Abstract
Since the discovery of the role of proprotein convertase subtilisin kexin 9 (PCSK9) in the regulation of low-density lipoprotein cholesterol (LDL-C) in 2003, a paradigm shift in the treatment of hypercholesterolaemia has occurred. The PCSK9 secreted into the circulation is a major downregulator of the low-density lipoprotein receptor (LDLR) protein, as it chaperones it to endosomes/lysosomes for degradation. Humans with loss-of-function of PCSK9 exhibit exceedingly low levels of LDL-C and are protected from atherosclerosis. As a consequence, innovative strategies to modulate the levels of PCSK9 have been developed. Since 2015 inhibitory monoclonal antibodies (evolocumab and alirocumab) are commercially available. When subcutaneously injected every 2–4 weeks, they trigger a ∼60% LDL-C lowering and a 15% reduction in the risk of cardiovascular events. Another promising approach consists of a liver-targetable specific PCSK9 siRNA which results in ∼50–60% LDL-C lowering that lasts up to 6 months (Phases II–III clinical trials). Other strategies under consideration include: (i) antibodies targeting the C-terminal domain of PCSK9, thereby inhibiting the trafficking of PCSK9-LDLR to lysosomes; (ii) small molecules that either prevent PCSK9 binding to the LDLR, its trafficking to lysosomes or its secretion from cells; (iii) complete silencing of PCSK9 by CRISPR-Cas9 strategies; (iv) PCSK9 vaccines that inhibit the activity of circulating PCSK9. Time will tell whether other strategies can be as potent and safe as monoclonal antibodies to lower LDL-C levels.
Keywords: PCSK9, LDL-C, Monoclonal antibodies, Gene silencing
1. PCSK9 biology
Proprotein convertase subtilisin kexin 9 (PCSK9) was discovered in 2003 as the last and unique inactive member of the family of subtilisin/kexin-like serine proteases.1 In the same year, two gain-of-function (GOF) mutations in its gene (S127R and F216L) were associated with autosomal dominant hypercholesterolaemia2 and, a few months later, its expression was shown to be downregulated by cholesterol in mice.3,4 Although PCSK9 and low-density lipoprotein receptor (LDLR) mRNA levels were co-regulated by cholesterol, Maxwell et al.5 established in 2004 the capacity of PCSK9 to trigger hepatic LDLR degradation, thus revealing a new level of regulation of hepatic LDLR levels. Subsequent studies confirmed and extended these data.6,7
Another breakthrough was the discovery in 2005 of loss-of-function (LOF) mutations in individuals with lifelong low levels of low-density lipoprotein cholesterol (LDL-C)8 and reduced risk of coronary heart disease,9 thereby making PCSK9 an attractive therapeutic target to reduce LDL-C levels.10 PCSK9 is highly expressed in the liver1 and secreted in the plasma. Following the binding of circulating PCSK9 to the EGF-A domain of the LDLR,11–13 the complex is internalized and the LDLR is targeted to lysosomes for degradation,14,15 thus resulting in a reduced LDLR expression on the hepatic cell surface, a reduced uptake of LDL particles from the blood, and a consequent rise in circulating LDL-C.16,17 The most deleterious GOF mutation D374Y,2,18 and others19,20 are characterized by the early occurrence of cardiovascular events. These findings led to the development of monoclonal antibodies targeting circulating PCSK9, which reduce LDL-C levels by ∼60% and substantially improve cardiovascular outcomes in a variety of high-risk patients.21–24
In addition to monoclonal antibodies,25 other strategies have been developed to target PCSK9, including the possibility to silence its mRNA expression, inhibit its mRNA translation, block the autocatalytic processing of proPCSK9 and alter the interaction between PCSK9 and the LDLR (Figure 1).26 Moreover, a vaccine strategy is under evaluation. The aim of this review is to first summarize the ongoing strategies targeting PCSK9 and, second, to discuss other promising innovative strategies.
Figure 1.
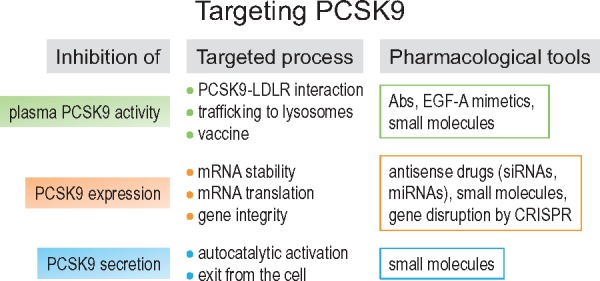
Strategies targeting PCSK9. PCSK9 activity can be inhibited at several levels. To date, only the PCSK9-LDLR interaction and PCSK9 mRNA stability are successfully targeted in humans with injectable mAbs (alirocumab, evolocumab) and siRNA (inclisiran), respectively.
2. Inhibition of the PCSK9-LDLR interaction by monoclonal antibodies
Anti-PCSK9 monoclonal antibodies (mAbs; alirocumab and evolocumab) represent the first pharmacological approach developed to target PCSK9 (Figure 2) and, so far, the only treatment approved by the regulatory agencies in many countries.
Figure 2.
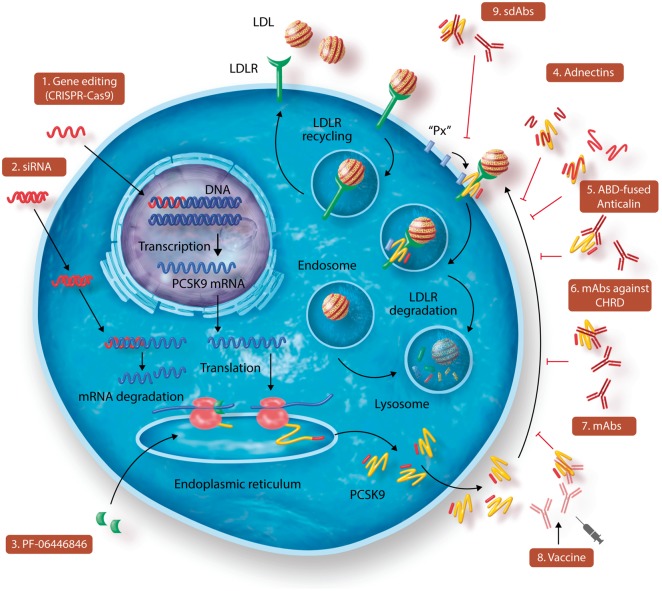
PCSK9 function and potential targets for inhibition. Following transcription and translation, PCSK9 is processed in the endoplasmic reticulum into the mature form and then secreted. In the absence of PCSK9, LDLR binds to circulating low-density lipoprotein (LDL) particle and the complex LDLR/LDL is internalized in the endosomes; LDL are shuttled in the lysosome for degradation while LDLR is recycled to the cell surface. When PCSK9 is present, it binds and escorts the LDLR/LDL complex for degradation in the lysosomes, with the net effect of reducing the number of LDLR on cell surface. PCSK9 can be inhibited at different levels including DNA gene editing (1); mRNA gene silencing (2); mRNA translational inhibition (3). In addition molecules targeting circulating PCSK9 are available or under development, these include adnectins (4), ABD-fused Anticalin (5), or selective antibodies (6–9) which, by binding PCSK9 prevent its interaction with LDLR (6–8) or the interaction of a hypothetical ‘Px’ protein to the complex LDLR-PCSK9 (9).
Following the positive results from Phase I and Phase II clinical trials,27 the efficacy and safety of two anti-PCSK9 mAbs have been evaluated in two major programs (PROFICIO for evolocumab and ODYSSEY for alirocumab) including several Phase III trials. These studies have evaluated the effects of inhibitory mAbs to PCSK9 either as monotherapy or in combination with other lipid-lowering drugs across a broad patient population, including high cardiovascular risk patients, patients with heterozygous familial hypercholesterolaemia (HeFH) who cannot reach the recommended LDL-C levels with current lipid-lowering therapies, as well as patients intolerant to statins. All these studies have indeed demonstrated that the inhibition of PCSK9 by the use of mAbs is overall safe; indeed, on top of maximally tolerated doses of statins, LDL-C levels are dramatically and significantly reduced (mean reduction: ∼60%) (Table 1). In addition, the treatment with mAbs to PCSK9 is associated with significant reductions of Lp(a) levels by about 20–30%38–40; the clinical relevance of this effect, however, is still uncertain.41 Finally, the addition of an anti-PCSK9 mAb to statin therapy results in the regression of coronary atherosclerosis42 without affecting plaque composition,43 with a continuous linear relation between achieved LDL-C levels and plaque regression. Although this study was not powered to assess clinical outcomes, a ∼20% relative and ∼3% absolute risk reduction for the first major adverse coronary event were observed.42 Based on the results of these clinical trials, the two mAbs evolocumab (Repatha; https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf) and alirocumab (Praluent; https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf) have been approved by the US FDA for subjects with HeFH or clinical atherosclerotic cardiovascular disease (ASCVD) under maximally tolerated statin doses and who still require additional lowering of LDL-C. Furthermore, evolocumab has received approval for use in homozygous FH. Similar indications were adopted by the European Medicines Agency (EMA) for the prescription of evolocumab (http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/003766/WC500246329.pdf) and alirocumab (http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/07/WC500190458.pdf). Outcome trials showed for evolocumab (FOURIER)28 and alirocumab (ODYSSEY OUTCOMES)24 that the reduction in plasma cholesterol levels translates into a significant reduction in the incidence of cardiovascular events (−15%). Based on the results of the FOURIER trial,28 evolocumab has been approved also for the treatment of adults with established cardiovascular disease to reduce the risk of myocardial infarction, stroke, and coronary revascularization.
Table 1.
LDL-C reduction through different PCSK9 inhibition approaches
| Type of inhibition | LDL-C reduction (%) | Relative CV reduction | Status | References |
|---|---|---|---|---|
| mAbs targeting circulating PCSK9 (evolocumab and alirocumab) | 55–60% | 15–20% | Approved by FDA and EMA | 24 , 28 |
| Gene silencing (siRNA and inclisiran) | 30–50% | Under evaluation in ORION-4 | Phase III | 29 , 30 |
| Gene editing (CRISPR-Cas9) | ∼30% (TC) | – | Preclinical | 31 |
| Inhibition of PCSK9 mRNA translation (PF-06446846) | ∼58% | – | Preclinical (halted) | 32 |
| Adnectins | ∼50% | – | Preclinical (halted) | 33 |
| ABD-fused Anticalin | ∼50–60% | – | Preclinical | 34 |
| mAb against PCSK9 CHRD | ∼40% | – | Preclinical | 35 |
| Single domain antibodies (sdAbs) | ∼50% | – | Preclinical | 36 , 37 |
| Vaccine | 13.3% | – | Phase I | Bauer et al., ESC Congress, 2018 Munich |
The clinical relevance of anti-PCSK9 mAbs administered as self-injection monthly or biweekly44 may be also related to a higher adherence to treatment compared with a daily orally administered statin. The adherence to statin therapy may be negatively affected by muscle symptoms,45 often leading to therapy discontinuation and consequent increase of cardiovascular risk. In addition, the variability in LDL-C levels during lipid-lowering treatment is a strong and independent predictor of cardiovascular events.46 One- or two-year-long treatment with evolocumab47,48 or alirocumab,49 respectively, provides a sustained LDL-C lowering, although LDL-C level variability appears to be lower with every-2-week dosing than with every-4-week dosing.50,51 A recent analysis of patients with statin-associated muscle symptoms and treated with evolocumab for up to 2 years showed that evolocumab provided persistent tolerability, adherence, safety, and efficacy in statin-intolerant patients.52. Of note, the treatment with mAbs targets circulating PCSK9, which exclusively originates from liver53,54 without inhibiting intracellular PCSK9 in liver or other tissues. This might increase PCSK9 production in the liver as well as maintain PCSK9 intracellular activities which contribute to other processes.1,10,55
The therapeutic safety of anti-PCSK9 mAbs has been evaluated in several trials. Overall, adverse event rates did not differ between subjects treated with mAbs or control, which is reassuring in terms of the safety of very low LDL-C levels reached with these drugs. Creatine kinase and liver enzyme increases were infrequent and comparable between groups, and no drug-induced liver injury or renal impairment were observed.56,57 A recent meta-analysis that investigated the effect of mAbs to PCSK9 on glycaemia and new-onset diabetes did not uncover drug-related changes in these parameters, independently of the mAb type, the characteristics of patients or treatment duration.58 In addition, no evidence of side effects including neurocognitive adverse events were found to be associated with alirocumab or evolocumab treatment,59,60 even in subjects who achieved very low levels of LDL-C. Noteworthy, no evidence for an increased risk of muscle-related adverse events was reported, despite the inclusion of statin-intolerant patients who experienced myalgia during statin therapy.22 Finally, meta-analyses of various outcome trials revealed that the cost–benefit ratio for the use of PCSK9 mAbs together with statins over more than 2-years is highest for patients with baseline levels of LDL-C higher than 100–130 mg/dL.61,62
3. PCSK9 gene silencing and CRISPR editing
Gene silencing is a physiological post-transcriptional process by which cells regulate gene expression via turning off a selected gene. This approach has been translated into selective pharmacological targeting with the development of small interfering RNA (siRNA) controlling the expression of specific genes playing key roles under different physiopathological conditions, including those involved in lipid and lipoprotein metabolism.63
Inclisiran is a double strand siRNA of the latest generation (Figure 2), with a specific chemistry designed to increase its half-life29 and a covalent linkage to a ligand containing three molecules of N-acetylgalactosamine (GalNAc). The latter confers liver specificity to inclisiran by binding the ASGR1/ASGR2 receptors, essentially localized at the hepatocyte surface.64
Results from Phase I study in healthy volunteers with LDL-C ≥ 100 mg/dL showed that a single inclisiran dose of 300 mg injected subcutaneously reduced PCSK9 levels by ∼75% and LDL-C levels by 50% on average for 6 months.29
The ORION-1 Phase 2 trial further explored the impact of single dose (200, 300, or 500 mg) or two doses (100, 200, or 300 mg) at Days 1 and 90.30 The two-dose regimen of inclisiran, 300 mg each, achieved a sustained 52.6% LDL-C reduction similar to that achieved by mAbs for 6 months (Table 1),30 and the reduction was independent of the diabetic status presence.65 Interestingly, although the siRNA inclisiran is expected to also reduce the intracellular levels of PCSK9, the generated drops in apoB, non-HDL-C, VLDL-C, and triglycerides were comparable to those obtained with mAbs, suggesting a limited impact of liver intracellular PCSK9 in cholesterol metabolism. Lp(a) levels were reduced by 26%, although not significantly, due to large inter-individual variations.66. A clinical Phase 3 trial is ongoing to evaluate the effects of inclisiran in hypercholesteraemic and heterozygous FH subjects, while the ORION-4 trial will examine clinical outcomes at 6 years or more.67 Time will tell whether the reduction of both circulating and intracellular PCSK9 generates unexpected/unwanted effects. So far, inclisiran was shown to lack side effects.29
Another approach is the delivery of CRISPR-Cas9 to the liver for the in vivo base editing of PCSK931,68,69 (Figure 2) as an alternative to siRNA therapy in liver. So far, the results in animal models revealed a ∼30% reduction in plasma cholesterol levels (Table 1), with no apparent evidence of off-target mutagenesis.31 More recently, as a proof-of-concept for treating genetic diseases before birth, CRISPR-mediated gene editing in utero resulted in adult mice expressing a W159X LOF-PCSK9 that exhibit a substantial reduction in serum cholesterol.70 Additional studies are needed to critically evaluate whether this approach might be translated into the clinic.
4. Inhibition of PCSK9 mRNA translation
A recent report reveals that translational inhibition of PCSK9 mRNA may represent an attractive approach to block PCSK9 synthesis71 (Figure 2). An orally active compound (PF-06446846) efficiently interrupted PCSK9 translation around codon 34, within the Leu stretch of the signal peptide coding region32. Although leucine/hydrophobic stretches are not known to cause translation stalls, the string of 9 to 11 CUG leucine codons72–74 present in the signal peptide coding region of PCSK9 was likely recognized by this inhibitor.32 Unfortunately, even though such translational inhibitors were optimized,75 the lack of PCSK9 specificity halted further development of this approach.
Another potential approach is represented by microRNA mimetics. Recently, miR-191, miR-222, and miR-224 were shown to post-transcriptionally down-regulate the levels of PCSK9 mRNA.76 However, it needs to be taken into account that these miRNAs are not PCSK9-specific, as exemplified by the ability of miR-222 to also down-regulate the expression of CD4 receptor.77
5. Targeting the autocatalytic processing of proPCSK9
An additional strategy to target PCSK9 is to interfere with the autocatalytic processing of proPCSK9 into PCSK9. This is based on the original observation that PCSK9 can exit the endoplasmic reticulum (ER) only following the autocatalytic cleavage of the zymogen proPCSK9 and the generation of a heterodimer of mature PCSK9 with its prodomain which remains non-covalently bound to the catalytic domain.1 Natural78,79 and engineered6,80,81 PCSK9 mutants were found unable to undergo auto-processing and were retained in the ER. Interestingly, these zymogen forms act as dominant-negatives, as they can dimerize with wild type PCSK9 forcing its retention in the ER.78–80 Of note the few heterozygous subjects carrying the LOF PCSK9-Q152H, which prevents zymogen processing and retains proPCSK9 in the ER, have all very low levels of circulating PCSK9 and LDL-C,78 supporting the potential relevance of this approach. Seemingly, they are in good health and do not exhibit any overt pathology associated with the lack of circulating PCSK9, indicating that complete retention of proPCSK9 in the ER may not result in unwanted side effects. Indeed, our recent data revealed that PCSK9 is protective against the induction of ER stress and that the Q152H mutant is poised to protect cells from the unfolded protein response inducing ER stress.82,83 Inhibition of the autocatalytic processing is thus an attractive approach to prevent PCSK9 secretion. However, engineering of a small molecule inhibitor turned out to be difficult because of the zero order kinetics of the autocatalytic processing of proPCSK9 into PCSK9 and the necessity to cross both plasma and ER membranes to reach the ER lumen.84,85
Finally, PCSK9 secretion may be reduced by inhibitors that would prevent the interaction of a recently reported ER resident cargo receptor, SURF4, with mature PCSK9, thereby facilitating its efficient exit from the ER into COP-II vesicles en route to the Golgi apparatus.86
6. Other inhibitors of PCSK9-LDLR binding
Within the strategies to inhibit the interaction between PCSK9 and the LDLR, a lot of interest is focused on EGF-A-like peptides or small molecule inhibitors. The first EGF-A-like peptide identified that effectively inhibited the PCSK9-LDLR binding was a Fc-fusion EGF66 that bound PCSK9 with a Kd of ∼70 nM and inhibited the PCSK9-induced LDLR degradation in HepG2 cells and in mice.87 Later on, shorter peptides able to bind PCSK9 with increased affinity have been generated, including the 13 amino acid (aa) Pep2-8, which however is 10-fold less active than EGF66.88 Efforts to further improve the potency of Pep2-8 led to the discovery of a targetable pocket region in PCSK9 structure very close to the EGFA-PCSK9 interaction surface89 that interacts with the N-terminal 10 aa P’ helix peptide (aa 153–162; SIPWNLERIT) of the catalytic domain of PCSK9. An intense engineering effort led to the design of a modestly active first generation 16-residue linear peptide MESFPGWNLV(homoR)IGLLR, which antagonizes PCSK9 activity.89 Efforts are underway to improve this structure and generate a potent orally active small molecule inhibitor.90
Another approach to disrupt the extracellular PCSK9-LDLR interaction is the use of engineered adnectins (Figure 2). These are ∼11 kDa fragments of fibronectin type III domain (BMS-962476) that bind the catalytic subunit of PCSK9. A single injection in cynomolgus monkeys led to a ∼50% reduction in LDL-C (Table 1).33 Although promising, the clinical development of this strategy has been abandoned as it could not favourably replace the mAb approach.
A recent alternative biologic approach was to use a ∼22 kDa albumin-binding domain (ABD)-fused Anticalin protein DS-9001a produced in bacteria (Figure 2).34 In cynomolgus monkeys, a single subcutaneous injection of such ABD-fused Anticalin protein was reported to have a somewhat longer half-life in plasma (∼120h)34 compared to mAbs (∼60–120 h)91 and BMS-962476 (∼74–108 h).33 Such treatment resulted in a sustained ∼50–60% reduction of LDL-C lasting up to 21 days (Table 1), and its effect was significantly potentiated by atorvastatin.34 We have to wait until these compounds are tested in clinical trials before drawing firm conclusions on the efficacy and safety of this promising new class of biologics.
7. Blockade of PCSK9-LDLR sorting to lysosomes by CHRD antibodies
Antibodies against PCSK9 were designed to recognize the C-terminal cysteine–histidine rich domain (CHRD), which is critical for sorting the PCSK9-LDLR complex to lysosomes/endosomes for degradation.10,15,92 Deletion of the CHRD does not impair PCSK9 folding and secretion, but results in an inactive secreted form of PCSK9 that still binds the LDLR but cannot sort it to degradation compartments.15 This result suggested that a so far unidentified ‘protein X (Px)’ is required to bind the CHRD and/or LDLR and to escort the PCSK9-LDLR complex to lysosomes for degradation (Figure 2).10,93 Thus, any approach that prevents the formation of the Px-PCSK9-LDLR trimeric complex could potentially inhibit PCSK9 function without necessarily preventing binding of PCSK9 to the LDLR. Indeed, a bulky Fab94 that binds the CHRD was shown to inhibit ∼50% of the extracellular PCSK9’s ability to enhance the degradation of the LDLR. Moreover, a CHRD-specific mAb also reduced LDL-C levels by ∼40% when injected to cynomolgus monkeys (Table 1).35
Recently, three single domain antibodies (sdAbs) raised in llamas that recognize exclusively the C-terminal M1/M3 domains11 of the CHRD of PCSK9 have been generated (Figure 2).36 When the antigen-binding nanobody domains are fused to a mouse Fc-sequence and injected in mice expressing exclusively human PCSK9,37 a sustained ∼50% reduction of LDL-C that lasted more than 17 days was observed (Table 1).36,37 Different from the mAbs that prevent the formation of the PCSK9-LDLR complex, the sdAbs did not inhibit such complex formation nor did they increase the levels of circulating PCSK9, but rather prevented PCSK9 activity on the LDLR. It could be hypothesized that the sdAb interfered with the binding of Px to the PCSK9-LDLR complex and hence prevented its intracellular sorting to lysosomes.37 Although these sdAbs do not reach the efficacy of the mAbs, they represent a unique tool to dissect out the sorting mechanism of the PCSK9-LDLR complex to endosomes/lysosomes. Further experiments based on the crystal structure of the sdAb-CHRD complexes and site directed-mutagenesis of the CHRD domain are ongoing to identify critical residues regulating such trafficking, which might result in more effective sdAbs.37
8. PCSK9 vaccine
A completely different approach to interfere with PCSK9 is to instruct the immune system to eliminate endogenous circulating PCSK9. This could be achieved by using PCSK9-peptide-based vaccines (Figure 2).95,96 So far preclinical studies in mice have shown that immunization induces a strong and long-lasting immune response resulting in reduced plasma levels of PCSK9, total cholesterol and non-HDL-C (VLDL-C and LDL-C), as well as systemic inflammation.97 Moreover, immunization resulted in reduced atherosclerotic lesion area and aortic inflammation compared with control mice.97 This vaccine has been tested in a Phase I clinical trial (https://clinicaltrials.gov/ct2/show/NCT02508896). Preliminary data showed that in healthy subjects immunization was safe and well tolerated; more than 90% of immunized subjects developed a PCSK9-specific antibody response that was reactivated after a second injection at Week 60; the mean LDL-C reduction was 13.3% at Week 70 (Table 1), and persisted for at least 30 weeks after the boost immunization (Bauer et al., Communication at ESC Congress, 2018 Munich). Although this approach seems attractive and more permanent, similar to the CRISPR approach, it is crucial to exclude the possibility of any serious unsuspected side effects due to the absence of PCSK9 expression in adult livers, especially in situations where liver function is compromised, such as during regeneration or viral infections.98
9. Conclusions and perspectives
These last 15 years of experimental and clinical research have demonstrated that the LOF of PCSK9 towards the LDLR associates with reduced levels of LDL-C and overall lower rate of cardiovascular complications and all-cause mortality,99 especially for patients starting with higher baseline levels of LDL-C.62 The beneficial effects of the loss of PCSK9 are independent from other risk factors such as diabetes and hypertension. Lower PCSK9 levels/activity also associate with a reduced risk of complications in sepsis and/or inflammation.100,101 Thus abolishing circulating PCSK9 seems to offer multiple advantages and minimal side effects.
PCSK9 is expected to have other functions in the developing liver and extrahepatic tissues, such as small intestine, cerebellum, pancreas, and kidney.10,55,102,103 A recent paper reported an association between a PCSK9 LOF and a reduced risk of abdominal aortic aneurism,104 an observation that may further extend the therapeutic indications for PCSK9 inhibition. Alirocumab and evolocumab reduce circulating PCSK9, which originates from the liver only.53,54 Although a few individuals lack functional PCSK9,79,105,106 only time will tell whether siRNA silencing of liver intracellular and secreted PCSK9 or lifelong deletion of PCSK9 in the above tissues by way of CRISPR/Cas9 is still as beneficial. It should be emphasized that, with regard to its impact on the LDLR, hepatocyte-derived PCSK9 mostly acts extracellularly after its secretion. This may not be the case for other functions or in other tissues where PCSK9 may act intracellularly, indeed some experimental evidence purports a role for intracellular PCSK9 in hepatic and non-hepatic cell metabolism.107
Although the mAb approach, that only targets circulating PCSK9, does not seem to enhance the onset of diabetes on the short run,23,58,108 it may on the long run increase the metabolic risk of developing a pre-diabetes state, possibly due to the increased ratio of apoB/PCSK9.109 Indeed, a higher apoB/PCSK9 ratio is associated with higher postprandial white adipose tissue macrophage infiltration and priming of the NLRP3 inflammasome, whose role in the aetiology of type 2 diabetes is well established.110
While much work is needed to unravel PCSK9 functions in extrahepatic tissues,103 its involvement in immune-inflammatory responses is emerging111 thanks to its ability to modulate the innate immune response during sepsis112 or to affect hepatitis C virus infectivity via the regulation of hepatic surface entry proteins.113 Of note, anti-PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia114 and improve vascular inflammation.115
Moreover, the observation that lower levels of PCSK9 are associated with reduced inflammation, especially in patients with the highest level of the inflammatory marker high sensitivity C-reactive protein (hs-CRP),116 will need to be properly validated in specific clinical trials on inflammation-associated pathologies, as was done with the mAb canakinumab targeting interleukin-1β in the CANTOS anti-inflammatory thrombosis outcome study.117 A recent meta-analysis reported a lack of effect of anti-PCSK9 therapy with mAbs on circulating hs-CRP levels, at least for the short-term treatment,118 and an analysis of the FOURIER trial showed that changes in hs-CRP levels were similar between evolocumab and placebo, even in subjects with a higher baseline hs-CRP level.119 Whether the combination of a PCSK9 inhibitor with canakinumab, or any other anti-inflammatory mAb,120 is clinically beneficial in patients with an elevated atherogenic profile will have to be carefully evaluated.
In conclusion, the discovery of PCSK9 in 2003 and its powerful regulation of LDL-C via the enhanced degradation of the LDLR has led the way towards the development of powerful new strategies to significantly enhance the reduction of LDL-C over and above the levels achieved with the more commonly used orally active statins or statins + ezetimibe. While some of the injectable PCSK9-targeting drugs are rapidly evolving, we may still witness the development of safe, orally active PCSK9-inhibitors in the future.89 Because of their anticipated lower cost, the latter may have a more widespread use worldwide in the treatment of various pathologies, benefiting from low levels of PCSK9.
Acknowledgement
We would like to thank Brigitte Mary for secretarial help.
Conflict of interest: N.G.S., A.Prat and A.P. have nothing to disclose. A.L.C. reports grants from Pfizer, Sanofi, Regeneron, Merck, Mediolanum, non-financial support from SigmaTau, Menarini, Kowa, Recordati, Eli Lilly, personal fees from Astrazeneca, Genzyme, Bayer, Menarini, Kowa, Eli Lilly, Recordati, Pfizer, Mediolanum, Merck, Sanofi, Aegerion, Amgen, outside the submitted work. G.D.N. has received research funding, and/or honoraria for consultancy or speaker bureau from Aegerion, Alnylam, Amgen, Novartis, Pfizer, Sanofi-Regeneron, outside the submitted work.
Funding
Canadian Institutes of Health Research grants Foundation Scheme 148369, a Canada Research Chair 231335, and a Fondation Leducq grant #13CVD03; Fondazione Cariplo 2015-0524 and 2015-0564 (A.L.C.), and 2016-0852 (G.D.N.); H2020 REPROGRAM PHC-03-2015/667837-2 (A.L.C); Ministero della Salute GR-2011-02346974 (G.D.N.); Aspire Cardiovascular Grant 2016-WI218287 (G.D.N.).
References
- 1. Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M.. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA 2003;100:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C.. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–156. [DOI] [PubMed] [Google Scholar]
- 3. Maxwell KN, Soccio RE, Duncan EM, Sehayek E, Breslow JL.. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J Lipid Res 2003;44:2109–2119. [DOI] [PubMed] [Google Scholar]
- 4. Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL.. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 2003;100:12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maxwell KN, Breslow JL.. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA 2004;101:7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, Asselin MC, Hamelin J, Varret M, Allard D, Trillard M, Abifadel M, Tebon A, Attie AD, Rader DJ, Boileau C, Brissette L, Chretien M, Prat A, Seidah NG.. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem 2004;279:48865–48875. [DOI] [PubMed] [Google Scholar]
- 7. Park SW, Moon YA, Horton JD.. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem 2004;279:50630–50638. [DOI] [PubMed] [Google Scholar]
- 8. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH.. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005;37:161–165. [DOI] [PubMed] [Google Scholar]
- 9. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH.. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 10. Seidah NG, Awan Z, Chretien M, Mbikay M.. PCSK9: a key modulator of cardiovascular health. Circ Res 2014;114:1022–1036. [DOI] [PubMed] [Google Scholar]
- 11. Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, Varghese AH, Ammirati MJ, Culp JS, Hoth LR, Mansour MN, McGrath KM, Seddon AP, Shenolikar S, Stutzman-Engwall KJ, Warren LC, Xia D, Qiu X.. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol 2007;14:413–419. [DOI] [PubMed] [Google Scholar]
- 12. Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH.. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat a of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem 2007;282:18602–18612. [DOI] [PubMed] [Google Scholar]
- 13. Surdo PL, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S, Ni YG, Hubbard B, Sitlani A, Carfi A.. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep 2011;12:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maxwell KN, Fisher EA, Breslow JL.. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci USA 2005;102:2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nassoury N, Blasiole DA, Tebon OA, Benjannet S, Hamelin J, Poupon V, McPherson PS, Attie AD, Prat A, Seidah NG.. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic 2007;8:718–732. [DOI] [PubMed] [Google Scholar]
- 16. Horton JD, Cohen JC, Hobbs HH.. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009;50 Suppl:S172–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seidah NG, Prat A.. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012;11:367–383. [DOI] [PubMed] [Google Scholar]
- 18. Timms KM, Wagner S, Samuels ME, Forbey K, Goldfine H, Jammulapati S, Skolnick MH, Hopkins PN, Hunt SC, Shattuck DM.. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum Genet 2004;114:349–353. [DOI] [PubMed] [Google Scholar]
- 19. Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, Krempf M, Reznik Y, Girardet JP, Fredenrich A, Junien C, Varret M, Boileau C, Benlian P, Rabes JP.. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat 2005;26:497–506. [DOI] [PubMed] [Google Scholar]
- 20. Homer VM, Marais AD, Charlton F, Laurie AD, Hurndell N, Scott R, Mangili F, Sullivan DR, Barter PJ, Rye KA, George PM, Lambert G.. Identification and characterization of two non-secreted PCSK9 mutants associated with familial hypercholesterolemia in cohorts from New Zealand and South Africa. Atherosclerosis 2008;196:659–666. [DOI] [PubMed] [Google Scholar]
- 21. Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R.. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 22. Karatasakis A, Danek BA, Karacsonyi J, Rangan BV, Roesle MK, Knickelbine T, Miedema MD, Khalili H, Ahmad Z, Abdullah S, Banerjee S, Brilakis ES.. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta-analysis of 35 randomized controlled trials. J Am Heart Assoc 2017;6:e006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni-Berthold I, Lewis BS, Handelsman Y, Pineda AL, Honarpour N, Keech AC, Sever PS, Pedersen TR.. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:941–950. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 25. Norata GD, Tibolla G, Catapano AL.. Targeting PCSK9 for hypercholesterolemia. Annu Rev Pharmacol Toxicol 2014;54:273–293. [DOI] [PubMed] [Google Scholar]
- 26. Seidah NG, Chretien M, Mbikay M.. The ever-expanding saga of the proprotein convertases and their roles in body homeostasis: emphasis on novel proprotein convertase subtilisin kexin number 9 functions and regulation. Curr Opin Lipidol 2018;29:144–150. [DOI] [PubMed] [Google Scholar]
- 27. Norata GD, Ballantyne CM, Catapano AL.. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J 2013;34:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 29. Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, Brooks A, Fernando C, Kauffman RS, Kallend D, Vaishnaw A, Simon A.. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med 2017;376:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ.. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017;376:1430–1440. [DOI] [PubMed] [Google Scholar]
- 31. Chadwick AC, Wang X, Musunuru K.. In vivo base editing of PCSK9 (proprotein convertase subtilisin/kexin type 9) as a therapeutic alternative to genome editing. Arterioscler Thromb Vasc Biol 2017;37:1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lintner NG, McClure KF, Petersen D, Londregan AT, Piotrowski DW, Wei L, Xiao J, Bolt M, Loria PM, Maguire B, Geoghegan KF, Huang A, Rolph T, Liras S, Doudna JA, Dullea RG, Cate JH.. Selective stalling of human translation through small-molecule engagement of the ribosome nascent chain. PLoS Biol 2017;15:e2001882.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell T, Chao G, Sitkoff D, Lo F, Monshizadegan H, Meyers D, Low S, Russo K, DiBella R, Denhez F, Gao M, Myers J, Duke G, Witmer M, Miao B, Ho SP, Khan J, Parker RA.. Pharmacologic profile of the Adnectin BMS-962476, a small protein biologic alternative to PCSK9 antibodies for low-density lipoprotein lowering. J Pharmacol Exp Ther 2014;350:412–424. [DOI] [PubMed] [Google Scholar]
- 34. Masuda Y, Yamaguchi S, Suzuki C, Aburatani T, Nagano Y, Miyauchi R, Suzuki E, Yamamura N, Nagatomo K, Ishihara H, Okuno K, Nara F, Matschiner G, Hashimoto R, Takahashi T, Nishizawa T.. Generation and characterization of a novel small biologic alternative to proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies, DS-9001a, albumin binding domain-fused anticalin protein. J Pharmacol Exp Ther 2018;365:368–378. [DOI] [PubMed] [Google Scholar]
- 35. Schiele F, Park J, Redemann N, Luippold G, Nar H.. An antibody against the C-terminal domain of PCSK9 lowers LDL cholesterol levels in vivo. J Mol Biol 2014;426:843–852. [DOI] [PubMed] [Google Scholar]
- 36. Weider E, Susan-Resiga D, Essalmani R, Hamelin J, Asselin MC, Nimesh S, Ashraf Y, Wycoff KL, Zhang J, Prat A, Seidah NG.. Proprotein convertase subtilisin/kexin type 9 (PCSK9) single domain antibodies are potent inhibitors of low density lipoprotein receptor degradation. J Biol Chem 2016;291:16659–16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Essalmani R, Weider E, Marcinkiewicz J, Chamberland A, Susan-Resiga D, Roubtsova A, Seidah NG, Prat A.. A single domain antibody against the Cys- and His-rich domain of PCSK9 and evolocumab exhibit different inhibition mechanisms in humanized PCSK9 mice. Biol Chem 2018;399:1363–1374. [DOI] [PubMed] [Google Scholar]
- 38. Gaudet D, Kereiakes DJ, McKenney JM, Roth EM, Hanotin C, Gipe D, Du Y, Ferrand AC, Ginsberg HN, Stein EA.. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol 2014;114:711–715. [DOI] [PubMed] [Google Scholar]
- 39. Stein EA, Raal F.. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014;65:417–431. [DOI] [PubMed] [Google Scholar]
- 40. Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA.. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol 2014;63:1278–1288. [DOI] [PubMed] [Google Scholar]
- 41. Boffa MB, Koschinsky ML.. The journey towards understanding lipoprotein(a) and cardiovascular disease risk: are we there yet? Curr Opin Lipidol 2018;29:259–267. [DOI] [PubMed] [Google Scholar]
- 42. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE.. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 43. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJP, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Honda S, Shishikura D, Scherer DJ, Borgman M, Brennan DM, Wolski K, Nissen SE.. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol 2018;72:2012–2021. [DOI] [PubMed] [Google Scholar]
- 44. Farnier M, Colhoun HM, Sasiela WJ, Edelberg JM, Asset G, Robinson JG.. Long-term treatment adherence to the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab in 6 ODYSSEY Phase III clinical studies with treatment duration of 1 to 2 years. J Clin Lipidol 2017;11:986–997. [DOI] [PubMed] [Google Scholar]
- 45. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN, Stroes E, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, Krauss RM, Laufs U, Santos RD, März W, Newman CB, John Chapman M, Ginsberg HN, John Chapman M, Ginsberg HN, de Backer G, Catapano AL, Hegele RA, Kees Hovingh G, Jacobson TA, Leiter L, Mach F, Wiklund O; European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH. Committee TNTS, Investigators. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol 2015;65:1539–1548. [DOI] [PubMed] [Google Scholar]
- 47. Hovingh GK, Raal FJ, Dent R, Stefanutti C, Descamps O, Masana L, Lira A, Bridges I, Coll B, Sullivan D.. Long-term safety, tolerability, and efficacy of evolocumab in patients with heterozygous familial hypercholesterolemia. J Clin Lipidol 2017;11:1448–1457. [DOI] [PubMed] [Google Scholar]
- 48. Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, Civeira F, Somaratne R, Nelson P, Liu T, Scott R, Wasserman SM, Sabatine MS.. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation 2014;129:234–243. [DOI] [PubMed] [Google Scholar]
- 49. El Shahawy M, Cannon CP, Blom DJ, McKenney JM, Cariou B, Lecorps G, Pordy R, Chaudhari U, Colhoun HM.. Efficacy and safety of alirocumab versus ezetimibe over 2 years (from ODYSSEY COMBO II). Am J Cardiol 2017;120:931–939. [DOI] [PubMed] [Google Scholar]
- 50. Stein EA, Giugliano RP, Koren MJ, Raal FJ, Roth EM, Weiss R, Sullivan D, Wasserman SM, Somaratne R, Kim JB, Yang J, Liu T, Albizem M, Scott R, Sabatine MS.. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J 2014;35:2249–2259. [DOI] [PubMed] [Google Scholar]
- 51. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA.. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 52. Cho L, Dent R, Stroes ESG, Stein EA, Sullivan D, Ruzza A, Flower A, Somaratne R, Rosenson RS.. Persistent safety and efficacy of evolocumab in patients with statin intolerance: a subset analysis of the OSLER open-label extension studies. Cardiovasc Drugs Ther 2018;32:365–372. [DOI] [PubMed] [Google Scholar]
- 53. Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H, Jin W, Davignon J, Seidah NG, Prat A.. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 2008;48:646–654. [DOI] [PubMed] [Google Scholar]
- 54. Roubtsova A, Munkonda MN, Awan Z, Marcinkiewicz J, Chamberland A, Lazure C, Cianflone K, Seidah NG, Prat A.. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol 2011;31:785–791. [DOI] [PubMed] [Google Scholar]
- 55. Cariou B, Si-Tayeb K, Le MC.. Role of PCSK9 beyond liver involvement. Curr Opin Lipidol 2015;26:155–161. [DOI] [PubMed] [Google Scholar]
- 56. Toth PP, Descamps O, Genest J, Sattar N, Preiss D, Dent R, Djedjos C, Wu Y, Geller M, Uhart M, Somaratne R, Wasserman SM, Investigators P.. Pooled safety analysis of evolocumab in over 6000 patients from double-blind and open-label extension studies. Circulation 2017;135:1819–1831. [DOI] [PubMed] [Google Scholar]
- 57. Jones PH, Bays HE, Chaudhari U, Pordy R, Lorenzato C, Miller K, Robinson JG.. Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am J Cardiol 2016;118:1805–1811. [DOI] [PubMed] [Google Scholar]
- 58. Cao YX, Liu HH, Dong QT, Li S, Li JJ.. Effect of proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies on new-onset diabetes mellitus and glucose metabolism: a systematic review and meta-analysis. Diabetes Obes Metab 2018;20:1391–1398. [DOI] [PubMed] [Google Scholar]
- 59. Harvey PD, Sabbagh MN, Harrison JE, Ginsberg HN, Chapman MJ, Manvelian G, Moryusef A, Mandel J, Farnier M.. No evidence of neurocognitive adverse events associated with alirocumab treatment in 3340 patients from 14 randomized Phase 2 and 3 controlled trials: a meta-analysis of individual patient data. Eur Heart J 2018;39:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR, Investigators E.. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017;377:633–643. [DOI] [PubMed] [Google Scholar]
- 61. Robinson JG, Huijgen R, Ray K, Persons J, Kastelein JJ, Pencina MJ.. Determining when to add nonstatin therapy: a quantitative approach. J Am Coll Cardiol 2016;68:2412–2421. [DOI] [PubMed] [Google Scholar]
- 62. Robinson JG, Watson KE.. Identifying patients for nonstatin therapy. Rev Cardiovasc Med 2018;19:S1–S8. [DOI] [PubMed] [Google Scholar]
- 63. Norata GD, Tibolla G, Catapano AL.. Gene silencing approaches for the management of dyslipidaemia. Trends Pharmacol Sci 2013;34:198–205. [DOI] [PubMed] [Google Scholar]
- 64. Peters DT, Henderson CA, Warren CR, Friesen M, Xia F, Becker CE, Musunuru K, Cowan CA.. Asialoglycoprotein receptor 1 is a specific cell-surface marker for isolating hepatocytes derived from human pluripotent stem cells. Development 2016;143:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leiter LA, Teoh H, Kallend D, Wright RS, Landmesser U, Wijngaard PLJ, Kastelein JJP, Ray KK.. Inclisiran lowers LDL-C and PCSK9 irrespective of diabetes status: the ORION-1 randomized clinical trial. Diabetes Care 2018;doi:10.2337/dc18-1491. [DOI] [PubMed] [Google Scholar]
- 66. Ray KK, Stoekenbroek RM, Kallend D, Leiter LA, Landmesser U, Wright RS, Wijngaard P, Kastelein JJP.. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins. Circulation 2018;138:1304–1316. [DOI] [PubMed] [Google Scholar]
- 67. Hadjiphilippou S, Ray KK.. PCSK9 inhibition and atherosclerotic cardiovascular disease prevention: does reality match the hype? Heart 2017;103:1670–1679. [DOI] [PubMed] [Google Scholar]
- 68. Thakore PI, Kwon JB, Nelson CE, Rouse DC, Gemberling MP, Oliver ML, Gersbach CA.. RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat Commun 2018;9:1674.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ding Q, Strong A, Patel KM, Ng SL, Gosis BS, Regan SN, Cowan CA, Rader DJ, Musunuru K.. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res 2014;115:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rossidis AC, Stratigis JD, Chadwick AC, Hartman HA, Ahn NJ, Li H, Singh K, Coons BE, Li L, Lv W, Zoltick PW, Alapati D, Zacharias W, Jain R, Morrisey EE, Musunuru K, Peranteau WH.. In utero CRISPR-mediated therapeutic editing of metabolic genes. Nat Med 2018;24:1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu S, Luo S, Zhu Z, Xu J.. Small molecules as inhibitors of PCSK9: current status and future challenges. Eur J Med Chem 2019;162:212–233. [DOI] [PubMed] [Google Scholar]
- 72. Yue P, Averna M, Lin X, Schonfeld G.. The c.43_44insCTG variation in PCSK9 is associated with low plasma LDL-cholesterol in a Caucasian population. Hum Mutat 2006;27:460–466. [DOI] [PubMed] [Google Scholar]
- 73. Mayne J, Ooi TC, Raymond A, Cousins M, Bernier L, Dewpura T, Sirois F, Mbikay M, Davignon J, Chretien M.. Differential effects of PCSK9 loss of function variants on serum lipid and PCSK9 levels in Caucasian and African Canadian populations. Lipids Health Dis 2013;12:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shioji K, Mannami T, Kokubo Y, Inamoto N, Takagi S, Goto Y, Nonogi H, Iwai N.. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J Hum Genet 2004;49:109–114. [DOI] [PubMed] [Google Scholar]
- 75. Londregan AT, Wei L, Xiao J, Lintner NG, Petersen D, Dullea RG, McClure KF, Bolt MW, Warmus JS, Coffey SB, Limberakis C, Genovino J, Thuma BA, Hesp KD, Aspnes GE, Reidich B, Salatto CT, Chabot JR, Cate JHD, Liras S, Piotrowski DW.. Small molecule proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: hit to lead optimization of systemic agents. J Med Chem 2018;61:5704–5718. [DOI] [PubMed] [Google Scholar]
- 76. Naeli P, Mirzadeh Azad F, Malakootian M, Seidah NG, Mowla SJ.. Post-transcriptional regulation of PCSK9 by miR-191, miR-222, and miR-224. Front Genet 2017;8:189.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lodge R, Ferreira Barbosa JA, Lombard-Vadnais F, Gilmore JC, Deshiere A, Gosselin A, Wiche Salinas TR, Bego MG, Power C, Routy JP, Ancuta P, Tremblay MJ, Cohen EA.. Host microRNAs-221 and -222 inhibit HIV-1 entry in macrophages by targeting the CD4 viral receptor. Cell Rep 2017;21:141–153. [DOI] [PubMed] [Google Scholar]
- 78. Mayne J, Dewpura T, Raymond A, Bernier L, Cousins M, Ooi TC, Davignon J, Seidah NG, Mbikay M, Chretien M.. Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French-Canadian family and with impaired processing and secretion in cell culture. Clin Chem 2011;57:1415–1423. [DOI] [PubMed] [Google Scholar]
- 79. Cariou B, Ouguerram K, ZaïR Y, Guerois R, Langhi C, Kourimate S, Benoit I, Le May C, Gayet C, Belabbas K, Dufernez F, Chétiveaux M, Tarugi P, Krempf M, Benlian P, Costet P.. PCSK9 dominant negative mutant results in increased LDL catabolic rate and familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol 2009;29:2191–2197. [DOI] [PubMed] [Google Scholar]
- 80. Benjannet S, Hamelin J, Chretien M, Seidah NG.. Loss- and gain-of-function PCSK9 variants: clevage specificity, dominant negative effects, and low density lipoprotein receptor (LDLR) degradation. J Biol Chem 2012;287:33745–33755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Benjannet S, Saavedra YG, Hamelin J, Asselin MC, Essalmani R, Pasquato A, Lemaire P, Duke G, Miao B, Duclos F, Parker R, Mayer G, Seidah NG.. Effects of the prosegment and pH on the activity of PCSK9: evidence for additional processing events. J Biol Chem 2010;285:40965–40978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lebeau P, Al-Hashimi A, Sood S, Lhotak S, Yu P, Gyulay G, Pare G, Chen SR, Trigatti B, Prat A, Seidah NG, Austin RC.. Endoplasmic reticulum stress and Ca2+ depletion differentially modulate the sterol regulatory protein PCSK9 to control lipid metabolism. J Biol Chem 2017;292:1510–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lebeau P, Platko K, Al-Hashimi AA, Byun JH, Lhotak S, Holzapfel N, Gyulay G, Igdoura SA, Cool DR, Trigatti B, Seidah NG, Austin RC.. Loss-of-function PCSK9 mutants evade the unfolded protein response sensor GRP78 and fail to induce endoplasmic reticulum stress when retained. J Biol Chem 2018;293:7329–7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chorba JS, Shokat KM.. The proprotein convertase subtilisin/kexin type 9 (PCSK9) active site and cleavage sequence differentially regulate protein secretion from proteolysis. J Biol Chem 2014;289:29030–29043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chorba JS, Galvan AM, Shokat KM.. Stepwise processing analyses of the single-turnover PCSK9 protease reveal its substrate sequence specificity and link clinical genotype to lipid phenotype. J Biol Chem 2018;293:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Emmer BT, Hesketh GG, Kotnik E, Tang VT, Lascuna PJ, Xiang J, Gingras AC, Chen XW, Ginsburg D.. The cargo receptor SURF4 promotes the efficient cellular secretion of PCSK9. eLife 2018;7:e38839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang Y, Zhou L, Beltran MK, Li W, Moran P, Wang J, Quan C, Tom J, Kolumam G, Elliott JM, Skelton N, Peterson A, Kirchhofer D.. Calcium-independent inhibition of PCSK9 by affinity-improved variants of the LDL receptor EGF(A) domain. J Mol Biol 2012;422:685–696. [DOI] [PubMed] [Google Scholar]
- 88. Zhang Y, Eigenbrot C, Zhou L, Shia S, Li W, Quan C, Tom J, Moran P, Di LP, Skelton NJ, Kong-Beltran M, Peterson A, Kirchhofer D.. Identification of a small peptide that inhibits PCSK9 binding to the low density lipoprotein receptor. J Biol Chem 2014;289:942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Y, Ultsch M, Skelton NJ, Burdick DJ, Beresini MH, Li W, Kong-Beltran M, Peterson A, Quinn J, Chiu C, Wu Y, Shia S, Moran P, Di Lello P, Eigenbrot C, Kirchhofer D.. Discovery of a cryptic peptide-binding site on PCSK9 and design of antagonists. Nat Struct Mol Biol 2017;24:848–856. [DOI] [PubMed] [Google Scholar]
- 90. Seidah N. Insights into a PCSK9 structural groove: a harbinger of new drugs to reduce LDL-cholesterol. Nat Struct Mol Biol 2017;24:785–786. [DOI] [PubMed] [Google Scholar]
- 91. Shen Y, Li H, Zhao L, Li G, Chen B, Guo Q, Gao B, Wu J, Yang T, Jin L, Su Y.. Increased half-life and enhanced potency of Fc-modified human PCSK9 monoclonal antibodies in primates. PLoS One 2017;12:e0183326.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang DW, Garuti R, Tang WJ, Cohen JC, Hobbs HH.. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc Natl Acad Sci USA 2008;105:13045–13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Butkinaree C, Canuel M, Essalmani R, Poirier S, Benjannet S, Asselin MC, Roubtsova A, Hamelin J, Marcinkiewicz J, Chamberland A, Guillemot J, Mayer G, Sisodia SS, Jacob Y, Prat A, Seidah NG.. Amyloid precursor-like protein 2 and sortilin do not regulate the PCSK9-mediated low density lipoprotein receptor degradation but interact with each other. J Biol Chem 2015;290:18609–18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ni YG, Condra JH, Orsatti L, Shen X, Di MS, Pandit S, Bottomley MJ, Ruggeri L, Cummings RT, Cubbon RM, Santoro JC, Ehrhardt A, Lewis D, Fisher TS, Ha S, Njimoluh L, Wood DD, Hammond HA, Wisniewski D, Volpari C, Noto A, Lo SP, Hubbard B, Carfi A, Sitlani A.. A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J Biol Chem 2010;285:12882–12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pan Y, Zhou Y, Wu H, Chen X, Hu X, Zhang H, Zhou Z, Qiu Z, Liao Y.. A therapeutic peptide vaccine against PCSK9. Sci Rep 2017;7:12534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kawakami R, Nozato Y, Nakagami H, Ikeda Y, Shimamura M, Yoshida S, Sun J, Kawano T, Takami Y, Noma T, Rakugi H, Minamino T, Morishita R.. Development of vaccine for dyslipidemia targeted to a proprotein convertase subtilisin/kexin type 9 (PCSK9) epitope in mice. PLoS One 2018;13:e0191895.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Landlinger C, Pouwer MG, Juno C, van der Hoorn JWA, Pieterman EJ, Jukema JW, Staffler G, Princen HMG, Galabova G.. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur Heart J 2017;38:2499–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Seidah NG. The PCSK9 revolution and the potential of PCSK9-based therapies to reduce LDL-cholesterol. J Glob Cardiol Sci Pract 2015;59:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Giugliano RP, Pedersen TR, Park J-G, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni-Berthold I, Lopez-Miranda J, Schiele F, Mach F, Ott BR, Kanevsky E, Pineda AL, Somaratne R, Wasserman SM, Keech AC, Sever PS, Sabatine MS.. Investigators F. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet (Lond Engl) 2017;390:1962–1971. [DOI] [PubMed] [Google Scholar]
- 100. Walley KR, Francis GA, Opal SM, Stein EA, Russell JA, Boyd JH.. The central role of PCSK9 in septic pathogen lipid transport and clearance. Am J Respir Crit Care Med 2015;192:1275–1286. [DOI] [PubMed] [Google Scholar]
- 101. Dwivedi DJ, Grin PM, Khan M, Prat A, Zhou J, Fox-Robichaud AE, Seidah NG, Liaw PC.. Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock 2016;46:672–680. [DOI] [PubMed] [Google Scholar]
- 102. Seidah NG, Abifadel M, Prost S, Boileau C, Prat A.. The proprotein convertases in hypercholesterolemia and cardiovascular diseases: emphasis on proprotein convertase subtilisin/kexin 9. Pharmacol Rev 2016;69:33–52. [DOI] [PubMed] [Google Scholar]
- 103. Norata GD, Tavori H, Pirillo A, Fazio S, Catapano AL.. Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc Res 2016;112:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, Gagnon DR, DuVall SL, Li J, Peloso GM, Chaffin M, Small AM, Huang J, Tang H, Lynch JA, Ho YL, Liu DJ, Emdin CA, Li AH, Huffman JE, Lee JS, Natarajan P, Chowdhury R, Saleheen D, Vujkovic M, Baras A, Pyarajan S, Di Angelantonio E, Neale BM, Naheed A, Khera AV, Danesh J, Chang KM, Abecasis G, Willer C, Dewey FE, Carey DJ, Global Lipids Genetics C, Myocardial Infarction Genetics C, Geisinger-Regeneron Discov Ehrc Program Vamv Concato J, Gaziano JM, O’Donnell CJ, Tsao PS, Kathiresan S, Rader DJ, Wilson PWF, Assimes TL.. Genetics of blood lipids among ∼300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet 2018;50:1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, Cohen JC, Hobbs HH.. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 2006;79:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR.. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007;193:445–448. [DOI] [PubMed] [Google Scholar]
- 107. Poirier S, Mayer G, Poupon V, McPherson PS, Desjardins R, Ly K, Asselin MC, Day R, Duclos FJ, Witmer M, Parker R, Prat A, Seidah NG.. Dissection of the endogenous cellular pathways of PCSK9-induced LDLR degradation: evidence for an intracellular route. J Biol Chem 2009;284:28856–28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kosmas CE, Sourlas A, Bouza KV, DeJesus E, Silverio D, Montan PD, Guzman E.. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition with evolocumab: powerful low-density lipoprotein cholesterol (LDL-C) lowering and improved cardiovascular outcomes without an increase in the risk of diabetes mellitus. Ann Transl Med 2018;6:130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wassef H, Bissonnette S, Saint-Pierre N, Lamantia V, Cyr Y, Chrétien M, Faraj M.. The apoB-to-PCSK9 ratio: a new index for metabolic risk in humans. J Clin Lipidol 2015;9:664–675. [DOI] [PubMed] [Google Scholar]
- 110. Cyr YBS, Lamantia V, Besse-Patin A, Meugnier E, Wabitsch M, Vidal H, Estall JL, Chrétien M, Faraj M.. ApoB-Lipoproteins and PCSK9 as Modulators of Human White Adipose Tissue Function and NLRP3 Inflammasome Activity. XVIII International Symposium on Atherosclerosis (ISA) Toronto, ON, Canada: Atherosclerosis Supplements, 2018. p. 117.
- 111. Paciullo F, Fallarino F, Bianconi V, Mannarino MR, Sahebkar A, Pirro M.. PCSK9 at the crossroad of cholesterol metabolism and immune function during infections. J Cell Physiol 2017;232:2330–2338. [DOI] [PubMed] [Google Scholar]
- 112. Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, Christie JD, Nakada TA, Fjell CD, Thair SA, Cirstea MS, Boyd JH.. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 2014;6:258ra143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pirro M, Bianconi V, Francisci D, Schiaroli E, Bagaglia F, Sahebkar A, Baldelli F.. Hepatitis C virus and proprotein convertase subtilisin/kexin type 9: a detrimental interaction to increase viral infectivity and disrupt lipid metabolism. J Cell Mol Med 2017;21:3150–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ, Stroes ESG.. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017;38:1584–1593. [DOI] [PubMed] [Google Scholar]
- 115. Kühnast S, van der Hoorn JWA, Pieterman EJ, van den Hoek AM, Sasiela WJ, Gusarova V, Peyman A, Schäfer H-L, Schwahn U, Jukema JW, Princen HMG.. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res 2014;55:2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ruscica M, Ferri N, Macchi C, Corsini A, Sirtori CR.. Lipid lowering drugs and inflammatory changes: an impact on cardiovascular outcomes? Ann Med 2018;1–24. [DOI] [PubMed] [Google Scholar]
- 117. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 118. Cao YX, Li S, Liu HH, Li JJ.. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018;8:e022348.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS.. Inflammatory and cholesterol risk in the FOURIER trial. Circulation 2018;138:131–140. [DOI] [PubMed] [Google Scholar]
- 120. Ueland T, Kleveland O, Michelsen AE, Wiseth R, Damas JK, Aukrust P, Gullestad L, Halvorsen B, Yndestad A.. Serum PCSK9 is modified by interleukin-6 receptor antagonism in patients with hypercholesterolaemia following non-ST-elevation myocardial infarction. Open Heart 2018;5:e000765.. [DOI] [PMC free article] [PubMed] [Google Scholar]