Abstract
A novel series of thiazole-based heterocycles was synthesized using 1,3-dipolar cycloaddition reactions in the presence of chitosan-grafted-poly(vinylpyridine) as an eco-friendly biopolymeric basic catalyst. The molecular structure of the synthesized compounds was illustrated by spectroscopic and elemental analysis. Various in vitro biological assays were performed to explore the potential antitumor, antimicrobial and hepatoprotective activities of the newly synthesized compounds. The cytotoxic activities were assessed against human hepatocellular carcinoma (HepG-2), colorectal carcinoma (HCT-116) and breast cancer (MCF-7) cell lines and results revealed that all compounds displayed antitumor activities with the chlorine-containing derivatives, 11c and 6g, being the most potent. The majority of the tested thiazole derivatives exhibited satisfactory antibacterial activity towards the used gram positive and gram-negative bacterial species. Moreover, many derivatives showed weak hepatoprotective activity against CCl4-induced hepatotoxicity.
Keywords: thiazoles, hydrazonoyl halides, hepatoprotective activity, anticancer activity, antimicrobial activity
1. Introduction
Synthesis of novel bioactive compounds using green methods that minimize the use and generation of hazardous substances, is a major aim for many researchers. Thiazole derivatives have gained considerable attention because of their broad biological activities that include antidiabetic, antimicrobial, anti-inflammatory, anticancer, anti-Alzheimer, antihypertensive, antioxidant and hepatoprotective activities [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. In addition, many thiazole-containing drugs such as Abafungin, Alagebrium, Acotiamide, Amiphenazole, Brecanavir, Cefepime, Carumonam, and Cefmatilen are commercially available.
Cancer is regarded as one of the dominant causes of mortality nowadays. The development of new antitumor agents represents an urgent need due to the increasing problems of various, sometimes, intolerable toxic side effects of the currently marketed drugs and the evolution of resistance to their actions [17,18]. Furthermore, liver diseases are viewed as one of the highly serious health issues globally [19]. The lack of satisfactory treatment strategies for these diseases with the occurrence of different side effects upon long term therapy, raise the demand for finding out new chemical entities that offer more efficient hepatoprotection and considerable safety. Moreover, there is a continuous compelling need for the development of new antibiotics to replace the current medications that are losing their efficacy and that could have higher efficiency or a wider spectrum.
In view of these precedents and together with our research concerns of developing new convenient approaches for the synthesis of different heterocyclic systems with auspicious pharmacological activities [20,21,22,23,24,25], we present in this report an efficient synthesis of some new series of novel thiazole derivatives using chitosan-grafted-poly(vinyl pyridine) as an eco-friendly biopolymeric basic catalyst. Additionally, we have assessed a variety of biological activities for the newly synthesized compounds that demonstrated their potential antitumor, antimicrobial and hepatoprotective effectiveness.
2. Results and Discussion
2.1. Chemistry
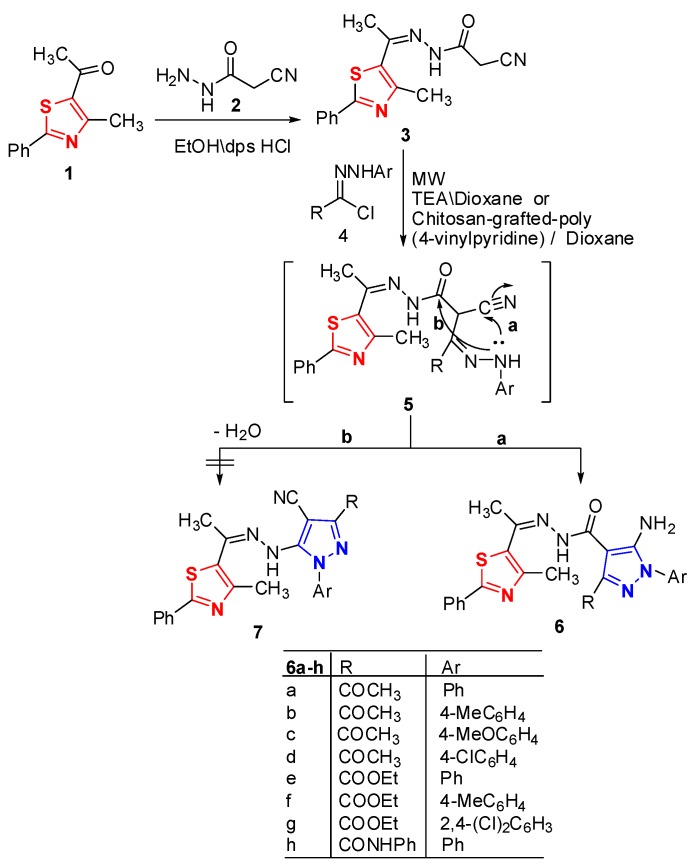
Refluxing of 5-acetyl-4-methyl-2-phenyl-thiazole (1) [26] and 2-cyanoacetohydrazide (2) [27] afforded a single product identified as 2-cyano-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)- acetohydrazide (3, Scheme 1).
Scheme 1.
Synthesis of thiazolyl pyrazoles 6a–h.
Its mass spectrum was compatible with the molecular formula C15H14N4OS and its IR spectrum showed absorption bands at 1643, 2338, and 3430 cm−1 due to amido carbonyl group, cyano and NH functions, respectively. Also, its 1H-NMR revealed signals at δ 2.49, 2.72, 3.30 and 10.6 due to two methyl, CH2, and NH protons, respectively. Moreover, its mass spectrum showed a molecular ion peak at m/z = 298.
Treatment of hydrazone derivative 3 with the appropriate hydrazonoyl halides 4a–h [28,29,30,31,32] using triethylamine or chitosan as a basic catalyst and under the same experimental conditions, afforded in each case the same products which are identified as the thiazole derivatives 6a–h rather than the other possible product 7 based on the spectral data (IR, MS and 1H-NMR) of the isolated products (Scheme 1, see Supporting Information). The distinction between the two possible products 6 and 7 was done based on the results of the spectral analysis. The IR spectra showed the absence of nitrile absorption band. Also, their 1H-NMR spectra revealed the presence of signals corresponds to NH2 protons. Moreover, their mass spectrum showed peaks corresponding to their molecular ions. The results of Table 1 indicated that high yield was obtained using chitosan as a basic catalyst.
Table 1.
Effect of nature of basic catalyst on the product yields 6a–h.
| No. | Time (min) | Yield % | |
|---|---|---|---|
| TEA | g-Chitosan | ||
| 6a | 4 | 67 | 80 |
| 6b | 6 | 69 | 82 |
| 6c | 9 | 68 | 84 |
| 6d | 5 | 73 | 85 |
| 6e | 10 | 73 | 84 |
| 6f | 8 | 68 | 83 |
| 6g | 7 | 76 | 88 |
| 6h | 7 | 73 | 81 |
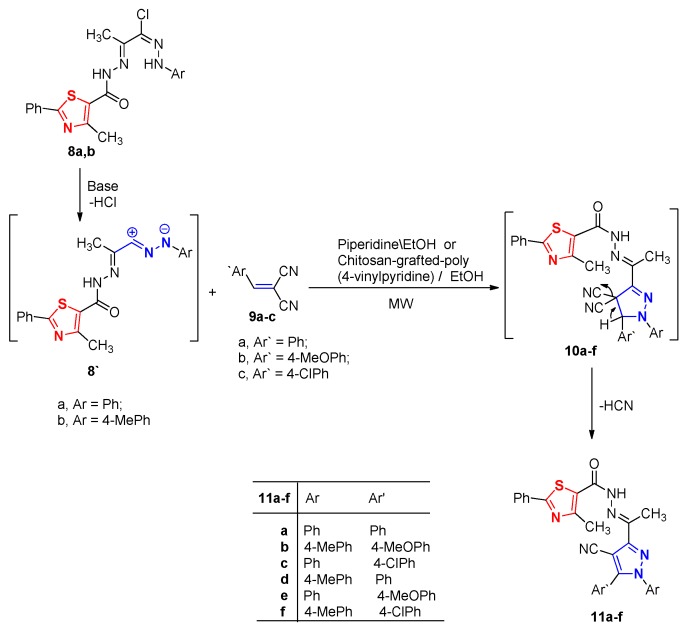
Heating a mixture of hydrazonoyl halides 8a or 8b [23] and the appropriate arylidine malononitriles 9a–c [33] in ethanol containing piperidine under irradiation by MW led to the formation of the thiazolyl pyrazoles 11a–f (Scheme 2). The structure of the latter products was established based on their elemental analysis and spectral data (cf. Experimental, see Supporting Information).
Scheme 2.
Synthesis of thiazolylpyrazoles 11a–f.
When the above reaction was repeated in presence of grafted-chitosan as a catalyst and under typical reaction conditions, the same products which are identical in all aspects (m.p., mixed m.p. and IR spectra) were obtained in good yields (Table 2).
Table 2.
Effect of nature of basic catalyst on the product yields 11a–f.
| No. | Time (min) | Yield % | |
|---|---|---|---|
| Piperidine | g-Chitosan | ||
| 11a | 5 | 69 | 83 |
| 11b | 7 | 71 | 81 |
| 11c | 5 | 74 | 86 |
| 11d | 8 | 72 | 81 |
| 11e | 3 | 71 | 84 |
| 11f | 7 | 73 | 85 |
To account for the formation of the product 11, it is suggested that the 1,3-dipolar cycloaddition of nitrile imine 8` generated in situ from hydrazonoyl halides 8 in the presence of base) to the arylidine derivative 9 to give the intermediate 10, followed by aromatization via losing of HCN molecule to give the final product 11 as illustrated in Scheme 2.
2.2. Biological Evaluation
2.2.1. Cytotoxic Activity
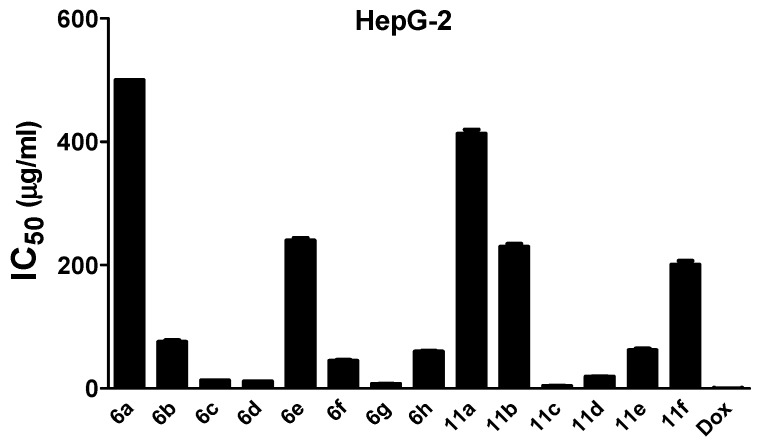
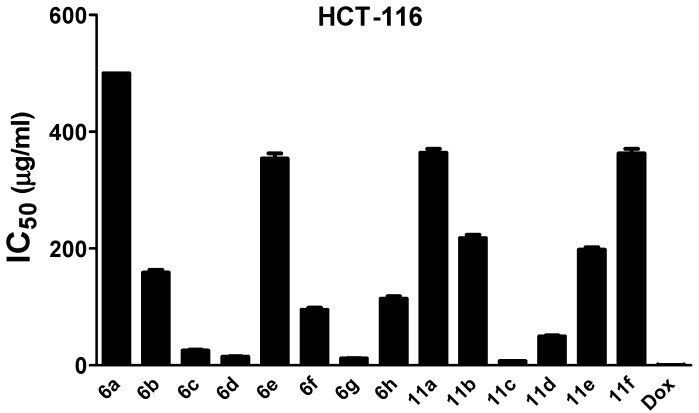
The in vitro antitumor activity of the newly-synthesized compounds 6a–h and 11a–f and the reference drug, Doxorubicin was investigated against three cancer cell lines, human hepatocellular carcinoma cell line (HepG-2), colon carcinoma cells (HCT-116), and human breast carcinoma cells (MCF-7 cell line). The cytotoxic potential was determined using the MTT (methyl thiazolyl tetrazolium) assay after 24 h of incubation [34]. The concentration of the tested compounds needed to inhibit 50% of the cells (IC50) was calculated and presented in Table 3 and Figure 1, Figure 2 and Figure 3.
Table 3.
Cytotoxic activity of the synthesized thiazolyl pyrazoles against HepG-2, HCT-116, and MCF-7 cell lines, expressed as IC50 values and compared to doxorubicin, the standard drug.
| Tested Compounds | IC50 (μg/mL) | ||
|---|---|---|---|
| HepG-2 | HCT-116 | MCF-7 | |
| 6a | >500 | >500 | >500 |
| 6b | 75.5 ± 2.7 | 159 ± 4.7 | 114 ± 1.2 |
| 6c | 13.1 ± 0.4 | 25.4 ± 1.3 | 13.9 ± 0.9 |
| 6d | 11.4 ± 0.2 | 14.8 ± 0.6 | 7.36 ± 0.4 |
| 6e | 240 ± 4.3 | 354 ± 8.9 | 231 ± 4.5 |
| 6f | 44.8 ± 1.3 | 95 ± 3.8 | 56.1 ± 0.7 |
| 6g | 7.4 ± 0.2 | 11.8 ± 0.5 | 3.77 ± 0.2 |
| 6h | 60 ± 1.1 | 114 ± 4.1 | 86.2 ± 1.1 |
| 11a | 413 ± 6.9 | 364 ± 6.9 | 276 ± 7.8 |
| 11b | 230 ± 4.6 | 218 ± 5.3 | 243 ± 4.9 |
| 11c | 4.24 ± 0.3 | 7.35 ± 0.4 | 2.99 ± 0.2 |
| 11d | 19.3 ± 0.8 | 49.6 ± 1.7 | 26.8 ± 0.8 |
| 11e | 62.1 ± 2.6 | 198 ± 4.2 | 110 ± 1.9 |
| 11f | 201 ± 5.9 | 363 ± 7.8 | 173 ± 3.5 |
| Doxorubicin | 0.36 ± 0.04 | 0.49 ± 0.07 | 0.35 ± 0.03 |
The analysis was performed using the MTT assay after 24 h of incubation. Values are shown as mean ± SD of three replicates.
Figure 1.
In vitro antitumor effect of synthesized thiazolyl pyrazoles (6a–h, 11a–f) against HepG-2. Dox: doxorubicin, the standard drug. The analysis was performed using the MTT assay after 24 h of incubation. Values are shown as mean ± SD of three replicates. All compounds exhibited cytotoxic effects and, 11c and 6g were the most potent. Compound 6a has IC50 > 500 µg/mL.
Figure 2.
In vitro antitumor effect of synthesized thiazolyl pyrazoles (6a–h, 11a–f) against HCT-116. Dox: doxorubicin, the standard drug. The analysis was performed using the MTT assay after 24 h of incubation. Values are shown as mean ± SD of three replicates. All compounds exhibited cytotoxic effects and, 11c and 6g were the most potent. Compound 6a has IC50 > 500 µg/mL.
Figure 3.
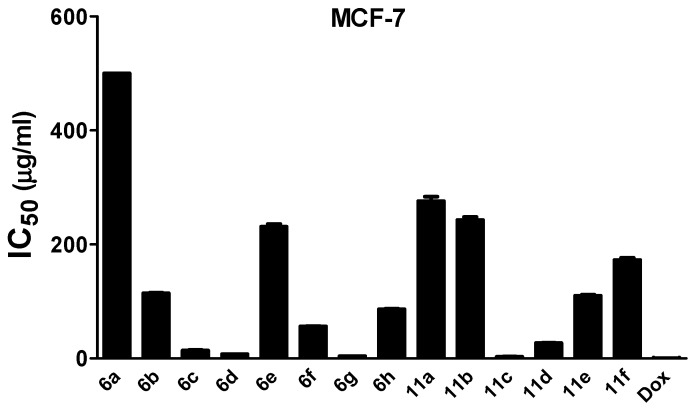
In vitro antitumor effect of synthesized thiazolyl pyrazoles (6a–h, 11a–f) against MFC-7. Dox: doxorubicin, the standard drug. The analysis was performed using the MTT assay after 24 h of incubation. Values are shown as mean ± SD of three replicates. All compounds exhibited cytotoxic effects and in particular, 11c and 6g were the most potent. Compound 6a has IC50 > 500 µg/mL.
Results of the MTT assay indicated that most of investigated compounds exhibited inhibitory activity against the tested cell lines, with some derivatives showing prominent antitumor activity. Thiazole derivatives 11c and 6g displayed the highest cytotoxic activities against the tested cell lines with IC50 values of about 4 µg/mL and 7 µg/mL for HepG-2, 3 µg/mL and 4 µg/mL for MCF-7, and 7 µg/mL and 12 µg/mL, for HCT-116 cells, respectively.
According to these results, we can suggest the following structure activity relationships:
A—In the thiazolylpyrazoles 6a–h:
-
(1)
Attachment of chlorine (6d) or methoxy group (6c) at position 4 in the aryl moiety of the pyrazole ring is important for cytotoxic activity with chlorine having the higher impact in compound (6d).
-
(2)
Addition of another chlorine atom in position 2 in the aryl moiety of compound (6g) increases the activity which reaches the double against MCF-7 cells.
B—In the thiazolylpyrazoles 11a–f:
-
(1)
Substitution on only one of the aryl moieties of the pyrazole ring in compounds (11c,d) induces cytotoxic activity, most prominently by chlorine in compound (11c).
-
(2)
Substitution on the second aryl moiety of the pyrazole ring by methyl group as in compounds (11b,f) induces great reduction (nearly abolishes) the cytotoxic activity.
2.2.2. Evaluation of the Antimicrobial Activity
The in vitro antimicrobial effectiveness of the newly synthesized thiazolyl pyrazoles 6a–h, 11a–f, and standard drugs were investigated using the inhibition zone technique and minimum inhibitory concentration (MIC) [35,36]. The antibacterial activities were tested against the gram-positive bacteria, Staphylococcus aureus (CMB010010) and Bacillus subtilis (RCMB 010067), and the gram-negative bacteria: Escherichia coli (RCMB 010052) and Proteus vulgaris (RCMB 004 (1) ATCC 13315), while the antifungal activities were tested against Aspergillus fumigatus (RCMB 002008 (4)) and Candida albicans (RCMB 05036). Gentamycin was used as the standard antibacterial drug while ketoconazole was used as the standard antifungal drug. The results are presented in Table 4 and Table 5 and Supplementary Figures S1–S6.
Table 4.
Antimicrobial activities of the new thiazole derivatives 6a–h and 11a–f expressed as inhibition zones diameter in millimeters (mm).
| Sample | Microorganisms | |||||
|---|---|---|---|---|---|---|
| Fungi | Gram Positive Bacteria | Gram Negative Bacteria | ||||
| AF | CA | SA | BS | EC | PV | |
| 6a | NA | NA | 12 ± 0.6 | 11 ± 0.5 | 10 ± 0.3 | NA |
| 6b | NA | NA | 13 ± 0.8 | 16 ± 0.7 | 12 ± 0.7 | NA |
| 6c | NA | NA | 14 ± 0.6 | 15 ± 0.4 | 14 ± 0.4 | NA |
| 6d | NA | NA | 12 ± 0.7 | 16 ± 0.9 | 13 ± 0.6 | NA |
| 6e | NA | NA | 11 ± 0.4 | 17 ± 0.8 | 12 ± 0.8 | NA |
| 6f | NA | NA | 20 ± 0.9 | 22 ± 1.3 | 17 ± 0.5 | 12 ± 0.9 |
| 6g | NA | NA | 14 ± 0.6 | 16 ± 0.4 | 13 ± 0.7 | NA |
| 6h | NA | NA | 12 ± 0.8 | 11 ± 0.6 | 16 ± 0.5 | 15 ± 0.7 |
| 11a | NA | NA | 10 ± 0.7 | 12 ± 0.8 | 11 ± 0.4 | 10 ± 0.3 |
| 11b | NA | NA | NA | 13 ± 0.5 | 9 ± 0.2 | 11 ± 0.4 |
| 11c | NA | NA | 16 ± 0.4 | 12 ± 0.7 | 15 ± 0.9 | 13 ± 0.5 |
| 11d | NA | NA | 14 ± 0.7 | 12 ± 0.4 | 13 ± 0.6 | 14 ± 0.7 |
| 11e | NA | NA | 15 ± 0.9 | 11 ± 0.6 | 12 ± 0.7 | 10 ± 0.2 |
| 11f | NA | NA | 9 ± 0.4 | NA | 10 ± 0.3 | NA |
| Ketoconazole | 17 ± 0.4 | 20 ± 0.8 | - | - | - | - |
| Gentamycin | - | - | 24 ± 1.2 | 26 ± 0.7 | 30 ± 0.9 | 25 ± 0.8 |
NA: No activity, results are shown as mean of inhibition zone diameter (mm) for different compounds done in triplicate ± SD; AF (Aspergillus fumigatus (RCMB 002008 (4)), CA (Candida albicans (RCMB 05036), SA (Staphylococcus aureus CMB010010)), BS (Bacillus subtilis (RCMB 010067)), EC (Escherichia coli (RCMB 010052)), PV (Proteus vulgaris RCMB 004 (1) ATCC 13315).
Table 5.
Antimicrobial activities of the newly synthesized thiazoles 6a–h and 11a–f was shown as minimum inhibitory concentration (MIC) in µg/mL of the tested microorganisms.
| Sample | Microorganisms | |||||
|---|---|---|---|---|---|---|
| Fungi | Gram Positive Bacteria | Gram Negative Bacteria | ||||
| AF | CA | SA | BS | EC | PV | |
| 6a | NA | NA | 625 | 5000 | 5000 | NA |
| 6b | NA | NA | 2500 | 312.5 | 625 | NA |
| 6c | NA | NA | 312.5 | 1250 | 625 | NA |
| 6d | NA | NA | 156.25 | 625 | 312.5 | NA |
| 6e | NA | NA | 5000 | 625 | 1250 | NA |
| 6f | NA | NA | 78.13 | 396 | 156.25 | 315 |
| 6g | NA | NA | 312.5 | 78.13 | 625 | NA |
| 6h | NA | NA | 1250 | 5000 | 156.25 | 312.5 |
| 11a | NA | NA | 5000 | 2500 | 2500 | 5000 |
| 11b | NA | NA | NA | 1250 | 10,000 | 5000 |
| 11c | NA | NA | 312.5 | 1250 | 625 | 1250 |
| 11d | NA | NA | 625 | 1250 | 1250 | 312.5 |
| 11e | NA | NA | 625 | 2500 | 2500 | 5000 |
| 11f | NA | NA | 10,000 | NA | 5000 | NA |
NA: No activity. Experiment was done using the diffusion agar method.
The results of the antimicrobial evaluation demonstrated that all the newly synthesized thiazoles exhibited good antibacterial effect towards the gram-positive bacteria Staphylococcus aureus (except 11b), and Bacillus subtilis (except 11f). With regards to the gram-negative bacteria, all compounds had antibacterial activity against Escherichia coli, while only 6f, 6h and 11a–e were effective against Proteus vulgaris. Of notice, the thiazole derivative 6f possessed the highest antibacterial activity compared to all other tested thiazoles against Staphylococcus aureus, Bacillus subtilis, and Escherichia coli. Interestingly, the antimicrobial activity of this derivative approaches the potency of gentamicin, against the tested gram-negative bacteria. However, the derivative 6h exerted the most prominent antibacterial activity against Proteus vulgaris. On the contrary, all synthesized compounds had no antifungal activity against Aspergillus fumigatus or Candida albicans. From these data, we can conclude that the presence of ethoxy carbonyl group and p-tolyl as substituents on the pyrazole ring increased the antimicrobial activity of compound 6f.
2.2.3. In Vitro Hepatoprotective Activity
The hepatoprotective potential of the newly synthesized thiazole derivatives was studied using an in vitro model of CCl4-induced hepatotoxicity. In vitro hepatoprotective activity was performed by assessing the viability of isolated rat hepatocytes treated with CCl4 in the presence and absence of the tested compounds [37]. Rat hepatocytes were isolated as previously described [38], and their viability was evaluated by the MTT reduction assay method [34,39] using silymarin as the reference standard drug. The concentration required to cure 50% of CCl4-exposed hepatocytes, EC50 was calculated and presented in Table 6. Results declared that compounds 6c, 6d, 6f, 6g, 6h, 11c, 11d, and 11e offered protection against CCl4-induced liver damage but lower than the standard drug. These results would suggest that these thiazole derivatives could be a candidate starting materials for the synthesis of more potent hepatoprotective drugs.
Table 6.
In vitro hepatoprotective activities of the investigated compounds and reference standard drug, presented as EC50 values.
| Tested Compounds | Hepatoprotective Activity (EC50 µg/mL) |
|---|---|
| 6a | NA |
| 6b | NA |
| 6c | 368 ± 14.6 |
| 6d | 972 ± 96.2 |
| 6e | NA |
| 6f | 1350 ± 87 |
| 6g | 456 ± 32 |
| 6h | 1324 ± 64.6 |
| 11a | NA |
| 11b | NA |
| 11c | 724 ± 31.7 |
| 11d | 936 ± 64 |
| 11e | 1980 ± 213 |
| 11f | NA |
| Silymarin | 34.9 ± 0.6 |
NA: No Hepatoprotective activity when tested at concentrations ranged from 1 to 6000 µg/mL. Values are shown as mean ± SD of four replicates.
3. Materials and Methods
3.1. Chemistry
General Information
Melting points were measured on an Electrothermal IA 9000 series digital melting point apparatus (Bibby Sci. Lim. Stone, Staffordshire, UK). IR spectra were recorded in potassium bromide discs on PyeUnicam SP 3300 (PyeUnicam Ltd., Cambridge, UK) and FTIR 8101 PC infrared spectrophotometers (Shimadzu, Tokyo, Japan). NMR spectra were measured on a Mercury VX-300 NMR spectrometer (Varian, Inc., Karlsruhe, Germany). 1H-NMR spectra were recorded at 300 MHz and 13C-NMR spectra were recorded at 75.46 MHz in deuterated dimethyl sulfoxide (DMSO-d6). Mass spectra were run on a Shimadzu GCMS-QP1000 EX mass spectrometer (Tokyo, Japan) at 70 eV. Elemental analyses were measured using Elementarvario LIII CHNS analyzer (GmbH & Co.KG, Hanau, Germany). Biological activities of the synthesized compounds were carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt. Irradiation was done in a domestic microwave oven (2500 MHz, 400 W). The reactions were carried out in a closed Teflon vessel which was placed at the center of the oven for irradiation. 5-Acetyl-4-methyl-2-phenyl-thiazole (1) [26], 2-cyanoacetohydrazide (2) [27], hydrazonoyl halides 4a [28,29], 4b–d [30], 4e–g [31], 4h [32], 8a, b [23] and arylidine malononitriles 9a–c [31] were prepared as described in the literature.
Synthesis of 2-cyano-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)acetohydrazide (3). A mixture of 5-acetyl-4-methyl-2-phenyl-thiazole 1 (2.17 g, 10 mmol) and 2-cyanoacetohydrazide 2 (0.99 g, 10 mmol) in 50 mL of EtOH containing catalytic amounts of HCl was refluxed for 6 h as monitored by TLC. The precipitated solid product was filtered, washed with ethanol and recrystallized from acetic acid to give pure product of thiazole derivative 3 as white solid (81%); mp 201–203 °C; IR (KBr) ν 3430 (NH), 3060, 2923 (C–H), 2338 (C≡N), 1643 (C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.49 (s, 3H, CH3), 2.72 (s, 3H, CH3), 3.30 (s, 2H, CH2), 7.51–8.03 (m, 5H, Ar-H), 10.60 (s, br, 1H, NH); MS m/z (%) 298 (M+, 83), 217 (96), 202 (100), 174 (53), 104 (69), 64 (72). Anal. Calcd: for C15H14N4OS (298.36): C, 60.38; H, 4.73; N, 18.78. Found: C, 60.45; H, 4.81; N, 18.66%.
General method for synthesis of 5-amino-1-aryl-3-substituted-N′-(1-(4-methyl-2-phenyl thiazol-5-yl) ethylidene)-1H-pyrazole-4-carbohydrazides 6a–h.
Method A. A mixture of hydrazone 3 (0.298 g, 1 mmol) and the appropriate hydrazonoyl halides 4 (1 mmol) in dioxane (20 mL) containing TEA (0.07 mL) was irradiated by MW at 400 Watt in a closed Teflon vessel until all the starting material was consumed (6–10 min as monitored by TLC). The hot reaction mixture was allowed to cool to room temperature and the precipitated solid was filtered off, washed with EtOH, dried and recrystallized from the suitable solvent to give the corresponding thiazole derivatives 6a–h.
Method B. A mixture of hydrazone 3 (0.298 g, 1 mmol) and the appropriate hydrazonoyl halides 4 (1 mmol) in dioxane (20 mL) containing grafted-chitosan (0.1 g) was irradiated by MW at 400 Watt in a closed Teflon vessel until all the starting material was consumed (6–10 min as monitored by TLC). The hot solution was filtered to remove grafted-chitosan and excess solvent was removed under reduced pressure. The reaction mixture was triturated with methanol and the product separated was filtered, washed with methanol, dried and recrystallized from the proper solvent to give the corresponding products, 6a–h which were identical in all aspects (m.p., mixed m.p. and IR spectra) with those obtained from method A. The physical constants of products 6a–h are provided below:
3-Acetyl-5-amino-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)-1-phenyl-1H-pyrazole-4-carbohydrazide (6a). Yellow solid; mp 163–165 °C (EtOH); IR (KBr) ν = 3432, 3264 (NH2 and NH), 3056, 2998, 2924 (C–H), 1694, 1643 (2C=O), 1601 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.49 (s, 3H, CH3), 2.58 (s, 3H, CH3), 2.71 (s, 3H, CH3), 7.50–8.01 (m, 12H, Ar-H and NH2), 10.63 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 16.80, 18.38, 25.25 (CH3), 113.00, 119.78, 120.51, 125.51, 127.53, 127.56, 128.32, 128.38, 130.14, 130.67, 136.11, 136.63, 143.46, 145.17, 145.58 (Ar-C and C=N), 167.58, 184.58 (C=O) ppm; MS, m/z (%) 458 (M+, 37), 390 (66), 329 (78), 80 (100), 64 (70). Anal. calcd for C24H22N6O2S (458.54): C, 62.86; H, 4.84; N, 18.33. Found: C, 62.77; H, 4.81; N, 18.24%.
3-Acetyl-5-amino-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)-1-(p-tolyl)-1H-pyrazole-4-carbo-hydrazide (6b). Yellow solid; mp 181–183 °C (EtOH); IR (KBr) ν = 3422, 3255 (NH2 and NH), 3059, 2920 (C–H), 1698, 1646 (2C=O), 1601 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.28 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.63 (s, 3H, CH3), 2.75 (s, 3H, CH3), 7.44–8.00 (m, 11H, Ar-H and NH2), 10.59 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 16.82, 18.30, 20.61, 25.25 (CH3), 114.80, 119.35, 121.83, 125.69, 127.00, 127.83, 128.37, 129.14, 132.36, 133.07, 136.49, 137.19, 143.49, 144.92, 146.41 (Ar-C and C=N), 167.25, 184.49 (C=O) ppm; MS, m/z (%) 472 (M+, 40), 430 (39), 214 (100), 121 (84), 71 (62). Anal. calcd for C25H24N6O2S (472.56): C, 63.54; H, 5.12; N, 17.78. Found: C, 63.37; H, 5.04; N, 17.55%.
3-Acetyl-5-amino-1-(4-methoxyphenyl)-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)-1H-pyrazole-4-carbohydrazide (6c). Yellow solid; mp 157–159 °C (EtOH); IR (KBr) ν = 3427, 3264 (NH2 and NH), 3064, 2928 (C–H), 1667, 1643 (2C=O), 1597 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.12 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.64 (s, 3H, CH3), 3.73 (s, 3H, OCH3), 6.67–7.72 (m, 11H, Ar-H and NH2), 10.73 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 17.03, 18.35, 25.58, 53.90 (CH3), 113.91, 119.04, 120.82, 123.94, 126.80, 127.06, 129.32, 129.74, 130.26, 132.37, 135.27, 137.04, 142.91, 143.48, 145.18 (Ar-C and C=N), 167.62, 184.97 (C=O) ppm; MS, m/z (%) 488 (M+, 51), 477 (72), 369 (84), 121 (70), 80 (71), 64 (100). Anal. calcd for C25H24N6O3S (488.56): C, 61.46; H, 4.95; N, 17.20. Found: C, 61.25; H, 4.74; N, 17.05%.
3-Acetyl-5-amino-1-(4-chlorophenyl)-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)-1H-pyrazole-4-carbo-hydrazide (6d). Yellow solid; mp 214–216 °C (DMF); IR (KBr) ν = 3424, 3252 (NH2 and NH), 3064, 2966 (C–H), 1668, 1623 (2C=O), 1596 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.46 (s, 3H, CH3), 2.64 (s, 3H, CH3), 2.74 (s, 3H, CH3), 7.31–8.12 (m, 11H, Ar-H and NH2), 10.77 (s, br, 1H, NH); MS, m/z (%) 494 (M+ + 2, 24), 492 (M+, 61), 440 (69), 369 (70), 212 (47), 142 (100), 127 (62), 64 (55). Anal. calcd for C24H21ClN6O2S (492.98): C, 58.47; H, 4.29; N, 17.05. Found: C, 58.26; H, 4.22; N, 16.93%.
Ethyl 5-amino-4-(2-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)hydrazinecarbonyl)-1-phenyl-1H-pyrazole-3-carboxylate (6e). Yellow solid; mp 177–179 °C (EtOH); IR (KBr) ν = 3433,3270 (NH2 and NH), 3041, 2922 (C–H), 1737, 1640 (2C=O), 1599 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 0.97 (t, J = 7.0 Hz, 3H, CH3CH2), 2.37 (s, 3H, CH3), 2.68 (s, 3H, CH3), 4.02 (q, J = 7.0 Hz, 2H, CH2CH3), 7.21–7.62 (m, 12H, Ar-H and NH2), 10.69 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 12.45, 15.93, 19.21 (CH3), 61.46 (CH2), 118.14, 118.99, 119.75, 120.74, 125.87, 127.36, 127.56, 128.13, 128.62, 128.97, 130.06, 130.11, 130.68, 146.20, 146.90 (Ar-C and C=N), 160.06, 166.11 (C=O) ppm; MS, m/z (%) 488 (M+, 75), 462 (69), 214 (100), 121 (47), 104 (47), 80 (73), 64 (99). Anal. calcd for C25H24N6O3S (488.56): C, 61.46; H, 4.95; N, 17.20. Found: C, 61.31; H, 4.73; N, 17.08%.
Ethyl 5-amino-4-(2-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)hydrazinecarbonyl)-1-(p-tolyl)-1H-pyrazole-3-carboxylate (6f). Pale yellow solid; mp 161–163 °C (EtOH); IR (KBr) ν = 3428, 3266 (NH2 and NH), 3061, 2919 (C–H), 1731, 1644 (2C=O), 1595 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 1.04 (t, J = 6.9 Hz, 3H, CH3CH2), 2.12 (s, 3H, CH3), 2.34 (s, 3H, CH3), 2.59 (s, 3H, CH3), 4.15 (q, J = 6.9 Hz, 2H, CH2CH3), 7.27–7.55 (m, 11H, Ar-H and NH2), 10.68 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 12.18, 16.03, 19.15, 20.73 (CH3), 61.83 (CH2), 117.93, 118.37, 119.58, 120.00, 125.69, 127.18, 128.09, 128.47, 129.36, 130.05, 130.83, 132.46, 133.04, 144.94, 146.55 (Ar-C and C=N), 161.77, 166.82 (C=O) ppm; MS, m/z (%) 502 (M+, 33), 408 (97), 356 (73), 217 (100), 202 (44), 104 (70), 71 (86). Anal. calcd for: C26H26N6O3S (502.59): C, 62.13; H, 5.21; N, 16.72. Found: C, 62.26; H, 5.22; N, 16.60%.
Ethyl 5-amino-1-(2,4-dichlorophenyl)-4-(2-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene) hydrazine-carbonyl)-1H-pyrazole-3-carboxylate (6g). Brown solid; mp 209–211 °C (DMF); IR (KBr) ν = 3429, 3253 (NH2 and NH), 3057, 2928 (C–H), 1738, 1643 (2C=O), 1603 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 1.27 (t, J = 7.9 Hz, 3H, CH3CH2), 2.49 (s, 3H, CH3), 2.74 (s, 3H, CH3), 4.32 (q, J = 7.9 Hz, 2H, CH2CH3), 7.36–8.16 (m, 10H, Ar-H and NH2), 10.57 (s, br, 1H, NH); MS, m/z (%) 557 (M+, 27), 498 (60), 347 (61), 202 (100), 111 (69), 80 (78), 64 (100). Anal. calcd for C25H22Cl2N6O3S (557.45): C, 53.86; H, 3.98; N, 15.08. Found: C, 53.75; H, 3.91; N, 14.88%.
5-Amino-4-(2-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)hydrazinecarbonyl)-N,1-diphenyl-1H-pyrazole-3-carboxamide (6h). White solid; mp 231–233 °C (DMF); IR (KBr) ν = 3426, 3239 (NH2 and 2NH), 3057, 2928 (C–H), 1671, 1627 (2C=O), 1598 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.12 (s, 3H, CH3), 2.65 (s, 3H, CH3), 7.13–7.83 (m, 17H, Ar-H and NH2), 10.81 (s, br, 1H, NH), 11.15 (s, br, 1H, NH); MS, m/z (%) 535 (M+, 73), 498 (60), 420 (51), 369 (79), 255 (100), 134 (66), 93 (100), 77 (98). Anal. calcd for C29H25N7O2S (535.62): C, 65.03; H, 4.70; N, 18.31. Found: C, 65.09; H, 4.64; N, 18.19%.
Synthesis of N′-(1-(4-cyano-1,4-diaryl-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenylthiazole-5-carbohydrazides 11a–f.
Method A: Equimolecular mixture of 2-(2-(4-methyl-2-phenylthiazole-5-carbonyl)hydrazono)-N′-arylpropane hydrazonoyl chlorides 8a,b (l mmol) and the appropriate arylidine malononitriles 9a–c (1 mmol) in absolute EtOH (10 mL) containing catalytic amounts of piperidine (0.50 mL) was irradiated by MW at 400 Watt in a closed Teflon vessel until all the starting material was consumed (4–8 min as monitored by TLC), then cooled to room temperature. The solid product was filtered off, washed with ethanol and recrystallized from the proper solvent to give the thiazole derivatives 11a–f, respectively.
Method B: A mixture of 8a,b (1 mmol) and the appropriate arylidine malononitriles 9a–c (1 mmol) in absolute EtOH (10 mL) containing grafted-chitosan (0.1 g) was irradiated by MW at 400 Watt in a closed Teflon vessel until all the starting material was consumed (4–8 min as monitored by TLC). The hot solution was filtered to remove grafted-chitosan and excess solvent was removed under reduced pressure. The reaction mixture was triturated with MeOH and the product separated was filtered, washed with MeOH, dried and recrystallized from the proper solvent to give the corresponding products, 11a–f which were identical in all aspects (m.p., mixed m.p. and IR spectra) with those obtained from method A. The physical constants of the products 11a–f are listed below.
N′-(1-(4-cyano-1,5-diphenyl-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenylthiazole-5-carbohydrazide (11a). Yellow solid; mp 201–203 °C (EtOH); IR (KBr) ν = 3439 (NH), 3056, 2926 (C–H), 2226 (C≡N), 1643 (C=O), 1599 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.31 (s, 3H, CH3), 2.73 (s, 3H, CH3), 7.22–8.01(m, 15H, Ar-H), 10.52 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 11.05, 16.93 (CH3), 103.73, 118.14, 118.99, 119.75, 120.74, 125.87, 127.36, 127.56, 128.13, 128.62, 128.97, 130.06, 130.11, 130.68, 131.58, 145.17, 145.56, 151.93, 156.20, 156.90 (Ar-C and C=N), 170.83 (C=O) ppm; MS, m/z (%) 502 (M+, 15), 462 (30), 273 (41), 202 (29), 80 (100), 64 (89). Anal.Calcd for C29H22N6OS (502.59): C, 69.30; H, 4.41; N, 16.72. Found C, 69.17; H, 4.27; N, 16.55%.
N′-(1-(4-Cyano-5-(4-methoxyphenyl)-1-(p-tolyl)-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenylthiazole-5-carbohydrazide (11b). Yellow solid; mp 227–229 °C (EtOH); IR (KBr) ν = 3313 (NH), 3041, 2917 (C–H), 2229 (C≡N), 1645 (C=O), 1588 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.30 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.70 (s, 3H, CH3), 3.77 (s, 3H, OCH3), 7.16–7.73 (m, 13H, Ar-H), 10.27 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 11.28, 17.16, 20.37, 54.06 (CH3), 102.93, 117.91, 119.38, 119.93, 121.49, 124.82, 126.66, 127.31, 128.00, 128.85, 129.48, 130.83, 131.46, 132.84, 135.08, 144.32, 145.59, 150.13, 153.91, 156.15 (Ar-C and C=N), 171.01 (C=O) ppm; MS, m/z (%) 546 (M+, 31), 479 (60), 399 (47), 338 (69), 149 (36), 80 (100). Anal.Calcd for C31H26N6O2S (546.64): C, 68.11; H, 4.79; N, 15.37. Found C, 68.04; H, 4.72; N, 15.25%.
N′-(1-(5-(4-Chlorophenyl)-4-cyano-1-phenyl-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenylthiazole-5-carbohydrazide (11c).Yellow solid; mp 237–239 °C (DMF); IR (KBr) ν = 3426 (NH), 3057, 2930 (C–H), 2190 (C≡N), 1642 (C=O), 1606 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.50 (s, 3H, CH3), 2.73 (s, 3H, CH3), 7.37–7.92 (m, 14H, Ar-H), 10.31 (s, br, 1H, NH); MS, m/z (%) 539 (M+ + 2, 6), 537 (M+, 23), 429 (55), 315 (40), 399 (47), 338 (69), 202 (100), 174 (51), 64 (73). Anal. Calcd for C29H21ClN6OS (537.03): C, 64.86; H, 3.94; N, 15.65. Found C, 64.66; H, 3.79; N, 15.47%.
N′-(1-(4-Cyano-5-phenyl-1-(p-tolyl)-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenylthiazole-5-carbohydrazide (11d).Yellow solid; mp 206–208 °C (EtOH); IR (KBr) ν = 3434 (NH), 3045, 2924 (C–H), 2229 (C≡N), 1644 (C=O), 1600 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.28 (s, 3H, CH3), 2.50 (s, 3H, CH3), 2.72 (s, 3H, CH3), 7.29–7.63 (m, 14H, Ar-H), 10.19 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 11.06, 16.94, 20.16 (CH3), 102.38, 117.36, 118.43, 119.56, 120.94, 122.84, 125.39, 127.06, 127.83, 128.29, 128.45, 130.17, 131.26, 132.39, 134.90, 143.79, 145.17, 149.85, 152.31, 155.96 (Ar-C and C=N), 170.92 (C=O) ppm; MS, m/z (%) 516 (M+, 16), 472 (31), 327 (28), 299 (27), 202 (100), 174 (44), 71 (62). Anal. Calcd for C30H24N6OS (516.62): C, 69.75; H, 4.68; N, 16.27. Found C, 69.69; H, 4.60; N, 16.11%.
N′-(1-(4-Cyano-5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenythiazole-5-carbohydrazide (11e). Yellow solid; mp 191–193 °C (EtOH); IR (KBr) ν = 3435 (NH), 3057, 2965 (C–H), 2196 (C≡N), 1643 (C=O), 1605 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.37 (s, 3H, CH3), 2.68 (s, 3H, CH3), 3.82 (s, 3H, OCH3), 6.96-7.58 (m, 14H, Ar-H), 10.68 (s, br, 1H, NH); 13C-NMR (DMSO-d6) δ 12.13, 17.72 (CH3), 52.10 (OCH3), 105.91, 119.97, 120.76, 124.19, 124.69, 127.40, 128.80, 129.68, 130.10, 130.87, 135.22, 137.33, 142.13, 145.06, 146.48, 150.06, 150.48, 155.20, 157.33, 160.18 (Ar-C and C=N), 170.20 (C=O) ppm; MS, m/z (%) 532 (M+, 17), 425 (29), 311 (23), 202 (100), 174 (37), 64 (49). Anal. Calcd for C30H24N6O2S (532.62): C, 67.65; H, 4.54; N, 15.78. Found C, 67.49; H, 4.46; N, 15.69%.
N′-(1-(5-(4-Chlorophenyl)-4-cyano-1-(p-tolyl)-1H-pyrazol-3-yl)ethylidene)-4-methyl-2-phenylthiazole-5-carbohydrazide (11f). Yellow solid; mp 237–239 °C (DMF); IR (KBr) ν = 3431 (NH), 3040, 2926 (C–H), 2197 (C≡N), 1641 (C=O), 1602 (C=N) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 2.32 (s, 3H, CH3), 2.50 (s, 3H, CH3), 2.76 (s, 3H, CH3), 7.31–7.83 (m, 13H, Ar-H), 10.38 (s, br, 1H, NH); MS, m/z (%) 553 (M+ + 2, 3), 551 (M+, 12), 380 (53), 202 (100), 109 (48), 64 (70). Anal.Calcd for C30H23ClN6OS (551.06): C, 65.39; H, 4.21; N, 15.25. Found C, 65.22; H, 4.15; N, 15.10%.
3.2. Biological Assays
3.2.1. In Vitro Cytotoxic Activity
The cytotoxic potential of the newly synthesized compounds was examined against three cancer cell lines HepG2, HCT-116, and MCF-7 using the MTT assay after 24 h of incubation [34]. For more details, see the Supporting Information file.
3.2.2. Antimicrobial Evaluation
Antifungal and antibacterial activities of the synthesized thiazoles were assessed towards different microbes using the agar diffusion method and were compared to standard reference drugs [35,36]. Refer to the Supporting Information file for more details.
3.2.3. Hepatoprotective Activity
In vitro hepatoprotective activity was done by assessing the viability of isolated rat hepatocytes exposed to 1% CCl4 along with or without the tested compounds [37]. Rat hepatocytes were isolated as described [38] and cell viability was evaluated by the MTT reduction assay [34,39] using silymarin as the reference standard drug. For further details, refer to the Supporting Information file.
4. Conclusions
We have efficiently synthesized a new series of thiazolylpyrazoles using hydrazonoyl halides and 2-cyano-N′-(1-(4-methyl-2-phenylthiazol-5-yl)ethylidene)acetohydrazide in the presence of chitosan-grafted-poly(vinylpyridine) as an eco-friendly biopolymeric basic catalyst. The structures of these novel compounds were determined using spectroscopic analyses (IR, NMR, and MS). All compounds were evaluated for their cytotoxic effectiveness against HepG-2, MCF-7, and HCT-116 cell lines. Our results indicated that most compounds exhibited a good anticancer activity and importantly, the thiazole derivatives 11c and 6g exhibited the greatest cytotoxic potential against the examined cell lines. In addition, antibacterial evaluation experiments illustrated that the thiazole derivative 6f has the most potent activity towards Staphylococcus aureus, Bacillus subtilis, and Escherichia coli. The antibacterial potency of 6f against the tested gram--positive bacteria even approaches that of gentamicin. However, the derivative 6h exerted the highest antibacterial activity against Proteus vulgaris. Some of the tested compounds showed a weak hepatoprotective effect against CCl4-induced liver damage suggesting their usage as a candidate starting materials for the synthesis of more potent hepatoprotective drugs. Taken together, the current work presents an eco-friendly approach for the synthesis of novel thiazole derivatives that have potential values in protection against cancer cells and bacterial infections and could be used to develop effective agents to guard against liver toxicity.
Supplementary Materials
The following are available online. Online supplementary information includes detailed methods of the cytotoxic, antimicrobial and hepatoprotective evaluations, Figures (S1–S6) of mean zone of inhibition of the newly synthesized compounds. Also 1H- and 13C-NMR spectra of some of the synthesized thiazolylpyrazoles derivatives are included.
Author Contributions
S.A.-M., M.M.E., N.A.K., H.H.S. and S.M.G. conceived and designed the experiments; S.A.-M., M.M.E. and M.R.A. carried out the experiments; S.M.G., N.A.K., M.M.E. and H.H.S. analyzed and interpreted the data; S.M.G., M.M.E., H.H.S. and N.A.K. prepared the manuscript. All authors have read and approved the final manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a General Research Project under grant number (GRP-74-39).
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Footnotes
Sample Availability: Samples of the compounds 6a–h and 11a–f are available from the authors.
References
- 1.Satoh A., Nagatomi Y., Hirata Y., Ito S., Suzuki G., Kimura T., Maehara S., Hikichi H., Satow A., Hata M., et al. Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorg. Med. Chem. Lett. 2009;19:5464–5468. doi: 10.1016/j.bmcl.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui N., Ahsan W. Synthesis, anticonvulsant and toxicity screening of thiazolyl–thiadiazole derivatives. Med. Chem. Res. 2011;20:261–268. doi: 10.1007/s00044-010-9313-6. [DOI] [Google Scholar]
- 3.Dawane B.S., Konda S.G., Mandawad G.G., Shaikh B.M. Poly(ethylene glycol) (PEG-400) as an alternative reaction solvent for the synthesis of some new 1-(4-(4′-chlorophenyl)-2-thiazolyl)-3-aryl-5-(2-butyl-4-chloro-1H-imidazol-5yl)-2-pyrazolines and their in vitro antimicrobial evaluation. Eur. J. Med. Chem. 2010;45:387–392. doi: 10.1016/j.ejmech.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Adibpour N., Khalaj A., Rajabalian S. Synthesis and antibacterial activity of isothiazolyloxazolidinones and analogous 3(2H)-isothiazolones. Eur. J. Med. Chem. 2010;45:19–24. doi: 10.1016/j.ejmech.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Sondhi S.M., Singh N., Lahoti A.M., Bajaj K., Kumar A., Lozach O., Meijer L. Synthesis of acridinyl-thiazolino derivatives and their evaluation for anti-inflammatory, analgesic and kinase inhibition activities. Bioorg. Med. Chem. 2005;13:4291–4299. doi: 10.1016/j.bmc.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Gomha S.M., Khalil K.D. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules. 2012;17:9335–9347. doi: 10.3390/molecules17089335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzina E.L., Popov A.V. Synthesis and anticancer activity of N-bis(trifluoromethyl)alkyl-N′-thiazolyl and N-bis(trifluoromethyl)alkyl-N’-benzothiazolylureas. Eur. J. Med. Chem. 2009;44:4944–4953. doi: 10.1016/j.ejmech.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Iino T., Hashimoto N., Sasaki K., Ohyama S., Yoshimoto R., Hosaka H., Hasegawa T., Chiba M., Nagata Y., Nishimura J.E.T. Structure activity relationships of 3,5-disubstituted benzamides as glucokinase activators with potent in vivo efficacy. Bioorg. Med. Chem. 2009;17:3800–3809. doi: 10.1016/j.bmc.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Rawal R.K., Tripathi R., Katti S.B., Pannecouque C., Clercq E.D. Design and synthesis of 2-(2,6-dibromophenyl)-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Eur. J. Med. Chem. 2008;43:2800–2806. doi: 10.1016/j.ejmech.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Shiradkar M.R., Akula K.C., Dasari V., Baru V., Chiningiri B., Gandhi S., Kaur R. Clubbed thiazoles by MAOS: A novel approach to cyclin-dependent kinase 5/p25 inhibitors as a potential treatment for Alzheimer’s disease. Bioorg. Med. Chem. 2007;15:2601–2610. doi: 10.1016/j.bmc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Gomha S.M., Edrees M.M., Altalbawy F.M.A. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Inter. J. Mol. Sci. 2016;17:1499. doi: 10.3390/ijms17091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turan-Zitouni G., Chevallet P., Kiliç F.S., Erol K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000;35:635–641. doi: 10.1016/S0223-5234(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 13.Shih M.H., Ke F.Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004;12:4633–4643. doi: 10.1016/j.bmc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Kheder N.A., Riyadh S.M., Asiry A.M. Azoles and bis-Azoles: Synthesis and Biological Evaluation as Antimicrobial and Anti-Cancer Agents. Chem. Pharm. Bull. 2013;61:504–510. doi: 10.1248//cpb.c12-00939. [DOI] [PubMed] [Google Scholar]
- 15.Gomha S.M., Salah T.A., Abdelhamid A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015;146:149–158. doi: 10.1007/s00706-014-1303-9. [DOI] [Google Scholar]
- 16.Chen Z.Z., Wang Z.L., Deng C.Y., Zheng H., Wang X.H., Ma L., Ye X., Ma Y.H., Xie C.F., Chen L.J., et al. (Z)-5-(4-methoxybenzylidene) thiazolidine-2,4-dione protects rats from carbon tetrachloride-induced liver injury and fibrogenesis. World J. Gastroenterol. 2012;18:654–661. doi: 10.3748/wjg.v18.i7.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa E., Zamora P., Feliu J., González Barón M. Classification of anticancer drugs—A new system based on therapeutic. Cancer Treat Rev. 2003;29:515–523. doi: 10.1016/S0305-7372(03)00116-6. [DOI] [PubMed] [Google Scholar]
- 18.Mansoori B., Mohammadi A., Davudian S., Shirjang S., Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017;7:339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcellin P., Kutala B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38:2–6. doi: 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- 20.Gomha S.M., Kheder N.A., Abdelaziz M.R., Mabkhot Y.N., Alhajoj A.M. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety. Chem. Central J. 2017;11:25. doi: 10.1186/s13065-017-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomha S.M., Riyadh S.M., Abbas I.M., Bauomi M.A. Synthetic utility of ethylidenethiosemi- carbazide: Synthesis and anti-cancer activity of 1,3-thiazines and thiazoles with imidazole moiety. Heterocycles. 2013;87:341–356. [Google Scholar]
- 22.Gomha S.M., Riyadh S.M., Abdalla M.M. Solvent-drop grinding method: Efficient synthesis, DPPH radical scavenging and anti-diabetic activities of chalcones, bis-chalcones, azolines, and bis-azolines. Curr. Org. Synth. 2015;12:220–228. doi: 10.2174/1570179412666150122230447. [DOI] [Google Scholar]
- 23.Gomha S.M., Abdelaziz M.R., Kheder N.A., Abdel-aziz H.M., Alterary S., Mabkhot Y.N. A facile access and evaluation of some novel thiazole and 1,3,4-thiadiazole derivatives incorporating thiazole moiety as potent anticancer agents. Chem. Central J. 2017;11:105. doi: 10.1186/s13065-017-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomha S.M., Salah T.A., Hassaneen H.M.E., Abdel-aziz H., Khedr M.A. Synthesis, characterization and molecular docking of novel bioactive thiazolyl-thiazole derivatives as promising cytotoxic antitumor drug. Molecules. 2016;21:3. doi: 10.3390/molecules21010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomha S.M., Eldebss T.M.A., Badrey M.G., Abdulla M.M., Mayhoub A.S. Novel 4-heteroaryl-antipyrines as DPP-IV Inhibitors. Chem. Biol. Drug Des. 2015;86:1292–1303. doi: 10.1111/cbdd.12593. [DOI] [PubMed] [Google Scholar]
- 26.Tazoo D., Oniga O., Bohle D.S., Chua Z., Dongo E. General two-step preparation of chalcones containing thiazole. J. Heterocycl. Chem. 2012;49:768–773. doi: 10.1002/jhet.853. [DOI] [Google Scholar]
- 27.Gorolets N.Y., Yousefi B.H., Belaj F., Kappe C.O. Rapid microwave-assisted solution phase synthesis of substituted 2-pyridone libraries. Tetrahedron. 2004;60:8633–8644. doi: 10.1016/j.tet.2004.05.100. [DOI] [Google Scholar]
- 28.Dieckmann W., Platz L. Ueber eine neue bildungsweise von osotetrazonen. Ber. Dtsch. Chem. Ges. 1905;38:2986–2990. doi: 10.1002/cber.190503803103. [DOI] [Google Scholar]
- 29.Abushamleh A.S., Al-Aqarbeh M.M., Day V. Transition metal complexes of derivatized chiral dihydro- 1,2,4-triazin-6-ones. Template synthesis of nickel (II) tetraaza-(4N-M) complexes incorporating the triazinone moiety. Am. J. Appl. Sci. 2008;5:750–754. doi: 10.3844/ajassp.2008.750.754. [DOI] [Google Scholar]
- 30.Eweiss N.F., Abdelhamid A.O. Synthesis of heterocycles. Part II. New routes to acetyl thiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980;17:1713–1717. doi: 10.1002/jhet.5570170814. [DOI] [Google Scholar]
- 31.Shawali A.S., Eweiss N.F., Hassaneen H.M., Al-gharib M.S. Synthesis and rearrangement of ethyl aryloxyglyoxalate arylhydrazones. Bull. Chem. Soc. Jpn. 1975;48:365–366. doi: 10.1246/bcsj.48.365. [DOI] [Google Scholar]
- 32.Shawali A.S., Abdelhamid A.O. Synthesis and reactions of phenylcarbamoylarylhydrazidic chlorides. Tetrahedron. 1971;27:2517–2528. doi: 10.1016/S0040-4020(01)90753-7. [DOI] [Google Scholar]
- 33.Rao P.S., Venkataratnam R.V. Zinc Chloride as a New Catalyst for Knoevenagel Condensation. Tetrahedron Lett. 1991;32:5821–5822. [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; CLSI Document M100-S22. CLSI; Wayne, PA, USA: 2012. [Google Scholar]
- 36.Ghorab M.M., Alsaid M.S., El-Gaby M.S.A., Safwat N.A., Elaasser M.M., Soliman A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016;124:299–310. doi: 10.1016/j.ejmech.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 37.Thirunavukkarasu P., Asha S., Ramanathan T., Balasubramanian T., Shanmogapriya R., Renugadevi G. In vitro Hepatoprotective activity of isolated fractions of Cressa cretica. Pharm. Chem. J. 2014;48:121–126. doi: 10.1007/s11094-014-1061-3. [DOI] [Google Scholar]
- 38.Reese J.A., Byard J.L. Isolation and culture of adult hepatocytes from liver biopsies. In Vitro. 1981;17:935–940. doi: 10.1007/BF02618417. [DOI] [PubMed] [Google Scholar]
- 39.Wilson A.P. Animal Cell Culture: A Practical Approach. 3rd ed. Oxford University Press; Oxford, UK: 2000. Cytotoxicity and viability assays; pp. 175–219. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.