Abstract
Background
It is estimated that 13% of arthropod species carry the heritable symbiont Cardinium hertigii. 16S rRNA and gyrB sequence divides this species into at least four groups (A–D), with the A group infecting a range of arthropods, the B group infecting nematode worms, the C group infecting Culicoides biting midges, and the D group associated with the marine copepod Nitocra spinipes. To date, genome sequence has only been available for strains from groups A and B, impeding general understanding of the evolutionary history of the radiation. We present a draft genome sequence for a C group Cardinium, motivated both by the paucity of genomic information outside of the A and B group, and the importance of Culicoides biting midge hosts as arbovirus vectors.
Methods
We reconstructed the genome of cCpun, a Cardinium strain from group C that naturally infects Culicoides punctatus, through Illumina sequencing of infected host specimens.
Results
The draft genome presented has high completeness, with BUSCO scores comparable to closed group A Cardinium genomes. Phylogenomic analysis based on concatenated single copy core proteins do not support Cardinium from arthropod hosts as a monophyletic group, with nematode Cardinium strains nested within the two groups infecting arthropod hosts. Analysis of the genome of cCpun revealed expansion of a variety of gene families classically considered important in symbiosis (e.g., ankyrin domain containing genes), and one set—characterized by DUF1703 domains—not previously associated with symbiotic lifestyle. This protein group encodes putative secreted nucleases, and the cCpun genome carried at least 25 widely divergent paralogs, 24 of which shared a common ancestor in the C group. The genome revealed no evidence in support of B vitamin provisioning to its haematophagous host, and indeed suggests Cardinium may be a net importer of biotin.
Discussion
These data indicate strains of Cardinium within nematodes cluster within Cardinium strains found in insects. The draft genome of cCpun further produces new hypotheses as to the interaction of the symbiont with the midge host, in particular the biological role of DUF1703 nuclease proteins that are predicted as being secreted by cCpun. In contrast, the coding content of this genome provides no support for a role for the symbiont in provisioning the host with B vitamins.
Keywords: Cardinium hertigii, Culicoides biting midges, Genome sequence, Phylogenomic analysis, Gene family expansion, Heritable symbionts
Introduction
Invertebrates form a diverse range of symbiotic associations with heritable bacteria, microbes that pass from a female to her progeny. Ranging from less-intimate to highly specialized, these associations can confer novel phenotypic traits on their individual host, and thus may represent major drivers of both ecological and evolutionary dynamics (McLean et al., 2016; Sudakaran, Kost & Kaltenpoth, 2017; Ferrari & Vavre, 2011). Heritable bacteria can supplement the nutritionally imbalanced diet of hematophagous or sap feeding species with vitamins or essential amino acids, thus expanding the niche of the species (Rio, Attardo & Weiss, 2016; Hansen & Moran, 2014). Other symbionts exert protective effects against biotic or abiotic stress, including natural enemies (predators, parasitoids, fungi, bacteria, and viruses) (Brownlie & Johnson, 2009; Hansen, Vorburger & Moran, 2012) and heat stress (Dunbar et al., 2007). Notably, some heritable bacteria are parasitic and have evolved to manipulate host reproduction to increase the frequency of infected females and facilitate their own transmission (Hurst & Frost, 2015). These effects have further prompted their application in vector and pest management (Iturbe-Ormaetxe, Walker & O’ Neill, 2011).
Cardinium (Bacteroidetes) is a bacterial genus found in a wide range of arthropod species that has a wide variety of impacts on host individuals, including feminization (Weeks, Marec & Breeuwer, 2001; Groot & Breeuwer, 2006), parthenogenesis induction (Zchori-Fein et al., 2001), and cytoplasmic incompatibility (CI) (Hunter, Perlman & Kelly, 2003; Gotoh, Noda & Ito, 2006; Perlman, Kelly & Hunter, 2008; Ros & Breeuwer, 2009), alongside the capacity to improve host fitness (Weeks & Stouthamer, 2004). First discovered in 1996 (Kurtti et al., 1996), it is now estimated that c. 13% of arthropod species carry the symbiont (Weinert et al., 2015). Cardinium infections are found in a diverse set of arthropods, but its incidence is heterogeneous, with pronounced “hotspots” in arachnids (including spiders, mites, and harvestmen), diaspidid scale insects, parasitoid wasps, planthoppers, whiteflies, and biting midges (Duron et al., 2008; Zchori-Fein & Perlman, 2004; Gruwell, Wu & Normark, 2009; Nakamura et al., 2009; Chang et al., 2010; Morag et al., 2012; Lewis et al., 2014; Mee et al., 2015). Further symbioses are observed with plant parasitic nematodes (Noel & Atibalentja, 2006; Denver et al., 2016), copepods (Edlund et al., 2012), non-marine ostracods (Schön et al., 2018), and oribatid mites (Konecka & Olszanowski, 2018) suggesting that the true diversity of the genus is yet to be appreciated. This wider clade Cardinium represents the sister group to the amoeba symbiont Amoebophilus asiaticus (Nakamura et al., 2009; Schmitz-Esser et al., 2010; Santos-Garcia et al., 2014).
Phylogenetic analyses of Cardinium based on two gene sequences (16S rRNA and gyrB) inferred the existence of at least four monophyletic groups designated as A, B, C, and D (Nakamura et al., 2009; Edlund et al., 2012), resembling Wolbachia super-groups in terms of host-affinities (Lo et al., 2002). Group A is the largest and the most studied of the three groups and has been found in various arthropod species. Group B has been found in plant parasitic nematodes (Noel & Atibalentja, 2006; Denver et al., 2016) and is represented by Cardinium strains cHgTN10, an endosymbiont of the soybean cyst nematode Heterodera glycines (Noel & Atibalentja, 2006) and cPpe, an endosymbiont of the plant parasitic nematode Pratylenchus penetrans (Brown et al., 2018). Group C consists of a phylogenetically distinct clade of Cardinium strains known only from species of Culicoides biting midges, an important group of hematophagous pests and vectors of arboviruses and parasites (Nakamura et al., 2009; Morag et al., 2012; Lewis et al., 2014; Mee et al., 2015). Finally, group D have been found as a constituent of the bacterial communities associated with the marine copepod Nitocra spinipes (Edlund et al., 2012).
To date, genomic characterization has been restricted to A and B group Cardinium strains. Three insect-associated A-group Cardinium strains have been sequenced. These include the CI-inducing Cardinium endosymbiont (cEper1) of the parasitic wasp Encarsia pergandiella (Penz et al., 2012), the Cardinium endosymbiont (cBtQ1) of the whitefly Bemisia tabaci (Santos-Garcia et al., 2014) and the Cardinium endosymbiont (cSfur) of the planthopper Sogatella furcifera (Zeng et al., 2018). These genome sequences have indicated that convergent phenotypes, like CI, have a divergent genetic basis in Cardinium from Wolbachia. Moreover, the cEper1 Cardinium genome suggests the symbiont may supplement B-vitamin provision (Penz et al., 2012), a phenotype that would be important in bloodsucking vectors. More recently, the genome sequences for two B group Cardinium strains from nematodes have been completed. These are the genomes of the Cardinium endosymbiont (cHgTN10) from H. glycines (Showmaker et al., 2018) and the Cardinium endosymbiont cPpe from P. penetrans (Brown et al., 2018). However, there is no available genome for the C clade Cardinium, which is particularly notable in the light of the pest and vector status of the host species.
In this paper, we present an annotated draft genome sequence for a Cardinium endosymbiont from clade C, carried by the biting midge Culicoides punctatus, hereafter cCpun, and use this genome data to estimate the relationship between C clade Cardinium and those of A and B groups; improving our understanding of strain relatedness that currently rest on the sequence of two loci. We further use the genome sequence to infer potential aspects of the symbiosis between this microbe and Culicoides biting midges. The study of midge symbionts is important, as the symbiosis may potentially have an impact on the physiology of a bloodsucking host, and (by parallel with Wolbachia) its vector competence for arboviruses and other pathogens. The difficulty of growing midges in insectary culture has presented a challenge to determining the effect of the symbiont on the host experimentally. Analysis of the cCpun genome and comparison to the previously sequenced Cardinium genomes as well as their sister species A. asiaticus (Schmitz-Esser et al., 2010) was therefore undertaken to provide insight into the evolution and life style of clade C Cardinium.
Materials and Methods
Genome sequencing, assembly, and annotation
Culicoides punctatus female midges were collected from Leahurst Campus, University of Liverpool, UK using UV light traps and identified from wing morphology and by cytochrome c oxidase subunit 1 barcoding as in Pilgrim et al. (2017). DNA was extracted from single individuals using the QIAGEN DNAeasy™ Blood & Tissue Kit following the protocol for purification of total DNA from Insect. All samples were tested for Cardinium infection using a PCR assay based on 16S rRNA Cardinium specific primers Car-sp-F 5′-CGGCTTATTAAGTCAGTTGTGAAATCCTAG-3′; Car-sp-R 5′-TCCTTCCTCCCGCTTACACG-3′ (Nakamura et al., 2009). Whole-genome sequencing was carried out by the Centre for Genomic Research, University of Liverpool using the Illumina TruSeq Nano library preparation protocol. Two short-insert (∼550 bp insert size) paired-end libraries were constructed from two pooled DNA samples of three individuals each. The libraries were multiplexed and sequenced using 2/3 of a lane on an Illumina HiSeq 2500 platform, yielding 2 × 125 bp paired reads. Adapter removal and quality trimming of the raw Illumina reads were performed with Cutadapt version1.2.1 (Martin, 2011) and Sickle version 1.2 (Joshi & Fass, 2011).
Identification and filtering of symbiont reads were performed using a similar approach to that used previously (Pilgrim et al., 2017). Briefly, a preliminary assembly of the quality trimmed dataset was performed using SPAdes version 3.7.0 (Nurk et al., 2013) using the following parameters (-k 21,33,55,77, –careful, –cov-cutoff 5). The initial contigs were visualized using taxon-annotated GC-coverage plots (Fig. S1) with Blobtools (Kumar et al., 2013; Laetsch, 2016). Additional tblastx searches (Altschul et al., 1997; Camacho et al., 2009) were conducted against a local genomic database consisting of Cardinium genomes—cBtQ1 and cEper1 endosymbionts of the whitefly B. tabaci and the parasitic wasp E. pergandiella, respectively (Santos-Garcia et al., 2014; Penz et al., 2012), that of Cardinium strain cHgTN10 from H. glycines (Showmaker et al., 2018) and the more distantly related Acanthamoeba endosymbiont A. asiaticus (Schmitz-Esser et al., 2010)—with an e-value cut-off of 1e−6. Cardinium contigs were extracted and checked for contamination by blastx searches against the non-redundant (nr) protein database. Cardinium-specific reads were subsequently retrieved using Bowtie2 (Langmead & Salzberg, 2012) and samtools (Li et al., 2009) and re-assembled de novo using SPAdes as described above. All contigs larger than 500 bp were checked for potential host or other bacteria contamination using blastx searches against nr database and all contaminant contigs were removed from the final assembly. Subsequently, we evaluated the quality of the assembled contigs using the reference-free assembly validation tool REAPR (Hunt et al., 2013). REAPR uses read pairs mapping information to identify potential assembly errors and assign quality scores on each base of the assembly. The error calls were then used to break the pre-assembled contigs at every potential miss-assembly position using the aggressive option “-a.” Finally, the broken assembly was scaffolded using SSPACE (Boetzer et al., 2011) using the default parameters.
The cCpun draft genome was annotated using Prokka version 1.12 (Seemann, 2014) and the completeness was assessed using BUSCO v3 based on the presence of 148 universal bacterial marker genes (Simão et al., 2015). Clusters of Orthologous Groups (COG) functional categories were assigned using the eggNOG database (Huerta-Cepas et al., 2016) while additional domains were assigned by searches against the Pfam protein database (Finn et al., 2016). The k-mer fraction of the filtered reads were computed with Jellyfish v2.2.3 (Marçais & Kingsford, 2011) and used to determine the repeat fraction of cCpun genome using GenomeScope (Vurture et al., 2017). Finally, comparison of the repeat density (repeats ≥ 200 bp and at least 95% identity) between the Amoebophilaceae genomes was performed using MUMmer-plots (Kurtz et al., 2004).
Ortholog identification, comparative, and phylogenetic analyses
The genome sequences of the three available arthropod-associated Cardinium strains Cardinium hertigii cEper1 (Penz et al., 2012), Cardinium hertigii cBtQ1 (Santos-Garcia et al., 2014) and Cardinium cSfur (Zeng et al., 2018), the two nematode-associated endosymbionts cHgTN10 and cPpe (Showmaker et al., 2018; Brown et al., 2018) and the Acanthamoeba endosymbiont A. asiaticus (Schmitz-Esser et al., 2010) were obtained from GenBank and used for comparative analyses (accession numbers GCF_000304455.1, GCF_000689375.1, GCA_003351905.1, GCA_003176915.1, and GCF_000020565.1, respectively). The genomes of Cyclobacterium marinum DSM 745 (GCF_000222485.1) and Marivirga tractuosa DSM 4126 (GCF_000183425.1), two free living Bacteroides species, were used as outgroup for the phylogenetic analyses (based on Santos-Garcia et al., 2014). All GenBank retrieved genomes were re-annotated using Prokka software as described above in order to mitigate the effect of inconsistencies due to alternative annotation practices. Orthologous groups of proteins were identified between cCpun, cEper1, cBtQ1, cSfur, cHgTN10, cPpe, and A. asiaticus using an all-vs-all BLAST search and Markov Cluster (MCL) clustering approach as implemented in OrthoFinder method (Emms & Kelly, 2015). Core, accessory and strain-specific orthogroups between the five genomes were visualized with an UpSet plot using the UpSetR package (Conway et al., 2017).
Phylogenetic reconstruction was performed on a set of 278 single copy core protein sequences shared between the six Cardinium genomes, the genome of A. asiaticus and two free living Bacteroides species (Cyclobacterium marinum and M. tractuosa) that were used as outgroup. To this end, a super-matrix was generated by concatenating the protein alignments of the 278 core proteins and trimmed with trimAl version 1.4 (Capella-Gutiérrez, Silla-Martínez & Gabaldón, 2009) using the “automated” option. The best substitution model (LG+F+R4) was selected using ModelFinder (Kalyaanamoorthy et al., 2017) and phylogenetic inference was performed using the maximum likelihood (ML) criterion as implemented in IQ-TREE v1.6.6 (Nguyen et al., 2015). The robustness of the inferred tree was finally assessed with the ultrafast bootstrap approximation method as implemented in IQ-TREE using 1,000 replicates (Hoang et al., 2018). Alternative phylogenetic hypotheses were tested by constrained tree searches using the approximately unbiased (AU) test (Shimodaira & Goldman, 2002) as implemented in IQ-TREE v1.6.6. Additionally, the distribution of the phylogenetic signal across the concatenated super-matrix was calculated as described in (Shen, Hittinger & Rokas, 2017). Briefly, for each of the 278 core protein alignments the log-likelihood score for the best ML tree topology under concatenation and an alternative conflicting topology was calculated under the same substitution model (LG+F+R4). The difference in the gene-wise log-likelihood scores (ΔGLS) between the two alternative topologies was used as a measure of the phylogenetic signal and to visualize the proportion of core genes supporting each conflicting phylogeny. Finally, an independent phylogenetic analysis was performed on a subset of 46 core ribosomal proteins in IQ-TREE v1.6.6 as described above in order to further test the robustness of our phylogenetic inference. Phylogenetic trees were drawn and annotated online using the EvolView tool (He et al., 2016).
Analyses of the DUF1703 gene family expansion
Genome analysis revealed an expansion of the DUF1703 gene family. To analyze this expansion further, a protein sequence alignment of the DUF1703 gene family from Cardinium together with selected Open Reading Frames (ORFs) with sequence similarity retrieved as best BLAST hits form NCBI’s nr database was performed using MAFFT v7 and default parameters (Katoh & Standley, 2013). Ambiguously aligned positions were subsequently removed using trimAl version 1.4 and the “automated” option. A ML phylogenetic analyses was performed with IQ-TREE version 1.6.6 and the phylogenetic tree were constructed and annotated as described above. Additionally, a neighbor-net phylogenetic network was inferred from the translated nucleotide alignment of the cCpun DUF1703 paralogs using SplitsTree version 4.12.6 (Huson & Bryant, 2006; Bryant & Moulton, 2004) and default parameters. A pairwise identity and similarity matrix of the cCpun DUF1703 amino acid sequence paralogs were constructed using the Needleman–Wunsch global alignment method and the BLOSUM62 substitution matrix as implemented in EMBOSS package (Rice, Longden & Bleasby, 2000). Putative signal peptides were predicted on the SignalP 4.1 Server (Petersen et al., 2011) using the sensitive D-cutoff settings. Detection of putative recombination events was performed using the RDP4 software package (Martin et al., 2015). Recombination Detection Program (RDP) implements several methods for detecting recombination signals including MaxChi (Smith, 1992), GENECONV (Padidam, Sawyer & Fauquet, 1999), BottScan (Salminen et al., 1995), Chimera (Posada & Crandall, 2001), and RDP (Martin & Rybicki, 2000). Global parameters were as follow: P-value cutoff was set to 0.001 using a Bonferroni correction and significance was evaluated from a permutation test based on 1,000 permutations. Detected signals were considered significant only when they were confirmed by multiple methods. Inference of recombination signals can be particularly misleading when diverse sequences are analyzed. To avoid such misalignment artefacts, the 25 complete DUF1703 paralogs were grouped into three groups on the bases of nucleotide sequences similarity (>65%) and the analyses was repeated for each group separately. Finally, the results were also confirmed with PhiPack implementing the pairwise homoplasy index (PHI) algorithm (Bruen, Philippe & Bryant, 2006). Residue composition and conservation within the core nuclease PD-(D/E)XK site of the DUF1703 homologs were illustrated with sequence logos using the Skylign tool (Wheeler, Clements & Finn, 2014).
Nucleotide sequence accession numbers
The raw reads and the cCpun draft genome assembly have been submitted to the DDBJ/EMBL/GenBank database under the BioProject accession number PRJNA487198 (WGS project QWJI00000000).
Results and Discussion
General features of cCpun draft genomes
The final assembly of the cCpun draft genome consists of 57 scaffolds larger than 500 bp (N50 = 41.6 kb, largest scaffold = 116 kb) comprising a total size of 1,137,634 bp (52 scaffolds ≥ 1,000 bp) with an average GC content of ∼33% and an average depth of coverage 90× (Table 1; Fig. S2). Overall, the cCpun genome shares many characteristics with those of the previously sequenced Cardinium strains cEper1, cBtQ1, cSfur, cHgTN10, and cPpe including similar genome size of around one Mb and comparable GC content (33.7–38%) (Table 1). No plasmids were inferred based on the presence of scaffolds with atypically higher read coverage compared with the average coverage of the complete assembly, presenting a contrast to the previously sequenced arthropod-associated Cardinium (cEper1 and cBtQ1) (Table 1; Fig. S2). Nevertheless, we were able to detect several regions with sequence similarity to elements of the two plasmids found in cEper1 and cBtQ1. Matching regions were mainly transposases, suggesting that these might be remnants of ancestral plasmid invasion/s. Although absence of plasmids has also been reported previously for A. asiaticus, the sister species of Cardinium clade (Schmitz-Esser et al., 2010), the presence of low-copy-number plasmids in cCpun cannot be ruled out.
Table 1. Genome Features of cCpun draft genome and its closest relatives.
| cCpun | cEper1** | cBtQ1** | cSfur | cHgTN10 | cPpe | A. asiaticus | |
|---|---|---|---|---|---|---|---|
| Number of scaffolds | 57* | 1 | 11 | 1 | 1 | 27 | 1 |
| Plasmids | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Total size in kb | 1,137 | 887 (58) | 1,013 (52) | 1,103 | 1,193 | 1,358 | 1,884 |
| GC content (%) | 33.7 | 36.6 (31.5) | 35 (32) | 39.2 | 38.2 | 35.8 | 35 |
| CDS | 917 | 841 (65) | 709 (30) | 795 | 974 | 1,131 | 1,557 |
| Avg. CDS length (bp) | 993 | 911 (733) | 1,033 (1,389) | 1,052 | 997 | 941 | 990 |
| Coding density (%) | 80 | 85.5 (82.1) | 79.7 (80.1) | 75.7 | 81.4 | 78.3 | 81.8 |
| rRNAs | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| tRNAs | 37 | 37 | 35 | 35 | 37 | 34 | 35 |
| Ankyrin repeat proteins | 46 | 18-19 | 26 | 29 | 27 | 32 | 54 |
| Reference | this study | a | b | c | d | e | f |
Notes:
contigs > 500 bp.
chromosome (plasmid).
A total of 917 protein coding genes were identified with an average length of 993 bp corresponding to a coding density of around 80% (Table 1; Table S1). cCpun harbors a single set of rRNA genes with the 16S separated from 5S and 23S and encode a complete set of 37 tRNA genes. The identification of 117 out of the 148 BUSCO marker genes (BUSCO score = C: 79% (S: 79%, D: 0%), F: 2.7%, M: 18.2%, n: 148) (Fig. S3) was comparable to that observed for the previously sequenced and complete cEper1 cSfur and cHgTN10 genomes, which suggests that cCpun is a near complete genome. Overall, the redundancy in cCpun as assessed through MUMmer-plots is lower than both A. asiaticus and cBtQ1 previously described as highly repetitive (Santos-Garcia et al., 2014) (Fig. S4). K-mer frequency analysis of the Illumina reads estimated the repetitive fraction of cCpun genome to be circa 13%.
Phylogenomic analyses place cCpun as an outgroup of both other insect and nematode Cardinium strains
Recently, a new family named Amoebophilaceae was proposed to include the Cardinium clades as well as the amoeba-associated A. asiaticus (Santos-Garcia et al., 2014). Currently, at least four major phylogenetic clades of Cardinium related bacteria have been described (Nakamura et al., 2009; Edlund et al., 2012) with possible evidence for additional clades (Chang et al., 2010). However, the phylogenetic (evolutionary) relationships between these clades are not clear. Previous phylogenetic studies based on partial 16S rRNA and gyrB sequences failed to provide a consistent phylogenetic placement for the arthropod and the nematode Cardinium clades (Morag et al., 2012; Nakamura et al., 2009).
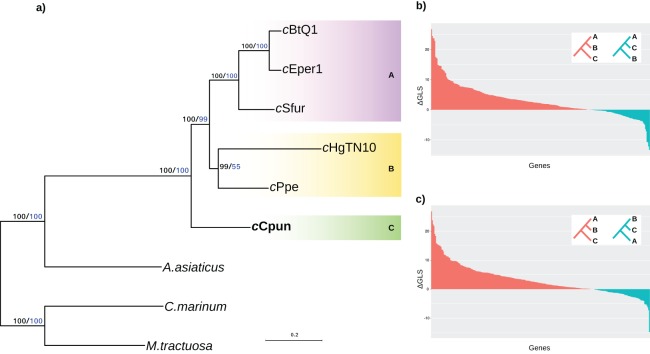
We established the relationship of this group across a concatenated set of 278 single copy core protein coding genes as well as a subset of 46 ribosomal protein genes shared between the seven Amoebophilaceae genomes. The results of both analyses clearly support the position of the midge Cardinium clade (C) as a sister group to both the other arthropod and nematode Cardinium clades (clades A and B) and confirm that the arthropod-associated Cardinium do not form a monophyletic group (Fig. 1A). Constrained tree tests for two alternative topologies (a) nematode Cardinium as sister group of all other arthropod Cardinium and (b) cCpun and nematode Cardinium as a monophyletic group resulted in significantly worse trees (AU test, p < 0.01). This inference was further supported by analysis of single protein phylogenies (Figs. 1B and 1C). A total of 157 out of the 278 single copy core genes (56%) support the monophyletic grouping of the B group Cardinium strains (cHgTN10, cPpe) with the A group (cEper1, cBtQ1 and cSfur) in exclusion of cCpun (p < 0.001, Fisher’s exact test). In contrast, only 68 genes (24%) support the monophyletic grouping of cCpun with the A group strains while a small subset of genes (n = 52; 19%) supports the monophyletic grouping of cCpun with cHgTN10 and cPpe.
Figure 1. Phylogenetic relationships of Cardinium strains.
(A) The phylogenetic tree was inferred from the concatenated analysis of 278 single copy core proteins and separately from a subset of 46 core ribosomal proteins using the Maximum Likelihood method as implemented in IQTRE v1.6.6 (model: LG+F+R4). Both datasets retrieved the same tree topology and here we present only the first one. The numbers on the branches represent support values based on 1,000 bootstrap replicates (black bold values: complete matrix; blue values: ribosomal dataset). The three major Cardinium groups A, B, and C are denoted with different color shading. Cyclobacterium marinum and Marivirga tractuosa, two free living members of Bacteroidetes were used as outgroups. (B, C) Distribution of the phylogenetic signal in Cardinium concatenated ML phylogeny. The gene-wise differences in log-likelihood scores (ΔGLS) between the concatenated Maximum likelihood tree in (A) versus two alternative topologies: A,C-groups monophyletic relative to B-group (B) and B,C-groups monophyletic relative to A-group (C) were calculated as described in (Shen, Hittinger & Rokas, 2017) and plotted in descending order. The red bars represent the genes supporting the Maximum likelihood tree while the blue bars represent the genes supporting each of the alternative topologies.
Genome content comparisons estimate both a core Cardinium genome, genes associated with an insect-symbiont lifestyle, and cCpun specific genes and gene families
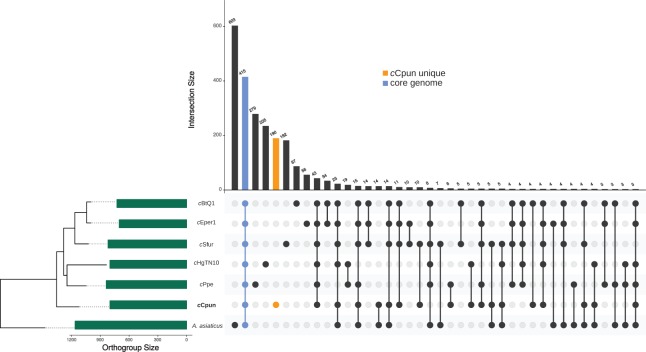
The OrthoFinder clustering algorithm identified a total of 2,015 ortholog protein clusters across the seven Amoebophilaceae genomes (A. asiaticus, cHgTN10, cPpe, cCpun, cEper1, cSfur, and cBtQ1). The seven genomes share a core of 415 ortholog clusters of which 278 consist of single-copy genes (Fig. 2). The cCpun genome codes for a substantial number of unique proteins (Fig. 2; Table S2). Specifically, among the 812 ortholog clusters predicted for cCpun, 190 clusters—including 204 protein coding genes—were assigned as strain-specific (Fig. 2). Of these genes, 40 were predicted to code for proteins of less than 70 amino acids and likely represent either annotation artefacts or pseudogenised gene fragments.
Figure 2. Genome content comparison across the seven Amoebophilaceae genomes.
UpSet plot showing unique and overlapping protein ortholog clusters across the seven Amoebophilaceae genomes cCpun, cEper1, cBtQ1, cSfur, cHgTN10, cPpe, and Amoebophilus asiaticus. The intersection matrix is sorted in descending order. Green bars represent the orthogroup size for each genome ordered by their phylogenetic relationships. Connected dots represent intersections of overlapping orthogroups while vertical bars shows the size of each intersection. The core orthogroup and the cCpun unique orthogroup cluster are shown with the blue and the orange bars respectively. The plot was generated using UpSetR package in R (Conway et al., 2017).
The majority of cCpun specific proteins, 138 (∼67%), had neither significant matches (blastp, e-value ≤ 10−10) in the NCBI-nr database, nor predicted functional domain. These were assigned as hypothetical proteins. Amongst the remaining 66 predicted cCpun-specific protein clusters, those with ankyrin-repeat domains were particularly well represented in the strain specific set (Table S2). The abundance, diversity and presumably eukaryotic origin ANK repeat containing proteins has long led them to be considered likely to be involved in symbiotic interactions, and this has been demonstrated in a few cases (Siozios et al., 2013; Nguyen, Liu & Thomas, 2014; Voth, 2011; Pan et al., 2008). A total of 46 ANK repeat proteins were present in the cCpun genome, which represents the largest expansion of this gene family in Cardinium, comparable to the expansion of this family in A. asiaticus (54 ANK proteins) (Schmitz-Esser et al., 2010). In total, 20 out of the 46 ankyrin repeat-containing proteins identified in cCpun were not found in the other Cardinium strains, suggesting potential host-specific functions. Among the remaining strain-specific protein clusters, 13 were assigned as putative mobile elements (transposases), three putative transporters, a DNA repair protein RecN, two putative GNAT-family acetyltransferases and a homologue of the hemolysin transporter protein ShlB (Table S2). Finally, a folylpolyglutamate synthase (FolC) homologue involved in the tetrahydrofolylpolyglutamate biosynthesis pathway and a putative riboflavin biosynthesis protein RibBA were also detected. Absence of the complete pathway for the de novo biosynthesis of folate in cCpun suggest that FolC probably participates in the folate salvage pathway (folate to polyglutamate) as suggested also by the presence of a dihydrofolate reductase homologue (De Crécy-Lagard et al., 2007).
Candidate proteins related to the adaptation of Cardinium to arthropod hosts (as opposed to Amoeba and nematode) were identified as being in the four arthropod-associated Cardinium strains (cCpun, cSfur, cEper1, and cBtQ1), and not Amoebophilus and the nematode-associated Cardinium strains (cHgTN10 and cPpe). The four strains from whitefly, wasp, planthopper and midge uniquely share 11 ortholog protein clusters (Fig. 2). Among them we observed the virulence-associated E family protein previously detected in the plasmids harbored by cEper1 and cBtQ1 (Penz et al., 2012; Santos-Garcia et al., 2014) and a nicotinamide mononucleotide transporter.
cCpun possesses both afp-like and type IX secretion systems
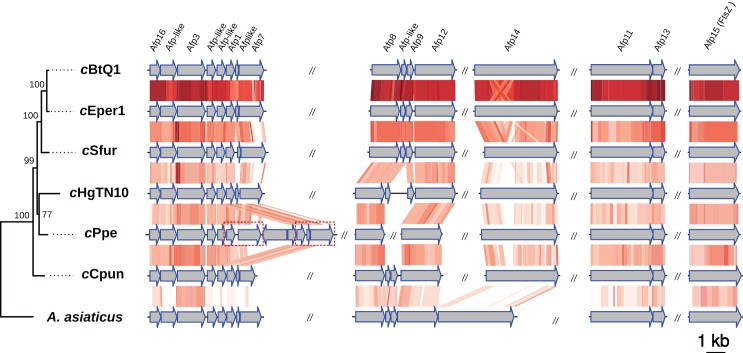
Intracellular microbes utilize a variety of specialized protein secretion systems in order to invade and interact with their eukaryote host (Tseng, Tyler & Setubal, 2009; Dale & Moran, 2006). A common characteristic of the Amoebophilaceae genomes is that all encode for a putative afp-like protein secretion system presumably involved in host-microbe interactions (Penz, Horn & Schmitz-Esser, 2010; Penz et al., 2012; Hurst et al., 2007). This system was also observed in the cCpun genome (Fig. 3) (Penz, Horn & Schmitz-Esser, 2010; Penz et al., 2012; Santos-Garcia et al., 2014). The organization of the AFP-like genes clusters is conserved between the four Amoebophilaceae genomes and suggests operon-like structures (Fig. 3).
Figure 3. Organization and comparison of the antifeeding prophage (Afp-like) genes clusters in the seven Amoebophilaceae genomes.
The phylogeny of the Afp-like secretion system was inferred with Maximum Likelihood based on the concatenated alignment of the 15 constituent protein sequences using IQTREE v1.6.6. Conserved regions are connected with a gradient of red shadings based on tblastx identities. The dash-line rectangles denote a duplicated region in cPpe strain described in Brown et al. (2018). The synteny and the phylogenetic tree of the Afp-like gene clusters were visualized using the genoPlotR package (Guy, Roat Kultima & Andersson, 2010).
We additionally identified seven components of the type IX secretion system (T9SS) in cCpun, a system related to gliding motility and pathogenicity in several members of the phylum Bacteroidetes (McBride & Zhu, 2013; McBride & Nakane, 2015). cCpun is the third Cardinium strain reported to retain components of the T9SS system (Santos-Garcia et al., 2014; Zeng et al., 2018). Four of these protein clusters with homology to the core components of the T9SS (GldK, GldL, GldM, GldN) are shared between cCpun, A. asiaticus, cBtQ1, and cSfur while an additional three proteins with homology to the lipoproteins GldD, GldJ, and GldH are uniquely shared between cCpun and A. asiaticus with exception the GldJ which was also found in the cSfur genome in two identical copies (Table S3). More recently, core components of the T9SS secretion system were found on the plasmid of Cardinium cBtQ1 (Santos-Garcia et al., 2014).
Originally described in Flavobacterium johnsoniae, the T9SS is unique among the phylum Bacteroidetes having important role in secretion of proteins involved both in gliding motility and pathogenicity (McBride & Nakane, 2015; Sato et al., 2010). The presence of the Gld homologs in cCpun as well as A. asiaticus supports an ancestral origin of the T9SS machinery which was subsequently lost from cEper1 and the nematode clade (cHgTN10 and cPpe). The functional role of the T9SS components in Cardinium is unknown. The gene set identified as present in the clade is small compared to that known for active T9SSs (which may have more than 18 components). The low number of genes identified may either reflect co-option of other (unidentified) genes into the secretion process, or a function outside of secretion. However, it is tempting to speculate that the T9SS machinery in Amoebophilaceae has progressively been replaced by the AFP-like protein secretion system. This hypothesis is supported by the complete absence of Gld homologs in both cEper1 and the nematode strains, which suggests that the T9SS is dispensable and likely undergoing gradual loss due to genome reduction processes (Toft & Andersson, 2010).
The cCpun genome contains an expansion of the DUF1703 gene family
Expansion and contraction of gene families in microbial genomes constitute a major source of both genetic and functional novelty, contributing to their adaptation to changing environments (Bratlie et al., 2010). Despite a tendency for evolution to eliminate redundancy and streamline genomes, endosymbiotic bacteria and intracellular pathogens often contain multi-gene families. Interestingly, the majority of the expanded gene families in these host-associated microbes encode putative effector proteins enriched in eukaryotic domains including ANK, LRR, and TPR repeats, F-box and U-box domains (Domman et al., 2014; Wu et al., 2004; Siozios et al., 2013; Schmitz-Esser et al., 2010).
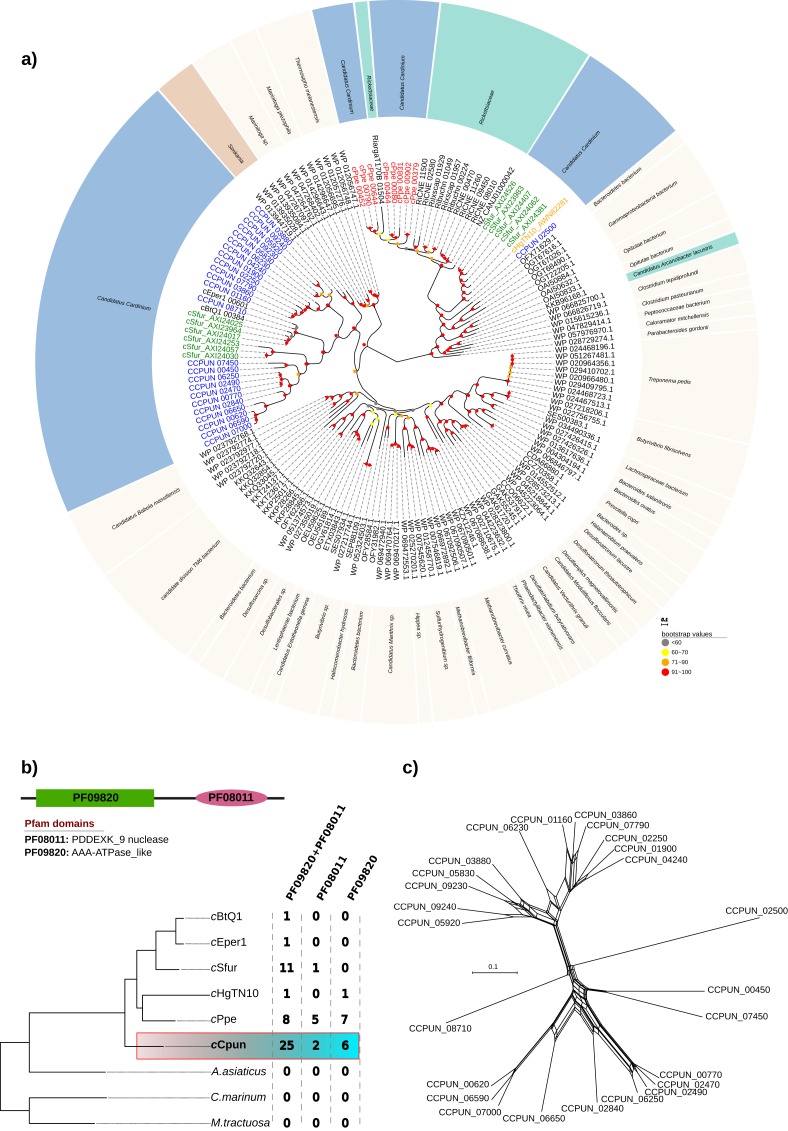
Inspection of the cCpun genome revealed the presence of an expansion of hypothetical proteins related to the DUF1703 protein family (Knizewski et al., 2007) not previously observed in other Cardinium genomes, or other heritable microbes. A total of 25 gene paralogs coding for hypothetical proteins of this family were identified (Fig. 4). The DUF1703 family contains a group of modular proteins consisting of an N-terminal AAA-ATPase like domain (Pfam ID: PF09820) and a C-terminal PDDEXK_9 nuclease domain (Pfam ID: PF08011). In addition to the 25 paralogs, six genes were found to contain only the AAA-ATPase like domain whilst two genes contained only the nuclease domain (Fig. 4B). All partial genes were detected near the borders of the cCpun scaffolds and may be artefactually truncated. Our estimate of gene family size is thus conservative.
Figure 4. DUF1703 expansion in cCpun genome.
(A) Phylogenetic analysis of the cCpun DUF1703 gene family. The unrooted phylogeny was inferred using maximum likelihood from the amino acid sequences of 156 DUF1703 homologs using IQ-TREE v1.6.6 (method: automated best model selection). Cardinium, Simkania, and Rickettsia homologs are shaded in blue, red, and green respectively. (B) The unique expansion of cCpun DUF1703 gene family within the Amoebophilaceae. (C) Phylogenetic network showing the reticulated evolution of the cCpun DUF1703 paralogs.
The members of the DUF1703 gene family display in cCpun are diverse, as attested by an average amino acid identity of just 39% amongst members (Fig. S5). This extensive divergence of paralogs suggests that the expansion of this gene family is not recent. Moreover, the pairwise comparison suggest at least three main expansion waves (Fig. S5). Phylogenetic analysis indicates that all but one of the Cardinium cCpun DUF1703 carrying protein sequences form a single cluster closely related to those found in Simkania, an intracellular bacterium member of Chlamidiales known to be associated with protozoa (Fig. 4A). Notably, two of the simkania’s paralogs are encoded on its pSn plasmid, suggesting possible roots for horizontal dissemination of the DUF1703 genes. The exception is the gene CCPUN_02500, which forms a distinct group with homologs identified in Cardinium strain cSfur and the only intact DUF1703 carrying homolog in cHgTN10, and which is closely related to homologs found in Rickettsia and metagenomically-recovered sequences belonging to uncultured members of the Bacteroidetes and Gammaproteobacteria (Anantharaman et al., 2016).
Although larger, the expansion of the DUF1703 gene family is not unique to the cCpun genome. Amongst the most recently sequenced Cardinium genomes (cSfur and cPpe) we identified smaller expansions of the DUF1703 family (Figs. 4A and 4B). In contrast, the genomes of cEper1, cBtQ1, and cHgTN10 contain only a single gene homolog whilst no homologs were detected in A. asiaticus or free-living relatives (Fig. 4B). Reconstruction of the phylogenetic relationships between the homologs clearly show that members from the same organism group together suggesting that independent expansions took place after divergence from the common ancestor. Surprisingly, the eight paralogs identified in cPpe genome are more closely related to their Rickettsia counterparts than the rest of the Cardinium homologs, indicating possible independent acquisition. Our results suggest that the DUF1703 genes have probably originated in Cardinium after they diverged from A. asiaticus, presumably by horizontal gene transfer (HGT) with later expansion in the lineage leading to cCpun, cSfur, and cPpe.
Phylogenetic network analyses revealed several reticulation events within the DUF1703 gene family in cCpun indicating frequent recombination among gene family members (Fig. 4C). We further investigated the extent of recombination using different methods implemented in RDP4 software (Martin et al., 2015). Due to the limited sequence similarity between the members of the DUF1703 family we restricted our analyses to group of sequences sharing at least 65–70% nucleotide similarities since misalignment artefacts can confound the identification of true recombination signals. We detected evidence of intragenic recombination in all examined groups with multiple methods (Table S4) suggesting that DUF1703 paralogs in cCpun readily recombine. Despite the extensive recombination, no apparent homogenization between the members of this gene family is observed as suggested by the limited sequence similarity and the absence of monophyletic clustering of cCpun paralogs. Overall, our results point to a HGT scenario for the origin of Cardinium DUF1703 gene family with subsequent expansion in the cCpun genome, and variation produced both by mutation and recombination.
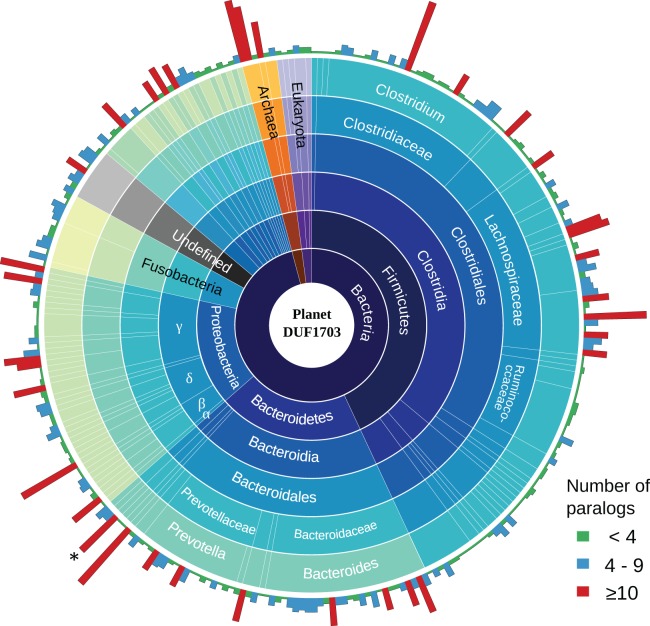
To gain a better insight into the role of DUF1703 proteins we sought to investigate the distribution and abundance of proteins containing the AAA-ATPase and PDDEXK_9 domains in other prokaryotes and eukaryotes. We searched the Pfam database for protein sequences containing the two domains and exhibited similar architecture with Cardinium homologs. In most cases, DUF1703 containing genes occurred in low copy number per genome. Most species carried fewer than four copies whilst only 9.8% of the species contained 10 copies or more (Fig. 5), ranking cCpun among the species with the largest number of DUF1703 paralogs. Species with higher abundance of DUF1703 paralogs are scattered across the prokaryotic taxonomy suggesting that DUF1703 protein expansion has occurred on multiple occasions within bacteria.
Figure 5. Planet DUF1703.
Abundance and taxonomic distribution of DUF1703 proteins in PFAM database. *: cCpun genome. The graph was constructed using Circos v0.69 (Krzywinski et al., 2009).
The reason for the expansion of the DUF1703 gene family in cCpun and its putative functional role is yet unknown. It is notable that DUF1703 genes have been also identified in the Rickettsia endosymbiont infecting biting midges (Pilgrim et al., 2017). Mirroring the pattern for midge Cardinium, the midge Rickettsia genome also contains multiple DUF1703 paralogs compared to other Rickettsia species with evidence of intragenic recombination (p < 0.001, PHI test, 1,000 permutations). However, Cardinium cCpun and Rickettsia DUF1703 carrying genes are phylogenetically unrelated (Fig. 4A) suggesting independent evolutionary histories, and independent expansion of this gene family in the two groups of midge symbionts. These data suggest this gene family may have a particular function in symbiosis with midges.
The biological role of the DUF1703 is still unclear. A recent transcriptomic study of the Cardinium strain cEper1 in its host E. suzannae showed that its only DUF1703 gene homolog is moderately transcribed in both sexes (Mann et al., 2017). Notably, a putative signal peptide cleavage site was predicted for 10 out of 25 DUF1703 paralogs in cCpun (Table S5) suggesting that they are potentially secreted, acting against DNA/RNA outside of the symbiont. Surprisingly, no signal peptides were detected in any of the paralogs identified in cSfur and cPpe (data not shown). It is noteworthy that an intact DUF1703 homolog of bacterial origin has been previously reported as component of the Maternal-Effect Dominant Embryonic Arrest (“MEDEA”) factor, a selfish genetic element reported in Tribolium castaneum (Lorenzen et al., 2008). PD-(D/E)XK nucleases constitute a large and functionally diverse superfamily of proteins which includes among others restriction endonucleases, Holliday junction resolvases, transposases, and DNA repair enzymes (Steczkiewicz et al., 2012). Recently, dual PD-(D/E)XK nuclease domains have been identified in a wide range of toxins from diverse intracellular bacteria (Gillespie et al., 2018; Lindsey et al., 2018). More interestingly, some of these domains have been directly linked with the induction of reproductive parasitism in the form of CI in Wolbachia (Beckmann, Ronau & Hochstrasser, 2017). Structural comparison of the PD-(D/E)XK core nuclease site from Cardinium and Rickettsia DUF1703 homologs and that of the CI-like toxins show considerable differences, especially in the sequence between the catalytic residues (Asp, Glu, and Lys) (Fig. S6). In addition, the AAA-ATPase domain associated with the DUF1703 nuclease is not found in the CI-like toxins of Wolbachia and related proteins (Gillespie et al., 2018) which might suggest these proteins have different functions. The biological role of Cardinium DUF1703 proteins remains to be determined.
Putative horizontal gene transfers as a source of genes in the cCpun genome
Horizontal gene transfer has been previously reported as the source of several genes in A. asiaticus, cEper1, and cBtQ1 (Penz et al., 2012; Santos-Garcia et al., 2014; Schmitz-Esser et al., 2010). Many of the HGT genes were found to be shared with members of the Alphaproteobacteria that have an intracellular lifestyle, especially species within the Rickettsiales order, consistent with HGT within the shared environment of the cell.
In accordance with previous observations of symbiont genomes, our results indicate that HGT has likely shaped the accessory genomes of cCpun (Table 2). The majority of the accessory genes of cCpun for which homologs could be assigned in the database are more similar to corresponding genes of bacterial species outside Bacteroidetes, with a bias to genes within the Proteobacteria having closest sequence similarity (Table 2; Fig. S7). For cCpun-specific genes, closest sequence matches lay within bacterial species known to be associated with other arthropods including Rickettsia and Wolbachia, as well as the amoeba-associated bacteria Candidatus Paracaedibacter acanthamoebae and Candidatus Jidaibacter acanthamoeba (Table 2). Four of these genes clustered with gene sequences from torix group Rickettsia, which are also found in midges. Three of these genes encode putative transposases, and one is a hypothetical protein that in other Rickettsia is located on a plasmid hypothesized to be important in determining the host-symbiont interaction (Gillespie et al., 2015).
Table 2. Example of cCpun genes likely originated from HGTs.
| Gene id | Length (AA) | Annotation | Taxonomy of the Best BLAST hit, (GenBank accession) | E-value | AA identity (%) |
|---|---|---|---|---|---|
| CCPUN_00040 | 308 | Hypothetical protein, putative transposase | Rickettsia endosymbiont of Culicoides newsteadi, (WP_094649760) | 2E-128 | 64 |
| CCPUN_00530 | 328 | Hypothetical protein, putative transposase | Rickettsia endosymbiont of Culicoides newsteadi, (WP_094649760) | 3E-124 | 62 |
| CCPUN_01090 | 346 | Hypothetical protein, putative transposase | Rickettsiales bacterium, (PCJ29205) | 6E-133 | 58 |
| CCPUN_02050 | 379 | Hypothetical protein, putative transposase | Rickettsiales bacterium, (PCJ24349) | 5E-55 | 44 |
| CCPUN_04150 | 328 | Hypothetical protein, putative transposase | Rickettsia endosymbiont of Culicoides newsteadi, (WP_094649760) | 9E-125 | 59 |
| CCPUN_04430 | 297 | Hypothetical protein, putative transposase | Rickettsiales bacterium, (PCJ25778) | 9E-136 | 65 |
| CCPUN_01120 | 218 | Carbonic anhydrase | Lysobacter sp. Root494, (WP_056131435) | 2E-95 | 59 |
| CCPUN_03570 | 551 | DNA repair protein RecN | Rickettsiales bacterium, (PCJ29272) | 2E-175 | 48 |
| CCPUN_03900 | 258 | Hypothetical protein, putative transposase | Candidatus Paracaedibacter acanthamoebae, (WP_038464592) | 3E-114 | 67 |
| CCPUN_06490 | 469 | Arginine/agmatine antiporter | Gammaproteobacteria bacterium 39-13, (OJV90723) | 4E-112 | 43 |
| CCPUN_07910 | 266 | Chromosome-partitioning protein Spo0J | Candidatus Phycorickettsia trachydisci, (WP_106874767) | 9E-101 | 57 |
| CCPUN_07920 | 327 | Sporulation initiation inhibitor protein Soj | Candidatus Phycorickettsia trachydisci, (WP_106874768) | 5E-135 | 62 |
| CCPUN_08840 | 436 | Folylpolyglutamate synthase | Wolbachia pipientis, (WP_010963010) | 0E+00 | 76 |
| CCPUN_08910 | 340 | Hypothetical protein | Rickettsia felis, (WP_039595314) | 2E-155 | 73 |
| CCPUN_03830 | 426 | Hypothetical protein | Aedes aegypti, (XP_001656120) | 2E-60 | 39 |
| CCPUN_08280 | 1,360 | Hypothetical protein | Aedes albopictus, (KXJ68548) | 5E-72 | 27 |
Among the putatively horizontally exchanged gene set were ORFs encoding a carbonic anydrase (CA), an amino acid permease, and a putative chromosome-partitioning protein. Finally, two cCpun-specific genes encoding hypothetical proteins had their closest homologs within Aedes mosquitoes (Table 2; Fig. S7). Notably, the two proteins also have partial similarities with a large ankyrin repeat containing protein (Aasi_1610) previously identified in A. asiaticus (Schmitz-Esser et al., 2010). Although both cCpun proteins had their ten top hits assigned to Aedes sequences, the partial similarities to A. asiaticus suggest that they might be fragments of an Aasi_1610 distant homolog. Note, the number of these genes derived from HGT may be even higher since the majority of the accessory genes did not have any significant matches on the GenBank database, and many of these likely represent HGT events from as yet uncharacterized genomes.
The presence of CAs gene is interesting. Among the Amoebophilaceae, CA homologs were detected only in cCpun, cSfur, and cHgTN10 and not in other Cardinium strains nor A. asiaticus, Notably, the three Cardinium homologs do not form a monophyletic group, with cHgTN10 and cSfur homologs being clustered together and more closely associated with a putative CA previously identified in the Rickettsia endosymbiont previously found in biting midges (Pilgrim et al., 2017) (Fig. S8). Our results suggest that the Cardinium CA homologs have independent evolutionary histories and probably originated from independent horizontal transfer events into the three genomes.
The function of these CAs is not clear. CAs are ancient and ubiquitous multi-class zinc-containing metalloenzymes that catalyze the interconversion of CO2 to bicarbonate (Smith & Ferry, 2000; Smith et al., 1999) and are involved in a variety of biochemical processes including respiration and pH homoeostasis (Gai et al., 2014). Studies have shown that CAs are essential for microbial growth in free living bacteria under ambient air with low levels of CO2 (Mitsuhashi et al., 2003; Merlin et al., 2003; Kusian, Sültemeyer & Bowien, 2002). However, whilst CAs are common in many bacterial groups, they are less commonly observed in the genomes of obligate intracellular bacteria (Ueda, Nishida & Beppu, 2012). Studies suggest that intracellular pathogens may rely on CAs for virulence and survival within the host cell (Valdivia & Falkow, 1997), possibly through regulating the phagosome pH during the infection (Nishimori et al., 2014). An intriguing hypothesis is whether CAs might actually play a role in the survival of Cardinium outside of the host in comparable way to the role of CAs in free living bacteria, and thus facilitating its horizontal transmission. Interestingly, the plant-mediated horizontal transmission of Cardinium bacteria between phloem sap-feeding insects has been previously reported, supporting such a scenario (Gonella et al., 2015).
cCpun lacks a biotin or other B-vitamin biosynthetic pathways, indicating it is unlikely to act as a source of these vitamins to its haematophagous host. Indeed, putative homologs of the complete biotin transport system (BioY: CCPUN_01590, BioM: CCPUN_08370, and BioN: CCPUN_08380) were detected, suggesting that cCpun may depend on external provision of biotin from the host. The presence of a complete biotin transporter gene set contrasts with other Cardinium genomes, which lack these transporters, but may carry complete operons for the synthesis of biotin, lipoeta and pyridoxal 5’-phosphate (vitamin B6) (Penz et al., 2012). Exception is the recently sequenced strain cSfur which encode for both a biotin transport system and a complete operon for biotin synthesis (Zeng et al., 2018).
Conclusions
In the present study, we expanded the current genomic information from Cardinium lineages by presenting a new Cardinium draft genome belonging to the divergent and poorly studied group C. Phylogenomic comparison clearly nests the B group nematode-associated Cardinium symbionts within the clade A and C symbionts derived from insect strains, indicating that inference previously made on the basis of two gene sequences can now be regarded as supported robustly. The lack of monophyly of strains of Cardinium symbiotic with arthropods resembles the pattern for Wolbachia, where nematode Wolbachia strains are nested within a diverse set of arthropod Wolbachia strains (Gerth et al., 2014). Heritable microbes occasionally switching between distant host phyla may be more common than previously considered, with the pattern observed in Wolbachia (nematode and arthropod infections), torix Rickettsia (leech and arthropod lineages) and here in Cardinium.
Comparative genomics also provides some insight into whether the three Cardinium clades consist different species. The assignment of systematic names in symbiotic bacteria has been a controversial field, owing to the intimate association with their hosts and their ability to exchange genetic material. Nakamura et al. (2009) had previously proposed the use of the single species name “Candidatus Cardinium hertigii” to describe the three Cardinium clades (A, B, C) based on morphological similarities and comparable substitutions in the 16S rRNA gene with other symbiotic bacteria. The paucity of Cardinium genomic data and the complete absence of phenotypic information on all but clade-A suggest that is still early to apply an accurate systematic framework. However, the extensive genomic diversity between Cardinium clades suggest that Cardinium clades may be best described as separate species. Future genomic and phenotypic data will allow us to revise the taxonomy within Cardinium lineage.
The presence of Rickettsia alongside Cardinium in midges presents an opportunity to examine whether the genomes show any convergent properties and if HGT has occurred. Comparison of the gene content of the cCpun Cardinium strain with the RiCNE Rickettsia symbiont of Culicoides newsteadi revealed some similarities. Expansion of the DUF1703 gene family and presence of a carbonic anhydrase gene were notable. However, neither case reflects HGT in the intracellular environment of midges, with the same pattern being independently derived. This separate derivation indicates the possession of these genes may be convergent properties, biologically related to symbiotic life in biting midge hosts, rather than HGT within a shared environment.
Finally, our data indicate that the Cardinium symbiont in biting midges is unlikely to serve as a source of B vitamins to its haematophagous host. Contrary to the cEper1 genome, a biotin synthesis system was not observed in the cCpun genome, and indeed the presence of a biotin transporter system indicates the symbiont may in fact be an importer of biotin, and thus a B vitamin sink rather than source. This result perhaps reflects the mixed trophic relationship of biting midges, where larval phases are aquatic and detritivores, and the adult phase either haematophagous (female) or reliant only on sugar sources (males). It is likely that B vitamins are acquired heterotrophically in the larval phase in sufficient quantities such that selection for symbiont-mediated supplementation is low. Given that a major vector species, including Culicoides imicola, harbours Cardinium (Morag et al., 2012), future work should likely focus on their effects on vectorial capacity alongside the putative facilitation of Cardinium-midge interactions from the DUF1703 gene family and carbonic anhydrases.
Supplemental Information
To enhance visualization the scaffolds were size sorted and concatenated into a pseudomolecule. The alternating grey and white strips highlight the scaffold borders. Inwards, the first, second and third circle are colour coded according to COG functional categories and represent a) the complete cCpun protein coding genes, b) the core genes between the four Amoebophilaceae genomes presented in Figure 1A, and c) the cCpun unique genes. In the fourth circle we show the genomic location of the genes coding for the Afp-like (magenta) and type IX (red) secretion systems as well as the DUF1703 gene paralogs. Finally, the two line plots represent genome coverage and GC% content across cCpun genome (1kb sliding window) respectively. An orange line indicates the mean coverage (90X) of the draft assembly.
BUSCO completeness assessment results for cCpun draft genome in comparison to the other Cardinium genomes and A. asiaticus. The Results are based on the presence or absence of 148 single-copy universal bacterial markers.
Mummer self-plots representing sequence repeat density in the seven Amoebaphilaceae genomes. Each dot represent a repeat (red=direct) and (blue=inverted) of at least 200bp and 95% similarity.
a) Multiple sequence alignment of the DUF1703 nuclease domain from Cardinium (blue outline) and Rickettsia (red outline) homologs described in Fig. 4. The core PD-(D/E)XK site is highlighted in yellow and the catalytic Asp, Glu and Lys residues are indicated with an asterisk. b) Residue conservation within the core PD-(D/E)XK site between the DUF1703 nuclease family depicted in (a) and the type II-IV CI-like toxins of Wolbachia and related proteins described in Gillespie et al. (2018). Sequence logos were prepared using the Skylign tool (Wheeler, Clements & Finn, 2014).
Phylogenetic relationships of cCpun HGTs with their closest homologs in the Genbank database was inferred using maximum likelihood with IQ-TREE v1.6.6 (method: automated best model selection). Branch support values are based on 1000 bootstrap replicates.
Maximum likelihood phylogenetic placement of cCpun, cSfur and cHgTN10 carbonic anhydrase (CAs) protein sequences compared with their closest homologs in the Genbank database. Members from the four clades forming the beta-class of CAs are presented. The positions of the Cardinium homologs and the CA homolog identified in Rickettsia (RiCNE) endosymbionds in biting midges are indicated in purple and red respectively. Phylogenetic relationships were inferred using IQ-TREE v1.6.6 (method: automated best model selection).
Acknowledgments
The sequencing was carried out at the Centre for Genomic Research, University of Liverpool, United Kingdom. We would like to thank Kenneth Sherlock and Dr Georgette Kluiters for their support with the collection of midge samples.
Funding Statement
This work was supported by a Marie Curie Individual Fellowship (H2020-MSCA-IF-2014) grant 657135 “MIDGESYM” to Stefanos Siozios and a BBSRC DTP studentship to Jack Pilgrim. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Stefanos Siozios conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Jack Pilgrim performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Alistair C. Darby analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Matthew Baylis conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Gregory D.D. Hurst conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The raw reads and the cCpun draft genome assembly have been submitted to the DDBJ/EMBL/GenBank database under the BioProject accession number PRJNA487198 (WGS project QWJI00000000).
Data Availability
The following information was supplied regarding data availability:
The supermatrix file used for the phylogenomic analysis (Fig. 1) and the alignment files used for the phylogenetic analyses of the DUF1703 and the Carbonic Anhydrase gene families (Fig. 3; Fig. S6, respectively) are provided as supplementary files. Both trimmed and untrimmed versions of the alignment files are provided.
References
- Altschul et al. (1997).Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman et al. (2016).Anantharaman K, Brown CT, Hug LA, Sharon I, Castelle CJ, Probst AJ, Thomas BC, Singh A, Wilkins MJ, Karaoz U, Brodie EL, Williams KH, Hubbard SS, Banfield JF. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nature Communications. 2016;7(1):13219. doi: 10.1038/ncomms13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, Ronau & Hochstrasser (2017).Beckmann JF, Ronau JA, Hochstrasser M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nature Microbiology. 2017;2(5):17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetzer et al. (2011).Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–579. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- Bratlie et al. (2010).Bratlie MS, Johansen J, Sherman BT, Huang DW, Lempicki RA, Drabløs F. Gene duplications in prokaryotes can be associated with environmental adaptation. BMC Genomics. 2010;11(1):588. doi: 10.1186/1471-2164-11-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al. (2018).Brown AMV, Wasala SK, Howe DK, Peetz AB, Zasada IA, Denver DR. Comparative genomics of Wolbachia–Cardinium dual Endosymbiosis in a Plant-Parasitic Nematode. Frontiers in Microbiology. 2018;9:2482. doi: 10.3389/fmicb.2018.02482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie & Johnson (2009).Brownlie JC, Johnson KN. Symbiont-mediated protection in insect hosts. Trends in Microbiology. 2009;17(8):348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Bruen, Philippe & Bryant (2006).Bruen TC, Philippe H, Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics. 2006;172(4):2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant & Moulton (2004).Bryant D, Moulton V. Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Molecular Biology and Evolution. 2004;21(2):255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Camacho et al. (2009).Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez, Silla-Martínez & Gabaldón (2009).Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang et al. (2010).Chang J, Masters A, Avery A, Werren JH. A divergent Cardinium found in daddy long-legs (Arachnida: Opiliones) Journal of Invertebrate Pathology. 2010;105(3):220–227. doi: 10.1016/j.jip.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Conway et al. (2017).Conway JR, Lex A, Gehlenborg N, Hancock J. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33(18):2938–2940. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale & Moran (2006).Dale C, Moran NA. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126(3):453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- De Crécy-Lagard et al. (2007).De Crécy-Lagard V, El Yacoubi B, De la Garza RD, Noiriel A, Hanson AD. Comparative genomics of bacterial and plant folate synthesis and salvage: predictions and validations. BMC Genomics. 2007;8(1):245. doi: 10.1186/1471-2164-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver et al. (2016).Denver DR, Brown AMV, Howe DK, Peetz AB, Zasada IA. Genome skimming: a rapid approach to gaining diverse biological insights into multicellular pathogens. PLOS Pathogens. 2016;12(8):e1005713. doi: 10.1371/journal.ppat.1005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domman et al. (2014).Domman D, Collingro A, Lagkouvardos I, Gehre L, Weinmaier T, Rattei T, Subtil A, Horn M. Massive expansion of ubiquitination-related gene families within the chlamydiae. Molecular Biology and Evolution. 2014;31(11):2890–2904. doi: 10.1093/molbev/msu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar et al. (2007).Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLOS Biology. 2007;5(5):e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron et al. (2008).Duron O, Hurst GDD, Hornett EA, Josling JA, Engelstädter J. High incidence of the maternally inherited bacterium Cardinium in spiders. Molecular Ecology. 2008;17(6):1427–1437. doi: 10.1111/j.1365-294X.2008.03689.x. [DOI] [PubMed] [Google Scholar]
- Edlund et al. (2012).Edlund A, Ek K, Breitholtz M, Gorokhova E. Antibiotic-induced change of bacterial communities associated with the copepod Nitocra spinipes. PLOS ONE. 2012;7(3):e33107. doi: 10.1371/journal.pone.0033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms & Kelly (2015).Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biology. 2015;16(1):157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari & Vavre (2011).Ferrari J, Vavre F. Bacterial symbionts in insects or the story of communities affecting communities. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1569):1389–1400. doi: 10.1098/rstb.2010.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn et al. (2016).Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research. 2016;44(D1):D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai et al. (2014).Gai CS, Lu J, Brigham CJ, Bernardi AC, Sinskey AJ. Insights into bacterial CO2 metabolism revealed by the characterization of four carbonic anhydrases in Ralstonia eutropha H16. AMB Express. 2014;4(1):2. doi: 10.1186/2191-0855-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth et al. (2014).Gerth M, Gansauge M-T, Weigert A, Bleidorn C. Phylogenomic analyses uncover origin and spread of the Wolbachia pandemic. Nature Communications. 2014;5(1):5117. doi: 10.1038/ncomms6117. [DOI] [PubMed] [Google Scholar]
- Gillespie et al. (2018).Gillespie JJ, Driscoll TP, Verhoeve VI, Rahman MS, Macaluso KR, Azad AF. A tangled web: origins of reproductive parasitism. Genome Biology and Evolution. 2018;10(9):2292–2309. doi: 10.1093/gbe/evy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie et al. (2015).Gillespie JJ, Driscoll TP, Verhoeve VI, Utsuki T, Husseneder C, Chouljenko VN, Azad AF, Macaluso KR. Genomic diversification in strains of rickettsia felis isolated from different arthropods. Genome Biology and Evolution. 2015;7(1):35–56. doi: 10.1093/gbe/evu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonella et al. (2015).Gonella E, Pajoro M, Marzorati M, Crotti E, Mandrioli M, Pontini M, Bulgari D, Negri I, Sacchi L, Chouaia B, Daffonchio D, Alma A. Plant-mediated interspecific horizontal transmission of an intracellular symbiont in insects. Scientific Reports. 2015;5(1):15811. doi: 10.1038/srep15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, Noda & Ito (2006).Gotoh T, Noda H, Ito S. Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity. 2006;98(1):13–20. doi: 10.1038/sj.hdy.6800881. [DOI] [PubMed] [Google Scholar]
- Groot & Breeuwer (2006).Groot TVM, Breeuwer JAJ. Cardinium symbionts induce haploid thelytoky in most clones of three closely related Brevipalpus species. Experimental and Applied Acarology. 2006;39(3–4):257–271. doi: 10.1007/s10493-006-9019-0. [DOI] [PubMed] [Google Scholar]
- Gruwell, Wu & Normark (2009).Gruwell ME, Wu J, Normark BB. Diversity and phylogeny of cardinium (Bacteroidetes) in armored scale insects (Hemiptera: Diaspididae) Annals of the Entomological Society of America. 2009;102(6):1050–1061. doi: 10.1603/008.102.0613. [DOI] [Google Scholar]
- Guy, Roat Kultima & Andersson (2010).Guy L, Roat Kultima J, Andersson SGE. genoPlotR: comparative gene and genome visualization in R. Bioinformatics. 2010;26(8):2334–2335. doi: 10.1093/bioinformatics/btq413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen & Moran (2014).Hansen AK, Moran NA. The impact of microbial symbionts on host plant utilization by herbivorous insects. Molecular Ecology. 2014;23(6):1473–1496. doi: 10.1111/mec.12421. [DOI] [PubMed] [Google Scholar]
- Hansen, Vorburger & Moran (2012).Hansen AK, Vorburger C, Moran NA. Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Research. 2012;22(1):106–114. doi: 10.1101/gr.125351.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2016).He Z, Zhang H, Gao S, Lercher MJ, Chen W-H, Hu S. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Research. 2016;44(W1):W236–W241. doi: 10.1093/nar/gkw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang et al. (2018).Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution. 2018;35(2):518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas et al. (2016).Huerta-Cepas J, Szklarczyk D, Forslund K, Cook H, Heller D, Walter MC, Rattei T, Mende DR, Sunagawa S, Kuhn M, Jensen LJ, Von Mering C, Bork P. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Research. 2016;44(D1):D286–D293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt et al. (2013).Hunt M, Kikuchi T, Sanders M, Newbold C, Berriman M, Otto TD. REAPR: a universal tool for genome assembly evaluation. Genome Biology. 2013;14(5):R47. doi: 10.1186/gb-2013-14-5-r47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, Perlman & Kelly (2003).Hunter MS, Perlman SJ, Kelly SE. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proceedings of the Royal Society of London B: Biological Sciences. 2003;270(1529):2185–2190. doi: 10.1098/rspb.2003.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst et al. (2007).Hurst MRH, Beard SS, Jackson TA, Jones SM. Isolation and characterization of the Serratia entomophila antifeeding prophage. FEMS Microbiology Letters. 2007;270(1):42–48. doi: 10.1111/j.1574-6968.2007.00645.x. [DOI] [PubMed] [Google Scholar]
- Hurst & Frost (2015).Hurst GDD, Frost CL. Reproductive parasitism: maternally inherited symbionts in a biparental world. Cold Spring Harbor Perspectives in Biology. 2015;7(5):a017699. doi: 10.1101/cshperspect.a017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson & Bryant (2006).Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution. 2006;23(2):254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe, Walker & O’ Neill (2011).Iturbe-Ormaetxe I, Walker T, O’ Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Reports. 2011;12(6):508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi & Fass (2011).Joshi NA, Fass JN. Sickle: a sliding-window, adaptive, quality-based trimming tool for FastQ files. Version 1.33https://github.com/najoshi/sickle 2011
- Kalyaanamoorthy et al. (2017).Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software Version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knizewski et al. (2007).Knizewski L, Kinch LN, Grishin NV, Rychlewski L, Ginalski K. Realm of PD-(D/E)XK nuclease superfamily revisited: detection of novel families with modified transitive meta profile searches. BMC Structural Biology. 2007;7(1):40. doi: 10.1186/1472-6807-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecka & Olszanowski (2018).Konecka E, Olszanowski Z. A new Cardinium group of bacteria found in Achipteria coleoptrata (Acari: Oribatida) Molecular Phylogenetics and Evolution. 2018;131:64–71. doi: 10.1016/j.ympev.2018.10.043. [DOI] [PubMed] [Google Scholar]
- Krzywinski et al. (2009).Krzywinski M, Schein J, Birol İ, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Research. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2013).Kumar S, Jones M, Koutsovoulos G, Clarke M, Blaxter M. Blobology: exploring raw genome data for contaminants, symbionts and parasites using taxon-annotated GC-coverage plots. Frontiers in Genetics. 2013;4:237. doi: 10.3389/fgene.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti et al. (1996).Kurtti TJ, Munderloh UG, Andreadis TG, Magnarelli LA, Mather TN. Tick cell culture isolation of an intracellular Prokaryote from the TickIxodes scapularis. Journal of Invertebrate Pathology. 1996;67(3):318–321. doi: 10.1006/jipa.1996.0050. [DOI] [PubMed] [Google Scholar]
- Kurtz et al. (2004).Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biology. 2004;5(2):R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusian, Sültemeyer & Bowien (2002).Kusian B, Sültemeyer D, Bowien B. Carbonic anhydrase is essential for growth of Ralstonia eutropha at Ambient CO2 concentrations. Journal of Bacteriology. 2002;184(18):5018–5026. doi: 10.1128/JB.184.18.5018-5026.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch (2016).Laetsch DR. blobtools:blobtools. v0.9.19.4http://doi.org/10.5281/zenodo.61799 2016
- Langmead & Salzberg (2012).Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis et al. (2014).Lewis SE, Rice A, Hurst GDD, Baylis M. First detection of endosymbiotic bacteria in biting midges Culicoides pulicaris and Culicoides punctatus, important Palaearctic vectors of bluetongue virus. Medical and Veterinary Entomology. 2014;28(4):453–456. doi: 10.1111/mve.12055. [DOI] [PubMed] [Google Scholar]
- Li et al. (2009).Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Subgroup 1000 Genome Project Data Processing The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey et al. (2018).Lindsey ARI, Rice DW, Bordenstein SR, Brooks AW, Bordenstein SR, Newton ILG. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in Prophage WO of Wolbachia. Genome Biology and Evolution. 2018;10(2):434–451. doi: 10.1093/gbe/evy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo et al. (2002).Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C. How many Wolbachia Supergroups exist? Molecular Biology and Evolution. 2002;19(3):341–346. doi: 10.1093/oxfordjournals.molbev.a004087. [DOI] [PubMed] [Google Scholar]
- Lorenzen et al. (2008).Lorenzen MD, Gnirke A, Margolis J, Garnes J, Campbell M, Stuart JJ, Aggarwal R, Richards S, Park Y, Beeman RW. The maternal-effect, selfish genetic element Medea is associated with a composite Tc1 transposon. Proceedings of the National Academy of Sciences of the United State of America. 2008;105(29):10085–10089. doi: 10.1073/pnas.0800444105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann et al. (2017).Mann E, Stouthamer CM, Kelly SE, Dzieciol M, Hunter MS, Schmitz-Esser S. Transcriptome sequencing reveals novel candidate genes for Cardinium hertigii-caused cytoplasmic incompatibility and host-cell interaction. mSystems. 2017;2(6):e00141-17. doi: 10.1128/mSystems.00141-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais & Kingsford (2011).Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27(6):764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin (2011).Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- Martin et al. (2015).Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evolution. 2015;1(1):vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin & Rybicki (2000).Martin D, Rybicki E. RDP: detection of recombination amongst aligned sequences. Bioinformatics. 2000;16(6):562–563. doi: 10.1093/bioinformatics/16.6.562. [DOI] [PubMed] [Google Scholar]
- McBride & Nakane (2015).McBride MJ, Nakane D. Flavobacterium gliding motility and the type IX secretion system. Current Opinion in Microbiology. 2015;28:72–77. doi: 10.1016/j.mib.2015.07.016. [DOI] [PubMed] [Google Scholar]
- McBride & Zhu (2013).McBride MJ, Zhu Y. Gliding motility and por secretion system genes are widespread among members of the phylum bacteroidetes. Journal of Bacteriology. 2013;195(2):270–278. doi: 10.1128/JB.01962-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean et al. (2016).McLean AHC, Parker BJ, Hrček J, Henry LM, Godfray HCJ. Insect symbionts in food webs. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1702):20150325. doi: 10.1098/rstb.2015.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee et al. (2015).Mee PT, Weeks AR, Walker PJ, Hoffmann AA, Duchemin J-B. Detection of low-level Cardinium and Wolbachia infections in Culicoides. Applied and Environmental Microbiology. 2015;81(18):6177–6188. doi: 10.1128/AEM.01239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin et al. (2003).Merlin C, Masters M, McAteer S, Coulson A. Why is carbonic Anhydrase essential to Escherichia coli? Journal of Bacteriology. 2003;185(21):6415–6424. doi: 10.1128/JB.185.21.6415-6424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi et al. (2003).Mitsuhashi S, Ohnishi J, Hayashi M, Ikeda M. A gene homologous to β-type carbonic anhydrase is essential for the growth of Corynebacterium glutamicum under atmospheric conditions. Applied Microbiology and Biotechnology. 2003;63(5):592–601. doi: 10.1007/s00253-003-1402-8. [DOI] [PubMed] [Google Scholar]
- Morag et al. (2012).Morag N, Klement E, Saroya Y, Lensky I, Gottlieb Y. Prevalence of the symbiont Cardinium in Culicoides (Diptera: Ceratopogonidae) vector species is associated with land surface temperature. FASEB Journal. 2012;26(10):4025–4034. doi: 10.1096/fj.12-210419. [DOI] [PubMed] [Google Scholar]
- Nakamura et al. (2009).Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, Kisimoto R, Yanase T, Matsumoto Y, Kageyama D, Noda H. Prevalence of Cardinium bacteria in Planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Applied and Environmental Microbiology. 2009;75(21):6757–6763. doi: 10.1128/AEM.01583-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, Liu & Thomas (2014).Nguyen MTHD, Liu M, Thomas T. Ankyrin-repeat proteins from sponge symbionts modulate amoebal phagocytosis. Molecular Ecology. 2014;23(6):1635–1645. doi: 10.1111/mec.12384. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2015).Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori et al. (2014).Nishimori I, Vullo D, Minakuchi T, Scozzafava A, Osman SM, AlOthman Z, Capasso C, Supuran CT. Anion inhibition studies of two new β-carbonic anhydrases from the bacterial pathogen Legionella pneumophila. Bioorganic & Medicinal Chemistry Letters. 2014;24(4):1127–1132. doi: 10.1016/j.bmcl.2013.12.124. [DOI] [PubMed] [Google Scholar]
- Noel & Atibalentja (2006).Noel GR, Atibalentja N. “Candidatus Paenicardinium endonii,” an endosymbiont of the plant-parasitic nematode Heterodera glycines (Nemata: Tylenchida), affiliated to the phylum Bacteroidetes. International Journal of Systematic and Evolutionary Microbiology. 2006;56(7):1697–1702. doi: 10.1099/ijs.0.64234-0. [DOI] [PubMed] [Google Scholar]
- Nurk et al. (2013).Nurk S, Bankevich A, Antipov D, Gurevich A, Korobeynikov A, Lapidus A, Prjibelsky A, Pyshkin A, Sirotkin A, Sirotkin Y, Stepanauskas R, McLean J, Lasken R, Clingenpeel SR, Woyke T, Tesler G, Alekseyev MA, Pevzner PA. Assembling genomes and mini-metagenomes from highly chimeric reads. In: Deng M, Jiang R, Sun F, Zhang X, editors. Research in Computational Molecular Biology. Springer Berlin Heidelberg: Lecture Notes in Computer Science; 2013. pp. 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padidam, Sawyer & Fauquet (1999).Padidam M, Sawyer S, Fauquet CM. Possible emergence of new geminiviruses by frequent recombination. Virology. 1999;265(2):218–225. doi: 10.1006/viro.1999.0056. [DOI] [PubMed] [Google Scholar]
- Pan et al. (2008).Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320(5883):1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penz, Horn & Schmitz-Esser (2010).Penz T, Horn M, Schmitz-Esser S. The genome of the amoeba symbiont “Candidatus Amoebophilus asiaticus” encodes an afp-like prophage possibly used for protein secretion. Virulence. 2010;1(6):541–545. doi: 10.4161/viru.1.6.13800. [DOI] [PubMed] [Google Scholar]
- Penz et al. (2012).Penz T, Schmitz-Esser S, Kelly SE, Cass BN, Müller A, Woyke T, Malfatti SA, Hunter MS, Horn M. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLOS Genetics. 2012;8(10):e1003012. doi: 10.1371/journal.pgen.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman, Kelly & Hunter (2008).Perlman SJ, Kelly SE, Hunter MS. Population biology of cytoplasmic incompatibility: maintenance and spread of cardinium symbionts in a parasitic wasp. Genetics. 2008;178(2):1003–1011. doi: 10.1534/genetics.107.083071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen et al. (2011).Petersen TN, Brunak S, Von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Pilgrim et al. (2017).Pilgrim J, Ander M, Garros C, Baylis M, Hurst GDD, Siozios S. Torix group Rickettsia are widespread in Culicoides biting midges (Diptera: Ceratopogonidae), reach high frequency and carry unique genomic features. Environmental Microbiology. 2017;19(10):4238–4255. doi: 10.1111/1462-2920.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada & Crandall (2001).Posada D, Crandall KA. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proceedings of the National Academy of Sciences of the United State of America. 2001;98(24):13757–13762. doi: 10.1073/pnas.241370698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, Longden & Bleasby (2000).Rice P, Longden I, Bleasby A. EMBOSS: the european molecular biology open software suite. Trends in Genetics. 2000;16(6):276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Rio, Attardo & Weiss (2016).Rio RVM, Attardo GM, Weiss BL. Grandeur alliances: symbiont metabolic integration and obligate arthropod hematophagy. Trends in Parasitology. 2016;32(9):739–749. doi: 10.1016/j.pt.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros & Breeuwer (2009).Ros VID, Breeuwer JAJ. The effects of and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. Heredity. 2009;102(4):413–422. doi: 10.1038/hdy.2009.4. [DOI] [PubMed] [Google Scholar]
- Salminen et al. (1995).Salminen MO, Carr JK, Burke DS, McCutchan FE. Identification of breakpoints in intergenotypic recombinants of HIV Type 1 by bootscanning. AIDS Research and Human Retroviruses. 1995;11(11):1423–1425. doi: 10.1089/aid.1995.11.1423. [DOI] [PubMed] [Google Scholar]
- Santos-Garcia et al. (2014).Santos-Garcia D, Rollat-Farnier P-A, Beitia F, Zchori-Fein E, Vavre F, Mouton L, Moya A, Latorre A, Silva FJ. The genome of Cardinium cBtQ1 provides insights into genome reduction, symbiont motility, and its settlement in Bemisia tabaci. Genome Biology and Evolution. 2014;6(4):1013–1030. doi: 10.1093/gbe/evu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato et al. (2010).Sato K, Naito M, Yukitake H, Hirakawa H, Shoji M, McBride MJ, Rhodes RG, Nakayama K. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proceedings of the National Academy of Sciences of the United State of America. 2010;107(1):276–281. doi: 10.1073/pnas.0912010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Esser et al. (2010).Schmitz-Esser S, Tischler P, Arnold R, Montanaro J, Wagner M, Rattei T, Horn M. The genome of the amoeba symbiont “Candidatus Amoebophilus asiaticus” reveals common mechanisms for host cell interaction among amoeba-associated bacteria. Journal of Bacteriology. 2010;192(4):1045–1057. doi: 10.1128/JB.01379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön et al. (2018).Schön I, Kamiya T, Van den Berghe T, Van den Broecke L, Martens K. Novel Cardinium strains in non-marine ostracod (Crustacea) hosts from natural populations. Molecular Phylogenetics and Evolution. 2018;130:406–415. doi: 10.1016/j.ympev.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Seemann (2014).Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Shen, Hittinger & Rokas (2017).Shen X-X, Hittinger CT, Rokas A. Contentious relationships in phylogenomic studies can be driven by a handful of genes. Nature Ecology & Evolution. 2017;1(5):126. doi: 10.1038/s41559-017-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira & Goldman (2002).Shimodaira H, Goldman N. An approximately unbiased test of phylogenetic tree selection. Systematic Biology. 2002;51(3):492–508. doi: 10.1080/10635150290069913. [DOI] [PubMed] [Google Scholar]
- Showmaker et al. (2018).Showmaker KC, Walden KKO, Fields CJ, Lambert KN, Hudson ME. Genome sequence of the soybean cyst nematode (Heterodera glycines) Endosymbiont “Candidatus Cardinium hertigii” Strain cHgTN10. Genome Announcements. 2018;6(26):e00624-18. doi: 10.1128/genomeA.00624-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão et al. (2015).Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(9):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Siozios et al. (2013).Siozios S, Ioannidis P, Klasson L, Andersson SGE, Braig HR, Bourtzis K. The diversity and evolution of Wolbachia ankyrin repeat domain genes. PLOS ONE. 2013;8(2):e55390. doi: 10.1371/journal.pone.0055390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith (1992).Smith JM. Analyzing the mosaic structure of genes. Journal of Molecular Evolution. 1992;34(2):126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- Smith & Ferry (2000).Smith KS, Ferry JG. Prokaryotic carbonic anhydrases. FEMS Microbiology Reviews. 2000;24(4):335–366. doi: 10.1111/j.1574-6976.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Smith et al. (1999).Smith KS, Jakubzick C, Whittam TS, Ferry JG. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proceedings of the National Academy of Sciences of the United State of America. 1999;96(26):15184–15189. doi: 10.1073/pnas.96.26.15184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steczkiewicz et al. (2012).Steczkiewicz K, Muszewska A, Knizewski L, Rychlewski L, Ginalski K. Sequence, structure and functional diversity of PD-(D/E)XK phosphodiesterase superfamily. Nucleic Acids Research. 2012;40(15):7016–7045. doi: 10.1093/nar/gks382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakaran, Kost & Kaltenpoth (2017).Sudakaran S, Kost C, Kaltenpoth M. Symbiont acquisition and replacement as a source of ecological innovation. Trends in Microbiology. 2017;25(5):375–390. doi: 10.1016/j.tim.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Toft & Andersson (2010).Toft C, Andersson SGE. Evolutionary microbial genomics: insights into bacterial host adaptation. Nature Reviews Genetics. 2010;11(7):465–475. doi: 10.1038/nrg2798. [DOI] [PubMed] [Google Scholar]
- Tseng, Tyler & Setubal (2009).Tseng T-T, Tyler BM, Setubal JC. Protein secretion systems in bacterial-host associations, and their description in the Gene Ontology. BMC Microbiology. 2009;9(Suppl 1):S2. doi: 10.1186/1471-2180-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, Nishida & Beppu (2012).Ueda K, Nishida H, Beppu T. Dispensabilities of carbonic anhydrase in Proteobacteria. International Journal of Evolutionary Biology. 2012;2012:e324549. doi: 10.1155/2012/324549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia & Falkow (1997).Valdivia RH, Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277(5334):2007–2011. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- Voth (2011).Voth D. ThANKs for the repeat. Cellular Logistics. 2011;1(4):128–132. doi: 10.4161/cl.1.4.18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurture et al. (2017).Vurture GW, Sedlazeck FJ, Nattestad M, Underwood CJ, Fang H, Gurtowski J, Schatz MC. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics. 2017;33(14):2202–2204. doi: 10.1093/bioinformatics/btx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks, Marec & Breeuwer (2001).Weeks AR, Marec F, Breeuwer JAJ. A mite species that consists entirely of haploid females. Science. 2001;292(5526):2479–2482. doi: 10.1126/science.1060411. [DOI] [PubMed] [Google Scholar]
- Weeks & Stouthamer (2004).Weeks AR, Stouthamer R. Increased fecundity associated with infection by a Cytophaga–like intracellular bacterium in the predatory mite, Metaseiulus occidentalis. Proceedings of the Royal Society of London Series B: Biological Sciences. 2004;271(suppl_4):S193–S195. doi: 10.1098/rsbl.2003.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert et al. (2015).Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1807):20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, Clements & Finn (2014).Wheeler TJ, Clements J, Finn RD. Skylign: a tool for creating informative, interactive logos representing sequence alignments and profile hidden Markov models. BMC Bioinformatics. 2014;15(1):7. doi: 10.1186/1471-2105-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2004).Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N, Wiegand C, Madupu R, Beanan MJ, Brinkac LM, Daugherty SC, Durkin AS, Kolonay JF, Nelson WC, Mohamoud Y, Lee P, Berry K, Young MB, Utterback T, Weidman J, Nierman WC, Paulsen IT, Nelson KE, Tettelin H, O’Neill SL, Eisen JA. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLOS Biology. 2004;2(3):e69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein et al. (2001).Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, Hunter MS. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proceedings of the National Academy of Sciences of the United State of America. 2001;98(22):12555–12560. doi: 10.1073/pnas.221467498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein & Perlman (2004).Zchori-Fein E, Perlman SJ. Distribution of the bacterial symbiont Cardinium in arthropods. Molecular Ecology. 2004;13(7):2009–2016. doi: 10.1111/j.1365-294X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- Zeng et al. (2018).Zeng Z, Fu Y, Guo D, Wu Y, Ajayi OE, Wu Q. Bacterial endosymbiont Cardinium cSfur genome sequence provides insights for understanding the symbiotic relationship in Sogatella furcifera host. BMC Genomics. 2018;19(1):688. doi: 10.1186/s12864-018-5078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To enhance visualization the scaffolds were size sorted and concatenated into a pseudomolecule. The alternating grey and white strips highlight the scaffold borders. Inwards, the first, second and third circle are colour coded according to COG functional categories and represent a) the complete cCpun protein coding genes, b) the core genes between the four Amoebophilaceae genomes presented in Figure 1A, and c) the cCpun unique genes. In the fourth circle we show the genomic location of the genes coding for the Afp-like (magenta) and type IX (red) secretion systems as well as the DUF1703 gene paralogs. Finally, the two line plots represent genome coverage and GC% content across cCpun genome (1kb sliding window) respectively. An orange line indicates the mean coverage (90X) of the draft assembly.
BUSCO completeness assessment results for cCpun draft genome in comparison to the other Cardinium genomes and A. asiaticus. The Results are based on the presence or absence of 148 single-copy universal bacterial markers.
Mummer self-plots representing sequence repeat density in the seven Amoebaphilaceae genomes. Each dot represent a repeat (red=direct) and (blue=inverted) of at least 200bp and 95% similarity.
a) Multiple sequence alignment of the DUF1703 nuclease domain from Cardinium (blue outline) and Rickettsia (red outline) homologs described in Fig. 4. The core PD-(D/E)XK site is highlighted in yellow and the catalytic Asp, Glu and Lys residues are indicated with an asterisk. b) Residue conservation within the core PD-(D/E)XK site between the DUF1703 nuclease family depicted in (a) and the type II-IV CI-like toxins of Wolbachia and related proteins described in Gillespie et al. (2018). Sequence logos were prepared using the Skylign tool (Wheeler, Clements & Finn, 2014).
Phylogenetic relationships of cCpun HGTs with their closest homologs in the Genbank database was inferred using maximum likelihood with IQ-TREE v1.6.6 (method: automated best model selection). Branch support values are based on 1000 bootstrap replicates.
Maximum likelihood phylogenetic placement of cCpun, cSfur and cHgTN10 carbonic anhydrase (CAs) protein sequences compared with their closest homologs in the Genbank database. Members from the four clades forming the beta-class of CAs are presented. The positions of the Cardinium homologs and the CA homolog identified in Rickettsia (RiCNE) endosymbionds in biting midges are indicated in purple and red respectively. Phylogenetic relationships were inferred using IQ-TREE v1.6.6 (method: automated best model selection).
Data Availability Statement
The following information was supplied regarding data availability:
The supermatrix file used for the phylogenomic analysis (Fig. 1) and the alignment files used for the phylogenetic analyses of the DUF1703 and the Carbonic Anhydrase gene families (Fig. 3; Fig. S6, respectively) are provided as supplementary files. Both trimmed and untrimmed versions of the alignment files are provided.