Alaska to Ecuador mega-experiment shows seeds are more likely to be munched toward the tropics and lowlands, as Darwin predicted.
Abstract
Species interactions have long been predicted to increase in intensity toward the tropics and low elevations because of gradients in climate, productivity, or biodiversity. Despite their importance for understanding global ecological and evolutionary processes, plant-animal interaction gradients are particularly difficult to test systematically across large geographic gradients, and evidence from smaller, disparate studies is inconclusive. By systematically measuring postdispersal seed predation using 6995 standardized seed depots along 18 mountains in the Pacific cordillera, we found that seed predation increases by 17% from the Arctic to the Equator and by 17% from 4000 meters above sea level to sea level. Clines in total predation, likely driven by invertebrates, were consistent across treeline ecotones and within continuous forest and were better explained by climate seasonality than by productivity, biodiversity, or latitude. These results suggest that species interactions play predictably greater ecological and evolutionary roles in tropical, lowland, and other less seasonal ecosystems.
INTRODUCTION
Few biological patterns are as striking as latitudinal and elevational changes in biotic communities. Biodiversity and ecosystem productivity increase dramatically toward low latitudes (1, 2) and elevations (3, 4). Biologists have long speculated that greater diversity and productivity should generate corresponding increases in the intensity of species interactions (5–7). However, tests for geographic gradients in interaction intensity (8–11) or their expected ecological and evolutionary signatures (12–14) find contradictory results. While latitude and elevation are often considered analogs, their effects on interaction strength are rarely tested together. This likely contributes to the variability of experimental results and limits our understanding of their joint effects on global patterns in species interactions.
More intense interactions toward low latitudes and elevations underpin iconic biogeographic hypotheses. Antagonistic species interactions are thought to maintain high tropical diversity by limiting species dominance [the Janzen-Connell hypothesis (15, 16)], amplify tropical diversity by accelerating speciation (7, 17), and play a predictably greater role in determining species’ warm (low latitude and elevation) versus cool range limits (5, 6). For example, stronger tropical seed predation, an interaction that shapes plant communities and distributions (18, 19), is proposed to explain the spectacular diversity of trees (12, 15, 16) and adaptations for seed defense (20) in the tropics. The strength and predictability of interaction gradients are therefore pivotal to understanding their role as macroevolutionary and biogeographic agents.
Despite an outsized role in theory, assessing the generality of interaction gradients is hampered by constraints of existing evidence (21). Most studies encompass a limited spatial scale, omitting one or both latitudinal extremes (tropics or tundra) (11, 22, 23). Particularly lacking are replicated elevational gradients across a sizeable latitudinal range. Another challenge is controlling for evolutionary responses that can mask underlying gradients, such as increased plant defenses to counter chronically high consumption (11, 13). Last, gradients in interaction strength can differ between taxa (e.g., invertebrates versus vertebrates), counteracting each other’s effects when combined (8, 9). Notably, large-scale experiments using standardized methods and materials have generated the most convincing and consistent examples of geographic gradients in species interaction (9, 24, 25). Here, we expand on previous work by explicitly testing both latitudinal and elevational gradients in a globally important plant-animal interaction—postdispersal seed predation.
While the generality of geographic patterns in interactions has been extensively debated, their causes have received less attention (9, 21). Coarse gradients could arise if seed predation differs among biomes (e.g., declines above treeline), and low-predation biomes are more common at high latitudes and elevations. This could explain why studies in single biomes sometimes fail to find latitudinal patterns (22, 23). Alternatively, seed predation could increase alongside factors that promote continuous increases in granivore populations or feeding rates toward tropical and lowland areas (26). Warmer, less variable temperatures can increase animal populations and activity, especially for invertebrates (27, 28). Greater vegetation productivity could increase granivore populations by enhancing available food and niche space (23). Although rarely tested, higher diversity of seed predator communities could increase total granivore numbers by minimizing competition and maximizing resource use (29).
In line with theory, we predicted that postdispersal seed predation would become more intense from high latitudes to the Equator and from high elevations to sea level. We expected invertebrate seed predation to show particularly strong patterns in relation to geography (latitude and elevation) and climate (biome and temperature), as temperature directly affects ectotherm activity (27, 28). If invertebrates drive geographic interaction gradients while vertebrates contribute a consistent amount of predation across latitudes and elevations [as in (9)], we expect total seed predation and invertebrate seed predation to change in parallel. We predict invertebrate-driven seed predation to decline both sharply above treeline, as major invertebrate groups “drop out” (30, 31), and continuously with falling temperature because of metabolic constraints.
We tested our predictions by systematically measuring predation on 6995 depots of standardized seeds (Fig. 1) placed along 18 elevational transects covering >4000 m of elevation and 64° of latitude (Fig. 2A and table S1). We used one oil-based and one starch-based agricultural seed species: sunflower (Helianthus annuus) seeds without shells and oat (Avena sativa) seeds with husks (fig. S1). We ran the experiment 56 times from 2015 to 2017, with each transect tested one to six times (median = 4), always during natural seed production and dispersal at each site, so any influence of background seed crop is incorporated in our estimates. To isolate invertebrate seed predation, we secured wire cages over three or four sunflower depots per site to exclude vertebrate seed predators during 25 experimental runs (Fig. 1 and fig. S2). After 24 hours, we counted the seeds that were intact or partially eaten. For each 24-hour assay, we calculated the mean fraction of seeds predated [(eaten + missing)/initial; 70 to 99% of seeds removed from the ground are eaten (19)] for each seed type and predator type (total or invertebrate) at each site. Postdispersal seeds are fully independent individuals; thus, this is the per-capita predation risk relevant to lifetime fitness and selection. We used generalized linear mixed-effects models (GLMMs) to quantify the effect of latitude, elevation, seed type, vegetation biome, and their interactions on seed predation. We used structural equation models (SEMs) to compare the explanatory power of continuous environmental gradients: climate (temperature and precipitation mean and annual variation), actual evapotranspiration (AET) and net primary productivity (NPP) (measures of ecosystem productivity), and vertebrate richness (a proxy for granivore diversity).
Fig. 1. Photos illustrating depot setup at field sites.

Depots were not overly visible from >1 m away (A; arrow), as care was taken not to disturb litter and vegetation around depots (B). Invertebrate seed predation was assessed by excluding vertebrates from some sunflower seed depots using wire mesh cages (C). Photos are from 49°N in Canada at 880 meters above sea level (masl) (A) and 80 masl (B) and from 5°N in Colombia at 2120 masl (C). Photo credits: (A and B) A. Hargreaves, McGill University, and (C) S. David, University of British Colombia.
Fig. 2. Latitudinal and elevational declines in seed predation.
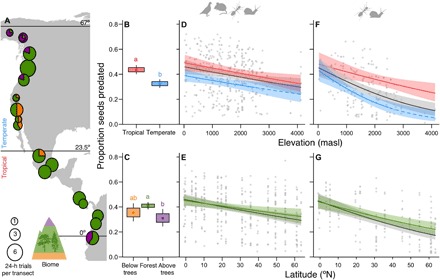
(A) Sampling transects from the Arctic to the Equator; the Tropic of Cancer (23.5°N) divides the temperate versus tropical zones. Circle area is proportional to the mean times the experiment was run at each site on the transect (1 to 6), and pie slices show the proportion of sites per biome: above upper treeline, forest, and below lower treeline. Seed predation differed among latitudinal zones (B; model 1) and biomes (C; model 4); different letters indicate significant differences; and dots, boxes, and whiskers show the means, 1 SE, and 95% confidence interval (CI), respectively, extracted from generalized linear mixed-effects models (GLMMs). (D to G) Continuous geographic trends in seed predation (±95% CIs) fitted by GLMMs. Elevational trends (D and F) are shown for the median latitude (31°N, black), median tropical latitude (10.5°N, red), and median temperate latitude (47.7°N, blue; models 2 and 3). Dashed trend lines show model extrapolations for temperate sites above 2500 masl. Latitudinal trends (E and G) are shown for the median elevation (1500 m) across biomes (black; models 2 and 3, respectively) and in forests specifically (green; model 5). Points are partial residuals for the all-site model (black) in each panel. (B to E) Total seed predation (56 experimental runs across 79 sites). (F and G) Seed predation by invertebrates only (25 experimental runs across 60 sites). Note that the steeper slopes in (F) and (G) compared to (D) and (E) are due to the different sites and dates included; among sites and dates where vertebrates were experimentally excluded, total and invertebrate predation showed the same geographic patterns (Fig. 3). Results are shown for sunflower seeds: Geographic patterns did not differ for oat seeds (fig. S3). Statistical results are shown in Table 1.
RESULTS
Consistent with long-standing predictions, seed predation increased toward the tropics and low elevations (Fig. 2; see full GLMM results in Table 1). Total seed predation was 10% higher in the tropics than in the temperate zone (south versus north of the Tropic of Cancer; Fig. 2B) for both oil- and starch-based seeds (Table 1, model 1; geographic patterns did not vary between sunflower and oat seeds in any analysis, fig. S3). Seed predation increased by 2.6% for every 10° of latitude closer to the Equator and by 0.4% for every 100 m decline in elevation, independent of latitude (i.e., no elevation × latitude interaction; Fig. 2, D and E, and Table 1, model 2). In total, seed predation increased by 17% from Alaska to Ecuador and by 17% from 4000 meters above sea level (masl) to sea level.
Table 1. Results from GLMMs analyzing latitudinal and elevational patterns in seed predation.
Models are described in the text. Model 4 (effect of biome) and model 5 (forests only) were run twice, first on total predation (uncaged depots only) and then on invertebrate predation (caged depots only). Initial models (gray rows) included all possible interactions (indicated by “×”), but nonsignificant interactions were dropped from final models (white rows), improving model convergence. Significance of factors and interactions was obtained from testing the final model against a model without that factor or interaction. Effect sizes are given for noninteracting effects in the final model; for categorical variables, these are categorical latitude = temperate versus tropical, seed type = sunflower versus oat, cage treatment = uncaged (total predation) versus caged (invertebrate predation), and biome = below trees versus above trees (top) and within forest versus above trees (bottom).
|
Model no.—predation type, biomes |
Fixed effects |
χ2(df) statistic from likelihood ratio tests Effect size ±SE in the final model (logit scale) |
Model χ2 deviance (residual df) |
R2GLMM† | ||||
|
Latitude (decimal degrees) |
Elevation (km asl) |
Seed species, df = 1 |
Lat × Elev, df = 1 |
Additional factor | ||||
| 1—Total, All | Initial: categorLat × elev × seed. sp |
|||||||
| Final: categorLat + elev + seed. sp |
20.6(1)*** −0.48 ± 0.10 |
8.0(1)** −0.18 ± 0.06 |
50.5*** 0.46 ± 0.07 |
1.6, P > 0.1 | — | 1500(464) | 0.053 | |
| 2—Total, All | Initial: lat × elev × seed.sp |
|||||||
| Final: lat + elev + seed.sp |
17.5(1)*** −0.01 ± 0.003 |
7.0(1)** −0.17 ± 0.06 |
55.2*** 0.48 ± 0.07 |
2.6, P > 0.1 | — | 1501(464) | 0.058 | |
| 3—Tot vs. Invert‡, All |
Initial: lat × elev × cage.treat |
Cage treat: | ||||||
| Final: lat × elev + cage.treat |
39.0(2)*** — |
21.8(2)*** — |
— | 7.5 ** −0.01 ± 0.004 |
13.7(1)*** 0.32 ± 0.09 |
704(195) | 0.083 | |
| 4t—Total, All | Initial: lat × elev × seed.sp. × biome |
Biome: | ||||||
| Final: lat + elev + seed.sp. + biome |
6.4(1)* −0.007 ± 0.003 |
2.6(1) P > 0.1 −0.11 ± 0.07 |
54.7*** 0.48 ± 0.07 |
2.6, P > 0.1 | 8.0(2)* 0.20 ± 0.23 0.44 ± 0.23 |
1493(462) | 0.057 | |
| 4i—Invert‡, All | Initial: lat × elev × biome |
Biome: | ||||||
| Final: lat + elev + biome |
10.0(1)** −0.02 ± 0.005 |
6.3(1)* −0.31 ± 0.13 |
— | 2.2, P > 0.1 | 8.1(2)* 0.78 ± 0.39 0.84 ± 0.30 |
350(94) | 0.123 | |
| 5 t—Total, Forest | Initial: lat × elev × seed.sp |
|||||||
| Final: lat × elev + seed.sp | 8.6(2)* — |
8.0(2)* — |
45.6*** −0.008 ± 0.004 |
4.3* 0.48 ± 0.07 |
— | 1177(361) | 0.039 | |
| 5i—Invert‡, Forest | Initial: lat × elev | |||||||
| Final: lat + elev | 10.9(1)*** −0.02 ± 0.005 |
5.4(1)* −0.28 ± 0.13 |
— | 3.7, P = 0.06 | — | 253(65) | 0.036 | |
*P < 0.05.
**P < 0.01.
***P < 0.001.
†Pseudo conditional R2 (variance explained by both fixed and random effects) for GLMMs with link-specific theoretical variances, calculated via the r.squaredGLMM function in the R MuMIn package (49) according to (50).
‡Sunflower seeds only, including only sites and dates that included the vertebrate exclusion treatment.
Invertebrate seed predation also increased toward low elevations and latitudes (Fig. 2, F and G), paralleling clines in total predation (Fig. 3). For the subset of sites and dates where we excluded vertebrates from some depots, elevational gradients in seed predation were steeper at high versus low latitudes (significant latitude × elevation interaction; Table 1, model 3), but patterns were consistent between total and invertebrate predation [Fig. 3; no interactions between vertebrate exclusion treatment and other variables: likelihood ratio test models with versus without exclusion treatment interactions χ2(3) = 2.1 (P = 0.5)]. Parallel gradients in total versus invertebrate seed predation suggest that vertebrate seed predation is relatively constant across the latitudes and elevations measured.
Fig. 3. Excluding vertebrate granivores reduced seed predation but did not change geographic patterns.
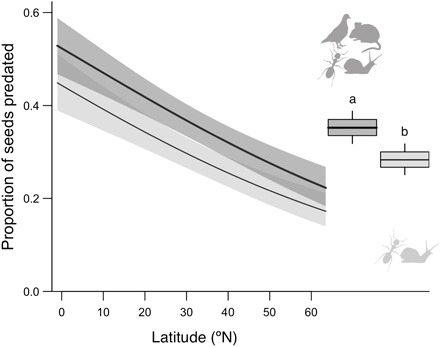
The figure compares invertebrate seed predation (light gray) and total seed predation (dark gray) from sites and dates that included the vertebrate exclusion treatment (25 experimental runs across 60 sites). The latitudinal trend in invertebrate seed predation is as strong as the trend in total seed predation (trends shown for the median site elevation ±95% CI; note that invertebrate predation is as shown in Fig. 2G), although vertebrate exclusion reduced seed predation overall (right); different letters indicate significant differences; and center line, boxes, and whiskers show the means, 1 SE, and 95% CI, respectively. Data were extracted from GLMM model 3 (Table 1).
Both categorical differences among biomes and continuous environmental gradients contributed to gradients in seed predation. Even after accounting for elevation and latitude, seeds above treeline experienced the lowest total predation (Fig. 2C) and invertebrate predation (Table 1, model 4). When we restricted our analyses to forests, the biome for which we have the best geographic coverage (72% of our sites; Fig. 2A), gradients in seed predation were shallower (Fig. 2, E and G), confirming that differences among biomes contribute to latitudinal and elevational patterns. However, significant latitudinal and elevational gradients existed within forests for both total and invertebrate seed predation (Fig. 2, E and G), with particularly strong effects of elevation at high latitudes (significant latitude × elevation interactions; Table 1, model 5). Persistent gradients in interaction intensity within forests demonstrate that lower alpine and tundra seed predation cannot fully explain latitudinal and elevational clines, implicating continuous environmental gradients as well.
Among the continuous ecological factors examined, annual temperature range explained total seed predation better than other climate variables, productivity, or diversity, particularly for sunflower seeds (model with annual temperature range + elevation: R2 = 0.366; Table 2). Total seed predation increased as temperature range declined and environments became less seasonal (Table 2). Contrary to our prediction of stronger temperature effects on invertebrates, latitude explained invertebrate seed predation better than more mechanistic predictors, including climate (Table 2 and table S2).
Table 2. Explanatory power of top SEMs.
Summary of top SEMs explaining predation intensity for each seed × predator type (from table S2). Data were arcsin-transformed and standardized to means = 0 and SD = 1 before analyses; hence, estimates are for relative comparison only. Full ranking of all 15 SEMs is shown in table S2.
|
Predator type, seed type |
SEM: independent variables |
Estimate (95% CI) |
R2 |
| Total predation, sunflower |
13: Annual temperature range Elevation |
−0.63 (−0.81, −0.44)**** −0.27 (−0.46, −0.09)** |
0.366 |
| Total predation, oats |
13: Annual temperature range Elevation |
−0.32 (−0.54, −0.10)** −0.14 (−0.36, 0.08) |
0.095 |
| 15: Latitude Elevation |
−0.21 (−0.44, 0.02)* −0.12 (−0.35, 0.11) |
0.042 | |
| Invert predation, sunflower |
15: Latitude Elevation |
−0.68 (−0.90, −0.46)**** −0.46 (−0.68, −0.24)**** |
0.39 |
*P < 0.05.
**P < 0.01.
****P < 0.0001.
DISCUSSION
Overall, our study provides strong evidence for latitudinal and elevational gradients in the intensity of an ecologically critical species interaction, postdispersal seed predation. Interaction intensity increased toward low latitudes and elevations for oil- and starch-based seed, total and invertebrate predation, and across biomes and within forests. Large-scale patterns emerged despite variation among elevational gradients (figs. S2 and S4) and even between replicated experiments on the same gradient. High heterogeneity in seed predation at the landscape scale has probably contributed to ambiguous or contradictory results among studies at smaller spatial scales and with less temporal replication (8). Our results provide important experimental support for long-standing speculation that interactions have a greater potential to shape tropical and lowland communities.
Latitudinal and elevational clines in invertebrate seed predation paralleled clines in total predation. While vertebrate granivores sometimes drive spatial [e.g., (25)] and temporal [e.g., (32)] patterns at smaller scales, our results suggest that potential vertebrate seed predation is relatively constant at the Arctic-to-Equator scale and that large-scale geographic patterns are driven primarily by invertebrates. This result is remarkably consistent with the only other standardized experiment on interaction intensity of comparable scale, which found that attack rates on model caterpillars increased toward low latitudes and—although not tested systematically—elevations, driven by invertebrates (9). Together, these experiments suggest that invertebrates play an outsized role in the community dynamics and evolutionary trajectories of tropical and lowland ecosystems at multiple trophic scales (8, 9, 20). The biological importance of invertebrates stands in stark contrast to our relative lack of knowledge about their diversity and dynamics in tropical ecosystems, particularly given their potentially dramatic demographic responses to climate change (33).
Our findings suggest testable hypotheses about the biogeographic importance of species interactions. Theory predicts that stronger interactions will produce stronger selection (26); thus, our finding of higher seed predation in the tropics supports a greater evolutionary role for interactions among tropical species (7, 17, 20). More intense interactions toward low elevations and in forest versus alpine habitat suggest stronger biotically mediated selection in these environments as well, comparisons that have received less attention than latitudinal contrasts. Plant-herbivore interactions are proposed to drive the impressive diversity of tropical leaf-eating insects and leaf defenses (20). Yet, seed predation affects plant fitness more directly than herbivory and so should impose even stronger selection and demographic effects (19). If defenses cannot fully compensate for increased predation, our results predict greater seed limitation of plant demography and migration rates (25) in high-predation (low latitude, low elevation, forested, and less seasonal) ecosystems.
Quantifying underlying gradients in interaction intensity is central to testing their predicted importance across latitude and elevation (9, 11, 21, 23, 25), but realized interaction strength also depends on exposure and adaptation (9, 34). We sampled predation throughout the active seed-producing period at each latitude, but some temperate seeds may escape predation under snow for much of the year (35). While testing geographic gradients in predation strength is beyond the scope of this study, we estimated these gradients for long-lived seeds by adjusting measured interaction intensity (Fig. 2) by the fraction of the year each site is snow-covered (Supplementary Methods). Many temperate and tropical species wait at least a year to germinate under natural conditions, although most take less time (table S3); thus, annual predation risk is ecologically relevant if only for a subset of species. For slow-germinating seeds, reduced predation under snow could as much as double the realized latitudinal and elevational trends in seed predation (fig. S5). Despite considerable potential to amplify (fig. S5) or dampen [e.g., (9)] gradients in interaction strength, geographic variation in exposure time remains relatively unexplored.
Realized interaction strength also depends on community and seed traits. Plants in high-predation environments should experience strong selection for antipredator strategies including escape (e.g., faster germination to reduce exposure), tolerance (e.g., increased seed production to saturate granivores), and defense (e.g., increased physical or chemical deterrents). Limited data suggest that tropical vegetation allocates >10× more energy to seed production (36), dwarfing the increase of up to 2× in seed predation (fig. S4). The shallower gradient in seed predation versus production could reflect a diluting effect of large seed crops, but it is unclear whether this is adaptive (i.e., whether species or individuals with large seed crops produce more surviving seeds). Although our two seed types were relatively similar in size and lack of defenses, they experienced a difference in predation rates (11%; fig. S3) approaching that of >7000 km of latitude or 4000 m of elevation (17% each), illustrating the impact of seed traits on predation. While latitudinal gradients in plant defenses remain controversial (37), a more nuanced theory suggests that seed defenses should vary with temperature, moisture, and their interaction with dormancy rather than latitude per se (38).
Elevational gradients in interaction strength were either independent of latitude (total seed predation across biomes) or stronger toward higher latitudes (invertebrate predation and predation in forests). Shallower elevational gradients could result if seed predation saturates at tropical lowland sites, constraining the maximum predation observed. Of the seven sites <1000 m and <20°N (all in forests), four had average total predation rates >90% for sunflower seeds (fig. S2). However, saturation effects would be strongest when seed predation is highest, that is, for sunflower versus oat seeds and for total versus invertebrate seed predation. In contrast, geographic patterns never varied between seed types and varied in the opposite direction than would be expected for predator type (shallower tropical gradients were found for invertebrate predation than for total predation; Table 1). Alternatively, shallower elevational gradients in tropical seed predation could indicate that the ecological factors determining seed predation intensity change more slowly up tropical versus temperate mountains. If true, this would contrast a well-known biogeographic paradigm that mountains are ecologically higher and steeper in the tropics (39, 40).
Geographic patterns in interaction strength have mostly been discussed in terms of their effects, rather than their causes. Seed predation intensity differed both between and within biomes, suggesting both discrete and continuous ecological causes. Temperature seasonality has previously been associated with seed predation intensity, but the direction of the association is inconsistent [negative in this study but positive in (23, 32)]. While we found no support for a direct role of biodiversity, this may be because global diversity estimates are limited to vertebrate species richness, whereas invertebrates seem to drive geographic patterns in interaction intensity (8, 9). The next step in understanding biogeographic patterns in interaction intensity will be to combine large-scale, standardized experiments with manipulative tests of potential mechanisms and fine-scale measurements of underlying gradients [e.g., (32)]. Identifying general mechanisms underlying interaction gradients would fundamentally improve our understanding of large-scale feedbacks between abiotic gradients and biotic communities (21). Generalizable relationships provide a mechanistic basis for predicting the relative ecological and evolutionary importance of biotic interactions in shaping species distributions, diversity, ecological networks, and resulting responses of ecological communities to global change.
MATERIALS AND METHODS
Experimental design
This distributed experiment was designed to maximize the potential to detect large geographic gradients in interaction intensity, if they existed, by standardizing as many components as possible and replicating in time and space. Each collaborator established a transect of four to five sites spanning at least 1000 m of elevation, or as much elevation as possible given the terrain (table S1). Site locations were occasionally adjusted between experimental runs (1 run = a 24-hour assay of seed predation along one transect); in these cases, a transect consisted of four sites during each run with five to six sites in total (table S1 and fig. S2). All sites were on the continental Americas within 300 km of the Pacific coast (Fig. 2A). This latitudinal gradient includes many protected areas along an unbroken mountain chain, minimizing differences in seed predator communities due to large-scale dispersal barriers (e.g., oceans). Sites were in natural areas, although most had experienced light human disturbance (e.g., logging >100 years ago, nearby park roads or trails). Experiments were conducted when some plant species were dispersing seed at each site along the transect; hence, predation rates on experimental seeds incorporate the background effect of differing seed productivity among sites. Experiments were conducted during a snow-free, rainless period or (at sites with daily rain) in typical weather for the transect. We could not fully standardize site phenology as tropical and temperate sites differ in seasonality, and phenology varies among elevations on any given date. To better capture “average” seed predation intensity, we ran the experiment multiple times at 15 of 18 transects (median replicates per transect = 4) from 2015 to 2017. Replicates were separated by at least 2 weeks and usually several months.
We used agricultural seed species to ensure that seeds were not local to any site, breaking potential coevolved or learned associations between seeds and granivores. Seeds bred for human consumption should have minimal chemical defenses, and our seeds had no (sunflowers without shells) or minimal (oats with thin husks) physical defenses, ensuring that seeds were edible by as wide a range of potential granivores as possible. Seeds weighed 61 mg (sunflower) and 46 mg (oat) on average, within the range of natural seed sizes from 7° to 60° absolute latitude (41) and the size range consumed by the smallest to largest vertebrate granivores globally [(42); size per se should not be an issue for invertebrates as they consume seeds in situ]. We bulk-purchased organic seeds from the same suppliers throughout the experiment: sunflower seeds from Community Natural Foods and oats from West Coast Seeds. To ensure that seeds would not germinate if dispersed by granivores, seeds were heat-sterilized at 110°C for 1 hour [modified from (23)]—a low enough temperature to prevent noticeable changes in color or smell. Sterilized seeds were mailed to collaborators and stored in a cool place in odor-proof containers until use.
Collaborators used a consistent protocol to quantify seed predation intensity. For each experimental run, we set out 10 depots of five oat seeds and 20 depots of eight sunflower seeds, alternating between species. We only used intact seeds, so that damage was unambiguously attributable to granivores. Depots were placed ≥5 m from walking trails, and ≥5 m apart in 2015 and ≥10 m apart in 2016 and 2017; otherwise, protocols did not change among years. Seeds were placed on bare ground in a shallow depression (depth, 0.5 to 3 cm; diameter, 5 to 10 cm), either natural or made by the experimenter. Care was taken not to disturb vegetation outside the depot. Depots were marked with a popsicle stick at the depot edge and green flagging tape 1 to 2 m from the depot (Fig. 1).
Beginning in 2016, during 25 of the 56 runs (60 of 79 sites along 14 of 18 transects), we excluded vertebrates from three to four sunflower depots per site, spaced evenly across the site (we used sunflower seeds only because oat seeds rarely showed signs of invertebrate predation). Conical exclusion cages (height, ~12 cm; diameter, 15 cm; 2.54 cm by 2.54 cm wire mesh) were secured over depots using metal pins (Fig. 1C). If a cage was found compromised (e.g., pins pulled out or cage dug under, moved, or trampled), then data from that depot were excluded.
We quantified predation 24 hours after depots were set out. Sites along a transect were generally set up in 1 day and checked 24 hours later in the same order, but three less accessible transects (in Mexico, Colombia, and Ecuador) were split into two groups of sites and the experiment was run over >2 days. After 24 hours, we photographed each depot and any seed remnants. We counted intact and partially eaten seeds and then removed all materials.
Data manipulation
We calculated seed predation as follows: proportion of seeds eaten = (seeds partially eaten + seeds missing)/seeds set out (23). This metric captures the combined population-level effect of granivore population size and per-capita effects (seed consumption/removal per granivore) (26).
Hypotheses relating biotic interaction intensity to large biogeographic patterns (e.g., latitudinal gradients in species diversity) are concerned with long-term averages. We therefore calculated average seed predation across depots of each seed type and vertebrate exclusion treatment (uncaged sunflower, caged sunflower, and uncaged oats) at each site for each experimental run. This generated one to six measures of mean seed predation per seed type per exclusion treatment per site, depending on how many times the experiment was run. Averaging across depots also accounted for nonindependence of depots within a site on a given date and made the data conform to a binomial distribution by resolving overdispersion. Models using averaged data converged better (no warnings) than models that used the unaveraged data (see the “Statistical analyses” section).
Environmental data
We compared the relative ability of climate, productivity, and biodiversity to explain spatial variation in seed predation using data from gridded databases extracted for each site location.
Climate
We tested the following climate parameters used in Orrock et al.’s (23) analysis of abiotic correlates of oat predation in North American grasslands: mean annual temperature, temperature annual range (maximum temperature of warmest month − minimum temperature of coldest month), annual precipitation, and precipitation seasonality (the coefficient of variation). Long-term averages (1950–2000) were downloaded from the WorldClim website (worldclim.org; accessed 2017) for each site at a resolution of 1 km × 1 km.
Productivity
We tested two measures of productivity: annual AET (the water entering the atmosphere via plant respiration and evaporation from soils) as in Orrock et al. (23) and NPP (biomass per unit area). AET and NPP data are for 2000–2013 at a resolution of 1 km × 1 km, downloaded from NASA’s MODIS Land Science Team (modis-land.gsfc.nasa.gov) and the NASA/USGS EOSDIS database (lpdaac.usgs.gov), respectively. For the four sites where NPP was unavailable (one site per transect on each California transect and the Jalisco transect), we used NPP from the nearest adjacent pixel (0.5 to 1.8 km away).
Biodiversity
To assess diversity, we extracted species richness data for 10 km by 10 km grid cells from Biodiversitymapping.org, a compilation of vertebrate range maps from the International Union for Conservation of Nature and BirdLife International (43). For sunflower seeds, we used vertebrate species richness as a proxy for total seed predator diversity because vertebrate ranges are well mapped and richness data are readily available. Many vertebrates do not consume seeds and many seed predators are not vertebrates, but for our study area—the Pacific coast of the Americas—vertebrate richness varies similarly to country- or state-level richness of ants (44), the most commonly noted invertebrate seed predator in our data. For oats, which are predominantly eaten by small mammals (45), we used species richness of rodents and shrew (Rodentia and Eulipotyphla).
Statistical analyses
Latitudinal and elevational patterns
We used GLMMs to quantify the effects of latitude, elevation, seed type, and their interactions on seed predation using the lme4 package in R (3.3.3). Because seed predation data are a binomial proportion, we used a binomial error distribution and a logit-link function. As sites on a transect may vary together temporally in seed predation (e.g., with regional pest outbreaks), and repeated measures of a single site are not independent, all models include a random intercept for the start date of the experiment (the mean date if the experiment was run over >2 days) and a random intercept for each site. We also analyzed raw data including the individual seed depots as the base level of the hierarchy and additional random factors for “site × date,” to account for nonindependence of depots at a given site on a given date, and “depot,” an individual-level random factor to resolve overdispersion. These models yielded the same fixed effects in the final model, the same ranking of factors within fixed effects, and the same slope direction for continuous variables as models on averaged data; however, they produced multiple convergence warnings. Hence, we present results from models on data averaged across depots within runs.
We ran one model per hypothesis with the following fixed effects. In model 1, we tested whether total seed predation differed between temperate and tropical zones, including transect latitude as a categorical variable (>23.5°N = temperate, <23.5°N = tropical), elevation, and seed type. Categorical latitude is consistent with biogeographic hypotheses that compare “the tropics” to “the temperate zone,” without necessarily invoking a continuous gradient (15). All other analyses consider continuous latitude. In model 2, we tested whether total seed predation declined continuously with increasing latitude and elevation, including site latitude (decimal degrees), elevation (masl), and seed type. In model 3, we tested whether invertebrate-only predation varied with latitude and elevation and whether these patterns differed from patterns in total predation. This model considers only sites and dates where invertebrate predation was measured and only sunflower seeds as we did not cage oats. Factors were latitude, elevation, and exclusion treatment (all predators versus invertebrates only).
Models initially included all possible interactions among factors. We assessed interaction significance using sequential likelihood ratio tests comparing models with and without the interaction using a χ2 distribution (46). Nonsignificant interactions (α = 0.05) were dropped from models, as model simplification improved mixed model convergence. Estimates of means and confidence intervals for different factor levels within categorical variables (e.g., categorical latitude and seed type) were extracted from reduced models using the R lsmeans package, while trend lines, confidence intervals, and partial residuals for continuous factors were extracted using the R visreg package. GLMM results are shown in Table 1.
Effect of biome (categorical mechanism)
To test whether latitudinal and elevational patterns were explained by differences in predation among biomes, we classified each site relative to local treelines: above upper treeline (alpine, tundra, and paramao), below lower treeline (grassland and desert), or between treelines (forest; table S1). In model 4, we first tested whether total seed predation (model 4t) and invertebrate seed predation (model 4i) differed among biomes, including latitude, elevation, and seed type as additional predictors [full model: seed predation ~ biome × latitude × elevation × seed type + (1|siteID) + (1|date)]. Model reduction and biome estimates were extracted from the reduced model as above. While we included latitude and elevation to account for their effects, we did not use these models to test for latitudinal and elevational effects within biomes, as we did not have even latitudinal coverage above treeline (only two tropical sites) or below treeline (only mid-latitudes; Fig. 2A). Instead, we ran separate models testing for latitudinal and elevational patterns in total (model 5t) and invertebrate (model 5i) seed predation in forested sites, for which we had good geographic coverage. GLMM results are shown in Table 1.
Continuous mechanisms (climate, productivity, and biodiversity)
We tested for correlations among mechanistic variables (climate variables, productivity, and species richness), latitude, and elevation. For both the entire dataset of 79 sites and for the 60 sites at which the vertebrate exclusion treatment was added, latitude was significantly correlated with most variables, which were also generally correlated with each other (fig. S6). We used structural equation modeling to test the mechanistic relationships among correlated predictor variables (47).
Additional manipulations made data suitable for structural equation modeling. First, to deal with repeated measures of individual sites, we averaged the data a second time to get one data point per seed type per caging treatment per site. We arcsin-transformed data to make errors normally distributed. This yielded n = 79 data points for total predation on sunflower seeds, n = 79 for total predation on oats, and n = 60 for invertebrate predation on sunflower seeds. We analyzed these three seed × predator types independently to allow for varying biogeographic patterns in consumption by different predator guilds (8, 9). To improve model fits, we standardized the response and predictors in each dataset to mean = 0 and SD = 1.
We first constructed a conceptual model, which was too complex to test with the collected data but clarified our understanding about how predictors affect each other and seed predation (fig. S5). From this conceptual model and results of an earlier analysis of climate and productivity on oat predation (23), we generated 15 simpler SEMs, representing biologically motivated simplifications of our conceptual model. These SEMs are illustrated in fig. S7, and the hypotheses that they represent are described fully in the Supplementary Materials. Briefly, latitude and elevation are always exogenous variables, whose values do not rely on values of other modeled variables. Climate variables were modeled either as a latent variable, “Climate,” or independently and in various combinations. Although productivity (AET and NPP) is positively correlated with species richness (fig. S6), global analyses suggest that high productivity does not cause high richness [e.g., (48)]; thus, we modeled them as affected by climate but independent of each other. Higher seed predator populations could arise from more productive ecosystems (more seeds or other food available) or more diverse predator assemblages (“species packing”); hence, we modeled direct effects of productivity and species richness on seed predation. All models include a direct effect of elevation on seed predation, as grid cells for climate, productivity, and richness data were large enough to encompass multiple elevational sites along steep gradients.
SEM1 to SEM8 tested various pathways of direct and indirect effects of multiple variables on seed predation intensity. SEM9 to SEM15 compared the simplest possible model for each variable thought to have direct effects on seed predation intensity (SEM9 to SEM12) or that significantly affected predation in Orrock et al. (23) (SEM13 and SEM14) to a model with latitude and no mechanistic predictors (SEM15). SEM9 to SEM15 also included elevation, with both variables modeled as exogenous (structure shown in fig. S7, SEM9). SEMs were run using the R package lavaan. We assessed model goodness of fit using the Tucker-Lewis Index and root mean square error of approximation. Model selection was done using the Akaike information criterion (AIC).
To assess whether results were affected by the additional data manipulations required for SEMs, we also compared binomial generalized linear models using our main dataset (i.e., 1 data point per date per site per seed type and caging treatment). We ran one model for each explanatory variable (mean annual temperature, AET, NPP, species richness, temperature annual range, annual precipitation, precipitation seasonality, and latitude) for total seed predation (models included seed type and elevation) and invertebrate predation (models included elevation). These models are equivalent to the simplest SEMs (SEM9 to SEM15). Models were compared using AIC modified for small sizes (AICc) and yielded the same model ranking as structural equation modeling; thus, we present SEMs only.
Supplementary Material
Acknowledgments
We thank C. Muir, V. Vásquez Reyes, S. Tremor, C. García-Jiménez, L. Bolin, J. Godlee, S. Angers-Blondin, J. Tolome, P. Sierra, H. Branch, J. Boyle, S. Lehtonen, C. Cosgrove, M. Little, L. Dyer, H. Barios, J. Assmann, and S. Chimbolema for help with fieldwork; A. Davis for help with structural equation modeling; and G. Langston for help with mapping. We thank G. Suarez of Fundación Colibrí, who passed away in 2017, for helping us establish sites in La Mesenia-Paramillo Reserve, Colombia. We acknowledge the Kluane First Nation, Gitdumden Clan of the Wet’suwet’en, Squamish Nation, Tsleil-Waututh Nation, Tübatulabal, Kumeyaay, Kiliwa, Voto, Emberá-Chamí, and other indigenous peoples on whose traditional territory this field work was carried out. Funding: This work was supported by a UBC Biodiversity Research Grant (A.L.H.), INECOL (proy. 20030-10796 to K.M.), an Universidad San Francisco de Quito Collaboration Grant (E.S.), NSF (grant DEB-1442103 to L. Dyer), and the San Diego Natural History Museum (to S. Tremor). Author contributions: Authorship is in order of contribution. A.L.H. designed and coordinated the study, analyzed the data, and wrote the manuscript. All authors contributed to data generation and editing. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the Supplementary Materials and/or Dryad digital repository at doi:10.5061/dryad.1723 h44. R code and additional data related to this paper may be requested from the corresponding author.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaau4403/DC1
Supplementary Methods
Fig. S1. Photos of field sites and seed predator signs.
Fig. S2. Mean seed predation by site.
Fig. S3. Geographic trends in total predation on sunflower versus oat seeds.
Fig. S4. Large-scale patterns emerged despite variation among the 18 transects.
Fig. S5. Snow cover could steepen latitudinal and elevational interaction gradients.
Fig. S6. Correlations between continuous environmental variables.
Fig. S7. Path diagrams of SEM1 to SEM9.
Table S1. Transect details.
Table S2. Relative performance of SEMs.
Table S3. Multispecies surveys of time to germination.
REFERENCES AND NOTES
- 1.Hillebrand H., On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Gillman L. N., Wright S. D., Cusens J., McBride P. D., Malhi Y., Whittaker R. J., Latitude, productivity and species richness. Glob. Ecol. Biogeogr. 24, 107–117 (2015). [Google Scholar]
- 3.Girardin C. A. J., Malhi Y., Aragão L. E. O. C., Mamani M., Haraca Huasco W., Durand L., Feeley K. J., Rapp J., Silva-Espejo J. E., Silman M., Salinas N., Whittaker R. J., Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 16, 3176–3192 (2010). [Google Scholar]
- 4.Rahbek C., The elevational gradient of species richness: A uniform pattern? Ecography 18, 200–205 (1995). [Google Scholar]
- 5.C. Darwin, On the Origins of Species by Means of Natural Selection (J. Murray, ed. 1, 1859). [Google Scholar]
- 6.R. MacArthur, in Geographical Ecology: Patterns in the Distribution of Species (Princeton Univ. Press, 1972), pp. 127–198. [Google Scholar]
- 7.Dobzhansky T., Evolution in the tropics. Am. Sci. 38, 209–221 (1950). [Google Scholar]
- 8.Peco B., Laffan S. W., Moles A. T., Global patterns in post-dispersal seed removal by invertebrates and vertebrates. PLOS ONE 9, e91256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roslin T., Hardwick B., Novotny V., Petry W. K., Andrew N. R., Asmus A., Barrio I. C., Basset Y., Boesing A. L., Bonebrake T. C., Cameron E. K., Dáttilo W., Donoso D. A., Drozd P., Gray C. L., Hik D. S., Hill S. J., Hopkins T., Huang S., Koane B., Laird-Hopkins B., Laukkanen L., Lewis O. T., Milne S., Mwesige I., Nakamura A., Nell C. S., Nichols E., Prokurat A., Sam K., Schmidt N. M., Slade A., Slade V., Suchanková A., Teder T., van Nouhuys S., Vandvik V., Weissflog A., Zhukovich V., Slade E. M., Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742–744 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Pennings S. C., Silliman B. R., Linking biogeography and community ecology: Latitudinal variation in plant–herbivore interaction strength. Ecology 86, 2310–2319 (2005). [Google Scholar]
- 11.Chen S.-C., Hemmings F. A., Chen F., Moles A. T., Plants do not suffer greater losses to seed predation towards the tropics. Glob. Ecol. Biogeogr. 26, 1283–1291 (2017). [Google Scholar]
- 12.Johnson D. J., Beaulieu W. T., Bever J. D., Clay K., Conspecific negative density dependence and forest diversity. Science 336, 904–907 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Rasmann S., Agrawal A. A., Latitudinal patterns in plant defense: Evolution of cardenolides, their toxicity and induction following herbivory. Ecol. Lett. 14, 476–483 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Moles A. T., Bonser S. P., Poore A. G. B., Wallis I. R., Foley W. J., Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct. Ecol. 25, 380–388 (2011). [Google Scholar]
- 15.Janzen D. H., Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 (1970). [Google Scholar]
- 16.J. Connell, in Dynamics of Populations, P. den Boer, G. Gradwell, Eds. (Centre for Agricultural Publications and Documentation, 1971), pp. 298–310. [Google Scholar]
- 17.D. Schemske, in Speciation and Patterns of Diversity, R. K. Butlin, J. R. Bridle, D. Schlutter, Eds. (Cambridge Univ. Press, 2009), pp. 219–239. [Google Scholar]
- 18.Brown C. D., Vellend M., Non-climatic constraints on upper elevational plant range expansion under climate change. Proc. R. Soc. B 281, 20141779 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulme P. E., Post-dispersal seed predation: Consequences for plant demography and evolution. Perspect. Plant Ecol. 1, 32–46 (1998). [Google Scholar]
- 20.Coley P. D., Kursar T. A., On tropical forests and their pests. Science 343, 35–36 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Schemske D. W., Mittelbach G. G., Cornell H. V., Sobel J. M., Roy K., Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (2009). [Google Scholar]
- 22.Andrew N. R., Hughes L., Herbivore damage along a latitudinal gradient: Relative impacts of different feeding guilds. Oikos 108, 176–182 (2005). [Google Scholar]
- 23.Orrock J. L., Borer E. T., Brudvig L. A., Firn J., MacDougall A. S., Melbourne B. A., Yang L. H., Baker D. V., Bar-Massada A., Crawley M. J., Damschen E. I., Davies K. F., Gruner D. S., Kay A. D., Lind E., McCulley R. L., Seabloom E. W., A continent-wide study reveals clear relationships between regional abiotic conditions and post-dispersal seed predation. J. Biogeogr. 42, 662–670 (2015). [Google Scholar]
- 24.McKinnon L., Smith P. A., Nol E., Martin J. L., Doyle F. I., Abraham K. F., Gilchrist H. G., Morrison R. I. G., Bety J., Lower predation risk for migratory birds at high latitudes. Science 327, 326–327 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Hillyer R., Silman M. R., Changes in species interactions across a 2.5 km elevation gradient: Effects on plant migration in response to climate change. Glob. Chang. Biol. 16, 3205–3214 (2010). [Google Scholar]
- 26.Benkman C. W., Biotic interaction strength and the intensity of selection. Ecol. Lett. 16, 1054–1060 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Bale J. S., Masters G. J., Hodkinson I. D., Awmack C., Bezemer T. M., Brown V. K., Butterfield J., Buse A., Coulson J. C., Farrar J., Good J. E. G., Harrington R., Hartley S., Jones T. H., Lindroth R. L., Press M. C., Symrnioudis I., Watt A. D., Whittaker J. B., Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 8, 1–16 (2002). [Google Scholar]
- 28.Zvereva E. L., Kozlov M. V., Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: A metaanalysis. Glob. Chang. Biol. 12, 27–41 (2006). [Google Scholar]
- 29.LaManna J. A., Mangan S. A., Alonso A., Bourg N. A., Brockelman W. Y., Bunyavejchewin S., Chang L.-W., Chiang J.-M., Chuyong G. B., Clay K., Condit R., Cordell S., Davies S. J., Furniss T. J., Giardina C. P., Gunatilleke I. A. U. N., Gunatilleke C. V. S., He F., Howe R. W., Hubbell S. P., Hsieh C.-F., Inman-Narahari F. M., Janík D., Johnson D. J., Kenfack D., Korte L., Král K., Larson A. J., Lutz J. A., McMahon S. M., McShea W. J., Memiaghe H. R., Nathalang A., Novotny V., Ong P. S., Orwig D. A., Ostertag R., Parker G. G., Phillips R. P., Sack L., Sun I.-F., Tello J. S., Thomas D. W., Turner B. L., Vela Díaz D. M., Vrška T., Weiblen G. D., Wolf A., Yap S., Myers J. A., Plant diversity increases with the strength of negative density dependence at the global scale. Science 356, 1389–1392 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Vaupel A., Matthies D., Abundance, reproduction, and seed predation of an alpine plant decrease from the center toward the range limit. Ecology 93, 2253–2262 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Longino J. T., Branstetter M. G., Colwell R. K., How ants drop out: Ant abundance on tropical mountains. PLOS ONE 9, e104030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis A. S., Raghu S., Weighing abiotic and biotic influences on weed seed predation. Weed Res. 50, 402–412 (2010). [Google Scholar]
- 33.Nelson W. A., Bjornstad O. N., Yamanaka T., Recurrent insect outbreaks caused by temperature-driven changes in system stability. Science 341, 796–799 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Bingham R. A., Orthner A. R., Efficient pollination of alpine plants. Nature 391, 238–239 (1998). [Google Scholar]
- 35.Shimano K., Masuzawa T., Effects of snow accumulation on survival of beech (Fagus crenata) seed. Plant Ecol. 134, 235–241 (1998). [Google Scholar]
- 36.Moles A. T., Wright I. J., Pitman A. J., Murray B. R., Westoby M., Is there a latitudinal gradient in seed production? Ecography 32, 78–82 (2009). [Google Scholar]
- 37.Moles A. T., Wallis I. R., Foley W. J., Warton D. I., Stegen J. C., Bisigato A. J., Cella-Pizarro L., Clark C. J., Cohen P. S., Cornwell W. K., Edwards W., Ejrnaes R., Gonzales-Ojeda T., Graae B. J., Hay G., Lumbwe F. C., Magaña-Rodríguez B., Moore B. D., Peri P. L., Poulsen J. R., Veldtman R., von Zeipel H., Andrew N. R., Boulter S. L., Borer E. T., Campón F. F., Coll M., Farji-Brener A. G., de Gabriel J., Jurado E., Kyhn L. A., Low B., Mulder C. P. H., Reardon-Smith K., Rodríguez-Velázquez J., Seabloom E. W., Vesk P. A., van Cauter A., Waldram M. S., Zheng Z., Blendinger P. G., Enquist B. J., Facelli J. M., Knight T., Majer J. D., Martínez-Ramos M., McQuillan P., Prior L. D., Putting plant resistance traits on the map: A test of the idea that plants are better defended at lower latitudes. New Phytol. 191, 777–788 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Dalling J. W., Davis A. S., Schutte B. J., Arnold A. E., Seed survival in soil: Interacting effects of predation, dormancy and the soil microbial community. J. Ecol. 99, 89–95 (2011). [Google Scholar]
- 39.McCain C. M., Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecol. Lett. 12, 550–560 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Janzen D. H., Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 (1967). [Google Scholar]
- 41.Moles A. T., Westoby M., Latitude, seed predation and seed mass. J. Biogeogr. 30, 105–128 (2003). [Google Scholar]
- 42.Chen S.-C., Moles A. T., A mammoth mouthful? A test of the idea that larger animals ingest larger seeds. Glob. Ecol. Biogeogr. 24, 1269–1280 (2015). [Google Scholar]
- 43.Jenkins C. N., Pimm S. L., Joppa L. N., Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. U.S.A. 110, E2602–E2610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins C. N., Guénard B., Diamond S. E., Weiser M. D., Dunn R. R., Conservation implications of divergent global patterns of ant and vertebrate diversity. Divers. Distrib. 19, 1084–1092 (2013). [Google Scholar]
- 45.B. C. Clifford, in The Oat Crop, R. W. Welch, Ed. (Chapman & Hall, 1995), pp. 252–278. [Google Scholar]
- 46.A. F. Zuur, E. N. Ieno, N. Walker, A. A. Saveliev, G. M. Smith, Mixed Effects Models and Extensions in Ecology with R. (Springer, 2009). [Google Scholar]
- 47.J. B. Grace, Structural Equation Modelling and Natural Systems (Cambridge Univ. Press, 2006). [Google Scholar]
- 48.Fine P. V. A., Ecological and evolutionary drivers of geographic variation in species diversity. Annu. Rev. Ecol. Evol. Syst. 46, 369–392 (2015). [Google Scholar]
- 49.K. Bartón, MuMIn: Multi-model inference. R package version 1.42.1. (2018).
- 50.Nakagawa S., Johnson P. C. D., Schielzeth H., The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radvanyi A., Small mammals and regeneration of white spruce forests in western Alberta. Ecology 51, 1102–1105 (1970). [Google Scholar]
- 52.Zhang Y., Chiew F. H. S., Peña-Arancibia J., Sun F., Li H., Leuning R., Global variation of transpiration and soil evaporation and the role of their major climate drivers. J. Geophys. Res. Atmos. 122, 6868–6881 (2017). [Google Scholar]
- 53.Monteith J. L., Solar radiation and productivity in tropical ecosystems. J. Appl. Ecol. 9, 747–766 (1972). [Google Scholar]
- 54.Belmaker J., Jetz W., Relative roles of ecological and energetic constraints, diversification rates and region history on global species richness gradients. Ecol. Lett. 18, 563–571 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Schluter D., Speciation, ecological opportunity, and latitude: American Society of Naturalists Address. Am. Nat. 187, 1–18 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Hargreaves A. L., Samis K. E., Eckert C. G., Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. Am. Nat. 183, 157–173 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Halbritter A. H., Alexander J. M., Edwards P. J., Billeter R., How comparable are species distributions along elevational and latitudinal climate gradients? Glob. Ecol. Biogeogr. 22, 1228–1237 (2013). [Google Scholar]
- 58.Norden N., Daws M. I., Antoine C., Gonzalez M. A., Garwood N. C., Chave J., The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Funct. Ecol. 23, 203–210 (2009). [Google Scholar]
- 59.Sautu A., Baskin J. M., Baskin C. C., Condit R., Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America. For. Ecol. Manag. 234, 245–263 (2006). [Google Scholar]
- 60.Toledo-Aceves T., Germination rate of endangered cloud forest trees in Mexico: Potential for ex situ propagation. J. For. Res. 22, 61–64 (2017). [Google Scholar]
- 61.Yu Y., Baskin J. M., Baskin C. C., Tang Y., Cao M., Ecology of seed germination of eight non-pioneer tree species from a tropical seasonal rain forest in southwest China. Plant Ecol. 197, 1–16 (2008). [Google Scholar]
- 62.Kyereh B., Swaine M. D., Thompson J., Effect of light on the germination of forest trees in Ghana. J. Ecol. 87, 772–783 (1999). [Google Scholar]
- 63.McIvor J. G., Howden S. M., Dormancy and germination characteristics of herbaceous species in the seasonally dry tropics of northern Australia. Austral Ecol. 25, 213–222 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waterton J., Cleland E. E., Trade-off between early emergence and herbivore susceptibility mediates exotic success in an experimental California plant community. Ecol. Evol. 6, 8942–8953 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barak R. S., Lichtenberger T. M., Wellman-Houde A., Kramer A. T., Larkin D. J., Cracking the case: Seed traits and phylogeny predict time to germination in prairie restoration species. Ecol. Evol. 8, 5551–5562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao S. Z., Liu K., Du G., Baskin J. M., Baskin C. C., Bu H., Qi W., Seedling emergence of 144 subalpine meadow plants: Effects of phylogeny, life cycle type and seed mass. Seed Sci. Res. 28, 93–99 (2018). [Google Scholar]
- 67.Bliss L. C., A comparison of plant development in microenvironments of arctic and alpine tundras. Ecol. Monogr. 26, 303–337 (1956). [Google Scholar]
- 68.Daws M., Burslem D. F. R. P., Crabtree L. M., Kirkman P., Mullins C. E., Dalling J. W., Differences in seed germination responses may promote coexistence of four sympatric Piper species. Funct. Ecol. 16, 258–267 (2002). [Google Scholar]
- 69.Xu J., Li W., Zhang C., Liu W., Du G., Variation in seed germination of 134 common species on the Eastern Tibetan Plateau: Phylogenetic, life history and environmental correlates. PLOS ONE 9, e98601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bu H., Chen X., Wang Y., Xu X., Liu K., Du G., Germination time, other plant traits and phylogeny in an alpine meadow on the eastern Qinghai-Tibet Plateau. Community Ecol. 8, 221–227 (2007). [Google Scholar]
- 71.Chen K., Burgess K. S., Yang X.-Y., Luo Y.-H., Gao L.-M., Li D.-Z., Functional trade-offs and the phylogenetic dispersion of seed traits in a biodiversity hotspot of the Mountains of Southwest China. Ecol. Evol. 8, 2218–2230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pivatto M. S., Funes G., Ferreras A. E., Gurvich D. E., Seed mass, germination and seedling traits for some central Argentinian cacti. Seed Sci. Res. 24, 71–77 (2014). [Google Scholar]
- 73.Amen R. D., The extent and role of seed dormancy in alpine plants. Q. Rev. Biol. 41, 271–281 (1966). [Google Scholar]
- 74.Tucker L. R., Lewis C., A reliability coefficient for maximum likelihood factor analysis. Psychometrika 38, 1–10 (1973). [Google Scholar]
- 75.J. J. Hox, T. M. Bechger, An introduction to structural equation modeling. (1998).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaau4403/DC1
Supplementary Methods
Fig. S1. Photos of field sites and seed predator signs.
Fig. S2. Mean seed predation by site.
Fig. S3. Geographic trends in total predation on sunflower versus oat seeds.
Fig. S4. Large-scale patterns emerged despite variation among the 18 transects.
Fig. S5. Snow cover could steepen latitudinal and elevational interaction gradients.
Fig. S6. Correlations between continuous environmental variables.
Fig. S7. Path diagrams of SEM1 to SEM9.
Table S1. Transect details.
Table S2. Relative performance of SEMs.
Table S3. Multispecies surveys of time to germination.


