Abstract
Aims
JNJ-64179375 (hereafter JNJ-9375) is a first-in-class, highly specific, large molecule, exosite 1 thrombin inhibitor. In preclinical studies, JNJ-9375 demonstrated robust antithrombotic protection with a wider therapeutic index when compared to apixaban. The purpose of the present study was to examine for the first time the antiplatelet, anticoagulant and antithrombotic effects of JNJ-9375 in a translational model of ex vivo human thrombosis.
Methods and results
Fifteen healthy volunteers participated in a double-blind randomized crossover study of JNJ-9375 (2.5, 25, and 250 μg/mL), bivalirudin (6 μg/mL; positive control), and matched placebo. Coagulation, platelet activation, and thrombus formation were determined using coagulation assays, flow cytometry, and an ex vivo perfusion chamber, respectively.
JNJ-9375 caused concentration-dependent prolongation of all measures of blood coagulation (prothrombin time, activated partial thromboplastin time, and thrombin time; P < 0.001 for all) and agonist selective inhibition of thrombin (0.1 U/mL) stimulated platelet p-selectin expression (P < 0.001) and platelet-monocyte aggregates (P = 0.002). Compared to placebo, JNJ-9375 (250 μg/mL) reduced mean total thrombus area by 41.1% (95% confidence intervals 22.3 to 55.3%; P < 0.001) at low shear and 32.3% (4.9 to 51.8%; P = 0.025) at high shear. Under both shear conditions, there was a dose-dependent decrease in fibrin-rich thrombus (P < 0.001 for both) but not platelet-rich thrombus (P = ns for both).
Conclusion
Exosite 1 inhibition with JNJ-9375 caused prolongation of blood coagulation, selective inhibition of thrombin-mediated platelet activation, and reductions in ex vivo thrombosis driven by a decrease in fibrin-rich thrombus formation. JNJ-9375 represents a novel class of anticoagulant with potential therapeutic applications.
Keywords: JNJ-9375, Exosite 1 thrombin, Thrombosis, Novel, Anticoagulant
Introduction
The coagulation cascade plays a central role in thrombosis and the pathophysiology of thrombo-embolic events, the leading cause of global mortality.1 Anticoagulants are of proven benefit in a wide range of thrombo-embolic disorders, but despite recent improvements, important limitations persist. All the currently licensed agents, including direct oral anticoagulants (DOACs), act to either inhibit thrombin generation or block the active site of the protease directly.2 Consequently, they provide broad inhibition of all thrombin activity, which although efficacious, invariably fails to discriminate between protease interactions relating to thrombosis and those essential to haemostasis. Treatment related bleeding remains a major concern and for many patients this leads to dosing restrictions or exclusion from anticoagulation altogether.3–9
JNJ-64179375 (hereafter JNJ-9375) is a first-in-class, recombinant, fully human, IgG4 monoclonal antibody anticoagulant that binds reversibly and with high affinity and specificity to the exosite 1 region of thrombin.10 Exosite 1 is a positively charged domain on the surface of thrombin that together with exosite 2 serves to regulate enzymatic activity of the protease by providing an initial binding site for substrates, co-factors, and inhibitors.11–13 JNJ-9375 therefore acts to inhibit the interaction of thrombin with its exosite 1 substrates, which include fibrinogen, but retains function of both the active site and exosite 2.10 This capacity to inhibit fibrinogen binding while preserving other (non-exosite 1) protease interactions offers the potential for a wider therapeutic index, and in preclinical animal models JNJ-9375 was associated with substantially less bleeding when compared to apixaban at doses of equivalent antithrombotic efficacy.10 In the present study, we sought to examine for the first time the anticoagulant and antithrombotic effects of exosite 1 thrombin inhibition with JNJ-9375 in human blood using a translational model of ex vivo thrombosis.
Methods
Study population
Healthy non-smoking male and female volunteers aged between 18 and 45 years (inclusive) with a body mass index (BMI) of 18–35 kg/m2 were enrolled in this study. All volunteers underwent a detailed screening assessment for eligibility. Exclusion criteria included women who were pregnant or still lactating, or any clinically significant coexisting condition including hypertension, hyperlipidaemia, diabetes mellitus, cardiovascular disease, recent infective or inflammatory condition, coagulopathy, known liver disease or screening blood tests indicative of renal, liver, clotting, thyroid, or haematological abnormality. Volunteers were not permitted to take any prescription or non-prescription medication (including acetylsalicylic acid, paracetamol, vitamins, and herbal supplements) within 14 days of an experimental visit. Prior to each visit, volunteers must have abstained from alcohol for 24 h and food including caffeine-containing products for 8 h. Informed written consent was obtained from all volunteers before enrolment. The study was approved by the local research ethics committee (reference 16-HV-025) and conducted in accordance with the Declaration of Helsinki.
Study design
This was a double-blind randomized controlled five-way crossover study conducted at a single site (Clinical Research Facility, Royal Infirmary of Edinburgh, Scotland) between the 24 May 2016 and 1 July 2016. Study measures were performed during extracorporeal infusion of JNJ-9375 (estimated final concentration of 2.5, 25, and 250 μg/mL), bivalirudin (positive control; estimated final concentration of 6 μg/mL; The Medicines Company, Abingdon, UK) at a dose equivalent to recommendations at the time of percutaneous coronary intervention (PCI), and matched placebo [10 mM phosphate, 8.5% (w/v) sucrose, 0.04% (w/v) polysorbate 20, 10 μg/mL ethylenediaminetetraacetic acid, pH 7.1; Janssen Research and Development] upstream of the perfusion chambers. Three perfusion chamber studies were performed at the first experimental visit and two perfusion chamber studies at the second experimental visit.
Study objectives
The primary objective was to assess the relationship of JNJ-9375 dose concentrations to ex vivo thrombus formation under conditions of both low and high shear stress, and to compare these effects with placebo under the same rheological conditions. Bivalirudin, which blocks both exosite 1 and the active site of thrombin, was used as a positive control. Secondary objectives included a similar comparison of compound effects on platelet activation, markers of coagulation, and the fibrin and platelet components of thrombus formation. Finally, correlations between measured chamber concentrations of study drug and pharmacodynamic endpoints were explored.
Perfusion chamber experiment
Thrombus formation was assessed using the Badimon chamber, a well-validated perfusion model for measuring the effect of study drugs on ex vivo human thrombus formation.14–21 In brief, a pump was used to draw native (unanticoagulated) blood from an antecubital vein directly through a series of three cylindrical perfusion chambers maintained at 37°C in a water bath. Each chamber contained a strip of porcine aorta from which the intima and a thin layer of media had been removed. Rheological conditions in the first chamber were set to simulate those of patent medium-sized arteries (inner lumen diameter 2.0 mm; vessel wall shear rate 212 s−1; mean blood velocity 5.3 cm/s; Reynolds number 30), whereas those in the second and third chambers were set to simulate those of mild to moderately stenosed coronary arteries (inner lumen diameter 1.0 mm; vessel wall shear rate 1690 s−1; mean blood velocity 21.2 cm/s; Reynolds number 60). Shear conditions at the vessel wall were calculated from the theoretical expression for shear rate given for a Newtonian fluid in tube flow.22,23 Each study lasted for exactly 5 min during which flow was maintained at a constant rate of 10 mL/min. All studies were performed using the same perfusion chamber and by the same operator.
Study outcome measures
Chamber concentrations of study drug
Blood samples for determination of serum JNJ-9375 and plasma bivalirudin concentrations were taken immediately distal to the perfusion chamber into 3.5 mL serum gel and 2.7 mL sodium citrate (3.2%) tubes (Becton-Dickinson, Cowley, UK). JNJ-9375 samples were allowed to clot for 30 min then centrifuged at 1500 g (20°C) for 20 min. Bivalirudin samples were centrifuged at 1500 g (15°C) for 15 min within 1 h of collection. Samples were then aliquoted and stored immediately at −70°C before analysis. Concentrations of JNJ-9375 were determined by electrochemiluminescence using the Meso Scale Discovery platform and plate reader (Rockville, MD, USA). JNJ-9375 concentrations were regressed from the standard curve in Watson LIMS (version 7.4.1, Thermo, PA, USA) using a five-parameter logistic regression model with 1/Y2 weighting.
Coagulations assays
Blood samples for coagulations assays [prothrombin time, activated partial thromboplastin time, and thrombin time (undiluted and diluted)] were collected immediately distal to the final perfusion chamber into 4.5 mL sodium citrate (0.38% final v/v) tubes (Becton-Dickinson). Samples were centrifuged at 1500 g (15°C) for 20 min within 1 h of collection. Plasma was then aliquoted and stored immediately at −70°C before analysis using a STA-Compact-Max analyser (Stago, Parsippany, NJ, USA). The following reagents were used, for prothrombin time, STA-Neoplastine CI Plus, for activated partial thromboplastin time, STA-PTT Automate, and for thrombin time, STA-Thrombin.
Platelet activation
Platelet p-selectin expression and platelet-monocyte aggregates are sensitive markers of in vivo platelet activation.24–26 Blood (2.7 mL) was collected immediately distal to the final perfusion chamber into tubes containing 0.3 mL of 3.8% sodium citrate and Pefabloc FG (final concentration 1.5 mg/mL; Quadratech Diagnostics, Surrey, UK). After 5 min, samples were aliquoted into Eppendorfs pre-filled with or without agonist [adenosine diphosphate (ADP) 20 μM, Sigma-Aldrich, Gillingham, UK; human alpha thrombin 0.1 U/mL, Enzyme Research Laboratories, Swansea, UK] and the following conjugated monoclonal antibodies: allophycocyanin (APC)-conjugated CD14, phycoerythrin (PE)-conjugated CD62P, and fluorescein isothiocyanate (FITC)-conjugated CD42a (Becton-Dickinson). All antibodies were diluted 1:10. Samples were incubated for 15 min at room temperature before fixing with 1% paraformaldehyde (p-selectin) or FACS-Lyse (Becton-Dickinson; platelet-monocyte aggregates). All samples were analysed within 24 h using a FACSCalibur flow cytometer (Becton-Dickinson). Data analysis was performed using FlowJo v10 (Treestar, Oregon, USA).
Thrombus formation
After each perfusion experiment, the porcine strips with attached thrombus were removed and fixed in 4% paraformaldehyde for 24 h at 4°C prior to being prepared for histological analysis. As thrombus forms longitudinally along the entire length of the exposed porcine aortic strip, the mean cross-sectional area gives a reliable representation of total thrombus formation.27 Following fixation, the proximal and distal 1 mm of the exposed substrate were discarded and the remainder cut into eight segments. Segments were embedded in paraffin wax and 4-μm sections prepared.
To detect total thrombus area, endogenous hydrogen peroxide activity was blocked using 3% hydrogen peroxide solution (Leica Microsystems GmbH, Wetzlar, Germany) for 5 min. Sections were then incubated at room temperature for 1 h with polyclonal rabbit anti-human fibrin(ogen) antibody (1.2 μg/mL, Dako, Glostrup, Denmark; Cat. No. A0080) and monoclonal mouse anti-human CD61 antibody (1.28 μg/mL, Dako; Cat. No. M0753). Antigen visualization was performed using a Bond Polymer refine detection kit (Leica Microsystems GmbH) and treatment with 3,3′-diaminobenzidine substrate chromogen (66 mM, Dako). Finally, sections were counterstained with a modified Masson’s trichrome (haematoxylin and sirius red 0.1%).
To examine the effect of study drug(s) on fibrin-rich and platelet-rich thrombus formation, endogenous hydrogen peroxide activity was blocked using 3% hydrogen peroxide solution (VWR, Radnor, PA, USA) for 10 min and non-specific binding blocked using 20% normal goat serum (Biosera, Nuaille, France) in Tris-Buffered Saline with 0.01% Tween (TBST). Sections were then incubated with polyclonal rabbit anti-human fibrin(ogen) antibody (1.2 μg/mL) to detect fibrin and CD61 monoclonal mouse anti-human antibody (0.32 μg/mL) to detect platelets. Following TBST washes, goat anti-rabbit peroxidase (1:500; Abcam, Cambridge, UK) was applied and the presence of antigen visualized with Tyramide Cy3 (1:50; Perkin Elmer, Boston, MA, USA; Cat. no. NEL744B001KT) and FITC (1:50; Perkin Elmer, Waltham, MA, USA; Cat. no. NEL741B001KT) before nuclear counterstaining with 4′,6-diamidino-2-phenylindole (5 μg/mL; Sigma-Aldrich; Cat. No. D9542).
A semi-automated slide scanner (Axioscan Z1; Zeiss, Jena, Germany) and image analysis software (Definiens, Munich, Germany) were used by a blinded operator to quantify thrombus area and composition. Digital images of each section were acquired at ×20 magnification. High-resolution classifiers based on colour were established to detect total thrombus area, fibrin-rich thrombus area, and platelet-rich thrombus area.
Statistical analysis
After study completion, the database was locked and all statistical analyses carried out by an independent statistician. Categorical variables are expressed as percentages, and continuous variables are expressed as mean ± standard deviation (SD). The effects of study compounds on study endpoints were assessed by general linear mixed effect models with period and study compound as fixed effects, subjects as random effects. Chamber endpoints were log-transformed and assessed separately by shear rate (low and high). From the models, point and interval estimates for means and mean differences vs. placebo (absolute and %) were generated and analysed using the Least Significance Difference (LSD) test. The correlation between plasma JNJ-9375 concentrations and study endpoints were determined by Pearson’s (r) or Spearman’s rank-order correlation (ρ) as appropriate. Two-sided P-values of ≤0.05 were considered statistically significant. All statistical calculations were performed using SAS version 9.4.
Results
All 15 enrolled volunteers (10 male) completed the study in full, with no safety concerns. Mean age of the volunteers was 26 ± 5 years with a BMI of 24 ± 3 kg/m2.
Chamber concentrations of study drug
Compound concentrations in the effluent of the perfusion chamber (JNJ-9375 1.93 ± 0.68, 22.3 ± 5.86, and 214.0 ± 20.8 μg/mL; bivalirudin 6.92 ± 11.3 μg/mL) closely matched the targeted concentrations (JNJ-9375 2.5, 25, and 250 μg/mL; bivalirudin 6 μg/mL).
Effect of JNJ-9375 on coagulation assays
JNJ-9375 caused dose-dependent prolongation of all measured blood coagulation markers, with thrombin time the most sensitive to the anticoagulant effect (Table 1). Pearson’s correlation coefficient between chamber plasma concentrations of JNJ-9375 and coagulation assays was 0.98 for prothrombin time, 0.87 for activated partial thromboplastin time, and 0.91 for thrombin time (P < 0.001 for all; Supplementary material online, Figure S1).
Table 1.
Effects of JNJ-64179375 on indices of thrombosis and coagulation
| Placebo | JNJ-9375 (2.5 μg/mL) | JNJ-9375 (25 μg/mL) | JNJ-9375 (250 μg/mL) | Bivalirudin (6 μg/mL) | |
|---|---|---|---|---|---|
| PT (s) | 13.7 (10.5, 16.9) | 13.9 (10.7, 17.1) | 15.8 (12.6, 19.0) | 30.0 (26.8, 33.2) | 36.6 (33.4, 39.8) |
| APTT (s) | 28.9 (23.3, 34.6) | 31.4 (25.7, 37.0) | 41.6 (35.9, 47.3) | 63.5 (57.8, 69.2) | 91.5 (85.8, 97.2) |
| TT (s) | 15.6 (−12.7, 43.8) | 24.9 (−3.3, 53.2) | 80.9 (52.6, 109.2) | 245.6 (217.3, 273.9) | 351.2 (323.0, 379.5) |
| Dilute TT (s) | <LLOQ | <LLOQ | <LLOQ | 151.5 (126.5, 176.6) | >501a |
| P-Selectin GMFI | |||||
| Unstimulated | 4.6 (3.6, 5.6) | 3.9 (2.9, 4.9) | 3.7 (2.7, 4.7) | 3.9 (2.9, 4.9) | 3.2 (2.2, 4.2) |
| ADP 20 μM | 17.8 (11.6, 24.0) | 12.1 (6.0, 18.1) | 17.8 (11.9, 23.8) | 15.6 (9.7, 21.6) | 15.3 (9.4, 21.2) |
| Thrombin 0.1 U/mL | 161.6 (100.3, 222.8) | 86.5 (28.0, 145.0) | 7.8 (−53.5, 69.1) | 1.6 (−62.8, 65.9) | −1.6 (−59.6, 56.3) |
| PMA GMFI | |||||
| Unstimulated | 33.0 (20.6, 45.4) | 35.6 (24.0, 47.3) | 27.6 (15.9, 39.3) | 25.0 (13.3, 36.7) | 25.0 (13.3, 36.7) |
| ADP 20 μM | 48.9 (28.2, 69.5) | 54.9 (35.8, 73.9) | 48.5 (30.1, 67.0) | 50.1 (31.7, 68.6) | 46.4 (28.0, 64.8) |
| Thrombin 0.1 U/mL | 401.0 (180.3, 621.6) | 414.6 (193.3, 635.8) | 175.3 (−45.7, 396.3) | 120.7 (−99.5, 340.9) | 85.0 (−123.2, 293.3) |
| Total thrombus area (μm2/mm) | |||||
| Low shear | 9571 (7669, 11 945) | 10 283 (8239, 12 834) | 8936 (7161, 11 153) | 5640 (4519, 7039) | 3318 (2659, 4141) |
| High shear | 14 367 (10 734, 19 229) | 12961 (9684, 17 347) | 13 898 (10 384, 18 602) | 9729 (7269, 13 022) | 6312 (4716, 8448) |
| Platelet-rich thrombus area (μm2/mm) | |||||
| Low shear | 1255 (834, 1889) | 1610 (1055, 2456) | 1117 (742, 1681) | 1200 (798, 1806) | 832 (545, 1269) |
| High shear | 7302 (4790, 11 131) | 5844 (3834, 8909) | 6405 (4202, 9763) | 5463 (3584, 8327) | 4111 (2697, 6267) |
| Fibrin-rich thrombus area (μm2/mm) | |||||
| Low shear | 10 349 (7535, 14 212) | 10 634 (7651, 14 782) | 9865 (7183, 13 547) | 4190 (3051, 5755) | 1162 (836, 1616) |
| High shear | 9598 (7997, 11 521) | 9176 (7645, 11 014) | 8100 (6749, 9722) | 4625 (3854, 5552) | 1776 (1480, 2132) |
Data shown are means with 95% confidence intervals.
ADP, adenosine diphosphate; APTT, activated partial thromboplastin time; GMFI, geometric mean fluorescent intensity; LLOQ, less than lower limit of quantification; PT, prothrombin time; TT, thrombin time.
14 of 15 results >501 s.
Effect of JNJ-9375 on ex vivo platelet activation
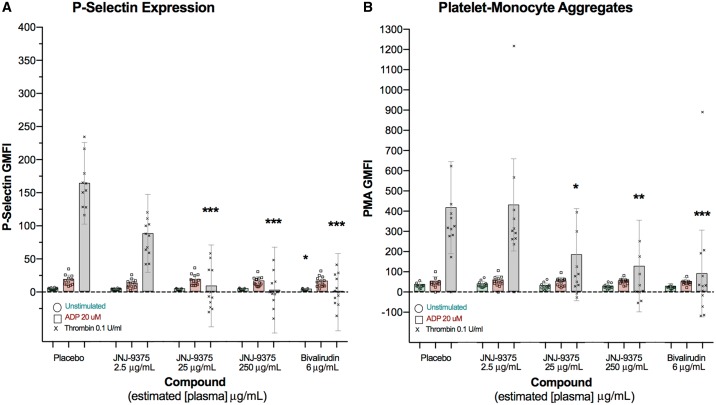
Compared to placebo, JNJ-9375 2.5, 25, and 250 μg/mL inhibited thrombin (0.1 U/mL) stimulated platelet p-selectin expression [geometric mean fluorescent intensity (GMFI)] by 46.5% [95% confidence intervals (CI) 4.6 to 97.5%; P = 0.07], 95.2% (95% CI 43.2 to 147.2%; P < 0.001), and 99.0% (95% CI 46.1 to 151.9%; P < 0.001) and platelet-monocyte aggregates (GMFI) by −3.4% (95% CI −56.1 to 49.4%; P = 0.90), 56.3% (95% CI 2.2 to 110.4%; P = 0.04), and 69.9% (95% CI 16.2 to 123.6%; P = 0.01). Chamber plasma concentrations of JNJ-9375 correlated with both platelet p-selectin expression (ρ = −0.83, P < 0.001) and platelet-monocyte aggregates (ρ = −0.64, P < 0.001). In contrast, JNJ-9375 had no effect on ADP (20 μM) stimulated platelet activation (P = ns for all). Bivalirudin exhibited a similar selective profile (Table 1; Figure 1).
Figure 1.
The effect of study compound on ex vivo platelet activation. Extra-corporeal administration of JNJ-9375 inhibited thrombin-simulated (A) p-selectin expression and (B) platelet-monocyte aggregates in a dose-dependent manner but had no effect on ADP activity. Data shown are the adjusted means (±95% confidence intervals) and individual points. Statistical comparisons (Least Significance Difference test) vs. placebo are represented above each plot: *P < 0.05, **P < 0.01, ***P < 0.001. ADP, adenosine diphosphate; GMFI, geometric mean fluorescent intensity; PMA, platelet-monocyte aggregates.
Effect of JNJ-9375 on ex vivo thrombus formation
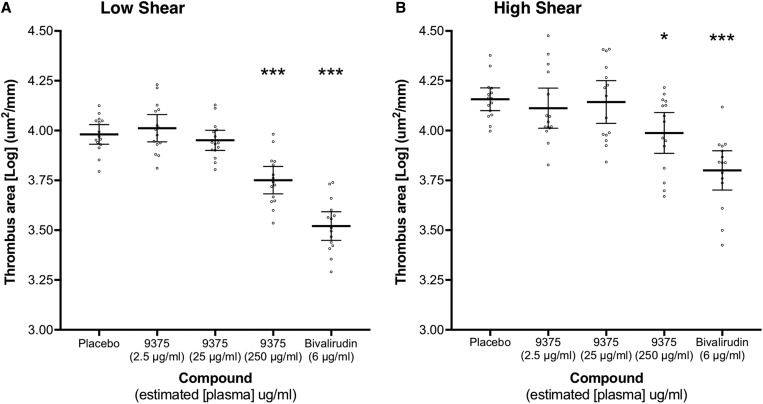
Ex vivo total thrombus formation was reduced at both low and high shear stress at the 250 μg/mL concentration (Figure 2). Compared to placebo, JNJ-9375 (2.5, 25, and 250 μg/mL) reduced mean total thrombus area by −7.4% (95% CI −41.6 to 18.5%; P = 0.60), 6.6% (95% CI −23.1 to 29.2%; P = 0.62), and 41.1% (95% CI 22.3 to 55.3%; P < 0.001) at low shear and by 9.8% (95% CI −26.6 to 35.7%; P = 0.54), 3.3% (95% CI −35.8 to 31.1%; P = 0.85), and 32.3% (95% CI 4.9 to 51.8%; P = 0.025) at high shear. Chamber plasma concentrations of JNJ-9375 correlated with total thrombus area at low (ρ = −0.56, P < 0.001) and high (ρ = −0.32, P = 0.03) shear (Supplementary material online, Figure S1).
Figure 2.
The effect of study compound on ex vivo total thrombus formation. Extra-corporeal administration of JNJ-9375 inhibited total thrombus formation in a dose-dependent manner at both (A) low shear stress (212 s−1) and (B) high shear stress (1690 s−1) shear stress. Data shown are the adjusted means (±95% confidence intervals) for [Log] total thrombus area (μm2/mm) and individual points. Statistical comparisons (Least Significance Difference test) vs. placebo are represented above each plot: *P < 0.05, **P < 0.01, ***P < 0.001. 9375, JNJ-9375.
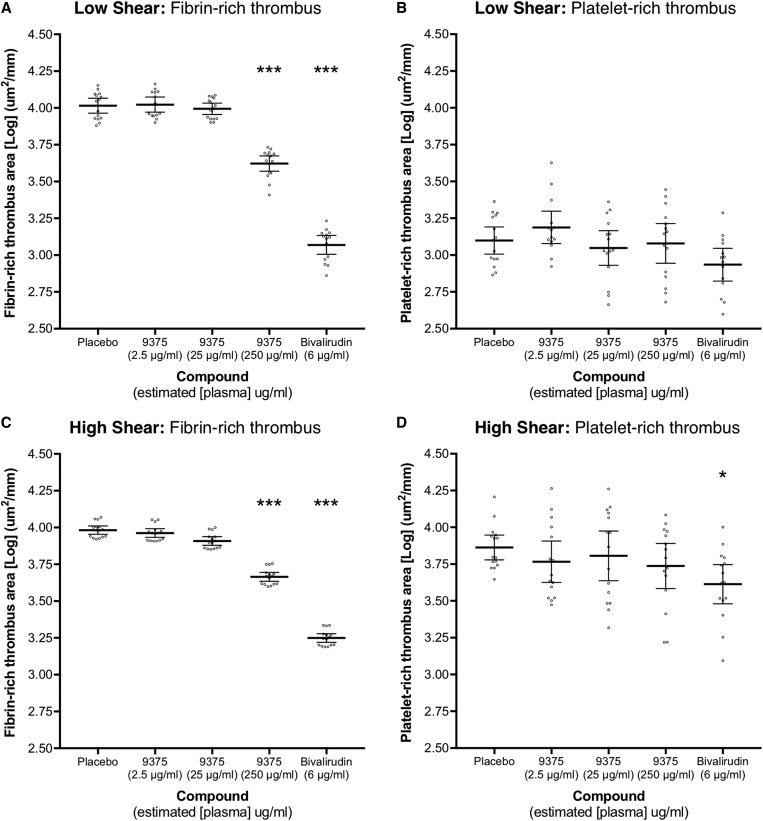
Reductions in total thrombus area were driven by a dose-dependent decrease in fibrin-rich thrombus deposition under both shear conditions (Figure 3). At peak dose (250 μg/mL), JNJ-9375 reduced fibrin-rich thrombus area by 59.5% (95% CI 37.8 to 73.7%; P < 0.001) at low shear and 51.8% (95% CI 37.7 to 62.7%; P < 0.001) at high shear. There was no reduction in platelet-rich thrombus area (P = ns for all). Chamber plasma concentrations of JNJ-9375 correlated with fibrin-rich thrombus area at low (ρ = −0.66, P < 0.001) and high (ρ = −0.70, P < 0.001) shear (Supplementary material online, Figure S1).
Figure 3.
The effect of study compound on the components of thrombus formation. Extra-corporeal administration of JNJ-9375 inhibited fibrin-rich thrombus deposition in a dose-dependent manner at both (A) low shear stress (212 s−1) and (C) high shear stress (1690 s−1) shear stress, as compared to placebo. JNJ-9375 had no effect on platelet-rich thrombus deposition at either shear stress. Bivalirudin reduced fibrin-rich thrombus deposition at low and high shear stress, and platelet-rich thrombus deposition at high shear stress. Data shown are the adjusted means (±95% confidence intervals) for [Log] fibrin- or platelet-rich thrombus area (μm2/mm) and individual points. Statistical comparisons (Least Significance Difference test) vs. placebo are represented above each plot: *P < 0.05, **P < 0.01, ***P < 0.001. 9375, JNJ-9375.
Effect of bivalirudin on ex vivo thrombus formation
Bivalirudin reduced total thrombus area at both low and high shear, also driven by a decrease in fibrin-rich thrombus formation (Figures 2 and 3). In contrast to JNJ-9375, there was a modest reduction (P = 0.01) in platelet-rich thrombus formation at high shear (Figure 3).
Discussion
In this double-blind randomized controlled crossover study, ex vivo administration of JNJ-9375, a highly specific exosite 1 thrombin inhibitor, resulted in dose-dependent prolongation of blood coagulation and selective inhibition of thrombin-stimulated platelet activation. Thrombosis was reduced under rheological conditions of both low and high shear stress, driven principally by a reduction in fibrin-rich thrombus formation. We conclude that JNJ-9375 holds promise as an anticoagulant for the prevention and treatment of thrombo-embolic events, and our results provide further insights into the role of exosite 1 in human thrombogenesis.
The outstanding challenge in anticoagulation is the development of drugs that can provide equivalent (or superior) antithrombotic efficacy but with a significantly lower bleeding risk. While the safety of JNJ-9375 has yet to be demonstrated in clinical trials, several lines of evidence indicate the potential for favourable outcomes. On a mechanistic level, selective inhibition of thrombin through exosite 1 specific antagonism is attractive because of the potential to inhibit fibrinogen binding without overly interfering with other (active site and exosite 2 dependent) protease interactions relating to haemostasis. For example, both the active site and exosite 2 are involved in catalytic feedback activation of clotting cofactors V, VIII, XI, and XIII, with deficiencies of each of these factors associated with bleeding diatheses.28–31
Thrombin is also a potent platelet agonist, and whilst over-aggregation may lead to pathological events, early platelet responses are central to haemostasis. Thrombin activates platelets through binding to platelet surface GPIb and protease-activated receptors 1 (PAR1) and 4 (PAR4).32 Exosite 1 interacts with PAR1 to facilitate efficient receptor cleavage,33 whereas PAR4 activation and GPIb binding are largely dependent on the active site and exosite 2, respectively.34,35 In the present study, JNJ-9375 selectively inhibited thrombin-stimulated platelet activation but was not associated with a reduction in platelet deposition. This is consistent with previous reports that exosite 1 inhibition only weakly inhibits thrombin-induced platelet aggregation and does not affect platelet collagen binding.36,37 Collectively, these results suggest potentially favourable differential effects on thrombin-platelet responses, that could be especially useful in clinical situations where combined treatment with an antiplatelet is required.38,39 This is speculative and requires further exploration. Future studies examining the effects of JNJ-9375 on platelet adhesion, thrombosis, and bleeding, alone and in combination with existing antiplatelet agents would be of interest.
Mechanistic evidence that exosite 1 thrombin inhibition may be associated with a low haemorrhagic potential is supported by data from animal studies of thrombosis and bleeding. Using a baboon arteriovenous shunt model, Cadroy et al.37 found that exosite 1 thrombin inhibition prevented thrombus formation but did not affect the ability to form haemostatic plugs. More recently, JNJ-9375 demonstrated a substantially wider therapeutic index when compared to apixaban in rats and cynomolgus monkeys.10 Further insight comes from the case report of an anti-exosite 1 thrombin IgA antibody (from which JNJ-937 was subsequently synthesized to mimic) identified in a patient presenting with a large traumatic subdural haematoma and persistently abnormal clotting studies.40 Despite evidence of intense anticoagulation (prothrombin time 40 s; activated partial thromboplastin time >240 s; thrombin time with bovine thrombin 173 s), the patient made a full recovery without surgical intervention and had no abnormal bleeding events during 8 years of follow-up.
Anticoagulants must in addition to avoiding unwanted bleeding provide clinically efficacious antithrombotic protection. Examination of the effect of exosite 1 thrombin inhibition on human thrombosis has previously been limited to studies using heparinised blood in a rabbit aortic angioplasty model41 and cone and plate chamber.42 This is the first description of the ex vivo antithrombotic effects of exosite 1 thrombin inhibition in native human blood under flow conditions. At a dose of 250 μg/mL, JNJ-9375 reduced total thrombus area by over 40% and 30% at low and high shear, respectively. Under the same conditions, high dose bivalirudin (equivalent to that used at the time of PCI) reduced thrombus formation by 65% at low shear and 56% at high shear; while in previous studies reductions of 14% with heparin (70 IU/kg bolus plus 15 IU/kg/h infusion),19 26–28% with oral edoxaban (60 mg)43 and up to 40% with serial dosing of the parenteral direct factor Xa inhibitor, DX-9065a,20 were reported. Importantly therefore, we have shown that exosite 1 thrombin antagonism alone with JNJ-9375 substantially reduces ex vivo human thrombus formation. Moreover, reductions were comparable (if not superior) in magnitude to the clinically approved anticoagulant edoxaban suggesting a high probability of in vivo antithrombotic efficacy.
JNJ-9375 resulted in dose-dependent prolongation of prothrombin time, activated partial thromboplastin time, and thrombin time. As expected, thrombin time was most sensitive to the anticoagulant effect. Although DOACs are licensed for use without the need for routine monitoring, there are clinical situations in which readily available assays to measure anticoagulant activity may be useful. Our data suggest that if indicated, thrombin time, and to a lesser extent prothrombin time and activated partial thromboplastin time, may provide a useful assay for measuring the effect of exosite 1 inhibition and JNJ-9375 activity.
Our study has some potential limitations. First, only a modest number of volunteers were studied. However, problems associated with intra-group variability were minimized by the crossover design that allowed each volunteer to serve as their own control. Second, although the exposed porcine aortic media used in the perfusion model presents many of the common constituents of an injured human blood vessel (including type I collagen), it is unlikely to contain tissue factor (TF).44–46 Tissue factor (TF) activates the coagulation cascade and is an important contributor to thrombogenicity.47,48 Nevertheless, this does not overly limit our model for the assessment of thrombosis because binding of blood borne circulating TF is sufficient to allow activation of the coagulation cascade and thrombus propagation.44,45,49–51 Indeed, previous studies have confirmed that thrombus formed from human blood perfused over porcine tunica media (devoid of TF) stains heavily for TF.44,45 Third, we used an anti-fibrin(ogen) antibody, which recognizes both fibrinogen and fibrin, to examine the fibrin component of thrombus formation. However, the chamber is perfused by saline at the end of the experiment washing away unbound cells, proteins, and other molecules, such as fibrinogen, leaving only adherent thrombus. Thus, histomorphometric quantification of fibrin-rich thrombus area is unlikely to be affected by this cross-reacting antibody, and our findings are consistent with previous studies using the same immunohistochemical approach.52–55 Fourth, while we have shown that exosite 1 thrombin inhibition reduces fibrin-rich thrombus formation, determining how JNJ-9375 alters the dynamics of clot development, stabilization, and dissolution might further inform therapeutic potential and are areas for future exploration. Finally, the study included ex vivo experiments only, and thus lacked hard clinical endpoints necessary to draw any conclusions regarding the safety or efficacy of this novel anticoagulant in practice. However, given this was a translational study designed to examine for the first time the effects of exosite 1 thrombin inhibition with JNJ-9375, we felt our study design appropriate.
In conclusion, JNJ-9375, a highly specific exosite 1 thrombin inhibitor, demonstrated substantial reductions in ex vivo thrombosis in native human blood under flow conditions. These reductions were driven by a decrease in fibrin-rich thrombus formation and were comparable in magnitude to clinically approved anticoagulants. Our findings suggest JNJ-9375 represents a promising novel class of anticoagulant, and that further clinical studies are warranted. A Phase 2 trial comparing the safety and efficacy of JNJ-9375 to apixaban in patients undergoing elective total knee replacement surgery is currently underway (ClinicalTrials.gov: NCT03251482).
Supplementary Material
Acknowledgements
We are grateful to the histology department of the Queen’s Medical Research Institute (Edinburgh, UK) for their support and expertise in conducting this study and to Cat Graham (Epidemiology and Statistics Core Manager, University of Edinburgh) for her assistance with outlining the statistical analysis plan and conducting the analysis, and to Deborah Preston, Janssen Preclinical Pathology Department.
Conflict of interest: S.J.W. and D.E.N. were supported by, and have undertaken consultancy for, Janssen. T.M.C., G.P., A.G., and M.J. are employees of Janssen.
Footnotes
Time for primary review: 13 days
Funding
D.E.N. is supported by the British Heart Foundation (CH/09/002, RM/13/2/30158, RE/13/3/30183) and is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). The Edinburgh Clinical Research Facility is supported by NHS Research Scotland. The Edinburgh Clinical Research Facility is supported by NHS Research Scotland. This study was funded by Janssen Research & Development, LLC.
References
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J-P, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh P-H, Yip P, Zabetian A, Zheng Z-J, Lopez AD, Murray CJL.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harter K, Levine M, Henderson SO.. Anticoagulation drug therapy: a review. WestJEM 2015;16:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, Davies PA, Bodalia PN, Bryden PA, Welton NJ, Hollingworth W, Caldwell DM, Savović J, Dias S, Salisbury C, Eaton D, Stephens-Boal A, Sofat R.. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017; doi: 10.1136/bmj.j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alamneh EA, Chalmers L, Bereznicki LR.. Suboptimal use of oral anticoagulants in atrial fibrillation: has the introduction of direct oral anticoagulants improved prescribing practices? Am J Cardiovasc Drugs 2016;16:183–200. [DOI] [PubMed] [Google Scholar]
- 5. Barra ME, Fanikos J, Connors JM, Sylvester KW, Piazza G, Goldhaber SZ.. Evaluation of dose-reduced direct oral anticoagulant therapy. Am J Med 2016;129:1198–1204. [DOI] [PubMed] [Google Scholar]
- 6. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM.. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2017;69:2475–2484. [DOI] [PubMed] [Google Scholar]
- 7. Franchini M, Mannucci PM.. Direct oral anticoagulants and venous thromboembolism. Eur Respir Rev 2016;25:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, Clifford T, Gandara E, Wells G, Carrier M.. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ 2013;347:f5133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bassand J-P, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KAA, Goldhaber SZ, Goto S, Haas S, Hacke W, Kayani G, Mantovani LG, Misselwitz F, ten Cate H, Turpie AGG, Verheugt FWA, Kakkar AK.. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J 2016;37:2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chintala M, Li Q, Huang-Devine Z, Andrade-Gordon P, Verrbeeck J, Baglin T, Huntington JA, Grainger D, Mason R, Conde-Knape K.. Improving the therapeutic index with JNJ-9375: a novel long acting exosite-1 thrombin inhibitor. Res Pract Thromb Haemost 2017;1:201. [Google Scholar]
- 11. Becker DL, Fredenburgh JC, Stafford AR, Weitz JI.. Exosites 1 and 2 are essential for protection of fibrin-bound thrombin from heparin-catalyzed inhibition by antithrombin and heparin cofactor II. J Biol Chem 1999;274:6226–6233. [DOI] [PubMed] [Google Scholar]
- 12. Lane DA. Directing thrombin. Blood 2005;106:2605–2612. [DOI] [PubMed] [Google Scholar]
- 13. Bock PE, Panizzi P, Verhamme IMA.. Exosites in the substrate specificity of blood coagulation reactions. J Thromb Haemost 2007;5:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chelliah R, Lucking AJ, Tattersall L, Daga S, Beresford-Cleary NJ, Cortas K, Fox KAA, Feuerstein GZ, Connolly TM, Newby DE.. P-selectin antagonism reduces thrombus formation in humans. J Thromb Haemost 2009;7:1915–1919. [DOI] [PubMed] [Google Scholar]
- 15. Lucking AJ, Chelliah R, Trotman AD, Connolly TM, Feuerstein GZ, Fox KA, Boon NA, Badimon JJ, Newby DE.. Characterisation and reproducibility of a human ex vivo model of thrombosis. Thromb Res 2010;126:431–435. [DOI] [PubMed] [Google Scholar]
- 16. Lucking AJ, Visvanathan A, Philippou H, Fraser S, Grant PJ, Connolly TM, Gardell SJ, Feuerstein GZ, Fox KAA, Booth NA, Newby DE.. Effect of the small molecule plasminogen activator inhibitor‐1 (PAI‐1) inhibitor, PAI‐749, in clinical models of fibrinolysis. J Thromb Haemost 2010;8:1333–1339. [DOI] [PubMed] [Google Scholar]
- 17. Lev EI, Marmur JD, Zdravkovic M, Osende JI, Robbins J, Delfin JA, Richard M, Erhardtsen E, Thomsen MS, Lincoff AM, Badimon JJ.. Antithrombotic effect of tissue factor inhibition by inactivated factor VIIa: an ex vivo human study. Arterioscler Thromb Vasc Biol 2002;22:1036–1041. [DOI] [PubMed] [Google Scholar]
- 18. Wåhlander K, Eriksson-Lepkowska M, Nyström P, Eriksson U, Sarich T, Badimon J, Kalies I, Elg M, Bylock A.. Antithrombotic effects of ximelagatran plus acetylsalicylic acid (ASA) and clopidogrel plus ASA in a human ex vivo arterial thrombosis model. Thromb Haemost 2006;95:447–453. doi: 10.1160/TH05-10-0664. [DOI] [PubMed] [Google Scholar]
- 19. Hayes R, Chesebro JH, Fuster V, Dangas G, Fallon JT, Sharma SK, Coller BS, Badimon L, Marmur JD, Badimon JJ.. Antithrombotic effects of abciximab. Am J Cardiol 2000;85:1167–1172. [DOI] [PubMed] [Google Scholar]
- 20. Shimbo D, Osende J, Chen J, Robbins J, Shimoto Y, Kunitada S, Fuster V, Badimon JJ.. Antithrombotic effects of DX-9065a, a direct factor Xa inhibitor: a comparative study in humans versus low molecular weight heparin. Thromb Haemost 2002;88:733–738. [PubMed] [Google Scholar]
- 21. Zafar MU, Ibanez B, Choi BG, Vorchheimer DA, Piñero A, Jin X, Sharma RK, Badimon JJ.. A new oral antiplatelet agent with potent antithrombotic properties: comparison of DZ-697b with clopidogrel a randomised phase I study. Thromb Haemost 2009;103:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Transport phenomena, Bird R. B., W. E Stewart, E. N Lightfoot, John Wiley and Sons, Inc., New York (1960). 780 pages. $11.50. AIChE J 1961;7:5J–6J. [Google Scholar]
- 23. Badimon L, Padro T, Vilahur G.. Extracorporeal assays of thrombosis In Platelets and Megakaryocytes, vol. 788 Methods in Molecular Biology. New York, NY: Springer New York; 2011. p43–57. [DOI] [PubMed] [Google Scholar]
- 24. Michelson AD, Furman MI.. Laboratory markers of platelet activation and their clinical significance. Curr Opin Hematol 1999;6:342–348. [DOI] [PubMed] [Google Scholar]
- 25. Michelson AD. Platelet activation by thrombin can be directly measured in whole blood through the use of the peptide GPRP and flow cytometry: methods and clinical applications. Blood Coagul Fibrinolysis 1994;5:121–131. [DOI] [PubMed] [Google Scholar]
- 26. Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI.. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001;104:1533–1537. [DOI] [PubMed] [Google Scholar]
- 27. Dangas G, Badimon JJ, Coller BS, Fallon JT, Sharma SK, Hayes RM, Meraj P, Ambrose JA, Marmur JD.. Administration of abciximab during percutaneous coronary intervention reduces both ex vivo platelet thrombus formation and fibrin deposition: implications for a potential anticoagulant effect of abciximab. Arterioscler Thromb Vasc Biol 1998;18:1342–1349. [DOI] [PubMed] [Google Scholar]
- 28. Levy JH, Greenberg C.. Biology of factor XIII and clinical manifestations of factor XIII deficiency. Transfusion 2013;53:1120–1131. [DOI] [PubMed] [Google Scholar]
- 29. Duckers C, Simioni P, Rosing J, Castoldi E.. Advances in understanding the bleeding diathesis in factor V deficiency. Br J Haematol 2009;146:17–26. [DOI] [PubMed] [Google Scholar]
- 30. Franchini M, Gandini G, Di Paolantonio T, Mariani G.. Acquired hemophilia A: a concise review. Am J Hematol 2005;80:55–63. [DOI] [PubMed] [Google Scholar]
- 31. Gomez K, Bolton-Maggs P.. Factor XI deficiency. Haemophilia 2008;14:1183–1189. [DOI] [PubMed] [Google Scholar]
- 32. Lundblad RL, White GC.. The interaction of thrombin with blood platelets. Platelets 2005;16:373–385. [DOI] [PubMed] [Google Scholar]
- 33. Jacques SL, LeMasurier M, Sheridan PJ, Seeley SK, Kuliopulos A.. Substrate-assisted catalysis of the PAR1 thrombin receptor. Enhancement of macromolecular association and cleavage. J Biol Chem 2000;275:40671–40678. [DOI] [PubMed] [Google Scholar]
- 34. Jacques SL, Kuliopulos A.. Protease-activated receptor-4 uses dual prolines and an anionic retention motif for thrombin recognition and cleavage. Biochem J 2003;376:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adam F, Guillin M-C, Jandrot-Perrus M.. Glycoprotein Ib-mediated platelet activation. A signalling pathway triggered by thrombin. Eur J Biochem 2003;270:2959–2970. [DOI] [PubMed] [Google Scholar]
- 36. Wu CC, Wang WY, Wei CK, Teng CM.. Combined blockade of thrombin anion binding exosite-1 and PAR4 produces synergistic antiplatelet effect in human platelets. Thromb Haemost 2010;105:88–95. [DOI] [PubMed] [Google Scholar]
- 37. Cadroy Y, Maraganore JM, Hanson SR, Harker LA.. Selective inhibition by a synthetic hirudin peptide of fibrin-dependent thrombosis in baboons. Proc Natl Acad Sci USA 1991;88:1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lip GYH, Windecker S, Huber K, Kirchhof P, Marin F, Berg Ten JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Document Reviewers, Storey RF, Bueno H, Collet J-P, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL.. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 2014;35:3155–3179. [DOI] [PubMed] [Google Scholar]
- 39. Moser M, Olivier CB, Bode C.. Triple antithrombotic therapy in cardiac patients: more questions than answers. Eur Heart J 2014;35:216–223. [DOI] [PubMed] [Google Scholar]
- 40. Baglin TP, Langdown J, Frasson R, Huntington JA.. Discovery and characterization of an antibody directed against exosite I of thrombin. J Thromb Haemost 2016;14:137–142. [DOI] [PubMed] [Google Scholar]
- 41. Li WX, Kaplan AV, Grant GW, Toole JJ, Leung LL.. A novel nucleotide-based thrombin inhibitor inhibits clot-bound thrombin and reduces arterial platelet thrombus formation. Blood 1994;83:677–682. [PubMed] [Google Scholar]
- 42. Mendelboum Raviv S, Horváth A, Aradi J, Bagoly Z, Fazakas F, Batta Z, Muszbek L, Hársfalvi J.. 4-Thio-deoxyuridylate-modified thrombin aptamer and its inhibitory effect on fibrin clot formation, platelet aggregation and thrombus growth on subendothelial matrix. J Thromb Haemost 2008;6:1764–1771. [DOI] [PubMed] [Google Scholar]
- 43. Zafar MU, Vorchheimer DA, Gaztanaga J, Velez M, Yadegar D, Moreno PR, Kunitada S, Pagan J, Fuster V, Badimon JJ.. Antithrombotic effects of factor Xa inhibition with DU-176b: phase-I study of an oral, direct factor Xa inhibitor using an ex-vivo flow chamber. J Thromb Haemost 2007;98:883–888. [DOI] [PubMed] [Google Scholar]
- 44. Giesen PL, Rauch U, Bohrmann B, Kling D, Roqué M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y.. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA 1999;96:2311–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rauch U, Nemerson Y.. Circulating tissue factor and thrombosis. Curr Opin Hematol 2000;7:273–277. [DOI] [PubMed] [Google Scholar]
- 46. Badimon L, Badimon JJ.. Mechanisms of arterial thrombosis in nonparallel streamlines: platelet thrombi grow on the apex of stenotic severely injured vessel wall. Experimental study in the pig model. J Clin Invest 1989;84:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Badimon JJ, Lettino M, Toschi V, Fuster V, Berrozpe M, Chesebro JH, Badimon L.. Local inhibition of tissue factor reduces the thrombogenicity of disrupted human atherosclerotic plaques: effects of tissue factor pathway inhibitor on plaque thrombogenicity under flow conditions. Circulation 1999;99:1780–1787. [DOI] [PubMed] [Google Scholar]
- 48. Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, Fernandez-Ortiz A, Chesebro JH, Badimon L, Nemerson Y, Fuster V, Badimon JJ.. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation 1997;95:594–599. [DOI] [PubMed] [Google Scholar]
- 49. Giesen PL, Nemerson Y.. Tissue factor on the loose. Semin Thromb Hemost 2000;26:379–384. [DOI] [PubMed] [Google Scholar]
- 50. Balasubramanian V. Platelets, circulating tissue factor, and fibrin colocalize in ex vivo thrombi: real-time fluorescence images of thrombus formation and propagation under defined flow conditions. Blood 2002;100:2787–2792. [DOI] [PubMed] [Google Scholar]
- 51. Rauch U, Bonderman D, Bohrmann B, Badimon JJ, Himber J, Riederer MA, Nemerson Y.. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 2000;96:170–175. [PubMed] [Google Scholar]
- 52. Vilahur G, Baldellou MI, Segalés E, Salas E, Badimon L.. Inhibition of thrombosis by a novel platelet selective S-nitrosothiol compound without hemodynamic side effects. Cardiovasc Res 2004;61:806–816. [DOI] [PubMed] [Google Scholar]
- 53. Vilahur G, Duran X, Juan-Babot O, Casaní L, Badimon L.. Antithrombotic effects of saratin on human atherosclerotic plaques. Thromb Haemost 2004;92:191–200. [DOI] [PubMed] [Google Scholar]
- 54. Vilahur G, Casaní L, Badimon L.. A thromboxane A2/prostaglandin H2 receptor antagonist (S18886) shows high antithrombotic efficacy in an experimental model of stent-induced thrombosis. Thromb Haemost 2007;98:662–669. [PubMed] [Google Scholar]
- 55. Wilson SJ, Ismat FA, Wang Z, Cerra M, Narayan H, Raftis J, Gray TJ, Connell S, Garonzik S, Ma X, Yang J, Newby DE.. PAR4 (Protease-Activated Receptor 4) antagonism with BMS-986120 inhibits human ex vivo thrombus formation. Arterioscler Thromb Vasc Biol 2018;38:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.