Abstract
The underlying mechanisms that regulate neonatal immune suppression are poorly characterized. CD31 (PECAM1) is highly expressed on neonatal lymphocytes and is a known modulator of TCR signaling. To further characterize the role of CD31 in the neonatal CTL response, 3-d and 7-d-old murine neonates were infected with influenza virus and compared to adults. The majority of the pulmonary viral-specific CTLs in the 3-d-old murine neonate retain CD31 expression, whereas adult CTLs have decreased CD31 expression. In addition, CD31+ neonatal viral-specific CTLs demonstrate decreased IFN-γ production, decreased proliferative capacity, and increased likelihood of death. At the peak of infection, sorted neonatal effector CTLs continue to transcribe CD31, indicating a developmental regulation of expression. To explore potential mechanisms for this reduced function, we compared the expression of the transcription factors Eomesodermin (Eomes) and T-bet; there was a significant increase in Eomes paired with a reduction in T-bet in CD31+ neonatal effector CTLs in the lung. Furthermore, in vitro stimulated neonatal CTLs significantly reduce IFN-γ production upon CD31 signaling. Altogether, these data indicate that neonatal CTLs may retain elevated levels of CD31 to maintain peripheral T cell suppression during the bridge to ex utero life.
Keywords: CD31, CD8+ T cell, infections: respiratory viruses, neonate
1 |. INTRODUCTION
Respiratory viral infections contribute substantially to global fetal and infant morbidity and mortality. Over 40% of children hospitalized for community-acquired pneumonia are less than the age of 2 and preterm infants are disproportionately affected.1 Of all deaths in the first year of life secondary to a respiratory viral infection, 55% occur in a neonate born before 30 wk gestation.2 CD8+ CTLs are critical for protection from viruses and intracellular bacteria.3,4 The CTL responses in neonates are less robust, which leads to reduced protection in this sensitive age group.5,6 Prematurity may further impair CTL responses and increase susceptibility to infections. Neonates display delayed T cell function due to a greater proportion of naïve T cells in the circulation and a low subpopulation of memory T cells.7 Most of this naïve, neonatal T cell pool is composed of recent thymic emigrants (RTEs). There is a massive efflux of RTEs from the thymus at the time of birth, and these RTEs display decreased effector functions.8
CD31 is a 130-kDa glycoprotein that is highly expressed on the surface of neonatal lymphocytes and plays a critical role in the regulation of the sensitivity of the TCR.9 Engagement of the extracellular protein CD31 during T cell activation leads to phosphorylation of cytoplasmic ITIMs, which leads to the recruitment of SH2-containing tyrosine phosphatases, namely SHP-2, which can dephosphorylate Zap-70 consequently inhibiting T cell activation.10,11 Previously, we demonstrated differences in trafficking of CD31+ effector CTLs to the lung during neonatal murine acute influenza viral infection, as compared to their adult counterparts.12 We hypothesized that neonatal viral-specific CD31+ CTLs would also preferentially traffic to the lung and have decreased function compared to CD31− CTLs during an acute pulmonary neonatal infection. To address this question, we infected 3-d-old murine neonates with influenza virus, which mirror a late preterm neonate (33–36 wk gestation).12,13
Here, we report murine neonatal CD31+ effector CTLs demonstrate decreased IFN-γ production during both in vivo influenza virus infection and in vitro T cell stimulation. This decreased effector function is coupled with decreased proliferation and differential transcriptional regulation. These results highlight a possible mechanism for differential regulation of effector CD8+ T cells in the neonate by an immunomodulatory receptor, which potentially contributes to a dampened response during acute respiratory viral infection.
2 |. MATERIALS AND METHODS
2.1 |. Mice and infections
Eight-week-old adult C57Bl/6 mice were purchased from Charles River Laboratory (Wilmington, MA, USA). Neonatal C57Bl/6 mice were generated using standard breeding procedures. The mice were housed under specific-pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care-certified barrier facility at the Drexel University College of Medicine New College Building Campus animal facility. Animal work was carried out according to approved Institutional Animal Care and Use Committee protocols.
For influenza viral infections, neonatal mice at 3 d of age (weight ~3 g) were infected intranasally (i.n.) with 0.12 TCID50 (0.04 TCID50/g) of influenza virus H1N1 strain PR8 (A/Puerto Rico/8/34) (generous gift of Dr. W. Gerhard, Wistar Institute, Philadelphia, PA, USA) in a 5 μl volume. Neonatal mice at 7 d of age (weight ~5 g) were infected i.n. with an equivalent dose on a per gram basis: 0.20 TCID50 (0.04 TCID50/g) of influenza virus in a 7 μl volume. Adult 8-wk-old C57Bl/6 mice (weight ~20 g) were infected i.n. with a sublethal dose of 3 TCID50 in a 20 μl volume (0.15 TCID50/g). Neonatal mice are more susceptible to influenza virus infection, and so their dose was titrated down such that neonates had equivalent viral loads to the adults at the peak CTL response 9 to 10 d postinfection as previously described.13 The mice were anesthetized with inhaled isoflurane before intranasal inoculations. Lungs and spleens were harvested on noted days postinfection.
2.2 |. Isolation of pulmonary leukocytes
Pulmonary leukocytes were isolated from individual mice by removing lungs and mincing into small pieces. The tissue was then digested for 2 h at 37°C with 3.0 mg/ml collagenase A and 0.15 μg/ml DNase I (Worthington Biochemical, Lakewood, NJ, USA) in RPMI 1640 (Corning) containing 5% heat-inactivated FBS (Gemini Bioproducts, West Sacramento, CA, USA), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Corning). The digested tissue was then run through a 40 μm cell strainer (Falcon) and washed in the same media as above. Cells were counted using trypan blue exclusion with light microscopy. All absolute cell numbers are calculated per 100 mg of lung tissue.
2.3 |. Intravascular CD8+ T cell staining
CD8+ T cells were labeled intravascularly using a previously described protocol modified for the neonate.14 In short, 3-d-old murine neonates and 8-wk-old adults were influenza virus infected, and 9 d postinfection were injected retro-orbitally with 0.3 μg (neonate) or 3 μg (adult) of anti-mouse CD8a monoclonal antibody Alexa Fluor 488 (Biolegend, San Diego, CA, USA) in a 10 uL volume diluted in normal saline. Following a 1 min (neonate) or 2 min (adult) incubation, lungs were quickly removed, and pulmonary lymphocytes were isolated as described above with an additional wash in HBSS containing 3% FBS (Gemini Bioproducts) to prevent leakage of labelled cells.
2.4 |. T cell activation and CD31 crosslinking
Splenocytes from naïve animals were processed to single cell suspension and the red blood cells were removed by ammonium chloride lysing solution. One million total splenocytes were incubated with plate bound 1 μg/ml anti-CD3ɛ functional grade (clone 145–2C11, eBioscience, Thermo Fisher Scientific, Philadelphia, PA, USA) and 5 μg/ml anti-CD28 functional grade (clone 37.51, eBioscience, Thermo Fisher Scientific) in T cell media (RPMI 1640 (Corning) supplemented with 10% heat-inactivated FBS (Gemini Bioproducts), 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin (Corning), 1X MEM nonessential amino acids (Sigma-Aldrich, St. Louis, MO, USA, M7145), 50 μM β-mercaptoethanol, 10 mM HEPES, pH 7.2 to 7.5, 1 mM sodium pyruvate, 0.075% sodium bicarbonate) for 48 h. Polyclonal rabbit anti-mouse CD31 antibody (#ab124431, Abcam, Cambridge, UK) was added to appropriate wells at 5 μg/mL concentration and incubated at 37°C for 30 min. Goat anti-rabbit IgG Fc secondary antibody (#SA5–10228, Thermo Fisher Scientific) was added to crosslink CD31 and Golgi plug (BD Biosciences, San Diego, CA, USA) was added to detect intracellular cytokines. The cells were incubated at 37°C for 5 h. Cells were harvested, washed, and stained as described below.
2.5 |. Flow cytometry
Cells were stained as previously described12 with the following anti-mouse monoclonal antibodies: anti-CD8α PerCP-efluor710 (eBioscience, Thermo Fisher Scientific), anti-CD44 BV605 (Biolegend), anti-CD62L BV421 (Biolegend), CD31 PE-Cy7 or BV421 (Biolegend), or PD-1 PE-Cy7 (eBioscience, Thermo Fisher Scientific, Philadelphia, PA, USA). MHC class I tetramers were prepared with biotinylated monomeric H-2b class I MHC molecules refolded in the presence of equimolar amount of β2-microglobulin and excess immunodominant NP(366–374) (ASNENMETM) peptide (generous gift of Dr. Peter Katsikis, Erasmus Medical Center, Rotterdam, Netherlands). Tetramers were prepared with allophycocyanin-labeled streptavidin (Molecular Probes, Eugene, OR, USA). Anti-16/32 (Fc Block) (Tonbo Biosciences, San Diego, CA, USA) was used in all stains. Cells were assessed for viability by incorporation of fixable viability dye efluor450 (eBioscience, Thermo Fisher Scientific). For staining of intracellular cytokines, GolgiPlug (BD Biosciences), IC fixation buffer (eBioscience, Thermo Fisher Scientific) and permeabilization buffer (eBioscience, Thermo Fisher Scientific) were used according to manufacturers’ instructions and stained with IFN-γ APC (eBioscience, Thermo Fisher Scientific). Cells were stimulated ex vivo with 1μM of the H2K- or H2D- associated influenza virus peptides NP(366–374), DbPA(224–233), or NS2(114–121) for 5 h prior to fixation, permeabilization and staining. For intranuclear stains, FoxP3 transcription factor kit (eBioscience, Thermo Fisher Scientific) was used according to manufacturers’ instructions and cells were stained with anti-mouse T-bet BV421 (Biolegend), anti-mouse Ki-67 AF647 (BD Biosciences), FoxP3 PE (eBioscience, Thermo Fisher Scientific) or CTLA-4 PE (eBioscience, Thermo Fisher Scientific). All stains were completed on ice to prevent internalization. Cells were fixed in 1% paraformaldehyde (Thermo Fisher Scientific) before flow cytometric analysis. Data were collected on a FACS LSR Fortessa using FACS Diva software (BD Biosciences). Analysis was performed using Flow Jo software (Tree Star). Lymphocytes were gated based on forward and side scatter, and then by CD8a+. Naïve cells were gated based on expression of CD62L+CD44− and activated cells were gated by CD44+CD62L− (Supplemental Fig. 1).
2.6 |. Cell sorting and real-time PCR
Live (fixable viability dye negative), naïve CD8+ T cells (CD8a+CD44−), or effector CD8+ T cells (CD8a+CD44+CD62L−), which were CD31+/− were sorted from the lungs of neonatal and adult animals 8 d postinfection (effector) or age-matched naïve animals on a FACSAria Fusion (BD Biosciences). Total RNA was isolated using RNeasy kit (Qiagen, Hilden, Germany). cDNA was synthesized using the High Capacity cDNA reverse transcription assay (Applied Biosystems, Waltham, MA, USA). The expression of PECAM-1, Tbx21, and Eomes was measured by RT-PCR using inventoried primers (Thermo Fisher Scientific) in an AB 7500 HT sequence detection system according to the manufacturer’s instructions. For relative quantitation of the different mRNA species, all values were normalized to measured levels of GAPDH transcripts and expressed relative to values for CD31− effector CTLs by the comparative cycling threshold (ΔΔCT) method.
2.7 |. Statistical analysis
Normality was assessed using the Shapiro-Wilk test for normality. Statistical analysis was performed using the 2-tailed unpaired t test for (parametric) or Mann-Whitney (nonparametric) for unpaired samples. For paired samples, a 2-tailed paired t test (parametric) or Wilcoxon matched-pairs signed rank test (nonparametric) was used. Analyses were performed using GraphPad PRISM version 6 for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). P values <0.05 were considered to be statistically significant.
3 |. RESULTS
3.1 |. Neonatal effector viral-specific CD8+ T cells preferentially express CD31+
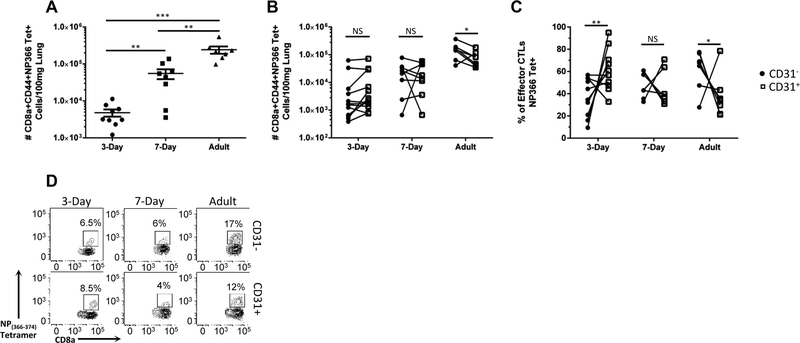
We have previously demonstrated that influenza virus-infected 3-d-old murine neonates have an increased number of CD31+ effector (CD44+CD62L−) CD8+ T cells within the lung at 6 and 9 d postinfection, the peak of the adult viral-specific response.12 This increase in CD31+ effector CTLs was specific to the lung as there was no difference in expression of CD31 in the neonatal periphery, indicating a differential regulation of trafficking of effector CTLs.12 Therefore, we questioned if there were also differences in trafficking of viral-specific effector CTLs. Animals were infected at 3 d, 7 d, and 8 wk of age; lungs were harvested at day 9 postinfection and analyzed by flow cytometry. A tetramer against the immunodominant epitope (NP(366–374)) was used to identify viral-specific cells. All absolute numbers are normalized per 100 mg of lung tissue to account for differences in lung size between different ages. Consistent with previous work from our lab characterizing the neonatal viral-specific CD8+ T cell response to influenza virus infection, there is a significant reduction in the number of viral-specific CD8+ T cells in both neonatal groups compared to adults (Fig. 1A).13
FIGURE 1. Characterization of viral-specificity of murine neonatal and adult CD31+ CTLs.
Lungs were harvested from animals on day 9 post-influenza virus infection. Number of NP(366–374) tetramer positive effector CTLs normalized per 100 mg of lung tissue is depicted (A). Each dot represents a single animal. (n = 8–12 animals per group, four independent experiments). (B) Number of CD31+ and CD31− NP(366–374) tetramer positive effector CTLs. (C) Percentage of NP(366–374) tetramer positive effector (CD44+CD62L−) CD31+ or CD31− cells of the total NP(366–374) viral-specific pool within the lung from absolute numbers in (B). (D) Representative flow plots depicting the tetramer positive population for CD31− (Top row) and CD31+ (Bottom row) from animals infected at day 3 of life (left column), day 7 of life (middle column) or as adults (right column). Unpaired t test (parametric) and Mann-Whitney (nonparametric) statistics were used for 1A and 1C to compare groups by CD31 expression/day of life. 1B was analyzed by a 2-tailed paired t test (7 d and adult) or Wilcoxon’s matched-pairs signed rank test (3 d) (*P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001)
Next, we questioned if there was differential CD31 expression on viral-specific CTLs at different ages. Within individual adult mice, there was a statistically significant decrease in the absolute number of CD31+ viral-specific CTLs, as compared to CD31− (Fig. 1B and D). Because of the relatively low absolute number of total viral-specific CTLs in the neonatal mouse, the percentage of viral-specific CD8+ T cells expressing CD31 was calculated for the respective groups by comparing the absolute number of either CD31 positive or negative NP(366–374)+ CD44+CD62L−CD8+ T cells to the total number of NP(366–374)+D44+CD62L−CD8+ T cells. Neonates infected on the third day of life have a significant increase in the frequency of CD31+ CTLs that comprise the viral-specific response (Fig. 1C). There is a stepwise decrease in CD31 expression as the mouse ages. Seven-day-old neonates have no difference in the percentage of CD31+ and CD31− CTLs, whereas adults have a statistically significant decrease in CD31+ expression in the NP(366–374) viral-specific pool (Fig. 1B and C). This may indicate CD31 is being preferentially retained or expressed by the neonatal viral-specific CTLs within the site of infection.
3.2 |. Neonatal CD31+ viral-specific CTLs demonstrate decreased IFN-γ production
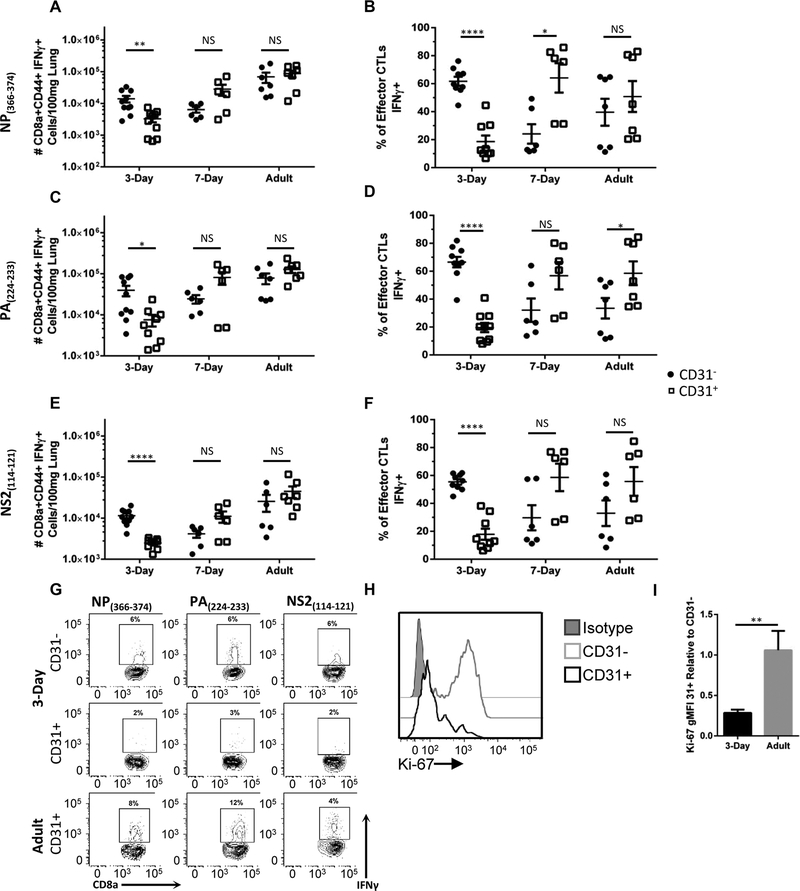
Given the increased proportion of CD31+ viral-specific CTLs in neonatal animals and the known regulatory function of CD31 in T cell function,10 we questioned whether there were differences in effector function between CD31+ and CD31− viral-specific CD8+ T cells. IFN-γ is a powerful antiviral cytokine that stimulates recruitment of T cells to the lung.15 In neonates, reduced CTL IFN-γ production is associated with reduced viral control.16 Therefore, we assessed IFN-γ production as a measure of CTL function. Again, animals were infected at 3 d, 7 d, and 8 wk; lungs were harvested at day 9 postinfection. Cells were stimulated ex vivo using the immunodominant peptide NP(366–374) and IFN-γ production measured. Unstimulated samples and samples stained with an isotype control were used as controls. Despite the increased proportion of CD31+ NP(366–374)+ CTLs in the 3 d neonate (Fig. 1C), these cells were significantly underrepresented in the IFN-γ+ effector CD8+CD31+ T cell population (Fig. 2A, B, and G). However, in the 7 d neonate, a greater proportion of the CD8+CD31+ T cells are IFN-γ+ (Fig. 2B). In the adult, there is no difference in the proportion of IFN-γ+ viral-specific CTLs in the CD31+ population compared to the CD31− population.
FIGURE 2. Neonatal CD31+ viral-specific CTLs demonstrate decreased IFN-γ production, as compared to their adult counterparts.
Lungs were harvested from animals on day 9 post-influenza virus infection. Cells were stimulated ex vivo with the immunodominant peptides NP(366–374) or PA(224–233) or the subdominant epitope NS2(114–121) and IFN-γ production was assessed via flow cytometry. The absolute number of IFN-γ effector CTLs were calculated for each peptide and normalized per 100 mg of lung tissue (A, C, and E, respectively). Each dot represents a single animal. (n = 6–10 animals per group, three independent experiments). (B, D, and F) Percentage of IFN-γ effector CD31+ or CD31− CD8+ T cells of the total IFN-γ effector CTL pool from absolute numbers in (A, C, and E). (G) Representative flow plots of IFN-γ producing effector CTLs following stimulation with NP(366–374) (left column), PA(224–233) (middle column), or NS2(114–121) (right column). From animals infected on day 3 of life from CD31− (Top row) or CD31+ (middle row) or from adult CD31+ (Bottom Row). (H) Representative histograms of intranuclear marker of proliferation, Ki-67, expression within effector CTLs infected on day 3 of life. (I) Geometric mean fluorescence intensity (gMFI) was calculated for Ki-67 expression within CD31+ and CD31− cells. The gMFI was standardized based on isotype gMFI subtraction and shown as the relative to own age groups CD31− Ki-67 gMFI. (n = 9 animals per group, four independent experiments). Unpaired t test (parametric) and Mann-Whitney (nonparametric) statistics were used to compare groups by CD31 expression/day of life (*P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001)
Neonatal CD8+ T cells can have a distinct epitope hierarchy.17 Therefore, we sought to determine if IFN-γ production would be similarly decreased in response to another immunodominant epitope (PA(224–233)) and a subdominant epitope (NS2(114–121)). Cells were stimulated ex vivo with these peptides and IFN-γ production was assessed. In agreement with the NP(366–374) data, there is a reduction in IFN-γ+CD31+ cells in neonatal animals in response to both PA(224–233) (Fig. 2C, D, and G) and NS2(366–374) (Fig. 2E, F, and G). However, adults produce equal amounts of IFN-γ independent of CD31 expression (Fig. 2A, B, C, E, and F). Taken together, these data indicate that the majority of the neonatal viral-specific CTLs express CD31 and have reduced production of a key anti-viral cytokine, IFN-γ.
3.3 |. Neonatal CD31+ CTLs have reduced viability and proliferation
Given the high number of nonfunctional CTLs within the neonatal mouse, we next sought to confirm the viability of these cells. Cells were harvested from the lungs of animals at day 9 postinfection, stained with a fixable viability dye and death was assessed via flow cytometry. Approximately 7% of CTLs are nonviable in the neonate and the adult. Of these nonviable CTLs, we questioned if CD31 was preferentially expressed. Indeed, most nonviable cells were CD31+ in both 3-d-old neonates and adults (Supplemental Fig. 2). However, there is a significant difference between CD31+ and CD31− populations within the 2 groups. An average of 80% of the nonviable CTLs are CD31+ in the neonate versus 60% in the adult, which indicates neonatal CD31+ effector CTLs are more susceptible to death.
Based on this increased susceptibility to death, we sought to determine if there were also differences in proliferation in CD31+ neonatal effector CTLs. To assess this, CTLs were stained for the intranuclear marker of proliferation Ki-67 at day 9 postinfection in animals infected at 3 d and 8 wk. Remarkably, neonatal CD31+ cells displayed a dramatic decrease in Ki-67 expression compared to the CD31− effector CTLs (Fig. 2H). The geometric mean fluorescence intensity (gMFI) of Ki-67 in neonatal effector CD31+ CTLs was quantified and assessed relative to the CD31− counterpart and compared to adults. We found a significant reduction in Ki-67 expression in the CD31+ viral-specific CTLs of neonatal animals (Fig. 2I). Therefore, neonatal CD31+ CTLs have a higher propensity for death and a decreased ability to proliferate within the lung.
3.4 |. Neonatal CD31+ CTLs have reduced IFN-γ production at the site of infection
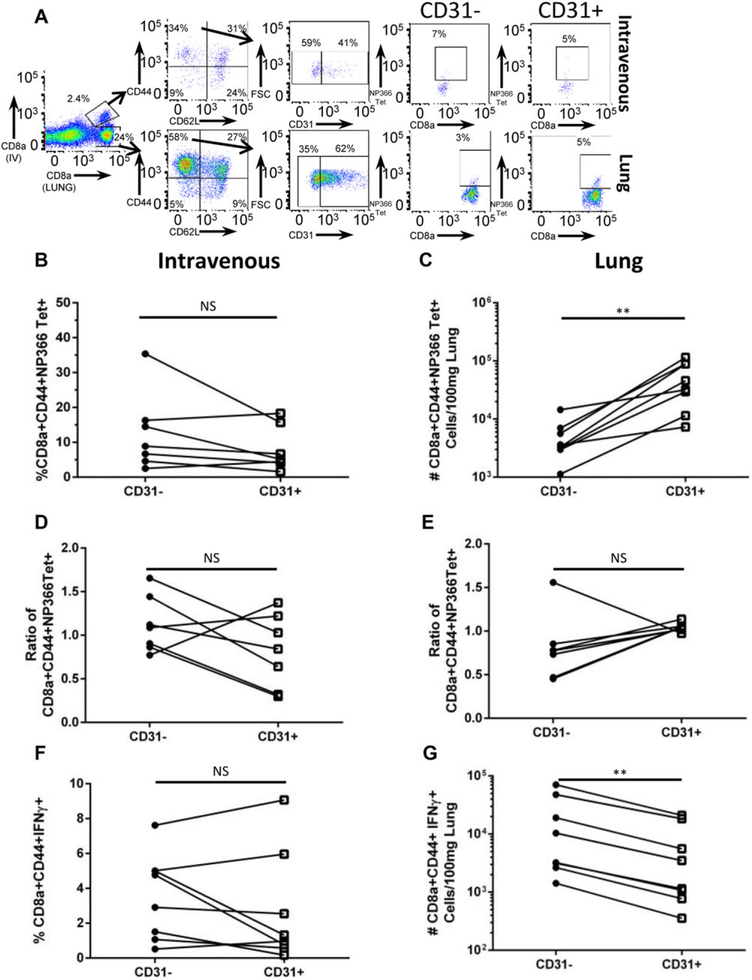
CD31 expression regulates both constitutive and inflammatory-based trafficking in vivo.18 Therefore, we questioned if the observed dysfunction in neonatal CTLs was systemic or restricted to the pulmonary compartment. To distinguish between those CD8+ T cells within the tissue of the lung versus those in circulation in the neonate, we used the previously characterized method of intravenous labelling of CD8+ T cells.14 Nine days post-influenza virus infection, neonatal mice received an anti-CD8 monoclonal antibody intravenously via retro-orbital injection and lungs were harvested after a brief incubation period (Fig. 3A). We first sought to determine the frequency of CD31+ and CD31− NP(366–374) effector CTLs in circulation and at the site of infection (Fig. 3B and C, respectively). There were no differences in the frequency of CD31+ and CD31− NP(366–374) effector CTLs within the circulation. However, within the lung we identified a significant increase in the number of CD31+ NP(366–374) effector CTL. Furthermore, the ratio of CD31+ to CD31− NP(366–374) effector CTLs at the site of infection favors CD31+viral-specific CTLs, like Fig. 1C (Fig. 3E). However, in direct contrast, there are no differences in the ratio of CD31+ viral-specific CTLs in the circulation (Fig. 3D).
FIGURE 3. Neonatal CD31+ CTLs produce less IFN-γ at the site of infection, as compared to circulating neonatal CTLs.
Day 9 post-influenza virus infection, neonatal CD8+ T cells were labelled via intravenous anti-CD8a monoclonal antibody labelling via retro-orbital injection to identify those cells within the lung tissue. Cells were stimulated ex vivo following intravenous labelling with immunodominant peptide NP(366–374). (A) Representative flow plots depicting IV versus Lung labeled CD8a+ T cells and gating strategy. (B) Frequency of NP(366–374) tetramer positive effector CTLs within the circulation. (C) Number of CD31+ and CD31− NP(366–374) tetramer positive effector CTLs within the lung. (D) Ratio of the frequency of NP(366–374) CD31+ or CD31− effector CTLs to the frequency of the total NP(366–374) positive effector CTLs within the circulation and within the lung (E). (F) Frequency of IFN-γ+ effector CTLs within the circulation. (G) Absolute number of IFN-γ+ effector CTLs within the lung tissue. (n = 5–8 animals per group, 2 independent experiments). Paired T test (nonparametric statistics were used to compare groups by CD31 expression (*P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001)
To assess function, labeled samples were stimulated ex vivo with the immunodominant peptide NP(366–374) and IFN-γ production was assessed. There is no difference in the frequency of CD31+ or CD31−CD8+CD44+IFN-γ+ CTLs in circulation (Fig. 3F). However, consistent with the previous data (Fig. 2A and B), there is a reduction in the number of neonatal CD31+ CTLs that produce IFN-γ in response to stimulation (Fig. 3G). These data provide evidence that CD31 expression on neonatal CTLs is associated with attenuation of function specifically at the site of infection.
3.5 |. CD31+ and CD31− effector CTLs have similar expression of PD-1, CTLA-4, and FoxP3
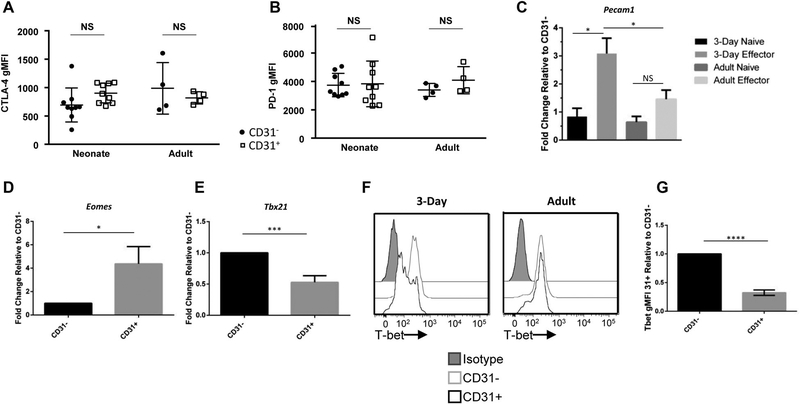
Next, we sought to determine temporal relationships between expression of CD31 with 2 other known inhibitory receptors, programmed cell death protein-1 (PD-1) and CTLA-4 and the master transcription factor for CD8+ regulatory T cells (FoxP3).20 Potentially, other inhibitory receptors were co-expressed with CD31 and contributed to a decrease in function. Nine days post-influenza virus infection, neonatal and adult animals were harvested and FoxP3, PD-1, and CTLA-4 expression was determined on CD31+ and CD31− effector CTLs. There is essentially no FoxP3 expression in either CD31+ or CD31− CTLs, with <0.1% of effector CTLs in the neonate or adult expressing FoxP3 (data not shown). CTLA-4 and PD-1 expression, as measured by geometric mean fluorescence, is similar among CD31+ and CD31− effector CTLs (Fig. 4A and B). This data indicates that CD31 is not coexpressed with some common inhibitory or suppressive factors.
FIGURE 4. CD31+ and CD31− effector CTLs have similar expression of PD-1, CTLA-4, and FoxP3, but increased transcription of CD31 and a different master transcription factor profile.
Lungs were harvested from animals on day 9 post-influenza virus infection. Corresponding geometric MFI of effector (CD44+CD62L−) CD31+ or CD31− cells that express (A) CTLA-4 and (B) PD-1. Each dot represents a single animal. (n = 4–9 animals per group, 2 independent experiments). Viable naïve and effector CTLs were sorted from the lungs of animals infected on day 3 of life or as adults based on CD31 expression. (C) Transcriptional expression of Pecam1 relative to the groups own CD31− CTLs. (n = 8–10 animals per group, three independent experiments) (P < 0.05). Transcription of master transcription factors (D) Tbx21 and (E) Eomes in effector CTLs relative to their own CD31− CTLs were analyzed by RT-PCR. (n = 6–8 animals per group, three independent experiments). (F) Representative histograms of intranuclear T-bet staining performed on animals infected at day 3 of life (left) or as adults (right) at day 9 postinfection. (G) Corresponding geometric MFI of neonatal T-bet expression (n = 6 animals per group, three independent experiments). Unpaired t test (parametric) statistics were used to compare groups by CD31 expression/day of life (*P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001)
3.6 |. Increased CD31 expression on neonatal effector CTLs is transcriptionally driven
Given the increase in CD31+ CTLs in the lungs of neonatal animals during influenza virus infection we questioned if this was simply due to the large thymic output at this young age, or if there was an ongoing transcriptional regulation of CD31 (Pecam1). There is conflicting data whether upon activation, CD31 is proteolytically cleaved from the surface of CD8+ T cells or transcriptionally regulated.20,21 First, baseline transcription of naïve CD31+ and CD31− CTLs was determined by sorting these CTLs from the lungs of neonatal and adult animals (Fig. 4C). Transcription of CD31 in CD31+ neonatal and adult CTLs is equivalent to their respective CD31− CTLs. Next, we sought to determine differences in transcription in activated CTLs. CD31+ and CD31− effector CTLs were sorted from the lungs of neonatal and adult animals day 8 postinfection. Interestingly, relative to their own CD31− CTLs, the neonatal animals have a threefold increase in the transcription of Pecam1 as compared to adult CD31+ CTLs (Fig. 4C). Therefore, neonates not only retain CD31 expression after activation, but also have a potentially developmentally regulated increase in transcription of CD31.
3.7 |. Neonatal CD31+ CTLs have a different master transcription factor profile
Given the lack of PD-1, CTLA-4, and FoxP3 co-expression, difference in transcription of CD31 (Pecam1) and the functional differences demonstrated above, we questioned if there would be differences in the master transcription factors T-bet (Tbx21) or Eomesodermin (Eomes). Again, CD31+ and CD31− effector CTLs were sorted from the lungs of influenza virus-infected 3-d- and 8-wk-old animals, day 8 postinfection. There was a significant increase in the transcription of Eomes in the CD31+ populations, with neonatal CD31+CD8+ T cells displaying a fourfold increase as compared to the CD31− effector CTLs (Fig. 4D). Additionally, there was a significant reduction in T-bet transcription in the CD31+ population relative to the CD31− effector population (Fig. 4E). T-bet is a known repressor of inhibitory receptor expression.22 Given our prior observation of a significant increase in Pecam1 transcription, we explored the expression of T-bet protein expression further. Intranuclear staining for T-bet was performed on both neonatal and adult effector CTLs 9 d postinfection (Fig. 4F and G). In agreement with the transcriptional data, we found a significant reduction in T-bet in the neonatal CD31+ CTLs, but no difference in the adult. These data demonstrate a differential master transcription factor profile in neonatal CD31+ effector CTLs.
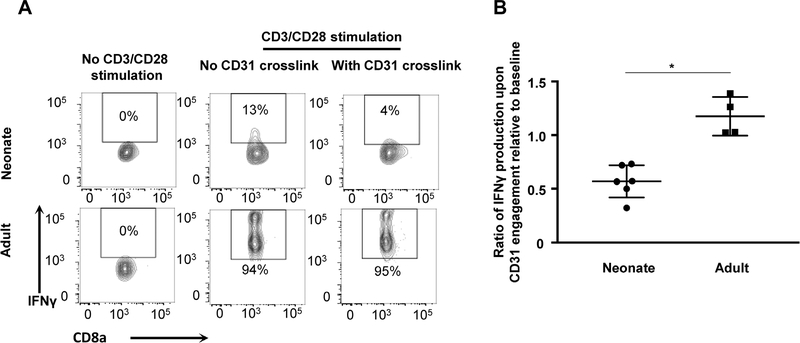
3.8 |. CD31 Signals Decrease IFN-γ production in neonatal CTLs upon in vitro stimulation
To determine if CD31 signaling can directly lead to reduced effector function in the neonate, an in vitro CD31 crosslink assay was performed.10 Naïve splenocytes were isolated from neonatal and adult mice and were activated by CD3 and CD28 co-stimulation. After 48 h in culture, CD31 was crosslinked for 5 h. Compared to age-matched cells in the absence of crosslinking, neonatal activated CTLs produced 50% less IFN-γ, as opposed to adults who had no change in IFN-γ production after CD31 crosslinking (Fig. 5A and B). Therefore, when an activated neonatal CD8+ T cell has CD31 crosslinked, there is a rapid decrease in production of IFN-γ indicating a direct role for CD31 in the regulation of IFN-γ production in neonates.
FIGURE 5. CD31 signals decreased IFN-γ production in neonatal CTLs upon in vitro stimulation.
Naïve splenocytes were isolated from neonatal and adult mice and were activated by CD3 and CD28 co-stimulation for 48 h and then CD31 crosslinking was performed. (A) Representative flow plots depicting the frequency of IFN-γ+ effector CTLs without stimulation (left), with CD3/28 stimulation and no CD31 crosslink (middle) and with CD3/28 stimulation and CD31 crosslink (right) for neonatal animals (top panels) and adult animals (bottom panels). (B) Ratio of IFN-γ production upon CD31 crosslink relative to baseline after in vitro T cell stimulation with anti-CD3/28 (n = 4–8 animals per group, 2 independent experiments). Mann-Whitney (nonparametric) statistics were used to compare neonate to adult (*P < 0.05)
4 |. DISCUSSION
Despite recognition of the severity and high mortality of neonatal respiratory viral infections the specific mechanism for this increased susceptibility remains unclear.1,2,23The mortality rate from influenza viral infection is highest in infants less than the age of 6 mo,23 currently an age group not eligible for the available influenza virus vaccine. Neonates are subjected to an immunological dilemma, where there is a need to be both tolerant to certain pathogens or antigens, but reactive toward others. This balance of suppression and reactivity is believed to stem from sustained suppression against the mother and rapid microbial colonization after birth.24
The underlying mechanisms that regulate suppression are poorly characterized. RTEs compose most of the peripheral neonatal T cell pool.7 RTEs have reduced effector functions compared to their mature naïve counterparts.8 Specifically, RTEs produce less IFN-γ, IL-2, and TNF-α, related to defects in aerobic glycolysis.25 CD31 is highly expressed on human RTEs, but this is lost during post-thymic peripheral expansion, potentially due to CD31 cleavage following homeo-static TCR:MHC engagement.12,26,27 Here, we report that neonatal effector CD8+ T cells have increased CD31 expression and decreased function specifically in the lung during an acute influenza viral infection. Neonatal viral-specific CTLs demonstrate normal phenotypic activation status, surface markers, and trafficking to the site of inflammation. However, once in the inflammatory setting, proliferation and IFN-γ secretion are primarily performed by CD31− virus-specific CTLs. Thus, this suggests a potential role for CD31 in the regulation of function and the maintenance of peripheral CD8+ T cell suppression in the neonate during acute infection.
CD31 is a known immunomodulator of T cell activation and effector responses.10,11,20,28 Loss of CD31 results in uncontrolled T cell-induced inflammation in the mouse.29 Additionally, polymorphisms in CD31 have been associated with an exacerbated human graft-versus-host and atherosclerosis severity.29 CD4+ T cells from human tuberculosis patients exhibit an inverse relationship between CD31 expression and IFN-γ production.30 Despite these known immunomodulatory roles, CD31 is understudied in comparison to other inhibitory receptors such as PD-1 or CTLA-4.9 Animals infected at 3 d of life had an increased percentage of CD31+ viral-specific cells. Seven-day-old animals had an intermediate number of viral-specific CTLs compared to adults, but in both the 7-d-old and 8-wk-old animals, there is no difference in CD31 expression on viral-specific effector CTLs.
Pulmonary CD31+ CTLs harvested from neonatal animals infected on the third day of life and stimulated with immunodominant or subdominant peptides demonstrated decreased IFN-γ production. Additionally, these cells have reduced expression of Ki-67. This dysfunctional phenotype is potentially related to diminished effector T cell response in the neonate and peripheral CD8+ T cell suppression. CTLs play little role in the immunopathology of RSV and influenza viral infections in human infants.31,32 To compensate for a diminished effector T cell response, there is a concomitant increase in neutrophil and macrophage influx, which is likely the source of immunopathology and high rates of morbidity associated with respiratory viruses in this vulnerable population.32 In accordance with the human data, 3-d-old murine neonates have a dampened effector response in those viral-specific CTLs that express CD31. CD31 expression on vascular endothelium functions to prevent CTL mediated lysis through homophilic engagement.11 Dendritic cells that receive sustained CD31 signaling during maturation develop a tolerogenic phenotype capable of suppressing IFN-γ, IL-2, and IL-17A production in CD4+ T cells via induction of Tregs.33 Potentially, homophilic engagement of the neonatal CTLs with endothelial or innate immune cell CD31 is inducing a suppressive environment within the lung.
To begin to address whether CD31 directly dampens effector function or is a marker of T cell dysfunction, we determined the expression of known modulators of the T cell response in conjunction with CD31. CTLA-4 is expressed immediately after initial T cell activation and limits the magnitude of naïve T cell responses.34 However, maximal inhibitory effects of CTLA-4 engagement are observed in memory T cells.35 At the peak of the effector response (9 d postinfection), we found no difference in CTLA-4 expression between age-matched CD31+ and CD31− effector CTLs. We next investigated PD-1, which is also immediately expressed following activation, but is most highly expressed following chronic exposure of T cells to cognate antigen.36 Human infants congenitally infected with CMV demonstrate increased expression of programmed death-1 (PD-1) and an exhaustion phenotype believed to reduce T cell mediated immunopathology.37 Additionally, in response to commensal bacterial colonization of the lungs during the first 2 wk of life, APCs increase PD-L1 expression.24 Our data demonstrates no difference in PD-1 expression on CD31+ or CD31− effector CTLs at the peak of the effector response in influenza virus infection. Therefore, PD-1 is likely not playing a critical role in the decreased effector response in CD31+ CD8+ T cells. However, this was not an exhaustive investigation of T cell inhibitory receptors. Other factors coupled with CD31 could be modulating the effector response in neonates. CD31 expression on T cells can enhance the suppressive effects of TGFβ selectively in T cells within the tumor microenvironment.38 Ongoing work in the laboratory is investigating the potential interplay of CD31 with other immunomodulatory factors.
Recently Newman and colleagues reported that transcriptional down-regulation, and not extracellular domain cleavage, is responsible for decreased levels of expression of CD31 in activated effector CTLs.21 In agreement with this, we found adult CD31+ effector CTLs produce very little CD31 transcript. However, neonatal effector CTLs have ongoing transcription and expression of CD31, as compared to neonatal naïve CTLs, which indicates both an age-related and infection-related regulation of CD31. Future studies could investigate the developmental and epigenetic regulation of CD31 expression.
The transcription factor T-bet is highly correlated with early CTL differentiation and acquisition of effector functions, whereas Eomes is associated with the development of a memory phenotype and the maintenance of IFN-γ production.39,40 This T-bet and Eomes axis is believed to dictate the functionality and differentiation of CTLs, with IFN-γ production dependent on both T-bet and Eomes.41,42 T-bet is also the master regulator of IFN-γ and Th1 commitment of CD4 T cells.43 Here, we have found a significant increase in Eomes transcription within the dysfunctional neonatal CD31+ viral-specific CTL population. Furthermore, we found that there was a significant reduction at both the transcription and protein level of T-bet in the neonatal CD31+ effector population. We were not able to definitively determine the complete relationship between decreased IFN-γ production, increased CD31 expression and decreased T-bet expression in neonatal effector CTLs. However, our findings of this dysfunctional transcriptional profile is consistent with models of chronic viral infection, where expression of inhibitory receptors, such as PD-1, CD160, and 2B4, is correlated with T-bet low, Eomes high virus specific CTLs.22,44,45 This transcriptional profile is also similar to that seen in self-tolerized CTLs,46 and so it is interesting to speculate how CD31 might be playing a role in not only suppressing effector function, but also modulating the bridge between fetal and neonatal life.
We propose that increased CD31 expression on neonatal CTLs is developmentally regulated and negatively regulates IFN-γ production at the site of infection, which leads to differential transcription factor regulation and reduced proliferation. Our data provide insight into a possible regulatory mechanism of the effector response to neonatal acute viral infection. We cannot say definitively if CD31+ CTLs preferentially migrate to the lung or CTLs up-regulate CD31 expression at the site of infection. Further investigation of CD31 ligand regulation and expression in the neonatal lung is being conducted to determine how the neonatal pulmonary environment drives up-regulation of CD31.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K08AI108791 to A.J.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, funding was provided by the Margaret Q. Landenberger Foundation to A.J.C. The authors would like to acknowledge Jennifer L. Hope, Ph.D. for her production of the NP(366–374) H-2b class I MHC monomers, and the Drexel University Laboratory Animal Resources staff for their aid in maintenance of the mice.
Abbreviations:
- Eomes,
Eomesodermin
- gMFI,
Geometric mean fluorescence intensity
- LCMV
Lymphocytic Choriomeningitis
- PD-1
Programmed Cell Death 1
- RSV
Respiratory syncytial virus
- RTEs
Recent thymic emigrants
- Treg
Regulatory T cell
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams EJ, Embleton ND, Clark JE, Bythell M, Ward Platt MP, Berrington JE. Viral infections: contributions to late fetal death, stillbirth, and infant death. J Pediatr. 2013;163:424–428. [DOI] [PubMed] [Google Scholar]
- 3.Kagi D, Ledermann B, Burki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24: 3068–3072. [DOI] [PubMed] [Google Scholar]
- 4.Bender BS, Croghan T, Zhang L. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med.1992;175:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez MA, Evans IA, Hassan EH, Carbone FR, Jones CA. Neonatal CD8+ T cells are slow to develop into lytic effectors after HSV infection in vivo. Eur J Immunol. 2008;38:102–113. [DOI] [PubMed] [Google Scholar]
- 6.Adkins B T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–335. [DOI] [PubMed] [Google Scholar]
- 7.Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, de Vries E. Development of lymphocyte subpopulations in preterm infants. Scand J Immunol. 2011;73:53–58. [DOI] [PubMed] [Google Scholar]
- 8.Fink PJ. The biology of recent thymic emigrants. Annu Rev Immunol. 2013;31:31–50. [DOI] [PubMed] [Google Scholar]
- 9.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-cells. J Cell Sci. 2013;126(Pt 11): 2343–2352. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Mauro C, Cornish GH, et al. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc Natl Acad Sci USA. 2010;107:19461–19466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung K, Ma L, Wang G, et al. CD31 signals confer immune privilege to the vascular endothelium. Proc Natl Acad Sci USA. 2015;112: E5815–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fike AJ, Nguyen LT, Kumova OK, Carey AJ. Characterization of CD31 expression on murine and human neonatal T lymphocytes during development and activation. Pediatr Res. 2017;82:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey AJ, Gracias DT, Thayer JL, et al. Rapid Evolution of the CD8+ TCR Repertoire in Neonatal Mice. J Immunol. 2016;196:2602–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson KG, Mayer-Barber K, Sung H, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan S, Thomas PG. Balancing Immune Protection and Immune Pathology by CD8(+) T-Cell Responses to Influenza Infection. Front Immunol. 2016;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You D, Ripple M, Balakrishna S, et al. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol. 2008;181:3486–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruckwardt TJ, Malloy AM, Gostick E, et al. Neonatal CD8T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7:e1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L, Cheung KC, Kishore M, Nourshargh S, Mauro C, Marelli-Berg FM. CD31 exhibits multiple roles in regulating T lymphocyte trafficking in vivo. J Immunol. 2012;189:4104–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reibke R, Garbi N, Ganss R, Hammerling GJ, Arnold B, Oelert T. CD8+ regulatory T cells generated by neonatal recognition of peripheral self-antigen. Proc Natl Acad Sci U S A. 2006;103:15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fornasa G, Groyer E, Clement M, et al. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunol. 2010;184: 5485–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman DK, Fu G, McOlash L, et al. Frontline Science: pECAM-1 (CD31) expression in naive and memory, but not acutely activated, CD8(+) T cells. J Leukoc Biol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao C, Oestreich KJ, Paley MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559–2567. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol. 2017;17:495–507. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham CA, Bergsbaken T, Fink PJ. Cutting edge: defective aerobic glycolysis defines the distinct effector function in antigen-activated CD8(+) recent thymic emigrants. J Immunol. 2017;198: 4575–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4T-cell maturation into Th1 or Th2 effector cells. Immunology. 1996;88: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimmig S, Przybylski GK, Schmidt CA, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clement M, Fornasa G, Loyau S, et al. Upholding the T cell immune-regulatory function of CD31 inhibits the formation of T/B immunological synapses in vitro and attenuates the development of experimental autoimmune arthritis in vivo. J Autoimmun. 2015;56:23–33. [DOI] [PubMed] [Google Scholar]
- 29.Kishore M, Ma L, Cornish G, Nourshargh S, Marelli-Berg FM. Primed T cell responses to chemokines are regulated by the immunoglobulin-like molecule CD31. PLoS One. 2012;7:e39433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiroga MF, Jurado JO, Martinez GJ, et al. Cross-talk between CD31 and the signaling lymphocytic activation molecule-associated protein during interferon- gamma production against Mycobacterium tuberculosis. J Infect Dis. 2007;196:1369–1378. [DOI] [PubMed] [Google Scholar]
- 31.Papin JF, Wolf RF, Kosanke SD, et al. Infant baboons infected with respiratory syncytial virus develop clinical and pathological changes that parallel those of human infants. Am J Physiol Lung Cell Mol Physiol. 2013;304:L530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welliver TP, Reed JL. Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J. 2008;27:S92–96. [DOI] [PubMed] [Google Scholar]
- 33.Clement M, Fornasa G, Guedj K. CD31 is a key coinhibitory receptor in the development of immunogenic dendritic cells. Proc Natl Acad Sci USA. 2014;111:E1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 35.Chambers CA, Kuhns MS, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4(+) T cell responses. Proc Natl Acad Sci U S A. 1999;96: 8603–8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8T cells during chronic viral infection. Nature. 2006;439: 682–687. [DOI] [PubMed] [Google Scholar]
- 37.Gibson L, Barysauskas CM, McManus M, et al. Reduced frequencies of polyfunctional CMV-specific T cell responses in infants with congenital CMV infection. J Clin Immunol. 2015;35:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman DK, Fu G, Adams T, et al. The adhesion molecule PECAM-1 enhances the TGF-beta-mediated inhibition of T cell function. Sci Signal. 2016;9:ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russ BE, Denton AE, Hatton L, Croom H, Olson MR, Turner SJ. Defining the molecular blueprint that drives CD8(+) T cell differentiation in response to infection. Front Immunol. 2012;3:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knox JJ, Cosma GL, Betts MR, McLane LM. Characterization of T-bet and eomes in peripheral human immune cells. Front Immunol. 2014;5:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray SM, Kaech SM, Staron MM. The interface between transcriptional and epigenetic control of effector and memory CD8(+) T-cell differentiation. Immunol Rev. 2014;261:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. [DOI] [PubMed] [Google Scholar]
- 43.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. [DOI] [PubMed] [Google Scholar]
- 44.Buggert M, Tauriainen J, Yamamoto T, et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10:e1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. [DOI] [PubMed] [Google Scholar]
- 46.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.