Abstract
Interpersonal attachment and drug addiction share many attributes across their behavioral and neurobiological domains including how they grow and decay within an individual’s motivational repertoire. Understanding the overlapping brain circuitry of attachment formation and addiction illuminates a deeper understanding of the pathogenesis of trauma-related mental illnesses and comorbid substance use disorders, and the extent to which ending an addiction is complicated by being a sort of mourning process. Attention to the process of addiction recovery—as a form of grieving— in which Kubler-Ross’s Stages of Grief and Prochaska’s Stages of Change are ultimately describing complimentary viewpoints on a general process of neural network and attachment remodeling, could lead to more effective and integrative psychotherapy and medication strategies.
Keywords: addiction, motivation, attachment, grief, recovery, stages of change, hippocampus, prefrontal cortex, nucleus accumbens
LIFE GOES ON
I can’t remember the first time we met…
But I do know that from the beginning
We formed a lifetime bond.
Maybe many life time bonds.
You were comfortable, familiar
As if I had known you forever.
It was so easy to just be with you.
I think everyone felt that way around you.
Actually when I think about it,
I think you were around much longer,
And before we even met
Maybe even before I was born.
When I look at old pictures of my family
I see you there too.
In the faces and in the places.
What a loyal friend you have been to all of us.
Showing up at every occasion:
Celebrations, quiet moments with friends, times of deep despair,
And even when no one else was there.
Never intrusive really, sometimes not even noticeable,
Sometimes you were the life of the party.
When I met you, I knew I had found a friend forever.
I knew you would never leave me,
And you always made me feel better no matter what.
When I felt lonely, or sad, anxious, angry, or even happy.
You were always there.
We had sooooo much fun together.
All of us loved you so much.
I’m not sure when all that changed;
You exited as subtly and quietly as you entered.
Your power to make me feel stronger slipped away gradually, almost without notice.
But in your wake you left your mark of betrayal and heartache.
You tried to take everything away.
But life goes on you see,
New generations are on the horizon,
And we’ll be ok. –SCW
INTRODUCTION
As suggested in this poetic dialogue with nicotine addiction, many parallels exist between the experiences individuals have in their attachments to loved ones, and their attachments to drugs in addiction. In the last decade, neuroscience has converged with clinical observation to suggest a new understanding of the biological and behavioral aspects of drug addiction as being not just a disease of motivation (Chambers, Bickel, & Potenza, 2007; Kalivas & Volkow, 2005)—but an illness of neural systems that normally generate and support our crucial attachments to each other (Burkett & Young, 2012).
Emerging data and theory from the fields of anthropological evolution and social neuroscience advanced by Harari and others (Harari, 2015; Sherwood, Subiaul, & Zawidzki, 2008) provide a compelling story of how humans have been so evolutionarily successful and dominant on our planet. Because of our uniquely powerful brain capacities for creating and maintaining social bonds and group cohesion— through the invention, communication, and projection of shared imaginary fictions—homo sapiens are able to generate concerted action on an incomparable scale and degree of potency, overcoming the limits of time and geography that constrain all other species. Clearly, our capabilities of language, abstract thinking, social cognition and our drive to form and keep social bonds—long before they made us so powerful that we could collectively destroy the planet through nuclear war, or climate change, or more optimistically, put people on the moon—have been keys to our evolutionary success. Now, understanding how our brain systems that allow our gifts of social cognition and attachment, could also be our ‘Achilles Heel’ in terms of vulnerabilities to mental illness and addiction, has become an important new frontier in psychiatric neuroscience.
This paper will explore new perspectives on addictive disease as informed by attachment neuroscience and theory that could inform methods for treatment and recovery, and open novel research avenues in addiction and dual diagnosis care. A brief overview of the similarities and overlaps between human attachment and addiction on behavioral and brain levels will set the stage for understanding what is happening in the struggle for recovery, and what needs to happen therapeutically to help patients more efficiently and successfully detach from their addictions. A reinterpretation and synthesis of two of the most influential staging theories in psychiatry: 1) the Stages of Grief by Kubler-Ross (Kubler-Ross, 1969), and, 2) Stages of Change by Prochaska & DiClemente (Prochaska & DiClemente, 2005), will inform this discussion. Exploring how the stages of grief and the stages of addiction recovery may actually be variants of a more general and unified process of attachment adaptation that is at once underpinned by the same brain biology and susceptible to shared pathological processes, opens new frontiers for designing more powerful treatments that integrate psychotherapies and medications.
ADDICTION AND ATTACHMENT: THE CLINICAL OVERLAP
Drug addiction is a neurodevelopmental disease primarily centered in the motivational circuits of the brain (Chambers et al., 2007; Chambers, Taylor, & Potenza, 2003; Kalivas & Volkow, 2005). Both DSM criteria and basic research on addiction point to cumulative pathological alterations in motivated behavior, and biological changes in the brain’s key motivational control center—the Nucleus Accumbens ((NAC) or ventral striatum)— as being core to the process of addiction (Self & Nestler, 1995; Volkow, 2004). The NAC can be understood as the principal neural network in the brain that stores, processes, and creates motivational representations that guide, sequence and select the behavioral programs we act out (Chambers et al., 2007). When we are healthy, our motivational system allows us to organize and execute behavioral sequences that optimally help us explore the world, procure resources, reproduce, take care of our children, and do it all over again. The NAC is the neurobiological seat of our ‘will power’ and it is a primary neurobiological engine that drives our will to survive.
Given the extent that our social behavior and motive for cohesion has been so crucial to our evolutionary success, it should be no surprise that a substantial portion of the function and biophysical real estate of the NAC and its connectivity with other brain regions is devoted to mediating social relationships, that is, the formation and maintenance of ‘conspecific’, i.e. human to human bonds (but let us not forget our beloved pets which are our inter-species attachments!) (Depue & Morrone-Strupinsky, 2005). In this way, we begin to appreciate that much in the same way people feel attached to their families of origin, or how they acquire new romantic partners, the acquisition and maintenance of drug addiction could likely be understood as a pharmacological exploitation of biological mechanisms that normally generate attachments between friends, family and lovers (Burkett & Young, 2012).
The parallels between falling in love vs. becoming addicted are remarkable. The early stages of both are marked by arousal, euphoria, and increasing preoccupations with the love object (person/drug) including a growing desire to be around, and, in some way or another, to consume, be consumed by, or merge with the ‘person/drug’. In more progressed stages, separation and withdrawal from the ‘person/drug’ correlates with a sense of loss, dysphoria, changes in sleep and appetite, and yearning, all congealing to vigorous efforts to re-establish access to the ‘person/drug’.
Recognizing these parallels brings us to taking a closer look at the extent to which addiction is not just a disease of the motivational system, but a disease of the social attachment system of the brain. In fact, could it be all that it is? Could addiction be a disease purely of systems that control attachment and mediate complex social behavior?
Not likely. For example, trans-species animal research on addiction shows that drugs that are addictive to humans, monkeys and other mammals, are also reinforcing for much lower order animals such as C. Elegans worms, which have brains with only 302 neurons (Engleman, Katner, & Neal-Beliveau, 2016; Katner, Neal-Beliveau, & Engleman, 2016) and show nothing close to the complexity and power of mammalian social behavior. Also, when animals or people do acquire drug addiction, the disease can be quite broad in terms of its destructive effects. Far more than social behavior and obligations are impacted. In severe addiction, the individual can experience a comprehensive loss of motivated behavior spanning social and occupational domains of function, not to mention incurring serious psychiatric and somatic damage. Finally, the biological mechanisms involved in addiction are clearly more general than those involved specifically in social behavior and attachment. For example, the pharmacological release of dopamine (DA) into the NAC produced by essentially all major addictive drug types, mimics endogenously activated DA release that occurs in response to a very wide variety of naturally reinforcing and motivating stimuli including food, sex, game winning, stress, novelty, and social interaction (Berke & Hyman, 2000; Wise, 1998).
But with these caveats in mind, the connection between addiction and social attachment becomes quite compelling even on the epidemiological level in considering how addictions spread or recede like contagious epidemics, how different sub-populations are differentially vulnerable to addiction, or differentially resistant to, or responsive to treatment (Christakis & Fowler, 2008). Peer pressure has long been known to propagate drug experimentation among adolescents who are also biologically primed to acquire addictive disease while their motivational-behavior repertoires are rapidly expanding to take on adult social, occupational and sexual roles (Chambers et al., 2007; Chambers et al., 2003). And, in drug users of all ages, using, sharing, and selling drugs often happens in a highly social context or network (e.g. at the party), or more exclusively, in self-selected subgroups that break off from the larger group. People not only like to use drugs and alcohol, but they like to do it together, and, they tend to spread these ‘resources’ to others they are attached to, or want to be attached to. Maybe, this is a manifestation of ancient mammalian behavior (e.g. where the clan comes together to share the consumable resources), and something more neurobiologically intricate, e.g. like a synergistic mixing of social or sexual brain reward processes with the reinforcement of drug use (Schneider & Irons, 2001). Regardless, drug use propagates readily across the scaffoldings of human social networks, so much so that the epidemiology of drug use ‘outbreaks’ can look remarkably similar to, and even go along with outbreaks of infectious diseases, which are also often propagated via social contacts.
These social dynamics also play into the level of success of individuals who are trying to stop using. Patients who persist in living with family members who are actively using are more treatment refractory (Lavee & Altus, 2001; Simmons & Singer, 2006). On the other hand, a major therapeutic mechanism of Alcoholics Anonymous (AA) and professionalized variants of 12-step Groups, likely involves their offering of social support and reward that comes with not using (Galanter, 1993; Moos, 2008). A key area of addiction treatment research that relates to patient’s social behavior focuses on the need for teams to be able to determine what patient indicators best predict clinical response. This area of addiction research, which has become increasingly important given the scarcity of treatment resources, infrastructure, and professionals that currently exist in proportion to the vast unmet clinical need, has produced a replicated observation that the attachment style of patients, and their capacity to attach to their treatment team is a major determinant of their ability to successfully recover (Borelli, Goshin, Joestl, Clark, & Byrne, 2010; Caspers, Yucuis, Troutman, & Spinks, 2006; Fowler, Groat, & Ulanday, 2013; Kassel, Wardle, & Roberts, 2007; Schindler, Thomasius, Petersen, & Sack, 2009; Vaillant, 1988). As we will explore next on a more neurobiological level, patients with a variety of mental illnesses that impact their attachment functions are more vulnerable biologically to acquiring addiction and developing more severe forms of the disease. At the same time, not all psychiatric illnesses that produce deficits in social interaction and/or attachment, always worsen addiction vulnerability, and in fact might have the opposite effect. For example, autism spectrum disorders may be somewhat protective against addiction. Clearly, the causal connections between addiction pathogenesis, mental illness, and attachment abnormalities are real, but quite complex and nuanced. Understanding the neurobiological substrates that connect these phenomena, as introduced next, will be important to developing better treatments for patients with dual diagnosis disorders that involve attachment abnormalities.
ADDICTION AND ATTACHMENT: THE NEUROBIOLOGICAL OVERLAP
The core circuitry of the brain involved in motivational learning and control, the NAC, is a key location where addiction pathogenesis takes place (Di Chiara, 2002; Kauer & Malenka, 2007; Self & Nestler, 1998). Understanding how motivation works, and what roles the NAC plays in relation to other key limbic structures involved in social behavior, can help us begin to link disturbances of attachment with the pathogenesis of addiction.
Motivational programming that takes place in the NAC is strongly regulated and informed by direct inputs from the Prefrontal cortex (PFC), Amygdala (AMY), and Ventral Hippocampus (VHIP) (Goto & Grace, 2005; O’Donnell, Greene, Pabello, Lewis, & Grace, 1999). In this architecture, executive decision-making and impulse control functions of the PFC are integrated with emotional states and affective memories perceived/generated/represented by the AMY, along with contextual memory information provided by the VHIP—all on behalf of generating an ‘optimal’ motivational representational code within the NAC (Finch, 1996). In turn, this motivational code, calls up, selects, sequences and directs, motor representational codes that are generated, stored, modified and played out within the dorsal striatum (Chambers et al., 2007; Haber, Fudge, & McFarland, 2000; Masterman & Cummings, 1997), which ultimately engages the pyramidal motor system (motor cortex/peripheral motor neurons) to output behavioral programs (Figure 1).
Figure 1.
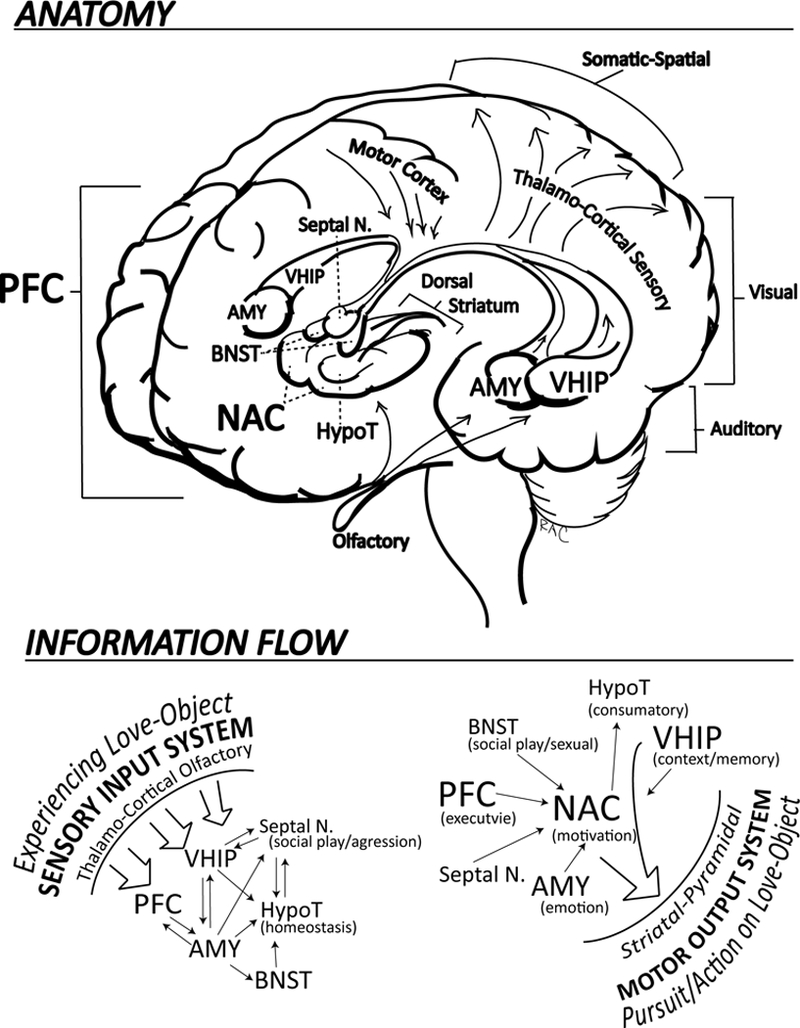
Social-Attachment-Motivation System
Within the NAC, there are a great number of motivational codes that are stored and can be ‘called into action.’ Some of these motivational codes are more oriented toward social behavior (e.g. the motivation to be nearby and interact with loved ones), while others are more oriented toward occupational behavior (e.g. the motivation to leave our family to go on a paid business trip). Thus, differential types or classes of motivation, may or may not be in some degree of competition with one another (Clithero, Reeck, Carter, Smith, & Huettel, 2011). In the course of drug addition, an accumulation of addictive drug effects within the NAC, produces a pathological introduction and progression of a strong desire (motivation) to procure and use the addictive drug(s). But, this motivation is almost always accompanied by some degree of still healthy motivation that the individual also harbors to stop using, e.g. consistent with the DSM criteria for addiction as continued drug use despite competing desires or attempts to cut back or quit. When these 2 motivations (to use vs. not to use) are in competition inside the brains of people with addiction, it is felt by the patient, and observed by the clinician, as ambivalence. Similarly, in people who are in a relationship that they have “mixed feelings” about, they carry a burning question with respect to the attachment, as the punk band The Clash so iconically sang: “Should I Stay or Should I Go.”
So, just as the formation and maintenance of interpersonal attachment resembles drug addiction, we should look at their shared neurocircuitry and neurotransmitter systems for discovering novel preventative and treatment interventions. Within the PFC-NAC-AMY-VHIP Circuit (Figure 1), which functional neuroimaging data indicates is involved in experiencing romantic love (Bartels & Zeki, 2000), we know that DA neurotransmission is very important, mediating the neuroplastic effects of essentially all addictive drugs (nicotine, alcohol, opioids, cocaine, amphetamines) (Di Chiara & Imperato, 1988; Wise, 1990). DA is a key facilitator and ‘sculptor’ of the molecular, electrical (i.e. physiological) and morphological (e.g. shapes and densities of dendrites and spines) aspects of neuroplasticity within the NAC (Nestler & Aghajanian, 1997; Robinson, Gorny, Mitton, & Kolb, 2001; Wolf, 2002). In other words, not only does DA efflux signal the presence of motivationally salient information in the NAC, but it changes the function, structure and connectivity architecture of NAC neurons. These changes in turn, impact the way the NAC ‘reads’ and processes inputs from the PFC, AMY, and VHIP, and thus, the way the NAC communicates with down-stream striatal-motor output structures. In essence, certain patterns of DA efflux into the NAC can produce enduring changes in motivation underlying the formation of relatively locked-in motor sequences, including habits and compulsions that are core to addiction (Chambers et al., 2007).
A number of excellent reviews with neurobiological depth have now been published outlining the evidence for DA and other neurotransmitter systems involved in both addiction and social attachment (Burkett & Young, 2012; Depue & Morrone-Strupinsky, 2005; Fletcher, Nutton, & Brend, 2015). In briefly summarizing this evidence, we know that DA is a general motivational and socially relevant reinforcement signal in the NAC. A host of other neurotransmitter and neuro-hormonal systems are also implicated including serotonin, oxytocin, vasopressin, corticosteroids, corticosterioid releasing factor, and the endogenous opioid system to name a few. Similarly, a broader collection of subcortical structures beyond the NAC, but interconnected with it (and the PFC-AMY-VHIP assembly), are invested in governing social motivation and behavior. These include the Septal Nuclei (Septal N. also called Lateral Septum; involved in social play and aggression) (Sheehan, Chambers, & Russell, 2004); the Bed Nucleus of the Stria Terminalis (BNST, also often considered as part of the ‘extended amygdala’; involved in social anxiety and sexual behavior) (Avery, Clauss, & Blackford, 2016; Petrulis, 2013), and of course the hypothalamus (HypoT; involved in a wide range of primitive homeostatic, consummatory and sexual behaviors) (Zha & Xu, 2015) (Figure 1). Although the complexities of these interacting systems do not yet permit us to unravel exactly how they govern social motivation and communication, new data-based theories are emerging to suggest how they might link attachment, addiction and stress resilience. For instance, oxytocin is strongly implicated in the formation and maintenance of intimate relationships and the maternal-fetal bond, while it also helps regulate hypothalamic-pituitary axis (HPA) and corticosteroid responsivity to external stressors and threats (e.g. creating the sense of relative safety among loved ones). The oxytocin system may serve these functions by facilitating the habit formation of behaviors surrounding exclusive relationships, by modulating the way the NAC interacts and influences the dorsal striatum (Tops, Koole, H, & Buisman-Pijlman, 2014).
Among all of these neurotransmitter systems, research on the endogenous opioid system offers some of the most compelling insights into the overlap between attachment and addiction. The endogenous opioid system is not only involved in drug reinforcement and pain perception via activity at μ-opiate receptors in the NAC and cortico-thalamic centers; it also regulates early attachment formation between infants and their primary care givers (Copeland et al., 2011). While injection of opioids or μ-opiate receptor blockers can modulate the formation or maintenance of these early attachments, cycles of separations and reunions between mother-infant pairs have biological effects on the endogenous opioid system, and have behavioral similarities with phases of opioid withdrawal and intoxication (Gustafsson, Oreland, Hoffmann, & Nylander, 2008; Kalin, Shelton, & Lynn, 1995).
These observations make it readily conceivable that abnormal patterns of early attachment, e.g. marked by chaotic inconsistencies, neglectful and/or abusive interactions, can cause a disturbance in the development and function of the endogenous opioid system itself, and/or downstream social and motivational networks that this system regulates. Hence, experience-induced ‘malformation’ of the endogenous opioid system in childhood may play a causal role in the emergence of borderline personality by early adulthood (Bandelow, Schmahl, Falkai, & Wedekind, 2010), which is of course, heavily comorbid with drug addictions (Trull, Sher, Minks-Brown, Durbin, & Burr, 2000). At the same time, early social-environmental trauma and chaotic attachment is a major risk factor for adult addiction in rodents and humans alike (Lawson, Back, Hartwell, Moran-Santa Maria, & Brady, 2013; Moffett et al., 2007). Interestingly, a recent study from our own dual diagnosis clinic has shown that that the likelihood a patient will be diagnosed with a personality disorder on initial psychiatric interview increases with the number of prescribers providing opioids to that patient in the year prior to the interview (Hackman et al., 2014).
The sadomasochism that pervades borderline spectrum illness is also highly suggestive of neurodevelopmental misalignments involving pleasure-pain perception, motivational systems, and social behavior (Bandelow et al., 2010; Pechtel & Pizzagalli, 2013). The way borderline patients often show a) cycles of impulsive sexuality and self-destructive behaviors in their compulsive engagement with abusive romantic partners (Carnes, 1997), and b) extreme binging patterns of drug use, can be remarkably similar and intertwined. Patients engaged in prostitution not only typically have childhood histories of sexual and emotional abuse and adult borderline symptoms, but their prostitution is often immersed in drug addiction, both as a means to afford addictive drugs, and as a context in which they use and dissociate.
Early attachment disruption, and early abuse/neglect are often interrelated phenomena that have been well known to be key root causes of adult psychopathology (Bowlby, 1988, 1995; Harlow, Dodsworth, & Harlow, 1965). New research is beginning to characterize how these experiences represent biologically potent and developmentally neurotoxic events that alter key limbic centers like the PFC, AMY and VHIP, leading to mental illness—and the functionality of the NAC, leading to increased risk of becoming drug addicted (Heim, Shugart, Craighead, & Nemeroff, 2010; Pechtel & Pizzagalli, 2013; Van Dam, Rando, Potenza, Tuit, & Sinha, 2014; Vela, 2014). For example trauma-spectrum disorders (encompassing PTSD, cluster B personality and affective disorders) are generally highly comorbid with drug addiction, while animal models of these mental illnesses show impairments in neuroplasticity and neurogenesis within the VHIP (Chambers, 2013). In turn, early neurodevelopmental damage to the VHIP increases motivational responsivity to addictive drugs (i.e. increasing the probability of acquiring addiction), by changing the way the NAC and dorsal striatum respond to their DA-induced neuroplastic effects (Chambers et al., 2013; Chambers & Self, 2002).
In summary, clinical and basic science are showing that brain mechanisms involved in attachment and addiction are overlapping and mutually engaged to such a high degree that the addicted patient experiences their attachment to their drug (s) as if they were loved ones; as if the drug(s) were people they are in a close relationship with. Pursuing this understanding further scientifically and translating it clinically, may improve outcomes in the care of addicted and dual diagnosis patients, as considered next.
ADDICTION RECOVERY: GRIEVING AND ATTACHMENT ADAPTATION
A helpful framework for understanding the illness that patients with addiction (and comorbid mental illness) have, is one that views their disease state as a trap that they need help getting out of. Over time, the addiction has created a pathological limitation of their free will and capacity to enact adaptive choices. It has limited their motivational-behavioral repertoire to an abnormally narrow set of ‘programs’ dedicated to acquiring and using drugs at the expense of healthy motivations and behaviors (Chambers, 2008). Mental illness, which is often present to some degree as a context for severe addiction, accelerates this process, because it not only changes the reinforcing power of addictive drugs (Chambers, Krystal, & Self, 2001), but it produces impulsive behavior and narrowing of the motivational-behavioral repertoire even before drug use starts to kindle addictive disease (Chambers et al., 2007). Then, as the drug addiction takes hold, drug use generates even more psychiatric symptoms (or worsens those already there), while further degrading the decision-making that is needed to perform adaptive occupational and social (family) functions. Notably, some of the earliest known usage of the term “addiction” was in Roman antiquity, referring to a bond of slavery, or the state of servitude of debtors to lenders or to those whom they owed restitution. Thus addiction is a trap, or state of servitude and enslavement of the afflicted person, in which wanting, desire, loyalty and behavior is constrained to and focused on the drug-object, at the expense of ‘free will’ and often, the health and longevity of the patient.
Reflecting this pathological behavioral change, neuroscience research has shown that primary motivational circuits (PFC/NAC/AMY/VHIP) impacted by addiction become pathologically inflexible in some of their structural and functional attributes (Chambers et al., 2007). Over time, addictive drug use literally begins to wear out and impair mechanisms and structures that allow normal neuroplasticity and motivational learning and memory, as reflected by multiple interactive pathological processes involving i) physical changes in cortical-striatal synaptic spines and dendrites; ii) abnormal regulation of dopamine and endogenous opioid neurotransmission; and iii) impaired hippocampal neurogenesis (Chambers, 2013; Hyman, 2005; Kalivas & O’Brien, 2008). More specifically, chronic addictive drug use causes abnormal growth in dendritic arborizations and synaptic connectivity in motivational (PFC-NAC) neural networks, likely making these systems both ‘forgetful’ of representing already-learned healthy motivations, and refractory to acquiring important new motivations. At the same time, the repetitive pharmacological stimulation of dopamine and/or opioid neurotransmission and receptors causes the brain to respond with a wide array of homeostatic changes that end up diminishing the brain’s capacity to properly use these same systems for signaling related to non-drug environmental cues and experiences. Finally, within the hippocampus (which is connected with core motivational circuity via VHIP to NAC axons), the chronic-toxic effects of addictive drugs to suppress neurogenesis and other forms of plasticity, causes a breakdown in the brain’s ability to integrate current and past experience, and to use this information to guide the formation of new adaptive motivational programs. Thus in addiction, the core motivational networks of the brain become structurally and functionally inflexible, much as the behavior of the patient is rigidly and compulsively stuck on the drug-turned love object.
In this framework, we can see the great difficulty of producing a therapeutic rescue and liberation of the patient from their imprisoning addiction, because it is also about a ‘love affair’ with the drug that is keeping them imprisoned. Indeed, encouraging a patient to move into recovery via Motivational Enhancement Therapy is practically equivalent to convincing them to kill a primary, intensively held, and yet pathological relationship. While reminding us of what a successful psychotherapy must sometimes help patients accomplish—the decisive ending of an intimate (but destructive) relationship— this framework of ending and mourning the ending of an important relationship is routinely evident when observing patients pursuing addiction recovery. For example, drug-relapse dreams are a common experience in early to middle stage abstinent patients that can produce a mixture of feelings (fear/anxiety/longing/relief) worth discussion in the treatment setting (Christo & Franey, 1996; Johnson, 2001). Similarly, recurrent dreams of loved ones lost are very common in the grieving process, and, while producing sadness and/or comfort in the short term, may be healing in the long term (Wray & Price, 2005). Given evidence that dreams, and more generally, REM sleep are involved in the consolidation of short and long-term memory via PFC-hippocampal intercommunication (Hutchison & Rathore, 2015), both grief dreams and relapse dreams may reflect a process of healthy adaption to a new state where the relationship with the person, or the drug, exists only in memory. Unfortunately, unlike the situation in grieving a dead loved one, it is very much in the power of the addicted patient to raise their love object from the dead, so to speak, via relapse! Because of this relapse potential, which typically happens many times in the course of addiction recovery, we can begin to appreciate addiction recovery as being like a condition of prolonged or pathological grief. Again, pathological-complicated grief and severe addictions share common risk factors of being associated with early adverse experiences, attachment disruptions and various forms of mental illness (Zisook & Shear, 2009). In these contexts, compulsive engagement in masochistic cycles of relapse, harm, withdrawal, relapse, harm, withdrawal, etc, with drug use, mirrors patterns of Trauma Bonding (Carnes, 1997).
The brain is a well-engineered adaptation machine that optimizes its adjustment during ‘constructive’ phases of relatively stable environments and relationship networks. But it can also undergo profound ‘deconstructive’ biophysical changes in response to very drastic changes in contexts and psychosocial networks (Liljenstrom, 2003). The VHIP, PFC, the HPA-corticosteroid axis and many other neurotransmitter systems including DA, 5-HT, glutamate and endogenous opioid system, are all implicated in these drastic change events. A key concept to consider is that optimizing adaptation to new environmental circumstances likely depends on the extent to which the neurobiological changes involving these systems can be made proportional to the degree and rapidity of environmental change. In other words, to best adapt to increasingly rapid and/or increasingly profound changes in relationships, occupations, geography, etc., the brain must literally break itself down to a greater degree, and build itself back up to a greater degree, in terms of neural network connection strengths and architectures within cortical-striatal limbic networks (Chambers & Conroy, 2007). Perhaps most concretely, we see evidence for this phenomena in terms of hippocampal neurogenesis. Prolonged stress provocation of the HPA axis induces corticosteroid and glutamate release that literally melts down axodendritic connectivity, and kills neurons in the hippocampus. This ‘burn down’ is then followed by a phase of re-growth of new and different connectivity patterns, underpinned by birth of new neurons in the hippocampus, that are capable of higher degrees of plasticity compared to older neurons (Chambers, Potenza, Hoffman, & Miranker, 2004).
An exciting implication of this ‘burn-down/build up’ model of adaptation, of which adult hippocampal neurogenesis is a center piece, is that it has broad explanatory power for understanding a variety of mental illnesses and comorbid addictions. Undershooting or overshooting the burn down or regenerative phases in proportion to the environmental change (or in proportion to each other) may explain differential aspects of PTSD, depression, bipolar disorders, personality disorders and schizophrenia (Chambers & Conroy, 2007). Or, a failure of appropriate regeneration produced by certain forms of mental illness and the pharmacological effects of addictive drugs may keep the patient trapped in the addicted state, where they cannot ‘adapt out’ of harmful and compulsive drug-seeking and taking (Chambers, 2013).
With respect to addiction as a pathological attachment that must be extinguished, repaired or replaced, the ‘burn-down/build up’ model of brain adaption leads us to consider two of the most important and clinically helpful stage models in psychiatry: Kubler-Ross’s stages of Grief (Kubler-Ross, 1969), and Prochaska & DiClemente’s Stages of Change (Prochaska & DiClemente, 2005) (Table 1). Comparing these 2 models side-by-side reveals their shared themes and process similarities. In ‘Denial/Precontemplation’ the environmental need for drastic change has presented itself, but the individual is only minimally aware. In ‘Anger/Contemplation’ the individual is aware of the need for change but also their investment in the status quo, and so is drawn into a consuming inner conflict, often with substantial emotional manifestations, about what to do. In ‘Bargaining/Preparation’ a decision has been made to enter into some kind of change, but the details are not yet worked out. In fact, the individual is often caught up in this phase trying to consider how they may retain and develop the ‘best of both worlds’ (which is actually not quite feasible with accepting either someone’s death or partial abstinence in addiction). At face value, ‘Depression/Action’ may seem like the comparison where Kubler-Ross and Prochaska are most dissonant, since a state of depression is often associated with psychomotor retardation, seemingly inconsistent with a state of ‘action’. However, in terms of the burn-down/build up model, we can appreciate that this phase of change on the brain level may be intensively active; now, the old connectivity pathways and neural networks are literally undergoing demolition as the blueprints and foundations of new connectivity architectures are being laid down. In some regards, the individual’s behavior becomes changed and restricted as if ‘battening’ down for the storm, while other people are moving in to support and protect via individual and group showing of empathy and advice (e.g. funerals or early stage group therapy). Then finally, we have the Acceptance/Maintenance phase where neural reconstruction is well underway, where the individual is now optimizing and fine tuning their adaptation to their post loved one/post drug using world. As shown in Table 1, this comparison suggests that Kubler-Ross’s and Prochaska’s stages can be synthesized into a more General Attachment Adaptation Model with a neuroscientific foundation. This general model understands grief and addiction recovery as quite similar and interlinked processes, underpinned by substantial neuroplastic revision and remodeling in the brain, consistent with the ‘burn-down/build-up model’ happening across components of the PFC-NAC-AMY-VHIP assembly.
Table 1.
Integrating Kubler-Ross and Prochaska’s Stages toward a General Attachment Adaptation Model
| Kubler-Ross | Prochaska | ||
|---|---|---|---|
|
Object of
Transition: |
loved one | drug addiction | Any major attachment |
|
Stages of
Transition |
Stages of Grief | Stages of Change | General Attachment Adaptation Model |
| Denial | Pre-Contemplation | Unaware of need to adapt | |
| Anger | Contemplation | Aware/Resisting need to adapt | |
| Bargaining | Preparation | Strategizing on how to adapt | |
| Depression | Action | Effort expended to adapt | |
| Acceptance | Maintenance | Significant adaptation has occurred |
DUAL DIAGNOSIS TREATMENT IMPLICATIONS OF THE GENERAL ATTACHMENT ADAPTATION MODEL
A key to implementing the General Attachment Adaptation Model in the treatment of addiction and dual diagnosis patients is understanding addiction recovery as a form of complex grief, and acting on this clinically. In terms of psychotherapies, this means incorporating approaches in the treatment of complicated/pathological grief (Rosner, Pfoh, & Kotoucova, 2011; Wetherell, 2012; Zisook & Shear, 2009) into individual and group psychotherapies for addiction. This incorporation might include recognition that the transition from addiction is a period of very hard emotional work, like grieving, where the individual (however obviously harmful the drug use was), is undergoing a substantial sense of loss. This loss can be quite profound particularly when patients must also give up close relationships and contexts tightly associated with drug using, encompassing family, friends and hometowns. Helping patients bear and mourn what are often tremendous and irreplaceable losses to their health, relationships and occupational aspirations, caused directly by addiction, is often critical to protecting them against future relapses and worsening depression. Bringing empathy, honoring patient’s humanity and need for connection, relieving them of shame, perhaps even using communal rituals, as in the funeral process, could all be valuable therapeutic ingredients to addiction recovery (Mate, 2008; Moore, 1992).
Clearly, a core strategy of grief therapy to facilitate growth of the individual into new healthy pre-occupations, habits and relationships is also critical to addiction recovery (Rosner et al., 2011; Wetherell, 2012). Part of this effort means that therapists, nurses and psychiatrists caring for addicted/dual diagnosis patients should operate professionally, not as detached figures, but as attachment surrogates, enacting and modeling healthy new relationships that these patients need (Lewis, Amini, & Lannon, 2000; Vaillant, 1988). In essence, a key to successful dual diagnosis and addiction care may be the ability of treatment teams to form strong therapeutic attachments with patients that can ‘over power’ their pathological attachment to addictive drugs. In this work, the clinical team (and researchers in the field of addiction psychotherapies) should be thinking about ways to individualize care by attending to the diversity of speeds and intensities by which different patients, based on their personalities, mental illness comorbidities, and attachment styles, are best able to form new therapeutic bonds. As illustrated by Brian Johnson’s, long-term psychoanalysis-facilitated recovery of a patient with heroin addiction, this work can be expected to take years for some individuals (Johnson, 2010). At any rate, acknowledging that addiction is a chronic, relapsing disease that needs evidence based-treatments involving sustained efforts to retrain, remodel and rebuild capacities for empathic human attachments, is an acknowledgement that dehumanizing, judging, disconnecting and brutalizing mentally ill/addicted people via criminalization and mass incarceration, is a catastrophic moral and public health failure.
While supporting the idea that outpatient longitudinal dual diagnosis treatment settings should be natural homes for practicing grief and trauma-informed psychotherapies, the General Attachment Adaptation Model, and its neuroscientific foundation also places importance on integrating pharmacotherapies (and other ‘mechanical’ brain interventions, like rTMS or brain stimulators) into psychotherapeutic interventions, as the norm, rather than exception for addiction recovery. Psychiatric medications are important not only for addressing mental illness comorbidities that are found in most patients with severe addictions, but they should facilitate cognitive and emotional stability needed for participation in psychotherapies aiming to facilitate attachment adaption. Similarly, medications for addictive disorders that have various motivational-brain effects that help patients safely replace or terminate addictive drug use, should be incorporated and synergistic with psychotherapies. An exciting new frontier of medication development in this area is the introduction and testing of novel medicines (e.g. ketamine, LSD or MDMA analogues) that may be considerably more potent (albeit more risky) in evoking neuroplastic responses in motivational-attachment centers of brain, than what our current repertoire of agents may provide. Such novel medicines might be developed particularly to enhance biological events in the ‘burn-down/build up’ transitions necessary for successful dual diagnosis recovery. In addition, new psychotherapeutic approaches such as the Circle of Security (Hoffman, Marvin, Cooper, & Powell, 2006), that directly focus on recovering, repairing or remodeling attachment behaviors in child-rearing adults who carry their own attachment injuries from childhood trauma, may by key to preventing the transgenerational transmission of addictions and dual diagnosis disorders within families.
ACKNOWLEDGEMETS and DISCLOSURES
Time spent for the conceptualization and authorship of this work was supported in part by NIAAA (R01 AA020396; RAC co-I with PI, Eric Engleman PhD). There were no commercial interests or other outside entities involved in the production of this paper, and the authors have no conflicts of interest to report. The authors would like to dedicate this collaborative work to the memory of their mother, their sister, and their mother’s sister who all died too early as a result of their relationship with nicotine addiction.
REFERENCES
- Avery SN, Clauss JA, & Blackford JU (2016). The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology, 41(1), 126–141. doi: 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Schmahl C, Falkai P, & Wedekind D (2010). Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychol Rev, 117(2), 623–636. doi: 10.1037/a0018095 [DOI] [PubMed] [Google Scholar]
- Bartels A, & Zeki S (2000). The neural basis of romantic love. Neuroreport, 11(17), 3829–3834. [DOI] [PubMed] [Google Scholar]
- Berke JD, & Hyman SE (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron, 25, 515–532. [DOI] [PubMed] [Google Scholar]
- Borelli JL, Goshin L, Joestl S, Clark J, & Byrne MW (2010). Attachment organization in a sample of incarcerated mothers: distribution of classifications and associations with substance abuse history, depressive symptoms, perceptions of parenting competency and social support. Attach Hum Dev, 12(4), 355–374. doi: 10.1080/14616730903416971923392854 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J (1988). A secure base : clinical applications of attachment theory. London: Routledge. [Google Scholar]
- Bowlby J (1995). Maternal care and mental health. Northvale, N.J.: J. Aronson. [Google Scholar]
- Burkett JP, & Young LJ (2012). The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl), 224(1), 1–26. doi: 10.1007/s00213-012-2794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes P (1997). The betrayal bond: breaking free of exploitative relationships. Deerfield Beach, FL: Health Communications, Inc. [Google Scholar]
- Caspers KM, Yucuis R, Troutman B, & Spinks R (2006). Attachment as an organizer of behavior: implications for substance abuse problems and willingness to seek treatment. Subst Abuse Treat Prev Policy, 1, 32. doi:1747-597X-1-32 [pii] 10.1186/1747-597X-1-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA (2008). Impulsivity, dual diagnosis, and the structure of motivated behavior in addiction. Behavioral and Brain Sciences, 31(4), 443–444. [Google Scholar]
- Chambers RA (2013). Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug Alcohol Depend, 130(1–3), 1–12. doi: 10.1016/j.drugalcdep.2012.12.005 S0376-8716(12)00481-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Bickel WK, & Potenza MN (2007). A scale-free systems theroy of motivation and addiction. Neurosciene and Biobehavioral Reviews, 31, 1017–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, & Conroy SK (2007). Network modeling of Adult Neurogensis: Shifting rates of neuronal turnover optimally gears network learning according to novelty gradient. Journal of Cognitive Neuroscience, 19, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JK, & Self DW (2001). A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry, 50, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, McClintick JN, Sentir AM, Berg SA, Runyan M, Choi KH, & Edenberg HJ (2013). Cortical-striatal gene expression in neonatal hippocampal lesion (NVHL)-amplified cocaine sensitization. Genes Brain Behav, 12(5), 564–575. doi: 10.1111/gbb.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN, Hoffman RE, & Miranker W (2004). Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology, 29, 747–758. [DOI] [PubMed] [Google Scholar]
- Chambers RA, & Self DW (2002). Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology, 27(6), 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, & Potenza MN (2003). Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry, 160, 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis NA, & Fowler JH (2008). The collective dynamics of smoking in a large social network. N Engl J Med, 358(21), 2249–2258. doi: 10.1056/NEJMsa0706154 358/21/2249 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christo G, & Franey C (1996). Addicts’ drug-related dreams: their frequency and relationship to six-month outcomes. Substance Use & Misuse, 31(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, & Huettel SA (2011). Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Front Hum Neurosci, 5, 87. doi: 10.3389/fnhum.2011.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Sun H, Costello EJ, Angold A, Heilig MA, & Barr CS (2011). Child mu-opioid receptor gene variant influences parent-child relations. Neuropsychopharmacology, 36(6), 1165–1170. doi: 10.1038/npp.2010.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, & Morrone-Strupinsky JV (2005). A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav Brain Sci, 28(3), 313–350; discussion 350–395. doi:S0140525X05000063 [pii] 10.1017/S0140525X05000063 [DOI] [PubMed] [Google Scholar]
- Di Chiara G (2002). Nucleus accumbens shell and core dopamine: differential role in bahavior and addiction. Beh Brain Res, 137, 75–114. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, & Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences, USA, 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Katner SN, & Neal-Beliveau BS (2016). Caenorhabditis elegans as a Model to Study the Molecular and Genetic Mechanisms of Drug Addiction. Prog Mol Biol Transl Sci, 137, 229–252. doi: 10.1016/bs.pmbts.2015.10.019 S1877-1173(15)00221-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM (1996). Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudat/putamen and nucleus accumbens. Hippocampus, 6, 495–512. [DOI] [PubMed] [Google Scholar]
- Fletcher K, Nutton J, & Brend D (2015). Attachment, a matter of substance: the potential of attachement theory in the treatment of addictions. Clinical Social Work Journal, 43, 109–117. [Google Scholar]
- Fowler JC, Groat M, & Ulanday M (2013). Attachment style and treatment completion among psychiatric inpatients with substance use disorders. Am J Addict, 22(1), 14–17. doi: 10.1111/j.1521-0391.2013.00318.x [DOI] [PubMed] [Google Scholar]
- Galanter M (1993). Network therapy for addiction: a model for office practice. Am J Psychiatry, 150(1), 28–36. doi: 10.1176/ajp.150.1.28 [DOI] [PubMed] [Google Scholar]
- Goto Y, & Grace A (2005). Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron, 47, 255–266. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Oreland S, Hoffmann P, & Nylander I (2008). The impact of postnatal environment on opioid peptides in young and adult male Wistar rats. Neuropeptides, 42(2), 177–191. doi: 10.1016/j.npep.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, & McFarland N (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci, 20(6), 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DT, Greene MS, Fernandes TJ, Brown AM, Wright ER, & Chambers RA (2014). Prescription drug monitoring program inquiry in psychiatric assessment: detection of high rates of opioid prescribing to a dual diagnosis population. J Clin Psychiatry, 75(7), 750–756. doi: 10.4088/JCP.14m09020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari YN (2015). Sapiens A Brief History of Human Kind (1st ed.). New York: Harper. [Google Scholar]
- Harlow HF, Dodsworth RO, & Harlow MK (1965). Total social isolation in monkeys. Proc Natl Acad Sci U S A, 54(1), 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, & Nemeroff CB (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol, 52(7), 671–690. doi: 10.1002/dev.20494 [DOI] [PubMed] [Google Scholar]
- Hoffman KT, Marvin RS, Cooper G, & Powell B (2006). Changing toddlers’ and preschoolers’ attachment classifications: the Circle of Security intervention. J Consult Clin Psychol, 74(6), 1017–1026. doi: 10.1037/0022-006X.74.6.1017 [DOI] [PubMed] [Google Scholar]
- Hutchison IC, & Rathore S (2015). The role of REM sleep theta activity in emotional memory. Front Psychol, 6, 1439. doi: 10.3389/fpsyg.2015.01439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE (2005). Addiction: A disease of learning memory. Am J Psychiatry, 162, 1414–1422. [DOI] [PubMed] [Google Scholar]
- Johnson B (2001). Drug dreams: a neuropsychoanalytic hypothesis. Journal of the American Psychoanalytic Association, 49(1), 75–96. [DOI] [PubMed] [Google Scholar]
- Johnson B (2010). The psychoanalysis of a man with heroin dependence: Implications for Neurobiological theories of attachment and drug craving. Neuropsychoanalysis, 12(2), 207–215. [Google Scholar]
- Kalin NH, Shelton SE, & Lynn DE (1995). Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology, 20(7), 735–742. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & O’Brien C (2008). Drug Addiction as a pathology of staged neuroplasticity. Neuoropsychophramacology, 33, 166–180. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Volkow ND (2005). The Neural Basis of Addiction: A pathology of motivation and choice. Am J Psychiatry, 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Wardle M, & Roberts JE (2007). Adult attachment security and college student substance use. Addict Behav, 32(6), 1164–1176. doi:S0306-4603(06)00270-X [pii] 10.1016/j.addbeh.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Katner SN, Neal-Beliveau BS, & Engleman EA (2016). Embryonic Methamphetamine Exposure Inhibits Methamphetamine Cue Conditioning and Reduces Dopamine Concentrations in Adult N2 Caenorhabditis elegans. Dev Neurosci, 38(2), 139–149. doi: 10.1159/000445761000445761 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, & Malenka RC (2007). Synaptic plasticity and addiction. Nat Rev Neurosci, 8, 844–858. [DOI] [PubMed] [Google Scholar]
- Kubler-Ross. (1969). On Death and Dying. New York: Simon & Schuster/Touchtone/Scribner. [Google Scholar]
- Lavee Y, & Altus D (2001). Family relationships as a predictor of post-treatment drug abuse relapse: a follow-up study of drug addicts and their spouses. Contemporary Family Therapy, 23(4), 513–530. [Google Scholar]
- Lawson KM, Back SE, Hartwell KJ, Moran-Santa Maria M, & Brady KT (2013). A comparison of trauma profiles among individuals with prescription opioid, nicotine, or cocaine dependence. Am J Addict, 22(2), 127–131. doi: 10.1111/j.1521-0391.2013.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, Amini F, & Lannon R (2000). A General Theory of Love. New York, NY: Random House. [Google Scholar]
- Liljenstrom H (2003). Neural flexibility and stability: a computational approach. Neuropschopharmacology, 28, S64–S73. [DOI] [PubMed] [Google Scholar]
- Masterman DL, & Cummings JL (1997). Frontal-subcortical circuits: the anatomical basis of executive, social and motivational behaviors. Journal of Psychopharmacology, 11(2), 107–114. [DOI] [PubMed] [Google Scholar]
- Mate G (2008). In the realm of hungry ghosts: Close encounters with addiction. Berkeley, California: Noeth Atlantic Books. [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, & Kuhar MJ (2007). Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol, 73(3), 321–330. doi: 10.1016/j.bcp.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T (1992). in everyday life Care of the soul: guide for cultivating depth and sacredness. New York, NY: Harper Collins. [Google Scholar]
- Moos RH (2008). Active ingredients of substance use-focused self-help groups. Addiction, 103(3), 387–396. doi: 10.1111/j.1360-0443.2007.02111.xADD2111 [pii] [DOI] [PubMed] [Google Scholar]
- Nestler EJ, & Aghajanian GK (1997). Molecular and cellular basis of addiction. Science, 278(5335), 58–63. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Greene J, Pabello N, Lewis BL, & Grace AA (1999). Modulation of cell firing in the nucleus accumbens. Annals of the New York Academy of Sciences, 877, 157–175. [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2013). Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: a high-density event-related potential study. JAMA Psychiatry, 70(5), 499–507. doi: 10.1001/jamapsychiatry.2013.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A (2013). Chemosignals and hormones in the neural control of mammalian sexual behavior. Front Neuroendocrinol, 34(4), 255–267. doi: 10.1016/j.yfrne.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Prochaska J, & DiClemente C (2005). The transtheoretical approach In Norcross J & Goldfried M (Eds.), Handbook of psychotherapy integration (2nd ed., pp. 147–171). New York: Oxfrod University Press. [Google Scholar]
- Robinson TE, Gorny G, Mitton E, & Kolb B (2001). Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse, 39, 257–266. [DOI] [PubMed] [Google Scholar]
- Rosner R, Pfoh G, & Kotoucova M (2011). Treatment of complicated grief. Eur J Psychotraumatol, 2. doi: 10.3402/ejpt.v2i0.7995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler A, Thomasius R, Petersen K, & Sack PM (2009). Heroin as an attachment substitute? Differences in attachment representations between opioid, ecstasy and cannabis abusers. Attach Hum Dev, 11(3), 307–330. doi: 10.1080/14616730902815009911415389 [pii] [DOI] [PubMed] [Google Scholar]
- Schneider JP, & Irons RR (2001). Assessment and treatment of addictive sexual disorders: relevance for chemical dependency relapse. Subst Use Misuse, 36(13), 1795–1820. [DOI] [PubMed] [Google Scholar]
- Self DW, & Nestler EJ (1995). Molecular mechanisms of drug reinforcement and addiction. Annual Review of Neuroscience, 18, 463–495. [DOI] [PubMed] [Google Scholar]
- Self DW, & Nestler EJ (1998). Relapse to drug-seeking:neural and molecular mechanisms. Drug and Alcohol Dependence, 51, 49–60. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, & Russell DS (2004). Regulation of affect by the lateral spetum: implications for neuropsychiatry. Brain Res Rev, 46, 71–117. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Subiaul F, & Zawidzki TW (2008). A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat, 212(4), 426–454. doi: 10.1111/j.1469-7580.2008.00868.xJOA868 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J, & Singer M (2006). I love you... and heroin: care and collusion among drug-using couples. Subst Abuse Treat Prev Policy, 1, 7. doi:1747-597X-1-7 [pii] 10.1186/1747-597X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Koole SL, H IJ, & Buisman-Pijlman FT (2014). Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacol Biochem Behav, 119, 39–48. doi: 10.1016/j.pbb.2013.07.015S0091-3057(13)00183-4 [pii] [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, & Burr R (2000). Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev, 20(2), 235–253. [DOI] [PubMed] [Google Scholar]
- Vaillant GE (1988). What can long-term follow-up teach us about relapse and prevention of relapse in addiction? Br J Addict, 83(10), 1147–1157. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Rando K, Potenza MN, Tuit K, & Sinha R (2014). Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry, 71(8), 917–925. doi: 10.1001/jamapsychiatry.2014.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela RM (2014). The effect of severe stress on early brain development, attachment, and emotions: a psychoanatomical formulation. Psychiatric Clinics of North America, 37(4), 519–534. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li T-K (2004). Drug addiction: The neurobiology of behavior gone awry. Nat Rev Neurosci, 5, 963–970. [DOI] [PubMed] [Google Scholar]
- Wetherell JL (2012). Complicated grief therapy as a new treatment approach. Dialogues Clin Neurosci, 14(2), 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1990). The role of reward pathways in the development of drug dependence In Balfour D (Ed.), Psychotropic Drugs of Abuse (pp. 23–57). Oxford: Pergamon Press. [Google Scholar]
- Wise RA (1998). Drug-activation of brain reward pathways. Drug & Alcohol Dependence, 51, 13–22. [DOI] [PubMed] [Google Scholar]
- Wolf ME (2002). Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv, 2(3), 146–157. doi: 10.1124/mi.2.3.1462/3/146 [pii] [DOI] [PubMed] [Google Scholar]
- Wray T, & Price A (2005). Grief Dreams: how they help us heal after the death of a loved one. San Francisco: Jossey-Bass/Wiley. [Google Scholar]
- Zha X, & Xu X (2015). Dissecting the hypothalamic pathways that underlie innate behaviors. Neuroscience Bulletin, 31(6), 629–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, & Shear K (2009). Grief and bereavement: what psychiatrists need to know. World Psychiatry, 8(2), 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


