Abstract
The recent decade has witnessed a growing demand to substitute synthetic materials with naturally-derived platforms for minimizing their undesirable footprints in biomedicine, environment, and ecosystems. Among the natural materials, cellulose, the most abundant biopolymer in the world with key properties, such as biocompatibility, biorenewability, and sustainability has drawn significant attention. The hierarchical structure of cellulose fibers, one of the main constituents of plant cell walls, has been nanoengineered and broken down to nanoscale building blocks, providing an infrastructure for nanomedicine. Microorganisms, such as certain types of bacteria, are another source of nanocelluloses known as bacterial nanocellulose (BNC), which benefit from high purity and crystallinity. Chemical and mechanical treatments of cellulose fibrils made up of alternating crystalline and amorphous regions have yielded cellulose nanocrystals (CNC), hairy CNC (HCNC), and cellulose nanofibrils (CNF) with dimensions spanning from a few nanometers up to several microns. Cellulose nanocrystals and nanofibrils may readily bind drugs, proteins, and nanoparticles through physical interactions or be chemically modified to covalently accommodate cargos. Engineering surface properties, such as chemical functionality, charge, area, crystallinity, and hydrophilicity, plays a pivotal role in controlling the cargo loading/releasing capacity and rate, stability, toxicity, immunogenicity, and biodegradation of nanocellulose-based delivery platforms. This review provides insights into the recent advances in nanoengineering cellulose crystals and fibrils to develop vehicles, encompassing colloidal nanoparticles, hydrogels, aerogels, films, coatings, capsules, and membranes, for the delivery of a broad range of bioactive cargos, such as chemotherapeutic drugs, anti-inflammatory agents, antibacterial compounds, and probiotics.
Keywords: Nanocellulose, cellulose nanocrystals, hairy nanocellulose, bacterial cellulose, cellulose nanofibrils, drug delivery, wound healing, cancer therapy
Graphical Abstract
Introduction
Cellulose is the most abundant biopolymer in the world, which is made up of linear glucose rings connected to each other through β(1 → 4) glycosidic bonds [1]. Bengt G. Rånby, whose early observations in 1951 pioneered the field of nanocelluloses [2], noted that the strong acid treatment of cellulose fibers almost always resulted in the formation of nanoscale needle-like colloidal particles (initially called micelles). The breakdown of cellulose fibers into dispersed nanowhiskers with width ~ 7 nm and varying length based on the degree of polymerization provides an indirect evidence for heterogeneity and the hierarchical structure of cellulose fibrils. This observation attests to the coexistence of regions prone to acid-mediated hydrolysis and regions resistant against the chemical modification. Later, this was attributed to the difference in crystallinity along the cellulose fibrils made up of alternating regions of crystalline and amorphous (disordered) regions [3]. The crystalline regions are difficult to penetrate; therefore, the reactants can only modify its surface, eventually resulting in the dissolution of the outer layer, whereas the amorphous layers are readily accessible [4] for facile chemical modification [5–9].
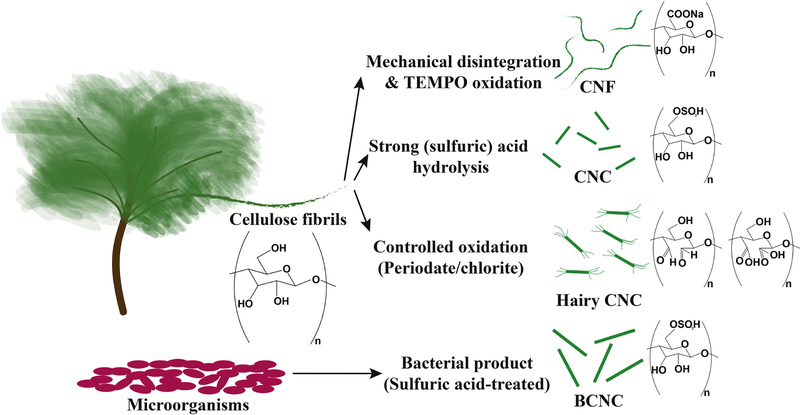
Nanocelluloses may be categorized into four main groups: cellulose nanofibrils (CNF), cellulose nanocrystals (CNC), hairy CNCs (HCNC), and bacterial CNCs (BCNC). Cellulose fibers undergoing rigorous mechanical shear experience defibrillation, yielding nanoscale fibrils with dimensions in the range of several nanometers in diameter and up to tens of microns in length, named cellulose nanofibrils [10–13]. Chemical modification of amorphous chains may be conducted through strong acid hydrolysis to remove the amorphous regions, resulting in CNC with width ~3–70 nm (typically less than 10 nm) and length ~ 25–500 nm (typically between 100200 nm) regulated by the fiber source [7]. The structure of CNF resembles spaghetti with a higher aspect ratio than rice grain-like CNCs. Recently, hairy cellulose nanocrystals have been produced by oxidizing and solubilizing the amorphous regions of fibrils. Periodate oxidation of fibers yields dialdehyde-modified cellulose fibrils, which can be heated at 50 ºC to increase the solubility of modified amorphous biopolymers. The disintegration of these fibrils into CNCs sandwiched between two layers of protruding dialdehyde cellulose chains yields neutral hairy cellulose nanocrystals, also known as sterically-stabilized cellulose nanocrystals. In another scenario, when the dialdehyde-modified cellulose fibrils are further oxidized with chlorite at room temperature, the fibrils readily fall apart in the solution, yielding CNCs attached to highly dicarboxylated protruding cellulose chains at both ends. This family of nanocelluloses has been named anionic hairy cellulose nanocrystals or electrosterically stabilized cellulose nanocrystals [8,9]. Another type of cellulose named bacterial cellulose (BC) is produced by organisms, such as bacteria [14,15]. BC is typically produced as fibers, which may chemically be treated by strong acids and disintegrated into nanocrystals known as bacterial CNC [16]. BC typically attains a high degree of crystallinity and longer crystalline body than conventional CNCs produced from wood fibers. Figure 1 summarizes nanocelluloses and their common synthesis methods.
Figure 1.
Nanocelluloses and examples of their production methods. Plants and microorganisms are two main sources of cellulose. Cellulose fibrils (often chemically pre-treated, e.g., with TEMPO) are disintegrated into nanoscale fibrils, named cellulose nanofibrils (CNF) through rigorous mechanical processing (such as microfluidization and high-pressure homogenization) [13]. When cellulose fibrils are treated with a strong acid, such as sulfuric acid, the amorphous regions of fibrils are hydrolyzed, yielding needle-shaped crystals known as cellulose nanocrystals (CNC) [17]. Moreover, instead of removing amorphous regions, when they are solubilized through oxidation, cellulose fibrils fall apart into CNCs sandwiched between two protruding layers of functionalized cellulose chains. This class of newly emerged nanocelluloses is called hairy CNCs [8,9,18]. Some microorganisms, such as bacteria Acetobacter xylinum, also produce cellulose with a high purity, which may further be processed with a strong acid to yield bacterial CNCs (BCNC) [14,16]. Note that other sources of nanocelluloses are tunicates and algae [19].
Nanocelluloses play promising roles in a broad range of applications [20,21], spanning from water treatment [22–24], environment and energy [25–27], catalysis [28], optics and photonics [29–31], sensing and biosensing [32], and biomedicine [33–38]. There was a limited literature on cellulose-based nanomedicine before 2014, which mainly focused on nanocellulose- and dissolved cellulose-base colloidal dispersions, colloidal hydrogels, and films for binding various types of molecules, such as chemotherapeutic agents to decrease their diffusion rate. Small-scale structural features of nanocelluloses have been the focus of research for developing drug delivery systems. In addition, biocompatibility and cytotoxicity [39] have sometimes been evaluated when new combinations of molecules have been added to nanocelluloses.
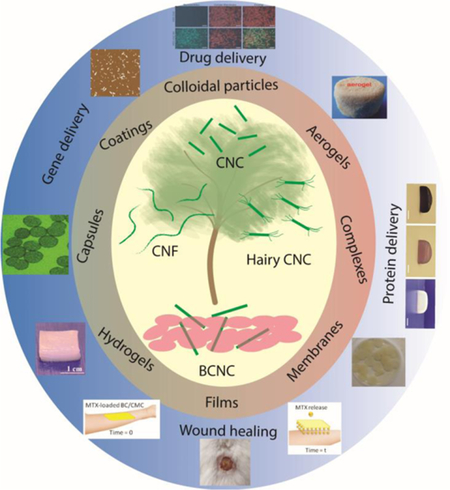
Here, we focus on the state-of-the-art nanocellulose-based cargo delivery systems, which have mainly emerged since 2014. The articles before 2014 are briefly summarized in each section, and relevant review papers are highlighted to provide a brief overview on the early cellulose-based nanomedicine research. We thoroughly review the recent advances in the synthesis, fabrication, and functionalization of biomaterials based on cellulose nanocrystals (non-hairy and hairy), bacterial cellulose, and cellulose nanofibrils for the delivery of a wide range of cargos, such as drugs, proteins, and antimicrobial agents and highlight various forms of nanocellulose-based delivery systems, including hydrogels, films, and aerogels. This paper provides a comprehensive review with a forward-looking approach on the nanocellulose-mediated cargo delivery platforms for nanomedicine, which have emerged in the past four years.
1. Nanocellulose properties
Physical, mechanical, chemical, and biological properties of nanocelluloses have been thoroughly reviewed in the literature. Here, we briefly explain the main characteristics of nanocelluloses to emphasize how this class of nanomaterials may be beneficial in biomedical applications, particularly for the controlled release of active substances. Depending on the cellulose source, key properties of nanocelluloses, such as shape, size, and crystallinity may vary [7,40–42]. Nanocelluloses sourced from organisms, such as bacteria and algae, are typically more pure (free from other polysaccharides, such as lignin and hemicellulose) and have a higher crystalline index (>90%) than those produced from plants (<70%) [5,43]. Accordingly, cellulose sources regulate the properties of nanocelluloses, such as density, porosity, hydrophilicity, and water absorption. An interesting example is porous Cladophora (green algae) cellulose with an extremely large surface area (> 100 m2 g−1) [44] compared to plant-derived nanocelluloses [45]. The aspect ratio (length-to-width ratio) of CNFs (which can be > 500) is noticeably higher than cellulose crystals (CNC, HCNC, or BCNC, typically < 200), while the width of all four types of nanocelluloses is typically in the order of 5–10 nm [5].
Hydrogen bonding plays an important role in the mechanical properties of nanocelluloses. According to the theoretical calculations, when hydrogen bonding is taken into consideration, the longitudinal modulus of cellulose I ~ 173 GPa, whereas it reduces to ~ 71 GPa without intramolecular hydrogen bonding [46,47]. Accordingly, hydrogels and aerogels [48] prepared from nanocelluloses typically benefit from high mechanical strength. Besides mechanical properties, the rheological characteristics of nanocelluloses are important in biomedical applications. Nanocelluloses have unique rheological properties, regulated by their surface chemistry, morphology, and colloidal ordering (e.g., liquid crystalline and gel states) [40,45,49–55]. At high enough concentrations, nanocelluloses form physical hydrogels, which are typically shear-thinning at particle concentrations lower than the critical phase transition to an ordered suspension [56].
The key contributions of nanocelluloses to nanomedicine would not be possible without the chemical modification of CNCs, HCNCs, BCNCs, and CNFs to modulate their functionality and the effective binding of target compounds for controlled release. An overview of the chemical reactions of nanocelluloses has been provided in literature [57], highlighting oxidation as one of the most common modification methods. By oxidizing the hydroxyl groups, which may be followed by reductive amination and esterification reactions, bioactive compounds may be covalently attached to nanocelluloses. Self-assembly of nanocelluloses is another, often less noticed, method to bind active molecules. For example, Varjonen et al., [58] and Valo et al., [59] attached cargos such as beclomethasone dipropionate (BDP) and itraconazole (ITR) to nanocelluloses using genetically engineered binding protein HFBI-DCBD composed of hydrophobin and showed their stability for several months.
Besides chemical conjugation of cargos, some chemical treatments, such as TEMPO (2,2,6,6tetramethylpiperidine-1-oxyl)- or chlorite-mediated oxidation may alter the nanocellulose charge, promoting the physical bonding of charged molecules to the carrier. In addition, HCNCs, decorated with highly functionalized cellulose polyelectrolytes, provide nanocarriers with tunable surface charge [60], which are able to regulate the chemical responses to carried drugs. Surface charge may have negative effects on the attached cargo, e.g., degrading aspirin over time [61]. The crystalline structure of HCNCs can be preserved by controlling the oxidation reaction condition. As innovations in nanocellulose-based therapeutics advance, thorough in vitro and in vivo characterizations are required to ensure that the product is a reliable molecular carrier for clinical applications.
In vivo behavior of nanocelluloses is rarely explored. Current literature, geared towards investigating the adhesion, proliferation, spreading, viability, and differentiation of a variety of cells in nanocellulose scaffolds, has attested to the biocompatibility of nanocelluloses when their concentrations are typically less than 1 mg mL−1. CNCs are ingestible and their gastrointestinal fate is associated with acid-mediated hydrolysis and interactions with gut flora [62]. Importantly, concentration-dependent apoptotic toxicity of CNFs at 2–5 mg mL−1 has been reported [63]. Cationic nanocelluloses, e.g., trimethylammonium-CNF have been reported to be less cytotoxic than the anionic counterparts, e.g., carboxymethylated CNF [64]. Similar to nanocelluloses, BC is non-toxic against endothelial and osteoblast cells [65–67]. Toxicological studies of nanocelluloses have been conducted based on in vitro multi-trophic assays, acute lethal tests, gene mutations, and chromosomal tests, as well as oral evaluation, neurocytotoxicity, and immunoreactivity. Both CNF and BC may contain endotoxin and (1,3)-β-D-glucan contaminants, confirmed by analyzing the hot water extracts of these nanocelluloses using ELISA assays, high-performance size exclusion chromatography-multiangle laser light scattering, gas chromatography, and nuclear magnetic resonance spectroscopy [68].
2. Cellulose nanocrystals (CNC and HCNC) and their composites for cargo delivery
Here, we discuss the contributions of CNCs to the controlled release of active molecules, including a brief insight into the potentials of HCNC, a newly emerged class of nanocelluloses, in nanomedicine. CNCs, sourced from plants, are rod-like particles with length ~ 100–200 nm, width ~ 5–10 nm, and a hydroxyl-rich surface, facilitating a wide range of chemical modification [59]. These nanocelluloses are being commercially produced in large scales (hundreds of tons per year). We review the recent advances in CNC-based cargo delivery and bioimaging.
The focus of CNC research for biomedical applications before 2014 was geared towards taking advantage of the mechanically strong crystalline body of CNC for reinforcing composite biomaterials [70–72]. For example, Wang and Chen utilized cellulose nanowhiskers to improve the mechanical properties of electrospun prolamin protein fibers and create a drug delivery system that could prolong riboflavin release in phosphate buffered saline (PBS) [73]. Controlled release of water-soluble drugs, such as doxorubicin (Dox) and tetracycline from unmodified CNC as well as hydrophobic anti-cancer drugs, such as docetaxel, paclitaxel, and etoposide from cetyltrimethylammonium bromide (CTAB) modified CNC have been demonstrated [74]. Xiang et al., [75] modified CNC and demonstrated that the diffusion rate of non-ionic molecules could be controlled by adding electrospun poly(lactic acid) (PLA) to the matrix, which induced the Fickian diffusion with reduced initial (burst) release for Columbia blue dye as a model drug.
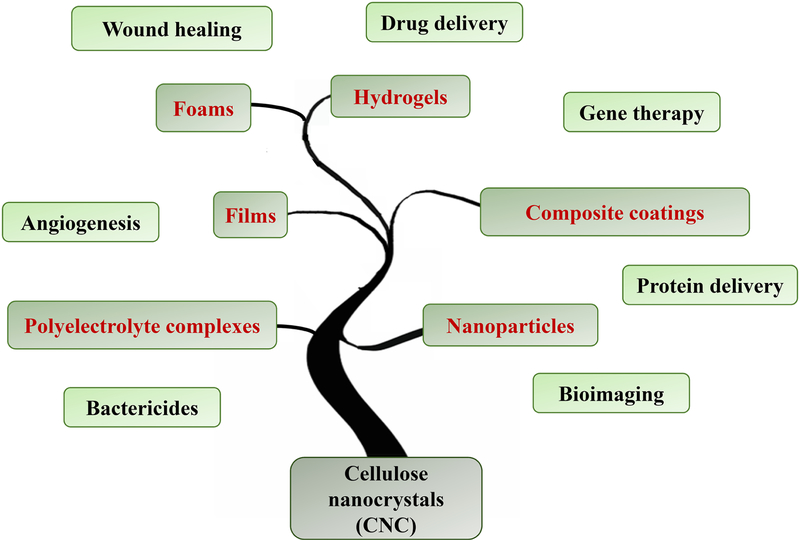
Despite the limited attempts for using CNC in biomedical engineering before 2014, the significant progress in synthesis, chemical modification, and production process optimization in the past four years has set the stage for the advanced medical applications of CNCs, in the forms of nanoparticles, hydrogels, films, and composite coatings. A summary of the main delivery applications of various forms of CNC-based biomaterials is presented in Figure 2. HCNC, however, has emerged recently, opening new horizons for producing CNCs that can accommodate ~ 1–7 mmol carboxylic acid groups on their surface [8,9], pushing the structural boundaries of conventional nanocelluloses. Applications of HCNC in the controlled release of active biomolecules has not been explored yet. The versatile chemical modality of this family of nanocelluloses may provide numerous possibilities for cargo loading and release.
Figure 2.
Various types of cellulose nanocrystal (CNC)-based biomaterials for nanomedicine and their main applications.
2.1. CNC for cargo delivery
Viable intracellular cargo delivery platforms must be nontoxic towards somatic and undifferentiated stem cells and pass through the cell membrane [76]. CNCs are currently being explored as carriers for drug delivery, because they can be inexpensively produced from widely available renewable resources [13,69,77,78] and attain large surface areas (~ 100 m2 per gram) [79] regulated by nano-morphology, particle size, and dispersity, tailored surface charge, and a high hydroxyl group content [19,42,69] for the physical and chemical binding of bioactive components. The bioactive components may be drugs for treating wounds or illnesses, or dyes to allow for visualizing the trajectory of carriers. Common drugs and contrast agents physically or chemically attached to CNCs are summarized in Table 1.
Table 1.
CNC-based cargo delivery: examples of recent cargos, carriers, applications, and loading and release mechanisms.
| Cargo | Carrier | Potential application | Loading mechanism | Release mechanism | Year [Reference] |
|---|---|---|---|---|---|
| Rhodamine B isothiocyanate (RBITC), Fluorescein-5isothiocyanate (FITC) | Colloidal CNC | Bioimaging | Covalent conjugation | NA | 2014 [80] |
| BSA-FITC | PLGA-doped PVA-CNC films | Biomacromolecule delivery to bone marrow | Physical entrapment | PLGA degradation | 2014 [81] |
| MTX | Fe3O4 nanoparticles decorated with amino-functionalized CNC | Cancer therapy | Hydrogen bonding and electrostatic interactions | pH dependent (maximum release at pH ~ 5.4) | 2016 [82] |
| 5-FU | Xanthan gum-chitosan-CNC hydrogels | Cancer therapy | Equilibrium swelling | Non-Fickian diffusion | 2017 [83] |
| Tetracycline hydrochloride (TH) | CNC-doped PLA/PEG nanofibers | Antibiotic | Hydrogen bonding and improved hydrophilicity | Composition regulated | 2017 [84] |
| Curcumin (Cur) | CNC-collagen-gelatin microspheres/porous scaffolds | Wound healing | Physical trapping | Gradual dissolution | 2017 [85] |
| Angiogenin (ANG) and Cur | PLGA/CNC/Cur/ pDNA-ANG composite nanofiber | Angiogenesis and infection prevention during wound healing | Electrostatic interactions | Expected to be pH dependent | 2017 [86] |
| siRNA | Cationic BCNC, PEI-CNC hybrids | Gene therapy | Electrostatic interactions | pH dependent (maximum release at pH ~ 4.0) | 2017 [87] |
| Bovine serum albumin (BSA), Human serum albumin (HSA) | CNC nanocomposites | Protein delivery for intracellular cholesterol efflux | Adsorption/chemical conjugation | pH dependent (maximum release at pH ~ 6.5–6.6) | 2017 [88] |
| Repaglinide | Chitosan-CNC | Oral drug | Electrostatic | pH dependent | 2018 [89] |
| (RPG) | polyelectrolyte macroion complexes (PMC) | delivery | interactions | (maximum release at acidic conditions) | |
| Chlorotoxin | Colloidal CNC | Brain tumor therapy | Covalent conjugation | NA (internalization) | 2018 [90] |
The fate of nanoparticles inside the body, regulated by protein adsorption, cellular uptake, and tissue translocation depends on numerous colloidal factors, including size, surface charge, and morphology [91,92]. Mechanisms of in vitro uptake and in vivo behavior of nanocelluloses as emerging platforms for cargo delivery have not been fully understood. Among the most important properties of nanocelluloses, surface charge plays a key role in regulating cellular uptake. Mahmoud et al., studied the effect of ζ-potential (the potential difference between the nanoparticle surface and the bulk electrolyte) on the bioactivity of CNCs conjugated with commonly used dyes, fluorescein-5-isothiocyanate (FITC) and rhodamine-B-isothiocyanate (RBITC) [93]. ζ-potential, defined as the potential difference between the nanocrystals and the surrounding fluid environment had a direct effect on cell viability and cytotoxicity. CNC-FITC uptake by human embryonic kidney 293 (HEK-293) cells and Spodoptera frugiperda (Sf9) cells was sensitive to pH, peaking at pH ~ 5 and dropping drastically at pH ~ 6.5. HEK-293 and Sf9 cells also absorbed CNC-RBITC mostly at pH ~ 5, and at the physiological pH, CNC-RBITC absorbance was much higher than CNC-FITC. ζ-potential analysis revealed that both neat and FITC-tagged CNCs have negative surface charges, −31.6 mV and −46.4 mV, respectively, while RBITC-tagged CNCs have a positive surface charge (~ +8.7 mV) at this pH range [93].
Increasing CNC-FITC concentration from 30 to 300 µg/mL decreased cell viability from 59% to 29%, whereas cell viability was ~66% at 300 µg/mL of CNC-RBITC. Electric cell-substrate impedance sensing (ECIS) was conducted to study the cytotoxicity mechanism of FITC-tagged nanocrystals. Toxic compounds inhibited current flow on a gold electrode coated with Sf9 cells cultivated with varying amounts of CNC, CNC-FITC, and CNC-RBITC. CNC and CNC-FITC inhibited the current flow with half-inhibition concentrations (ECIS50) ~ 61 ± 2.4 µg/mL and 45 ± 4.6 µg/mL, respectively. ECIS50 for CNC-RBITC was 224 ± 6.3 µg/mL, indicating that CNC-RBITC inhibited cell growth less than other tested nanocrystals [93]. While cellulosic nanocrystals, often decorated with negatively-charged moieties, are potential materials for targeted drug delivery, the cytotoxicity studies suggest that functionalizing nanocelluloses with positively-charged groups may improve their uptake and reduce the toxicity, which must be well understood before these nanoparticles can serve for clinical applications.
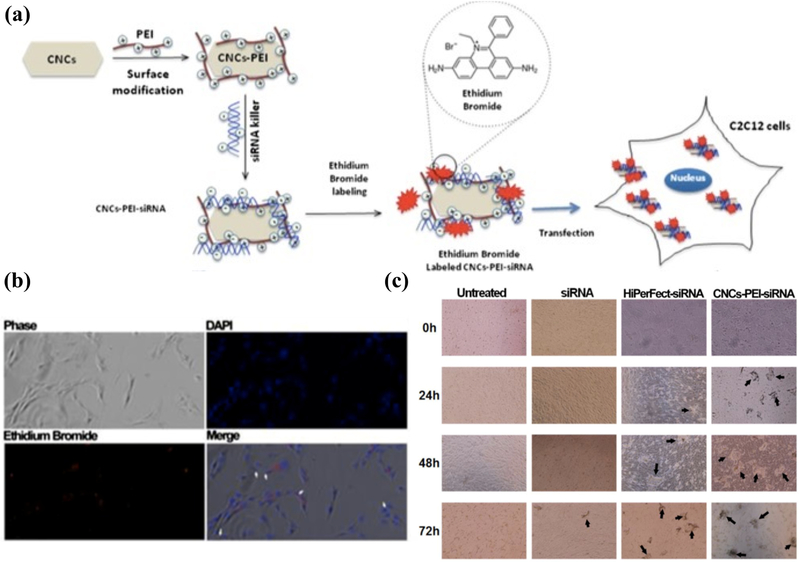
The interactions between CNCs and cargo molecules are regulated by chemical and/or physical modifications. Beside simple mixing, e.g., to produce CNC-alginate nanoparticles in honey (as a surfactant and stabilizer) for rifampicin delivery [94], a facile method to engineer the surface charge of CNCs is through the adsorption of polycations. Sulfuric acid-hydrolyzed negatively charged CNC surfaces can be coated with positively-charged polyethylenimine (PEI) to enhance small interfering RNA (siRNA) binding [87]. siRNA is a double-stranded RNA which is introduced into a cell to silence a specific gene. While siRNA serves as a potent method to alter various disease-causing agents, its susceptibility to enzymatic degradation and lack of cell target specificity are obstacles that require improvement. To this end, PEI has been used to facilitate siRNA delivery (Figure 3) via the high density of positively charged functional groups. This strategy not only enhances the electrostatic binding of siRNA to CNC but also improves the endocytosis and further enables endosomal escape as a result of the so-called “proton sponge effect” [87]. The high buffering capacity of the polycation increases the influx of counterions and water into the endosomes, disrupting the endosomal membrane due to elevated osmotic pressure, which results in releasing the cargo in the cytosol [95].
Figure 3.
(a) CNCs are readily decorated with polycations (e.g., polyethylenimine, PEI), imparting a positive charge to their surface, which promotes the electrostatic (physical) binding of siRNA killer. These self-assembled nanoparticles are internalized by C2C12 myoblasts (b), resulting in gene editing, silencing the expression of cell cycle genes, and promoting apoptosisdriven cell death [87]. (c) Within 24–72 h, cell death becomes evident by the formation of debris (shown with arrows), which is more pronounced for CNC-PEI-siRNA complexes, similar to the commercial transfection reagent (HiPerFect, Qiagen). Carbohydr. Polym., 164: 258–267, Copyright (2017), with permission from Elsevier.
The formation of CNC-PEI has been confirmed by the change in ζ-potential from ~−40 mV to ~+37 mV [87]. Subsequent siRNA adsorption decreases ζ-potential to ~ +2.5 mV. The CNC-PEI-siRNA complexes have been assayed for cytotoxicity, cellular uptake, siRNA protection, and apoptosis induction [87]. Cytotoxicity assays reported no toxic effect of CNCs-PEI on the murine C2C12 myoblast cell line for certain doses and culture conditions within 72 h. Cellular uptake was evaluated by 4′,6-diamidino-2-phenylindole (DAPI) and ethidium bromide fluorescence, which showed the localization of siRNA complexes in the cytoplasm. The degree of siRNA protection was tested by exposing the CNC-PEI-siRNA to RNase A for a limited time followed by confirming the indicative bands of siRNA in gel electrophoresis after the separation of siRNA from the complex using sodium dodecyl sulfate. The gel assay demonstrated that siRNA was protected in the presence and absence of RNase A. In addition, the presence of siRNA was not confirmed in complexed siRNA that was only exposed to RNase A, indicating that siRNA encapsulation is preserved even after the exposure to nuclease. Finally, the expression levels of extrinsic and intrinsic apoptotic genes, Casp 8 and Casp 9, respectively, were measured as indicators for apoptotic induction. Casp 8 was measured as an indicator of apoptotic induction via external stimuli (e.g., carrier attachment to the periphery of the cell) while Casp 9 was used to indicate the internal stimuli (carrier internalization). Casp 9 mRNA expression levels, measured by the quantitative analysis of cDNA produced from extracted mRNA, were significantly upregulated while Casp 8 expression was almost unchanged. This data supported that the CNC-PEI-siRNA was successfully endocytosed by the cells and delivered to the cytoplasm, followed by the siRNA-induced internal apoptosis (Figure 3) [87].
Drug delivery systems must preferably be nontoxic and stable in the circulatory system while remaining stealth against immune system. While carbon nanotubes [96] and self-assembled nanocarriers [97], such as lipid nanoparticles and vesicles [98] have been dealing with this challenge, CNCs with proper surface modifications may serve as a biocompatible biopolymer carriers for bioactive molecules. Hydroxyl groups on CNCs provide active sites for bioconjugation and promote hydrophilic interactions, decelerating phagocytosis. Needle-shaped nanoparticles, i.e., CNCs, of 50–200 nm length, may provide optimal evasion of both renal and immune system regulation [99].
Low cytotoxicity and uptake of unmodified CNCs are desirable properties for targeted drug delivery. It has been reported that CNCs are not cytotoxic against human brain microvascular endothelial cells (HBMECs) and have minimal uptake [99]. The viability of HBMECs, indicated by the formazan product of MTT assay, was not significantly different from the CNC-free control at CNC concentrations ~ 10, 25, and 50 µg/mL of media after 24, 48, or 72 h. Cellular uptake of FITC-tagged CNCs was time and dose dependent in 24 h exposure to 50 µg/mL of CNC; however, the uptake was minimal as the delivery system was non-specific. Because CNC uptake was minimal, these nanoparticles may be used as targeted drug delivery systems, which are able to minimize non-specific drug uptake.
Non-specific pharmaceutical cancer drugs (chemotherapeutic agents) typically cause damages to healthy tissues and organs. To limit these detrimental side effects, current research and development are driven towards creating specific targeting drug carriers using nontoxic materials. CNCs at optimum concentrations may not have toxic effects on the body and are not associated with specific cellular uptake. Additionally, the hydroxyl groups along the CNC body provide attachment sites for agents, such as fluorescent dyes, targeting materials, and therapeutic drugs. Typical chemical conjugation pathways rely on the facile oxidation, amination/amidation, esterification, etherification, and carbamation [100] of CNCs, enabling a broad range of surface modifications.
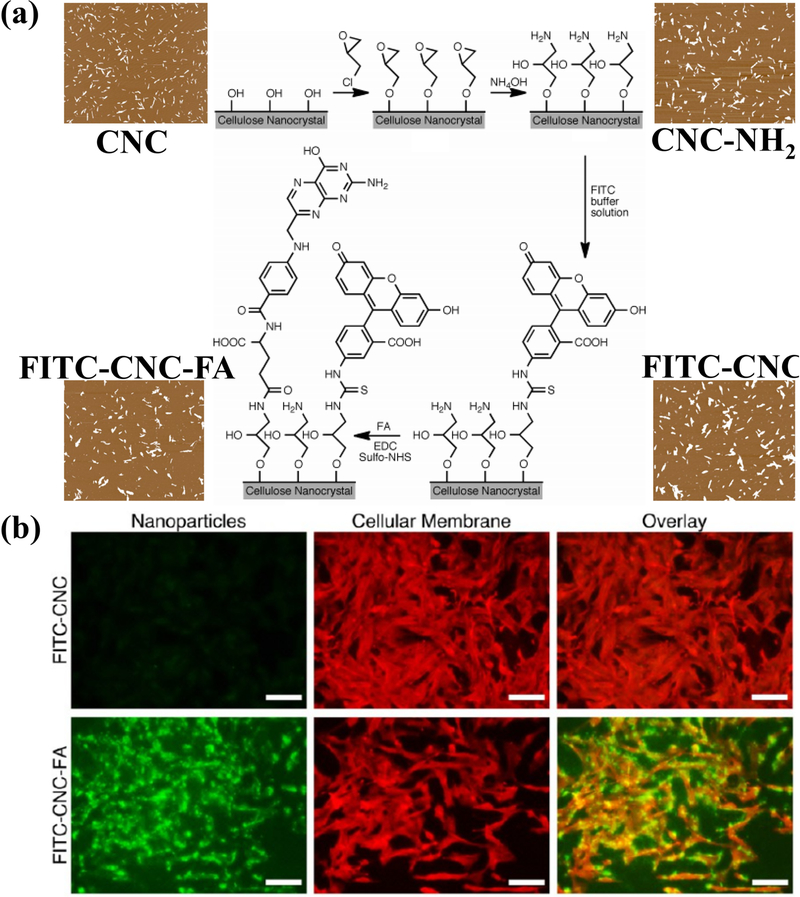
Targeted delivery may be achieved through the surface modification of CNCs with cell-specific receptors via amination. Targeting has been successful in brain tumor cells, whereby the targeting mechanism relies on folic acid (FA)/folate receptors that are overexpressed in cancer cells. Dong et al. [80] utilized these attachment sites to have CNCs bind specifically to three types of brain tumor cells: DBTRG-05MG and H4 (human brain tumor cells) and C6 (rat brain tumor cells). By adding FA to CNCs tagged with FITC, the endocytosis of FITC-CNC-FA drastically increased. X-ray diffraction and Fourier-transform infrared spectroscopy showed that the crystallinity and crystal lattice structure of CNCs were not affected by attaching FITC or FA. All three brain tumor cells endocytosed the FITC-CNC-FA, where fluorescence intensities revealed that the uptake was 1472, 975, and 46 times greater than those of FITC-CNC for DBTRG-05MG, H4, and C6 cells, respectively. Further investigation revealed that DBTRG-05MG and C6 cells, both non-neuronal cells, operate on a different uptake mechanism than H4 ganglioma cells. Genistein, an inhibitor of caveolae-mediated endocytosis, inhibited 85% and 55% of FITC-CNC-FA uptake in DBTRG-05MG and C6 cells, respectively, but had no significant effect on the H4 uptake. Conversely, chlorpromazine, an inhibitor of clathrinmediated endocytosis, inhibited 48% of FITC-CNC-FA uptake in H4 human brain tumor cells. FA-mediated cellular uptake of CNCs has also been used for enhanced tumor ablation [101], wherein the selective enhancement of the electroporation-based therapy of human breast cancer cells is achieved via defect formation on the cell membrane as a result of CNC-FA endocytosis as well as the distribution of electrically non-conductive CNCs throughout the tissue. Figure 4 presents the enhanced cellular uptake of CNC-FA for targeted delivery.
Figure 4.
(a) Chemical modification of CNC surface with FITC and folic acid (FA) for visualizing the enhanced receptor-mediated uptake of the nanocelluloses by human DBTRG05MG, which is shown in (b) the fluorescence images of stained cells [80]. Reprinted with permission from S. Dong, H.J. Cho, Y.W. Lee, M. Roman, Synthesis and cellular uptake of folic acid-conjugated CNCs for cancer targeting. Reprinted with permission from (Biomacromolecules, 15:1560–1567). Copyright (2014) American Chemical Society.
Besides cell-specific receptors, recent studies of protein functions have revealed that albumins play a critical role in cell specific drug delivery through their specificity for ligand binding [102–107], which have been used as endogenous cargo carriers [108,109]. Common ligands for albumin include fatty acids, hormones, and toxic metabolites. The degree of cholesterol production from endothelial cells in the vascular arteries is a major factor that contributes to the formation of atherosclerotic plaques. In an attempt to enhance cholesterol efflux from these endothelial cells, the effect of cellular exposure to bovine serum albumin (BSA)/human serum albumin (HSA)-conjugated CNCs on cholesterol deposition was studied. CNCs were conjugated to each type of albumin in two ways: physical adsorption and chemical conjugation. Physically adsorbed BSA/HSA CNCs were prepared by direct addition and incubation of dried CNCs in BSA and HSA solutions overnight. Chemical conjugation was conducted through the TEMPOmediated formation of carboxylic acid groups on CNCs, followed by EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride)/NHS (N-hydroxysuccinimide)-mediated addition of BSA/HSA [88].
Both negatively and positively charged CNCs adsorb albumins. Binding between the cationic pyridinium-grafted CNCs and BSA is endothermic, which is driven by charge neutralization, whereas negatively charged (e.g., carboxylated and sulfated) CNCs show a weaker protein binding [110]. The efficiency of protein loading may be studied through the analysis of the supernatant after exposing CNC dispersions to BSA/HSA. Physical adsorption has yielded 40% and 36% loading efficiency while the chemical conjugation has led to 54% and 46% loading for BSA and HSA, respectively [88]. Interestingly, microscopic observations suggested that the topology of the CNC particles differed upon protein binding. Albumin-free CNCs were observed to have rod-like structures while albumin attached CNCs appeared to have more porous structures and loose arrangements so as to demonstrate a decent capacity to encapsulate various cargo. Secondary and tertiary structures along with general protein integrity were confirmed via SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) and CD (circular dichroism) spectropolarimetry, suggesting that CNC does not have adverse effects on the cargo proteins before or after binding. This was also supported by quantifying the bioactivity of proteins released from the CNCs, which reached up to 90% as compared to the activity of BSA/HSA in their native states [88]. Fluorimetric cholesterol efflux assays have revealed that while cholesterol efflux is constantly high in human coronary artery endothelial cells (HCAECs) independent of the concentrations and conjugation method, human umbilical vein endothelial cells (HUVECs) experienced a gradual increase in cholesterol efflux with increasing the concentrations of both BSA- and HAS-conjugated CNCs. Notable differences were observed for chemically-conjugated CNCs as opposed to the physically-adsorbed CNCs, whereby the chemically conjugated BSA/HSA demonstrated higher efflux. Accordingly, while albumins may serve as shuttles for cholesterol efflux, CNCs are also able to perform as sinks, carrying the effluxed cholesterol to the intestinal lumen and out of circulation.
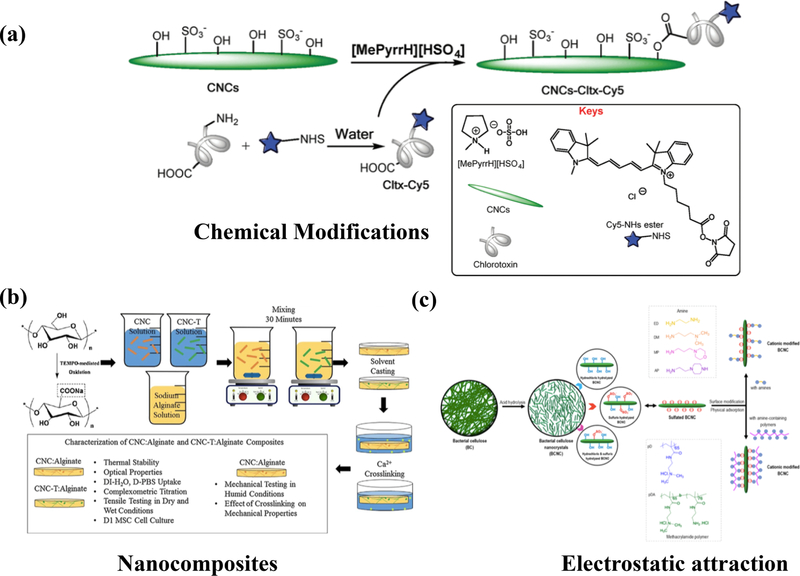
Common surface modifications of nanocelluloses involve steps that demand organic solvents, which often renders these nanomaterials less biocompatible and more difficult to produce at large scales. To address these issues, ionic liquids have been designed to replace organic solvents. Ionic liquids are a desirable alternative due to their significantly low vapor pressure and ease of separation [111]. One-step esterification using Bronsted acid ionic liquid N-methylpyrrolidinium hydrogen sulfate (MePyrrHSO4) [112] is an alternative to organic solvents. CNCs may chemically bind to chlorotoxin (Cltx) via a one-step esterification process, yielding Cltx-CNCs. Cltx is a protein that allows targeting brain tumors, such as glioblastoma multiforme (GBM) via specific binding to MMP-2/ClC3. Subsequent cellular studies using the U87MG GBM cell line shed light on the efficacy of Cltx-CNC as a target-specific drug delivery system against brain tumors [112].
Cytotoxicity of the functionalized Cltx-CNC was evaluated by an MTS assay upon the exposure of seeded cells to various concentrations of Cltx-CNC (up to 200 µg/mL) and compared to varying concentrations of non-functionalized CNCs [112], showing no significant toxicity. The internalization assay was conducted by observing the cellular uptake of Cy5-conjugated Cltx-CNC (Cy5-Cltx-CNC) via fluorescence staining. U87MG GBM, secreting high levels of MMP2 (matrix metalloproteinase 2), internalized the Cltx-CNC the most. Fluorescence staining of Cy5-Cltx-CNC indicated the nanoparticle localization in the cytoplasm and nuclei surrounding. On the other hand, MCF7 with low levels of MMP2 expression indicated no intracellular Cy5 fluorescence. The demonstrated biocompatibility and specificity for MMP2 targeting indicate the potential of Cltx-CNC as a brain tumor targeting drug delivery system.
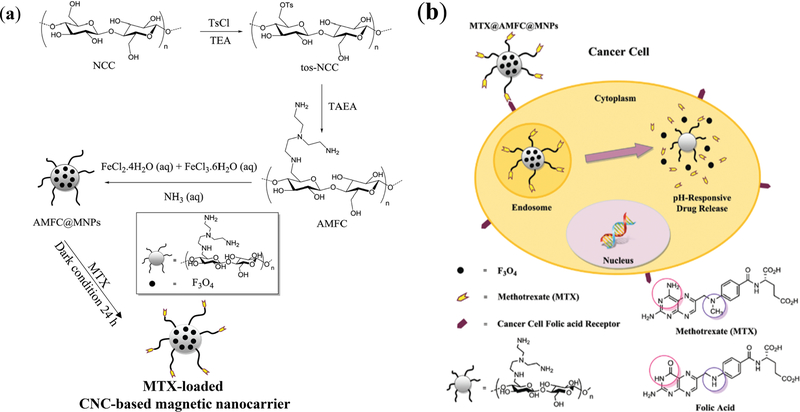
CNC may also be used as a coating material for the enhancement of colloidal stability, biocompatibility, biodegradability, and chemical functionality of nanoparticles. Modified CNCs with tris(2-aminoethyl)amine (AMFC) have been used to coat Fe3O4 magnetic nanoparticles (AMFC-MNPs) (Figure 5) [82]. The nanocellulose was treated with tosylchloride for the functionalization with tris(2-aminoethyl)amine, which was selected to provide amino groups and cationic properties. AMFC was able to effectively bind to the carboxyl groups of methotrexate (MTX, an anticancer immunosuppressive drug), overcoming the downsides of MTX by maximizing its anticancer efficacy while minimizing the off-target side effects to healthy cells. While the drug-free AMFC-MNP showed a high cell viability, the nanoparticles loaded with MTX had comparable cytotoxicity to free MTX, demonstrating that the efficacy of the bound drug is not inhibited by the nanoparticle. Hemolysis assays performed at 0–5000 µg/mL of blood showed less than 2.6% hemolytic activity for the drug-free AMFC-MNPs. The MTX-loaded nanoparticles had significantly higher cellular uptake than AMFC-MNP. This may be attributed to the chemical similarity of MTX to FA, facilitating receptor-mediated cellular internalization, which attests to the potential of MTX for enhanced cell-targeting (Figure 5) [82]. In the MTXAMFC-MNP system, drug encapsulation efficiency was ~ 91.2% along with a decent, 30.4%, drug loading efficiency. The pH-regulated controlled release is achieved based on the protonation states of MTX carboxylic groups. At physiological pH (7.4), up to 29% of the drug was released over 5 days, while up to 79% was released in acidic conditions (pH ~ 5.4). Magnetic CNCs have also been prepared using direct co-precipitation of Fe2+ and Fe3+ in CNC dispersions containing NaOH [113].
Figure 5.
(a) Synthesis of pH-responsive magnetic CNC-based nanocarrier. The nanocellulose was reacted with tosylchloride for the functionalization with tris(2-aminoethyl)amine (AMFC), providing amino groups to effectively attract the carboxyl groups of methotrexate (MTX, an anticancer drug). The magnetic CNC-based MTX delivery system, fabricated by an in situ reaction with FeCl2 and FeCl3, provides AMFC-coated magnetic nanoparticles (AMFC@MNPs), which are responsive to pH. (b) MTX-loaded magnetic CNC carriers, benefitting from the similarity of MTX to folic acid, faciliatet the folate receptor-mediated cell internalization [82]. Reproduced from [82] with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry.
2.2. CNC hydrogels, foams, and complexes
One of the main motivations for using CNCs in drug delivery scaffolds is to benefit from the stiff body of nanocrystals (elastic modulus > 100 GPa [79]) to reinforce the otherwise mechanically weak carriers. Biocompatible and biodegradable polysaccharide-based nanodrug delivery systems, such as chitosan (CS), which often lack mechanical resilience may be reinforced with CNCs [114–116]. Chitosan-based bionanocomposites reinforced with CNCs for tissue engineering and drug delivery are among the most common delivery scaffolds [117–119]. Other non-cytotoxic biopolymers, such as xanthan gum (XG), which are able to provide controlled drug release, have been used as additives in the therapeutic scaffolds loaded with chemotherapeutic drugs, such as 5-fluorouracil (5-FU) [83]. To evaluate the in vitro performance of CNC-based drug delivery hydrogels, encapsulation efficiency, cumulative release, and cytotoxicity have mainly been evaluated.
Typically, both compressive strength and stiffness increase with increasing CNC concentration. For example, XG-CS had compressive strength and stiffness ~ 36 kPa and 1432 N/m, respectively, whereas for XG-CS-CNC, they ranged from 40 kPa-58 kPa and 1525 N/m-2398 N/m for 2–10% CNC concentrations [83]. The cumulative drug (5-FU) release was regulated by the CNC: while the CNC-free system released more than 75% of the drug within the first four hours, adding 10% CNC was able to slow down the kinetics and provide ~ 60% release in ~ 30 h. This may be explained by the increased structural rigidity and enhanced physical binding between the 5-FU and hydrogel scaffold induced upon the incorporation of CNCs. Cytotoxicity assays for the hydrogels indicated that the drug delivery system was not toxic against the cells (NIH/3T3), which was concluded based on more than 95% cell viability at all concentrations of CNCs used for the preparation of hydrogels. These results suggest that CNC-reinforced biocompatible nanocomposite hydrogels may provide controlled release platforms, benefitting from decent biocompatibility and improved mechanical and chemical properties.
Besides reinforcement, CNCs may impart hydrophilicity to polymeric matrices, enabling the dispersion of hydrophobic agents, such as curcumin (Cur) in aqueous media. Cur provides antioxidant, anti-inflammatory, and antibacterial activity, which may potentially be effective for wound infection treatment; however, it is not trivial to maintain it in physiological systems due to its hydrophobicity. Porous collagen-cellulose nanocrystal (Coll-CNC) composites have been used to synthesize foam scaffolds loaded with curcumin-gelatin microspheres (GM) for prolonged Cur release [85]. This biomaterial has exhibited antibacterial activity in addition to dermis regeneration and inflammation inhibition as tested in mouse wound-infection models. The GM carriers embedded in the CNC scaffolds release Cur over a significantly longer period as compared to the GM or the CNC scaffolds alone [85], providing sustainable antimicrobial activity against various strains of bacteria. In the mouse model, the Cur/GM/Coll-CNC demonstrated accelerated dermal repair without significant wound hemorrhage or inflammation.
Increasing the dispersibility of drugs in aqueous media may not necessarily increase the circulation time in which case sudden increase in the drug concentration followed by its elimination significantly decrease the therapeutic effects and increase the side effects. Drug complex formation with nanoengineered polymeric networks may enhance the circulation time. Conventional oral drugs come into effect upon diffusion in the body fluids, such as intestinal fluid, followed by the uptake into the bloodstream. Oral drugs are known to have increased efficacy upon meeting certain criteria, among which drug residence time in the intestine is a key factor. Polyelectrolyte macroion complexes (PMC), synthesized from CNCs and chitosan have provided a long residence time in the small intestine due to the attraction between the negatively charged intestinal mucosa and positively-charged chitosan, providing an efficient platform for oral drug delivery [120].
2.3. CNC composite films
CNCs have typically been added to polymeric films to increase their Young’s modulus, elongation at break, and dissolution/release time. CNCs have been incorporated into composite electrospun nanofibers for wound care and skin regeneration. Typically, CNCs are electrospun with polymers/nanoparticles and target drugs, such as poly (D, L-lactic-co-glycolic acid) (PLGA), Cur, and polyethyleneimine (PEI)-carboxymethyl chitosan (CMCS)/plasmid DNA (pDNA)-angiogenin (ANG) [86]. ANG stimulates angiogenesis in wounded areas, facilitating skin regeneration by improving oxygen and nutrient delivery. Since ANG is highly unstable and has a short half-life in serum, drug delivery systems, such as chitosan-based nanoparticles, have been engineered to introduce ANG into cells via the transfection of pDNA. To examine the effectiveness of this nanofiber scaffold in skin regeneration, multiple tests have been conducted, including the measurement of pDNA release, ANG expression, and cell viability and proliferation, followed by in vivo evaluations using rat models [86]. PLGA/CNC/pDNA-ANG provided a significantly higher ANG expression as compared to controls with only PLGA/CNCs, recording 1.6-fold higher expression at day 3 and 2.8-fold higher expression by day 7. In vivo analyses showed that the pDNA-ANG nanocomposite-exposed rats had faster wound recovery and re-epithelialization [86].
CNCs have also been used as reinforcing agents in electrospun sheets, such as poly(3hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) [121] to increase their mechanical strength and hydrophilicity, allowing for long-term sustained release of cargos, such as tetracycline hydrochloride (TH). Similarly, PLA, a widely used nanofiber drug delivery system, has been doped with CNCs. In fact, PLA is not a suitable material for drug delivery purposes considering its hydrophobicity and subsequent poor control of hydrophilic drug release; however, CNC has been able to overcome the unfavorable properties of PLA by imparting hydrophilicity to the electrospun nanofibers while enabling sustained control of drug release as a result of drug binding [84]. Polyethylene glycol (PEG) may often be selected as an additional compatibilizer to increase biocompatibility and further enhance the hydrophilicity through its hydroxyl groups. A typical process of preparing these fibrous platforms consists of mixing the PLA solution with CNC/PEG, followed by electrospinning.
Drug release levels and cytotoxicity are two key properties of composite fibers that must be assessed before animal studies and clinical trials. Compared to additive-free PLA nanocomposites, PLA/CNC/PEG provides a two-step release profile, depending on the amount of loaded drug. When a low concertation of drug is loaded (e.g., TH concentration ~ 3%), almost all of it will be released within a few hours. On the other hand, the nanocomposites loaded with ~ 10% TH provide ~ 60% burst drug release by the first hour followed by ~ 80%−95% release after ~ 43 days. Interestingly, a biodegradability assay revealed that the drug accelerated the scaffold degradation as a result of increased hydrophilicity [84]. These drug-eluting systems have been cytocompatible against MG-63 cells (human osteosarcoma).
Hybrid spherical CNC-colloidal titania patches for the sustained delivery of antibacterial and antifungal agents, such as triclosan [122], CNC-alginate films for ampicillin delivery [123], and gold-CNC-doped guar gum and poly(vinyl alcohol) (PVA) for transdermal diltiazem hydrochloride administration [124] are among other examples of CNC-based cargo delivery films. Interestingly, the controlled release of carvacrol and Cur from TEMPO-oxidized CNC-cyclodextrin films may hold promise for antibacterial food packaging [125].
Biodegradable polymers have offered new platforms for administering proteins and other biological macromolecules that demand additional structural support. PLGA has widely been tested as a delivery polymer with tunable degradability and mechanical properties to control the administration rate of biomacromolecules. PVA, a commonly used water-soluble and biodegradable polymer, is molecularly large enough to perform as a matrix for therapeutic cargos. Nanocomposites of PVA and CNCs have a higher Young’s modulus than PVA alone, providing mechanically-robust structures [81]. PVA films doped with CNC have acquired up to 195% increase in the elongation at break at only 1% CNC. Besides mechanical reinforcement, thermal stability may also be improved by CNCs. Thermogravimetric analysis (TGA) of the polymer films has confirmed that the CNC nanocomposites have higher thermal stability than CNC-free PVA [81]. Some examples of common methods to make CNC-based cargo delivery systems are presented in Figure 6.
Figure 6.
Examples of common methods to make cellulose nanocrystal (CNC)-based cargo delivery systems. (a) Direct chemical modifications of CNC surface to conjugate Cltx via the Fisher esterification of the hydroxyl groups of CNC and the carboxylic acid groups of a drug in a Brønsted acid ionic liquid [90], reproduced from [90] with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry. (b) Solvent casting of CNC-alginate nanocomposites crosslinked using calcium ions. Carbohydr. Polym., 1:186–195, Copyright (2018), with permission from Elsevier [126]. This platform may be used to encapsulate active molecule of choice. (c) Self-assembly through the electrostatic adsorption of sulfuric acid-hydrolyzed CNC (bacterial) with amine-bearing polymers [127]. Reprinted with permission from [127]. Copyright (2018) American Chemical Society.
3. Bacterial cellulose (BC) and its composites for cargo delivery
Bacterial cellulose and nanocellulose (BNC) are crystalline biopolymers synthesized by specific types of bacteria, such as Gluconacetobacter xylinus [105,106]. Colloidal BNC, prepared from BC are typically negatively charged, which in concentrated BNC dispersions, form physical hydrogels with decent structural integrity, biocompatibility, and water binding capacity, providing a desirable microenvironment for selective cargo delivery [130,131]. Biocompatibility, purity, and malleability of BC make it suitable for biomedical applications [131], such as topical treatment, as it can fit the epidermal surfaces and deliver antimicrobial or dermal therapeutics. Topical products typically do not require strict sterilization processes; however, challenges associated with emulsion stability, e.g., in cream manufacturing, require careful process design. Additive selection to engineer the physical properties (e.g., rheology) of nanocellulose-based formulations is of utmost translational importance. The topical products may be in the forms of creams, sprays, drops, and patches. Biocompatibility assays of topical BC patches over 24 h have resulted in clinical scores of zero, indicating no major health risks [132]. In the past decade, BC has mostly been used for wound dressing [133].
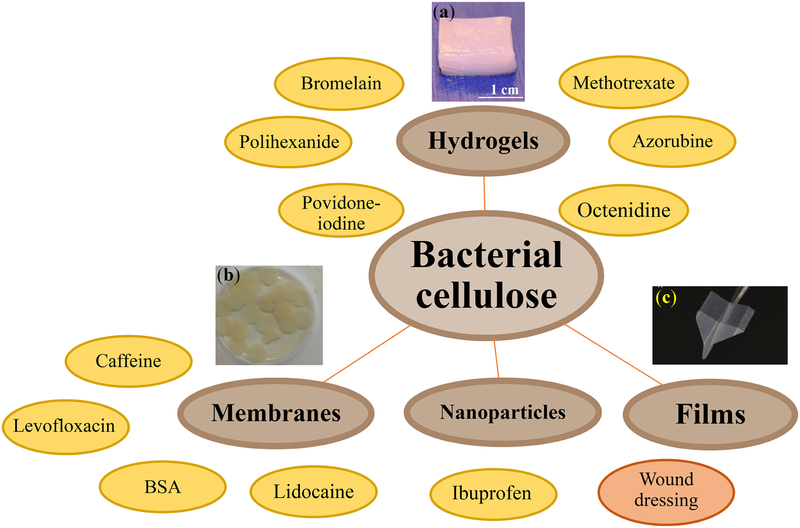
BC demonstrates desirable properties, including high tensile strength, crystallinity, and structural integrity, which are attributed to the intramolecular interactions of the hydroxyl groups on the BC surfaces [134]. BC has been integrated in various forms of medical treatment, including wound dressings, hydrogel-based oral drug delivery, and cargo scaffolds. Table 2 and Figure 7 summarize some of the most recent cargo delivery systems based on BC.
Table 2.
Cargo delivery systems based on BC: carriers, applications, and loading and release mechanisms
| Drug/bioactive agent | Carrier | Potential application | Loading mechanism | Release mechanism | Year [Reference] |
|---|---|---|---|---|---|
| Azorubine | Supplemented BC fleeces | Safe-to-store oral delivery and targeted drug delivery | Immersion in solution (absorption) | Diffusion | 2014 [135] |
| Bovine serum albumin | BNC fleece | Protein delivery | High-speed vortexing | Diffusion | 2014 [136] |
| Octenidine | BNC fleece | Wound treatment | Immersion in solution under shaking | Diffusion | 2014 [137] |
| Octenidine | BNC/poloxamer micellar hydrogels | Dermal drug delivery | Immersion under shaking | Sustained gel dissolution | 2016 [138] |
| Ibuprofen | BNC/graphene oxide composite hydrogels | Delivery of poorly watersoluble drugs | Immersion in drug solution | Non-Fickian, pH- regulated diffusion | 2017 [139] |
| Bromelain | BNC membranes | Antimicrobial wound dressing | Immersion in solution under shaking | Diffusion | 2017 [140] |
| Diclofenac sodium salt (DCF) | BNC-Poly(Nmethacryloyl glycine) membrane | Dermal and oral drug delivery | Immersion in solution | pHdependent (high release at pH ~ 7.4) | 2017 [141] |
| NA | BNC-ZnO nanocomposite films | Antimicrobial burn wound dressing | Immersion under shaking and lyophilization | Dermal application to burn as a bandage | 2017 [142] |
| Methotrexate | BC/CMC composite membrane | Topical treatment for psoriasis | Immersion in solution | Dissolution in vitro | 2018 [143] |
| Silymarin | Zein nanoparticle/BC nanofiber composite films | Antioxidant/a ntibacterial | Encapsulatio n in nanoparticles | Improved solubility | 2018 [144] |
| Cells (Keratinocytes and fibroblasts) | BC/acrylic acid hydrogels | burn wounds | encapsulation | NA | 2018 [145] |
Figure 7.
Bacterial cellulose (BC)-based biomaterials, including colloidal nanoparticles, films, membranes, and hydrogels for the delivery of a broad range of cargos. Panel (a) is reprinted with permission from [146], Copyright © 2015, Springer Nature, Springer Science Business Media New York. Panel (b) is reprinted from [140] under Creative Commons Attribution 4.0 International License for the Open Access content. Panel (c) is reprinted with permission from [147]. © 2018 WILEY- VCH Verlag GmbH & Co. KGaA, Weinheim.
3.1. BC for cargo delivery
One of the challenges in designing drug delivery systems is to control the exposure location and delivery time [148]. Engineering the spatiotemporal release profile of cargos may reduce side effects and enable targeted clinical treatments [149]. BC has been studied as an infrastructure to warrant controlled drug delivery, which benefits from stability, biocompatibility, and a large surface area decorated with hydroxyl groups that allow for attaching bioactive components. BCs attain various desirable properties that are comparable to CNCs without plant-derivative byproducts (impurities), including hemicellulose, lignin, and pectin (P) [150]. While BNCs do not possess any antimicrobial or anti-inflammatory characteristics, upon functionalization and drug loading, they may be an excellent platform for wound dressing.
Sustained release of lidocaine hydrochloride, a drug typically used for numbing, and ibuprofen (IBU, a nonsteroidal anti-inflammatory drug) are among the examples of BC-mediated drug delivery [151,152]. BC may be modified with a glycerol plasticizer [153] to increase the flexibility and swelling ratio of scaffolds, while providing the sustained release of bioactive molecules, such as caffeine for cellulite treatment. Glycerol have safely been added to the BC to soften the treated regions without any adverse effect on the biocompatibility. Another interesting cargo for BC is bromelain, a protein derived from pineapple fruit, leaves, and stem, which is a proteolytic enzyme that has antibacterial capacity for treating diabetic ulcers [154], rheumatoid arthritis [155], angina pectoris [156], and bronchitis [157]. BNC loaded with bromelain has been used for wound healing [140]. The antibacterial and antioxidant activities of bromelain-loaded BNC biomaterials attest to their applicability as a drug delivery system for treating common pathogens. Loading the cargo in the BNC hydrogels typically demands the incubation of BNC membrane in the drug (e.g., bromelain) solution for 4 to 24 h (e.g., absorbing 7.94 mg/mL of the protein). Hydrogels immersed in bromelain for 4, 8, 12, and 24 h showed identical enzymatic activity. The antibacterial properties of bromelain-attached CNC against Staphylococcus aureus, E. coli and P. aeruginosa., characterized by the agar plate method, showed that all treated colonies were eradicated. Released bromelain solution enzymatic activity was higher than initial and residual enzymatic activity, and the total protein concentration in the released solution was the lowest. The BNC successfully adsorbed and delivered the proteolytic enzyme bromelain, which exhibited high enzymatic and bactericidal activities towards gram-negative and grampositive bacteria [140].
Current BNC wound dressing technology relies on combinatorial treatments, encompassing regeneration and vascularization promoters as well as bactericidal agents. Povidone-iodine (PI)-loaded BNC wound dressing has been able to improve the currently available polyhexanide-(PHMB)-loaded BNC dressing technology [146]. Using BNC, extracted from Komagataeibacter xylinus, drug loading is readily achieved by the incubation of nanocellulose in PI or PHMB solutions. While the uptake of the PI and PHMB by BNC dressings only reached to ~ 12%, the amount of PI loaded in terms of drug mass per unit mass, surface area, and volume of the wound dressing for BNC were about 10 times higher than PHMB [146]. An initial burst drug release was observed in the first 8 h whereby the burst release for PHMB was significantly higher than that of PI, recording 67% and 46%, respectively, and the PI-loaded BNCs exhibited a more prolonged drug release than PHMB. PHMB-loaded BNCs recorded a cumulative drug release of approximately 85% after 24 h, which plateaued after 48 h. The cumulative PI release was at around 73%, which progressively increased. Drastic cytotoxicity differences were observed for the PI- and PHMB-loaded BNCs against HaCaT keratinocytes. While both PI and PHMB exhibited some degrees of cytotoxicity, the PI-loaded BNCs exhibited significantly lower toxicity, observed at concentrations ~ 15 and 20 mg/mL, whereas a pronounced toxicity was measured at 2 mg/mL of PHMB-loaded BNCs. Antimicrobial activities against S. aureus showed a log-reduction greater than 7. These tests allowed for the quantification of half maximal lethal concentrations (LC) and half maximal inhibitory concentrations (IC), whereby the LC50/IC50 of PI-loaded BNC was 4.8 while PHMB-loaded BNC recorded a ratio of 229 [146].
Recently-developed wound dressings are engineered to maintain moist conditions, attain high efficacy and selective antimicrobial effects, and promote rapid healing. In one of the attempts to design effective wound dressings, octenidine has been incorporated in BNC. Octenidine is an antiseptic drug with desirable characteristics, such as biocompatibility, skin compatibility, non-resistance-inducing bactericidal properties, and 24 h-long skin remnant. BNCs, prepared from K. xylinus, were submersed in an octenidine solution for loading [137]. An initial burst release for all BNC fleeces was desirable to achieve a therapeutically-relevant level. Equilibrium conditions were reached after 24 h, followed by a slow release. The biocompatibility of octenidine-loaded BNCs was tested against keratinocytes, a major cell type in human skin, indicating that no significant cytotoxicity was observed at 0.1–1% octenidine/BNC after 1, 24, and 48 h. However, octenidine/BNC ~ 10% drastically decreased the cell viability after 24 h. The antimicrobial activity against cultures of S. aureus, a common bacterial strain involved in wound infection, was also confirmed. Interestingly, the antimicrobial potency was preserved for BNC fleeces that were prepared and used after 6 months of storage [137]. Importantly, antimicrobial activity against the S. aureus was more pronounced than the cytotoxicity against HaCaT keratinocytes. The octenidine-loaded BNCs may serve as a powerful wound dressing with practical commercial benefits, such as potency after prolonged shelf storage [137].
BC has also been used for the delivery of nucleic acids. Singhsa et al. [127] have prepared bacterial celluloses using three different bacterial strains of K. xylinus, followed by hydrolysis using three different acids: hydrochloric acid, sulfuric acid, and a mixture of both. These BCNCs were tested for cytotoxicity and efficacy for nucleic acid delivery post-cationic (amino) functionalization. The modified cationic BCNC was simply mixed with siRNA to yield BCNC-siRNA complexes. The BCNC-siRNA complex formation was assessed using agarose gel electrophoresis, which indicated that modified BCNCs with a net negative ζ-potential failed to completely form a complex with the siRNA, while those with a net positive ζ-potential successfully yielded full complexation with siRNA. These results attest to the promising potential of surface charge modified BCNCs as cargo delivery vehicles for nucleic acids and proteins [127].
3.2. BC composite hydrogels and membranes
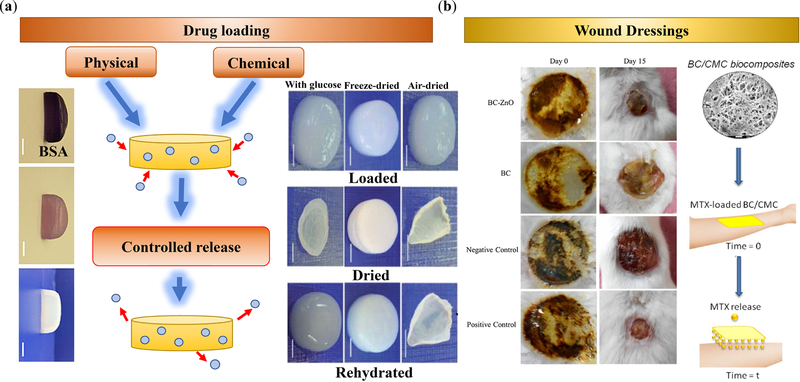
Hydrogels made up of bacterial nanocellulose have emerged as potential platforms for targeted drug delivery with remarkable biocompatibility, structural stability, water retention, and customizability. BNC hydrogels can be fabricated using as low as 1% nanocelluloses, which may benefit form a large water content. Conventional methods for loading drugs in BNC hydrogels and other BNC-integrated matrices and scaffolds involve the immersion of the BNC delivery system in the drug solution. This allows for the drugs to be loaded via diffusion and adsorption; however, the downside of this process is the prolonged loading time, which is not a practical for clinical and industrial purposes. Composite BNC/poly(N-methacryloyl glycine) membranes have been incubated with diclofenac sodium salt (DCF) for 48 h, resulting in ~ 100% drug loading (5 mg cm−2) and a pH-regulated controlled release with the highest release at the neutral pH [141]. An alternative method for drug loading involves vortexing for a few minutes, resulting in the high-speed loading of cargos, e.g., bovine serum albumin as a model protein (Figure 8a) [136]. Interestingly, the BNCs loaded through the conventional method showed a much faster BSA release as compared to the vortexing technique. Significant differences were observed after 24 h, and by 72 h, the model drug loaded with the conventional technique recorded a 91.2% release as opposed to a 62.4% release by the vortexing technique. Scanning electron microscopy (SEM) images revealed interesting outcomes that could serve as explanations for the observed drug release profile. As compared to the untreated BNC, the BNC treated with conventional drug loading methods exhibited a slight increase in fiber thickness and pore size. On the other hand, BNC treated with the vortex exhibited a significantly denser network of fibers with thicker fibers and partial pore closures [136]. Besides physical loading, drugs, such as amoxicillin may be chemically conjugated to BCs, e.g., to produce wound dressing sponges [158].
Figure 8.
(a) Mechanisms of cargo (e.g., BSA [136]) loading in BC-based carriers, including physical absorption/adsorption and chemical conjugation. The carrier may be used in hydrated or dry states. Supplementing BC with additives, such as glucose, provides remarkable shape recovery after rehydration [135]. (b) BC-based materials have been widely used for wound dressing, enabling the controlled release of bioactive (e.g., MTX) molecules [143]. BC may also be combined with antibacterial nanomaterials, such as ZnO for burn wounds [128]. Images in Panel (a, left) are reprinted from [136] Copyright (2014), with permission from Elsevier. Images in panel (a, right) are reprinted from [135] under Creative Commons Attribution 3.0 Unported Licence. Panel (b, left) is adapted from [128] Copyright (2017), with permission from Elsevier. Panel (b, right) is adapted from [143] Copyright (2018), with permission from Elsevier.
High swelling capacity is beneficial to load cargos in BCs. BC/acrylamide hydrogels may be prepared using microwave radiation under a basic condition (NaOH/urea), which demonstrate a higher theophylline (a drug for respiratory medical conditions) loading capacity than BC-free polyacrylamide scaffolds, providing controlled release in 24 h [159]. While clinical drug delivery demands hydrated hydrogels, long-term storage of hydrated materials raises issues regarding storage and the risk of bacterial contamination. Air-drying hydrogels results in microstructure collapse, which may deteriorate their bioactivity [135]. Lyophilization, supercritical carbon dioxide, ethanol drying, or hot-pressing are all expensive, resource-dependent operations that result in low-quality BNC hydrogels. To preserve the desired BNC microstructure and functionality for drug delivery, the dehydration of crystalline hydrogels may be conducted using several supplements, encompassing glucose, sucrose, lactose, trehalose, sorbitol, mannitol, PEG, magnesium chloride salt, and sodium chloride salt [135].
Supplementing 10% (w/v) magnesium chloride (MgCl2), glucose, and sucrose resulted in the highest re-swelling and drug delivery properties, which offered the best dehydration methods for preserving BNC structure and function [135]. Magnesium chloride supplement retained the largest amount of water after dehydration relative to never-dried BNC. After 168 h of rehydration, MgCl2-supplemented BNC reswelled to ~93% of its initial volume before drying and ~ 88% of its original weight. Glucose-, sucrose-, and sorbitol-treated biomaterials reswelled to above ~ 60% of their original volumes. SEM images revealed that the top and bottom surfaces as well as the cross-section of glucose-, sucrose-, and magnesium chloride-supplemented biomaterials retained their original three-dimensional structure after dehydration as compared to air-dried biomaterials that suffered from fiber aggregation and structural failure [135]. Interestingly, glucose-supplemented BNC released ~ 52% of a model drug (azorubine) in 1 h and ~ 95% in 4 h, which has a better control of the cargo release than the air-dried BNC releasing ~ 98% of the drug in the first hour (Figure 8a). Accordingly, supplementing BNC with carbohydrates or inorganic salts may facilitate the storage and transportation of BNC while maintaining the nanocrystal structure and function [135].
Interestingly, the physicochemical properties of BC have been engineered by simple drying methods. The integration of BC into single-excipient based BC matrices undergoing various drying methods, such as freeze-drying and oven-drying before and/or after drug loading (famotidine or tizanidine) has been investigated [160]. Higher drug loading was obtained for hydrated and partially hydrated BC matrices as compared to the freeze-dried ones. Hydrophilicity and porous fibrous network facilitated drug diffusion through the matrix as opposed to the freeze-dried matrix, wherein lacking these properties resulted in noticeable fiber aggregation and reduced water retention capacity. BC matrices loaded with famotidine or tizanidine had more than 80% burst drug release in the first 15 min in the simulated gastric fluid.
3.3. BC composite films
One of the main motivations to prepare BC films is their applications in wound healing, wherein functionalized substrates are required to remain on the lesion and provide healing and antibacterial effects. Traditionally, burn wounds have been covered with gauze and cotton wool; however, they adhere to the wound and are difficult to remove. Accordingly, alternative biomaterials, such as collagen, chitosan, and alginates have emerged for wound dressing [161,162]. The focus of research has been on the wound dressing biomaterials with drug releasing properties that leverage the healing process [163,164]. While various types of transdermal patches have been developed for the strategic and controlled delivery of drugs, increased microbial resistance has imposed unmet challenges to effective wound healing and wound infection prevention. To address this issue, novel approaches have been explored to achieve the dual release of proteins and antibiotics, leveraging bio-functionalities against antimicrobial resistance [165–167].
In a recent effort, the dual release of proteins and antibiotics from hybrid BC films has been investigated. BC, extracted from Komagataeibacter hansenii, was mixed with high methoxylated pectin (HMP) and modified with HSA and levofloxacin (Levo, an antibiotic agent). BC-HMP provided a lower burst drug release, enabling the controlled release of both Levo and HSA as compared to the unmodified BC. Cytotoxicity studies have suggested that this dual drug release system is biocompatible as Levo toxicity was attenuated upon loading in HMP, recording ~ 90% cell viability at 200 µg/mL (as compared to ~ 71% for free Levo) and ~ 100% cell viability at 100 µg/mL (as compared to ~ 89% for free Levo) [168]. Importantly, Levo-HSA-HMP had an antimicrobial activity against S. aureus and demonstrated promising outcomes not only for infection prevention but also for enhanced cell proliferation and tissue regeneration [168].
Another antimicrobial agent that can be delivered using BNC films is fusidic acid (FuA). FuA is a commonly used bactericidal drug, which is effective against drug-resistant bacteria. FuA can be loaded in BNC films by immersing the films in a sodium fusidate solution. The antibacterial effect of FuA-loaded BNC against S. aureus, tested using the disk diffusion method wherein the biomaterials were placed on S. aureus lawns on an agar plate and examined for the degree of clearance of the lawn surrounding the composite [169], demonstrated that the BNC films loaded with 0.4 g/L of FuA resulted in a 34 mm-diameter inhibition zone. Recorded inhibition zone diameters were 27, 30, and 32 mm for loading concentrations ~ 0.1, 0.2, and 0.3 g/L, respectively, showing the high potency of FuA-doped BNC nanocomposite films as a bactericide wound dressing.
PVA-BC hydrogels have been produced in monolayer and multilayer forms to load and release vanillin as an antimicrobial agent in a controlled manner that is regulated by the film composition [170]. Huang et al., [171] compared the release of berberine (a plant-based quaternary ammonium salt) from BC in different simulated fluids and found that the slowest berberine release occurred in simulated gastric fluid (SGF), containing sulfuric acid, and the highest release rate took place in the simulated intestinal fluid (SIF). SEM imaging confirmed that BC nanofibers became thicker in acidic solutions, resulting in smaller pore sizes that restricted the cargo diffusion rate.
Alternatively, BC may be impregnated with antimicrobial zinc oxide (ZnO) nanoparticles (Figure 8b, left panel) [172] or silver nanoparticles [173] for wound dressing. The BC-ZnO nanocomposites can be synthesized via the incubation of BC sheets in a ZnO nanoparticle dispersion [142]. These nanocomposites have antimicrobial activity against E Coli, Citrobacter freundii, Pseudomonas aeruginosa, and Staphylococcus aureus, the first three of which are Gram negative bacteria and the last Gram positive. Treatment with BC-ZnO showed a zone of inhibition that was similar in diameter to that of silver sulfadiazine (SD), a standard antimicrobial drug. Percent inhibition was calculated by dividing the BC-ZnO zone of inhibition by that of SD, all of which had approximately 90% inhibition for all four bacterial species. The effectiveness of wound healing and antibacterial activity were also confirmed in the in vivo tests conducted on burn mouse models. The average wound area was observed over 0, 5, 10, and 15 days, in which the BC-ZnO always showed significant wound healing effects (p ≤ 0.001) [142]. In fact, the recorded average wound area for the BC-ZnO-treated mice (269.3, 195.6, and 98.3 mm2 for 5, 10, and 15 days, respectively) was comparable to those of the SD-treated mice (287, 148.3, and 66.6 mm2, for 5, 10, and 15 days, respectively), supporting the potency of BC-ZnO nanocomposites.
BC may be combined with synthetic nanomaterials to overcome their limitations. BC/graphene oxide (GO) nanocomposites overcome the non-specific binding of GO to proteins in biological systems. BC/GO nanocomposites can readily be prepared in situ by adding GO to the culture media of cellulose-producing bacteria (e.g., Komagataibacter xylinus X-2) [139], providing a higher capacity for loading drugs than neat BC. Despite increasing the surface area and improving the loading capacity, added GO may have adverse effects on cell viability. As an example, 0.48% GO was not suitable for drug delivery as it demonstrated inhibitory or cytotoxic effects. Ibuprofen delivery using BC/GO and GO-free BC was tested at near neutral pH (~ 7.4) and acidic pH (~ 1.2) at the physiological temperature (37.0 ± 0.5°C), which showed a non-Fickian diffusion-regulated release and a zero-order transport rate, respectively. For both acidic and near-neutral pH, BC/GO released IBU at a more controlled manner than neat BC over 24 h [139].
To increase the hydration capacity of BC, dissolved cellulose additives, such as carboxymethyl cellulose (CMC), have been used without significantly compromising the crystallinity and thermal stability of BC. Modifying BC with polyanions has enabled the regulation of physical and chemical interactions with target molecules, e.g., water and drugs, to design drug delivery systems with desirable swelling, loading, and release rates [174–178]. BC can be modified with solubilized celluloses in situ, for example, via adding 1% CMC to the bacteria culture medium (Figure 8b, right panel) [143]. Increasing the degree of carboxylic acid substitution (DS) of CMC had a positive effect on hydration capacity and swelling ratio; however, at high concentrations, the crystallinity index and storage modulus of BC composites decreased. BC/CMC (DS ~ 0.7) swelled more than neat BC, BC/CMC (DS ~ 0.9), and BC/CMC (DS ~ 1.2); however, the liquid intake for neat BC was 490%, and BC/CNC composites with DS ~ 0.7, 0.9, and 1.2 absorbed 958%, 86%, and 578% water, respectively [143]. Interestingly, the high porosity of BC/CMC (DS ~ 0.7) resulted in the highest swelling ratio. Hydroxyl-ester interactions in BC/CMC (DS ~ 0.9) composites increased the matrix stiffness, which decreased the liquid absorption and the swelling capacity. In all samples, 70–80% MTX was released in the first 15 min, followed by a steady drug release thereafter. BC/CMC (DS ~ 0.9) provided the lowest MTX release rate, yielding 80% release after 180 min, whereas BC/CMC (DS ~ 0.7) released 96% of MTX over the similar period [143].
4. Cellulose nanofibrils (CNF) for cargo delivery
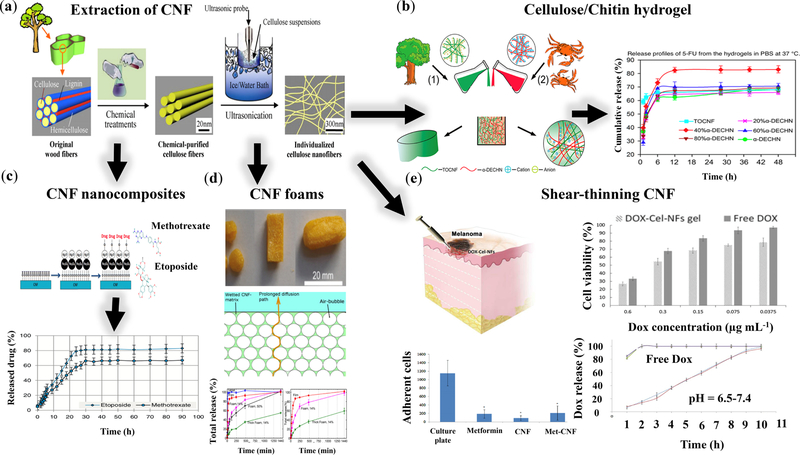
Biomedical research on CNFs has mainly revolved around studying the mechanical, chemical, thermal, and simulated loading/release properties of the nanocellulose composites. Recently, immense attention has been drawn towards biobased materials to design and produce a broad range of environmentally-benign materials with added values. The abundancy of plant biomass and the tunable mechanical and rheological properties of CNF have rendered this nanocellulose an attractive additive for a variety of polymer nanocomposites [117,179]. Different forms of CNF-based cargo delivery systems, encompassing colloidal particles, hydrogels, shells/capsules, aerogels/foams, and films are summarized in Table 3 and Figure 9.
Table 3.
Examples of recent CNF-based cargo delivery: carriers, applications, and loading and release mechanisms
| Drug/bioimaging agent | Carrier | Potential application | Loading mechanism | Release mechanism | Year [Reference] |
|---|---|---|---|---|---|
| Methotrexate | Resistant starch/pectin/CNF electrospun nanofibers | Colon inflammation | Absorption and electrostatic attraction | pH dependent, enzymatic degradation | 2016 [180] |
| Beclomethasone dipropionate, indomethacin, itraconazol | CNF microparticles and capsules | Drug delivery of poorlysoluble drugs (broad range of applications) | Mixing/filtration/drying | Diffusion | 2017 [181] |
| Cisplatin | CNF-PVA hydrogel | Cancer treatment | Mixing | pH dependent release (maximum release at 7.4) | 2017 [182] |
| Metformin | CNF gel | Anti-cancer (melanoma) drug delivery and metastasis prevention | Electrostatic adsorption | pH dependent | 2017 [183] |
| N/A | Ca2+-mediated crosslinked CNF hydrogel, kaolinCNF, and collagen-CNF | Hemostatic wound dressing | N/A | N/A | 2017 [184] |
| Etoposide, methotrexate | Fe3O4-Ag2O quantum dots decorated cellulose nanofibers | Chemotherapy for lung, breast, and skin cancer | Binding to Ag2O | Drug solubility in water | 2017 [185] |
| Bendamustine hydrochloride | CNF aerogel | Gastroretentive oral drug delivery | Absorption, electrostatic attraction | pH dependent | 2017 [186] |
| Dextran | Microcapsules of CNF, hemicellulose, and pectin | Model drug delivery | Physical encapsulation | Ion-mediated release | 2017, 2018 [187,188] |
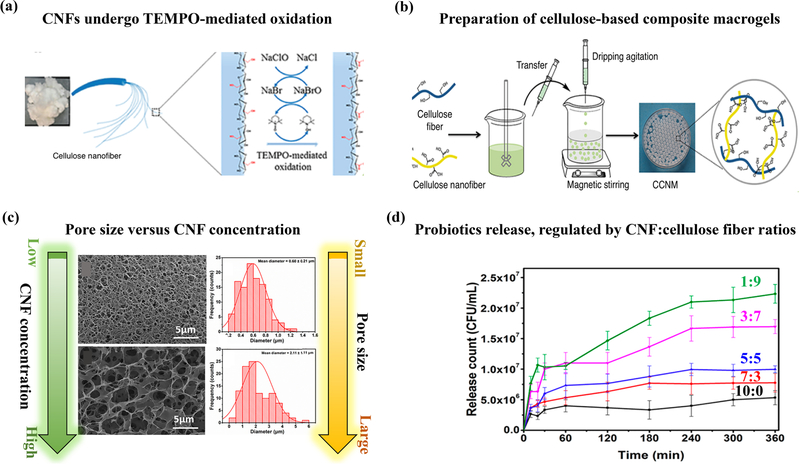
| 5-fluorouracil | α-chitin/TEMPO-oxidized CNF hydrogel | Anti-cancer oral drug delivery | Electrostatic adsorption through swelling | pH and swelling dependent | 2018 [189] |
| L. plantarum | Cellulose fiber and nanofibril hydrogel macroparticle | Probiotic delivery | Physical encapsulatio n | pH responsive pore size | 2018 [190] |
| Doxorubicin | Injectable CNF gel | Cancer (Melanoma) treatment and metastasis prevention | Electrostatic attraction | pH dependent | 2018 [191] |
| Tetracycline hydrochloride | CNF/ polydopamine hydrogel | Wound healing | Electrostatic attraction | pH dependent, high release at low pH | 2018 [192] |
Figure 9.
Cellulose nanofibril (CNF)-based biomaterials, including hydrogels, aerogels, capsules, films, and inorganic nanocomposites for cargo delivery. Images of hydrogel, capsules, aerogel, composite films, and inorganic nanocomposites are reprinted from [193], [188], [194], [195], and [185] with permissions from The Royal Society of Chemistry, Copyright (2017) American Chemical Society, Copyright (2015) American Chemical Society, Copyright (2017) American Chemical Society, and Elsevier, respectively.
4.1. CNF for drug delivery
Formulation and development of drug delivery systems have faced several challenges in the past decade. Poor aqueous solubility of cargos remains a common obstacle in developing effective delivery systems [196,197]. To overcome the limitations of sparingly soluble drugs, CNFs have recently been noticed in the pharmaceutical industry [181,198]. Unmodified CNFs are amphiphilic nanocelluloses decorated with hydrophilic and hydrophobic surfaces, which are able to adsorb hydrophobic drug molecules and nanoparticles and increase their colloidal stability. In addition, the nanocellulose-mediated mechanical reinforcement of drug carriers, such as starch, may leverage the controlled release of active molecules [199]. These properties have been verified by multiple studies confirming the favorable molecular interactions between CNF and poorly-soluble drugs [181]. Small poorly-soluble surfactant-like drug molecules can be adsorbed by CNF and change the surface energy of nanocellulose. This can provide the Pickering stabilization of surfactant-free nanocellulose-based emulsions [200]. CNF, produced as microparticles with tight fibrillar network, has been loaded with drugs via spray drying. This strategy has resulted in the sustained release of drugs for several months, regulated by the drug solubility.
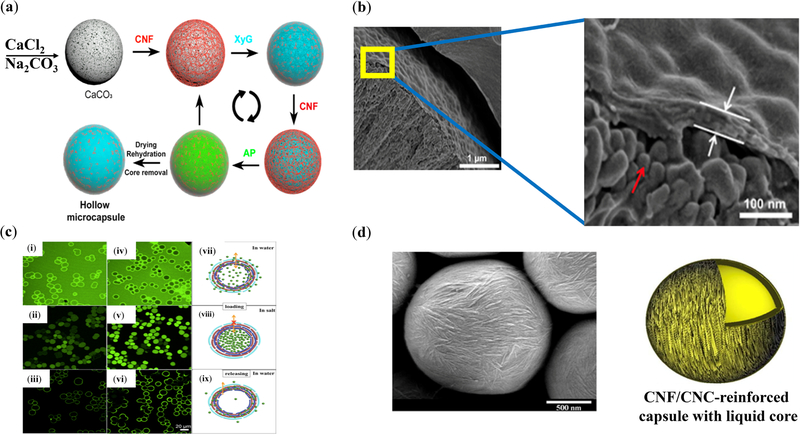
Capsules with versatile core functionality and tunable mechanical stability have been explored for potential applications in drug delivery [201,202]. Fiber-based reinforcement is inspired by the nature’s architectural solutions for building mechanically resilient constructs [203]. Mechanically-robust CNF capsules have been prepared using the layer-by-layer (LbL) technique (Figure 10) to fabricate small-scale scaffolds with on/off permeability regulated by salinity (ionic strength) for the on-demand loading/unloading of cargos, such as model fluorescent-labelled dextran [187,188]. This platform also holds promise in delivering drugs in a time-dependent manner. The modulus of wood-derived CNF is in the order of 150 GPa [204], supporting the mechanical integrity of CNF nanocomposites, such as capsules. Tunable oil core of CNF-based capsules may successfully contribute to the delivery of poorly-soluble drugs (Figure 10) [188,205].
Figure 10.
Cellulose nanofibril capsules. (a) Fabrication of CNF microcapsules from colloidal CNF dispersions using sacrificial calcium carbonate cores undergoing the layer-by-layer (LbL) assembly of CNF, XyG, CNF, and AP [187]. (b) The cross section of capsules [188], indicating a calcium carbonate core (red arrow) coated with the multilayer CNF nanocomposite (white arrows). (c) Capsule pores change size as a response to ionic strength variation, providing a stimuli-responsive cargo carrier platform with tailored loading (in water)/trapping (in salt)/release (in water) capacities [187]. (d) Mechanically-stable liquid-core capsules [205] made up of a covalently crosslinked CNF/CNC shell with an inner aromatic polyurea layer. These capsules are about six times stiffer than aromatic polyurea capsules. Images of panels (a) and (c) are reprinted from [187], copyright (2018), with permission from Elsevier. Panel (b) is reprinted with permission from [188]. Copyright (2017) American Chemical Society. Panel (d) is reprinted with permission from [205]. Copyright (2014) American Chemical Society.
Although CNF is a commercially available nanomaterial with potential applications in food industry, e.g., for decreasing the intestinal digestion and absorption of fat [206], a few studies have been conducted on its safety and the potential hazards post-accidental exposure [207,208]. Biological reactions through inhalation and dermal contact caused by wood-derived carboxymethylated or cationic CNF have been simulated in vitro [209]. Antibody assay (AB) and lactate dehydrogenase (LDH) assays show that CNFs do not have adverse effects on the metabolic activity of immune, dermal, and lung cells. THP-1 cells, as an in vitro cell model for immune modulation, had a significantly high metabolic activity when treated with CNF. When cell culture media were incubated with unmodified CNF, a significant increase in the pro-inflammatory cytokines, such as TNF-α and IL1-β, was observed; however, the chemically-modified CNFs did not significantly affect the cytokine levels. Furthermore, CNFs did not result in a significant increase in reactive oxygen species levels, suggesting that the oxidative potential of the cells was not affected by the CNFs. Transmission electron microscopy of CNF-exposed cells indicated no significant change in the cell shape and no CNF uptake by macrophages [210].
4.2. CNF composite hydrogels
Naturally-derived hydrogels have been gaining popularity; these hydrophilic polymer networks with widespread applications across multiple fields benefit from biocompatibility, easily adjustable chemical and physical properties, and high water contents [211–213]. Hydrogels are commonly used as drug delivery systems. In a simple approach, hydrogels undergo swelling inside a drug suspension to capture the drug and subsequently release it with programmed temporal and/or spatial precision. To this end, CNFs may form the backbone of hydrogels or be used as an additive. Common CNF-based hydrogels have been reviewed in literature [48].
CNF may be physically self-crosslinked, e.g., using two oppositely-charged CNF batches [214], or be incorporated in a wide range of polymeric matrices to increase their mechanical integrity, hydrophilicity, and thermal stability, and control the cargo loading and release rates. A broad range of polymers, including PVA, a biocompatible and non-toxic polymer, may be blended well with CNF to form reinforced hydrogels. These hybrid hydrogels may have applications in delivering chemotherapeutic compounds, such as cisplatin, an antineoplastic drug to treat tumors [182]. The CNF-PVA hydrogels attain a significantly high swelling ratio, exhibit a tunable drug release profile (e.g., from 0.5 h to 7 h at 1 wt% CNF), and are able to reduce the side effects of cisplatin, such as nephrotoxicity. Increasing the pH (from 1.2 to 7.4) results in an increased cumulative drug release, indicating that the slightly basic solution found in the small intestine is an ideal environment for maximum drug release.
Several types of cancers, such as melanoma (skin cancer), have undergone increased resistance against chemotherapeutic drugs, such as Dox, demanding localized chemotherapy to increase the drug concentration at the tumor site [215–219]. One solution under investigation is the use of, often electrospun, nanofibers to benefit from their similarity to the fibrils (e.g., collagen) in the extracellular matrix (ECM). Nanofibrous cellulosic biomaterials may enhance interfacial cell-matrix interactions, perform as a physical barrier to prevent tumor cell invasion, and provide a high-capacity drug depot [220,221]. Cellulose nanofibers may also provide injectable nanofibrous biocompatible biomaterials decorated with hydroxyl and carboxylic acid groups that undergo electrostatic or hydrogen bonding with drugs [191]. The adsorption of Dox to the surface of CNFs increases the localized cytotoxicity against the cancer cells compared to free Dox, which decreases the required drug dose for chemotherapy.
The ECM degradation and remodeling are crucial factors in inhibiting the migration and metastasis of cancer cells [222–225]. Novel treatments to reduce the risk of metastasis are highly demanded for treating aggressive malignant cancers, such as melanoma. The properties of nanofibers such as high surface area, high drug loading capacity, sustained drug release, and similarity to the ECM may offer effective treatments to reduce the risk of metastasis by promoting the adhesion of cancer cells to the barrier biomaterial and blocking the cell migration [183]. Metformin, an anti-cancer and anti-metastatic drug, is more effective if delivered directly to the tumor site. This drug is positively charged at physiological pH, enabling it to electrostatically adsorb to negatively-charged CNF. The amount of drug attached to the nanofiber surface is independent of the drug concentration and can be manipulated by changing pH to protonate/deprotonate the carboxylic acid groups of CNF. The rheological properties of these gels are not significantly affected by the drug loading, providing an injectable drug delivery platform with suitable injection forces for minimally-invasive clinical procedures [183]. An MTT assay showed that loading CNF with 17.6 mg/mL metformin was able to partially inhibit the growth of melanoma cancer cells without affecting non-cancerous cells. Cell attachment occurred in the drug-modified cellulose nanofiber (Met-CNF) gel for nanofibers with a diameter greater than 400 nm. Drug-eluting biomaterials that promote cell adhesion may hold promise in preventing cell migration [183].
Natural hydrogels, such as those consisting of deacetylated α-chitin nanofibers may be combined with drug (e.g., 5-FU)-loaded negatively charged TEMPO-oxidized cellulose nanofibrils (TOCNF). TOCNF retains the original crystallinity of CNF and disperses in water [226]. α-chitin fibrils have a high aspect ratio, strong mechanical properties, and excellent biocompatibility and degradability [227,228]. 5-FU is an anticancer drug with low solubility, which induces multiple undesirable side effects when intravenously injected or infused. Thus, administering this chemotherapeutic drug orally may help minimize the side effects [189]. Both TOCNF and α-chitin not only reinforce the structure of carriers but also impart a slow drug release profile. When mixed, physical crosslinking occurred due to the electrostatic interactions between the anionic TOCNF and cationic chitin. The lowest ζ-potential of these polyplexes was found in a hydrogel containing 40% partially deacetylated α-chitin nanofibers, which indirectly indicates that this composition has the highest crosslinking density. Increasing the chitin content beyond the optimum value increases equilibrium swelling ratio as a result of the free amine and hydroxyl groups undergoing hydrogen bonding, which promote water absorption in the nanofiber networks. The hydrogel containing 40% chitin had the lowest surface charge and provided the highest 5-FU loading capacity and efficiency (~53%) in PBS at 37 °C. When the ratio was changed, the hydrogel loading capacity along with drug release percentage declined [189].
An emerging application of CNF is probiotics delivery [190]. A composite macrogel made up of cellulose fibers and nanofibers functionalized with carboxyl groups (Figure 11a, b) performed as a suitable platform to deliver probiotics to a specific region of small intestine. To encapsulate and protect the probiotics from early deactivation by gastric fluids, carboxylic groups on the cellulose surface are favorable, imparting pH-responsive properties to the nanocellulose carrier. The carboxyl groups on CNF, typically yielded by TEMPO-mediated oxidation, regulate the pore size of hydrogel carriers, rendering them suitable for the controlled release of probiotics (Figure 11c). CNF macrogels exposed to SIF and SGF exhibited tunable swelling behavior and pH responsiveness. Increasing the number of carboxyl groups on the nanofibers resulted in a slight shrinkage in SGF and increased swelling when transferred to SIF. Furthermore, the carboxyl groups performed as a pore size regulator while the cellulose backbone strengthened the porous structure. The maximum release of viable L. plantarum (model probiotic) from the carrier was more than 2.23 × 107 CFU/mL (Figure 11d) [190]. Porosity and the pore size of macrogels leveraged the loading and amplified the colonization of L. plantarum cells. CNF gels prepared from dietary fibers with selective permeability may be an excellent prospect for targeted delivery in the gastrointestinal tract.
Figure 11.
CNF-based hydrogel beads for the controlled release of probiotics. (a) Cellulose fibrils undergo TEMPO-mediated oxidation, resulting in the carboxylate conjugation to the C6 of monomers (glucose). (b) The oxidized nanofibrils are mixed with cellulose fibers in an aqueous solution containing LiOH/urea/water and maintained at −20°C, followed by freeze-thawing and centrifugation. The product is added to a dilute hydrochloric acid solution to form the beads with various CNF-to-cellulose fiber ratios. (c) The bead pore size may be regulated by changing the concentration of CNF; the higher the CNF, the larger the pores. (d) The engineered porosity of gel beads and their pH-responsiveness originated from the carboxylic acid groups enable the controlled release of probiotics [190]. Reprinted with permission from [190]. Copyright (2018) American Chemical Society.
In addition to drug delivery, CNF composite hydrogels have been used as hemostatic wound dressing materials [229–231]. Calcium-mediated crosslinked anionic CNF, kaolin-CNF, and collagen-CNF composite hydrogels have been explored as potential hemostatic wound dressing biomaterials. CNF biocompatibility and ability to induce “good” hemostasis, defined as the rapid initiation of the coagulation cascade, platelet adhesion, and complement system activation, have been highlighted [184]. Kaolin, an aluminum silicate, was chosen as a hemostatic agent to activate the intrinsic pathway of coagulation. Collagen, commonly used as a hemostatic agent, contributed to the initial activation of platelets leading to hemostasis and promoting later phases of wound healing. TEMPO-mediated oxidation yielded highly charged CNFs, which were physically crosslinked with calcium ions to produce self-standing hydrogels.
CNF hydrogels resulted in C3a and sC5b-9 activation; however, the amounts were labeled as non-significant compared to the commercial dressing (AQUACEL® Extra), showing that the gels were not able to promote complement system activation [184]. However, the low complement activation by CNF hydrogels were beneficial for chronic wound healing and the treatment of early-stage wounds. Coagulation cascade was studied by incubating the hydrogels in whole blood, followed by measuring the platelet number and the level of thrombin-antithrombin (TAT), which showed significant reduction in platelet number and considerable TAT formation compared to the control (AQUACEL® Extra). The CNF-kaolin hydrogel displayed the most efficient coagulation activation through platelet reduction and TAT formation. All three hydrogels in comparison to the control showed greater thrombin peak height and faster time-to-thrombin-peak, indicating the endogenous thrombin potential correlated with a fast coagulation [184]. The hydrogels displayed an accelerated onset of thrombin formation compared to untreated platelet-free plasma (PFP) and AQUACEL® Extra. Thrombin and factor XII formation and platelet reduction attested to the potential of CNF-based hydrogels for effective blood coagulation and rapid clot formation. These biomaterials may be used for advance therapeutic wound dressing.
CNF has also been used to decrease the cytotoxicity of polymers, such as chitosan. The addition of CNF may help decrease the gel formation time, increase porosity, and improve the biocompatibility of hydrogels both in vitro and in vivo [232]. Natural thermosensitive injectable hydrogels can be prepared for minimally-invasive procedures using TOCNF and chitosan/glycerophosphate (CS/GP). A high GP content have cytotoxic effects on cells, however, adding TOCNF to the CS/GP hydrogels enhanced the proliferation and adhesion of fibroblast (L929) and pre-osteoblast (MC3T3-E1) cells. Subcutaneous injections in vivo showed that these hydrogels led to an inflammatory response in rats. After 14 days, tissue remodeling was evident with an increased M2 type macrophages, which indicated the commencement of wound healing. The hydrogels containing TOCNF accelerated wound healing by inducing a pro-inflammatory response after seven days of implantation [232].
4.3. CNF composite films
CNF films are mainly fabricated by spinning [233], filtration/drying [234], spray coating [235,236], electrospinning [237], and layer-by-layer assembly techniques [235]. Electrospinning produces nanofibrous scaffolds from a polymer base, which attain nanoporous structures, flexibility, and a high surface area-to-volume ratio, all of which are suitable for drug delivery purposes. CNF films may entrap poorly-soluble drugs and provide enhanced drug loading and sustained release for medical conditions such as inflammation and cancer. An important example is the inflammatory bowel diseases [237], demanding colon-specific drug delivery to overcome the challenges associated with the reduced proteolytic activity. In this case, an extended residence time is a favorable property for the release and absorption of drugs and proteins.
Film coating used for colonic drug delivery relies on colonic enzyme-degradable biomaterials, such as resistant starch (RS) produced by the hydrothermal modification of starch [238], which often lacks structural integrity. Polymer composites are commonly used to leverage the performance of film-based cargo delivery agents. Pectin is a biodegradable, biocompatible, and nontoxic heteropolysaccharide that protects the films from amylase- and protease-induced digestion [239]. Nanocelluloses, such as CNF and BNC, are a viable solution to leverage the performance of RS/P nanocomposite films. Macroscopic evaluation of CNF and BNC-RS/P nanocomposites showed that the films with a higher cellulose nanofiber concentration were thicker [180]. Field emission gun SEM indicated that the BNC covered the surface of the samples, while the CNF films showed more homogenous mixing. However, CNF was more effective than BNC in increasing the elongation without breaking, yielding elongation at break ~ 5.4 to 9.2 times higher than the control [180]. These improved properties are likely a result of the hydrogen bonds between the film and the crystalline/amorphous regions of the nanocelluloses. Additionally, water vapor permeability (WVP) measurements showed that the films provided effective water protection with 3.9–5.8 times reduced permeability due to the nanocellulose-filled pores [180]. The incorporation of nanocelluloses in the films may also contribute to the sustained release of drugs. For example, MTX had the highest release from RS/P control film and the lowest from the RS/P-0.5CNF, verifying that the CNF and BNC films with reduced porosity yielded prolonged drug release [180]. These findings suggest that nanocellulose-reinforced RS/P films may be a cost-efficient and viable oral drug delivery system for insoluble drugs to treat bowel-inflammatory diseases. Furthermore, the transdermal delivery of drugs, such as Ketorolac tromethamine (KT, a non-steroidal analgesic) using CNF-based nanocomposite films (e.g., with chitosan) has alternatively been explored [240].
Ion-responsive drug delivery platforms, such as microcapsules, have been fabricated from composites of CNF with hemicellulose (xyloglucan) and pectin. These polysaccharides are typically non-immunogenic, non-toxic, biocompatible, and often biodegradable. Ion-mediated changes in permeability enables these microcapsules to perform as on/off switches, which may be engineered by tuning the doping concentration of CNF and hemicellulose. For example, increasing hemicellulose content decreases the ion sensitivity of CNF-based systems [187]. To overcome the poor binding of hemicellulose and pectin (sourced from apple, AP) to CNF, multilayer CNF/Xyloglucan-amyloid (XyG)/AP with various compositions and layers have been coated on sacrificial spherical calcium carbonate microparticles. To decrease the AP/XyG incompatibility, pectin was used to increase the stability of microcapsule walls. In water, capsule walls swell and absorb dextran (e.g., 20 kDa and 70 kDa) [187,188]. The pore size and permeability may be controlled using salts; for example, a NaCl solution decreases the permeability as a result of pore size shrinkage.
CNF films provide a prolonged, sustained release (e.g., up to several months) of poorly water-soluble drugs, such as beclomethasone dipropionate, indomethacin, and itraconazole [234]. Typically, a CNF suspension is mixed with poorly water-soluble drugs, followed by filtration to collect the layered CNF network embedding the undissolved drugs. Next, CNF undergoes hornification (irreversible inter-fiber binding), forming a drug-loaded film. Compared to spray drying, this approach provides higher drug loading and entrapment efficacies (> 90%). Interestingly, the films attain excellent mechanical properties and readily hold a desirable shape [234]. The immediate release of drugs, such as indomethacin, from CNF nanopapers is also plausible. Engineering the fiber structures, a short release time (~ 10–20 min) has been achieved [241]. This has been attributed to the CNF-mediated drug solubility improvement, large pores, and the low tortuosity of the composites.
CNF nanocomposites for drug delivery may be fabricated using inorganic nanoparticles, such as titanium oxide [242] and magnetite. Magnetite (Fe3O4)-silver(I) oxide (Ag2O) quantum dots/cellulose fiber nanocomposites have been fabricated to deliver a chemotherapeutic cocktail of etoposide and MTX for lung, breast, and skin cancer [185]. The drugs were dissolved in water and added to the aqueous dispersion of Fe3O4-Ag2O quantum dots/cellulose fibers, followed by drying. The release of etoposide and MTX was then evaluated at physiological pH. Etoposide was released at a constant rate, recording ~ 79% in the buffer after 24 h, and the MTX was released at a prolonged constant rate, requiring a longer incubation time to reach equilibrium: ~ 64% release after 30 h [185]. The different release kinetics of drugs may be explained by the difference in the solubilities: etoposide is more soluble than methotrexate and can readily be released. This nanocomposite was a safe drug delivery system that targeted melanoma cancer cells.
Solubilized celluloses, such as CMC and hydroxypropyl cellulose (HPC), are an important class of celluloses, which play a key role in developing nanocellulose films [243]. As an example, for the delivery of sulfisoxazole (SFS), a weakly acidic and insoluble sulfonamide antibacterial drug, HPC has been added to the inclusion complex (IC) of cyclodextrin (CD), a non-covalent complex that binds the poorly soluble drug to the hydrophobic cavity of the water-soluble CD structure [185]. The release profile was biphasic in which an initial fast release followed by a slower, constant release over 12 h were achieved. Because of the large surface area of nanofibers, the total amount of SFS released from the HPC/SFS/HPβCD-IC-nanofibers was significantly greater than the amount released from both HPC/SFS/HPβCD-IC films and SFS powder samples. Furthermore, the HPC/SFS/HPβCD-IC-nanofibers initially released SFS slower than the nanofiber-free film [185].
4.4. CNF aerogels/foams
CNF aerogels/foams (also known as nanofoams) provide sustainable drug delivery platforms with positive buoyancy [244,245]. Drugs, often demonstrating poor solubility in aqueous media with a short absorption window, may be readily loaded in CNF aerogels. The rheological, barrier, and physicochemical properties, readily formable structures (particles, capsules), and the ability to stabilize oil/water and air/water interfaces along with large surface area-to-volume ratios enable CNF to perform as a decent excipient of poorly water-soluble drugs [246]. This can induce a tunable and prolonged residence time of drugs and improve their bioavailability. Dry CNF-based aerogels/foams are macroporous materials, fabricated from hydrogels in which the liquid has been replaced by gas (air) [48]. Typically, the hydrogel undergoes freezing, solvent-exchange, or foaming in the presence of surfactants, followed by the direct removal of frozen solvent, e.g., through sublimation. The release profile may be controlled by tailoring the physicochemical interactions between the cellulose matrix and nanoparticles. Different sources of CNF have different release capability, which may be used to engineer aerogels for immediate or sustained release.
Wet foams may be prepared using cationic CNF and lauric acid sodium salt (surfactant) to bind drugs, such as riboflavin, which can be followed by drying to obtain dry foams [247]. This platform absorbs drugs in its flexible structure that may be molded/cut into different shapes and sizes. Maintaining drug loading capacity and carrier weight constant, the drug release kinetics may be modified by changing the foam size. For example, after 24 h, an 8 mm thick rectangular foam released ~ 59% of the drug, whereas a 0.6 mm thick foam was able to release 78% of the drug in 8 h. The release rate may be accelerated by using a thinner foam: a 0.009 mm thick foam with the same outer surface area and total mass released ~ 72% of the drug within 1 h. CNF aerogels have been explored as gastroretentive drug delivery vehicles [186]. Important properties of CNF aerogels that need to be characterized for optimal drug delivery encompass surface morphology, swelling ratio at different pH, floating behavior (the time that the material remains buoyant), tensile strength, mucoadhesion, and pH-dependent drug release.
Cargos are typically loaded in the aerogels by physical absorption/adsorption, which is directly correlated to the fiber concentration: the higher the fiber content, the higher the loading capacity. The swelling ratio of CNF aerogels in physiological media depends on the nanocellulose charge content. As an example, a swelling ratio ~ 200% at physiological pH has been recorded after 24 h, which was affected by the accessibility of hydrophilic sites [186]. The drug-loaded CNF may remain floated for several hours due to the air entrapment in the aerogel. Drug loading may decrease the mucoadhesive strength of the aerogels: drug-loaded CNF aerogels had an adhesion strength ~ 18.19±3.64 g/cm2, which was lower than the CNF aerogels (36.9±3.8 g/cm2). This was attributed to the decrease in the surface area of drug-loaded CNF [186]. Similarly, tensile strength was lower in drug-loaded CNF. After 24 h, ~ 69% of the drug was released at pH ~ 1.2, whereas ~ 78% was released at pH ~ 7.4. A pharmacokinetic study showed that the aerogels were able to improve the oral bioavailability of drug by controlling the release rate [186]. The high surface area, unique swelling tendency, and hygroscopic nature of CNF enhance physical drug loading and controlled release. The excellent floating capacity and suitable mucoadhesion renders CNF aerogels an ideal candidate for gastroretentive drug delivery. Some examples of CNF-based cargo delivery platforms are summarized in Figure 12.
Figure 12.
An example of CNF production methods and CNF-based biomaterials for cargo delivery. (a) Extraction of CNF from trees via the physical disintegration of chemically-modified cellulose fibrils [248], Copyright (2011), with permission from Elsevier. (b) Hydrogels made up of carboxylated CNF and chitosan for the controlled release of 5-FU [189], reprinted with permission from [189], copyright (2018) American Chemical Society. (c) CNF nanocomposites for chemotherapeutic delivery via direct drug binding to Fe3O4-Ag2O quantum dot-decorated CNF [185], copyright (2017), with permission from Elsevier. (d) CNF foams, providing a sustained release of active molecules (riboflavin) as a result of increased diffusion path [247], copyright (2016), with permission from Elsevier. (e) Concentrated CNF dispersions readily form a shear-thinning hydrogel, which may be used for the delivery of anti-cancer drugs, such as metformin (Met) [183] (Copyright (2017), with permission from Elsevier) and Dox [191] (Copyright (2018), with permission from John Wiley and Sons) for treating melanoma.
5. Translational aspects of nanocellulose-based cargo delivery
Despite numerous in vitro and a few in vivo studies focused on characterizing nanoengineered celluloses crystals and fibers for cargo delivery applications, translating these classes of nanomaterials from bench to bedside is contingent upon (i) gaining a thorough understanding of the physical and biological challenges associated with in vivo colloidal stability, targeting, permeability and uptake, cytotoxicity, hemocompatibility, and bioaccumulation, (ii) large-scale production, processing, analysis, sterilization, and quality control, (iii) cost optimization and competitiveness against current technologies, and (iv) regulatory criteria. Nanocelluloses are produced in a variety of morphologies, sizes, crystal structures and content, and surface chemistry [249] each of which may have a unique in vivo behavior. Provided the high cost of animal studies and clinical trials, the choice of a base nanocellulose platform that meets the translational criteria is pivotal for the successful commercialization of this technology. Besides the comprehensive in vivo evaluation of commercially available nanocelluloses, their production must be revisited to enable good manufacturing practice (GMP), followed by a cost estimation for characterizations [250], such as handling, drying, sterilization, redispersion, surface charge determination, elemental analysis, and imaging.
Safety and life cycle are other important translational aspects nanocellulose-based delivery technologies. Cellulose is typically not absorbed systemically following oral administration, therefore it may have insignificant toxic potential, which renders it a generally-recognized-as-safe (GRAS)-listed material for oral use. GRAS designation may not apply to parenteral or pulmonary drug products. Pulmonary toxicity must be thoroughly assessed as a risk for instilled/inspired/inhaled nanocellulosic materials in diverse forms [208,251–255]. Despite some reports on the dose-dependent toxicity of nanocelluloses, this class of nanomaterials are mainly distinguished as non-cytotoxic under regulated dosages. As an example, bacterial cellulose has been identified non-irritating and biocompatible, which does not trigger a harsh inflammatory reaction and has a longstanding history of safe human consumption in Pacific countries [256]. However, according to the ISO-10993 standard for Biological Evaluation of Medical Devices [257], the extractables/leachables of nanocelluloses must be assessed with respect to biological and chemical risks. Immunogenic substances have been listed as the products of nanocelluloses, regulated by the source and surface functionality [68], which must be thoroughly assessed to ensure safe biomedical translation.
6. Conclusions
Advances in nanoengineering cellulose, the most abundant biopolymer in the world, have leveraged the controlled delivery of a broad range of bioactive cargos, including chemotherapeutic agents, anti-inflammatory drugs, and antimicrobial compounds. Nanocelluloses, commonly synthesized from the physical and/or chemical treatment of cellulose fibrils, one of the main building blocks of plant cell walls, or produced by microorganisms, such as certain bacteria, may provide cost-effective, accessible, easy to use, biocompatible, and green delivery platforms, which often benefit from hierarchical structures, ready-to-functionalize surfaces, tunable morphologies, shapes, and aspect ratios, high surface area, adjustable surface charges, mechanically strong backbone, and low or even no toxicity. Depending on the target cargo and organ, nanocelluloses may be used as individual crystals, such as CNC, bacterial CNC, and HCNC, or nanofibrils, e.g., CNF. Hydrated or dry networks of nanocelluloses with engineered mechanical properties, stability, and cargo loading/release profiles may be fabricated in the forms of hydrogels, films, and aerogels individually or combined with other polymers and nanoparticles. Besides accommodating poorly soluble drugs, nanocelluloses have been able to regulate the hydrophilicity, swelling ratio, and pH- and ion-responsiveness of carriers, which are all favorable for the effective loading and controlled release of bioactive molecules. Challenges ahead of this field encompass understanding the behavior of nanocelluloses inside the body in terms of circulation efficiency, immunomodulation, biodegradation, biodistribution, and long-term toxicity. We speculate that the emergence of the novel types of nanocelluloses, including hairy cellulose nanocrystals bearing crystalline (resistant against biodegradation) and amorphous (readily biodegradable) cellulosic regions opens new horizons for customizable cargo delivery with applications in precision medicine.
Synopsis.
Engineering certain types of microorganisms as well as the hierarchical structure of cellulose fibers, one of the main building blocks of plant cell walls, has yielded unique families of cellulose-based nanomaterials, which have leveraged the effective delivery of bioactive molecules.
Acknowledgments
A.S. thanks the financial support from the Canadian Institutes of Health Research (CIHR) through a post-doctoral fellowship. A.K. would like to acknowledge funding from the National Institutes of Health (EB021857, AR066193, AR057837, CA214411, HL137193, EB024403, EB023052).
List of abbreviations
- CNC
Cellulose nanocrystals
- CNF
cellulose nanofibrils
- BC
bacterial cellulose
- HCNC
hairy cellulose nanocrystals
- BNC
bacterial nanocellulose
- BCNC
bacterial cellulose nanocrystals
- Dox
doxorubicin
- BDP
beclomethasone dipropionate
- ITR
itraconazole
- PBS
phosphate buffered saline
- CTAB
cetyltrimethylammonium bromide
- PLA
poly(lactic acid)
- RBITC
rhodamine B isothiocyanate
- FITC
fluorescein-5-isothiocyanate
- BSA
bovine serum albumin
- HSA
human serum albumin
- MTX
methotrexate
- 5-FU
5-Fluorouracil
- TH
tetracycline hydrochloride
- Cur
curcumin
- KT
ketorolac tromethamine
- DCF
diclofenac sodium salt
- ANG
angiogenin
- siRNA
small (or short) interfering RNA
- RPG
repaglinide
- HEK-293
human embryonic kidney 293
- Sf9
spodoptera frugiperda
- RNase
ribonuclease
- HBMEC
human brain microvascular endothelial cells
- HUVEC
human umbilical vein endothelial cells
- FA
folic acid
- MePyrrHSO4
N-methylpyrrolidinium hydrogen sulfate
- Cltx
chlorotoxin
- GBM
glioblastoma multiforme
- MMPs
matrix metalloproteinases
- DAPI
4′,6-diamidino-2-phenylindole
- MCF-7
Michigan Cancer Foundation-7
- AMFC
Tris(2-aminoethyl)amine
- MNP
magnetic nanoparticles
- XG
xanthan gum
- CS
chitosan
- Coll
collagen
- GM
gelatin microspheres
- PMC
polyelectrolyte macroion complexes
- PLGA
poly (D L-lactic-co-glycolic acid)
- PEI
polyethyleneimine
- CMCS
carboxymethyl chitosan
- pDNA
plasmid DNA
- PEG
polyethylene glycol
- PVA
poly(vinyl alcohol)
- TGA
thermogravimetric analysis
- PI
povidone-iodine
- PHMB
polihexanide
- LC
lethal concentration
- IC
inhibitory concentration
- HMP
high-methoxy pectin
- Levo
levofloxacin
- FuA
fusidic acid
- SGF
simulated gastric fluid
- SIF
simulated intestinal fluid
- SEM
scanning electron microscopy
- SD
sulfadiazine
- IBU
ibuprofen
- GO
graphene oxide
- CMC
carboxymethyl cellulose
- LbL
layer-by-layer
- AB
antibody assay
- TNF-α
tumor necrosis factor alpha
- IL1-β
interleukin 1 beta
- ECM
extracellular matrix
- Met
metformin
- Met-CNF
metformin-modified cellulose nanofiber
- TEMPO
2,2,6,6-tetramethylpiperidine-1-oxyl
- TOCNF
TEMPO-oxidized cellulose nanofibrils
- PFP
platelet-free plasma
- GP
glycerophosphate
- P
pectin
- WVP
water vapor permeability
- XyG
xyloglucan-amyloid
- AP
pectin from apple
- HPC
hydroxypropyl cellulose
- IC
inclusion complex
- CD
cyclodextrin
- SFS
sulfisoxazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].O’sullivan A, Cellulose: the structure slowly unravels, Cellulose 4 (1997) 173–207. doi: [Google Scholar]
- [2].Rånby BG, The Colloidal Properties of Cellulose Micelles, Discuss. Faraday Soc 11 (1951) 158–164. [Google Scholar]
- [3].Hon DN-S, Cellulose: a random walk along its historical path, Cellulose 1 (1994) 1–25. [Google Scholar]
- [4].Fan L, Gharpuray MM, Lee Y-H, Nature of Cellulosic Material, in: Fan L, Gharpuray MM, Lee Y-H (Eds.), Cellul. Hydrolysis. Biotechnol. Monogr, Springer Berlin Heidelberg, Berlin, Heidelberg, 1987: pp. 5–20. doi: 10.1007/978-3-642-72575-3_2. [DOI] [Google Scholar]
- [5].Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J, Cellulose nanomaterials review: structure, properties and nanocomposites., Chem. Soc. Rev 40 (2011) 3941–3994. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- [6].Medronho B, Lindman B, Competing forces during cellulose dissolution: From solvents to mechanisms, Curr. Opin. Colloid Interface Sci 19 (2014) 32–40. doi: 10.1016/j.cocis.2013.12.001. [DOI] [Google Scholar]
- [7].Habibi Y, Lucia LA, Rojas OJ, Cellulose Nanocrystals : Chemistry , Self-Assembly , and Applications, d (2010) 3479–3500. doi: 10.1021/cr900339w. [DOI] [PubMed] [Google Scholar]
- [8].van de Ven TGM, Sheikhi A, Hairy cellulose nanocrystalloids: A novel class of nanocellulose, Nanoscale 8 (2016). doi: 10.1039/c6nr01570k. [DOI] [PubMed] [Google Scholar]
- [9].Sheikhi A, van de Ven TGM, Colloidal aspects of Janus-like hairy cellulose nanocrystalloids, Curr. Opin. Colloid Interface Sci 29 (2017) 21–31. doi: 10.1016/j.cocis.2017.02.001. [DOI] [Google Scholar]
- [10].Nechyporchuk O, Belgacem MN, Bras J, Production of cellulose nanofibrils: A review of recent advances, Ind. Crops Prod 93 (2016) 2–25. doi: 10.1016/j.indcrop.2016.02.016. [DOI] [Google Scholar]
- [11].Zimmermann T, Bordeanu N, Strub E, Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential, Carbohydr. Polym 79 (2010) 1086–1093. doi: 10.1016/j.carbpol.2009.10.045. [DOI] [Google Scholar]
- [12].Siró I, Plackett D, Microfibrillated cellulose and new nanocomposite materials: a review, Cellulose 17 (2010) 459–494. doi: 10.1007/s10570-010-9405-y. [DOI] [Google Scholar]
- [13].Abdul Khalil HPS, Davoudpour Y, Islam MN, Mustapha A, Sudesh K, Dungani R, Jawaid M, Production and modification of nanofibrillated cellulose using various mechanical processes: A review, Carbohydr. Polym 99 (2014) 649–665. doi: 10.1016/j.carbpol.2013.08.069. [DOI] [PubMed] [Google Scholar]
- [14].Iguchi M, Yamanaka S, Budhiono A, Bacterial cellulose—a masterpiece of nature’s arts, J. Mater. Sci 35 (2000) 261–270. [Google Scholar]
- [15].Reiniati I, Hrymak AN, Margaritis A, Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals, Crit. Rev. Biotechnol 37 (2017) 510–524. doi: 10.1080/07388551.2016.1189871. [DOI] [PubMed] [Google Scholar]
- [16].Vasconcelos NF, Feitosa JPA, da Gama FMP, Morais JPS, Andrade FK, de Souza Filho M. de S.M., Rosa M. de F., Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features, Carbohydr. Polym 155 (2017) 425–431. doi: 10.1016/j.carbpol.2016.08.090. [DOI] [PubMed] [Google Scholar]
- [17].Brinchi L, Cotana F, Fortunati E, Kenny JM, Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications, Carbohydr. Polym 94 (2013) 154–169. doi: 10.1016/j.carbpol.2013.01.033. [DOI] [PubMed] [Google Scholar]
- [18].Yang H, Tejado A, Alam N, Antal M, van de Ven TGM, Films prepared from electrosterically stabilized nanocrystalline cellulose, Langmuir 28 (2012) 7834–7842. doi: 10.1021/la2049663. [DOI] [PubMed] [Google Scholar]
- [19].Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A, Nanocelluloses: A new family of nature-based materials, Angew. Chem. Int. Ed 50 (2011) 5438–5466. doi: 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- [20].Kontturi E, Laaksonen P, Linder MB, Gröschel AH, Rojas OJ, Ikkala O, Advanced Materials through Assembly of Nanocelluloses, Adv. Mater (2018) 1703779. [DOI] [PubMed] [Google Scholar]
- [21].Sheikhi A, Emerging Cellulose-Based Nanomaterials and Nanocomposites, in: Nanomater. Polym. Nanocomposites, Elsevier, 2019: pp. 307–351. [Google Scholar]
- [22].Voisin H, Bergström L, Liu P, Mathew AP, Nanocellulose-based materials for water purification, Nanomaterials 7 (2017) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carpenter AW, de Lannoy C-F, Wiesner MR, Cellulose Nanomaterials in Water Treatment Technologies, Environ. Sci. Technol 49 (2015) 5277–5287. doi: 10.1021/es506351r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Putro JN, Kurniawan A, Ismadji S, Ju Y-H, Nanocellulose based biosorbents for wastewater treatment: Study of isotherm, kinetic, thermodynamic and reusability, Environ. Nanotechnology, Monit. Manag 8 (2017) 134–149. [Google Scholar]
- [25].Zhu H, Luo W, Ciesielski PN, Fang Z, Zhu JY, Henriksson G, Himmel ME, Hu L, Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications, Chem. Rev 0 (n.d.) null. doi: 10.1021/acs.chemrev.6b00225. [DOI] [PubMed] [Google Scholar]
- [26].Muhd Julkapli N, Bagheri S, Nanocellulose as a green and sustainable emerging material in energy applications: a review, Polym. Adv. Technol (2017). [Google Scholar]
- [27].Soriano ML, Ruiz- Palomero C, Nanocellulose as Promising Material for Environmental Applications, Nanotechnol. Environ. Sci (2018) 579–598. [Google Scholar]
- [28].M.K. and Moores A, Review: nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis, Green Chem (2016) 622–637. doi: 10.1039/c5gc02500a. [DOI] [Google Scholar]
- [29].Wilts BD, Dumanli AG, Middleton R, Vukusic P, Vignolini S, Invited Article: Chiral optics of helicoidal cellulose nanocrystal films, APL Photonics 2 (2017) 40801. [Google Scholar]
- [30].Wu T, Li J, Li J, Ye S, Wei J, Guo J, A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties, J. Mater. Chem. C 4 (2016) 9687–9696. [Google Scholar]
- [31].Korolovych VF, Cherpak V, Nepal D, Ng A, Shaikh NR, Grant A, Xiong R, Bunning TJ, V Tsukruk V, Cellulose nanocrystals with different morphologies and chiral properties, Polymer (Guildf). (n.d.) doi: 10.1016/j.polymer.2018.04.064. [DOI] [Google Scholar]
- [32].Golmohammadi H, Morales-Narváez E, Naghdi T, Merkoçi A, Nanocellulose in Sensing and Biosensing, Chem. Mater 29 (2017) 5426–5446. doi: 10.1021/acs.chemmater.7b01170. [DOI] [Google Scholar]
- [33].Jorfi M, Foster EJ, Recent advances in nanocellulose for biomedical applications, J. Appl. Polym. Sci 132 (2015). [Google Scholar]
- [34].Lin N, Dufresne A, Nanocellulose in biomedicine: Current status and future prospect, Eur. Polym. J 59 (2014) 302–325. doi: 10.1016/j.eurpolymj.2014.07.025. [DOI] [Google Scholar]
- [35].Pachuau L, Application of Nanocellulose for Controlled Drug Delivery, Nanocellulose Nanohydrogel Matrices Biotechnol. Biomed. Appl (2017) 1–19. [Google Scholar]
- [36].Pushpamalar J, Veeramachineni AK, Owh C, Loh XJ, Biodegradable Polysaccharides for Controlled Drug Delivery, Chempluschem (2016). [DOI] [PubMed] [Google Scholar]
- [37].Shelke NB, James R, Laurencin CT, Kumbar SG, Polysaccharide biomaterials for drug delivery and regenerative engineering, Polym. Adv. Technol 25 (2014) 448–460. [Google Scholar]
- [38].Ullah H, Santos HA, Khan T, Applications of bacterial cellulose in food, cosmetics and drug delivery, Cellulose 23 (2016) 2291–2314. [Google Scholar]
- [39].Roman M, Toxicity of Cellulose Nanocrystals: A Review, Ind. Biotechnol 11 (2015) 25–33. doi: 10.1089/ind.2014.0024. [DOI] [Google Scholar]
- [40].Elazzouzi-Hafraoui S, Nishiyama Y, Putaux J-L, Heux L, Dubreuil F, Rochas C, The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose, Biomacromolecules 9 (2007) 57–65. [DOI] [PubMed] [Google Scholar]
- [41].D. Beck-Candanedo S; Roman M; Gray, Effect of conditions on the properties behavior of wood cellulose nanocrystals suspensions, Biomacromolecules 6 (2005) 1048–1054. [DOI] [PubMed] [Google Scholar]
- [42].Lin N, Huang J, Dufresne A, Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review, Nanoscale 4 (2012) 3274. doi: 10.1039/c2nr30260h. [DOI] [PubMed] [Google Scholar]
- [43].Jonoobi M, Oladi R, Davoudpour Y, Oksman K, Dufresne A, Hamzeh Y, Davoodi R, Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review, Cellulose 22 (2015) 935–969. [Google Scholar]
- [44].Le Bras D, Strømme M, Mihranyan A, Characterization of dielectric properties of nanocellulose from wood and algae for electrical insulator applications, J. Phys. Chem. B 119 (2015) 5911–5917. [DOI] [PubMed] [Google Scholar]
- [45].Lee K-Y, Nanocellulose and Sustainability: Production, Properties, Applications, and Case Studies, CRC Press, 2018. [Google Scholar]
- [46].Tashiro K, Kobayashi M, Calculation of crystallite modulus of native cellulose, Polym. Bull 14 (1985) 213–218. [Google Scholar]
- [47].Jaswon MA, Gillis PP, Mark RE, The elastic constants of crystalline native cellulose, Proc. R. Soc. Lond. A 306 (1968) 389–412. [Google Scholar]
- [48].De France KJ, Hoare T, Cranston ED, Review of hydrogels and aerogels containing nanocellulose, Chem. Mater 29 (2017) 4609–4631. [Google Scholar]
- [49].Oguzlu H, Danumah C, Boluk Y, Colloidal behavior of aqueous cellulose nanocrystal suspensions, Curr. Opin. Colloid Interface Sci 29 (2017) 46–56. doi: 10.1016/j.cocis.2017.02.002. [DOI] [Google Scholar]
- [50].Liu D, Chen X, Yue Y, Chen M, Wu Q, Structure and rheology of nanocrystalline cellulose, Carbohydr. Polym 84 (2011) 316–322. doi: 10.1016/j.carbpol.2010.11.039. [DOI] [Google Scholar]
- [51].Lenfant G, Heuzey MC, van de Ven TGM, Carreau PJ, Intrinsic viscosity of suspensions of electrosterically stabilized nanocrystals of cellulose, Cellulose (2015) 1109–1122. doi: 10.1007/s10570-015-0573-7. [DOI] [Google Scholar]
- [52].Shafiei-Sabet S, Hamad WY, Hatzikiriakos SG, Rheology of nanocrystalline cellulose aqueous suspensions, Langmuir 28 (2012) 17124–17133. [DOI] [PubMed] [Google Scholar]
- [53].Mendoza L, Gunawardhana T, Batchelor W, Garnier G, Effects of fibre dimension and charge density on nanocellulose gels, J. Colloid Interface Sci 525 (2018) 119–125. [DOI] [PubMed] [Google Scholar]
- [54].Mendoza L, Batchelor W, Tabor RF, Garnier G, Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective, J. Colloid Interface Sci 509 (2018) 39–46. [DOI] [PubMed] [Google Scholar]
- [55].Nordenström M, Fall A, Nystörm G, Wågberg L, Formation of Colloidal Nanocellulose Glasses and Gels, Langmuir 33 (2017) 9772–9780. [DOI] [PubMed] [Google Scholar]
- [56].George J, Sabapathi SN, Cellulose nanocrystals: synthesis, functional properties, and applications, Nanotechnol. Sci. Appl 8 (2015) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Habibi Y, Key advances in the chemical modification of nanocelluloses, Chem. Soc. Rev 43 (2014) 1519–1542. doi: 10.1039/c3cs60204d. [DOI] [PubMed] [Google Scholar]
- [58].Varjonen S, Laaksonen P, Paananen A, Valo H, Hähl H, Laaksonen T, Ben Linder M, Self-assembly of cellulose nanofibrils by genetically engineered fusion proteins, Soft Matter 7 (2011) 2402–2411. [Google Scholar]
- [59].Valo H, Kovalainen M, Laaksonen P, Häkkinen M, Auriola S, Peltonen L, Linder M, Järvinen K, Hirvonen J, Laaksonen T, Immobilization of protein-coated drug nanoparticles in nanofibrillar cellulose matrices—Enhanced stability and release, J. Control. Release 156 (2011) 390–397. doi: 10.1016/j.jconrel.2011.07.016. [DOI] [PubMed] [Google Scholar]
- [60].Hosseinidoust Z, Alam MN, Sim G, Tufenkji N, van de Ven TGM, Cellulose nanocrystals with tunable surface charge for nanomedicine, Nanoscale 7 (2015) 16647–16657. doi: 10.1039/x0xx00000x. [DOI] [PubMed] [Google Scholar]
- [61].Carlsson DO, Hua K, Forsgren J, Mihranyan A, Aspirin degradation in surface-charged TEMPO-oxidized mesoporous crystalline nanocellulose, Int. J. Pharm 461 (2014) 74–81. [DOI] [PubMed] [Google Scholar]
- [62].Koshani R, Madadlou A, A viewpoint on the gastrointestinal fate of cellulose nanocrystals, Trends Food Sci. Technol 71 (2018) 268–273. [Google Scholar]
- [63].Pereira MM, Raposo NRB, Brayner R, Teixeira EM, Oliveira V, Quintão CCR, Camargo LSA, Mattoso LHC, Brandão HM, Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers, Nanotechnology 24 (2013) 75103. [DOI] [PubMed] [Google Scholar]
- [64].Hua K, Carlsson DO, Ålander E, Lindström T, Strømme M, Mihranyan A, Ferraz N, Translational study between structure and biological response of nanocellulose from wood and green algae, Rsc Adv 4 (2014) 2892–2903. [Google Scholar]
- [65].Kim G-D, Yang H, Park HR, Park C-S, Park YS, Lee SE, Evaluation of immunoreactivity of in vitro and in vivo models against bacterial synthesized cellulose to be used as a prosthetic biomaterial, BioChip J 7 (2013) 201–209. [Google Scholar]
- [66].Chen YM, Xi T, Zheng Y, Guo T, Hou J, Wan Y, Gao C, In vitro cytotoxicity of bacterial cellulose scaffolds used for tissue-engineered bone, J. Bioact. Compat. Polym 24 (2009) 137–145. [Google Scholar]
- [67].Il Jeong S, Lee SE, Yang H, Jin Y-H, Park C-S, Park YS, Toxicologic evaluation of bacterial synthesized cellulose in endothelial cells and animals, Mol. Cell. Toxicol 6 (2010) 370–377. [Google Scholar]
- [68].Liu J, Bacher M, Rosenau T, Willför S, Mihranyan A, Potentially Immunogenic Contaminants in Wood-based and Bacterial Nanocellulose: Assessment of Endotoxin and (1, 3)-β-D-glucan Levels, Biomacromolecules 19 (2017) 150–157. [DOI] [PubMed] [Google Scholar]
- [69].Klemm D, Cranston ED, Fischer D, Gama M, Kedzior SA, Kralisch D, Kramer F, Kondo T, Lindström T, Nietzsche S, Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state, Mater. Today (2018). [Google Scholar]
- [70].Dufresne A, Polysaccharide nano crystal reinforced nanocomposites, Can. J. Chem 86 (2008) 484–494. [Google Scholar]
- [71].Kalia S, Dufresne A, Cherian BM, Kaith BS, Avérous L, Njuguna J, Nassiopoulos E, Cellulose-based bio-and nanocomposites: a review, Int. J. Polym. Sci. 2011 (2011). [Google Scholar]
- [72].Xu X, Liu F, Jiang L, Zhu JY, Haagenson D, Wiesenborn DP, Cellulose nanocrystals vs. cellulose nanofibrils: a comparative study on their microstructures and effects as polymer reinforcing agents, ACS Appl. Mater. Interfaces 5 (2013) 2999–3009. [DOI] [PubMed] [Google Scholar]
- [73].Wang Y, Chen L, Cellulose nanowhiskers and fiber alignment greatly improve mechanical properties of electrospun prolamin protein fibers, ACS Appl. Mater. Interfaces 6 (2014) 1709–1718. [DOI] [PubMed] [Google Scholar]
- [74].Jackson JK, Letchford K, Wasserman BZ, Ye L, Hamad WY, Burt HM, The use of nanocrystalline cellulose for the binding and controlled release of drugs., Int. J. Nanomedicine 6 (2011) 321–330. doi: 10.2147/IJN.S16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Xiang C, Taylor AG, Hinestroza JP, Frey MW, Controlled release of nonionic compounds from poly (lactic acid)/cellulose nanocrystal nanocomposite fibers, J. Appl. Polym. Sci 127 (2013) 79–86. [Google Scholar]
- [76].Cooper DL, Conder CM, Harirforoosh S, Nanoparticles in drug delivery: mechanism of action, formulation and clinical application towards reduction in drug-associated nephrotoxicity, Expert Opin. Drug Deliv 11 (2014) 1661–1680. doi: 10.1517/17425247.2014.938046. [DOI] [PubMed] [Google Scholar]
- [77].Hong F, Zhu YX, Yang G, Yang XX, Wheat straw acid hydrolysate as a potential cost- effective feedstock for production of bacterial cellulose, J. Chem. Technol. Biotechnol 86 (2011) 675–680. [Google Scholar]
- [78].Jozala AF, de Lencastre-Novaes LC, Lopes AM, de Carvalho Santos-Ebinuma V, Mazzola PG, Pessoa A Jr, Grotto D, Gerenutti M, Chaud MV, Bacterial nanocellulose production and application: a 10-year overview, Appl. Microbiol. Biotechnol 100 (2016) 2063–2072. [DOI] [PubMed] [Google Scholar]
- [79].Dufresne A, Nanocellulose: A new ageless bionanomaterial, Mater. Today 16 (2013) 220–227. doi: 10.1016/j.mattod.2013.06.004. [DOI] [Google Scholar]
- [80].Dong S, Cho HJ, Lee YW, Roman M, Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting, Biomacromolecules 15 (2014) 1560–1567. doi: 10.1021/bm401593n. [DOI] [PubMed] [Google Scholar]
- [81].Rescignano N, Fortunati E, Montesano S, Emiliani C, Kenny JM, Martino S, Armentano I, PVA bio-nanocomposites: A new take-off using cellulose nanocrystals and PLGA nanoparticles, Carbohydr. Polym 99 (2014) 47–58. doi: 10.1016/j.carbpol.2013.08.061. [DOI] [PubMed] [Google Scholar]
- [82].Rahimi M, Shojaei S, Safa KD, Ghasemi Z, Salehi R, Yousefi B, Shafiei-Irannejad V, Biocompatible magnetic tris(2-aminoethyl)amine functionalized nanocrystalline cellulose as a novel nanocarrier for anticancer drug delivery of methotrexate, New J. Chem 41 (2017) 2160–2168. doi: 10.1039/C6NJ03332F. [DOI] [Google Scholar]
- [83].Madhusudana Rao K, Kumar A, Han SS, Polysaccharide based bionanocomposite hydrogels reinforced with cellulose nanocrystals: Drug release and biocompatibility analyses, Int. J. Biol. Macromol 101 (2017) 165–171. doi: 10.1016/j.ijbiomac.2017.03.080. [DOI] [PubMed] [Google Scholar]
- [84].Yu HY, Wang C, Abdalkarim SYH, Cellulose nanocrystals/polyethylene glycol as bifunctional reinforcing/compatibilizing agents in poly(lactic acid) nanofibers for controlling long-term in vitro drug release, Cellulose 24 (2017) 4461–4477. doi: 10.1007/s10570-017-1431-6. [DOI] [Google Scholar]
- [85].Guo R, Lan Y, Xue W, Cheng B, Zhang Y, Wang C, Ramakrishna S, Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair, J. Tissue Eng. Regen. Med 11 (2017) 3544–3555. doi: 10.1002/term.2272. [DOI] [PubMed] [Google Scholar]
- [86].Mo Y, Guo R, Zhang Y, Xue W, Cheng B, Zhang Y, Controlled Dual Delivery of Angiogenin and Curcumin by Electrospun Nanofibers for Skin Regeneration, Tissue Eng. Part A 23 (2017) 597–608. doi: 10.1089/ten.tea.2016.0268. [DOI] [PubMed] [Google Scholar]
- [87].Ndong Ntoutoume GMA, Grassot V, Brégier F, Chabanais J, Petit JM, Granet R, Sol V, PEI-cellulose nanocrystal hybrids as efficient siRNA delivery agents—Synthesis, physicochemical characterization and in vitro evaluation, Carbohydr. Polym 164 (2017) 258–267. doi: 10.1016/j.carbpol.2017.02.004. [DOI] [PubMed] [Google Scholar]
- [88].Singla R, Soni S, Padwad YS, Acharya A, Yadav SK, Sustained delivery of BSA/HSA from biocompatible plant cellulose nanocrystals for in vitro cholesterol release from endothelial cells, Int. J. Biol. Macromol 104 (2017) 748–757. doi: 10.1016/j.ijbiomac.2017.06.068. [DOI] [PubMed] [Google Scholar]
- [89].Abo-Elseoud WS, Hassan ML, Sabaa MW, Basha M, Hassan EA, Fadel SM, Chitosan nanoparticles/cellulose nanocrystals nanocomposites as a carrier system for the controlled release of repaglinide, Int. J. Biol. Macromol 111 (2018) 604–613. doi: 10.1016/j.ijbiomac.2018.01.044. [DOI] [PubMed] [Google Scholar]
- [90].Cellante L, Costa R, Monaco I, Cenacchi G, Locatelli E, One-step esterification of nanocellulose in a Brønsted acid ionic liquid for delivery to glioblastoma cancer cells, New J. Chem 42 (2018) 5237–5242. doi: 10.1039/C7NJ04633B. [DOI] [Google Scholar]
- [91].Kettiger H, Schipanski A, Wick P, Huwyler J, Engineered nanomaterial uptake and tissue distribution: from cell to organism, Int. J. Nanomedicine 8 (2013) 3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fröhlich E, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int. J. Nanomedicine 7 (2012) 5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mahmoud KA, Mena JA, Male KB, Hrapovic S, Kamen A, Luong JHT, Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals, ACS Appl. Mater. Interfaces 2 (2010) 2924–2932. doi: 10.1021/am1006222. [DOI] [PubMed] [Google Scholar]
- [94].Thomas D, Latha MS, Thomas KK, Synthesis and in vitro evaluation of alginate-cellulose nanocrystal hybrid nanoparticles for the controlled oral delivery of rifampicin, J. Drug Deliv. Sci. Technol 46 (2018) 392–399. doi: 10.1016/j.jddst.2018.06.004. [DOI] [Google Scholar]
- [95].Zakeri A, Kouhbanani MAJ, Beheshtkhoo N, Beigi V, Mousavi SM, Hashemi SAR, Karimi Zade A, Amani AM, Savardashtaki A, Mirzaei E, Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon, Nano Rev. Exp 9 (2018) 1488497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kumar S, Rani R, Dilbaghi N, Tankeshwar K, Kim K-H, Carbon nanotubes: a novel material for multifaceted applications in human healthcare, Chem. Soc. Rev 46 (2017) 158–196. [DOI] [PubMed] [Google Scholar]
- [97].Sabra S, Abdelmoneem M, Abdelwakil M, Taha MM, Anwar D, Mohamed R, Khattab S, Bekhit A, Elkhodairy K, Freag M, Self-assembled nanocarriers based on amphiphilic natural polymers for anti-cancer drug delivery applications., Curr. Pharm. Des (2017). [DOI] [PubMed] [Google Scholar]
- [98].Maeki M, Kimura N, Sato Y, Harashima H, Tokeshi M, Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems, Adv. Drug Deliv. Rev (2018). [DOI] [PubMed] [Google Scholar]
- [99].Roman M, Dong S, Hirani A, Lee YW, Products F, Cellulose Nanocrystals for Drug Delivery, (2010) 81–91. [Google Scholar]
- [100].Eyley SS, Thielemans W, Surface modification of cellulose nanocrystals., Nanoscale 6 (2014) 7764–79. doi: 10.1039/c4nr01756k. [DOI] [PubMed] [Google Scholar]
- [101].Colacino KR, Arena CB, Dong S, Roman M, V Davalos R, Lee YW, Folate conjugated cellulose nanocrystals potentiate irreversible electroporation-induced cytotoxicity for the selective treatment of cancer cells, Technol. Cancer Res. Treat 14 (2015) 757–766. [DOI] [PubMed] [Google Scholar]
- [102].Neumann E, Frei E, Funk D, Becker MD, Schrenk H-H, Müller-Ladner U, Fiehn C, Native albumin for targeted drug delivery, Expert Opin. Drug Deliv 7 (2010) 915–925. [DOI] [PubMed] [Google Scholar]
- [103].Kragh-Hansen U, Chuang VTG, Otagiri M, Practical aspects of the ligand-binding and enzymatic properties of human serum albumin, Biol. Pharm. Bull 25 (2002) 695–704. [DOI] [PubMed] [Google Scholar]
- [104].Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P, The extraordinary ligand binding properties of human serum albumin, IUBMB Life 57 (2005) 787–796. [DOI] [PubMed] [Google Scholar]
- [105].Maciążek-Jurczyk M, Szkudlarek A, Chudzik M, Pożycka J, Sułkowska A, Alteration of human serum albumin binding properties induced by modifications: A review, Spectrochim. Acta Part A Mol. Biomol. Spectrosc 188 (2018) 675–683. [DOI] [PubMed] [Google Scholar]
- [106].Kragh-Hansen U, Molecular aspects of ligand binding to serum albumin., Pharmacol. Rev 33 (1981) 17–53. [PubMed] [Google Scholar]
- [107].Kragh-Hansen U, Structure and ligand binding properties of human serum albumin., Dan. Med. Bull 37 (1990) 57–84. [PubMed] [Google Scholar]
- [108].Parayath NN, Amiji MM, Therapeutic targeting strategies using endogenous cells and proteins, J. Control. Release 258 (2017) 81–94. [DOI] [PubMed] [Google Scholar]
- [109].Bhushan B, Khanadeev V, Khlebtsov B, Khlebtsov N, Gopinath P, Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery, Adv. Colloid Interface Sci 246 (2017) 13–39. doi: 10.1016/j.cis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- [110].Lombardo S, yley S, Sch tz C, Van Gorp H, Rosenfeldt S, Van den Mooter G, Thielemans W, Thermodynamic study of the interaction of bovine serum albumin and amino acids with cellulose nanocrystals, Langmuir 33 (2017) 5473–5481. [DOI] [PubMed] [Google Scholar]
- [111].Mallakpour S, Dinari M, Ionic liquids as green solvents: progress and prospects, in: Green Solvents II, Springer, 2012: pp. 1–32. [Google Scholar]
- [112].Cellante L, Costa R, Monaco I, Cenacchi G, Locatelli E, One-step esterification of nanocellulose in a Brønsted acid ionic liquid for delivery to glioblastoma cancer cells, New J. Chem 42 (2018) 5237–5242. [Google Scholar]
- [113].Supramaniam J, Adnan R, Kaus NHM, Bushra R, Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system, Int. J. Biol. Macromol In press (2018). doi: 10.1016/j.ijbiomac.2018.06.043. [DOI] [PubMed] [Google Scholar]
- [114].Gumrah Dumanli A, Nanocellulose and its composites for biomedical applications, Curr. Med. Chem 24 (2017) 512–528. [DOI] [PubMed] [Google Scholar]
- [115].HPS AK, Saurabh CK, Adnan AS, Fazita MRN, Syakir MI, Davoudpour Y, Rafatullah M, Abdullah CK, Haafiz MKM, Dungani R, A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications, Carbohydr. Polym 150 (2016) 216–226. [DOI] [PubMed] [Google Scholar]
- [116].Tummala GK, Joffre T, Rojas R, Persson C, Mihranyan A, Strain-induced stiffening of nanocellulose-reinforced poly (vinyl alcohol) hydrogels mimicking collagenous soft tissues, Soft Matter 13 (2017) 3936–3945. [DOI] [PubMed] [Google Scholar]
- [117].Kargarzadeh H, Mariano M, Huang J, Lin N, Ahmad I, Dufresne A, Thomas S, Recent developments on nanocellulose reinforced polymer nanocomposites: A review, Polymer (Guildf) 132 (2017) 368–393. doi: 10.1016/j.polymer.2017.09.043. [DOI] [Google Scholar]
- [118].Sampath UGTM, Ching YC, Chuah CH, Singh R, Lin P-C, Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel, Cellulose 24 (2017) 2215–2228. [Google Scholar]
- [119].Udeni Gunathilake TMS, Ching YC, Chuah CH, Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel, Polymers (Basel) 9 (2017) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wang H, Roman M, Formation and properties of chitosan− cellulose nanocrystal polyelectrolyte− macroion complexes for drug delivery applications, Biomacromolecules 12 (2011) 1585–1593. [DOI] [PubMed] [Google Scholar]
- [121].Cheng M, Qin Z, Hu S, Dong S, Ren Z, Yu H, Achieving Long-Term Sustained Drug Delivery for Electrospun Biopolyester Nanofibrous Membranes by Introducing Cellulose Nanocrystals, ACS Biomater. Sci. Eng 3 (2017) 1666–1676. [DOI] [PubMed] [Google Scholar]
- [122].Evdokimova OL, Svensson FG, V Agafonov A, Håkansson S, Seisenbaeva GA, Kessler VG, Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties, Nanomaterials 8 (2018) 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Poonguzhali R, Basha SK, Kumari VS, Synthesis of alginate/nanocellulose bionanocomposite for in vitro delivery of ampicillin, Polym. Bull (2018) 1–9. [Google Scholar]
- [124].Anirudhan TS, Nair SS, Deposition of gold-cellulose hybrid nanofiller on a polyelectrolyte membrane constructed using guar gum and poly (vinyl alcohol) for transdermal drug delivery, J. Memb. Sci 539 (2017) 344–357. [Google Scholar]
- [125].de Castro DO, Tabary N, Martel B, Gandini A, Belgacem N, Bras J, Controlled release of carvacrol and curcumin: bio-based food packaging by synergism action of TEMPO-oxidized cellulose nanocrystals and cyclodextrin, Cellulose (2017) 1–15. [Google Scholar]
- [126].Smyth M, M’Bengue MS, Terrien M, Picart C, Bras J, Foster EJ, The effect of hydration on the material and mechanical properties of cellulose nanocrystal-alginate composites, Carbohydr. Polym 179 (2018) 186–195. doi: 10.1016/j.carbpol.2017.09.002. [DOI] [PubMed] [Google Scholar]
- [127].Singhsa P, Narain R, Manuspiya H, Bacterial Cellulose Nanocrystals (BCNC) Preparation and Characterization from Three Bacterial Cellulose Sources and Development of Functionalized BCNCs as Nucleic Acid Delivery Systems, ACS Appl. Nano Mater 1 (2018) 209–221. doi: 10.1021/acsanm.7b00105. [DOI] [Google Scholar]
- [128].Esa F, Tasirin SM, Rahman NA, Overview of Bacterial Cellulose Production and Application, Agric. Agric. Sci. Procedia 2 (2014) 113–119. doi: 10.1016/j.aaspro.2014.11.017. [DOI] [Google Scholar]
- [129].Islam MU, Ullah MW, Khan S, Shah N, Park JK, Strategies for cost-effective and enhanced production of bacterial cellulose, Int. J. Biol. Macromol 102 (2017) 1166–1173. [DOI] [PubMed] [Google Scholar]
- [130].Abeer MM, Amin M, Iqbal MC, Martin C, A review of bacterial cellulose- based drug delivery systems: their biochemistry, current approaches and future prospects, J. Pharm. Pharmacol 66 (2014) 1047–1061. [DOI] [PubMed] [Google Scholar]
- [131].Picheth GF, Pirich CL, Sierakowski MR, Woehl MA, Sakakibara CN, de Souza CF, Martin AA, da Silva R, de Freitas RA, Bacterial cellulose in biomedical applications: A review, Int. J. Biol. Macromol 104 (2017) 97–106. [DOI] [PubMed] [Google Scholar]
- [132].Almeida IF, Pereira T, Silva N, Gomes FP, Silvestre AJD, Freire CSR, Lobo JMS, Costa PC, Bacterial cellulose membranes as drug delivery systems: an in vivo skin compatibility study, Eur. J. Pharm. Biopharm 86 (2014) 332–336. [DOI] [PubMed] [Google Scholar]
- [133].Naseri-Nosar M, Ziora ZM, Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites, Carbohydr. Polym (2018). [DOI] [PubMed] [Google Scholar]
- [134].Douglass EF, Avci H, Boy R, Rojas OJ, Kotek R, A review of cellulose and cellulose blends for preparation of bio-derived and conventional membranes, nanostructured thin films, and composites, Polym. Rev 58 (2018) 102–163. [Google Scholar]
- [135].Müller A, Zink M, Hessler N, Wesarg F, Müller FA, Kralisch D, Fischer D, Bacterial nanocellulose with a shape-memory effect as potential drug delivery system, RSC Adv 4 (2014) 57173–57184. doi: 10.1039/C4RA09898F. [DOI] [Google Scholar]
- [136].Müller A, Wesarg F, Hessler N, Müller FA, Kralisch D, Fischer D, Loading of bacterial nanocellulose hydrogels with proteins using a high-speed technique, Carbohydr. Polym 106 (2014) 410–413. doi: 10.1016/j.carbpol.2014.01.038. [DOI] [PubMed] [Google Scholar]
- [137].Moritz S, Wiegand C, Wesarg F, Hessler N, Müller FA, Kralisch D, Hipler UC, Fischer D, Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine, Int. J. Pharm 471 (2014) 45–55. doi: 10.1016/j.ijpharm.2014.04.062. [DOI] [PubMed] [Google Scholar]
- [138].Alkhatib Y, Dewaldt M, Moritz S, Nitzsche R, Kralisch D, Fischer D, Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system, Eur. J. Pharm. Biopharm 112 (2017) 164–176. doi: 10.1016/j.ejpb.2016.11.025. [DOI] [PubMed] [Google Scholar]
- [139].Luo H, Ao H, Li G, Li W, Xiong G, Zhu Y, Wan Y, Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system, Curr. Appl. Phys 17 (2017) 249–254. doi: 10.1016/j.cap.2016.12.001. [DOI] [Google Scholar]
- [140].Ataide JA, De Carvalho NM, Rebelo MDA, Chaud MV, Grotto D, Gerenutti M, Rai M, Mazzola PG, Jozala AF, Bacterial Nanocellulose Loaded with Bromelain: Assessment of Antimicrobial, Antioxidant and Physical-Chemical Properties, Sci. Rep 7 (2017) 2–10. doi: 10.1038/s41598-017-18271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Saïdi L, Vilela C, Oliveira H, Silvestre AJD, Freire CSR, Poly (N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac, Carbohydr. Polym 169 (2017) 357–365. [DOI] [PubMed] [Google Scholar]
- [142].Khalid A, Khan R, Ul-Islam M, Khan T, Wahid F, Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds, Carbohydr. Polym 164 (2017) 214–221. doi: 10.1016/j.carbpol.2017.01.061. [DOI] [PubMed] [Google Scholar]
- [143].de Lima Fontes M, Meneguin AB, Tercjak A, Gutierrez J, Cury BSF, dos Santos AM, Ribeiro SJL, Barud HS, Effect of in situ modification of bacterial cellulose with carboxymethylcellulose on its nano/microstructure and methotrexate release properties, Carbohydr. Polym 179 (2018) 126–134. doi: 10.1016/j.carbpol.2017.09.061. [DOI] [PubMed] [Google Scholar]
- [144].Tsai Y-H, Yang Y-N, Ho Y-C, Tsai M-L, Mi F-L, Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films, Carbohydr. Polym 180 (2018) 286–296. [DOI] [PubMed] [Google Scholar]
- [145].Mohamad N, Loh EYX, Fauzi MB, Ng MH, Amin MCIM, In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds, Drug Deliv. Transl. Res (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [146].Wiegand C, Moritz S, Hessler N, Kralisch D, Wesarg F, Müller FA, Fischer D, Hipler UC, Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine, J. Mater. Sci. Mater. Med 26 (2015). doi: 10.1007/s10856-015-5571-7. [DOI] [PubMed] [Google Scholar]
- [147].Wang S, Li T, Chen C, Kong W, Zhu S, Dai J, Diaz AJ, Hitz E, Solares SD, Li T, Transparent, anisotropic biofilm with aligned bacterial cellulose nanofibers, Adv. Funct. Mater (2018) 1707491. [Google Scholar]
- [148].Delcassian D, Patel AK, Cortinas AB, Langer R, Drug delivery across length scales, J. Drug Target (2018) 1–15. [DOI] [PubMed] [Google Scholar]
- [149].Ramasamy T, Ruttala HB, Gupta B, Poudel BK, Choi H-G, Yong CS, Kim JO, Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review, J. Control. Release 258 (2017) 226–253. [DOI] [PubMed] [Google Scholar]
- [150].Gama M, Gatenholm P, Klemm D, Bacterial nanocellulose: a sophisticated multifunctional material, CRC Press, 2012. [Google Scholar]
- [151].Trovatti E, Silva NHCS, Duarte IF, Rosado CF, Almeida IF, Costa P, Freire CSR, Silvestre AJD, Neto CP, Biocellulose membranes as supports for dermal release of lidocaine, Biomacromolecules 12 (2011) 4162–4168. [DOI] [PubMed] [Google Scholar]
- [152].Trovatti E, Freire CSR, Pinto PC, Almeida IF, Costa P, Silvestre AJD, Neto CP, Rosado C, Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: in vitro diffusion studies, Int. J. Pharm 435 (2012) 83–87. [DOI] [PubMed] [Google Scholar]
- [153].Silva NHCS, Drumond I, Almeida IF, Costa P, Rosado CF, Neto CP, Freire CSR, Silvestre AJD, Topical caffeine delivery using biocellulose membranes: a potential innovative system for cellulite treatment, Cellulose 21 (2014) 665–674. [Google Scholar]
- [154].Zielins ER, Brett EA, Luan A, Hu MS, Walmsley GG, Paik K, Senarath-Yapa K, Atashroo DA, Wearda T, Lorenz HP, Emerging drugs for the treatment of wound healing, Expert Opin. Emerg. Drugs 20 (2015) 235–246. [DOI] [PubMed] [Google Scholar]
- [155].Brien S, Lewith G, Walker A, Hicks SM, Middleton D, Bromelain as a treatment for osteoarthritis: a review of clinical studies, Evidence-Based Complement. Altern. Med 1 (2004) 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Pavan R, Jain S, Kumar A, Properties and therapeutic application of bromelain: a review, Biotechnol. Res. Int. 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Das PK, Sahoo R, Bromelain: Applications and Purification Strategies, PharmaTutor 5 (2017) 40–48. [Google Scholar]
- [158].Ye S, Jiang L, Wu J, Su C, Huang C, Liu X, Shao W, Flexible Amoxicillin Grafted Bacterial Cellulose Sponges for Wound Dressing: in Vitro and in Vivo Evaluation, ACS Appl. Mater. Interfaces (2018). [DOI] [PubMed] [Google Scholar]
- [159].Pandey M, Amin M, Iqbal MC, Ahmad N, Abeer MM, Rapid synthesis of superabsorbent smart-swelling bacterial cellulose/acrylamide-based hydrogels for drug delivery, Int. J. Polym. Sci. 2013 (2013). [Google Scholar]
- [160].Badshah M, Ullah H, Khan SA, Park JK, Khan T, Preparation, characterization and in-vitro evaluation of bacterial cellulose matrices for oral drug delivery, Cellulose 24 (2017) 5041–5052. [Google Scholar]
- [161].Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, Lajevardi SS, Li Z, Maitz PK, Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring, Adv. Drug Deliv. Rev 123 (2018) 3–17. [DOI] [PubMed] [Google Scholar]
- [162].Han G, Ceilley R, Chronic wound healing: A review of current management and treatments, Adv. Ther 34 (2017) 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Lee EJ, Huh BK, Kim SN, Lee JY, Park CG, Mikos AG, Bin Choy Y, Application of materials as medical devices with localized drug delivery capabilities for enhanced wound repair, Prog. Mater. Sci 89 (2017) 392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Castaño O, Pérez-Amodio S, Navarro-Requena C, Mateos-Timoneda MÁ, Engel E, Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms, Adv. Drug Deliv. Rev (2018). [DOI] [PubMed] [Google Scholar]
- [165].Simões D, Miguel SP, Ribeiro MP, Coutinho P, Mendonça AG, Correia IJ, Recent advances on antimicrobial wound dressing: a review, Eur. J. Pharm. Biopharm (2018). [DOI] [PubMed] [Google Scholar]
- [166].Jiao Y, Niu L, Ma S, Li J, Tay FR, Chen J, Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance, Prog. Polym. Sci 71 (2017) 53–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX, Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing, Biomaterials 122 (2017) 34–47. [DOI] [PubMed] [Google Scholar]
- [168].Cacicedo ML, Islan GA, Drachemberg MF, Alvarez VA, Bartel LC, Bolzán AD, Castro GR, Hybrid bacterial cellulose–pectin films for delivery of bioactive molecules, New J. Chem 42 (2018) 7457–7467. [Google Scholar]
- [169].Liyaskina E, Revin V, Paramonova E, Nazarkina M, Pestov N, Revina N, Kolesnikova S, Nanomaterials from bacterial cellulose for antimicrobial wound dressing, in: J. Phys. Conf. Ser, IOP Publishing, 2017: p. 12034. [Google Scholar]
- [170].Stroescu M, Stoica-Guzun A, Jipa IM, Vanillin release from poly (vinyl alcohol)-bacterial cellulose mono and multilayer films, J. Food Eng 114 (2013) 153–157. [Google Scholar]
- [171].Huang L, Chen X, Nguyen TX, Tang H, Zhang L, Yang G, Nano-cellulose 3D-networks as controlled-release drug carriers, J. Mater. Chem. B 1 (2013) 2976–2984. doi: 10.1039/C3TB20149J. [DOI] [PubMed] [Google Scholar]
- [172].Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D, Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism, Nano-Micro Lett 7 (2015) 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wu C-N, Fuh S-C, Lin S-P, Lin Y-Y, Chen H-Y, Liu J-M, Cheng K-C, TEMPO-oxidized bacterial cellulose pellicle with silver nanoparticles for wound dressing, Biomacromolecules 19 (2018) 544–554. [DOI] [PubMed] [Google Scholar]
- [174].Treesuppharat W, Rojanapanthu P, Siangsanoh C, Manuspiya H, Ummartyotin S, Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems, Biotechnol. Reports 15 (2017) 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Pötzinger Y, Kralisch D, Fischer D, Bacterial nanocellulose: the future of controlled drug delivery?, Ther. Deliv 8 (2017) 753–761. [DOI] [PubMed] [Google Scholar]
- [176].Khamrai M, Banerjee SL, Kundu PP, Modified bacterial cellulose based self-healable polyeloctrolyte film for wound dressing application, Carbohydr. Polym 174 (2017) 580–590. [DOI] [PubMed] [Google Scholar]
- [177].Shao W, Wu J, Liu H, Ye S, Jiang L, Liu X, Novel bioactive surface functionalization of bacterial cellulose membrane, Carbohydr. Polym 178 (2017) 270–276. [DOI] [PubMed] [Google Scholar]
- [178].Tekade RK, Maheshwari R, Tekade M, Biopolymer-based nanocomposites for transdermal drug delivery, in: Biopolym. Compos, Elsevier, 2018: pp. 81–106. [Google Scholar]
- [179].Khalil HPSA, Bhat AH, Bakar AA, Tahir PM, Zaidul ISM, Jawaid M, Cellulosic nanocomposites from natural fibers for medical applications: A review, in: Handb. Polym. Nanocomposites. Process. Perform. Appl, Springer, 2015: pp. 475–511. [Google Scholar]
- [180].Meneguin AB, Ferreira Cury BS, dos Santos AM, Franco DF, Barud HS, da Silva Filho EC, Resistant starch/pectin free-standing films reinforced with nanocellulose intended for colonic methotrexate release, Carbohydr. Polym 157 (2017) 1013–1023. doi: 10.1016/j.carbpol.2016.10.062. [DOI] [PubMed] [Google Scholar]
- [181].Löbmann K, Svagan AJ, Cellulose nanofibers as excipient for the delivery of poorly soluble drugs, Int. J. Pharm 533 (2017) 285–297. doi: 10.1016/j.ijpharm.2017.09.064. [DOI] [PubMed] [Google Scholar]
- [182].Azhar FF, Shahbazpour E, Olad A, pH sensitive and controlled release system based on cellulose nanofibers-poly vinyl alcohol hydrogels for cisplatin delivery, Fibers Polym 18 (2017) 416–423. doi: 10.1007/s12221-017-6958-5. [DOI] [Google Scholar]
- [183].Nurani M, Akbari V, Taheri A, Preparation and characterization of metformin surface modified cellulose nanofiber gel and evaluation of its anti-metastatic potentials, Carbohydr. Polym 165 (2017) 322–333. doi: 10.1016/j.carbpol.2017.02.067. [DOI] [PubMed] [Google Scholar]
- [184].Basu A, Hong J, Ferraz N, Hemocompatibility of Ca2+-Crosslinked Nanocellulose Hydrogels: Toward Efficient Management of Hemostasis, Macromol. Biosci 17 (2017) 1–9. doi: 10.1002/mabi.201700236. [DOI] [PubMed] [Google Scholar]
- [185].Fakhri A, Tahami S, Nejad PA, Preparation and characterization of Fe3O4-Ag2O quantum dots decorated cellulose nanofibers as a carrier of anticancer drugs for skin cancer, J. Photochem. Photobiol. B Biol 175 (2017) 83–88. doi: 10.1016/j.jphotobiol.2017.08.032. [DOI] [PubMed] [Google Scholar]
- [186].Bhandari J, Mishra H, Mishra PK, Wimmer R, Ahmad FJ, Talegaonkar S, Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery, Int. J. Nanomedicine 12 (2017) 2021–2031. doi: 10.2147/IJN.S124318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Paulraj T, Riazanova AV, Svagan AJ, Bioinspired capsules based on nanocellulose, xyloglucan and pectin – The influence of capsule wall composition on permeability properties, Acta Biomater 69 (2018) 196–205. doi: 10.1016/j.actbio.2018.01.003. [DOI] [PubMed] [Google Scholar]
- [188].Paulraj T, Riazanova AV, Yao K, Andersson RL, Müllertz A, Svagan AJ, Bioinspired Layer-by-Layer Microcapsules Based on Cellulose Nanofibers with Switchable Permeability, Biomacromolecules 18 (2017) 1401–1410. doi: 10.1021/acs.biomac.7b00126. [DOI] [PubMed] [Google Scholar]
- [189].Xu J, Liu S, Chen G, Chen T, Song T, Wu J, Shi C, He M, Tian J, Engineering Biocompatible Hydrogels from Bicomponent Natural Nanofibers for Anticancer Drug Delivery, J. Agric. Food Chem 66 (2018) 935–942. doi: 10.1021/acs.jafc.7b04210. [DOI] [PubMed] [Google Scholar]
- [190].Luan Q, Zhou W, Zhang H, Bao Y, Zheng M, Shi J, Tang H, Huang F, Cellulose-Based Composite Macrogels from Cellulose Fiber and Cellulose Nanofiber as Intestine Delivery Vehicles for Probiotics, J. Agric. Food Chem 66 (2018) 339–345. doi: 10.1021/acs.jafc.7b04754. [DOI] [PubMed] [Google Scholar]
- [191].Alizadeh N, Akbari V, Nurani M, Taheri A, Preparation of an injectable doxorubicin surface modified cellulose nanofiber gel and evaluation of its anti-tumor and anti-metastasis activity in melanoma, Biotechnol. Prog (2018) 1–9. doi: 10.1002/btpr.2598. [DOI] [PubMed] [Google Scholar]
- [192].Liu Y, Sui Y, Liu C, Liu C, Wu M, Li B, Li Y, A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing, Carbohydr. Polym 188 (2018) 27–36. [DOI] [PubMed] [Google Scholar]
- [193].Saito T, Uematsu T, Kimura S, Enomae T, Isogai A, Self-aligned integration of native cellulose nanofibrils towards producing diverse bulk materials, Soft Matter 7 (2011) 8804–8809. [Google Scholar]
- [194].Zhao J, Lu C, He X, Zhang X, Zhang W, Zhang X, Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery, ACS Appl. Mater. Interfaces 7 (2015) 2607–2615. [DOI] [PubMed] [Google Scholar]
- [195].Dai L, Long Z, Chen J, An X, Cheng D, Khan A, Ni Y, Robust guar gum/cellulose nanofibrils multilayer films with good barrier properties, ACS Appl. Mater. Interfaces 9(2017) 5477–5485. [DOI] [PubMed] [Google Scholar]
- [196].Spencer DS, Puranik AS, Peppas NA, Intelligent nanoparticles for advanced drug delivery in cancer treatment, Curr. Opin. Chem. Eng 7 (2015) 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Zhang S, Langer R, Traverso G, Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease, Nano Today (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Lombardo S, Chen P, Larsson PA, Thielemans W, Wohlert J, Svagan AJ, Toward Improved Understanding of the Interactions between Poorly Soluble Drugs and Cellulose Nanofibers, Langmuir (2018). [DOI] [PubMed] [Google Scholar]
- [199].Patil M, Patil V, Sapre A, Ambone T, Torris AT A, Shukla P, Shanmuganathan K, Tuning Controlled Release Behaviour of Starch Granules using Nanofibrillated Cellulose Derived from Waste Sugarcane Bagasse, ACS Sustain. Chem. Eng In Press (2018). [Google Scholar]
- [200].Fujisawa S, Togawa E, Kuroda K, Nanocellulose-stabilized Pickering emulsions and their applications, Sci. Technol. Adv. Mater 18 (2017) 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Craig M, Mo SS, Mazdiyasni H, Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy, Nat. Commun 9 (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R, Advances in Biomaterials for Drug Delivery, Adv. Mater (2018) 1705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Cosgrove DJ, Growth of the plant cell wall, Nat. Rev. Mol. Cell Biol 6 (2005) 850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- [204].Iwamoto S, Kai W, Isogai A, Iwata T, Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy, Biomacromolecules 10 (2009) 2571–2576. [DOI] [PubMed] [Google Scholar]
- [205].Svagan AJ, Musyanovych A, Kappl M, Bernhardt M, Glasser G, Wohnhaas C, Berglund LA, Risbo J, Landfester K, Cellulose nanofiber/nanocrystal reinforced capsules: a fast and facile approach toward assembly of liquid-core capsules with high mechanical stability, Biomacromolecules 15 (2014) 1852–1859. [DOI] [PubMed] [Google Scholar]
- [206].DeLoid GM, Sohal IS, Lorente LR, Molina RM, Pyrgiotakis G, Stevanovic A, Zhang R, McClements DJ, Geitner NK, Bousfield DW, Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose., ACS Nano In Press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Alexandrescu L, Syverud K, Gatti A, Chinga-Carrasco G, Cytotoxicity tests of cellulose nanofibril-based structures, Cellulose 20 (2013) 1765–1775. [Google Scholar]
- [208].Pitkänen M, Kangas H, Laitinen O, Sneck A, Lahtinen P, Peresin MS, Niinimäki J, Characteristics and safety of nano-sized cellulose fibrils, Cellulose 21 (2014) 3871–3886. [Google Scholar]
- [209].Lopes VR, Sanchez-Martinez C, Strømme M, Ferraz N, In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect, Part. Fibre Toxicol 14 (2017) 1–13. doi: 10.1186/s12989-016-0182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Lopes VR, Sanchez-Martinez C, Strømme M, Ferraz N, In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: surface chemistry aspect, Part. Fibre Toxicol 14 (2017) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Vieira S, da Silva Morais A, Silva- Correia J, Oliveira JM, Reis RL, Natural- Based Hydrogels: From Processing to Applications, Encycl. Polym. Sci. Technol (2017). [Google Scholar]
- [212].Singh MR, Patel S, Singh D, Natural polymer-based hydrogels as scaffolds for tissue engineering, in: Nanobiomaterials Soft Tissue Eng, Elsevier, 2016: pp. 231–260. [Google Scholar]
- [213].Li J, Mooney DJ, Designing hydrogels for controlled drug delivery, Nat. Rev. Mater 1 (2016) 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Hujaya SD, Lorite GS, Vainio SJ, Liimatainen H, Polyion complex hydrogels from chemically modified cellulose nanofibrils: Structure-function relationship and potential for controlled and pH-responsive release of doxorubicin, Acta Biomater (2018). doi: 10.1016/j.actbio.2018.06.013. [DOI] [PubMed] [Google Scholar]
- [215].Kakkar A, Traverso G, Farokhzad OC, Weissleder R, Langer R, Evolution of macromolecular complexity in drug delivery systems, Nat. Rev. Chem 1 (2017) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Lu Y, Aimetti AA, Langer R, Gu Z, Bioresponsive materials, Nat. Rev. Mater 2(2017) 16075. [Google Scholar]
- [217].Mitchell MJ, Jain RK, Langer R, Engineering and physical sciences in oncology: challenges and opportunities, Nat. Rev. Cancer 17 (2017) 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Webber MJ, Langer R, Drug delivery by supramolecular design, Chem. Soc. Rev 46(2017) 6600–6620. [DOI] [PubMed] [Google Scholar]
- [219].Park NH, Cheng W, Lai F, Yang C, Florez de Sessions P, Periaswamy B, Wenhan Chu C, Bianco S, Liu S, Venkataraman S, Addressing Drug Resistance in Cancer with Macromolecular Chemotherapeutic Agents, J. Am. Chem. Soc 140 (2018) 4244–4252. [DOI] [PubMed] [Google Scholar]
- [220].Norouzi M, Recent advances in brain tumor therapy: application of electrospun nanofibers, Drug Discov. Today 23 (2018) 912–919. [DOI] [PubMed] [Google Scholar]
- [221].Chen S, Boda SK, Batra SK, Li X, Xie J, Emerging roles of electrospun nanofibers in cancer research, Adv. Healthc. Mater 7 (2018) 1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Leight JL, Drain AP, Weaver VM, Extracellular matrix remodeling and stiffening modulate tumor phenotype and treatment response, (2017). [Google Scholar]
- [223].Skhinas JN, Cox TR, The interplay between extracellular matrix remodelling and kinase signalling in cancer progression and metastasis, Cell Adh. Migr (2017) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Erdogan B, Webb DJ, Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis, Biochem. Soc. Trans 45 (2017) 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Rajesh Y, Mandal M, Regulation of Extracellular Matrix Remodeling and Epithelial-Mesenchymal Transition by Matrix Metalloproteinases: Decisive Candidates in Tumor Progression, in: Proteases Physiol. Pathol, Springer, 2017: pp. 159–194. [Google Scholar]
- [226].Saito T, Kimura S, Nishiyama Y, Isogai A, Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose, Biomacromolecules 8 (2007) 2485–2491. [DOI] [PubMed] [Google Scholar]
- [227].Zargar V, Asghari M, Dashti A, A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications, ChemBioEng Rev 2 (2015) 204–226. [Google Scholar]
- [228].Bamba Y, Ogawa Y, Saito T, Berglund LA, Isogai A, Estimating the Strength of Single Chitin Nanofibrils via Sonication-Induced Fragmentation, Biomacromolecules 18 (2017) 4405–4410. [DOI] [PubMed] [Google Scholar]
- [229].Sukul M, Ventura RD, Bae SH, Choi HJ, Lee SY, Lee BT, Plant-derived oxidized nanofibrillar cellulose-chitosan composite as an absorbable hemostat, Mater. Lett 197(2017) 150–155. [Google Scholar]
- [230].Wu Y, Wang F, Huang Y, Comparative evaluation of biological performance, biosecurity, and availability of cellulose-based absorbable hemostats, Clin. Appl. Thromb 24 (2018) 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Liu R, Dai L, Si C, Zeng Z, Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers, Carbohydr. Polym 195 (2018) 63–70. [DOI] [PubMed] [Google Scholar]
- [232].Nguyen THM, Abueva C, Van Ho H, Lee SY, Lee BT, In vitro and in vivo acute response towards injectable thermosensitive chitosan/TEMPO-oxidized cellulose nanofiber hydrogel, Carbohydr. Polym 180 (2018) 246–255. doi: 10.1016/j.carbpol.2017.10.032. [DOI] [PubMed] [Google Scholar]
- [233].Vuoriluoto M, Orelma H, Lundahl M, Borghei M, Rojas OJ, Filaments with Affinity Binding and Wet Strength Can Be Achieved by Spinning Bifunctional Cellulose Nanofibrils, Biomacromolecules 18 (2017) 1803–1813. [DOI] [PubMed] [Google Scholar]
- [234].Kolakovic R, Peltonen L, Laukkanen A, Hirvonen J, Laaksonen T, Nanofibrillar cellulose films for controlled drug delivery, Eur. J. Pharm. Biopharm 82 (2012) 308–315. [DOI] [PubMed] [Google Scholar]
- [235].Shanmugam K, Varanasi S, Garnier G, Batchelor W, Rapid preparation of smooth nanocellulose films using spray coating, Cellulose 24 (2017) 2669–2676. [Google Scholar]
- [236].Kolakovic R, Laaksonen T, Peltonen L, Laukkanen A, Hirvonen J, Spray-dried nanofibrillar cellulose microparticles for sustained drug release, Int. J. Pharm 430 (2012) 47–55. [DOI] [PubMed] [Google Scholar]
- [237].López-Córdoba A, Castro GR, Goyanes S, Cellulose- Containing Scaffolds Fabricated by Electrospinning: Applications in Tissue Engineering and Drug Delivery, Handb. Compos. from Renew. Mater. Nanocomposites Adv. Appl 8 (2017) 361. [Google Scholar]
- [238].Meneguin AB, Cury BSF, Evangelista RC, Films from resistant starch-pectin dispersions intended for colonic drug delivery, Carbohydr. Polym 99 (2014) 140–149. [DOI] [PubMed] [Google Scholar]
- [239].Cury BSF, Meneguin AB, Cardoso VMO, Prezotti FG, Oral drug release systems based on pectin, in: Pectin Chem. Prop. Uses Heal. Benefits, Nova Science Publishers New York, 2014: pp. 65–81. [Google Scholar]
- [240].Sarkar G, Orasugh JT, Saha NR, Roy I, Bhattacharyya A, Chattopadhyay AK, Rana D, Chattopadhyay D, Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine, New J. Chem 41 (2017) 15312–15319. [Google Scholar]
- [241].Löbmann K, Wohlert J, Müllertz A, Wågberg L, Svagan AJ, Cellulose Nanopaper and Nanofoam for Patient- Tailored Drug Delivery, Adv. Mater. Interfaces 4 (2017). [Google Scholar]
- [242].Galkina OL, Ivanov VK, V Agafonov A, Seisenbaeva GA, Kessler VG, Cellulose nanofiber–titania nanocomposites as potential drug delivery systems for dermal applications, J. Mater. Chem. B 3 (2015) 1688–1698. [DOI] [PubMed] [Google Scholar]
- [243].Karki S, Kim H, Na S-J, Shin D, Jo K, Lee J, Thin films as an emerging platform for drug delivery, Asian J. Pharm. Sci 11 (2016) 559–574. doi: 10.1016/j.ajps.2016.05.004. [DOI] [Google Scholar]
- [244].Ulker Z, Erkey C, An emerging platform for drug delivery: Aerogel based systems, J. Control. Release 177 (2014) 51–63. [DOI] [PubMed] [Google Scholar]
- [245].Bhandari J, Mishra H, Mishra PK, Wimmer R, Ahmad FJ, Talegaonkar S, Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery, Int. J. Nanomedicine 12 (2017) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Svagan AJ, Müllertz A, Löbmann K, Floating solid cellulose nanofibre nanofoams for sustained release of the poorly soluble model drug furosemide, J. Pharm. Pharmacol 69 (2017) 1477–1484. [DOI] [PubMed] [Google Scholar]
- [247].Svagan AJ, Benjamins J-W, Al-Ansari Z, Shalom DB, Müllertz A, Wågberg L, Löbmann K, Solid cellulose nanofiber based foams–Towards facile design of sustained drug delivery systems, J. Control. Release 244 (2016) 74–82. [DOI] [PubMed] [Google Scholar]
- [248].Chen W, Yu H, Liu Y, Chen P, Zhang M, Hai Y, Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments, Carbohydr. Polym 83 (2011) 1804–1811. [Google Scholar]
- [249].Sacui IA, Nieuwendaal RC, Burnett DJ, Stranick SJ, Jorfi M, Weder C, Foster EJ, Olsson RT, Gilman JW, Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods, ACS Appl. Mater. Interfaces 6 (2014) 6127–6138. [DOI] [PubMed] [Google Scholar]
- [250].Foster EJ, Moon RJ, Agarwal UP, Bortner MJ, Bras J, Camarero-Espinosa S, Chan KJ, Clift MJD, Cranston ED, Eichhorn SJ, Current characterization methods for cellulose nanomaterials, Chem. Soc. Rev (2018). [DOI] [PubMed] [Google Scholar]
- [251].Cullen RT, Miller BG, Jones AD, Davis JMG, Toxicity of cellulose fibres, Ann. Occup. Hyg 46 (2002) 81–84. [Google Scholar]
- [252].Endes C, Camarero-Espinosa S, Mueller S, Foster EJ, Petri-Fink A, Rothen-Rutishauser B, Weder C, Clift MJD, A critical review of the current knowledge regarding the biological impact of nanocellulose, J. Nanobiotechnology 14 (2016) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Shatkin JA, Kim B, Environmental Health and safety of cellulose nanomaterials and composites, Handb. Nanocellulose Cellul. Nanocomposites 2 (2017) 683–729. [Google Scholar]
- [254].Li Q, McGinnis S, Sydnor C, Wong A, Renneckar S, Nanocellulose life cycle assessment, ACS Sustain. Chem. Eng 1 (2013) 919–928. [Google Scholar]
- [255].Cullen RT, Miller BG, Clark S, Davis JMG, Tumorigenicity of cellulose fibers injected into the rat peritoneal cavity, Inhal. Toxicol 14 (2002) 685–703. [DOI] [PubMed] [Google Scholar]
- [256].Dourado F, Gama M, Rodrigues AC, A Review on the toxicology and dietetic role of bacterial cellulose, Toxicol. Reports (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].ISO 10993–1:2009 Standard for Biological Evaluation of Medical Devices. In Part 1: Evaluation and Testing within a Risk Management Process, International Organization for Standardization, 2009. [Google Scholar]