Significance
Rho is an essential protein that promotes transcription termination at specific regions of the genome. Its activity is important not only at the end of genes, but also within leader regions where it has regulatory functions. This protein was shown to be involved in the regulation of rho mRNA promoting its premature transcription termination. In this study, we included an additional player in the complex pathway of regulation of this protein. Using in vivo and in vitro experiments, the small noncoding RNA SraL was shown to directly interact with a specific region in the 5′-UTR of rho mRNA protecting this transcript against the action of its own protein Rho.
Keywords: posttranscriptional control, functional regulatory RNA, prokaryotes, small noncoding RNA, transcription termination
Abstract
Transcription termination is a critical step in the control of gene expression. One of the major termination mechanisms is mediated by Rho factor that dissociates the complex mRNA-DNA-RNA polymerase upon binding with RNA polymerase. Rho promotes termination at the end of operons, but it can also terminate transcription within leader regions, performing regulatory functions and avoiding pervasive transcription. Transcription of rho is autoregulated through a Rho-dependent attenuation in the leader region of the transcript. In this study, we have included an additional player in this pathway. By performing MS2-affinity purification coupled with RNA sequencing (MAPS), rho transcript was shown to directly interact with the small noncoding RNA SraL. Using bioinformatic in vivo and in vitro experimental analyses, SraL was shown to base pair with the 5′-UTR of rho mRNA upregulating its expression in several growth conditions. This base pairing was shown to prevent the action of Rho over its own message. Moreover, the results obtained indicate that both ProQ and Hfq are associated with this regulation. We propose a model that contemplates the action of Salmonella SraL sRNA in the protection of rho mRNA from premature transcription termination by Rho. Note that since the interaction region between both RNAs corresponds to a very-well-conserved sequence, it is plausible to admit that this regulation also occurs in other enterobacteria.
In prokaryotes, two distinct mechanisms of transcription termination are known: intrinsic termination (or Rho-independent termination), which involves terminator sequences in the RNA indicating RNA polymerase where to stop, and Rho-dependent termination, which relies on the action of Rho factor to stop RNA synthesis at specific sites (1). Rho factor is a very-well-conserved protein across bacteria, and its corresponding gene is present in >90% of sequenced bacterial genomes (2). Rho is a helicase protein with RNA-dependent ATPase activity that catalyzes the disassociation of nascent mRNA from genomic DNA and RNA polymerase, promoting transcription termination. This protein is essential in many bacterial organisms, namely Escherichia coli, Salmonella enterica, Shigella flexneri, and Pseudomonas aeruginosa (3–6). In fact, Rho is responsible for termination of about half of the transcription events in E. coli (7). Rho-dependent termination plays a significant role even in organisms in which it is not essential (4). For instance, Rho inactivation in Bacillus subtilis affects gene expression of important pathways related to cell motility, biofilm formation, and sporulation (8).
The advent of the high-throughput techniques enabled the discovery of small noncoding RNAs (sRNAs), RNA molecules whose function and importance were underestimated. Since their discovery, sRNAs have been broadly described as important regulators of gene expression in both prokaryotes and eukaryotes. Most of the sRNAs known are trans-encoded and act by base pairing with their target(s) with short and imperfect complementary sequence, leading to changes in translation and/or mRNA degradation (9–11). However, distinct mechanisms of action have been increasingly reported in the literature (11). For instance, the Salmonella sRNA ChiX was shown to induce premature transcription termination within the coding sequence of chiP as a result of its interaction with 5′-UTR of the operon chiPQ, thus affecting the expression of both genes of the operon (12). Conversely, it was also very recently established that some sRNAs are able to prevent premature transcription termination by interfering with Rho-mediated termination in the 5′-UTR of many genes (13). These and other studies seem to indicate that interference of sRNAs in Rho-dependent termination is a more common mechanism of regulation than previously envisioned.
SraL is a very-well-conserved trans-encoded sRNA that was initially identified in E. coli (14–16). Subsequently, the expression of this sRNA was also detected and studied in detail in Salmonella enterica serovar Typhimurium (15, 17). Although the transcriptional and posttranscriptional regulation of SraL sRNA have been described, only one target is known for this sRNA (15, 17). The ribosome-associated chaperone Trigger factor (TF), encoded by tig mRNA, is repressed by SraL binding to the tig 5′-UTR during late stationary phase of growth (15).
In this report, we aimed to identify new biological targets of Salmonella SraL. Notably, we have established the role of SraL in the regulation of expression of the important transcription termination factor Rho. Previously, rho mRNA expression was shown to be autogenously regulated by attenuation of transcription and consequent premature transcription termination (18–20). By mutational analysis, SraL was revealed to directly base pair with the 5′-UTR of rho mRNA in a region upstream of the previously reported attenuators. This interaction protects rho mRNA from premature transcription termination by Rho protein.
As mentioned above, sRNAs can base pair in 5′-UTR of transcripts to preclude premature transcription termination by Rho factor (13). It is noteworthy that the regulator can be also modulated by the same mechanism, since SraL sRNA is responsible for protection of rho mRNA from premature transcription termination. This finding adds one level of complexity to the network of control of gene expression by termination, showing that SraL sRNA acts upstream of a regulatory cascade and regulates the regulator.
Results
MS2 Affinity Purification Coupled with RNA Sequencing to Identify Targets of SraL.
Several studies were performed regarding Salmonella SraL sRNA expression and transcriptional and posttranscriptional regulation (14, 15, 17, 21, 22). However, the only biological function known for this sRNA is the downregulation of chaperone Trigger factor (15).
To identify new targets of SraL, we used the recently developed in vivo technology MS2-affinity purification coupled with RNA sequencing (MAPS) (23–26). For this, SraL was fused to an MS2 RNA aptamer, which binds the MS2 coat protein with high affinity, enabling copurification of SraL with its mRNA(s) target(s). Two different conditions were selected for the application of this technology: late stationary phase of growth in LB medium (OD600 of 2 plus 6 h), the condition in which this sRNA is more expressed (15, 17); and anaerobic shock, a condition reported to increase the expression of SraL sRNA (27) (SI Appendix, Fig. S1). MAPS was performed in an sraL− background to avoid interference of native copies of the sRNA. After normalization of the read counts by coverage (28), enrichment of the putative RNA binding partners was determined by comparing the number of reads obtained from tagged MS2-SraL and untagged sRNA control. Enriched RNA partners are listed in Dataset S1.
SraL sRNA Is a Positive Regulator of rho mRNA.
One of the top candidates enriched in MS2-SraL sample was the transcription termination factor Rho. Rho is a well-conserved homohexameric protein ubiquitous throughout the bacterial domain (2, 29, 30), and it is essential for the viability of many bacterial species, including Salmonella (5, 31). Due to its major importance in regulation of gene expression we decided to pursue the validation of the result obtained by MAPS.
Expression of rho mRNA was analyzed using three strains with different SraL sRNA expression levels: a wild-type, a sraL null mutant, and a complemented sraL null mutant strain in which sraL was cloned into a constitutive expression plasmid (15). Since MAPS technology was applied in cells grown until stationary phase (STAT) and upon anaerobic shock (AS), expression level of rho mRNA was initially accessed in these conditions.
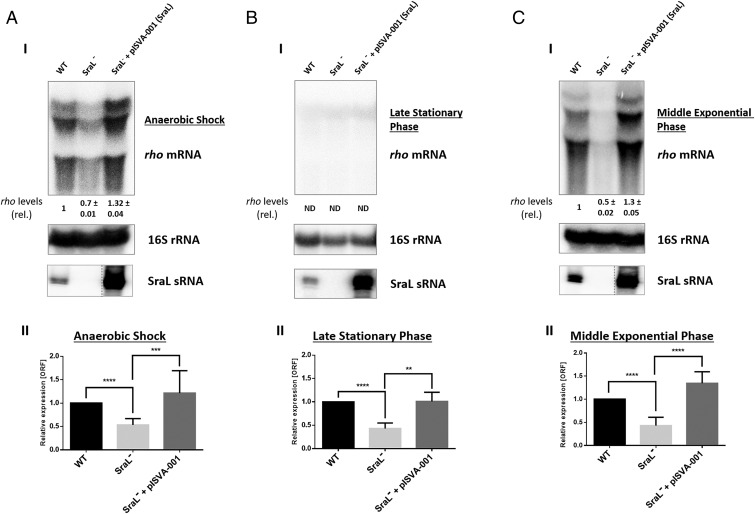
In contrast to what happens with the other known target of SraL (15), the absence of this sRNA in the cell produced a negative impact in rho mRNA expression. Quantification of rho transcripts using both Northern blots and quantitative real time-PCR (qRT-PCR) revealed a decrease of ∼50% of rho mRNA in the absence of SraL, compared with the wild-type strain (Fig. 1 A, I and II and Fig. 1 B, I and II). Consistently, rho mRNA expression levels were restored in sraL null mutant upon ectopic expression of wild-type sraL from a constitutive promoter (Fig. 1 A, I and II and Fig. 1 B, I and II). The low expression level of rho mRNA in late stationary phase did not allow a proper detection of this transcript by Northern blot analysis, but it was quantified by qRT-PCR.
Fig. 1.
rho mRNA regulation by SraL sRNA in different growth conditions. Total cellular RNA was extracted from Salmonella strains indicated in the figure. The following conditions were used: (A) Cells were grown in LB at 37 °C and 220 rpm to an OD600 of 0.3 and then placed in a filled 50-mL Falcon tube and incubated at 37 °C without agitation during 30 min (AS). (B) Cells were grown in LB at 37 °C and 220 rpm until 6 h after OD600 of 2 (late stationary phase). (C) Cells were grown in LB at 37 °C and 220 rpm until OD600 of 1 (middle exponential phase). (I) (Upper) Twenty micrograms of total RNA were separated on a 1.3% formaldehyde/agarose gel. The gel was then blotted to a Hybond-N+ membrane and hybridized with the corresponding rho riboprobe. The transcripts were quantified using ImageQuant software. The amount of RNA in the wild type was set as one. The ratio between the amounts of RNA of each strain and the wild type is represented (relative levels). A representative membrane is shown, and the values indicated correspond to the average of several Northern blot experiments with RNAs from at least two independent extractions. The membrane was stripped and then probed with 16S rRNA as loading control. (Lower) Fifteen micrograms of total RNA were separated in a 6% PAA/8.3 M urea to determine the expression level of SraL; contrast of SraL−+pISVA-001 was adjusted separately as indicated by the dashed line. (II) The transcriptional level of rho was also determined by quantitative real time-PCR analysis using Rotor-Gene 3000 (Corbett) system and with cDNA synthesized from 1 µg of purified RNA. Values are shown relative to the expression levels in wild-type strain. Results were normalized with the expression of the housekeeping gene 23S rRNA and represent at least three independent experiments. ****P < 0.0001, ***P ≤ 0.0005, **P < 0.001 by Student’s t test.
Afterward, this regulation was also tested in middle exponential growth (MEP; OD600 of 1), a condition in which SraL sRNA is only slightly expressed (15). Despite the relatively low expression level of SraL sRNA, the same type of regulation, as in the other conditions, was obtained (Fig. 1 C, I and II).
Hereupon, SraL sRNA positively regulates rho mRNA expression level in Salmonella in all conditions tested.
SraL sRNA Directly Interacts with rho mRNA.
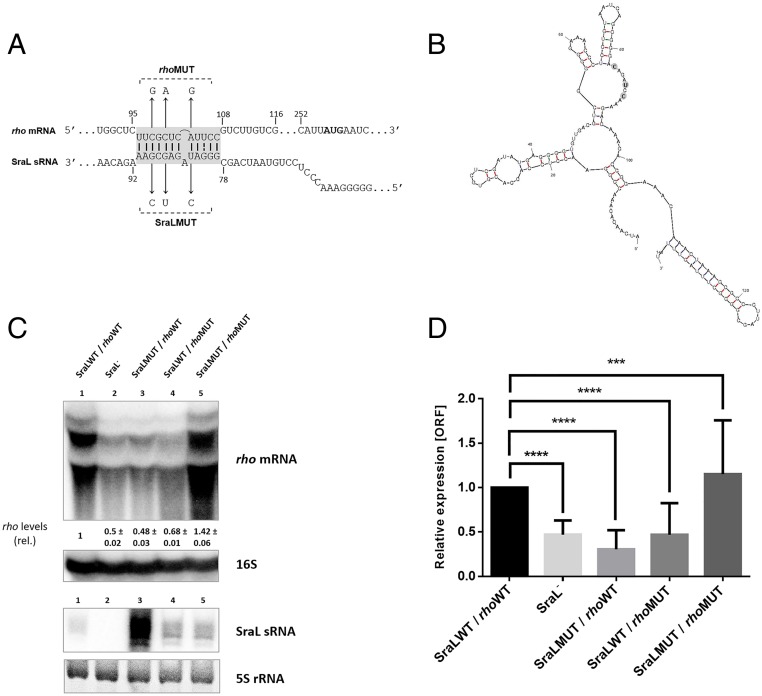
To study in more detail the role of SraL in the regulation of rho mRNA and verify whether pairing is direct or indirect, an in silico prediction of possible interaction regions between the sRNA and its target was performed using IntaRNA and RNA Hybrid software (32, 33). Both algorithms predicted the same interaction, corresponding to a region of 12 nucleotides in length located in the 5′-UTR of rho mRNA (Fig. 2A).
Fig. 2.
Interaction between SraL sRNA and rho mRNA. (A) Predicted interaction region between rho mRNA and SraL sRNA by IntaRNA software (32) and RNA Hybrid (33). Chromosomal point mutations to generate rhoMUT and SraLMUT alleles are indicated; rho mRNA AUG is also indicated in bold. (B) S. Typhimurium SraLMUT sRNA structure predicted by Mfold program (54). (C) Total cellular RNA was extracted from the S. Typhimurium strains indicated in the figure, grown in LB at 37 °C until OD600 of 1. (Upper) The expression level of rho mRNA was determined by Northern blot using 20 μg of total RNA separated in a 1.3% formaldehyde/agarose gel. The amount of RNA in wild type was set as one. The ratio between the RNA amount of each strain and wild type is represented below each strain (relative levels). A representative membrane is shown, and values indicated correspond to the average of several Northern blot experiments with RNAs from at least two independent extractions. The membrane was stripped and then probed for 16S rRNA as loading control. (Lower) Fifteen micrograms of total RNA were separated in a 6% PAA/8.3 M urea to determine the expression level of both SraLWT and SraLMUT sRNAs; 5S rRNA was used as loading control. (D) The transcriptional level of rho was also determined by quantitative RT-PCR analysis with the Rotor-Gene 3000 (Corbett) system, using cDNA synthesized from 1 µg of purified RNA. Values are shown relative to the expression levels in wild-type strain. Results were normalized with the expression of the housekeeping gene 23S rRNA and represent at least three independent experiments. ****P < 0.0001, ***P ≤ 0.0005 by Student’s t test.
Since the effect of SraL sRNA over rho mRNA was observed in all of the aforementioned conditions, we decided to proceed using cells grown until middle exponential phase. To test whether the predicted region is required for SraL–rho interaction, three point mutations were introduced in sraL chromosomal region (SraLMUT) (Fig. 2A), which cause disruption of the predicted interaction between the RNAs (cf. SI Appendix, Fig. S2 A and C). We tried to ensure that these mutations did not compromise the secondary structure of the sRNA by predicting the secondary structure of SraLMUT using the Mfold program (Fig. 2B). We verified by Northern blot and qRT-PCR analysis that point mutations introduced in SraL abolished the effect of the sRNA over rho mRNA (Fig. 2C, Upper, cf. lanes 1 and 3; Fig. 2D), presenting a similar effect to the one observed in sraL null mutant (Fig. 2C, Upper, cf. lanes 2 and 3; Fig. 2D). To validate this effect, point mutations were inserted in rho chromosomal region (rhoMUT) at the positions corresponding to the mutations introduced in the sRNA (Fig. 2A). Full complementarity should be restored when using the mutated version of both RNAs. In this condition rho mRNA expression level should be similar to the one obtained when using the wild-type background. By Northern blot and qRT-PCR, we confirmed that the wild-type version of SraL (SraLWT) was only able to modulate the wild-type rho mRNA levels (rhoWT) and not the mutated version (rhoMUT) (Fig. 2C, Upper, cf. lanes 1 and 4; Fig. 2D). The same effect was observed when using the mutated version of SraL, which is only able to regulate rhoMUT (Fig. 2C, Upper, cf. lanes 5 and 3; Fig. 2D). When comparing SraL expression levels between the constructed strains, there was a clear increase of SraL expression in the strain containing the SraL chromosomal mutations (Fig. 2C, Lower, lane 3). This result was a surprise since the secondary structure of the sRNA was maintained and did not change its promoter region. Thus, another level of regulation would have been affected by the point mutations, such as the inactivation of a ribonuclease cleavage site. However, this increase was not sufficient to increase the expression of rhoWT, which reinforces the results that SraLMUT was only able to interact with rhoMUT. Curiously, the increase in SraL expression levels was not detected when both SraL and rho chromosomal mutations were combined in the same strain (SraLMUT/rhoMUT) (Fig. 2C, Lower, lane 5).
These results confirmed that in fact SraL sRNA is a positive regulator of rho mRNA and revealed that this regulation is mediated through base pairing between sRNA and mRNA.
SraL Does Not Affect rho mRNA Stability.
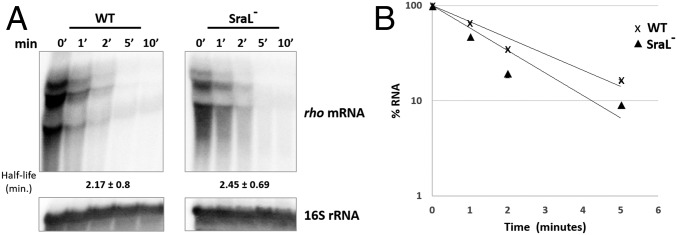
So far, our study revealed that SraL sRNA somehow upregulates the mRNA expression of the crucial transcription termination factor Rho. Furthermore, this regulation occurs through the base pairing between the sRNA and the 5′-UTR of rho mRNA. There are some mechanisms that might contribute to a regulation of this type (34). One of the most commonly described is related to the protective effect of sRNAs against ribonucleases, giving rise to an increased stability of the mRNA targets. To test if this is the case, stability of rho mRNA was accessed by measuring its half-life in the presence and absence of SraL sRNA. After addition of rifampicin, rho mRNA presented a fast decay rate of ∼2.2 min in the wild-type strain (Fig. 3). Surprisingly, there was no significant difference in rho mRNA half-life with or without SraL sRNA expression, despite the already observed difference in steady levels (Fig. 3). These results indicate that SraL does not affect rho mRNA stability.
Fig. 3.
Analysis of rho mRNA stability. Wild-type and SraL− strains grown in LB medium at 37 °C and 220 rpm until OD600 of 1. At this OD, a mixture of rifampicin and nalidixic acid was added to the cultures and samples were taken out at the indicated times. (A) Total RNA was extracted, and 20 μg of RNA was separated on a 1.3% formaldehyde/agarose gel. A representative membrane is shown, and the half-life values indicated correspond to the average of several Northern blot experiments with RNAs from at least two independent extractions. (B) The quantification of the transcripts was plotted versus time of extraction (in minutes) to calculate the half-life of the mRNA.
SraL Affects Rho-Dependent Termination at the 5′-UTR of rho mRNA.
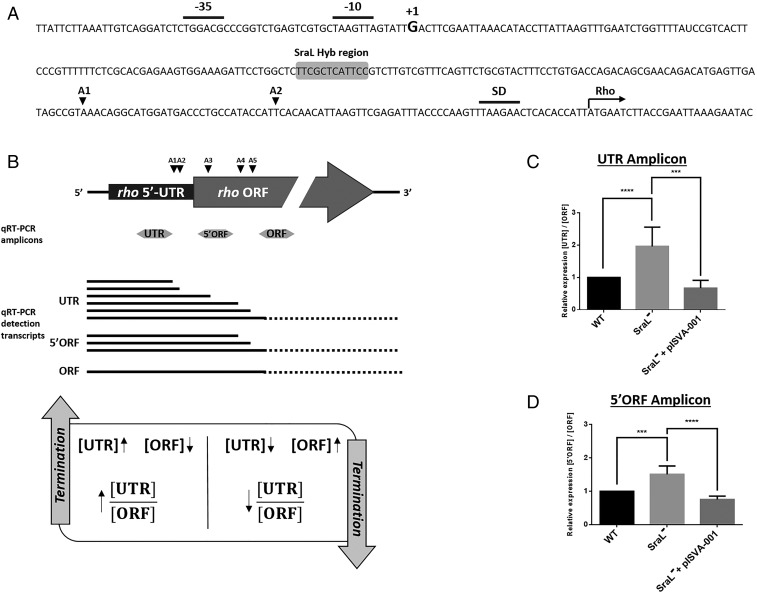
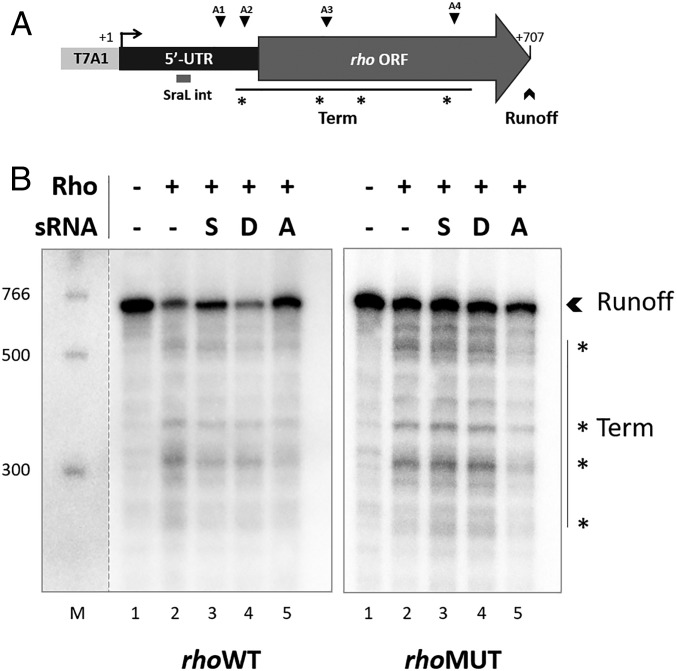
It has been reported by several authors that the expression of rho is autogenously regulated at the transcriptional level, via at least five attenuators localized both in rho 5′-UTR and in the upstream region of its ORF (18–20) (Fig. 4 A and B). This autoregulation results in premature transcription termination of rho, giving rise to the appearance of smaller mRNA transcripts containing only the 5′ region of rho (20). Since SraL base pairs with rho in its 5′-UTR and near two of the already identified attenuators (Fig. 4A), we hypothesized that the sRNA could somehow be involved in this mechanism.
Fig. 4.
SraL sRNA affects rho mRNA at the transcription termination level. (A) Nucleotide sequence of the upstream region of rho gene. The −35 and −10 sites, Shine–Dalgarno box (5), as well as the +1 (G) of the rho mRNA (20) and the interaction region where SraL sRNA hybridizes are indicated. A1 and A2 correspond to the attenuation sites described in ref. 20. (B, Upper) Scheme of rho 5′-UTR and ORF regions with the location of the five attenuation sites described in ref. 20. The locations of the three qRT-PCR amplicons are indicated below. (B, Middle) Transcripts detected with the different amplicons used in the qRT-PCR. (B, Lower) Diagram with the approach used for the estimation of Rho-dependent termination within rho leader. An increase in the [UTR]/[ORF] ratio corresponds to an increase in the termination efficiency and vice versa. An increase of the termination efficiency implies the increase of [UTR] and the decrease of [ORF] and vice versa. (C and D) Total cellular RNA was extracted from the S. Typhimurium strains indicated in the graphics, grown in LB at 37 °C until OD600 of 1. Transcript levels of the [UTR] amplicon (C) and of the [5′ORF] amplicon (D) were quantified by qRT-PCR. The values were normalized to that of the ORF amplicon and represent at least three independent experiments. ****P < 0.0001, ***P ≤ 0.0005 by Student’s t test.
To test this hypothesis, three rho regions were selected to evaluate quantitatively the transcription termination and sRNA-mediated anti-termination by qRT-PCR: a segment in the 5′-UTR, upstream of the two predicted attenuators (“UTR” amplicon); the upstream region of the rho ORF (“5′ORF” amplicon); and a segment in the middle of rho ORF (“ORF” amplicon) (Fig. 4B). Using the “ORF” amplicon, qRT-PCR only detects transcripts which were not targeted by premature transcription termination (Fig. 4B). The qRT-PCR of the “UTR” amplicon detects not only transcripts subjected to premature transcription termination, but also the ones detected using the “ORF” amplicon (Fig. 4B). The internal normalization of the qRT-PCR values to the amount of “ORF” allowed us to discriminate the smaller transcripts mentioned above and to directly compare the termination efficiency within rho UTR among the different strains. The highest ratio of [UTR]/[ORF] detected in the sraL null mutant when comparing with the wild-type strain indicates a more efficient Rho-dependent termination in rho 5′-UTR in the absence of the sRNA (Fig. 4C). Curiously, the same tendency was obtained for [5′ORF]/[ORF] ratio (Fig. 4D). The effect obtained in the sraL null mutant was reversed to the wild-type situation when using the complemented sraL null mutant strain, corroborating the previous results (Fig. 4 C and D).
Hereupon, the results obtained seem to indicate that SraL sRNA protects rho mRNA from premature transcription termination.
SraL sRNA Does Not Regulate rho mRNA in the Absence of Rho Activity.
To determine whether SraL sRNA and Rho regulate rho mRNA expression in the same pathway, both wild-type and sraL null mutant strains were exposed to bicyclomycin (BCM). This antibiotic is highly specific to Rho and acts by disrupting its ATPase and translocase activity, making Rho an inactive protein (31). BCM has been used to mimic the absence of Rho in the cell, due to the impossibility of obtaining rho null mutants in Salmonella and other bacterial species (12, 13, 35, 36).
As expected, rho mRNA expression was increased more than threefold in the samples treated with BCM, reflecting the absence of Rho activity in its autoregulation (Fig. 5A, cf. WT −BCM and WT +BCM). When Rho was active (without BCM), a 50% reduction was obtained in rho mRNA expression in the absence of SraL (Fig. 5A, cf. WT and SraL− in −BCM). However, when Rho activity was inhibited by the addition of BCM this reduction was no longer observed (Fig. 5A, cf. WT and SraL− in +BCM). Therefore, rho mRNA positive regulation by SraL seems to be dispensable in the absence of Rho activity.
Fig. 5.
SraL sRNA is not necessary for rho mRNA regulation in the absence of Rho. Total cellular RNA was extracted from the S. Typhimurium strains indicated in the graphics, grown in LB at 37 °C until an OD600 of 1. Samples were taken before and after treatment with BCM (10 μg/mL, 15 min). (A) Transcript levels of the [ORF] amplicon were quantified by qRT-PCR. The values were normalized to that of the housekeeping 23S rRNA and represent at least three independent experiments. ****P < 0.0001, ns indicates nonsignificant by Student’s t test. (B and C) Transcript levels of the [UTR] amplicon (B) and of the [5′ORF] amplicon (C) were quantified by qRT-PCR. The values were normalized to that of the ORF amplicon and represent at least three independent experiments. ****P < 0.0001, *P ≤ 0.05, ns indicates nonsignificant by Student’s t test.
To further validate our hypothesis, we used the same approach as in the previous point to compare the termination efficiency within rho among the different strains, with and without BCM treatment. As expected, in the wild-type strain there was less premature transcription termination upon addition of BCM (inactivation of Rho), which was reflected by the decrease in the [UTR]/[ORF] ratio (Fig. 5B, cf. WT −BCM and WT +BCM). In agreement with the previous results, the difference obtained in [UTR]/[ORF] ratio between the wild-type and sraL null mutant strains when Rho was active (−BCM) was no longer observed when Rho was inhibited (Fig. 5B). Although there was a lower difference, the same tendency was obtained for [5′ORF]/[ORF] ratio (Fig. 5C). Thus, the protective effect of rho mRNA by SraL sRNA was only important when Rho is active in the cell.
These data indicate that SraL acts by base pairing with rho mRNA protecting it from the action of Rho in its 5′-UTR.
SraL sRNA Inhibits Rho Termination in Vitro.
To further study the mechanism of action of SraL over rho mRNA, in vitro transcription termination experiments were performed using both rhoWT and rhoMUT as DNA templates, purified Salmonella Rho protein and purified SraL sRNA (Fig. 6). As expected, comparing reactions in the absence and presence of Rho protein (Fig. 6B, cf. lanes 1 and 2), it was possible to observe a decrease in the intensity of the runoff band (which corresponds to the transcription of the entire template) with concomitant appearance of smaller transcripts resultant from premature transcription termination. In agreement with in vivo results, upon addition of SraL sRNA there was an increment of the runoff transcript resultant from SraL protective effect against premature termination by Rho (Fig. 6B, Left gel, cf. lanes 2 and 3). Additionally, this effect was abolished when using rhoMUT as a template (Fig. 6B, Right gel, cf. lanes 2 and 3), which corroborates the importance of the predicted interaction region in this regulation (Fig. 2). Two different controls were used in these experiments. DsrA sRNA was shown to be involved in the suppression of Rho-dependent transcription termination of rpoS (13); in this case it was used as a negative control (Fig. 6B, cf. lanes 2 and 4). Moreover, as a positive control we utilized a small oligonucleotide (Anti; 12 nucleotides) corresponding to the SraL sequence predicted to interact with rhoWT mRNA (Fig. 2A). The result obtained (Fig. 6B, Left gel, cf. lanes 2 and 5) also confirmed the importance of this region since it was very effective in blocking Rho’s action, leading to the increase of the runoff transcript’s level and to the reduction of smaller transcripts’ levels.
Fig. 6.
rho in vitro transcription termination is also affected by SraL sRNA. (A) Scheme of rho DNA template used in the in vitro transcription termination assay. T7A1 corresponds to the promoter sequence recognized by E. coli RNA polymerase inserted in the templates by two consecutive PCRs. The approximate site where SraL interacts and the location of the four attenuation sites (A1–A4) described in ref. 20 are indicated. Termination products represented in B as the most prominent bands are indicated by asterisks (*). (B) In vitro transcription termination assay was performed using both rhoWT (Left gel) and rhoMUT (Right gel) as DNA template and in the absence (lanes 1 and 2) or presence of different sRNAs: SraL sRNA (S; lane 3), DsrA (D; lane 4; serving as a negative control), and Anti (A; lane 5; synthetic oligonucleotide fully complementary to rhoWT mRNA sequence targeted by SraL, serving as a positive control). The runoff transcripts (Runoff) and the transcription termination region are indicated (Term). The bands identified by asterisks (*) represent the most prominent termination transcripts in this experiment. The contrast of the DNA ladder lane (M; PCR Marker, New England Biolabs) was adjusted separately as indicated by the dashed line. Representative gels are shown; the experiment was performed more than 3 times with each DNA template.
In conclusion, in vitro transcription termination experiments confirmed in vivo results obtained so far.
Influence of Chaperones in SraL-Rho Regulation.
RNA-binding proteins that act in conjunction with sRNAs to regulate its targets have been widely studied (22, 37). In a previous study, chaperone Hfq was shown to be important for stabilization of SraL sRNA in stationary phase of growth (17). More recently, this sRNA was also shown to coimmunoprecipitate with ProQ, and this chaperone was also very important for the stability of SraL (22).
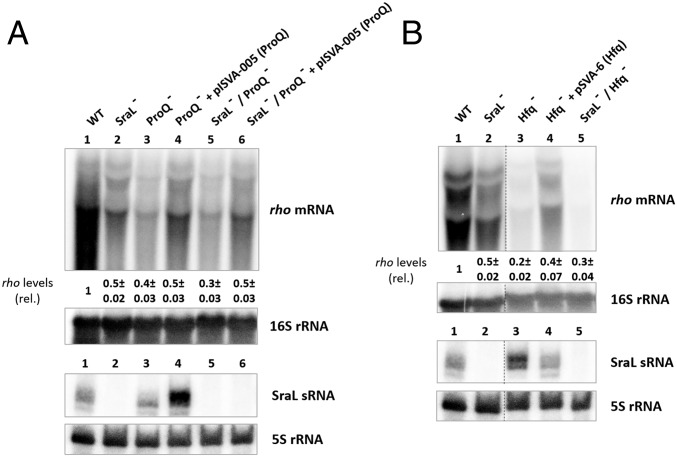
To study the importance of these chaperones for SraL-rho regulation Northern blot experiments were performed using strains lacking proQ and hfq (Fig. 7). Regarding the effect of ProQ, a 60% reduction was obtained in rho transcripts’ level in proQ null mutant (Fig. 7A, Upper, cf. lanes 1 and 3) that was only partially restored when expressing proQ in trans (Fig. 7A, Upper, cf. lanes 3 and 4). To test if ProQ and SraL affect rho message through the same pathway, the effect of the double mutant SraL−/ProQ− in rho transcripts level was evaluated. The level obtained was comparable to the one of proQ null mutant (Fig. 7A, Upper, cf. lanes 3 and 5). Moreover, rho mRNA expression levels were restored to the same values obtained with sraL null mutant upon ectopic expression of proQ from a constitutive promoter (Fig. 7A, Upper, cf. lanes 2 and 6). These results seem to indicate that even though SraL and ProQ affect rho mRNA through the same pathway, ProQ is also affecting rho mRNA through an additional pathway.
Fig. 7.
Importance of the chaperones ProQ (A) and Hfq (B) in SraL–rho regulation. (A and B) Total cellular RNA was extracted from the S. Typhimurium strains, grown in LB at 37 °C until an OD600 of 1. (Upper) The rho mRNA expression level was determined by Northern blot using 20 μg of total RNA separated in a 1.3% formaldehyde/agarose gel. The amount of RNA in wild type was set as one. The ratio between the RNA amount of each strain and wild type is represented below each strain (relative levels); 16S rRNA was used as loading control. (Lower) Thirty micrograms of total RNA were separated in a 6% PAA/8.3 M urea to determine the expression level of SraL sRNA; 5S rRNA was used as loading control. A representative membrane is shown, and values indicated correspond to the average of several Northern blot experiments with RNAs from at least two independent extractions. Dashed lines indicate noncontiguous lanes.
The absence of the other chaperone, Hfq, showed to be more critical since the level of rho transcript decreased 80% when compared to the wild-type strain (Fig. 7B, Upper, cf. lanes 1 and 3). Contrarily to what happens in stationary phase (17), Hfq seems to somehow inhibit the expression of SraL in exponential phase (Fig. 7B, Lower, cf. lanes 1 and 3). Although SraL level was increased in hfq null mutant, the sRNA was not able to counteract the effect caused by the absence of hfq. Moreover, in the double mutant lacking both sraL and hfq the effect on rho mRNA level was not cumulative compared with the two single mutants (Fig. 7B, Upper, cf. lanes 2, 3 and 5). These results seem to indicate that SraL and Hfq are affecting this transcript through the same pathway. However, like ProQ, Hfq also seems to be affecting rho level through another pathway of regulation.
Impact of SraL in Rho Protein Level and Activity.
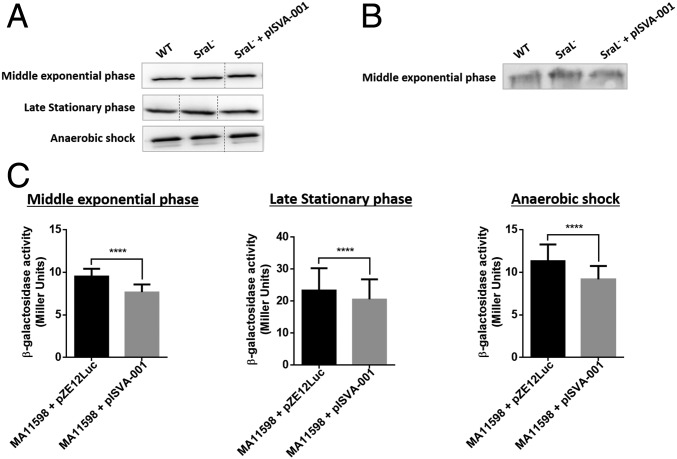
So far, our results revealed the importance of the sRNA SraL for the protection of rho mRNA against the action of its own protein. The next step was to investigate whether the difference obtained for rho mRNA level in the absence of SraL would be also reflected at protein level. For that, Western blot analysis was performed using the same strains used in Northern blot analysis. Differences in Rho monomer expression level were assessed running total protein extracts in denaturing conditions (Fig. 8A). On the other hand, variations at the level of Rho hexamer were investigated using native conditions (Fig. 8B). In both cases, there was no significative difference in Rho protein level between the strains used (Fig. 8 A and B).
Fig. 8.
The influence of SraL in Rho protein expression and activity. (A) Detection of Rho protein under denaturing conditions was performed by Western blot from 5 μg of total protein extracts obtained in middle exponential phase, stationary phase, and upon anaerobic shock. Dashed lines indicate noncontiguous lanes. (B) Native Western blot to evaluate Rho protein expression in middle exponential phase was done from 40 μg of total protein cellular extracts. For both denaturing and native conditions Rho expression was determined in strains expressing SraL sRNA at different levels using anti-Rho antibody (MyBioSource). (C) β-Galactosidase activity was accessed in a strain carrying a fusion of LacZ reporter under the control of pgaABDC operon promoter and rut site, using o-nitrophenyl-β-d-galactopyranoside (Sigma) as substrate. Rho protein activity in pgaABDC promoter was evaluated in middle exponential phase, stationary phase, and upon anaerobic shock. Results are expressed in Miller units and are representative of at least three biological replicates. Statistical analysis was carried out using GraphPad Prism 6 software. ****P < 0.001 (Paired t test).
The next step was to measure the activity of the protein. For that, we performed β-galactosidase activity assays using a Salmonella LT2 strain with E. coli pgaABDC promoter and rut site fused to lacZ reporter gene (MA11598). This construct was used since the existence of a Rho-dependent terminator in pgaA 5′-UTR was previously reported (38). Due to experimental constraints, the assay was only performed using the strains MA11598 and MA11598 overexpressing SraL in trans (MA11598 + pISVA001). As shown in Fig. 8C, there was a slight but significant decrease of β-galactosidase activity in the strain with high levels of SraL (MA11598 + pISVA-001) in all of the conditions tested. This decrease was a consequence of the increased transcription termination in pgaA Rho-dependent terminator mediated by Rho protein.
In conclusion, although we were not able to detect differences in Rho protein level by Western blot, the slight effect obtained in Rho activity upon overexpression of SraL shows that this regulation has a biological repercussion.
Discussion
There are several studies regarding SraL regulation in Salmonella, but only one biological function has been assigned for this sRNA as of yet. SraL was shown to downregulate the expression of the important chaperone Trigger factor in a mechanism that involves the base pairing of the sRNA with the 5′-UTR of tig mRNA (15). In the present study, we have uncovered the role of SraL as a positive regulator of the transcription termination factor Rho. SraL sRNA constitutes an example of the versatility of the sRNAs since it interacts with both of its targets via two different regions leading to negative or positive regulation (SI Appendix, Fig. S4) (15).
Rho is known to be crucial for the prokaryotic termination of transcription either at the end of genes and/or operons or in the leader sequence of several mRNAs, performing regulatory functions (3, 13, 31, 39). The transcription of E. coli rho was shown to be autogenously regulated, via several attenuators located in its leader region (18–20). The existence of Rho-dependent termination within rho mRNA was very recently corroborated in E. coli. Using a very elegant experiment, the Rho termination score was calculated for several transcripts, and rho mRNA was used as a control (35). The high percentage of homology between Salmonella and E. coli rho sequence, namely in its 5′-UTR, suggests that the mechanism is similar in both organisms (SI Appendix, Fig. S3). In fact, the expression of Salmonella rho mRNA was shown to increase more than threefold upon the addition of the specific inhibitor of Rho protein BCM to the cell culture (Fig. 5A), revealing that in this pathogenic organism Rho also acts as a negative regulator of its own transcription.
The findings made in the current study suggest the existence of an additional player in the already described mechanism. Using the recently developed MAPS technology, rho mRNA was one of the top candidates present in the list of the RNAs predicted to base pair with the sRNA SraL (Dataset S1). Due to the high importance of this protein in transcription termination process, we decided to investigate this regulation in detail. Accordingly, SraL showed to be a positive regulator of rho mRNA expression not only when the sRNA is highly expressed (anaerobic shock and late stationary phase), but also in conditions where SraL is only slightly expressed (middle exponential phase) (Fig. 1). This fact corroborates the importance of the sRNA in rho mRNA regulation. The interaction region between SraL and the 5′-UTR of rho mRNA was predicted in silico and confirmed through the insertion of specific chromosomal mutations that abolish the interaction between both RNAs (Fig. 2). Curiously, analyzing the RNA-seq results obtained after MS2-SraL enrichment, the most enriched region of rho mRNA was indeed its 5′-UTR.
Since SraL was shown to directly base pair with the 5′-UTR of rho mRNA, we hypothesized that the sRNA would protect this mRNA from degradation by ribonucleases. However, the stability of rho mRNA was not affected by the absence of the sRNA (Fig. 3). Due to the previously described autogenous regulation of rho mRNA transcription, we decided to investigate the role of SraL in this mechanism. In the absence of the sRNA, we have obtained a more efficient Rho-dependent termination not only in the 5′-UTR, but also in the upstream region (5′ORF) of rho mRNA. Moreover, this difference was abolished when Rho factor activity was inhibited by the addition of BCM. Thus, SraL sRNA positively regulates the expression of rho mRNA by affecting Rho-dependent termination through base pairing with its 5′-UTR in a mechanism that is only important in the presence of an active Rho factor (Figs. 4 and 5).
There are five different attenuation regions described in rho mRNA that are positioned not only in its 5′-UTR, but also in its 5′ORF (Fig. 4B, Upper) (20). In the approach used to investigate in vivo Rho-dependent termination, when measuring the UTR amplicon by qRT-PCR not only was the full-length transcripts detected, but also the smaller transcripts resulting from premature transcription termination in all of the attenuators. However, only the two longer transcripts resulting from premature transcription termination (4, 5) were detected when measuring the 5′ORF. This fact explains the lower difference obtained in the wild-type strain for [5′ORF]/[ORF] ratio compared with the [UTR]/[ORF] ratio upon the addition of BCM and consequent inhibition of Rho activity (Fig. 5 B and C). The attenuation regions described for E. coli rho (20) were also identified in Salmonella by performing an in vitro transcription termination assay. In this experiment we were able to detect the appearance of most of the attenuation regions (A1 to A4) when comparing the samples without and with Rho protein (Fig. 6). The attenuation region A5 is located downstream of the 3′-end of the DNA template used, and for that reason was not detected in this assay. In line with the in vivo results, the addition of SraL in the reaction gave rise to an increment of runoff transcripts and to a decrease in termination products, which confirms the protective effect of SraL over rho mRNA. In addition, this effect was specific to rhoWT confirming in vitro the predicted interaction region between the two RNA species (Figs. 2 and 6).
The influence of chaperones in this type of riboregulation has been widely studied (22, 37). Indeed, SraL was already associated with two of the major RNA-binding proteins, Hfq and ProQ (17, 22). The results obtained seem to indicate that these chaperones are involved in the regulation of rho by SraL (Fig. 7). However, both chaperones seem to affect rho mRNA in an alternative pathway in which SraL is not involved. In fact, Hfq was reported to be directly associated with Rho protein, inhibiting its ATPase, helicase, and transcription termination activities (40). Since Rho protein negatively affects rho transcript, in the absence of hfq, the level of rho mRNA should be increased. In fact, this was not the case since in hfq null mutant rho mRNA level decreased 80%. These data, together with the results obtained regarding the level of Rho protein in the absence of sraL (Fig. 8), show the complexity behind the regulation of such an important protein like Rho.
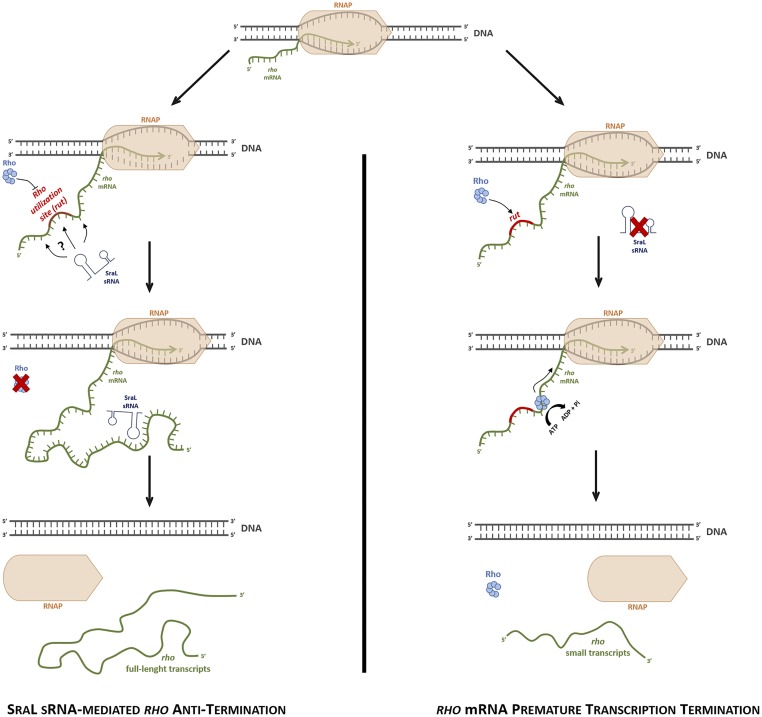
With all of the results obtained in this study, we propose a model for rho mRNA regulation that contemplates the action of SraL sRNA (Fig. 9). When expressed in the cell, SraL binds to the 5′-UTR of rho mRNA, an interaction that can be mediated by ProQ and/or Hfq. The formation of sRNA/5′-UTR complex may cause various effects depending on the base-pairing region. If the binding between both RNAs occurs in the Rho utilization site (rut), Rho is not able to bind this region leading to the inhibition of Rho loading; the same effect is obtained if the sRNA interacts upstream or downstream of rho rut site leading to a rearrangement of the RNA secondary structure and consequent occlusion of rut. Additionally, the sRNA interaction can interfere with Rho translocation along RNA inhibiting also its action in rho mRNA autoregulation. Thus, by interacting with rho mRNA SraL protects this transcript from premature transcription termination (Fig. 9, Left). In the absence of sraL expression, Rho factor recognizes the rut site in the 5′-UTR of rho mRNA, and after loading and translocating along RNA, catches up to the elongating RNA polymerase (RNAP) causing premature transcription termination (Fig. 9, Right).
Fig. 9.
Proposed model of transcription termination control of rho mRNA by SraL sRNA. (Left) Upon binding of the sRNA to the 5′-UTR of rho mRNA, rut site becomes inaccessible to Rho factor or Rho translocation is inhibited. This “protective” effect of SraL over rho mRNA allows the transcription to proceed until the complete synthesis of the full-length rho transcripts. (Right) In the absence of SraL sRNA, the rut sequence(s) localized in the upstream region of rho mRNA is recognized by Rho factor. After the loading onto the rut site, Rho translocates on RNA (in a reaction dependent on ATP) catching up to the elongating RNAP complex (the other factors involved in the transcription process are omitted in this model). Upon binding of Rho to RNAP, the unwinding of the RNA:DNA hybrid occurs and the release of the nascent transcripts from RNAP complex, and the premature transcription termination occurs.
Interestingly, sRNA-mediated anti-termination was also recently described for the general stress σS subunit of RNA polymerase (13). The sRNAs DsrA, ArcZ, and RprA were previously known to relieve the translational inhibition by base pairing with the rpoS leader sequence, rendering the ribosome binding site (RBS) available to the ribosome (41–43). Beyond this mechanism, these three sRNAs also suppress premature Rho-dependent transcription termination stimulating the transcription of rpoS during the transition to the stationary phase of growth (13). By using the specific Rho inhibitor BCM and an RNA-seq approach, the authors also verified that Rho functions as a global attenuator of gene expression, and that sRNA-mediated anti-termination is a widespread mode of bacterial gene regulation (13). Curiously, most of the riboswitches from E. coli and B. subtilis were shown to control premature transcription termination. In B. subtilis, riboswitches modulate the formation Rho-independent terminators (44, 45). In turn, E. coli riboswitches control transcription termination by using Rho transcription factor (35, 46). This mechanism was also described for a Salmonella riboswitch (46). Taken together, all these studies indicate that the mechanism of anti-termination mediated by riboregulators seem to be widespread in several bacterial organisms.
Using a combination of experimental and bioinformatic approaches, we have described in detail the role of Salmonella SraL sRNA in the control of the expression of the important transcription termination factor Rho. This sRNA was shown to interact with the 5′-UTR of rho mRNA protecting the transcript against premature transcription termination by its own protein. Curiously, the interaction region in both RNA species corresponds to a very-well-conserved sequence in enterobacteria (SI Appendix, Figs. S3 and S4), which may indicate that this regulation also occurs in other bacterial organisms.
Materials and Methods
Oligonucleotides.
The oligonucleotides used in this work are listed in SI Appendix, Table S1 and were synthesized by STAB Vida (Portugal).
Bacterial Strains and Plasmids.
All bacterial strains and plasmids used throughout this study are listed in SI Appendix, Tables S2 and S3, respectively. Unless stated, Salmonella strains used are isogenic derivatives of the wild-type Salmonella enterica serovar Typhimurium strain SL1344. Strains and plasmids constructions are detailed in SI Appendix, Supplementary Materials and Methods.
Bacterial Growth.
All strains were grown in LB at 37 °C and 220 rpm. Electroporation was used for transformation of S. Typhimurium. The SOC (super optimal broth with catabolite repression) medium was used to recover transformants after electroporation procedure.
To apply anaerobic shock, cells were first grown at 37 °C and 220 rpm to an OD600 of 0.3. Then, they were placed in a completely filled 50-mL Falcon tube and incubated at 37 °C without agitation for 30 min (27). For treatment with BCM, cultures were grown to middle exponential phase (OD600 of 1), and then BCM was added to a final concentration of 10 μg/mL for 15 min before harvesting.
When appropriate, antibiotics were used at the following concentrations: 100 µg/mL ampicillin, 90 µg/mL streptomycin, 100 μg/mL kanamycin, and 25 µg/mL chloramphenicol.
MS2-Affinity Purification Coupled with RNA Sequencing.
Salmonella Typhimurium SL1344 strains were grown in LB medium at 37 °C (100 mL). Cells were harvested in (i) exponential phase after anaerobic shock and (ii) late stationary phase. MAPS was performed as previously described in refs. 24 and 26 and are detailed in SI Appendix, Supplementary Materials and Methods. The cDNA libraries were prepared with ScriptSeq v3 RNA-Seq Library Preparation Kit (Illumina) and sequenced (MiSeq sequencing system; Illumina). We used Galaxy Project (47) to analyze data. The whole list of genes enriched is available in Dataset S1. The GEO accession number is GSE108234.
RNA Extraction and Northern Blot.
Overnight cultures were diluted 1/100 in fresh medium and grown to the indicated cell densities (growth conditions are detailed in the respective figure legends). Culture samples were collected, mixed with 1 volume of stop solution (10 mM Tris pH 7.2, 25 mM NaNO3, 5 mM MgCl2, 500 μg/mL chloramphenicol), and harvested by centrifugation (10 min, 6,000 g, 4 °C) (48). For stability experiments, rifampicin (500 μg/mL) and nalidixic acid (20 μg/mL) were added to cells grown in LB at 37 °C, 220 rpm, until an OD600 of 1. Incubation was continued, and culture aliquots were withdrawn at the times indicated in the respective figure.
RNA was isolated using the phenol/chloroform extraction method, precipitated with ethanol and resuspended in RNase free water (48). The integrity of the RNA samples was accessed by 1.5% Agarose gel and the samples were quantified on a Nanodrop 1000 machine (NanoDrop Technologies).
For Northern blot analysis, total RNA was separated under denaturing conditions either by 8.3 M urea/6% polyacrylamide gel in TBE (Tris/borate/EDTA) buffer or by 1.3% agarose Mops/formaldehyde gel. For polyacrylamide gels, transfer of RNA onto Hybond-N+ membranes (GE Healthcare) was performed by electroblotting in TAE (Tris/acetic acid/EDTA) buffer. For agarose gels, RNA was transferred to Hybond-N+ membranes by capillarity using 20× SSC as transfer buffer. In both cases, RNA was UV cross-linked to the membranes immediately after transfer. Membranes were then hybridized with PerfectHyb buffer (Sigma) at 68 °C for riboprobes and 43 °C for oligoprobes. Signals were visualized by PhosphorImaging (Fujifilm FLA-5100, FUJIFILM Life Science) and analyzed and quantified using ImageQuant software (Molecular Dynamics).
Hybridization Probes.
Oligonucleotides for templates amplification are listed in SI Appendix, Table S1. Labeling of the probes was performed as previously described (17). The riboprobes were obtained using the primers pair pIS-021/pIS-022 for SraL riboprobe and pIS-049/pIS-051 for rho riboprobe; 5S rRNA and 16S rRNA were detected by the 5′-end-labeled oligonucleotides pIS-023 and pIS-024, respectively.
Quantitative Real Time-PCR Analysis.
The transcriptional levels of transcripts were determined with the Rotor-Gene 3000 (Corbett) system, using SensiFast SYBR kit (Bioline) (according to the supplier’s instructions), cDNA synthesized from 1 µg of purified RNA with SensiFAST cDNA synthesis kit (Bioline), and the primers pair pIS-050/pIS-051 for rho ORF, pIS-052/pIS-053 for rho 5′ORF, pIS-054/pIS-055 for rho UTR, and pIS-056/pIS-057 for 23S rRNA. For each pair of primers, the efficiency of real-time PCR amplification was estimated using the standard curve method in one color detection system (49). Relative quantification of gene expression was calculated using the threshold cycle (ΔΔCT) method (50).
Rho Protein Expression and Purification.
Protein purification was performed using a Novagen E. coli BL21 (DE3) strain carrying a pET28a plasmid containing Rho coding sequence (pISVA-004). Rho expression was induced in exponential phase using 1 mM of IPTG. After 2 h of induction, bacterial cells were harvested, and total protein extracts were obtained by lysis using French press cell. After clarification by centrifugation, the soluble extract was collected, and Rho protein purified with an imidazole gradient using AKTA purifier system (GE Healthcare) by histidine affinity chromatography with HiTrap HP columns (GE Healthcare). The fractions containing pure Rho protein were selected. To perform transcription termination assay, buffer exchange was done to Buffer 2 (100 mM KCl, 0.1 mM EDTA, 0.1 mM DTT, 10 mM Tris⋅HCl, 50% glycerol, pH 7.9), using PD-10 columns (GE Healthcare). Protein purity was accessed in a 10% SDS/PAGE gel (SI Appendix, Fig. S5). Quantification was performed according to Bradford method (51).
In Vitro Transcription Termination Assays.
Transcription termination experiments were performed as described previously (52) and are detailed in SI Appendix, Supplementary Materials and Methods. Signals were visualized by PhosphorImaging (Fujifilm FLA-5100, FUJIFILM Life Science). The analysis and quantification were performed using ImageQuant software (Molecular Dynamics).
Western Blot.
For Western blot analysis, total cellular extracts were collected in middle exponential phase, stationary phase and under anaerobic shock conditions. To determine Rho expression under denaturing conditions, total protein was obtained using Bugbuster reagent (Novagen) and denatured at 100 °C for 5 min. Protein quantification was performed according to Bradford method (51). Five micrograms of total protein were analyzed in a denaturing 10% SDS/PAGE gel and transferred to a nitrocellulose membrane (Hybond ECL; GE Healthcare). For native conditions, total protein extracts were obtained by cell resuspension in Lysis buffer [50 mM Tris⋅HCl pH 8, 150 mM NaCl, 0.03 mg of Lysosyme, 1 mM PMSF, 1× Halt Protease & Phosphatase Inhibitor Mixture (Thermo Scientific), and 125 U Benzonase (Sigma)] followed by freeze–thaw cycles. Forty micrograms of total protein were separated in a 10% native polyacrylamide gel and transferred to an Immun-Blot PVDF membrane (Biorad). In both conditions, membranes were probed with anti-Rho antibody (MyBioSource) with a dilution of 1:10,000 and anti-rabbit IgG with the same dilution as secondary antibody. Immunoblot signal was detected using Western Lightning Plus-ECL Reagents (PerkinElmer).
β-Galactosidase Activity Assays.
β-Galactosidase activity was assayed as previously described by Miller (53) and is expressed in Miller units. The details of the assay are described in SI Appendix, Supplementary Materials and Methods. Paired t test statistical analysis was carried out using GraphPad Prism 6 software.
Supplementary Material
Acknowledgments
We thank Teresa Batista da Silva for technical assistance. The authors also thank Vânia Pobre for help in the analyses of RNA sequencing results and Lionello Bossi for critical reading of the manuscript and helpful discussions. This work was financially supported by: Project LISBOA-01-0145-FEDER-007660 (Microbiologia Molecular, Estrutural e Celular) funded by FEDER (Fundo Europeu de Desenvolvimento Regional) funds through COMPETE2020 - Programa Operacional Competitividade e Internacionalização (POCI) and by national funds through Fundação para a Ciência e a Tecnologia (FCT); Project PTDC/BIA-MIC/1399/2014 also through FCT; European Union’s Horizon 2020 Research and Innovation Programme [Grant agreement 635536]; Canadian Institutes of Health Research (CIHR) Grant MOP69005 (to E.M.); ANR-13-BSV-0005 from the French “Agence National de la Recherche” (to N.F.-B.). This work has been supported by an operating grant from the CIHR (to E.M.). I.J.S. [SFRH/BPD/84086/2012] and S.B. [PD/BD/113983/2015] were recipients of a FCT Post-Doctoral and Doctoral Fellowship, respectively. A.E. was supported by a “Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke” (CRCHUS) postdoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE108234).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811589116/-/DCSupplemental.
References
- 1.Ray-Soni A, Bellecourt MJ, Landick R. Mechanisms of bacterial transcription termination: All good things must end. Annu Rev Biochem. 2016;85:319–347. doi: 10.1146/annurev-biochem-060815-014844. [DOI] [PubMed] [Google Scholar]
- 2.D’Heygère F, Rabhi M, Boudvillain M. Phyletic distribution and conservation of the bacterial transcription termination factor Rho. Microbiology. 2013;159:1423–1436. doi: 10.1099/mic.0.067462-0. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale CJ, et al. Termination factor Rho and its cofactors NusA and NusG silence foreign DNA in E. coli. Science. 2008;320:935–938. doi: 10.1126/science.1152763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grylak-Mielnicka A, Bidnenko V, Bardowski J, Bidnenko E. Transcription termination factor rho: A hub linking diverse physiological processes in bacteria. Microbiology. 2016;162:433–447. doi: 10.1099/mic.0.000244. [DOI] [PubMed] [Google Scholar]
- 5.Miloso M, et al. Characterization of the rho genes of Neisseria gonorrhoeae and Salmonella typhimurium. J Bacteriol. 1993;175:8030–8037. doi: 10.1128/jb.175.24.8030-8037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita Y, Narita S, Tomida J, Tokuda H, Kawamura Y. Application of an inducible system to engineer unmarked conditional mutants of essential genes of Pseudomonas aeruginosa. J Microbiol Methods. 2010;82:205–213. doi: 10.1016/j.mimet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Ciampi MS. Rho-dependent terminators and transcription termination. Microbiology. 2006;152:2515–2528. doi: 10.1099/mic.0.28982-0. [DOI] [PubMed] [Google Scholar]
- 8.Bidnenko V, et al. Termination factor Rho: From the control of pervasive transcription to cell fate determination in Bacillus subtilis. PLoS Genet. 2017;13:e1006909. doi: 10.1371/journal.pgen.1006909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman S, Storz G. Bacterial small RNA regulators: Versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol. 2011;3:a003798. doi: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: Expanding frontiers. Mol Cell. 2011;43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner EGH, Romby P. Small RNAs in bacteria and archaea: Who they are, what they do, and how they do it. Adv Genet. 2015;90:133–208. doi: 10.1016/bs.adgen.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Bossi L, Schwartz A, Guillemardet B, Boudvillain M, Figueroa-Bossi N. A role for rho-dependent polarity in gene regulation by a noncoding small RNA. Genes Dev. 2012;26:1864–1873. doi: 10.1101/gad.195412.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedlyarova N, et al. sRNA-mediated control of transcription termination in E. coli. Cell. 2016;167:111–121.e13. doi: 10.1016/j.cell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argaman L, et al. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11:941–950. doi: 10.1016/s0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 15.Silva IJ, Ortega AD, Viegas SC, García-Del Portillo F, Arraiano CM. An RpoS-dependent sRNA regulates the expression of a chaperone involved in protein folding. RNA. 2013;19:1253–1265. doi: 10.1261/rna.039537.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001;15:1637–1651. doi: 10.1101/gad.901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viegas SC, et al. Characterization of the role of ribonucleases in Salmonella small RNA decay. Nucleic Acids Res. 2007;35:7651–7664. doi: 10.1093/nar/gkm916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barik S, Bhattacharya P, Das A. Autogenous regulation of transcription termination factor rho. J Mol Biol. 1985;182:495–508. doi: 10.1016/0022-2836(85)90236-0. [DOI] [PubMed] [Google Scholar]
- 19.Brown S, Albrechtsen B, Pedersen S, Klemm P. Localization and regulation of the structural gene for transcription-termination factor rho of Escherichia coli. J Mol Biol. 1982;162:283–298. doi: 10.1016/0022-2836(82)90527-7. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto Y, Shigesada K, Hirano M, Imai M. Autogenous regulation of the gene for transcription termination factor rho in Escherichia coli: Localization and function of its attenuators. J Bacteriol. 1986;166:945–958. doi: 10.1128/jb.166.3.945-958.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega AD, Gonzalo-Asensio J, García-del Portillo F. Dynamics of Salmonella small RNA expression in non-growing bacteria located inside eukaryotic cells. RNA Biol. 2012;9:469–488. doi: 10.4161/rna.19317. [DOI] [PubMed] [Google Scholar]
- 22.Smirnov A, et al. Grad-seq guides the discovery of ProQ as a major small RNA-binding protein. Proc Natl Acad Sci USA. 2016;113:11591–11596. doi: 10.1073/pnas.1609981113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalaouna D, Carrier MC, Massé E. The shock absorber: Preventing sRNA transcriptional noise. Cell Cycle. 2015;14:2539–2540. doi: 10.1080/15384101.2015.1060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalaouna D, et al. A 3′ external transcribed spacer in a tRNA transcript acts as a sponge for small RNAs to prevent transcriptional noise. Mol Cell. 2015;58:393–405. doi: 10.1016/j.molcel.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Lalaouna D, Massé E. Identification of sRNA interacting with a transcript of interest using MS2-affinity purification coupled with RNA sequencing (MAPS) technology. Genom Data. 2015;5:136–138. doi: 10.1016/j.gdata.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalaouna D, Prévost K, Eyraud A, Massé E. Identification of unknown RNA partners using MAPS. Methods. 2017;117:28–34. doi: 10.1016/j.ymeth.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Kröger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Oshlack A, Robinson MD, Young MD. From RNA-seq reads to differential expression results. Genome Biol. 2010;11:220. doi: 10.1186/gb-2010-11-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opperman T, Richardson JP. Phylogenetic analysis of sequences from diverse bacteria with homology to the Escherichia coli rho gene. J Bacteriol. 1994;176:5033–5043. doi: 10.1128/jb.176.16.5033-5043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washburn RS, Marra A, Bryant AP, Rosenberg M, Gentry DR. Rho is not essential for viability or virulence in Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1099–1103. doi: 10.1128/AAC.45.4.1099-1103.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra P, Ghosh G, Hafeezunnisa M, Sen R. Rho protein: Roles and mechanisms. Annu Rev Microbiol. 2017;71:687–709. doi: 10.1146/annurev-micro-030117-020432. [DOI] [PubMed] [Google Scholar]
- 32.Busch A, Richter AS, Backofen R. IntaRNA: Efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24:2849–2856. doi: 10.1093/bioinformatics/btn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papenfort K, Vanderpool CK. Target activation by regulatory RNAs in bacteria. FEMS Microbiol Rev. 2015;39:362–378. doi: 10.1093/femsre/fuv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastet L, et al. Translational control and Rho-dependent transcription termination are intimately linked in riboswitch regulation. Nucleic Acids Res. 2017;45:7474–7486. doi: 10.1093/nar/gkx434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters JM, et al. Rho directs widespread termination of intragenic and stable RNA transcription. Proc Natl Acad Sci USA. 2009;106:15406–15411. doi: 10.1073/pnas.0903846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavita K, de Mets F, Gottesman S. New aspects of RNA-based regulation by Hfq and its partner sRNAs. Curr Opin Microbiol. 2018;42:53–61. doi: 10.1016/j.mib.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueroa-Bossi N, et al. RNA remodeling by bacterial global regulator CsrA promotes Rho-dependent transcription termination. Genes Dev. 2014;28:1239–1251. doi: 10.1101/gad.240192.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedlyarova N, et al. Natural RNA polymerase aptamers regulate transcription in E. coli. Mol Cell. 2017;67:30–43.e36. doi: 10.1016/j.molcel.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabhi M, et al. The Sm-like RNA chaperone Hfq mediates transcription antitermination at rho-dependent terminators. EMBO J. 2011;30:2805–2816. doi: 10.1038/emboj.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majdalani N, Hernandez D, Gottesman S. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol Microbiol. 2002;46:813–826. doi: 10.1046/j.1365-2958.2002.03203.x. [DOI] [PubMed] [Google Scholar]
- 43.Mandin P, Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29:3094–3107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breaker RR. Riboswitches and the RNA world. Cold Spring Harb Perspect Biol. 2012;4:a003566. doi: 10.1101/cshperspect.a003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. doi: 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollands K, et al. Riboswitch control of Rho-dependent transcription termination. Proc Natl Acad Sci USA. 2012;109:5376–5381. doi: 10.1073/pnas.1112211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goecks J, Nekrutenko A, Taylor J. Galaxy Team Galaxy: A comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010;11:R86. doi: 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viegas SC, Silva IJ, Saramago M, Domingues S, Arraiano CM. Regulation of the small regulatory RNA MicA by ribonuclease III: A target-dependent pathway. Nucleic Acids Res. 2011;39:2918–2930. doi: 10.1093/nar/gkq1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaffl MW. 2004. Quantification strategies in real-time PCR. A-Z of Quantitative PCR, ed Bustin SA [International University Line (IUL), La Jolla, CA] pp 87–112.
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 52.Nadiras C, Schwartz A, Delaleau M, Boudvillain M. Evaluating the effect of small RNAs and associated chaperones on rho-dependent termination of transcription in vitro. Methods Mol Biol. 2018;1737:99–118. doi: 10.1007/978-1-4939-7634-8_7. [DOI] [PubMed] [Google Scholar]
- 53.Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Springs Harbor Laboratory Press; Plainview, NY: 1972. [Google Scholar]
- 54.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.