative | Negative | Negative | Negative |
| September 98 | Reactive changes | ND | Negative | Negative | Negative | ND | Negative | |||
| 4 | 68 | October 2002 | December 93 | Negative | Negative | Reactive changes | Negative | Negative | Negative | Negative |
| July 01 | Reactive changes | ND | Negative | Negative | Negative | ND | Negative | |||
| 5 | 35 | July 1995 | October 93 | Negative | Negative | Reactive changes | Negative | Negative | Negative | Negative |
| 6 | 78 | October 2004 | October 93 | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 7 | 75 | October 2009 | April 94 | ND | ND | ND | Atypical | Negative | ND | ND |
| 8 | 45 | July 2000 | January 94 | Reactive changes | NA | HSIL | ND | Negative | Negative | HPV 31, 70 |
| 9 | 21 | April 2010 | October 93 | Inadequate | negative | ASCUS | Atypical | Negative | Negative | Negative |
| May 95 | Reactive changes | negative | ASCUS | Negative | Negative | ND | HPV16, 18, 31, 51, 52, 53, 58, 59, 66, 81 | |||
| October 96 | Reactive changes | ND | ASCUS | Negative | Negative | ND | Negative | |||
| November 97 | Reactive changes | ND | LSIL | LSIL | Negative | ND | HPV 59 | |||
| July 98 | Reactive changes | ND | Negative | LSIL | Negative | ND | Negative | |||
| May 99 | ASCUS | ND | Negative | Negative | Negative | ND | Negative | |||
| February 2000 | Reactive changes | ND | Negative | LSIL | Negative | ND | HPV 59 | |||
| April 2000 | Reactive changes | ND | Negative | Negative | Negative | ND | Negative | |||
| October 2000 | Reactive changes | ND | Negative | LSIL | Negative | ND | HPV 59 | |||
Somewhat arbitrarily, we grouped these three cases as a screening step failure. Case 7, refused pelvic sample collection at enrollment and was diagnosed with ICC several years later. Case 8 was referred to colposcopy at enrollment but refused to attend the colposcopy clinic and had an ICC diagnosed 6 years later. Case 9 was 21 years old at enrollment, attended multiple screening visits and all her screening results were below the colposcopy referral threshold; therefore, she was never referred for colposcopic evaluation. Ten years after study completion, she was diagnosed with a microinvasive cervical cancer.
Discussion
We observed an additional 31% ICC risk reduction among cohort women compared to all women living in Guanacaste who showed a decreasing age-standardized ICC rate trend for the 1993–2011 period. Despite the expensive and concerted emphasis on application of repeated screening using multiple methods which were considered either state of the art at the time (e.g., liquid-based cytology) or experimental methods (e.g., HCT later supplanted by HC2® with a lower positivity threshold and more HPV types targetted), a substantial number of cervical cancers that did occur among the cohort women were not diagnosed during the study effort (18 out of 38). Moreover, some cancers detected during the study by screening and treated were still fatal. In terms of the traditional three steps of cervical cancer screening programs, half of the Guanacaste effort failures were missed at the screening step, and referral to colposcopy and treatment did not absolutely prevent cancer. Of note, if restricted to the ideal screening age, 30–50 years old, only 4 of the 5,986 women were diagnosed with ICC after study participation and only 2 were HPV negative. Thus, the a priori hypothesis was supported, in that testing negative for carcinogenic HPV in the premenopausal years past the peak of HPV acquisition did predict very low risk of subsequent ICC. However, the real-life performance of the screening program in preventing ICC was less profound than the ideal efficacy of HPV screening, because of several factors, including: the diagnosis at enrollment of already prevalent cancers; less sensitive HPV detection of precancer/cancer postmenopause due to endocervical positioning of the cervical transformation zone; and failures of colposcopy and treatment among eventual cases of ICC that tested HPV positive.
It is evident, although we cannot precisely estimate the details, that this population-based project achieved substantial “downstaging” among women diagnosed with ICC during study follow-up. The median age among cohort women at ICC diagnosis during the study was 6 years younger than that of all Guanacaste province women diagnosed with ICC between 1993 and 2011. The project likely prevented numerous cancers that otherwise would have occurred among the 246 women with a final CIN2/3 diagnosis during the study who were treated, but we cannot know which ones would have progressed. There is no comparable cohort without screening to directly estimate the ultimate benefit of the Guanacaste project. It is beyond the scope of this manuscript to discern whether and how programmatic elements combined to influence risk of ICC (e.g., age range, HPV DNA detection method, immediate treatment, and screening interval). Nonetheless, overall, the risk reduction observed with the study (31%) is within the range of 25–36% or even the 27–34% range estimated by mathematical models when HPV DNA screen-and-treat interventions are provided three times per lifetime (a woman’s life in a particular population).20,21
A trial in rural India found a hazard of 0.47% (95% CI 0.32–0.69) for 8-year ICC risk after comparing a cluster of women, 30–49 years old, who received a single HPV DNA test with colposcopy and treatment to a similar cluster of women who did not receive any screening during those years.30 Our study results are not directly comparable with those reported by Sankaranarayanan et al. but in spite of the major design differences both studies show an evident ICC risk reduction 8 and 18 years after the studies started, respectively. Nonetheless, to determine a particular intervention as cost-efficient, cost-beneficial or with an acceptable cost-utility margin is society dependent. More real life long-term experiences are needed to confirm predictions.
We confirm the utility of cervical cancer screening programs albeit their imperfect nature. Refusal to collect the cervical sample and refusal to undergo colposcopic evaluation were rare under the study protocol, although are more common in cervical screening programs.11 Referral to colposcopy and even treatment within the study did not absolutely prevent cancer; it is likely that no program would do this. We confirmed that screening and colposcopy are less sensitive among older women, especially after menopause.31 We also found a possible indication of a learning curve after the introduction of the LEEP technique since all treatment failures occurred among women treated early on; as an alternative explanation, these prevalent cases might have been intrinsically more severe than later cases.
The strengths of this study include the large population-based cohort with very high recruitment and retention rates, the use of multiple screening methods during the study years and the long passive follow-up through a good quality cancer registry that provide a unique opportunity to determine the impact in reducing the cervical cancer risk within a population18-years after beginning the intervention. Another strength is the good quality of the identification variables in both datasets that allowed a clear stratification between those real matching pairs and look-alikes.
The main limitation of this analysis is the lack of a national cervical precancerous lesions registration system; therefore, we cannot estimate how many ICC were prevented through the national screening program in the years after the study was completed. Out migration is not that frequent among women in Costa Rica therefore its effect on our estimate would be minimal. In contrast, some overestimation is possible due to lack of pathology confirmation or expert histopathology review of ICC cases diagnosed outside.
We consider it is important to recognize the impact likely to be achieved when public health officials in low-resource settings decide on health program priorities. Even though cervical screening programs are difficult and imperfect by nature, cervical cancer is one of the best targets for secondary prevention by screening, especially if directed to women in the 30–50 years old range. In this consideration, it is worth noting that screening program methods do continue to improve with better tests,32,33 improved colposcopic biopsy strategies,34 and improved treatments not necessarily requiring prior histopathologic confirmation.35 Thus, we remain cervical screening advocates where resources permit.
What’s new?
Vaccines against human papillomavirus (HPV) help prevent infection with carcinogenic HPV types. HPV vaccination programs, however, generally target preadolescent girls, leaving older individuals dependent on secondary prevention via cervical cancer screening. This study, based on 18 years of follow-up in the Guanacaste Natural History Study Cohort, highlights the importance of secondary prevention efforts in high-risk regions. More than 10,000 women in Guanacaste, Costa Rica, underwent repeated cervical screening with three kinds of cytology, visual methods, PCR-based HPV testing, close follow-up, and excisional treatment. Screening was associated with substantial but incomplete reduction in cervical cancer risk with likely downstaging of cancers.
Acknowledgements
The authors thank the women who participated in this study and Dr. Rebeca Ocampo from the Guanacaste Project who helped with some data gathering aspects. This work was supported by the National Institutes of Health (N01-CP-21081, N01-CP-33061, N01-CP-40542, N01-CP-50535, N01-CP-81023, and intramural program CA78527 to RB) and (HHSN261201200474P). The Guanacaste cohort (design and conduct of the study, sample collection, management, and analysis and interpretation of the data) for the enrollment and follow-up phases were supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest to declare.
References
- 1.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, Group WHOIAfRoCMW. A review of human carcinogens—part B: biological agents. Lancet Oncol 2009;10:321–2. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C. American cancer society guideline for the early detection of cervical neoplasia and cancer. J Low Genit Tract Dis 2003;7: 67–86. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarella S, Franceschi S, Engholm G, Lonnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer 2014;111: 965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrero R, Gonzalez P, Markowitz LE. Present status of human papillomavirus vaccine development and implementation. Lancet Oncol 2015;16: e206–16. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Siegel RL, Ward EM, Jemal A, Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016;25: 16–27. [DOI] [PubMed] [Google Scholar]
- 6.Stewart BW, Bray F, Forman D, Ohgaki H, Straif K, Ullrich A, Wild CP. Cancer prevention as part of precision medicine: ‘plenty to be done’. Carcinogenesis 2016;37: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Group HPS. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PAT-RICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374: 301–14. [DOI] [PubMed] [Google Scholar]
- 8.Munoz N, Manalastas R, Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, Bautista O, Bryan J, Taddeo FJ, Esser MT, Vuocolo S, Haupt RM, Barr E, Saah A, Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet 2009;373: 1949–57. [DOI] [PubMed] [Google Scholar]
- 9.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/ 18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 2007;2987: 43–53. [DOI] [PubMed] [Google Scholar]
- 10.Agosti JMG, S. J. Introducing HPV vaccine in developing countries-key challenges and issues. N Engl J Med 2007;356: 1908–10. [DOI] [PubMed] [Google Scholar]
- 11.Almonte M, Murillo R, Sanchez GI, Jeronimo J, Salmeron J, Ferreccio C, Lazcano-Ponce E, Herrero R. New paradigms and challenges in cervical cancer prevention and control in Latin America. Salud Publica Mex 2010;52: 544–59. [DOI] [PubMed] [Google Scholar]
- 12.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: screening for cervical cancer in developing countries. Vaccine 2006;24: S3/71–7. [DOI] [PubMed] [Google Scholar]
- 13.Murillo R, Almonte M, Pereira A, Ferrer E, Gamboa OA, Jeronimo J, Lazcano-Ponce E. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine 2008;26: 1L37–48. [DOI] [PubMed] [Google Scholar]
- 14.Denny L, Prendiville W. Cancer of the cervix: early detection and cost-effective solutions. Int J Gynaecol Obstet 2015;131: S28–32. [DOI] [PubMed] [Google Scholar]
- 15.Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R, Peto J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;5: F88–99. [DOI] [PubMed] [Google Scholar]
- 16.Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJ, International HPVswg. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383: 524–32. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007;370: 890–07. [DOI] [PubMed] [Google Scholar]
- 18.Santesso N, Mustafa RA, Schunemann HJ, Arbyn M, Blumenthal PD, Cain J, Chirenje M, Denny L, De Vuyst H, Eckert LO, Forhan SE, Franco EL, Gage JC, Garcia F, Herrero R, Jeronimo J, Lu ER, Luciani S, Quek SC, Sankaranarayanan R, Tsu V, Broutet N, Guideline Support G. World Health Organization Guidelines for treatment of cervical intraepithelial neoplasia 2–3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynaecol Obstet 2016;132: 252–8. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Lorincz A, Schussler JE, Burk RD, Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol 2002;68: 417–23. [DOI] [PubMed] [Google Scholar]
- 20.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, Wright TC, Alliance for Cervical Cancer Prevention Cost Working G. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 2005;353: 2158–68. [DOI] [PubMed] [Google Scholar]
- 21.Campos NG, Kim JJ, Castle PE, Ortendahl JD, O’Shea M, Diaz M, Goldie SJ. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int J Cancer 2012;130: 2672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman ME, Greenberg M, Cardenas F, Gomez V, Helgesen K, Morales J, Hutchinson M, Mango L, Alfaro M, Potischman NW, Wacholder S, Swanson C, Brinton LA. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salud Publica 1997;1: 362–75. [DOI] [PubMed] [Google Scholar]
- 23.Bratti MC, Rodriguez AC, Schiffman M, Hildesheim A, Morales J, Alfaro M, Guillen D, Hutchinson M, Sherman ME, Eklund C, Schussler J, Buckland J, Morera LA, Cardenas F, Barrantes M, Perez E, Cox TJ, Burk RD, Herrero R. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica 2004;15: 75–89. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, Cheung L, Wacholder S, Burk RD. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010;10:2315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, Alfaro M, Sherman ME, Wacholder S, Chen S, Rodriguez AC, Burk RD. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis 2005;191: 1796–07. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008;100: 513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, Pineros M, Steliarova-Foucher E, Swaminathan R, Antoni S, Soerjomataram I, Forman D. Cancer Incidence in Five Continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer 2015;137: 2060–71. [DOI] [PubMed] [Google Scholar]
- 28.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality world-wide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 29.dos Santos Silva I Cancer epidemiology: principles and methods Barcelona, Spain: International Agency for Research on Cancer, 1999. [Google Scholar]
- 30.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A, Chinoy R, Kelkar R, Kane S, Desai S, Keskar VR, Rajeshwarkar R, Panse N, Dinshaw KA. HPV screening for cervical cancer in rural India. N Engl J Med 2009;360: 1385–94. [DOI] [PubMed] [Google Scholar]
- 31.Castle PE, Qiao YL, Zhao FH, Chen W, Valdez M, Zhang X, Kang LN, Bansil P, Paul P, Bai P, Peck R, Li J, Chen F, Jeronimo J. Clinical determinants of a positive visual inspection after treatment with acetic acid for cervical cancer screening. BJOG 2014;121: 739–46. [DOI] [PubMed] [Google Scholar]
- 32.Schiffman M, Boyle S, Raine-Bennett T, Katki HA, Gage JC, Wentzensen N, Kornegay JR, Apple R, Aldrich C, Erlich HA, Tam T, Befano B, Burk RD, Castle PE. The role of human papillomavirus genotyping in cervical cancer screening: a large-scale evaluation of the cobas HPV test. Cancer Epidemiol Biomarkers Prev 2015;24: 1304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffman M, Hyun N, Raine-Bennett TR, Katki H, Fetterman B, Gage JC, Cheung LC, Befano B, Poitras N, Lorey T, Castle PE, Wentzensen N. A cohort study of cervical screening using partial HPV typing and cytology triage. Int J Cancer 2016;139: 2606–15. [DOI] [PubMed] [Google Scholar]
- 34.Wentzensen N, Walker JL, Gold MA, Smith KM, Zuna RE, Mathews C, Dunn ST, Zhang R, Moxley K, Bishop E, Tenney M, Nugent E, Graubard BI, Wacholder S, Schiffman M. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol 2015;33: 83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolman L, Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: a systematic review. BJOG 2014;121: 929–42. [DOI] [PubMed] [Google Scholar]


