Abstract
Despite considerable effort aimed at decreasing the incidence of spontaneous preterm birth, it remains the leading cause of perinatal morbidity and mortality. Screening strategies are imperfect. Approaches used to identify women considered by historical factors to be low risk for preterm delivery (generally considered to be women with singleton pregnancies without a history of a previous preterm birth) as well as those at high risk for preterm birth (those with a previous preterm birth, short cervix, or multiple gestation) continue to evolve. Herein, we review the current evidence and approaches to screening women for preterm birth, and examine future directions for clinical practice. Further research is necessary to better identify at-risk women and provide evidence-based management.
Keywords: Cervical length measurement, Premature birth, Clinical prediction, Perinatal morbidity
1. Introduction
Preterm birth (PTB) continues to be one of the leading causes of perinatal morbidity and mortality worldwide [1–3]. The majority (two-thirds) of PTB cases are attributed to spontaneous PTB (SPTB); the remaining one-third are medically indicated, due to maternal or fetal complications [4]. SPTB is classically defined as birth between 200/7 and 370/7 weeks gestation following the spontaneous onset of labor, preterm prelabor rupture of membranes, or premature dilation of the cervix (cervical insufficiency) [5]. Recently, however, some experts have recognized that some spontaneous deliveries late in the mid-second trimester (e.g. 160/7 to 196/7), classically considered to be miscarriages, may also be SPTB due to similarities to classical SPTB with regard to risk factors, presentation, and recurrence. Infants born preterm require prolonged hospitalizations and are at high risk of adverse outcomes, including respiratory difficulty, neurodevelopmental sequelae, necrotizing enterocolitis, feeding difficulties, blindness, deafness, and intraventricular hemorrhage. Preterm infants are also at a higher risk of death both during the neonatal period and up to age five years when compared to infants delivered at term [2,6]. Hence, the health needs of premature infants can be extensive and lifelong, both for the family and society as a whole, and PTB constitutes a major public health problem.
Roughly 11% of infants worldwide are born preterm; of these, the majority of cases occur in low-income countries [7]. PTB continues to be one of the most common pregnancy-related complications in the USA. Though the rate of preterm birth declined modestly in the USA from 2008 to 2014 to 9.57%, this rate rose between 2014 and 2015 to 9.63% [8]. This recent rise was most significant among non-Hispanic black women – a group with an already substantially higher rate of PTB compared to other races. Despite its overall recent downtrend, the rate of PTB remains high, and neonatal and infant mortality associated with PTB and subsequent low birth weight is estimated at 104.6 infant deaths per 100,000 in 2014 in the USA alone [1].
Given the significant societal implications of PTB in the USA and worldwide, considerable attention has been placed on identifying those women at highest risk, focusing on SPTB because it constitutes the majority of premature deliveries. Unfortunately, SPTB is a heterogeneous condition, with multiple underlying etiologies. The greatest risk factor for SPTB is a history of previous SPTB. However, beyond this, due to the heterogeneity of the condition and variety of underlying etiologies and risk factors, the prediction of PTB is challenging. Known epidemiologic risk factors for SPTB, along with the odds of PTB based on each risk factor, are shown in Table 1 [4,9,10]. Though some demographic and baseline patient characteristics provide insight into women that may benefit from closer surveillance, maternal history and historical risk factors traditionally have poor efficacy at identifying women destined to deliver preterm [21]. The objective of this review is to evaluate the current literature surrounding screening modalities for prediction of SPTB in singleton pregnancies. Screening for PTB in multiple gestations encompasses different underlying pathophysiology and is therefore outside the scope of this article. Early detection of pregnancies at highest risk for SPTB may hold promise in the implementation of therapeutic management options and secondary prevention of morbidities associated with SPTB.
Table 1.
Risk factors associated with spontaneous preterm birth.
| Risk factor | RR for PTB <37 weeks gestation (95% CI as applicable) | Ref. |
|---|---|---|
| Previous preterm birth of a singleton gestation | 2.62 (1.99–3.44)a | [11] |
| Short interpregnancy interval (<6 months) | 1.40 (1.24–1.58) | [12] |
| Underweight pre-pregnancy BMI | 1.32 (1.10–1.57) | [13] |
| Low socio-economic status | 1.66 (1.06–2.61) | [11] |
| Non-Hispanic black race | 1.68 (1.06–2.67) | [11] |
| Congenital uterine malformation – canalization defects (e.g. uterine septum) | 2.14 (1.48–3.11) | [14] |
| Congenital uterine malformation – unification defects (e.g. unicornuate, bicornuate) | 2.97 (2.08–4.23) | [14] |
| Maternal smoking | 1.27 (1.21–1.33) | [15] |
| Cocaine abuse | 3.53 (1.65–7.56) | [16] |
| Opioid abuse | 2.86 (1.11–7.36) | [16] |
| Family history of PTB | 1.35 (1.12–1.63)b | [17,18] |
| Pregnancy-specific risk factors | ||
| Shortened mid-trimester cervical length <2.50 cm | 6.9 (4.3–11.1)c | [19] |
| Placental abruption or vaginal bleeding in the first or second trimester | 1.62 (1.22–2.17) | [11] |
| Carriage of male fetus | 1.51 (1.02–2.24)b | [20] |
RR, relative risk; PTB, preterm birth; CI, confidence interval; BMI, body mass index.
Risk depends on other factors that cannot be characterized in the table, such as number of prior preterm births and gestational age of previous preterm births.
Data presented are odds ratios (95% CI).
For preterm birth <35 weeks gestation.
2. Current methods of screening for preterm birth
2.1. Ultrasonographic cervical length assessment
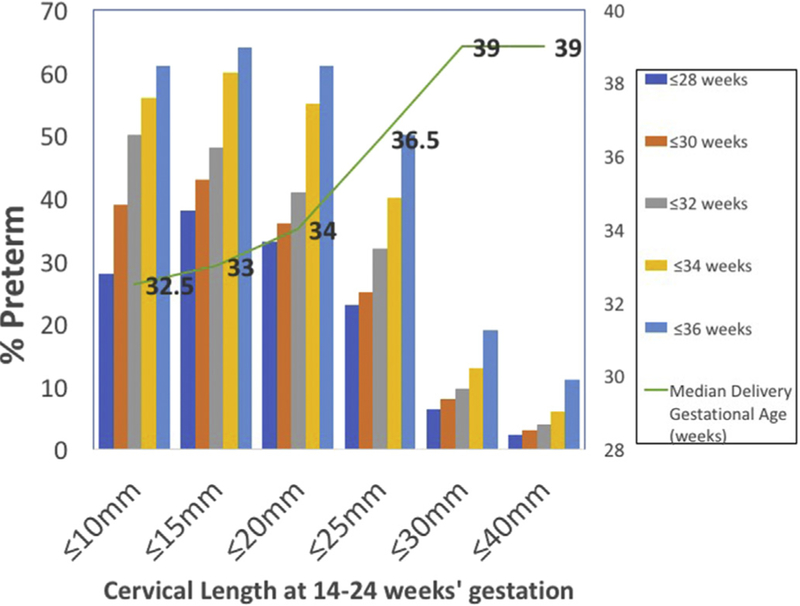
A short mid-trimester cervical length is one of the strongest risk factors for SPTB, as studies have consistently shown that the risk of SPTB is inversely proportional to the length of the cervix (Fig. 1) [11,22]. Transvaginal ultrasound measurement of cervical length is safe, reliable, and highly reproducible when performed by trained providers [23]. Formal training and certification is available through several online educational programs (e.g. the Perinatal Quality Foundation's Cervical Length Education and Review (CLEAR) program (https://clear.perinatalquality.org/), the Fetal Medicine Foundation's cervical assessment certificate of competence, and others [24]). Though proponents of transvaginal ultrasound argue that this approach more accurately identifies a short cervical length compared with transabdominal ultrasound [25,26] and that image quality does not vary with fetal position and maternal body habitus [24,27,28], others argue that a two-step approach where the cervix is visualized transabdominally first and transvaginal cervical length is performed only if it appears <30 or 35 mm may also be adequate [29].
Fig. 1.
Proportion of women delivering preterm at various gestational age cut-offs according to the mid-trimester cervical length (based on n = 6877 women) [12].
In the mid-trimester (16–24 weeks gestation), a transvaginal cervical length <25 mm is considered “short,” as 25 mm corresponds to the 10th percentile for this gestational age [22]. Even among women who have a “normal” cervical length, the risk of SPTB remains inversely proportional to the length of the cervix in mid-pregnancy. Mercer et al. estimated that for each increase of 1 mm in the length of the cervix, the odds for SPTB was 0.91 (RR: 0.89—0.93) [11]. Furthermore, the risk of SPTB is higher if the cervix is found to be short earlier in pregnancy (e.g. a short cervix first detected at 18 weeks gestation carries a higher risk for SPTB compared with a short cervix first detected at 22 weeks gestation). Ultimately, the risk of SPTB in the setting of a short cervical length depends on the a-priori risk of SPTB. The risk is therefore highest among those with a prior SPTB and a short cervix [30]; this combination confers a relative risk for SPTB of 3.3 [31]. By contrast, in women without a prior SPTB, the risk is lower, but still significant.
Because the resultant risk for SPTB if a short cervical length is detected differs by pregnancy history, cervical length screening recommendations set forth by professional societies also differ based on these baseline characteristics. In women with a prior SPTB <37 weeks gestation, both the Society for Maternal–Fetal Medicine and the American College of Obstetricians and Gynecologists recommend screening with serial cervical length from 160/7 to 240/7 weeks gestation. Unfortunately, evidence is conflicting regarding the utility, feasibility, and cost-effectiveness of universal transvaginal cervical length screening in low-risk populations. Though many institutions have implemented universal cervical length screening protocols, evidence regarding the effectiveness of this approach continues to evolve.
Most recently, Esplin et al. [32] reported results from a multicenter, prospective observational cohort that included 9410 nulliparous singleton pregnancies. Transvaginal cervical length assessments were performed twice, at least 4 weeks apart, between 160/7 weeks and 226/7 weeks and again between 220/7 weeks and 306/7 weeks. Overall, 5.0% of nulliparas delivered <370/7 weeks gestation due to SPTB; of these, only 23.3% were identified by transvaginal cervical length screening in the mid-trimester [32]. Therefore, these authors concluded that the low incidence of short cervix limits the utility of transvaginal cervical length measurement as a screening test for subsequent SPTB in low-risk, nulliparous American women. These findings are consistent with those from other smaller studies, suggesting that only a small proportion of those without a history of a prior SPTB can be identified by transvaginal cervical length screening. For example, Boelig et al. performed a secondary analysis of a prospective cohort of singleton pregnancies without a prior history of SPTB undergoing universal cervical length screening between 180/7 and 236/7 weeks [33]. Though women with a short cervical length (≤25 mm) were found to deliver at a significantly earlier gestational age (250/7 ± 1.1 vs 345/7 ± 3.1 weeks, P < 0.01) when compared to women who delivered preterm spontaneously with cervical length >25 mm in the midtrimester, the majority of women (82%) who developed SPTB did not have a short cervical length during screening transvaginal ultrasound [33].
2.2. Fetal fibronectin
Fetal fibronectin (fFN) is an extracellular matrix glycoprotein found at the maternal–fetal interface, between the chorion and decidua [34]. Though elevated prior to 220/7 weeks gestation and after 350/7 weeks gestation, under normal conditions, very low levels of fFN are found in cervico-vaginal secretions (<50 ng/mL) in mid-pregnancy. Levels >50 ng/mL at or after 220/7 weeks gestation are associated with an increased risk of SPTB [35]. This glycoprotein has been extensively studied to predict SPTB in both symptomatic and asymptomatic women. False-positive test results have been associated with sexual intercourse, digital cervical examination, vaginal bleeding, and vaginal lubrication or douching [36].
The fFN test is currently available only as a qualitative test in the USA (positive ≥50 ng/mL). Unfortunately, despite initial enthusiasm for the qualitative fFN evaluation for the prediction of SPTB, a positive fFN test (≥50 ng/mL) has limited predictive ability, as its sensitivity and positive predictive value are low [19,37]. However, a negative fFN test has a high negative predictive value up to 340/7 weeks gestation and strongly suggests that SPTB will not occur within the following weeks [38]. At present, the American College of Obstetricians Gynecologists discourages the use of this test as a screening strategy in asymptomatic women, as there is a lack of evidence for improved perinatal outcomes, decreased utilization of health care resources, or improvements in other metrics after fFN use [23].
Whereas only the qualitative fFN test is available in the USA, the quantitative fFN assay is currently in use in other nations to aid in screening and prediction of SPTB. Several studies have demonstrated improved clinical performance for SPTB screening with the measurement of fFN concentrations in cervico-vaginal fluid of women at high risk of preterm delivery. For example, Abbot et al. [39] prospectively evaluated the predictive accuracy of quantitative fFN for SPTB in asymptomatic high-risk women between 220/7 and 276/7 weeks gestation. They demonstrated that the rate of SPTB at <34 weeks gestation increased with increasing concentration of fFN; moreover, the fFN concentration threshold of 200 ng/mL had a positive predictive value of 37.7%, a specificity of 96%, and an area under the curve of 0.78 (95% confidence interval (CI): 0.73–0.84) to predict SPTB <34 weeks. The authors concluded that the use of quantitative fetal fibronectin measurements enhances the accuracy and risk stratification of women at risk of preterm delivery.
Some researchers have theorized that there may be improved test characteristics with the combination of fFN with other risk factors for SPTB, such as in the setting of a transvaginal cervical length. In the prospective, multicenter, NICHD-funded Preterm Birth Prediction Study [40], 2706 women underwent cervical length screening in the mid-trimester as well as fFN measurements at four points throughout gestation; at each timepoint, the authors noted a significantly increased risk of SPTB if both a positive fFN and a short cervical length (≤25 mm) were concurrently present. Despite this, researchers were unable to determine the sequence of events leading up to the SPTB – whether a short cervix developed prior to a positive fFN, or vice versa. A secondary analysis from this same cohort [19], which included only low-risk women, noted that fFN had a poor sensitivity to detect SPTB (23.4%) by itself, and sensitivity decreased further if fFN was followed by cervical length screening (15.6%). The authors therefore concluded that combined fFN assay and cervical length screening had low sensitivity to predict SPTB before 35 weeks gestation. These findings were corroborated in a systematic review by Berghella et al. [34] and two other recent prospective observational studies [32,41]. However, these studies used quantitative fFN evaluations rather than qualitative measurements.
At present, further research is needed to determine whether widespread use of the quantitative fFN assay, in combination with other screening modalities such as cervical length, can optimize neonatal outcomes for women at risk of SPTB, regardless of previous history of SPTB.
2.3. Maternal serum, amniotic fluid, and cervico-vaginal fluid inflammatory biomarkers
Considerable attention has been placed on identifying other potential biomarkers aimed at screening for SPTB. Of particular interest are biomarkers involved in inflammation and infection, as SPTB is thought to be strongly mediated by an inflammatory response [42]. Specific pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1α, IL-8, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) are hypothesized to respond to infection at the maternal–fetal interface and stimulate the release of prostaglandins and matrix metalloproteinases, thereby causing uterine contractility and subsequent cervical change [43]. Hence, these markers warrant investigation as potential screening tools for SPTB.
A systematic review and meta-analysis by Wei et al. [44] evaluated the association of these cytokines with SPTB in asymptomatic women. The authors selected studies that collected maternal biologic fluid samples (amniotic fluid, cervico-vaginal fluid, blood) before 37 weeks gestation and before delivery, studies that had SPTB as an outcome measure, and studies whose participants were asymptomatic women. They found that elevated IL-6 and CRP in amniotic fluid, but not plasma, were strongly correlated with increased risk of SPTB among asymptomatic women. Furthermore, elevated cervico-vaginal fluid IL-6 was also strongly associated with SPTB <37 weeks gestation. However, the other inflammatory biomarkers lacked sufficient and consistent evidence of association with SPTB. The authors concluded that elevated levels of IL-6 and CRP in amniotic and cervico-vaginal fluids may be evidence of impending SPTB and may hold future promise as a biomarker screening option. However, their clinical use has not been standardized and would be limited to non-invasive specimen collection of cervico-vaginal fluid, which limits the generalizability of using this as an effective screening modality. Other published literature evaluating the association between these cytokines and SPTB confirms these findings [45,46].
2.4. Serum proteomics
Recently, attention has turned to the field of “omics” – proteomic, transcriptomic, genomic, and metabolomics – and its contribution to the understanding of and screening for SPTB. Notably, proteomic research has sought to develop non-invasive testing methods for early identification of women at risk for SPTB, regardless of prior pregnancy history.
Saade et al. [47] reported results from the multicenter Proteomic Assessment of Preterm Risk (PAPR) study, conducted between 2011 and 2013. The aim of this study was to develop a proteomic profile to aid in prediction of SPTB in asymptomatic women. Following Institute of Medicine guidelines for best practices in “omics” research, the authors validated maternal serum insulin-like growth factor-binding protein 4 (IBP4):sex hormone binding globulin (SHBG) predictor levels obtained from maternal serum at 191/7–206/7 weeks gestation in women with a body mass index (BMI) ranging >22 and ≤ 37 kg/m2. The maternal serum IBP4:SHBG ratio identified 75% of women destined to deliver preterm <37 weeks gestation, regardless of parity. However, women with a history of prior PTB receiving progesterone were excluded from the cohort, and the performance of the proteomic predictor was not tested in combination with clinical variables, such as BMI and other demographic characteristics known to be risk factors for SPTB. Nonetheless, these findings are promising and lead the way for further investigative efforts to identify serum proteomic pathways that detect asymptomatic women at risk of SPTB.
2.5. Genetic factors
SPTB is the result of a complex interaction between multiple causative pathways, including inflammation, socio-economic factors, environmental influences, demographic characteristics, maternal medical conditions, and genetic predispositions. Among its numerous risk factors, the genetic contribution to SPTB has gained considerable attention as a future pathway for early identification of women at risk of SPTB. Genetics may be used as screening for SPTB in two ways, as follows.
First, traditional case–control analyses of genomic DNA, performed after delivery outcomes are known, may be used to screen for – and inform – future PTB risk. The advantage to genomic DNA analyses (e.g. candidate gene studies and genome wide analyses) is that genomic DNA is static and results do not vary from pregnancy to pregnancy or with environmental exposures or other acute changes. One resource, the “Database for Preterm Birth (dbPTB),” is a web-based aggregation tool to organize the genes, genetic variations, and pathways involved in PTB. This database was generated using semantic data mining and natural language processing to extract published literature related to PTB. The dbPTB is publicly available as a resource for investigators interested in the genetics of PTB (www.dbptb.cs.brown.edu) [48]. Genes involved in inflammatory mechanisms, such as IL6, NOS1, TLR3, IFNG (among others) are most consistently implicated in SPTB; these variants are included in dbPTB. Altogether, 617 genes are included in the dbPTB database; the majority have a-priori biological evidence for association with PTB. Though this is a valuable resource and these data are promising, many of the genetic variants in the database have not successfully been validated [49]. Further, genetic screening tests are not yet ready for clinical practice, as the interpretation of results is challenging and there are no known interventions for treating women with specific genetic ‘at-risk’ variants.
Second, screening for functional genetic and epigenetic variants in mid-pregnancy is an evolving area of research in obstetrics. In contrast to the study of genomic DNA variation, epigenetics is the study of genetic changes resulting in modification of gene expression. Epigenetics is considerably less static than genomic DNA and is prone to influence by environmental factors (e.g. smoking, medication exposure), and may differ between pregnancies. The most common epigenetic modification is CpG methylation – that is, the placement of a methyl group on the 5′ position of a cytosine ring to form 5-methylcytosine. In most cases, this hypermethylation results in gene silencing (reduced transcription). In contrast, hypomethylation in a gene promoter region will result in gene activation (increased transcription). Evaluation of gene transcription and gene expression may provide critical insights into the underlying interactions between the maternal genome and the environment and/or interactions with fetal signaling that ultimately contribute to pregnancy maintenance or PTB. In one study of 78 women, DNA methylation in two candidate genes – the prostaglandin E receptor 2 (PTGER2) gene and long interspersed nuclear element-1 Homo sapiens-specific (LINE 1-HS) – repetitive element of cervical cells obtained from vaginal swabs collected between 160 and 196 weeks gestation predicted the length of gestation [50]. Additionally, increased methylation of LINE1-HS DNA was appreciated among women exposed to tobacco smoke, a known risk factor for PTB [50]. DNA methylation patterns vary in maternal blood, umbilical cord blood and placentas of women who deliver preterm; whether these changes are also apparent earlier in pregnancy is unknown and is an active area of research [51,52]. Methylation and gene expression in maternal blood or cervical samples can easily be assessed during early and mid-pregnancy, and may provide the basis for PTB screening tests in the future.
3. Intervention strategies to reduce spontaneous preterm birth in high-risk women
Given the multifactorial nature of SPTB, several preventive interventions have been studied to reduce its incidence and recurrence. Herein we briefly describe these interventions.
Progesterone is arguably the most well-studied medication for PTB prevention. It is thought to have anti-inflammatory and immunomodulatory properties at the maternal–fetal interface, thereby decreasing uterine contractility, prostaglandin release, and subsequent cervical dilation and effacement; however, its full mechanism of action at SPTB prevention is poorly understood. Progesterone supplementation remains the best-known strategy to prevent SPTB in women with a history of prior PTB. This recommendation is based on strong evidence from several randomized trials [53–55] demonstrating a statistically significant reduction in the rate of SPTB in women with a prior history of PTB who received either intramuscular (17α-hydroxyprogesterone caproate) or vaginal progesterone starting in the mid-trimester to 37 weeks gestation. Subsequent systematic reviews and meta-analyses have confirmed these results. Based on level I evidence, the use of progesterone starting in the mid-trimester in women at high risk of PTB has become standard of care in many countries [56].
Moreover, in women without a prior history of PTB who are incidentally found to have a short cervix (≤20 or 25 mm on transvaginal ultrasonography in the mid-trimester), the addition of vaginal progesterone has been shown to reduce the risk of SPTB. This practice is based on evidence by Hassan et al. [55] and Fonseca et al. [57], who separately demonstrated statistically significant reduced rates of SPTB in patients with a sonographic short cervix. On the contrary, more recent data from the OPPTIMUM study [58] – a multicenter, double-blind, randomized trial evaluating the effects of vaginal progesterone on long-term infant outcomes – demonstrated no benefit in SPTB risk reduction in women at risk of SPTB who received vaginal progesterone versus placebo. Further research is needed to better identify women who will benefit from progesterone, and which route of progesterone administration will better serve them based on their genetics, phenotype, and obstetric history.
Finally, cervical cerclage is often used as a surgical modality for management of cervical insufficiency or prevention of recurrent SPTB [59]. During a normal pregnancy, the cervix acts as a physical and structural barrier to ascending pathogens from the vaginal canal [60,61]. Cerclage placement is thought to provide structural support to the poorly functional cervix in women at highest risk of delivering a very preterm infant; however, some women successfully achieve delivery >32 weeks after cerclage, regardless of indication, whereas others fail treatment and still deliver a very preterm infant. Hence, women with a prior SPTB <34 weeks may be offered the options of prophylactic cerclage in the late first trimester or cervical length screening in the mid-second trimester, with subsequent discussion of cerclage placement if the cervix shortens without symptoms of preterm labor based on serial transvaginal ultrasound assessments. As previously described, a shortened mid-trimester cervical length is associated with an increased risk of preterm birth. In women with a prior SPTB <34 weeks and a cervical length <15 mm, cerclage has been shown to prevent birth <35 weeks and reduce pre-viable birth and perinatal mortality [62]. Further studies are needed to address this hypothesis and the underlying mechanism of action of cerclage at preventing SPTB.
Incorporation of these multiple modalities may provide the best success for PTB prevention. Growing evidence demonstrates improvements in outcomes among women receiving care in specialized prematurity clinics or prematurity programs. Newnham et al. [63] performed a prospective population-based cohort study of perinatal outcomes before and after implementation of a comprehensive preterm birth prevention program in Western Australia. They found a statistically significant reduction in SPTB by stipulating an outreach management program for obstetric health care providers, in conjunction with media campaigns for women and their families. Similarly, reductions in the rates of recurrent PTB and perinatal morbidity were observed among women cared for in a prematurity prevention clinic in Salt Lake City, Utah [64]. And lastly, efforts by the Ohio Perinatal Quality Collaborative to begin prenatal care earlier have been associated with increased use and earlier initiation of 17-α-hydroxyprogesterone caproate, with a corresponding lower rate of PTB [65]. These multidisciplinary approaches provide great promise in the field of PTB prevention and will require further validation in other high- and low-income settings.
4. Continued challenges and future directions
4.1. Challenges in phenotyping and the heterogeneous nature of PTB
It has been theorized that heterogeneity of the studied populations – both with regard to maternal and paternal race/ethnicity and PTB phenotype – may contribute to negative study findings, poor reproducibility, and continued challenges in finding optimal screening methods and biomarkers for the detection of SPTB. For example, non-Hispanic black race is a recognized risk factor for all PTB; rates of PTB in black women in the USA are nearly doubled compared to women of other races. Minor allele frequencies are known to vary by race, and studies including women of multiple races or ethnic groups will therefore have results biased towards the null. When studies are restricted to women of only one race or ethnicity, they result in a more homogeneous study population and findings may differ. An example of how race affects results is demonstrated in the analysis evaluating the association between interleukin-6 (IL-6) polymorphisms and SPTB performed by Wu and colleagues [66]. They investigated the association between the IL6 single nucleotide polymorphism rs1800795 genotype and the PTB phenotype, stratified by population (comparing women of European descent to women of other races, including those of heterogeneous origin or admixed populations). They concluded that the CC genotype for IL6 SNP rs1800795 is protective against PTB in women of European descent, but not in heterogeneous or admixed populations in whom the CC genotype frequency is low. These differences by race are also seen in biomarker concentrations. Brou et al. [67] performed a nested case-control study to evaluate racial disparities in biomarker concentrations in maternal plasma, fetal plasma, and amniotic fluid between non-Hispanic blacks and non-Hispanic white women with SPTB, and normalterm birth controls. Thirty-six biomarkers, including markers of inflammation, were analyzed using a protein microarray approach. Biomarker concentrations in maternal, fetal, and intra-amniotic compartments differed between cases and controls, consistent with racial disparities in the biomarker profile in each of the compartments. Interestingly, they concluded that maternal pro-inflammatory changes dominated SPTB in African-Americans.
The above results illustrate that within the already heterogeneous pathophysiology of SPTB, there are also pronounced differences in populations, highlighting the difficulties in identifying a single biomarker to screen for SPTB. Similarly, poor phenotypic definitions may also contribute to negative findings related to SPTB screening studies, as women with SPTB may fall under multiple mechanisms of clinical phenotypes [68,69]. Finally, PTB serves as a final common pathway, and is the downstream effect of many possible interactions between genetics, the environment, fetal signaling, etc. – many aspects of which remain understudied.
4.2. Future research directions
Recently, some researchers have evaluated the utility of screening women with additional cervical imaging parameters as potential predictors of SPTB in addition to the length of the cervix. These include elements such as the ‘cervical consistency index’ and the ‘uterocervical angle.’ The cervical consistency index is a measurement of the compressibility of the cervix. Limited studies have found a correlation between assessment of this parameter in mid-pregnancy (190/7 to 246/7) and gestational age at delivery. They suggest that the cervical consistency index may perform better than cervical length alone, or may serve as an important adjunct to traditional cervical length assessment [70,71]. The utero-cervical angle is a measurement of the intersection of two lines drawn post-imaging – the first line is drawn along the cervical canal and the second is drawn parallel to the lower aspect of the anterior uterine wall at the level of the internal cervical os. One case–control study of 275 women (including 34 women who delivered <34 weeks gestation) found that women who eventually delivered preterm had a wider mean utero-cervical angle during mid-pregnancy (assessed between 180/7 and 236/7 weeks gestation) [72]. These techniques and others evaluating other properties of the cervix are active areas of investigation but not yet routinely integrated into clinical care.
In addition to epigenetic studies and genomic DNA analysis, the association between cell-free fetal DNA (cfDNA) and the onset of parturition has gained considerable attention, and although it is not currently clinically validated, it provides a potential future avenue for PTB screening. In one prospective study, women with spontaneous term labor were found to have a greater cfDNA methylation ratio compared with term controls who did not labor [73]. Phillippe [74] summarized this association in a recent article, which describes the proposed pathway behind these findings. He theorized that levels of cell-free fetal DNA increase throughout gestation and peak at the end of pregnancy. This peak subsequently leads to activation of an immune response involving DNA-sensing pattern-recognition receptors, thereby initiating the parturition cascade. Similarly, SPTB may be triggered by cell-free fetal DNA. A recent study by Dugoff et al. [75] investigated this hypothesis. The authors performed a retrospective cohort study of 1349 women who had undergone cfDNA testing at 10–20 weeks gestation. They found that elevated cfDNA fetal fraction ≥95th percentile at 14.1–20 weeks gestation was significantly associated with SPTB <37 weeks (adjusted odds ratio: 4.59; 95% CI: 1.39–15.2) and <34 weeks (22.0; 5.02–96.9). Future research may involve using this knowledge to screen for SPTB with known putative genetic variants prior to conception or early in gestation, aiming at finding cost-effective genetic screening modalities for SPTB.
The additional avenues for PTB research are endless, and include the vaginal and oral microbiome, further exploration of the fetal contribution to PTB, and more. Hence, given the heterogeneous nature of this condition, multidimensional and multidisciplinary approaches are necessary to gain groundwork on the prediction and prevention of SPTB. As Heyborne recently described in his commentary [76], SPTB must be thought of and studied as a syndrome rather than a “one size fits all” disease. Similar to management of patients with cancer, women with a prior history of SPTB may have different phenotypes, obstetric histories, genomics, and sociodemographic and physical characteristics that will inevitably alter their response to different treatment modalities.
5. Conclusions
SPTB is a complex, multifactorial condition characterized by varying clinical presentation. Whereas advances have been made to identify women at increased risk of SPTB, these practices are best applied to women with a history of prior preterm birth. There remains a lack of consistent and cost-effective screening modalities for early recognition of low-risk patients who subsequently develop SPTB. As knowledge regarding the pathophysiology of SPTB evolves, further research must take into consideration the heterogeneity of this condition and its inherently diverse phenotypic profile.
5.1. Practice points
Historic and current pregnancy risk factors can aid in counseling women regarding their a-priori risk of SPTB and assist clinicians in knowing which women require increased vigilance during pregnancy.
- For women with a prior spontaneous preterm birth <37 weeks gestation, it is recommended to offer supplemental progesterone therapy beginning in mid-pregnancy and to perform screening with serial transvaginal cervical length assessments from 16 to 24 weeks gestation.
- If a short cervix is < 20 mm in an otherwise asymptomatic woman with a prior SPTB history, risks and benefits of cerclage should be discussed with the patient. 17α-Hydroxyprogesterone caproate for preterm birth prevention should be continued.
The use of qualitative fetal fibronectin as a screening modality for spontaneous preterm birth in asymptomatic women is discouraged. By contrast, a negative fetal fibronectin test, when used in symptomatic women at risk for spontaneous preterm birth up to 34 weeks gestation, has a high negative predictive value and strongly suggests that SPTB will not occur within the following weeks.
Other screening modalities, including epigenetic, proteomic, and genomic studies, are under investigation and provide promising prospects for SPTB research.
5.2. Research directions
Clinical phenotyping and its role in cost-effective screening modalities for SPTB.
The use of proteomics and epigenetics to find novel markers to screen asymptomatic women at risk of SPTB.
Refinement of, and combination of, historical and clinical risk factors with biomarkers and genetic factors to provide the most accurate SPTB risk assessment.
Footnotes
Conflict of interest statement
None declared.
References
- [1].Mathews TJ, Driscoll AK. Trends in infant mortality in the United States, 2005–2014. NCHS Data Brief 2017:1–8. [PubMed] [Google Scholar]
- [2].Platt MJ. Outcomes in preterm infants. Public Health 2014;128:399–403. [DOI] [PubMed] [Google Scholar]
- [3].Howson CP, Kinney MV, McDougall L, Lawn JE. Born too soon: preterm birth matters. Reprod Health 2013;10(Suppl. 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].American College of Obstetricians and Gynecologists. Practice bulletin No. 171: management of preterm labor. Obstet Gynecol 2016;128:e155–64. [DOI] [PubMed] [Google Scholar]
- [6].Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005;352:9–19. [DOI] [PubMed] [Google Scholar]
- [7].Morisaki N, Togoobaatar G, Vogel JP, et al. Risk factors for spontaneous and provider-initiated preterm delivery in high and low human development index countries: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. Br J Obstet Gynaecol 2014;121(Suppl. 1):101–9. [DOI] [PubMed] [Google Scholar]
- [8].Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep 2017;66:1. [PubMed] [Google Scholar]
- [9].Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol 2011;35:234–9. [DOI] [PubMed] [Google Scholar]
- [10].Klerman LV, Cliver SP, Goldenberg RL. The impact of short interpregnancy intervals on pregnancy outcomes in a low-income population. Am J Public Health 1998;88:1182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miller ES, Tita AT, Grobman WA. Second-trimester cervical length screening among asymptomatic women: an evaluation of risk-based strategies. Obstet Gynecol 2015;126:61–6. [DOI] [PubMed] [Google Scholar]
- [12].Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000;182:1458–67. [DOI] [PubMed] [Google Scholar]
- [13].Mercer BM, Goldenberg RL, Das A, et al. The preterm prediction study: a clinical risk assessment system. Am J Obstet Gynecol 1996;174:1885–93. discussion 93–5. [DOI] [PubMed] [Google Scholar]
- [14].American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol 2012;120: 964–73. [DOI] [PubMed] [Google Scholar]
- [15].Kagan KO, Sonek J. How to measure cervical length. Ultrasound Obstet Gynecol 2015;45:358–62. [DOI] [PubMed] [Google Scholar]
- [16].Friedman AM, Srinivas SK, Parry S, Elovitz MA, Wang E, Schwartz N. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol 2013;208:190. e1–7. [DOI] [PubMed] [Google Scholar]
- [17].Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. J Matern Fetal Neonatal Med 2012;25:1682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khalifeh A, Berghella V. Not transabdominal! Am J Obstet Gynecol 2016;215: 739–44. e1. [DOI] [PubMed] [Google Scholar]
- [19].Owen J, Iams JD. National institute of child health and human development, maternal–fetal medicine units network. What we have learned about cervical ultrasound. Semin Perinatol 2003;27:194–203. [DOI] [PubMed] [Google Scholar]
- [20].Cho HJ, Roh HJ. Correlation between cervical lengths measured by transabdominal and transvaginal sonography for predicting preterm birth. J Ultrasound Med 2016;35:537–44. [DOI] [PubMed] [Google Scholar]
- [21].Iams JD, Berghella V. Care for women with prior preterm birth. Am J Obstet Gynecol 2010;203:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA 2001;286:1340–8. [DOI] [PubMed] [Google Scholar]
- [23].Esplin MS, Elovitz MA, Iams JD, et al. Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA 2017;317:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boelig RC, Orzechowski KM, Berghella V. Cervical length, risk factors, and delivery outcomes among women with spontaneous preterm birth. J Matern Fetal Neonatal Med 2016;29:2840–4. [DOI] [PubMed] [Google Scholar]
- [25].Berghella V, Hayes E, Visintine J, Baxter JK. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev 2008;(4). CD006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goldenberg RL, Mercer BM, Iams JD, et al. The preterm prediction study: patterns of cervicovaginal fetal fibronectin as predictors of spontaneous preterm delivery. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Am J Obstet Gynecol 1997;177: 8–12. [DOI] [PubMed] [Google Scholar]
- [27].Lukes AS, Thorp JM Jr, Eucker B, Pahel-Short L. Predictors of positivity for fetal fibronectin in patients with symptoms of preterm labor. Am J Obstet Gynecol 1997;176:639–41. [DOI] [PubMed] [Google Scholar]
- [28].Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol 2001;184:652–5. [DOI] [PubMed] [Google Scholar]
- [29].Goepfert AR, Goldenberg RL, Mercer B, et al. The preterm prediction study: quantitative fetal fibronectin values and the prediction of spontaneous preterm birth. The National Institute of Child Health and Human Development, Maternal–Fetal Medicine Units Network. Am J Obstet Gynecol 2000;183: 1480–3. [DOI] [PubMed] [Google Scholar]
- [30].Goldenberg RL, Iams JD, Mercer BM, et al. What we have learned about the predictors of preterm birth. Semin Perinatol 2003;27:185–93. [DOI] [PubMed] [Google Scholar]
- [31].Abbott DS, Hezelgrave NL, Seed PT, et al. Quantitative fetal fibronectin to predict preterm birth in asymptomatic women at high risk. Obstet Gynecol 2015;125:1168–76. [DOI] [PubMed] [Google Scholar]
- [32].Goldenberg RL, Klebanoff M, Carey JC, et al. Vaginal fetal fibronectin measurements from 8 to 22 weeks' gestation and subsequent spontaneous preterm birth. Am J Obstet Gynecol 2000;183:469–75. [DOI] [PubMed] [Google Scholar]
- [33].Jwala S, Tran TL, Terenna C, et al. Evaluation of additive effect of quantitative fetal fibronectin to cervical length for prediction of spontaneous preterm birth among asymptomatic low-risk women. Acta Obstet Gynecol Scand 2016;95: 948–55. [DOI] [PubMed] [Google Scholar]
- [34].Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–7. [DOI] [PubMed] [Google Scholar]
- [35].Bastek JA, Elovitz MA. The role and challenges of biomarkers in spontaneous preterm birth and preeclampsia. Fertil Steril 2013;99:1117–23. [DOI] [PubMed] [Google Scholar]
- [36].Wei SQ Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 2010;116:393–401. [DOI] [PubMed] [Google Scholar]
- [37].Lucaroni F, Morciano L, Rizzo G, et al. Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. J Maternal–Fetal Neonatal Med 2017:1–9. [DOI] [PubMed] [Google Scholar]
- [38].Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. Br J Obstet Gynecol 2011;118:1042–54. [DOI] [PubMed] [Google Scholar]
- [39].Saade GR, Boggess KA, Sullivan SA, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol 2016;214:633. e1–24. [DOI] [PubMed] [Google Scholar]
- [40].Uzun A, Dewan AT, Istrail S, Padbury JF. Pathway-based genetic analysis of preterm birth. Genomics 2013;101:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang H, Baldwin DA, Bukowski RK, et al. A genome-wide association study of early spontaneous preterm delivery. Genet Epidemiol 2015;39:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Burris HH, Baccarelli AA, Motta V, et al. Association between length of gestation and cervical DNA methylation of PTGER2 and LINE 1-HS. Epigenetics 2014;9:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hong X, Sherwood B, Ladd-Acosta C, et al. Genome-wide DNA Methylation associations with spontaneous preterm birth in US Blacks: findings in maternal and cord blood samples. Epigenetics 2017. February 6:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Parets SE, Conneely KN, Kilaru V, Menon R, Smith AK. DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics 2015;10:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003;188:419–24. [DOI] [PubMed] [Google Scholar]
- [46].Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- [47].Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2011;38:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chang HH, Larson J, Blencowe H, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2013;381:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;357:462–9. [DOI] [PubMed] [Google Scholar]
- [50].Norman JE, Marlow N, Messow CM, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet 2016;387:2106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wood SL, Owen J. Vaginal cerclage: preoperative, intraoperative, and postoperative management. Clin Obstet Gynecol 2016;59:270–85. [DOI] [PubMed] [Google Scholar]
- [52].Becher N, Adams Waldorf K, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand 2009;88: 502–13. [DOI] [PubMed] [Google Scholar]
- [53].Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol 2002;187:137–44. [DOI] [PubMed] [Google Scholar]
- [54].Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 2009;201:375. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Newnham JP, White SW, Meharry S, et al. Reducing preterm birth by a statewide multifaceted program: an implementation study. Am J Obstet Gynecol 2017;216:434–42. [DOI] [PubMed] [Google Scholar]
- [56].Manuck TA, Henry E, Gibson J, et al. Pregnancy outcomes in a recurrent preterm birth prevention clinic. Am J Obstet Gynecol 2011;204:320. e1–6. [DOI] [PubMed] [Google Scholar]
- [57].Markham KB, Walker H, Lynch CD, Iams JD. Preterm birth rates in a prematurity prevention clinic after adoption of progestin prophylaxis. Obstet Gynecol 2014;123:34–9. [DOI] [PubMed] [Google Scholar]
- [58].Wu W, Clark EA, Stoddard GJ, et al. Effect of interleukin-6 polymorphism on risk of preterm birth within population strata: a meta-analysis. BMC Genet 2013;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brou L, Almli LM, Pearce BD, et al. Dysregulated biomarkers induce distinct pathways in preterm birth. Br J Obstet Gynecol 2012;119:458–73. [DOI] [PubMed] [Google Scholar]
- [60].Esplin MS, Manuck TA, Varner MW, et al. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol 2015;213:429. e1 –9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Manuck TA, Esplin MS, Biggio J, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol 2015;212:487. e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Parra-Saavedra M, Gomez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol 2011;38:44–51. [DOI] [PubMed] [Google Scholar]
- [63].Banos N, Murillo-Bravo C, Julia C, et al. Mid-trimester sonographic cervical consistency index to predict spontaneous preterm birth in a low-risk population. Ultrasound Obstet Gynecol 2017. March 31. PMID 28370687, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [64].Farras Llobet A, Regincos Marti L, Higueras T, et al. The uterocervical angle and its relationship with preterm birth. J Matern Fetal Neonatal Med 2017:1–11. [DOI] [PubMed] [Google Scholar]
- [65].Herrera CA, Stoerker J, Carlquist J, et al. Cell-free DNA, inflammation, and the initiation of spontaneous term labor. Am J Obstet Gynecol 2017;217:583. e1–8. [DOI] [PubMed] [Google Scholar]
- [66].Phillippe M Cell-free fetal DNA – a trigger for parturition. N Engl J Med 2014;370:2534–6. [DOI] [PubMed] [Google Scholar]
- [67].Dugoff L, Barberio A, Whittaker PG, Schwartz N, Sehdev H, Bastek JA. Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol 2016;215:231. e1–7. [DOI] [PubMed] [Google Scholar]
- [68].Heyborne KD. 17-Alpha hydroxyprogesterone caproate for the prevention of recurrent preterm birth: one size may not fit all. Obstet Gynecol 2016;128: 899–903. [DOI] [PubMed] [Google Scholar]
- [69].Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006;295:1809–23. [DOI] [PubMed] [Google Scholar]
- [70].Han Z, Mulla S, Beyene J, Liao G, McDonald SD. Knowledge Synthesis Group. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol 2011;40:65–101. [DOI] [PubMed] [Google Scholar]
- [71].Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound Obstet Gynecol 2011;38:371–82. [DOI] [PubMed] [Google Scholar]
- [72].Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol 2000;182:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Quesada O, Gotman N, Howell HB, Funai EF, Rounsaville BJ, Yonkers KA. Prenatal hazardous substance use and adverse birth outcomes. J Matern Fetal Neonatal Med 2012;25:1222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Porter TF, Fraser AM, Hunter CY, Ward RH, Varner MW. The risk of preterm birth across generations. Obstet Gynecol 1997;90:63–7. [DOI] [PubMed] [Google Scholar]
- [75].Bhattacharya S, Raja EA, Mirazo ER, et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol 2010;115:1125–33. [DOI] [PubMed] [Google Scholar]
- [76].Manuck TA, Stoddard GJ, Fry RC, Esplin MS, Varner MW. Nonresponse to 17-alpha hydroxyprogesterone caproate for recurrent spontaneous preterm birth prevention: clinical prediction and generation of a risk scoring system. Am J Obstet Gynecol 2016;215:622. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]