Significance
The recent decline of the monarch butterfly has attracted a great deal of attention. One of the leading hypotheses blames genetically modified (GM) crops, ostensibly because of the impact of GM-related herbicide use on the monarch’s food plants, milkweeds. Here, we use museum specimen records to chart monarch and milkweed occurrence over the past century (1900 to 2016), dating well before previous datasets begin (in 1993). We show that monarch and milkweed declines begin around 1950 and continue until the present day. Whatever factors caused milkweed and monarch declines prior to the introduction of GM crops may still be at play, and, hence, laying the blame so heavily on GM crops is neither parsimonious nor well supported by data.
Keywords: genetically modified crops, monarch butterfly, milkweed, herbarium, specimen records
Abstract
Monarch butterfly (Danaus plexippus) decline over the past 25 years has received considerable public and scientific attention, in large part because its decline, and that of its milkweed (Asclepias spp.) host plant, have been linked to genetically modified (GM) crops and associated herbicide use. Here, we use museum and herbaria specimens to extend our knowledge of the dynamics of both monarchs and milkweeds in the United States to more than a century, from 1900 to 2016. We show that both monarchs and milkweeds increased during the early 20th century and that recent declines are actually part of a much longer-term decline in both monarchs and milkweed beginning around 1950. Herbicide-resistant crops, therefore, are clearly not the only culprit and, likely, not even the primary culprit: Not only did monarch and milkweed declines begin decades before GM crops were introduced, but other variables, particularly a decline in the number of farms, predict common milkweed trends more strongly over the period studied here.
The monarch butterfly (Danaus plexippus) is a large, showy Nymphalid butterfly best known for its migration, in which monarchs from a small overwintering area in Mexico recolonize breeding grounds across eastern North America over the course of several summer generations, followed by a single migration back to Mexico in the autumn (1, 2). Over the past 25 y, this migratory population of the monarch has experienced a drastic decline, as much as 80%, as measured at the overwintering area in Mexico (3, 4). Surveys of both immature and adult stages suggest a decline at the breeding grounds as well (5). Several hypotheses have been put forward to explain this decline: loss of overwintering habitat (6); severe weather, both at the overwintering grounds and along the migratory corridor (6, 7); insecticide use (7); and loss of nectar plants along the migration corridor (7). Probably the best known hypothesis, however, is that of habitat loss in the summer breeding grounds, driven by the expansion of herbicide-tolerant genetically modified (GM) crops. This is commonly proposed in both the scientific (3, 6, 8, 9) (but see ref. 7) and public (10, 11) literature. Because there are few described instances of GM crops causing declines in species outside of agricultural fields (12), the monarch has become a touchpoint for debates over GM crops.
The proposed link between GM crops and monarch declines is this. Previous work has identified the decline of milkweed (Asclepias spp.), monarch’s food source and egg nursery, as a likely culprit in monarch decline (8). GM crops, in turn, have been identified as the major cause of milkweed decline (8, 13): Because GM crops are frequently engineered to be resistant to glyphosate or other herbicides, herbicides are sprayed indiscriminately across crop fields, killing all nonresistant plants. This is especially harmful to common milkweed, Asclepias syriaca. Although D. plexippus caterpillars are able to feed on at least 30 species of milkweed (14), currently the most important host species for D. plexippus in their summer breeding grounds is A. syriaca (15), likely because of its former abundance in agricultural fields (16, 17).
It is clear that herbicide treatments kill milkweed; however, the importance of GM crops in milkweed and monarch declines is not yet clear, with some evidence pointing to other factors as more important drivers of the observed decline. The best evidence for this is that the decline of monarch butterflies appears to predate the use of GM crops. The monarch population size has been recorded in the overwintering grounds since 1993 (4), and the population decline is thought to be either linear or exponential over this period (5, 6). However, herbicide-resistant crops were not introduced until 1996, and initially accounted for only 2% of US cropland (SI Appendix, Fig. S2). Herbicide-resistant GM varieties are available for corn, soy, and cotton; half of the acreage of these crops was herbicide-resistant in 2004, and half of all crops were herbicide-resistant by 2013 (SI Appendix, Fig. S2). Since few acres were planted with herbicide-resistant crops during the beginning of the monarch decline, monarch and milkweed declines may have begun some time before the advent of herbicide-tolerant crops. However, because monarchs, like many insects, exhibit substantial year-to-year variation in population size (4), it is challenging to test this hypothesis using the currently available datasets, which include only 10 or so data points from before the widespread use of GM crops. Here, we use natural history collections to test this hypothesis across a much longer period, spanning the 117-y period from 1900 to 2016.
Results
Abundance Trends in the Genus Asclepias and Species Danaus plexippus from 1900 to 2016.
We extracted digitized collection information for 1,191 specimens of D. plexippus and 39,510 specimens of Asclepias collected from 1900 to 2016 (Table 1). Since collection effort has varied over this time period (SI Appendix, Fig. S1), we accounted for collection effort by calculating “relative abundance” for both groups. To do so, we divided the number of milkweed and monarch specimens collected each year by the total number of vascular plant and lepidoptera specimens, respectively, collected within the same geographic range and year. Our dataset does not include records from states west of the continental divide as these states are home to a population of monarchs that is geographically distinct from the eastern migratory population (18). We present these data in Fig. 1A, alongside a smoothed mean and 95% confidence interval, done using Loess smoothing, with the default smoothing span as implemented in ggplot2 (19).
Table 1.
Records used in this study
| Data source | Plants | Insects | |||
| Asclepias records | A. syriaca records (1950 to 2006) | Total plant records (1950 to 2006) | D. plexippus records | Total lepidoptera records | |
| Global Biodiversity Information Facility | 14,458 | 627 | 1,264,715 (452,073) | ||
| Consortium of Midwest Herbaria | 21,458 | 1,251 | 3,208,245 (1,065,608) | ||
| Online Virtual Flora of Wisconsin | 2,496 | 384 | 362,789 (288,291) | ||
| Minnesota Biodiversity Atlas | 1,098 | 93 | 144,254 (98,919) | ||
| Total | 39,510 | 2,355 | 4,980,003 (1,904,891) | ||
| Symbiota Collections of Arthropods Network | 1,191 | 323,611 | |||
For each plant data source, we downloaded all available records as of March 2018. For insects, we downloaded all records matching a higher taxonomy search for “Lepidoptera” as of April 2018. In both cases, we then filtered records to produce these sample sizes. For Asclepias, we provide the number of records for all Asclepias in the eastern United States from 1900 to 2016 (used in the analyses presented in Figs. 1 and 2 and SI Appendix, Fig. S6), as well as the number of records of A. syriaca used for multimodel inference (i.e., those records from 1950 to 2006 collected in the regions shown in SI Appendix, Fig. S9). The first number for total plant records is the number of records 1900 to 2016; the value in parentheses is the number of records used in multimodel inference.
Fig. 1.
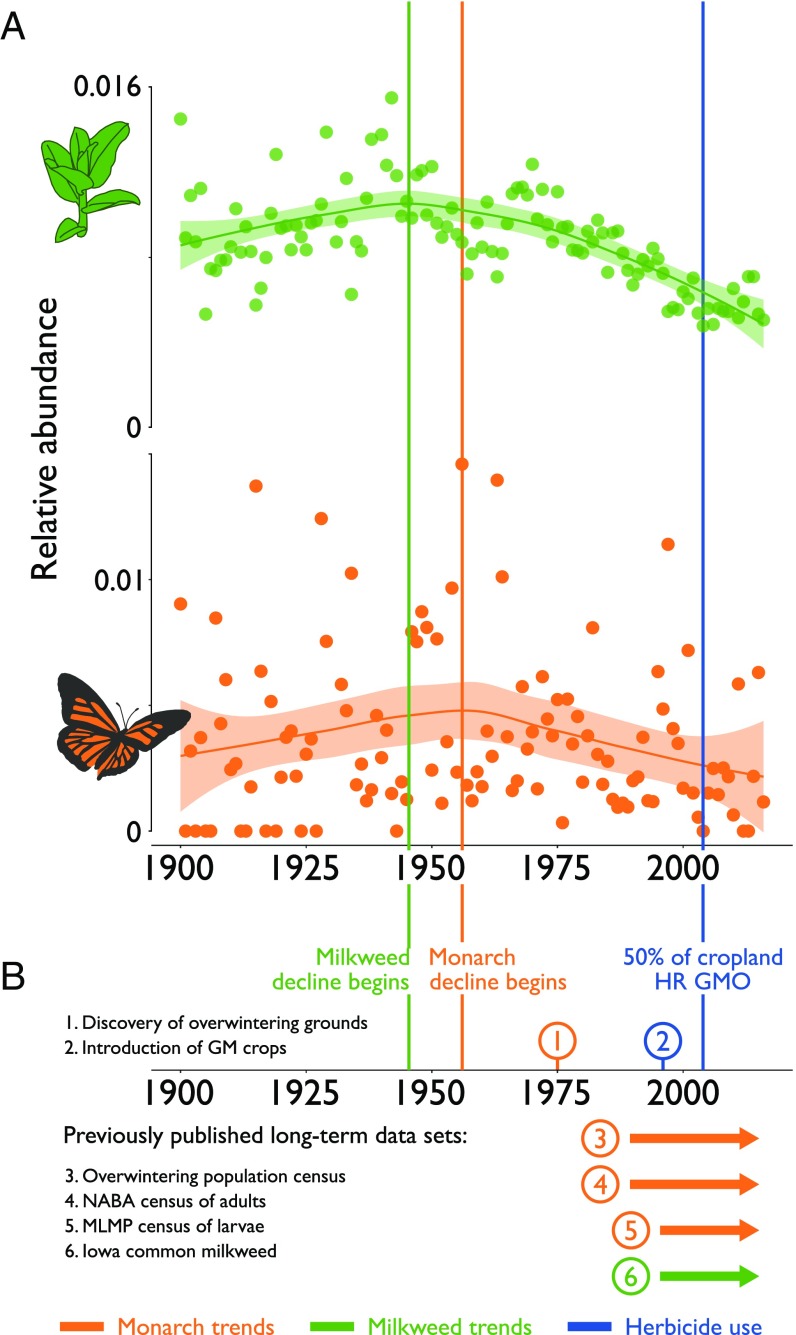
Museum specimens reveal long-term trends in monarchs and milkweed. (A) Green points show annual abundance for milkweed spp.; orange points show annual abundance for monarchs; and lines and shading indicate smoothed mean and 95% confidence intervals, calculated using the Loess smoothing method implemented in ggplot2 (19), with the default smoothing span. Green and orange vertical lines indicate the approximate beginning of the decline for milkweed and monarchs, respectively. The blue vertical line indicates the point at which half of all corn, soybeans, and cotton are herbicide resistant (HR) GM varieties. (B) Indicates (1) the discovery of the monarch overwintering grounds in Mexico; (2) the introduction of GM crops; (3) the winter population census at the Mexican overwintering grounds (20); (4) the summer NABA census of adults (available from ref. 7); (5) the summer MLMP census of eggs and larvae (available from ref. 5); and (6) the summer census of Iowa A. syriaca abundance (available from ref. 5).
For both monarchs and milkweed, relative abundance shows substantial year-to-year variation, but the trend over the 20th century is nevertheless clear. In both cases, monarch and milkweed abundance increases early in the century, milkweeds peak slightly before monarchs (around 1945, and 1955, respectively), and both suffer a twofold decline between then and 2016.
The monarch data show substantially more year-to-year variation than the milkweed data. In part, this is likely because the annual variation in monarch populations is considerable: for instance, the overwintering population size commonly experiences two- to fivefold changes from year to year (4). Part of the variability is likely also because the milkweed data are based on nearly 30 times as many records. We tested whether the trends we observed were artifacts of small record numbers. Monarch and milkweed trends are largely robust to small changes in the underlying datasets, with the exception that monarch trends between 1900 and 1930 are quite sensitive to the addition of new records (SI Appendix, Fig. S3).
Comparison of Our Trends with Other Datasets.
Year-to-year variation in both monarchs and milkweed meant that there was no statistically significant correlation between them (r = 0.16, P = 0.08), but our estimates of abundance correlate with other monarch butterfly datasets over the ∼20-y period over which they overlap (data from refs. 5, 7, and 20). In particular, our monarch abundance correlated with estimates of egg abundance from the Monarch Larvae Monitoring Project (MLMP) (r = 0.65, P < 0.01). It likewise correlates, though not statistically significantly, with the North American Butterfly Association (NABA) estimates of adult abundances, once the NABA abundances were corrected for land cover-based biases in survey sites using the method of ref. 5 (r = 0.48, P = 0.06), and likewise to adult abundance in the Mexican overwintering area the following winter (r = 0.40, P = 0.07). The correlation between milkweed abundance and the size of the Mexican overwintering population the following winter is not statistically significant (r = 0.34, P = 0.14). To further investigate this relationship, we restricted our analysis to those states that comprise the larval range of a relatively large proportion of the eastern migratory monarch population (21). Milkweed abundance in this core area does predict the size of the subsequent overwintering monarch population (r = 0.45, P = 0.04). Further comparisons, alongside plots of the relationships between the different estimates of monarch and milkweed abundances, are provided in SI Appendix.
Abundance Trends in Individual Asclepias Species.
Particular species may be more or less likely to be collected as herbarium specimens (22); therefore, changing communities within the genus could bias the genus-wide trends in Asclepias. To exclude this possibility, we also looked at the population trends for the 10 most abundant individual species as the particular collection biases for each species should be relatively constant over time.
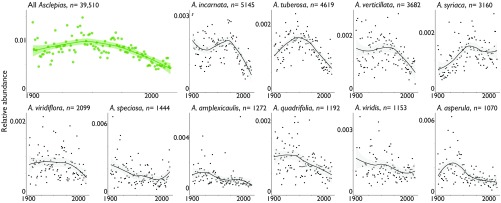
We found that each of the 10 most abundant milkweed species showed a trend similar to the genus-wide trend (Fig. 2). In particular, all 10 species showed a marked decline toward the end of the 20th century. The beginning of this decline was species-specific. For instance, Asclepias speciosa and Asclepias viridis declined over most of the studied period; Asclepias amplexicaulis and Asclepias asperula had brief periods of increase before a decline beginning around the 1920s; while the declines for Asclepias incarnata and Asclepias verticillata began much later, around the 1970s.
Fig. 2.
Genus-level milkweed decline over the 20th century is recapitulated in the 10 most common Asclepias species. The total number of specimens collected is shown next to each species. Points indicate abundance for each year, and lines and shading indicate smoothed mean and 95% confidence intervals. Smoothing was done using the Loess smoothing method implemented in ggplot2 (19), with the default smoothing span. Because different species have different ranges, the abundances for each species do not add up to the abundance for the genus as a whole.
While each species declined over the latter part of the 20th century, these declines were relatively slow for A. syriaca and A. speciosa, and, in fact, both species showed signs of slight increase in population size after 2000. This may be an artifact of noise in the data as there are fewer records digitized after 2000 (SI Appendix, Fig. S1). However, it may also show increases in milkweed due to monarch conservation efforts that encourage the planting of milkweed (e.g., refs. 23 and 24).
The relatively slow declines in these two species mean that these species now account for a greater proportion of the total milkweed records than they did at the beginning of our study period (SI Appendix, Fig. S6).
Multimodel Inference.
To investigate which changes in farming practice predict monarch and milkweed decline, we focused on the primary host plant of the monarch, A. syriaca, the common milkweed. Over the period of 1950 to 2006, for which we have good data on US agricultural practices, A. syriaca increased in abundance from 1950 to 1970, and then subsequently declined (SI Appendix, Fig. S10). We performed multimodel inference to test whether changing agricultural practices in the United States predict A. syriaca abundance. In every selected model, A. syriaca abundance was negatively correlated with number of farms, which declined over the period 1950 to 2006 as smaller farms consolidated. Most selected models included at least one other factor: Total area of farmland, fertilizer use, and glyphosate use all appeared in at least one selected model, although their effect sizes were much smaller than “number of farms,” and confidence intervals overlapped zero for these three traits, leaving open the possibility that they do not predict A. syriaca abundance.
Discussion
Both monarchs and milkweeds increase at the beginning of our study period before declining to their present abundance. The reliability of these trends depends on the assumption that the number of museum specimens collected in a given year is proportional to population size. With the recent increase in the availability of digitized specimen records, this assumption has now received some attention, at least with respect to plant records (22, 25). Perhaps the most obvious biases are spatiotemporal: Collection effort is well-known to vary widely across time and space (22, 25, 26), and this bias is clearly seen in our data as well (SI Appendix, Fig. S1). Our method accounts for this bias by using the total number of lepidoptera or vascular plant records in a given time and region to estimate collection effort, similar to the approach of, for example, refs. 27–29. Furthermore, a number of species-specific biases, such as trait-specific or phylogenetic biases, have also been described: For instance, Daru et al. (22) found that fewer annual specimens (per annual species) are found in herbaria records than are perennial specimens. Such biases remain constant over time and thus should not affect our results here. For the purposes of our study, the most concerning possible biases are those that change over time within a species. For instance, we investigated the possibility that biases in collection effort on different land covers could produce spurious results. While we saw no evidence for this, other potential sources of bias are harder to rule out: For instance, increases in the number of monarch specimen records could represent increases in monarch populations, or, instead, increases in scientific interest in monarchs, or even collectors’ increased interest in rare species as monarchs declined! It is almost certain that our dataset contains error attributable to these biases: For instance, the highest D. plexippus relative abundance was over 0.03 in 1931, largely due to 47 specimens collected by a L. W. Orr over 2 d at Itasca State Park, likely taken for a specific study, as he has no records from other species during the same time period. While this value was removed by our screen for outlier points, more modest studies may not have been; in addition, as monarchs and milkweeds have become rarer, they may have attracted more interest from collectors. As scientific interest has increased, and monarchs and milkweeds have become rarer, it is possible that they have become overrepresented in collections, and thus we underestimate recent declines: This however, means that our findings of an overall twofold decline in monarchs and milkweeds since the mid-1900s may be conservative. Furthermore, despite possible biases, museum data are unique among the available data in this system in that they allow us to reconstruct monarch and milkweed trends before scientists began to notice their decline: The oldest previously published data in this system date back to 1993 (7, 20), 2 y after Brower and Malcolm (30) named the monarch migration an “endangered phenomenon.” Museum records are useful in this, and other similar systems, because they give us insight into the dynamics of systems before scientists decided to study them specifically.
Regarding the trends shown in Fig. 1, the increase of both groups in the early 1900s is interesting as some authors suggest that milkweeds and monarchs experienced a range expansion in the late 1800s, driven by the conversion of eastern forests to farmlands (2, 31). Our early-20th-century increases in monarch and milkweed may reflect the tail end of such a trend, although the number of records at the beginning of the century is probably too small to be certain about the degree or precise timing of such an increase.
The decline for both monarchs and milkweed appears monotonic, suggesting that the well-studied decline from 1993 to date is part of a larger trend beginning in the middle of the last century. The overall trend for the monarch is very similar to the trend for milkweed, although its decline begins later (maximum value for the smoothed mean is in 1956, compared with 1946 for milkweeds) (Fig. 1). Because our data are correlational, it is difficult to distinguish between several competing hypotheses. It could be the case that the declines in milkweed cause monarch declines (the “milkweed limitation hypothesis” of, e.g., refs. 5 and 6), or monarch declines may be caused by some other factor which is correlated with milkweed declines, such as severe weather and changing climate (6, 32), changes in farming practice that destroy habitat for both milkweed and other plants that provide nectar resources for adult monarchs (7, 33), or more than one of the above.
When looking at the declines for each individual Asclepias species, these declines were the least marked in A. syriaca and A. speciosa (Fig. 2 and SI Appendix, Fig. S6). These two species are the host plants of the majority of monarch larvae in the central and northern United States (15, 34, 35). However, Martin and Lynch (35) and Brower (2) both hypothesized that monarchs’ reliance on A. syriaca and A. speciosa is a recent phenomenon, caused by the (relative) increase of these disturbance-loving species on a human-disturbed landscape. Our results provide quantitative evidence that A. syriaca and A. speciosa have recently increased their share of the Asclepias community: These two species account for an increasing proportion of the total Asclepias records over time, almost doubling from 9% in 1900 to 1909 to 17% in 2010 to 2016 (SI Appendix, Fig. S6). A. syriaca and A. speciosa are relatively poorly chemically defended (35); since monarchs get their chemical defenses from their larval host plants, current populations of monarchs may be less chemically well-defended than were monarchs 50 to 100 y ago, as suggested by refs. 2 and 35.
Based on our multimodel inference results, we suggest a preliminary hypothesis to explain the rise and fall of A. syriaca abundance after 1950: Over the course of the 1950s and 1960s, many small farms rapidly consolidated into fewer, larger farms (SI Appendix, Fig. S10). We suggest that this likely reduced the area of uncultivated divisions between different properties, benefitting A. syriaca, which thrived in the relatively competitor-free areas between crop rows in the fields themselves (36). However, beginning in the 1970s, the rate of farm consolidation greatly slowed, and this was no longer enough to buoy A. syriaca populations against threats such as a decline in total area of farms, or increasing use of glyphosate (and other herbicides) in the fields themselves. Our global model explains about 18% of the variation of common milkweed, leaving much to be done in explaining its changing abundance patterns, especially outside of agricultural land.
Looking at the trends in monarchs and milkweeds in general, a number of hypotheses are consistent with their steady declines over the last 60 or more years. It is possible that, as is widely hypothesized, herbicide use associated with GM crops is the current cause of milkweed declines. While some other factor must have caused them to decline before GM crops were introduced, perhaps this previous stressor has relaxed in recent years, but monarch and milkweed populations have not rebounded because of this new pressure relating to GM crop usage. Another, more parsimonious possibility is that monarch and milkweed declines are related to herbicide use generally (or even more broadly, other agrochemical use). Agricultural use of herbicides (even glyphosate) long predates herbicide-resistant crops, to such a degree that it is not even clear whether adoption of herbicide-resistant crops increases or decreases total herbicide use (12). Indeed, the timing of monarch and milkweed declines roughly corresponds to the midcentury agricultural revolution in the United States which saw greatly increased mechanization and chemical inputs to farmland (37). A third possibility is that milkweed declines are driven by other factors entirely, such as broad changes in other agricultural practices, land use, etc., over the twentieth century, such as those which we suggested above may explain the trends in A. syriaca specifically.
These hypotheses need not be mutually exclusive, and some combination of them may well be the best explanation for recent declines in monarchs and milkweeds. Whatever the case, however, it is clear that we cannot pin the blame on GM crops alone. By no means do our results absolve from blame agrochemical use in general, or herbicide use in particular; nevertheless, it is clear that focusing on GM crops in particular at the expense of other potential drivers will hinder our ability to address and reverse the worrying declines in these species.
Materials and Methods
Plant and Insect Records.
We gathered online herbaria records of preserved plant specimens from four sources: the Global Biodiversity Information Facility (38), the Consortium of Midwest Herbaria (39), the University of Minnesota Bell Museum of Natural History (40), and the Online Virtual Flora of Wisconsin (41) (Table 1). Records were cleaned to include only tracheophytes (i.e., all vascular plants) collected in the contiguous United States between 1900 and 2016, inclusively. Records from several institutions were found in more than one of our data sources: In these cases, the duplicated records were deleted from all but one of the data sources. We also gathered online museum records of insects from the Symbiota Collections of Arthropods Network (42) (Table 1). Records were cleaned to include only preserved specimens of lepidoptera (i.e., butterflies and moths) collected in the same time period and location as the plant records. Data cleaning and all statistical analyses described below were done in R version 3.4.2 (43), and the scripts used are available on Dryad.
Monarch butterflies are found throughout North and Central America and, within North America, are divided into two distinct migratory populations: east and west. We focused on an area which has numerous museum records available, alongside relatively fine-scale data on agricultural practices. We limited our study to the eastern population of butterflies and their host plants within the United States, and so we excluded records from other countries and US states west of the continental divide (Washington, Oregon, Colorado, Idaho, Nevada, Utah, and Arizona) from our dataset.
Abundance Trends in the Genus Asclepias from 1900 to 2016.
Our cleaned, eastern dataset included 39,510 records of Asclepias species. However, raw number of Asclepias specimens is a poor metric of abundance because collection effort has varied over the course of our study period. To control for varying collection effort from year to year, we calculated relative abundance of Asclepias by taking the quotient of the number of Asclepias records collected each year divided by the total number of tracheophyte records collected within the range of Asclepias species. We estimated the genus-wide range of Asclepias with a bounding box containing all Asclepias records except for the most extreme 1% in each direction: north, south, east, and west. We did this analysis within each of the four data sources and then combined the four by calculating the average, weighting each dataset by the number of Asclepias records in that dataset in that year. When visualizing the trends for Asclepias, we removed some years which were substantial outliers (1930 and 1939): i.e., falling greater than three SDs away from the mean annual abundance.
Because collection effort was low during certain time periods, we tested whether our observed trends were robust to our making small (artifactual) changes in the number of monarch or milkweed records. As described in SI Appendix, our trends were largely robust to these changes, with the exception that the monarch trends in the early 1900s showed marked sensitivity to the addition of small numbers of records across multiple years (SI Appendix, Fig. S3).
To confirm the sensitivity of this relative abundance metric to real changes in population size, we followed a similar procedure for several other herbaceous plants which are known to have invaded the United States during the time period of this study. As described in SI Appendix, these invasive species showed marked increases in their relative abundance over the 20th century (SI Appendix, Fig. S4).
Shorter-term trends in milkweed decline appear to vary by land cover category; e.g., declines in crop fields land may be much steeper than declines in nonagricultural land, like roadsides (44). We investigated whether this was the case for our long-term trends. As described in SI Appendix, Asclepias showed a decline in abundance across all studied land cover types (SI Appendix, Fig. S5).
Abundance Trends in Individual Asclepias Species.
Particular species may be more or less likely to be collected as herbarium specimens (22): For instance, an Asclepias species that is commonly found near road sides may be collected more commonly than a second Asclepias species that is equally common, but found in less convenient locations. Therefore, any decreasing trends in Asclepias abundance could have two explanations: first, that there were fewer Asclepias plants found over time; second, that the total number of Asclepias plants remained constant, but that the more easily collected species declined while the less easily collected species increased, leading to an apparent decline when looking at all Asclepias records at once.
To test between these two possibilities, we also looked at the population trends for individual species as the particular collection biases for each species should be relatively constant over time. As described further in SI Appendix, we looked at individual trends for the 10 most abundant milkweed species in our dataset, which combined make up 63% of the total dataset.
Abundance Trends for D. plexippus from 1900 to 2016.
Our cleaned, eastern dataset included 1,191 records of Danaus plexippus. As with Asclepias, we calculated the geographic range of D. plexippus as described above and then estimated the abundance of D. plexippus for each year by comparing the number of D. plexippus specimens collected with the total number of lepidoptera specimens collected within the D. plexippus range. When visualizing the trends for D. plexippus, we removed some years which were substantial outliers (1930 and 1931): i.e., falling greater than three SDs away from the mean annual abundance.
Comparison of Our Trends with Other Datasets.
Using Pearson’s correlation coefficient, we compared the abundance of milkweeds and monarchs from our museum data both with each other, and also with estimates of monarch and milkweed abundance from other datasets. We examined three other datasets: estimates of the size of the monarch overwintering population from 1994 to 2014 (20), Monarch Larva Monitoring Project (MLMP) estimates of immature (egg stage) monarch population sizes in the summer breeding grounds from 1999 to 2014 (5), and North American Butterfly Association (NABA) estimates of adult monarch population sizes in the summer breeding grounds from 1993 to 2014 (7). For the latter two datasets, we also employed the corrections for changes in land cover described by Pleasants et al. (5).
A relatively small number of states contribute disproportionately to the monarch butterfly population (figure 3 of ref. 21). Therefore, we also calculated milkweed abundance in these states alone, using the methods described above, but including only records from Texas, Oklahoma, Missouri, Illinois, Indiana, and Ohio. We compared these estimates of milkweed abundance from the core area with the size of the overwintering population.
Agricultural Data.
We gathered data on selected agricultural practices in the United States: namely, the number of farms and other agricultural operations, such as ranches and tree nurseries (45), the total area of farmland (46), the amount of nitrogen and phosphorus fertilizers used (47, 48), and the amount of glyphosate herbicide used (49). State-level data for all of these were available between 1950 and 2006, except glyphosate, for which we used national level data; for each variable, state-level data were combined across regions consisting of states with similar agricultural practices. We also determined the common milkweed, Asclepias syriaca, abundance in each region. Nitrogen and phosphorus fertilizer use were highly correlated with each other so we combined them into a single variable. The remaining variables were much less strongly correlated with each other. The correlation coefficients between predictor variables and/or A. syriaca abundance, as well as more detailed methods, are presented in SI Appendix.
Multimodel Inference.
To test which changes in agricultural practice had an effect on A. syriaca abundance, we performed multimodel inference using the MuMIn package (50). We ran 16 different mixed-effects models, each with A. syriaca abundance as the response variable. The fixed-effects variables were some combination of total area of farmland, number of farms, fertilizer used, and glyphosate herbicide used, and a random-effect of geographic region on the y intercept was also used. The 16 models included every possible combination of the four fixed-effect variables, including a null model with only an intercept term.
We calculated the relative quality of each model using the Akaike information criterion (AIC), retaining in our analysis any model within four AIC units of the highest quality model. The effect of each variable on A. syriaca abundance was averaged across all of the retained models, weighting by the relative likelihood of each model. When a variable was not found in a model, it was considered to have an effect of zero. The results of this analysis are shown in Table 2 and discussed above. In addition, we tested the sensitivity of our results to noise in the dataset. As discussed in SI Appendix, our results appear robust to individual sources of error albeit that the addition of noise across the dataset reduced our ability to distinguish the predictive power of the different agricultural variables.
Table 2.
Results of multimodel inference
| Variable | Model | Importance | Estimate | 95% CI | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| Number of farms | −4.4 | −3.6 | −4.3 | −4.3 | −3.6 | −3.7 | 1.00 | −4.04 | −6.36, −1.72 |
| Area farmed | 1.9 | 2.3 | 1.9 | 0.55 | 1.10 | −1.50, 3.70 | |||
| Fertilizer use | −0.8 | 0.1 | 0.21 | −0.09 | −1.30, 1.11 | ||||
| Glyphosate use | 0.2 | 0.2 | 0.19 | 0.04 | −0.91, 0.98 | ||||
| ΔAIC | 0 | 0.38 | 1.93 | 2.36 | 2.65 | 2.68 | |||
| Model weight | 0.28 | 0.23 | 0.11 | 0.09 | 0.07 | 0.07 | |||
Rows show the estimates for the effect of each scaled agricultural variable on A. syriaca abundance, along with the difference in AIC between each model and the best model, and the weight assigned each model. Blank cells in the rows for each agricultural variable indicate that the variable was not considered in the given model. “Importance” gives the relative weight of the models containing that variable. “Estimate” and “95% CI” give the mean and confidence interval for the effect of each agricultural variable, averaged across the six models according to their model weight. All estimates are multiplied by 104 for readability.
Data Availability.
Data tables used for all analyses, along with R scripts for each analysis, are available on Dryad.
Supplementary Material
Acknowledgments
We thank the insect collections staff at the University of Minnesota and University of Kansas, who aided us by providing D. plexippus records through the Symbiota Collections of Arthropods Network; D. S. De La Mater and the Milkweed Research Group (William & Mary) for feedback on the manuscript; and the anonymous reviewers whose helpful comments improved the manuscript throughout. J.H.B. was supported by a postdoctoral fellowship in the William & Mary Environmental Science and Policy Program, funded by the Andrew W. Mellon Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data tables used for all analyses, along with R scripts for each analysis, have been deposited in the Dryad Digital Repository, https://datadryad.org/ (doi: 10.5061/dryad.sk37gd2).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811437116/-/DCSupplemental.
References
- 1.Urquhart FA, Urquhart NR. Autumnal migration routes of the eastern population of the monarch butterfly (Danaus p. plexippus L.; Danaidae; Lepidoptera) in North America to the overwintering site in the Neovolcanic Plateau of Mexico. Can J Zool. 1978;56:1759–1764. [Google Scholar]
- 2.Brower LP. Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857-1995. J Lepid Soc. 1995;49:304–385. [Google Scholar]
- 3.Pleasants JM. Milkweed restoration in the Midwest for monarch butterfly recovery: Estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conserv Divers. 2017;10:42–53. [Google Scholar]
- 4.Vidal O, Rendón-Salinas E. Dynamics and trends of overwintering colonies of the monarch butterfly in Mexico. Biol Conserv. 2014;180:165–175. [Google Scholar]
- 5.Pleasants JM, et al. Interpreting surveys to estimate the size of the monarch butterfly population: Pitfalls and prospects. PLoS One. 2017;12:e0181245. doi: 10.1371/journal.pone.0181245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower LP, et al. Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conserv Divers. 2012;5:95–100. [Google Scholar]
- 7.Inamine H, Ellner SP, Springer JP, Agrawal AA. Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos. 2016;125:1081–1091. [Google Scholar]
- 8.Pleasants JM, Oberhauser KS. Milkweed loss in agricultural fields because of herbicide use: Effect on the monarch butterfly population. Insect Conserv Divers. 2013;6:135–144. [Google Scholar]
- 9.Saunders SP, Ries L, Oberhauser KS, Thogmartin WE, Zipkin EF. Local and cross-seasonal associations of climate and land use with abundance of monarch butterflies Danaus plexippus. Ecography. 2018;41:278–290. [Google Scholar]
- 10.Smith LN. 2014 Monarch butterfly’s reign threatened by milkweed decline. Available at https://news.nationalgeographic.com/news/2014/08/140819-monarch-butterfly-milkweed-environment-ecology-science. Accessed May 27, 2018.
- 11.Fears D. 2015 The monarch massacre: Nearly a billion butterflies have vanished. Available at https://www.washingtonpost.com/news/energy-environment/wp/2015/02/09/the-monarch-massacre-nearly-a-billion-butterflies-have-vanished/?utm_term=.a6f714fd0e0b. Accessed May 27, 2018.
- 12.National Academies of Science, Engineering, and Medicine . Genetically Engineered Crops: Experiences and Prospects. National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- 13.Stenoien C, et al. Monarchs in decline: A collateral landscape-level effect of modern agriculture. Insect Sci. 2016;25:528–541. doi: 10.1111/1744-7917.12404. [DOI] [PubMed] [Google Scholar]
- 14.Lynch SP, Martin RA. Biology and Conservation of the Monarch Butterfly. Natural History Museum of Los Angeles County; Los Angeles: 1993. Milkweed host plant utilization and cardenolide sequestration by monarch butterflies in Louisiana and Texas. [Google Scholar]
- 15.Malcolm SB, Cockrell BJ, Brower LP. Biology and Conservation of the Monarch Butterfly. Natural History Museum of Los Angeles County; Los Angeles: 1993. Spring recolonization of eastern North America by the Monarch butterfly: Successive brood or single sweep migration? [Google Scholar]
- 16.Cramer G, Burnside O. Distribution and interference of common milkweed (Asclepias syriaca) in Nebraska. Weed Sci. 1982;30:385–388. [Google Scholar]
- 17.Hartzler RG, Buhler DD. Occurrence of common milkweed (Asclepias syriaca) in cropland and adjacent areas. Crop Prot. 2000;19:363–366. [Google Scholar]
- 18.Dingle H, Zalucki MP, Rochester WA, Armijo-Prewitt T. Distribution of the monarch butterfly, Danaus plexippus (L.) (Lepidoptera: Nymphalidae), in western North America. Biol J Linn Soc Lond. 2005;85:491–500. [Google Scholar]
- 19.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York: 2009. [Google Scholar]
- 20.Monarch Watch 2016 Monarch population status. Available at https://monarchwatch.org/blog/2016/02/26/monarch-population-status-26/. Accessed May 27, 2018.
- 21.Flockhart DTT, et al. Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proc Biol Sci. 2013;280:20131087. doi: 10.1098/rspb.2013.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daru BH, et al. Widespread sampling biases in herbaria revealed from large-scale digitization. New Phytol. 2018;217:939–955. doi: 10.1111/nph.14855. [DOI] [PubMed] [Google Scholar]
- 23.Monarch Joint Venture 2018 Create habitat for monarchs. Available at https://monarchjointventure.org/get-involved/create-habitat-for-monarchs. Accessed May 31, 2018.
- 24.Xerces Society 2018 Project milkweed. Available at https://xerces.org/milkweed/. Accessed May 31, 2018.
- 25.Meyer C, Weigelt P, Kreft H. Multidimensional biases, gaps and uncertainties in global plant occurrence information. Ecol Lett. 2016;19:992–1006. doi: 10.1111/ele.12624. [DOI] [PubMed] [Google Scholar]
- 26.Küper W, Sommer JH, Lovett JC, Barthlott W. Deficiency in African plant distribution data–missing pieces of the puzzle. Bot J Linn Soc. 2006;150:355–368. [Google Scholar]
- 27.Carpaneto GM, Mazziotta A, Valerio L. Inferring species decline from collection records: Roller dung beetles in Italy (Coleoptera, Scarabaeidae) Divers Distrib. 2007;13:903–919. [Google Scholar]
- 28.Colla SR, Packer L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers Conserv. 2008;17:1379–1391. [Google Scholar]
- 29.Cameron SA, et al. Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA. 2011;108:662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brower LP, Malcolm SB. Animal migrations: Endangered phenomena. Am Zool. 1991;31:265–276. [Google Scholar]
- 31.Vane-Wright RI. Biology and Conservation of the Monarch Butterfly. Natural History Museum of Los Angeles County; Los Angeles: 1993. The Columbus hypothesis: An explanation for the dramatic 19th century range expansion of the monarch butterfly. [Google Scholar]
- 32.Oberhauser K, Peterson AT. Modeling current and future potential wintering distributions of eastern North American monarch butterflies. Proc Natl Acad Sci USA. 2003;100:14063–14068. doi: 10.1073/pnas.2331584100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal A. Monarchs and Milkweed: A Migrating Butterfly, a Poisonous Plant, and Their Remarkable Story of Coevolution. Princeton Univ Press; Princeton: 2017. [Google Scholar]
- 34.Malcolm SB, Cockrell BJ, Brower LP. Cardenolide fingerprint of monarch butterflies reared on common milkweed, Asclepias syriaca L. J Chem Ecol. 1989;15:819–853. doi: 10.1007/BF01015180. [DOI] [PubMed] [Google Scholar]
- 35.Martin RA, Lynch SP. Cardenolide content and thin-layer chromatography profiles of monarch butterflies, Danaus plexippus L., and their larval host-plant milkweed, Asclepias asperula subsp. capricornu (Woods.) Woods., in north central Texas. J Chem Ecol. 1988;14:295–318. doi: 10.1007/BF01022548. [DOI] [PubMed] [Google Scholar]
- 36.Bhowmih PC, Bandeen JD. The biology of Canadian weeds 19. Asclepias syriaca L. Can J Plant Sci. 1976;56:579–589. [Google Scholar]
- 37.Conkin PK. A Revolution Down on the Farm: The Transformation of American Agriculture Since 1929. University Press of Kentucky; Lexington, KY: 2008. [Google Scholar]
- 38.GBIF.org 2018 doi: 10.15468/dl.irezsw. GBIF occurrence download. Available at . . Accessed March 5, 2018. [DOI]
- 39.Consortium of Midwest Herbaria 2018 Consortium of Midwest Herbaria. Available at midwestherbaria.org/portal/index.php. Accessed March 6, 2018.
- 40.University of Minnesota Bell Museum of Natural History 2018 Minnesota Biodiversity Atlas. Available at bellatlas.umn.edu/index.php. Accessed March 5, 2018.
- 41.Online Virtual Flora of Wisconsin 2018 Welcome to the Online Virtual Flora of Wisconsin. Available at wisflora.herbarium.wisc.edu. Accessed March 5, 2018.
- 42.Symbiota Collections of Arthropods Network 2018 Collections. Available at scan-bugs.org/portal/collections. Accessed April 3, 2018.
- 43.R Core Team 2017. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.4.2.
- 44.Hartzler RG. Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Prot. 2010;29:1542–1544. [Google Scholar]
- 45.United States Department of Agriculture-National Agricultural Statistics Service (USDA-NASS) 2017 Quick stats. Available at quickstats.nass.usda.gov/results/6C18B50F-38B5-3927-B020-E8C4D80EBAB2. Accessed December 4, 2017.
- 46.United States Department of Agriculture-National Agricultural Statistics Service (USDA-NASS) 2017 Quick stats. Available at https://quickstats.nass.usda.gov/results/41CCCE77-42EB-3F9B-A943-5A69AB57EFCB. Accessed December 4, 2017.
- 47.Alexander RB, Smith RA. 1990. County-level estimates of nitrogen and phosphorus fertilizer use in the United States, 1945-1985 (United States Geological Survey, Reston, VA), Report number 90-130.
- 48.Gronberg JAM, Spahr NE. 2012. County-level estimates of nitrogen and phosphorus from commercial fertilizer for the conterminous United States, 1987-2006 (United States Geological Survey, Reston, VA), Report number 2012-5207.
- 49.Benbrook CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur. 2016;28:3. doi: 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barton K. 2017 MuMIn: Multi-Model Inference. R Package Version 1.40.0. Available at https://cran.r-project.org/src/contrib/Archive/MuMIn/. Accessed October 19, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data tables used for all analyses, along with R scripts for each analysis, are available on Dryad.