Abstract
Inflammatory immune disorders such as inflammatory bowel disease and multiple sclerosis are major health problems. Currently, the intestinal whipworm Trichuris suis is being explored in clinical trials to reduce inflammation in these diseases; however, the mechanisms by which the parasite affects the host immune system are not known. Here we determined the effects of T. suis soluble products (SPs) on Toll-like receptor-4 (TLR4)-stimulated human dendritic cells (DCs) using Illumina bead chip gene arrays. Pathway analysis of lipopolysaccharide-stimulated DCs with or without T. suis treatment showed that co-stimulation with T. suis SPs resulted in a downregulation of both the myeloid differentiation primary response gene 88-dependent and the TIR-domain-containing adaptor-inducing interferon-β-dependent signalling pathways triggered by TLR4. These data were verified using quantitative real-time PCR of several key genes within these pathways and/or defining their protein levels. In addition, T. suis SPs induce Rab7b, a negative regulator of TLR4 signalling that interferes with its trafficking, which coincided with a reduced surface expression of TLR4. These data indicate that the mechanism by which T. suis SPs reduce inflammatory responses is through suppression of both TLR4 signalling and surface expression on DCs.
Introduction:
Infection with parasitic helminths (worms) suppresses inflammation in a variety of human inflammatory disorders. Clinical trials have shown that therapy with living eggs of the porcine nematode whipworm Trichuris suis can improve the outcomes of inflammatory bowel diseases.1,2 Whereas initial trials of helminth therapy in autoimmune diseases were very encouraging, the recent results of larger clinical trials in inflammatory bowel diseases have somewhat tempered the initial expectations.3 On the other hand, clinical tests in multiple sclerosis and observational studies in individuals with autoimmune disease who were naturally infected by a helminth are proving encouraging and indicate that the setup of future clinical trials may have to be adapted.3–5 The variable results in the clinical trials also warrant a closer look into the mechanisms by which the parasites affect the host immune system.
Insights into the mechanisms that helminths use to modulate specific immune cells may give clues about their ability to protect against inflammatory diseases.6,7 In this respect, it is important to note that several studies indicate that soluble, aqueous products (SPs) isolated from helminths are also effective in protection to inflammatory diseases in animal models, showing that infection with worms may not be necessary to induce the helminth-mediated protective effects.8–10 For example, we have recently shown that T. suis SPs are able to suppress the development of murine experimental autoimmune encephalomyelitis and can modulate the function of human immature dendritic cells (DCs)11,12 and macrophages.13
Inflammatory disorders, such as inflammatory bowel diseases and multiple sclerosis, have their origin in an overactive immune response against harmless components or self molecules. DCs have a crucial role in determining the type of immune response that is induced via the sampling and recognition of antigens and have a crucial regulatory role in the control of inflammatory diseases. The Toll-like receptor (TLR)-mediated recognition of pathogens by DCs is a central event in the activation of inflammatory innate and adaptive immune responses.14 Pertussis toxin, the main agent inducing inflammation in experimental autoimmune encephalomyelitis, interacts with TLR4 to induce inflammation.15 In addition, several TLR4 antagonists reduce inflammation in animal models of inflammatory diseases.16 Thus, TLR signalling, and specifically TLR4 signalling, may have an important role in the overactive immune response found in inflammatory diseases.
The most commonly used compound for in vitro stimulation of TLR4 is lipopolysaccharide (LPS) derived from Escherichia coli. LPS binding to TLR4 induces two main signalling pathways: (a) the myeloid differentiation primary response gene 88 (MyD88)-dependent and (b) the TIR-domain-containing adaptor protein inducing interferon-β (TRIF)-dependent pathways.17 MyD88-dependent signalling is induced via tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6), IκB kinases (IKKs) and mitogen-activated protein kinases (MAPKs), which results in nuclear translocation of nuclear factor (NF)-κB and AP-1 subunits, thereby inducing transcription of pro-inflammatory genes. In contrast, TRIF-dependent signalling is regulated via TRIF, TLR4-adaptor protein (TRAM) and interferon regulatory factor 3 (IRF3), resulting in type I interferon (IFN) gene transcription.17
Several studies have shown that production of proinflammatory cytokines by DCs in response to TLR stimulation is markedly reduced upon direct contact with helminth antigens.18–20 We have recently demonstrated that T. suis SPs are able to suppress pro-inflammatory cytokines and chemokines, including TNFα, interleukin (IL)-6, IL-12, C-C motif chemokine ligand 2 (CCL2) and C-X-C motif chemokine ligand 10, from TLR4-activated human DCs.11,12
Here we use Illumina bead chip arrays to determine the effects of T. suis SPs on LPS-induced TLR4 signalling. Pathway analysis of the mRNA data showed that stimulation with T. suis SPs resulted in a downregulation of both the MyD88- and the TRIF-dependent signalling pathways triggered by TLR4. In addition, our data showed that T. suis SPs enhance the expression level of Rab7b, a late endosomal marker involved in TLR4 trafficking, which coincided with a reduced surface expression of TLR4. In conclusion, these data indicate that T. suis SPs are able to suppress TLR4 signalling on several levels and thereby contribute to our understanding of the mechanisms by which this helminth suppresses inflammation.
Results:
Bead chip arrays of DCs primed with T. suis SPs and LPS
To identify the role of T. suis SPs in the suppression of TLR4-induced inflammatory pathways in human DCs, we analysed Illumina bead chip arrays of DCs stimulated for 24 h with LPS or co-stimulated with T. suis SPs+LPS. The data showed that DCs treated with T. suis SPs+LPS, vs DCs treated with LPS alone, showed significant (q<5) downregulation of 106 genes and significant upregulation of 111 genes. Table 1 shows the 32 positively regulated genes and 38 negatively regulated genes at⩾2-fold difference in expression. In addition, we analysed Illumina bead chip arrays of control experiments (unstimulated DCs and DCs stimulated with T. suis SPs alone). The data showed that T. suis SPs alone induce a ⩾2-fold upregulation of 44 genes and downregulation of 4 genes, compared with unstimulated DCs (Supplementary Table S1).
Table 1.
List of upregulated and downregulated genes in dendritic cells (DCs) stimulated with T. suis soluble products (SPs) and LPS vs LPS alone.
| Gene | q-Value | FI |
|---|---|---|
| Upregulated | ||
| INSM1 | 0 | 5.41 |
| STAT4 | 0 | 4.97 |
| ABP1 | 0 | 4.41 |
| ITPR1 | 0 | 3.69 |
| RAB7B | 0 | 3.57 |
| TSPAN13 | 0 | 3.18 |
| LPS387882 | 0 | 2.91 |
| PDCL3 | 0 | 2.71 |
| PECR | 0 | 2.68 |
| PDE4B | 0 | 2.66 |
| BID | 0 | 2.58 |
| SLC5A6 | 0 | 2.57 |
| CDK2 | 0 | 2.52 |
| TRIM69 | 0 | 2.51 |
| GRASP | 0 | 2.42 |
| MS4A7 | 0 | 2.37 |
| PIM2 | 0 | 2.19 |
| POLS | 0 | 2.09 |
| CIEC4F | 0 | 2.08 |
| IL2RA | 0 | 2.02 |
| MYLIP | 0 | 2.02 |
| GPR160 | 0.71 | 2.73 |
| DSG2 | 0.71 | 2.47 |
| TRIM69 | 0.71 | 2.45 |
| TNFSF4 | 0.71 | 2.40 |
| CXCR4 | 1.02 | 2.97 |
| ALOX5AP | 1.02 | 2.57 |
| RHOB | 1.02 | 2.28 |
| SEL1L3 | 1.02 | 2.17 |
| NAV1 | 1.02 | 2.08 |
| SLA | 1.38 | 2.43 |
| TARP | 1.38 | 2.05 |
| Down regulated | ||
| CCL3L3 | 0 | 6.84 |
| CCL19 | 0 | 5.63 |
| CXCL9 | 0 | 5.32 |
| EMP1 | 0 | 4.70 |
| LTA | 0 | 4.21 |
| ClQB | 0 | 4.10 |
| CXCL10 | 0 | 3.98 |
| CCL13 | 0 | 3.92 |
| SOD2 | 0 | 3.82 |
| C1QC | 0 | 3.70 |
| FCER1A | 0 | 3.59 |
| ANKRD22 | 0 | 3.26 |
| AQP9 | 0 | 3.24 |
| SPRED1 | 0 | 3.19 |
| C5orf13 | 0 | 2.92 |
| CCL5 | 0 | 2.67 |
| TNFSF10 | 0 | 2.62 |
| HSD11B1 | 0 | 2.59 |
| C21orf42 | 0 | 2.55 |
| GBP5 | 0 | 2.53 |
| HS011BI | 0 | 2.45 |
| FG12 | 0 | 2.42 |
| P2RX7 | 0 | 2.37 |
| PITX1 | 0 | 2.20 |
| ACTA2 | 0 | 2.11 |
| PABPC4 | 0 | 2.10 |
| NOD2 | 0 | 2.02 |
| SOD2 | 0.79 | 2.96 |
| LOC73008 | 0.79 | 2.74 |
| TIMP1 | 0.79 | 2.16 |
| IRF8 | 1.27 | 3.39 |
| TNF | 1.27 | 2.74 |
| ARHGAP22 | 1.27 | 2.27 |
| STAMPBPL1 | 1.27 | 2.14 |
| ZNF366 | 1.27 | 2.05 |
| cDNA FLJ33375 clone | 1.27 | 2.02 |
| FCER1G | 1.38 | 2.94 |
| MNDA | 1.38 | 2.16 |
Abbreviations: FI, fold induction; LPS, lipopolysaccharide. All genes with a q-value <1.5 and an FI>2 are shown.
To gain more insight into the role of genes that modulate TLR4-induced activation of DCs, pathway analysis was performed on the LPS vs T. suis SPs+LPS data set by gene set enrichment analysis,21 provided by the Broad Institute. Using a false discovery rate of q⩽ 0.25 and P ⩽0.05, 65 pathways in the LPS vs T. suis SPs +LPS data set were significantly downregulated, which are shown in Table 2.
Table 2.
List of downregulated pathways, as determined by gene set enrichment analysis (GSEA), in dendritic cells (DCs) stimulated with T. suis soluble products (SPs) and LPS vs LPS alone.
| Affected genes | NOM P-value | FDR q-value | |
|---|---|---|---|
| Inflammation | |||
| Biocarta-Eryth pathway | 7/15 | 0.000 | 0.013 |
| Biocarta-LAIR pathway | 7/17 | 0.014 | 0.067 |
| Reactome NOD 1,2 signalling pathway | 11/27 | 0.023 | 0.144 |
| Reactome-activated TAK1 mediates p38 MAPK activation | 6/15 | 0.018 | 0.072 |
| Biocarta-cytokine pathway | 8/21 | 0.000 | 0.001 |
| Biocarta-CDMAC pathway | 6/16 | 0.048 | 0.197 |
| KEGG-prion diseases | 13/35 | 0.000 | 0.001 |
| PID-Toll endogenous pathway | 9/25 | 0.029 | 0.147 |
| PID-anthrax pathway | 6/17 | 0.014 | 0.074 |
| PID-IL-27 pathway | 9/26 | 0.008 | 0.097 |
| PID-IL-1 pathway | 11/32 | 0.047 | 0.197 |
| Biocarta-HSP 27 pathway | 5/15 | 0.012 | 0.096 |
| Biocarta DC pathway | 6/24 | 0.040 | 0.187 |
| Biocarta RelA pathway | 5/16 | 0.055 | 0.198 |
| Reactome-interferon α,β signalling | 19/63 | 0.000 | 0.014 |
| Biocarta IL-1 R pathway | 9/32 | 0.004 | 0.032 |
| Reactome IL-1 signalling | 10/36 | 0.012 | 0.134 |
| Reactome initial triggering of complement | 4/15 | 0.002 | 0.006 |
| PID IL-6, 7 pathway | 12/47 | 0.011 | 0.074 |
| PID AP1 pathway | 18/69 | 0.002 | 0.032 |
| Biocarta NTHI pathway | 6/24 | 0.024 | 0.106 |
| Reactome chemokine receptors bind chemokines | 14/55 | 0.000 | 0.000 |
| Reactome interferon signalling | 39/157 | 0.000 | 0.014 |
| Reactome cytokine signalling in immune system | 63/264 | 0.000 | 0.032 |
| Biocarta IL-6 pathway | 5/22 | 0.053 | 0.213 |
| KEGG-RIG l-like receptor signalling pathway | 16/69 | 0.034 | 0.215 |
| Biocarta-cardiac EGF pathway | 4/18 | 0.065 | 0.243 |
| Biocarta-inflam pathway | 6/29 | 0.000 | 0001 |
| Biocarta comp pathway | 4/19 | 0.000 | 0.013 |
| KEGG-natural killer cell-mediated cytotoxicity | 27/137 | 0.002 | 0.152 |
| SA-MMP-cytokine connection | 3/15 | 0.000 | 0.032 |
| PID-GM-CSF pathway | 7/37 | 0.000 | 0.033 |
| KEGG-NOD-like receptor signalling pathway | 11/59 | 0.000 | 0.001 |
| KEGG-chemokine signalling pathway | 34/188 | 0.000 | 0.000 |
| KEGG-Toll-like receptor signalling pathway | 18/100 | 0.000 | 0.000 |
| KEGG-type I diabetes mellitus | 8/44 | 0.011 | 0.118 |
| Reactome-interferon α,β signalling | 11/62 | 0.000 | 0.023 |
| Reactome-innate immune system | 48/265 | 0.000 | 0.098 |
| Reactome-G-α-l signalling events | 33/192 | 0.000 | 0.025 |
| KEGG-complement and coagulation cascades | 12/69 | 0.000 | 0.049 |
| PID-Fra pathway | 6/36 | 0.008 | 0.076 |
| Biocarta-NKT pathway | 5/29 | 0.006 | 0.067 |
| Reactome-signalling by lls | 18/103 | 0.013 | 0.208 |
| KEGG-hematopoietic cell lineage | 14/87 | 0.002 | 0.096 |
| KEGG-cytosolic DNA sensing pathway | 9/55 | 0.000 | 0.001 |
| Reactome-nucleotide binding domain leucine-rich repeat-containing receptor NLR signalling pathways | 7/43 | 0.023 | 0.110 |
| Reactome-class A1 rhodopsin-like receptors | 45/298 | 0.000 | 0.001 |
| Reactome-GPCR ligand binding | 60/401 | 0.000 | 0.012 |
| Miscellaneous | |||
| Reactome-mRNA decay by 5–3 exoribonuclease | 7/15 | 0.019 | 0.120 |
| Reactome-posttranslational modification synthesis of GPI-anchored proteins | 10/26 | 0.045 | 0.224 |
| KEGG-lysosome | 39/121 | 0.004 | 0.121 |
| Reactome-synthesis of glycosylphosphatidylinositol GPI | 6/17 | 0.041 | 0.189 |
| KEGG-other glycan degradation | 5/15 | 0.043 | 0.152 |
| Reactome-transcriptional regulation of white adipocyte differentiation | 22/70 | 0.002 | 0.083 |
| Reactome-Fanconi anaemia pathway | 7/24 | 0.013 | 0.117 |
| PID-integrin 2 pathway | 8/29 | 0.012 | 0.117 |
| Biocarta-MTA3 pathway | 5/18 | 0.002 | 0032 |
| Reactome-eicosanoid ligand binding receptors | 4/15 | 0.047 | 0.189 |
| Reactome-signalling by Hippo | 5/20 | 0.054 | 0.192 |
| KEGG-amino sugar and nucleotide sugar metabolism | 11/44 | 0.016 | 0.119 |
| Reactome-steroid hormones | 7/29 | 0.006 | 0.038 |
| KEGG-fructose and mannose metabolism | 8/34 | 0.012 | 0.106 |
| PID-syndecan 1 pathway | 9/46 | 0.028 | 0.210 |
| Reactome-metabolism of steroid hormones and vitamins A and D | 7/35 | 0.000 | 0.050 |
| KEGG-glycolysis gluconeogenesis | 12/62 | 0.006 | 0.119 |
Abbreviations: FDR, false discovery rate; LPS, lipopolysaccharide; NOM, nominal.
T. suis SPs suppress TLR4 signalling pathways
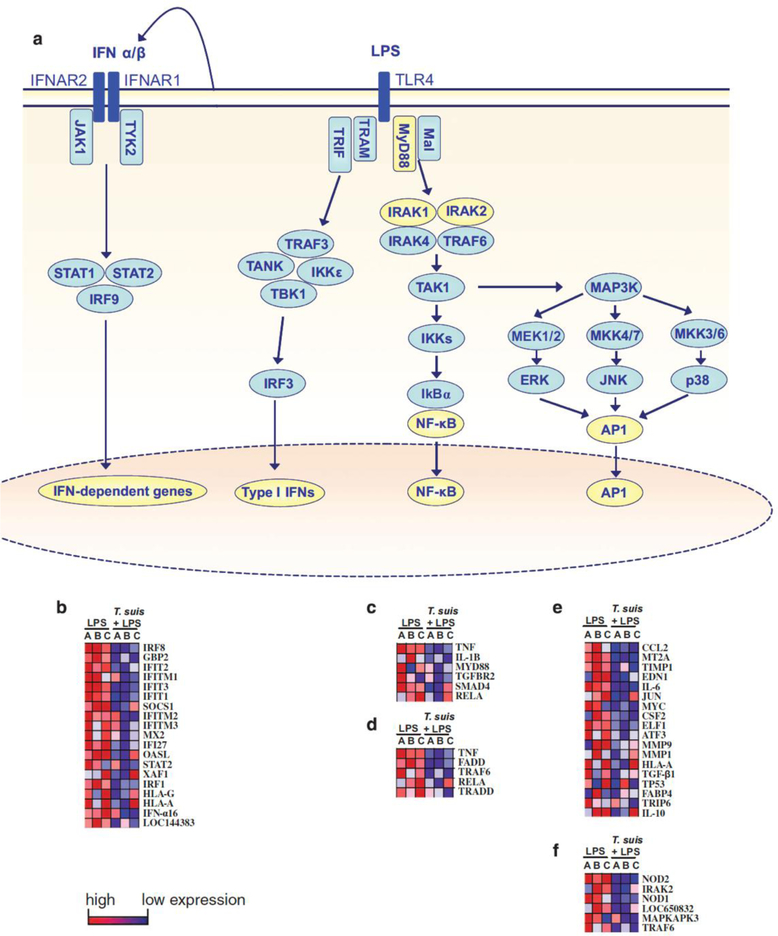
Table 2 shows that most of the pathways downregulated in DCs by T. suis SPs+LPS vs LPS alone, as determined by gene set enrichment analysis, are pathways involved in inflammation. Specifically, several different pathways within the TLR4 signalling cascade are reduced in response to T. suis SPs. TLR4 has two different signalling pathways: the TRIF- and the MyD88-dependent pathways. The TRIF-dependent TLR4 pathway results in induction of type I IFNs. The MyD88-dependent TLR4 signalling is divided into two separate pathways: the NF-κB and the MAPK signaling pathway (Figure 1a). The TRIF-dependent reactome IFN α/β pathway was significantly reduced in response to addition of T. suis SPs (Figure 1b). The MyD88-dependent NF-κB signaling pathways biocarta NTHI pathway, which represents NF-κB signalling in response to Haemophilus influenzae infection, and the biocarta RelA pathway, which represents signalling induced by p65, are both reduced in response to T. suis SPs+LPS vs LPS (Figures 1c and d). In addition, the MyD88-dependent MAPK pathway is significantly reduced as shown by a significant reduction of the PID AP1 pathway and the reactome-activated TAK1-mediated p38 MAPK pathway in response to addition of T. suis SPs (Figures 1e and f).
Figure 1.
T. suis SPs suppress the TLR4 signalling pathways. (a) The TLR4 signalling pathway consists of the MyD88- and the TRIF-dependent signalling pathways. In the TRIF-dependent pathway, TRIF and TRAM signal via IRF3 resulting in the induction of type I IFNs. In turn, these interact with IFN receptors to induce IFN-dependent gene expression. In MyD88 signalling, Mal and MyD88 induce IRAK phosphorylation and TAK1 signalling, after which the pathway splits into the NF-κB and the MAPK pathway. Both these MyD88-dependent pathways contribute to the production of proinflammatory cytokines. (b–f) TLR4 pathway analyses of DCs stimulated with LPS vs T. suis SPs+LPS for 24 h. The data shown include all genes in the pathways indicated that are significantly suppressed by T. suis pretreatment. The data are presented as heat maps, with high expression in red and low expression in blue. The reactome IFN α/β signalling pathway (b), NTHI pathway (c), biocarta RelA pathway (d), PID AP1 pathway (e) and reactome-activated TAK1-mediated p38 MAPK pathway (f) are shown.
T. suis SPs suppress type I IFN signalling in LPS-primed DCs
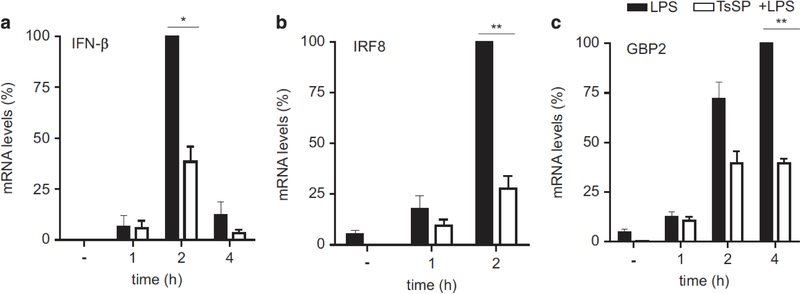
From all the significantly reduced genes within the reactome IFN α/β pathway (Figure 1b), we selected several genes for real-time PCR (RT-PCR) validation. To determine the levels of α/β IFNs, the type I IFNs that induce this signalling pathway, we determined the mRNA expression levels of IFN-β1 (Figure 2a) and IFN-α1 (data not shown) in DCs stimulated with LPS or T. suis SPs+LPS for various time points. IFN-α1 did not show induction in response to LPS. In contrast, suppression of IFN-β1 mRNA expression levels was found in T. suis SPs+LPS-stimulated DCs when compared with DCs stimulated with LPS alone. In addition, IRF8 and IFN-induced guanylate-binding protein 2 (GBP2) were significantly reduced in the type I IFN pathway (Figure 1b). PCR validation of these results showed that, in early time points, mRNA expression levels for both IRF8 (Figure 2b) and GBP2 (Figure 2c) were significantly reduced by addition of T. suis SPs+LPS vs LPS alone.
Figure 2.
T. suis SPs suppress IFN signalling pathways in LPS-activated DCs. RT-PCR validation was carried out for gene expression of the TRIF signalling-dependent genes IFN-β (a), IRF8 (b) and IFN-induced guanlylate-binding protein 2 (GBP2) (c) in DCs stimulated with LPS vs T. suis SPs+LPS for the time periods as indicated, using GAPDH as a reference gene. Data were normalized against the highest LPS value and are shown as mean of duplicate assays of three separate donors ± s.e.m. *P<0.05 and **P<0.01.
T. suis SPs reduce TLR4 MyD88-dependent signaling
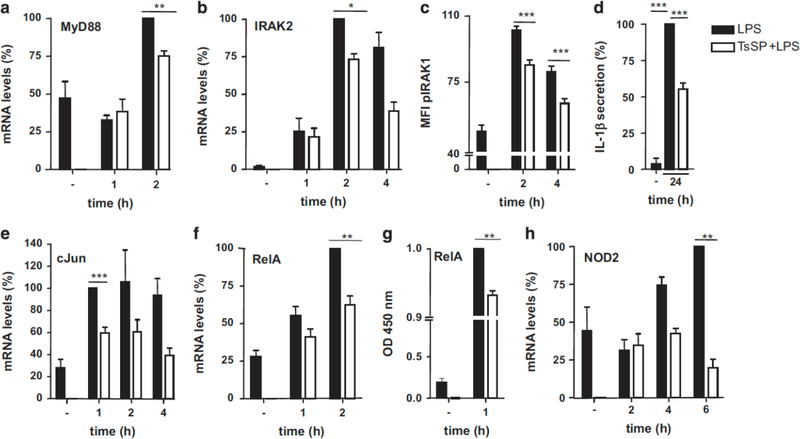
The MAPK signalling pathway can be regulated via phosphorylation of extracellular signal–regulated kinase, c-Jun N-terminal kinase or p38, leading to AP-1 formation, translocation to the nucleus and subsequent transcription of pro-inflammatory genes, which includes induced expression of cytokines and chemokines. AP-1 consists of the subunits cFos and cJun. In the NF-κB pathway, IKKs are activated, leading to NF-κB translocation into the nucleus. Five subunits of NF-κB have been described: p50, p52, RelA (p65), RelB, and c-Rel that can form heterodimers or homodimers.22 The most abundantly expressed dimers upon LPS stimulation are heterodimers of p50 with RelA.23 MyD88-dependent signalling is initiated by MyD88, followed by interaction with and phosphorylation of IL-1 receptor-associated kinases (IRAKs) and TRAF6. RTPCR validations showed that mRNA levels of MyD88 and IRAK2 are reduced in T. suis SPs+LPS vs LPS (Figures 3a and b). In addition, phosphorylation of IRAK1 is reduced upon addition of T. suis SPs to LPS-stimulated DCs at several time points (Figure 3c).
Figure 3.
T. suis SPs suppress the TLR4-induced MyD88 signalling pathways in LPS-activated DCs. RT-PCR validation was carried out on DCs treated with LPS vs T. suis SPs+LPS for the time periods as indicated. MyD88 (a) and IRAK2 (b). IRAK1 phosphorylation (pIRAK1) was determined by flow cytometry (c). mRNA expression levels of transcription factors of the MAPK and NF-κB signalling pathways, c-Jun (e) and RelA (f), respectively, were determined by RT-PCR. Nuclear localization of RelA was determined by nuclear extraction of RelA and was detected using a specific antibody (g). mRNA levels of NOD2, target gene of the MAPK pathway (h) and 24-h protein levels of IL-1β, a target gene of the NF-κB pathway were determined (d). In all RT-PCRs, GAPDH was used as a reference gene. All data were normalized against the highest LPS value and are shown as mean of duplicate assays of three separate donors ±s.e.m. *P<0.05, **P<0.01 and ***P<0.001.
The transcription factors of the MAPK and NF-κB pathways, cJun and RelA, respectively, showed reduced mRNA expression levels upon addition of T. suis SPs as well (Figures 3e and f). In addition, nuclear protein levels of RelA of T. suis SPs+LPS vs LPS were significantly reduced (Figure 3g). Target genes of the MAPK pathway include CCL2, IL-6 and NOD2 (nucleotide-binding oligomerization domain-containing protein 2). Target genes of NF-κB pathway include TNFα and IL-1β. We have shown previously12 that T. suis SPs suppress TNFα, CCL2 and IL-6 mRNA and protein expression in LPS-stimulated DCs. In addition, we observed that IL-1β protein levels and NOD2 mRNA expression levels are reduced in T. suis SPs+LPS-stimulated DCs vs LPS stimulated DCs (Figures 3d and h, respectively). These data show that the MyD88-dependent signalling pathway is reduced and results in lower expression of target genes.
T. suis SPs induce the expression of Rab7b
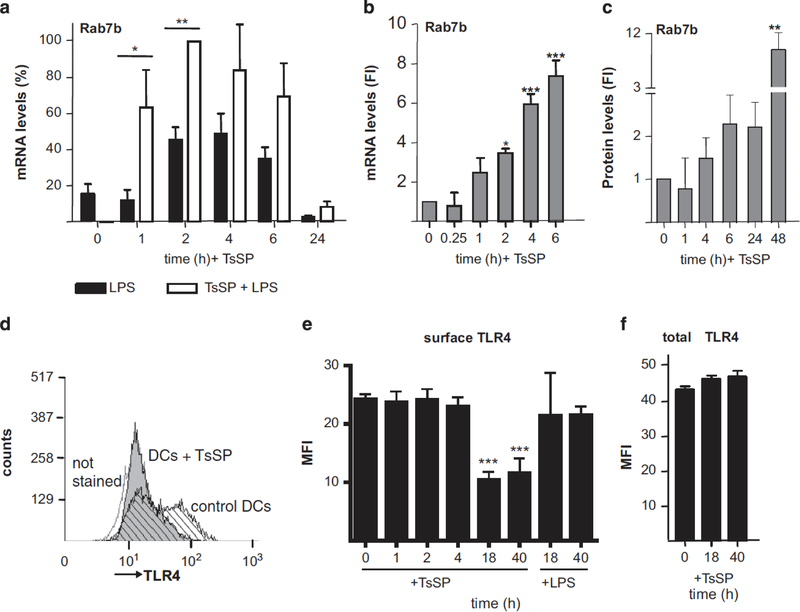
Remarkably, one of the genes induced by T. suis SPs alone (Supplementary Table S1) and T. suis SPs+LPS vs LPS (Table 1) is Ras related in brain 7b (Rab7b), a small guanosine triphosphatase (GTPase). Rab7b, by affecting TLR4 trafficking, has been shown to serve as a negative regulator for TLR4 signalling in macrophages.24 For validation of the array results, we determined the Rab7b mRNA levels in DCs stimulated with T. suis SPs+LPS vs LPS alone by RT-PCR. The results confirmed that addition of T. suis SPs significantly induced Rab7b expression (Figure 4a). Interestingly, DC priming with T. suis SPs alone also elevated Rab7b mRNA expression levels (Figure 4b) and protein levels (Figure 4c). To determine the effects of Rab7b induced by T. suis SPs on TLR4 expression levels, surface and intracellular expression of TLR4 were determined. Flow cytometry analysis showed that addition of T. suis SPs reduced surface expression of TLR4 on DCs after 18 h (Figures 4d and e), which coincided with the increased expression of Rab7b. By contrast, treatment of DCs with LPS did not change TLR4 surface levels (Figure 4e). Intracellular staining showed that total TLR4 staining was not significantly changed after 40 h of incubation with T. suis SPs (Figure 4f). These data show a simultaneous induction of Rab7b and reduction of surface TLR4 in DCs in response to T. suis SPs, which suggests that T. suis-induced Rab7b expression has a role in reducing TLR4 surface expression on DCs and thereby reducing the ability of cells to signal via TLR4.
Figure 4.
T. suis SPs (TsSP) induce the expression of Rab7b and reduce surface TLR4 expression in DCs. Rab7b mRNA expression was determined by RT-PCR, using GAPDH as a reference gene, in DCs stimulated with LPS, vs T. suis SPs+LPS (a) and T. suis SPs (b), respectively. Fold induction (FI) of Rab7b protein levels was determined by western blotting using GAPDH as a reference protein in T. suis SP-stimulated DCs (c). Surface expression (d and e) and total expression (f) of TLR4, respectively, was determined by flow cytometry in DCs stimulated with T. suis SPs or LPS. In panel (d), a histogram of a representative experiment of TLR4 surface expression, after 40-h incubation with T. suis SPs, is shown. Data were normalized against the highest T. suis SPs+LPS value (a) or against unstimulated cells (b, c and e) and are shown as mean of duplicate assays of three separate donors ±s.e.m. *P<0.05, **P<0.01 and ***P<0.001.
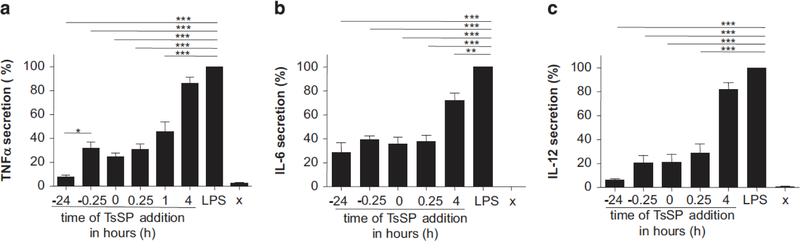
T. suis SPs suppress LPS-induced cytokine secretion
T. suis SPs have previously been shown to suppress LPS-induced cytokine production in human DCs in a concentration-dependent manner when administered 15 min prior to LPS exposure.11,12 We observed that the secretion of TNFα, IL-6 and IL-12 by DCs is suppressed by T. suis SPs when it is administered as long as 24 h before LPS-exposure. Administration of T. suis SPs 24 h before LPS stimulation resulted in a significantly reduced secretion of TNFα when compared with T. suis SPs administration 15 min before LPS stimulation. When T. suis SPs were administered 1 h after LPS stimulation, they were still able to reduce the secretion of TNFα, and the secretion of IL-6 was still significantly reduced when T. suis SPs were given 4 h after LPS stimulation (Figures 5a–c). These results demonstrate that T. suis SPs can reduce TLR4-dependent inflammation resulting in cytokine secretion for prolonged times when present before TLR4 is activated.
Figure 5.
T. suis SPs suppress cytokine secretion in TLR4-activated DCs for prolonged times. Immature DCs were stimulated with LPS (10 ng ml− 1) for 24 h while T. suis SPs (40 μgml − 1) were added at different time points before and after LPS addition, respectively. TNFα (a), IL-6 (b) and IL-12p70 (c) secretion were determined in culture supernatants using ELISA. Results are shown as mean of duplicate assays of four experiments using different blood donors ± s.e.m. For each donor, the value for LPS-activation was set at 100%, to correct for donor variation in cytokine secretion levels. Between the donors, secreted cytokine concentrations in LPS-stimulated DCs varied from 18 to 120 ng ml− 1 for TNFα, 11–60 ng ml− 1 for IL-6 and 1–4 ng ml − 1 for IL-12. *P<0.05, **P<0.01 and ***P<0.001.
Discussion:
The data presented in this paper provide novel insights into the mechanisms by which T. suis products modulate TLR4-induced inflammatory responses in human DCs. Several types of helminths have been shown to alter TLR4-induced signalling. Tegumental coat protein of Fasciola hepatica (FhTeg) was shown to reduce TLR4-induced MAPK signalling as well as reduce the subsequent secretion of pro-inflammatory cytokines.25 The product ES-62, derived from the helminth Acanthocheilonema viteae, was shown to cause degradation of the adaptor MyD88 upon TLR4 stimulation.26 In contrast, Schistosoma mansoni worm glycolipids induce inflammatory responses in a TLR4-depedent manner.27 Here we determined the effects of T. suis SPs on TLR4 signalling in DCs by gene array expression analysis in TLR4-stimulated DCs with or without treatment with T. suis SPs. The results showed a broad suppression of TLR4-induced inflammatory genes in response to co-stimulation with T. suis SPs.
Previously, we have shown that T. suis SPs induce the expression of P2RX7, a purinergic receptor in inflammation, in macrophages and DCs.13 The DC data were confirmed in the Illumina array done here. In addition, suppression of TLR4-induced TNFα secretion was observed 2 h after T. suis SP stimulation, indicating a relatively fast effect.12 We here show that many signalling and target molecules of the TLR4 pathway are reduced on mRNA and protein levels in the first few hours of T. suis SP treatment. Treatment with T. suis SPs results in a relatively rapid downregulation of mRNA levels of MyD88 as well as phosphorylation of IRAK1. Both of these regulators have roles at the initiation of the MyD88-dependent signalling cascade, which splits downstream into the NF-κB and MAPK pathways. Therefore, it is likely that co-stimulation with T. suis SPs results in downregulation of the complete NF-κB and MAPK signalling cascades, due to the reduced signalling events at the start of the cascades.
However, we did not observe across-the-board reductions in mRNA levels throughout the TRIF- and MyD88-dependent signalling pathways in response to treatment with T. suis SPs. As these signalling pathways also depend on phosphorylation and ubiquitination of proteins, defining T. suis SP’s effects using RT-PCR could result in underestimation of the effects. For example, IRAK1 mRNA expression levels were not significantly reduced in response to treatment with T. suis SPs (data not shown); however, pIRAK1 levels were clearly reduced, indicating that determination of mRNA expression levels of IRAK1 do not reflect the activation of this signalling molecule. In addition, IRF3 mRNA expression remained unaltered by LPS and/or T. suis SPs, indicating that suppression of TRIF-dependent signalling occurs either downstream of IRF3 or that phosphorylation has a significant role. In addition to the relatively rapid effects of T. suis SPs on TLR4 signalling, longer (pre)treatment with T. suis SPs results in a stronger suppression of cytokine production in DCs. Pretreatment of DCs with T. suis SPs for 24 h resulted in a significantly reduced secretion of TNFα when compared with addition of T. suis SPs 15 min prior to LPS stimulation. These strong effects of a long pretreatment with T. suis SPs may be due to a reduction of TLR4 on the surface of DCs, which was observed upon overnight (pre) treatment with T. suis SPs. In turn, the reduced surface TLR4 levels may be due to the induction of Rab7b in response to DC treatment with T. suis SPs. Rab proteins are a family of small GTPases that regulate vesicular formation, movement and fusion processes. In addition, Rabs can be involved in signal transduction via the regulation of membrane trafficking of receptors.28 TLR4 can be localized in several intracellular compartments, including the plasma membrane, endosomes, Golgi apparatus and lysosomes.24 It has been shown that Rab7b can serve as a negative regulator of both TLR4 and TLR9 function in macrophages by transporting these receptors from the endosome to the lysosome, thereby accelerating TLR degradation and reducing their surface expression.24,29 By contrast, another study showed in a human monocytic cell line that Rab7b does not enhance lysosomal degradation but instead is involved in the transport of TLR4 between endosomes and the trans-Golgi network.30 Thus further work is needed to fully understand the exact mechanism of action of Rab7b.
Our data show that T. suis SPs induce Rab7b levels in DCs and simultaneously reduce the surface expression of TLR4 but not total TLR4 levels (Figure 4d and e). These data suggest that Rab7b is involved in the lowering of surface TLR4 expression by T. suis SPs and indicate that TLR4 is not degraded but that its recycling to the cell surface is affected.
It has been demonstrated that MyD88-dependent and TRIF dependent signalling in the TLR4 pathway do not occur simultaneously.31 Instead, the pathway that is induced depends on the localization of TLR4, which trafficks between the cell surface and endosomal compartments. When TLR4 is expressed on the cell surface, MyD88 signalling occurs. Once TLR4 is internalized, MyD88 is likely to be released, leading to the termination of the MyD88-dependent signalling cascade.31 On the other hand, the TRIF-dependent signalling cascade is induced by endosome-localized TLR4.32 Thus a reduction in surface expression of TLR4 would result in the reduction of the MyD88-dependent signalling cascades, leading to a reduced expression of target genes, including TNFα, IL-6 and IL-12. This corresponds with our data, as we observed that 18 h (pre)treatment of DCs with T. suis SPs results in reduced surface TLR4 expression, and when this stimulation is followed by 24 h of LPS stimulation, it is associated with significantly lower secretion of TNFα levels when compared with pretreatment with T. suis SPs for 15 min.
The suppression of TLR4 signalling by T. suis SPs on the short and long term may be due to interaction of the SPs with different immune receptors. T. suis SPs may be able to interact with many different glycan- or protein-binding immune receptors. However, we have previously shown that suppression of LPS-induced proinflammatory cytokine secretion is prevented by periodate treatment of the T. suis SPs, indicating that in particular periodate-sensitive molecules such as glycans have a role.12 In addition, T. suis SPs have been shown to interact with the glycan binding C-type lectin receptors (CLRs) mannose receptor, macrophage galactose-type receptor and DC-specific ICAM3-grabbing non-integrin.12 Several CLRs have been described to influence TLR signalling.33–35 Therefore, it may be that one or more of these CLRs interact with or internalize T. suis SPs to allow suppression of TLR4-induced inflammation.
Currently, 28 clinical trials of helminth therapy, using either T. suis ovalbumin (OVA) or the human hookworm, Necator americanus, in different immunopathological diseases have been planned, started or completed.3 In our studies, we have used T. suis SPs to identify mechanisms by which soluble compounds of T. suis induce pronounced anti-inflammatory reactions. These may include compounds that differ from the products that are beneficial in the T. suis OVA therapy, as recent transcriptomic analyses of different life stages of T. suis show differences in gene expression in the various parasitic stages.36 It is not unthinkable, however, that treatment of autoimmune diseases will be more effective with purified helminth-derived immune-regulatory molecules, or mimics thereof, compared with helminth infection. The T. suis OVA/larvae may include ‘pathogenic’ compounds that counteract the immunoregulatory action, and there may be adult worm molecules missing that could be effective as anti-inflammatory compounds.
Here we have shown that T. suis SPs suppress many inflammatory pathways in DCs, including TLR4 signalling pathways. In addition, T. suis SPs reduce TLR4 surface expression, likely via the induction of Rab7b. These data increase our insights into the mechanisms that T. suis SPs exploit to suppress TLR4 signalling in human DCs, thereby reducing inflammation.
Materials and Methods:
Preparation of T. suis soluble products (SPs)
T. suis worms were collected from the caecum and colon of infected pigs and washed extensively with 0.98% NaCl. SPs were prepared by freezing whole worms in liquid nitrogen and crushing them with mortar and pestle into powder. The powder was dissolved in MilliQ water (EMD Millipore, Billerica, MA, USA) and centrifuged to remove the insoluble fraction. After 30 min of centrifugation at 10 000 g, 4 °C, supernatants were filtered (0.45 μm) and kept at − 80 °C until use. T. suis SPs were tested for LPS contamination by Limulus Amoebocyte Lysate assay and showed o1 ngml − 1 of endotoxin contamination.
Cell isolation, culture and differentiation
Human monocytes were isolated from buffy coats of healthy donors (Sanquin, Amsterdam, The Netherlands) or from fresh blood of healthy volunteers. All blood donors gave informed consent. Monocytes were isolated from peripheral blood mononuclear cells using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) as previously described37 and differentiated into immature DCs in RPMI in the presence of 10% fetal calf serum, 10.000 Uml − 1 penicillin and streptomycin (BioWhittaker, Walkersville, MD, USA), 10.000 Uml − 1 glutamine (Invitrogen, Thermo Scientific, Waltham, MA, USA) and 250 IU ml− 1 IL-4 and 120 IU ml− 1 granulocyte-macrophage colony-stimulating factor (Immunotools, Friesoythe, Germany). For all experiments, monocyte-derived DCs were used after 4 days of differentiation, and E. coli LPS (Sigma, St Louis, MO, USA) was used at a final concentration of 10 ng ml− 1. All experiments were performed at least three times, in separate experiments using DCs derived from different donors.
Cytokine enzyme-linked immunosorbent assay (ELISA)
Culture supernatants were harvested and frozen at − 20 °C until further analysis. TNF-α, IL-8 and IL-12p70 secretion levels were determined using the respective Cytoset ELISA Kits (Invitrogen). IL-6 and IL-1β secretion were determined using the respective human Pelipair ELISA Kits (Sanquin, Amsterdam, The Netherlands). All ELISA assays were done according to the manufacturer’s protocol.
Micro-array
After 24 h of stimulation with T. suis SPs (40 μgml − 1) and/or LPS (10 ng ml− 1), DCs derived from fresh blood-isolated monocytes of three different donors were harvested and snap-frozen at − 80 °C. Total RNA was isolated using RNeasy spin columns (Qiagen, Venlo, The Netherlands). RNA of the samples was amplified and biotinylated using the Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX, USA) as previously described.38 The samples, having passed quality control, were hybridized to Sentrix HumanHT-12v4 Expression BeadChip arrays (Illumina, San Diego, CA, USA), followed by scanning and feature extraction, all performed at ServiceXS (Leiden, The Netherlands). Bead summary intensities were log2-transformed and normalized using quantile normalization.39 For identification of differentially expressed genes between the two groups (DCs stimulated with LPS and LPS+T. suis SPs), we used Significance Analysis of Microarrays.40 Pathway-level analysis of gene expression data was performed by gene set enrichment analysis, using gene set permutation of C2v3.1 canonical pathways.21
cDNA synthesis and quantitative RT-PCR
DCs were harvested at the indicated time points and snap-frozen at − 80 °C. mRNA was isolated using the mRNA Capture Kit (Roche Diagnostics GmbH, Mannheim, Germany) and subsequently transcribed into cDNA using the Reverse Transcription System Kit (Promega, Madison, WI, USA), as described previously.41 PCR reactions were performed with the SYBR Green method as previously described.42 Oligonucleotides were designed using the Primer Express 2.0 (Applied Biosystems, Bedford, MA, USA) computer software. All oligonucleotides used are shown in Table 3 and were synthesized by Invitrogen.
Table 3.
Oligonucleotide primers for quantitative RT-PCR.
| Name | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|
| IFN-β1 | TCTGCATTACCTGAAGGCCAA | TGACTATGGTCCAGGCACAGTG |
| IRF8 | GCGCACCATTCAGCATTCTC | CCAGCTTGCCCCCATAGTAG |
| GBP2 | CTACCAGGTGCCAAGGAAGG | ACGTTCCACTTCAATCGCTTT |
| MyD88 | TTTGCACTCAGCCTCTCTCCA | TGATGAACCTCAGGATGCTGG |
| IRAK2 | GCTACATCTACCAGCTGCCC | CGTAGGAGGCGAACTCCATC |
| cJun | CGCCTGATAATCCAGTCCA | TTCTTGGGGCACAGGAACT |
| ReIA | TCCCATCTTTGACAATCGTGC | TTCACTCGGCAGATCTTGAGC |
| NOD2 | AAGGCTCTGTATTTGCGCGA | CATTCAATGAGCTTGCAGATGC |
| Rab7b | TGCTCATGAACGTTTTAGGAGC | CCTCCAAGCCCATGAACAA |
| TLR4 | ATAGCGAGCCACGCATTCA | CATCCTCACTGCTTCTGTGAGC |
| GAPDH | CCATGTTCGTCATGGGTGTG | GGTGCTAAGCAGTTGGTGGTG |
Abbreviation: RT-PCR, real-time PCR.
Western blotting
Mouse-anti-human monoclonal antibodies against Rab7b (Abnova, Taipei City, Taiwan) and GAPDH (Abcam, Cambridge, UK) were detected using horseradish peroxidase-labelled rabbit anti-mouse immunoglobulins (Dako, Agilent Technologies, Santa Clara, CA, USA). Staining was detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) in an EpiChemi II Darkroom (UVP LCC, Analytik Jena, Jena, Germany).
Flow cytometry
DCs were stained with anti-human CD284-PE (eBioscience, Affymetrix, Santa Clara, CA, USA) to determine the presence of TLR4. Flow cytometric analysis of pIRAK1 was done using rabbit polyclonal to IRAK (phosphor T209; Abcam) and donkey-anti-rabbit IgG (H+L) 488 (Dako). For intracellular cell staining, cells were fixed and permeabilized using 2% paraformaldehyde and 0.5% saponin (Sigma) prior to staining. Cell staining was measured by flow cytometry using Calibur (Becton Dickinson, Franklin Lakes, NJ, USA). Data were analysed using the Summit software (Dako).
Detection of nuclear RelA
Nuclear extracts of DCs stimulated with LPS or T. suis SPs+LPS were made using the Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer’s protocol. Nuclear RelA levels were determined from the nuclear extracts using the TransAM NF-κB Family Transcription Factor Assay Kit (Active Motif) according to the manufacturer’s protocol.
Statistical analysis
Results are expressed as the mean ± s.e.m. Statistical analyses were performed using the SPSS 20 software (IBM, Armonk, NY, USA). For paired comparisons of two groups, a paired sample T-test was performed (Figures 2, 3a–c, e–h and 4a). For other parametric data, a one-way analysis of variance was performed followed by Dunnett’s (Figures 3d and b–f) or Tukey’s (Figure 5) multiple comparisons test. *P<0.05, **P<0.01, ***P<0.001.
Supplementary Material
ACKNOWLEDGEMENTS
Part of this work was supported by NIH Grant AI101982 to RDC.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut 2005; 54: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis 2009; 15: 128–133. [DOI] [PubMed] [Google Scholar]
- 3.Fleming JO, Weinstock JV. Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol 2015; 37: 277–292. [DOI] [PubMed] [Google Scholar]
- 4.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol 2007; 61: 97–108. [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol 2011; 233: 6–11. [DOI] [PubMed] [Google Scholar]
- 6.Kuijk LM, van Die I. Worms to the rescue: can worm glycans protect from autoimmune diseases? IUBMB Life 2010; 62: 303–312. [DOI] [PubMed] [Google Scholar]
- 7.McKay DM. The therapeutic helminth? Trends Parasitol 2009; 25: 109–114. [DOI] [PubMed] [Google Scholar]
- 8.Harnett W, McInnes IB, Harnett MM. ES-62, a filarial nematode-derived immunomodulatory with anti-inflammatory potential. Immunol Lett 2004; 94: 27–33. [DOI] [PubMed] [Google Scholar]
- 9.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol 2003; 15: 59–69. [DOI] [PubMed] [Google Scholar]
- 10.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol 2003; 33: 1439–1449. [DOI] [PubMed] [Google Scholar]
- 11.Kuijk LM, Klaver EJ, Kooij G, van der Pol SM, Heijnen P, Bruijns SC, Kringel H et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 2012; 51: 210–218. [DOI] [PubMed] [Google Scholar]
- 12.Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G et al. Trichuris suis induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol 2013; 43: 191–200. [DOI] [PubMed] [Google Scholar]
- 13.Ottow MK, Klaver EJ, van der Pouw Kraan TC, Heijnen PD, Laan LC, Kringel H et al. The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun 2014; 15: 477–486. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675–680. [DOI] [PubMed] [Google Scholar]
- 15.Racke MK, Hu W, Lovett-Racke AE. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol 2005; 26: 289–291. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 2010; 9: 293–307. [DOI] [PubMed] [Google Scholar]
- 17.Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004; 113: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton CM, Dowling DJ, Loscher CE, Morphew RM, Brophy PM, O’Neill SM. The Fasciola hepatica tegumental antigen suppresses dendritic cell maturation and function. Infect Immun 2009; 77: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol 2007; 37: 1887–1904. [DOI] [PubMed] [Google Scholar]
- 20.van Liempt E, van Vliet SJ, Engering A, Garcia Vallejo JJ, Bank CM, Sanchez-Hernandez M et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol 2007; 44: 2605–2615. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen FE, Kempiak S, Huang DB, Phelps C, Ghosh G. Construction, expression, purification and functional analysis of recombinant NFkappaB p50/p65 heterodimer. Protein Eng 1999; 12: 423–428. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 2006; 25: 6706–6716. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Chen T, Han C, He D, Liu H, An H et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 2007; 110: 962–971. [DOI] [PubMed] [Google Scholar]
- 25.Vukman KV, Adams PN, O’Neill SM. Fasciola hepatica tegumental coat antigen suppresses MAPK signalling in dendritic cells and up-regulates the expression of SOCS3. Parasite Immunol 2013; 35: 234–238. [DOI] [PubMed] [Google Scholar]
- 26.Puneet P, McGrath MA, Tay HK, Al-Riyami L, Rzepecka J, Moochhala SM et al. The helminth product ES-62 protects against septic shock via Toll-like receptor 4-dependent autophagosomal degradation of the adaptor MyD88. Nat Immunol 2011; 12: 344–351. [DOI] [PubMed] [Google Scholar]
- 27.van Stijn CM, Meyer S, van den Broek M, Bruijns SC, van Kooyk Y, Geyer R et al. Schistosoma mansoni worm glycolipids induce an inflammatory phenotype in human dendritic cells by cooperation of TLR4 and DC-SIGN. Mol Immunol 2010; 47: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 28.Aloisi AL, Bucci C. Rab GTPases-cargo direct interactions: fine modulators of intracellular trafficking. Histol Histopathol 2013; 28: 839–849. [DOI] [PubMed] [Google Scholar]
- 29.Yao M, Liu X, Li D, Chen T, Cai Z, Cao X. Late endosome/lysosome-localized Rab7b suppresses TLR9-initiated proinflammatory cytokine and type I IFN production in macrophages. J Immunol 2009; 183: 1751–1758. [DOI] [PubMed] [Google Scholar]
- 30.Progida C, Cogli L, Piro F, De Luca A, Bakke O, Bucci C. Rab7b controls trafficking from endosomes to the TGN. J Cell Science 2010; 123: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 31.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol 2009; 9: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal 2013; 6: ra71. [DOI] [PubMed] [Google Scholar]
- 33.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 2007; 26: 605–616. [DOI] [PubMed] [Google Scholar]
- 34.van Vliet SJ, Bay S, Vuist IM, Kalay H, Garcia-Vallejo JJ, Leclerc C et al. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-alpha secretion. J Leukoc Biol 2013; 94: 315–323. [DOI] [PubMed] [Google Scholar]
- 35.Gringhuis SI, Kaptein TM, Wevers BA, Mesman AW, Geijtenbeek TB. Fucose-specific DC-SIGN signalling directs T helper cell type-2 responses via IKKepsilon- and CYLD-dependent Bcl3 activation. Nat Commun 2014; 5: 3898. [DOI] [PubMed] [Google Scholar]
- 36.Jex AR, Nejsum P, Schwarz EM, Hu L, Young ND, Hall RS et al. Genome and transcriptome of the porcine whipworm Trichuris suis. Nat Genet 2014; 46: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Stijn CM, van den Broek M, van de Weerd R, Visser M, Tasdelen I, Tefsen B et al. Regulation of expression and secretion of galectin-3 in human monocyte-derived dendritic cells. Mol Immunol 2009; 46: 3292–3299. [DOI] [PubMed] [Google Scholar]
- 38.Schirmer SH, Fledderus JO, van der Laan AM, van der Pouw-Kraan TC, Moerland PD, Volger OL et al. Suppression of inflammatory signaling in monocytes from patients with coronary artery disease. J Mol Cell Cardiol 2009; 46: 177–185. [DOI] [PubMed] [Google Scholar]
- 39.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003; 19: 185–193. [DOI] [PubMed] [Google Scholar]
- 40.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001; 98: 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Vallejo JJ, van Liempt E, da Costa Martins P, Beckers C, van het Hof B, Gringhuis SI et al. DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through LewisY antigen expressed on ICAM-2. Mol Immunol 2008; 45: 2359–2369. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Vallejo JJ, Gringhuis SI, van Dijk W, van Die I. Gene expression analysis of glycosylation-related genes by real-time polymerase chain reaction. Methods Mol Biol 2006; 347: 187–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.