Significance
Glycan-lectin recognition is vital to embryogenesis, and progression in common diseases such as cancer and osteoarthritis. However, the rules governing specificity for the functional pairing and postbinding events are not yet understood, even though human lectins have been thoroughly characterized structurally. Herein, we employ protein engineering to produce new types of variants via redesign of modular lectin architecture. Comparative assays with cells (for growth regulation) and surface-programmable nanovesicles (for bridging) revealed qualitative effector/antagonist changes caused by topological switching, using human galectins-1 and -3 as a test case. In addition to identifying galectin-3’s unique chimera-type design as a means to enable functional antagonism between galectins, this approach generates new promising tools for imaging studies on lattices and for innovative therapeutic approaches.
Keywords: glycoconjugate, lectin, parasite, tumor
Abstract
Glycan-lectin recognition is assumed to elicit its broad range of (patho)physiological functions via a combination of specific contact formation with generation of complexes of distinct signal-triggering topology on biomembranes. Faced with the challenge to understand why evolution has led to three particular modes of modular architecture for adhesion/growth-regulatory galectins in vertebrates, here we introduce protein engineering to enable design switches. The impact of changes is measured in assays on cell growth and on bridging fully synthetic nanovesicles (glycodendrimersomes) with a chemically programmable surface. Using the example of homodimeric galectin-1 and monomeric galectin-3, the mutual design conversion caused qualitative differences, i.e., from bridging effector to antagonist/from antagonist to growth inhibitor and vice versa. In addition to attaining proof-of-principle evidence for the hypothesis that chimera-type galectin-3 design makes functional antagonism possible, we underscore the value of versatile surface programming with a derivative of the pan-galectin ligand lactose. Aggregation assays with N,N′-diacetyllactosamine establishing a parasite-like surface signature revealed marked selectivity among the family of galectins and bridging potency of homodimers. These findings provide fundamental insights into design-functionality relationships of galectins. Moreover, our strategy generates the tools to identify biofunctional lattice formation on biomembranes and galectin-reagents with therapeutic potential.
Having set as an aim that “after the genetic code was deciphered, the next important code to solve will be the one for cellular recognition” (1), attention is turning to cellular glycoconjugates and to the extraordinary talent of carbohydrates for building structural diversity. In fact, these “letters” of the third alphabet of life are endowed with the capacity to form oligomers that store information at an unsurpassed high-level density (2, 3), an ideal prerequisite to let their “functions pervade biology at all levels” (4).
Following the route of the flow of their information, the sugar-encoded messages are then “read” and translated into responses by lectins, letting the “sugar code” govern a broad variety of activities in adhesion, outside-in signaling, host defense, or glycoconjugate routing and transport relevant for embryology, homeostasis, and manifestation/progression of common diseases such as cancer or osteoarthritis (5, 6). Reflecting glycome complexity and a broad range of lectin-dependent processes, the specificity of this type of recognition process, i.e., glycan-protein binding, and the nature of the triggered effects are most likely not only determined by the ligand–receptor contact, as is the case for hormones or peptide motifs. Of fundamental importance, the molecular architecture of glycan presentation and of lectin design appears to matter somehow, and the emergence of this paradigm is calling for implementing strategies to help delineating definitive topology—activity relationships of biomedical significance. As a test model for this study, human adhesion/growth-regulatory galectins are selected to illustrate the power of rational design engineering, teamed up with functional assays on cells and on surface-programmable vesicle-like binding partners.
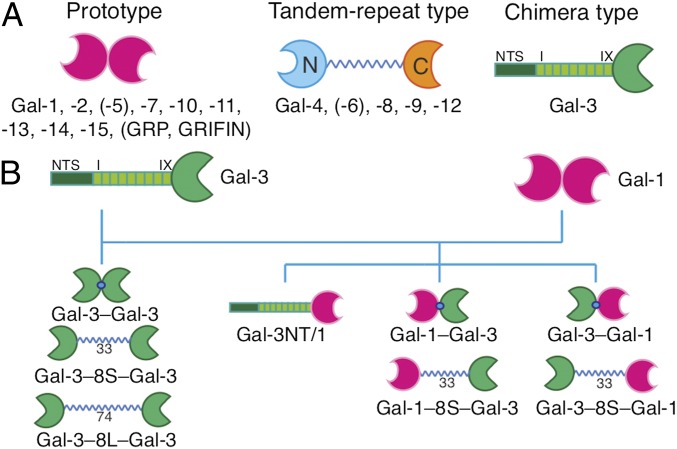
Galectins are being detected to be involved in a steadily growing number of aspects of cell sociology by bridging cognate glycans (7–12). In terms of modular architecture, the presentation of the common contact site for the ligand [i.e., the central part of the carbohydrate recognition domain (CRD)] occurs in three modes in vertebrates, i.e., as (i) a noncovalently associated homodimer (prototype), (ii) a heterodimer of two different CRDs connected by a linker peptide, or (iii) a modular protein constituted by a CRD and a further part of similar length but entirely different sequence, thus termed chimera type (Fig. 1A) (9, 13). Whereas structural aspects of the individual human CRDs and their glycan preferences have already been well studied (14, 15), our understanding of why these three special forms acquired their common status is much less advanced.
Fig. 1.
The three types of modular architecture of galectins (A) and the design of the panel of engineered variants to let CRD presentation switch between classes (B).
Among the members of the galectin family, the chimera-type galectin-3 (Gal-3) is unique owing to its trimodular structure: an N-terminal peptide with two sites for serine phosphorylation, the following nontriple helical collagen-like repeat section (nine repeats in human Gal-3), both establishing the N-terminal tail (NT), completed by the CRD (Fig. 1A) (16, 17). Monomeric in solution, it can form aggregates when associating with mono- or oligovalent ligands via the NT, the CRD, or both (for exemplary studies, see refs. 18–22); for review of the literature, see ref. 23). Up to now, protein engineering of Gal-3 has focused on trimming the NT up to complete truncation (trGal-3) to pinpoint determinants critical, for example, for secretion and growth regulation (24–26). We here fundamentally change the design of chimera-type Gal-3. Explicitly, it is hereby turned into a homobivalent protein [without/with insertion of a 33-aa (or 74-aa) linker known from galectin-8 (Gal-8)]. Pair building of WT and variant proteins for comparative testing is ideal to pinpoint design-dependent functionality of Gal-3’s CRD. The proteins were first thoroughly characterized structurally after recombinant production by mass spectrometry, gel filtration, and small-angle X-ray scattering (SAXS). As a further sensor for structural changes, hydrogen–deuterium exchange (HDX) was applied. Next, maintained activity for ligand binding was ascertained by HDX, in the absence and in the presence of lactose (Lac), by isothermal titration calorimetry (ITC) and carbohydrate-inhibitable cell binding of fluorescent proteins. Having passed these experimental series, WT and variant proteins were tested comparatively for cis– and trans–cross-linking using human neuroblastoma cells and glycodendrimersomes (GDSs) as sensitive assay platforms (26–32).
Broadening the scope of experiments, the feasibility for bottom-up surface programming of GDSs was exploited to change their glycan display in a rational manner, illustrating its versatility. In addition to the pan-galectin ligand Lac, we here established a parasite (schistosome) glycan signature by presenting N,N′-diacetyllactosamine (GalNAcβ1, 4GlcNAc, LacdiNAc), a known ligand for Gal-3 that mediates phagocytosis by macrophages with comparatively small binding capacity for galectin-1 (Gal-1) (33–35). Cell surface LacdiNAc is also a factor in self-renewal of mouse embryonic stem cells (36) and a potential cancer glycobiomarker (37). This reported difference in binding of Gal-1 and -3 inspired an inverse engineering of the homobivalent Gal-1, i.e., converting WT Gal-1 into a chimera-type–like hybrid composed of the Gal-1 CRD and the NT of Gal-3 (Gal-3NT/1). Strategically, by crossing borders in terms of design and valency, this protein panel fundamentally goes beyond the application of engineering by covalent CRD linkage using various methods and domain transfer among heterobivalent proteins (38–43) as well as linker tailoring (44–47). Our experiments with variants obtained by design-class switching, together with WT proteins as a standard, identify the natural Gal-3 design as a means to attain functional antagonism among galectins.
Results and Discussion
The Galectin Toolbox.
Natural Gal-3 forms its own class of galectin architecture by its unique trimodular design (Fig. 1A). Engineering on the level of cDNA enables a galectin to switch its class. We first turned the chimera-type protein into a homodimer by directly joining two Gal-3 CRDs (Gal-3–Gal-3) or by inserting a linker of a tandem repeat-type galectin, i.e., 33-aa (S) or 74-aa (L) linkers of tandem repeat-type Gal-8, yielding the Gal-3–8S/L–Gal-3 variants (Fig. 1B). In reverse direction, homodimeric Gal-1 is reshaped into a chimera-type protein by attaching its CRD to Gal-3’s NT (Fig. 1B). Together with the WT proteins, these two sets of protein pairs are ideal to test the hypothesis on architecture-dependent functionality.
Prompted by the recent discovery of heterodimer formation in mixtures of a prototype galectin and the Gal-3 CRD (48), we also engineered covalently connected heterodimers. In this case, the spatial order of the CRD, i.e., Gal-1/-3 or Gal-3/-1 (from the N to C terminus), and absence or presence of a linker are the structural variables, as illustrated in Fig. 1B. These eight proteins were all obtained by recombinant production and purified by affinity chromatography (for yields under optimal conditions, see SI Appendix, Table S1) so that their structures and characteristics of ligand binding could be characterized in detail. This comparison is especially important for the homobivalent variants of Gal-3.
The Variant Proteins: Structure and Ligand Binding.
To exclude a sequence deviation or posttranslational processing, each variant was systematically processed by MALDI-TOF (Dataset S1) and N-/C-terminal sequencing [reflectron and linear in-source decay (re/lin ISD)] (Dataset S1). Having passed these quality controls, the solution structure was first analyzed by gel filtration in the absence and in the presence of Lac. Homodimers of Gal-3 maintained the monomer status of the WT protein in solution with a tendency for a shift to a higher molecular weight induced by Lac presence (SI Appendix, Figs. S1A and S2A). When increasing the galectin concentration to up to 10 mg/mL under the conditions of SAXS, no evidence for aggregation was seen (SI Appendix, Figs. S1B and S2B). The same is true for the Gal-3NT/1 variant in gel filtration and SAXS analysis (SI Appendix, Fig. S3 A and B). Class switching of CRD presentation did not change the quaternary structure.
The situation becomes different when both types of CRD are combined in heterodimers. As shown for Gal-3–8S–Gal-1 in SI Appendix, Fig. S4 A and B, a second form appears that has elution/scattering properties of a dimer of dimers (see SI Appendix, Figs. S5–S7 for the other three heterodimers; profiles for WT Gal-1 and -3 are added as standards in SI Appendix, Fig. S8). The summary of the SAXS-derived characteristics (SI Appendix, Tables S2–S4) underlines this conclusion, and structural models depict a plausible orientation of the CRDs in such complexes (SI Appendix, Fig. S9 A–C). These models show similarity to a dimer of dimer detected for Gal-1 dissolved in dimethyl sulfoxide by small-angle neutron scattering (49). Whereas class switching had no impact on the quaternary structure of these variants, heterodimers have this inherent tendency for dimer-of-dimer formation. When joining CRDs in the Gal-3 homodimers without or with a linker, binding properties may or may not be changed. This question is answered by examining the effect of ligand binding on (i) the level of all amino acids, (ii) the thermodynamics of ligand binding, and (iii) the capacity of carbohydrate-inhibitable association to cell surfaces.
To set the stage for applying HDX as sensitive tool for detecting structural changes by CRD conjugation, binding of Lac and any ensuing alteration in the capacity for exchanging protons by deuterium as an indicator for a structural change by ligand binding, full-sequence coverage of the Gal-3 CRD and the homodimers was first achieved at redundancy between 7.32 and 9.1 (SI Appendix, Fig. S10 A–C). Invariably, significantly decreased deuterium uptake was found in the region of the canonical binding site (amino acids 42–84) (SI Appendix, Fig. S11 A–C). In addition, two sequence stretches at the N/C termini showed respective increases (SI Appendix, Fig. S11 A–C), documenting rather similar response profiles.
On the level of thermodynamics, no deviations between WT and variant proteins were apparent, in each case, reaching nearly full loading of the binding site by N-acetyllactosamine (LacNAc) (in monomeric WT Gal-3 at n = 1.01; in the homodimers at n = 1.89/1.93; Table 1; titration curves are given in SI Appendix, Fig. S12 A–C; for data on Lac as ligand, see SI Appendix, Table S5). Thus, based on these criteria, the Gal-3 CRD is not visibly affected by the conjugation process so that its mode of topological presentation will govern its association to polyvalent ligands, as presented on a cell surface. To measure surface binding, galectins were made fluorescent at a similar labeling efficiency measured spectrophotometrically (SI Appendix, Table S6). Using Chinese hamster ovary (CHO) cells (WT cells and cells of the Lec13 mutant with their reduced level of core fucosylation) as a model, this binding is dependent on glycans as ligand (SI Appendix, Fig. S13). When comparatively analyzed by flow cytometry using fluorescent galectins, the homodimers produced a higher response in mean fluorescence intensity/percentage of positive cells than WT Gal-3, an exchange between Gal-3/-1 CRDs in heterodimers causing a smaller effect (Fig. 2). The Gal-1 CRD as part of the new chimera-type protein was more effective to mediate binding than WT Gal-3 (Fig. 2), as also seen for Lec13 mutant cells when increasing the concentration (SI Appendix, Fig. S14). To proceed to biomedically relevant functional testing of postbinding effects of galectins, human neuroblastoma (SK-N-MC) cells offer an attractive assay platform. For these cells, homodimeric Gal-1 is a negative growth regulator by virtue of binding to the monosialylated pentasaccharide of ganglioside, whereas Gal-3 is an antagonist (50). Both proteins bind with similar affinity to their ganglioside counterreceptor, likely presented in microdomains (51). However, the organization of the formed lattice is assumed to be different to explain the activity difference (52).
Table 1.
Data of calorimetric measurements using LacNAc (6.0 mM) as ligand
| Protein | Concentration, μM | n sites | −ΔG, kcal/mol | −ΔH, kcal/mol (means ± SD) | −TΔS, kcal/mol | Kd, μM (means ± SD) |
| Gal-3 | 88 | 1.01 | 6.06 | 13.4 ± 0.170 | 7.33 | 36.1 ± 0.71 |
| Gal-3–Gal-3 | 50 | 1.89 | 5.95 | 12.1 ± 0.665 | 6.17 | 43.6 ± 3.05 |
| Gal-3–8S–Gal-3 | 33 | 1.93 | 5.86 | 13.2 ± 0.033 | 7.33 | 51.2 ± 0.33 |
Fig. 2.
Cytofluorimetric cell staining using fluorescent galectins at 1 µg/mL and CHO WT cells (A) as well as at 0.1 µg/mL and CHO Lec13 mutant cells (B).
The Variant Proteins: Cell Binding and Growth Regulation.
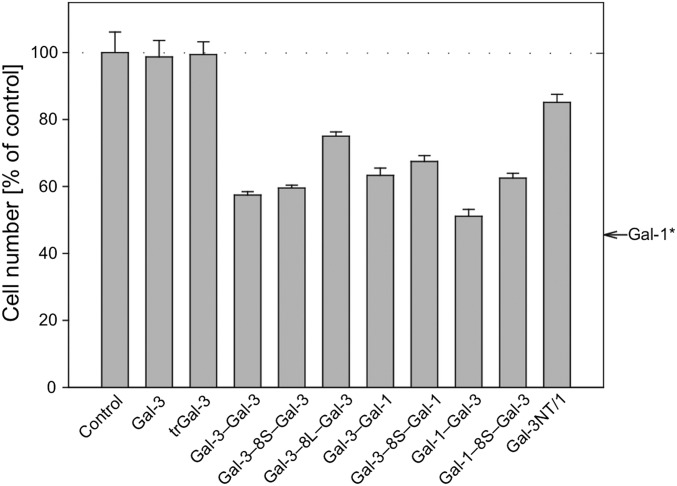
Radioiodinated proteins bind to the neuroblastoma cells in a carbohydrate-inhibitable manner up to saturation (as in flow cytometry), and the algebraic conversion of data of the titrations of specific binding yielded linear Scatchard plots (SI Appendix, Figs. S15 A and B and S16). The calculated Kd values for binding to these cells were rather similar, in the case of the homodimers, indicating a tendency of affinity decrease by increasing length of the linker (Table 2). When including WT Gal-3 and Gal-3 CRD as controls in proliferation assays, the Gal-3 homodimers clearly proved active (Fig. 3). The same applies to the heterodimers, linker presence leading to slight reductions (Fig. 3). Similarly intriguing, while WT Gal-1 was the most effective cell growth regulator (see arrow on right side of Fig. 3), the Gal-1–based chimera-type variant was much less active (Fig. 3). Obviously, the bivalent design conveys growth-inhibitory activity to the Gal-3 CRD. As shown for Gal-1 (50), WT Gal-3 is a functional antagonist for this activity (SI Appendix, Fig. S17), as it is also for prototype galectins-2 and -7 (Gal-2/-7) (SI Appendix, Fig. S18). Since galectins are physiologically active as molecular bridges not only on the surface of a cell (cis–cross-linking in lattice formation) but also between cells (trans-binding in aggregation), we proceeded to examine the variant proteins by using surface-programmable GDSs as robust binding partners. Their galectin-dependent aggregation leads to a turbidity increase.
Table 2.
Binding of various galectins to neuroblastoma cells
| Lectin | Kd, nM | Bmax × 104 |
| Gal-3* | 940 ± 44 | 270 ± 31 |
| Gal-3–Gal-3 | 911 ± 34 | 257 ± 24 |
| Gal-3–8S–Gal-3 | 962 ± 38 | 271 ± 27 |
| Gal-3–8L–Gal-3 | 1,189 ± 45 | 270 ± 31 |
| Gal-3–Gal-1 | 994 ± 32 | 276 ± 23 |
| Gal-3–8S–Gal-1 | 891 ± 29 | 246 ± 21 |
| Gal-1–Gal-3 | 847 ± 36 | 249 ± 25 |
| Gal-1–8S–Gal-3 | 859 ± 31 | 237 ± 23 |
| Gal-3NT/1 | 1,371 ± 49 | 255 ± 33 |
| Gal-1* | 980 ± 47 | 260 ± 32 |
| Gal-1–GG–Gal-1** | 684 ± 18 | 210 ± 19 |
| Gal-1–8S–Gal-1** | 1,694 ± 35 | 213 ± 13 |
Fig. 3.
The effect of galectin presence on cell proliferation of SK-N-MC cells at 100 µg/mL (n = 6; means ± SD). *Data for Gal-1 (arrow) are from ref. 31.
The Variant Proteins: Effectors of GDS Aggregation.
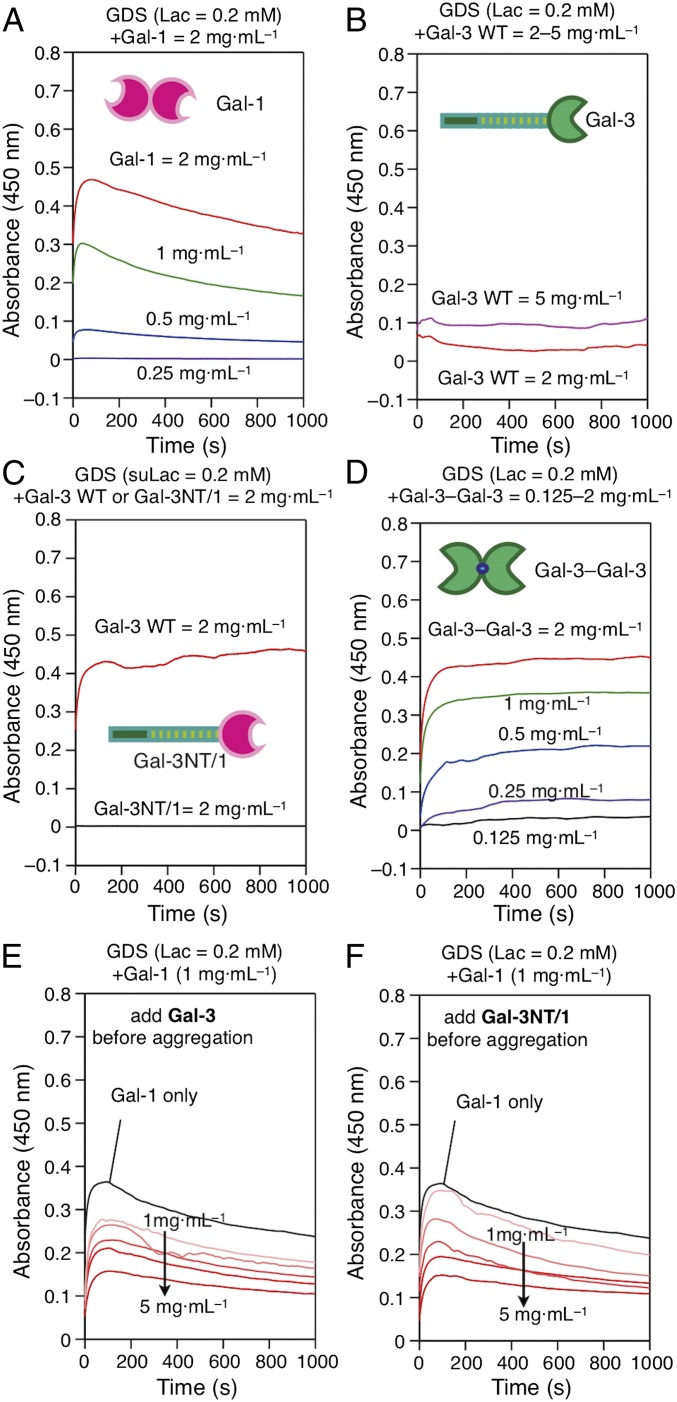
Using WT Gal-1 as positive and WT Gal-3 as negative controls, potent cross-linking activity of homo- and heterodimers was revealed, whereas the Gal-1 CRD did not convey activity to Gal-3’s NT (Fig. 4 A–D, see below). Mimicking competitive inhibition on neuroblastoma and pancreatic cancer cell surfaces (50, 53), the presence of this variant negatively affected GDS aggregation by Gal-1. Of note, this variant (Gal-3NT/1) was less active as inhibitor of Gal-1–dependent aggregation than WT Gal-3 at a low concentration (Fig. 4 E and F). When testing antagonism to covalently associated Gal-1, Gal-3 homodimers and heterodimers, no effect was observed (SI Appendix, Fig. S19 A–C). Thus, these measurements unveil the structural requirement of noncovalent CRD association in Gal-1 for functional antagonism between WT Gal-1/-3.
Fig. 4.
Aggregation of Lac-presenting GDSs (A, B, and D) or suLac-presenting GDSs (C) with test proteins given in each panel in the regular mode (A–D) and in the competitive mode using WT Gal-3 (E) and Gal-3NT/1 variant (F) as competitor of Gal-1–dependent aggregation.
The concept of the sugar code ascribes different biological meanings to structurally different glycans. By entering a formally subtle change to LacNAc to generate LacdiNAc, a difference in binding between Gal-3 and -1 appears to be implemented (34, 36). To test LacdiNAc as surface signal on GDS, we first prepared it in a form of the suited head-group derivative for GDS surface programming by the resulting GD (SI Appendix, Figs. S20 and S21). Characterization of GDS preparations revealed similar properties independent of the sugar head group (SI Appendix, Fig. S22). As expected, no activity was detectable for the two WT proteins (SI Appendix, Fig. S23 A and B). Gal-3 as homodimer and also the heterodimers, in contrast, were equally active to aggregate GDSs, irrespective of the type of ligand (LacdiNAc or Lac) (SI Appendix, Fig. S24 A–L). Fittingly, the covalently connected Gal-1 homodimer aggregated these GDSs, albeit slightly less potent than with Lac as ligand (SI Appendix, Fig. S23 C and D). Obviously, the type of CRD presentation matters, the conjugation of two Gal-1 CRDs by a GG linker leading to activity.
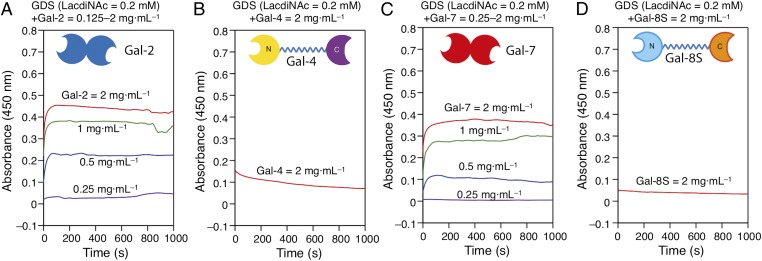
Equally important to variant testing with the canonical ligand, GDS surface programming makes tools available for general WT protein testing. The data presented in Fig. 5 illustrate the inherent differences between four human galectins, all active with Lac (SI Appendix, Fig. S25 A–D), when facing LacdiNAc. WT Gal-2 and -7, homodimers as Gal-1 is, but not tandem repeat-type Gal-4 and -8 can thus cooperate with Gal-3 (and likely Gal-1/-3 heterodimers) in situ in host defense against LacdiNAc-presenting parasites, Gal-2 and -7 by cross-link formation. Of note, the results emphasize occurrence of divergent functionality of closely related galectins, here Gal-1 and -2, so far inferred on the level of caspase activation profiles of T cells (54) also apparent in lack of susceptibility to Gal-3/Gal-3NT/1 presence in aggregation assays (SI Appendix, Fig. S26 A–C). In addition to surface engineering of cells, this chemical protocol with full control on glycan density and complexity is thus likely to find wide application, to study in detail galectin teamworking. Of note, immunohistochemical analysis of the complete galectin family underscores occurrence of coexpression of galectins as a general phenomenon (55) so that elucidating details of teamwork is emerging as a current challenge.
Fig. 5.
Aggregation of LacdiNAc-presenting GDSs by comparative galectin panel testing.
Conclusion and Perspectives
Reading sugar-encoded information is of pivotal significance for development, host defense and (patho)physiological processes such as inflammation or malignancy (56–58). Accurate information transfer depends on a lectin’s CRD, its translation into bioresponses on topological aspects of CRD presentation. Looking at the history of galectins, electrolectin’s homobivalency made the detection of the first galectin possible by measuring hemagglutination (59), and crystallographic analysis of bovine galectin-1 revealed evidence for lattice formation with N-glycans, the structural basis for triggering outside-in signaling on cells (60). After having gained a clear view on the range of diversity within the galectin family, we switched design of the CRD presentation fundamentally in both directions for monomeric (chimera-type) Gal-3 and homobivalent Gal-1, thereby, affecting the way cell surface ligands become either organized (in cis) or bridged (in trans). As a consequence, we applied a combined strategy for measuring protein activity of glycan binding, teaming up cell assays with galectin-dependent clustering of biomimetic nanoscale chemically programmed vesicles.
Hereby, we provide definitive proof for the validity of the hypothesis of the central importance of the modular architecture: proto- or chimera-type design underlies activity either as neuroblastoma cell growth inhibitor/bridging factor or as antagonist for both activities, regardless of the nature of the CRD. On the side of the glycan, the subtle structural change from Lac to LacdiNAc on the GDS surface uncovered selectivity among WT galectins and between WT Gal-1 and its covalently linked variant. These results imply structural changes in the canonical CRD attained by diversification of the galectin family (Fig. 1A) and the type of CRD association both appear to matter so that respective permutations broaden the functional spectrum of these lectins, as also attested by demonstration of bioactivity of Gal-1/-3 heterodimers. Strikingly, as our results reveal, the chimera-type galectin structure can now be interpreted as inhibitor (antagonist) design. Its activity is modulated in a versatile manner by proteolytic cleavage within the tail (50, 61, 62). In fundamental terms, our results add an aspect to glycan binding by modular lectin structures, in selectins and bacterial adhesins constituting the structural basis for catch bonds (63, 64). Of note, considering especially intracellular functions of Gal-3 via protein binding such as bcl-2 (8) or its nuclear effect on gene expression (18), availability of these variants opens the door, too, to exploring impact of protein design on these activities.
In addition to these insights, the availability of the variants enables comparative high-resolution analysis of complexes with glycoconjugates in solution and in membranes. Working with synthetic N-glycans and testing efficiency of binding depending on peculiar arrangements, as inferred to be important in the case of differential gp120 recognition by galectins-1 and -3 in HIV infectivity (65), becomes a viable perspective. Due to the increasing realization of the functional teamwork between galectins in situ, ranging from antagonism to cooperation (66), the variants of human galectins described herein can be envisioned to reliably dampen or enhance bioactivities of the WT proteins with clinical benefit. In this aspect, they may well be superior to synthetic glycan-based inhibitors, which exhibit cross-reactivity among galectins. Since Gal-1 as a multifunctional, context-dependent effector is not only a growth inhibitor but can also favor tumor progression, e.g., in glioblastoma or pancreas carcinoma (67, 68), the availability of the Gal-3NT/1 variant enables testing this hypothesis. Lastly, the herein-described access to heterodimers recently detected to occur in galectin mixtures, and the data on their activity, inspire further functional analysis of these engineered members of the galectin family, a route to let a Gal-3 CRD acquire heterobivalency (patho)physiologically.
Methods
Preparation of Variants.
Engineering of cDNAs and recombinant production followed protocols developed for homooligomer generation of Gal-1 (31, 45, 47), all proteins purified by affinity chromatography on lactose-binding Sepharose 4B resin.
Characterization of Protein Panel.
Mass spectra were obtained on an Ultraflex TOFTOF I instrument (Bruker Daltonic); re/lin ISD spectra were recorded in a positive-ion reflectron mode; gel filtration was carried out on a Superose HR10/30 column at 0.5 mL/min; and SAXS analysis was performed on Beamline 29 (BM29) at the European Synchrotron Radiation Facility (ESRF) with synchrotron radiation at λ = 0.1 nm, with computational generation of models as described (23, 31, 47).
Ligand Binding.
The extent of HDX was measured on quenched undeuterated and deuterated sample solution (320 pmol) after nanoAcquity ultraperformance liquid chromatography system-based fractionation (Waters Corporation) in a Synapt G2 high-definition MS mass spectrometer equipped with a lock spray electrospray ionization source (Waters), using the MSE mode (69). ITC titrations were performed in a PEAQ-ITC calorimeter in 20 mM phosphate buffer (pH 7.2) with 150 mM NaCl and 10 mM β-mercaptoethanol using the company’s software for calculations. Flow cytometry using fluorescent proteins labeled by fluorescein isothiocyanate at similar incorporation yield measured spectrometrically (70) was performed after incubation for 30 min at 4 °C with 5 × 104 cells per sample suspended in Dulbecco’s PBS, followed by careful washing steps to remove labeled probe, in parallel with processing mock controls (31). Radioiodinated proteins were incubated with cells grown for 5 d to reach confluency in serum-free Eagle’s medium for 2 h at 37 °C without/with cognate glycan as an inhibitor, and cell-bound radioactivity was measured by liquid scintillation counting (27, 31).
Functional Assays.
Cell proliferation was measured with a commercial kit (CellTiter 96; Promega) after an experimental period of 48 h (31, 50). GDS aggregation by the proteins was monitored in semimicro cuvettes at 23 °C at 450 nm using a Shimadzu UV-1601 UV-vis spectrophotometer with Shimadzu/UV Probe software in the kinetic mode (28–32).
Supplementary Material
Acknowledgments
Inspiring discussions with Drs. B. Friday and A. Leddoz are gratefully acknowledged, as is the support of the staff of the BM29 beamline at the ESRF (Grenoble, France). We also express our gratitude to the thoughtful advice of the reviewers. Financial support from NSF Grants DMR-1066116, DMR-1720530, and DMR-1807127 (to V.P.), the P. Roy Vagelos Chair at the University of Pennsylvania (V.P.), the Alexander von Humboldt Foundation (V.P.), NSF Grant DMR-1120901 (to M.L.K. and V.P.), NIH National Cancer Institute Grant R21-CA178754 (to M.C.), a China Scholarship Council PhD scholarship (to H.M.), Science Foundation Ireland Grant 13/IA/1959 (S.O.), and Spanish Grant BFU2016-77835R (to A.R.) is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813515116/-/DCSupplemental.
References
- 1.Barondes SH. Galectins: A personal review. Trends Glycosci Glycotechnol. 1997;9:1–7. [Google Scholar]
- 2.Winterburn PJ, Phelps CF. The significance of glycosylated proteins. Nature. 1972;236:147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]
- 3.Gabius H-J, Roth J. An introduction to the sugar code. Histochem Cell Biol. 2017;147:111–117. doi: 10.1007/s00418-016-1521-9. [DOI] [PubMed] [Google Scholar]
- 4.Hart GW. Thematic minireview series on glycobiology and extracellular matrices: Glycan functions pervade biology at all levels. J Biol Chem. 2013;288:6903. doi: 10.1074/jbc.R113.453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabius H-J, Manning JC, Kopitz J, André S, Kaltner H. Sweet complementarity: The functional pairing of glycans with lectins. Cell Mol Life Sci. 2016;73:1989–2016. doi: 10.1007/s00018-016-2163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning JC, et al. Lectins: A primer for histochemists and cell biologists. Histochem Cell Biol. 2017;147:199–222. doi: 10.1007/s00418-016-1524-6. [DOI] [PubMed] [Google Scholar]
- 7.Harrison FL, Chesterton CJ. Factors mediating cell–Cell recognition and adhesion. Galaptins, a recently discovered class of bridging molecules. FEBS Lett. 1980;122:157–165. doi: 10.1016/0014-5793(80)80428-5. [DOI] [PubMed] [Google Scholar]
- 8.Harazono Y, Nakajima K, Raz A. Why anti-Bcl-2 clinical trials fail: A solution. Cancer Metastasis Rev. 2014;33:285–294. doi: 10.1007/s10555-013-9450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaltner H, et al. Galectins: Their network and roles in immunity/tumor growth control. Histochem Cell Biol. 2017;147:239–256. doi: 10.1007/s00418-016-1522-8. [DOI] [PubMed] [Google Scholar]
- 10.Kasai K-i. Galectins: Quadruple-faced proteins. Trends Glycosci Glycotechnol. 2018;30:SE221–SE223. [Google Scholar]
- 11.Nangia-Makker P, Hogan V, Raz A. Galectin-3 and cancer stemness. Glycobiology. 2018;28:172–181. doi: 10.1093/glycob/cwy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato S. Cytosolic galectins and their release and roles as carbohydrate-binding proteins in host-pathogen interaction. Trends Glycosci Glycotechnol. 2018;30:SE129–SE135. [Google Scholar]
- 13.Kasai K, Hirabayashi J. Galectins: A family of animal lectins that decipher glycocodes. J Biochem. 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- 14.Iwaki J, Hirabayashi J. Carbohydrate-binding specificity of human galectins: An overview by frontal affinity chromatography. Trends Glycosci Glycotechnol. 2018;30:SE137–SE153. [Google Scholar]
- 15.Kamitori S. Three-dimensional structures of galectins. Trends Glycosci Glycotechnol. 2018;30:SE41–SE50. [Google Scholar]
- 16.Hughes RC. Mac-2: A versatile galactose-binding protein of mammalian tissues. Glycobiology. 1994;4:5–12. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605–610. doi: 10.1007/s10555-007-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu DK, Zuberi RI, Liu F-T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992;267:14167–14174. [PubMed] [Google Scholar]
- 19.Massa SM, Cooper DNW, Leffler H, Barondes SH. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- 20.Yang R-Y, Hill PN, Hsu DK, Liu F-T. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37:4086–4092. doi: 10.1021/bi971409c. [DOI] [PubMed] [Google Scholar]
- 21.Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem. 2007;282:1374–1383. doi: 10.1074/jbc.M604506200. [DOI] [PubMed] [Google Scholar]
- 22.Ippel H, et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology. 2016;26:888–903. doi: 10.1093/glycob/cww021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores-Ibarra A, Vértesy S, Medrano FJ, Gabius H-J, Romero A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci Rep. 2018;8:9835. doi: 10.1038/s41598-018-28235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong HC, et al. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–6245. [PubMed] [Google Scholar]
- 25.Menon RP, Hughes RC. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur J Biochem. 1999;264:569–576. doi: 10.1046/j.1432-1327.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 26.Kopitz J, et al. Human chimera-type galectin-3: Defining the critical tail length for high-affinity glycoprotein/cell surface binding and functional competition with galectin-1 in neuroblastoma cell growth regulation. Biochimie. 2014;104:90–99. doi: 10.1016/j.biochi.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Kopitz J, von Reitzenstein C, Burchert M, Cantz M, Gabius HJ. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J Biol Chem. 1998;273:11205–11211. doi: 10.1074/jbc.273.18.11205. [DOI] [PubMed] [Google Scholar]
- 28.Percec V, et al. Modular synthesis of amphiphilic Janus glycodendrimers and their self-assembly into glycodendrimersomes and other complex architectures with bioactivity to biomedically relevant lectins. J Am Chem Soc. 2013;135:9055–9077. doi: 10.1021/ja403323y. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, et al. Glycodendrimersomes from sequence-defined Janus glycodendrimers reveal high activity and sensor capacity for the agglutination by natural variants of human lectins. J Am Chem Soc. 2015;137:13334–13344. doi: 10.1021/jacs.5b08844. [DOI] [PubMed] [Google Scholar]
- 30.Sherman SE, Xiao Q, Percec V. Mimicking complex biological membranes and their programmable glycan ligands with dendrimersomes and glycodendrimersomes. Chem Rev. 2017;117:6538–6631. doi: 10.1021/acs.chemrev.7b00097. [DOI] [PubMed] [Google Scholar]
- 31.Kopitz J, et al. Reaction of a programmable glycan presentation of glycodendrimersomes and cells with engineered human lectins to show the sugar functionality of the cell surface. Angew Chem Int Ed Engl. 2017;56:14677–14681. doi: 10.1002/anie.201708237. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Q, et al. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc Natl Acad Sci USA. 2018;115:E2509–E2518. doi: 10.1073/pnas.1720055115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg TK, et al. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J Immunol. 2004;173:1902–1907. doi: 10.4049/jimmunol.173.3.1902. [DOI] [PubMed] [Google Scholar]
- 34.Krzeminski M, et al. Human galectin-3 (Mac-2 antigen): Defining molecular switches of affinity to natural glycoproteins, structural and dynamic aspects of glycan binding by flexible ligand docking and putative regulatory sequences in the proximal promoter region. Biochim Biophys Acta. 2011;1810:150–161. doi: 10.1016/j.bbagen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Laaf D, Bojarová P, Pelantová H, Křen V, Elling L. Tailored multivalent neo-glycoproteins: Synthesis, evaluation, and application of a library of galectin-3-binding glycan ligands. Bioconjug Chem. 2017;28:2832–2840. doi: 10.1021/acs.bioconjchem.7b00520. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki N, Shinomi M, Hirano K, Ui-Tei K, Nishihara S. LacdiNAc (GalNAcβ1-4GlcNAc) contributes to self-renewal of mouse embryonic stem cells by regulating leukemia inhibitory factor/STAT3 signaling. Stem Cells. 2011;29:641–650. doi: 10.1002/stem.615. [DOI] [PubMed] [Google Scholar]
- 37.Haji-Ghassemi O, et al. Molecular basis for recognition of the cancer glycobiomarker, LacdiNAc (GalNAcβ1-4GlcNAc), by Wisteria floribunda agglutinin. J Biol Chem. 2016;291:24085–24095. doi: 10.1074/jbc.M116.750463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato M, et al. Functional analysis of the carbohydrate recognition domains and a linker peptide of galectin-9 as to eosinophil chemoattractant activity. Glycobiology. 2002;12:191–197. doi: 10.1093/glycob/12.3.191. [DOI] [PubMed] [Google Scholar]
- 39.Bättig P, Saudan P, Gunde T, Bachmann MF. Enhanced apoptotic activity of a structurally optimized form of galectin-1. Mol Immunol. 2004;41:9–18. doi: 10.1016/j.molimm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Bi S, Earl LA, Jacobs L, Baum LG. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem. 2008;283:12248–12258. doi: 10.1074/jbc.M800523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cedeno-Laurent F, et al. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J Immunol. 2010;185:4659–4672. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romaniuk MA, et al. Human platelets express and are activated by galectin-8. Biochem J. 2010;432:535–547. doi: 10.1042/BJ20100538. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig A-K, et al. Studying the structural significance of galectin design by playing a modular puzzle: Homodimer generation from human tandem-repeat-type (heterodimeric) galectin-8 by domain shuffling. Molecules. 2017;22:1572. doi: 10.3390/molecules22091572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy Y, et al. It depends on the hinge: A structure-functional analysis of galectin-8, a tandem-repeat type lectin. Glycobiology. 2006;16:463–476. doi: 10.1093/glycob/cwj097. [DOI] [PubMed] [Google Scholar]
- 45.van der Leij J, et al. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol. 2007;44:506–513. doi: 10.1016/j.molimm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Earl LA, Bi S, Baum LG. Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology. 2011;21:6–12. doi: 10.1093/glycob/cwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vértesy S, et al. Structural significance of galectin design: Impairment of homodimer stability by linker insertion and partial reversion by ligand presence. Protein Eng Des Sel. 2015;28:199–210. doi: 10.1093/protein/gzv014. [DOI] [PubMed] [Google Scholar]
- 48.Miller MC, et al. Adhesion/growth-regulatory galectins tested in combination: Evidence for formation of hybrids as heterodimers. Biochem J. 2018;475:1003–1018. doi: 10.1042/BCJ20170658. [DOI] [PubMed] [Google Scholar]
- 49.He L, et al. Detection of ligand- and solvent-induced shape alterations of cell-growth-regulatory human lectin galectin-1 in solution by small angle neutron and x-ray scattering. Biophys J. 2003;85:511–524. doi: 10.1016/S0006-3495(03)74496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopitz J, et al. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem. 2001;276:35917–35923. doi: 10.1074/jbc.M105135200. [DOI] [PubMed] [Google Scholar]
- 51.Kopitz J, Bergmann M, Gabius H-J. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: Case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life. 2010;62:624–628. doi: 10.1002/iub.358. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad N, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Ruderisch H, et al. Tumor suppressor p16INK4a: Downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010;277:3552–3563. doi: 10.1111/j.1742-4658.2010.07764.x. [DOI] [PubMed] [Google Scholar]
- 54.Sturm A, et al. Human galectin-2: Novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol. 2004;173:3825–3837. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- 55.Manning JC, García Caballero G, Knospe C, Kaltner H, Gabius H-J. Network analysis of adhesion/growth-regulatory galectins and their binding sites in adult chicken retina and choroid. J Anat. 2017;231:23–37. doi: 10.1111/joa.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 2001;20:245–277. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- 57.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 58.Thiemann S, Baum LG. Galectins and immune responses - just how do they do those things they do? Annu Rev Immunol. 2016;34:243–264. doi: 10.1146/annurev-immunol-041015-055402. [DOI] [PubMed] [Google Scholar]
- 59.Teichberg VI, Silman I, Beitsch DD, Resheff G. A β-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci USA. 1975;72:1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharon N. When lectin meets oligosaccharide. Nat Struct Biol. 1994;1:843–845. doi: 10.1038/nsb1294-843. [DOI] [PubMed] [Google Scholar]
- 61.Ochieng J, et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 62.Sundqvist M, et al. Galectin-3 type-C self-association on neutrophil surfaces; the carbohydrate recognition domain regulates cell function. J Leukoc Biol. 2018;103:341–353. doi: 10.1002/JLB.3A0317-110R. [DOI] [PubMed] [Google Scholar]
- 63.Sokurenko EV, Vogel V, Thomas WE. Catch-bond mechanism of force-enhanced adhesion: Counterintuitive, elusive, but ... widespread? Cell Host Microbe. 2008;4:314–323. doi: 10.1016/j.chom.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldron TT, Springer TA. Transmission of allostery through the lectin domain in selectin-mediated cell adhesion. Proc Natl Acad Sci USA. 2009;106:85–90. doi: 10.1073/pnas.0810620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.St-Pierre C, et al. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol. 2011;85:11742–11751. doi: 10.1128/JVI.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weinmann D, et al. Galectin-8 induces functional disease markers in human osteoarthritis and cooperates with galectins-1 and -3. Cell Mol Life Sci. 2018;75:4187–4205. doi: 10.1007/s00018-018-2856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rorive S, et al. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia. 2001;33:241–255. doi: 10.1002/1098-1136(200103)33:3<241::aid-glia1023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 68.Orozco CA, et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc Natl Acad Sci USA. 2018;115:E3769–E3778. doi: 10.1073/pnas.1722434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruiz FM, et al. Chicken GRIFIN: Structural characterization in crystals and in solution. Biochimie. 2018;146:127–138. doi: 10.1016/j.biochi.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rinderknecht H. A new technique for the fluorescent labelling of proteins. Experientia. 1960;16:430–431. doi: 10.1007/BF02178856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.