Abstract
Chlorine dioxide (ClO2) can be used as an alternative disinfectant for controlling fungal contamination during postharvest storage. In this study, we tested the in vitro and in vivo inhibitory effects of gaseous ClO2 against Diaporthe batatas SP-d1, the causal agent of sweetpotato dry rot. In in vitro tests, spore suspensions of SP-d1 spread on acidified potato dextrose agar were treated with various ClO2 concentrations (1–20 ppm) for 0–60 min. Fungal growth was significantly inhibited at 1 ppm of ClO2 treatment for 30 min, and completely inhibited at 20 ppm. In in vivo tests, spore suspensions were drop-inoculated onto sweetpotato slices, followed by ClO2 treatment with different concentrations and durations. Lesion diameters were not significantly different between the tested ClO2 concentrations; however, lesion diameters significantly decreased upon increasing the exposure time. Similarly, fungal populations decreased at the tested ClO2 concentrations over time. However, the sliced tissue itself hardened after 60-min ClO2 treatments, especially at 20 ppm of ClO2. When sweetpotato roots were dip-inoculated in spore suspensions for 10 min prior to treatment with 20 and 40 ppm of ClO2 for 0–60 min, fungal populations decreased with increasing ClO2 concentrations. Taken together, these results showed that gaseous ClO2 could significantly inhibit D. batatas growth and dry rot development in sweetpotato. Overall, gaseous ClO2 could be used to control this fungal disease during the postharvest storage of sweetpotato.
Keywords: chlorine dioxide, Diaporthe batatas, dry rot, sweetpotato
Sweetpotato (Ipomoea batatas Lam.) is one of the most important food crops worldwide, especially in developing countries. The crop currently ranks seventh among staple food crops after rice, wheat, potatoes, maize, barley, and cassava. (Sanusi et al., 2016). The world production of sweetpotato was estimated at 105,190 Mt, in which 74.7% was produced in China and other Asian countries, according to the Food and Agriculture Organization of the United Nations in 2016 (http://www.fao.org/faostat/en/#data/QC/). Recently, the consumption of sweetpotato as a healthy food has been increasing; however, postharvest diseases of the crop are among the significant limitations for its production (estimated loss, 15–65%), in terms of both quantity and quality (Ray and Ravi, 2005). Fungal contamination is one of the most serious problems during sweetpotato storage (Ray and Ravi, 2005; Scruggs and Quesada-Ocampo, 2016). Recently, Chakraborty et al. (2017) reported that Botryodiplodia theobromae, Certocystis fimbriata, Fusarium spp., and Rhizopus oryzae are the most significant fungi to sweetpotato storage contamination. These fungal pathogens cause local discoloration and disruption of surrounding tissues in infected sweetpotato, resulting in changes involving deterioration of texture and flavor (Amienyo and Ataga, 2007). Because sweetpotato stored in enclosed spaces, fungal contamination may spread throughout the storage facility, resulting in significantly contaminated sweetpotato (Wu and Rioux, 2010). Therefore, it is important to apply appropriate measures to limit fungal infection of sweetpotato during storage as described in other crops (Mannaa and Kim, 2018). Fungicides, such as thiabenda-zole, benomyl, and iprodione, are generally used to control fungal contamination in sweetpotato (Afek et al., 1998; Singh and Sharma, 2018). However, postharvest application of fungicides is generally avoided because sweetpotato roots are directly used as a food source.
Chlorine has long been used as an effective chemical to control postharvest diseases of fruits and vegetables (Zoffoli et al., 1999). This chemical can react with many organic nitrogen compounds, unsaturated organic compounds, and phenols. Thus, microorganisms can be destroyed through the oxidative action of chlorine on cellular constituents, and in part via direct combinations of the compound with membrane proteins and enzymes (Tweddell et al., 2003). In this regard, chlorine dioxide (ClO2) gas has been shown to effectively reduce food-borne pathogens in many storage crops such as apples, green peppers, lettuce, tomatoes, cabbage, carrots, and peaches, as well as blueberries, raspberries, and strawberries (Du et al., 2002; Han et al., 2001, 2004; Lee et al., 2004; Sy et al., 2005a, 2005b). Thus, ClO2 gas can be used as an alternative disinfectant to control fungal contamination. Moreover, ClO2 gas has low toxicity in humans, reacts rapidly, and is effective at low concentrations (Vaid et al., 2010; Wang et al., 2016). ClO2 gas has been approved for use as a sanitizer for agricultural, industrial, commercial, and medical uses by the U.S. Environmental Protection Agency (2006). Although both aqueous and gaseous ClO2 are effective as sanitizing agents, gaseous ClO2 is more effective than aqueous ClO2 because the gaseous form can penetrate small spaces that the liquid form cannot reach (Han et al., 2001; Lee et al., 2004).
Recently, sweetpotato dry rot caused by D. batatas in Korea was reported by Lee et al. (2016). This was the first report of the disease on sweetpotato in the world besides in the USA, where it was first described (Harter and Field, 1912). The disease may, therefore, cause significant reduction in sweetpotato production worldwide, including in Korea. Although ClO2 gas may inhibit the activity of various deleterious storage fungi, D. batatas as an emerging fungal pathogen of sweetpotato was examined in this study. Thus, the objectives of this study were to evaluate the in vitro and in vivo inhibitory effects of various concentrations and treatment times of gaseous ClO2 against growth of D. batatas and dry rot development in sweetpotato.
D. batatas SP-d1, isolated from a diseased sweetpotato root (cv. Juwhangmi) and supplied by the Bioenergy Crop Research Center, National Institute of Crop Science, Rural Development Administration, Muan, Korea in 2015, was used in this study (Lee et al., 2016). Sweetpotatoes (cv. Juwhangmi) obtained from the same center were used in this study. The sweetpotatoes were stored in a moist chamber with 60% relative humidity (RH) at 15°C until use.
To test the in vitro inhibitory effect of gaseous ClO2 against D. batatas, 200 μl of spore suspensions was spread on acidified potato dextrose agar (APDA) (three plates per treatment as replicate). For inoculum preparation, spores from cultures of isolate SP-d1 grown on potato dextrose agar (PDA) at 25°C for 14 days were harvested with 0.03% Tween 20 and then adjusted to 5 × 103 spores/ml using a hemocytometer. The inoculated plates were treated with 1, 5, 10, and 20 ppm of ClO2 gas for 0, 1, 10, 30, and 60 min. The gas was generated by a ClO2 generator (PurgoFarm Co. Ltd., Hwasung, Korea) (Supplementary Fig. 1) using an electrochemical method (Gates, 1998). Briefly, aqueous NaClO2 was electrolyzed and the cleaved sodium ions migrated to the cathode through a patented multi-porous membrane electrode assembly, leaving highly pure ClO2 (> 99%) in the anode chamber. The evaporating ClO2 gas was blown out through a vent into a test chamber [54 (length) × 44 (width) × 46 (height) cm]. Gas entry to the chamber was manually controlled depending on the preset concentrations (1, 5, 10, and 20 ppm) of ClO2, which were monitored at 0, 1, 10, 30, and 60 min by a PortaSens II gas leak detector (Analytical Technology, Collegeville, PA, USA) (Supplementary Fig. 1). The ClO2 gas-treated plates were incubated at 25°C for 2 days and then colony-forming units (cfus) were determined.
To test the in vivo inhibitory effect of gaseous ClO2 against D. batatas via slice test, healthy sweetpotato roots were first washed with tap water and then with distilled water. The clean roots were surface-sterilized by spraying with 70% ethanol. After drying for 5 min, these roots were rinsed with distilled water twice and then dried for 10 min at room temperature. Next, the roots were cut into 1-cm thick slices and placed in Petri plates (90 mm in diameter). The centers of these slices (three slices per treatment) were drop-inoculated with 10 μl of the spore suspension (5 × 106 spores/ml) harvested as described above. The inoculated slices were then treated with 5, 10, and 20 ppm of ClO2 gas for 0, 10, 30, and 60 min as described above. After ClO2 gas treatment, the slices were put in containers [23 (length) × 13 (width) × 16 (height) cm] containing three layers of wet paper towels (100% RH) and further incubated at 28°C. After 10 days of incubation, lesion diameters on the inoculated slices were measured as follows: [longest lesion length (mm) + shortest lesion length (mm)] was divided by 2 (Oh et al., 2016). In addition, fungal populations from the lesions on the slices were assessed. Samples from lesions were obtained using a sterile cork borer and were finely ground using an analytical mill (IKA A11 basic, IKA Works, Wilmington, DE) in sterile distilled water (SDW). After appropriate dilutions, samples were spread on APDA, and cfus were counted 2 days after incubation at 25°C.
To test the in vivo inhibitory effect of gaseous ClO2 against D. batatas via the root test, sweetpotato roots were dipped in spore suspensions (5 × 106 spores/ml) or 0.03% Tween 20 (uninoculated control) for 10 min (three roots per treatment). The inoculated roots were treated with 20 and 40 ppm of ClO2 gas for 0, 30, and 60 min as described above. The concentrations of ClO2 gas in this root tests were examined to consider practical applications during postharvest storage. After the ClO2 gas treatment, surface layers of the roots were peeled using a vegetable peeler. Two grams of the samples (approximately 1.5 mm in thickness) were finely ground using an analytical mill (IKA Works) in 20 ml of SDW. After appropriate dilutions, the samples were spread on APDA. After 2 days of incubation at 25°C, colonies were counted and expressed as cfu per g of dry weight.
Experiments were established using factorial designs to observe the effect of ClO2 concentration and treatment time on lesion diameter and fungal population. All experiments were performed twice with three replicates per treatment. In vitro fungal population data from repeated experiments were pooled after confirmation of homogeneity of variances, using Levene’s test (Levene, 1960). For analysis of fungal populations, data were analyzed after logarithmic transformation. ANOVA was conducted using general linear model procedures, and the means were separated using least significant difference tests at P < 0.05. Statistical analysis of the data was conducted using Statistical Analysis Systems software (SAS Institute, Cary, NC, USA).
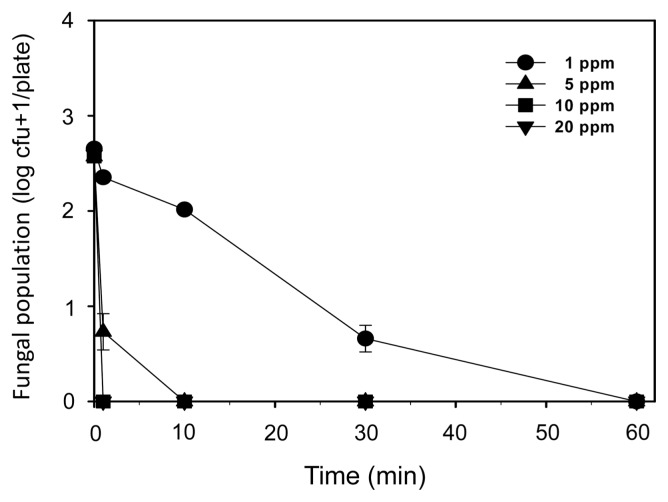
The inhibitory effect of gaseous ClO2 on D. batatas SP-d1 was significant (Fig. 1 and Supplementary Fig. 2). Fungal growth was significantly inhibited at 1 ppm of ClO2 for 1 min and was almost completely suppressed at 1 ppm for 30 min, as well as at other concentrations of ClO2 treatments, regardless of time (Fig. 1 and Supplementary Fig. 2).
Fig. 1.
Populations of Diaporthe batatas SP-d1 on acidified potato dextrose agar treated with various ClO2 concentrations (1, 5, 10, and 20 ppm) for 0, 1, 10, 30, and 60 min. Colony-forming units (cfus) were counted 2 days after incubation. Isolate SP-d1 (200 μl of 5 × 103 spores/ml) was spread on APDA before ClO2 gas treatments. Error bars are the standard deviations of the means (n = 6).
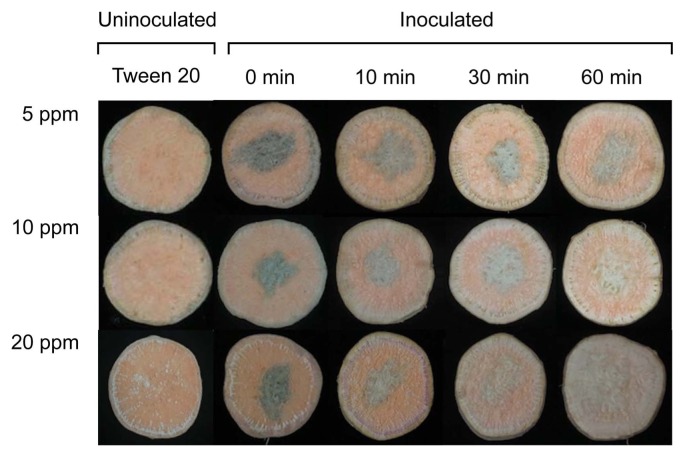
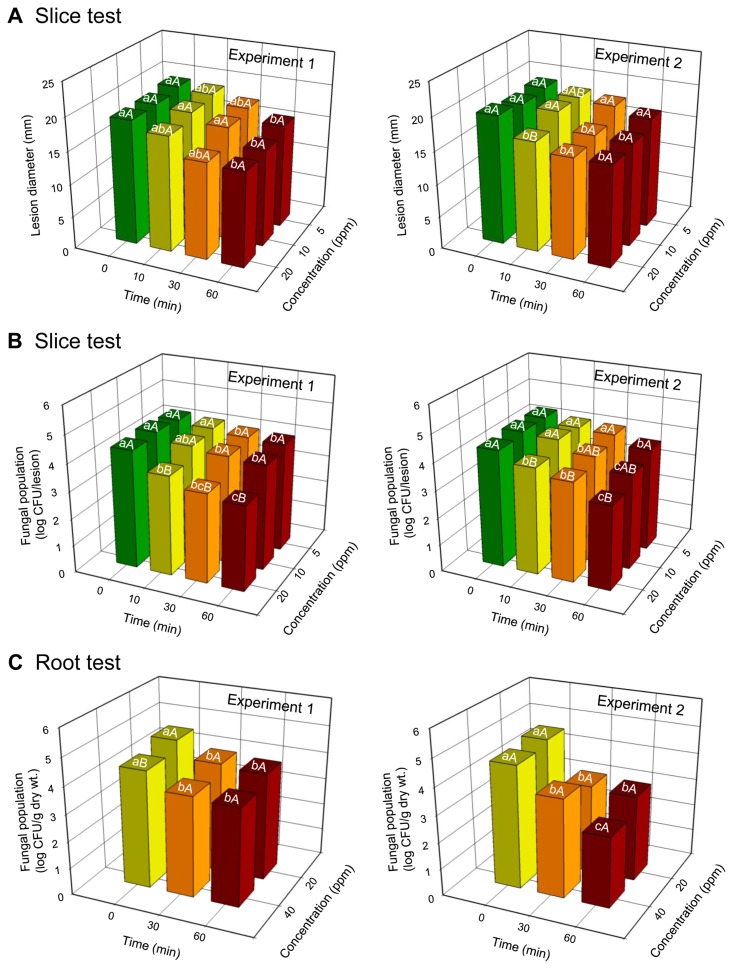
Lesion diameters of D. batatas SP-d1 did not change significantly at the concentrations of ClO2 tested; however, lesion diameters decreased with an increase in treatment time in both experiments (Fig. 2, Fig. 3A, and Table 1). The concentration significantly (P = 0.0352) affected lesion diameters in one experiment (Table 1). Treatment time significantly (P = 0.0046 for experiment 1 and P < 0.0001 for experiment 2) affected lesion diameters on sweetpotato slices in both experiments. Interactions between concentration and time for lesion diameters were not significant (P > 0.05) in both experiments (Table 1). In addition, the degree of darkness of lesions was reduced over time, regardless of gas concentration, and sweetpotato tissues hardened after gas treatment for 60 min (Fig. 2). However, disease symptoms were not observed in uninoculated sweetpotato slices (Fig. 2).
Fig. 2.
Photographs of sweetpotato slices drop-inoculated with Diaporthe batatas SP-d1 (10 μl of 5 × 106 spores/ml) following treatments with various ClO2 concentrations (5, 10, and 20 ppm) for 0, 10, 30, and 60 min. These photographs were taken 10 days after inoculation.
Fig. 3.
(A) Lesion diameters and (B) populations of Diaporthe batatas SP-d1 on inoculated slices of sweetpotatoes treated with gaseous chlorine dioxide (ClO2). (C) Populations of isolate SP-d1 in the surface layers of sweetpotato roots treated with ClO2 gas. Slices were inoculated with isolate SP-d1 (10 μl of 5 × 106 spores/ml) and then treated with various ClO2 concentrations (5, 10, and 20 ppm) for 0, 10, 30, and 60 min. Roots were dipped in spore suspension (5 × 106 spores/ml) for 10 min and then treated with different ClO2 concentrations (20 and 40 ppm) for 0, 30, and 60 min. Different lowercase and uppercase letters on bars (n = 3) are significantly different between time at a given concentration and between concentrations at a given time according to the least significant difference test at P < 0.05, respectively. Repeated experiments are indicated as experiments 1 and 2.
Table 1.
Analysis of variance components including the degrees of freedom (df), sum of squares (SS), F ratio, and P value for lesion diameters and fungal populations of Diaporthe batatas SP-d1 on inoculated slices of sweetpotatoes treated with gaseous chlorine dioxide (ClO2)
| Source of variation | Lesion diameter (mm)a | Fungal population (log cfu/lesion)a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |||||||||||||
|
|
|
|
|
|||||||||||||
| df | SS | F | P | df | SS | F | P | df | SS | F | P | df | SS | F | P | |
| Concentration | 2 | 16.0 | 1.5 | 0.2460 | 2 | 12.2 | 3.9 | 0.0352 | 2 | 2.2 | 32.3 | < 0.0001 | 2 | 0.9 | 15.6 | < 0.0001 |
| Time | 3 | 90.9 | 5.6 | 0.0046 | 3 | 58.0 | 12.3 | < 0.0001 | 3 | 2.4 | 23.9 | < 0.0001 | 3 | 3.9 | 43.5 | < 0.0001 |
| Concentration × time | 6 | 7.2 | 0.2 | 0.9653 | 6 | 11.2 | 1.2 | 0.3495 | 6 | 0.8 | 3.9 | 0.0073 | 6 | 0.4 | 2.2 | 0.0843 |
Sweetpotato slices were drop-inoculated with isolate SP-d1 (10 μl of 5 × 106 spores/ml) and then treated with various ClO2 concentrations (5, 10, and 20 ppm) for 0, 10, 30, and 60 min. Lesion diameters and colony-forming units (cfus) were evaluated 10 days after incubation at 28°C. Repeated experiments are indicated as experiments 1 and 2.
The population of D. batatas SP-d1 in the inoculated slices decreased with an increase in ClO2 concentration and treatment time, regardless of experiment (Fig. 3B and Table 1). Fungal inhibition was higher at 20 ppm of ClO2 than 5 ppm, regardless of time, and at 30- and 60-min gas treatments compared with 0-min gas treatment, regardless of ClO2 concentration in both experiments (Fig. 3B). ClO2 concentrations and treatment times independently resulted in significantly (P < 0.0001) reduced fungal growth on sweetpotato slices in both experiments (Table 1). There were significant (P = 0.0073) interactions between concentration and time for fungal populations in one experiment (Table 1).
The growth of D. batatas SP-d1 did not vary between ClO2 concentrations; however, it decreased with increasing exposure time in both experiments (Fig. 3C and Table 2). Fungal growth was inhibited at 20 and 40 ppm of ClO2 with the 60-min gas treatment, compared with the 0-min gas treatment (Fig. 3C). The concentration of ClO2 resulted in a significant (P = 0.0012) reduction in the fungal population in one experiment; the treatment time produced a significant (P < 0.0001) reduction in the fungal population in both experiments (Table 2). However, significant interactions between concentration and time for fungal populations were not observed in both experiments (Table 2).
Table 2.
Analysis of variance components including the degrees of freedom (df), sum of squares (SS), F ratio, and P value for fungal populations of Diaporthe batatas SP-d1 in surface layers of sweetpotato roots treated with gaseous chlorine dioxide (ClO2)
| Source of variation | Fungal population (log cfu/g dry wt.)a | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Experiment 1 | Experiment 2 | |||||||
|
|
|
|||||||
| df | SS | F | P | df | SS | F | P | |
| Concentration | 1 | 0.6 | 17.8 | 0.0012 | 1 | 0.1 | 0.4 | 0.5614 |
| Time | 2 | 1.8 | 29.2 | < 0.0001 | 2 | 9.6 | 30.3 | < 0.0001 |
| Concentration × time | 2 | 0.0 | 0.0 | 0.9971 | 2 | 0.7 | 2.2 | 0.1584 |
Sweetpotato roots were dipped in spore suspension of isolate SP-d1 (5 × 106 spores/ml) for 10 min and then treated with various ClO2 concentrations (20 and 40 ppm) for 0, 30, and 60 min. After ClO2 gas treatment, colony-forming units (cfus) on the surface layers of the roots were determined. Repeated experiments are indicated as experiments 1 and 2.
In this study, we tested whether gaseous ClO2 could effectively control infection by D. batatas, the causal agent of dry rot on sweetpotato. Consequently, we found that gaseous ClO2 had significant inhibitory activity against the fungal infection on sweetpotato. Furthermore, we detected that treatment time had a greater effect than the concentration of ClO2 gas on the growth of D. batatas SP-d1 and dry rot development in the crop.
In the in vitro test, growth of D. batatas SP-d1 was partially inhibited at 1 ppm ClO2 treatment for 30 min. With the 10-and 20-ppm ClO2 treatments, the tested fungus was completely inhibited. Similarly, there have been several studies that demonstrated the in vitro inhibitory efficiency of ClO2 on fungal pathogens. For example, the radial growth of Alternaria alternata and Stemphylium vesicarium were completely inhibited after a 3 min treatment with 10 ppm of ClO2 gas (Trinetta et al., 2013). In other studies, the impact of atmospheric chlorine on spore viability and mycelial growth of Botrytis cinerea and Rhizopus stolonifer were evaluated (Avis et al., 2006). A chlorine concentration of 10 ppm was sufficient to inhibit fungal spore germination, while mycelial growth of the fungi was completely inhibited at concentrations of 5 and 20 ppm at 6 h after treatment (Avis et al., 2006).
In the in vivo tests, ClO2 gas consistently restricted lesion diameters for the 60-min treatment, but not for the 10- and 30-min treatments, at concentrations of 10 and 20 ppm in repeated experiments. However, lesion color (indicating fungal growth) on the slices changed over time, and the tissues hardened after gas treatment for 60 min, especially at 20 ppm of ClO2. Therefore, the fungal populations of infected tissues were evaluated for a more accurate assessment of inhibitory activity of ClO2 gas. Populations of D. batatas SP-d1 greatly decreased at the tested ClO2 concentrations over time, especially at 20 ppm of ClO2 for 30–60 min. However, considering tissue hardening after 60 min of ClO2 treatment, a 30-min treatment may be appropriate. On the other hand, the root dip tests for fungal populations also showed similar results compared with the slice tests as observed in other studies (Jin-Hua et al., 2007; Lee et al., 2004). For example, when green bell peppers were treated with 0–50 ppm of ClO2 gas at 10 ± 0.5°C for 40 days, pepper rot was inhibited at all of the tested ClO2 gas concentrations (Jin-Hua et al., 2007). Similarly, reductions in Escherichia coli, Salmonella typhimurium, and Listeria monocytogenes were observed when inoculated lettuce was exposed to ClO2 gas for 0.5–3 h (Lee et al., 2004).
Taken together, our results in this study showed that gaseous ClO2 could significantly inhibit D. batatas growth and dry rot development on sweetpotato. In particular, 20 ppm of ClO2 gas treatment for 30 min may be appropriate to inhibit fungal growth and disease development on the crop. Hence, gaseous ClO2 could be used to control this fungal disease during the postharvest storage of sweetpotato roots.
Supplementary Information
Acknowledgments
This work was supported by a grant from the Rural Development Administration (PJ011332) in Korea. J.-J. Jeong was supported by the Global Ph.D. program through the National Research Foundation of Korea funded by the Ministry of Education (2015-034526) in Korea.
References
- Afek U, Orenstein J, Nuriel E. Increased quality and prolonged storage of sweet potatoes in Israel. Phytoparasitica. 1998;26:307–312. doi: 10.1007/BF02981445. [DOI] [Google Scholar]
- Amienyo CA, Ataga AE. Use of indigenous plant extracts for the protection of mechanically injured sweet potato [Ipomoea batatas (L.) Lam] tubers. Sci Res Essays. 2007;2:167–170. [Google Scholar]
- Avis TJ, Martinez C, Tweddell RJ. Effect of chlorine atmospheres on the development of Rhizopus rot (Rhizopus stolonifer) and gray mold (Botrytis cinerea) on stored strawberry fruits. Can J Plant Pathol. 2006;28:526–532. doi: 10.1080/07060660609507330. [DOI] [Google Scholar]
- Chakraborty C, Roychowdhury R, Chakraborty S, Chakravorty P, Ghosh D. A review on post-harvest profile of sweet potato. Int J Curr Microbiol App Sci. 2017;6:1894–1903. doi: 10.20546/ijcmas.2017.605.210. [DOI] [Google Scholar]
- Du J, Han Y, Linton RH. Inactivation by chlorine dioxide gas (ClO2) of Listeria monocytogenes spotted onto different apple surfaces. Food Microbiol. 2002;19:481–490. doi: 10.1006/fmic.2002.0501. [DOI] [Google Scholar]
- Gates DJ. The chlorine dioxide handbook. American Water Works Association; Denver, CO, USA: 1998. [Google Scholar]
- Han Y, Linton RH, Nielsen SS, Nelson PE. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7°C. J Food Prot. 2001;64:1730–1738. doi: 10.4315/0362-028X-64.11.1730. [DOI] [PubMed] [Google Scholar]
- Han Y, Selby TL, Schultze KK, Nelson PE, Linton RH. Decontamination of strawberries using batch and continuous chlorine dioxide gas treatments. J Food Prot. 2004;67:2450–2455. doi: 10.4315/0362-028X-67.11.2450. [DOI] [PubMed] [Google Scholar]
- Harter LL, Field EC. Diaporthe, the ascogenous form of sweet potato dry rot. Phytopathology. 1912;2:121–124. [Google Scholar]
- Jin-Hua DU, Mao-Run FU, Miao-Miao LI, Wei XIA. Effects of chlorine dioxide gas on postharvest physiology and storage quality of green bell pepper (Capsicum frutescens L. var. Longrum) Agr Sci China. 2007;6:214–219. doi: 10.1016/S1671-2927(07)60037-6. [DOI] [Google Scholar]
- Lee SY, Costello M, Kang DH. Efficacy of chlorine dioxide gas as a sanitizer of lettuce leaves. J Food Prot. 2004;67:1371–1376. doi: 10.4315/0362-028X-67.7.1371. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Mannaa M, Jeong JJ, Lee HU, Kim W, Kim KD. First report of dry rot of sweetpotato (Ipomoea batatas) caused by Diaporthe batatas in Korea. Plant Dis. 2016;100:1786. doi: 10.1094/PDIS-02-16-0249-PDN. [DOI] [Google Scholar]
- Levene H. Robust tests for equality of variances. In: Olkin I, Ghurye SG, Hoeffding W, Madow WG, Mann HB, editors. Contributions to Probability and Statistics. Stanford Univ. Press; Stanford, CA, USA: 1960. pp. 278–292. [Google Scholar]
- Mannaa M, Kim KD. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: study II. Mycobiology. 2018;46:52–63. doi: 10.1080/12298093.2018.1454015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Mannaa M, Han GD, Chun S-C, Kim KD. First report of Aspergillus awamori as a fungal pathogen of garlic (Allium sativum L.) Crop Prot. 2016;85:65–70. doi: 10.1016/j.cropro.2016.03.019. [DOI] [Google Scholar]
- Ray RC, Ravi V. Post-harvest spoilage of sweet potato in tropics and control measures. Crit Rev Food Sci Nutr. 2005;45:634–644. doi: 10.1080/10408390500455516. [DOI] [PubMed] [Google Scholar]
- Sanusi MM, Lawal OI, Sanusi RA, Adesogan AO. Profitability of sweet potato production in derived savannah zone of Ogun State, Nigeria. J Agric Soc Res. 2016;16:16–27. [Google Scholar]
- Scruggs AC, Quesada-Ocampo LM. Etiology and epidemiological conditions promoting Fusarium root rot in sweetpotato. Phytopathology. 2016;106:909–919. doi: 10.1094/PHYTO-01-16-0009-R. [DOI] [PubMed] [Google Scholar]
- Singh D, Sharma RR. Postharvest diseases of fruits and vegetables and their management. In: Siddiqui MW, editor. Postharvest Disinfection of Fruits and Vegetables. Academic Press; 2018. pp. 1–52. [Google Scholar]
- Sy KV, McWatters KH, Beuchat LR. Efficacy of gaseous chlorine dioxide as a sanitizer for killing Salmonella, yeasts, and molds on blueberries, strawberries, and raspberries. J Food Prot. 2005a;68:1165–1175. doi: 10.4315/0362-028X-68.6.1165. [DOI] [PubMed] [Google Scholar]
- Sy KV, Murray MB, Harrison MD, Beuchat LR. Evaluation of gaseous chlorine dioxide as a sanitizer for killing Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, yeasts, and molds on fresh and fresh-cut produce. J Food Prot. 2005b;68:1176–1187. doi: 10.4315/0362-028X-68.6.1176. [DOI] [PubMed] [Google Scholar]
- Trinetta V, Linton RH, Morgan MT. Use of chlorine dioxide gas for the postharvest control of Alternaria alternata and Stemphylium vesicarium on Roma tomatoes. J Sci Food Agric. 2013;93:3330–3333. doi: 10.1002/jsfa.6180. [DOI] [PubMed] [Google Scholar]
- Tweddell RJ, Boulanger R, Arul J. Effect of chlorine atmospheres on sprouting and development of dry rot, soft rot and silver scurf on potato tubers. Postharvest Biol Technol. 2003;28:445–454. doi: 10.1016/S0925-5214(02)00205-3. [DOI] [Google Scholar]
- U.S. Environmental Protection Agency. [16 August 2018];Pesticides - Reregistration eligibility decision (RED) for chlorine dioxide and sodium chlorite (Case 4023) 2006 URL https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-020503_3-Aug-06.pdf.
- Vaid R, Linton RH, Morgan MT. Comparison of inactivation of Listeria monocytogenes within a biofilm matrix using chlorine dioxide gas, aqueous chlorine dioxide and sodium hypochlorite treatments. Food Microbiol. 2010;27:979–984. doi: 10.1016/j.fm.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Wang T, Qi J, Wu J, Hao L, Yi Y, Lin S, Zhang Z. Response surface modeling for the inactivation of Bacillus subtilis subsp. niger spores by chlorine dioxide gas in an enclosed space. J Air Waste Manage Assoc. 2016;66:508–517. doi: 10.1080/10962247.2016.1150365. [DOI] [PubMed] [Google Scholar]
- Wu VC, Rioux A. A simple instrument-free gaseous chlorine dioxide method for microbial decontamination of potatoes during storage. Food Microbiol. 2010;27:179–184. doi: 10.1016/j.fm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Zoffoli JP, Latorre BA, Rodrıguez EJ, Aldunce P. Modified atmosphere packaging using chlorine gas generators to prevent Botrytis cinerea on table grapes. Postharvest Biol Technol. 1999;15:135–142. doi: 10.1016/S0925-5214(98)00078-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.