Abstract
Background: Antibiotic resistance in human and animal pathogens is mainly the outcome of human use of antibiotics. However, bacteria are also exposed to thousands of other antimicrobial agents. Increasingly those exposures are being investigated as co-selective agents behind the rapid rise and spread of resistance in bacterial pathogens of people and our domesticated animals.
Methods: We measured the sub-lethal effects on antibiotic tolerance of the human pathogen/commensal Escherichia coli caused by exposure to three common biocide formulations based on either copper, pyrethrins, or atrazine as active ingredients. The influence of the efflux pump AcrAB-TolC was investigated using deletion strains, and the persistence of observed effects was determined.
Results: Some effects were seen for all biocides, but the largest effects were observed with copper in combination with the antibiotic tetracycline. The effect was caused by both the induction of the adaptive efflux system and by chelation of the antibiotic by copper. Finally, persistence of the adaptive response was measured and found to persist for about two generations.
Conclusions: Through a combination of microbe-chemical and chemical-chemical interactions, humanity may be creating micro-environments in which resistance evolution is accelerated.
Keywords: biocides, antibiotic resistant bacteria, antibiotics, copper, pyrethrins, atrazine
Introduction
Besides antibiotics, a growing number of anthropogenic products are being found to affect antibiotic resistance in microorganisms ( Heinemann & Kurenbach, 2017; Knöppel et al., 2017; Molina-González et al., 2014). These include non-antibiotic therapeutics ( Kristiansen, 1992; Maier et al., 2018), food sweeteners ( Wang et al., 2018), food perservatives ( Capita & Alonso-Calleja, 2013; Capita et al., 2014), emulsifiers used in food and medicine ( Kurenbach et al., 2017), paints, and cleaning products ( Buffet-Bataillon et al., 2016; Molina-González et al., 2014).
The world’s industrial capacity to produce, distribute and consume manufactured chemical products is at an all time high and growing ( American Chemistry Council, 2016). In the United States alone, 13,000 kg of industrial chemicals are produced per capita per year, and over 11,000 kg of 8,000 chemicals are produced or imported per capita per year ( Wang et al., 2018).
Manufactured chemicals contribute to pollution, which is the leading cause of disease and premature death worldwide ( Landrigan et al., 2018). The Lancet Commission on Pollution and Health said that less than half of “high-production volume chemicals have undergone any testing for safety or toxicity, and rigorous pre-market evaluation of new chemicals has become mandatory in only the past decade and in only a few high income countries. The result is that chemicals and biocides whose effects on human health and the environment were never examined have repeatedly been responsible for episodes of disease, death, and environmental degradation” ( Landrigan et al., 2018).
To our knowledge, pre-market assessments of biocides that include tests of sub-lethal effects on microorganisms have not been performed yet ( Kurenbach et al., 2015), although this may be changing, at least in Europe ( Buffet-Bataillon et al., 2016). For every human exposure to a biocide, there may be 10s of trillions of exposures in our personal microbiota, not to mention microbiota exposures in soil, water and air, and on plants, livestock, companion animals and insects ( Claus et al., 2016; Imfeld & Vuilleumier, 2012; Motta et al., 2018).
We have previously shown that active ingredients and commercial formulations based on dicamba, glyphosate, and 2,4-D induced changes in the response of Escherichia coli and Salmonella enterica to five different antibiotics from different classes. Increases in tolerance to antibiotics could be attributed in part to increased production of efflux pumps from the resistance-nodulation-division (RND) family ( Kurenbach et al., 2015; Kurenbach et al., 2017). Unfortunately, the diversity of active and adjuvant ingredients of the tested herbicides provide little basis to produce general predictions of effects on different bacteria because of a common chemistry. Thus, at present, products must be tested on a case-by-case basis to determine whether or not there are sub-lethal responses in bacteria of interest.
The aim of the work described here was to determine whether other biocides used in agriculture and urban environments could induce a similar response in E. coli. The biocides used in the experiments were commercial formulations of a fungicide (copper ammonium acetate), an insecticide (pyrethrins) and an herbicide (atrazine).
We measured the initial response of bacteria to chemical exposures by the adaptive changes in the expression of TolC, an efflux pump component that controls transport across membranes ( Corona & Martinez, 2013). This response is reversible in time, but may be heritable through epigenetic transmission ( Bootsma et al., 2012; Motta et al., 2015). We used one biocide-antibiotic combination to attempt to empirically measure the transgenerational longevity of the adaptive response.
Methods
Strains and chemicals
Strains used in this study are detailed in Table 1. Liquid cultures were grown in LB Lennox (Invitrogen, Auckland, NZ) at 37°C in a rotary incubator. Antibiotics used were tetracycline (Tet, Sigma, Auckland, NZ), streptomycin (Str, Sigma, Auckland, NZ), kanamycin (Kan, Gibco, Auckland, NZ), and ciprofloxacin (Cip, Pentex, Auckland, NZ). Biocides were commercial formulations Yates Liquid Copper Fungicide (Yates, Auckland, NZ) containing 92.8 g/L of Copper (Cu 2+) in the form of copper ammonium acetate, Pyrethrum Natural Insect Spray (Yates, Auckland, NZ) containing pyrethrins (14 g/L) and 56.5 g/L of piperonyl butoxide, and Atranex WG (Adama, Nelson, NZ), containing 900g/kg atrazine. Relevant concentrations are given in the main text or Figure legends.
Table 1. E. coli strains used in this study.
| E. coli strain | Genotype/relevant characteristic | Reference/Source |
|---|---|---|
| BW25113 | Wild type. F-, λ-, Δ(
araD-
araB)567, Δ
lacZ4787(::
rrnB-3),
rph-1, Δ( rhaD-rhaB)568, hsdR514 |
( Baba et al., 2006) |
| CR7000 | BW25113 Δ acrA | ( Ruiz & Levy, 2014) |
| CR5000 | BW25113 Δ acrB | ( Ruiz & Levy, 2014) |
| JW5503 | BW25113 Δ tolC:: kan, Kan R | ( Baba et al., 2006) |
| BW25113 (pHJ01) | BW25113 pHJ01 (pFru - P tolC_mScarlet) | This work |
Minimum inhibitory concentration (MIC) and antibiotic response
Antibiotic responses were determined as described previously ( Kurenbach et al., 2015). In brief, E. coli was grown to saturation (ca. 2 x 10 9 cfu/mL) in LB, and serial dilutions were plated on LB in the presence of antibiotics and/or biocides. When added, biocide concentrations were constant, while antibiotic concentrations varied. Plates were incubated at 37°C and examined daily for up to 10 days, at which point no new colonies emerged. To account for day to day variability, cfu counts were normalised to growth on nonselective medium. The efficiency of plating (EoP) is the ratio of a culture’s titre (cfu/mL) on treatment plates to the titre on LB [(cfu/mL) treatment/ (cfu/mL) LB] ( Rosner, 1985). The detection range was an EoP of ca. 1 to 10 -7.
Dose response
The concentration of biocide that caused a significantly different response to an antibiotic (“dose response”) was determined as described previously ( Kurenbach et al., 2015). In brief, E. coli were grown to saturation in LB and a serial dilution was plated on LB agar plates supplemented with varying concentrations of biocide and a constant concentration of antibiotic. The antibiotic concentration used was the one causing the greatest difference in EoP in the antibiotic response experiments. The inducing concentration of a biocide was defined as the lowest concentration for which a change occurred that was a) statistically significant and b) showed an at least 100-fold difference in EoP compared to the control containing only antibiotic. Plates were incubated at 37°C and examined daily for up to 10 days, at which point no new colonies emerged.
Plasmid construction
To construct plasmid pHJ01, E. coli BW25113 (GenBank accession number CP009273) was used as a template for the 204 bp upstream of the start codon of tolC. The tolC promoter was amplified by PCR and fused to mScarlet-I which was amplified from pTriEx-RhoA-wt_mScarlet-I_SGFP2 (Addgene plasmid #85071) and HindIII digested pFru97 ( Tecon & Leveau, 2012) by isothermal assembly ( Gibson et al., 2009; Schlechter et al., 2018). Touchdown PCRs were performed as described previously ( Schlechter et al., 2018) using Phusion High-Fidelity DNA polymerase (Thermo Scientific, Auckland, NZ). Primers used were FWD_TolC (5' CAG GAC GCC CGC CAT AAA CTG CCA GGA ATT GGG GAT CGG ATG TTA ATG TCC TGG CAC TAA TAG TGA ATT AAA TGT 3’; Tm: 60°C), REV_TolC (5' TCG CCC TTG CTC ACC ATG GT T TGC ATT CCT TGT GGT GAA GCA G 3'; Tm: 60°C), TolC_mScarlet_FWD (5' CTT CAC CAC AAG GAA TGC AAA CCA TGG TGA GCA AGG GC 3’; Tm: 70°C), and mScarlet_REV (5' TTA CTG GAT CTA TCA ACA GGA GTC CAA GCT CAG CTA ATT ACT TGT ACA GCT CGT CCA TGC 3'; Tm: 71°C), where nucleotides shown in bold font are complementarity to the vector, and nucleotides shown in italics overlap with other primers. pHJ01 transformands of BW25113 were selected on kanamycin.
Microscopy
Prior to microscopy, cells grown for 180 min either in LB or in LB + 450 µg/mL copper were fixed using 4% paraformaldehyde as described previously and stored at -20°C in 1:1 ethanol:phosphate buffered saline ( Akkermans et al., 1996; Kowalchuk et al., 2004). Fixed cells were examined with an Axio Imager. M1 (Zeiss, Oberkochen, Germany) using an EC Plan-Neofluar 100x objective (NA 1.30) and Zeiss filter set 43HE (BP 550/25 (HE); FT 570 (HE); BP 605/70 (HE)). Multichannel images were acquired using an AxioCam 506 mono camera (Zeiss) in differential interference contrast (DIC) and Zeiss filter set 43HE. Single cell fluorescence was determined as described previously ( Remus-Emsermann et al., 2016).
Tetracycline chelation
Seven Erlenmeyer flasks (50 mL) containing LB (10 mL) were supplemented with copper (450 µg/mL) and tetracycline (35 μg/mL) (Flasks 1–4), tetracycline (35 μg/mL) without copper (Flasks 5 and 6), or copper (450 µg/mL) without tetracycline (Flask 7) at t 0. All flasks were incubated continuously at 37°C with aeration. E. coli BW25113 was grown to saturation without selection and approximately 10 4 cfu were used to inoculate flasks 1, 6 and 7 at t 0, and flasks 2 and 3 at t 24 and t 48, respectively. Flasks 4 and 5 were inoculated at t 96. The culture in each flask was monitored for growth every 24 hours by plating appropriate dilutions onto LB agar plates.
Measuring the persistence of copper-induced tetracycline resistance
E. coli was grown to saturation with aeration at 37°C in liquid LB medium supplemented with both copper (450 µg/mL) and tetracycline (15 µg/mL) for 3 days. This culture was diluted 100-fold into 10 mL LB medium supplemented with only tetracycline (15 µg/mL) and incubated at 37°C for 12 hours with aeration. The concentration of E. coli at the start and end of the experiment was determined using a haemocytometer.
Statistical analysis
R (version 3.2.0) was used for all statistical analyses ( R Core Team, 2013). In experiments testing the responses to antibiotics during exposure to biocides we were interested in effects on EoP that were different in antibiotic+biocide combinations compared to either substance in isolation. We therefore tested the log-transformed EoP scores using a multifactor analysis of variance (ANOVA) by evaluating the significance of the antibiotic by biocide interaction term. Antibiotic concentrations were treated as separate categories in the ANOVA. Plots of residuals were used to test for violations of assumptions. We fit these models using the lm function.
Since many data points used for the determination of the concentration of biocide that caused a significantly different response to an antibiotic were near or below the detection limit, residuals from a standard ANOVA were not normally distributed. We therefore used the equivalent non-parametric Kruskal-Wallis one-way ANOVA to test for differences in log-transformed EoP/EoP (0) scores among biocide concentrations. The P-value reported is derived for a null model where EoP/EoP (0) is the same across all biocide concentrations versus and alternative model where the ratio differs among some concentrations.
We tested whether cfu scores depended on “flask” using a single factor ANOVA at 24 and 48 hours post inoculation. In each case, we first used an analysis of covariance (ANCOVA) to test if cfu scores post inoculation were confounded with the cfu count and the time of inoculation. Cfu scores at inoculation did not influence final scores (data not shown). Cfu scores were transformed to log (cfu +0.0001) to ensure normality of the residuals. We used a sequential Bonferroni contrast to test for differences among treatments (Flasks 1–4) and between treatments and controls (flasks 1 and 6, 1 and 6, and 4 and 5). Residuals were used to check assumptions. With the exception of two low-influence outliers, the data matched our expectations under normality.
Where fluorescence was measured, differences between median fluorescence values of the reporter strain grown under two conditions (+/- copper) were determined using a non-parametric Mann-Whitney U T-test because residuals were not normally distributed. Violin plots were created using ggplot2 ( Wickham, 2016).
Results
Effects of biocides on antibiotic response
MIC was defined as the minimum concentration of agent in an agar plate at which no growth was observed after ca. 10 8 cfu were applied to the surface. It was not possible to determine the MIC for atrazine because E. coli BW25113 survived to the limit of solubility of atrazine in our standard culture medium, LB. The No Observable Effect Level (NOEL) was defined as the highest concentration of a substance that had no effect on the EoP ( Table 2).
Table 2. Relevant biocide NOEL and MIC values.
| E. coli strain | Biocide | NOEL (µg/ml) | MIC (µg/ml) |
|---|---|---|---|
| BW25113 (WT) | Copper | 450 | 1635 |
| Pyrethrins | 140 | 2300 | |
| Atrzine | 1000 | > 2000 | |
| CR7000 (Δ acrA) | Copper | 450 | 1430 |
| CR5000 (Δ acrB) | Copper | 450 | 1230 |
| JW5503 (Δ tolC) | Copper | 450 | 700 |
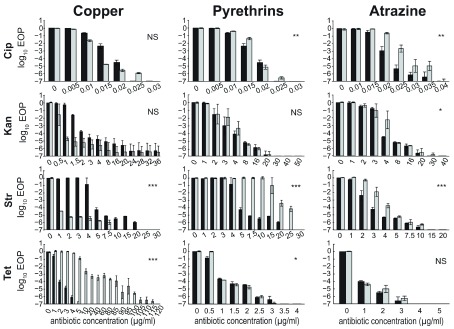
Bacteria were cultured on LB agar supplemented with one of the three commercial formulations of biocide (at respective NOEL concentrations) as well as different concentrations of selected antibiotics. Changes in response to particular concentrations of antibiotic because of exposure to the biocide are revealed as a differential EoP ( Figure 1). As reported for other biocide*antibiotic combinations, the observed responses were specific for the combination of biocide and antibiotic used ( Kurenbach et al., 2015; Kurenbach et al., 2017). We observed increases and decreases in tolerance to different antibiotics as well as no effect in some cases. As a conservative threshold, we used the antibiotic concentration for which we saw an at least 10 3-fold decrease in EoP as the cut-off point to determine the fold-change in survival ( Table 3).
Figure 1. Change in EoP when E. coli BW25113 is (grey) or is not (black) exposed to biocides.
The x-axes scale is antibiotic concentrations in µg/mL. Biocide concentrations used were 450 µg/mL for copper ammonium acetate, 140 µg/mL for pyrethrin, and 1000 µg/mL for atrazine. Values are means of at least three independent experiments; error bars are standard errors (SEM, with SEM=standard deviation/√n). Asterisks indicate P-values for antibiotic*herbicide interaction terms (see Materials and Methods). * P<0.05; ** P<0.01; *** P<0.001; ns, not significant.
Table 3. Fold change shift in antibiotic effectiveness following exposure to biocides.
aWhile EoP dropped below our threshold at the same concentration in the presence and absence of biocides, the ANOVA showed a statistically significant interaction term for this combination.
bThe ANOVA did not show a statistically significant interaction term, despite the drop in EoP below our threshold at different concentrations.
Copper significantly increased the EoP over a 40-fold concentration range of tetracycline (from 2 to 80 µg/mL) and decreased it on a 5-fold concentration range of streptomycin (from 5 to 1 µg/mL). Copper caused non-statistically significant decreases in the EoP on either ciprofloxacin or kanamycin.
Pyrethrins increased EoP over a 5-fold streptomycin concentration range. They caused a statistically significant difference in EoP on ciprofloxacin, but no change in MIC. This has been sometimes observed for various herbicide-antibiotic combinations ( Kurenbach et al., 2015; Kurenbach et al., 2018).
Atrazine caused statistically significant but small increases in EoP on ciprofloxacin, kanamycin, and streptomycin.
Determining the minimum biocide concentration causing a change in the response to antibiotics
In the experiments described above, the concentration of antibiotic was varied while the biocide concentration was constant. To determine the minimum biocide concentration necessary to cause the observed effects, we chose an antibiotic concentration for which there was a maximum resolution between treatments and decreased the biocide concentration for each biocide.
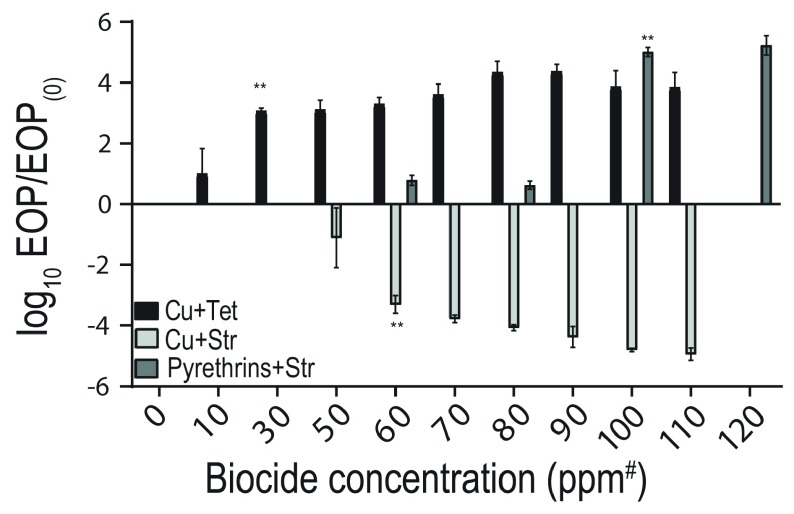
As a conservative measure, we report the biocide concentration that caused a statistically significant and at least a 100-fold change in the EoP compared to the EoP of the antibiotic-only plate (EoP (0)). To aid visualization, we calculated log EoP/EoP (0) ( Figure 2). A value >0 indicates that the biocide increases EoP of bacteria on higher concentrations of the antibiotic. Our threshold of a 100-fold change was reached at 120 µg/mL copper with tetracycline, 250 µg/mL copper with streptomycin, and 100 µg/mL pyrethrins with streptomycin.
Figure 2. Dose response curves for E. coli BW25113.
Antibiotic concentrations used (in µg/mL) were as follows: Tet: 10 µg/mL for copper; Str: 2 µg/mL for copper and 10 µg/mL for pyrethrins. Values are means of at least 3 independent experiments; error bars are standard errors (SEM, with SEM=standard deviation/√n). Asterisks indicate the lowest biocide concentration for which a statistically significant change in EoP by at least 100-fold compared to the antibiotic only occurred. * P<0.05; ** P<0.01; *** P<0.001; ns, not significant.
The minimum biocide concentration was not determined for some other statistically significant combinations shown in Figure 1. This was because the affected antibiotic concentrations were of such a small range. As a consequence, we also concentrated on copper exposures in the remainder of the study.
Reversibility of phenotype as evidence of an adaptive response
We previously found that the herbicidal formulations based on 2,4-D, dicamba and glyphosate ( Kurenbach et al., 2015), as well as the corresponding purified active ingredients ( Kurenbach et al., 2017) caused changes in the expression pattern of genes that may alter antibiotic susceptibility. This response is phenotypic, resulting from a change in gene expression rather than genotype. It is distinguished from the outgrowth of rare spontaneous mutants by the uniform reversion on the population level when the environment changes ( Motta et al., 2015).
We further characterized the copper-induced tetracycline response as an adaptive response by following the phenotype of induced clones. Randomly chosen colonies from cultures plated on LB, LB+Tet, or LB+Tet+Cu were transferred to plates containing 35 μg/mL tetracycline or LB. At this tetracycline concentration, E. coli survived only when simultaneously exposed to copper.
Regardless of whether the colonies were transferred from LB or LB+Tet+Cu, they all again formed colonies on LB. However, none of the colonies transferred from either LB or LB+Tet+Cu grew on LB+Tet plates, indicating that the response to tetracycline was reversible and dependent upon ongoing stimulation by copper.
Dependence on the AcrAB-TolC efflux pump as evidence of an adaptive response
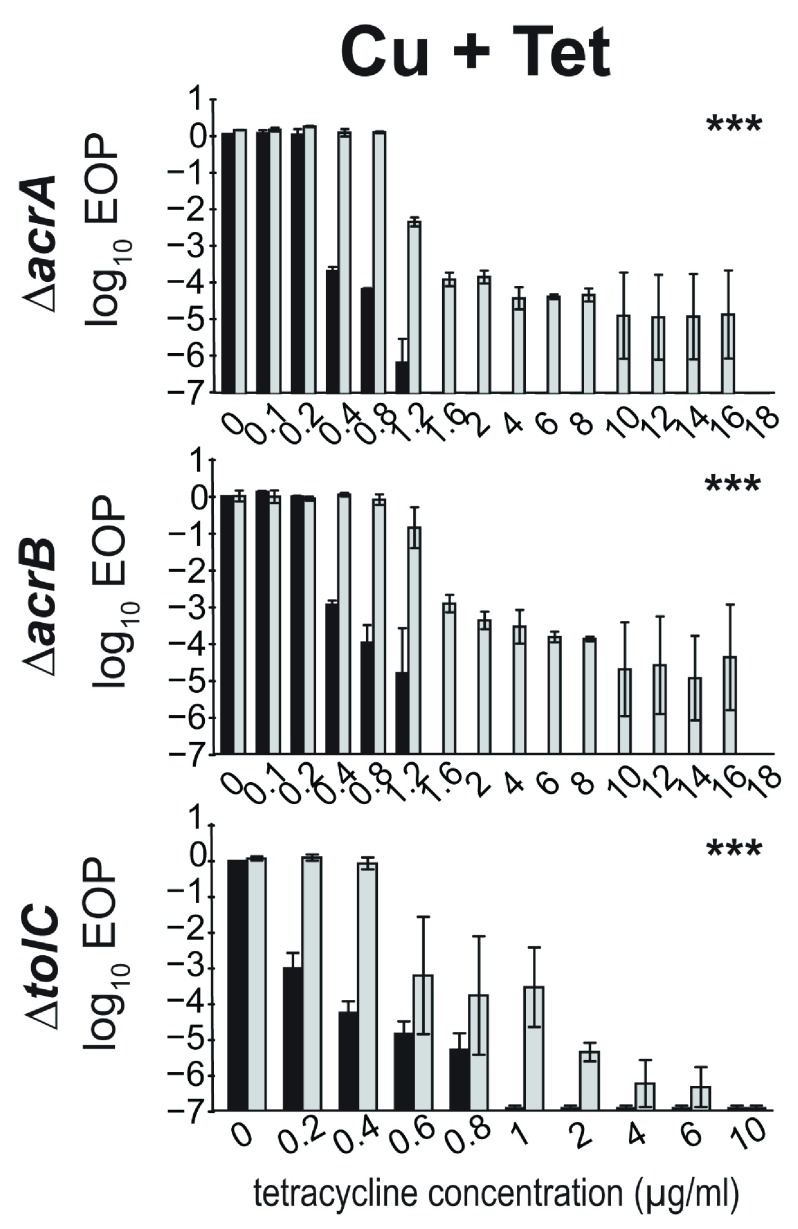
The efflux pump AcrAB-TolC was shown to contribute to the altered EoP of E. coli on different antibiotics when simultaneously exposed to various herbicides ( Kurenbach et al., 2017). Here, the same set of strains from an isogenic series carrying single gene deletions, Δ acrA , Δ acrB and Δ tolC, were used to test whether copper induced an adaptive response via this pump. NOEL and MIC of copper were determined for all three strains ( Table 2). Changes in EoP on tetracycline-supplemented media were measured as described above, using the NOEL copper concentration ( Figure 3).
Figure 3. Change in EoP when E. coli deletion strains are (grey) and are not (black) exposed to Cu in the presence of Tetracycline.
The x-axis indicates antibiotic concentration in µg/mL. Copper was added at 450 µg/mL. Error bars are standard errors (SEM, with SEM=standard deviation/√n). Asterisks indicate P values for interaction terms (see Materials and Methods). * P<0.05; ** P<0.01; *** P<0.001; ns, not significant.
The MIC but not the NOEL of tetracycline was lower in all three deletion strains compared to the wildtype BW25113. This is consistent with the observations of others ( de Cristóbal et al., 2006) and suggests that the AcrAB-TolC efflux pump is responding to copper and contributing to tetracycline resistance. Concurrent copper exposure significantly increased tolerance to tetracycline in all strains. However, with increases of 4-fold for Δ acrA, 22.5-fold for Δ acrB, and 5-fold for Δ tolC these effects were smaller than those observed for the parental strain (40-fold). This suggests that the AcrAB-TolC efflux pump is responding to copper and contributing to tetracycline resistance, but it is not the only mechanism involved.
TolC was induced by copper
Accumulation of copper directly effects the transcription factor MarR, derepressing the MarRAB operon ( Hao et al., 2014). Increased production of the transcription factor MarA leads to increased transcription of among others the acrAB and tolC genes ( Weston et al., 2018). We chose to investigate this further by using a tolC reporter strain.
The E. coli strain BW25113 (pHJ01) has the mScarlet fluorescent protein open reading frame transcriptionally fused to the tolC promoter region. This technique was used previously to demonstrate e.g. the accessibility of fructose to bacterial cells on leaves and the availability of phenol to bacteria on leaves ( Leveau & Lindow, 2001; Sandhu et al., 2007).
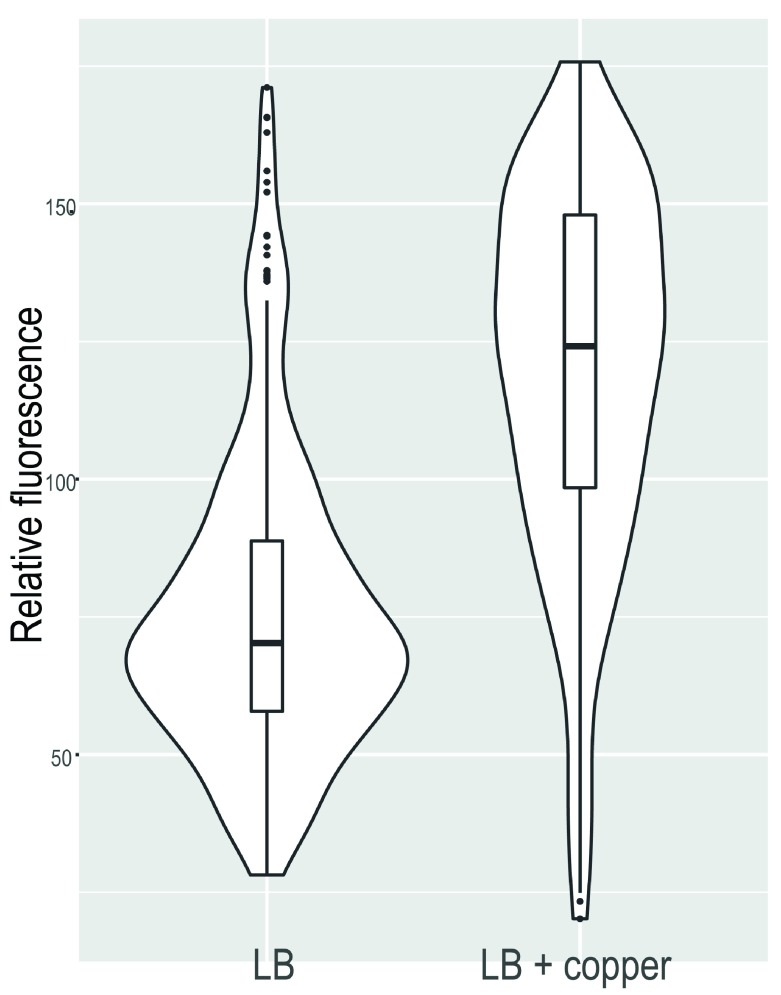
The fluorescence of BW25113 (pHJ01) was statistically significantly lower (p < 0.001) when cultured in LB compared to LB + 450 µg/mL copper ( Figure 4). The median relative fluorescence of the reporter strain increased 77% from 70 arbitrary fluorescence units (afu) when cultured in LB to 124 afu when cultured with additional copper. This indicates induction of tolC by the copper fungicide.
Figure 4. Single-cell fluorescence intensity of E. coli BW25113 (pHJ101) expressing red fluorescent mScarlet protein under the control of the tolC promoter without (left) or with (right) copper exposure.
The violin plots show the distribution of the single-cell fluorescence within the cell population. The median is depicted by the bar in the box; the box represents the 25% and 75% quartiles.
Copper directly reduced available tetracycline
Copper had a large effect on the EoP of E. coli exposed to tetracycline, increasing the concentration necessary to decrease EoP by >10 3-fold from 2 to 80 µg/mL tetracycline. This was the largest effect of any biocide on any antibiotic that we have observed. When cultured in a combination of copper and tetracycline at copper-induced sub-lethal concentrations of tetracycline, we observed a significant delay in the growth of the culture. This could be due to copper chelation of tetracycline ( Tong et al., 2015), or to the outgrowth of rare tetracycline resistant mutants. Since we have not detected the latter (see above), we tested the former hypothesis.
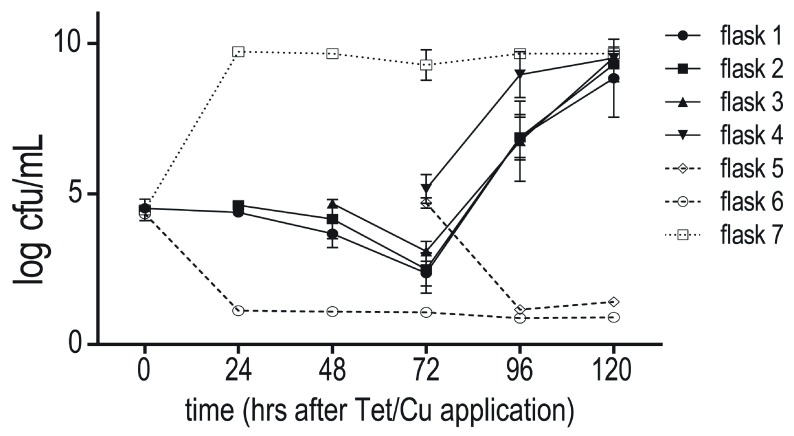
A series of E. coli BW25113 cultures were used to estimate tetracycline bioavailability. The series was composed of four flasks with a medium supplemented with copper and tetracycline and incubated at 37°C with aeration. The medium in the flasks was inoculated with bacteria in successive 24 hour intervals (flask 1 at t 0 – flask 4 at t 72) and the titre of each culture was determined at the same intervals by plating dilutions of samples on LB. Control cultures with medium supplemented with only tetracycline were started in parallel with flasks 1 and 4, and a positive control culture using medium only supplemented with copper was inoculated in parallel to flask 1. These controls showed that tetracycline alone, even after 92 hours of pre-incubation, prevented growth of the culture, and that the copper concentration was sub-lethal. Cultures began to grow only after the medium with a mixture of copper and tetracycline was over 72 hours old ( Figure 5).
Figure 5. Chelation of tetracycline by copper.
X-axis is time in hrs after adding copper and tetracycline (flasks 1–4, solid lines), tetracycline (flasks 5 & 6, dashed lines), or copper (flask 7, dotted line). The first data point in each series indicates time of inoculation with E. coli BW25113. Values are means of three independent experiments ± SD.
Using ANOVAs, we tested for significant differences between flasks in cfu counts 24 and 48 hours after inoculation with bacteria (see Underlying data: ‘Chelation experiment_ANOVA tables’; https://doi.org/10.17605/OSF.IO/RZKWU ( Kurenbach, 2018)). At 24 hrs, the cfus of flask 4, inoculated at t 72, was significantly different to either flasks 1, 2, or 3. At 24 hrs, these flasks had not passed the t 72 point. At 48 hrs, flasks 3 and 4, now both past t 72, were not significantly different from each other. Flask 4 was still different from flasks 1 and 2, while differences between 3 and 1 and 2 were not significant or marginally significant, respectively. This is in general agreement with the interpretation that bacteria start growing after t 72 regardless of the point in time at which they were inoculated (see Underlying data: ‘Chelation experiment_ANOVA tables’; https://doi.org/10.17605/OSF.IO/RZKWU ( Kurenbach, 2018)).
This observation is consistent with the notion that copper forms a complex with tetracycline ( Tong et al., 2015), or facilitates degradation of tetracycline, over time. Because tetracycline is bacteriostatic, the bacteria are able to recover once the effective concentration of tetracycline falls to sub-inhibitory levels.
Measuring persistence of the adaptive phenotype
E. coli’s response to copper exposure was consistent with an adaptive response through a change in efflux pump levels rather than a change in DNA sequence conferring antibiotic resistance. We therefore hypothesized that the tetracycline-resistant physiotypes created by the adaptive response should continue to reproduce in medium supplemented with tetracycline above the MIC until the number of efflux pumps, and possibly other contributing factors, per cell fell below an efficacious threshold ( Bootsma et al., 2012; Motta et al., 2015).
This hypothesis could be tested by determining the number of generations E. coli was able to reproduce after removal of copper but not tetracycline from a previously induced (Cu+Tet) culture. A complication was encountered when we observed a reversible filamentation of the bacteria after their transfer from a medium with both copper and tetracycline. E. coli are known to form filaments when stressed ( Justice et al., 2006). Filamentation made the determination of growth by measuring OD 600 inaccurate. To address this, densities of bacteria were determined visually using a haemocytometer.
Immediately after transfer to tetracycline-supplemented medium, the concentration of bacteria was determined. The cultures were then incubated at 37°C for 16 hours. Testing the limits of our method, we were consistently able to distinguish 4-fold differences in population growth, i.e. two generations. Our experimental data fell below that threshold, with populations growing by ca. 3-fold, or just over one generation. We therefore estimate that the adaptive phenotype in this experiment was heritable for less than two generations.
Discussion
About 2 million metric tons of the 30 most commonly used commercial pesticides are released into the environment annually worldwide. Of these, 55.4% are herbicides, 28.6% are fungicides and 5.7% are insecticides ( Casida & Bryant, 2017). Despite their long and widespread use, to our knowledge they have never been tested for sub-lethal effects on potential human or animal pathogenic bacteria.
We have tested three common pesticides for sub-lethal effects on the bacterium E. coli. Copper-based formulations are the third largest fungicide usage group. The triazine herbicide ingredient atrazine is by amount used the third most commonly used herbicide in the world. Pyrethroids are medium use insecticides, occupying positions of 11, 12, 16 and 26 of the top 30 insecticides used worldwide ( Casida & Bryant, 2017).
Similar to our previous findings for the herbicides based on glyphosate, dicamba, and 2,4-d active ingredients, the three biocides tested here did alter the response of the human and animal commensal and potential pathogen E. coli to some clinical antibiotics. The concentrations of biocide that caused the change in response to antibiotics were at or below label-recommended application rates, which are 30 - 2320 µg/mL for copper, 70 µg/mL for pyrethrins, and 500 µg/mL for atrazine.
Streptomycin resistance was most affected by pyrethroids, while effects on other antibiotics tested were small. Moreover, results were not statistically significant for the other tested aminoglycoside antibiotic, kanamycin. Likewise, atrazine caused only small effects for all antibiotics tested. We observed the largest changes using copper, which increased survival on 40 times higher concentrations of tetracycline.
The response seen to tetracycline from copper exposure was the largest we have observed from a biocide and antibiotic combination. Some of this is attributed to the chelation of copper by tetracycline, resulting in a decrease in effective concentration of both agents. Nevertheless, some of the response was confirmed to be adaptive because as shown by use of the strains with gene deletions, it depended in part on acrA, acrB and tolC. Moreover, exposure to copper was directly observed to increase the expression of the red fluorescent protein gene mScarlet under the control of the tolC promoter, and the fully susceptible phenotype was uniformly restored to the population when induced bacteria were transferred to LB+Tet medium. Because the gene deletion strains continued to respond to copper and tetracycline, the full effect of copper was not explained only through the expression of the AcrAB-TolC efflux pump.
Copper is a common supplement for animal feeds which can also contain traces of copper from biocide residues. In a European Union survey of copper content in animal feed used in member countries, copper was found over a broad range of concentrations (in the mg/kg range) and mean concentrations of 8 to ~20 mg/kg in the feed of most surveyed animals, including pets such as dogs and cats. The highest mean was 119 mg/kg for piglets ( EFSA, 2016). The lowest statistically significant tetracycline-resistance inducing concentration of copper in our study was 120 mg/L, just above routine piglet exposures. Other exposures to copper from use of biocides would be in addition to these.
Animal feed can also be unintentionally contaminated with antibiotics. Tetracycline-class antibiotics are approved for use in animal feed and are among the most frequently used. This alone resulted in concentrations of chlortetracycline and doxycycline at concentrations of 10 mg/kg and 4 mg/kg, respectively, in the feces of pigs. The level was high enough to select for resistance ( Gavilán et al., 2015; Granados-Chinchilla & Rodríguez, 2017).
A study in Vietnam that examined nearly 1500 chicken and pig feed formulations estimated that 77.4 mg and 286.7 mg, respectively, of antimicrobials were used to raise each 1 kg of animal. The level of antimicrobial agent in the feed ranged from 25.7–62.3 mg/kg. Chlortetracycline was among the most common additives in chicken and pig feed ( Van Cuong et al., 2016). Thus it is not unusual to find both copper and tetracycline in the same environments.
Conclusion
Preservation of antibiotics as useful medicines requires stewardship of populations of bacteria that remain susceptible to them. It is imprudent to base stewardship on frequency of resistance because even low numbers of resistant bacteria will dominate a population when antibiotics are used. Environments that maintain phenotypes caused by adaptive resistance or genotypes with a fitness advantage during antibiotic exposure thus could contribute to the rate at which populations of pathogens evolve resistance ( Kurenbach et al., 2018).
The number of circulating high use commercial chemicals being associated with sub-lethal effects on bacteria is growing, as are the number of environments that are being contaminated with antibiotics themselves. Exposure to herbicides and antibiotics simultaneously accelerates the evolution of genotypically resistant bacteria ( Kurenbach et al., 2018). The effects seen for atrazine, copper and pyrethrins were more limited than for some other herbicide active ingredients and commercial formulations, but may contribute to the overall burden of resistance.
Data availability
Underlying data
All underlying data is available on the Open Science Framework: Effects of sub-lethal concentrations of copper ammonium acetate, pyrethrins and atrazine on the response of Escherichia coli to antibiotics, https://doi.org/10.17605/OSF.IO/RZKWU ( Kurenbach, 2018).
The following files are available:
-
Effects of biocides on antibiotic response.Antibiotic resistance in the presence and absence of biocide. Data presented in Figure 1.
-
○
Atrazine+Cip_killing curves.csv
-
○
Atrazine+Kan_killing curves.csv
-
○
Atrazine+Str_killing curve.csv
-
○
Atrazine+Tet_killing curve.csv
-
○
Cu+Cip_killing curves.csv
-
○
Cu+Kan_killing curves.csv
-
○
Cu+Str_killing curves.csv
-
○
Cu+Tet_killing curves.csv
-
○
Pyrethrins+Cip_killing curves.csv
-
○
Pyrethrins+Kan_killing curves.csv
-
○
Pyrethrins+Str_killing curves.csv
-
○
Pyrethrins+Tet_killing curves.csv
-
○
-
Minimum inducing concentration. Data presented in Figure 2.
-
○
Cu+Str_Minimum inducing concentration.csv
-
○
Cu+Tet_Minimum inducing concentration.csv
-
○
Pyrethrins+Str_Minimum inducing concentration.csv
-
○
-
Dependence on the AcrAB-TolC efflux pump as evidence of an adaptive response. Antibiotic resistance response in the presence and absence of copper. Data presented in Figure 3.
-
○
AcrA_Cu+Tet_killig curves.csv
-
○
AcrB_Cu+Tet_killig curves.csv
-
○
TolC_Cu+Tet_killig curves.csv
-
○
tolC was induced by copper. Relative fluorescence data for BW21003(pHJ101) in the absence and presence of copper. Data presented in Figure 4.
Fluorescense_PtolC induction.csv
-
Copper directly reduced available tetracycline. Data presented in Figure 5.
-
○
Chelation_all timepoints.csv
-
○
Chelation experiment_ANOVA tables.docx
-
○
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
We are grateful to Stuart Levy for the gift of Keio strains and Dorus Gadella for the gift of pTriEx-RhoA-wt_mScarlet-I_SGFP2. We also acknowledge Lynn Clark for support with the violin plots.
Funding Statement
Funding was received from a variety of sources none of whom played any role in the study, preparation of the article, or decision to publish. This project received funding from the Brian Mason Trust (Grant # 2015/08 to JAH) and donations to the UC Foundation (JAH) including from, inter alia, donors Third World Network (Malaysia) and the Sustainable Food Trust (UK).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved
References
- Akkermans ADL, van Elsas JD, de Bruija FJ: Molecular Microbial Ecology Manual. (Kluwer Academic Publishers: Dortrecht, The Netherlands)1996. 10.1007/978-94-009-0215-2 [DOI] [Google Scholar]
- American Chemistry Council: US Chemical industry continues to outpace industrial output; Accounts for more than one-half of construction spending by manufacturing sector.2016. Reference Source [Google Scholar]
- Baba T, Ara T, Hasegawa M, et al. : Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2(1): 2006.0008. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma MC, van der Horst MA, Guryeva T, et al. : Modeling non-inherited antibiotic resistance. Bull Math Biol. 2012;74(8):1691–1705. 10.1007/s11538-012-9731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffet-Bataillon S, Tattevin P, Maillard JY, et al. : Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016;11(1):81–92. 10.2217/fmb.15.131 [DOI] [PubMed] [Google Scholar]
- Capita R, Alonso-Calleja C: Antibiotic-resistant bacteria: a challenge for the food industry. Crit Rev Food Sci Nutr. 2013;53(1):11–48. 10.1080/10408398.2010.519837 [DOI] [PubMed] [Google Scholar]
- Capita R, Riesco-Peláez F, Alonso-Hernando A, et al. : Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl Environ Microbiol. 2014;80(4):1268–80. 10.1128/AEM.02283-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Bryant RJ: The ABCs of pesticide toxicology: amounts, biology, and chemistry. Toxicol Res (Camb). 2017;6(6):755–763. 10.1039/c7tx00198c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus SP, Guillou H, Ellero-Simatos S: The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. 10.1038/npjbiofilms.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona F, Martinez JL: Phenotypic Resistance to Antibiotics. Antibiotics (Basel). 2013;2(2):237–255. 10.3390/antibiotics2020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cristóbal RE, Vincent PA, Salomón RA: Multidrug resistance pump AcrAB-TolC is required for high-level, Tet(A)-mediated tetracycline resistance in Escherichia coli. J Antimicrob Chemother. 2006;58(1):31–6. 10.1093/jac/dkl172 [DOI] [PubMed] [Google Scholar]
- EFSA: Revision of the currently authorised maximum copper content in complete feed. EFSA J. 2016;14:4563 10.2903/j.efsa.2016.4563 [DOI] [Google Scholar]
- Gavilán RE, Nebot C, Miranda JM, et al. : Analysis of Tetracyclines in Medicated Feed for Food Animal Production by HPLC-MS/MS. Antibiotics (Basel). 2015;5(1): pii: E1. 10.3390/antibiotics5010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, et al. : Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–5. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Granados-Chinchilla F, Rodríguez C: Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem. 2017;2017:1315497. 10.1155/2017/1315497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Lou H, Zhu R, et al. : The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat Chem Biol. 2014;10(1):21–8. 10.1038/nchembio.1380 [DOI] [PubMed] [Google Scholar]
- Heinemann JA, Kurenbach B: Post-Antibiotic Era is Business as Usual. Alliance for the prudent use of antibiotics (APUA) Newsletter. 2017;35(1):7–9. [Google Scholar]
- Imfeld G, Vuilleumier S: Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur J Soil Biol. 2012;49:22–30. 10.1016/j.ejsobi.2011.11.010 [DOI] [Google Scholar]
- Justice SS, Hunstad DA, Seed PC, et al. : Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc Natl Acad Sci U S A. 2006;103(52):19884–9. 10.1073/pnas.0606329104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöppel A, Näsvall J, Andersson DI: Evolution of Antibiotic Resistance without Antibiotic Exposure. Antimicrob Agents Chemother. 2017;61(11): pii: e01495-17. 10.1128/AAC.01495-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalchuk GA, de Bruijn FJ, Head IM, et al. eds.: Molecular Microbial Ecology Manual. Dordrecht: Springer Netherlands.2004. Reference Source [Google Scholar]
- Kristiansen JE: The antimicrobial activity of non-antibiotics. Report from a congress on the antimicrobial effect of drugs other than antibiotics on bacteria, viruses, protozoa, and other organisms. APMIS Suppl. 1992;30:7–14. [PubMed] [Google Scholar]
- Kurenbach B: “Effects of Sub-Lethal Concentrations of Copper Ammonium Acetate, Pyrethrins and Atrazine on the Response of Escherichia Coli to Antibiotics.” OSF. 2018. 10.17605/OSF.IO/RZKWU [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenbach B, Gibson PS, Hill AM, et al. : Herbicide ingredients change Salmonella enterica sv. Typhimurium and Escherichia coli antibiotic responses. Microbiology. 2017;163(12):1791–1801. 10.1099/mic.0.000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenbach B, Hill AM, Godsoe W, et al. : Agrichemicals and antibiotics in combination increase antibiotic resistance evolution. PeerJ. 2018;6:e5801. 10.7717/peerj.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenbach B, Marjoshi D, Amábile-Cuevas CF, et al. : Sublethal exposure to commercial formulations of the herbicides dicamba, 2,4-dichlorophenoxyacetic acid, and glyphosate cause changes in antibiotic susceptibility in Escherichia coli and Salmonella enterica serovar Typhimurium. mBio. 2015;6(2): pii: e00009-15. 10.1128/mBio.00009-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, et al. : The Lancet Commission on pollution and health. Lancet. 2018;391(10119):462–512. 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- Leveau JH, Lindow SE: Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc Natl Acad Sci U S A. 2001;98(6):3446–3453. 10.1073/pnas.061629598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, et al. : Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. 10.1038/nature25979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-González D, Alonso-Calleja C, Alonso-Hernando A, et al. : Effect of sub-lethal concentrations of biocides on the susceptibility to antibiotics of multi-drug resistant Salmonella enterica strains. Food Control. 2014;40:329–334. 10.1016/j.foodcont.2013.11.046 [DOI] [Google Scholar]
- Motta EVS, Raymann K, Moran NA: Glyphosate perturbs the gut microbiota of honey bees. Proc Natl Acad Sci U S A. 2018;115(41):10305–10310. 10.1073/pnas.1803880115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta SS, Cluzel P, Aldana M: Adaptive resistance in bacteria requires epigenetic inheritance, genetic noise, and cost of efflux pumps. PLoS One. 2015;10(3):e0118464. 10.1371/journal.pone.0118464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team: R: a language and environment for statistical computing.2013. Reference Source [Google Scholar]
- Remus-Emsermann MN, Gisler P, Drissner D: MiniTn7-transposon delivery vectors for inducible or constitutive fluorescent protein expression in Enterobacteriaceae. FEMS Microbiol Lett. 2016;363(16): pii: fnw178. 10.1093/femsle/fnw178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner JL: Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985;82(24):8771–4. 10.1073/pnas.82.24.8771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C, Levy SB: Regulation of acrAB expression by cellular metabolites in Escherichia coli. J Antimicrob Chemother. 2014;69(2):390–399. 10.1093/jac/dkt352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu A, Halverson LJ, Beattie GA: Bacterial degradation of airborne phenol in the phyllosphere. Environ Microbiol. 2007;9(2):383–392. 10.1111/j.1462-2920.2006.01149.x [DOI] [PubMed] [Google Scholar]
- Schlechter RO, Jun H, Bernach M, et al. : Chromatic Bacteria - A Broad Host-Range Plasmid and Chromosomal Insertion Toolbox for Fluorescent Protein Expression in Bacteria. Front Microbiol. 2018;9:3052 10.3389/fmicb.2018.03052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecon R, Leveau JH: The mechanics of bacterial cluster formation on plant leaf surfaces as revealed by bioreporter technology. Environ Microbiol. 2012;14(5):1325–1332. 10.1111/j.1462-2920.2012.02715.x [DOI] [PubMed] [Google Scholar]
- Tong F, Zhao Y, Gu X, et al. : Joint toxicity of tetracycline with copper(II) and cadmium(II) to Vibrio fischeri: effect of complexation reaction. Ecotoxicology. 2015;24(2):346–355. 10.1007/s10646-014-1383-7 [DOI] [PubMed] [Google Scholar]
- Van Cuong N, Nhung NT, Nghia NH, et al. : Antimicrobial consumption in medicated feeds in vietnamese pig and poultry production. Ecohealth. 2016;13(3):490–498. 10.1007/s10393-016-1130-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QP, Browman D, Herzog H, et al. : Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS One. 2018;13(7):e0199080. 10.1371/journal.pone.0199080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Gerona RR, Schwartz JM, et al. : A Suspect Screening Method for Characterizing Multiple Chemical Exposures among a Demographically Diverse Population of Pregnant Women in San Francisco. Environ Health Perspect. 2018;126(7):077009. 10.1289/EHP2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston N, Sharma P, Ricci V, et al. : Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res Microbiol. 2018;169(7–8):425–431. 10.1016/j.resmic.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Wickham H: "ggplot2: Elegant graphics for data Analysis."In. New York, Springer Verlag.2016. 10.1007/978-0-387-98141-3 [DOI] [Google Scholar]