Abstract
Differences in time to heading that remain after photoperiod and vernalisation requirements have been saturated are classified as earliness per se (Eps) effects. It has been commonly assumed that Eps genes are purely constitutive and independent of environment, although the likely effect of temperature on Eps effects in hexaploid wheat has never been tested. We grew four near isogenic lines (NILs) for the Eps gene located in chromosome 1D (Eps-D1) at 6, 9, 12, 15, 18, 21 and 24 °C. In line with expectations we found that lines carrying the Eps-late allele were always later than those with Eps-early alleles. But in addition, we reported for the first time that the magnitude of the effect increased with decreasing temperature: an Eps x temperature interaction in hexaploid wheat. Variation in heading time due to Eps x temperature was associated with an increase in sensitivity to temperature mainly during late reproductive phase. Moreover, we showed that Eps alleles exhibited differences in cardinal (base, optimum, maximum) temperatures and that the expression of ELF3, (the likely candidate for Eps-D1) also interacted with temperature.
Introduction
Differences in time to flowering are critical for wheat adaptation and the improvement of yield potential1–4. Genotypic variation in the developmental rate during phases preceding anthesis is mainly caused by differences in sensitivity to photoperiod and vernalisation5,6, the genetic pathways of which are interconnected7, and allow coarse-tuning of adaptation8.
However, there are still relatively minor variations in flowering time once requirements of vernalisation and photoperiod are totally satisfied. These differences are regulated by earliness per se (Eps) genes9,10. Their effects are usually small8,11, and critical for the fine-tuning of developmental patterns10,12,13. There is a large degree of variation in Eps genes in germplasm adapted to different regions14,15. The genetics of Eps is not as well understood as it is for Ppd and Vrn effects10, and the underlying genes and causal polymorphisms have only been identified very recently in hexaploid wheat. In Triticum monococcum L., a cereal ortholog of Arabidopsis thaliana circadian clock regulator LUX ARRHYTHMO/PHYTOCLOCK 1 (LUX/PCL1) was proposed as a promising candidate gen for the earliness per se 3 (Eps-3Am) locus16 and the ortholog circadian clock regulator EARLY FLOWERING 3 (ELF3) was identified as a candidate gene for the earliness per se Eps-Am1 locus17. ELF3 was suggested to be the best candidate gene within the Eps-D1 locus in Triticum aestivum as a deletion containing ELF3 is associated with advanced flowering and altered expression of an evening loop member GIGANTEA (GI)18. In this work, we used the same near isogenic lines (NILs) developed in that study18 and studied as well the expression of both ELF3 and GI, as explained in Materials and Methods (see below).
It has been assumed that earliness per se is “constitutive” and, therefore, independent of the environment10,14 (that is why it was termed ‘per se’). However, it was hypothesised that these Eps effects would likely be temperature sensitivity genes9. Temperature has a universal effect on wheat development in that all phases and all cultivars develop faster under high than under low temperatures19. This universal effect can be seen well beyond wheat in developmental rates of other plant species as well as of other living organisms not controlling their own temperature20,21. Therefore, all phases are sensitive to temperature but this does not necessarily imply that there is no variation in sensitivity22. Working with an Eps gene in Triticcum monococcum (that had an unusually large effect on time to heading; c. 60 d difference between lines with the Eps-Am1-late and -early alleles)12, the Eps x temperature interaction was confirmed12,23,24. Furthermore, in genetic terms it has been shown that ELF3 may have an important role in temperature entrainment25,26 which suggested that changes in clock gene expression in a 24 hours period were associated with daily temperature changes26,27.
More recently, we grew several Eps NILs derived from Spark x Rialto hexaploid wheats under field conditions28, that had also been tested in the UK11,29. It was seen that the magnitude of the Eps effects were stronger in the experiments conducted in the UK than in Spain. This was interpreted as a possible effect of temperature on the action of Eps alleles in hexaploid wheat, as it had been shown for Eps-Am1 gene in T. monococcum (see above).
Although there is evidence that ambient temperature affects different developmental traits22,30, the specific interaction between Eps genes and temperature beyond time to heading in hexaploid wheat has not been reported. A more comprehensive understanding on whether particular sub-phases of “time to heading” are sensitive to Eps and Eps x temperature is essential, because it is during these pheno-phases when sources and sinks contributing to yield are being determined2.
In this study we aimed to quantify Eps x temperature interactions, for the first time (i) in hexaploid wheat considering Eps alleles (i.e. shown to possess significant effects on time to heading)11,28, (ii) considering independently the effects on different developmental processes, using NILs derived from the same Spark x Rialto cross from which we identified performance differences in Spain and UK, and (iii) estimating the cardinal temperatures (maximum, optimum and minimum) for lines carrying the Eps-D1 early and late alleles. In addition, we also studied in two of the temperature regimes, the expression of the circadian clock gene ELF3, which had been identified as the candidate gene for Eps-D1 in these NILs18 and GI, which is thought to be the major target of ELF3 in the flowering time pathway.
Results
Abnormalities in development
When plants were grown at the lowest and two highest temperature regimes (6, 21 or 24 °C) the patterns of development exhibited abnormalities, none of which were visible plants grown under the other thermal conditions (9, 12, 15, and 18 °C) (Fig. 1).
Figure 1.

Abnormalities in development in the plants grown at 6, 21 and 24 °C. At the lowest temperatures plants headed but anthers were not extruded, at 21 °C more than half of the plants of NILs with early alleles headed (left image) while none of the plants reached heading in the NILs with the late allele (right image) and at 24 °C none of the plants progressed in their development to heading. Pictures were taken at the end of the experiment.
Plants grown at 6 °C never reached anthesis in spite of developing quite normally, though expectedly slowly, until heading. This remained the case 89 d after heading of the NILs carrying the Eps-late alleles. Lack of anthesis was not an effect of temperature on the genes controlling anther extrusion. Florets inside the spikelets grown under this thermal regime developed abnormally: e.g. the anthers lost their colour and the florets seemed to have been dehydrated before they could become receptive for pollination (dehydration is a major criterion used to establish that a floret has died)31. In addition, apical spikelets started to lose their normal green colour after heading. Consequently, although 100% of the plants reached heading normally, none reached anthesis when grown at the lowest temperature of the study (Supplementary Fig. S1).
When grown at 21 °C none of the plants with Eps-late alleles reached heading, whilst c. 60% of those with Eps-early alleles did, though only less than half of them continued developing normally to reach anthesis (Fig. S1). However, all plants of both genotypes (with Eps-early and –late alleles) reached the terminal spikelet stage normally; indicating that the impairment of development occurred during the late reproductive phase. At 24 °C all plants, regardless of the Eps allele they carried developed normally up until the appearance of the first 5–6 leaves. Leaves produced after that appeared extremely slowly and did not reach full size. These events were concurrent with premature senescence of the earlier leaves. In terms of floral development, none of the plants reached the double ridge stage after 55 days (equivalent to a 1320 °C d assuming a Tb = 0 °C) since the onset of the experiments and at this point, the plants were dying.
In the rest of the results section, we considered only the plants that exhibited normal development for the particular trait considered. Due to the relatively large failure in reaching anthesis from plants that had reached heading normally, we used heading (rather than anthesis) as the most integrative developmental trait. However, for all conditions in which plants reached anthesis normally, heading time was very well correlated with time to anthesis with a slope higher than 1 (1.38 ± 0.10) due to the fact that the durations are in days and all phases, including that from heading to anthesis, are delayed at lower temperatures (Fig. S2). Although the very high coefficient of determination is due to the clear effect of temperature, differences between NILs also contributed.
Phenology
The plants having the same Eps alleles from the two pairs of NILs, derived from either SR9 or SR23, displayed exactly the same behaviour. Considering time to heading as the overarching trait embracing developmental processes in this study, it is easy to see that the Eps-early NILs of each of the two families (SR9 and SR23) reached heading almost simultaneously at each of the temperature conditions, and the same was true for the Eps-late NILs of both pairs (Fig. 2). This shows that genetic background effects of the NIL pair (whether lines were derived from SR9 or SR23), as well as its interaction with temperature, were not significant (Fig. 2, inset). In fact, the linear regression of the relationship in Fig. 2 was not only very highly significant but also had an intercept (−2.23 ± 3.01 d) and a slope (1.03 ± 0.03) which were not significantly different from 0 d and 1, respectively. Therefore, in the rest of the paper the results of the two Eps-early and the two Eps-late NILs are shown as an average.
Figure 2.
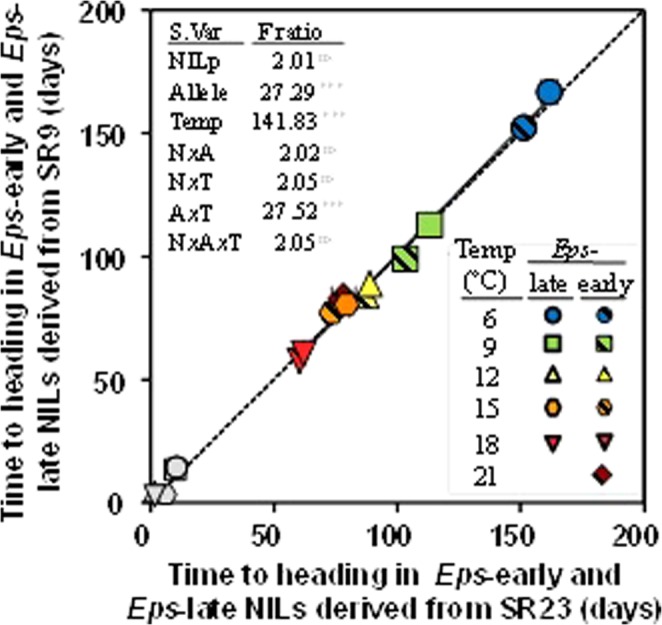
Time from the initiation of the experiment to heading for the NILs carrying the same Eps alleles in each of the two NIL pairs (NILs derived from either SR9 or SR23, see Materials and Methods) under the temperature regimes in which heading was reached. Dashed line is the 1:1 relationship. Solid line fitted by linear regression (R2 = 0.993, P < 0.001). Grey smaller data-points (bottom left corner) stand for the differences in time to heading between lines with the Eps-late or -early alleles (i.e. the delay produced by the Eps-late respect to its Eps-early counterpart); which are not considered in the regression. Inset are the F-ratio values of the main factors and their interactions (*** and ns indicates that the F-ratio was significant, P < 0.001, and not significant, respectively). Segments on each symbol stand for the SEs (if not seen is because the magnitude was smaller than the size of the symbol).
In general, it was clear that both higher temperatures and the Eps-early alleles accelerated development, as reflected by their F-ratios (with the effect of temperature much larger than that of the Eps alleles and Eps x temperature interaction; Fig. 2, inset). In order to illustrate these results visually, the dissected apexes observed by microscope at regular intervals in NILs carrying the Eps-early or -late alleles at 12 and 18 °C were shown in the Fig. 3. We found that apexes developed at 18 °C were more advanced than those grown at 12 °C within each of the same lines. Moreover, within the same temperature regime, lines carrying the Eps-early allele were more developed than those with the Eps-late allele (Fig. 3).
Figure 3.
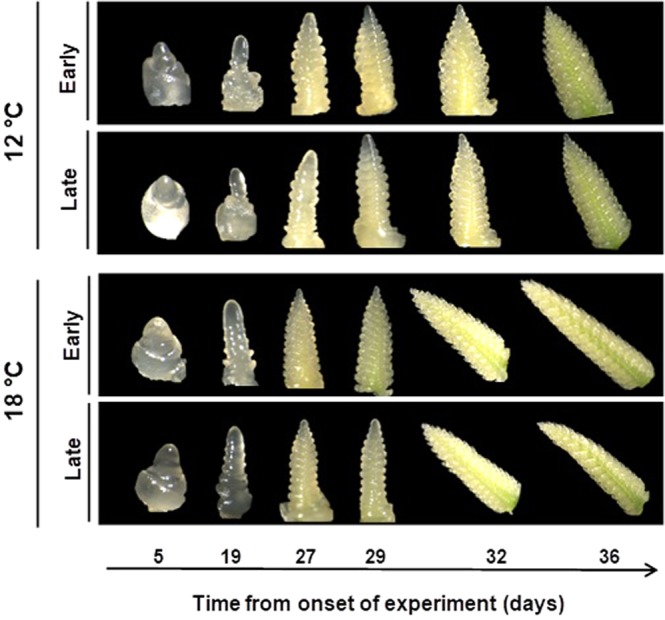
Illustration of apex development in selected lines representative of each sample from 5 to 36 d after the onset of the experiment for lines carrying Eps-early and -late alleles grown at constant temperature of 12 (top panel) and 18 °C (bottom panel).
Between 6 °C and 18 °C, increased temperature accelerated the developmental processes and consequently reduced the period from onset of the experiment to heading (Fig. 4). However, when comparing the time to heading under 21 and 18 °C, the opposite was true: plants of NILs with the Eps-early allele headed earlier at 18 than at 21 °C (Fig. 4) and, as already stated, those carrying the late allele did not reach heading at all.
Figure 4.
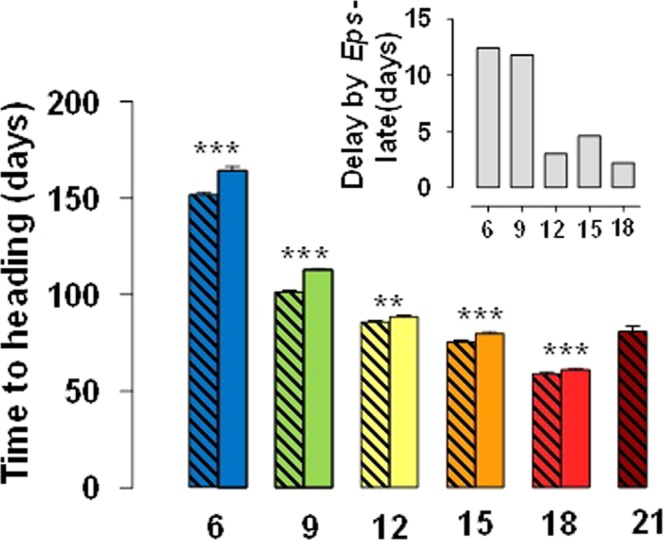
Days from the onset of the experiment to heading for lines carrying Eps-early (hatched bars) or Eps-late (solid bars) alleles grown under constant temperatures of 6, 9, 12, 15, 18, and 21 °C (treatment at 24 °C was not included as none of the plants at that condition reached heading). Inset is a detail of the difference between lines with the Eps-late or Eps-early alleles (for the range of temperatures, 6 to 18 °C, in which plants of both types of NILs reached heading). Asterisks indicate the statistical significance of the differences between lines with late and early alleles at each temperature from the LSmeans contrast (**P < 0.01, ***P < 0.001).
It was also clear that lines carrying the Eps-late allele were consistently later than those with the Eps-early allele (Fig. 4). However, the magnitude of the difference was not the same across temperatures: at low temperatures the effect of the Eps gene was more pronounced than at mild and warm temperatures (Fig. 4, inset); and this interaction was still evident if the delay produced by Eps-late allele is considered in relative terms. At 21 °C the difference was even qualitative as early lines reached heading while late lines did not.
This revealed a clear Eps x temperature interaction of quantitative nature: i.e. there was a difference in the magnitude of the effect, but the interaction was not crossover in nature as lines with the Eps-late allele were never earlier than those carrying the Eps-early allele (Fig. 4). In addition, the no crossover interaction was reflected by the fact that the F-ratio of the Eps x temperature interaction was much smaller than that of temperature (Fig. 2, inset).
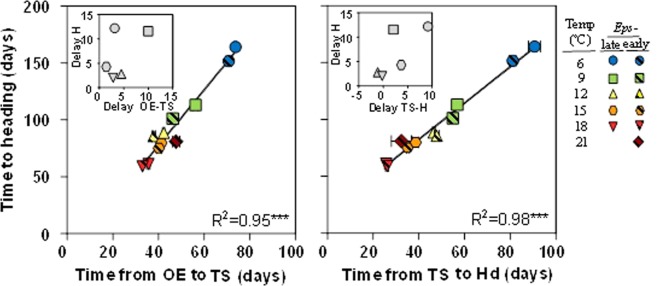
Across all treatments, time to heading was well explained by the two component phases considered in the study: from the onset of the experiment to terminal spikelet (Fig. 5, left panel), and from then to heading (Fig. 5, right panel). Naturally, the very high coefficients of determination are due to the universal effect of temperature: as all phases are sensitive. However, the sensitivity of both phases was not identical: the time elapsed between the onset of the experiment and terminal spikelet ranged from 33 to c. 74 d (an increase of 220% in duration of the leaf and spikelet initiation phase from 18 to 6 °C), whilst duration of the period from terminal spikelet to heading ranged from c. 26 to c. 91 d (an increase of 350% in duration of the late reproductive phase from 18 to 6 °C).
Figure 5.
Relationship between time to heading and either the period from the onset of the experiment (OE) to terminal spikelet (TS) (left panel) or the late reproductive phase from TS to heading (Hd) (right panel) for lines carrying Eps-early (hatched symbols) or Eps-late (solid symbols) alleles grown under constant temperatures of 6, 9, 12, 15, 18 and 21 °C. Lines fitted by linear regression (*P < 0.001). Inset each panel is a detail of the delay produced by the Eps-late allele (difference between lines with the Eps-late or Eps-early alleles) in time to heading respect to the delay in the component phases considered in the study.
In addition, the delay produced by the Eps-late allele (difference between plants carrying Eps-late and Eps-early alleles) in time to heading was better explained by the delay in the late reproductive phase (Fig. 5, inset right panel) than by that of the period from the onset of the experiment to terminal spikelet (Fig. 5, inset left panel).
Thus, the effect of the main factors (temperature and Eps alleles) as well as that of the Eps x temperature interaction shown in time to heading was more clearly reflected in the effect these treatments had on the rate of development during the late reproductive phase than during the period from the onset of the experiment to terminal spikelet.
Developmental responses to temperature
The significant Eps gene x temperature interaction in duration of phases implies that the temperature sensitivity of developmental rates during different phases must vary between NILs carrying Eps-early and -late alleles.
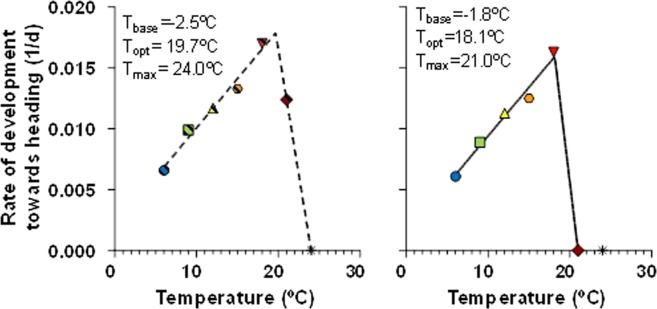
We found that the rate of development towards heading was very well fitted to a bi-linear regression (R2 = 0.993 and 0.807 for Eps-early and -late alleles, respectively). The relationship had a first positive slope (indicating that plants develop faster at higher temperatures, within the range from the base and the optimum thresholds) followed by a second negative one (indicating decline in developmental rates with increasing temperatures between the optimum and maximum thresholds) (Fig. 6). The parameters of the relationship revealed slight changes in base, optimum and maximum temperatures, with the NILs carrying Eps-late alleles showing slightly lower optimum temperature, lower maximum temperature and slightly higher base temperature (Fig. 6). Moreover, the initial slopes (i.e. sensitivity to temperature between base and optimum values) were clearly different, indicating that the rate of development towards heading was more sensitive in the Eps-early (0.807 10−3 [d °C]−1, whose reciprocal is 1239 °C d) than in the Eps-late NILs (0.720 10−3 [d °C]−1, whose reciprocal is 1389 °C d). This differential sensitivity to temperature seemed to be the mechanistic bases for the delay produced by the Eps-late alleles in reaching heading.
Figure 6.
Relationship between the rate of development between the onset of the experiment and heading and temperature for lines carrying Eps-early (left panel) or Eps-late (right panel) alleles grown under constant temperatures of 6, 9, 12, 15, 18, 21 and 24 °C. To avoid a biased estimate of parameters, lines were fitted by bi-linear regression fitted with data at temperatures between the base and the maximum thresholds (for lines carrying Eps-early 6–24 °C and for Eps-late 6–21 °C). Insets are the calculated cardinal temperatures (base, Tbase; optimum, Topt; and maximum, Tmax) for each group of NILs.
Regarding the rates of development during the two phases considered in this study as components of time to heading we found that the general differences between Eps-late and Eps-early lines for time to heading were maintained (Fig. S3). Interestingly, the base temperature was noticeably increased from the early phase until terminal spikelet to the later phase from then to heading. The base temperature estimated for the whole period from the onset of the experiment to heading (Fig. 6) reflected well a weighted average of the base temperatures estimated for two phases composing time to heading (Fig. S3).
The difference between NILs in slope was negligible for the phase from the onset of the experiment to terminal spikelet (c. 1.19 10−3 [d °C]−1, whose reciprocal is c. 840 °C d for both Eps-late and -early NILs; Fig. S3A,B). On the other hand, the rate of development during the late reproductive phase was much more sensitive to temperature in the Eps-early (2.08 10−3 [d °C]−1, whose reciprocal is c. 481 °C d; Fig. S3C) than in the Eps-late NILs (1.69 10−3 [d °C]−1, whose reciprocal is c. 592 °C d; Fig. S3D).
Gene expression over a 24 h period
Although in this study, we analysed the level of expression of ELF3 from the three (A, B and D) genomes, we still found differences of expression between Eps-D1 early lines with ELF3-loss function and late lines with alleles from Rialto. Hence, the expression of ELF3 related with the A and B genomes did not compensate the expression linked to the D genome.
Virtually no significant differences in the expression of ELF3 or GI were found between late and early flowering NILs at 18 °C (Fig. 7). However, at 12 °C the lines are clearly differentiated by the circadian expression profiles of these genes (Fig. 7). ELF3 expression at 12 °C actually shows an increase in total ELF3 transcript at six hours after dawn in early lines, this is reversed by hour 15 at which time ELF3 expression reaches its lowest level of expression in early flowering lines, a state which was maintained into the dark period and is consistent with an early flowering phenotype as ELF3 is a floral repressor. This pattern is mirrored in the GI expression profiles at 12 °C; this time with 6 hours significantly increased expression levels of GIA and GIB in late lines and followed by an increase in GIB for early flowing lines at 12 hours.
Figure 7.
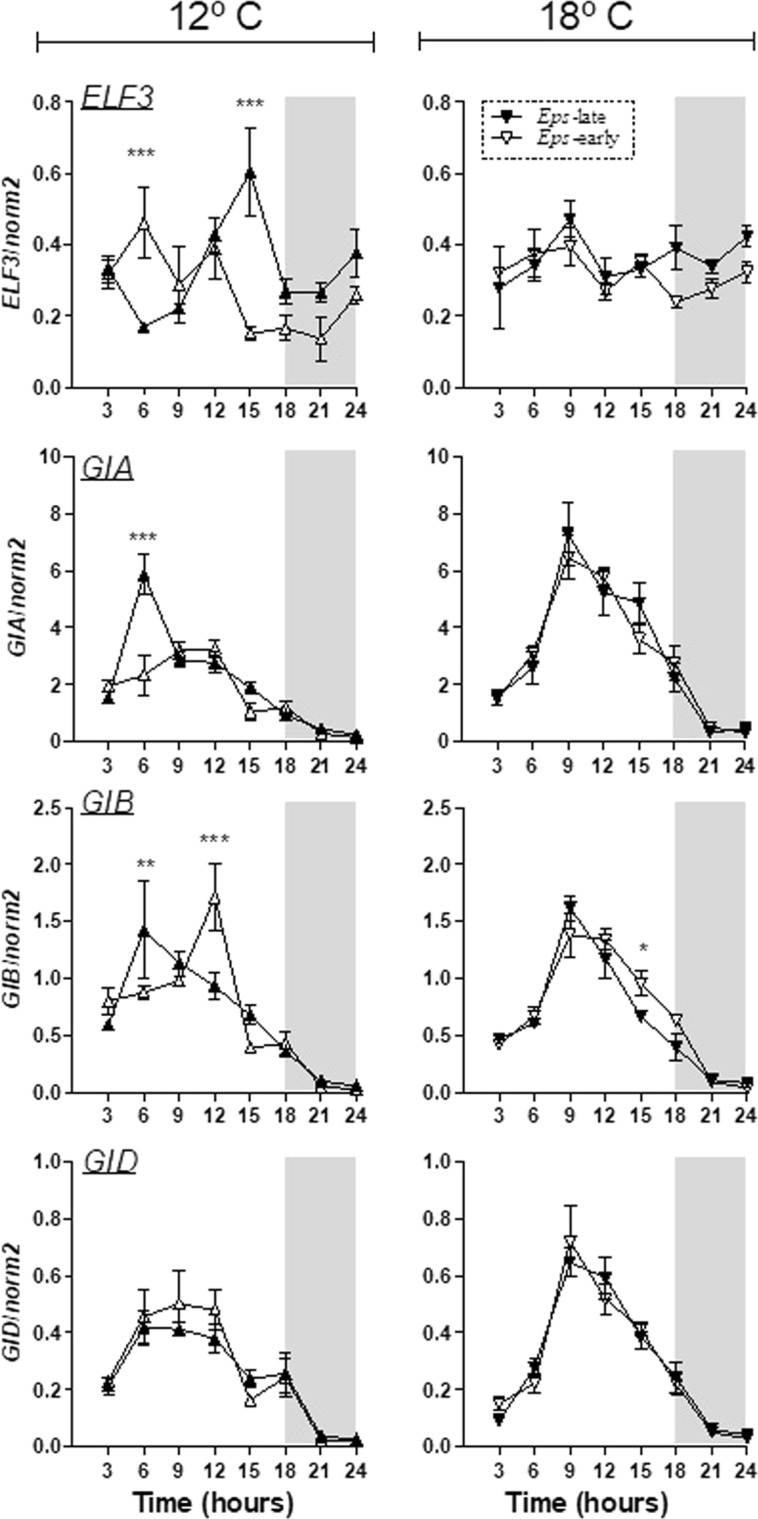
Expression patterns of genes ELF3 (generic primer amplifying all three A, B and D homoeologues), GIA, GIB and GID homoeologues Eps- early (open symbols) and Eps-late (closed symbols) genotypes. The vertical axis represents the relative expression of the genes against the house keeping gene norm2. Dark period is indicated by grey square. Asterisks indicate the statistical significance of the differences between lines with late and early alleles at each temperature from the LSmeans contrast (*P < 0.05, **P < 0.01, ***P < 0.001 and no asterisks means that the differences was not significant).
Discussion
The fact that temperature, between the base and the optimum thresholds, accelerated development was expected, as there is a huge body of literature reporting this effect in wheat19,32 as well as not only in other crops33 but also in other completely unrelated organisms20,21. The overall delay in time to heading produced by the introgression of Eps-late alleles was also expected, as these lines were selected because of their previous reported effects in field conditions both in the UK11 and in Spain28; conditions in which the actual effect of Eps alleles can be challenged, as they can be masked by the presence of other major developmental genes11,34. But the work described here contributes more specifically by providing the first time direct evidence of the Eps x temperature interaction in hexaploid wheat. Previous reports indicated that cultivars may differ in their sensitivity to temperature22,30 as well as Eps x temperature interactions but working with an Eps gene of T. monococcum usually with strong effects12,23,24; but to the best of our knowledge this interaction have never been shown before for an Eps gene with an expected subtle effect on time to heading in bread wheat.
Indeed the magnitude of the deceleration of development produced by introgressing Eps-late alleles was stronger at relatively low temperatures below the optimum threshold. The fact that the difference in time to reach heading between NILs carrying the Eps-late and -early alleles was affected by temperature was not due to a simple effect of temperature itself on the “extra time” required by the Eps-late NILs. Actually, if we analyse the results in thermal time using a general base temperature of 0 °C (Fig. 8), the same interaction is still noticeable. Naturally the effect of temperature is lost for the range 6–18 °C, and the thermal time increases at 21 °C because this temperature was higher than the optimum.
Figure 8.
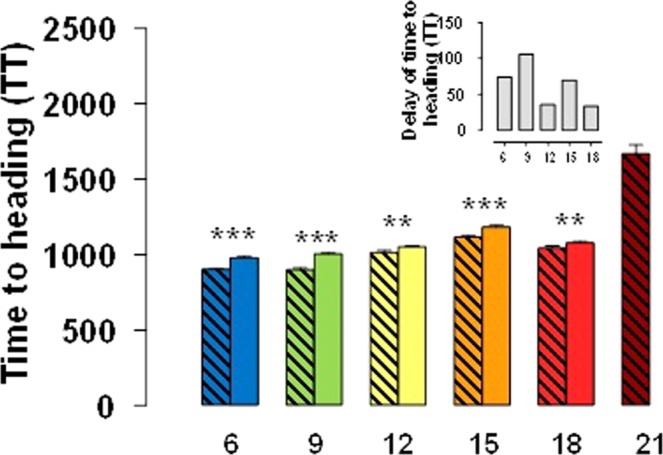
Thermal time from the onset of the experiment to heading (estimated using 0 °C as base temperature) for lines carrying Eps- early (hatched bars) or Eps-late (solid bars) alleles grown under constant temperatures of 6, 9, 12, 15, 18, and 21 °C. Inset is a detail of the difference (in °C d) between lines with the Eps-late or Eps-early alleles. Asterisks indicate the statistical significance of the differences between lines with late and early alleles at each temperature from the LSmeans contrast (**P < 0.01, ***P < 0.001).
This is in agreement with the apparent inconsistency of the Eps genes effects on wheat development under field conditions of Spain28 and UK11. When facing the fact that the effects of Eps-D1 were stronger in relatively cool British conditions than in rather warmer Spanish fields, we hypothesised that the reason was the difference between locations in ambient temperature28. In the present study, we provided evidences that support that hypothesis.
It was demonstrated then that the Eps-D1 gene studied in this work was likely to control sensitivity to temperature; and the Eps-early and –late alleles determine different degrees of sensitivity to this environmental cue, rather than being an intrinsic factor modifying the rate of developmental processes independent of the environment. Furthermore, the fact that the sort of interaction we reported did not involve crossover offers some confidence that this allele could be an important tool for wheat breeding programs in which pursue to fine-tuning time to heading (at least it would not be expected to result in changes in rank order of expected results, as suggested may be the case with other Eps genes, which might exhibit stronger interactions)9.
It seemed clear that the reduction in time to heading produced by the introgression of the Eps-early allele from chromosome 1DL of Spark into that of Rialto was mainly associated with an increase in sensitivity to temperature during the late reproductive phase. We are not aware of other studies reporting these effects for Eps genes in hexaploid wheat, but the finding is consistent with the fact that different phases of wheat and barley development may respond to temperature with varying degrees of sensitivity22,30. This capacity of Eps-D1 to modify time to heading through modifying development during stem elongation is important, as in most cases it is assumed that the effects of Eps genes are limited to (or at least are stronger in) the earlier phases of crop development9,12. Deployment of alleles that affect the developmental rates of the late reproductive phase may be instrumental as they can be used to not only fine-tuning heading time but also the likely fertility of the spikes. Optimizing the duration of this developmental phase may be an avenue for further improving the likelihood of floret primordia to become fertile florets35 that would further improve the number of grains36,37, which is the yield component most largely affecting yield2,38,39.
The earliness per se locus (Eps-D1) studied in this research was suggested to have as candidate gene, the circadian clock gene, ELF318. For this reason, we analysed the expression of this gene under two different temperatures in order to explore if expression of this circadian clock gene was modulated by temperature. In fact, we ascertained that the deletion on 1D that contained ELF3 was associated always to “Spark” allele and linked with early flowering genotypes in both temperatures. These results are in agreement with previous evidences that ELF3 repressed the gibberellin (GA) biosynthesis40, which are able to promote the transition from vegetative to reproductive development. In addition, we also confirm ELF3 repressed the expression of the evening loop gene GI18,41,42 and that these differences in expression were clear at 12 °C but largely absent at 18 °C. It was shown that ELF3 expression was decreased throughout the diurnal cycle in early flowering NILs under a temperature regime of 16–18 °C in the light and 13–15 °C in the dark period18. The current study might indicate that the cooler dark temperatures might have been crucial for the induction of differences in heading date and gene expression in that study and that gene expression studies in more dynamic field conditions might shed more light on the observed Eps x temperature interaction. Previous studies have reported that ELF3 plays a role on temperature entrainment of the clock in Arabidopsis25 and in barley26. In temperature entrainment, the daily rhythms of the circadian clock are synchronized with daily changes of the temperature. In our studies, daily temperature changes were inexistent because we applied constant temperatures (12 or 18 °C) but we found different responses of ELF3 to temperature, level of expression increased as temperature increased. This might indicate that ELF3, besides having an effect on the temperature entrainment, as it was described previously, also has a role in temperature compensation43. Another study showed that the binding of ELF3 to target promoters was dependent on temperature44 providing a plausible mechanism for the temperature interaction effects seen here.
Materials and Methods
Growth conditions
The experiments were carried out in the facilities of both the University of Lleida (UdL, Spain) and John Innes Centre (JIC, UK) using growth chambers (GER-1400 ESP, Radiber SA, Spain and controlled environment room, respectively). One single seed was sown in pots (200 or 400 cm3, at the UdL and JIC, respectively) filled with a mixture of 30% peat and 70% soil (UdL) or cereal mix compost composed of 40% peat and 40% soil and 20% grit (JIC). Before starting the experiments in the growth chambers, all seedlings were vernalised at 4 °C for 49 days. To maximise uniformity at the onset of the experiment (i.e. when pots were transferred from the vernalisation room to the growth chambers), we sowed 50–100% (depending on availability of seeds) extra plants of each line. Then, at the end of the vernalisation period, we selected those that showed the same stage to avoid noise caused by variability arising from uneven development among plants at the commencement of the study. After vernalisation, the plants were transferred to the corresponding growth chambers. At this time, the seedlings were at stage 1.27 ± 0.21 leaves of the scale of Haun45.
Chambers were always set under long day conditions (18 hours). Pots inside the chambers were rearranged approximately once a week to minimise the effects of likely differences in microenvironment at different positions within each chamber.
Treatments
The treatments consisted of a factorial combination of two sets of near isogenic lines (NILs) carrying different Eps alleles and seven temperature regimes.
NILs were developed at John Innes Centre (Norwich, UK) from two double haploid lines derived from the cross Spark × Rialto (SR9 and SR23, both carrying the Eps-early allele of Spark in chromosome 1DL) as described in Zikhali et al.11. In brief, each of these doubled haploid lines was back-crossed twice with the recurrent parent Rialto (which carries the Eps-late allele in 1DL) producing, within each of the two families, BC2 progenies (equivalent to BC3 in terms of recurrent parent background composition). These BC2 lines were self-pollinated producing BC2F3 families, and within these families lines homozygous for Eps alleles on chromosome 1DL (carrying the Eps-early or -late) were selected using SSR markers. Each NIL pair was then composed of a NIL carrying Eps-late allele and another carrying Eps-early allele, within the families derived from the SR9 and SR23 lines.
Temperature treatments were kept constant in for each chamber and covered a range from 6 to 24 °C at intervals of 3 °C. The temperature regimes of 24, 21, 15, 9 and 6 °C were imposed at the UdL whilst those of 18 and 12 °C were at JIC.
Fifty-five (UdL) or 63 (JIC) pots per genotype were arranged inside each chamber (temperature regime) in a completely randomised design with three replicates. Each replicate was formed by 1/3 of the pots corresponding to each NIL, that is, 18 (UdL) and 21(JIC) pots per replicate and per genotype, resulting a total of 220 (UdL) and 252 (JIC) pots at each temperature regime.
Gene expression analyses over a 24 hours period
Expression analysis of ELF3 (with generic primer amplifying all three A, B and D homoeologues), GIA, B and D homoeologues were measured in both NILs pairs (Eps-early and -late alleles) on plants grown at 12 and 18 °C. The authors studied the expression of GI as well as ELF3 because, in Arabidopsis at least, the influence of ELF3 on flowering time is achieved by direct repression of GI46,47. Samples were collected every 3 hours over a 24-hours period. Three whole plants per genotype and treatment were immediately frozen in liquid nitrogen and crushed. Crushed plant material were store at −80 °C until the RNA extraction step. RNA extraction was carried out by using RNEasy Plant Mini Kit (Quiagen) with TRI reagent according with John Innes Centre Standard Operating Procedure. The concentration of RNA extracted was measured through spectrophotometry by NanoDrop 1000 (Thermo Scientific). From the concentration of RNA (ng/µl) obtained by NanoDrop 1000, the volume (µl) needed to get 5 ng of RNA was calculated for each sample. H2O was added to make up to 8 µl of total volume. The DNA was removed from the RNA sample by adding 1 µl of buffer DNase I recombinant RNase-free, 1 µl of DNase I recombinant RNase-free, 1 µl of dNTPs at a concentration of 10 Mm (Promega UK LDT) and 1 µl of random primer mix were then added and a standard PCR was run by thermal cycler MJ Research PTC-225 Peltier with forty cycles that included denaturation for 20 s at 95 °C, annealing for 20 s at 55 °C and polymerization at 72 °C for 1 min. After the forty cycles, the PCR was held at 72 °C for 5 mins. Of the resulting cDNA we took 2.5 µl to which it was added 10 µl of GoTaq qPCR Master Mix (Promega UK LDT), 1 µl of forward primer and 1 µl of reverse primer whose sequences were AGCGATTTCCAGCTGCCTTC and TGCGAAGAGGCCAGTCAGTC (the primer is termed as norm2 and it was used previously)18. Analysis of relative transcription levels of the respective sequences was performed by using LightCycler software.
Measurements
Three plants per replicate of each genotype (all in all 9 plants per genotype) were labelled at the beginning of the experiments. On all plants we measured (i) the phenological stages of seedling emergence (stage DC10), heading (DC59) and anthesis (DC65) following the Decimal Code48. Additionally, timing of terminal spikelet49 was determined from periodical dissection under binocular microscope sampling at each time one plant per treatment and replicate from the remaining plants (15 and 18 plants used for periodic dissections at UdL and JIC, respectively).
Statistical analysis
Data of variables were subjected to analysis of variance and the relationships between traits were analysed through regression analyses. All analyses were performed using the statistical software JMP® Pro Version 12.0 (SAS Institute Inc. Cary, NC, USA). Differences among Eps alleles in each temperature treatment were tested using ANOVA and a post-hoc analysis of LSMeans Contrast.
Supplementary information
Acknowledgements
Funding was provided by ADAPTAWHEAT (a project funded by the European Commission under the 7th Framework Programme for Research and Technological Development) and by project AGL2012-35300 funded by the Ministry of Economy and Competitiveness of Spain. HO held a FPI scholarship from the Spanish Ministry of Science and Innovation and PP held a PhD scholarship from the University of Lleida. The authors thank Andreu Casellas (UdL) for his excellent technical assistance and Prof. Roxana Savin (UdL) and Dr. Laura Dixon (JIC) for their support in the organisation of the work in the UdL and JIC chambers, respectively.
Author Contributions
G.A.S. and S.G. conceived the research; H.O. and P.P. performed the experiments, collected and organised the data; H.O., P.P. and G.A.S. analysed and interpreted the data on developmental traits; H.O. and M.Z. performed the analyses of gene expression; H.O. and S.G. interpreted the analyses of gene expression; H.O. and G.A.S. drafted the original manuscript; all authors reviewed the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39201-6.
References
- 1.Worland AJ, Sayers E. The influence of flowering time genes on environmental adaptability in European wheats. Euphytica. 1996;89:49–57. doi: 10.1007/BF00015718. [DOI] [Google Scholar]
- 2.Slafer GA. Genetic Basis of Yield as Viewed From a Crop Physiologist’s Perspective. Ann. Appl. Biol. 2003;142:117–128. doi: 10.1111/j.1744-7348.2003.tb00237.x. [DOI] [Google Scholar]
- 3.Reynolds M, et al. Achieving yield gains in wheat. Plant, Cell Environ. 2012;35:1799–1823. doi: 10.1111/j.1365-3040.2012.02588.x. [DOI] [PubMed] [Google Scholar]
- 4.Fjellheim S, Boden S, Trevaskis B. The role of seasonal flowering responses in adaptation of grasses to temperate climates. Front. Plant Sci. 2014;5:431. doi: 10.3389/fpls.2014.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay RKM, Kirby EJM. Convergence and Synchrony - a Review of the Coordination of Development in Wheat. Aust. J. Agric. Res. 1991;42:661–700. doi: 10.1071/AR9910661. [DOI] [Google Scholar]
- 6.Slafer GA, Rawson HM. Sensitivity of wheat phasic development to major environmental factors: a reexamination of some assumptions made by physiologists and modellers. Aust. J. Plant Physiol. 1994;21:393–426. [Google Scholar]
- 7.Zikhali M, Wingen LU, Leverington M, Specel S, Griffiths S. The identification of new candidate genes Triticum aestivum FLOWERING LOCUS T3-B1 (TaFT3-B1) and TARGET OF EAT1 (TaTOE1-B1) controlling the short-day photoperiod response in bread wheat. Plant, Cell Environ. 2017;40:2678–2690. doi: 10.1111/pce.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths S, et al. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor. Appl. Genet. 2009;119:383–395. doi: 10.1007/s00122-009-1046-x. [DOI] [PubMed] [Google Scholar]
- 9.Slafer GA. Differences in phasic development rate amongst wheat cultivars independent of responses to photoperiod and vernalization. A viewpoint of the intrinsic earliness hypothesis. J. Agric. Sci. 1996;126:403–419. doi: 10.1017/S0021859600075493. [DOI] [Google Scholar]
- 10.Snape. JW, Butterworth K, Whitechurch E, Worland AJ. Waiting for fine times: Genetics of flowering time in wheat. Euphytica. 2001;119:185–190. doi: 10.1023/A:1017594422176. [DOI] [Google Scholar]
- 11.Zikhali M, et al. Validation of a 1DL earliness per se (eps) flowering QTL in bread wheat (Triticum aestivum) Mol. Breed. 2014;34:1023–1033. doi: 10.1007/s11032-014-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis S, Faricelli ME, Appendino ML, Valárik M, Dubcovsky J. The chromosome region including the earliness per se locus Eps-A m1 affects the duration of early developmental phases and spikelet number in diploid wheat. J. Exp. Bot. 2008;59:3595–3607. doi: 10.1093/jxb/ern209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zikhali, M. & Griffiths, S. The effect of Earliness per se (Eps) genes on flowering time in bread wheat. In: Ogihara, Y., Takumi, S., Handa, H., Eds Advances in Wheat Genetics: From Genome to Field, Springer Japan, Tokio, pp. 339–345 (2015).
- 14.Worland AJ, Appendino ML, Sayers EJ. The distribution, in European winter wheats, of genes that influence ecoclimatic adaptability whilst determining photoperiodic insensitivity and plant height. Euphytica. 1994;80:219–228. doi: 10.1007/BF00039653. [DOI] [Google Scholar]
- 15.Appendino ML, Bartoloni N, Slafer GA. Vernalization response and earliness per se in cultivars representing different eras of wheat breeding in Argentina. Euphytica. 2003;130:61–69. doi: 10.1023/A:1022376711850. [DOI] [Google Scholar]
- 16.Gawronski P, et al. A distorted circadian clock causes early flowering and temperature-dependent variation in spike development in the Eps-3Ammutant of einkorn wheat. Genetics. 2014;196:1253–1261. doi: 10.1534/genetics.113.158444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez MA, Tranquilli G, Lewis S, Kippes N, Dubcovsky J. Genetic and physical mapping of the earliness per se locus Eps-A m 1 in Triticum monococcum identifies EARLY FLOWERING 3 (ELF3) as a candidate gene. Funct. Integr. Genomics. 2016;16:365–382. doi: 10.1007/s10142-016-0490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zikhali M, Wingen LU, Griffiths S. Delimitation of the Earliness per se D1 (Eps-D1) flowering gene to a subtelomeric chromosomal deletion in bread wheat (Triticum aestivum) J. Exp. Bot. 2015;67:287–299. doi: 10.1093/jxb/erv458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slafer, G. A. et al. Genetic and environmental effects on crop development determining adaptation and yield. In: “Crop Physiology: Applications for Genetic Improvement and Agronomy” (V. O Sadras and D. F. Calderini, Eds), Elsevier, Amsterdam, pp. 285–319 (2015).
- 20.Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. Effects of size and temperature on developmental time. Nature. 2002;417:70–73. doi: 10.1038/417070a. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor MI, et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1266–71. doi: 10.1073/pnas.0603422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slafer GA, Rawson HM. Base and optimum temperatures vary with genotype and stage of development in wheat. Plant, Cell Environ. 1995;18:671–679. doi: 10.1111/j.1365-3040.1995.tb00568.x. [DOI] [Google Scholar]
- 23.Bullrich L, Appendino ML, Tranquilli G, Lewis S, Dubcovsky J. Mapping of a thermo-sensitive earliness per se gene on Triticum monococcum chromosome 1Am. Theor. Appl. Genet. 2002;105:585–593. doi: 10.1007/s00122-002-0982-5. [DOI] [PubMed] [Google Scholar]
- 24.Appendino ML, Slafer GA. Earliness per se and its dependence upon temperature in diploid wheat lines differing in the major gene Eps-A(m)1 alleles. J. Agric. Sci. 2003;141:149–154. doi: 10.1017/S0021859603003472. [DOI] [Google Scholar]
- 25.Thines, B. & Harmon, F. G. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proceedings of the National Academy of Sciences, USA 107, 3257–3262 (2010). [DOI] [PMC free article] [PubMed]
- 26.Ford B, et al. Barley (Hordeum vulgare) circadian clock genes can respond rapidly to temperature in an EARLY FLOWERING 3-dependent manner. J. Exp. Bot. 2016;67:5517–5528. doi: 10.1093/jxb/erw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salome PA, McClung CR. Pseudo-response regulator 7 and 9 are partially redundant genes essential for the temperature responsiveness of the arabidopsis circadian clock. Plant Cell. 2005;17:791–803. doi: 10.1105/tpc.104.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochagavía, H., Prieto, P., Savin, R., Griffiths, S. & Slafer G. A. Earliness per se effects on developmental traits in hexaploid wheat grown under field conditions. Eur. J. of Agron., submitted (2018).
- 29.Farré A, et al. Application of a library of near isogenic lines to understand context dependent expression of QTL for grain yield and adaptive traits in bread wheat. BMC Plant Biol. 2016;16:161. doi: 10.1186/s12870-016-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karsai I, et al. Developmental patterns of a large set of barley (Hordeum vulgare) cultivars in response to ambient temperature. Ann. Appl. Biol. 2013;162:309–323. doi: 10.1111/aab.12023. [DOI] [Google Scholar]
- 31.González FG, Miralles DJ, Slafer GA. Wheat floret survival as related to pre-anthesis spike growth. J. Exp. Bot. 2011;14:4889–4901. doi: 10.1093/jxb/err182. [DOI] [PubMed] [Google Scholar]
- 32.Porter JR, Gawith M. Temperatures and the growth and development of wheat: a review. Eur. J. Agron. 1999;10:23–36. doi: 10.1016/S1161-0301(98)00047-1. [DOI] [Google Scholar]
- 33.Parent B, Tardieu F. Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species. New Phytol. 2012;194:760–774. doi: 10.1111/j.1469-8137.2012.04086.x. [DOI] [PubMed] [Google Scholar]
- 34.Sukumaran S, et al. Identification of earliness per se flowering time locus in spring wheat through a genome-wide association study. Crop Sci. 2016;56:2962–2972. doi: 10.2135/cropsci2016.01.0066. [DOI] [Google Scholar]
- 35.Gonzalez-Navarro OE, Griffiths S, Molero G, Reynolds MP, Slafer GA. Variation in developmental patterns among elite wheat lines and relationships with yield, yield components and spike fertility. Field Crops Res. 2016;196:294–304. doi: 10.1016/j.fcr.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slafer GA, Abeledo LG, Miralles DJ, Gonzalez FG, Whitechurch EM. Photoperiod sensitivity during stem elongation as an avenue to raise potential yield in wheat. Euphytica. 2001;119:191–197. doi: 10.1023/A:1017535632171. [DOI] [Google Scholar]
- 37.Miralles DJ, Slafer GA. Sink limitations to yield in wheat: how could it be reduced? J. Agric. Sci. 2007;145:139–149. doi: 10.1017/S0021859607006752. [DOI] [Google Scholar]
- 38.Fischer RA. The importance of grain or kernel number in wheat: a reply to Sinclair and Jamieson. Field Crops Res. 2008;105:15–21. doi: 10.1016/j.fcr.2007.04.002. [DOI] [Google Scholar]
- 39.Slafer GA, Savin R, Sadras VO. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014;157:71–83. doi: 10.1016/j.fcr.2013.12.004. [DOI] [Google Scholar]
- 40.Boden SA, et al. EARLY FLOWERING3 regulates flowering in spring barley by mediating gibberellin pro- duction and FLOWERING LOCUS T expression. Plant Cell. 2014;26:1557–1569. doi: 10.1105/tpc.114.123794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins JA, Bailey PC, Laurie DA. Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PloS ONE. 2010;5:e10065. doi: 10.1371/journal.pone.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faure S, et al. Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc. Natl. Acad. Sci. 2012;109:8328–8333. doi: 10.1073/pnas.1120496109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould PD, et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell. 2006;18:1177–1187. doi: 10.1105/tpc.105.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Box MS, et al. ELF3 controls thermoresponsive growth in Arabidopsis. Curr.Biol. 2015;25:194–199. doi: 10.1016/j.cub.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 45.Haun JR. Visual quantification of wheat development. Agron. J. 1973;65:116–119. doi: 10.2134/agronj1973.00021962006500010035x. [DOI] [Google Scholar]
- 46.Mizuno T, et al. Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:958–976. doi: 10.1093/pcp/pcu030. [DOI] [PubMed] [Google Scholar]
- 47.Ezer D, et al. The evening complex coordinates environmental and endogenous signals in Arabidopsis. NaturePlants. 2017;3:17087. doi: 10.1038/nplants.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stage of cereals. Weed Res. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- 49.Waddington SR, Cartwright PM, Wall PC. A Quantitative Scale of Spike Initial and Pistil Development in Barley and Wheat. Ann. Bot. 1983;51:119–130. doi: 10.1093/oxfordjournals.aob.a086434. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.