Key Points
Question
Is the GNAQ R183Q mutation present in the choroidal vessels of a patient with Sturge-Weber syndrome and choroidal hemangioma?
Findings
In this case study, laser-capture microdissection followed by droplet digital polymerase chain reaction analysis showed that the GNAQ R183Q mutation was present in the patient’s choroidal tissue. The tissue tested negative for GNAQ mutations found in congenital hemangioma and did not express the infantile hemangioma marker glucose transporter-1.
Meaning
Based on a single case, a choroidal hemangioma was identified as a choroidal capillary malformation, laying the foundation to accurately classify this vascular anomaly to better guide treatment decisions.
Abstract
Importance
Choroidal hemangiomas are defined by a thickened choroid owing to vessel overgrowth, which may increase the intraocular pressure and lead to glaucoma. Choroidal hemangioma and glaucoma often co-occur in patients with Sturge-Weber syndrome, a rare neurocutaneous disorder characterized by capillary malformations.
Objective
To determine whether the mutation found in most capillary malformations, GNAQ R183Q (c.548G>A), was present in the choroidal hemangioma of a patient with Sturge-Weber syndrome.
Design, Setting, and Participant
Using laser-capture microdissection, choroidal blood vessels were isolated from paraffin-embedded tissue sections, and genomic DNA was extracted for mutational analysis. Choroidal sections were analyzed in parallel. A patient with choroidal hemangioma and Sturge-Weber syndrome who had undergone enucleation was analyzed in this study at Boston Children’s Hospital. Negative controls were choroidal tissue from an eye with retinoblastoma and unaffected lung tissue; brain tissue from a different patient with Sturge-Weber syndrome served as a positive control. Infantile hemangioma was analyzed as well. Data were analyzed in 2018.
Main Outcomes and Measures
The mutant allelic frequency of GNAQ R183 and GNAQ Q209L/H/P was determined by droplet digital polymerase chain reaction on isolated genomic DNA. The infantile hemangioma marker glucose transporter-1 was visualized by immunofluorescent staining of tissue sections.
Results
The GNAQ R183Q mutation was present in the patient’s choroidal vessels (21.1%) at a frequency similar to that found in brain tissue from a different patient with Sturge-Weber syndrome (25.1%). In contrast, choroidal vessels from a case of retinoblastoma were negative for the mutation (0.5%), as was lung tissue (0.2%). The patient’s choroidal tissue was negative for the 3 GNAQ mutations associated with congenital hemangioma and for the infantile hemangioma marker glucose transporter-1.
Conclusions and Relevance
The results suggest that a more accurate description for choroidal hemangioma in patients with Sturge-Weber syndrome is choroidal capillary malformation. This finding may explain why propranolol, used to treat infantile hemangiomas, has been largely ineffective in patients with choroidal hemangioma. Further studies are needed to corroborate this finding.
This case study investigates the presence of the GNAQ R183Q mutation in the choroidal vessels of a patient with Sturge-Weber syndrome.
Introduction
Capillary malformations (CMs, also known as port-wine stains) are aberrant clusters of capillary-venule–like blood vessels involving the skin, usually in the head and neck area; they occur sporadically in 0.3% of newborns.1 Sturge-Weber syndrome (SWS) is a neurocutaneous disorder with at least 2 of these features: CM affecting the face, the brain leptomeninges, and ocular abnormalities.2 The risk of brain involvement is elevated in infants with hemifacial, bilateral, or forehead CM.3 Half of patients with SWS have ocular involvement, and the most common ocular complication is glaucoma. Glaucoma may arise at different stages of the disease, although its etiology is still unclear. Glaucoma in SWS is associated with choroidal hemangioma in 30% to 70% of patients,4 defined by choroidal vessel overgrowth and thickening. Overabundant small vessels may contribute to increased intraocular pressure and increased risk of glaucoma.2 The high coincidence of CM and choroidal hemangioma and their morphological resemblance (both consist of tortuous capillaries) prompted us to speculate that these 2 conditions have shared origins.
In the literature, there is an assumed similarity between choroidal hemangioma and infantile hemangioma, a common benign vascular tumor that occurs after birth. The frontline medical therapy for infantile hemangioma, propranolol,5 has been tested on 2 choroidal hemangioma cases without noticeable improvement over 6 months.6 This finding suggests that a different molecular mechanism drives choroidal hemangioma.
In 2013, Shirley et al7 identified a somatic activating point mutation in GNAQ in 88% of CM specimens and 92% of brain specimens from patients with SWS, providing a genetic basis for diagnosis. It has since been shown that the activating R183Q mutation is enriched in endothelial cells of CM and SWS lesions.8,9 Different somatic mutations in GNAQ and its homologue GNA11 have been reported in congenital hemangiomas.10 In contrast, to our knowledge, no somatic variants have been definitely associated with infantile hemangioma.
The goal of this study was to investigate choroidal hemangioma in SWS. We hypothesized that choroidal hemangioma in patients with SWS is associated with GNAQ R183Q (c.548G>A), the same mutation found in CMs of most patients with SWS.
Methods
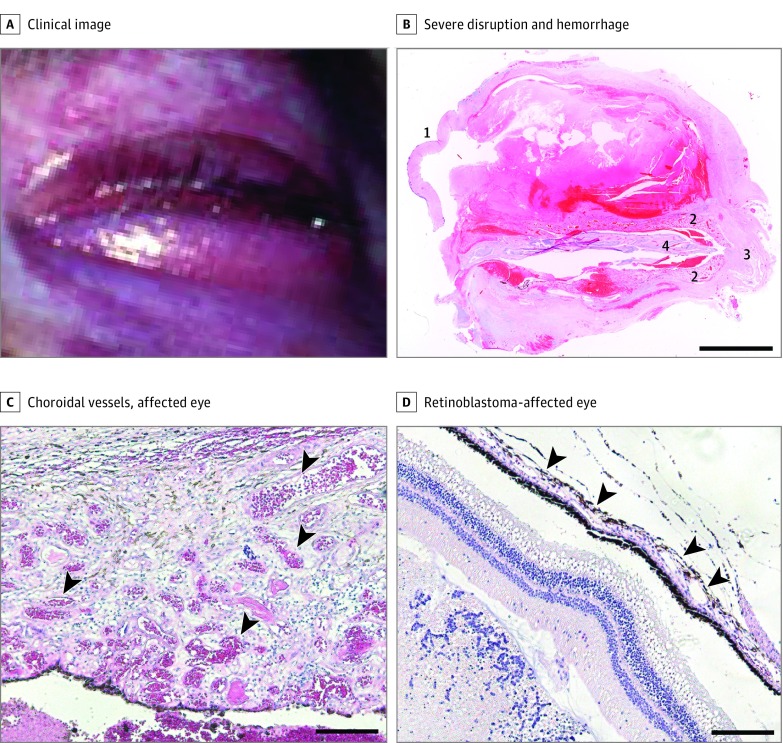
We analyzed an ocular tissue specimen from a patient with SWS, choroidal hemangioma, and severe glaucoma. The Committee on Clinical Investigation at Boston Children’s Hospital approved this study, and patient consent was not obtained owing to an inability to locate the patient’s family. The patient had an extensive CM affecting the face and eyes with leptomeningeal involvement (Figure 1A). The left eye had been surgically removed following severe hemorrhage and retinal detachment. Figure 1B shows the aberrant eye morphology, extensive intraocular hemorrhage, and thickened choroid. Figure 1C and D illustrates the microscopic differences between the patient’s choroidal vessels compared with a specimen affected by a retinoblastoma that exhibits a histologically normal choroid.
Figure 1. Patient With Sturge-Weber Syndrome and Choroidal Capillary Malformation.
A, Clinical image of the patient with choroidal capillary malformation showing diffuse capillary stain affecting the face and eye. B, Hemotoxylin-eosin–stained ocular tissue section showing severe disruption and hemorrhage. Major discernible structures are the cornea (1), the enlarged choroid (2), the optic nerve (3), and retinal tissue (4). Scale bars: 5 mm. C and D, Hemotoxylin-eosin–stained choroidal tissue sections. The affected eye is thickened and shows an abundance of blood-filled choroidal vessels (C, arrowheads) compared with the retinoblastoma-affected eye (D, where choroidal vessels are flat and close to the retinal pigment epithelium [arrowheads]). Scale bars: 200 μm.
Formalin-fixed paraffin-embedded tissue from this patient’s enucleation was processed according to the eMethods in the Supplement. Briefly, choroidal tissue was isolated by laser capture microdissection (eFigures 1 and 2 in the Supplement), and genomic DNA was isolated for droplet digital polymerase chain reaction (PCR).
Results
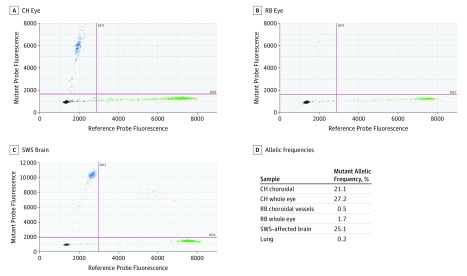
The droplet digital PCR assay revealed that the GNAQ R183Q mutation was present throughout the patient’s eye (Figure 2). The mutant allelic frequency in the patient’s eye (27.2%) was in the same range as brain tissue from a different patient with SWS (25.1%). The choroidal vessels also showed a high mutant allelic frequency (21.1%). In contrast, there were only 5 positive droplets (0.5%) in choroidal vessels from the retinoblastoma-affected eye. The mutant allelic frequency was also low for the DNA isolated from the whole retinoblastoma-affected eye (1.7%) and unrelated lung tissue (0.2%). The few positive droplets in the retinoblastoma and lung tissue samples are likely attributable to deamination events that sporadically occur in formalin-fixed paraffin-embedded tissues and can lead to C:G>T:A base changes, which could produce false-positives.
Figure 2. GNAQ R183Q Allelic Frequency in the Patient’s Choroidal Blood Vessels.
Representative droplet digital polymerase chain reaction (PCR) results of patient whole-eye tissue (A), retinoblastoma-affected whole-eye tissue (B), and Sturge-Weber syndrome (SWS) brain tissue (C). The dot plots show the droplet fluorescence for the wild-type allele (x-axis) and GNAQ R183Q allele (y-axis). D, GNAQ R183Q allelic frequencies determined by droplet digital PCR in choroidal hemangioma (CH), retinoblastoma (RB), SWS-affected brain, and normal lung tissue. Choroidal vessels were isolated with laser-capture microdissection.
Furthermore, we tested for 3 somatic GNAQ mutations associated with congenital hemangioma,10 GNAQ Q209L, Q209P, and Q209H. The patient’s choroidal hemangioma tissue was negative for each of the 3 mutations (eFigure 3 in the Supplement).
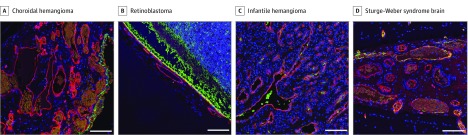
Endothelial cells lining infantile hemangioma vessels as well as normal brain vessels express glucose transporter-1 (GLUT1).11 Immunofluorescent staining of the patient’s tissue sections showed choroidal endothelial cells lacked GLUT1 (Figure 3A; eFigure 4 and eMethods in the Supplement). Choroidal blood vessels from the retinoblastoma-affected eye were also negative for GLUT1 (Figure 3B). In contrast, infantile hemangioma was positive for GLUT1 (Figure 3C). Brain leptomeningeal blood vessels from an SWS and an unaffected brain showed GLUT1-positive endothelial cells (Figure 3D; eFigure 4 in the Supplement), as expected for brain endothelial cells.
Figure 3. Tissue Sections Stained for the Infantile Hemangioma Marker Glucose Transporter-1 (GLUT1).
Formalin-fixed paraffin-embedded sections stained for GLUT1 (green), the endothelial marker Ulex Europaeus Agglutinin I (red), the nuclear stain Hoechst (blue) in the patient’s choroidal vessels (A), retinoblastoma choroidal vessels (B), infantile hemangioma (C), and Sturge-Weber syndrome -affected leptomeninges (D). Scale bars: 100 μm.
Discussion
This case study shows that GNAQ R183Q is present in the choroidal vascular bed of a patient with SWS and choroidal hemangioma. Given that the GNAQ R183Q is established as a causative somatic mutation for capillary malformations,7,8,12 this finding shows that the choroidal vascular malformation is akin to a capillary malformation of the choroidal blood vessels. The malformation is further distinguished from hemangioma insofar as the 3 GNAQ mutations found in some congenital hemangiomas were not detected. Immunofluorescent staining for the infantile hemangioma marker GLUT1 was negative in the patient’s choroidal vascular bed, further indicating that this ocular malformation is distinct from infantile hemangioma. Therefore, we suggest that the term choroidal hemangioma is a misnomer and propose to replace it with the more accurate term of choroidal CM.
Although this is a single-case study, there are indications that choroidal CM could be present in other cases that are diagnosed as choroidal hemangioma and CM/SWS. Indeed, studies show an association between facial CMs involving the upper eyelid and an increased risk to develop glaucoma, which is often indicative of increased intraocular pressure that can result from a choroidal vascular lesion.13 Waelchli et al14 noted that CMs coincide with embryonic vascular branching patterns rather than innervation. In 2016 and 2017, we showed that the CM-driving GNAQ R183Q mutation is enriched in endothelial cells,8,9 further linking the development of CM to the vascular compartment. Therefore, a CM in proximity of the choroid could indicate an increased risk of CM in the choroidal vascular bed. Similarly, children with CMs on the upper forehead have an increased risk of SWS.15
Before drawing definitive conclusions on the etiology of choroidal CM, more specimens should be tested for the GNAQ R183Q mutation. It would be beneficial to study choroidal CMs from patients with and without SWS. Because enucleation is rare, such studies are limited by tissue availability, but they are the only way for researchers to obtain choroidal tissue.
Finally, the findings here may explain why the β-blocker propranolol was largely ineffective in patients with choroidal CM. Propranolol is proposed to target the mitogen-activated kinase (MAPK) pathway that is overactive in proliferating infantile hemangiomas.5 Conversely, only a modest increase in signaling activity has been shown in the MAPK pathway in cells with GNAQ R183Q,7 indicating that different pathways are likely altered in CM. Therefore, propranolol is likely to be ineffective in patients affected by a choroidal capillary malformation. An accurate diagnostic test and the identification of molecular therapeutic targets for CM should improve the patients’ treatment and outcome.
Limitations
A limitation of the study is the rarity of archival choroidal tissue from patients with SWS because enucleation of the eye seldom occurs. A second limitation is the possibility of deamination events in formalin-fixed paraffin-embedded specimens, which could lead to false positives in the droplet digital ddPCR assay.
Conclusions
Our study shows that GNAQ R183Q was present in the choroidal vascular bed of a patient with SWS with severe glaucoma. The presence of the GNAQ R183Q mutation, combined with a lack of distinguishing features for hemangioma, such as GLUT1-positve vessels, indicates the abnormal choroidal vasculature in SWS is akin to CM. We propose the term choroidal CM to replace choroidal hemangioma.
eMethods.
eFigure 1. The Patient’s Choroidal Tissue Before and After Laser-Capture Microdissection
eFigure 2. Macroscopic View of the Patient’s Eye and the Retinoblastoma-Affected Eye
eFigure 3. The Patient’s Ocular Tissue is Negative for GNAQ Q209L, Q209P and Q209H
eFigure 4. Tissue Sections Stained for the Infantile Hemangioma Marker GLUT1
References
- 1.Kanada KN, Merin MR, Munden A, Friedlander SF. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161(2):240-245. doi: 10.1016/j.jpeds.2012.02.052 [DOI] [PubMed] [Google Scholar]
- 2.Mantelli F, Bruscolini A, La Cava M, Abdolrahimzadeh S, Lambiase A. Ocular manifestations of Sturge-Weber syndrome: pathogenesis, diagnosis, and management. Clin Ophthalmol. 2016;10:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zallmann M, Leventer RJ, Mackay MT, Ditchfield M, Bekhor PS, Su JC. Screening for Sturge-Weber syndrome: a state-of-the-art review. Pediatr Dermatol. 2018;35(1):30-42. doi: 10.1111/pde.13304 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 1992;29(6):349-356. [DOI] [PubMed] [Google Scholar]
- 5.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. . A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372(8):735-746. doi: 10.1056/NEJMoa1404710 [DOI] [PubMed] [Google Scholar]
- 6.Krema H, Yousef YA, Durairaj P, Santiago R. Failure of systemic propranolol therapy for choroidal hemangioma of Sturge-Weber syndrome: a report of 2 cases. JAMA Ophthalmol. 2013;131(5):681-683. doi: 10.1001/jamaophthalmol.2013.2879 [DOI] [PubMed] [Google Scholar]
- 7.Shirley MD, Tang H, Gallione CJ, et al. . Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368(21):1971-1979. doi: 10.1056/NEJMoa1213507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couto JA, Huang L, Vivero MP, et al. . Endothelial cells from capillary malformations are enriched for somatic GNAQ mutations. Plast Reconstr Surg. 2016;137(1):77e-82e. doi: 10.1097/PRS.0000000000001868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Couto JA, Pinto A, et al. . Somatic GNAQ mutation is enriched in brain endothelial cells in sturge-weber syndrome. Pediatr Neurol. 2017;67:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayturk UM, Couto JA, Hann S, et al. . Somatic activating mutations in GNAQ and GNA11 are associated with congenital hemangioma. Am J Hum Genet. 2016;98(4):789-795. doi: 10.1016/j.ajhg.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North PE, Waner M, Mizeracki A, Mihm MC Jr. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31(1):11-22. doi: 10.1016/S0046-8177(00)80192-6 [DOI] [PubMed] [Google Scholar]
- 12.Nakashima M, Miyajima M, Sugano H, et al. . The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge-Weber syndrome. J Hum Genet. 2014;59(12):691-693. doi: 10.1038/jhg.2014.95 [DOI] [PubMed] [Google Scholar]
- 13.Enjolras O, Riche MC, Merland JJ. Facial port-wine stains and Sturge-Weber syndrome. Pediatrics. 1985;76(1):48-51. [PubMed] [Google Scholar]
- 14.Waelchli R, Aylett SE, Robinson K, Chong WK, Martinez AE, Kinsler VA. New vascular classification of port-wine stains: improving prediction of Sturge-Weber risk. Br J Dermatol. 2014;171(4):861-867. doi: 10.1111/bjd.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piram M, Lorette G, Sirinelli D, Herbreteau D, Giraudeau B, Maruani A. Sturge-Weber syndrome in patients with facial port-wine stain. Pediatr Dermatol. 2012;29(1):32-37. doi: 10.1111/j.1525-1470.2011.01485.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. The Patient’s Choroidal Tissue Before and After Laser-Capture Microdissection
eFigure 2. Macroscopic View of the Patient’s Eye and the Retinoblastoma-Affected Eye
eFigure 3. The Patient’s Ocular Tissue is Negative for GNAQ Q209L, Q209P and Q209H
eFigure 4. Tissue Sections Stained for the Infantile Hemangioma Marker GLUT1