Abstract
Here we review present understanding of sources and trends in human exposure to poly- and perfluoroalkyl substances (PFASs) and epidemiologic evidence for impacts on cancer, immune function, metabolic outcomes, and neurodevelopment. More than 4000 PFASs have been manufactured by humans and hundreds have been detected in environmental samples. Direct exposures due to use in products can be quickly phased out by shifts in chemical production but exposures driven by PFAS accumulation in the ocean and marine food chains and contamination of groundwater persist over long timescales. Serum concentrations of legacy PFASs in humans are declining globally but total exposures to newer PFASs and precursor compounds have not been well characterized. Human exposures to legacy PFASs from seafood and drinking water are stable or increasing in many regions, suggesting observed declines reflect phase-outs in legacy PFAS use in consumer products. Many regions globally are continuing to discover PFAS contaminated sites from aqueous film forming foam (AFFF) use, particularly next to airports and military bases. Exposures from food packaging and indoor environments are uncertain due to a rapidly changing chemical landscape where legacy PFASs have been replaced by diverse precursors and custom molecules that are difficult to detect. Multiple studies find significant associations between PFAS exposure and adverse immune outcomes in children. Dyslipidemia is the strongest metabolic outcome associated with PFAS exposure. Evidence for cancer is limited to manufacturing locations with extremely high exposures and insufficient data are available to characterize impacts of PFAS exposures on neurodevelopment. Preliminary evidence suggests significant health effects associated with exposures to emerging PFASs. Lessons learned from legacy PFASs indicate that limited data should not be used as a justification to delay risk mitigation actions for replacement PFASs.
1. Introduction
Poly- and perfluoroalkyl substances (PFASs) are a family of more than 4000 highly fluorinated aliphatic compounds manufactured for diverse applications.1 They have been widely used for their hydrophobic and oleophobic properties in consumer products such as disposable food packaging, cookware, outdoor gear, furniture, and carpet. They are also one of the main components (1–5% w/w)2 of aqueous film forming foams (AFFF) used frequently at airports and military bases for firefighting and training activities.3 AFFF contamination of groundwater is a major source of drinking water contamination and has been identified as a nationally significant challenge in countries such as the United States and Sweden.4, 5 Releases of PFASs to the environment can occur next to chemical manufacturing locations, at industrial sites where PFASs are used, and at various stages of product use and disposal. The carbon-fluorine bond in these compounds is extremely strong and thus many PFASs are not appreciably degraded under environmental conditions.6 This has resulted in their accumulation in the environment since the onset of production in the late 1940s.7
International concern regarding potential health effects associated with PFAS exposure began in the early 2000s when perfluorooctanesulfonate (PFOS) was detected in the blood of polar bears in the Arctic and wildlife in other remote regions.8 Early data on PFOS bioaccumulation in aquatic food webs indicated the propensity for human exposure to these compounds through seafood.9 The U.S. Centers for Disease Control and Prevention (CDC) later reported these compounds are detectable in the blood of virtually all Americans (98%).10–12 Between 2000–2002, the main global manufacturer of PFASs (3M) voluntarily discontinued manufacturing of the parent chemical used to produce PFOS and its precursors.13 The United States (U.S.) introduced a variety of programs to curb use of the most abundant environmental PFASs, including the PFOA Stewardship Program enacted in 2006 to end production of the longest chained compounds by 2015. PFOS was added to the Stockholm Convention’s list of globally restricted Persistent Organic Pollutants (POPs) in 2009.
Human exposures to PFOS and PFOA have been declining in western countries and Japan over the last decade14–16 due to these regulatory interventions while understanding of their adverse effects on human health has been rapidly advancing.17 At the same time, a proliferation of new PFASs have been reported in the environmental literature as industry has rapidly replaced PFOS and PFOA with shorter chain length PFASs and new chemicals that are difficult to detect using standard methods.3 Emerging evidence from animal experiments suggests some of these alternative PFASs can be equally hazardous.18 Environmental health scientists thus face a considerable challenge in understanding the relative importance of diverse exposure pathways to PFASs in different human populations and their potential effects on human health in a rapidly changing chemical landscape.
Here we review current understanding of: 1) the predominant exposure pathways for PFASs for different populations, 2) health impacts associated with exposure, and 3) critical research needs for the future. We focus on four health effects: cancer, immune effects, metabolic effects, and neurodevelopment. We use this review to summarize key knowledge gaps and future research needs.
PFAS nomenclature
All PFASs contain at least one perfluoroalkyl moiety (CnF2n+1-).19 Fully fluorinated aliphatic carbon chains are known as perfluoroalkyl substances while those with incomplete replacement of hydrogen atoms by fluorine are referred to as polyfluoroalkyl substances. Perfluoroalkyl acids (PFAAs) include perfluoroalkyl carboxylic, sulfonic, phosphonic, and phosphinic acids, which are differentiated by their functional groups. Most research has focused on perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) with between four and sixteen (C4-C16) carbons. Long-chain PFASs are defined as PFCAs with seven or more perfluorinated carbons and PFSAs with six or more perfluorinated carbons. The fluorinated carbon chain of these chemicals is both hydrophobic and oleophobic but the head group for many PFASs is easily deprotonated, resulting in high stability in solution. High water solubility of some PFASs has led to their accumulation in groundwater, rivers, and the ocean and contamination of drinking water resources, fish and marine mammals.
PFAA precursors, hereon referred to as “precursors,” are compounds that can biotically, and sometimes abiotically, degrade to PFAAs.6, 20 Volatile precursors can be transported long distances in the atmosphere prior to deposition in regions remote from pollution sources.21, 22 Precursors are often not measured during standard PFAA analysis, which can result in an underestimate of human exposure because these precursors can be metabolized to terminal PFAAs in the human body.23, 24
2. Human exposure pathways
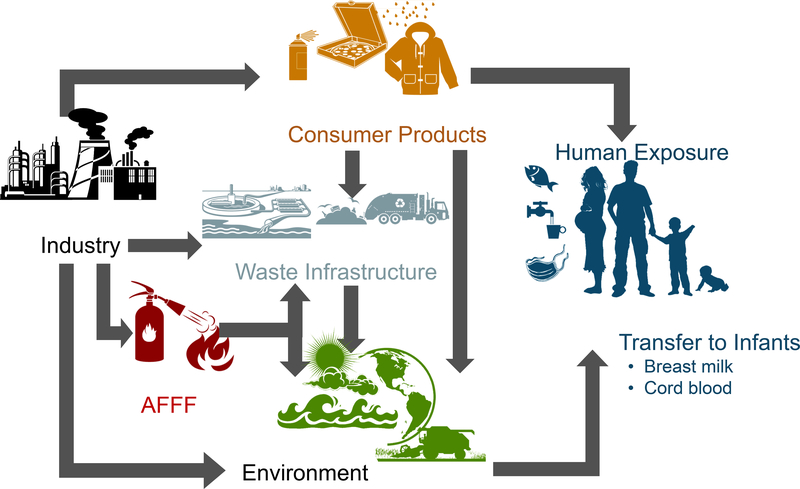
Figure 1 provides an overview of the pathways for human exposure to PFASs. Human exposure to PFASs occurs through ingestion of contaminated drinking water and seafood, inhalation of indoor air, and contact with other contaminated media.25 PFASs are often used for their “non-stick” and surface-tension lowering properties, which makes them useful for repelling oil and water (preventing stains) and modifying surface chemistry. The latter includes applications such as aqueous film-forming foams (AFFF), processing aids for fluoropolymer manufacture, metal plating, and the production of semi-conductors.29, 30 Direct exposures due to use in products can be quickly phased out by shifts in chemical production but exposures driven by PFAS accumulation in the ocean and marine food chains and AFFF contamination of groundwater persist over long timescales.26, 27 Understanding the relative importance of these different exposure pathways is thus critical for interpreting drivers of temporal differences in serum PFAS concentrations measured in biomonitoring studies,26, 28 and for anticipating future exposure risks.
Figure 1.
Overview of PFAS exposure pathways for different human populations outside of occupational settings.
2.1. Consumer products, indoor air and dust
PFASs have been detected in jackets, upholstery, carpets, papers, building materials, food contact materials, impregnation agents, cleansers, polishes, paints, and ski waxes, among many other items commonly found in offices, households, and cars.31–40 PFASs can migrate from fluorochemical-treated food contact papers into food-simulants such as butter, water, vinegar, and water/ethanol mixtures, indicating a direct exposure route to humans.36, 41, 42 Dermal exposure to PFOS and PFOA from products is thought to be low.25 In a study of 41 Norwegian women, Haug et al.23 reported that food is typically the dominant exposure pathway, although the indoor environment (dust, air) could account for up to ~50% of the total PFAS intake.
Precursor compounds in many consumer products can be biotransformed in the human body to PFAAs, leading to additional uncertainty regarding the significance of exposures from this source.23, 24 Inhalation of volatile precursors is known to occur and these precursors have been measured in indoor environments where PFAS containing products are used.43, 44 The phase out of PFOS and PFOA and their precursors has led to the increased production of short chain compounds and structurally similar alternative compounds,3, 6 requiring a more holistic approach to determining human exposure from fluorinated compounds. To address this challenge, Robel et al.32 measured total fluorine concentrations and determined the fraction of fluorine that can migrate from a select group of consumer products and is available for human exposure. The authors reported that typical measurement techniques for PFASs only account for up to 16% of the total fluorine measured using particle-induced gamma ray emission (PIGE).32 Additional research is thus needed to establish the link between the PFAS concentrations in products and the concentrations in dust, air, and food and their overall contributions to human exposure in populations with diverse product use patterns.
2.2. Drinking water
Drinking water has been identified as a substantial source of PFAS exposure for many populations, particularly those living near contaminated sites.4, 5 The United States Environmental Protection Agency (U.S. EPA) proposed a lifetime health advisory level for PFOS+PFOA of 70 ng/L in drinking water in 201645 In 2018, the Agency for Toxic Substances and Disease Registry (ATSDR) in the United States further lowered the Minimum Risk Levels (MRLs) for PFOS and PFOA by approximately an order of magnitude compared to the reference dose (RfD) used by the U.S. EPA to develop the 2016 lifetime advisory.46 Drinking water advisory levels corresponding to the MRLs used by ATSDR would be 11 ng/L for PFOA and 7 ng/L for PFOS. Some lifetime drinking water advisories proposed by other state and international agencies include up to 11 or 12 PFASs (Sweden and Denmark) and range from less than 10 ng/L up to hundreds to thousands of ng/L for different PFASs in Canada.47 Notably, Grandjean and Burdz-Jorgensen48 estimated the lifetime drinking water advisory level should be less than 1 ng/L based on the benchmark dose for immunotoxicity associated with PFAS exposure for children in the Faroe Islands.
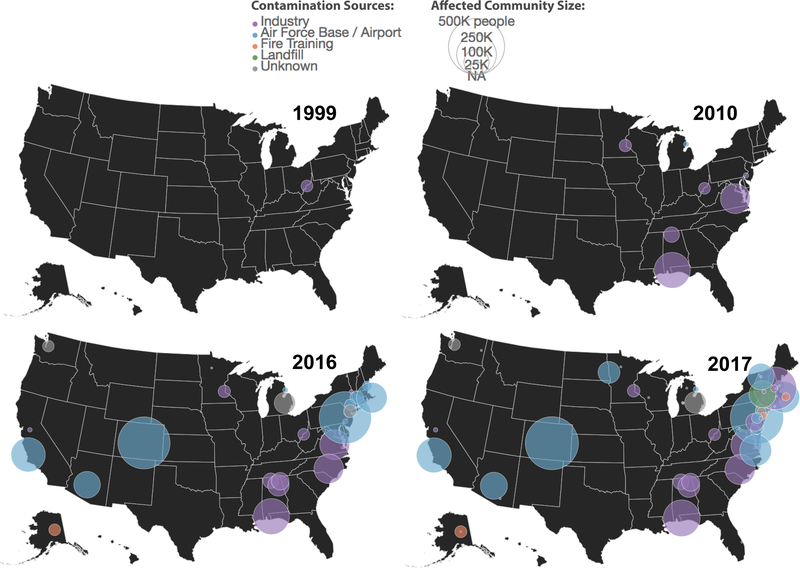
Figure 2 shows the growth in identification of sites contaminated by PFASs across the U.S. between 1999 and 2017. PFAS contamination of drinking water was first reported in the U.S. in public and private drinking water supplies near a fluoropolymer manufacturing facility in Washington, West Virginia in 1999.49 The average PFOA in one public water supply, the Little Hocking water system, was 3,550 ng L−1 (range 1,500 ng L−1 to 7,200 ng L−1) between 2002 and 2005. Drinking water contamination near a military base was first discovered in Michigan in 2010. Many additional cases of high concentrations of PFASs in finished drinking water across the U.S. have since been reported (Figure 2).
Figure 2.
Discovery of sites contaminated by PFASs leading to elevated concentrations in drinking water across the United States. Figure adapted from data compiled by Northeastern University’s Social Science Environmental Health Research Institute (SSEHRI) that was last updated 12/17/17.166 Colors of circles represent different types of pollution source, and magnitudes indicate sizes of local communities.
Most of these cases focus on single communities or small areas with a known point source of contamination. The first statewide study of PFAS occurrence in U.S. drinking water was conducted by New Jersey, where PFOA was detected in 59% of the public water supplies and maximum concentrations reached 190 ng L-1.50 The first nationwide occurrence survey of PFASs in public water supplies was conducted between 2013 and 2015 by the U.S. EPA under the third Unregulated Contaminant Monitoring Rule (UMCR3).51 Hu et al4 noted that drinking water concentrations of PFOS and/or PFOA exceeding the U.S. EPA 2016 health advisory levels were detected in large public water supplies serving approximately six million Americans. Further, there are no data for approximately 100 million Americans who obtain their water from small public water supplies serving less than 10,000 individuals and private wells, representing a critical research need for the future.
Following the shift in PFAS production away from PFOS, PFOA and their precursors, different PFASs may now be accumulating in drinking water and become relevant for human exposure. Newer PFASs, such as GenX, have been detected at high concentration (hundreds of ng L−1) in the Cape Fear River watershed in North Carolina, downstream of a PFAS manufacturing plant.52 The large-scale implications of such findings have yet to be evaluated and knowledge of the international significance of drinking water contamination by PFASs continues to advance at a rapid pace.
2.3. Seafood
Elevated serum concentrations of PFASs have been reported for a number of seafood consuming populations, including Inuit men in Greenland who frequently consume seafood and marine mammals,53 whaling men in the Faroe Islands,54 and commercial fishery employees in China.55 Seafood PFAS concentrations vary considerably with highest concentrations measured next to contaminated sites.56, 57 Environmental concentrations of long-chain compounds appear to be the main driver of variability in tissue concentrations across sites and species. 56, 58, 59 Long-chained compounds and PFSAs bioaccumulate to a greater degree than shorter chain length compounds and PFCAs.60, 61 However, early studies of bioaccumulation potential were based on assays designed for highly lipophilic substances and therefore do not provide comprehensive information on all PFASs presently in use.58
There is considerable variability in the contribution of seafood to overall exposure of humans to PFASs. Cooking has been shown to reduce concentrations of some PFASs such as PFOS.59 Christensen et al.62 found higher concentrations of serum PFASs among high-frequency fish consumers in the U.S. National Health and Nutrition Exam Survey between 2007 and 2014. The European Food Safety Authority (EFSA) recently estimated that “fish and other seafood” account for up to 86% of dietary PFAS exposure in adults.57 Hu et al.63 showed that the presence of elevated serum concentrations of PFASs with C≥9 chain-length in humans is useful for identifying when seafood is a dominant exposure source. Birth cohort data from the Faroe Islands confirmed this observation by showing strong associations between serum concentrations of perfluoroundecanoic acid (PFUnDA, C11) and hair mercury concentrations, which are a strong tracer of seafood consumption.28 Concentrations of legacy PFASs in marine biota have lagged shifts in production away from these compounds, resulting in increased significance of seafood as an exposure source.28
2.4. Biosolids and agriculture
Many PFASs used in products or in industry enter the waste stream and are channeled to wastewater treatment plants. Wastewater treatment plants themselves are thus point sources for PFAS pollution.57 The presence of greater than three treatment plants within a catchment has been associated with increased likelihood of PFAS detection in drinking water.63 Data on the full suite of PFASs present in wastewater plumes are limited and this is expected to change temporally as chemical production and use in products shifts.
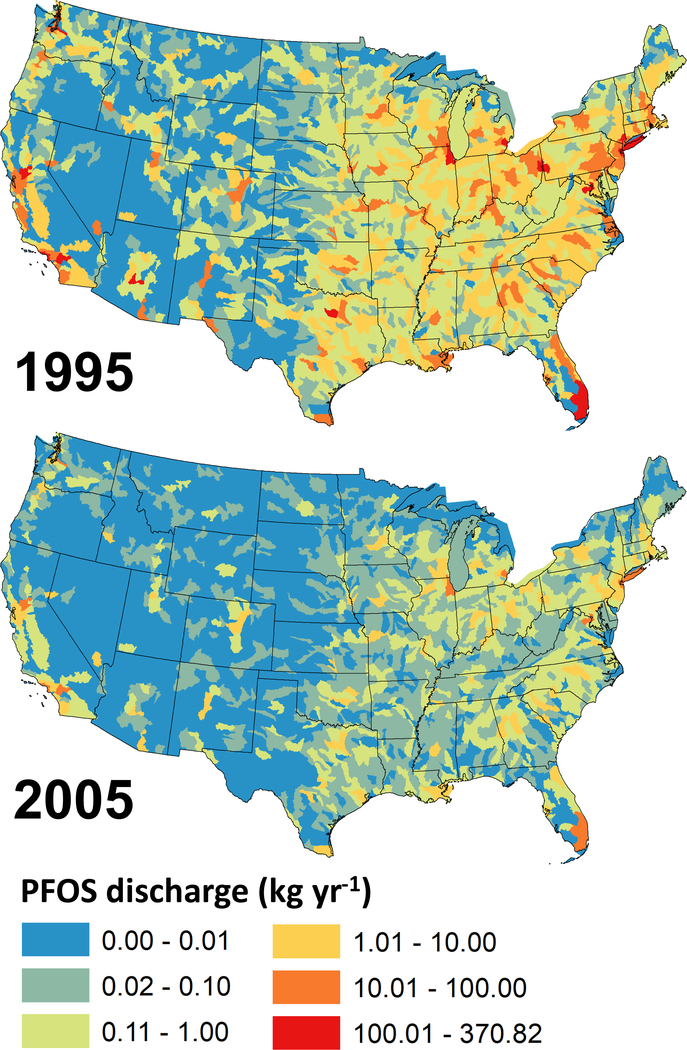
Figure 3 shows temporal changes in catchment level discharges of PFOS from wastewater treatment plants across the U.S. between 1995 and 2005.27 PFOS discharges were modeled based on wastewater flow rates (m3 d−1) from the Clean Watersheds Needs Survey (CWNS) 2008 Report to Congress and an empirical relationship between population served by wastewater treatment plants and PFOS concentrations, as described in Zhang et al.27 Higher levels of PFOS discharges from wastewater treatment plants are apparent in 1995 prior to the phase out between 2000 and 2002.27, 29 Discharges from wastewater enter regional river networks and ultimately result in large inputs to marine ecosystems as the terminal sink. For PFOS, wastewater is thought to account for approximately 85% of releases on a continental scale, while industrial sites can be most significant at the local scale.64, 65
Figure 3.
PFOS discharges from wastewater treatment plants into streams and rivers across the United States in 1995 and 2005. Adapted from data presented in Zhang et al.27
Sewage sludge from wastewater treatment plants is often used for fertilizer in agriculture, presenting another potential vector for human exposure. Several studies have detected PFASs in such biosolids.66–68 The 2001 U.S. EPA National Sewage Sludge Survey suggested that the load of PFASs in U.S. biosolids is 2749 – 3450 kg yr−1 based on the 13 PFASs measured. Of this total U.S. load, an estimated 1375 – 2070 kg yr−1 is applied for agriculture and 467 – 587 kg yr −1 is transported to landfills.68 Several studies have also investigated the uptake of PFASs into crops and earthworms from biosolids application.69–71 In one study, concentration factors for roots relative to soil up to 4.7 and 10.3 were found for PFOS and PFOA, respectively, and all seven plants investigated displayed root concentration factors greater than one.71 Elevated PFAS concentrations in meat and dairy products has also been reported,57, 72 suggesting PFAS uptake from biosolids contaminated agriculture is a source of dietary exposure for farm animals. Additional research on the significance of human exposures to PFASs originating from biosolids and agriculture is needed.
3. Approaches for quantifying exposure sources
Table 1 presents some literature estimates of source contributions to overall PFAS exposures for adults. There is general agreement that dietary intake is the largest source of PFAS exposure rather than inhalation or dermal contact. However, the relative importance of different source categories varies dramatically across demographic groups and populations (Table 1). Next to contaminated sites, drinking water has been reported to account for up to 75% of total PFAS exposure.73, 74 Using a compilation of numerous food samples, dietary survey data and toxicokinetic modeling, EFSA estimated that fish and other seafood dominate the chronic dietary exposure of adults to PFOS (up to 86% of total exposure). For the elderly, EFSA estimated meat and meat products account for up to 52% of PFOS exposure, while eggs and egg products account for up to 42% of infant exposure.57 For PFOA, EFSA suggested the most important sources of chronic exposure were milk and dairy products for toddlers (up to 86% of exposure), drinking water (up to 60% for infants), and fish and other seafood (up to 56% in elderly).
Table 1.
Literature estimates of sources contributions (%) to adult PFAS exposures.
| PFAS | Diet | Dust | Tap water | Food Pkg. | Inhalation | Dermal | Other | Reference |
|---|---|---|---|---|---|---|---|---|
| PFOA | 16 | 11 | 56 | 14 | 2a | Trudel et al.25 | ||
| PFOA | 85 | 6 | 1 | 3b | 4c | Vestergren and Cousins74 | ||
| PFOA | 77 | 8 | 11 | 4 | Haug et al.76 | |||
| PFOA | 66 | 9 | 24 | <1 | <1 | Lorber and Egeghy77 | ||
| PFOA | 41 | 37 | 22d | Tian et al.163 | ||||
| PFOA | 99 | <1 | Shan et al.164 | |||||
| PFOS | 66 | 10 | 7 | 2 | 16d | Gebbink et al.165 | ||
| PFOS | 72 | 6 | 22 | <1 | <1 | Egeghy and Lorber75 | ||
| PFOS | 96 | 1 | 1 | 2 | Haug et al76 | |||
| PFOS | 81 | 15 | 4a | Trudel et al.25 | ||||
| PFOS | 93 | 4 | 3d | Tian et al.163 | ||||
| PFOS | 100 | <1 | Shan et al.164 | |||||
| PFBA | 4 | 96 | Gebbink et al.165 | |||||
| PFHxA | 38 | 4 | 38 | 8 | 12d | Gebbink et al.165 | ||
| PFOA | 47 | 8 | 12 | 6 | 27d | Gebbink et al.165 | ||
| PFDA | 51 | 2 | 4 | 15 | 28d | Gebbink et al.165 | ||
| PFDoDA | 86 | 2 | 2 | 4 | 5d | Gebbink et al.165 |
Carpet
Consumer goods
Precursors
Indirect.
Human exposures to PFASs (blood PFAS concentrations) are typically estimated using data on measured concentrations in exposure media, contact frequency, and toxicokinetic parameters.25, 74–77 The reliability of this approach depends on the accuracy of data needed to convert an external dose to internal concentrations. Many of these parameters for PFASs are poorly understood or hard to measure, resulting in large uncertainties about exposure sources (Table 1). For example, Vestergren and Cousins74 relied on exposure estimates from multiple geographic regions to estimate total PFAS intake from the combination of dietary sources (German data), dust (data from the U.S. and Spain) and inhalation (northwest Europe). Trudel et al.25 tested a series of scenarios for chemical concentrations and contact frequencies across populations in Europe and North America and found plausible ranges in PFAS exposures spanned two orders of magnitude.
Uncertainty in such estimates motivates an alternative solution that uses measured serum concentrations to identify predominant exposure sources. The ratio between two chemical homologues and the correlation among multiple chemical homologues in environmental samples, including human serum, contains information on their origin. This process is referred to as “chemometrics” and has been applied to polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) 78, 79 Applying such techniques to PFASs is complicated by dramatic shifts in production over time and the complex metabolism of PFAS precursors. In prior work, researchers have used PFAS isomer profiles to assess the relative contributions from electrochemical fluorination (ECF) and telomere manufacturing to measured PFOA concentrations in the environment.80, 81 Zhang et al.82 showed that the measured PFAS composition in surface water provides useful information on sources of environmental pollution. Hu et al.63 extended this approach to human biomarkers by comparing human serum samples collected at similar time periods and controlling for physiological differences. Using cohort data from the Faroe Islands and the U.S. National Health and Nutrition Examination Survey (NHANES), the authors showed that elevated C9-C12 PFCAs were associated with predominant exposures through seafood consumption. Further, PFHxS and N-EtFOSAA were linked to exposure from consumer products such as carpet and food packaging.63
Serum samples are routinely collected during epidemiological studies, but environmental samples pertinent to multiple exposure pathways such as drinking water, diet, air and dust samples are not.83 Information on contact frequency is often collected using self-reported questionnaires with known recall bias.84 In addition, there are limited data on chemical half-lives in the human body (ti/2) and distribution volumes (VD) for PFASs other than PFOS, PFOA and PFHxS. This means that traditional exposure modeling is limited to only a few relatively well- characterized individual PFASs and cannot be easily applied to the PFAS mixtures that are more relevant for human exposures.
The results presented in Hu et al.63 are mostly qualitative and cannot quantify the percentage of PFAS exposure from different exposure pathways. This preliminary approach can be enhanced by expanding the list of PFAS analytes. Regular epidemiological studies usually report six legacy PFASs (branched and linear PFOS, PFOA, PFHxS, PFNA, PFDA) but exposure analyses would be enhanced by including additional PFASs that are increasingly relevant to current production patterns. In addition, a total mass balance is needed to provide quantitative assessments of the relative importance of different exposure sources.85 Routine measurements of extractable organic fluorine (EOF) in human sera would thus complement data on individual PFASs and allow such quantitative inferences from the chemometric approach.86, 87
4. Temporal trends in human exposure to PFASs
The presence of organic fluorine in human blood was first detected by Taves88 in the 1960s. Data on specific forms of organic fluorine such as PFOS and PFOA in human sera were not published until 1990.89 Grandjean90 pointed out that there has been a lag of more than two decades between industry information on exposures and health effects of PFASs and academic research and regulatory action.
Declines in serum concentrations of PFASs following the phase out in production of the parent chemical to PFOS and its precursors between 2000–2002 have been reported across diverse populations worldwide and provide a success story for the effectiveness of industrial shifts and regulatory actions. These include children from the Faroe Islands28 and eastern U.S.,91 adult women from the western U.S.92 and Sweden,93 the general Australian population,94 and Norwegian men.95 However, declines in PFOS and PFOA have primarily driven decreasing legacy PFAS concentrations. Concentrations of total PFASs or EOF in human serum that include newer PFASs in production and precursors have not been measured for most populations. One study that examined EOF in human serum in China found the legacy PFASs measured in standard epidemiologic studies only comprised between 30–70% of the total fluorine.96 These results suggest unquantified PFASs may be exhibiting different trends than legacy compounds.
Following the phase outs in use of PFOS and PFOA in many products, C6-based fluorocarbons (including perfluorohexanesulfonic acid: PFHxS and perfluorohexanoic acid: PFHxA ) were used as an initial replacement. 97, 98 Concentrations of PFHxS and PFCAs with 9–14 carbons in human serum have not decreased concomitantly with PFOS, PFOA and their precursors. No change and some increases in exposures to these compounds have been observed across populations. For example, significant increases in PFNA, PFDA and PFUnDA and no change in PFHxS was observed in Swedish and Danish women through 2015.93, 99 Blood concentrations of PFNA, PFDA, PFUnDA and PFDoDA from multiple countries show no significant change.13 Similarly, PFHxS concentrations in the blood of Mexican American NHANES participants showed no significant trend between 1999–2004 and increased from 2005–2008.12, 100
Increasing trends in concentrations of PFHxS and long-chain PFCAs are noteworthy since they significantly contribute to the overall body burden of PFASs and have longer half-lives than both PFOS and PFOA. Additionally, exposures to the C9-C11 PFCAs for some individuals are primarily from seafood consumption.28, 62, 63 C9-C11 PFCAs exhibit different temporal patterns than PFOS and PFOA. They are bioaccumulative and concentrations in some seafood have been increasing, as discussed in Dassuncao et al.28 This suggests that while exposures to PFOS and PFOA have been successfully reduced by product phase-outs for many populations, exposures to C9-C11 PFCAs have not followed the same trends.
5. Health Effects associated with exposure to PFASs
The 3M Company was the major global manufacturer of PFASs in the 1990s and conducted most of the early studies on the health effects of PFAS exposures in animals and humans.29, 101 Many of these studies were not published in the peer-reviewed literature but can be found in the U.S. EPA public docket AR-226, and are reviewed in the section below.
5.1. Early industry studies
Before 1980, 3M conducted multiple studies of acute animal toxicity associated with exposure to legacy PFASs.102 Serum PFAS concentrations measured as organic fluorine in 3M workers were ten times higher than the general population in 1980.103 Shortly after this, 3M carried out a series of subacute and chronic studies in various animal models such as rats, mice, and monkeys.104–106 Results showed N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE) was carcinogenic in rats after a two-year chronic study concluded in 1988. However, the results were first misinterpreted as a null finding and only corrected a decade later.107, 108 In a 90-day rhesus monkey study, all monkeys in all treatment groups died after 20 days and the study had to be aborted.105 In later monkey studies with lower doses, reductions in total cholesterol, increased liver weight, and toxicity on the reticuloendothelial system (immune system) were observed.104
Health surveillance of 3M workers produced inconsistent results, mainly due to small sample sizes and a scenario known in epidemiology literature as the “healthy worker effect”.109 A doctoral thesis that focused on a cohort of 3M workers reported in 1992 that PFOA exposure may significantly alter male reproductive hormones and leukocyte counts.110 Later investigations published by 3M did not find the same associations.111 Differences between these findings may be caused by the exposure assessment methods used: Gilliland110 measured serum total organic fluorine while Olsen111 measured serum PFOA concentrations. This suggests adverse effects observed in Gilliland’s work110 may have been contributed by fluorochemicals other than PFOA.
5.2. Academic studies
Most academic research on PFASs was initiated in the early 2000s after the voluntary phase-out in production of the parent chemical to PFOS and its precursors by 3M, the major global manufacturer at the time. Results from experimental studies in rodents can be challenging to translate directly to human health impacts because of differences in peroxisome proliferation expression, which is one of the main mechanisms of PFASs toxicity.112 The most comprehensive longitudinal evidence for adverse health effects associated with PFAS exposure (C8 Health Project) is from the population living near the West Virginia DuPont Washington Works fluorotelomer plant. Probable links between PFOA exposure and six diseases have been identified: high cholesterol, thyroid disease, pregnancy-induced hypertension, ulcerative colitis, and kidney and testicular cancer.113–116
Children may be more vulnerable to PFAS exposures because they often have higher body burdens than adults and are going through sensitive windows for development. A recent systematic review of the children’s health literature identified positive associations between PFAS exposures and dyslipidemia, immunity, renal function and age at menarche.117 Some health effects such as immunotoxicity can be detected at lower exposure levels than others. For example, Grandjean et al.118 examined the impact of serum PFAS concentrations on serum antibody production in children at ages 5 and 7 years following routine vaccinations for tetanus and diphtheria. A doubling of serum PFOS, PFOA and PFHxS concentrations at age 5 was associated with a 50% decline in antibody concentrations at age 7. If this effect is causal, average serum concentrations in the general population of most countries with biomonitoring data greatly exceed the benchmark doses of 1.3 ng/mL for PFOS and 0.3 ng/mL for PFOA calculated based on immunotoxicity in children.48
5.3. Cancer
Numerous studies have investigated PFAS carcinogenicity, mainly focusing on PFOA and PFOS. PFHxA is the only other PFAS that has been investigated in an animal study and null findings were reported.119 Human studies for PFOS and PFOA include chemical workers, communities with contaminated drinking water, and the general population. A 3.3-fold increase (95% CI, 1.02 to 10.6) in prostate cancer mortality was reported for each month spent in the chemical division with PFOA production was observed among occupationally exposed workers, but the number of cases was small.120 Later data from this occupational cohort did not support an association between occupational exposure and cancer mortality or incidence.121 The strongest evidence for increased cancer risk has been reported by studies among community members whose drinking water was contaminated by PFOA. Barry et al113 and Vieira et al122 showed a positive association between PFOA levels and kidney and testicular cancers among participants in the C8 Health Project. These studies form the foundation of the overall conclusion from the C8 Health Project. Results among studies conducted in general population are inconsistent. Eriksen et al123 was a the first to examine PFOA exposure and cancer in the general population and they did not find an association between plasma PFOA or PFOS concentration and prostate, bladder, pancreatic or liver cancer. The International Agency for Research on Cancer (IARC) classified PFOA as a possibly carcinogenic to humans (Group 2B). No IARC evaluation is available for PFOS.
5.4. Immune effects
Immunotoxicity of PFASs has been demonstrated in multiple animal models, including rodents, birds, reptiles and other mammalian and non-mammalian wildlife. Epidemiological data is relatively sparse but mounting evidence suggests that the immunotoxic effects in laboratory animal models occur at serum concentrations that are comparable to body burden of highly exposed humans and wildlife.124
Table 2 shows findings from a review of 25 epidemiological studies published between 2008–2018. Cohort data were from China, Denmark, the Faroe Islands, Japan, Norway, Taiwan and the U.S. and 14 out of the 25 studies reviewed were longitudinal. Two studies focused on occupational exposures and the remaining 23 were based on environmental exposures. Infants and children were the most studied demographic group for this health endpoint and accounted for 16 out of the 25 studies. Three studies considered data from teenagers in the U.S. NHANES survey. Six studies were based on either residents or workers from the C8 health project near a fluorotelomer plant in West Virginia. One study examined a group of healthy adults who received vaccination. The most widely used exposure assessment method is to measure serum PFAS concentrations, accounting for 22 out of 25 studies. Four studies from the C8 health project used job-exposure matrix or residential history to estimate lifetime cumulative exposures.
Table 2.
Summary of the epidemiologic literature on PFAS exposures and metabolic outcomes.a
| Outcome | # of total studies | # of studies by results | Other PFASs | |||
|---|---|---|---|---|---|---|
| PFOA | PFNA | PFHxS | PFOS | |||
| Lipid profileb | 39 | 21/10/1c | 8/1/2 | 4/4/2 | 20/9/3 | Inconsistent results for PFDA, PFUnDA, PFTeDA |
| Insulin resistance and Diabetes |
18 | 6/9/1 | 3/5/0 | 1/2/1 | 7/4/1 | Mostly null for PFDA, PFUnDA, PFDoDA, N-EtFOSAA, N- MeFOSAA; One positive finding for PFDoDA and insulin resistance |
| Hypertension, vascular disease and stroke | 10 | 3/5/1 | 3/0/1 | 0/3/1 | 1/3/1 | Only one study reported null for PFDA and PFUnDA |
| Thyroid disease |
8 | 4/3/0 | 1/2/0 | 1/2/0 | 1/3/0 | Positive finding for PFDA and PFUnDA in two studies. Null for PFTrDA |
| Cardiovascular disease | 6 | 1/4/1 | 1/0/0 | 0/1/0 | 0/1/0 | No other PFASs have been investigated |
| Uric acid | 5 | 4/0/0 | 0/0/0 | 0/1/0 | 2/2/0 | No other PFASs have been investigated |
| Overweight and obese | 4 | 1/3/0 | 1/1/0 | 1/1/0 | 3/1/0 | Positive finding for PFDA in only one study (Liu et al.135) |
Details of the studies examined are provided in the Supporting Information Table S1.
Lipid profile includes low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol, and triglycerides.
Number of studies with adverse/null/protective results
The health outcomes related to PFAS immunotoxicity include both molecular-level (i.e. antibody concentrations) and organ/system-level (i.e. infection of respiratory system). In general, more consistent results across different studies were reported for molecular-level health endpoints such as vaccine antibody or other immune markers such as immunoglobulin (Table 1).
Five studies examined the association between PFAS exposure and suppression of antibody response to vaccination among children, adolescents or adults. Four out of the five found statistically significant associations between higher PFAS exposure and suppressed immune response. Grandjean et al.118 was the first to link PFAS exposure in children to deficits in immune function. The authors reported a 2-fold increase of major PFASs in child serum was associated with a −49% (95% confidence interval (CI), −67% to −23%) decline in tetanus and diphtheria antibody concentrations. This effect size is larger than later studies and can be attributed to different exposure levels, different vaccine strains, and different times elapsed since vaccination (peak antibodies vs residual antibodies). Other studies have not examined tetanus and diphtheria, but similar associations have been found in PFAS exposure and other childhood vaccinations such as rubella and mumps,125, 126 and adult influenza vaccination such as FluMist127 and anti-H3N2.128
Five out of seven studies that examined associations between PFAS exposure and immune markers found statistically significant evidence of immunosuppression. The strongest evidence has been generated for PFOA and PFOS with few data for other PFASs. One example for other PFASs is from a case-control study in Taiwan129 that reported that among children with asthma, nine out of the ten PFASs evaluated were positively associated with at least two of the three immunological biomarkers (immunoglobulin E (IgE), absolute eosinophil counts (AEC), and eosinophilic cationic protein (ECP)). However, this study did not account for the fact that multiple PFASs serum concentrations are positively correlated and therefore did not distinguish whether all PFASs or a subset of PFASs were associated with immune suppression.
Results with organ/system-level outcomes such as asthma, infection and allergies are more inconsistent. Slightly more than half of the studies on asthma and infection show statistically significant results. Similar to the molecular-level outcomes, stronger evidence has been established for PFOS and PFOA than other more minor PFASs. Buser et al130 found serum levels of PFASs were associated with higher odds of self-reported food allergies among teenagers in NHANES 2007 – 2010. This is the only study out of the six studies reviewed with a statistically significant finding, but the cross-sectional design of this study necessitates further investigation using longitudinal studies. Existing studies have limitations such as outcome measurement error. For example, some studies measure asthma using a self-reported questionnaire but did not validate these data with medical records. Some studies used hospitalization due to infection as an outcome but hospitalization may not be necessary for most infections. In addition, since infection and allergy be caused by food and airborne allergens, it is challenging to identify the contribution of PFAS exposures in a low signal-to-noise setting.
5.5. Metabolic effects
We reviewed 69 epidemiological studies published between 1996–2018 based on human populations in Australia, Canada, China, several European countries, Japan, South Korea, Taiwan, UK and the U.S. We identified 26 out of 69 studies as longitudinal and 59 out of 69 studies were based on environmental exposures. Diverse demographic groups have been studied for this health endpoint, including infants, mother-child pairs, children, teenagers, adults, workers, and special subpopulations such as diabetic patients and obese individuals in randomized clinical trials. Measured serum PFAS concentrations were the most widely used exposure assessment method (65 out of 69 studies). Two occupational studies used job-exposure matrix and work history to estimate lifetime cumulative exposures. Gilliland131 was the earliest study and used total serum fluorine to quantify the exposure. Only one study132 examined the different isomers of PFOA and PFOS (linear vs. branched) using data from NHANES 2013 – 2014.
There is relatively consistent evidence of modest positive associations with lipid profiles such as total cholesterol and triglycerides, although the magnitude of the cholesterol effect is inconsistent across different exposure levels. There is some but much less consistent evidence of a modest positive correlation with metabolic diseases such as diabetes, overweight, obesity and heart diseases (Table 3). The majority of studies are cross-sectional, which have limited causal interpretation.133 A few studies provided stronger evidence than observational studies, such as Diabetes Prevention Program Trial134 and a diet-induced weight-loss trial.135
Table 3.
Summary of the epidemiologic literature on PFAS exposures and immunotoxicity.a
| Outcome | # of total studies | # of significant studies |
# of significant studies by each PFAS |
|---|---|---|---|
| Vaccine antibody | 5 | 4 | Mixture: 1; PFOA: 2; PFNA: 1; PFHxS: 1; PFOS: 2 |
| Immune markers | 7 | 5 | PFHpA: 1; PFOA: 5; PFNA: 2; PFDA: 1; PFTeDA: 1; PFDoA: 1; PFBS: 1; PFHxS: 2; PFOS: 4 |
| Asthma and biomarker of asthma | 9 | 5 | PFHpA: 1; PFOA: 5; PFNA: 3; PFDA: 3; PFDoDA: 1; PFBS: 1; PFHxS: 2; PFOS: 4 |
| Infection and other autoimmune diseases | 13 | 8 | PFOA: 6; PFOS: 4; PFDA: 1; PFDoDA: 1; PFNA: 2; PFUnDA: 1; PFHxS: 2; PFOSA: 1 |
| Allergy | 6 | 1 | PFOA: 1; PFHxS: 1; PFOS: 1 |
Details of the studies examined are provided in the Supporting Information Table S2.
The majority of the studies examined found associations between elevated serum PFASs and detrimental lipid profiles, such as elevated total cholesterol and low-density lipoprotein cholesterol (LDL-C), or reduced high-density lipoprotein cholesterol (HDL-C). PFOS and PFOA exhibit the most consistent finding across studies. The effect size varies across studies, which can be a result of different exposure levels. Increases in serum PFOA and PFOS from the lowest to the highest quintiles among children in C8 health project was associated with 4.6 and 8.5 mg/dL total cholesterol (reference level for children is <170 mg/dL).136 Among NHANES 2003–2004 participants, increases in serum PFOA and PFOS from the lowest to the highest quartiles were associated with 9.8 and 13.4 mg/dL total cholesterol (reference level for adults is <200 mg/dL).137 Maisonet et al.138 reported a non-linear relationship between prenatal PFOA concentrations and total cholesterol at ages 7 and 15 of the child.
Eighteen studies have examined the associations between PFAS exposures and glucose metabolism, insulin resistance and diabetes. Overall the results across different studies are inconclusive. Lin et al.139 was the first to report a positive association between serum PFAS concentrations and glucose homeostasis among adults and adolescents in NHANES. They reported a considerable effective size - doubling serum PFNA concentrations was associated with hyperglycemia odds ratio (OR) of 3.16 (95% CI 1.39 −7.16). Later studies tend to report smaller effect sizes. Exposure during pregnancy may affect the mother and child during gestation and later in life. In a small pregnancy cohort in the U.S., each standard deviation of increase in PFOA was associated with a 1.87-fold increase of gestational diabetes risk (95% CI 1.14–3.02).140 In a larger Spanish cohort, a null result was reported for PFOA, but PFOS, PFHxS and gestational diabetes had positive associations: Odds Ratio (OR) per log10-unit increase=1.99 (95% CI: 1.06, 3.78) and OR=1.65 (95% CI: 0.99, 2.76), respectively.141
Results for hypertension and other vascular diseases including stroke are also inconsistent. Two of the earliest studies examined the relationship between PFAS exposure and hypertension among NHANES and found different results for children and adults. Adjusted OR=2.62 for hypertension comparing 80th vs. 20th percentiles serum PFOA among NHANES adults in the U.S.142, while among children a null finding was reported.143 In some later cohort studies, null results and even protective effects associated with PFAS exposure and hypertension were reported.144, 145 A cross-sectional study on carotid artery intima-media thickness in adolescents reported increased risks with increase in plasma PFOS.146 However, a more recent study on artery stiffness found protective effects of PFOA and PFNA among children and adolescents enrolled in the World Trade Center Health Registry.147
Other metabolic endpoints include thyroid disease (which could also be considered an endpoint for endocrine disruption), cardiovascular diseases, uric acid metabolism, and body weight. Except for uric acid metabolism, most results are inconclusive. An increase in hyperuricemia risks and PFOA exposure was observed in all four studies (two from NHANES and two from C8 Health Project).
In summary, the strongest evidence for a relationship between PFAS exposure and metabolic outcome is in the area of dyslipidemia. Animal studies have found decreases in serum cholesterol levels associated with increased PFAS exposures, which contradicts epidemiological findings. The difference may lie in different levels of expression for nuclear receptors involved in the toxicological pathway, such as peroxisome proliferator-activated receptor (PPAR)-alpha. It may also be related to differences in exposure levels. Dietary factors can influence metabolic outcomes,148 introducing bias into observed relationships if not controlled for properly. Explanations for null findings include healthy worker effects and non-linear relationships, such as a decreasing slopes as exposure increases (log-linear relationships).149
5.6. Neurodevelopmental effects
In vitro studies suggest PFOS can trigger the “opening” of tight junction in brain endothelial cells and increase the permeability of the blood brain barrier.150 There has therefore been some interest in investigating the neurotoxic effects associated with PFAS exposures. In laboratory animals, it has been reported that PFOS, PFOA and PFHxS exposures during the peak time of rapid brain growth in mice resulted in an inability to habituate in unfamiliar environment.151 Liew et al152 reviewed 21 epidemiological studies in 2018 and concluded that evidence is mixed regarding neurodevelopmental effects of PFAS exposures. Health outcomes examined included developmental milestones in infancy, attention-deficit/hyperactivity disorder (ADHD) and behaviors in childhood, and neuropsychological functions such as IQ and other scales or scores. Neurodevelopmental trajectories are highly complicated and there is great heterogeneity in the instruments and methods to evaluate neurodevelopmental endpoints. Additional research is needed to establish a link between neurodevelopmental outcomes and PFAS exposures.
6. Future directions
Challenges associated with quantifying the full-diversity of individual PFASs present in environmental samples and a paucity of toxicity data highlight the need for data and tools to better understand new and emerging fluorinated compounds. EOF provides an estimate of all combustible organofluorine compounds present and provides a proxy measure for unquantified PFASs.87 Yeung and Mabury153 reported that quantifiable PFASs accounted for 52 – 100% of EOF in human plasma samples collected between 1982 and 2009 in two German cities. The amount and proportion of unidentified organofluorine in human plasma increased after 2000 in one city. This study hypothesized that humans are exposed to many new and unidentified organofluorine compounds, which is consistent with the environmental exposure literature.3, 74,154, 155
The toxicity of new and emerging PFASs for ecosystems and humans is poorly understood. This is problematic because in communities with high concentrations of alternative PFASs, the magnitude of potential health impacts associated with exposures has not been quantified and such information is generally considered necessary to engage in risk mitigation actions. Chemical manufacturers have claimed that replacement PFASs are not associated with adverse health effects and that shorter-chain homologues with shorter half-lives in the human body are not likely to bioaccumulate.156, 157 However, ongoing work suggests shorter chain compounds have a higher potential to interact with biomolecules due to less steric hindrance than the longer chain homologues.158, 159 For example, fluorinated carbon chains in perfluoroalkyl ether carboxylic acids (PFECAs), an important new class of PFASs, are broken into shorter units by the insertion of oxygen molecules that are thought to make them more reactive.160 One known PFOA alternative is the ammonium salt of perfluoro-2-propoxypropanoic acid, a PFECA that has been produced since 2010 with the trade name “GenX”.161 A recent hazard assessment based on the internal dose of GenX suggests it has higher toxicity than PFOA after accounting for toxicokinetic differences.18 The extreme environmental persistence, bioaccumulation, and potential toxicity of the entire class of PFASs have led some researchers to question the use the any highly fluorinated chemicals and call for a class approach in managing them.162
In summary, additional research is needed to better understand the exposure pathways and health outcomes associated with emerging PFASs and to understand the timescales of exposures to legacy PFASs associated with drinking water and seafood contamination. Risk mitigation measures require new technology for reducing PFAS concentrations at contaminated sites and in drinking water supplies. Delayed action on legacy PFASs has resulted in widespread human exposures and risks and lessons should be learned from this example and not repeated for the newer PFASs entering the market.90 Although much additional data is needed to understand the full extent of impacts of PFAS exposures on human health, particularly at sensitive life stages, we assert that this should not be used as a justification for delaying risk mitigation actions. The phase out in PFOS and its precursors between 2000–2002 was extremely effective at rapidly reducing exposures of humans and wildlife globally to these compounds and provides an example of the potential benefits from coordinated global action.
Acknowledgements
Financial support for this work was provided by the NIH Superfund Research Program P42ES027706 and the Harvard National Institute of Environmental Health and Sciences (NIEHS) Center Grant (P30 ES000002).
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References:
- 1.OECD (The Organisation for Economic Co-operation and Development). Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per and Polyfluoroalkyl Substances (PFASs)., 2018.
- 2.Vecitis CD, Wang Y, Cheng J, Park H, Mader BT, Hoffmann MR Sonochemical degradation of perfluorooctane sulfonate in aqueous film-forming foams. Environmental Science & Technology 2010; 44: 432–438. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, DeWitt JC, Higgins CP, Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental Science & Technology 2017; 51: 2508–2518. [DOI] [PubMed] [Google Scholar]
- 4.Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environmental Science & Technology Letters 2016; 3: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banzhaf S, Filipovic M, Lewis J, Sparrenbom C, Barthel R A review of contamination of surface-, ground, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs). Ambio 2017; 46: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Cousins IT, Scheringer M, Hungerbuehler K Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environment International 2015; 75: 172–179. [DOI] [PubMed] [Google Scholar]
- 7.Armitage J, Cousins IT, Buck RC, Prevedouros K, Russell MH, MacLeod M et al. Modeling Global-Scale Fate and Transport of Perfluorooctanoate Emitted from Direct Sources. Environmental Science & Technology 2006; 40: 6969–6975. [DOI] [PubMed] [Google Scholar]
- 8.Giesy J, Kannan K Global distribution of perfluoroctane sulfonate in wildlife. Environmental Science & Technology 2001; 35: 1339–1342. [DOI] [PubMed] [Google Scholar]
- 9.Tomy G, Budakowski W, Halldorson T, Helm P, Stern G, Friesent K et al. Fluorinated organic compounds in an eastern Arctic marine food web. Environmental Science & Technology 2004; 38: 6475–6481. [DOI] [PubMed] [Google Scholar]
- 10.Lewis RC, Johns LE, Meeker JD Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. Int J Environ Res Public Health 2015; 12: 6098–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Fourth National Report on Human Exposure to Environmental Chemicals; Centers for Disease Control and Prevention: Atlanta, 2015. [Google Scholar]
- 12.Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environmental health Perspectives 2007; 115: 1696–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Land M, Wit CAd, Cousins IT, Herzke D, Johansson J, Martin JW What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review protocol. Environ Evidence 2015; 4: 3. [Google Scholar]
- 14.Gomis MI, Vestergren R, MacLeod M, Mueller JF, Cousins IT Historical human exposure to perfluoroalkyl acids in the United States and Australia reconstructed from biomonitoring data using population-based pharmacokinetic modelling. Environment International 2017; 108: 92–102. [DOI] [PubMed] [Google Scholar]
- 15.Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environment international 2013; 60: 89–96. [DOI] [PubMed] [Google Scholar]
- 16.NØst TH, Vestergren R, Berg V, Nieboer E, Ödland JØ, Sandanger TM Repeated measurements of per-and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environment international 2014; 67: 43–53. [DOI] [PubMed] [Google Scholar]
- 17.Ritscher A, Wang Z, Scheringer MJ, et al. Zürich Statement on Future Actions on Per-and Polyfluoroalkyl Substances (PFASs). Environmental Heath Perspectives 2018; 10.1289/EHP4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomis MI, Vestergren R, Borg D, Cousins IT Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environment International 2018; 113: 1–9. [DOI] [PubMed] [Google Scholar]
- 19.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment and Management 2011; 7: 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt CM, Muir DCG, Mabury SA Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: A review. Environmental Toxicology and Chemistry 2014; 33: 243–267. [DOI] [PubMed] [Google Scholar]
- 21.Young C, Mabury S Atmospheric perfluorinated acid precursors: chemistry, occurrence, and impacts. Reviews of Environmental Contamination and Toxicology 2010; 208: 1–109. [DOI] [PubMed] [Google Scholar]
- 22.Pickard H, Criscitiello A, Spencer C, Sharp M, Muir D, De Silva A et al. Continuous non-marine inputs of per- and polyfluoroalkyl substances to the High Arctic: a multi-decadal temporal record. Atmospheric Chemistry and Physics 2018; 18: 5045–5058. [Google Scholar]
- 23.Haug LS, Huber S, Becher G, Thomsen C Characterisation of human exposure pathways to perfluorinated compounds — Comparing exposure estimates with biomarkers of exposure. Environment International 2011; 37: 687–693. [DOI] [PubMed] [Google Scholar]
- 24.Vestergren R, Cousins IT, Trudel D, Wormuth M, Scheringer M Estimating the contribution of precursor compounds in consumer exposure to PFOS and PFOA. Chemosphere 2008; 73: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 25.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K Estimating Consumer Exposure to PFOS and PFOA. Risk Analysis 2008; 28: 251–269. [DOI] [PubMed] [Google Scholar]
- 26.Dassuncao C, Hu XC, Zhang X, Bossi R, Dam M, Mikkelsen B et al. Temporal shifts in poly-and perfluoroalkyl substances (PFASs) in North Atlantic pilot whales indicate large contribution of atmospheric precursors. Environmental Science & Technology 2017; 51: 4512–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Zhang Y, Dassuncao C, Lohmann R, Sunderland EM North Atlantic deep water formation inhibits high Arctic contamination by continental perfluorooctane sulfonate discharges. 2017. 2017; Global Biogeochemical Cycles: 8. [Google Scholar]
- 28.Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environmental Science & Technology 2018; doi 10.1021/acs.est.7b06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.3M Company. Fluorochemical Use, Distribution and Release Overview. US EPA Public Docket AR226–0550: 3M Company: St Paul, MN, 1999.
- 30.Kissa E Fluorinated surfactants and repellents. CRC Press, 2001. [Google Scholar]
- 31.Favreau P, Poncioni-Rothlisberger C, Place BJ, Bouchex-Bellomie H, Weber A, Tremp J et al. Multianalyte profiling of per- and polyfluoroalkyl substances (PFASs) in liquid commercial products. Chemosphere 2017; 171: 491–501. [DOI] [PubMed] [Google Scholar]
- 32.Robel AE, Marshall K, Dickinson M, Lunderberg D, Butt C, Peaslee G et al. Closing the Mass Balance on Fluorine on Papers and Textiles. Environmental Science & Technology 2017; 51: 9022–9032. [DOI] [PubMed] [Google Scholar]
- 33.Herzke D, Olsson E, Posner S Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway - A pilot study. Chemosphere 2012; 88: 980–987. [DOI] [PubMed] [Google Scholar]
- 34.Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME et al. Fluorinated Compounds in U.S. Fast Food Packaging. Environmental Science & Technology Letters 2017; 4: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Z, Liu X, Krebs AK. Perfluorocarboxylic acid content in 116 articles of commerce: EPA?600/R-09/−33; Environmental Protections Agency: Research Triangle Park, NC, 2009. [Google Scholar]
- 36.Yuan G, Peng H, Huang C, Hu J Ubiquitous Occurrence of Fluorotelomer Alcohols in Eco-Friendly Paper-Made Food-Contact Materials and Their Implication for Human Exposure. Environmental Science & Technology 2016; 50: 942–950. [DOI] [PubMed] [Google Scholar]
- 37.Zabaleta I, Bizkarguenaga E, Bilbao D, Etxebarria N, Prieto A, Zuloaga O Fast and simple determination of perfluorinated compounds and their potential precursors in different packaging materials. Talanta 2016; 152: 353–363. [DOI] [PubMed] [Google Scholar]
- 38.Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environmental Science and Pollution Research 2015; 22: 14546–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye F, Zushi Y, Masunaga S Survey of perfluoroalkyl acids (PFAAs) and their precursors present in Japanese consumer products. Chemosphere 2015; 127: 262–268. [DOI] [PubMed] [Google Scholar]
- 40.Bečanová J, Melymuk L, Vojta Š, Komprdová K, Klánová J Screening for perfluoroalkyl acids in consumer products, building materials and wastes. Chemosphere 2016; 164: 322–329. [DOI] [PubMed] [Google Scholar]
- 41.Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA Perfluorochemicals: Potential sources of and migration from food packaging. Food Additives & Contaminants 2005; 22: 1023–1031. [DOI] [PubMed] [Google Scholar]
- 42.Begley TH, Hsu W, Noonan G, Diachenko G Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Additives & Contaminants: Part A 2008; 25: 384–390. [DOI] [PubMed] [Google Scholar]
- 43.Harrad S, de Wit C, Abdallah M, Bergh C, Bjorklund J, Covaci A et al. Indoor contamination with hexabromocyclododecanes, polybrominated diphenyl ethers, and perfluoroalkyl compounds: an important exposure pathway for people? Environmental Science & Technology 2010; 44: 3221–3231. [DOI] [PubMed] [Google Scholar]
- 44.Fromme H, Dreyer A, Dietrich S, Fembacher L, Lahrz T, Völkel W Neutral polyfluorinated compounds in indoor air in Germany - The LUPE 4 study. Chemosphere 2015; 139: 572–578. [DOI] [PubMed] [Google Scholar]
- 45.US EPA. Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate; Environmental Protection Agency: Washington, DC: https://www.epa.gov/sites/production/files/2016-05/documents/pfoapfosprepub508.pdf [Accessed 31 August 2018], 2016. [Google Scholar]
- 46.ATSDR. Toxicological Profile for Perfluoroalkyls: Draft for Public Comment, June 2018 Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Atlanta, GA: Available: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [Accessed: 31 August, 2018], 2018. [Google Scholar]
- 47.Health Canada. Health Canada’s Drinking Water Screening Values for Perfluoroalkylated Substances (PFASs). February 2016, Ottawa, ON. Available: http://scottreid.ca/wp-content/uploads/2016/03/Health-Canada-PFAS-Screening-Values-Fact-Sheet-EN.pdf [Accessed: 20 August, 2018]. 2016.
- 48.Grandjean P, Budtz-Jorgensen E Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environmental Health 2013; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM Community Exposure to Perfluorooctanoate: Relationships Between Serum Concentrations and Exposure Sources. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 2006; 48: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Post GB, Louis JB, Cooper KR, Boros-Russo BJ, Lippincott RL Occurrence and potential significance of perfluorooctanoic acid (PFOA) detected in New Jersey public drinking water systems. Environmental science & technology 2009; 43: 4547–4554. [DOI] [PubMed] [Google Scholar]
- 51.U.S. EPA. The Third Unregulated Contaminant Monitoring Rule (UCMR 3): Data Summary, April 2016. In. Office of Water, EPA 815-S-16–002, 2016.
- 52.Sun M, Arevalo E, Strynar M, Linstrom A, Richardson M, Kearns B et al. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants int he Cape Fear River watershed of North Carolina. Environmental Science & Technology Letters 2016; 3: 415–419. [Google Scholar]
- 53.Lindh CH, Rylander L, Toft G, Axmon A, Rignell-Hydbom A, Giwercman A et al. Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere 2012; 88: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 54.Weihe P, Kato K, Calafat AM, Nielsen F, Wanigatunga A, Needham L et al. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environmental Science & Technology 2008; 42: 6291–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Z, Shi Y, Vestergren R, Wang T, Liang Y, Cai Y Highly elevated serum concentrations of perflouroalkyl substances in fishery employees from Tangxun Lake, China. Environmental Science & Technology 2014; 48: 3864–3874. [DOI] [PubMed] [Google Scholar]
- 56.Berger U, Glynn A, Holmstrom K, Berglund M, Ankarberg E, Tomkvist A Fish consumption as a source of human exposure to perfluorinated alkyl substances in Sweden - Analysis of edible fish from Lake Vattern and the Baltic Sea. Chemosphere 2009; 76: 799–804. [DOI] [PubMed] [Google Scholar]
- 57.European Food Safety Authority Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food (draft).. EFSA J 2018; 16: 1–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stahl L, Snyder B, Olsen A, Kinkaid T, Wathen J, McCarty H Perfluorinated compounds in fish from U.S. urban rivers and the Great Lakes. Science of The Total Environment 2014; 499: 185–195. [DOI] [PubMed] [Google Scholar]
- 59.Del Gobbo L, Tittlemier S, Diamond M, Pepper K, Tague B, Yeudall F et al. Cooking decreases observed perfluorinated compound concentrations in fish. Journal of Agricultural and Food Chemistry 2008; 56: 7551–7559. [DOI] [PubMed] [Google Scholar]
- 60.Kelly B, Ikonomou M, Blair J, Surridge B, Hoover D, Grace R et al. Perfluoroalkyl contaminants in an Arctic marine food web: Trophic magnification and wildlife exposure. Environmental Science & Technology 2009; 43: 4037–4043. [DOI] [PubMed] [Google Scholar]
- 61.Condor J, Hoke R, de Wolf W, Russell MH, Buck RC Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophiic Compoiunds. Environmental Science & Technology 2008; 42: 995–1003. [DOI] [PubMed] [Google Scholar]
- 62.Christensen K, Raymond M, Blackowicz M, Liu Y-J, Thompson B, Anderson H et al. Perfluoroalkyl substances and fish consumption. Environmental Research 2017; 154: 145–151. [DOI] [PubMed] [Google Scholar]
- 63.Hu X, Dassuncao C, Zhang X, Grandjean P, Weihe P, Webster G et al. Can profiles of poly- and perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources. Environmental Health 2018; 17: DOI 10.1186/s12940-12018-10355-12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buser A, Morf L. Substance flow analysis of PFOS and PFOA, in Perfluorinated Surfactants Perfluorooctanesulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Switzerland, Federal Office for the Environment (FOEN), Berne, Switz, 2009.
- 65.Earnshaw MR, Paul AG, Loos R, Tavazzi S, Paracchini B, Scheringer M et al. Comparing measured and modelled PFOS concentrations in a UK freshwater catchment and estimating emission rates. Environment International 2014; 70: 25–31. [DOI] [PubMed] [Google Scholar]
- 66.Sepulvado JG, Blaine AC, Hundal LS, Higgins CP Occurrence and fate of perfluorochemicals in soil following the land application of municipal biosolids. Environ Sci Technol 2011; 45: 8106–8112. [DOI] [PubMed] [Google Scholar]
- 67.Washington JW, Yoo H, Ellington JJ, Jenkins TM, Libelo EL Concentrations, Distribution, and Persistence of Perfluoroalkylates in Sludge-Applied Soils near Decatur, Alabama, USA. Environmental Science & Technology 2010; 44: 8390–8396. [DOI] [PubMed] [Google Scholar]
- 68.Venkatesan AK, Halden RU National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. Journal of Hazardous Materials 2013; 252–253: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro I, de la Torre A, Sanz P, Pro J, Carbonell G, Martínez MdlÁ Bioaccumulation of emerging organic compounds (perfluoroalkyl substances and halogenated flame retardants) by earthworm in biosolid amended soils. Environmental Research 2016; 149: 32–39. [DOI] [PubMed] [Google Scholar]
- 70.Navarro I, de la Torre A, Sanz P, Porcel MÁ, Pro J, Carbonell G et al. Uptake of perfluoroalkyl substances and halogenated flame retardants by crop plants grown in biosolids-amended soils. Environmental Research 2017; 152: 199–206. [DOI] [PubMed] [Google Scholar]
- 71.Wen B, Wu Y, Zhang H, Liu Y, Hu X, Huang H et al. The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environmental Pollution 2016; 216: 682–688. [DOI] [PubMed] [Google Scholar]
- 72.Domingo JL, Nadal M Per- and Polyfluoroalkyl Substances (PFASs) in Food and Human Dietary Intake: A Review of the Recent Scientific Literature.. Journal of Agricultural and Food Chemistry 2017; 56: 533–543. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman K, Webster T, Bartell S, Weisskopf M, Fletcher T, Vieira V Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environmental Health Perspectives 2010; 119: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vestergren R, Cousins IT Tracking the Pathways of Human Exposure to Perfluorocarboxylates. Environmental Science & Technology 2009; 43: 5565–5575. [DOI] [PubMed] [Google Scholar]
- 75.Egeghy PP, Lorber M An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. Journal of exposure science & environmental epidemiology 2011; 21: 150–168. [DOI] [PubMed] [Google Scholar]
- 76.Haug LS, Huber S, Becher G, Thomsen C Characterisation of human exposure pathways to perfluorinated compounds—comparing exposure estimates with biomarkers of exposure. Environment International 2011; 37: 687–693. [DOI] [PubMed] [Google Scholar]
- 77.Lorber M, Egeghy PP Simple intake and pharmacokinetic modeling to characterize exposure of Americans to perfluoroctanoic acid, PFOA. Environmental science & technology 2011; 45: 8006–8014. [DOI] [PubMed] [Google Scholar]
- 78.Johnson GW, Ehrlich R, Full W, Ramos S. Principal components analysis and receptor models in environmental forensics. Academic Press: San Diego, 2002. [Google Scholar]
- 79.Wang Z, Stout SA, Fingas M Forensic Fingerprinting of Biomarkers for Oil Spill Characterization and Source Identification. Environmental Forensics 2006; 7: 105–146. [Google Scholar]
- 80.Benskin JP, Phillips V, St Louis VL, Martin JW Source elucidation of perfluorinated carboxylic acids in remote alpine lake sediment cores. Environ Sci Technol 2011; 45: 7188–7194. [DOI] [PubMed] [Google Scholar]
- 81.D’eon JC, Mabury SA Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat. Environmental health perspectives 2011; 119: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD et al. Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environmental Science & Technology Letters 2016; 3: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao X-L et al. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. Journal of agricultural and food chemistry 2007; 55: 3203–3210. [DOI] [PubMed] [Google Scholar]
- 84.Li M, von Stackelberg K, Rheinberger CM, Hammitt JK, Krabbenhoft DP, Yin R et al. Insights from mercury stable isotopes into factors affecting the internal body burden of methylmercury in frequent fish consumers. Elem Sci Anth 2016; 4. [Google Scholar]
- 85.Thurston GD, Spengler JD A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmospheric Environment (1967) 1985; 19: 9–25. [Google Scholar]
- 86.Yeung LW, Miyake Y, Taniyasu S, Wang Y, Yu H, So MK et al. Perfluorinated compounds and total and extractable organic fluorine in human blood samples from China. Environmental science & technology 2008; 42: 8140–8145. [DOI] [PubMed] [Google Scholar]
- 87.Miyake Y, Yamashita N, So MK, Rostkowski P, Taniyasu S, Lam PK et al. Trace analysis of total fluorine in human blood using combustion ion chromatography for fluorine: a mass balance approach for the determination of known and unknown organofluorine compounds. Journal of Chromatography A 2007; 1154: 214–221. [DOI] [PubMed] [Google Scholar]
- 88.Taves DR Evidence that there are two forms of fluoride in human serum. Nature 1968; 217: 1050. [DOI] [PubMed] [Google Scholar]
- 89.Hansen KJ, Clemen LA, Ellefson ME, Johnson HO Compound-Specific, Quantitative Characterization of Organic Fluorochemicals in Biological Matrices. Environmental Science & Technology 2001; 35: 766–770. [DOI] [PubMed] [Google Scholar]
- 90.Gradjean P Delayed discovery, dissemination, and decisions on intervention in environmental health: A case study on immunotoxicity of perfluorinated alkylated substances. Environmental Health 2018; 17: 10.1186/s12940-12018-10405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF et al. Predictors of Per- and Polyfluoroalkyl Substance (PFAS) Plasma Concentrations in 6–10 Year Old American Children. Environmental Science & Technology 2017; 51: 5193–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L et al. Time Trends in Per- and Polyfluoroalkyl Substances (PFASs) in California Women: Declining Serum Levels, 2011–2015.. Environmental Science & Technology 2018; 52: 277–287. [DOI] [PubMed] [Google Scholar]
- 93.Glynn A, Benskin J, Lignell S, Gyllenhammar I, Aune M, Cantillana T et al. Temporal trends of perfluoroalkyl substances in pooled serum samples from first-time mothers in Uppsala 1997–2014. Report to the Swedish EPA; 2015., 2015. [Google Scholar]
- 94.Eriksson U, Mueller JF, Toms LL, Hobson P, Karrman A Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environmental Pollution; 220. [DOI] [PubMed] [Google Scholar]
- 95.Haug LS, Thomsen C, Becher GB Time Trends and the Influence of Age and Gender on Serum Concentrations of Perfluorinated Compounds in Archived Human Samples. Environmental Science & Technology 2009; 43: 2131–2136. [DOI] [PubMed] [Google Scholar]
- 96.Yeung LW, Miyake Y, Taniyasu S, Wang Y, Yu H, So MK et al. Perfluorinated Compounds and Total and Extractable Organic Fluorine in Human Blood Samples from China. Environmental Science & Technology 2008; 42: 8140–8145. [DOI] [PubMed] [Google Scholar]
- 97.Mohsin M, Sarwar N, Ahmad S, Rasheed A, Ahmad F, Afzal A et al. Maleic acid crosslinking of C-6 fluorocarbon as oil and water repellent finish on cellulosic fabrics. Journal of Cleaner Production 2016; 112: 3525–3530. [Google Scholar]
- 98.Ritter SK FLUOROCHEMICALS GO SHORT. Chemical & Engineering News Archive 2010; 88: 12–17. [Google Scholar]
- 99.Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH et al. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environment International 2016; 91. [DOI] [PubMed] [Google Scholar]
- 100.Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM Trends in exposure to polyfluoroalkyl chemicals in the US population: 1999– 2008. Environmental Science & Technology 2011; 45: 8037–8045. [DOI] [PubMed] [Google Scholar]
- 101.Paul AG, Jones KC, Sweetman AJ A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environmental Science & Technology 2009; 43: 386–392. [DOI] [PubMed] [Google Scholar]
- 102.Griffith FD, Long JE Animal toxicity studies with ammonium perfluorooctanoate. American Industrial Hygiene Association journal 1980; 41: 576–583. [DOI] [PubMed] [Google Scholar]
- 103.Ubel FA, Sorenson SD, Roach DE Health status of plant workers exposed to fluorochemicals--a preliminary report. American Industrial Hygiene Association journal 1980; 41: 584–589. [DOI] [PubMed] [Google Scholar]
- 104.Goldenthal EI, Jessup DC, Geil RG, Mehring JS. 90-day subacute rhesus monkey toxicity study No. 137–092. Available on USEPA Public Docket AR-226–0137: International Research and Development Corp., Mattawan, MI, 1978. [Google Scholar]
- 105.Goldenthal EI, Jessup DC, Geil RG, Mehring JS. 90-day subacute rhesus monkey toxicity study (aborted) No. 137–087. Available on USEPA Public Docket AR-226–0138: International Research and Development Corp., Mattawan, MI, 1978. [Google Scholar]
- 106.RIKER laboratories. Two year oral (diet) toxicity/carcinogenicity study of fluorochemical FM-3924 in rats. RIKER Laboratoreis, Inc. / 3M Company; Available on USEPA Public Docket AR-226–0257: St. Paul, Minnesota, 1983. [Google Scholar]
- 107.Sibinski LJ, Allen JL, Elrod SV. Two year oral (diet) toxicity—oncogenicity study of fluorocarbon FM-3924 in rats Available on USEPA Public Docket AR-226–0262: St. Paul, MN, 1988. [Google Scholar]
- 108.Pathology Associates International. Pathology Review of Reported Tumorigenesis in a Two Year Study of FM-3924 in Rats. Available on U.S. EPA public docket AR-226–0264.: West Chester, Ohio, 1998. [Google Scholar]
- 109.McMichael AJ Standardized mortality ratios and the” healthy worker effect”: Scratching beneath the surface. Journal of occupational medicine: official publication of the Industrial Medical Association 1976; 18: 165–168. [DOI] [PubMed] [Google Scholar]
- 110.Gilliland FD. Fluorocarbons and Human health: Studies in an Occupational Cohort. University of Minnesota Available on U.S. EPA Public Docket AR-226–0473, 1992.
- 111.Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. Journal of occupational and environmental medicine 1998; 40: 614–622. [DOI] [PubMed] [Google Scholar]
- 112.Palmer CN, Hsu M-H, Griffin KJ, Raucy JL, Johnson EF Peroxisome proliferator activated receptor-α expression in human liver. Molecular pharmacology 1998; 53: 14–22. [PubMed] [Google Scholar]
- 113.Barry V, Winquist A, Steenl Kand Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environmental health perspectives 2013; 121: 1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez-Espinosa M-J, Mondal D, Armstrong B, Bloom MS, Fletcher T Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environmental health perspectives 2012; 120: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steenland K, Zhao L, Winquist A, Parks C Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environmental health perspectives 2013; 121: 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Darrow LA, Stein CR, Steenland K Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environmental health perspectives 2013; 121: 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rappazzo K, Coffman E, Hines E Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. International Journal of Environmental Research and Public Health 2017; 14: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grandjean P, Andersen E, Budtz-Jorgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012; 307: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klaunig JE, Shinohara M, Iwai H, Chengelis CP, Kirkpatrick JB, Wang Z et al. Evaluation of the Chronic Toxicity and Carcinogenicity of Perfluorohexanoic Acid (PFHxA) in Sprague-Dawley Rats. Toxicologic Pathology 2014; 43: 209–220. [DOI] [PubMed] [Google Scholar]
- 120.Gilliland FD, Mandel JS Mortality among employees of a perfluorooctanoic acid production plant. Journal of occupational medicine : official publication of the Industrial Medical Association 1993; 35: 950–954. [DOI] [PubMed] [Google Scholar]
- 121.Raleigh KK, Alexander BH, Olsen GW, Ramachandran G, Morey SZ, Church TR et al. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occupational and Environmental Medicine 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect 2013; 121: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eriksen KT, Sorensen M, McLaughlin JK, Lipworth L, Tjonneland A, Overvad K et al. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. Journal of the National Cancer Institute 2009; 101: 605–609. [DOI] [PubMed] [Google Scholar]
- 124.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR Immunotoxicity of perfluorinated compounds: recent developments. Toxicologic pathology 2012; 40: 300–311. [DOI] [PubMed] [Google Scholar]
- 125.Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatric research 2016; 79: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. Journal of immunotoxicology 2013; 10: 373–379. [DOI] [PubMed] [Google Scholar]
- 127.Stein CR, Ge YC, Wolff MS, Ye XY, Calafat AM, Kraus T et al. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environmental Research 2016; 149: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG et al. Influenza Vaccine Response in Adults Exposed to Perfluorooctanoate and Perfluorooctanesulfonate. Toxicological Sciences 2014; 138: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dong G-H, Tung K-Y, Tsai C-H, Liu M-M, Wang D, Liu W et al. Serum Polyfluoroalkyl Concentrations, Asthma Outcomes, and Immunological Markers in a Case-Control Study of Taiwanese Children. Environmental Health Perspectives 2013; 121: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buser MC, Scinicariello F Perfluoroalkyl substances and food allergies in adolescents. Environment International 2016; 88: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gilliland FD, Mandel JS Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men. American journal of industrial medicine 1996; 29: 560–568. [DOI] [PubMed] [Google Scholar]
- 132.Liu HS, Wen LL, Chu PL, Lin CY Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environmental pollution (Barking, Essex : 1987) 2018; 232: 73–79. [DOI] [PubMed] [Google Scholar]
- 133.Steenland K, Fletcher T, Savitz DA Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 2010; 118: 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM et al. Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ Health Perspect 2017; 125: 107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Medicine 2018; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T et al. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Archives of pediatrics & adolescent medicine 2010; 164: 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nelson JW, Hatch EE, Webster TF Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 2010; 118: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maisonet M, Näyhä S, Lawlor DA, Marcus M Prenatal exposures to perfluoroalkyl acids and serum lipids at ages 7 and 15 in females. Environment International 2015; 82: 49–60. [DOI] [PubMed] [Google Scholar]
- 139.Lin CY, Chen PC, Lin YC, Lin LY Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes care 2009; 32: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertility and Sterility 2015; 103: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matilla-Santander N, Valvi D, Lopez-Espinosa MJ, Manzano-Salgado CB, Ballester F, Ibarluzea J et al. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environ Health Perspect 2017; 125: 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Min JY, Lee KJ, Park JB, Min KB Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in US adults. Occupational and environmental medicine 2012; 69: 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Geiger SD, Xiao J, Shankar A. No association between perfluoroalkyl chemicals and hypertension in children. In, 2014. pp 1–7. [DOI] [PMC free article] [PubMed]
- 144.Conway B, Costacou T Perfluoroalkyl acids and stroke risk in persons with and without diabetes: A salutatory effect of high oxygen carrying capacity environmental contaminants. Circulation 2016; 133. [Google Scholar]
- 145.Steenland K, Zhao L, Winquist A A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med 2015; 72: 373–380. [DOI] [PubMed] [Google Scholar]
- 146.Lin CY, Lin LY, Wen TW, Lien GW, Chien KL, Hsu SHJ et al. Association between levels of serum perfluorooctane sulfate and carotid artery intima-media thickness in adolescents and young adults. International Journal of Cardiology 2013; 168: 3309–3316. [DOI] [PubMed] [Google Scholar]
- 147.Koshy TT, Attina TM, Ghassabian A, Gilbert J, Burdine LK, Marmor M et al. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environment International 2017; 109: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Olsen GW, Ehresman DJ, Buehrer BD, Gibson BA, Butenhoff JL, Zobel LR Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 2012; 54: 974–983. [DOI] [PubMed] [Google Scholar]
- 149.Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. The American journal of gastroenterology 2010; 105: 1354–1363. [DOI] [PubMed] [Google Scholar]
- 150.Wang X, Li B, Zhao W-D, Liu Y-J, Shang D-S, Fang W-G et al. Perfluorooctane sulfonate triggers tight junction “opening” in brain endothelial cells via phosphatidylinositol 3-kinase. Biochemical and Biophysical Research Communications 2011; 410: 258–263. [DOI] [PubMed] [Google Scholar]
- 151.Johansson N, Fredriksson A, Eriksson P Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 2008; 29: 160–169. [DOI] [PubMed] [Google Scholar]
- 152.Liew Z, Goudarzi H, Oulhote Y Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Current Environmental Health Reports 2018; 5: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yeung LW, Mabury SA Are humans exposed to increasing amounts of unidentified organofluorine? Environmental Chemistry 2016; 13: 102–110. [Google Scholar]
- 154.Gomis MI, Vestergren R, Nilsson H, Cousins IT Contribution of Direct and Indirect Exposure to Human Serum Concentrations of Perfluorooctanoic Acid in an Occupationally Exposed Group of Ski Waxers. Environmental Science & Technology 2016; 50: 7037–7046. [DOI] [PubMed] [Google Scholar]
- 155.Rotander A, Karrman A, Toms LM, Kay M, Mueller JF, Gomez Ramos MJ Novel fluorinated surfactants tentatively identified in firefighters using liquid chromatography quadrupole time-of-flight tandem mass spectrometry and a case-control approach. Environ Sci Technol 2015; 49: 2434–2442. [DOI] [PubMed] [Google Scholar]
- 156.Olsen GW, Chang S-C, Noker PE, Gorman GS, Ehresman DJ, Lieder PH et al. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 2009; 256: 65–74. [DOI] [PubMed] [Google Scholar]
- 157.Fujii Y, Niisoe T, Harada KH, Uemoto S, Ogura Y, Takenaka K et al. Toxicokinetics of perfluoroalkyl carboxylic acids with different carbon chain lengths in mice and humans. Journal of occupational health 2015; 57: 1–12. [DOI] [PubMed] [Google Scholar]
- 158.Ritter SK Fluorochemicals go short. Chemical & engineering news 2010; 88: 12–17. [Google Scholar]
- 159.Butt CM, Muir DC, Mabury SA Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: a review. Environmental toxicology and chemistry / SETAC 2014; 33: 243–267. [DOI] [PubMed] [Google Scholar]
- 160.Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E et al. Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ Sci Technol 2015; 49: 11622–11630. [DOI] [PubMed] [Google Scholar]
- 161.Wang Z, Cousins IT, Scheringer M, Hungerbühler K Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environment international 2013; 60: 242–248. [DOI] [PubMed] [Google Scholar]
- 162.Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT et al. The Madrid statement on poly-and perfluoroalkyl substances (PFASs). Environmental health perspectives 2015; 123: A107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tian Z, Kim S-K, Shoeib M, Oh J-E, Park J-E Human exposure to per- and polyfluoroalkyl substances (PFASs) via house dust in Korea: Implication to exposure pathway. Science of The Total Environment 2016; 553: 266–275. [DOI] [PubMed] [Google Scholar]
- 164.Shan G, Wang Z, Zhou L, Du P, Luo X, Wu Q et al. Impacts of daily intakes on the isomeric profiles of perfluoroalkyl substances (PFASs) in human serum. Environment International 2016; 89–90: 62–70. [DOI] [PubMed] [Google Scholar]
- 165.Gebbink WA, Berger U, Cousins IT Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor) exposure. Environment International 2015; 74: 160–169. [DOI] [PubMed] [Google Scholar]
- 166.Boxer E, Chaves N, Niwa Y. Northestern SSEHRI PFAS Contamination Site Tracker. In, 2018.