Abstract
BACKGROUND:
Familial hypercholesterolemia (FH) and other extreme elevations in low-density lipoprotein cholesterol significantly increase the risk of atherosclerotic cardiovascular disease; however, recent data suggest that prescription rates for statins remain low in these patients. National rates of screening, awareness, and treatment with statins among individuals with FH or severe dyslipidemia are unknown.
METHODS:
Data from the 1999 to 2014 National Health and Nutrition Examination Survey were used to estimate prevalence rates of self-reported screening, awareness, and statin therapy among US adults (n=42 471 weighted to represent 212 million US adults) with FH (defined using the Dutch Lipid Clinic criteria) and with severe dyslipidemia (defined as low-density lipoprotein cholesterol levels ≥190 mg/dL). Logistic regression was used to identify sociodemographic and clinical correlates of hypercholesterolemia awareness and statin therapy.
RESULTS:
The estimated US prevalence of definite/probable FH was 0.47% (standard error, 0.03%) and of severe dyslipidemia was 6.6% (standard error, 0.2%). The frequency of cholesterol screening and awareness was high (>80%) among adults with definite/probable FH or severe dyslipidemia; however, statin use was uniformly low (52.3% [standard error, 8.2%] of adults with definite/probable FH and 37.6% [standard error, 1.2%] of adults with severe dyslipidemia). Only 30.3% of patients with definite/probable FH on statins were taking a high-intensity statin. The prevalence of statin use in adults with severe dyslipidemia increased over time (from 29.4% to 47.7%) but not faster than trends in the general population (from 5.7% to 17.6%). Older age, health insurance status, having a usual source of care, diabetes mellitus, hypertension, and having a personal history of early atherosclerotic cardiovascular disease were associated with higher statin use.
CONCLUSIONS:
Despite the high prevalence of cholesterol screening and awareness, only ≈50% of adults with FH are on statin therapy, with even fewer prescribed a high-intensity statin; young and uninsured patients are at the highest risk for lack of screening and for undertreatment. This study highlights an imperative to improve the frequency of cholesterol screening and statin prescription rates to better identify and treat this high-risk population. Additional studies are needed to better understand how to close these gaps in screening and treatment.
Keywords: cardiovascular diseases, diagnosis, dyslipidemias, hydroxymethylglutaryl-CoA reductase inhibitors, hyperproteinemia type II
Elevations of serum low-density lipoprotein cholesterol (LDL-C) are associated with increased cardiovascular disease morbidity and mortality,1,2 and severe elevations of LDL-C ≥190 mg/dL can indicate forms of familial hypercholesterolemia (FH).3,4 Statins are the first-line therapy in all disorders of elevated cholesterol, including FH, because they have been shown to reduce the number of coronary heart disease (CHD) events and mortality in individuals with severe dyslipidemia.5–8 However, recent data suggest that prescription rates for statins remain low in young adults with severe dyslipidemia or diagnosed FH.9–12 With the advent of new treatments like PCSK9 (proprotein convertase sub-tilisin/kexin type 9) inhibitors for severe dyslipidemia, it is imperative to understand the current gaps in the diagnosis and treatment of FH and severe dyslipidemia.
FH is an autosomal dominant genetic disorder that is characterized by elevated LDL-C, sometimes accompanied by cutaneous manifestations (eg, xanthoma) and significantly increased risk of atherosclerotic cardiovascular disease (ASCVD).13,14 Current estimates place the prevalence of FH at ≈1 in 250 individuals,15 and studies have demonstrated that FH increases the risk of CHD by 13-fold.16 With proper treatment, the risk of ASCVD is greatly reduced, and possibly even equal to that of the unaffected population17; however, many people with FH are never diagnosed or treated. Cascade screening can help to identify individuals with FH using lipid screening combined with family history and, ideally, genotyping.18 The term FH phenotype has also been used in epidemiological studies to describe individuals with severely elevated LDL-C, typically with levels >190 mg/dL, in the absence of available information about genotype or family history.9,13,14 Although FH is a known genetic disorder, severe dyslipidemia can be attributable to other acquired factors and is associated with a 5-fold increase in the risk of CHD over adults with LDL-C levels <130 mg/dL.14
Data from small samples of ambulatory care centers have demonstrated that over one third of patients with either severe dyslipidemia (LDL-C ≥190 mg/dL) or heterozygous FH are not prescribed statins, and only half of these patients are adequately treated.9,11,19 The presence of concurrent ASCVD or diabetes mellitus increased the rate of statin treatment only slightly.9 Although these studies have provided important insights into treatment rates among individuals with severe dyslipidemia, they have focused largely on patients treated at ambulatory care clinics, thereby excluding individuals without a regular source of care. In addition, no study has examined national rates of screening or awareness in conjunction with statin treatment in individuals with FH or characterized sociodemographic correlates of statin therapy in this population to understand which subgroups are at the highest risk of being untreated.
Accordingly, the aim of this study was to use a nationally representative sample to quantify the prevalence rates of self-reported screening, awareness, and statin therapy among US adults with FH and severe dyslipidemia and to identify correlates of self-reported awareness and treatment in this group. Quantifying national rates and predictors of screening, awareness, and treatment is essential for understanding the gaps in screening and treatment to inform interventions that may increase the rates of diagnosis and treatment and, in turn, improve cardiac outcomes.
METHODS
Data Source
Data from the National Health and Nutrition Examination Survey (NHANES) were used for this study. Detailed information on data collection, processing, and weighting procedures can be found online.20 In brief, NHANES is conducted by the National Center for Health Statistics to assess the health and nutritional status of the US population. It uses a stratified, multistage sampling design to obtain a nationally representative sample of the noninstitutionalized civilian population living in the United States. Examining ≈5000 persons annually, NHANES has been continuously operating since 1999. Data are collected through in-home interviews and physical examinations performed at mobile examination centers and include detailed information on participant demographic, socioeconomic, dietary, and health-related characteristics. For this study, we used data from NHANES 1999 to 2014 to maximize the number of participants with FH and to examine time trends in statin treatment. We included all nonpregnant adults aged ≥20 years. All participants provided written informed consent. The study protocol was approved by the National Center for Health Statistics Institutional Review Board. This analysis was deemed exempt under federal regulation 45 CFR §46.101(b). The data, analytic methods, and study materials have been made available to other researchers for purposes of reproducing the results or replicating the procedures.20a
Laboratory Values
Detailed descriptions about blood collection and processing are provided in the NHANES Laboratory/Medical Technologists Procedures Manuals. Specimens for lipid, glycolated hemoglobin, and serum glucose measurement were stored under appropriate refrigerated conditions and shipped weekly to the respective processing centers.
Lipid levels were measured from morning peripheral blood draws with 95% of participants reporting fasting for at least 8 hours. Serum total cholesterol and triglycerides were measured enzymatically; high-density lipoprotein cholesterol was measured by direct immunoassay or by precipitation. LDL-C was calculated using the Friedewald equation if the triglycerides level was ≤4.5 mmol/L (400 mg/dL).21
Definition of FH
We used a modified version of the Dutch Lipid Clinic (DLC) criteria to identify individuals having definite or probable FH. We chose to use the DLC criteria because they are the most easily adaptable for use in population surveys and to allow for comparisons with other studies using the DLC.10,15,22,23 The DLC is a validated algorithm for the diagnosis of clinical FH that classified individuals having definite or probable FH on the basis of LDL-C levels, physical examination findings (xanthoma, arcus cornea), known gene defect causative for FH, and personal and family history of premature ASCVD. Because NHANES lacks the genetic and physical examination findings included in the full DLC, we used a modified version that assigned points based on LDL-C levels (ranging from 8 points for LDL-C >8.5 mmol/L [330 mg/dL] down to 1 point for LDL-C 4.0–4.9 mmol/L [155–190 mg/dL]), personal history of ASCVD (2 points), and family history of early ASCVD in a first-degree relative (1 point). These modified criteria have been used in other population-based surveys to assess the prevalence of FH.12,15 For these criteria, personal ASCVD was defined as having a self-reported history of CHD, angina, heart attack, or stroke before the age of 55 years in men and the age of 60 years in women. Family history of ASCVD was defined as having a first-degree relative (mother, father, sisters, or brother) with a heart attack or angina before the age of 50 years.
For individuals who reported taking lipid-lowering medications or who brought statins to the mobile examination center, we multiplied LDL-C levels by 1.43 to estimate untreated LDL-C levels for inclusion in the DLC criteria.10,15 The prevalence of definite/probable FH varied slightly depending on whether only self-reported lipid-lowering agent or documented statin use was used (Table I in the online-only Data Supplement). In addition to adults with definite/probable FH, we also examined adults with severe dyslipidemia separately, not including the other DLC criteria. Severe dyslipidemia was defined as having LDL-C ≥190 mg/dL after adjustment for lipid-lowering medication use, regardless of FH status.
Variable Definitions
Height and weight were measured with a digital scale and stadiometer. Body mass index was calculated as weight in kilograms divided by height in meters squared. Criteria from the Centers for Disease Control and Prevention were used to define body mass index categories as normal weight (≤24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2).
Blood pressure was measured by auscultation 3 consecutive times after individuals had been seated for a minimum of 5 minutes. Hypertension was defined using the average blood pressure across the 3 measurements of ≥140 mm Hg systolic or ≥90 mm Hg diastolic, or self-reported use of an antihypertensive medication. Prehypertension was defined as an average blood pressure of 120 to 139 mm Hg systolic or 80 to 89 mm Hg diastolic.
Given the large number of participants with missing fasting plasma glucose, we used both fasting plasma glucose and hemoglobin A1c to identify individuals with diabetes mellitus and prediabetes. Diabetes mellitus was defined as either fasting plasma glucose ≥126 mg/dL or hemoglobin A1c ≥6.5%, or self-reported use of insulin or oral agents. Prediabetes was defined as fasting plasma glucose 100 to 125 mg/dL or hemoglobin A1c 5.7% to 6.4%.24
We examined the association of specific sociodemographic and clinical characteristics with self-reported awareness of hypercholesterolemia and statin use. Variables were selected based on prior literature and included age, sex, race/ethnicity, poverty (defined as the ratio of family income to geographic poverty level <133%),25 insurance status, education, place of birth, usual source of care, concurrent hypertension or diabetes mellitus, body mass index, and smoking status.
Outcome Definitions
The primary outcome was current statin use. Participants were asked to bring all medications they were currently taking to the mobile examination center. The medications were recorded as free text without dosing. Participants who did not bring a statin to the examination were assumed to not be taking a statin. Because we lacked information on statin dosage, we defined atorvastatin and rosuvastatin as higher-intensity statins and simvastatin, pravastatin, lovastatin, or fluvastatin as lower-intensity statins. Individual statins and other lipidlowering medications (fibrates, ezetimibe, cholestyramine, niacin) were reported separately. In addition to documented statin use, we also reported rates of self-reported use of any lipid-lowering medication based on participants’ responses to the following questions: “To lower your blood cholesterol, have you ever been told by a doctor or other health professional to take prescribed medicine?” and “Are you now following this advice to take prescribed medicine?” Self-reported awareness of hypercholesterolemia was assessed by affirmative responses to the following question: “Have you ever been told by a doctor or other health professional that your blood cholesterol level was high?” Cholesterol screening within the past 5 years was determined via self-report.
Missing Data
Of the 42 471 individuals included in the sample, 58.5% were missing LDL-C levels and 0.3% and 36.1% were missing data on personal and family history of premature ASCVD, respectively. Participants with missing LDL-C levels were more likely to have normal glucose testing (63.6% versus 44.5%) but less likely to be normotensive (33.7% versus 37.4%) than participants with nonmissing LDL-C. Differences in other demographic and clinical characteristics were minimal. In addition, there were no differences in the prevalence of cholesterol screening or self-reported awareness between participants with missing and nonmissing LDL-C.
Missing covariate data were otherwise minimal with 14.2% of participants missing data on 1 variable, 2.5% on 2 variables, and 5.5% on ≥3 variables. Because of incomplete data for some of the DLC criteria, in particular, LDL-C levels and family history of early ASCVD, missing data were multiply imputed (n=10 data sets) by using a Markov chain Monte Carlo method to account for missing values for the DLC criteria and for model covariates. All variables included in the multivariable analyses with any missing data were imputed by using the same strategy. Given the large number of adults with missing LDL-C criteria, we also repeated the analyses restricted to those with available LDL-C (see Statistical Analyses below). Outcome data were not imputed and were missing in 29.7%, 23.3%, 0%, and 34% of the screening, awareness, documented statin, and self-reported lipid-lowering medication variables.
Statistical Analyses
Data were analyzed by using the survey procedures in SAS version 9.4 (SAS Institute Inc) to account for the complex survey design used in NHANES.20 We estimated the prevalence of definite/probable FH and severe dyslipidemia in the general US population. Rates of cholesterol screening, awareness, and lipid-lowering medication use (documented and self-reported) were calculated among 3 groups: (1) individuals with definite/probable FH, (2) individuals with severe dyslipidemia, and (3) the general population. Rates of screening, awareness, and medication use were compared between individuals with definite/probable FH and the other 2 groups by using Rao-Scott modified χ2 tests. Trends in statin use from 1999 to 2000 to 2013 to 2014 were examined for the 3 comparison groups.
Multivariable logistic regression was used to examine sociodemographic and clinical correlates of hypercholesterolemia awareness and statin treatment among individuals with severe dyslipidemia (LDL-C ≥190 mg/dL) regardless of FH. Given the small number of adults with definite/probable FH, we were unable to examine predictors of hypercholesterolemia awareness and statin use separately in this population. Covariates were selected for inclusion in the model using a backward elimination approach and retained in the model if P<0.05. For all analyses, we used the survey procedures (PROC SURVEYFREQ, SURVEYMEANS, SURVEYLOGISTIC) in the imputed data sets and then used PROC MIANALYZE to combine the results with adjusted variances on the 10 imputed data sets. Results were extrapolated to the US population by using the Current Population Survey according to the NHANES analytic guidelines.20 Sensitivity analyses included (1) restricting the analysis to those with available LDL-C and excluding participants with missing personal (n=12) or family (n=15 315) history of early ASCVD, and (2) varying the LDL-C multiplier for statin therapy based on whether a lower- or higher-intensity statin was used (1.21 and 1.63, respectively).26 For both sensitivity analyses, we repeated analyses for definite/probable FH, hypercholesterolemia awareness, and statin use.
RESULTS
The sample included 42 471 NHANES participants representing 212 million US adults aged ≥20 from 1999 to 2014. Approximately 14 million adults, or 6.6% (standard error [SE], 0.2%) of the population, had severe dyslipidemia (LDL-C ≥190 mg/dL), and of these, 1 million adults (7.2% [SE, 0.5%]) met the DLC criteria for definite/probable FH (Figure I in the online-only Data Supplement). Overall, individuals with definite/probable FH accounted for 0.47% (SE, 0.03%) of the total population (definite, 0.03% [SE, 0.01%]; probable, 0.44% [SE, 0.04%]) (Table I in the online-only Data Supplement).
Sociodemographic characteristics and cardiovascular risk factors for the population stratified by LDL-C levels and FH criteria are provided in Table 1. In comparison with the general population, adults with definite/probable FH were more likely to be born in the United States and to have comorbid diabetes mellitus or hypertension. Consistent with the DLC criteria, they were also more likely to have a personal or family history of early ASCVD. Adults with severe dyslipidemia (with or without FH) were more likely to be older and to have comorbid obesity relative to the general population.
Table 1.
Characteristics of US Adults With Definite/Probable Familial Hypercholesterolemia, Severe Dyslipidemia (Adjusted LDL-C ≥190 mg/dL), and Nonelevated LDL-C
| Characteristic | Definite/Probable FH (n=1 007 020)% (SE) |
Severe Dyslipidemia (LDL-C ≥190 mg/dL) (n=13 999 990) % (SE) |
General Population (N=212 438 580) % (SE) |
P Value* | P Value† |
|---|---|---|---|---|---|
| Age, y | 0.98 | 0.005 | |||
| 20–39 | 13.9 (5.3) | 14.8 (1.0) | 37.0 (0.5) | ||
| 40–59 | 49.3 (7.7) | 49.4 (1.2) | 38.6 (0.4) | ||
| ≥60 | 36.8 (7.0) | 35.8 (1.0) | 24.4 (0.4) | ||
| Female sex | 53.1 (8.0) | 49.3 (1.1) | 51.3 (0.2) | 0.63 | 0.83 |
| Race | 0.04 | 0.046 | |||
| Non-Hispanic white | 72.1 (6.6) | 73.0 (1.4) | 69.5 (1.1) | ||
| Non-Hispanic black | 18.5 (5.4) | 10.2 (0.8) | 11.2 (0.6) | ||
| Mexican American | 8.5 (3.3) | 11.4 (1.0) | 13.2 (0.9) | ||
| Other | 1.0 (1.0) | 5.4 (0.6) | 6.1 (0.3) | ||
| Education | 0.77 | 0.59 | |||
| Less than high school | 19.3 (4.8) | 22.4 (0.9) | 18.9 (0.5) | ||
| High school graduate | 30.1 (7.2) | 26.1 (1.2) | 24.2 (0.4) | ||
| Some college or more | 50.6 (7.5) | 51.5 (1.3) | 57.0 (0.7) | ||
| Insurance status | 0.64 | 0.31 | |||
| Uninsured | 9.6 (3.6) | 14.6 (1.0) | 18.9 (0.4) | ||
| Intermittently insured | 6.3 (4.5) | 5.0 (0.5) | 4.8 (0.1) | ||
| Fully insured | 84.1 (5.7) | 80.4 (1.1) | 76.3 (0.48) | ||
| Poverty (<133% poverty line) | 31.9 (6.6) | 22.9 (1.2) | 22.4 (0.6) | 0.13 | 0.10 |
| US-born | 91.8 (2.9) | 83.3 (1.1) | 83.4 (0.8) | 0.022 | 0.027 |
| Usual source of care | 0.38 | 0.05 | |||
| None | 7.3 (3.4) | 10.7 (0.8) | 17.1 (0.4) | ||
| Hospital outpatient or emergency | 13.8 (4.7) | 18.8 (1.0) | 18.6 (0.7) | ||
| Doctor’s office or clinic | 78.9 (5.4) | 70.5 (1.1) | 64.3 (0.7) | ||
| Body mass index | 0.39 | 0.04 | |||
| Normal weight (≤24.9 kg/m2) | 16.7 (5.7) | 17.4 (0.9) | 32.1 (0.4) | ||
| Overweight (25–29.9 kg/m2) | 29.8 (8.6) | 39.7 (1.3) | 33.6 (0.4) | ||
| Obese (≥30 kg/m2) | 53.5 (8.6) | 42.9 (1.2) | 34.4 (0.4) | ||
| Smoking status | 0.88 | 0.79 | |||
| Never | 47.4 (8.5) | 46.8 (1.3) | 52.7 (0.5) | ||
| Past | 27.5 (6.8) | 30.7 (1.1) | 24.5 (0.4) | ||
| Current | 25.1 (6.6) | 22.4 (1.0) | 22.8 (0.4) | ||
| Glucose tolerance | 0.018 | <0.001 | |||
| Normal | 26.0 (7.2) | 46.8 (1.2) | 62.1 (0.4) | ||
| Prediabetic | 53.3 (8.2) | 35.3 (1.2) | 27.4 (0.4) | ||
| Diabetic | 20.7 (5.8) | 17.9 (0.9) | 10.5 (0.2) | ||
| Blood pressure | <0.001 | <0.001 | |||
| Normal | 21.7 (6.5) | 22.4 (1.0) | 41.1 (0.5) | ||
| Prehypertensive | 2.6 (1.6) | 26.8 (1.2) | 27.7 (0.3) | ||
| Hypertensive | 75.7 (6.7) | 50.8 (1.2) | 31.2 (0.4) | ||
| Personal history of early ASCVD | 49.2 (8.4) | 7.8 (0.6) | 3.6 (0.1) | <0.001 | <0.001 |
| Family history of early ASCVD | 45.8 (8.6) | 16.4 (1.0) | 13.4 (0.3) | <0.001 | <0.001 |
ASCVD indicates atherosclerotic cardiovascular disease; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; and SE, standard error.
P value for comparison between adults with definite/probable FH and adults with severe dyslipidemia.
P value for comparison between adults with definite/probable FH and the general adult population.
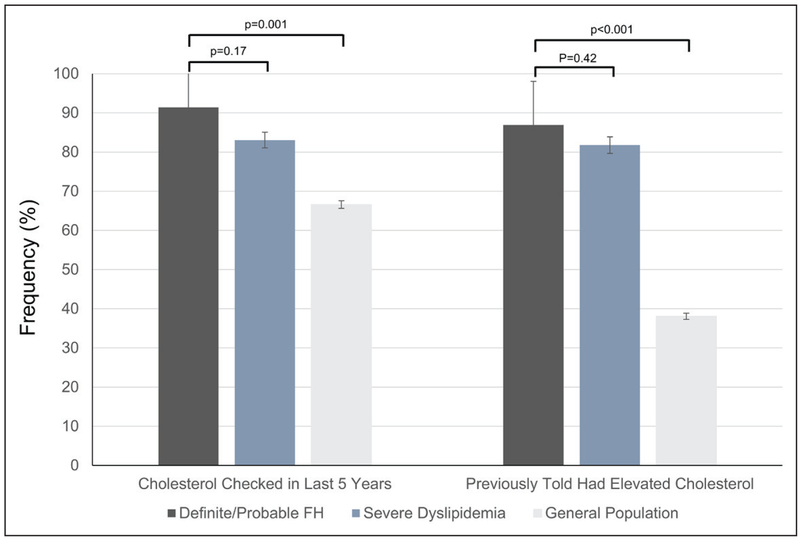
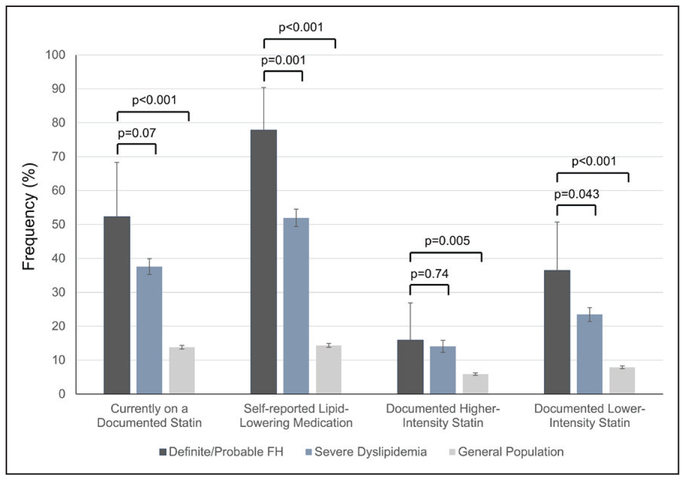
Rates of cholesterol screening and awareness were high (>80%) among adults with definite/probable FH and adults with severe dyslipidemia, but moderate in the general population (Figure 1). Adults with definite/ probable FH were more likely to have been screened and to be aware of their hyperlipidemia than those with severe dyslipidemia. In contrast, statin use was uniformly low; only 52.3% (SE, 8.2%) of adults with definite/probable FH and 37.6% (SE, 1.2%) of adults with severe dyslipidemia were on a documented statin (Figure 2). Less than half of those on statins (30.3% for definite/probable FH and 37.4% for severe dyslipidemia) were prescribed a higher-intensity statin. Rates of individual statin use and documented nonstatin lipidlowering medications are provided in Figures II and III in the online-only Data Supplement. Although rates of self-reported lipid-lowering medication use were higher (77.8% [SE, 6.4%] for adults with definite/probable FH and 52.0% [SE, 1.3%] for adults with severe dyslipidemia) than the general population, it is unclear which medications (ie, statin, nonstatin, or over-the-counter supplement) were included in this determination.
Figure 1. Prevalence (95% confidence interval) of self-reported cholesterol screening and awareness among individuals with definite/probable familial hypercholesterolemia, severe dyslipidemia (adjusted LDL-C ≥190 mg/dL), and the general population.
FH indicates familial hypercholesterolemia; and LDL-C, low-density lipoprotein cholesterol.
Figure 2. Prevalence (95% confidence interval) of documented statin and self-reported lipid-lowering medication use among individuals with definite/probable familial hypercholesterolemia, severe dyslipidemia (adjusted LDL-C ≥190 mg/dL), and the general population.
FH indicates familial hypercholesterolemia; and LDL-C, low-density lipoprotein cholesterol.
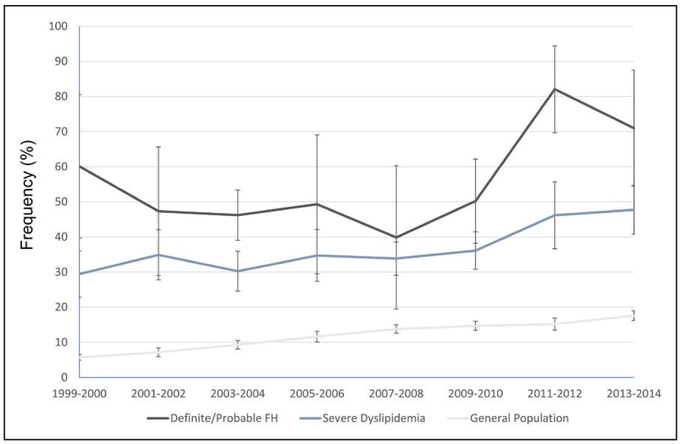
The prevalence of statin use in adults with definite/probable FH, in adults with severe dyslipidemia, and in the general population increased over time (Figure 3). Among individuals with severe dyslipidemia, only 29.4% (SE, 3.4%) were taking statins in 1999 to 2000 in comparison with 47.7% (SE, 3.6%) in 2013 to 2014. There was significant variability in the prevalence of statin use over time among adults with definite/probable FH, likely because of the small sample size; however, there appeared to be a trend toward increasing statin use over time.
Figure 3. Trends in the prevalence (95% confidence interval) of documented statin use among individuals with definite/probable familial hypercholesterolemia, severe dyslipidemia (adjusted LDL-C ≥190 mg/dL), and the general population from 1999 to 2014.
FH indicates familial hypercholesterolemia; and LDL-C, low-density lipoprotein cholesterol.
Sociodemographic and clinical factors associated with cholesterol awareness and statin use in adults with severe dyslipidemia are shown in Tables 2 and 3. Because of the small sample size, we were unable to repeat these analyses for adults with definite/probable FH. Factors independently associated with hypercholesterolemia awareness included older age, absence of poverty, having a usual source of health care, obesity, diabetes mellitus, and having a personal history of early ASCVD (Table 2). Similar factors were associated with statin use including older age, being fully insured, having a usual source of care, diabetes mellitus, hypertension, and having a personal history of early ASCVD (Table 3). It is notable that race, education, and birthplace were not associated with either hypercholesterolemia awareness or statin use.
Table 2.
Sociodemographic and Clinical Characteristics Associated With Hypercholesterolemia Awareness in US Adults With Severe Dyslipidemia (Adjusted LDL-C ≥190 mg/dL)
| Unadjusted | Multivariable Adjusted | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | ||||
| 20–39 | 1.0 | — | 1.0 | — |
| 40–59 | 2.9 (2.0–4.4) | <0.001 | 2.5 (1.6–3.8) | <0.001 |
| ≥60 | 3.3 (2.2–4.9) | <0.001 | 2.7 (1.8–4.0) | <0.001 |
| Female sex | 1.1 (0.9–1.4) | 0.44 | — | — |
| Race | — | — | ||
| Non-Hispanic white | 1.0 | — | ||
| Non-Hispanic black | 1.0 (0.7–1.3) | 0.79 | ||
| Mexican American | 0.9 (0.7–1.2) | 0.53 | ||
| Other | 0.8 (0.5–1.2) | 0.23 | ||
| Education | — | — | ||
| Less than high school | 1.0 | — | ||
| High school graduate | 1.3 (0.9–1.9) | 0.20 | ||
| Some college or more | 1.0 (0.8–1.4) | 0.89 | ||
| Insurance status | — | — | ||
| Uninsured | 1.0 | — | ||
| Intermittently insured | 1.5 (0.8–3.0) | 0.24 | ||
| Fully insured | 1.8 (1.3–2.7) | 0.002 | ||
| Poverty (<130% poverty line) | 0.7 (0.6–1.0) | 0.019 | 0.7 (0.5–0.9) | 0.013 |
| US-born | 1.1 (0.8–1.4) | 0.74 | — | — |
| Usual source of care | ||||
| None | 1.0 | — | 1.0 | — |
| Hospital outpatient or emergency | 2.4 (1.5–3.8) | 0.001 | 1.9 (1.2–3.1) | 0.010 |
| Doctor’s office or clinic | 2.3 (1.5–3.4) | <0.001 | 1.7 (1.1–2.6) | 0.026 |
| Body mass index | ||||
| Normal weight (≤24.9 kg/m2) | 1.0 | — | 1.0 | — |
| Overweight (25–29.9 kg/m2) | 1.1 (0.8–1.6) | 0.67 | 1.1 (0.7–1.6) | 0.83 |
| Obese (≥30 kg/m2) | 1.5 (1.1–2.0) | 0.006 | 1.4 (1.0–1.9) | 0.043 |
| Smoking status | — | — | ||
| Never | 1.0 | — | ||
| Past | 1.1 (0.8–1.5) | 0.43 | ||
| Current | 0.9 (0.6–1.3) | 0.59 | ||
| Glucose tolerance | ||||
| Normal | 1.0 | — | 1.0 | — |
| Prediabetic | 1.7 (1.3–2.2) | 0.001 | 1.5 (1.1–2.0) | 0.006 |
| Diabetic | 1.9 (1.4–2.7) | <0.001 | 1.6 (1.2–2.3) | 0.006 |
| Blood pressure | — | — | ||
| Normal | 1.0 | — | ||
| Prehypertensive | 1.1 (0.8–1.6) | 0.53 | ||
| Hypertensive | 1.9 (1.3–2.6) | <0.001 | ||
| Personal history of early ASCVD | 2.5 (1.3–4.7) | 0.005 | 2.2 (1.2–4.1) | 0.014 |
| Family history of early ASCVD | 1.4 (1.0–2.0) | 0.28 | — | — |
ASCVD indicates atherosclerotic cardiovascular disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; and OR, odds ratio.
Table 3.
Sociodemographic and Clinical Characteristics Associated With Statin Use in US Adults With Severe Dyslipidemia (Adjusted LDL-C ≥190 mg/dL)
| Unadjusted | Multivariable Adjusted | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | ||||
| 20–39 | 1.0 | — | 1.0 | — |
| 40–59 | 3.5 (2.4–5.1) | <0.001 | 2.5 (1.7–3.7) | <0.001 |
| ≥60 | 6.7 (4.7–9.6) | <0.001 | 3.7 (2.6–5.5) | <0.001 |
| Female sex | 1.4 (1.1–1.6) | 0.002 | — | — |
| Race | — | — | ||
| Non-Hispanic white | 1.0 | — | ||
| Non-Hispanic black | 1.1 (0.9–1.3) | 0.56 | ||
| Mexican American | 0.6 (0.5–0.8) | <0.001 | ||
| Other | 0.7 (0.5–1.0) | 0.08 | ||
| Education | — | — | ||
| Less than high school | 1.0 | — | ||
| High school graduate | 1.1 (0.9–1.4) | 0.41 | ||
| Some college or more | 1.1 (0.9–1.4) | 0.27 | ||
| Insurance status | ||||
| Uninsured | 1.0 | — | 1.0 | — |
| Intermittently insured | 2.0 (1.1–3.5) | 0.025 | 1.6 (0.8–2.9) | 0.16 |
| Fully insured | 3.9 (2.6–5.8) | <0.001 | 2.3 (1.5–3.6) | <0.001 |
| Poverty (<133% poverty line) | 0.8 (0.6–0.9) | 0.006 | — | — |
| US-born | 0.8 (0.6–1.0) | 0.017 | — | — |
| Usual source of care | ||||
| None | 1.0 | — | 1.0 | — |
| Hospital outpatient or emergency | 5.7 (3.5–9.4) | <0.001 | 3.6 (2.2–6.0) | <0.001 |
| Doctor’s office or clinic | 6.7 (4.1–10.9) | <0.001 | 3.8 (2.3–6.2) | <0.001 |
| Body mass index | — | — | ||
| Normal weight (≤24.9 kg/m2) | 1.0 | — | ||
| Overweight (25–29.9 kg/m2) | 0.9 (0.7–1.2) | 0.59 | ||
| Obese (≥30 kg/m2) | 1.1 (0.8–1.4) | 0.75 | ||
| Smoking status | — | — | ||
| Never | 1.0 | — | ||
| Past | 1.3 (1.0–1.6) | 0.06 | ||
| Current | 0.7 (0.5–0.9) | 0.008 | ||
| Glucose tolerance | ||||
| Normal | 1.0 | — | 1.0 | — |
| Prediabetic | 1.4 (1.1–1.7) | 0.005 | 1.2 (0.9–1.5) | 0.24 |
| Diabetic | 1.9 (1.5–2.4) | <0.001 | 1.4 (1.1–1.8) | 0.005 |
| Blood pressure | — | |||
| Normal | 1.0 | — | 1.0 | |
| Prehypertensive | 1.1 (0.8–1.4) | 0.72 | 0.9 (0.7–1.3) | 0.69 |
| Hypertensive | 2.2 (1.7–2.8) | <0.001 | 1.4 (1.0–1.9) | 0.036 |
| Personal history of early ASCVD | 2.5 (1.7–3.6) | <0.001 | 2.0 (1.3–2.9) | 0.001 |
| Family history of early ASCVD | 1.2 (0.9–1.5) | 0.28 | — | — |
ASCVD indicates atherosclerotic cardiovascular disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; and OR, odds ratio.
Rates of cholesterol screening, awareness, and lipid-lowering medication use among adults with severe dyslipidemia stratified by those factors associated with statin use (age, insurance status, usual source of health care, diabetes mellitus, and personal history of early ASCVD) are presented in Figure IV in the online-only Data Supplement. The discrepancy between cholesterol screening and treatment rates was most pronounced in younger patients, uninsured patients, and patients without a usual source of care. Among adults aged 20 to 39 years, 62% reported cholesterol screening in the past 5 years, and 64% reported awareness of having hypercholesterolemia, but only 13% were on a documented statin. In contrast, >85% of adults aged ≥60 years had been screened and were aware of their hypercholesterolemia, and 51% were on a documented statin. Similarly, only 29% of uninsured adults reporting recent screening were taking a statin in comparison with 48% of fully insured adults (Figure IV in the online-only Data Supplement).
Sensitivity analyses resulted in findings similar to the primary analyses. Limiting the analyses to those with measured LDL-C and nonmissing personal or family history of early ASCVD yielded a lower prevalence of definite/probable FH (0.33% [SE, 0.06%]) and severe dyslipidemia (5.1% [SE, 0.3%]). However, rates of cholesterol screening, awareness, and statin use were similar to those in the full analysis (Table II in the online-only Data Supplement). When analyses were repeated varying the LDL-C multiplier for lower- versus higher-intensity statin use, the prevalence of definite/probable FH (0.48% [SE, 0.04%]) and severe dyslipidemia (6.4% [SE, 0.2%]) was similar to the primary analyses. Like-wise, rates of cholesterol screening, awareness, and statin use were similar to the primary analyses, but a greater percentage of adults were taking a higher-intensity versus lower-intensity statin as expected (Table III in the online-only Data Supplement).
DISCUSSION
In this nationally representative, cross-sectional analysis of the US adult population, we found that 0.47% of the adult US population has definite/probable FH (or 1 in 212 individuals) but observed a large disconnect between screening and treatment rates in adults with definite/probable FH and adults with severe dyslipidemia. Although rates of cholesterol screening and awareness approached 90% in adults with definite/probable FH, only about half of these patients were taking a documented statin at the time of assessment, and only 30% of those on statins were taking a higher-intensity statin. Rates of statin use were even lower among adults with severe dyslipidemia. Although rates of statin use in adults with FH and severe dyslipidemia appear to be increasing over the past decade, there remains substantial room for improvement with younger and uninsured adults at greatest risk of being undertreated.
Our findings are consistent with those of prior studies demonstrating suboptimal rates of statin prescription in patients with heterozygous FH or severe dyslipidemia. Using data from a single Midwest healthcare system, Knickelbine et al11 found that only two thirds of patients with heterozygous FH had been prescribed a statin, and only half of those on statins were adequately treated. Moreover, they found that among those not prescribed statins, 73% had no documented statin intolerance, suggesting significant room for improvement in both initiating and augmenting statin therapy to therapeutic levels. Similarly, another study performed in ambulatory care centers throughout the United States found that only two thirds of patients with a documented LDL-C level ≥190 mg/dL had been prescribed a statin, and statin prescriptions were highly dependent on other comorbid conditions such as diabetes mellitus or prior ASCVD.9 Rates of statin use in our study were lower than those of studies in ambulatory care settings, likely because we were able to capture adults without a usual source of care who were less likely to be screened for hypercholesterolemia or to have received statin prescriptions. Indeed, we found that having a usual source of care was a significant predictor of statin use among patients with severe dyslipidemia.
To our knowledge, only 2 studies have documented the prevalence of lipid-lowering medication use among adults with FH in the general population. Benn et al10 used data from the Copenhagen General Population Study to identify rates of self-reported lipid-lowering medication use in adults with FH, defined using the DLC criteria. Consistent with our study, they found that only about half of adults with definite/probable FH were taking a lipid-lowering medication. In contrast, data from the CASCADE registry (Cascade Screening for Awareness and Detection) reported higher rates of statin (74.8%) and high-intensity statin (42.0%) therapy among patients with heterozygous FH treated at lipid clinics in the United States.27 The higher rates reported by the CASCADE registry likely stem from the select catchment area from which participants were drawn. Our study extends these findings to all US adults, explores rates of screening and hypercholesterolemia awareness in conjunction with statin treatment, and identifies sociodemographic correlates of hypercholesterolemia awareness and statin use in this high-risk population.
We observed a disconnect between screening and treatment rates in adults with FH and adults with severe dyslipidemia. Over 80% of adults with FH or severe dyslipidemia reported recent cholesterol screening; however, only 52% of adults with FH and 38% of adults with severe dyslipidemia were taking a documented stain. Although the low statin rates may be attributable in part to lower screening rates in certain populations (eg, younger and uninsured adults), the disconnect between screening and treatment rates was most pronounced in these populations. Studies have repeatedly shown that severe dyslipidemia and FH increase the rate of CHD by 5- to 13-fold, respectively14,16; however, with appropriate statin therapy, the risk of ASCVD is greatly reduced.17 In addition, cost-effectiveness analyses have shown sizeable cost savings with initiating statins across all levels of risk in patients with LDL-C >160 mg/dL.28 The low rates of statin therapy, particularly among young adults, is of particular concern given the potential for long-term atherosclerotic plaque buildup and coronary events if LDL-C remains untreated.
Young adults aged 20 to 39 years may be at particularly high risk of being undertreated given their lower rates of insurance (70.6% versus 87.3%) and having a usual source of care (40.3% versus 63.2%) in this study. In addition, young adults may be less likely to think that they are at risk of cardiovascular disease, and clinicians may be less likely to initiate statin therapy in this population. Although the 2013 American College of Cardiology and American Heart Association cholesterol guidelines in addition to FH-specific guidelines recommend aggressive pharmacological treatment including immediate initiation of statins in adults with LDL-C ≥190 mg/dL,29–32 it is possible that lifestyle modifications continue to be prescribed as an initial treatment before initiating statin therapy. Indeed, prior studies have shown that young adults are more likely to be prescribed lifestyle interventions than older adults, which may explain, in part, the large discrepancy between screening and treatment rates in this population.33 In addition, although we excluded pregnant women, many women aged 20 to 39 years may be planning pregnancy or lactating. Many providers may be reluctant to prescribe statins to women who may become pregnant given their contraindications with pregnancy.
Other explanations for the low statin use despite widespread screening may include statin intolerance or interactions with other medications; however, other studies have shown that statin intolerance accounts for only a small percentage of adults with FH not taking statins.11 In addition, poor follow-up and disjointed care likely explain much of the poor treatment rate in this population. Adults without insurance or who lacked a usual source of care were significantly less likely to be taking statins than fully insured adults or those receiving care in either a hospital or clinic setting, respectively. Nevertheless, statin rates remained low even among those who were fully insured or had a usual source of care (42.3% [SE, 1.3%] and 41.8% [SE, 1.4%], respectively), suggesting that other factors besides inadequate follow-up contribute to suboptimal treatment rates.
Not surprisingly, we found that adults with severe dyslipidemia and either comorbid diabetes mellitus or a history of early ASCVD were significantly more likely to be prescribed statins or other lipid-lowering medications than those without other cardiovascular risk factors. Although both the original ATP III guidelines (Adult Treatment Panel III) and current guidelines for hypercholesterolemia management recommend initiation of statin therapy in patients with LDL-C ≥190 mg/dL regardless of other risk factors, they also emphasize the importance of drug therapy for secondary prevention of coronary events in patients with CHD or CHD risk equivalents such as diabetes mellitus and recommend initiating lipid-lowering medications at lower LDL-C levels for patients with these conditions.34 Moreover, the American Diabetes Association recommends the initiation of statin therapy in all adults with diabetes mellitus over the age of 40 and adults <40 years of age with even mild elevations in LDL-C.35 This increased emphasis on early initiation of statins in adults with diabetes mellitus or early ASCVD, combined with close follow-up and comprehensive care programs for individuals with diabetes mellitus, likely explains the higher rates of statin therapy in these patients.
Limitations of this study include the following. First, the definition of definite/probable FH was based on modified clinical criteria rather than genotyping. Although FH is a known genetic disorder, between 20% and 70% of patients are mutation-negative.36–38 Both the full DLC criteria and the modified version used in this study have previously been used in population studies to estimate the prevalence and burden of FH.12,15 As with any clinical criteria used to estimate a genetic diagnosis, the possibility for misclassification exists. Indeed, we found that adults with concurrent diabetes mellitus or hypertension were more likely to be identified as having definite/probable FH over unlikely/possible FH, suggesting that there may be some degree of misclassification across groups. Nevertheless, we estimate that the degree of misclassification is minimal given that other studies found slightly higher rates of diabetes mellitus and obesity in patients with DLC-defined FH with genetic analysis adjudication.10 Second, cholesterol screening, awareness, and treatment rates were estimated from a self-reported questionnaire that may be subject to imperfect recall or social desirability bias. Third, statin use may be underestimated if participants neglected or refused to show medications to the interviewer. To guard against this, NHANES interviews are conducted in the home. Fourth, we lacked information on statin dosages and therefore were unable to differentiate high-dose atorvastatin (40–80 mg) and rosuvastatin (20–40 mg) from low-dose atorvastatin (10–20 mg) and rosuvastatin (5–10 mg). As a result, we may have overestimated the percentage of adults on these higher-intensity statins. In addition, several adults may have been taking higher doses of simvastatin (80 mg) before the 2011 Food and Drug Administration warnings. Fifth, we were unable to differentiate between failure to prescribe statins and participant nonadherence among those participants not taking statins. Sixth, we assessed participant access to care including health insurance, income, and healthcare use at a single time point, which may not accurately reflect healthcare utilization and access over time as participants move in and out of insurers and providers. Seventh, we did not include children or adolescents <20 years in the sample. Screening and treatment may be even lower in this age group. Finally, >50% of the population were missing data on LDL-C. To address this issue, we first imputed the data using a multiple imputations approach and then repeated the analyses excluding participants with missing data. The restricted analyses showed nearly identical rates of cholesterol screening, awareness, and statin treatment as the full analyses. Although multiple imputations do not account for systematic missingness, there were minimal differences between adults with missing and nonmissing LDL-C for the demographic and clinical variables examined. Nevertheless, some individuals may have been misclassified as having severe dyslipidemia or definite/probable FH in the primary analyses.
In a nationally representative population of US adults with FH and severe dyslipidemia, the rates of statin and other lipid-lowering medications remain suboptimal despite high rates of self-reported cholesterol screening and awareness. This study highlights an opportunity and an imperative to improve statin treatment rates in this high-risk population. Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates. Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care. The low rate of statin use in young adults is of particular relevance given the early onset of ASCVD in adults with FH. Additional studies are needed to better understand how to close the gap between screening and treatment in this high-risk population and improve treatment rates among individuals with limited access to care.
Supplementary Material
Clinical Perspective
What Is New?
Familial hypercholesterolemia (FH) and severe dyslipidemia increase the risk of coronary heart disease and mortality; yet, the national prevalence of screening, awareness, and statin treatment for individuals with these conditions is unknown.
Using data from the National Health and Nutrition Examination Survey, we demonstrated a high prevalence of screening and awareness (>80%) but relatively low rates of statin use (52.3%) among individuals with FH and even lower with severe dyslipidemia (37.6%).
The discrepancy between the prevalence of cholesterol screening and treatment was most pronounced in younger patients, uninsured patients, and patients without a usual source of health care.
What Are the Clinical Implications?
Given the cardiovascular morbidity and mortality associated with FH and other severe dyslipidemias and the benefits of statin therapy among such patients, current rates of statin therapy in US adults with FH/severe dyslipidemia are too low.
Further studies need to investigate strategies to improve treatment among adults with FH and severe dyslipidemia, particularly among younger adults and those with limited access to care.
Acknowledgments
Dr Bucholz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
Dr de Ferranti receives royalties from UpToDate for topics related to pediatric and adolescent preventive cardiology, and research funding from the Pediatric Heart Network.
Footnotes
Disclosures
None.
ARTICLE INFORMATION
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.032321/-/DC1.
Contributor Information
Emily M. Bucholz, Department of Medicine, Boston Children’s Hospital, MA. Harvard Medical School, Boston, MA.
Angie Mae Rodday, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA.
Katherine Kolor, Office of Public Health Genomics, Centers for Disease Control and Prevention, Atlanta, GA.
Muin J. Khoury, Office of Public Health Genomics, Centers for Disease Control and Prevention, Atlanta, GA.
Sarah D. de Ferranti, Department of Cardiology, Boston Children’s Hospital, MA. Harvard Medical School, Boston, MA.
REFERENCES
- 1.Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch intern Med. 2001;161:1413–1419. [DOI] [PubMed] [Google Scholar]
- 2.Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 3.Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160:407–420. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 4.Youngblom E, Pariani M, Knowles JW. Familial hypercholesterolemia. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, eds. Genere-views. Seattle, WA: University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Besseling J, Hovingh GK, Huijgen R, Kastelein JJP, Hutten BA. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol. 2016;68:252–260. doi: 10.1016/j.jacc.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–2346. [DOI] [PubMed] [Google Scholar]
- 9.Al-Kindi SG, DeCicco A, Longenecker CT, Dalton J, Simon DI, Zidar DA. Rate of statin prescription in younger patients with severe dyslipidemia. JAMA Cardiol. 2017;2:451–452. doi: 10.1001/jamacardio.2016.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 11.Knickelbine T, Lui M, Garberich R, Miedema MD, Strauss C, VanWormer JJ. Familial hypercholesterolemia in a large ambulatory population: Statin use, optimal treatment, and identification for advanced medical therapies. J Clin Lipidol. 2016;10:1182–1187. doi: 10.1016/j.jacl.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Nanchen D, Gencer B, Auer R, Räber L, Stefanini GG, Klingenberg R, Schmied CM, Cornuz J, Muller O, Vogt P, Jüni P, Matter CM, Windecker S, Lüscher TF, Mach F, Rodondi N. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J. 2015;36:2438–2445. doi: 10.1093/eurheartj/ehv289. [DOI] [PubMed] [Google Scholar]
- 13.Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes-Virella M, Watts GF, Wierzbicki AS; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 14.Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 16.Wong B, Kruse G, Kutikova L, Ray KK, Mata P, Bruckert E. Cardiovascular disease risk associated with familial hypercholesterolemia: a systematic review of the literature. Clin Ther. 2016;38:1696–1709. doi: 10.1016/j.clinthera.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29:2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles JW, Rader DJ, Khoury MJ. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318:381–382. doi: 10.1001/jama.2017.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJ, Sijbrands EJ. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013:2;1–24. [PubMed] [Google Scholar]
- 20a.National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. October 31, 2017. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed December 18, 2017.
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Guglielmi V, Bellia A, Pecchioli S, Medea G, Parretti D, Lauro D, Sbraccia P, Federici M, Cricelli I, Cricelli C, Lapi F. What is the actual epidemiology of familial hypercholesterolemia in Italy? Evidence from a National Primary Care Database. Int J Cardiol. 2016;223:701–705. doi: 10.1016/j.ijcard.2016.08.269. [DOI] [PubMed] [Google Scholar]
- 23.Pajak A, Szafraniec K, Polak M, Drygas W, Piotrowski W, Zdrojewski T, Jankowski P. Prevalence of familial hypercholesterolemia: a meta-analysis of six large, observational, population-based studies in Poland. Arch Med Sci. 2016;12:687–696. doi: 10.5114/aoms.2016.59700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Classification and diagnosis of diabetes. Diab Care. 2017;40:S11–S24. [DOI] [PubMed] [Google Scholar]
- 25.Medicaid and CHIP eligibility levels. Baltimore, MD: Centers for Medicare & Medicaid Services; 2016. https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-eligibility-levels/index.html. Accessed June 3, 2017. [Google Scholar]
- 26.Cholesterol Treatment Trialists Collaboration, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, LaRosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 27.deGoma EM, Ahmad ZS, O’Brien EC, Kindt I, Shrader P, Newman CB, Pokharel Y, Baum SJ, Hemphill LC, Hudgins LC, Ahmed CD, Gidding SS, Duffy D, Neal W, Wilemon K, Roe MT, Rader DJ, Ballantyne CM, Linton MF, Duell PB, Shapiro MD, Moriarty PM, Knowles JW. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH Registry. Circ Cardiovasc Genet. 2016;9:240–249. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–153. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 29.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, Daniels SR, Gidding SS, de Ferranti SD, Ito MK, McGowan MP, Moriarty PM, Cromwell WC, Ross JL, Ziajka PE. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:133–140. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, Livingston M, Mata P, Parhofer KG, Raal FJ, Santos RD, Sijbrands EJ, Simpson WG, Sullivan DR, Susekov AV, Tomlinson B, Wiegman A, Yamashita S, Kastelein JJ. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. J Clin Lipidol. 2014;8:148–172. doi: 10.1016/j.jacl.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 33.Davis SK, Ahn DK, Fortmann SP, Farquhar JW. Determinants of cholesterol screening and treatment patterns. Insights for decision-makers. Am J Prev Med. 1998;15:178–186. [DOI] [PubMed] [Google Scholar]
- 34.National Cholesterol Education Program. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). National Heart, Lung, and Blood Institute; NIH Publication No. 02–5215. September 2002. https://www.nhlbi.nih.gov/files/docs/resources/heart/atp-3-cholesterol-full-report.pdf. Accessed June 28, 2017. [Google Scholar]
- 35.American Diabetes Association. Cardiovascular disease and risk management. Sec. 9. In Standards of Medical Care in Diabetes-2017. Diab Care. 2017;40(suppl 1):S75–S87. [DOI] [PubMed] [Google Scholar]
- 36.Taylor A, Wang D, Patel K, Whittall R, Wood G, Farrer M, Neely RD, Fairgrieve S, Nair D, Barbir M, Jones JL, Egan S, Everdale R, Lolin Y, Hughes E, Cooper JA, Hadfield SG, Norbury G, Humphries SE. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin Genet. 2010;77:572–580. doi: 10.1111/j.1399-0004.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 37.Grenkowitz T, Kassner U, Wühle-Demuth M, Salewsky B, Rosada A, Zemojtel T, Hopfenmüller W, Isermann B, Borucki K, Heigl F, Laufs U, Wagner S, Kleber ME, Binner P, März W, Steinhagen-Thiessen E, Demuth I. Clinical characterization and mutation spectrum of German patients with familial hypercholesterolemia. Atherosclerosis. 2016;253:88–93. doi: 10.1016/j.atherosclerosis.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 38.Kirke AB, Barbour RA, Burrows S, Bell DA, Vickery AW, Emery J, Watts GF. Systematic detection of familial hypercholesterolaemia in primary health care: a community based prospective study of three methods. Heart Lung Circ. 2015;24:250–256. doi: 10.1016/j.hlc.2014.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.