Abstract
Stress can influence the secretion of neuroendocrine mediators, thereby exposing immune cells to altered signaling and interactions. Here we investigated the synergetic effect of stress and environmental enrichment on the immune response of Long–Evans rats. Subjects (n = 46) were assigned to 5 treatment groups: acute compared with chronic stress with or without environmental enrichment, plus an unmanipulated control group. Animals also were classified as active, passive, and flexible copers according to back-test assessment. Rats were exposed to enrichment in an open-field containing objects in different areas for 30 min 3 times each week, thus modeling the effects of a temporary increase in environmental stimuli. Animals assigned to chronic stress groups were exposed to predator sound stressors for 30 min daily, whereas animals assigned to acute stress groups were exposed once each week. After 7 wk, a dermal punch biopsy was administered to activate the immune response, after which rats were challenged through a forced swim test. Biologic samples were collected to measure corticosterone, dehydroepiandrosterone (DHEA), oxytocin, testosterone, and the cytokines IL6 and IL10. Rats exposed to chronic stress had lower DHEA:corticosterone ratios, suggesting increased allostatic load. Enrichment exposure modulated these effects, lowering overall corticosterone and testosterone levels and increasing DHEA and oxytocin levels in animals exposed to the predator sound. The immune response was decreased in rats exposed to chronic stress, but the effect of environmental enrichment helped to mitigate the negative influence on cells producing IL6. Combining acute stress and exposure to an enriched environment returned a healthier profile in terms of both immune activation and stress regulation. By using a multidimensional scaling model, we found that a combination of ‘good’ stress and exposure to brief sessions of enriching stimuli can reliably predict health in Long–Evans rats.
Abbreviations: DHEA, dehydroepiandrosterone; EP, enrichment park; FST, forced swim task
The immune system is crucial to the survival of mammals. In the presence of a compromised immune system, even a minor infection can take hold and prove fatal. Left untreated, immunocompromised children, such as those affected by severe combined immunodeficiency, usually die in their early life due to the complication of common infections.23,24 It takes time for organisms to build and maintain an efficient immunity to common pathogens. When infants are born, they are exposed to microbes from the mother's cervix and, after parturition, from the surrounding environment. Within a matter of hours, the gastrointestinal tract of newborns, initially relatively free of microbes, is heavily colonized with bacteria.38 As development progresses, insults arising from the environment can compromise the optimal functioning of the immune system.44 To better cope with a continuously changing physical and social environment, mammals have developed a dynamic interaction among the various cell types that comprise the immune system and other key neuroendocrine regulatory systems, including the HPA axis.2 Compelling evidence suggests that the cells of the immune system operate similarly to a sensory organ, informing the brain of inflammatory conditions.11 As a consequence, in a time in which global changes to the environment occur daily and stress levels are skyrocketing, it is imperative to better understand the dynamic communication between behavioral plasticity and immune functions.
Many neuroendocrine mediators are released in the peripheral circulation during the stress response, thereby exposing cells of the immune system to altered signaling networks. Levels of glucocorticoids, catecholamines, opiates, and prolactin tend to increase in response to stress, whereas growth hormone, oxytocin, and testosterone levels decrease.35,48 Several cell types in the immune system have receptors for each of the mediators just mentioned. Conversely, proinflammatory cytokines (for example, IL6) and antiinflammatory cytokines (for example, IL10) can activate the HPA axis and contribute to behavioral modification, such as altered mood and impaired emotional regulation.12,15,31 The biologic communication (that is, crosstalk) between the HPA axis and the immune system is particularly important because evidence shows that chronic stress can create significant ‘wear and tear’ (that is, allostatic load) on organisms, reducing their ability to maintain normal physiologic functions, including immunologic activation.17,34 The effects of acute stress on the immune response have not been studied as extensively, although several recent studies indicated that moderate concentrations of glucocorticoids exercise a stimulatory effect on many cell types of the immune system and thus exert a short-term beneficial effect on immune functioning.3 Considering that other studies have found an increase in inflammation during acute stress,37 the role of acute stress must be clarified.
To complicate matters further, organisms don't live in a vacuum. Changes in the environment can, directly and indirectly, modify the crosstalk between the neuroendocrine and immune systems. For example, it is well-known that raising animals in an enriched environment can decrease stress and improve neural plasticity in laboratory rats.5 The positive effects of a natural environment on the immune response of patients recovering from trauma has been known for quite some time, but surprisingly very little research has been conducted to further explore this critical topic. Clinical studies suggest that patients recover from surgery more quickly when the view from their hospital room window is a natural environment.49 Animal models have identified natural enrichment as a way to improve resilience, reduce stress, and increase the general behavioral plasticity of animals.5,28 Clearly, stress levels and environmental characteristics are highly interrelated. However, no studies have investigated the synergic effects of natural enrichment and acute stress on the immune response of an organism, especially when the exposure to environmental enrichment is relatively brief.
A possible way to differentiate between the positive and negative effects of HPA axis activation is by monitoring chemicals such as dehydroepiandrosterone (DHEA). During a stress response, DHEA is released to inhibit catecholamine upregulation in the adrenal medulla and many of the negative effects of glucocorticoids in various tissues.14 Potentially related to the changes in the crosstalk between the HPA axis and immune response, DHEA can act centrally to decrease glucocorticoid-induced neuronal death in the hippocampus and to promote neurogenesis in the dentate gyrus of the hippocampus and sensory dorsal root ganglion neurons.40 Furthermore, the ratio between DHEA and cortisol has been found to be a reliable index of neuroprotection.32 Very little is known about the potential role of DHEA in protecting neurons from inflammation.
In addition, HPA axis activity is critically intertwined with several other biomarkers of the overall allostatic load of animals. Of primary importance are the hormones testosterone and oxytocin. Testosterone plays a crucial role in behavioral modifications related to the reproductive effort of males, and it well known that peripheral concentrations of testosterone often plunge in response to acute stress.13 Evidence also shows that testosterone levels can moderate immune response activation through the androgen receptors expressed in many immune cells.8 The oxytocin-secreting system recently has emerged a pivotal factor in the neuroendocrine mediation of the immune response.30 Oxytocin can regulate immune function directly and indirectly through sympathetic activation.9 In response to immune challenges, IL6 and IL1β can activate oxytocin neurons in the paraventricular and supraoptic nuclei.9
The main goal of the current study was to use a rodent model to investigate the synergic effects of acute or chronic stress and exposure to brief environmental enrichment for 30 min every other day on the behavioral, physiologic, and immunologic responses of Long Evans rats. To investigate the neuromodulation of stress and environmental enrichment, peripheral concentration of oxytocin, testosterone, cortisone, and DHEA were assessed. Proinflammatory (IL6) and antiinflammatory (IL10) interleukins were measured also. Detailed behavioral analyses in 2 different settings (a forced swim task for all animals and during exposure to environmental enrichment for the animals in those groups) allowed for the comparison of the physiologic and behavioral responses to different paradigms. Considering that a complete, dramatic change in the environment is highly improbable for most human populations living in impoverished conditions and given that most animal studies have focused on housing enrichments,5,28 which model a continuous and dramatic increase in environmental stimulation, we decided to assess the effect of moderate environmental enrichment, in the form of exposure to an ‘enrichment park’ (EP) for just 30 min, 3 times each week. We hypothesized that even moderate exposure to the EP would facilitate neuroendocrine and behavioral adaption to stress, including boosting the immune response. We also hypothesized that acute stress would be beneficial to the long-term immune response of rats. Finally, we expected significant interaction between the type of stressor and the type of environmental enrichment, with the most notable improvement to the immune response associated with animals exposed to acute stress and the enriched environment. More specifically, we expected (1) that rats exposed to chronic stress would spend more time engaging in anxiety-like behaviors (such as hiding and freezing) and less time in exploratory behaviors and (2) that the interaction between EP exposure and stress would result in higher levels of DHEA than corticosterone, higher levels of oxytocin, and lower levels of IL6 in comparison to IL10. Our results could provide the basis for future translational research in the effort to improve the treatment of patients in clinical settings.
Materials and Methods
Animals.
Male Long–Evans rats (Rattus norvegicus; n = 46; age, 21 to 24 d) were purchased from Envigo RMS (Indianapolis, IN). Animal were purchased pathogen free (45 different species were tested, including Hantaan virus, Kilham rat virus, rat minute virus, rat Theiler virus, CAR bacillus, Salmonella spp, Streptobacillus monoliformis, enteric protozoan, and so forth) and maintained in this status for the remaining of the study. After 14 d of habituation to the laboratory, animals were pair-housed in static standard laboratory cages (48 cm × 26 cm × 21 cm) containing corncob bedding, with rodent chow (catalog no. T.2018.15, Teklad Global 18% Protein Rodent Diet, Envigo, Indianapolis, IN) and water freely available. The rats were on a 12:12-h light:dark cycle (lights on, 0800; lights off, 2000 h). Rats were maintained in accordance with the Randolph–Macon College IACUC (protocol no. 17-01). Appropriate analgesia (buprenorphine, 1 mL/kg) was provided for all procedures that were potentially painful or harmful across all treatments.
Rats were assigned randomly to 5 treatment groups (approximately 10 animals per group, to achieve a statistical power of approximately 0.8; the observed power calculation option in the GLM function, SPSS 25.0, was used) by using a randomization program (Excel, Microsoft, Redmond, WA). The timeline of the experiment and a description of each treatment is given in Figure 1. The first group (CSE; n = 10) was exposed to chronic stress in the form of predator sounds for 30 min daily, 5 d each week, and to environmental enrichment (that is, EP) for 30 min daily, 3 d each week (CSE). The second group (ASE; n = 8) was exposed to acute stress due to predator sounds for 30 min daily, once each week, and to environmental enrichment as detailed earlier. The third group (CSS; n = 10) was exposed to chronic stress and no enrichment (that is, static). The fourth group (ASS; n = 10) was exposed to acute predator sound stress and no enrichment. The final group was the control group (NSS; n = 8); these rats lived in standard conditions, with no additional stress or enrichment. The EP was a circular (diameter, 1 m), open-field area with standard corncob bedding and contained 3 designated areas: a play area, a hiding area, and a climbing area (Figure 2). The climbing area included ropes and vertical objects; the hiding area had shelters and other objects in which rats could hide from view; and the play area contained several small objects which rats could interact with, touch, and move. Pilot behavioral data showed that rats used the different areas according to their design. Exposure to the EP was limited to 30 min daily, 3 times each week, to simulate a discontinuous and moderate environmental enrichment. Rats were exposed to the EP in the morning, between 0800 and 1200 h
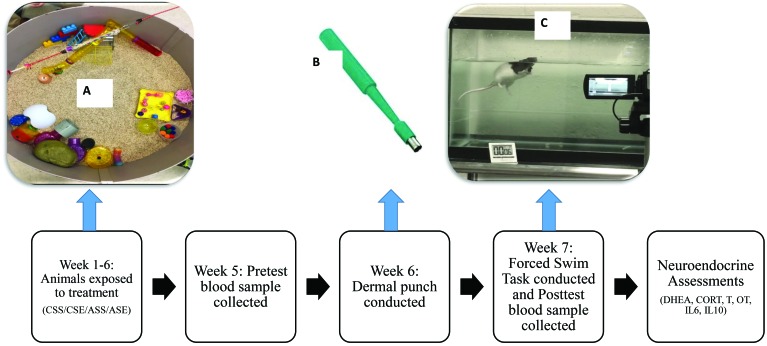
Figure 1.
Diagram of the timeline and procedures of the experiment. Rats were pair-housed in standard cages for 7 wk and underwent treatments daily (A). During week 5, a baseline blood sample was collected from each animal. During week 6, dermal punches were conducted to activate the immune response (B) During week 7, the 2 trials of the forced swim task were conducted over the course of 2 d, and a blood sample was collected (C). After 7 wk of treatment, neuroendocrine assessments were conducted, including hormone assays for dehydroepiandrosterone (DHEA), corticosterone (CORT), testosterone (T), oxytocin (OT), and the cytokines IL6 and IL10.
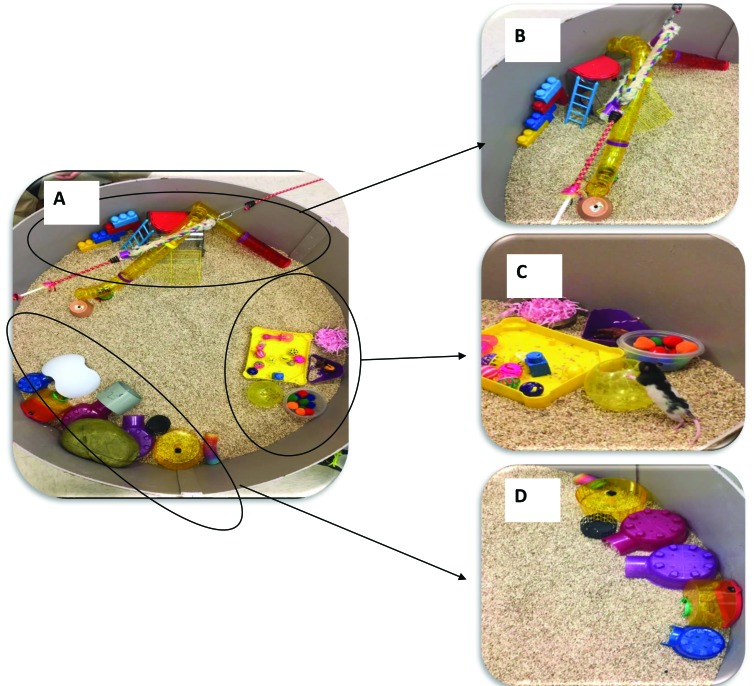
Figure 2.
Enrichment park (EP) apparatus. (A) The EP was a circular (diameter, 1 m), open-field area with standard corncob bedding. It was divided in 3 distinct areas: (B) a climbing area, (C) a play area, and (D) a hiding area. Activity in the empty center of the field was recorded also.
To prevent other animals from hearing the sounds, rats were exposed to predator sound stressors in a separate room; stressor predator sounds rotated daily between a distressed rat sound, metal chain sound, hawk sound, and cougar sound and were played over speakers (HK206, Computer Speakers, Harman Kardon, Stamford, CT). The predator sound was selected to simulate an ecologically relevant stressor. Rats in the chronic stress conditions were exposed to the sounds for 30 min daily, 5 days each week. All rats were transported in their home cage to the treatment room, and they were all exposed at the same time in the afternoon, between 1500 and 1800 h. Animals in acute stress were exposed to similar sounds during the same time range but only once each week. Because rats can recover from stress rather quickly, exposing them to stress once each week was an ecologically valid approximation of acute stress.6,7,25
Coping profile assessment.
Three days after the rats’ arrival to the laboratory, coping profile assessments were conducted in the morning (between 0800 and 1200 h), with each session videotaped for subsequent confirmation of behavior. During the assessment, each animal was gently restrained on its back for 1 min so that the number of escape attempts (or wiggles) could be quantified.25 The same assessment was repeated 7 d later, but the animals were tested in a different order to avoid any confounding sequencing effects. Once the 2 sessions were observed and scored, the number of escape attempts for each session was used as the criterion score for the determination of coping profiles. The greatest number of escape attempts for either session was 16 responses, whereas the lowest number was 2 (average escape attempts per session, 6.88). Rats with 7 or fewer attempts in each session were assigned to the passive coping group and those with 8 or more responses were categorized as active copers. When the rat's response number remained in the same category during the second assessment, then its final placement was in that respective category. However, when the animal switched from one category to another (in either direction), it was classified as a flexible coper. After the final assessment, 17 rats were classified as active copers, 13 as flexible, and 16 as passive. Animals were assigned to the treatment conditions on the basis of their coping skills to create groups of similar size.
Behavioral assessment.
To evaluate the behavioral responses to the EP, rats in the enrichment treatment groups (CSE, n = 10; ASE, n = 8) were rotated through the EP every other day for 30-min sessions each day. Rats were recorded continuously during each trial, and assessments were conducted throughout the entire session. Before starting the actual data collection, animals were exposed once to the EP for 1 h. Animals were transported in their home cages from the housing colony to the EP by using a cart. All rooms were adjacent to each other, so transportation time was negligible (that is, a few seconds). Animals were placed individually in the EP, by using a randomization process to select the order. After each use, the EP was sanitized by using disinfecting wipes. Bedding was inspected after each animal and cleaned when excreta were found. At the end of each day, the EP was cleaned and reset for the following day. The frequency, duration, and latency to first enter each zone was tracked and calculated (Ethovision, Noldus, Asheville, NC) for the climbing zone (that is, ladders, Lego stairs, and climbing tubes), play zone (that is, cat toy balls, cotton balls, a running ball, and a bowl of paper stuffing), and hiding zone (that is, plastic hide-a-ways, caves, and tubing; Figure 2).
In addition, animals in the EP were videorecorded (model HMX-F90, Samsung, Seoul, South Korea) to collect the frequency and duration of the behaviors listed in Figure 3. All behavioral coding was done by 2 observers who were blinded to the treatment conditions and trained by the principal investigator (MB; interreliability, greater than 90%).
Figure 3.
List of behaviors recorded during the exposure of rats to the enrichment park (EP).
Forced swim task (FST).
To evaluate emotional regulation during a life-threating challenge, the FST was conducted after 7 wk of treatment. A tank (62 × 32 × 53 cm) was filled with water 1 d before testing, so that it could reach room temperature (21 °C). Each rat was placed individually into the tank and was allowed 5 min to swim. The task was conducted between 1800 and 2100 h. The numbers of dives, shakes, and fecal pellets were recorded. Subsequently, the duration spent floating and duration spent swimming were recorded. Once the trial was over, all rats were dried and returned to their assigned cages. A second FST was conducted 24 h after the first one, at the same time (between 1800 and 2100 h) and with the same procedure. We selected 2 trials to assess the reaction of animals after having experienced the challenge once, to minimize the effects due to the novelty of the experience. Once again, observers were blinded to the treatment conditions of the animals.
Dermal biopsy punch procedure and Measurements.
To activate the immune response of the rats,42 a dermal punch biopsy was administered after 7 wk of treatment. After aseptic preparation of the skin, each rat was placed inside a small chamber and anesthetized by using 1 mL of halothane ((Sigma Aldrich, St Louis, MO) on cotton balls; after anesthesia was induced, a small dorsal area was shaved by using an electric pet razor. This method was preferred to alternative methods of anesthesia because we have used it effectively in our laboratory previously. Subsequently, each rat received a dermal punch biopsy wound from a 5-mm biopsy punch (Mountainside Medical Equipment, Marcy, NY), followed by wound disinfection with alcohol. Wounds were disinfected and measured over the course of 14 d by using a digital caliper. Local analgesic (bupivacaine, 0.5% solution) was administered as needed (9 rats in total). All wounds were healed by day 15.
Collection of biologic samples.
Fresh fecal samples were collected at baseline, prior to any treatment, from all animals when they arrived at the laboratory. After that, fecal samples were collected every week and before and after the FST. This procedure allowed us to monitor the physiologic stress response of the rats throughout the experimental procedures. All samples were collected between 0900 and 1000 h, to account for circadian changes in hormonal levels, including before the FST (in the morning of the day the FST was given) and in the morning after the FST. Previous work in our lab revealed that the peak concentrations of fecal steroid metabolites occur approximately 12 h after exposure to the stressor.5-7 During fecal collection, rats were briefly isolated (less than 10 min for each rat) and fecal boli (0.1 g each) were collected and frozen unmixed in sealed containers at −70 °C until analysis. Metabolized steroid hormones (that is, corticosterone, DHEA, and testosterone) were assayed by using fecal samples.
At 3 d before and 1 d after the FST, blood samples were collected from all animals. Samples were taken in the morning, between 800 and 1100 h. Blood samples were taken from the saphenous vein, a vascular bundle located in the back of the hindleg that can provide an effective and consistent source of blood, by using lancets (point length, 6.5 mm; Goldenrod Animal Lancets, MEDIpoint, Mineola, NY). Before venipuncture, the rats were placed inside a small chamber and anesthetized by exposing them to cotton balls treated with 0.5 mL of halothane. The anesthetized animal then was retrieved by an experimenter, who shaved the back of the rat's hindleg by using electric trimmers until the saphenous vein was visible. Slight pressure was applied gently above the knee joint to facilitate blood dripping. When necessary, bleeding was stopped by using a cotton-tipped swab. By using this technique, as much as 0.5 mL blood was collected from most of the animals. The entire process, including anesthetization, was fairly quick, with the total collection time averaging less than approximately 3 min for each animal. During the duration of the experiment, animals were constantly monitored during the procedure and were observed to recover quickly. Blood samples were transferred into 1.5-mL microcentrifuge tubes (Fisher Scientific, Pittsburgh PA) impregnated with EDTA as an anticoagulant. The samples were stored at 4 °C, centrifuged (1500 × g for 10 min) for serum separation, and then stored at –20 °C until evaluated. Blood samples were used to assay oxytocin, IL6, and IL10 levels.
Extraction of biologic samples.
Prior to extraction, stored fecal samples were thawed at room temperature, placed in a glass tube containing 1 mL of 100% methanol, mixed (Vortex Genie 2, Scientific Industries, Bohemia, NY) for approximately 30 s, and centrifuged for 15 min at 1000 × g. By using a transfer pipette, the sample was transferred to a 13×100-mm glass test tube. The final step of extraction procedures was to dilute the methanol-containing sample 1:20 in the manufacturer-provided assay buffer. Assays were performed by using the materials and protocols provided in each hormone-specific ELISA kit (Enzo Life Science, Farmingdale, NY). Samples were read by using an automated microplate reader (Synergy model, BioTek, Winooski, VT) and Gen5 software (version 2.04.11, BioTek). Readings were assessed at a wavelength of 405 nm with correction at 490 nm. Log–logit transformations of the data were analyzed through least-squares regression analysis. Accuracy was demonstrated at each point on the standard curve. Quality-control pools were assayed in triplicate on each plate.
Plasma concentrations of oxytocin, IL6, and IL10 were determined by using commercially available assay kits (Enzo Life Sciences). Chemicals were extracted by adding 1 mL diethyl ether to each sample and then mixing the contents rapidly (Vortex Genie 2, Scientific Industries). Tubes were allowed to settle, and the aqueous phase was removed by freezing and pouring. Ether was evaporated away by placing the tubes in a 42 °C water bath inside a fume hood. The samples were reconstituted through the addition of assay buffer; 1:20 dilution for oxytocin and 1:30 for IL6 and IL10. Samples, controls, and standards were prepared in duplicate and added in 100-μL aliquots to the appropriate wells of the assay plates. Samples were read by using an automated microplate reader (Synergy model, BioTek) and Gen5 software (version 2.04.11, BioTek). Readings were assessed at a wavelength of 405 nm, with correction at 490 nm.
Assay validation.
To demonstrate the parallelism and accuracy of the assays, each of 5 randomly selected extracted samples was serially diluted (1:2 to 1:16). Percentage binding data from the standard curve were plotted against logarithmic transformations of their dosages, and the resulting regression equation was compared with those of the dilution sequences. To assess dose response, another 5 extracted samples were randomly selected, and unlabeled target medium (corticosterone, DHEA, testosterone, oxytocin, IL6, and IL10) was added to 10-μL sample aliquots in increasing doses (that is, 0, 2.5, 10, 40, and 160 pg per tube). The dose–response studies generated curves with similar slopes around the expected 1.0 (± 0.05). All mean recoveries exceeded the optimal 90% threshold. The slopes generated from the serially diluted samples in the parallelism study did not differ from the standard curve slope (P > 0.85 for all comparisons). Intra- and interassay coefficients of variations were 5.0% to 8.1% and 6.6% to 11.1%, respectively.
Statistical analysis.
A 2-way general linear model analysis was used to determine the effects of the independent variables stress and EP (5 levels: acute or chronic stress; exposure to enrichment compared with standard cage; control animals with no stress; and no exposure) and coping profiles (3 levels: active, flexible, and passive animals). When the response variable was measured several times (such as, time spent floating on days 1 and 2), we used a repeated-measures mixed model with the same independent variables as factors. The α value was set at 0.05. A sample size of 10 animals per group was set to achieve a statistical power of about 0.80. We used eta-squared values (η2) to provide measures of effect size; this parameter indicates the percentage of variance in the dependent variable that is attributable to a particular independent variable and it is calculated as η2 = SSbetween / SStotal, where SS is the total sum of squares. Multivariate ANOVA was used to test the effects of the independent variables on groups of variables highly correlated, such as anxiety-like behaviors (hiding, freezing, grooming interrupted) and exploratory behaviors (rearing, exploring, object interaction).
To assess the independent associations among selected variables, we used multidimensional scaling analysis, a data reduction technique that reveals the similarities among variables and individual cases in a set of data.27 Distances between variables were derived by looking at partial correlations (that is, proximities) among variables, which subsequently were used to create a matrix of distance could be displayed graphically. The closer 2 or more variables are on the map, the more highly correlated they are, whereas the farther apart they are, the less correlated they are. To arrange the variable into a map sensitive to each individual contribution, a limited lack of fit between the data and the model is inevitable. This lack of fit is known as the s-stress. The values of s-stress range from 0 (perfect fit) to 1 (worst possible fit). Thus, the aim of multidimensional scaling is to find a map of the variables that minimize the s-stress. The number of dimensions in a map is linked to the number of latent underlying factors in the dataset, similarly to other procedures like factor analysis. As a consequence, the optimal number of dimensions to represent the data is dependent on several factors: (1) the number of variables in the model, (2) the lack of fit (s-stress value) given the number of dimensions, (3) an index of fit of the model (r2 value), and (4) interpretability of the dimensions.27 Typically, r2 values of 0.8 or higher are considered acceptable.
All analyses were performed by using SPSS 25.0 (IBM, Armonk, NY).
Results
Behavioral responses in the EP.
There were no significant differences in the time spent in the 3 of the 4 areas of the EP (play, climb, and center) between rats exposed to acute and chronic stress (Table 1). The only significant difference was in the time spent in the hiding area, where rats exposed to chronic stress stayed longer than those exposed to acute stress (210 compared with 171 seconds per session, respectively; P = 0.05; Table 1). Coping style did not affect the time spent in any of the 4 areas (Table 1), nor was there any significant interaction effect between stress and coping (Table 1).
Table 1.
Time spent in the different areas of the enrichment park (EP) according to treatment (acute compared with chronic stress) and coping style
| Area | Overall timea | F1,12 | P | η2 |
| Chronic compared with acute stress | ||||
| Hiding | 192 | 4.12 | 0.05 | 0.25 |
| Play | 446 | 0.27 | 0.61 | 0.02 |
| Climbing | 836 | 0.07 | 0.79 | 0.01 |
| Center | 112 | 0.89 | 0.36 | 0.07 |
| Coping style (active, flexible, or passive) | ||||
| Hiding | 192 | 0.17 | 0.84 | 0.03 |
| Play | 446 | 0.67 | 0.53 | 0.1 |
| Climbing | 836 | 0.65 | 0.54 | 0.09 |
| Center | 112 | 2.31 | 0.14 | 0.28 |
| Interaction effect (copingxstress) | ||||
| Hiding | 192 | 1.09 | 0.37 | 0.15 |
| Play | 446 | 0.66 | 0.53 | 0.09 |
| Climbing | 836 | 2.86 | 0.09 | 0.32 |
| Center | 112 | 0.09 | 0.91 | 0.01 |
Rats were exposed to the EP for 30 min.
Data are given as the average no. of seconds per session.
While in the EP, rats exposed to chronic stress froze more than animals exposed to acute stress (10.9 compared with 25.6 seconds per session; F1,12 = 10.04; P = 0.003; η2 = 0.456). There were no significant differences relative to coping strategy (F2,12 = 0.234; P = 0.795; η2 = 0.038) or due to coping×stress interaction (F2,12 = 1.36; P = 0.293; η2 = 0.185).
Exposure to chronic stress reduced the time that rats spent interacting with objects while in the EP (chronic stress group, 274.5 seconds per session; acute stress group, 333.3 seconds per session; F1,12 = 6.57; P = 0.025; η2 = 0.354). There were no significant differences according to coping strategy (F2,12 = 2.819; P = 0.099; η2 = 0.32) or due to coping×stress interaction (F2,12 = 0.533; P = 0.6; η2 = 0.082).
Chronic stress decreased significantly the time spent in both rearing (chronic stress group, 48.7 seconds per session; acute stress group, 101.7 seconds per session; F1,12 = 12.55; P = 0.004; η2 = 0.511) and assisted rearing (chronic stress group,78.7 seconds per session; acute stress group,121.9 seconds per session; F1,12 = 8.06; P = 0.015; η2 = 0.402) during the EP sessions. No effects of coping or an interaction effect of coping×stress were found for either behavior (P > 0.28 for all comparisons).
Both grooming and grooming interrupted during the EP sessions were affected by chronic stress. Grooming was lower in rats exposed to chronic stress (chronic stress group, 42.3 seconds per session; acute stress group, 100.6 seconds per session; F1,12 = 16.12; P = 0.002; η2 = 0.573) and grooming interrupted was higher (chronic stress group,41.1 seconds per session; acute stress group, 16.2 seconds per session; F1,12 = 19.85; P = 0.001; η2 = 0.623). No effects of coping or the interaction of coping×stress were found for either behavior (P > 0.19 for all comparisons).
Using multivariate ANOVA, we found that chronic stress increased anxiety-like behaviors (that is, freezing, hiding, grooming interrupted; Pillai Trace = 0.676; F3,10 = 6.96; P = 0.008) and decreased exploratory behaviors (that is, rearing, assisted rearing, exploring, object interactions; Pillai Trace = 0.744; F4,9 = 6.99; P = 0.009) in the EP.
Behavioral responses during the FST.
For all groups combined, time spent floating was significantly different between days 1 and 2 (5.07 compared with 9.65 seconds per session; F1,31 = 13.23; P = 0.001; η2 = 0.299). In addition, there was a significant time×coping interaction effect (F2,31 = 3.36; P = 0.048; η2 = 0.178): flexible copers had the largest difference in time spent floating between the 2 sessions, whereas active copers spent significant less time floating during both sessions (Figure 4 A). Moreover, the EP–stress treatment had a significant effect on time spent floating as well (F4,31 = 3.70; P = 0.014; η2 = 0.323): animals exposed to both acute stress and the EP had the least floating, whereas rats in the control group had the most (Figure 4 B). Time and EP–stress treatment did not show an interaction effect (F4,31 = 1.37; P = 0.267; η2 = 0.150).
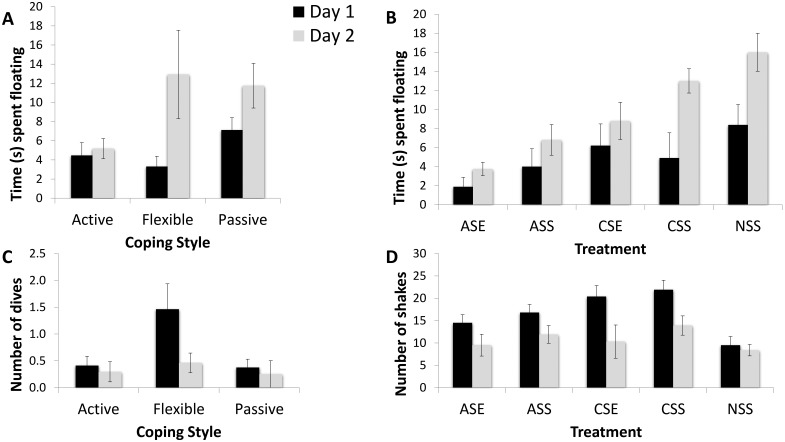
Figure 4.
Behavioral responses during the forced swim task (FST); data are shown as mean ± 2 SEM (95% intervals). *, P < 0.05. Animals were tested twice by using the FST, on day 1 (black bars) and day 2 (gray bars). The independent variables were coping profile (animals were classified as active, passive, or flexible on the basis of the back test) and treatment (ASE, exposed to acute stress and the enrichment park; ASS, acute stress but not EP; CSE, chronic stress and the EP; CSS, chronic stress but not EP; NSS, no stressor or environmental enrichment). (A) Time spent floating showed a significant interaction effect with coping style (F2,31 = 3.36; P = 0.048; η2 = 0.178): flexible copers had the largest difference in time spent floating between the 2 sessions, whereas active copers spent less time floating during both sessions. (B) Treatment influenced the time spent floating also (F4,31 = 3.70; P = 0.014; η2 = 0.323); animals exposed to acute stress and the EP had the lowest levels of floating, whereas animals in the control group had the highest levels. (C) Coping style had a significant effect on the number of diving attempts (F2,31 = 4.32; P = 0.028; η2 = 0.206): flexible copers attempted to dive more than both active and passive copers. (D) EP–stress exposure had a significant effect on shakes (F4,31 = 2.93; P = 0.037; η2 = 0.274): exposure to chronic stress was related to more shakeseconds per session.
The number of diving attempts for all groups combined was significantly different between days 1 and 2 (0.70 compared with 0.33 attempts per session; F1,31 = 4.36; P = 0.045; η2 = 0.123). Coping style had a significant effect on diving attempts (F2,31 = 4.32; P = 0.028; η2 = 0.206): flexible copers attempted to dive significantly often than both active and passive copers (Figure 4 C). There was no interaction between time and coping strategy (F2,31 = 2.68; P = 0.084; η2 = 0.148) or an effect of exposure to the EP–stress treatment (F4,31 = 1.34; P = 0.276; η2 = 0.148) on the number of diving attempts.
For all groups combined, the number of shakes was significantly different between days 1 and 2 (17.02 compared with 10.96 per session; F1,31 = 22.44; P < 0.001; η2 = 0.420). EP–stress exposure had a significant effect on the number of shakes (F4,31 = 2.93; P = 0.037; η2 = 0.274): exposure to chronic stress was related to a higher frequency of shakes per session (Figure 4 D). There was no time×coping interaction effect (F2,31 = 1.37; P = 0.224; η2 = 0.163) or an effect of coping (F2,31 = 0.85; P = 0.437; η2 = 0.052) on shake frequency.
Steroid hormone metabolites.
Metabolized corticosterone levels were affected by both EP–stress treatment (F4,31 = 15.60; P < 0.001; η2 = 0.668) and coping style (F2,31 = 9.91; P < 0.001; η2 = 0.390). Corticosterone varied significantly during the 3 phases of the experimental procedure, showing a significant interaction with both EP–stress treatment (F8,62 = 39.49; P < 0.001; η2 = 0.836) and with coping (F4,62 = 3.71; P = 0.008; η2 = 0.197). At baseline, there were no differences in corticosterone concentration among all treatment groups (Figure 5 A). After 1 mo of treatment, corticosterone levels for animals exposed to acute stress and the EP were significantly lower than for any other group, including the controls, and corticosterone was significantly higher in rats exposed to chronic stress in both EP groups (exposed and not exposed to EP). After the FST, corticosterone levels were the highest in the control rats and in the group exposed to acute stress but not EP exposure groups and the lowest in the animals exposed to chronic stress. Focusing on coping style, flexible copers had the lowest levels of corticosterone after 1 mo of treatment and after the FST (Figure 5 B).
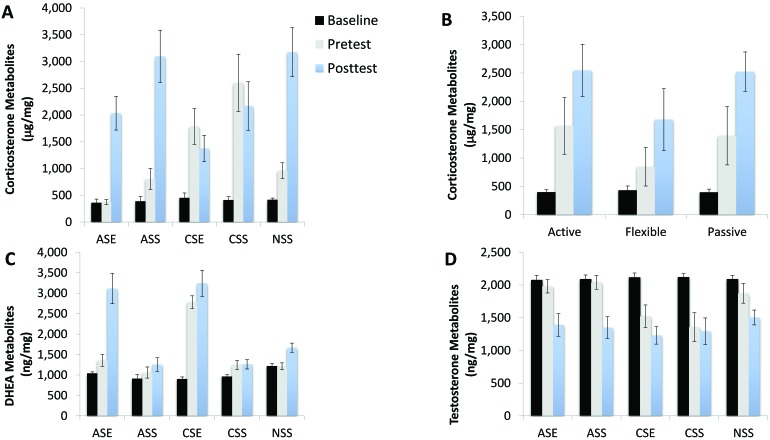
Figure 5.
Metabolized steroid hormones; data are shown as mean ± 2 SEM. *, P < 0.05. Rats were tested 3 times during the experimental procedures: at baseline (black bars), 1 mo after the experiment started (pretest, gray bars), and after the FST (posttest, light-blue bars). The independent variables were coping profiles (rats were classified as active, passive, or flexible on the basis of the back test) and treatment (ASE, exposed to acute stress and the enrichment park; ASS, acute stress but not EP; CSE, chronic stress and the EP; CSS, chronic stress but not EP; NSS, no stressor or environmental enrichment). (A) Metabolized corticosterone varied during the 3 phases of the experimental procedure, showing a significant interaction with EP–stress treatment (F8,62 = 39.49; P < 0.001; η2 = 0.836). (B) Metabolized corticosterone varied significantly by coping style: flexible copers had the lowest levels of CORT after 1 mo of treatment (pretest) and after the FST (posttest). (C) Metabolized DHEA showed significant interaction with EP–stress treatment (F8,62 = 7.45; P < 0.001; η2 = 0.490). (D) Metabolized testosterone levels were affected by the EP–stress treatment (F4,31 = 9.31; P < 0.001; η2 = 0.546); at baseline, there were no differences among all treatment groups. After 1 mo of treatment, testosterone levels deceased significantly in animals exposed to chronic stress. After the FST, testosterone levels were significantly lower than baseline across all treatment groups.
Metabolized DHEA levels were affected by both EP–stress treatment (F4,31 = 39.39; P < 0.001; η2 = 0.836) and coping style (F2,31 = 4.76; P = 0.016; η2 = 0.235). DHEA varied significantly during the 3 phases of the experimental procedure, showing a significant interaction with EP–stress treatment (F8,62 = 7.45; P < 0.001; η2 = 0.490) but not with coping style (F4,62 = 0.43; P = 0.785; η2 = 0.027). At baseline, there were no differences in DHEA concentration among all treatment groups (Figure 5 C). After 1 mo of treatment, DHEA levels increased significantly for animals exposed to both chronic stress and the EP. After the FST, DHEA levels were significantly (P < 0.01) higher in both groups exposed to the EP (acute and chronic stress). Focusing on coping style, flexible copers had the highest levels of DHEA after 1 mo of treatment and after the FST (P = 0.002).
Metabolized testosterone levels were affected by the EP–stress treatment (F4,31 = 9.31; P < 0.001; η2 = 0.546) but not by coping style (F2,31 = 2.28; P = 0.125; η2 = 0.126). Testosterone varied significantly during the 3 phases of the experimental procedure, showing a significant interaction with both EP–stress treatment (F8,62 = 28.14; P < 0.001; η2 = 0.784) and coping style (F4,62 = 3.23; P = 0.018; η2 = 0.172). At baseline, there were no differences among all treatment groups (Figure 5 D). After 1 mo of treatment, testosterone levels decreased significantly (P = 0.013) in animals exposed to chronic stress. After the FST, testosterone levels were significantly (P = 0.011) lower than baseline across all treatment groups.
Immunologic profiles.
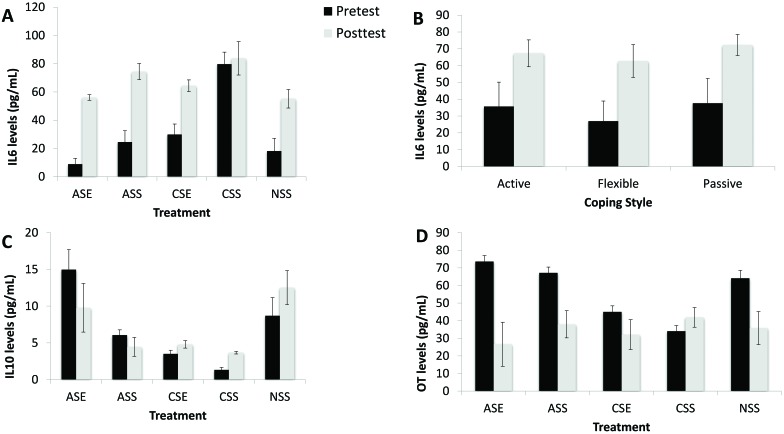
Peripheral IL6 levels were affected by both EP–stress treatment (F4,31 = 45.19; P < 0.001; η2 = 0.854) and coping style (F2,31 = 7.71; P = 0.003; η2 = 0.314). IL6 levels varied significantly before and after the FST (F1,31 = 49.60; P < 0.001; η2 = 0.901), showing a significant interaction with EP–stress treatment (F4,31 = 29.60; P < 0.001; η2 = 0.854) but not with coping strategy (F2,31 = 0.23; P = 0.794; η2 = 0.015). After 1 mo of treatment, IL6 levels were significantly (P < 0.001) higher in chronically stressed rats not exposed to the EP (that is, CSS animals) than any other group (Figure 6 A). Animals exposed the EP and to acute stress had significantly (P < 0.01) lower IL6 levels than all other groups (Figure 6 A). After the FST, IL6 levels increased in all groups, but animals not exposed to the EP had the highest levels in comparison the other 3 groups (Figure 6 A). IL6 was also affected by coping profiles: flexible animals tended to have the lowest IL6 levels at both times, whereas passive animals had the highest (Figure 6 B).
Figure 6.
Immunologic profiles; data are shown as mean ± 2 SEM. *, P < 0.05. Rats were tested twice during the experimental procedures: 1 mo after the experiment started (pretest, black bars) and after the FST (posttest, gray bars). The independent were coping profiles (rats were classified as active, passive, or flexible on the basis of the back test) and treatment (ASE, exposed to acute stress and the enrichment park; ASS, acute stress but not EP; CSE, chronic stress and the EP; CSS, chronic stress but not EP; NSS, no stressor or environmental enrichment). (A) IL6 levels were significantly higher in chronic stressed animals not exposed to the EP (CSS) than any other groups. (B) Flexible animals tended to have the lowest IL6 levels at both times, whereas passive animals had the highest. (C) IL10 levels were significantly lower in chronic stressed animals not exposed to the EP (CSS) than any other groups. (D) Animals exposed to chronic stress had the lowest levels of oxytocin (OT). After the FST, all groups showed a significant decrease in oxytocin levels.
Peripheral IL10 levels changed according to EP–stress treatment (F4,31 = 66.46; P < 0.001; η2 = 0.896) but not coping style (F2,31 = 1.05; P = 0.363; η2 = 0.063). IL10 levels did not change significantly before and after the FST (F1,31 = 0.27; P = 0.870; η2 = 0.001), but there was a significant interaction with EP–stress treatment (F4,31 = 7.54; P < 0.001; η2 = 0.493). We did not find a significant interaction with coping type (F2,31 = 0.87; P = 0.429; η2 = 0.053). After 1 mo of treatment, IL10 levels were significantly (P < 0.01) lower in chronic stressed animals not exposed to the EP (that is, CSS group) than any other group (Figure 6 C). In addition, rats exposed the EP and to acute stress had higher IL10 levels than all other groups. After the FST, IL10 levels decreased in animals exposed to chronic stress (regardless of whether [or not] they were exposed to the EP) and in those under to acute stress only when not exposed to the EP (Figure 6 C). Rats exposed to acute stress and environmental enrichment (that is, the ASE group) did not differ significantly from the control group.
Peripheral oxytocin levels were not affected by the EP–stress treatment (F4,31 = 2.27; P = 0.084; η2 = 0.226) or by coping style (F2,31 = 0.68; P = 0.540; η2 = 0.039). Oxytocin varied significantly during the experimental procedure, showing a significant interaction with the EP–stress treatment (F4,31 = 4.21; P = 0.008; η2 = 0.352) but not with coping (F2,31 = 2.31; P = 0.453; η2 = 0.050). After 1 mo in the experimental protocol, animals exposed to chronic stress had the lowest levels of oxytocin. After the FST, all groups showed a significant decrease in oxytocin levels (Figure 6 D)
Integrative multivariate models (multidimensional scaling).
To evaluate the independent association among the response variables (behavioral, physiologic, and immunologic profiles), we evaluated a series of models by using multidimensional scaling, with the treatment (EP–stress) as the grouping variable. Overall levels of corticosterone, DHEA, testosterone, oxytocin, IL6, and IL10 were entered in the model as physiologic and immunologic variables. For the behavior, we selected average floating time during the FST, together with the number of dive attempts and shakes.
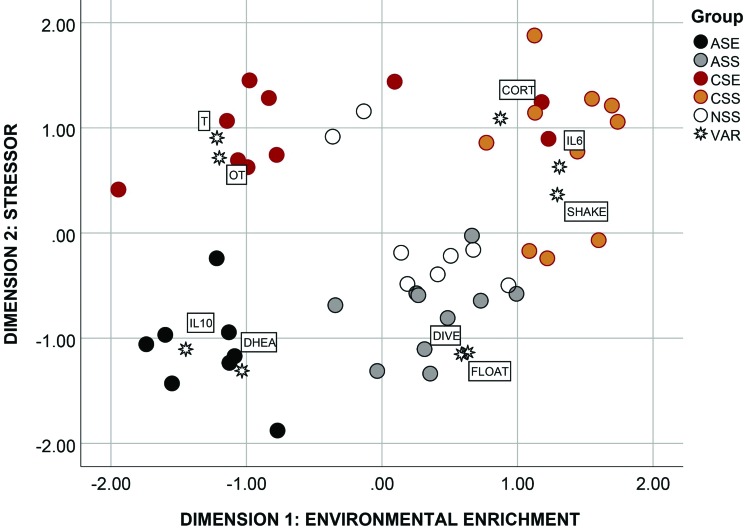
The model that best fit the data returned a Kruskal stress index value of 0.14, indicating an acceptable fit between the dimensions and the mapped distances. The percentage of variance explained by the model was very good, exceeding 90% (R2 = 90.1%). According to the 2 dimensions selected in the model, animals in the 5 treatment conditions (ASE, ASS, CSE, CSS, and NSS) were clustered in different areas of the map, with only a few animals associated away from the rest of the group (4 of 46; 91% correct classification; Figure 7). The first dimension (x-axis) discriminated primarily between animals exposed (left quadrants) and not exposed (right quadrants) to the EP; and therefore the x-axis was named ‘environmental enrichment’. The second dimension (y-axis) discriminated between rats exposed to acute (bottom quadrants) and chronic stress (top quadrants), and thus it was named ‘stressor’. The map showed that rats in the ASE group were closely associated with high levels of DHEA and IL10, thereby indicating that exposure to the EP and acute stress are compatible with biomarkers of good health. Rats in the ASS group were mostly associated with time spent floating and number of diving attempts during the FST, thus suggesting acute stress without environmental enrichment is conducive to a higher level of behavioral coping. Animals in the control group (NSS) were scattered around the middle of the map with no clear association with any major response variable, and overall they were the closest group to ASS rats, thus suggesting that acute stress without environmental enrichment was the closet state to the neutral condition.
Figure 7.
Multidimensional scaling map of association among the response variables (VAR) and individual rats according to experimental treatment (ASE, acute stress and EP; ASS, acute stress but not EP; CSE, chronic stress and EP; CSS, chronic stress but not EP; NSS, control animals not exposed to any stressor or environmental enrichment). ASE animals were characterized by high testosterone (T) and oxytocin (OT) levels. ASS rats showed increased levels of floating (FLOAT) and diving (DIVE) during the forced swim task (FST). CSE animals had high levels of DHEA and IL10. CSS animals were characterized by high levels of corticosterone (CORT) and the proinflammatory cytokine IL6.
Chronic stress returned a quite different behavioral and physiologic profile than acute stress, according to the multidimensional scaling map. Animals in the CSE condition were characterized by completely different biomarkers—high oxytocin and low testosterone levels—which suggests that animals under chronic stress but exposed to an enriched environment were able to cope with the stressor due to increases in biomarkers of sociability. Finally, the last group (CSS rats) returned behavioral (high number of shakes), physiologic (high levels of glucocorticoids), and immunologic (high levels of IL6) markers usually associated with a compromised overall health status.
Discussion
Although past research using animal models points to the importance of an enriched environment in increasing resiliency to the negative effect of stress,5,28 the vast majority of studies compared animals living in enriched housing compared with standard cages, a condition that can be viewed as a drastic and continuous change in the exposure to enriched environmental stimulation. Even studies focused on the short-term effects of environmental enrichment used housing enrichment as the primary manipulation. For example, one research team39 shortened the exposure to housing enrichment to just 14 d, and yet this brief change was sufficient to alter the behavioral and neural characteristic of the rats. In another study,10 the time of exposure was reduced even more, to just 10 d of environmental enrichment (for 6 h each day), which still led to notable behavioral, cognitive, and neural improvement for animals under chronic stress. Trying to translate these results to humans can be very difficult because often this paradigm cannot be easily applied to most people living in impoverished conditions. For these reasons, we exposed young male rats to environmental enrichment (in the setting of an ‘enrichment park’) for just 90 min each week (3 sessions of 30 min), to better understand the effects of a moderate and discontinuous state on the animals’ health. Our results clearly showed that even at these low levels, environmental enrichment can generate tangible effects on the behavioral and physiologic responses of developing male rats. Moreover, our results also showed that environmental enrichment and acute stress have a synergic effect, with rats exposed to both short-term predatory sounds and the EP having healthier behavioral and physiologic profiles (Figure 6).
Exposure to the EP reduced the levels of proinflammatory cytokine (IL6), both before and after a challenging task (the FST), and increased the levels of antiinflammatory cytokine (IL10). The combined action of IL6 and IL10 has clearly been associated with depressive-like symptoms in both humans and animal models.12,49 IL6 stimulates the HPA axis and can act centrally to increase tryptophan levels and serotonin metabolism.19,50,51 Moreover, the inflammatory status of the hippocampus is directly implicated in affective disorders in humans26 and anxiogenic behavior in animal models,4 whereas IL10 expression is reduced in the cortex and hippocampus of chronically stressed animals.49 Taken together, these results suggest a potential mechanism of action explaining how changes in the immune activation can influence emotional resilience. Our study suggests that even a moderate change in the daily routine can have significant effects on the immune system. Recording of behavioral data confirmed that rats were active and exploratory while in the EP, perhaps suggesting that physical exercise played a mediating role as well. It is well known that exercise can alter crosstalk between the HPA axis and immune system in both humans16 and animal models.43 Therefore, the synergic effect of a new and enriched environment coupled with many chances for physical activity appears to be an effective way to improve immune activation, even when exposure is limited to a few sessions each week.
In addition, rats exposed to the EP were characterized by low corticosterone and high DHEA levels, 2 biomarkers of the stress response and coping mechanisms.6,7,14 Physiologic resilience, defined as the ability of the organism to adapt to allostatic loads without permanent negative consequences,35 has been associated with an enhanced negative feedback system regulating stress neurochemicals, as well as a form of cognitive reappraisal of stressful events.21 In particular, DHEA is released in parallel with glucocorticoids (including corticosterone) during physical stress,22 and has been associated with buffering against the negative effects of chronic stress.40 Potentially related to the enhanced emotional resilience we found in the current study is that DHEA can act centrally to decrease glucocorticoid-induced neuronal death in the hippocampus and to promote neurogenesis in the dentate gyrus of the hippocampus.32 Through exposure to the EP, increased levels of DHEA relative to circulating glucocorticoids may have been instrumental as a neurobiologic mechanism that activate a more adaptive response to predator sound. Therefore, it is possible that the behavioral and neurobiologic effects observed in the current study are factors in the emergence and maintenance of neurobiologic aspects of resilience—which is particularly interesting considering the timing and nature of the exposure to environmental enrichment. In other words, even moderate and discontinuous environmental enrichment conditions may have a long-term protective effect on emotional regulation, given that the mitigated stress response would likely lead to fewer chances of inflammation in the hippocampal neurocircuitry associated with emotional regulation.36 In the multidimensional scaling model, in which multivariate associations were taken into account, both IL10 and DHEA were highly associated with animals exposed to both the EP and acute stress, thus indicating that the combination of environmental enrichment and short-term stress can have a key role in increasing emotional resilience and wellbeing. This finding is not surprising, as many models suggest that even moderate stressors can be beneficial, because they activate physiologic responses to promote resiliency against chronic, high-level stressors.45
Exposure to the EP influenced other biomarkers of resilience as well, because rats in these groups had generally higher levels of oxytocin and testosterone. It is well-known that the hippocampus is enriched with oxytocin receptors and that oxytocin can reduce the negative effects of chronic or unpredictable stress on hippocampal synaptic plasticity and memory by acting on oxytocin receptors.29 In addition, both oxytocin and testosterone and their receptors in the brain are involved in the regulation of various social behaviors.18,41 Furthermore, both are also closely related to physical experience and stress levels, which in turn can trigger profound neuroendocrine changes in synaptic plasticity and adult neurogenesis.47 In our current study, the positive effects of both oxytocin and testosterone were particularly evident in rats exposed to the EP and chronic stress, possibly indicating that exposure to the EP would facilitate positive social behavior when rats were back in their home cages (Figure 6). This interpretation supports results from a previous study, in which we showed that environmental enrichment increases positive arousal and, as a consequence, positive affiliation among animals.5 Considering reports on different main effects of oxytocin and the arginine vasopressin peptides in males and females,1 it would be extremely interesting to monitor the effects of the EP and acute–chronic stress on arginine vasopressin peptides as well.
Furthermore the synergic effect between type of stress and environmental enrichment was clear from our data as well. The response to acute stress under environmental enrichment is another topic vastly understudied in animal models, because most experimental paradigms use chronic stress.5 All stressors are not created equal: stress can be generally divided into ‘good’ (or ‘tolerable’) stress and ‘bad’ (or ‘maladaptive’) stress.45 Acute stress is generally classified as good stress and chronic or unpredictable stress as maladaptive. In other words, animals have evolved behavioral and neuroendocrine mechanisms to cope with stress that is either short-lived or predictable, but because they can benefit only from limited resources, persistent insults create an imbalance that is difficult to overcome.33,46 Although in our current study the separation between chronic and acute stress was artificially imposed, animals exposed to acute stress without environmental enrichment had behavioral and physiologic profiles very similar to animals in the control group, thus confirming that acute stress in itself is neither bad nor good, rather mostly tolerable. In contrast, when acute stress was coupled with exposure to the EP, animals had the high levels of both DHEA and IL10, thus confirming that the synergic effect of acute stress and environmental enrichment can create a condition resulting in enhanced emotional resilience. These results, if confirmed in other species, could be essential to establish behavioral guidelines for supporting healthy life choices in humans, especially when addressing the effect of moderate levels of environmental enrichment on the wellbeing of individuals. Global skyrocketing levels of stress and economic disparity tend to exert a significant toll on human health. This impoverished exposure to surrounding biodiversity together with increased stress might have a negative effect on neurobiologic systems. Furthermore, less interaction with the environmental enrichment leads to diminished physical activity and effective contingency-building opportunities that may be important for emotional and cognitive resilience. Based on prior research in our laboratory, the reduction in physical effort directed toward securing necessary resources that likely accompanies impoverished environments leads to reduced activation of brain areas critical for reward, pleasure, motivation, problem-solving, and effective coping strategies.5-7
In terms of coping styles, as defined by the back test and assessed during an early stage of development, our results confirmed that flexible copers had the most adaptive behavioral transition from the first to the 2nd day of the FST. As noted previously in several other studies,6,25 flexible copers exhibited an adaptive adaption to the FST by conserving more energy, swimming less frantically, and spending more time floating, whereas both active and passive copers tended to show similar responses on both days. Coping style did not seem to interact with treatments (EP and acute or chronic stress). Other interesting results were related to the lower levels of IL6 for flexible copers, in both pre- and posttest measures, and lower levels of corticosterone metabolites after 4 wk of treatment and after the FST. Together, these results suggest that flexible copers, compared with rats with other coping styles, tend to have greater physiologic and immunologic protection against the negative effects of stress, irrespectively of any possible advantage received from environmental enrichment. Clearly, these results need to be confirmed by further studies aimed to increase the number of subjects evaluated in each of the possible conditions.
In conclusion, even moderate exposure to environmental enrichment, especially when coupled with activities that can stimulate the HPA axis in a positive way (acute stress), can potentially strengthen the crosstalk between the immune system and HPA axis, thus boosting health profiles and facilitating effective interactions with the environment. These interactions with the environment may provide a greater sense of control over events, contributing to activation of the neurobiologic mechanisms that build resilience against affective disorders, such as anxiety and the onset of depression. Translational models can help to increase understanding of the responses or outcomes that are predictive of such healthy behaviors. A heightened sense of mastery over the environment represents an important aspect of mental health in humans,20 which can be connected directly with increased immune activation and thus, in the long run, to overall improved physical health as well. In that context, the fate of organisms ultimately may lie in their critical ability to adapt to a rapidly changing environment that is characterized by an unpredictable and continuous stream of biologic and psychologic insults. Further investigations of the long-term effects of moderate exposure to environmental enrichment are essential to understand whether the beneficial effects revealed in the current study are far-reaching. In the end, and especially for humans that are challenged by an impoverished environment, assessing the neural effect of small modifications in their everyday routine will be essential in future studies. Consequently, the current rat model offers a novel means to assess environmental enrichment to determine how lifestyle changes associated with an impoverished environment may be associated with high stress level, compromised immune function as indicated by altered cytokine levels, and increases in the incidence of affective disorders, such as anxiety and depression.
References
- 1.Albers HE. 2015. Species, sex, and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol 36:49–71. 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anisman H. 2009. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci 34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Aschbacher K, O'Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. 2013. Good stress, bad stress, and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38:1698–1708. 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T. 2009. Impaired hippocampus-dependent and ‑independent learning in IL6-deficient mice. Behav Brain Res 200:192–196. 10.1016/j.bbr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Bardi M, Kaufman C, Franssen C, Hyer MM, Rzucidlo A, Brown M, Tschirhart M, Lambert KG. 2016. Paper or plastic? Exploring the effects of natural enrichment on behavioural and neuroendocrine responses in Long–Evans rats. J Neuroendocrinol 28 10.1111/jne.12383. [DOI] [PubMed] [Google Scholar]
- 6.Bardi M, Rhone AP, Franssen CL, Hampton JE, Shea EA, Hyer MM, Huber J, Lambert KG. 2012. Behavioral training and predisposed coping strategies interact to influence resilience in male Long–Evans rats: implications for depression. Stress 15:306–317. 10.3109/10253890.2011.623739. [DOI] [PubMed] [Google Scholar]
- 7.Bardi M, True M, Franssen CL, Kaufman C, Rzucidlo A, Lambert KG. 2013. Effort-based reward (EBR) training enhances neurobiological efficiency in a problem-solving task: insights for depression therapies. Brain Res 1490:101–110. 10.1016/j.brainres.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Baschant U, Tuckermann J. 2010. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol 120:69–75. 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 9.Benrick A, Schele E, Pinnock SB, Wernstedt-Asterholm I, Dickson SL, Karlsson-Lindahl L, Jansson JO. 2009. Interleukin 6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol 21:620–628. 10.1111/j.1365-2826.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhagya VR, Srikumar BN, Veena J, Rao S, Shankaranarayana Rao BS. 2017. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res 95:1602–1610. 10.1002/jnr.23992. [DOI] [PubMed] [Google Scholar]
- 11.Blalock JE, Smith EM. 2007. Conceptual development of the immune system as a 6th sense. Brain Behav Immun 21:23–33. 10.1016/j.bbi.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Blume J, Douglas SD, Evans DL. 2011. Immune suppression and immune activation in depression. Brain Behav Immun 25:221–229. 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charmandari E, Tsigos C, Chrousos G. 2005. Endocrinology of the stress response. Annu Rev Physiol 67:259–284. 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 14.Charney DS. 2004. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161:195–216. 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Gianferante D, Hanlin L, Fiksdal A, Breines JG, Thoma MV, Rohleder N. 2017. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology 78:168–176. 10.1016/j.psyneuen.2017.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper DM, Nemet D, Galassetti P. 2004. Exercise, stress, and inflammation in the growing child: from the bench to the playground. Curr Opin Pediatr 16:286–292. 10.1097/01.mop.0000126601.29787.39. [DOI] [PubMed] [Google Scholar]
- 17.Dhabhar FS. 2014. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 58:193–210. 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 18.Dumais KM, Veenema AH. 2016. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol 40:1–23. 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn AJ. 2000. Cytokine activation of the HPA axis. Ann N Y Acad Sci 917:608–617. 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 20.Evans GW, English K. 2002. The environment of poverty: multiple-stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev 73:1238–1248. 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 21.Feder A, Nestler EJ, Charney DS. 2009. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci 10:446–457. 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncharova ND, Vengerin AA, Chigarova OA. 2012. Repeated moderate stress stimulates the production of dehydroepiandrosterone sulfate (DHEAS) and reduces corticosteroid imbalance in old Macaca mulatta. Bull Exp Biol Med 153:750–753. 10.1007/s10517-012-1817-2. [DOI] [PubMed] [Google Scholar]
- 23.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, Fischer A, Davies EG, Kuis W, Leiva L, Cavazzana-Calvo M. 2002. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 346:1185–1193. 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 24.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, Bertrand Y, Fasth A, Porta F, Cant A, Espanol T, Müller S, Veys P, Vossen J, Fischer A. 1998. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell–depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood 91:3646–3653. [PubMed] [Google Scholar]
- 25.Hawley DF, Bardi M, Everette AM, Higgins TJ, Tu KM, Kinsley CH, Lambert KG. 2010. Neurobiological constituents of active, passive, and variable coping strategies in rats: integration of regional brain neuropeptide Y levels and cardiovascular responses. Stress 13:172–183. 10.3109/10253890903144621. [DOI] [PubMed] [Google Scholar]
- 26.Khairova RA, Machado-Vieira R, Du J, Manji HK. 2009. A potential role for proinflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol 12:561–578. 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruskal JB, Wish M. 1978. Multidimensional scaling. Newbury Park (CA): Sage; 10.4135/9781412985130 [DOI] [Google Scholar]
- 28.Lambert K, Hyer M, Bardi M, Rzucidlo A, Scott S, Terhune-Cotter B, Hazelgrove A, Silva I, Kinsley C. 2016. Natural-enriched environments lead to enhanced environmental engagement and altered neurobiological resilience. Neuroscience 330:386–394. 10.1016/j.neuroscience.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Park SH, Chung C, Kim JJ, Choi SY, Han JS. 2015. Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Sci Rep 5:1–9. 10.1038/srep18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T, Wang P, Wang SC, Wang YF. 2017. Approaches mediating oxytocin regulation of the immune system. Front Immunol 7:1–9. 10.3389/fimmu.2016.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. 2016. Balancing the immune response in the brain: IL10 and its regulation. J Neuroinflammation 13:1–10. 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30:65–91. 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwen BS. 2001. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933:265–277. 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS. 2016. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann N Y Acad Sci 1373:56–64. 10.1111/nyas.13020. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. 2017. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry 74:551–552. 10.1001/jamapsychiatry.2017.0270. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS, Eiland L, Hunter RG, Miller MM. 2012. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62:3–12. 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrzad J, Shajari M, Saleh-moghaddam M, Sarmad-Nabavi M. 2015. Stressed (acute) mice display neuroimmunodysregulation and defective innate immune response against coliform infection. Int Immunopharmacol 28:168–174. 10.1016/j.intimp.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Mellander L, Carlsson B, Jalil F, Söderström T, Hanson LÅ. 1985. Secretory IgA antibody response against Escherichia coli antigens in infants in relation to exposure. J Pediatr 107:430–433. 10.1016/S0022-3476(85)80528-X. [DOI] [PubMed] [Google Scholar]
- 39.Mitra R, Sapolsky RM. 2012. Short-term enrichment makes male rats more attractive, more defensive and alters hypothalamic neurons. PLoS One 7:1–8. 10.1371/journal.pone.0036092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan CA, 3rd, Rasmusson A, Pietrzak RH, Coric V, Southwick SM. 2009. Relationships among plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans exposed to underwater navigation stress. Biol Psychiatry 66:334–340. 10.1016/j.biopsych.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Opendak M, Briones BA, Gould E. 2016. Social behavior, hormones, and adult neurogenesis. Front Neuroendocrinol 41:71–86. 10.1016/j.yfrne.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Park JE, Barbul A. 2004. Understanding the role of immune regulation in wound healing. Am J Surg 187:11S–16S. 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen BK. 2000. Exercise and cytokines. Immunol Cell Biol 78:532–535. 10.1111/j.1440-1711.2000.t01-11-.x. [DOI] [PubMed] [Google Scholar]
- 44.Pruett SB. 2003. Stress and the immune system. Pathophysiology 9:133–153. 10.1016/S0928-4680(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 45.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. 2008. Exercise, oxidative stress, and hormesis. Ageing Res Rev 7:34–42. 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Shonkoff JP, Boyce WT, McEwen BS. 2009. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA 301:2252–2259. 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 47.Song J, Christian KM, Ming G-L, Song H. 2012. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol 72:1032–1043. 10.1002/dneu.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soto-Tinoco E, Guerrero-Vargas NN, Buijs RM. 2016. Interaction between the hypothalamus and the immune system. Exp Physiol 101:1463–1471. 10.1113/EP085560. [DOI] [PubMed] [Google Scholar]
- 49.Ulrich RS. 1984. View through a window may influence recovery from surgery. Science 224:420–421. 10.1126/science.6143402. [DOI] [PubMed] [Google Scholar]
- 50.Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. 2013. Prolonged restraint stress increases IL6, reduces IL10, and causes persistent depressive-like behavior that is reversed by recombinant IL10. PLoS One 8:1–11. 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Dunn AJ. 1998. Mouse interleukin 6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int 33:143–154. 10.1016/S0197-0186(98)00016-3. [DOI] [PubMed] [Google Scholar]