Abstract
Graves' ophthalmopathy (GO), a complication of Graves' disease (GD), is typified by orbital inflammation, ocular tissue expansion and remodeling and, ultimately, fibrosis. Orbital fibroblasts are key effectors of GO pathogenesis exhibiting exaggerated inflammatory and fibroproliferative responses to cytokines released by infiltrating immune cells. Activated orbital fibroblasts also produce inflammatory mediators that contribute to disease progression, facilitate the orbital trafficking of monocytes and macrophages, promote differentiation of matrix-producing myofibroblasts and stimulate accumulation of a hyaluronan-rich stroma, which leads to orbital tissue edema and fibrosis. Proteomic and transcriptome profiling of the genomic response of ocular and non-ocular fibroblasts to INF-γ and TGF-β1 focused on identification of translationally-relevant therapeutic candidates. Induction of plasminogen activator inhibitor-1 (PAI-1, SERPINE1), a clade E member of the serine protease inhibitor (SERPIN) gene family and a prominent regulator of the pericellular proteolytic microenvironment, was one of the most highly up-regulated proteins in INF-γ- or TGF-β1-stimulated GO fibroblasts as well as in severe active GD compared to patients without thyroid disease. PAI-1 has multifunctional roles in inflammatory and fibrotic processes that impact tissue remodeling, immune cell trafficking and survival as well as signaling through several receptor systems. This review focuses on the pathophysiology of the GO fibroblast and possible targets for effective drug therapy.
Keywords: Graves’ disease, orbitopathy, biomarkers, inflammatory cytokines, TGF-β, PAI-1, SERPINs, fibrosis, plasmin cascade, tissue remodeling
1. Introduction and clinical manifestations of orbitopathy
Graves’ ophthalmopathy (GO), which affects approximately 50 percent of Graves’ disease (GD) patients, exhibits a prominent female gender bias (Wiersinga and Bartalena, 2002). Ocular involvement varies occurring before, with or after the onset of overt thyroid disease and presents as swelling, inflammation, redness, dryness, proptosis, eyelid retraction, foreign body sensation and stromal remodeling which can be extensive (Dik et al., 2016; (Wiersinga and Bartalena, 2002; Wiersinga et al., 1998; Bahn, 2010; Piantanida et al., 2013; Chng et al., 2012). Severely affected individuals (3-5% of those with ocular pathology) experience pain, diplopia, compression of the optic nerve and subsequent loss of vision largely as a consequence of connective tissue and muscular expansion within the confines of the boney orbit (Bahn, 2015, 2016). The most common ocular symptom of early mild GO is upper eyelid retraction coincident with complaints of foreign body sensation, photophobia and tearing. Lagging of the upper eyelid in downgaze (Von Graefe’s sign) and inability to close the eyelids completely (lagophthalmos) are further diagnostic hallmarks as is the presence of corneal erosions and superior limbic keratoconjunctivitis (Dolman, 2018). In moderate GO, inflammation and edema may lead to gaze abnormalities and myopathy; the patient may experience vertical diplopia in upgaze with compromised extraocular muscle (EOM) movement secondary to fibrosis (reviewed in Khalilzadeh et al., 2011). In severe disease, EOM/orbital fat expansion and progressive scarring within the orbit, concomitant with connective tissue and glycosaminoglycan accumulation, manifests a worsening proptosis and predisposes the optic nerve to compression (Dik et al., 2016). Chronic complaints of acute dry eye and pain, elevated intraocular pressure, visual field deficits or vision loss are common when the optic nerve is impinged (Bahn 2010) and may warrant surgery to prevent irreversible blindness (Dolman, 2018). The management of mild GO consists of artificial tears and other topical lubricating options; Tarsorrhaphy is considered an alternative for chronic dry eye complaints. Steroids or radiotherapy can reduce inflammation in patients with more advanced GO and ophthalmic surgery is an option in cases of severe or emergent orbitopathy.
2. Cellular pathophysiology
Most patients with GO have EOM and/or adipose tissue enlargement (Dik et al., 2016). Patients under the age of 40 usually exhibit fat expansion while those over 60 present with muscle involvement (Forbes et al., 1986; Anderson et al., 1989). The EOM fibers in GO are separated by an amorphous, granular material consisting of collagen fibrils and non-sulfated glycosaminoglycans (GAGs), with the most abundant and highly-hydrophilic GAG being hyaluronan (HA) (Smith et al., 1989; Dik et al., 2016). HA synthesis is up-regulated in the GO fibroblast in response to IL-2 and TGF-β1 where it accumulates in the orbit connective tissue (Smith et al., 1989; Bahn, 2003; Khalilizadah et al., 2011). In active disease, the polyanionic charge and high osmotic pressure of this hydrated HA-rich matrix results in edema while likely exacerbating cellular growth, migration and inflammatory cell influx (Bahn, 2010; Hufnagel et al., 1984; Natt and Bahn, 1997; Smith et al., 1989; Bahn, 2010; Guo et al., 2011; Evanko et al., 2012; Itano et al., 2002). Although the specific HA synthase involved in orbital disease is uncertain (Galgoczl et al., 2016), earlier findings implicated HAS2 (Kaback and Smith, 1999; Zhang et al., 2009) and ocular fibroblasts derived from a mouse model of GO express significant levels of HAS2 (Gortz et al., 2016) supporting an association between HAS2 and GO pathogenesis.
Orbital fibroblasts are both the major targets of inflammatory cytokines released by infiltrating immune cells as well as active participants in pathological progression (Bahn, 2010, 2015, 2016; van Steensel et al., 2012a,b; Feng et al., 2017; reviewed in Bahn, 2015; Dik et al., 2016). In early GO, diffuse infiltration of primarily CD4+ T cells are the predominant microenvironmental effectors in the orbit but CD8+ T cells, macrophages, plasma cells and B cells are also evident in the EOM and adipose tissue (Pappa et al., 1997; Eckstein et al., 2004). Type 1 helper T cells produce inflammatory cytokines including IL-2, interferon-γ and TNFα the initial stages (Bahn, 2010). As disease progresses to a more chronic stage, type 2 helper T cells produce additional inflammatory cytokines including IL-4, IL −5, IL-6 and IL-10 (Aniszewski et al., 2000) while macrophages, fibroblasts and adipocytes synthesize and release IL-1, IL-6, IL-16 and TGF-α (Kumar and Bahn, 2003; Hiromatsu et al., 2000; Bahn 2010; Pawlowski et al., 2014). In GD-associated CD34+ T cell dysfunction, recent findings suggest that elevated levels of miR-4443 results in the increased expression of IL-1, IL-6, IL-17 and INF-γ (Qi et al., 2017). The increased expression of this cohort of proinflammatory effectors would be expected to impact virtually all orbit-resident cells including stromal fibroblasts and the vascular system. Orbit fibroblasts exhibit exuberant inflammatory responses when compared with fibroblasts from other anatomical sites as well as contribute to immune cell recruitment and activation (Smith et al., 2008; Dik et al., 2016). Indeed, stimulation of orbital fibroblasts with IL-1β, leukoregulin, INF-γ, or TNF-α results in a greater induction of cytokines, HA, prostaglandins and profibrotic factors compared to dermal cells (reviewed in Dik et al., 2016). It is not immediately obvious, however, whether this reflects their anatomical or developmental uniqueness relative to mesenchymal-derived fibroblasts. An increased number of T helper17 cells, moreover, which synthesize IL-17A in response to stimulation with IL-23, traffic to the orbit during development of thryoid-associated orbitopathy where the secreted IL-17A amplifies the proinflammatory and fibrotic response of resident fibroblasts promoting their differentiation into matrix-producing myofibroblasts and exacerbating disease progression (Fang et al., 2016, 2017; Zhao et al., 2018).
Human ocular cells can also differentiate into high thyrotropin-expressing mature adipocytes (Sorisky et al., 1996; Valyasevi et al., 1999; Starkey et al., 2003) which may, in part, explain the enlargement of orbital fat in GO. A significant fraction of fibroblasts isolated from the EOM of GO patients express the surface marker Thy-1 (Khoo et al., 2008), as opposed to GO orbital fat fibroblasts in which only 50% are Thy-1+ (Khoo et al., 2008). Elevated expression of Thy-1 (CD90) defines a fibroblast subpopulation that produces prostaglandin E2, IL-8 and HA (Khoo et al., 2008). When exposed to TGF-β which is strongly expressed in the orbit of patients with mild and severe GO (Pawlowski et al., 2014), these fibroblasts differentiate into myofibroblasts which participate in repair and fibrosis (Smith et al., 2002; Bahn, 2010). The duration and level of TGF-β exposure, coupled with the intrinsic heterogeneity of the ocular fibroblast cohort, may well dictate disease severity (Smith et al., 2002). TGF-β also increases expression of sphingosine-1-phosphate (S1P), a profibrotic effector for various cell types including GO fibroblasts (Ko et al., 2017). Since S1P receptor blockade attenuates expression of fibrotic and tissue remodeling factors in GO cells (Ko et al., 2017), TGF-β signaling may activate several pathways that contribute to ocular inflammation and fibrosis (e.g., Fang et al., 2016; Dik et al., 2016).
Numerous CD34+ fibrocytes are evident in the orbit of GO patients but not in healthy individuals (Douglas et al., 2010; Peng et al., 2012). These cells may play a pivotal role in the pathogenesis of GO, secondary to their expression of IGF-1 and thyroid stimulating hormone receptors, two well-known autoantigens in GO (e.g., Smith, 2015; Bahn, 2015). Orbital fibroblasts in GO patients, in fact, express higher levels of IGF-1R than non-diseased controls (Smith 2003). Receptor-activating antibodies stimulate signaling in orbital fibroblasts to release inflammatory cytokines, including IL-6 and TNF-α (Douglas et al., 2010). Therapeutic targeting of the IGF-1R with teprotumumab, an inhibiting antibody, may provide a therapeutic option for patients with active GO (Mohyi and Smith, 2017).
3. Molecular basis of orbital disease
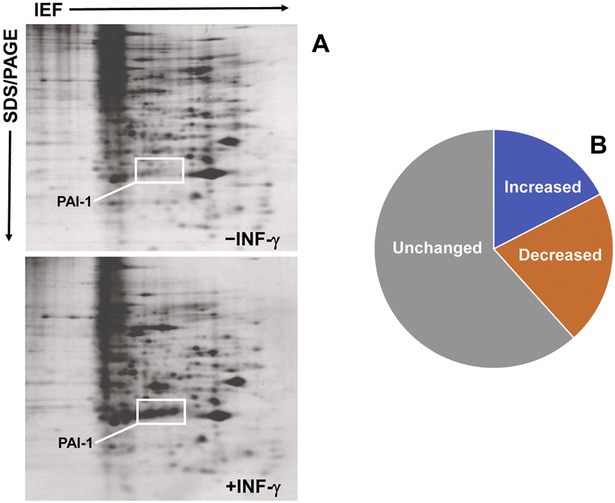
Expression profiling of orbital tissue and ocular fibroblasts from GO patients revealed significant up-regulation of several immediate-early genes including those encoding the inflammation/fibrosis inducers CYR61, connective tissue growth factor (CTGF) and the serine protease inhibitor plasminogen activator inhibitor-1 (PAI-1) (Lantz et al., 2005; Tsai et al, 2015; Smith et al., 1992; Higgins and Smith, 1993) suggesting involvement in disease initiation and/or progression. Since the proinflammatory cytokines interferon-γ (INF-γ) and IL-1α and the potent profibrotic factor TGF-β1 are implicated in Graves’ orbitopathy (Wakelkamp et al., 2003), proteomic and transcriptome analysis of the genomic response of ocular and non-ocular fibroblasts to INF-γ and TGF-β1 focused on identification of potential disease-relevant targets. Of 129 individual proteins resolved in 2-D gel separations of cutaneous fibroblasts suitable for quantitative analysis, the relative abundance of 14% changed in response to INF-γ (Smith and Higgins, 1993a,b). In contrast, 38% of the de novo-synthesized proteins resolved in 2-D gel separations of GO fibroblasts were significantly influenced by exposure to INF-γ (Smith et al., 1992; Higgins and Smith, 1993) with an approximately equal number partitioning between the up- and down-regulated sets (Figure 1). This differential sensitivity to INF-γ reprogramming evident between GO and dermal fibroblasts underscores the exacerbated response of diseased orbital cells to proinflammatory stimuli. Although alterations in specific proteins involved in inflammation and remodeling were also resolved by MALDI mass spectrometry of orbital tissue obtained from GO patients compared to non-thyroid involved controls, more significant up-regulations were evident in GO patients not previously treated with steroids (Matheis et al., 2015). Induction of plasminogen activator inhibitor-1 (PAI-1, SERPINE1), a clade E member of the serine protease inhibitor (SERPIN) gene family and a prominent regulator of the pericellular proteolytic microenvironment (Figure 2), was one of the most highly up-regulated proteins in INF-γ-stimulated GO fibroblasts (Smith et al., 1992). By comparison, INF-γ only modestly increased (5-fold) or attenuated PAI-1 levels in dermal fibroblasts. PAI-1 was virtually undetectable in unstimulated orbital cells compared to expression levels under basal conditions in all dermal fibroblast strains (Smith et al., 1992; Higgins and Smith, 1993). Similarly, large-scale mRNA expression profiling of confirmed that PAI-1 transcript abundance was markedly increased (28-fold) in the intraorbital adipose/connective tissue collected from severe active Graves’ disease patients by lateral decompression surgery compared to that obtained from patients without thyroid disease undergoing cosmetic procedures (Planck et al., 2011). Tear PAI-1 levels, moreover, were also significantly increased in GO patients relative to GD patients without orbitopathy or to normal controls (Ujhelyi et al., 2012). Data analysis confirmed, in fact, that PAI-1 was the only protein to exhibit a statistically increased release in GO relative to GD patients with non-ocular involvement. These findings collectively suggest that the regulation of pericellular proteolysis may be fundamentally different between cutaneous and ocular fibroblasts (Smith et al., 1992).
Figure 1.
Two-dimensional electrophoretic separations of de novo-synthesized 35S-methionine-labeled cellular proteins from control and INF-γ-stimulated GO fibroblasts (A) confirmed a significant up-regulation (>16- to >40-fold) in the induced expression of the various distinct isoelectric variants of PAI-1 described previously (Higgins and Smith, 1993). PAI-1 map coordinates were confirmed using combined immunoblotting and 2-D gel separation criteria established previously (Higgins and Ryan, 1992). Individual protein spots were detected by fluorography and quantified with a Zeiss MOPS III digital image analyzer (Smith et al., 1992). I An approximately equal number of the resolved INF-γ-responsive GO fibroblast protein complement partitioned between the up- and down-regulated sets (B).2IndividuMM-dimensional electrophoretic protein maps derived from 2 individual strains of gamma- spot 55s and 65s-69s induction by interferon gamma in human orbital fibroblasts. Cells were incubated in control
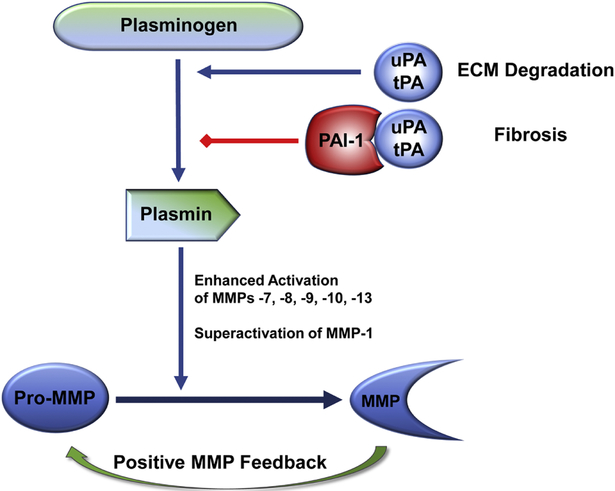
Figure 2.
PAI-1 is a critical factor in the regulation of pericellular proteolysis and tissue fibrosis. Plasminogen activators (urokinase, uPA; tissue-type, tPA) are the physiologically relevant plasmin-generating proteinases that impact ECM homeostasis through a complex and interdependent proteolytic cascade. uPA-stimulated conversion of plasminogen to plasmin leads to an increased downstream activation of matrix metalloproteinases (MMPs). Collectively, plasmin and MMPs dictate the locale and extent of ECM remodeling. Increased PAI-1 expression and/or activity facilitates ECM accumulation and attenuates ECM degradation which, if prolonged or chronic, results in the onset and progression of fibrotic disease (reviewed in Flevaris and Vaughan, 2016; Rabieian et al., 2018; Milenkovic et al., 2017; Higgins et al., 2018).
While TGF-β1 is highly-expressed in the GO orbit (Pawlowski et al., 2014), analysis of differentially-expressed genes indicates that the fibroblast response to TGF-β1 is considerably more complex compared to the rather restricted reprogramming induced by INF-γ (Gardner et al., 2004; Planck et al., 2011). Moreover, as is the case for INF-γ, the TGF-β1-stimulated increase in PAI-1 mRNA and protein levels was significantly greater in GO vs. dermal fibroblasts (Cao et al., 1995) and likely contributes to the matrix expansion characteristic of active Graves’ disease. Whether this is impacted by the elevated HA levels in the GO orbit (Wang et al. 2005, Guo et al. 2011) is unknown, however HA increases PAI-1 expression in human vascular smooth muscle cells (Marutsuka et al. 1998) and a positive correlation exists between HA and PAI-1 produced by human aortic endothelial cells exposed to inflammatory stimuli (Devaraj et al. 2009). In human umbilical vein endothelial cells, moreover, high molecular weight HA both activates the type I TGF-β receptor and induces PAI-1 expression (Park et al. 2012). It is reasonable to assume that similar vascular consequences of HA exposure may occur in the microenvironment of Graves’ orbitopathy.
4. Multifunctional roles of PAI-1 in inflammatory/fibrotic disease
Among its varied functions, PAI-1 negatively regulates the plasmin-dependent pericellular proteolytic cascade, largely through inhibition of the urokinase/tissue-type plasminogen activators (uPA/tPA), effectively limiting ECM degradation and fibrinolytic activity (Figure 2) contributing, thereby, to the initiation and/or progression of fibrotic disease (Ghosh and Vaughan, 2012; Flevaris and Vaughan, 2017). Plasmin targets several ECM proteins directly while also activating various proenzymes of the matrix-metalloproteinase (MMP) family creating a proteolytic stromal remodeling cascade. PAI-1 restricts this process of proteinase activation, thus controlling the locale and extent of ECM degradation by (1) direct inactivation of PAs attenuating, thereby, plasmin generation/MMP activation which increases matrix deposition and promotes fibrosis and (2) targeting uPA receptor-bound uPA complexes for endocytotic clearance via members of the LDL-receptor family (Ghosh and Vaughan 2012). Development of gene-deficient animals confirmed that PAI-1 null mice are, in fact, protected from excessive ECM accumulation as well as lung, liver, kidney and vascular fibrosis and PAI-1 uPA/tPA domain decoys reduced both injury-initiated and established interstitial fibrosis (Gonzalez et al., 2009). Plasmin levels and activity, however, are not affected by PAI-1 deficiency in certain tissues (e.g., kidney) suggesting the involvement of other pathways impacted by PAI-1 knockout (e.g., Flevaris and Vaughan, 2016). Indeed, illumina-based microarray analysis revealed that a number of genes involved in diverse biological processes (e.g., immune system processing, stress response, cytokine and growth factor signaling, cell growth, migration and death, ECM organization and transcriptional regulation) were up- or down-regulated in several organ systems by the genetic absence of PAI-1 (Ghosh et al., 2013). Clearly, the role of PAI-1 in fibrotic disease is complex and likely transcends its function as a regulator of the pericellular proteolytic microenvironment. In this regard, increased PAI-1 levels in the Graves’ disease orbit may well impact various cell types (e.g., endothelial and immune cells, activated pericytes) as well as resident fibroblasts. PAI-1 is, in fact, a key contributor to intravascular coagulopathy, endothelial dysfunction and metabolic syndrome (Aso, 2007) each of which may be exacerbated by the effects of thyroid disease on glucose/insulin metabolism, Insulin is a potent inducer of PAI-1 expression in vivo (Nordt et al., 1995) and hyperinsulinemia is a major factor in PAI-1 elevation (Aso, 2007; De Taeye et al., 2005) where this SERPIN may promote vascular luminal fibrin accumulation. and creation of a procoagulant state (Cozma et al., 2007). Color doppler imaging revealed significant vascular anomalies in dysthyroid ophthalomopathy involving the superior ophthalmic vein (SOV), likely a consequence of optic nerve compression by the expanding EOM (Walasik-Szemplinska et al., 2015; Nakase et al., 1994; Kurioka et al., 2001; Yanik et al., 2005; Perez-Lopez et al., 2011). SOV thrombosis, while uncommon, is a pathophysiologically important complication of GO (Sorrentino et al., 2018) and may well reflect a state of PAI-1-induced coagulopathy and vessel pathology. Small molecule PAI-1 inhibitors (e.g., TM5441, TM5007, Tiplaxtinin) significantly attenuate the development of insulin resistance, intravascular coagulopathy, vascular thrombosis, and fibrosis in several mouse model systems (Lee et al., 2017; Isuhara et al., 2008; Rouch et al., 2015; Hennan et al., 2008; Baxi et al., 2008; Smith et al., 2006). Given the relative ease of PAI-1 inhibitor systemic administration, these findings suggest that the use of anti-PAI-1 functional therapeutics may have efficacy in the management of the orbital consequences of GD.
Recent findings also highlight an unexpected involvement of PAI-1 in innate immunity. PAI-1-deficient mice develop an attenuated inflammatory/fibrotic response following tissue injury while transgenic PAI-1 over-expressing animals exhibit increased macrophage and T-cell infiltration and/or immune cell tissue residence time (Oda et al., 2001; Gupta et al., 2016). In the aorta, monocyte adhesion to the intima is significantly reduced in streptozotocin-treated PAI-1−/− mice reflecting decreases in the inflammatory mediators TNF-α and monocyte chemotactic protein-1 (Zhao et al., 2017). Since PAI-1 provides a “don’t eat me” signal, effectively inhibiting neutrophil efferocytosis (Park et al., 2008; Chao et al., 2011), it appears that this SERPIN may affect cellular influx as well as the intensity and/or duration of the injury-initiated inflammatory phase. Indeed, elevated PAI-1 levels closely mirror systemic and localized inflammation while exogenously-delivered PAI-1 stimulates expression of proinflammatory cytokines (e.g., TNFα and macrophage inflammatory protein-2) in primary bone marrow macrophages (Gupta et al., 2016). The protease inhibitory as well as the vitronectin- or LRP1-binding properties of PAI-1, however, are not necessary for macrophage activation but TLR4 is required, at least in part, since TLR4 neutralizing antibodies or the genetic depletion of TLR4 attenuated PAI-1-induced tissue inflammation (Gupta et al., 2016) suggesting that PAI-1 may function as a matricellular damage-associated molecular pattern (DAMP) TLR ligand (Marquerlot et al., 2006; Cartier-Michaud et al., 2012). PAI-1 appears involved, in fact, in lipopolysaccharide (LPS) signaling and PAI-1 knockdown attenuates LPS-induced increases in macrophage TLR4, MD-2, MyD88, TNF-α, IL-1β and NF-κB levels while vector-driven PAI-1 over-expression enhanced these responses (Ren et al., 2015; Wang et al., 2014). Recent findings implicate specific TLR4 and TLR9 polymorphisms in the pathogenesis of GD and thyroid-associated ophthalmopathy (Cho et al., 2017). Although the mechanism is unclear, PAI-1 participates in host inflammatory responses via TLR4, at least in macrophages (Gupta et a., 2016). This is likely to have a significant impact on fibrogenic outcomes following tissue injury and/or prolonged inflammation as exogenous PAI-1 treatment increased TGF-β1, collagen 1α1, collagen 1α2 and MCP-1 transcripts in non-ocular cells (Nicholas et al., 2005; Jeong et al., 2016; Seo et al., 2009) and may well do so in the orbit. The TLR4/RAGE DAMP-type ligand HMGB1 also activates a subset of genes in the TGF-β1 profibrotic signature that includes PAI-1, CTGF and TGF-β1 (Cheng et al., 2015) suggesting that DAMPs and LPS utilize common and unique signaling pathways that may be exploited in the design of interventional strategies. Collectively, it appears that TLR4 may function as a molecular “switch”, activated by endogenous DAMPs to initiate repair while stimulating TGF-β1 signaling (by down-regulating the TGF-β pseudoreceptor BAMBI) promoting the persistent expression of TGF-β target genes to create and maintain a progressive fibrotic microenvironment (Bhattacharyya and Varga, 2015; Bhattacharyya et al., 2013). This is particularly relevant to the cytokine-driven inflammatory microenvironment and extensive matrix remodeling that typifies the onset and progression of orbitopathy in GD patients. DAMPs, including various fragments of proteoglycans and ECM components, are released from damaged tissues and subsequently activate TLR2, 4, 6, and 9 to initiate downstream signaling triggering and prolonging the inflammatory response (Frevert et al., 2018). Low molecular weight HA, moreover, is a potent stimulator of both TLR2 and TLR4 resulting in the activation of the NF-κB pathway and mobilization of a matrix-active remodeling cascade that includes increased expression of PAI-1 (Frevert et al., 2018). The marked up-regulation of HA in GO fibroblasts may exacerbate disease progression, through up-regulation of TGF-β signaling and PAI-1 expression (Wang et al., 2005; Guo et al., 2011; Marutsuka et al., 1998; Devaraj et al., 2009; Park et al., 2012), facilitating creation of a fibrotic stroma in the confines of the orbit.
5. Conclusions
Expression profiling of orbital tissue and ocular fibroblasts from GO patients revealed significant up-regulation of several potentially disease-relevant genes in response to INF-γ or TGF-β including that encoding the serine protease inhibitor PAI-1, a downstream effector of the fibrotic response. PAI-1 limits matrix degradation by negatively impacting the plasmin-activated pericellular proteolytic cascade to promote tissue fibrosis while promoting the duration and amplitude of the inflammatory response by inhibiting neutrophil efferocytosis. Exogenously-delivered PAI-1, moreover, stimulates TGF-β1 synthesis in several cell types which could be attenuated by small molecule PAI-1 functional inhibitors, suggesting the existence of a PAI-1/TGF-β1-positive feedback mechanism. These findings suggest that PAI-1 may initiate, perhaps maintain, a potential pro-fibrogenic “loop” (Nicholas et al., 2005; Seo et al., 2009) consistent with recent observations that engineered PAI-1 over-expression is sufficient to promote the development of a fibrogenic phenotype (Lian et al., 2018). PAI-1 also stimulates myofibroblast differentiation, a transition blocked by pretreatment with small molecule functional inhibitors (Omori et al., 2016). Several such compounds (e.g., SK-216, TM5275) similarly attenuated bleomycin- and TGF-β1-induced lung fibrosis in mice (Huang et al., 2012; Omori et al., 2016). It is tempting to speculate, therefore, that targeted pharmacological down-modulation of PAI-1 expression or function (Rouch et al., 2015) may provide multi-level therapeutic opportunities to inhibit the onset and progression of tissue inflammatory and fibrotic disease, particularly in the context of the accessible GO orbit.
Acknowledgements
Supported by NIH grant GM057242, the Roach Family Foundation, the Graver Family Endowment, the Friedman Cancer Research Fund and the Butler Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Anderson RL, Tweeten JP, Patrinely JR, Garland PE, Thiese SM, 1989. Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg. 20, 568–574. [PubMed] [Google Scholar]
- Aniszewski JP, Valyasevi RW, Bahn RS, 2000. Relationship between disease duration and predominant orbital T cell subset in Graves' ophthalmopathy. J. Clin. Endocrinol. Metab 85, 776–780. [DOI] [PubMed] [Google Scholar]
- Aso Y, 2007. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front. Biosci 12, 2957–2966. [DOI] [PubMed] [Google Scholar]
- Bahn RS, 2003. Clinical review 157: pathophysiology of Graves' ophthalmopathy: the cycle of disease. J. Clin. Endocrinol. Metab 88, 1939–1946. [DOI] [PubMed] [Google Scholar]
- Bahn RS, 2010. Graves’ ophthalmopathy. New Eng. J. Med 362, 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn RS, 2015. Current insights into the pathogenesis of Graves’ ophthalmopathy. Horm. Metab. Res 47, 773–778. [DOI] [PubMed] [Google Scholar]
- Bahn RS, 2016. Graves’ disease syndrome: A need for unified therapy. J. Translational Int. Med 4, 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxi S, Crandall DL, Meier TR, Wrobleski S, Hawley A, Farris D, Elokdah H, Sigler R, Schaub RG, Wakefield T, Myers D, 2008. Dose-dependent thrombus resolution due to oral plasminogen activator inhibitor (PAI)-1 inhibition with tiplaxtinin in a rat stenosis model of venous thrombosis. Thromb. Haemost 99, 749–758. [DOI] [PubMed] [Google Scholar]
- Bhattacharrya S, Kelley K, Melichian DS, Tamaki Z, Fang F, Su Y, Feng G, Pope RM, Budinger GR, Mutlu GM, Lafyatis R, Radstake T, Feghali-Bostwick C, Varga J, 2013. Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Phys 182, 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Varga J, 2015. Emerging role of innate immune signaling and toll-like receptors in fibrosis and systemic sclerosis. Curr. Rheumatol. Rep 17, 474. [DOI] [PubMed] [Google Scholar]
- Cao HJ, Hogg MG, Martino LJ, Smith TJ, 1995. Transforming growth factor-β induces plasminogen activator inhibitor Type-1 in cultured human orbital fibroblasts. Invest. Ophthalmol. Vis. Sci 36, 1411–1419. [PubMed] [Google Scholar]
- Cartier-Michaud A, Malo M, Charriere-Bertrand C, Gadea G, Anquille C, Supiramaniam A, Lesne A, Delaplace F, Hutzler G, Roux P, Lawrence DA, Barlovatz-Meimon G, 2012. Matrix-bound PAI-1 supports cell blebbing via RhoA/ROCK1 signaling. PLoS One 7, e32204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Majeti R, Weissman IL, 2011. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer 12, 58–67. [DOI] [PubMed] [Google Scholar]
- Cheng M, Liu H, Zhang D, Liu Y, Wang C, Liu F, Chen J, 2015. HMGB1 enhances the AGE-induced expression of CTGF and TGF-β via RAGE-dependent signaling in renal tubular epithelial cells. Am. J. Nephrol 41, 257–266. [DOI] [PubMed] [Google Scholar]
- Chng C-L, Seah LL, Choo DHC, 2012. Ethnic differences in the clinical presentation of Graves’ ophthalmopathy. Best Practice Res. Clin. Endocrinol. Metab 26, 349–258. [DOI] [PubMed] [Google Scholar]
- Cho W, Jang J-P, Choi E-J, Ahn M, Kim SH, Cho KS, Park SH, Back IC, Jung MH, Kim T-G, Suh B-K, 2017. Association of polymorphisms in toll-like receptors 4 and 9 with autoimmune thyroid disease in Korean pediatric patients. Int. J. Endocrinol 2017, article ID 234042188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozma A, Orasan O, Sampelean D, Fodor A, Vlad C, Negrean V, Rednic N, Zdrenghea D, 2009. Endothelial dysfunction in metabolic syndrome. Rom. J. Int. Med 42(2): 133–140. [PubMed] [Google Scholar]
- Davaraj S, Yun JM, Adamson G, Galvez J, Jialal I, 2009. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovasc. Res 84, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Taeye B, Smith Harris L, Vaughan DE, 2005. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes, and cardiovascular disease. Current Opin Pharmacol 5: 149–154. [DOI] [PubMed] [Google Scholar]
- Dik WA, Virakul S, van Steensel L, 2016. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp. Eye Res 142, 83–91. [DOI] [PubMed] [Google Scholar]
- Dolman PJ., 2018. Grading severity and activity in thryoid eye disease. Ophthalmic Plastic Reconst. Surg 34, S34–S40. [DOI] [PubMed] [Google Scholar]
- Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ, 2010. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab 95, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein AK, Quadbeck B, Tews S, Mann K, Kruger C, Mohr CH, Steuhl KP, Esser J, Gieseler RK, 2004. Thyroid associated ophthalmopathy: evidence for CD4(+) γδ T cells, de novo differentiation of RFD7(+) macrophages, but not of RFD1(+) dendritic cells, and loss of γδ and αβ T cell receptor expression. Br. J. Ophthalmol 88, 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN, 2011. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 31, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Huang Y, Wang S, Zhang Y, Luo X, Lu L, Zhong S, Liu X, Li D, Liang R, Miranda P, Gu P, Zhou H, Fan X, Li B, 2016. IL-17A exacerbates fibrosis by promoting the proinflammatory and profibrotic function of orbital fibrobalsts in TAO. J. Clin. Endocrinol. Metab 101, 2955–2965. [DOI] [PubMed] [Google Scholar]
- Fang S, Huang Y, Zong S, Li Y, Zhang Y, Li Y, Sun, Liu X, Wang Y, Zhang S, Xu T, Sun X, Gu P, Li D, Zhou H, Li B, Fan X, 2017. Regulation of orbital fibrosis and adipogenesis by pathogenic Th17 cells in Graves orbitopathy. J. Clin. Endocrinol. Metab 102, 4273–4283. [DOI] [PubMed] [Google Scholar]
- Flevaris P, Vaughan D, 2017. The role of plasminogen activator inhibitor Type-1 in fibrosis. Semin. Thromb. Hemost 43, 169–177. [DOI] [PubMed] [Google Scholar]
- Forbes G, Gorman CA, Brennan MD, Gehring DG, Ilstrup DM, Earnest F IV, 1986. Ophthalmopathy of Graves' disease: computerized volume measurements of the orbital fat and muscle. AJNR Am. J. Neuroradiol 7, 651–656. [PMC free article] [PubMed] [Google Scholar]
- Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L, 2018. Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J. Histochem. Cytochem 66, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgoczi E, Jeney F, Gazdag A, Erdei A, Katko M, Nagy DM, Ujhelyi B, Steiber Z, Gyory F, Berta E, Nagy EV, 2016. Cell density-dependent stimulation of PAI-1 and hyaluronan synthesis by TGF-β in orbital fibroblasts. J. Endocrin 229, 187–196. [DOI] [PubMed] [Google Scholar]
- Gardner H, Strehlow D, Bradley L, Widom R, Farina A, de Fougerolles A, Peyman J, Koteliansky V, Korn JH, 2004. Global expression analysis of the fibroblast transcriptional response to TGFβ. Clin. Exp. Rheumatol 22(3 Suppl 33), S47–S57. [PubMed] [Google Scholar]
- Ghosh AK, Vaughan DE, 2012. PAI-1 in tissue fibrosis. J. Cell. Physiol 227, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Murphy SB, Kishore R, Vaughan DE, 2013. Global gene expression profiling in PAI-1 knockout murine heart and kidney: molecular basis of cardiac-selective fibrosis. PLoS One 8, e63825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Klein J, Chauhan SD, Neau E, Calise D, Nevoit C, Chaaya R, Miravette M, Delage C, Bascands JL, Schanstra JP, Buffin-Meyer B, 2009. Delayed treatment with plasminogen activator inhibitor-1 decoys reduces tubulointerstitial fibrosis. Exp. Biol. Med. (Maywood) 234, 1511–1518. [DOI] [PubMed] [Google Scholar]
- Gortz GE, Moshkelgosha S, Jesenek C, Edelmann B, Horstmann M, Banga JP, Eckstein A, Berchner-Pfannschmidt U, 2016. Pathogenic phenotype of adipogenesis and hyaluronan in orbital fibroblasts from female Graves’ orbitopathy mouse model. Endocrinology 157, 3771–3778. [DOI] [PubMed] [Google Scholar]
- Guo N, Woeller CF, Feldon SE, Phipps RP, 2011. Peroxisome proliferator-activated receptor γ ligands inhibit transforming growth factor-β-induced, hyaluronan-dependent, T cell adhesion to orbital fibroblasts. J. Biol. Chem 286, 11856–11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KK, Xu Z, Castellino FJ, Ploplis VA, 2016. Plasminogen activator inhibitor-1 stimulates macrophage activation through toll-like receptor 4. Biochem. Biophys. Res. Commun 477, 503–508. [DOI] [PubMed] [Google Scholar]
- Hennan JK, Morgan GA, Swillo RE, Antrilli TM, Mugford C, Vlasuk GP, Gardell SJ, Crandall DL, 2008. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. J. Thromb. Haemost 6(9): 1558–1564. [DOI] [PubMed] [Google Scholar]
- Higgins PJ, Smith TJ, 1993. Pleotrophic action of interferon gamma in human orbital fibroblasts. Biochim. Biophys. Acta 1181, 23–30. [DOI] [PubMed] [Google Scholar]
- Higgins PJ and Ryan MP, 1992. Identification of the 52 kDa cytoskeletal-like protein of cytochalasin D-stimulated normal rat kidney (NRK/CD) cells as substrate-associated glycoprotein p52 [plasminogen-activator inhibitor type-1 (PAI-1)]. Expression of p52 (PAi-1) in NRK/CD cells is regulated at the level of mRNA abundance. Biochem. J. 284, 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins SP, Tang Y, Higgins CE, Mian B, Zhang W, Czekay RP, Samarakoon R, Conti DJ, Higgins PJ, 2018. TGF-β1/p53 signaling in renal fibrogenesis. Cell. Signal. 43, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y, 2000. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab 85, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Huang WT, Vayalil PK, Miyata T, Hagood J, Liu RM, 2012. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am. J. Respir. Cell Mol. Biol 46, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel TJ, Hickey WF, Cobbs WH, Jakobiec FA, Iwamoto T, Eagle RC, 1984. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves’ disease. Ophthalmol. 91, 1411–1419. [DOI] [PubMed] [Google Scholar]
- Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, Hamaguchi M, Kimata K, 2002. Abnormal accumulation of hyaluronan matrix diminished contact inhibition of cell growth and promotes cell migration. Proc. Natl. Acad. Sci. USA 99, 3609–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuhara Y, Takahashi S, Nangaku M, Takizawa S, Ishida H, Kurokawa K, van Ypersele de Strihou C, Hirayama N, Miyata T, 2008. Inhibition of plasminogen activator inhibitor-1. Its mechanism and effectiveness on coagulation and fibrosis. Arterioscler. Thromb. Vasc. Biol 28, 672–677. [DOI] [PubMed] [Google Scholar]
- Jeong BY, Uddin MJ, Park JH, Lee JH, Lee HB, Miyata T, Ha H, 2016. Novel plasminogen activator inhibitor-1 and transforming growth factor-beta1 during renal fibrosis in diabetes. Am. J. Nephrol 30, 481–490. [DOI] [PubMed] [Google Scholar]
- Kaback LA, Smith TJ, 1999. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1β in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab 84, 4079–4084. [DOI] [PubMed] [Google Scholar]
- Khalilzadeh O, Noshad S, Rashidi A, Amirzargar A, 2011. Graves’ ophthalmopathy: A review of immunogenetics. Current Genomics 12, 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo TK, Coenen MJ, Schiefer AR, Kumar S, Bahn RS, 2008. Evidence for enhanced Thy-1 (CD90) expression in orbital fibroblasts of patients with Graves' ophthalmopathy. Thyroid 18, 1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Chae MK, Lee JH, Lee EJ, Yoon JS, 2017. Sphingosine-1-phosphate mediates fibrosis in orbital fibroblasts in Graves’ orbitopathy. Invest. Ophthalmol. Vis. Sci 58, 2544–2553. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bahn RS, 2003. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with Graves' ophthalmopathy. J. Clin. Endocrinol. Metab 88, 4246–4250. [DOI] [PubMed] [Google Scholar]
- Kurioka Y, Inaba M, Kawagishi T, Emoto M, Kumeda Y, Inoue Y, Morii H, Nishizawa Y, 2001. Increased retinal blood flow in patients with Graves’ disease: influence of thyroid function and ophthalmopathy. Eur. J. Endocrinol 144(2), 99–107. [DOI] [PubMed] [Google Scholar]
- Lantz M, Vondrichova T, Parikh H, Fernander C, Ridderstrale M, Asman P, Aberg M, Groops L, Hallengren B, 2005. Overexpression of immediate early genes in active Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab 90, 4784–4791. [DOI] [PubMed] [Google Scholar]
- Lee SM, Dorotea D, Jung I, Nakabayashi T, Miyata T, Ha H, 2017. TM5441, a plasminogen activator inhibitor-1 inhibitor, protects against high fat diet-induced non-alcoholic fatty liver disease. Oncotarget 8(52), 89746–89760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian F, Tang J, Armouk A, Samarakoon R, Higgins PJ, 2018. Plasminogen activator inhibitor-1 expression promotes fibrotic renal tubular dysfunction. J. Urol. 199, e115. [Google Scholar]
- Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughan DE, 2004. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 53(2), 336–346. [DOI] [PubMed] [Google Scholar]
- Marquerlot F, Galiacy S, Malo M, Guignabert C, Lawrence DA, d’Ortho MP, Barlovatz-Meimon G, 2006. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. Am. J. Pathol 169, 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutsuka K, Woodcock-Mitchell J, Sakamoto T, Sobel BE, Fujii S, 1998. Pathogenic implications of hyaluronan-induced modification of smooth muscle cell fibrinolysis in diabetes. Coron. Artery Dis 9, 177–184. [DOI] [PubMed] [Google Scholar]
- Matheis N, Lantz M, Grus FH, Ponto KA, Wolters D, Brorson H, Planck T, Shahida B, Pitz S, Pfeiffer N, Gahaly GJ, 2015. Proteomics of orbital tissue in thyroid-associated orbitopathy. J. Clin. Endocrinol. Metab 100, E1523–E1530. [DOI] [PubMed] [Google Scholar]
- Milenkovic J, Milojkovic M, Stoimenov TJ, Djindjic B, Milijkovic E, 2017. Mechanisms of plasminogen activator inhibitor 1 in stromal remodeling and related diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 161, 339–347. [DOI] [PubMed] [Google Scholar]
- Mohyi M, Smith TJ, 2018. IGF-I receptor and thyroid-associated ophthalmopathy. J. Mol. Endocrinol 61, T29–T43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y, Osanai T, Yoshikawa K, Inoue Y, 1994. Color Doppler imaging of orbital venous flow in dysthyroid optic neuropathy. Jpn. J. Ophthallmol 38(1), 80–86. [PubMed] [Google Scholar]
- Natt N, Bahn RS, 1997. Cytokines in the evolution of Graves’ ophthalmopathy. Autoimmunity 26, 129–136. [DOI] [PubMed] [Google Scholar]
- Nichols SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA, 2005. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Inst. 67, 1297–1307. [DOI] [PubMed] [Google Scholar]
- Nordt TK, Sawa H, Fujii S, Sobel BE, 1995. Induction of plasminogen activator inhibitor type-1 (PAI-1) by proinsulin and insulin in vivo. Circulation 91(3), 764–770. [DOI] [PubMed] [Google Scholar]
- Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA, 2001. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 60, 587–596. [DOI] [PubMed] [Google Scholar]
- Omori K, Hattori N, Senoo T, Takayama Y, Masuda T, Nakashima T, Iwamoto H, Fujitaka K, Hamada H, Kohno N, 2016. Inhibition of plasminogen activator inhibitor-1 attenuates transforming growth factor-β-dependent epithelial mesenchymal transition and differentiation of fibroblasts to myofibroblasts. PloS One 11, e0148969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa A, Calder V, Ajjan R, Fells P, Ludgate M, Weetman AP, Lightman S, 1997. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO). Clin. Exp. Immunol 109, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Kim Y, Kim H, Kim K, Lee YS, Choe J, Hahn JH, Lee H, Jeon J, Choi C, Kim YM, Jeoung D, 2012. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFβ receptor interaction via CD44-PKCδ. Mol. Cells 33, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Liu G, Lorne EF, Zhao X, Wang J, Tsuruta Y, Zmijewski J, Abraham E, 2008. PAI-1 inhibits neutrophil efferocytosis. Proc. Natl. Acad. Sci. U.S.A, 105, 11784–11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski P, Reszec J, Eckstein A, Johnson K, Grzybowski A, Chydzewski L, Mysliwiec J, 2014. Markers of inflammation and fibrosis in the orbital fat/connective tissue of patients with Graves’ orbitopathy: clinical implications. Mediators Inflamm 2014, article ID 412158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Li C, Wang X, Liu X, Han C, Jin T, Liu S, Zhang X, Zhang H, He X, Xie X, Yu X, Wang C, Shan L, Fan C, Shan Z, Teng W, 2016. Increased toll-like receptors activity and TLR ligands in patients with autoimmune thyroid diseases. Frontiers Immunol. 7, article 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lopez M, Sales-Sanz M, Rebolleda G, Casas-Liera P, Gonzalez-Gordaliza C, Jarrin E, Munoz-Negrete FJ, 2011. Retrobulbar ocular blood flow changes after orbital decompression in Graves’ ophthalmopathy measured by color Doippler imaging. Invest. Ophthalmol. Vis. Sci 52(8), 5612–5617. [DOI] [PubMed] [Google Scholar]
- Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L, 2013: Prevalence and natural history of Graves’ orbitopathy in the XXI century. J. Endocrinol. Invest 36, 444–449. [DOI] [PubMed] [Google Scholar]
- Planck T, Parikh H, Brorson H, Martensson T, Asman P, Groop L, Hallengren B, Lantz M, 2011. Gene expression in Graves’ ophthalmopathy and arm lymphedema: similarities and differences. Thyroid 21, 663–674. [DOI] [PubMed] [Google Scholar]
- Qi Y, Zhou Y, Chen X, Ye L, Zhang Q, Huang F, Cui B, Lin D, Ning G, Wang W, Wang S, 2017. MicroRNA-4443 causes CD4+ T cells dysfunction by targeting TNFR-associated Factor 4 in Graves’ disease. Front. Immunol 8, 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabieian R, Boshtam M, Zareei M, Kouhpayeh S, Masoudifar A, Mirzaei H, 2018. Plasminogen activator inhibitor type-1 as a regulator of fibrosis. J. Cell. Biochem 119, 17–27. [DOI] [PubMed] [Google Scholar]
- Ren W., Wang Z, Hua F, Zhu L, 2015. Plasminogen activator inhibitor-1 regulates LPS-induced TLR4/MD-1 pathway activation and inflammation in alveolar macrophages. Inflammation 38, 384–393. [DOI] [PubMed] [Google Scholar]
- Rouch A, vanucci-Bacque C, Bedos-Belval F, Baltas M, 2015. Small molecules inhibitors of plasminogen activator inhibitor-1 – an overview. Europ. J. Med. Chem 92, 619–636. [DOI] [PubMed] [Google Scholar]
- Seo JY, park J, Yu MR, Kim YS, Ha H, Lee HB, 2009. Positive feedback loop between plasminogen activator inhibitor-1 and transforming growth factor-β1 during renal fibrosis in diabetes. Am. J.Nephrol 30, 481–490. [DOI] [PubMed] [Google Scholar]
- Smith LH, Dixon JD, Stringham JR, Eren M, Elokdah H Crandall DL, Washington K, Vaughan DE, 2006. Pivotal role of PAI-1 in a murine model of hepatic vein thrombosis. Blood 107(1): 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, 2003. The putative role of fibroblasts in the pathogenesis of Graves' disease: evidence for the involvement of the insulin-like growth factor-1 receptor in fibroblast activation. Autoimmunity 36, 409–415. [DOI] [PubMed] [Google Scholar]
- Smith TJ, 2015. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat. Rev. Endocrinol 11, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Ahmed A, Hogg MG, Higgins PJ, 1992. Interferon-gamma is an inducer of plasminogen activator inhibitor type 1 in human orbital fibroblasts. Am. J. Physiol 23, C24–C29. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Bahn RS, Gorman CA, 1989. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr. Rev 10, 366–391. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Higgins PJ, 1993a. Interferon gamma regulation of de novo protein synthesis in human dermal fibroblasts in culture is anatomic site dependent. J. Invest. Dermatol 100, 288–292. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Higgins PJ, 1993b. Bidimensional gel electrophoretic analysis of protein synthesis and response to interferon-gamma in cultured human dermal fibroblasts. Biochim. Biophys. Acta 1181, 300–306. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A, 2002. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab 87, 385–392. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Tsai CC, Shih MJ, Tsui S, Chen B, Naik V, King CS, Press C, Kamat S, Goldberg RA, Phipps RP, Douglas RS, Gianoukakis AG, 2008. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid 18, 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorisky A, Pardasani D, Gagnon A, Smith TJ, 1996. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J. Clin. Endocrinol. Metab 81, 3428–3431. [DOI] [PubMed] [Google Scholar]
- Sorrentino D, Taubenslag KJ, Bodily LM, Duncan K, Stefko T, Yu JY, 2018. Superior ophthalmic vein thrombosis: A rare complication of Graves’ orbitopathy. Orbit 37(3), 175–178. [DOI] [PubMed] [Google Scholar]
- Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M, 2003. Adipose thyrotrophin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J. Mol. Endocrinol 30, 369–380. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Wu SB, Chang PC, Wei YH, 2015. Alteration of connective tissue growth factor (CTGF) expression in orbital fibroblasts from patients with Graves’ ophthalmopathy. PLoS One 10: e0143514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujhelhy B, Gogolak P, Erdei A, Nagy V, Balazs E, Rajnavolgyi E, Berta A, Nagy EV, 2012. Graves’ orbitopathy results in profound changes in tear composition: A study of plasminogen activator inhibitor-1 and seven cytokines. Thyroid 22, 407–414. [DOI] [PubMed] [Google Scholar]
- Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS, 1999. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J. Clin. Endocrinol. Metab 84, 2557–2562. [DOI] [PubMed] [Google Scholar]
- van Steensel L, Paridaens D, van Meurs M, van Hagen PM, van den Bosch WA, Kuijpers RW, Dexhage HA, Hooijkaas H, Dik WA, 2012a. Orbit-inflitrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab 97, E400–E408. [DOI] [PubMed] [Google Scholar]
- van Steensel L, Hooijkaas H, Paridaens D, van den Bosch WA, Kuijpers RW, Dexhage HA, van Hagen PM, Dik WA, 2012b. PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves’ ophthalmopathy patients. J. Clin. Endocrinol.Metab 97, E944–E953. [DOI] [PubMed] [Google Scholar]
- Walasik-Szemplinska D, Pauk-Domanska M, Sanocka U, Sudol-Szopinska I, 2015. Doppler imaging of orbital vessels in the assessment of the activity and severity of thyroid-associated orbitopathy. J. Ultrason 15(63), 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Tung WH, Tang KT, Huang GJ, Wu JC, Chen CC, 2005. TGF-β-induced hyaluronan synthesis in orbital fibroblasts involves protein kinase CβII activation in vitro. J. Cell. Biochem 95, 256–267. [DOI] [PubMed] [Google Scholar]
- Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF, 2003. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves’ opthalmopathy patients. Clin. Endocrinol 58, 280–287. [DOI] [PubMed] [Google Scholar]
- Wang ZH, Ren WUY, Zhu L, Hu LJ, 2014. Plasminogen activator inhibitor-1 regulates LPS induced inflammation in rat macrophages through autophagy activation. Scientific World J 2014, Article ID 189168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WM, Bartalena L, 2002. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid 12, 855–860. [DOI] [PubMed] [Google Scholar]
- Wiersinga WM, Smit T, van der Gaag R, Koornneef L, 1988. Temporal relationship between onset of Graves' ophthalmopathy and onset of thyroidal Graves' disease. J. Endocrinol. Invest 11, 615–619. [DOI] [PubMed] [Google Scholar]
- Yanik B, Conkbayir I, Acaroglu G, Hekimoglu B, 2005. Graves’ ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J. Clin. Ultrasound 33(8), 375–380. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bowen T, Grennan-Jones F, Paddon C, Giles P, Webber J, Steadman R, Ludgate M, 2009. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J. Biol. Chem 284, 26447–26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Le K, Moghadasian MH, Shen GX, 2017. Reduced monocyte adhesion to aortae of diabetic plasminogen activator inhibitor-1 knockout mice. Inflamm. Res 66, 783–792. [DOI] [PubMed] [Google Scholar]
- Zhao J, Lin B, Deng H, Zhi X, Li Y, Liu Y, Bible PW, Li Q, Xu B, Wei L, Yang H, Huang D, 2018. Decreased expression of TIM-3 on Th17 cells associated with ophthalmopathy in patients with Graves’ disease. Curr. Mol. Med 18(2), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]