Abstract
The ribosome is the enzymatic macromolecular machine responsible for protein synthesis. The rates of protein synthesis are primarily dependent on translational efficiency and capacity. Ribosome biogenesis has emerged as an important regulator of skeletal muscle growth and maintenance by altering the translational capacity of the cell. Here, we provide evidence to support a central role for ribosome biogenesis in skeletal muscle growth during postnatal development and in response to resistance exercise training. Furthermore, we discuss the cellular signaling pathways regulating ribosome biogenesis, discuss how myonuclear accretion affects translational capacity, and explore future areas of investigation within the field.
Introduction
Cell growth is defined as an increase in cell mass (85). Cell growth is often conflated with cell proliferation because the two processes are typically coordinated to ensure cell size is maintained upon division (85). The uncoupling of cell growth from cell division can lead to an increase in cell size; this hypertrophic growth has been observed in differentiated, post-mitotic cells such as those of the liver, kidney, adipose, heart, and skeletal muscle (35, 45, 63, 72, 129). In particular, adult skeletal muscle cells are capable of dramatically increasing their size in response to increased mechanical loading, stretch, and growth factor signaling (1, 8, 10, 45, 128). A defining characteristic of skeletal muscle hypertrophy in both humans and rodents is a robust increase in the rate of protein synthesis (43, 138).
A primary determinant of skeletal muscle mass is the relationship between protein synthesis and protein degradation; a sustained, net increase in the rate of protein synthesis will ultimately lead to the accumulation of cellular protein resulting in muscle fiber hypertrophy (43, 44, 144). In general, the rate of protein synthesis is determined by two factors: translational efficiency and translational capacity. Translational efficiency is defined as protein synthesis per unit RNA, whereas translational capacity is determined by the total ribosomal content and often is expressed as the total RNA content per unit tissue (79, 83). Translational efficiency and capacity are coordinately regulated with the emerging notion (borrowed from the cardiac field) that the initial increase in translational efficiency observed following a hypertrophic stimulus is required for the subsequent increase in translational capacity, which allows for the sustained increase in protein synthesis necessary for skeletal muscle hypertrophy (53, 136). The purpose of this review is to provide a brief overview on ribosome biogenesis, its regulation during muscle growth, and the evidence demonstrating that ribosome biogenesis is an important mediator of skeletal muscle hypertrophy.
The Ribosome
The ribosome is a macromolecular machine responsible for the translation of mRNA into protein, i.e., protein synthesis. The mature ribosome (80S in eukaryotes) is composed of a small subunit (40S) and a large subunit (60S). Each subunit contains distinct ribosomal RNAs (rRNAs) and associated ribosomal proteins (r-proteins); the large 60S subunit is formed by 28S, 5.8S, and 5S rRNAs, and 47 r-proteins with the small 40S subunit composed of a 18S rRNA and 33 ribosomal proteins (7, 61).
Even though ribosome is composed of 80 r-proteins, it is the four rRNAs that confer the peptide bond formation activity (86, 96). The small subunit is responsible for the interaction between the mRNA codons and the tRNA anticodons, whereas the large subunit contains the rRNAs that interact to create the actual site of peptidyl transferase within the ribosome (86, 122). Although r-proteins are not strictly required for peptidyl transferase activity, they have been shown to be required for rRNA processing, maturation, assembly, and stabilization, as well as to affect translational fidelity (68, 95, 106, 145).
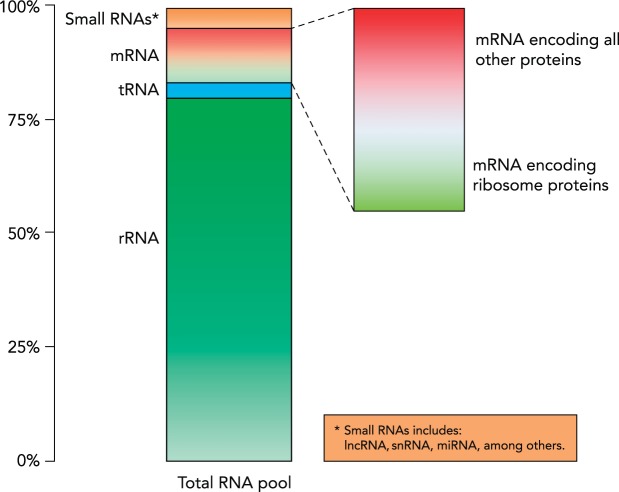
Although it may be argued that the ribosome is not in the stricto sensus an organelle, due to its lack of a membrane, it is a comparatively small organelle of 2.9 Å (61). Despite the relatively small size of the ribosome, ribosomes are found in high concentrations within the cell, from as little as several thousand per μm3, up to 14,000 ribosomes per μm3 in proliferating eukaryotic cells, occupying roughly 20% of the cell volume (21). Indeed, in any given cell, the rRNAs compose the vast majority of total cellular RNA (ranging from 70 to 90%), and thus any change in total cellular RNA content, as the result of ribosome biogenesis, is generally attributed to a change in rRNA abundance and considered to be indicative of an increase in the translational capacity of the cell (25, 29, 97, 134, 141). Given the abundance of rRNA, it is not surprising then that r-protein mRNAs make up a significant proportion of the total mRNA pool in many cell types, including in skeletal muscle tissue (4, 11, 135). Likewise, in growing yeast, ~50% of the total cellular RNA polymerase II transcription (protein-coding genes) is devoted to the transcription of r-protein mRNAs (see FIGURE 1) (134).
FIGURE 1.
RNA composition of the transcriptome
The total RNA pool within the cell is composed of ~80% ribosomal RNA (rRNA), with mRNA accounting for ~12% of the total RNA pool; a significant portion of mRNA encodes for ribosomal proteins. The remainder of the RNA content of the cell is composed of small noncoding RNAs such as transfer RNA (tRNA), long noncoding RNAs, and microRNAs.
Ribosome Biogenesis
Ribosome biogenesis is a complex, multistep process involving the de novo synthesis of ribosomes. It involves ribosomal DNA (rDNA) transcription with subsequent processing, maturation, and assembly of rRNAs and r-proteins. Ribosome biogenesis occurs primarily within a substructure within the nucleus, the nucleolus, either being synthesized in the nucleolar region or shuttled from other compartments into nucleolus. Ribosome biogenesis requires the activity of all three RNA polymerases I, II, and III (Pol I, Pol II, and Pol III). Pol I exclusively transcribes rDNA, whereas Pol II is responsible for the transcription of all mRNAs, including the r-protein mRNAs, with Pol III transcribing the 5S rRNA gene and small RNAs such a tRNAs (see FIGURE 1) (77).
The de novo synthesis of ribosomes is initiated by transcription of rDNA to produce the 47S pre-rRNA, which is considered the primary point of regulation in ribosome biogenesis (100). Transcription of rDNA by Pol I first requires the assembly of the pre-initiation complex (PIC) at the rDNA promoter (28, 109). The PIC is formed at the rDNA promoter by the upstream binding factor (UBF), selectivity factor 1 (SL-1) complex [formed by several proteins: the TATA-binding protein (TBP) and four TBP-associated factors (TAFs)], transcription initiation factor TIF-IA (encoded by Rrn3), and the RNA Pol I complex (28, 47, 50). The rDNA promoter contains two control sequences, the core promoter region (Core) and the upstream promoter element (UPE) (88). Specifically, UBF and SL-1 are responsible for DNA binding, with TIF-IA acting as bridge between these factors and Pol I (108); however, a recent study reported that TIF-IA has DNA binding activity (122a). As it will be discussed below, studies have provided clear evidence the PIC is a major signaling hub, allowing for the integration of different signaling pathways to affect ribosome biogenesis through modulation of rDNA transcription (67, 77). Following initiation, PIC components, UBF, and SL-1 complex, remain bound to rDNA promoter elements, whereas Pol I transcribes the rDNA until it encounters the termination elements (see FIGURE 2).
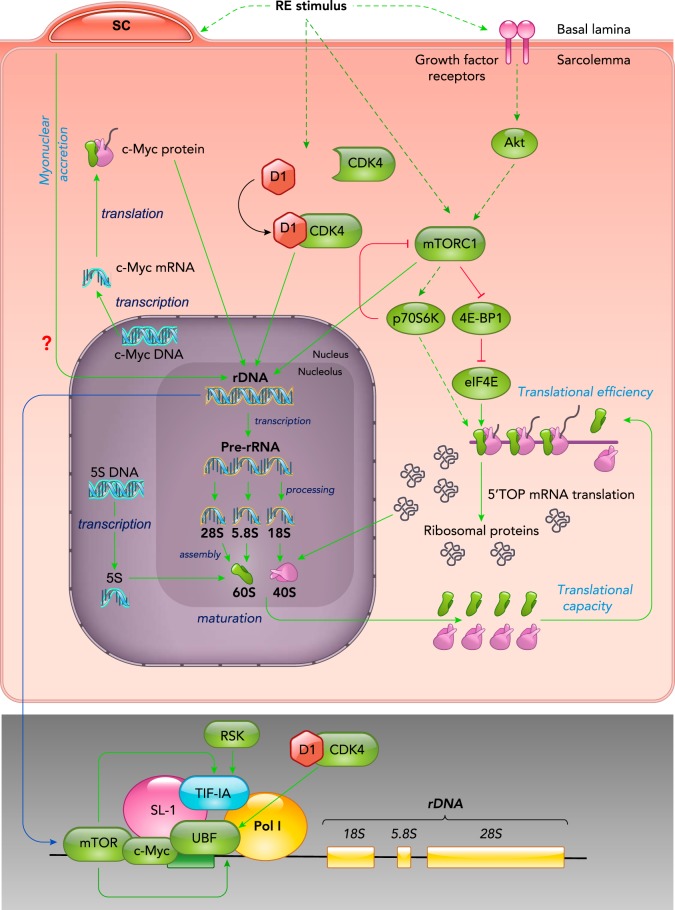
FIGURE 2.
Regulation of ribosome biogenesis during skeletal muscle hypertrophy
Relevant signaling transduction pathways to ribosome biogenesis are shown along with the pre-initiation complex (PIC) formed in the nucleolus, where active rRNA genes are being transcribed leading to processing, maturation, and assembly of ribosomes. This will result in increased number of ribosomes and increased translational capacity. Efficiency also contributes with translational capacity by promoting the specific translation of key proteins involved in ribosome biogenesis, such as the ribosomal proteins. The role of satellite cell by donating rDNA template is shown with a question mark to denote the need for further investigation.
Following synthesis of the 47S pre-rRNA, the long transcript is processed intro three mature rRNAs, 18S, 5.8S, and 28S, in a controlled manner by a series of cleavage enzymes (55). The processing involves the removal of external and internal transcribed spacers (ETS and ITS, respectively) from the pre-rRNA (56). In addition to these rRNAs, the mature ribosome requires a fourth rRNA, the 5S, which is transcribed by Pol III, outside the nucleolus (140). Altogether, these four rRNAs form the backbone of the mature ribosomes, along with 80 r-proteins.
Unlike protein-coding genes, there are hundreds of copies of rDNA genes found within the genome; on average, humans have ~300 copies, ranging from as low as 60 copies up to 1,590 copies (40, 101). The rDNA copies are arranged in tandem and located on five different chromosomes (101). These rDNA clusters form chromosomal loops known as nucleolus organizer regions, which interact through hundreds of nucleolar-specific proteins to form the nucleolus, thereby facilitating transcription of the 47S pre-rRNA (111, 123). In addition to pre-rRNA synthesis, the nucleolus is the site where pre-rRNA processing and ribosome assembly occurs. Following translation in the cytoplasm, r-proteins are imported into the nucleolus, where they associate with their respective ribosomal subunit; thus ribosome maturation takes place in both the nucleolus and nucleoplasm, whereas the final steps occurs in the cytoplasm (55, 88).
Ribosome Biogenesis During Postnatal Skeletal Muscle Hypertrophy
The significant increase in skeletal muscle mass after birth in mammals occurs primarily through muscle fiber hypertrophy given that fiber number appears to be established during early postnatal development (71, 139). The roughly sevenfold increase in fiber size during postnatal development is accompanied by a fivefold increase in myonuclear number as the result of satellite cell fusion (114, 139). The increase in muscle DNA content during this rapid period of growth is paralleled by a similar increase in total RNA and protein content, suggesting that increased myonuclear accretion promotes growth by yielding increased translational capacity (60, 81). In fact, the concentration of ribosomes in skeletal muscle, based on total RNA per milligram of tissue, was found to be the greatest at early stages of postnatal development; however, although the concentration of ribosomes progressively decreases during skeletal muscle growth and maturation, the total translation capacity of the muscle (as assessed by total RNA content) is much higher in adulthood (see FIGURE 3) (36, 37, 60, 102, 146). The importance of ribosome biogenesis to skeletal muscle growth during postnatal development is supported by studies in which diet is manipulated during this period. Young and colleagues found that pups placed on a low protein diet showed a decrease in skeletal muscle total RNA concentration and blunted growth (146). Similarly, nutritional restoration in undernourished pups restored muscle mass, which was associated with an increase in UBF levels and translational capacity (36).
FIGURE 3.
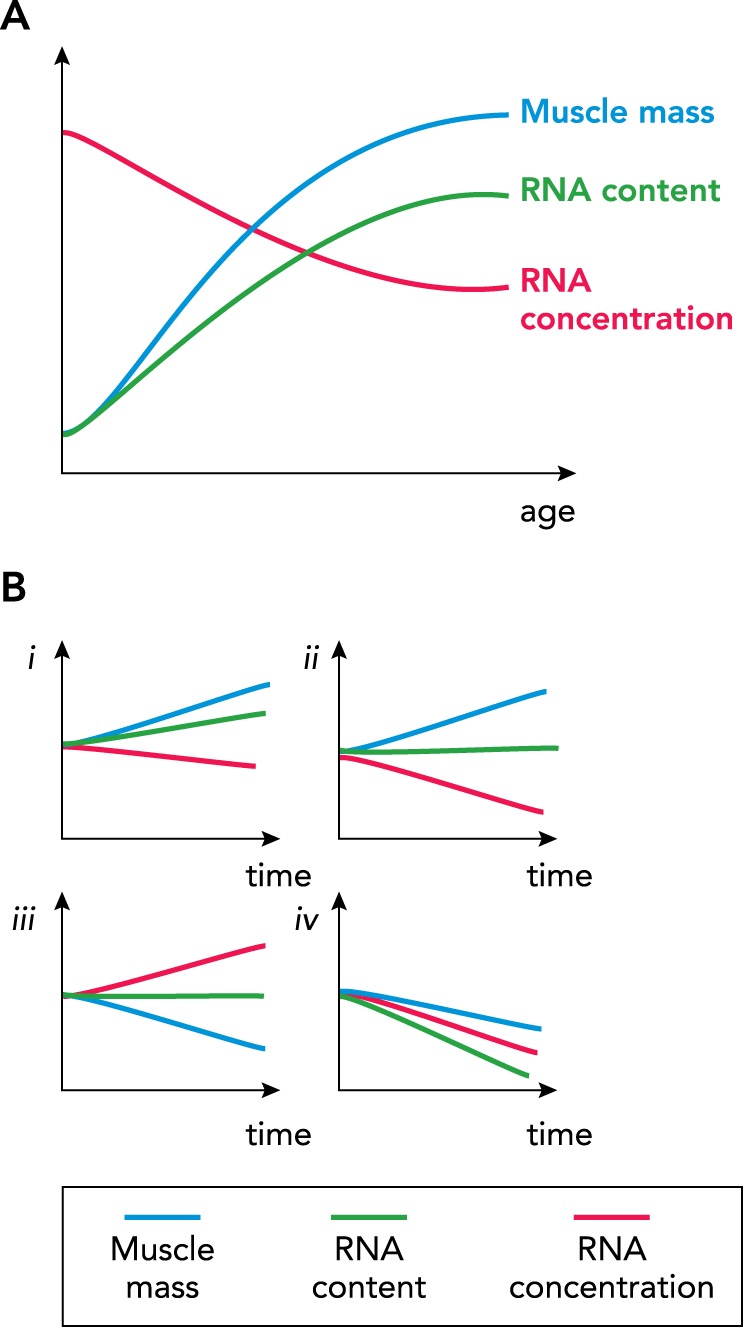
RNA concentration vs. RNA content
The concept of RNA concentration vs. RNA content is important for understanding the role of translational capacity in the regulation of skeletal muscle hypertrophy. A: postnatal skeletal muscle growth is characterized by a high concentration of total RNA immediately after birth. During postnatal development, both skeletal muscle mass and RNA content are significantly increased; however, the concentration of total RNA decreases because the increase in mass is greater than the increase in the total amount of RNA. B: different hypothetical scenarios are presented to show how the relationship between muscle mass, RNA content, and RNA concentration can change with hypertrophy and atrophy: i, in growing muscle, ribosome biogenesis results in an increase translational capacity (total RNA content) but a modest decrease in RNA concentration because growth outpaces rRNA production; ii, there is muscle growth but in the absence of ribosome biogenesis such that translational capacity remains unchanged but with a significant decrease in RNA concentration; iii, during muscle atrophy, translational capacity remains unchanged such that RNA concentration is significantly increased; iv, during muscle atrophy, there is a significant loss of translational capacity such that RNA concentration is also significantly reduced.
Ribosome Biogenesis During Adult Skeletal Muscle Hypertrophy
In Vitro Studies
In vitro experiments provided the first evidence linking ribosome biogenesis to skeletal muscle hypertrophy. Pioneering work from Nader and colleagues showed the treatment of myotubes with fetal bovine serum (FBS; a serum enriched with growth factors) activated mTOR signaling and cyclin-dependent kinase 4 (CDK4) activity, which, in turn, promoted UBF activity and rDNA transcription leading to higher rRNA abundance and myotube hypertrophy (91).
More recent studies using the specific Pol I inhibitor CX-5461 have provided further evidence supporting a requirement for ribosome biogenesis, i.e., increased translational capacity in muscle hypertrophy (27, 51, 118, 133). The findings of these studies showed inhibition of Pol I activity by CX-5461 significantly blunted serum-stimulated myotube hypertrophy, which was associated with a diminished increase in 47S pre-rRNA expression, total RNA abundance, and protein synthesis (118, 133). Contrary to these findings, Crossland and coworkers found IGF-1-induced myotube hypertrophy still occurred independent of ribosome biogenesis (20).
Studies in cell culture have also demonstrated a novel role for mTOR protein in the regulation of ribosome biogenesis. The well-established role of mTOR in cell growth is most often attributed to its cytoplasmic role in regulating translational efficiency, von Walden and colleagues reported that mTOR associates with rDNA promoter in muscle cells and is involved in rRNA transcription, confirming an earlier study in non-muscle cells (91, 127, 133). This novel finding shows that mTOR, independent of its well-known cell signaling function, is able to promote cell growth through direct regulation of ribosome biogenesis.
It is important to highlight, however, that studies in cell culture may have inherent limitations that preclude its translation to in vivo conditions, especially in an adult organism where the number of neighboring myoblasts (or satellite cells) near myofibers are very low. Even though most of these studies have attempted to remove myoblasts following cell differentiation via cytosine arabinoside (AraC) treatment, this does not remove all myoblasts, and the role of myoblast fusion into the existing myotubes cannot be completely ruled out. In particular, the use of CX-5461 likely decreases ribosome biogenesis in myoblasts as well, reducing proliferation and, consequently, myoblast fusion. Despite the mechanism, in vitro studies have demonstrated that a growth stimulus promotes ribosome biogenesis and translational capacity, leading to myotube hypertrophy. We positioned that the role of myonuclei accretion in ribosome biogenesis is better answered via in vivo studies that have been depleted of satellite cells, which will be discussed in Influence of Satellite Cells and Myonuclear Accretion on Ribosome Biogenesis.
Rodents Models of Muscle Hypertrophy
Since its original description over 50 years ago, the synergist ablation model has been shown to be a useful tool to study the molecular and cellular mechanisms underlying skeletal muscle hypertrophy (42). An early cell-free study hinted at the possibility that the increase in total RNA observed in hypertrophied muscle may be responsible for the higher rate of protein synthesis associated with muscle growth (52). Since then, numerous studies using synergist ablation have firmly established the increase in total RNA per milligram of tissue in response to a hypertrophic stimulus (2, 16, 45, 46, 48, 54, 64, 117). It is only more recently, however, that studies have begun to uncover the mechanisms regulating ribosome biogenesis that lead to the increase in rRNA with mechanical loading in rodents and humans.
The Nader laboratory has been at the forefront of understanding the role of ribosome biogenesis in skeletal muscle hypertrophy. Von Walden and colleagues provided the first evidence showing an increase in 47S pre-rRNA expression in response to a hypertrophic stimulus induced by synergist ablation (132). The increase in 47S pre-rRNA expression preceded fiber hypertrophy and was associated with higher UBF binding at the rDNA promoter as well as c-Myc, a major driver of cell growth, and Pol I itself (132). The enrichment of Pol I regulon factors at the rDNA promoter, along with evidence of chromatin remodeling, further suggested the intriguing possibility that, during hypertrophy, additional copies of rDNA become open, available for transcription (132). Later studies have confirmed these initial findings, showing an increase in ribosome biogenesis, as assessed by 47S pre-rRNA expression, following synergist ablation (49, 64, 65). More recently, Nakada and coworkers provided compelling evidence showing a strong correlation between the magnitude of hypertrophy and translational capacity but not markers of translational efficiency (93).
Resistance Exercise Training Studies
Although rodent studies have shown a clear relationship between skeletal muscle hypertrophy and ribosome biogenesis, it has only been very recently that studies were undertaken to determine whether ribosome biogenesis occurs in humans following resistance exercise and its potential role in promoting skeletal muscle hypertrophy.
Several recent studies have now investigated the acute and chronic effects of resistance exercise on 47S pre-rRNA levels (30–33, 38, 92, 104, 119). Based on these studies, summarized in Table 1, the increase in 47S pre-rRNA expression following a single resistance exercise bout occurs only after several hours. In particular, 47S pre-rRNA expression was increased above baseline levels at 4 h and continues to increase until 24 h, remaining elevated at 48 h (FIGURE 4A) (32, 92, 119). Analysis of pre-rRNA expression at earlier time points, e.g., at 1, 2, and 3 h, showed no change in pre-rRNA levels (30, 33, 38). Although pre-rRNA expression is not increased at these earlier time points, activation of key events, such as increased TIF-IA phosphorylation at an ERK-dependent site, suggests that, following an acute bout of resistance exercise, Pol I regulon factors become primed to promote PIC formation and subsequent rDNA transcription (30, 32).
Table 1.
Pre-rRNA synthesis analysis following acute and chronic resistance exercise in humans
| Reference | Time Point Post-RE | Biopsy Site | Subjects | 47S Change |
|---|---|---|---|---|
| 30 | 1 h and chronic | VL | Young men | ↔1 h, ↑ chronic |
| 92 | 4 h | Biceps brachii | Young men and women | ↑4 h |
| 32 | 2, 24, and 48 h | VL | Young men trained | ↔2, ↑24 and 48 h |
| 119 | 24 h | VL | Young and elder men and women | ↑24 h |
| 38 | 3 h and chronic | VL | Young men | ↔3 h and chronic |
| 33 | 2 and 4 h | VL | Elder men | ↔2 and 4 h |
Note that only the control groups are included. Experimental groups that received different nutritional interventions or non-resistance training protocol were not included. VL, vastus lateralis; RE, resistance exercise.
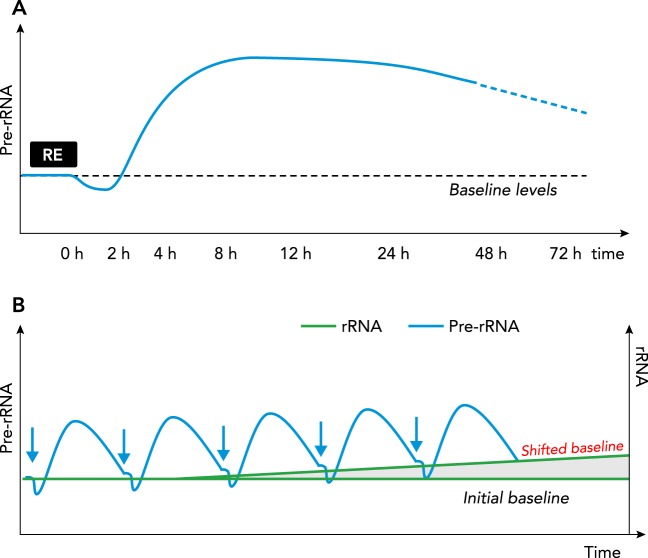
FIGURE 4.
Resistance exercise increases the translational capacity of the muscle
Based on the findings from human studies, a working model is presented showing the change in translational capacity following a single bout vs. multiple bouts of resistance exercise. A: a single bout of resistance exercise results in a significant increase in ribosome biogenesis, as assessed by 47S pre-rRNA expression, starting 4 h postexercise and maintained until at least 48 h with no detectable change in translational capacity of the muscle. B: in response to multiple bouts of resistance exercise, there is a progressive increase in the translational capacity of the muscle.
Although a single bout of resistance exercise increased 47S pre-rRNA levels, it does not appear to be sufficient to increase the abundance of mature rRNA significantly (32, 99). The reason for this disconnect between 47S pre-rRNA and rRNA levels remains to be determined, but one possible explanation is the inability to precisely measure minor changes in the rRNA, given it makes up ~85% of the total RNA pool of the cell. However, cumulative bouts of resistance exercise eventually lead to the accumulation of mature rRNAs, leading to increased concentration of total RNA (13, 30, 104, 118) (FIGURE 4B). This increase in translation capacity following resistance exercise in humans was significantly correlated with the change in muscle mass (12, 30, 104). The importance of ribosome biogenesis in human skeletal muscle hypertrophy is further underscored by studies showing the increase in muscle size in extreme responders to resistance exercise training was associated with a greater change in total RNA concentration, a finding consistent with an earlier rodent study (84, 93, 118).
Total RNA content of muscle has been shown to be correlated with the in vitro rate of protein synthesis (137), indicating a possible mechanism in vivo. Moreover, given that the magnitude of rRNA accumulation following chronic resistance training has been associated with muscle growth in vivo (12, 30, 93, 118), the increase in translational capacity should result in a higher basal rate of protein synthesis. In support of this view, several studies have found that muscle protein synthesis was increased at resting conditions following resistance exercise training (6, 62, 87, 104, 142). Moreover, the change in the basal rate of protein synthesis was recently shown to be significantly correlated with the degree of muscle hypertrophy (104). Although the magnitude of the change in the basal rate of protein synthesis is relatively minor, it may have a greater impact on growth than the relatively transitory increase in translational efficiency, given it occurs over a much longer period of time. These findings, together with previous results showing an association between total RNA and rRNA levels with muscle growth, provide compelling evidence that translational capacity is a determining factor driving skeletal muscle growth induced by resistance exercise training (12, 30).
Regulation of rDNA Transcription During Skeletal Muscle Hypertrophy
Studies investigating cell growth and proliferation have revealed the intimate connection between the cell cycle and ribosome biogenesis. An important challenge in skeletal muscle research is to verify whether the signaling pathways shown to regulate ribosome biogenesis during cell growth are conserved in adult skeletal muscle. Although there has been considerable effort to describe the signaling pathways involved in mediating the hypertrophic response of skeletal muscle to resistance exercise, most of these studies have focused on the regulation of translational efficiency. Although these studies have been designed to capture changes in cell signaling thought to regulate translational efficiency, i.e., early time points, we have, when appropriate, inferred the observed changes in cell signaling may also regulate ribosome biogenesis.
In particular, UBF is a central player in the regulation of rDNA transcription given that it possesses DNA-binding activity via several high mobility group (HMG) box domains, which receive input from different signaling pathways regulated by hormonal, nutritional, and cellular energy (67, 108). Of relevance for skeletal muscle, growth factors, such as IGF-1, and amino acids, such as leucine, may regulated UBF activity (67).
UBF is considered to be the master regulator of rDNA transcription because of its direct contact with the promoter regions of rDNA to form active nucleolar organizer regions, in addition to its central role in the recruitment and stabilization of SL1 (24, 98, 121). UBF receives input via phosphorylation from MAPK and mTOR signaling pathways as well as cell cycle regulators. There are several known serine phosphorylation sites (Ser388, Ser484, and Ser637) within the UBF protein, with Ser388 and Ser484 phosphorylation required for upregulation of rDNA transcription (130, 131). Along with phosphorylation status, the total level of UBF protein has been shown to influence Pol I recruitment and rDNA transcription rates (110, 131). In addition to UBF, TIF-IA is also required for PIC formation and rDNA transcription, and also receives cues from different signaling pathways (14, 82, 113, 147). As mentioned before, TIF-IA participates in the PIC formation by recruitment of the Pol I complex, acting as a bridge between the SL-1 and Pol I complex via protein-protein interaction (9, 14, 82, 103, 147).
MAPK Signaling and Cell Cycle Regulators
The MAPK signaling pathway regulates ribosome biogenesis at multiple levels by 1) promoting translation of specific cell cycle proteins such as cyclin D1, 2) enhancing the stabilization of c-Myc protein, and 3) phosphorylation of PIC proteins, UBF, and TIF-IA (69, 73, 115, 120, 148). Cyclin D1 expression is primarily regulated at the level of translation by eukaryotic translation initiation factor 4E (eIF4E). eIF4E promotes cyclin D1 translation following MNK1 (MAP kinase interacting serine/threonine kinase 1) activation of eIF4E via Ser209 phosphorylation (73, 107, 126). Once translated, cyclin D1 binds and activates CDK4, which allows CDK4 to directly phosphorylate UBF, thereby promoting rDNA transcription (5, 66, 131). MAPK signaling has also been shown to promote ribosome biogenesis through phosphorylation of TIF-IA at Ser649 via RSK1 (ribosomal protein S6 kinase polypeptide 1) (148).
Numerous studies have investigated activation of the MAPK signaling pathway in response to exercise. In particular, kinases such as ERK 1/2, p38, MNK1, and p90RSK have been shown to be highly phosphorylated immediately after exercise as well as a few hours postexercise (22, 30, 32, 39, 75, 143). Following resistance exercise, there is an increase in both the expression and the MAPK phosphorylation of eIF4E (30, 32, 78, 142). Thus these studies suggest that resistance exercise induces activation of the MNK1-eIF4E-cyclin D1 axis, leading to UBF phosphorylation and rDNA transcription. Similarly, RSK1 phosphorylation of TIF-IA Ser649 has been reported 1–3 h following resistance exercise but not at 24 or 48 h (30, 32, 38).
PI3K-AKT-mTOR Pathway
Growth factors have been shown to be potent stimulators of ribosome biogenesis (58, 77). Growth factors, such as IGF-1, affect muscle cells via both the PI3K-AKT-mTOR and MAPK signaling pathways (112). This in part could be attributed to Akt activity, since Akt has been shown to be a potent stimulator of ribosome biogenesis in many cells types via growth factors; however, Akt regulation of ribosome biogenesis in skeletal muscle in response to a hypertrophic stimulus has yet to be investigated (17, 23). mTOR signaling, on the other hand, is known to regulate ribosome biogenesis through multiple mechanisms. mTOR promotes the translation of the r-proteins and other accessory proteins through a 5′-TOP (5′-terminal oligopyrimidine tract) mechanism (94, 125). Furthermore, mTOR was shown to be associated with rDNA promoter (127, 133). mTOR may regulate the formation of transcriptionally engaged PIC via TIF-IA activation and nucleolar chromatin structures (19, 70, 133).
A number of studies in rodents and muscle cells have shown that mTOR plays a role in muscle growth via ribosome biogenesis (74, 99, 133). However, the mechanisms through which mTOR promotes ribosome biogenesis is not fully clear, since rapamycin-sensitive but apparent mTOR-independent mechanism may exist (48, 99). More studies are required to elucidate how mTOR affects ribosome biogenesis and muscle growth, and whether the different complexes, mTORC1 and mTORC2, have roles in regulating the translational capacity of skeletal muscle.
Two of the best-known downstream targets of mTORC1, 4E-BP1 and p70S6K, affect translation initiation and elongation. 4E-BP1 is directly involved in promoting the translation of TOP 5′ mRNA such as r-proteins, which are required for 47S pre-rRNA processing and ribosome assembly (125). As for p70S6K, it does not appear to be necessary for rDNA transcription of 47S pre-rRNA but may instead may be involved in rRNA processing, maturation, assembly, and ribosome export (18, 74, 133). So, although it has been clearly established that mTOR activation following resistance exercise enhances translational efficiency, it remains to be determined whether the acute activation of mTOR postexercise promotes its association with, or activity of, rDNA promoters (3, 26, 30, 75, 105, 124).
Combined, these data demonstrated that a bout of resistance exercise promotes signaling transduction pathways associated with PIC formation and transcription of rDNA, along with increased translation of ribosomal proteins (see FIGURE 2). Interestingly, a few upstream kinases and proteins can be upregulated shortly after a bout of resistance exercise, such as phosphorylation of TIF-IA, and levels of cyclin D1, although increased pre-RNA synthesis is not yet observed. It is possible that these effects are priming PIC formation at the rDNA promoter region to activate pre-rRNA synthesis at a later time point.
Influence of Satellite Cells and Myonuclear Accretion on Ribosome Biogenesis
Determining the role of satellite cells in adult skeletal muscle hypertrophy has been an area of intense interest for the skeletal muscle community for some time (76, 89). Satellite cells are thought to be required for hypertrophy through fusion with existing myofibers; this fusion event leads to an increase in the number of myonuclei, which is considered to be necessary for maintaining the myonuclear domain within the growing fiber. Indeed, each nucleus added to the myofiber results in the myofiber possessing a greater number of rDNA copies for transcription, which, in theory, would increase the capacity of the cell to promote ribosome biogenesis. Although this is a reasonable hypothesis, the myofiber contains hundreds of nuclei, with each nucleus having ~300 copies of rDNA, as previously mentioned. Furthermore, most of the rDNA copies are not available for transcription at a given time, i.e., the rDNA copies exist in both active and inactive states, suggesting that the fiber has a sufficient reserve of rDNA copies available for transcription (57, 110). In mice, the increase in rDNA transcription occurs significantly earlier (~3–5 days post-synergistic ablation) than satellite cell fusion (7–10 days), indicating that the addition of nuclei is not required for substantial increase in 47S pre-rRNA expression (65, 132). Similarly in humans, pre-47S pre-RNA expression appears to be maximally stimulated within 1 day following a single session of resistance exercise (Table 1), whereas myonuclear accretion can only be observed following months of repeated bouts of resistance training (116).
To determine the necessity of myonuclear accretion for skeletal muscle hypertrophy, we developed the Pax7-DTA mouse model, which allows for the conditional ablation of satellite cells in adult skeletal muscle (80). Using the Pax7-DTA mouse, we demonstrated that significant muscle growth can occur in the absence of satellite cell fusion in adult mice (65, 80, 90). These findings provide evidence that myonuclear addition via satellite cell fusion is not required for muscle hypertrophy and that resident myonuclei are capable of supporting the transcriptional demand necessary for muscle growth. In support of this idea, Tyler and colleagues used metabolic labeling to show that resident myonuclei in satellite cell-depleted muscle are capable of enhanced transcription during hypertrophy (65). The enhanced transcription in satellite cell-depleted muscle was paralleled by a significant increase in 47S pre-rRNA expression, indicating the resident myonuclei were able to compensate by further upregulating ribosome biogenesis compared with muscle with satellite cells (65). Older subjects have been reported to have a higher number of myonuclei than their young counterparts (59) but yet show blunted ribosome biogenesis in response to a hypertrophic stimulus (12, 64, 119). These findings support the notion that it is not necessarily the number of myonuclei per fiber that is important for hypertrophy but rather the ability of resident myonuclei to activate rDNA transcription, thereby promoting ribosome biogenesis and the subsequent increase in translational capacity of the myofiber.
Although our studies using the Pax7-DTA mouse have provided convincing evidence to show adult (>4 mo of age) skeletal muscle is capable of hypertrophic growth independent of satellite cells, more recent analysis has revealed an age-dependent requirement for satellite cells for muscle hypertrophy (90). When satellite cells were ablated in still maturing 2-mo-old mice, the muscle was unable to hypertrophy in response to 10 days of synergist ablation (90). Given actively growing mice have a high translational capacity (as measured by total RNA per milligram of tissue), we hypothesize that, during postnatal growth, the transcriptional output is maximized, and thus the additional transcription demand induced by mechanical overload cannot occur without satellite cell fusion (FIGURE 5). Thus myonuclei accretion promotes a transcriptional capacity reserve that can be further utilized in a fully grown animal. These findings, together with the well-established need for satellite cells during postnatal muscle hypertrophy, support the concept of a continuum in the requirement for satellite cells that progressively diminishes from postnatal development to adulthood. More research is necessary to confirm the concept of an age-dependent requirement for satellite cells during skeletal muscle and the underlying mechanism.
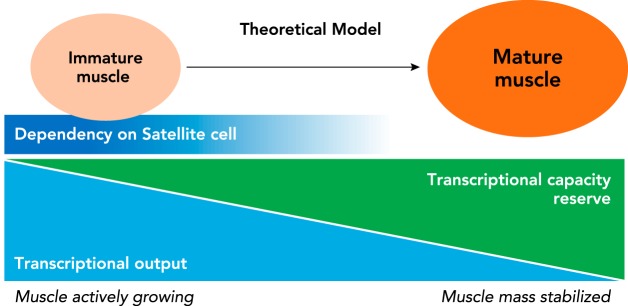
FIGURE 5.
Age-dependent requirement for satellite cells for skeletal muscle hypertrophy
A theoretical model is proposed in which there is a strict requirement for satellite cells, i.e., myonuclear accretion during postnatal growth to meet the high transcription demand necessary for muscle hypertrophy. As the muscle reaches adulthood and the rate of growth levels off, the dependence on satellite cells for hypertrophic growth disappears as resident myonuclei have sufficient reserve capacity to meet the transcriptional output required for hypertrophic growth.
Future Directions and Conclusions
Ribosome biogenesis, as stated before, appears to be largely regulated at rDNA transcription. Because of that, and because most of the current knowledge accumulated from the recent studies in skeletal muscle have focused on this step, the present review has largely also focused at this cellular event. However, the possibility that other steps governing ribosome biogenesis are also altered by exercise and during growth is promising and should receive attention. In addition, to better understand the mechanisms of rDNA transcription following an anabolic stimulus, such as mechanical overload or resistance exercise training, there are other avenues of research regarding ribosome biogenesis and translational capacity, described below, that have yet to be fully explored.
Ribophagy
Ribophagy is the autophagic process that results in the degradation of ribosomes. Whether ribophagy is altered following a bout of resistance exercise or mechanical overload induced by synergist ablation remains to be fully investiagted. A change in ribosome turnover could have a significant impact on the translational capacity of the cell and its response to an anabolic stimulus. Furthermore, determining whether ribophagy is affected by age may provide insight into why older individuals have comparatively more ribosomes than younger individuals.
rRNA Processing
Although most of the focus of recent studies has been on the initiation of ribosome biogenesis, i.e., rDNA transcription, there is a paucity of data regarding the regulation pre-rRNA processing and maturation. We anticipate that resistance exercise and/or mechanical overload may also increase the machinery involved in rRNA processing, given that genes encoding rRNA processing factors have been reported to be upregulated in rodent and human skeletal muscle during hypertrophic growth (15, 32).
rDNA Copy Number
The number of rDNA copies varies considerably within the population (40, 41). Humans have on average ~300 copies of rDNA per genome, ranging from 60 up to 1,590 copies (101). Thus, within the population, it is possible to find subjects with 25 times the number of rDNA copies of another individual. An unanswered question is whether such a dramatic difference in rDNA copy has an impact on ribosome biogenesis in response to a hypertrophic stimulus (FIGURE 4). Are individuals with a higher rDNA copy number more responsive to anabolic stimulus compared with persons with a lower number of rDNA copies?
In addition to a possible impact on anabolic response, it may be that rDNA copy number has an influence on the requirement for myonuclear accretion during muscle growth. For instance, an individual with a low rDNA copy number may benefit from myonuclear accretion as a way to increase the number of rDNA copies available for transcription; alternatively, a person with a high rDNA copy number might not require myonuclear accretion because their myofibers already possess a sufficient number of rDNA copies to support ribosome biogenesis. This may also help explain some of the controversy regarding the requirements for satellite cell and muscle hypertrophy found in the literature. Although this is a provocative idea, it remains highly speculative and requires further investigation.
Over the past few years, there has been considerable progress in better understanding the role of ribosome biogenesis in skeletal muscle mass regulation. Findings from numerous studies support an important role for an increase in translational capacity as a central regulator of muscle hypertrophy; however, there are still open questions to be answered. Future studies will need to use loss- and gain-of-functions strategies to provide definitive evidence for the necessity and sufficiency, respectively, of ribosome biogenesis for skeletal muscle hypertrophy. If such studies provide confirmatory evidence, it will support ribosome biogenesis as a therapeutic target to prevent and/or restore the loss of skeletal muscle mass associated with various wasting conditions, aging, and cancer cachexia.
Acknowledgments
We thank the anonymous reviewers who have critically evaluated this manuscript.
J.J.M. was supported by National Institutes of Health Grants AG-049806 and AR-060701.
No conflicts of interest, financial or otherwise, are declared by the author(s).
V.C.F. prepared figures; V.C.F. and J.J.M. drafted manuscript; V.C.F. and J.J.M. edited and revised manuscript; V.C.F. and J.J.M. approved final version of manuscript.
References
- 1.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol (1985) 81: 2509–2516, 1996. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 2.Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985) 87: 1705–1712, 1999. doi: 10.1152/jappl.1999.87.5.1705. [DOI] [PubMed] [Google Scholar]
- 3.Apró W, Wang L, Pontén M, Blomstrand E, Sahlin K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 305: E22–E32, 2013. doi: 10.1152/ajpendo.00091.2013. [DOI] [PubMed] [Google Scholar]
- 4.Ardlie KG, DeLuca DS, Segrè AV, Sullivan TJ, Young TR, Gelfand ET, Trowbridge CA, Maller JB, Tukiainen T, Lek M, Ward LD, Kheradpour P, Iriarte B, Meng Y, Palmer CD, Esko T, Winckler W, Hirschhorn JN, Kellis M, MacArthur DG, Getz G, Shabalin AA, Li G, Zhou YH, Nobel AB, Rusyn I, Wright FA, Lappalainen T, Ferreira PG, Ongen H, Rivas MA, Battle A, Mostafavi S, Monlong J, Sammeth M, Melé M, Reverter F, Goldmann JM, Koller D, Guigó R, McCarthy MI, Dermitzakis ET, Gamazon ER, Im HK, Konkashbaev A, Nicolae DL, Cox NJ, Flutre T, Wen X, Stephens M, Pritchard JK, Tu Z, Zhang B, Huang T, Long Q, Lin L, Yang J, Zhu J, Liu J, Brown A, Mestichelli B, Tidwell D, Lo E, Salvatore M, Shad S, Thomas JA, Lonsdale JT, Moser MT, Gillard BM, Karasik E, Ramsey K, Choi C, Foster BA, Syron J, Fleming J, Magazine H, Hasz R, Walters GD, Bridge JP, Miklos M, Sullivan S, Barker LK, Traino HM, Mosavel M, Siminoff LA, Valley DR, Rohrer DC, Jewell SD, Branton PA, Sobin LH, Barcus M, Qi L, McLean J, Hariharan P, Um KS, Wu S, Tabor D, Shive C, Smith AM, Buia SA, Undale AH, Robinson KL, Roche N, Valentino KM, Britton A, Burges R, Bradbury D, Hambright KW, Seleski J, Korzeniewski GE, Erickson K, Marcus Y, Tejada J, Taherian M, Lu C, Basile M, Mash DC, Volpi S, Struewing JP, Temple GF, Boyer J, Colantuoni D, Little R, Koester S, Carithers LJ, Moore HM, Guan P, Compton C, Sawyer SJ, Demchok JP, Vaught JB, Rabiner CA, Lockhart NC, Ardlie KG, Getz G, Wright FA, Kellis M, Volpi S, Dermitzakis ET; GTEx Consortium . Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348: 648–660, 2015. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayrault O, Andrique L, Fauvin D, Eymin B, Gazzeri S, Séité P. Human tumor suppressor p14ARF negatively regulates rRNA transcription and inhibits UBF1 transcription factor phosphorylation. Oncogene 25: 7577–7586, 2006. doi: 10.1038/sj.onc.1209743. [DOI] [PubMed] [Google Scholar]
- 6.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab 280: E203–E208, 2001. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- 7.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289: 905–920, 2000. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 8.Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167: 301–305, 1999. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 9.Blattner C, Jennebach S, Herzog F, Mayer A, Cheung ACM, Witte G, Lorenzen K, Hopfner K-P, Heck AJR, Aebersold R, Cramer P. Molecular basis of Rrn3-regulated RNA polymerase I initiation and cell growth. Genes Dev 25: 2093–2105, 2011. doi: 10.1101/gad.17363311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 11.Boivin V, Deschamps-Francoeur G, Couture S, Nottingham RM, Bouchard-Bourelle P, Lambowitz AM, Scott MS, Abou-Elela S. Simultaneous sequencing of coding and noncoding RNA reveals a human transcriptome dominated by a small number of highly expressed noncoding genes. RNA 24: 950–965, 2018. doi: 10.1261/rna.064493.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594: 7399–7417, 2016. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29: 4485–4496, 2015. doi: 10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh AH, Evans A, Rothblum LI. Mammalian Rrn3 is required for the formation of a transcription competent preinitiation complex containing RNA polymerase I. Gene Expr 14: 131–147, 2008. [PMC free article] [PubMed] [Google Scholar]
- 15.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol 229: 1584–1594, 2014. doi: 10.1002/jcp.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 115: 1065–1074, 2013. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JC, Hannan KM, Riddell K, Ng PY, Peck A, Lee RS, Hung S, Astle MV, Bywater M, Wall M, Poortinga G, Jastrzebski K, Sheppard KE, Hemmings BA, Hall MN, Johnstone RW, McArthur GA, Hannan RD, Pearson RB. AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci Signal 4: ra56, 2011. doi: 10.1126/scisignal.2001754. [DOI] [PubMed] [Google Scholar]
- 18.Chauvin C, Koka V, Nouschi A, Mieulet V, Hoareau-Aveilla C, Dreazen A, Cagnard N, Carpentier W, Kiss T, Meyuhas O, Pende M. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene 33: 474–483, 2014. doi: 10.1038/onc.2012.606. [DOI] [PubMed] [Google Scholar]
- 19.Claypool JA, French SL, Johzuka K, Eliason K, Vu L, Dodd JA, Beyer AL, Nomura M. Tor pathway regulates Rrn3p-dependent recruitment of yeast RNA polymerase I to the promoter but does not participate in alteration of the number of active genes. Mol Biol Cell 15: 946–956, 2004. doi: 10.1091/mbc.e03-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crossland H, Timmons JA, Atherton PJ. A dynamic ribosomal biogenesis response is not required for IGF-1-mediated hypertrophy of human primary myotubes. FASEB J 31: 5196–5207, 2017. doi: 10.1096/fj.201700329R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delarue M, Brittingham GP, Pfeffer S, Surovtsev IV, Pinglay S, Kennedy KJ, Schaffer M, Gutierrez JI, Sang D, Poterewicz G, Chung JK, Plitzko JM, Groves JT, Jacobs-Wagner C, Engel BD, Holt LJ. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell 174: 338–349.e20, 2018. doi: 10.1016/j.cell.2018.05.042. New results to this article are available at . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104: 57–65, 2008. doi: 10.1007/s00421-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 23.Devlin JR, Hannan KM, Ng PY, Bywater MJ, Shortt J, Cullinane C, McArthur GA, Johnstone RW, Hannan RD, Pearson RB. AKT signalling is required for ribosomal RNA synthesis and progression of Eμ-Myc B-cell lymphoma in vivo. FEBS J 280: 5307–5316, 2013. doi: 10.1111/febs.12135. [DOI] [PubMed] [Google Scholar]
- 24.Diesch J, Hannan RD, Sanij E. Perturbations at the ribosomal genes loci are at the centre of cellular dysfunction and human disease. Cell Biosci 4: 43, 2014. doi: 10.1186/2045-3701-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, Mortensen B, Appel EV, Jørgensen N, Kristiansen VB, Hansen T, Workman CT, Zierath JR, Barrès R. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab 23: 369–378, 2016. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A, Streiner N, Quin JE, Sanij E, Bywater MJ, Hannan RD, Ryckman D, Anderes K, Rice WG. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res 71: 1418–1430, 2011. doi: 10.1158/0008-5472.CAN-10-1728. [DOI] [PubMed] [Google Scholar]
- 28.Drygin D, Rice WG, Grummt I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol 50: 131–156, 2010. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 29.Ecker RE, Kokaisl G. Synthesis of protein, ribonucleic acid, and ribosomes by individual bacterial cells in balanced growth. J Bacteriol 98: 1219–1226, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309: E72–E83, 2015. doi: 10.1152/ajpendo.00050.2015. [DOI] [PubMed] [Google Scholar]
- 32.Figueiredo VC, Roberts LA, Markworth JF, Barnett MPG, Coombes JS, Raastad T, Peake JM, Cameron-Smith D. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep 4: e12670, 2016. doi: 10.14814/phy2.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figueiredo VC, Zeng N, D’Souza RF, Markworth JF, Della Gatta PA, Petersen A, Barnett MPG, Cameron-Smith D. High dose of whey protein after resistance exercise promotes 45 S preribosomal RNA synthesis in older men. Nutrition 50: 105–107, 2018. doi: 10.1016/j.nut.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Fine LG, Norman J. Cellular events in renal hypertrophy. Annu Rev Physiol 51: 19–32, 1989. doi: 10.1146/annurev.ph.51.030189.000315. [DOI] [PubMed] [Google Scholar]
- 36.Fiorotto ML, Davis TA, Sosa HA, Villegas-Montoya C, Estrada I, Fleischmann R. Ribosome abundance regulates the recovery of skeletal muscle protein mass upon recuperation from postnatal undernutrition in mice. J Physiol 1–18, 2014. doi: 10.1113/jphysiol.2014.279067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorotto ML, Davis TA, Reeds PJ. Regulation of myofibrillar protein turnover during maturation in normal and undernourished rat pups. Am J Physiol Regul Integr Comp Physiol 278: R845–R854, 2000. doi: 10.1152/ajpregu.2000.278.4.R845. [DOI] [PubMed] [Google Scholar]
- 38.Fyfe JJ, Bishop DJ, Bartlett JD, Hanson ED, Anderson MJ, Garnham AP, Stepto NK. Enhanced skeletal muscle ribosome biogenesis, yet attenuated mTORC1 and ribosome biogenesis-related signalling, following short-term concurrent versus single-mode resistance training. Sci Rep 8: 560, 2018. doi: 10.1038/s41598-017-18887-6. New results for this article are available at https://doi.org/10.1101/115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galpin AJ, Fry AC, Chiu LZF, Thomason DB, Schilling BK. High-power resistance exercise induces MAPK phosphorylation in weightlifting trained men. Appl Physiol Nutr Metab 37: 80–87, 2012. doi: 10.1139/h11-131. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons JG, Branco AT, Godinho SA, Yu S, Lemos B. Concerted copy number variation balances ribosomal DNA dosage in human and mouse genomes. Proc Natl Acad Sci USA 112: 2485–2490, 2015. doi: 10.1073/pnas.1416878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons JG, Branco AT, Yu S, Lemos B. Ribosomal DNA copy number is coupled with gene expression variation and mitochondrial abundance in humans. Nat Commun 5: 4850, 2014. doi: 10.1038/ncomms5850. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg AL. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol 213: 1193–1198, 1967. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg AL. Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol 36: 653–658, 1968. doi: 10.1083/jcb.36.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg AL. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem 244: 3217–3222, 1969. [PubMed] [Google Scholar]
- 45.Goldberg AL, Etlinger JD, Goldspink DF, Jablecki C. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports 7: 185–198, 1975. [PubMed] [Google Scholar]
- 46.Goldspink DF. The influence of activity on muscle size and protein turnover. J Physiol 264: 283–296, 1977. doi: 10.1113/jphysiol.1977.sp011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodfellow SJ, Zomerdijk JC. Basic mechanisms in RNA polymerase I transcription of the ribosomal RNA genes. Subcell Biochem 61: 211–236, 2013. doi: 10.1007/978-94-007-4525-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You J-S, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon BS, Liu C, Steiner JL, Nader GA, Jefferson LS, Kimball SR. Loss of REDD1 augments the rate of the overload-induced increase in muscle mass. Am J Physiol Regul Integr Comp Physiol 311: R545–R557, 2016. doi: 10.1152/ajpregu.00159.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorski JJ, Pathak S, Panov K, Kasciukovic T, Panova T, Russell J, Zomerdijk JCBM. A novel TBP-associated factor of SL1 functions in RNA polymerase I transcription. EMBO J 26: 1560–1568, 2007. doi: 10.1038/sj.emboj.7601601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haddach M, Schwaebe MK, Michaux J, Nagasawa J, O’Brien SE, Whitten JP, Pierre F, Kerdoncuff P, Darjania L, Stansfield R, Drygin D, Anderes K, Proffitt C, Bliesath J, Siddiqui-Jain A, Omori M, Huser N, Rice WG, Ryckman DM. Discovery of CX-5461, the first direct and selective inhibitor of RNA polymerase I, for cancer therapeutics. ACS Med Chem Lett 3: 602–606, 2012. doi: 10.1021/ml300110s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamosch M, Lesch M, Baron J, Kaufman S. Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science 157: 935–937, 1967. doi: 10.1126/science.157.3791.935. [DOI] [PubMed] [Google Scholar]
- 53.Hannan RD, Jenkins A, Jenkins AK, Brandenburger Y. Cardiac hypertrophy: a matter of translation. Clin Exp Pharmacol Physiol 30: 517–527, 2003. doi: 10.1046/j.1440-1681.2003.03873.x. [DOI] [PubMed] [Google Scholar]
- 54.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol (1985) 102: 573–581, 2007. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- 55.Henras AK, Plisson-Chastang C, O’Donohue MF, Chakraborty A, Gleizes PE. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip Rev RNA 6: 225–242, 2015. doi: 10.1002/wrna.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henras AK, Soudet J, Gérus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci 65: 2334–2359, 2008. doi: 10.1007/s00018-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang S, Rothblum LI, Chen D. Ribosomal chromatin organization. Biochem Cell Biol 84: 444–449, 2006. doi: 10.1139/o06-089. [DOI] [PubMed] [Google Scholar]
- 58.James MJ, Zomerdijk JCBM. Phosphatidylinositol 3-kinase and mTOR signaling pathways regulate RNA polymerase I transcription in response to IGF-1 and nutrients. J Biol Chem 279: 8911–8918, 2004. doi: 10.1074/jbc.M307735200. [DOI] [PubMed] [Google Scholar]
- 59.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29: 120–127, 2004. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- 60.Kelly FJ, Lewis SE, Anderson P, Goldspink DF. Pre- and postnatal growth and protein turnover in four muscles of the rat. Muscle Nerve 7: 235–242, 1984. doi: 10.1002/mus.880070309. [DOI] [PubMed] [Google Scholar]
- 61.Khatter H, Myasnikov AG, Natchiar SK, Klaholz BP. Structure of the human 80S ribosome. Nature 520: 640–645, 2015. doi: 10.1038/nature14427. [DOI] [PubMed] [Google Scholar]
- 62.Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568: 283–290, 2005. doi: 10.1113/jphysiol.2005.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S, Li Q, Dang CV, Lee LA. Induction of ribosomal genes and hepatocyte hypertrophy by adenovirus-mediated expression of c-Myc in vivo. Proc Natl Acad Sci USA 97: 11198–11202, 2000. doi: 10.1073/pnas.200372597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol (1985) 119: 321–327, 2015. doi: 10.1152/japplphysiol.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirby TJ, Patel RM, McClintock TS, Dupont-Versteegden EE, Peterson CA, McCarthy JJ. Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol Biol Cell 27: 788–798, 2016. doi: 10.1091/mbc.e15-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene 25: 1620–1628, 2006. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- 67.Kusnadi EP, Hannan KM, Hicks RJ, Hannan RD, Pearson RB, Kang J. Regulation of rDNA transcription in response to growth factors, nutrients and energy. Gene 556: 27–34, 2015. doi: 10.1016/j.gene.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 68.de la Cruz J, Karbstein K, Woolford JL Jr. Functions of ribosomal proteins in assembly of eukaryotic ribosomes in vivo. Annu Rev Biochem 84: 93–129, 2015. doi: 10.1146/annurev-biochem-060614-033917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landon AL, Muniandy PA, Shetty AC, Lehrmann E, Volpon L, Houng S, Zhang Y, Dai B, Peroutka R, Mazan-Mamczarz K, Steinhardt J, Mahurkar A, Becker KG, Borden KL, Gartenhaus RB. MNKs act as a regulatory switch for eIF4E1 and eIF4E3 driven mRNA translation in DLBCL. Nat Commun 5: 5413, 2014. doi: 10.1038/ncomms6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee D, An J, Park Y-U, Liaw H, Woodgate R, Park JH, Myung K. SHPRH regulates rRNA transcription by recognizing the histone code in an mTOR-dependent manner. Proc Natl Acad Sci USA 114: E3424–E3433, 2017. doi: 10.1073/pnas.1701978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li M, Zhou X, Chen Y, Nie Y, Huang H, Chen H, Mo D. Not all the number of skeletal muscle fibers is determined prenatally. BMC Dev Biol 15: 42, 2015. doi: 10.1186/s12861-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lompre AM, Schwartz K, d’Albis A, Lacombe G, Van Thiem N, Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature 282: 105–107, 1979. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- 73.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E--from translation to transformation. Oncogene 23: 3172–3179, 2004. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 74.Marabita M, Baraldo M, Solagna F, Ceelen JJM, Sartori R, Nolte H, Nemazanyy I, Pyronnet S, Kruger M, Pende M, Blaauw B. S6K1 is required for increasing skeletal muscle force during hypertrophy. Cell Reports 17: 501–513, 2016. doi: 10.1016/j.celrep.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 75.Markworth JF, Vella LD, Figueiredo VC, Cameron-Smith D. Ibuprofen treatment blunts early translational signaling responses in human skeletal muscle following resistance exercise. J Appl Physiol (1985) 117: 20–28, 2014. doi: 10.1152/japplphysiol.01299.2013. [DOI] [PubMed] [Google Scholar]
- 76.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25: 6384–6391, 2006. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- 78.Mayhew DL, Kim J-S, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985) 107: 1655–1662, 2009. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCarthy JJ, Esser KA. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care 13: 230–235, 2010. doi: 10.1097/MCO.0b013e32833781b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mesires NT, Doumit ME. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am J Physiol Cell Physiol 282: C899–C906, 2002. doi: 10.1152/ajpcell.00341.2001. [DOI] [PubMed] [Google Scholar]
- 82.Miller G, Panov KI, Friedrich JK, Trinkle-Mulcahy L, Lamond AI, Zomerdijk JC. hRRN3 is essential in the SL1-mediated recruitment of RNA Polymerase I to rRNA gene promoters. EMBO J 20: 1373–1382, 2001. doi: 10.1093/emboj/20.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Millward DJ, Garlick PJ, James WPT, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241: 204–205, 1973. doi: 10.1038/241204a0. [DOI] [PubMed] [Google Scholar]
- 84.Mobley CB, Haun CT, Roberson PA, Mumford PW, Kephart WC, Romero MA, Osburn SC, Vann CG, Young KC, Beck DT, Martin JS, Lockwood CM, Roberts MD. Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training. PLoS One 13: e0195203, 2018. doi: 10.1371/journal.pone.0195203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montagne J. Genetic and molecular mechanisms of cell size control. Mol Cell Biol Res Commun 4: 195–202, 2000. doi: 10.1006/mcbr.2001.0284. [DOI] [PubMed] [Google Scholar]
- 86.Moore PB, Steitz TA. The roles of RNA in the synthesis of protein. Cold Spring Harb Perspect Biol 3: a003780, 2011. doi: 10.1101/cshperspect.a003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moro T, Brightwell CR, Deer RR, Graber TG, Galvan E, Fry CS, Volpi E, Rasmussen BB. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr 148: 900–909, 2018. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moss T, Langlois F, Gagnon-Kugler T, Stefanovsky V. A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell Mol Life Sci 64: 29–49, 2007. doi: 10.1007/s00018-006-6278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murach KA, Fry CS, Kirby TJ, Jackson JR, Lee JD, White SH, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Physiology (Bethesda) 33: 26–38, 2018. doi: 10.1152/physiol.00019.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murach KA, White SH, Wen Y, Ho A, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle 7: 14, 2017. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 289: C1457–C1465, 2005. doi: 10.1152/ajpcell.00165.2005. [DOI] [PubMed] [Google Scholar]
- 92.Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE, Gordon PM. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 116: 693–702, 2014. doi: 10.1152/japplphysiol.01366.2013. [DOI] [PubMed] [Google Scholar]
- 93.Nakada S, Ogasawara R, Kawada S, Maekawa T, Ishii N. Correlation between ribosome biogenesis and the magnitude of hypertrophy in overloaded skeletal muscle. PLoS One 11: e0147284, 2016. doi: 10.1371/journal.pone.0147284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nandagopal N, Roux PP. Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation (Austin) 3: e983402, 2015. doi: 10.4161/21690731.2014.983402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nikolay R, van den Bruck D, Achenbach J, Nierhaus KH. Ribosomal proteins: role in ribosomal functions. J Mol Cell Biol 7: 92–104, 2015. doi: 10.1093/jmcb/mjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science 289: 920–930, 2000. doi: 10.1126/science.289.5481.920. [DOI] [PubMed] [Google Scholar]
- 97.O’Neil D, Glowatz H, Schlumpberger M. Ribosomal RNA depletion for efficient use of RNA-Seq capacity. In: Current Protocols in Molecular Biology. Hoboken, NJ: John Wiley & Sons, Inc, 2013, p. 19 [DOI] [PubMed] [Google Scholar]
- 98.O’Sullivan AC, Sullivan GJ, McStay B. UBF binding in vivo is not restricted to regulatory sequences within the vertebrate ribosomal DNA repeat. Mol Cell Biol 22: 657–668, 2002. doi: 10.1128/MCB.22.2.657-668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ogasawara R, Fujita S, Hornberger TA, Kitaoka Y, Makanae Y, Nakazato K, Naokata I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep 6: 31142, 2016. doi: 10.1038/srep31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panov KI, Friedrich JK, Zomerdijk JCBM. A step subsequent to preinitiation complex assembly at the ribosomal RNA gene promoter is rate limiting for human RNA polymerase I-dependent transcription. Mol Cell Biol 21: 2641–2649, 2001. doi: 10.1128/MCB.21.8.2641-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parks MM, Kurylo CM, Dass RA, Bojmar L, Lyden D, Vincent CT, Blanchard SC. Variant ribosomal RNA alleles are conserved and exhibit tissue-specific expression. Sci Adv 4: eaao0665, 2018. doi: 10.1126/sciadv.aao0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pereira MG, Dyar KA, Nogara L, Solagna F, Marabita M, Baraldo M, Chemello F, Germinario E, Romanello V, Nolte H, Blaauw B. Comparative analysis of muscle hypertrophy models reveals divergent gene transcription profiles and points to translational regulation of muscle growth through increased mTOR signaling. Front Physiol 8: 968, 2017. doi: 10.3389/fphys.2017.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peyroche G, Milkereit P, Bischler N, Tschochner H, Schultz P, Sentenac A, Carles C, Riva M. The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. EMBO J 19: 5473–5482, 2000. doi: 10.1093/emboj/19.20.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reidy PT, Borack MS, Markofski MM, Dickinson JM, Fry CS, Deer RR, Volpi E, Rasmussen BB. Post-absorptive muscle protein turnover affects resistance training hypertrophy. Eur J Appl Physiol 117: 853–866, 2017. doi: 10.1007/s00421-017-3566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberts LA, Raastad T, Markworth JF, Figueiredo VC, Egner IM, Shield A, Cameron-Smith D, Coombes JS, Peake JM. Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. J Physiol 593: 4285–4301, 2015. doi: 10.1113/JP270570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robledo S, Idol RA, Crimmins DL, Ladenson JH, Mason PJ, Bessler M. The role of human ribosomal proteins in the maturation of rRNA and ribosome production. RNA 14: 1918–1929, 2008. doi: 10.1261/rna.1132008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenwald IB, Lazaris-Karatzas A, Sonenberg N, Schmidt EV. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol 13: 7358–7363, 1993. doi: 10.1128/MCB.13.12.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russell J, Zomerdijk JCBM. RNA-polymerase-I-directed rDNA transcription, life and works. Trends Biochem Sci 30: 87–96, 2005. doi: 10.1016/j.tibs.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Russell J, Zomerdijk JCBM. The RNA polymerase I transcription machinery. Biochem Soc Symp 73: 203–216, 2006. doi: 10.1042/bss0730203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanij E, Poortinga G, Sharkey K, Hung S, Holloway TP, Quin J, Robb E, Wong LH, Thomas WG, Stefanovsky V, Moss T, Rothblum L, Hannan KM, McArthur GA, Pearson RB, Hannan RD. UBF levels determine the number of active ribosomal RNA genes in mammals. J Cell Biol 183: 1259–1274, 2008. doi: 10.1083/jcb.200805146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scherl A, Couté Y, Déon C, Callé A, Kindbeiter K, Sanchez JC, Greco A, Hochstrasser D, Diaz JJ. Functional proteomic analysis of human nucleolus. Mol Biol Cell 13: 4100–4109, 2002. doi: 10.1091/mbc.e02-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1: 4, 2011. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schnapp A, Schnapp G, Erny B, Grummt I. Function of the growth-regulated transcription initiation factor TIF-IA in initiation complex formation at the murine ribosomal gene promoter. Mol Cell Biol 13: 6723–6732, 1993. doi: 10.1128/MCB.13.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786, 2000. doi: 10.1016/S0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 115.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14: 2501–2514, 2000. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Snijders T, Smeets JSJ, van Kranenburg J, Kies AK, van Loon LJC, Verdijk LB. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol (Oxf) 216: 231–239, 2016. doi: 10.1111/apha.12609. [DOI] [PubMed] [Google Scholar]
- 117.Sobel BE, Kaufman S. Enhanced RNA polymerase activity in skeletal muscle undergoing hypertrophy. Arch Biochem Biophys 137: 469–476, 1970. doi: 10.1016/0003-9861(70)90464-9. [DOI] [PubMed] [Google Scholar]
- 118.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 119: 851–857, 2015. doi: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stefanovsky VY, Langlois F, Bazett-Jones D, Pelletier G, Moss T. ERK modulates DNA bending and enhancesome structure by phosphorylating HMG1-boxes 1 and 2 of the RNA polymerase I transcription factor UBF. Biochemistry 45: 3626–3634, 2006. doi: 10.1021/bi051782h. [DOI] [PubMed] [Google Scholar]
- 121.Stefanovsky VY, Pelletier G, Bazett-Jones DP, Crane-Robinson C, Moss T. DNA looping in the RNA polymerase I enhancesome is the result of non-cooperative in-phase bending by two UBF molecules. Nucleic Acids Res 29: 3241–3247, 2001. doi: 10.1093/nar/29.15.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steitz TA, Moore PB. RNA, the first macromolecular catalyst: the ribosome is a ribozyme. Trends Biochem Sci 28: 411–418, 2003. doi: 10.1016/S0968-0004(03)00169-5. [DOI] [PubMed] [Google Scholar]
- 122a.Stepanchick A, Zhi H, Cavanaugh AH, Rothblum K, Schneider DA, Rothblum LI. DNA binding by the ribosomal DNA transcription factor rrn3 is essential for ribosomal DNA transcription. J Biol Chem 288: 9135–9144, 2013. doi: 10.1074/jbc.M112.444265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tafforeau L, Zorbas C, Langhendries JL, Mullineux ST, Stamatopoulou V, Mullier R, Wacheul L, Lafontaine DLJ. The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of Pre-rRNA processing factors. Mol Cell 51: 539–551, 2013. doi: 10.1016/j.molcel.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 124.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- 125.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485: 109–113, 2012. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Topisirovic I, Ruiz-Gutierrez M, Borden KLB. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res 64: 8639–8642, 2004. doi: 10.1158/0008-5472.CAN-04-2677. [DOI] [PubMed] [Google Scholar]
- 127.Tsang CK, Liu H, Zheng XFS. mTOR binds to the promoters of RNA polymerase I- and III-transcribed genes. Cell Cycle 9: 953–957, 2010. doi: 10.4161/cc.9.5.10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vandenburgh H, Kaufman S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203: 265–268, 1979. doi: 10.1126/science.569901. [DOI] [PubMed] [Google Scholar]
- 129.Vázquez-Vela MEF, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res 39: 715–728, 2008. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 130.Voit R, Grummt I. Phosphorylation of UBF at serine 388 is required for interaction with RNA polymerase I and activation of rDNA transcription. Proc Natl Acad Sci USA 98: 13631–13636, 2001. doi: 10.1073/pnas.231071698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Voit R, Hoffmann M, Grummt I. Phosphorylation by G1-specific cdk-cyclin complexes activates the nucleolar transcription factor UBF. EMBO J 18: 1891–1899, 1999. doi: 10.1093/emboj/18.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.von Walden F, Casagrande V, Östlund Farrants AK, Nader GA. Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. Am J Physiol Cell Physiol 302: C1523–C1530, 2012. doi: 10.1152/ajpcell.00460.2011. [DOI] [PubMed] [Google Scholar]
- 133.von Walden F, Liu C, Aurigemma N, Nader GA. mTOR signaling regulates myotube hypertrophy by modulating protein synthesis, rDNA transcription, and chromatin remodeling. Am J Physiol Cell Physiol 311: C663–C672, 2016. doi: 10.1152/ajpcell.00144.2016. [DOI] [PubMed] [Google Scholar]
- 134.Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 24: 437–440, 1999. doi: 10.1016/S0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 135.Welle S, Bhatt K, Thornton CA. Inventory of high-abundance mRNAs in skeletal muscle of normal men. Genome Res 9: 506–513, 1999. [PMC free article] [PubMed] [Google Scholar]
- 136.Wen Y, Alimov AP, McCarthy JJ. Ribosome Biogenesis is Necessary for Skeletal Muscle Hypertrophy. Exerc Sport Sci Rev 44: 110–115, 2016. doi: 10.1249/JES.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.West DWD, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC, Baar K. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol 594: 453–468, 2016. doi: 10.1113/JP271365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.West DWD, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol 42: 1371–1375, 2010. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 139.White RB, Biérinx A-S, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol 10: 21, 2010. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.White RJ. RNA polymerase III transcription and cancer. Oncogene 23: 3208–3216, 2004. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- 141.White RJ. RNA polymerases I and III, non-coding RNAs and cancer. Trends Genet 24: 622–629, 2008. doi: 10.1016/j.tig.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 142.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547: 977–987, 2003. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wong TS, Booth FW. Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol (1985) 69: 1709–1717, 1990. doi: 10.1152/jappl.1990.69.5.1709. [DOI] [PubMed] [Google Scholar]
- 145.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol 13: 355–369, 2012. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Young VR, Alexis SD. In vitro activity of ribosomes and RNA content of skeletal muscle in young rats fed adequate or low protein. J Nutr 96: 255–262, 1968. doi: 10.1093/jn/96.2.255. [DOI] [PubMed] [Google Scholar]
- 147.Yuan X, Zhao J, Zentgraf H, Hoffmann-Rohrer U, Grummt I. Multiple interactions between RNA polymerase I, TIF-IA and TAF(I) subunits regulate preinitiation complex assembly at the ribosomal gene promoter. EMBO Rep 3: 1082–1087, 2002. doi: 10.1093/embo-reports/kvf212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao J, Yuan X, Frödin M, Grummt I. ERK-dependent phosphorylation of the transcription initiation factor TIF-IA is required for RNA polymerase I transcription and cell growth. Mol Cell 11: 405–413, 2003. doi: 10.1016/S1097-2765(03)00036-4. [DOI] [PubMed] [Google Scholar]