Abstract
The JAK-STAT pathway critically regulates T-cell differentiation, and STAT1 is postulated to regulate several immune-mediated diseases by inducing proinflammatory subsets. Here we show that STAT1 enables CD4+ T-cell-mediated intestinal inflammation by protecting them from natural killer (NK) cell-mediated elimination. Stat1−/− T cells fail to expand and establish colitis in lymphopenic mice. This defect is not fully recapitulated by the combinatorial loss of type I and II IFN signaling. Mechanistically, Stat1−/− T cells have reduced expression of Nlrc5 and multiple MHC class I molecules that serve to protect cells from NK cell-mediated killing. Consequently, the depletion of NK cells significantly rescues the survival and spontaneous proliferation of Stat1−/− T cells, and restores their ability to induce colitis in adoptive transfer mouse models. Stat1−/− mice however have normal CD4+ T cell numbers as innate STAT1 signaling is required for their elimination. Overall, our findings reveal a critical perspective on JAK-STAT1 signaling that might apply to multiple inflammatory diseases.
The JAK-STAT signaling pathway is important for cytokine responses and CD4 T-cell differentiation. Here the authors show that Stat1 also serves to protect CD4 T cells from natural killer cell-mediated killing, potentially by promoting the expression of Nlrc5 and MHC-I, to preserve the induction of experimental colitis via the adoptive transfer of CD4 T cells.
Introduction
The JAK-STAT signaling pathway plays a critical role in transducing signals from various cytokines to achieve distinct transcriptional outcomes1. In T cells, this pathway has been well studied in terms of their regulation of T-cell differentiation2. Among the seven mammalian signal transducer and activator of transcription (STAT) family members, STAT1 is known to be important for the induction of Th1 cells downstream of IFNγ due to its induction of the transcription factor T-bet3,4. STAT1 has also been shown to suppress regulatory T-cell differentiation5. These proinflammatory properties of STAT1 are important for controlling infections, where patients with loss-of-function mutations in Stat1 develop susceptibility to viral/mycobacterial infections6. They are also important for promoting inflammatory diseases like graft-vs-host-disease (GvHD)5. However, STAT1 also suppresses Th17 differentiation7, and Stat1−/− mice develop aggravated Th17-mediated autoimmune diseases including experimental autoimmune encephalomyelitis (EAE)8,9.
Inflammatory bowel diseases (IBD) likely arise from an aberrant immune response toward intestinal microbes in a genetically susceptible host10. Crohn’s disease in particular is characterized by a skewing of the CD4+ T cell profile toward the proinflammatory Th1 and Th17 subsets, which are believed to be critical for disease pathogenesis11. Patients with Crohn’s disease display higher STAT1 expression, albeit only modestly in CD4+ T cells12. However, the mechanism by which STAT1 modulates CD4+ T cells in IBD is currently unclear and presumed to be through altering differentiation states13.
IL-10 is a critical anti-inflammatory cytokine for maintaining intestinal immune homeostasis, as evidenced in mice and humans deficient in IL-10 or IL-10 receptor (IL-10R) that develop spontaneous colitis14–16. We and others recently described the importance of IL-10R signaling in macrophages in the prevention of colitis, with Il10rb−/−Rag1−/− mice but not Rag1−/− mice developing colitis upon reconstitution with WT CD4+ T cells17,18. Subsequent studies in our model and others pointed to a role for pathogenic Th17 cells in driving the disease19–24. As STAT1 is a critical regulator of Th1/Th17 differentiation, we further investigated its role in the ability of CD4+ T cells to induce colitis.
Here we describe a role for STAT1 in enabling T cells to induce colitis by protecting them from NK cell-mediated cytotoxicity. Stat1−/− T cells fail to expand and induce colitis in vivo unless NK cells are depleted. This is because STAT1 is required to induce sufficient levels of Nlrc5 and the inhibitory NK ligand MHC class I to enable evasion of rejection by host NK cells. Surprisingly, this requirement for STAT1 is largely independent of both Type I and II IFN signaling, the classical activators of STAT1. Moreover, this mechanism is specific to Stat1−/− T cells undergoing spontaneous proliferation and requires STAT1 expression in the innate compartment. Altogether, our study reveals a critical role of STAT1 that is distinct from T-cell differentiation and adds a new perspective to studies on T-cell-mediated inflammatory disease.
Results
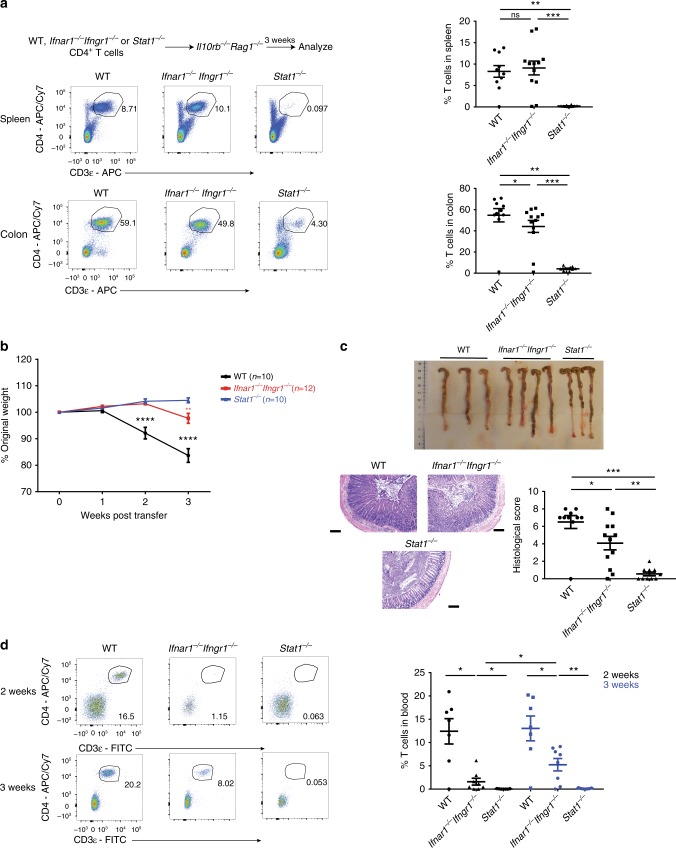
T cells require STAT1 to expand and induce colitis in vivo
To investigate the role of STAT1 signaling in T-cell driven colitis, we adoptively transferred unfractionated WT or Stat1−/− CD4+ T cells into Il10rb−/−Rag1−/− mice (Fig. 1a). WT T cells induced severe colitis in Il10rb−/−Rag1−/− recipient mice as expected17. In contrast, mice transferred with Stat1−/− T cells displayed no signs of intestinal inflammation as evidenced by the lack of weight loss, colonic thickening and histological inflammation (Fig. 1a, b). Flow cytometric analysis of the colonic lamina propria revealed a marked reduction of Stat1−/− T cells compared to WT T cells (Fig. 1c). This was not due to aberrant homing of Stat1−/− T cells to the intestine, as a similar reduction of T cells was observed in the spleen (Fig. 1d).
Fig. 1.
Stat1−/− T cells fail to induce colitis due to defective expansion. Il10rb−/−Rag1−/− mice were injected i.p. with 1 × 106 unfractionated WT or Stat1−/− CD4+ T cells. a Mean % original body weights ± SEM following T-cell transfer. Source data are provided as a Source Data file. b Representative images of colons, as well as representative H&E images of distal colon sections with mean histological scores ± SEM at 3 weeks post transfer. Scale bar represents 200 µm. c, d Representative flow cytometry plots of CD4+ T cells (gated on live CD45+ cells, Supplementary Fig. 4a) in the c colon and d spleen followed by their mean frequencies ± SEM at 3 weeks post transfer. All data are pooled from two to three independent experiments, with each point representing an individual mouse. ****p < 0.0001, ***p < 0.001 by a two-way ANOVA with Bonferroni’s correction or b–d two-tailed Mann–Whitney test
We next asked if the reduction of Stat1−/− T cells was dependent on colonic inflammation by transferring unfractionated WT or Stat1−/− CD4+ T cells into Rag1−/− mice, a strain that does not develop colitis when reconstituted with unfractionated WT T cells17. Similar to Il10rb−/−Rag1−/− mice, Stat1−/− T cells were markedly reduced in the colons and spleens of Rag1−/− mice, indicating that STAT1 is required for robust in vivo T-cell expansion independent of colonic inflammation and innate IL-10R expression (Fig. 2a, b).
Fig. 2.
Stat1−/− T cells fail to expand in Rag1−/− mice. Rag1−/− mice were injected i.p. with 1 × 106 WT or Stat1−/− unfractionated CD4+ T cells and analyzed 3 weeks post transfer. a, b Representative flow cytometry plots of CD4+ T cells (gated on live CD45+ cells) in the colon (a) and spleen (b) followed by their mean frequencies ± SEM. Data are pooled from three independent experiments, with each point representing an individual mouse. ****p < 0.0001 by two-tailed Mann–Whitney test
Partial dependency of STAT1 on Type I + II IFN signaling
IFNs are the classical inducers of STAT1 signaling with both Type I and Type II IFN individually reported to regulate T cell function3,25–27. We therefore sought to determine if the impaired expansion of Stat1−/− T cells was due to the lack of both type I and type II IFN signaling by transferring Ifnar1−/−Ifngr1−/− CD4+ T cells into Il10rb−/−Rag1−/− mice (Fig. 3a). Surprisingly, the abrogation of both Type I and Type II IFN receptors failed to recapitulate STAT1 deficiency, as Ifnar1−/−Ifngr1−/− CD4+ T cells expanded to similar levels as WT T cells in the spleen and colon 3 weeks post transfer (Fig. 3a).
Fig. 3.
Type I + II IFN signaling do not explain the defective expansion of Stat1−/− T cells. Il10rb−/−Rag1−/− mice were injected i.p. with 1 × 106 WT, Ifnar1−/−Ifngr1−/− or Stat1−/− CD4+ T cells. a Representative flow cytometry plots of CD4+ T cells (gated on live CD45+ cells, Supplementary Fig. 4b) in the spleen and colon followed by their mean frequencies ± SEM at 3 weeks post transfer. b Mean % initial body weights ± SEM following T-cell transfer. Source data are provided as a Source Data file. c Representative images of colons, as well as representative H&E images of distal colon sections with mean histological scores ± SEM at 3 weeks post transfer. Scale bar represents 200 µm. d Representative flow cytometry plots of CD4+ T cells (gated on CD45+ cells) in the blood followed by their mean frequencies ± SEM at 2 and 3 weeks post transfer. All data are pooled from two to three independent experiments, with each point representing an individual mouse. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by b two-way ANOVA with Bonferroni’s correction (WT compared to Ifnar1−/− Ifngr1−/− or Stat1−/−, Ifnar1−/− Ifngr1−/− compared to Stat1−/−) or by a, c, d two-tailed Mann–Whitney test
Ifnar1−/−Ifngr1−/− T cells were also able to induce colitis unlike Stat1−/− T cells (Fig. 3b, c). However, the severity of colitis induced by Ifnar1−/−Ifngr1−/− T cells was reduced compared to WT T cells (Fig. 3b, c), which correlated with a reduced rate of expansion of Ifnar1−/−Ifngr1−/− T cells in the blood (Fig. 3d). As expected, Stat1−/− T cells did not expand in the blood (Fig. 3d). These data suggest that while Type I+II IFN partially contribute to the STAT1-dependent signaling, the impaired expansion of Stat1−/− T cells is predominantly an IFN-independent process at later time points.
Cell-intrinsic role for STAT1 in in vivo T-cell expansion
To understand the mechanisms linking STAT1 to T-cell expansion in vivo, we first asked if the presence of WT T cells could rescue the defective expansion of Stat1−/− T cells by transferring equal ratios of congenically marked WT (CD45.1+) and Stat1−/− (CD45.2+) T cells into Rag1−/− mice (Fig. 4a). Notably, all of the T cells identified three weeks post transfer were WT, indicating that the defective expansion of Stat1−/− T cells is cell-intrinsic (Fig. 4b).
Fig. 4.
The defective expansion of Stat1−/− T cells is cell-intrinsic. WT (CD45.1+) or Stat1−/− (CD45.2+) unfractionated CD4+ T cells were injected i.p. at a 1:1 ratio (0.8–1 × 106/type) into Rag1−/− mice. a Schematic of experiment and representative plot of cells injected. b Representative images of CD45.1+ vs CD45.2+ cells (gated on live CD45+ CD3ε+ CD4+ T cells) from various organs followed by their mean frequencies ± SEM. Data is pooled from two independent experiments, with each point representing an individual mouse. ***p < 0.001 by two-tailed Mann–Whitney test. Accompanied by Supplementary Fig. 1
We next asked if the defective expansion of Stat1−/− T cells could be recapitulated in vitro. In contrast with the in vivo defect, Stat1−/− T cells displayed a hyperproliferative phenotype compared to WT T cells upon in vitro stimulation (Supplementary Fig. 1), consistent with earlier reports5,28. This indicates that the expansion defect of Stat1−/− T cells is not cell autonomous and requires an in vivo environment.
Reduced expression of the MHC-I pathway in Stat1−/− T cells
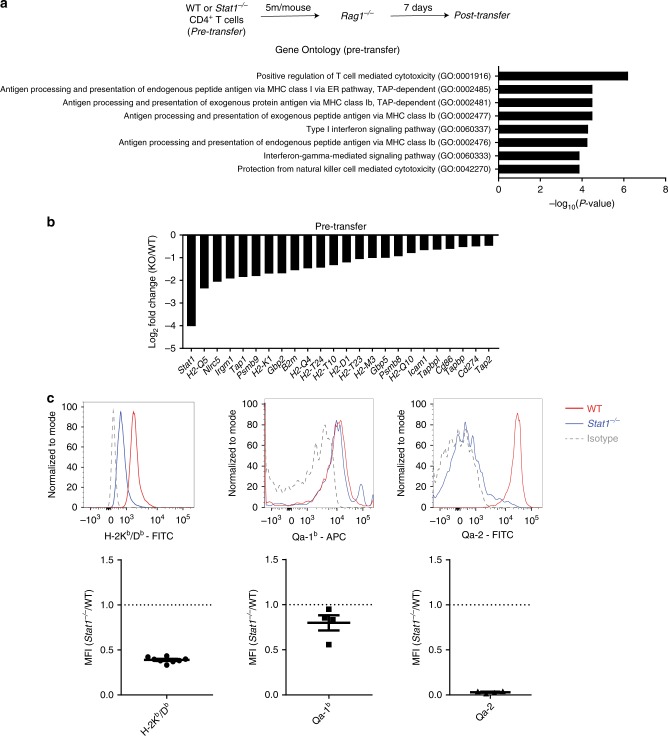
To investigate whether a dysregulated transcriptional profile might account for the observed defect, we performed gene expression analysis on Stat1−/− T cells pre and post transfer into Rag1−/− mice by RNA-seq. As Stat1−/− T cells failed to expand to appreciable amounts after 3 weeks, we transferred a larger number of cells and analyzed gene expression at 1 week post transfer, a time point where Stat1−/− T cells were beginning to decline (Fig. 5a and Supplementary Fig. 2a). Gene ontology (GO) analysis of genes differentially regulated between WT and Stat1−/− T cells revealed, as expected, categories related to Type I and II IFN signaling. Interestingly, categories related to the MHC class I (MHC-I) antigen presentation pathway were significantly enriched in both pre and post-transfer settings (Fig. 5a and Supplementary Fig. 2b). Consistent with the GO analysis, Stat1−/− T cells had reduced expression of Nlrc5, MHC-I (H2-K1, H2-D1, B2m, H2-T23) and various genes involved in MHC-I antigen presentation (Tap1, Tap2, Psmb8, Psmb9) (Fig. 5b and Supplementary Fig. 2c).
Fig. 5.
Downregulation of the MHC class I antigen presentation machinery in Stat1−/− T cells. RNA-seq was performed on WT or Stat1−/− T cells pre-transfer. a Schematic of experimental setup and selected Gene ontology terms (PANTHER) showing differential expression of the MHC class I pathway in Stat1−/− T cells. b Downregulation of various genes involved in MHC class I antigen presentation in Stat1−/− T cells compared to WT T cells by RNA-seq. All genes displayed are significantly different between WT and Stat1−/− T cells (n = 3 biological replicates, p < 0.05 with correction for multiple testing by Benjamini-Hochberg procedure). Accompanied by Supplementary Fig. 2 where similar analyses were performed in T cells post transfer. c Representative flow cytometry plots showing surface expression of classical and non-classical MHC class I molecules on CD4+ T cells from the spleens of WT or Stat1−/− mice, followed by their cumulative enumeration expressed as a ratio of Median Fluorescence Intensity (Stat1−/− / WT) ± SEM. Data is pooled from three or more independent experiments, with each point representing an individual mouse. Similar numbers of WT and Stat1−/− mice were used for the comparison
NLRC5 is a critical transactivator of multiple MHC-I genes, and STAT1 is required to induce its expression by binding to the Nlrc5 promoter in response to IFNγ29–32. Consistent with our RNA-seq data and with earlier reports31,33, Stat1−/− T cells displayed reduced surface levels of the classical MHC-I molecules H-2Kb/H-2Db. Interestingly, surface levels of the non-classical molecule Qa-1 was only mildly affected by STAT1 deficiency whereas levels of Qa-2 were severely reduced (Fig. 5c).
NK depletion rescues Stat1−/− T-cell expansion and colitis
The MHC-I molecule is the classic inhibitory ligand for NK cells, and cellular expression of MHC-I protects cells from NK mediated killing34. Tumors or virally infected cells can reduce MHC-I expression to evade CD8+ T-cell recognition, but this renders them susceptible to NK mediated killing—a phenomenon described as missing self34,35. The reduced expression of MHC-I on Stat1−/− T cells led us to hypothesize that their defective expansion was due to elimination by NK cells, which are present and more active in Rag1−/− mice36. This hypothesis was supported by the GO analysis, which revealed the category: Protection from natural killer mediated cytotoxicity (Fig. 5a and Supplementary Fig. 2b).
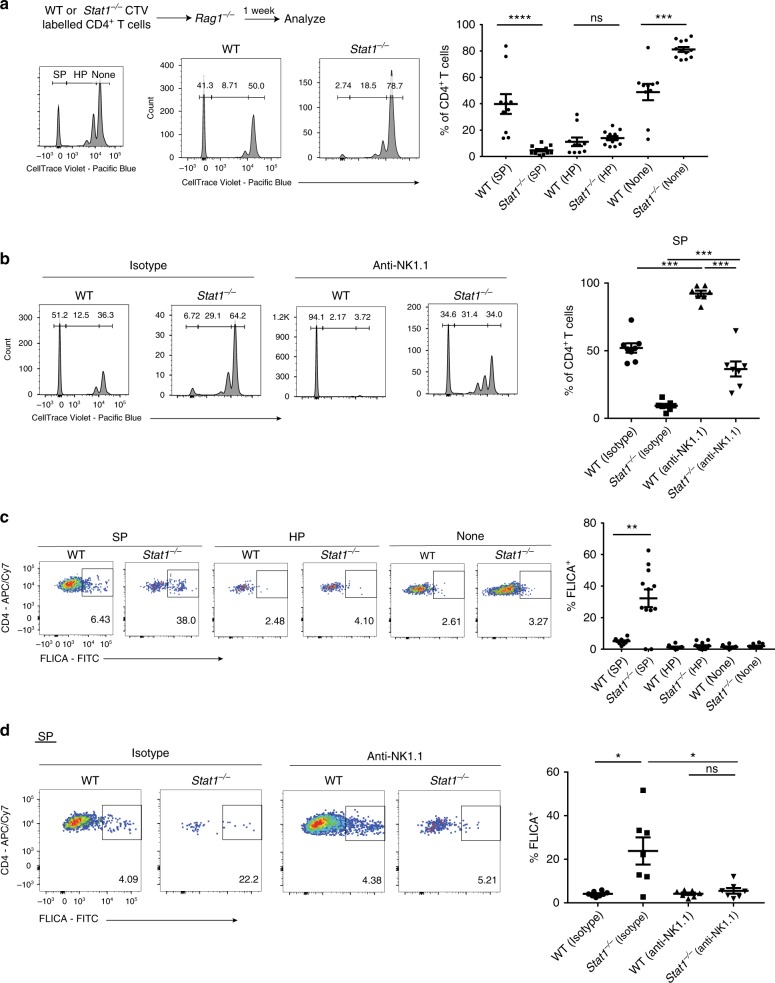
To test the hypothesis that Stat1−/− T cells were eliminated in vivo by NK cells, we depleted NK cells in Rag1−/− mice at the time of T-cell transfer by employing an anti-NK1.1 antibody (Fig. 6a and Supplementary Fig. 3a). In support of the hypothesis, the depletion of NK cells significantly rescued the survival of Stat1−/− T cells (Fig. 6a). We next asked if Stat1−/− T cells were able to induce colitis in the absence of NK cells by transferring them into NK cell-depleted Il10rb−/−Rag1−/− mice. Strikingly, Stat1−/− T cells were able to induce disease in NK cell-depleted Il10rb−/−Rag1−/− mice unlike their control-treated NK cell-replete counterparts (Fig. 6b, c). The induction of disease correlated with a restored expansion of Stat1−/− T cells in the spleen and the colon (Fig. 6d). In agreement with previous reports7, Stat1−/− T cells displayed an enhanced Th17 differentiation profile in vivo (Supplementary Fig. 3b). This differentiation profile was however seen in both control and NK cell-depleted Il10rb−/−Rag1−/− mice, suggesting that the primary role of STAT1 in T-cell-driven colitis is to protect the T cells from NK mediated elimination, rather than to repress their intrinsic Th17 differentiation potential.
Fig. 6.
Depletion of NK cells restores Stat1−/− T-cell expansion and colitis. a 1 × 106 WT or Stat1−/− CD4+ T cells were injected i.p. into Rag1−/− mice that were treated with NK depleting antibody (or isotype control). Schematic of experimental setup, as well as representative flow cytometry plots of CD4+ T cells (gated on live CD45+ non-NK cells, Supplementary Fig. 4c) in the spleen and colon of Rag1−/− mice with their mean frequencies ± SEM at 3 weeks post transfer. b–d Similar to a, but in Il10rb−/−Rag1−/− mice instead of Rag1−/− mice. b Mean % initial body weights ± SEM following T-cell transfer. Source data are provided as a Source Data file. c Representative images of colons, as well as representative H&E images of distal colon sections with mean histological scores ± SEM at 3 weeks post transfer. d Representative flow cytometry plots of CD4+ T cells (gated on live CD45+ non-NK cells, Supplementary Fig. 4c) in the spleen and colon followed by their mean frequencies ± SEM at 3 weeks post transfer. Scale bar represents 200 µm. Data pooled from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by b two-way ANOVA with Bonferroni’s correction (Stat1−/− anti-NK1.1 compared to Stat1−/− Isotype) or by a, c, d two-tailed Mann–Whitney test. ✝ Two mice (WT anti-NK1.1) were sacrificed before the 3-week time point due to excessive weight loss thus their weights only apply till week 2. Accompanied by Supplementary Fig. 3
NK cells target spontaneously proliferating Stat1−/− T cells
T cells undergo two distinct modes of proliferation upon transfer into chronically lymphopenic hosts (e.g., Rag1−/− mice)—slow, true homeostatic proliferation (HP) that is driven primarily by IL-7, as well as rapid, spontaneous proliferation (SP) that is driven by the microbiota and IL-637–41. We asked whether the NK cell-mediated elimination of Stat1−/− T cells requires T-cell proliferation by transferring CellTrace Violet (CTV) labeled WT or Stat1−/− CD4+ T cells into Rag1−/− mice. Stat1−/− T cells displayed a reduction in the SP population compared to WT T cells, with no difference in the HP population (Fig. 7a). Importantly, the depletion of NK cells significantly rescued the Stat1−/− SP population, suggesting that NK cells specifically restrict Stat1−/− T cells undergoing SP (Fig. 7b). Interestingly, this rescue was not complete as we also observed an increase in the SP of WT T cells upon NK cell depletion, which is consistent with the incomplete rescue of Stat1−/− T-cell expansion as well as the degree of colitis induced in Il10rb−/−Rag1−/− mice (Fig. 6).
Fig. 7.
NK cells specifically eliminate Stat1−/− T cells undergoing spontaneous proliferation. Rag1−/− mice were injected with equal numbers (3–4 × 106) of WT or Stat1−/− CTV labeled unfractionated CD4+ T cells and analyzed after 1 week. a Schematic of experiment, as well as representative flow cytometry plots of T cells in the spleen + lymph nodes (gated on live CD45+ CD3ε+ CD4+ cells, Supplementary Fig. 4d) followed by their mean frequencies ± SEM. b, d Similar to a, but with 400μg anti-NK1.1 antibody or Isotype Control injected 1 day prior to T-cell transfer. b CTV profiles of the T cells are shown as well as the mean frequencies ± SEM of the SP population. c Representative images of FLICA staining from the T cell SP, HP and non-proliferating populations in a, as well as their mean frequencies ± SEM. d FLICA staining in the SP population from b shown with mean frequencies ± SEM shown. Pooled from three to four independent experiments, with each point representing an individual mouse. *p < 0.05, **p < 0.01, ***p < 0.001, ***p < 0.0001 by two-tailed Mann–Whitney test
We also assessed for cell death in these populations by staining for activated caspases using FAM-FLICA, a fluorescently conjugated pan-caspase inhibitor. Compared to WT T cells, Stat1−/− T cells displayed increased cell death specifically in the SP population, with no differences in the HP or non-proliferating populations (Fig. 7c). Importantly, the increased cell death in Stat1−/− SP T cells was reversed by NK cell depletion (Fig. 7d). Taken together, these data strongly suggest that NK cells eliminate Stat1−/− T cells when they undergo SP.
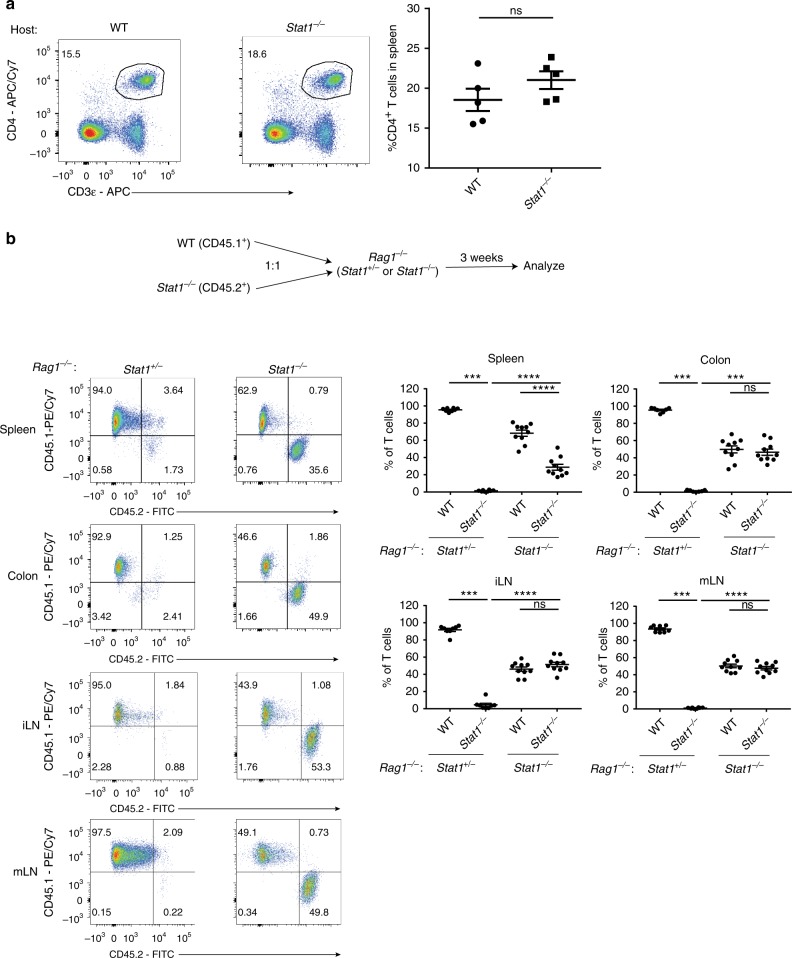
Innate STAT1 expression is needed to reject Stat1−/− T cells
Despite the potent elimination of Stat1−/− T cells upon adoptive transfer into lymphopenic hosts, Stat1−/− mice had normal levels of CD4+ T cells, suggesting additional mechanism(s) in place to prevent their elimination by NK cells (Fig. 8a). As STAT1 is required for NK cells to achieve optimal cytotoxicity42,43, we hypothesized that these T cells were not eliminated in Stat1−/− mice due to a defect in killing by Stat1−/− NK cells. To test this hypothesis, we deleted Stat1 in the innate compartment by generating Stat1−/−Rag1−/− mice and transferred congenically marked WT (CD45.1+) and Stat1−/− (CD45.2+) T cells into them. Whereas Stat1−/− T cells were efficiently depleted in the Stat1+/− Rag1−/− littermate controls, deletion of Stat1 in the innate compartment restored the expansion of Stat1−/− T cells (Fig. 8b). This indicates that the elimination of Stat1−/− T cells is dependent on innate STAT1 signaling.
Fig. 8.
Innate STAT1 expression is required to eliminate Stat1−/− T cells. a Representative flow cytometry plots of CD4+ T cells in the spleen of WT and Stat1−/− mice followed by their mean frequencies ± SEM. b WT (CD45.1+) or Stat1−/− (CD45.2+) CD4+ T cells were injected i.p. at a 1:1 ratio (1 × 106/type) into Stat1−/−Rag1−/− mice or their Stat1+/−Rag1−/− littermate controls and analyzed after 3 weeks. Representative images of CD45.1+ vs CD45.2+ cells (gated on live CD45+ CD3ε+ CD4+ T cells) from various organs are shown followed by their mean frequencies ± SEM. Pooled from three independent experiments, with each point representing an individual mouse. ***p < 0.001, ****p < 0.0001 by two-tailed Mann–Whitney test
Discussion
In this study we have identified a critical role for STAT1 in T-cell survival, where STAT1 signaling, through the upregulation of Nlrc5 and MHC-I, protects T cells from NK cell-mediated elimination in vivo. We also show that this is important in the setting of T-cell-mediated immunopathology, as Stat1−/− T cells can induce colitis if allowed to survive and expand in a NK-deficient environment.
In T cells, most studies on the JAK-STAT pathway have focused on its effects on T-cell differentiation, with STAT1 promoting Th1 differentiation (through the induction of T-bet) and inhibiting Th17 differentiation3,4,7. In IBD, previous studies on STAT1 signaling in T cells focused on the STAT1-dependent transcription factor T-bet13,23. While it was noted that Stat1−/− T cells were unable to cause colitis, the profile of Stat1−/− T cells in vivo was not analyzed and STAT1 was assumed to act in a similar fashion as T-bet13. We observe that in our model of colitis17, STAT1 modulates the disease outcome primarily by promoting T-cell survival rather than altering differentiation, as Stat1−/− T cells displayed similar differentiation profiles in both control and NK-depleted Il10rb−/−Rag1−/− hosts (Supplementary Fig. 3b).
While we have focused mainly on IBD, we believe that the STAT1-mediated protection from NK cell killing is relevant for several T-cell-mediated inflammatory disorders. STAT1 induces MHC-I expression by inducing the transcription of Nlrc529–32, and deletion of Nlrc5 in T cells to reduce MHC-I expression leads to their rejection by host NK cells during viral infection44. In the case of STAT1 deficiency, while several groups have reported a reduced expansion of Stat1−/− T cells in animal models of autoimmune disease and GvHD, the mechanistic basis for this reduction was unclear5,9. We propose that, similar to IBD, NK cells might also play a prominent role in restraining the ability of Stat1−/− T cells to induce these inflammatory disorders. This is analogous to studies in tumor biology, where tumor cells utilize STAT1 expression to avoid rejection in vivo43. Our finding that only Stat1−/− T cells undergoing SP are targeted by NK cells suggests that NK cells restrict T cells only when they are activated (Fig. 7). This is in agreement with earlier studies where Ifnar1−/− antiviral T cells are only eliminated by NK cells when the mice are virally infected26,45 and suggests that a similar mechanism might be used to regulate commensal-driven T-cell responses. Recently, it was reported that overexpression of STAT1 in T cells inhibits their expansion in lymphopenic mice, which led to the suggestion of targeting STAT1 to enhance T-cell numbers in clinical settings of lymphopenia like bone marrow transplantation and HIV infection46. Our findings suggest that this approach will have to be balanced with ensuring that there is sufficient MHC-I expression to protect T cells from being targeted by NK cells.
The upstream signal(s) that activates the STAT1-NLRC5-MHC class I axis in T cells in vivo has not been fully elucidated. In vitro, Nlrc5 expression in T cells is primarily triggered by autocrine IFNγ signaling31. In vivo, type I IFN has been reported to protect antiviral T cells and NK cells from NK mediated elimination during LCMV infection26,45,47. Our data stands in contrast with these studies, showing that deletion of both Type I and Type II IFN receptors fails to fully recapitulate the defective survival of Stat1−/− T cells in the setting of IBD (Fig. 3). This is consistent with an earlier report showing that type I IFN signaling is not required for naïve T cells to induce colitis in Rag−/− hosts48, but we further extend this observation to include Type II IFN signaling. What are the IFN-independent signals that might account for the discrepancy between Ifnar1−/−Ifngr1−/− and Stat1−/− T cells? IL-7 has been proposed as a possible candidate, being able to induce STAT1 activation in T cells in vitro and in vivo33,46 as well as MHC-I in vitro33. Therefore, in addition to the conventional STAT5-driven proliferative and pro-survival response49, IL-7 might activate STAT1 signaling to induce MHC-I for protection from NK cells. However, our finding that NK cells specifically eliminate Stat1−/− T cells undergoing SP and not the IL-7 driven HP argues against this hypothesis (Fig. 7). IL-6, which has been reported to be important in driving SP, also activates STAT140,41,50. However, a recent report showed that IL-6R deficient T cells only display defective expansion in Rag1−/− mice when there is colonic inflammation51, which is in contrast to our observations with Stat1−/− T cells (Fig. 2). It is possible that the STAT1-dependent signal is provided by multiple cytokines, including IL-6 and type I+II IFN. Alternatively, the maintenance of MHC-I levels might be driven by tonic STAT1 signaling that is independent of any upstream cytokine engagement.
The ability of NK cells to restrict T-cell expansion has been noted in mouse models of infection52 and IBD53,54, and our data and others26,45 suggest that STAT1 signaling plays a critical role in this regulation. However, the extent of STAT1 dependency might be different in different disease contexts. While Stat1−/− T cells are very efficiently eliminated in our study and others9, the degree of reduction in expansion of these T cells are not as pronounced in GvHD5. Moreover, in a recent report employing a mouse model of EAE, Stat1−/− T cells induced worse disease than WT T cells, although it is unclear whether they had an expansion defect55. It is thus possible that in certain disease contexts the inflammatory environment can provide STAT1-independent signals to the T cells to protect them from NK cells. Alternatively, the NK cells might be altered in these settings toward reduced cytotoxicity.
We observe that Stat1−/− T cells fail to expand in both Rag1−/− and Il10rb−/−Rag1−/− mice (Figs. 1, 2), suggesting that IL-10Rβ signaling is not required for NK cells to eliminate Stat1−/− T cells. However, earlier work has shown roles for IL-10 and IFNλ/IL-28—both of which signal via the IL-10Rβ chain—in stimulating NK cells56–58. It is possible that Il10rb−/− NK cells might still have sufficient cytotoxic ability to eliminate Stat1−/− T cells, as IL-28R deficient NK cells are only partially defective58. Alternatively, there might be other mechanisms in Il10rb−/−Rag1−/− mice that compensate for this defect, such as IL-10R deficiency in macrophages which promotes a proinflammatory environment17 and/or RAG1 deficiency which has been shown to lead to NK cell hyperresponsiveness36.
In our study, we also show that innate STAT1 signaling is required to eliminate Stat1−/− T cells, which would explain why Stat1−/− mice have normal levels of T cells (Fig. 8). We believe that this is likely due to the impaired cytotoxic capability of Stat1−/− NK cells as previously reported42,43. However, we do not exclude the possibility that this can also be due to altered NK education, where NK cells are educated to recognize the low level of MHC-I on Stat1−/− T cells as normal.
In summary, we describe a critical role for STAT1 in promoting T-cell survival by maintaining sufficient MHC class I expression to evade NK cell-mediated killing. This mechanism is largely IFN-independent and is critical in enabling T cells to induce intestinal inflammation. Our findings shed a new light on JAK-STAT signaling in T cells, adding critical functions for this pathway beyond T-cell differentiation that have potential therapeutic implications for IBD and other T-cell-mediated inflammatory disorders.
Methods
Mouse strains
C57BL/6J (Strain 000664), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1, Strain 002014), B6.129S7-Rag1tm1Mom/J (Rag1−/−, Strain 002216), B6.129S(Cg)-Stat1tm1Dlv (Stat1−/−, Strain 012606), B6.Cg-Ifngr1tm1AgtIfnar1tm1.2Ees/J (Ifnar1−/−Ifngr1−/−, Strain 029098) mice were purchased from Jackson Labs. Il10rb−/−Rag1−/− mice were generated by crossing Il10rb−/− mice (a gift from Thaddeus Stappenbeck, Washington University) with Rag1−/− mice. Stat1−/−Rag1−/− mice were generated by crossing Stat1−/− mice with Rag1−/− mice. All mice were on the B6 background and maintained in a specific pathogen-free animal facility in Boston Children’s Hospital. All experiments were conducted after approval from the Animal Resources at Children’s Hospital and according to regulations by the Institutional Animal Care and Use Committee (IACUC).
Adoptive T-cell transfer and colitis induction
In T-cell transfer experiments, unfractionated CD4+ T cells were isolated from the spleens and lymph nodes of donor mice (WT, CD45.1, Stat1−/−, Ifnar1−/−Ifngr1−/−) by negative selection (Miltenyi Biotec CD4+ T-cell isolation Kit, Cat No. 130-104-454). 1 × 106 T cells (92.7–98.6% pure) were then adoptively transferred into recipient mice (Rag1−/−, Il10rb−/−Rag1−/−) by i.p. injection in PBS unless otherwise stated. In some experiments, CD45.1+ T cells and Stat1−/− T cells were mixed at a 1:1 ratio before being transferred into recipient mice (Rag1−/−, Stat1−/−Rag1−/−). In some experiments, T cells were labeled with 5 μM CellTrace Violet (Thermo Fisher) in PBS + 0.1% FBS for 10 min at 37 °C prior to injection. All recipient mice were at least 6 weeks old and matched for sex, age, and housing between groups. Il10rb−/−Rag1−/− mice were monitored weekly for body weight changes post T cell transfer. For NK depletion assays, each mouse was first injected with 400 µg anti-NK1.1 (or isotype control) 1 day prior to T-cell transfer. For experiments lasting beyond 1 week, depletion of NK cells was maintained by injections of 200 µg anti-NK1.1 (or isotype control) at 1 and 2 weeks post transfer. All antibody injections were administered i.p in InVivoPure pH 7.0 Dilution Buffer (BioXCell).
Histological scoring
To evaluate signs of histological inflammation, sections of distal colons were stained in haematoxylin and eosin and scored in a blinded fashion. Scoring was based on histological evidence of crypt hyperplasia (0–3), inflammatory cell infiltration (0–3) and presence of crypt abscesses (0–2), summed up to give the overall score (0–8). Representative images were acquired using an Olympus BX41 upright microscope with DP70 color CCD (Fig. 1) or a Keyence automated epifluorescent microscope (Figs. 3, 6).
Isolation of colonic lamina propria cells
Cells were isolated from the lamina propria as described17. Briefly, the large intestine (colon + cecum) was removed, cut open longitudinally and then into small sections before being incubated in Hank’s balanced salt solution (HBSS) containing 0.5% fetal bovine serum (FBS), 10 mM EDTA, 1.5 mM dithiothreitol and 10 mM HEPES at 37 °C for 35 min with agitation to remove the epithelial cell layer. After the removal of the epithelial cells, tissues were washed in PBS, finely diced and incubated in HBSS buffer (w Ca/Mg) containing 20% FBS, 10 mM HEPES, 1.5 mM CaCl2 and collagenase VIII (200 U/ml) at 37 °C for 40 min with agitation. Tissues were then repeatedly flushed through a 10 ml syringe and further incubated for 15 min. Digested tissues were filtered, washed in PBS and used for flow cytometry.
In vitro T-cell proliferation
Unfractionated CD4+ T cells were first labeled with 5 µM CFSE for 5 min at room temperature and washed repeatedly with PBS containing FBS. They were then cultured in 96-well flat-bottom plates containing plate-bound anti-CD3ε (3 µg/ml, eBioscience) and soluble anti-CD28 (1 µg/ml, eBioscience) for 3 days. T cells were cultured in DMEM containing 10% FBS, L-glutamine, pyruvate, non-essential amino acids, MEM vitamins, L-arginine, L-asparagine, folic acid, β-mercaptoethanol and pen/strep.
Reagents
For flow cytometric staining, antibodies against the following were used (Clone name, dilution, manufacturer and catalog number in brackets): CD3ε (145-2C11, 1:300–400, Biolegend #100312/100306), TCRβ (H57-597, 1:400, Biolegend #109222), CD4 (GK1.5, 1:300 Biolegend #100414), NKp46 (29A1.4, 1:50, Biolegend #137604), CD49b (HMα2, 1:200 Biolegend #103517), CD45 (30-F11, 1:500, Biolegend #103140), H2-Kb/Db (28-8-6, 1:100, Biolegend #114606/114607), Qa-2 (695H1-9-9, 1:100, Biolegend #121709), Qa-1b (6A8.6F10.1A6, 1:10, Miltenyi Biotec #130-104-220), CD16/32 (93, 0.5 μg/106 cells, Biolegend #101302), IL-17A (TC11-18H10.1, 1:125, Biolegend 506904), IFNγ (XMG1.2, 1:200, Biolegend #505809/eBioscience #17-7311-82), Mouse IgG2a, κ Isotype Ctrl (MOPC-173, 1:100, Biolegend #400207/400211), Mouse IgG1, κ Isotype Ctrl (MOPC-21, 1:66.7, Biolegend #400119), CD45.1 (A20, 1:300, Biolegend #110729), CD45.2 (104, 1:300, Biolegend #109806). For T-cell stimulation, antibodies against CD3ε (145-2C11, 3 μg/ml, eBioscience #16-0031-86) and CD28 (37.51, 1 μg/ml, eBioscience #16-0281-85) were used. For NK depletion assays, antibodies against NK1.1 (PK136, BioXCell #BP0036) or Isotype Control (C1.18.4, BioXCell #BP0085) were used.
Flow cytometry
For flow cytometry and sorting experiments, cells were stained in flow cytometric staining buffer (2% FBS plus 0.1% NaN3 in PBS) and MACS buffer (0.5% BSA and 2 mM EDTA in PBS), respectively. For antibody staining of surface markers, cells were incubated with anti-CD16/32 (Biolegend) for 10 min at room temperature to block Fc receptors, before being incubated with antibodies for 20–30 min at 4 °C. Cells were also incubated with Zombie Violet Fixable Viability Dye (1:400, Biolegend) or 7-AAD (1:20, BD Biosciences) according to the manufacturer’s instructions to identify and exclude dead cells. For intracellular cytokine staining, cells were incubated with PMA (50 ng/ml), ionomycin (500 ng/ml), and GolgiStop (1:1000, BD Biosciences) for 4 h at 37 °C. After staining for surface markers, cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), followed by staining in Perm/Wash buffer (BD Biosciences) according to the manufacturer’s instructions. For assessment of cell death, cells were stained with the FAM-FLICA Poly Caspase Kit (ImmunoChemistry Technologies) for 1 h at 37 °C in T-cell media prior to antibody surface staining. All samples were acquired with a BD Canto II or LSRFortessa Flow Cytometer (BD Biosciences) and analyzed with FlowJo (FlowJo, LLC).
RNA sequencing
In the post-transfer setting, WT or Stat1−/− T cells (gated as CD45+ CD3ε+ CD4+, Supplementary Fig. 2d) were FACS sorted from the spleen and lymph nodes of Rag1−/− mice post transfer directly into RLT lysis buffer (Qiagen) and RNA extracted using the RNeasy Micro kit (Qiagen). As Stat1−/− T cells showed reduced survival/expansion in vivo, it was not technically feasible to acquire sufficient cells for purity analysis by flow cytometry, hence purity was determined by confirming the downregulation of Stat1 in the Stat1−/− T cells. Library preparation, RNA-seq and analysis were performed at the Molecular Biology Core Facility (MBCF) of Dana-Farber Cancer Institute, Boston, using the Clontech SMARTer v4 kit for mRNA library generation and the Illumina NextSeq 500 Platform (Single-end 75 bp) for sequencing. The data was analyzed using the VIPER algorithm59, with reads aligned to the mouse mm9 genome using STAR, transcripts assembled with Cufflinks and differential analysis performed with DESeq2. Gene Ontology analysis was performed using the PANTHER Overrepresentation test (http://www.geneontology.org/). The raw and processed data for RNA sequencing are deposited in the NCBI GEO database under GSE116475.
Statistical analysis
Statistical analyses were performed with GraphPad Prism software using two-way ANOVA with Bonferroni’s multiple comparisons test, two-tailed Mann–Whitney test or two-tailed t-test as indicated in the figure legends. Significance was defined as p-value < 0.05 using the following notations: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Jeremy Goettel for assistance in histological scoring, Ryan Kelly for technical assistance with the experiments and Jon Kagan, Ivan Zanoni and Bruce Horwitz for helpful discussions. We also thank the Dana-Farber Cancer Institute Flow Cytometry Core for cell sorting and the DFCI Molecular Biology Core Facilities - Genomics for RNA-seq and analysis. Y.H.K. is supported by an A*STAR National Science Scholarship, Singapore. A.B. is supported by the CCFA Career Development Award (327200) and a NIH KO1 award (K01DK109026). S.B.S. is supported by NIH Grants DK034854, and AI50950, the Helmsley Charitable Trust, and the Wolpow Family Chair in IBD Treatment and Research.
Author contributions
Y.H.K. and S.B.S. conceived the study. Y.H.K., A.B., and S.B.S. designed the experiments. Y.H.K., M.F. and A.B. performed the experiments, acquired and analyzed the data. Y.H.K. and S.B.S. wrote the manuscript.
Data availability
All data in this study are available from the corresponding author upon reasonable request. RNAseq data has been deposited in the GEO under GSE116475. A reporting summary for this Article is available as a Supplementary Information file, as well as a Source Data file with the source data underlying the weight curves in Figs. 1a, 3b, and 6b where individual data points are not displayed.
Competing interests
S.B.S. declares the following interests: Scientific advisory board participation for Pfizer, Janssen, Celgene, IFM therapeutics, and Pandion Inc. Grant support from Pfizer, Janssen, Merck. Consulting for Hoffman La Roche and Amgen. The remaining authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-019-08743-8.
References
- 1.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgoffe GM, Vignali DAA. STAT heterodimers in immunity A mixed message or a unique signal? JAK-STAT. 2013;2:e23060. doi: 10.4161/jkst.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M, et al. T-bet is a STAT1-induced regulator for IL-12R expression in naïve CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 4.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 5.Ma H, et al. Absence of Stat1 in donor CD4+ T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J. Clin. Invest. 2011;121:2554–2569. doi: 10.1172/JCI43706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarino AV, Gallo E, Abbas AK. STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J. Immunol. 2010;185:6461–6471. doi: 10.4049/jimmunol.1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neurath MF. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Cho JH. Inflammatory bowel disease. N. Engl. J. Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mudter J, et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 13.Neurath MF, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 15.Spencer SD, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. IL-10 and IL-10 receptor defects in humans. Ann. N. Y. Acad. Sci. 2011;1246:102–107. doi: 10.1111/j.1749-6632.2011.06339.x. [DOI] [PubMed] [Google Scholar]
- 17.Shouval DS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigmond E, Bernshtein B, Friedlander G. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Shouval DS, et al. Interleukin 1β mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterology. 2016;151:1100–1104. doi: 10.1053/j.gastro.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coccia M, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, et al. IL-10 engages macrophages to shift Th17 cytokine dependency and pathogenicity during T-cell-mediated colitis. Nat. Commun. 2015;6:1–13. doi: 10.1038/ncomms7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shouval DS, et al. Enhanced TH17 responses in patients with IL10 receptor deficiency and infantile-onset IBD. Inflamm. Bowel Dis. 2017;23:1950–1961. doi: 10.1097/MIB.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 23.Krausgruber T, et al. T-bet is a key modulator of IL-23-driven pathogenic CD4+ T cell responses in the intestine. Nat. Commun. 2016;7:11627. doi: 10.1038/ncomms11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava S, Koch LK, Campbell DJ. IFNαR signaling in effector but not regulatory T cells is required for immune dysregulation during type I IFN-dependent inflammatory disease. J. Immunol. 2014;193:2733–2742. doi: 10.4049/jimmunol.1401039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crouse J, et al. Type I interferons protect T cells against NK Cell Attack Mediated by the Activating Receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Overacre-Delgoffe, A. E. et al. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell169, 1130–1141 (2017). [DOI] [PMC free article] [PubMed]
- 28.Lee CK, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-γ. J. Immunol. 2000;164:1286–1292. doi: 10.4049/jimmunol.164.3.1286. [DOI] [PubMed] [Google Scholar]
- 29.Meissner TB, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl Acad. Sci. USA. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuenzel S, et al. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J. Immunol. 2010;184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 31.Staehli F, et al. NLRC5 deficiency selectively impairs MHC class I-dependent lymphocyte killing by cytotoxic T cells. J. Immunol. 2012;188:3820–3828. doi: 10.4049/jimmunol.1102671. [DOI] [PubMed] [Google Scholar]
- 32.Tong Y, et al. Enhanced TLR-induced NF-κB signaling and type I interferon responses in NLRC5 deficient mice. Cell Res. 2012;22:822–835. doi: 10.1038/cr.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BC, et al. Differential regulation of constitutive major histocompatibility complex class I expression in T and B lymphocytes. J. Exp. Med. 1999;190:1451–1464. doi: 10.1084/jem.190.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 35.Yewdell J, Bennink JR. Mechanisms of viral interference with MHC class I antigen processing and presentation. Annu. Rev. Cell. Dev. Biol. 1999;15:579–606. doi: 10.1146/annurev.cellbio.15.1.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karo JM, Schatz DG, Sun JC. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell. 2014;159:94–107. doi: 10.1016/j.cell.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Booki Min P, Yamane H, Hu-Li J, Min B, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 39.Kieper WCW, et al. Cutting edge: recent immune status determines the source of antigens that drive homeostatic T cell expansion. J. Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 40.Tajima M, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8 + T cells. J. Exp. Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CK, et al. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- 43.Kovacic B, et al. STAT1 acts as a tumor promoter for leukemia development. Cancer Cell. 2006;10:77–87. doi: 10.1016/j.ccr.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Ludigs, K. et al. NLRC5 shields T lymphocytes from NK-cell-mediated elimination under inflammatory conditions. Nat. Commun. 7, 10554 (2016). [DOI] [PMC free article] [PubMed]
- 45.Xu HC, et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Le Saout, C. et al. IL-7-dependent STAT1 activation limits homeostatic CD4+ T cell expansion. JCI Insight2, 1–18 (2017). [DOI] [PMC free article] [PubMed]
- 47.Madera S, et al. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 2016;213:225–233. doi: 10.1084/jem.20150712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kole A, et al. Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis. J. Immunol. 2013;191:2771–2779. doi: 10.4049/jimmunol.1301093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin. Immunol. 2012;24:209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lütticken C, et al. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- 51.Li B, Jones LL, Geiger TL. IL-6 promotes T cell proliferation and expansion under inflammatory conditions in association with low-level RORγt expression. J. Immunol. 2018;201:2934–2946. doi: 10.4049/jimmunol.1800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fort MM, Leach MW, Rennick DM. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J. Immunol. 1998;161:3256–3261. [PubMed] [Google Scholar]
- 54.Yamaji O, et al. The development of colitogenic CD4+ T cells is regulated by IL-7 in collaboration with NK cell function in a murine model of colitis. J. Immunol. 2012;188:2524–2536. doi: 10.4049/jimmunol.1100371. [DOI] [PubMed] [Google Scholar]
- 55.Meyer zu Horste G, et al. Fas promotes T helper 17 cell differentiation and inhibits T helper 1 cell development by binding and sequestering transcription factor STAT1. Immunity. 2018;48:556–569.e7. doi: 10.1016/j.immuni.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur. J. Immunol. 1999;29:2658–2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 57.Mocellin S, et al. IL-10 stimulatory effects on human NK cells explored by gene profile analysis. Genes Immun. 2004;5:621–630. doi: 10.1038/sj.gene.6364135. [DOI] [PubMed] [Google Scholar]
- 58.Souza-Fonseca-Guimaraes F, et al. NK cells require IL-28R for optimal in vivo activity. Proc. Natl Acad. Sci. USA. 2015;112:E2376–E2384. doi: 10.1073/pnas.1424241112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornwell MI, et al. VIPER: visualization pipeline for RNA-seq, a Snakemake workflow for efficient and complete RNA-seq analysis. BMC Bioinforma. 2018;19:1–14. doi: 10.1186/s12859-018-2139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this study are available from the corresponding author upon reasonable request. RNAseq data has been deposited in the GEO under GSE116475. A reporting summary for this Article is available as a Supplementary Information file, as well as a Source Data file with the source data underlying the weight curves in Figs. 1a, 3b, and 6b where individual data points are not displayed.