Abstract
Clinical studies frequently report that patients with major mental illness such as schizophrenia and bipolar disorder have co-morbid physical conditions, suggesting that systemic alterations affecting both brain and peripheral tissues might underlie the disorders. Numerous studies have reported elevated levels of anti-T. gondii antibodies in patients with major mental illnesses, but the underlying mechanism was unclear. Using multidisciplinary epidemiological, cell biological, and gene expression profiling approaches, we report here multiple lines of evidence suggesting that a major mental illness-related susceptibility factor, Disrupted in schizophrenia (DISC1), is involved in altered host immune responses against Toxoplasma gondii (T. gondii) infection. Specifically, our cell biology and gene expression studies have revealed that DISC1 Leu607Phe variation, which changes DISC1 interaction with activating transcription factor 4 (ATF4), modifies gene expression patterns upon T. gondii infection. Our epidemiological data have also shown that DISC1 607 Phe/Phe genotype was associated with higher T. gondii antibody levels in sera. Although further studies are required, our study provides mechanistic insight into one of the few well-replicated serological observations in major mental illness.
Introduction
Mechanism(s) underlying many mental illnesses are poorly understood 1, in contrast to physical disorders, such as diabetes and cancer. Although the biology of mental illnesses tends to be discussed only in the context of brain dysfunction, recent epidemiological studies have indicated shorter lifespans and increased mortality in patients with schizophrenia and bipolar disorder 2. Many factors, such as differences in lifestyle, adverse effects of medication, and unnatural death can influence lifespan and mortality 3; however, beyond these confounding factors, it is possible that intrinsic factors associated with mental illness might underlie both mental and physical susceptibility.
The Disrupted in schizophrenia (DISC1) gene was originally reported in a Scottish pedigree, in which major mental illnesses (e.g., depression and schizophrenia) were clearly segregated with a hereditary chromosomal translocation disrupting this gene 4, 5. Although the genetic association of DISC1 polymorphisms with major mental illness per se is under debate, biological studies clearly demonstrate the significance of DISC1 protein as a “hub” molecule in neurobiology related to mental conditions 5–12. In addition, in the general population, the DISC1 Leu607Phe (rs6675281) and Ser704Cys (rs821616) polymorphisms have been reported to be associated with a wide range of mental conditions, and to influence brain morphology and connectivity 13–22. Recent genetic association studies from the Psychiatry Genomic Consortium (PGC) highlight the link between schizophrenia and genes related to immune function 23. These results suggest that aberrant immune and inflammatory responses may underlie the pathophysiology of schizophrenia and related disorders. Given that the etiology of these mental disorders includes significant influences of environmental stressors 24, intrinsic susceptibility in immune/inflammatory responses to environmental stressors may play a role in the pathophysiology of the disease.
Toxoplasma gondii (T. gondii) is a protozoan parasite that infects about one-third of the human population worldwide 25–29. The parasite’s natural life cycle in the feline definitive host encompasses three stages: oocysts, tachyzoites, and bradyzoites, with the last stage found in tissue cysts 25–29. Although T. gondii infection is usually asymptomatic, it can cause cervical lymphadenopathy and chorioretinitis in immunocompetent individuals 29. Furthermore, in immunocompromised individuals, this infection can result in life-threatening conditions, including encephalitis and pneumonia 26, 29.
Epidemiological and clinical studies have suggested an involvement of infection by pathogenic microorganisms in the pathology of devastating major mental disorders 30–35. In particular, elevated levels of serum antibodies against T. gondii have been reproducibly reported in schizophrenia and bipolar disorder, and some studies show its association with more severe positive psychopathology 30, 33–38.
Host genetic variations play a major role in determining susceptibility to various infectious diseases 39, 40 and thus could explain altered host responses against T. gondii in a specific subgroup of the general population. Nonetheless, the influence of host genetic variations relevant to major mental illness on T. gondii infection has not yet been investigated 41, 42. Our recent study using a DISC1 animal model shows that behavioral abnormalities appear only in the presence of psychosocial isolation stress 43, suggesting that genetic variation at DISC1 might participate in the pathophysiology or biological processes underlying mental illness in the context of host susceptibility to environmental stressors.
In the present study, we hypothesized that variation at DISC1 may underlie the host response to T. gondii infection, possibly associated with major mental illness. We have observed that a DISC1 low frequency variant that alters the encoded DISC1 protein (Leu607Phe) influences host immune responses against T. gondii. Our data also show that anti-T. gondii IgG serum levels are elevated in subjects homozygous for the DISC1 607 Phe/Phe variant in a cohort in which the majority of subjects are Caucasian.
Materials and Methods
Epidemiological study
Two independent cohorts from Baltimore (n=650) and Pittsburgh (n=652) were used in this study (Supplementary Table 1). The Baltimore cohort was recruited from a clinical program affiliated with the Sheppard Pratt Health System and other agencies in central Maryland, with inclusion/exclusion criteria as previously described 44. Participants included individuals with schizophrenia and related (schizophrenia), bipolar disorder, or recent onset psychosis, and individuals with no history of a mental illness. The Pittsburgh cohort was recruited at the University of Pittsburgh as part of a schizophrenia genetics research study; individuals were assessed using semi-structured interviews and consensus diagnoses assigned using DSM-IV criteria as described 45. Participants included individuals with schizophrenia and related (schizophrenia and schizoaffective disorder), major depression, substance abuse/dependence (alcohol, nicotine, cannabis), and those with no diagnosis or condition on Axis II. Sera were collected and stored at −80ºC until tested. Levels of anti-T. gondii IgG and antibodies against other pathogens were measured by enzyme immunoassay (EIA) as previously described 46–49. Genotypes for [DISC1 Leu607Phe (rs6675281), Ser704Cys (rs821616), COMT Val158Met (rs4680), BDNF Val66Met (rs6265), and GRM3 (rs6465084)] were determined TaqMan SNP genotyping assays (Life technologies) as previously reported 50, 51. This study was approved by Institutional Review Boards at Johns Hopkins University.
Cell biology experiments
In vitro T. gondii infection:
Epstein Barr Virus (EBV)-transformed B lymphoblastoid cells were obtained from the cohorts that were reported in previous studies 52, 53 and are independent of the two cohorts (Baltimore and Pittsburgh) used in the current epidemiological study. DISC1 607 Leu/Leu and Phe/Phe genotyping was performed as previously described 51. Individual profiles are provided in Supplementary Table 2. Primary mouse glial cell cultures were prepared from the cortices of postnatal day 3 (P3) pups of C57BL/6 mice as described previously 54–56. Human lymphoblastoid cells (Leu/Leu, n=8; Phe/Phe, n=7) and mouse glial cells (two independent cultures) were infected with tachyzoites of T. gondii strain 2F [RH-2F], which constitutively expresses cytoplasmic β-galactosidase 57, at a multiplicity of infection (MOI) of 1, and then harvested for RNA collection at 48 h. T. gondii tachyzoite growth was determined by CPRG (chlorophenol red-β-D-galactopyranoside) assay as previously reported 57 and described in Supplementary Methods.
Gene expression assay:
Total RNA was purified using RNeasy kit (Qiagen) from lymphoblastoid cells (Leu/Leu, n=3; Phe/Phe, n=3) with or without 48 h infection with T. gondii. Gene expression levels were determined using Illumina Human HT-12 V4 BeadChip arrays (Illumina) at the Microarray Core Facility at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University. Data were extracted using GenomeStutio v1.0 Expression Module and statistical analysis was performed using GeneSpring GX 11.0.2. Because of the small sample size, we used unadjusted p < 0.05 as cut-off for differential gene expression. Gene ontology analysis of overrepresented gene classes was performed with the DAVID (Database for Annotation, Visualization and Integrated Discovery) software (http://david.abcc.ncifcrf.gov). Quantitative real-time PCR was carried out with SYBR GreenER reagent (Invitrogen) on an ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems) following a standard protocol 58, 59. qPCR data were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels. Gene-specific primers are shown in Supplementary Methods.
Statistical analysis
Statistical analysis was performed by two-tailed Student’s t-test, one-way ANOVA, Kruskal-Wallis test, and simple χ2 test. Post-hoc multiple correction was performed with Bonferroni’s method. GraphPad Prism 6 (GraphPad) and R were used for these analyses.
Results
Altered host responses and T. gondii growth in DISC1 Phe607 homozygotes exposed to T. gondii
To address whether DISC1 is involved in immune responses against T. gondii, we studied primary cortical glial cell culture from mice in which the major isoforms of the Disc1 gene are genetically depleted (Disc1 LI homozygous mice) 60. Glial cells were used because they play a critical role in brain immune responses against T. gondii infection 61 and because it has been suggested that chronic infection of Toxoplasma affecting the brain is linked to the development and severity of psychiatric disorders 47, 62, 63. Our data showed that reduced DISC1 levels in primary mouse cortical glial cells resulted in an enhancement of Tnf mRNA induction in response to T. gondii infection (Figure 1A, B). In contrast, Disc1 LI homozygous mice did not show any obvious immune/inflammation-related phenotypes or loss in the subsets of T, B, CD11b+, CD11c+, and NK cells under a resting condition (Supplementary Figure 1).
Figure 1: Increased anti-T. gondii immune response in primary cortical glial cells from mice with loss of function of Disc1 gene.
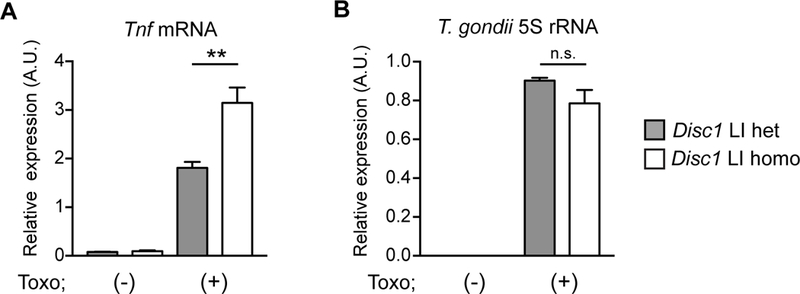
Primary cortical glial cells were prepared from postnatal day 2 (P2) pups of mating pairs of Disc1 LI heterozygous mice. Disc1 LI heterozygous and homozygous pups of the same litters were used for the study. On day 10, glial cells were infected with T. gondii tachyzoites and harvested at 48 h post-infection for qRT-PCR analysis. A. Enhanced induction of Tnf mRNA in Disc1 LI homozygous glial cells compared to Disc1 LI heterozygous glial cells. B. No difference in the amount of intracellular T. gondii between Disc1 LI mice and their heterozygous littermates. T. gondii 5S rRNA levels were measured by qRT-PCR in glial cell lysates to estimate the number of T. gondii. **p<0.01. n.s., not significant. Error bars show s.e.m.
Based on this observation that DISC1 levels altered glial response to T. gondii infection, we investigated whether the DISC1 Leu607Phe low frequency variant might influence human immune responses to T. gondii. This SNP was selected because it is located at the interaction domain of DISC1 with activating transcription factor 4 (ATF4), a transcription factor controlling the expression of genes related to cellular stress and inflammation 64–66. Indeed, our two-hybrid and co-immunoprecipitation experiments revealed that DISC1-ATF4 interaction was attenuated in the presence of 607Phe variation (Figure 2A, B). In addition, over-expression of DISC1 in cultured cells showed that the nuclear localization of DISC1–607Phe was markedly reduced compared with that of DISC1–607Leu (Figure 2C), consistent with findings from the Millar/Porteous lab 67. Thus, together with our previous observation of the role of DISC1 in gene transcription 68, 69, we hypothesized that cellular responses against T. gondii infection are dysregulated in the cells from individuals with DISC1 607Phe/Phe (homozygous for low frequency variants) via ATF4-related mechanisms.
Figure 2: Altered DISC1 function by the 607Phe variation.
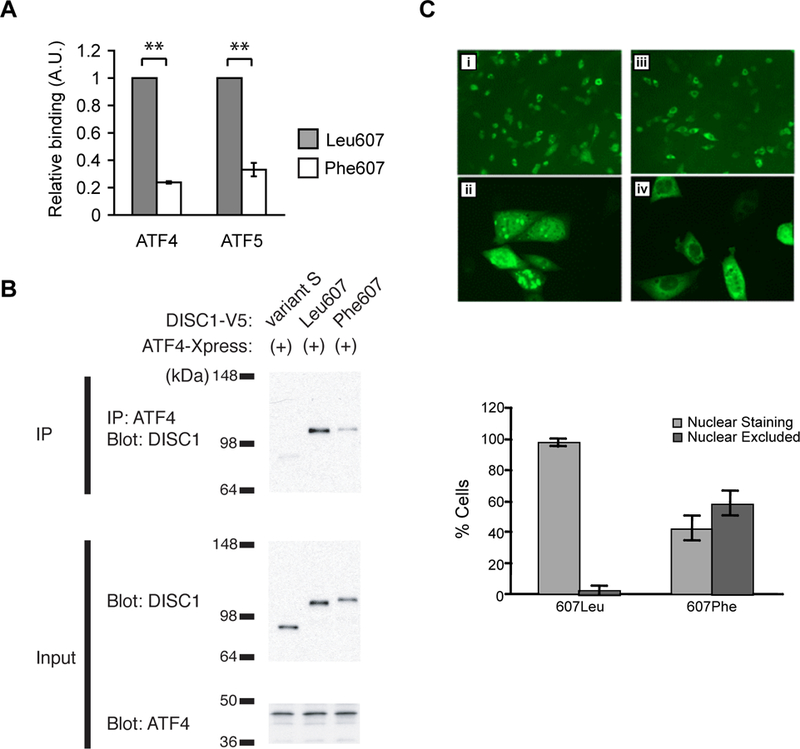
A. Reduced interaction of DISC1 607Phe variant to ATF4 in mammalian two-hybrid assay. CHO cells were transfected with pBIND-DISC1 together with pACT-prey and pGL5luc. Twenty-four hours after transfection, the cells were harvested for luciferase assays. Binding activity of DISC1 to ATF4 and ATF5 was severely impaired by DISC1 607Phe construct compared to that of the 607Leu. Experiments were performed in triplicate and data from four independent transfections were combined for analysis. Error bars show s.e.m. **ATF4, p=8.42×10−12; ATF5, p=1.74×10−7 (one-tailed t-test). B. Decreased binding of ATF4 to DISC1 607Phe in co-immunoprecipitation experiment. COS-7 cells were co-transfected with plasmids expressing V5-tagged DISC1 and ATF4. Twenty to thirty hours later, cell lysates were immunoprecipitated with anti-ATF4 antibody and analyzed by Western blot using anti-V5 antibody. ATF4 did not efficiently precipitate DISC1 607Phe compared to DISC1 607Leu. C. Reduced nuclear distribution of DISC1 protein by the 607Phe variant. CHOk1 cells transfected with the 607Phe variant showed reduced nuclear DISC1 protein (iii and iv) compared to cells transfected with the 607Leu variant (i and ii). Bar graph shows a reduction in the percentage of cells with nuclear DISC1 localization in 607Phe-transfected cells. Error bars show s.e.m. Difference in intracellular localization of DISC1 between 607Leu and 607 Phe variant was significant at p< 2.2×10−16 (Fisher’s Exact test).
We utilized EBV-transformed lymphoblastoid cells as surrogate cells because previous studies successfully used these cells to address the role of genetic variations in immune responses against pathogen infections in humans 70, 71. These lymphoblastoid cells readily expressed DISC1 protein and the genotype did not affect the expression level of DISC1 proteins (Supplementary Figure 2A). We infected lymphoblastoid cells from individuals with DISC1 607 Leu/Leu (homozygous for high frequency variant) or Phe/Phe in vitro with T. gondii tachyzoites for 48 h. As expected, DISC1 607 genotype influenced DISC1-ATF4 protein binding (Supplementary Figure 2B).; DISC1 607 Phe/Phe genotype reduced endogenous DISC1-ATF4 binding. In contrast, T. gondii infection did not cause a marked change in DISC1-ATF4 interaction across the genotypes.
Microarray-based gene expression profiling (n=3 for Leu/Leu, n=3 for Phe/Phe) of T. gondii-infected cells revealed that 38 and 46 genes were changed more than 1.5-fold in DISC1 607 Leu/Leu and Phe/Phe cells respectively, with 71 genes showing differential expression patterns and 13 genes showing similar expression changes between the two genotypes (Figure 3A, B). Notably, 61 genes out of 71 genes that were differentially expressed between DISC1 607 Leu/Leu and Phe/Phe cells by T. gondii infection contained predicted cAMP response element (CRE)-binding in their promoters (Supplementary Table 3). These findings suggest that DISC1 607 variation alters gene expression changes in response to T. gondii infection by modifying ATF4-mediated transcriptional mechanisms through CRE-binding sites in the promoters. Gene ontology analysis revealed several functional gene groups that are uniquely overrepresented in either Leu/Leu or Phe/Phe cells upon infection (Figure 3C). For example, the groups for “regulation of programmed cell death” and “transcription factor binding” were specifically underscored in cells with DISC1 Leu/Leu, whereas the groups for “plasma membrane” and “defense response” were specific to cells with DISC1 Phe/Phe. In contrast, groups for “cytokine-cytokine receptor interaction” and “immune responses” were represented similarly in cells of both genotypes.
Figure 3: Altered gene expression patterns in cells with DISC1 607 Phe/Phe variant during T. gondii infection.

A. Heat map of the mRNAs that were changed more than 1.5-fold upon T. gondii infection in DISC1 607 Leu/Leu cells. B. Heat map of the mRNAs that were changed more than 1.5-fold upon T. gondii infection in DISC1 607 Phe/Phe lymphoblastoid cells. Data were normalized and plotted on a log2 color scale. Welch t-test (unpaired and unequal variance) was used to select differentially expressed genes between two groups with p<0.05. Expression pattern similarity was visualized by hierarchical clustering with Euclidean as distance metric and Centroid as linkage rule. C. Venn diagram indicating overlap of gene expression changes upon T. gondii infection in DISC1 607 Leu/Leu and Phe/Phe lymphoblastoid cells. D. Ontology profiling of genes that are changed upon T. gondii infection in DISC1 607 Leu/Leu and Phe/Phe lymphoblastoid cells. Genes listed in A and B were analyzed using DAVID Bioinformatics Database (http://david.abcc.ncifcrf.gov/).
To overcome the limitations of using microarray with such a small sample size (n=3 per group), we confirmed the findings using quantitative reverse-transcription PCR (qRT-PCR) with a larger number of samples (n=7–8 per group). We observed that the extent of mRNA upregulation for JUNB and BATF3, both of which encode transcription factors known to be involved in the host response to T. gondii infection 72, 73, were significantly smaller in Phe/Phe cells in response to T. gondii infection than in Leu/Leu cells (Figure 4A). The increase of JUNB mRNA induction was significantly higher in Leu/Leu cells than Phe/Phe cells after T. gondii infection. In the case of BATF3 mRNA, there was no significant increase in Phe/Phe cells after T. gondii infection, in contrast to significant increases in Leu/Leu cells. Both of these genes contain CRE-binding sites in their promoter, and therefore represent potential targets of ATF4 regulation (Supplementary Figure 3).
Figure 4: Altered response of DISC1 607 Phe/Phe cells to T. gondii infection.
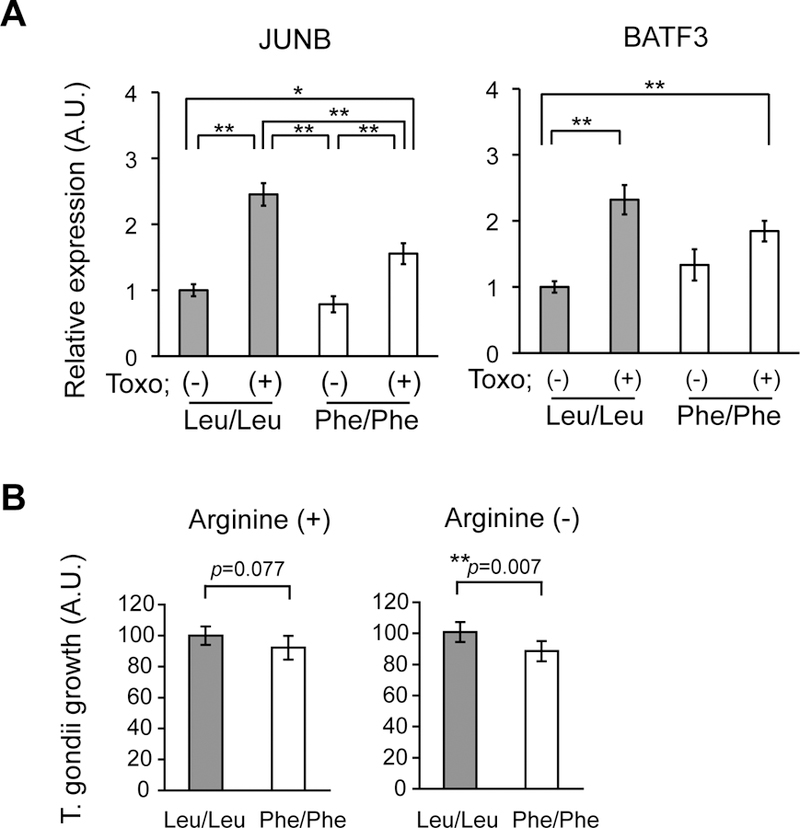
A. qRT-PCR analysis of JUNB and BATF3 mRNA expression before and after T. gondii infection in DISC1 607 Leu/Leu and Phe/Phe lymphoblastoid cells. **p<0.01, *p<0.05. B. Reduced growth of T. gondii tachyzoites in DISC1 607 Phe/Phe lymphoblastoid cells. **p<0.01. Error bars show s.e.m.
To further link DISC1 Leu607Phe variation and altered responses of lymphoblastoid cells to T. gondii infection, we examined the growth of T. gondii tachyzoites after infection of DISC1 607 Leu/Leu or Phe/Phe lymphoblastoid cells. Because T. gondii tachyzoites rely on arginine for their growth 74, limiting arginine availability can enhance host resistance to the parasite and result in lower growth rates and higher conversion into bradyzoites. We therefore tested the growth of T. gondii under both normal and arginine-free conditions. Under normal conditions DISC1 genotype did not significantly alter tachyzoite growth, however, we observed a significant decrease in the growth of T. gondii tachyzoites in DISC1 607 Phe/Phe cells when cells were cultured under arginine-free medium (Figure 4B).
Effects of DISC1 Leu607Phe variation on anti-T. gondii IgG level
We then examined the effects of the DISC1 607Phe variant on the levels of anti-T. gondii IgG in serum in two different cohorts of human subjects. In the first cohort (n=650) (Supplementary Table 1), we found that the 607Phe variant is associated with the levels of anti-T. gondii IgG in serum; individuals homozygous for the DISC1 Phe607 variant (DISC1 607 Phe/Phe) showed a >3-fold elevation in serum anti-T. gondii IgG levels compared to carriers of the DISC1 Leu607 variant (DISC1 607 Leu/Leu and Leu/Phe) (Figures 5A, 5B). Notably, T. gondii IgG levels were associated with DISC1 607 Phe/Phe by logistic regression analysis (p=0.01) adjusting for age, gender, race, and clinical diagnosis (Table 1). We did not observe any influence of the Ser704Cys variation on the serum levels of anti-T. gondii IgG (Figure 5C). We also examined T. gondii seropositivity among these cohorts, but high seropositivity for anti-T. gondii IgG was observed at trend levels both for individuals with the 607Phe and Ser704Cys variants (Figures 5D, E). Variations in the genes coding for catechol-O-methyltransferase (COMT at Val158Met: rs4680), metabotropic glutamate receptor 3 (GRM3: rs6465084), and brain-derived neurotrophic factor (BDNF at Val66Met: rs6265), all of which are relevant to major mental illness 45, 75–78, did not show any association with T. gondii seropositivity (Supplementary Figures 4A-4C). Finally, we found that the association of DISC1 Leu607Phe variation to serum IgG levels is specific to T. gondii, as the same variation did not show any significant differences in serum levels of antibodies against other infectious agents, including cytomegalovirus (CMV), varicella zoster virus (VZV), and herpes simplex virus type1 (HSV-1) (Supplementary Figure 4D-4F).
Figure 5: Levels of anti-T. gondii IgG in serum from individuals with specific DISC1 SNP genotypes.
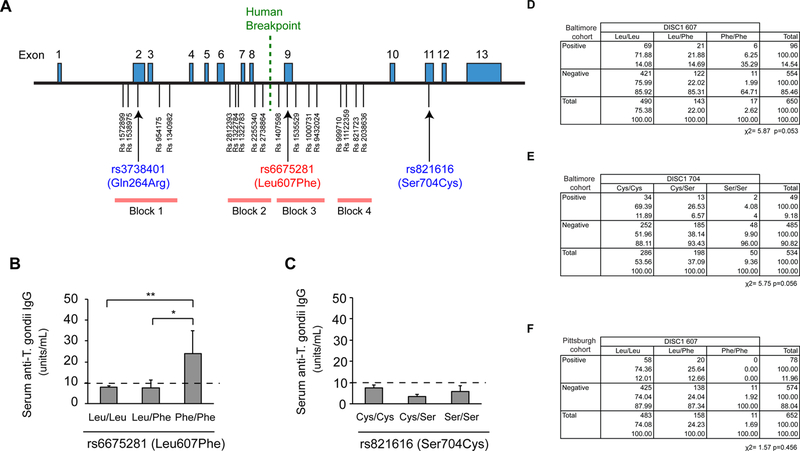
A. Human DISC1 gene and known single nucleotide polymorphisms (SNPs). Human DISC1 gene covering exons 1 to 13 is shown, with the positions of SNPs and the haplotype blocks defined in a previous study 68, 69, 72, 73, 75. B. Increase in serum anti-T. gondii IgG levels in individuals with DISC1 607 Phe/Phe (n=17) compared to those with DISC1 607 Leu/Leu (n=490) or Leu/Phe genotype (n=143). Anti-T. gondii IgG levels in the serum were measured by ELISA. Error bars show s.e.m. *p<0.05, **p<0.01. C. No difference in serum anti-T. gondii IgG level among individuals with DISC1 704 Ser/Ser, Ser/Cys and Cys/Cys. D. Higher T. gondii seropositivity (antibody levels ≥10 units/mL) rate in individuals with DISC1 607 Phe/Phe genotype compared to those with DISC1 607 Leu/Leu. Note that T. gondii IgG levels were well predicted by DISC1 Phe/Phe in multinomial logistic regression analysis with age, gender, race, and disease diagnosis as variables (p=0.010) (Table 1). E. No difference in T. gondii seropositivity (antibody levels ≥10 units/mL) among individuals with DISC1 704 Ser/Ser, Ser/Cys and Cys/Cys. F. T. gondii seropositivity rate in another cohort (n=652). No individuals with DISC1 607 Phe/Phe (n=11) showed increase in serum anti-T. gondii IgG levels.
Table 1:
Multinominal logistic regression analysis for Baltimore sample
| DISC1 607 variant | Variable | Coefficiency | Standard Errors | z-score | P>|z| | [95% Confidence Interval] | |
|---|---|---|---|---|---|---|---|
| Leu/Leu | (base outcome) | ||||||
| Leu/Phe | T. gondii IgG (units) | −0.0007434 | 0.0045311 | −0.16 | 0.870 | 0.0096241 | 0.0081373 |
| Age | 0.0011233 | 0.0083343 | 0.13 | 0.893 | 0.0152116 | 0.0174582 | |
| Gender | 0.1421207 | 0.1986375 | 0.72 | 0.474 | 0.2472018 | 0.5314431 | |
| Race | −0.3015441 | 0.1943543 | −1.55 | 0.121 | 0.6824716 | 0.0793834 | |
| Diagnosis | |||||||
| Schizophrenia | 0.2269713 | 0.2337690 | 0.97 | 0.332 | 0.2312076 | 0.6851503 | |
| Bipolar Disorder | −0.0570001 | 0.2885047 | −0.20 | 0.843 | 0.6224589 | 0.5084587 | |
| Recent Onset Psychosis | 0.0182294 | 0.5322108 | 0.03 | 0.973 | 1.024885 | 1.061343 | |
| Cons | −1.0899000 | 0.5290639 | −2.06 | 0.039 | 2.126847 | −0.0529542 | |
| Phe/Phe | T. gondii IgG (units) | 0.0169319 | 0.0065574 | 2.58 | 0.010 | 0.0040796 | 0.0297842 |
| Age | −0.0166023 | 0.0217365 | −0.76 | 0.445 | 0.0592051 | 0.0260004 | |
| Gender | 0.5696324 | 0.5437728 | 1.05 | 0.295 | 0.4961427 | 1.635408 | |
| Race | −0.5836219 | 0.5036877 | −1.16 | 0.247 | 1.570832 | 0.4035879 | |
| Diagnosis | |||||||
| Schizophrenia | 0.6237704 | 0.5954931 | 1.05 | 0.295 | 0.5433746 | 1.790916 | |
| Bipolar Disorder | −13.89627 | 658.48050 | −0.02 | 0.983 | 1304.494 | 1276.702 | |
| Recent Onset Psychosis | 0.5512231 | 1.1538960 | 0.48 | 0.633 | 1.710371 | 2.812818 | |
| Cons | −3.1783410 | 1.4435280 | −2.20 | 0.028 | 6.007604 | −0.3490788 | |
We examined the DISC1 Leu607Phe variation in an independent sample (n=652) (Supplementary Table 1), but no seropositive subjects among those with DISC1 607 Phe/Phe genotype having T. gondii seropositivity were found in this cohort. Thus, we were unable to evaluate changes in the seropositivity for anti-T. gondii IgG in individuals with DISC1 607 Phe/Phe in the Pittsburgh cohort. Indeed, with the current sample size, the data showed no significance (Figure 5F). Notably, these two cohorts were significantly different in terms of age, sex, race, clinical diagnosis, and T. gondii seropositivity (Supplementary Table 1). In summary, although the data from one cohort clearly showed that the DISC1 607Phe variation is specifically associated with elevated serum anti-T. gondii IgG levels, the lack of T. gondii seropositive individuals with DISC1 607 Phe/Phe genotype in another independent cohort prevented us from conducting confirmation studies.
Discussion
In this study, we have provided evidence that variation at DISC1 can regulate immune responses. Our data suggest that impaired DISC1 function can modify host immune responses against T. gondii infection. To our knowledge, no previous study has addressed the involvement of DISC1 in host-pathogen interactions. Although our findings in cell biology do not directly address either the mechanisms underlying elevated anti-T. gondii IgG or a potential causal role of T. gondii infection in psychiatric disorders, this study provides an important first step to investigate the biological link between psychiatric genetics and epidemiological findings. The current study needs to be followed up by multi-institutional larger studies in the near future.
With respect to mechanisms, the Leu607Phe variation is likely to modify ATF4-mediated gene transcription upon T. gondii infection by altering nuclear targeting of DISC1 and thereby DISC1-ATF4 interaction. Indeed, recent studies provide evidence that DISC1 modulates ATF4-dependent gene transcription 67–69. ATF4 is a key transcription factor that regulates genes carrying CRE binding sites in their promoters and is known to regulate the unfolded protein response (UPR) and, more recently, immune responses 64–66. Thus, DISC1/ATF4-mediated modulation of immune/stress gene expression provides a promising working hypothesis to link DISC1 607 variation and host immune responses to T. gondii infection. Loss of DISC1-ATF4 interaction in cells of DISC1 607 Phe/Phe genotype or Disc1 LI mouse cell culture may result in enhanced innate immune responses, which can decrease T. gondii tachyzoite growth by forcing their conversion into bradyzoites and simultaneously induce strong T and B cell-mediated adaptive immune responses, including higher IgG production. This scenario may be supported by our Disc1 LI mouse cell culture data, gene expression and cell biology findings in DISC1 607 Phe/Phe cells upon T. gondii infection, and epidemiological findings on the association of higher anti-T. gondii IgG levels with DISC1 607 Phe/Phe genotype, but further mechanistic studies are essential to reinforce the hypothesis.
Intriguingly, accumulating evidence suggests that UPR-related molecules are involved in host responses against pathogens 79–81. Although the role of ATF4 in T. gondii infection is not yet known, mice with a genetic deficiency of ATF6β, one of the UPR initiators, showed altered immune responses against T. gondii infection via altered function of dendritic cells 81. This implicated a potential role of ATF4 and UPR in antigen presentation and cytokine production in dendritic cells, macrophages, and B lymphocytes against T. gondii infection. Although DISC1/ATF4-mediated modulation of immune responses is a promising hypothesis, we acknowledge that DISC1 has many binding partners 5 and even the Leu607Phe variation affects its interaction with another protein, FEZ1 68. Despite the paucity of evidence supporting the role of FEZ1 in immune responses, further studies are necessary to determine the immune functions of DISC1 through other binding partners.
DISC1 expression is found in both neurons and glia in the brain and also in peripheral non-neuronal cells, including lymphocytes 82, 83. Therefore, cell populations that are mainly affected by DISC1 607 SNP need to be identified. Although it is beyond the scope of this study, once induced pluripotent stem cells from subjects carrying DISC1 607Phe/Phe and 607Leu/Leu are established, such cells can be further differentiated into neurons and/or glia for further validation of the working hypothesis proposed in the present study.
A limitation of our findings was that only one cohort revealed the effects of DISC1 607 variation on the levels of anti-T. gondii IgG in serum, the other cohort lacking any seropositive DISC1 607 Phe/Phe homozygotes, which prevented replication. These cohorts represent two independent populations with distinct features (Supplementary Table 1), and such differences in demographic background (e.g., gender, race and genetic background, and disease profiles) may underlie the different patterns of association in the results between the two cohorts. Furthermore, the diversity of T. gondii infection in different geographical locations may affect the results, because infection is influenced by lifestyle. Thus, we have estimated the sample size that can overcome the heterogeneity of cohorts. Our power analysis suggests that we need at least 8,091 samples to obtain a significant difference (α=0.05) in the levels of anti-T. gondii IgG between individuals with DISC1 607 Phe/Phe and others (DISC1 607 Leu/Leu and Leu/Phe) with a power of 80%. This number is very large, given that the study requires simultaneous measurement of the levels of proteins in sera and SNP genotypes. We hope that the present study will serve as a platform to design larger comprehensive studies in the near future. Alternatively, by forming multi-institutional consortium for larger sample studies in the near future, we might be able to identify another cohort where sufficient DISC1 607 Phe/Phe individuals with high T. gondii IgG levels are available. The concept of interactions between host and infectious agents for mental illness may be extended not only to T. gondii, but also more generally to other infectious and non-infectious agents such as bacteria, parasites, and intestinal microbiomes. Future studies will also address the link between the levels of anti-T. gondii IgG and disease severity, which requires a larger sample size.
Cell biology and molecular findings of this study are limited by the small sample size and the heterogeneous demographic data of EBV-transformed lymphoblastoid cells from individuals with DISC1 607 Leu/Leu and Phe/Phe genotypes. At this moment, demographically-matched lymphoblastoid cells are not available for both DISC1 607 Leu/Leu and Phe/Phe genotypes. In the current study, many of the cells with DISC1 607 Phe/Phe genotype were derived from individuals with substance abuse. Thus, unclear differences in exposure to substance may have affected our findings. In future studies where a larger cohort will be used, we expect to recruit these cells from individuals with DISC1 607 Phe/Phe genotype and no psychiatric history. Another limitation of cell biology studies is that the data has been compared only between cells with DISC1 Leu/Leu and Phe/Phe genotype. The effects of DISC1 607 variation on anti-T. gondii immune responses may be recessive at least in our epidemiology data (Figure 5B). Analysis of EBV-transformed lymphoblastoid cells from individuals with DISC1 607 Leu/Phe on matched demographic information would be important to address this points in the future. Our study is also limited in that we could only examine primary infection of T. gondii. Clinical findings related to psychiatric disorders have suggested that either chronic infection or reactivation of a latent, quiescent infection plays a role in brain dysfunction and behavioral changes 47, 62, 63. Currently, cell culture models cannot recapitulate these important biological phenomena. Recent advances in human brain organoids 84–86 may be useful in addressing these more clinically related questions in future studies.
Recent clinico-epidemiological studies indicate a high rate of comorbid physical conditions (e.g., autoimmune diseases and metabolic disorders) as well as shorter lifespans and increased mortality in patients with schizophrenia and bipolar disorder 66, 67. Here we have provided a strategy to address these issues by utilizing human cells from genetically defined populations in the context of gene and environmental interactions. This approach may open a window for further validation of the concept that schizophrenia and bipolar disorder are systemic disorders involving immune alterations. This novel concept, if true, would dramatically change future strategies for drug discovery and help overcome the social stigma that accompanies mental illness.
Supplementary Material
Acknowledgements
We thank Dr. Wayne Yu and microarray facility at Sidney Kimmel Comprehensive Cancer Center (Johns Hopkins University) for microarray experiments; Drs. J-C Xiao, Teppei Tanaka, Minori Koga for technical advice; Indigo VL Rose, Pamela Talalay, Nao Gamo, and Noah Elkins for critically reading the manuscript. This work was supported by U.S. Public Health Service Grants MH-069853 (A.S.), Silvio O. Conte Center grant MH-094268 (A.S.), MH-105660 (A.S.), MH-107730 (A.S.), K99/R00MH-093458 (S.K.); by foundation grants from Stanley Medical Research Institute (A.S., F.D.), S-R (A.S.), RUSK (A.S.), NARSAD (A.S., S.K.), JSPS (S.K.); by the Hammerschlag family (S.K.).
Footnotes
Conflict of Interests
There are no competing financial interests in relation to the current work.
References
- 1.Insel TR. Rethinking schizophrenia. Nature 2010; 468(7321): 187–193. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PM et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry 2005; 66(2): 183–194; quiz 147, 273–184. [DOI] [PubMed] [Google Scholar]
- 3.Montejo AL. The need for routine physical health care in schizophrenia. Eur Psychiatry 2010; 25 Suppl 2: S3–5. [DOI] [PubMed] [Google Scholar]
- 4.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 2000; 9(9): 1415–1423. [DOI] [PubMed] [Google Scholar]
- 5.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci 2011; 12(12): 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathieson I, Munafo MR, Flint J. Meta-analysis indicates that common variants at the DISC1 locus are not associated with schizophrenia. Mol Psychiatry 2012; 17(6): 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PF. Questions about DISC1 as a genetic risk factor for schizophrenia. Mol Psychiatry 2013; 18(10): 1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porteous DJ, Thomson PA, Millar JK, Evans KL, Hennah W, Soares DC et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry 2014; 19(2): 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O’Donovan MC et al. Evaluating historical candidate genes for schizophrenia. Mol Psychiatry 2015; 20(5): 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su P, Li S, Chen S, Lipina TV, Wang M, Lai TK et al. A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron 2014; 84(6): 1302–1316. [DOI] [PubMed] [Google Scholar]
- 11.Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ et al. Disc1 point mutations in mice affect development of the cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011; 31(9): 3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AH, Josselyn SA. Caution When Diagnosing Your Mouse With Schizophrenia: The Use and Misuse of Model Animals for Understanding Psychiatric Disorders. Biological psychiatry 2016; 79(1): 32–38. [DOI] [PubMed] [Google Scholar]
- 13.Di Giorgio A, Blasi G, Sambataro F, Rampino A, Papazacharias A, Gambi F et al. Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur J Neurosci 2008; 28(10): 2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprooten E, Sussmann JE, Moorhead TW, Whalley HC, Ffrench-Constant C, Blumberg HP et al. Association of white matter integrity with genetic variation in an exonic DISC1 SNP. Mol Psychiatry 2011; 16(7): 685, 688–689. [DOI] [PubMed] [Google Scholar]
- 15.Raznahan A, Lee Y, Long R, Greenstein D, Clasen L, Addington A et al. Common functional polymorphisms of DISC1 and cortical maturation in typically developing children and adolescents. Mol Psychiatry 2011; 16(9): 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carless MA, Glahn DC, Johnson MP, Curran JE, Bozaoglu K, Dyer TD et al. Impact of DISC1 variation on neuroanatomical and neurocognitive phenotypes. Mol Psychiatry 2011; 16(11): 1096–1104, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomppo L, Hennah W, Miettunen J, Jarvelin MR, Veijola J, Ripatti S et al. Association of variants in DISC1 with psychosis-related traits in a large population cohort. Arch Gen Psychiatry 2009; 66(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A 2005; 102(24): 8627–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry 2005; 62(11): 1205–1213. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Liu B, Hou B, Qin W, Wang D, Yu C et al. Less Efficient Information Transfer in Cys-Allele Carriers of DISC1: A Brain Network Study Based on Diffusion MRI. Cereb Cortex 2012. [DOI] [PubMed]
- 21.Eastwood SL, Walker M, Hyde TM, Kleinman JE, Harrison PJ. The DISC1 Ser704Cys substitution affects centrosomal localization of its binding partner PCM1 in glia in human brain. Hum Mol Genet 2010; 19(12): 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eastwood SL, Hodgkinson CA, Harrison PJ. DISC-1 Leu607Phe alleles differentially affect centrosomal PCM1 localization and neurotransmitter release. Mol Psychiatry 2009; 14(6): 556–557. [DOI] [PubMed] [Google Scholar]
- 23.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511(7510): 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010; 468(7321): 203–212. [DOI] [PubMed] [Google Scholar]
- 25.Boothroyd JC. Toxoplasma gondii: 25 years and 25 major advances for the field. Int J Parasitol 2009; 39(8): 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter CA, Remington JS. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis 1994; 170(5): 1057–1067. [DOI] [PubMed] [Google Scholar]
- 27.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol 2012; 10(11): 766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laliberte J, Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci 2008; 65(12): 1900–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363(9425): 1965–1976. [DOI] [PubMed] [Google Scholar]
- 30.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167(3): 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 2011; 168(12): 1303–1310. [DOI] [PubMed] [Google Scholar]
- 32.Prasad KM, Watson AM, Dickerson FB, Yolken RH, Nimgaonkar VL. Exposure to herpes simplex virus type 1 and cognitive impairments in individuals with schizophrenia. Schizophr Bull 2012; 38(6): 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tedla Y, Shibre T, Ali O, Tadele G, Woldeamanuel Y, Asrat D et al. Serum antibodies to Toxoplasma gondii and Herpesvidae family viruses in individuals with schizophrenia and bipolar disorder: a case-control study. Ethiop Med J 2011; 49(3): 211–220. [PubMed] [Google Scholar]
- 34.Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biological psychiatry 2012; 72(4): 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamdani N, Daban-Huard C, Lajnef M, Richard JR, Delavest M, Godin O et al. Relationship between Toxoplasma gondii infection and bipolar disorder in a French sample. J Affect Disord 2013; 148(2–3): 444–448. [DOI] [PubMed] [Google Scholar]
- 36.Holub D, Flegr J, Dragomirecka E, Rodriguez M, Preiss M, Novak T et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand 2013; 127(3): 227–238. [DOI] [PubMed] [Google Scholar]
- 37.Torrey EF, Yolken RH. Schizophrenia and toxoplasmosis. Schizophr Bull 2007; 33(3): 727–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 2007; 33(3): 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alcais A, Abel L, Casanova JL. Human genetics of infectious diseases: between proof of principle and paradigm. J Clin Invest 2009; 119(9): 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vannberg FO, Chapman SJ, Hill AV. Human genetic susceptibility to intracellular pathogens. Immunol Rev 2011; 240(1): 105–116. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Wong SY, Grumet FC, Fessel J, Montoya JG, Zolopa AR et al. Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. J Infect Dis 1996; 173(1): 265–268. [DOI] [PubMed] [Google Scholar]
- 42.Jamieson SE, de Roubaix LA, Cortina-Borja M, Tan HK, Mui EJ, Cordell HJ et al. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS One 2008; 3(6): e2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 2013; 339(6117): 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickerson F, Boronow JJ, Stallings C, Origoni AE, Cole SK, Yolken RH. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res 2004; 129(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 45.Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H et al. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet 2008; 17(5): 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight BC, Kissane S, Falciani F, Salmon M, Stanford MR, Wallace GR. Expression analysis of immune response genes of Muller cells infected with Toxoplasma gondii. J Neuroimmunol 2006; 179(1–2): 126–131. [DOI] [PubMed] [Google Scholar]
- 47.Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr Bull 2007; 33(3): 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am J Psychiatry 2008; 165(1): 99–106. [DOI] [PubMed] [Google Scholar]
- 49.Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry 2003; 60(5): 466–472. [DOI] [PubMed] [Google Scholar]
- 50.Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Cole S, Leister F et al. The catechol O-methyltransferase Val158Met polymorphism and herpes simplex virus type 1 infection are risk factors for cognitive impairment in bipolar disorder: additive gene-environmental effects in a complex human psychiatric disorder. Bipolar disorders 2006; 8(2): 124–132. [DOI] [PubMed] [Google Scholar]
- 51.Rastogi A, Zai C, Likhodi O, Kennedy JL, Wong AH. Genetic association and post-mortem brain mRNA analysis of DISC1 and related genes in schizophrenia. Schizophr Res 2009; 114(1–3): 39–49. [DOI] [PubMed] [Google Scholar]
- 52.Horiuchi Y, Kano S, Ishizuka K, Cascella NG, Ishii S, Talbot CC Jr., et al. Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: utility and limitation of the surrogate tissues in research for brain disorders. Neurosci Res 2013; 77(4): 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL et al. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry 1998; 55(11): 989–994. [DOI] [PubMed] [Google Scholar]
- 54.de Vellis J, Cole R. Preparation of mixed glial cultures from postnatal rat brain. Methods Mol Biol 2012; 814: 49–59. [DOI] [PubMed] [Google Scholar]
- 55.Lee JK, Tansey MG. Microglia isolation from adult mouse brain. Methods Mol Biol 2013; 1041: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozeki Y, Pickard BS, Kano S, Malloy MP, Zeledon M, Sun DQ et al. A novel balanced chromosomal translocation found in subjects with schizophrenia and schizotypal personality disorder: altered l-serine level associated with disruption of PSAT1 gene expression. Neurosci Res 2011; 69(2): 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res 2003; 62(3): 237–244. [DOI] [PubMed] [Google Scholar]
- 58.Kano S, Sato K, Morishita Y, Vollstedt S, Kim S, Bishop K et al. The contribution of transcription factor IRF1 to the interferon-gamma-interleukin 12 signaling axis and TH1 versus TH-17 differentiation of CD4+ T cells. Nat Immunol 2008; 9(1): 34–41. [DOI] [PubMed] [Google Scholar]
- 59.Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry 2013; 18(7): 740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shahani N, Seshadri S, Jaaro-Peled H, Ishizuka K, Hirota-Tsuyada Y, Wang Q et al. DISC1 regulates trafficking and processing of APP and Abeta generation. Mol Psychiatry 2014. [DOI] [PMC free article] [PubMed]
- 61.Blanchard N, Dunay IR, Schluter D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol 2015; 37(3): 150–158. [DOI] [PubMed] [Google Scholar]
- 62.Flegr J Effects of toxoplasma on human behavior. Schizophr Bull 2007; 33(3): 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 2007; 33(3): 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang Q, Deng H, Sun CW, Townes TM, Zhu F. Negative regulation of IRF7 activation by activating transcription factor 4 suggests a cross-regulation between the IFN responses and the cellular integrated stress responses. J Immunol 2011; 186(2): 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8(7): 519–529. [DOI] [PubMed] [Google Scholar]
- 66.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 2009; 11(12): 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malavasi EL, Ogawa F, Porteous DJ, Millar JK. DISC1 variants 37W and 607F disrupt its nuclear targeting and regulatory role in ATF4-mediated transcription. Hum Mol Genet 2012; 21(12): 2779–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry 2008; 13(12): 1138–1148, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soda T, Frank C, Ishizuka K, Baccarella A, Park Y, Flood Z et al. DISC1-ATF4 transcriptional repression complex: dual regulation of the cAMP-PDE4 cascade by DISC1. Molecular Psychiatry 2013; In press. [DOI] [PMC free article] [PubMed]
- 70.Bogunovic D, Byun M, Durfee LA, Abhyankar A, Sanal O, Mansouri D et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science 2012; 337(6102): 1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med 2015; 212(10): 1641–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem 2001; 276(26): 24223–24231. [DOI] [PubMed] [Google Scholar]
- 73.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS et al. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 2011; 35(2): 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox BA, Gigley JP, Bzik DJ. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol 2004; 34(3): 323–331. [DOI] [PubMed] [Google Scholar]
- 75.Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A 2004; 101(34): 12604–12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012; 37(1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry 2007; 164(12): 1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological psychiatry 2006; 60(2): 141–151. [DOI] [PubMed] [Google Scholar]
- 79.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 2010; 11(5): 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 2010; 463(7284): 1092–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E et al. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med 2011; 208(7): 1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seshadri S, Kamiya A, Yokota Y, Prikulis I, Kano S, Hayashi-Takagi A et al. Disrupted-in-Schizophrenia-1 expression is regulated by beta-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc Natl Acad Sci U S A 2010; 107(12): 5622–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005; 310(5751): 1187–1191. [DOI] [PubMed] [Google Scholar]
- 84.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME et al. Cerebral organoids model human brain development and microcephaly. Nature 2013; 501(7467): 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017; 545(7652): 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017; 545(7652): 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


