Abstract
Background
We compared new Aspergillus Galactomannan Lateral Flow Assay with the newly-formatted Aspergillus-specific Lateral Flow device tests for the diagnosis of invasive pulmonary aspergillosis (IPA) in non-neutropenic patients,
Methods
We performed both tests in 82 bronchoalveolar lavage fluid samples from 82 patients at risk for IPA but without underlying hematologic malignancy. Samples were collected between September 2016 and September 2018 at the University of California San Diego, United States. IPA was classified following two published consensus criteria.
Results
Classification of cases varied widely between the two consensus criteria. When using criteria established for the intensive care unit, 26/82 patients (32%) met criteria for proven or putative IPA. Both point-of-care assays showed sensitivities ranging between 58% and 69%, with specificities between 68% and 75%. Sensitivity increased up to 81% when both tests were combined.
Conclusion
The study outlines the need for updated, unified and more broadly applicable consensus definitions for classifying IPA in non-neutropenic patients, a work that is currently in progress. Both point-of-care tests showed comparable performance, with sensitivities and specificities in the 60–70% range when used alone and increasing to 80% when used in combination. The new point-of-care tests may serve a role at the bedside in those with clinical suspicion of IPA.
Keywords: Galactomannan, BAL, Aspergillus Galactomannan Lateral Flow Assay (LFA), Aspergillus-specific Lateral Flow device Tests (LFD), intensive care, respiratory diseases, solid organ transplantation, autoimmune diseases, HIV
Introduction
Invasive pulmonary aspergillosis (IPA) is a serious opportunistic infection with high mortality rates of 30–60% (1–3). Early diagnosis of IPA and treatment initiation is the single most important factor in reducing morbidity and mortality from IPA (1, 4, 5), but diagnosis can be difficult to establish. In patients with traditional risk factors for IPA, such as those with hematological malignancies and prolonged neutropenia, the use of mold-active prophylaxis has been associated with a decrease in prevalence of IPA (6, 7). Conversely, the prevalence of IPA continues to increase in non-neutropenic patients with other severe underlying diseases, including patients in the intensive care unit (ICU) where prevalence rates vary between 0.33–19% (8–11), solid organ transplant (SOT) recipients (12), patients receiving systemic glucocorticoids (13), patients with underlying respiratory conditions (3, 8, 14), patients with solid cancers (8, 15), and other patient groups (8, 16). Given this increase and the importance of early treatment to improve survival, there is an unmet need for better tests for early diagnosis IPA in non-neutropenic patients, including those critically ill in the ICU.
Diagnosis of IPA is based on compatible signs and symptoms of infection in an appropriate host with supportive radiological and mycological findings (17). Importantly, the pathogenesis of IPA differs between neutropenic and non-neutropenic patients (18), impacting clinical presentation, radiological findings and diagnostic test results in the mycology laboratory. For example, serum galactomannan (GM) testing is the gold-standard test that is used in consensus definitions for diagnosing IPA in neutropenic patients with angioinvasive disease, but sensitivities decrease to 30% and less in non-neutropenic patients. As a result, GM testing of bronchoalveolar lavage fluid (BALF) is preferable (19–21). In addition, “typical” signs of IPA on computed tomography (CT) in neutropenic patients, such as the “halo sign” or “air crescent sign”, are atypical in non-neutropenic patients and rarely occur (8, 22, 23). Despite these important differences, revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions focus primarily on neutropenic patients with underlying hematological malignancies and “typical” presentation of IPA, and have been shown to have limited applicability in non-neutropenic patients who frequently do not fulfill radiological and host criteria (17). While an alternative clinical algorithm for diagnosing IPA in the ICU setting seems to overcome some of those limitations including attempting to distinguish colonization from true infection/disease (22), that algorithm relies on clinical signs that typically occur during later stages of IPA in non-neutropenic patients (8), and is applicable only to those with a positive BALF culture for Aspergillus spp., which is an entry criteria in this algorithm (20, 24).
Given the absence of widely-accepted criteria for defining IPA in non-neutropenic patients, studies on new diagnostic tests for IPA focus mostly on patients with underlying hematological malignancies (21, 25–27), where broadly accepted classification criteria exist, while diagnostic studies in non-neutropenic patients are scarce. Importantly, two novel point-of care diagnostic tests, both European conformity (CE) marked for diagnosis of IPA in BALF, have recently become commercially available, but to date have been only validated in patients with underlying hematological malignancies (25, 28). These POC tests may allow earlier diagnosis and initiation of anti-mold treatment compared to GM testing, given the longer and variable turnaround time of GM, and may thereby improve survival.
The objective of this study was to evaluate the new Aspergillus Galactomannan Lateral Flow Assay (LFA) and compare performance with the newly formatted Aspergillus-specific Lateral Flow device Tests (LFD) for the diagnosis of IPA in patients at risk for IPA but without neutropenia or underlying hematologic malignancy.
Methods
A total of 82 BALF samples obtained from 82 patients without underlying hematological malignancies, but with clinical suspicion of IPA, who had bronchoscopy performed and BALF GM ordered between September 2016 and September 2018 at the University of California San Diego, United States were included in this analysis.
IPA was classified according to two criteria: i.) the revised EORTC/MSG criteria (17), and ii.) a slightly modified version of the clinical algorithm described by Blot and colleagues (22, 29). The Blot algorithm was broadened by adding BALF GM >1.0 ODI as entry criterion, given that BALF culture was previously shown to have a sensitivity of only 58% for proven IPA in ICU patients, while BALF GM > 1.0 ODI had a sensitivity of 85% (with a specificity of >90%) (19).
GM (Platelia Aspergillus Ag ELISA; Bio-Rad Laboratories, Munich, Germany) and culture were performed prospectively in all BALF samples. GM positive and randomly selected GM negative samples were stored at −20°C and tested between August and September 2018 for the Aspergillus-specific LFD (OLM Diagnostics, Newcastle upon Tyne, UK), and the Aspergillus Galactomannan LFA (IMMY, Norman, Oklahoma, USA). Stored BALF samples where thawed, vortexed, and tested according to the manufacturer’s instructions. For the Aspergillus-specific LFD, clear BALF was centrifuged only, while milky, or turbid BALF was pretreated according to the manufacturer’s instructions, and 70 μL of supernatant was added to the test. Results were read 15 and 25 minutes later and scored as either -, +, ++, or +++. For the Aspergillus Galactomannan LFA, BALF samples were pretreated, heated, and centrifuged. Test strips were then inserted into 80 μL of sample following the manufacturer’s instructions. Results were read after 30 minutes and scores given ranging from 0 (i.e. negative), to 4 (highly positive). Results of both the LFD and LFA were each read by two interpreters who were blinded to IPA status, GM ELISA, and culture results.
Statistical analyses were performed using SPSS 25 (SPSS Inc., Chicago, IL, USA). A two-sided P-value of less than 0.05 was considered statistically significant. The Human Research Protections Program at the University of California, San Diego approved the study protocol and all study-related procedures.
Results
A total of 82 samples from unique patients were included in the analysis. Demographic characteristics and underlying diseases of the study population are displayed in Table 1.
Table 1.
Demographic data and underlying diseases of the study population
| Classification according to revised EORTC/MSG criteria | Classification according to Blot criteria | |||
|---|---|---|---|---|
| Probable or proven IPA (n=13) | No evidence for IPA (n=65) | Possible IPA (n=4) | Putative/Proven IPA (n=26) | |
| Female (n, %) | 5 (38%) | 26 (40%) | 1 (25%) | 8 (31%) |
| Age, years (median, range) | 58 (41–79) | 56 (19–80) | 66 (49–67) | 57 (19–79) |
| Primary Underlying diseases / conditions (n, %) | ||||
| Lung Transplant | 2 (15%) | 19 (29%) | 3 (75%) | 7 (27%) |
| Other Transplant | 1 (8%) | 2 (3%) | - | 1 (4%) |
| Oncological malignancy | 1 (8%) | 5 (8%) | 1 (25%) | 2 (8%) |
| Liver Cirrhosis | - | 5 (8%) | - | 3 (12%) |
| Interstitial Lung Disease | 1 (8%) | 5 (8%) | - | 2 (8%) |
| Rheumatoid/autoimmune diseases with lung involvement | 1 (8%) | 4 (6%) | - | 1 (4%) |
| HIV/AIDS | 1 (8%) | 3 (5%) | - | 2 (8%) |
| Tuberculosis | 1 (8%) | 1 (2%) | - | - |
| Asthma | - | 3 (5%) | - | 1 (4%) |
| Cystic fibrosis | - | 3 (5%) | - | - |
| Others | 1 (8%) | 4 (6%) | - | 2 (8%) |
| ICU COPD | 1 (8%) | 3 (5%) | - | 1 (4%) |
| ICU Septic Shock | 3 (23%) | 1 (2%) | - | 3 (12%) |
| ICU others | - | 7 (11%) | - | 1 (4%) |
| Overall Mortality 30-days | 2 (15%) | 14 (22%) | 1 (25%) | 8 (31%) |
| Overall Mortality 90-days | 3/12 (25%) | 14/63 (22%) | 1 (25%) | 9/25 (36%) |
= In one case of probable IPA and putative IPA, an additional BALF samples drawn 14 days earlier was available (only the later sample was included in the analysis). The patient fulfilled probable IPA criteria throughout the 14days. Patients first BALF sample (not included in this analysis) resulted positive for GM (2.38 ODI), grew Aspergillus terreus in culture and gave positive results with both the LFA and LFD, but the patient did not fulfill criteria of putative IPA according to Blot because of lack of typical symptoms. Two weeks later, at the time of the second BALF sample, this patient had still positive GM, positive LFD and positive LFA, negative culture, but symptoms as defined by Blot criteria and therefore fulfilled putative IPA criteria.
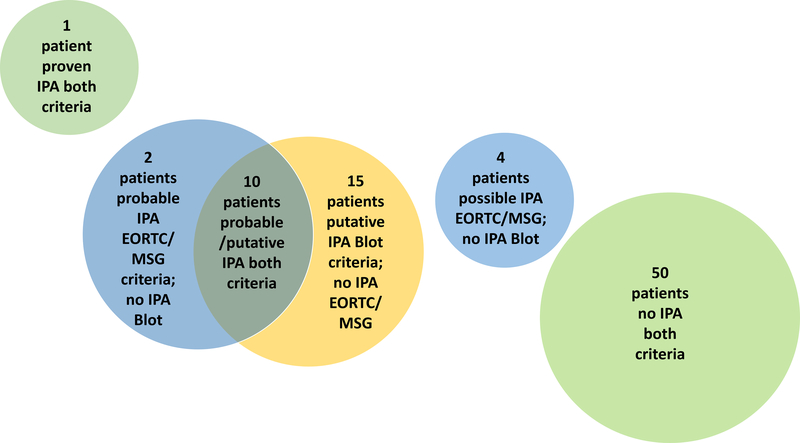
Classification of IPA using the two criteria is displayed in Figure 1. One patient was classified as proven IPA by EORTC/MSG criteria, while the remaining patients were classified as probable IPA (n=12), possible IPA (n=4), and no evidence for IPA (n=65). When using modified Blot criteria, one fulfilled proven IPA, 25 patients fulfilled criteria of putative IPA, while the remaining 56 patients (including 2 who fulfilled probable IPA and 4 who fulfilled possible IPA according to EORTC/MSG definitions) did not fulfill Blot IPA criteria. Overall 10 of those 56 patients fulfilled Blot entry criteria (i.e., BALF GM >1.0 ODI and/or positive BALF culture with Aspergillus spp. growth; median BALF GM 1.91, range 0–6.55 ODI), but did not fulfill other criteria for putative IPA. Of those 10, eight did not have at least one of the seven predefined compatible signs and symptoms, while two did not fulfill predefined host risk factors or additional mycology criteria. In addition, a total of 7 patients had BALF GM levels between 0.5 and 1 ODI but negative culture and therefore did not fulfill modified Blot entry criteria.
Figure 1:
Categorization of IPA based on EORTC/MSG modified Blot Criteria. Green bubbles indicate classification agreement of both criteria, while yellow and blue bubbles outline cases where classification differed between criteria.
Performance of the Aspergillus-specific LFD, Aspergillus Galactomannan LFA, BALF culture, Galactomannan ELISA, as well as combinations of the point-of-care assays for probable/proven versus no IPA and putative/proven versus no IPA are depicted in Table 1. In addition, positivity rates in patients with possible IPA according to EORTC/MSG, patients who fulfilled Blot entry criteria but not clinical or host criteria, and those with BALF GM levels <1 ODI but above 0.5 ODI are displayed in Table 2. BALF GM and culture were used as criteria for probable and putative IPA, and actual performance could therefore not be evaluated. Overall 9 cases with probable and/or putative IPA had Aspergillus spp. growth in BALF culture; among those the LFD resulted positive in 4/9 after 15 minutes and in 6/9 after 25 minutes, the LFA resulted positive in 5/9, GM>1.0ODI in 5/9, and GM>0.5ODI in 7/9. The single case with proven IPA (tissue invasion in lung biopsy) had negative BALF GM but Aspergillus fumigatus growth in BALF culture and positive Aspergillus Galactomannan LFA and Aspergillus-specific LFD test results.
Table 2.
Performance of the Aspergillus-specific Lateral Flow Device Test (LFD), the Aspergillus Galactomannan Lateral Flow Assay (LFA), Galactomannan (GM), and fungal culture in bronchoalveolar lavage (BALF) for diagnosis of invasive pulmonary aspergillosis (IPA) in patients without hematological malignancies. Sensitivity and specificity for probable/proven IPA versus no IPA, putative/proven IPA versus no IPA as well as test positivity in cases of possible IPA and those who fulfilled Blot entry criteria but not symptom or host criteria, as well as those with BALF GM levels between 0.5 and 1.0 are displayed.
| Biomarkers/tests and combinations | Revised EORTC/MSG Criteria | BLOT Criteria | |||||
|---|---|---|---|---|---|---|---|
| Sensitivity probable/proven IPA versus no IPA | Specificity probable/proven IPA versus no IPA | Positivity in possible IPA (n=4) | Sensitivity putative/proven IPA versus no IPA | Specificity putative/proven IPA versus no IPA | Positivity in those fulfilling Entry criteria but not symptoms or host criteria (n=10) | Positivity in those with BALF GM >0.5 and <1 ODI (n=7) | |
| Aspergillus-specific LFD 15 Minutes | 46% (6/13) | 66% (43/65) | 25% (1/4) | 58% (15/26) | 75% (42/56) | 60% (6/10) | 43% (3/7) |
| Aspergillus-specific LFD 25 Minutes | 62% (8/13) | 63% (41/65) | 50% (2/4) | 69% (18/26) | 71% (40/56) | 60% (6/10) | 43% (3/7) |
| Aspergillus Galactomannan LFA 30 Minutes | 69% (9/13) | 62%% (40/65) | 25% (1/4) | 65% (17/26) | 68% (38/56) | 50% (5/5) | 43% (3/7) |
| BALF GM 0.5 ODI cut-off * | 85% (11/13) | 66% (37/65) | 0% (0/4) | 92% (24/26) | 73% (41/56) | 80% (8/10) | 100% (7/7) |
| BALF GM 1.0 ODI cut-off * | 77% (10/13) | 71% (46/65) | 0% (0/4) | 85% (22/26) | 88% (49/56) | 70% (7/10) | 0% (0/7) |
| BAL culture * | 31% (4/13) | 88% (57/65) | 0% (0/4) | 35% (9/26) | 95% (53/56) | 30% (3/10) | 0% (0/7) |
| Aspergillus-specific LFD 15 Minutes AND/OR Aspergillus Galactomannan LFA 30 Minutes | 69% (9/13) | 54% (35/65) | 25% (1/4) | 77% (20/26) | 64% (36/56) | 60% (6/10) | 57% (4/7) |
| Aspergillus-specific LFD 25 Minutes AND/OR Aspergillus Galactomannan LFA 30 Minutes | 77% (10/13) | 52% (34/65) | 50% (2/4) | 81% (21/26) | 61% (34/56) | 60% (6/10) | 57% (4/7) |
The study could not evaluate performance of GM and culture as test results used as mycological standard for defining probable and putative IPA.
No difference in mortality was seen between EORTC/MSG categories of IPA (Table 1). Patients with putative or proven IPA according to Blot criteria had a trend towards higher 90-day overall mortality than those without IPA (9/25 vs. 9/54; p=0.057), with a weaker trend observed for 30-day overall mortality (8/26 vs. 9/56; p=0.127).
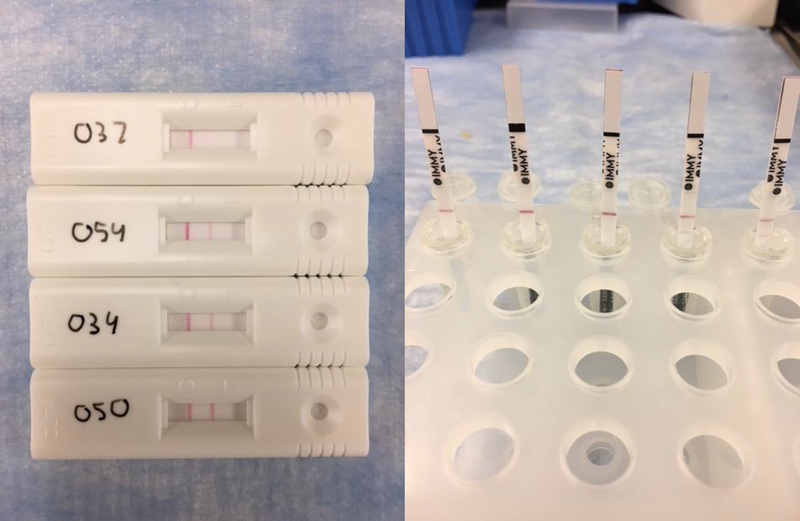
For both the LFD and LFA, the strength of the positive result (Figure 2) correlated with BALF GM levels. BALF GM levels were significantly higher in those with at least a ++ positive test result (n=14) versus + positive test results (n=15) with the Aspergillus-specific LFD 15 min (median 5.51 ODI, IQR 1.19–6.15 ODI versus median 1.59 ODI, IQR 0.6–2.2 ODI; p=0.029; BALF GM levels for those with negative LFD results were median <0.5 ODI; IQR <0.5 – 0.64 ODI). There was a similar trend observed for the Aspergillus Galactomannan LFA, with a tendency towards higher results in those with scores of 2 or higher (n=11) versus those with a score of 1 (n=24) (median 5.62 ODI, IQR 0.61–6.09 ODI versus median 1.14 ODI, IQR <0.5–2.39 ODI; p=0.068; BALF GM levels for those with negative LFD results were median <0.5 ODI ODI; IQR <0.5 – 0.6 ODI).
Figure 2:
Left: Results with the CE-marked Aspergillus-specific Lateral Flow Device Test lateral-flow device test ranging from negative (–) to strong positive (+++). Right: Results with the CE-marked Aspergillus Galactomannan Lateral Flow Assay, ranging from negative (0) to strong positive (4).
Discussion
We evaluated the diagnostic performance of two new BALF point-of care diagnostic tests for patients at risk for IPA but without neutropenia or underlying hematologic malignancy. Our study reports two major findings. First, classification of IPA differed substantially when utilizing revised EORTC/MSG or Blot criteria, outlining the need for broader and improved consensus definitions for classifying IPA in non-neutropenic patients. Second, both point-of-care tests for IPA showed comparable performance, with sensitivities and specificities in the 60–70% range when used alone, increasing to 80% when used in combination.
Non-neutropenic patients who develop IPA continue to suffer from poor diagnostic algorithms and delayed access to novel diagnostics. A critical reason is the missing gold-standard for defining IPA in non-neutropenic patients, where different pathogenesis (i.e., airway invasive growth of Aspergillus spp. versus angioinvasive growth in neutropenic patients) (18) results in “atypical” clinical and radiological presentations of IPA, deviating significantly from what has been defined as “typical presentation” in neutropenic patients (17). For example, fever is only present in 70% of non-neutropenic patients compared to over 95% of neutropenic patients, and cough and chest pain are significantly less commonly observed in non-neutropenic patients (8). “Typical” signs of IPA on computed tomography (CT) such as the “halo sign” or “air crescent sign” are atypical in non-neutropenic patients and occur in only 30%−50% of cases (8, 22, 23).
In our study, 17 patients fulfilled either criteria of putative IPA according to Blot or probable IPA according to EORTC/MSG but not both, while 10 patients fulfilled both criteria. Overall, putative IPA classification according to Blot criteria showed some correlation with higher mortality, while probable IPA classification according to EORTC/MSG did not. While Blot criteria seem to be preferable to revised EORTC/MSG criteria in non-neutropenic patients, there are two important limitations to note. First, the original Blot criteria are applicable only to a small proportion of non-neutropenic patients with IPA, namely those with Aspergillus spp. detection in BALF culture. Sensitivity of BALF culture for IPA, however, only ranges from 29% - 58% in previous studies of proven IPA (3, 19). BALF culture was only 31% sensitive in our study. To overcome this limitation, we tried to broaden applicability of Blot definitions by including BALF GM > 1.0 ODI as entry criterion in this study. However, future studies are needed to evaluate this approach, as well as evaluate whether introduction of GM with a lower cut-off of 0.5 ODI may yield superior performance. Second, symptoms as defined by Blot criteria may occur late in the course of disease, making them less applicable for earlier stages of IPA in non-neutropenic patients, where 31% do not have fever and 72% lack cough (8). This is outlined by one of our cases, who fulfilled probable IPA criteria first (plus had positive results with both the LFD and LFA and Aspergillus terreus growth in BALF culture) but initially did not fulfill Blot criteria due to lack of symptoms, although they did 14 days later when symptoms began. In addition, 80% of cases who fulfilled Blot entry criteria, but not criteria of putative IPA, did not have predefined “typical” signs and symptoms according to Blot, while fulfilling all other criteria and also having very frequently positive test results with the LFA and/or LFD.
Overall performance of the LFD and LFA showed sensitivities ranging between 58% and 69%, with specificities between 68% and 75%. In particular, the LFD test correlated well with BALF GM levels. This performance may be deemed acceptable given the imperfect definitions of IPA in non-neutropenic patients. In contrast, a recent smaller study reported markedly higher sensitivities (78%−89%) and specificities (88%−100%) for both the LFD and the LFA in patients with hematological malignancies (28), where better definitions of IPA exist. Overall sensitivity and specificity observed in this study were below those published for the LFD prototype test in comparable patient collectives (3, 11), which was surprising, given that the newly formatted LFD was previously shown to be equally sensitive but more specific than the prototype (25). Importantly, when the LFA and LFD were combined, sensitivity increased to 81% while specificity remained just above 60%, making this a reasonable approach in patients with high clinical suspicion of IPA. Importantly, this study could not evaluate performance of conventional GM EIA and culture as they were both used as mycological standard for defining probable and putative IPA. In patients with probable and/or putative IPA and Aspergillus spp. growth in culture, positivity rates for the LFD and LFA were comparable to those of BALF GM. Only one patient in this study had histologically proven IPA, and had negative BALF GM, negative Aspergillus LFA, but positive Aspergillus LFD.
Our study has several limitations, including its single-center design, which meant that the numbers of putative/probable and proven cases of IPA were low. While larger cohorts of proven IPA cases may overcome some of the limitations of the current IPA classification and may allow for evaluation of BALF GM against the new point-of-care assays, proven IPA mostly represents advanced disease stages so the diagnostic performances of biomarkers and tests in proven IPA may not translate to similar performance in earlier stages of disease.
In conclusion, our study indicates that both the LFA and the LFD tests may be useful for point-of-care diagnosis of IPA in BALF in non-neutropenic patients when IPA is clinically suspected. Due to imperfect specificities, these point-of-care tests may be more useful for targeted testing in those patients in whom there is clinical suspicion of IPA, rather than for general screening of all patients with BALF. Our study also outlines the need for updated and more broadly applicable consensus definitions for classification of IPA in non-neutropenic patients. Work on these new definitions is currently in progress (30).
Acknowledgments
Transparency Declaration
Aspergillus-specific LFDs were provided by OLM Diagnostics, Newcastle upon Tyne, UK, while Aspergillus Galactomannan LFAs were provided by IMMY, Norman, Oklahoma, USA. Neither company had a role in study design, data collection, analysis, interpretation, decision to publish, in the writing of the manuscript, or in the decision to submit the manuscript for publication. The work was also partially supported by grants from the National Institutes of Health: MH113477, and P30MH062512. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- LFD
Aspergillus-specific Lateral Flow device Tests
- BALF
Bronchoalveolar lavage fluid
- CT
Computed tomography
- (ELIZA)
Enzyme-linked immunosorbent assay
- EORTC/MSG
European Organization for Research and Treatment of Cancer/Mycoses Study Group
- GM
Galactomannan
- LFA
Galactomannan Lateral Flow Assay
- IPA
Invasive pulmonary aspergillosis
- SOT
Solid organ transplant
Footnotes
Potential conflicts of interest
M. Hoenigl received research grants from Gilead.
R. Taplitz served as a consultant for Merck.
All other authors no conflict.
References
- 1.Cornely OA, Lass-Florl C, Lagrou K, Arsic-Arsenijevic V, Hoenigl M. Improving outcome of fungal diseases - Guiding experts and patients towards excellence. Mycoses. 2017;60(7):420–5. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Vidal C, Peghin M, Cervera C, Gudiol C, Ruiz-Camps I, Moreno A, et al. Causes of death in a contemporary cohort of patients with invasive aspergillosis. PLoS One. 2015;10(3):e0120370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prattes J, Flick H, Pruller F, Koidl C, Raggam RB, Palfner M, et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. American journal of respiratory and critical care medicine. 2014;190(8):922–9. [DOI] [PubMed] [Google Scholar]
- 4.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2018;24 Suppl 1:e1–e38. [DOI] [PubMed] [Google Scholar]
- 5.Hoenigl M, Gangneux JP, Segal E, Alanio A, Chakrabarti A, Chen SC, et al. Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: New leadership is about working together. Mycoses. 2018;61(11):885–894. [DOI] [PubMed] [Google Scholar]
- 6.Lenczuk D, Zinke-Cerwenka W, Greinix H, Wolfler A, Prattes J, Zollner-Schwetz I, et al. Antifungal Prophylaxis with Posaconazole Delayed-Release Tablet and Oral Suspension in a Real-Life Setting: Plasma Levels, Efficacy, and Tolerability. Antimicrobial agents and chemotherapy. 2018;62(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duarte RF, Sanchez-Ortega I, Cuesta I, Arnan M, Patino B, Fernandez de Sevilla A, et al. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(12):1696–702. [DOI] [PubMed] [Google Scholar]
- 8.Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43(5):577–84. [DOI] [PubMed] [Google Scholar]
- 9.Bassetti M, Peghin M, Vena A. Challenges and Solution of Invasive Aspergillosis in Non-neutropenic Patients: A Review. Infectious diseases and therapy. 2018;7(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. The Lancet Respiratory medicine. 2018;6(10):782–92. [DOI] [PubMed] [Google Scholar]
- 11.Eigl S, Prattes J, Lackner M, Willinger B, Spiess B, Reinwald M, et al. Multicenter evaluation of a lateral-flow device test for diagnosing invasive pulmonary aspergillosis in ICU patients. Critical care (London, England). 2015;19:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(8):1101–11. [DOI] [PubMed] [Google Scholar]
- 13.Lewis RE, Kontoyiannis DP. Invasive aspergillosis in glucocorticoid-treated patients. Medical mycology. 2009;47 Suppl 1:S271–81. [DOI] [PubMed] [Google Scholar]
- 14.Guinea J, Torres-Narbona M, Gijon P, Munoz P, Pozo F, Pelaez T, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2010;16(7):870–7. [DOI] [PubMed] [Google Scholar]
- 15.Yan X, Li M, Jiang M, Zou LQ, Luo F, Jiang Y. Clinical characteristics of 45 patients with invasive pulmonary aspergillosis: retrospective analysis of 1711 lung cancer cases. Cancer. 2009;115(21):5018–25. [DOI] [PubMed] [Google Scholar]
- 16.Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–9. [DOI] [PubMed] [Google Scholar]
- 17.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(12):1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergeron A, Porcher R, Sulahian A, de Bazelaire C, Chagnon K, Raffoux E, et al. The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood. 2012;119(8):1831–7; quiz 956. [DOI] [PubMed] [Google Scholar]
- 19.Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. American journal of respiratory and critical care medicine. 2008;177(1):27–34. [DOI] [PubMed] [Google Scholar]
- 20.Cordonnier C, Botterel F, Ben Amor R, Pautas C, Maury S, Kuentz M, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(1):81–6. [DOI] [PubMed] [Google Scholar]
- 21.Eigl S, Hoenigl M, Spiess B, Heldt S, Prattes J, Neumeister P, et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Medical mycology. 2017;55(5):528–34. [DOI] [PubMed] [Google Scholar]
- 22.Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. American journal of respiratory and critical care medicine. 2012;186(1):56–64. [DOI] [PubMed] [Google Scholar]
- 23.Nucci M, Nouer SA, Grazziutti M, Kumar NS, Barlogie B, Anaissie E. Probable invasive aspergillosis without prespecified radiologic findings: proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(11):1273–80. [DOI] [PubMed] [Google Scholar]
- 24.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(3):265–73. [DOI] [PubMed] [Google Scholar]
- 25.Hoenigl M, Eigl S, Heldt S, Duettmann W, Thornton C, Prattes J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses. 2018;61(1):40–3. [DOI] [PubMed] [Google Scholar]
- 26.Hoenigl M, Orasch T, Faserl K, Prattes J, Loeffler J, Springer J, et al. Triacetylfusarinine C: A urine biomarker for diagnosis of invasive aspergillosis. The Journal of infection. 2018. 10.1016/j.jinf.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heldt S, Prattes J, Eigl S, Spiess B, Flick H, Rabensteiner J, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: Performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. The Journal of infection. 2018;77(3):235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenks JD, Mehta SR, Taplitz R, Law N, Reed SL, Hoenigl M. Bronchoalveolar Lavage Aspergillus Galactomannan Lateral Flow Assay versus Aspergillus-specific Lateral Flow Device Test for Diagnosis of Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies. J Infect. 2018. 10.1016/j.jinf.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Salzer HJF, Lange C, Honigl M. [Aspergillus in airway material : Ignore or treat?]. Der Internist. 2017;58(11):1150–62. [DOI] [PubMed] [Google Scholar]
- 30.Bassetti M, Scudeller L, Giacobbe DR, Lamoth F, Righi E, Zuccaro V, et al. Developing definitions for invasive fungal diseases in critically ill adult patients in intensive care units. Mycoses. 2018. In Press. [DOI] [PubMed] [Google Scholar]