Abstract
Parasitic interactions are so ubiquitous that all multicellular organisms have evolved a system of defences to reduce their costs, whether the parasites they encounter are the classic parasites which feed on the individual, or brood parasites which usurp parental care. Many parallels have been drawn between defences deployed against both types of parasite, but typically, while defences against classic parasites have been selected to protect survival, those against brood parasites have been selected to protect the parent's inclusive fitness, suggesting that the selection pressures they impose are fundamentally different. However, there is another class of defences against classic parasites that have specifically been selected to protect an individual's inclusive fitness, known as social immunity. Social immune responses include the anti-parasite defences typically provided for others in kin-structured groups, such as the antifungal secretions produced by termite workers to protect the brood. Defences against brood parasites, therefore, are more closely aligned with social immune responses. Much like social immunity, host defences against brood parasitism are employed by a donor (a parent) for the benefit of one or more recipients (typically kin), and as with social defences against classic parasites, defences have therefore evolved to protect the donor's inclusive fitness, not the survival or ultimately the fitness of individual recipients This can lead to severe conflicts between the different parties, whose interests are not always aligned. Here, we consider defences against brood parasitism in the light of social immunity, at different stages of parasite encounter, addressing where conflicts occur and how they might be resolved. We finish with considering how this approach could help us to address longstanding questions in our understanding of brood parasitism.
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: bird, cuckoo, defences, kleptoparasite, fish, social insect
1. Introduction
Parasitic interactions, where one organism uses the resources of another to the detriment of the host, are so ubiquitous that all individuals can be expected to face a threat from a parasite at some point in their lives. The effects that parasites exert on hosts can range from minor reductions in fitness to rapid death. Therefore, parasites represent a widespread source of natural selection that operates across all stages of development, from egg traits [1] to secondary sexual traits [2]. The intensity of selection arising from parasitism has resulted in all multicellular organisms evolving a variety of defence mechanisms that counterbalance the fitness costs of parasitism, whether the parasites they encounter are the classic parasites which feed on the individual [3], or brood parasites which usurp parental care [4]. Both forms of parasitism provide the bedrock for theoretical and empirical work on addressing when parasites attack and how hosts respond with adaptive defences that vary extensively [3,4]. Nevertheless, researchers studying brood parasites rarely also study classic parasites, and vice versa. In this synthesis paper we take the novel step of placing defences against brood parasitism under the umbrella of ‘social immunity', a concept from classic parasitology, whereby the defences have been selected in a donor, to benefit a recipient, which is the host (or potential host) of the parasite [5]. It is not our intention to review the brood parasite literature in detail, as this has been done elsewhere [4,6]; rather we select examples of defences from the classic and brood parasite literature to illustrate our points. We first reflect on the costs of classic versus brood parasitism, and then compare the social defences displayed against classic versus brood parasites at different stages of encounter. We conclude by considering how setting the evolution of host defences against brood parasitism in the ‘social immunity' framework may give us new insights into the brood parasitism phenomenon, and vice versa.
2. What are the costs of parasitism?
(a). Classic parasitism
Classic parasites usurp an individual's resources that are destined for the use of that individual, and typically reduce the survival of their hosts as a consequence (virulence) [7]. Although the effects of virulence on host mortality can be direct (especially from microparasites like bacterial or viral infections), many parasites rarely induce mortality directly. This is particularly true for macroparasites (e.g. gastrointestinal worms), but also true for many microparasites, such as the cold virus. Instead, these parasites reduce foraging efficiency or competitiveness for territories, for example, and as a consequence reduce mortality indirectly. Therefore, the extent of damage caused by a parasitic interaction can depend strongly on the host's condition, such that parasites that cause little damage in a high-quality host may be heavily detrimental to a host suffering from malnourishment, for example [8]. Although virulence is generally defined in terms of host mortality e.g. [7], parasite-induced mortality does not necessarily reduce host fitness. Mortality can occur post-reproduction, for example, when the organism has already achieved its lifetime reproductive success (LRS). However, mortality of individuals at a pre-reproductive developmental stage will completely wipe out LRS, and mortality at any point during the reproductive life stage is predicted to drive fitness decays below those of non-parasitized individuals. Low-virulence parasites can also directly or indirectly impact LRS through mechanisms other than mortality. Many trematodes that infect snails, for example, are ‘castrating parasites' which cause a diversion of the resources that would be allocated by the host into reproduction, to growth or survival, increasing the chances that the parasite will be transmitted to the next host [9]. Like the indirect effects on mortality from low-virulence parasites, there can also be indirect effects on LRS via reduced competitiveness for mates [2]. It is, therefore, the relative difference in fitness among parasitized and non-parasitized hosts that determines the strong selection pressure to resist (reduce the numbers of) parasites or tolerate them (reduce their negative impact).
(b). Brood parasitism
The inclusive fitness of many animal species benefits from provision of resources to their offspring after they have detached from the parental body (‘narrow-sense parental care' [10]), which increases the indirect fitness element of an individual's inclusive fitness (see [11] for a detailed discussion on assigning fitness to parents versus offspring). Parental care thereby provides a further source of energy that a parasite can exploit. These episodes of resource availability drive the origin of brood parasitism. Parents may invest resources in the egg, and/or provide a more secure environment through direct protection from predators or parasites [12]. Parents may also protect their young against adverse environmental conditions by provisioning food, either by stocking the ‘larder', for example nest provisioning in mason bees (Osmia spp.) [13], or by directly feeding offspring [10,11,14]. Parental care is extremely costly and wherever large amounts of costly resources are delivered by parents to their offspring, there is an opportunity for cheats to try to usurp those resources. The eponymous example of this parasitism of reproductive investment is performed by the common cuckoo (Cuculus canorus), which targets passerine hosts with post-hatching parental care. She removes a host egg upon laying her own in the nest, which hatches more rapidly than its adopted nestmates and promptly forcibly ejects all of the host's own offspring from the nest, ensuring that all subsequent parental investment is directed exclusively towards the parasite [15]. Interspecific brood parasitism has evolved seven times independently in the birds alone [16], and brood parasitism in its diverse forms is widespread across animal taxa that display parental care [17].
In contrast with classic parasitism, brood parasitism is a direct attack on the indirect fitness of the parent. However, like classic parasitism, the costs of brood parasitism vary depending on the strategy of the parasite. For brood parasites, the magnitude of the costs are also a function of the level of parental investment in post-hatching care provided by the host. For example, in many cases of avian brood parasitism by cuckoos (Cuculus spp.) the combination of high levels of parental investment and an extremely virulent attack strategy by the parasite results in high parasite-induced inclusive fitness costs for hosts [16]. However, some avian parasites are less virulent, either because they do not eject the host's eggs or chicks, or because pre-hatching investment can be shared among the brood and the parasite has lower requirements for post-hatching care [16]. There is a comparable range of fitness costs associated with brood parasites in non-avian systems. For example, inquiline social parasites of social hymenopteran colonies can completely replace the colony queen prior to the production of reproductives, thus reducing her LRS to zero [18], which is more extreme than even the most virulent avian brood parasites [16]. Other parasites are less virulent, typically reducing the overall success of the brood, but not destroying it completely, for example the cuckoo fungus in termites, Fibularhizoctonia sp. [19], the inquiline thrips, Akainothrips francisi (and see table 1 in [20] for further examples within the Thysanoptera), cuckoo wasps, e.g. Sapyga pumella [13], slavemaking ants, Temnothorax americanus ([21] and references therein), and Maculinea caterpillars ([22] and references therein).
3. Social immunity—a framework to understand defences against classic versus brood parasites
(a). What is social immunity?
Parasite defences, in the classic understanding of host–parasite interactions, are directed by the host individual against the parasite to protect the host's survival and therefore its direct fitness. In other words, they are ‘personal'. However, there is a class of defences that increase the fitness of the individual producing that defence and one or more conspecific recipients—that is, social defences. This is known as ‘social immunity’ in the broad sense [5,23], and is the definition we use throughout this paper, but see [24,25] for a narrow-sense definition, which is restricted to anti-parasite defences occurring in eusocial species. Social immune responses include the anti-parasite defences typically provided for others in kin-structured groups, such as socially breeding vertebrates, sub-social and social invertebrates, and even potentially plants and microbes [5,23]. Instances of social anti-parasite defences abound, most notably in the highly social Hymenoptera, whose colonial living and close relatedness make them especially valuable [24,25]. Fever, for example, is employed by an individual for personal protection against parasites [26–28]. However, there is a social equivalent in honeybees, whereby workers collectively raise the temperature of the brood when they are infected with Ascosphaera apis, a heat sensitive fungus which causes chalkbrood disease [29]. This defence is generated by uninfected workers for the defence of the offspring and so constitutes a social defence. Collective behaviours are an extreme example of social immune responses and are typically only seen in eusocial species. There are, however, many examples of social immune responses that do not require collective action and are present both in eusocial species and in groups with lower levels of social organization, such as nuclear families. For example, there are many cases of the provisioning of immune molecules from one individual to another, at a cost to the producer for the benefit of the receiver. This typically occurs between parents and offspring, for example the maternal transfer of antibodies/other immune components in milk or eggs [30,31], or between siblings, for example the transfer of antifungal secretions between termite workers [32]. A key aspect of social immunity is that selection operates through traits that maximize the indirect fitness of the donor by protecting its kin from infection. By contrast, personal immunity has typically been selected to protect an individual's direct fitness via survival [5]. A consequence of this is that social immunity sets up potential conflicts between donors and receivers, much in the same way that the provisioning of resources to a brood sets up parent–offspring conflict [33]. For example, with social immune responses, a donor's indirect fitness might best be maximized by killing one infected offspring to protect the remaining brood. However, this wipes out the direct fitness of the sacrificed individual (though it may still gain some indirect fitness via the survival of its kin).
The most specialized social immune responses are, unsurprisingly, found in the most developed social systems, namely the eusocial insects [24,25]. In those societies, there has been a separation of brood into workers and reproductives, such that the colony functions much like that of an individual, where workers (functionally equivalent to somatic cells) can be sacrificed to protect the reproductives (germline). Here, we see many examples of workers being killed, isolated, excluded or even excluding themselves from the colony when infected, to protect their kin (see examples in [24,25]). In many cases workers are sterile, and where they can reproduce to a certain extent (e.g. the laying of unfertilized male eggs in Hymenoptera), policing by the queen or other workers reduces the success of this strategy [34]. As such, there is little conflict between their own fitness and that of the colony, because their fitness is primarily indirect [25]. However, eusocial colonies are at the extreme end of social organization and this relative lack of conflict over the response to parasites is not typical [35]. Social immune responses occur at multiple social levels, including nuclear families [5,23], in which conflicts are rife [33]. Social living, therefore, provides both the ideal environment for parasites to thrive and a network of interactions between individuals whose response to those parasites is shaped by their own selfish interests. The concept of social immunity may, therefore, allow new insights into host–parasite interactions, whereby the infected individual is not necessarily at the centre of the defensive response, and the defences employed on its behalf are not necessarily in its best interests. So can the concept of social immunity be applied to brood parasitism?
(b). Is defence against brood parasitism a form of social immunity?
Much like social immunity, host defences against brood parasitism are employed by a donor (a parent) for the benefit of one or more recipients (typically kin), and as with social defences against classic parasites, selection acts on the donor, not the recipient. Defences have therefore evolved to protect the donor's indirect fitness, not the survival or ultimately the direct fitness of individual recipients. If the response that best maximizes the donor's inclusive fitness does not necessarily maximize the inclusive fitness of all recipients, this will create conflicts within the social group. If we take the example of a reed warbler threatened with parasitism by the common cuckoo, the best response to finding a suspicious egg in the nest could be rejection, and the best threshold for rejection could be quite low. This is because the mistaken rejection of a host's own egg is significantly less costly to the host than the potential loss of an entire brood, should a cuckoo successfully parasitize the nest [36]. From the recipient brood's perspective, the remaining siblings will benefit from an increased share of their parents' resources, but the consequences are catastrophic for the mistakenly rejected reed warbler egg. In the brood parasite literature, the parent (for nuclear families) is typically considered the ‘host' as it is its effort that is being parasitized, and this seems in conflict with the definition of social immunity. However, the offspring could also be considered hosts, as the brood parasite is more like a classic parasite that threatens the offspring's direct fitness. Defences against brood parasitism, therefore, fit the paradigm of social immunity, and are subject to the same conflicts between donor and recipients that are present for the response to classic parasites. Much like classic parasites, these conflicts will be reduced or resolved for eusocial colonies that encounter brood parasites, owing to the reproductive division of labour. So how do the responses of hosts to brood parasites compare with examples of social defences against classic parasites?
(c). How do social immune defences against classic and brood parasites compare across different stages of parasite encounter?
Defences can be employed at any stage, from before parasites have been detected through employing risk-averse behavioural strategies, to immediately upon detection in the environment, at the point where parasites directly threaten the body/nest and even post-invasion where the damage they cause can be controlled. Here, we compare the types of social defences displayed by donors against classic or brood parasites at each of these stages.
(i). Parasite avoidance
The most basic form of social defence against parasitism is to employ mechanisms that reduce the likelihood of encountering parasites in the first place. Avoidance behaviours can be employed against classic parasites in a social immune context, for example by avoiding laying eggs or raising young in contaminated locations. Carrion-breeding dung beetles have been shown to roll the carrion balls that they use to provision their young a distance from the carcass, either horizontally, or by digging to depths of up to 1 m, at which the concentration of microbes, particularly those that cause infection, is greatly reduced [37]. The removal of corpses from a communal nest is a social defence found in ants [38], bees and termites [39], thus reducing the risk of infection to other colony mates. As brood parasites are mobile and able to actively seek out their hosts, parasite avoidance is less straightforward. However, it would be possible to avoid risky locations, such as those that are known to host a brood parasite, or that have been host to brood parasites in the past [40–42].
A large number of potential hosts can facilitate parasitism, either by reducing search costs for an actively searching parasite, or by facilitating the transmission of parasites passively contracted from the environment [43]. Social living is therefore subject to increased risks of parasitism. In beewolves, cuckoo activity is positively density dependent, suggesting that cuckoos do indeed target sites with higher nest density [41]. Similarly, in this issue, Medina & Langmore [44] perform a comparative analysis across 242 species of host and non-host species of birds. This analysis reveals that species with smaller breeding areas (and thus, with higher breeding densities) are more likely to be hosts of brood parasites. Another mechanism of parasite avoidance could, therefore, be to avoid nesting near other conspecifics, but this outcome would be driven by the balance between the costs of the increased risk of parasitism against the benefits of social living, which can also include increased defences against parasitism (see next section).
(ii). Defending the body/nest
Once parasites have successfully located their host, they need to enter or attach to the body, or enter the nest, in the case of brood parasites, so that the host's resources can be exploited. Hosts have an array of behavioural, physical or chemical defences that can be employed to resist parasite ingression. Behaviours include allogrooming with antimicrobial chemicals in termites [45] and ants [46], and excluding infected individuals from the nest, which is fatal for the individuals but protects the colony ([24,25] and references therein). Behavioural defences can also prevent brood parasites from accessing nests. These so-called frontline defences are now the focus of active research, especially against avian brood parasites [47], after it was discovered that alarm calls and physical mobbing of cuckoos and cowbirds can reduce parasitism [48,49] even to the point that attacks can kill the parasite [49,50]. For some hosts, these attacks can become collaborative, where multiple individuals join to drive the parasite from the nest. Cooperatively breeding fairy-wrens, for example, mob as a group [47], and otherwise non-cooperative oriental reed warblers will join in attacking cuckoos at neighbours' nests [51]. Solitary bees also aggressively defend their provisioned nests against brood parasites, and indeed against parasites attempting to attack nearby nests [52], generating group defences similar to those in the avian examples. More broadly, observing the mobbing behaviour of neighbours (social information) can also act to upregulate defences at the nest, even if it does not increase the number of active defenders beyond the nest-owners (e.g. [53]). After reed warblers, for example, witness neighbours attacking cuckoos they increase mobbing attacks back at their own nests [54]. This use of social information is thought to shift the reed warbler's recognition threshold of cuckoos versus the hawks that cuckoos mimic [55], thus allowing hosts to fine-tune defence of their nest. Vigilance behaviours and aggression towards brood parasites have also been shown in social insects e.g. aggression towards slavemaking Temnothorax ants by defending hosts [21], although the effects of social information on modulating expression of these defences have not been explored in detail.
Classic parasites can also be repelled through physical defences. For example, many social insects live in defensible nests, which provide physical protection from a range of threats, including detection by mobile parasites [56]. They also allow for effective vigilance, with entrance guards able to screen nestmates for parasitic infection. Both ants and birds have been shown to protect their nests by collecting, respectively, antiparasitic resins [57] and plants [58]. Several animal species also use self-produced antimicrobials in the fabric of a nesting structure, e.g. túngara frogs [59] and several nest-building fish [60–62], termites [63], and burying beetles [64], and some even cooperate with bacteria to deter parasites, e.g. bark beetles [65] and burying beetles [66–68], all of which reduce the likelihood of parasite ingression. Are there similar physical barriers to defend against brood parasites before they become established in the nest? There is evidence that the physical architecture of the nest may have evolved to reduce the likelihood of brood parasites accessing the brood, for example, the woven access tubes in the nests of Ploceus weaverbirds [69] and the capping of brood cells, or addition of empty brood cells in solitary bees [13,70]. However, as these physical defences are likely to work against predators too it is possible that they evolved for that purpose and act secondarily against brood parasites. The use of a defensible nest by social insects can also provide protection against brood parasites, which need to gain access and avoid detection by guards [56]. By contrast, there is no evidence for the use of collected or self-produced antiparasitic substances to protect the nest against brood parasites. This may be inhibited by the taxonomic similarity between brood parasites and their hosts, particularly avian parasites, such that substances repellent to, or detrimental to the health of, brood parasites are likely to have a similar effect on their hosts.
(iii). Reducing parasite success post-invasion
If the parasite breaches the first line of defences it still needs to become established in the host's body, or in the nest, to be successful. This hinges on two key elements: the donor's ability to recognize the parasite as non-self, and then to respond to it by either resisting (i.e. reducing parasite fitness), or tolerating its presence (i.e. reducing the parasite's negative impact on the host).
Recognition. The mechanisms by which hosts can recognize classic parasites in their own bodies are now well understood and involve the detection of pathogen-associated molecular patterns (PAMPs) via pathogen recognition receptors (PRRs) in both vertebrate and invertebrate hosts (see [3,71] and references therein). For a social immune response, however, recognition is complicated by the fact that the donor of the defence has to recognize infection concealed inside the recipient's body. As such, PAMPs may not be detectable to the donor. Instead, the donor has to rely on visual or chemical signatures of infection being displayed by the infected individual, e.g. ants [72–74] and termite [75], or even active transfer of information on infection status by the recipient, e.g. warning dances in termites [76,77]. Recognition of brood parasites in the nest should be more straightforward as the parasites are not concealed inside another individual's body. However, direct molecular recognition is unlikely owing to a lack of interaction between parasite and host at the cellular level. Much like social immunity, recognition instead relies primarily on visual (birds and insects) or olfactory (insects) cues [4].
This visual apparency has selected for visual mimicry or camouflage of brood parasites to avoid detection [19,78–80]. Hosts of avian brood parasites, for example, discriminate against parasite eggs when there is a mismatch in their colour and patterning with the host's own eggs. Research into how host ‘signatures' on the egg help identify non-self has exploded in recent years as appropriate analysis tools incorporating avian vision have been developed, e.g. [81–86], or as the taxonomic range of brood parasite hosts has expanded [87]. We now know, for example, that signature elements are likely to interact in terms of the information they provide to hosts and allow more fine-scaled recognition [81,88]. We also have evidence that the context of signatures matters, as both the location and developmental stage of the parasite can determine how readily it is recognized. For example, Yang et al. [89] show that poorly mimetic eggs placed outside of the nest cup will be retrieved, but later rejected once they are alongside the host's own eggs. Similarly, cuckoo catfish, Synodontis multipunctatus, eggs are readily collected by host cichlids, despite being visually non-mimetic [90,91] but then recognized as non-self and rejected once in the host's mouth [87], potentially via chemical cues. Interestingly, eggs that are rejected can survive outside of their cichlid host and re-infect successfully as juveniles, at which stage recognition does not seem to occur [91].
Insect hosts of brood parasites, on the other hand, tend to have distinctive cuticular hydrocarbon profiles that can be used to discriminate kin or social partners [92–95], leading to chemical mimicry [96] or camouflage [97]. Kaur et al. [21] measured the aggressive responses displayed by hosts to slavemaking ants across a number of populations. Surprisingly, the best predictor of the host response, both behaviourally and in terms of gene expression in the brain, was the ecological success of the parasite. Parasites from some populations were just better at avoiding recognition, potentially owing to their altered cuticular chemical profiles [21]. Brood parasite hosts, therefore, use external chemical or visual cues to recognize parasites as non-self, much as social immune donors use when recognizing recipients infected with classic parasites.
Resistance. For classic parasites, the post-infection social immune responses of the donor can cure the recipient e.g. social fever in honeybees (see §3a) or directly kill the parasite, e.g. ants [72] and termites [75], thus protecting other kin sharing the social environment. In the most extreme cases, hosts can even abandon a nest that is heavily infected with parasites, as has been shown in ants [38] and termites [39], thus killing the parasite by depriving it of hosts. Similarly, brood parasite hosts can also show resistance by ejecting the parasite from the nest [98] or directly killing it [21]. There is also evidence that some hosts will abandon infected nests after brood parasites have been detected. For example, brown-headed cowbird (Molothrus ater) hosts have been shown to desert parasitized nests [99] and mason bees (Osmia spp.) have been shown to abandon the tunnels they have been provisioning once one of the brood cells becomes parasitized [21]. Similarly, Myrmica ants have been shown to abandon nests where a virulent species of Maculinea caterpillar has become established [100].
Resistance against both classic and brood parasites can be costly, however, either energetically or by inflicting damage to self. Spottiswoode & Busch [101] compare the vertebrate MHC parasite recognition system with the egg recognition systems of birds and conclude that both are selected to find the best balance between the inevitable and costly type I and type II errors. Type I errors occur when hosts wrongly attack self: body cells in the case of classic parasites and rejection of the hosts' own eggs/offspring in the case of brood parasites. Type II errors occur when the host fails to recognize a parasite as non-self. Any mechanism that can ameliorate these costs will, therefore, be selected for. One such mechanism is self-medication, whereby an individual changes its diet upon infection to reduce the success of the parasite [102]. There are examples of self-medication in social parasite defence. For example, monarch butterflies infected with a protozoan parasite that can be transmitted vertically to their offspring choose to lay eggs on milkweeds containing high levels of cardenolides, the consumption of which can reduce parasite load and virulence [103]. The potential to use self- or brood-medication against brood parasites is less clear. However, if we consider self-medication simply as a shift in diet that favours the host in the host–parasite interaction, then it is a possibility. As brood parasites can only survive in the host nest if the resources they are usurping are suitable, then there could be selection for a shift in host diet, either temporarily or permanently away from the diet preferred by the parasite. For example, the common European cuckoo parasitizes insectivorous passerines [104] but is unable to parasitize the Asian flycatcher, which provisions its chicks with hard to digest beetles and grasshoppers, because the cuckoo cannot thrive on that diet [105]. Selection could, therefore, act on heavily parasitized hosts to adjust their diet away from that preferred by the cuckoo to one on which the cuckoo cannot survive.
Tolerance. Another cost-reducing mechanism of immune defence is tolerance, whereby the negative fitness effects of a given parasite load on the host are reduced [106]. Tolerance mechanisms have long been studied in plants, but have only recently found their way into the animal host–parasite literature [107]. Tolerance against classic parasites is typically measured as a fitness reaction norm across host genotypes for a range of parasite loads, to estimate genetic variation in the trait, or as differential fitness effects across environments of a given parasite load to measure environmental influences on tolerance (table 1 in [108]). Mechanisms differ across host–parasite combinations, but can include reducing the damage to self from a strong immune response [109], reducing the virulence of parasites, for example by mopping up the cell-damaging toxins produced by pathogenic bacteria [110], or by altering reproductive responses, for example fecundity compensation can be a tolerance mechanism as it maintains fitness better for a given parasite load than the original reproductive schedule, which is likely to be curtailed by parasite-induced mortality or sterility [111]. The presence of tolerance mechanisms in social immunity are hypothesized, but have not been explicitly tested [25], though there is some evidence for fecundity compensation to replace workers lost to parasitism in termites [112].
Can tolerance work in host–brood parasite interactions? Evidence to date suggests that the last mechanism described above, altering reproductive responses, can be employed to reduce the costs of brood parasitism to the host (e.g. [113]). For example, spreading broods over more clutches by reducing the number of offspring per brood could reduce the costs if the likelihood of a single host being parasitized remained the same, as a single parasitism event would impact fewer offspring. This strategy would work for both highly virulent (e.g. those that destroy entire broods) and less virulent parasites (i.e. those that are reared alongside the host's brood). By contrast, increasing clutch size is a tolerance mechanism that can only work against parasites that share parental care with the brood. There is evidence for both of these strategies from a number of studies of avian brood parasite hosts (see table 1 in [114]), but studies on tolerance to brood parasites have typically been correlational and other interpretations of the host response than tolerance could be invoked [114]. Potential tolerance responses in brood parasite hosts are hard to disentangle from resistance without direct experimental manipulation. For example, a larger clutch size might reduce the impact of a single low-virulence parasite in the nest, but the parasite might also suffer a reduced growth rate due to greater competition with its foster siblings, such that this approach could also be a resistance mechanism. It is clear that more work is required to understand the potential role of tolerance in host–brood parasite interactions (for a more in-depth discussion of this topic see [114]).
4. Conflict in social defences
(a). How does the potential for conflict in social defences compare across the stages of parasite encounter?
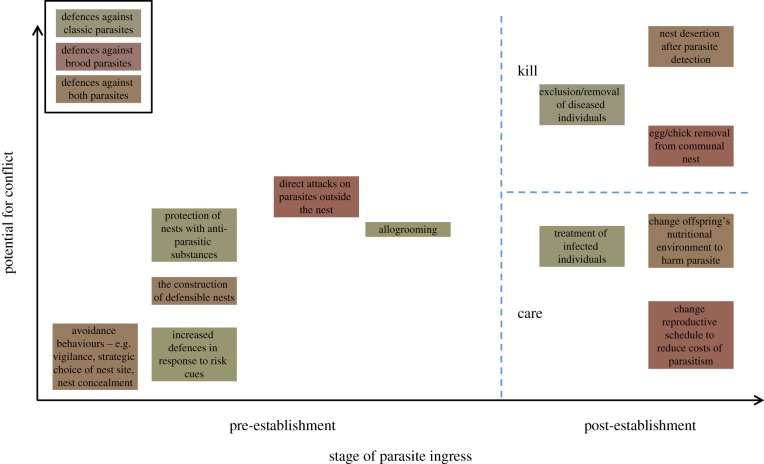
As with all apparently altruistic acts, unless the interests of donor and recipient are perfectly aligned, there is a potential for conflict, and social defences against parasites are no exception. The point at which the parasite is encountered and the defences employed has a strong bearing on the potential levels of conflict the defences could induce (figure 1). Early stage defences, such as avoiding parasites in the environment by avoiding risky nesting locations, for example, are unlikely to induce much conflict, because the interests of the donor and the recipient are aligned. Both benefit from avoiding parasitism in the first place, whether that is from classic or brood parasites. More direct defences employed prior to parasite encounter, such as the construction of defensible [56,69] or concealed nests [13,37,69,70], or the collection/production of anti-parasite substances [57–64] have the potential to induce some conflict, because the donor is paying high energetic and time costs for the construction/protection of the nest/kin, and may have to do this on multiple occasions for future broods. The donor must balance the costs of investing now against its residual reproductive value, and therefore recipients will value greater levels of investment in protection than donors will be selected to provide [10,14,33].
Figure 1.
The potential for conflict between donors and recipients is estimated across the stages of parasite encounter for defences against classic parasites (yellow), brood parasites (red) or both (orange). The potential for conflict typically increases as the threat from the parasite increases. Post-establishment, the donor can choose to care or kill, with the latter option providing the greatest potential conflicts between donor and recipients.
The point at which the potential for conflict is greatest is after the parasite has successfully established itself in the nest/host (figure 1). At this point, whether it is dealing with a classic or brood parasite, the donor has to determine whether to kill or cure. For classic parasites, this choice is likely driven by the stage in the process at which the infection is detected, the virulence of the parasite and the cost to the donor of providing a cure, e.g. [72,75]. If the host is terminally infected, then its best interests are served by being killed, as this may protect its indirect fitness by reducing the likelihood that it will infect its kin. However, if it can be cured, but the cost to the donor of treating the infection is too high, or the risk to other individuals in the social group is too great, the donor would be under selection to kill, in conflict with the interests of the recipient. For brood parasites, this dilemma is different. Killing could be targeted specifically at the parasite, e.g. by egg or chick rejection, and in this endeavour, the interests of the donor and the recipient are aligned. However, selection for mimicry in brood parasites means that rejection is prone to type 1 errors, whereby donors fail in their recognition and accidentally reject their own kin [101]. As discussed above, this leads to conflicts as the threshold for rejection could be very different for donors and recipients. Finally, for both classic and brood parasites, donors can respond by nest abandonment, thus killing their entire brood/colony [13,99,100]. This has the highest potential for conflict as only in cases of irretrievable, terminal infection of all individuals by a classic parasite, or the presence of a highly virulent brood parasite against which the donor has no defence, would this response also serve the interests of the recipients.
(b). When are these conflicts resolved?
Here we suggest two potential mechanisms that could lead to the resolution of conflicts associated with social defences in non-eusocial systems. (i) Selection could favour defences with the least conflict, for example, by focussing efforts on defences that occur early in the sequence of host–brood parasite interactions, such as nest placement and vigilance (figure 1). Another possibility is the evolution of reduced-cost care defences in response to parasitism, such as changing the food provided to offspring as a form of medication [103], or to increase their condition such that they can better tolerate low-virulence classic, or brood parasites. Other tolerance mechanisms, such as changing the reproductive schedule in response to brood parasites, can also reduce the potential for conflict, as the donor would be selected to reproduce in the way that maximizes both its own and its offspring's survival in the face of parasitism. (ii) Donor(s) and recipient(s) could coevolve ‘united' defences. The costs to the donor can be reduced by donors working together; collective defences against cuckoos in fairy-wrens are more effective than individual mobbing, thereby increasing the success, and so reducing the cost of the defence [47]. Alternately, recipients could take on the role of donor by contributing to the social defence themselves. For example, in burying beetles, parents produce antimicrobial secretions that reduce the presence of classic parasites on their offspring's food [64], at a substantial cost to themselves [115]. However, larvae also produce these secretions collectively [116,117], reducing the cost to the parent and so reducing potential conflict over this social immune response [118]. These collective defences, or reciprocal actions where individuals take on the role of both donor and recipient, are frequent occurrences in eusocial insect colonies, where conflicts over defence are typically reduced owing to the reproductive division of labour [23–25]. However, whether these behaviours are a consequence of eusociality [25], or one of its drivers [23], has yet to be resolved.
A final potential outcome is where the conflicts are not resolved, but one of the parties ‘wins', as can happen in cases of parent–offspring [33] or sexual conflict [119]. This is most probably to be the parent for both classic and brood parasites owing to the imbalance of power in the social relationship [120]. At the egg stage, in particular, offspring have no power to defend themselves against rejection or eviction from the parent, and juveniles are physically weaker and dependent on parents for protection and food, and so unlikely to be able to defend themselves, should selection favour the parent to sacrifice them owing to infection.
5. Future directions
(a). Why do defences vary?
Despite decades of research on host–brood parasite interactions, we still lack a satisfactory explanation for why defences vary within and across host species. In contrast with many of the examples above, some hosts show comparatively weak defences (e.g. redstarts may abandon nests, but rarely remove eggs even though this is likely to be a less costly strategy [121]), or more puzzling still, they express no resistance against brood parasites (e.g. dunnocks do not reject even the most non-mimetic of eggs [122]). This may be evolutionary lag (there has been insufficient time for natural selection to act [123]), hosts without defences may represent systems at an evolutionary equilibrium [123], or the costs of mounting defences are too great relative to the fitness benefit of avoiding parasitism [124]. Alternative hypotheses based on spatial population and habitat structure have also been suggested, where defences vary because gene flow from non-parasitized populations reduces the likelihood that genetic mechanisms underpinning behavioural defences will reach fixation [125]. Distinguishing between these potential explanations has thus far been challenging [125] and limited largely to understanding egg rejection defences in avian hosts [6]. Can the social immunity framework, as we have applied it here, provide some insight into this problem?
Theory predicts that the presence and strength of social immune defences produced on behalf of kin will vary because of the balance of costs (c) versus benefits (b), modified by the relatedness (r) of the donor to the recipient (Hamilton's rule: r × b > c [126]) (also see [127] for a similar approach from the brood parasite's perspective). As discussed above, any mechanisms that could reduce the costs of social defence could therefore shift the balance towards defence against, rather than acceptance of, brood parasitism. One possibility is the evolution of personal defences against the parasite by the brood, as we see in burying beetle larvae, which cooperate with their parents in the production of antimicrobial secretions [116,117]. In the case of brood parasitism, the parasite could be considered a classic parasite from the brood's perspective, as it directly affects the brood's survival (see §2a), but evidence for direct defence against parasites by the brood is lacking from well-studied avian systems. Instead, in some cases there appears to be a transition to mutualism as the presence of a brood parasite in the nest can even enhance survival of host young against predators [128], though this effect might be population- or context-specific [129]. It may be that selection fails to act on the brood because they don't have the mechanisms to recognize parasites in the nest. However, it has been shown that offspring of species that suffer a higher incidence of parasitism by the brown-headed cowbird (Molothrus ater) tend to beg louder [130], and grow more rapidly [131], potentially reducing the costs of parasitism, much like the parental-driven tolerance responses covered above (see §3c(iii)). Furthermore, there is increasing evidence that offspring can cooperate to exploit parental resources, and so, in theory, the brood could potentially evolve effective defences of their own. Perhaps this helps to explain why many avian brood parasites attempt to evict or kill host nestmates within hours of hatching, and often before host eggs themselves have hatched [15,16].
In this review, we have stressed that brood parasitism exerts detrimental effects on host fitness via a reduction in indirect fitness across the host's lifetime. In terms of defences, selection acts on the donor to protect its lifetime indirect fitness rather than prioritizing the current reproductive attempt. Studies attempting to empirically quantify the costs of defences, or the costs of parasitism across the host's lifespan, remain few, however, and rely instead on assessing costs only in terms of the current brood (but see [132]). This is largely because inclusive fitness across the life course is difficult to measure in the field for many of the favoured brood parasite study systems, where hosts migrate or show high natal dispersal, and most of these are not amenable to experiments in the laboratory. Recent studies with captive cichlid fish and their catfish cuckoos (e.g. [87,90,91,133]) may provide a new avenue for replicating the advances in understanding resistance and tolerance against classic parasites, and the fitness benefits and costs of social immunity in particular, that have come from using invertebrate systems easily manipulated in the laboratory (e.g. [115,134]).
(b). Plasticity in immune defences—do social environments promote ‘density-dependent prophylaxis'?
Many insect species that undergo boom and bust population cycles, for example, locusts [135] and armyworm caterpillars [136,137], have been shown to use population density as a cue to increase investment in their immune systems, known as density-dependent prophylaxis (DDP) [138,139]. This anticipates the increased risk of infection when living in close quarters with conspecifics and ensures that costly immune investment is targeted to high-risk conditions. This response has been shown to occur across invertebrate taxa in response to classic parasites [139–142], and some studies provide evidence supporting its evolution in some vertebrate taxa, e.g. rodents [143–145] and birds [146], but it has not explicitly been considered for brood parasites.
In this issue, Medina & Langmore [44] found that fairy-wrens suffered greater levels of brood parasitism as their density increased, though at very high densities this risk again reduced, such that hosts at intermediate densities suffered the most when parasitism levels were high [44]. High densities should increase the risk of parasitism as discussed above (see §3c(i) Parasite avoidance), but fairy-wrens in larger colonies mob cuckoo models more than those in low-density colonies [147]. This suggests an upregulation of this defence in conditions where parasite risk is increased, which could arguably be considered a form of DDP. However, rather than the new phenotype being induced directly by density cues, as occurs in Lepidoptera [136,137] and Orthoptera [148], it is thought to be driven by social learning, whereby individuals in larger colonies have more opportunities to learn the correct defensive response to potential parasites [149,150]. Social cues could also act more broadly in a prophylactic manner if they enhance vigilance against brood parasites. For example, if a male reed warbler witnesses a cuckoo at its nest during the female's egg-laying period, then it guards the nest more closely [36]. Females, on the other hand, do not increase their nest attendance. Presumably, this is because the opportunity costs of increased vigilance against cuckoos are too high when females need to forage to recoup the loss of resources incurred from producing eggs [36]. Social information reduces uncertainty about parasitism risk [151], because it reduces the relative cost of mistakes against the benefits of accurate defences when collecting personal information is costly [55,150]. Perhaps females with increased social information about the risk of parasitism would also increase their nest attendance, but it is unknown if witnessing the aggressive behaviour of neighbours towards brood parasites influences vigilance per se, or indeed if nest attendance varies with host density.
(c). Towards a macroecology of host–brood parasite dynamics?
A final reflection on the integrative expansions of the field of evolution of host–brood parasite dynamics points toward the need for comparative analyses at larger spatial and phylogenetic scales—that is, towards a macroecology of host–brood parasite interactions. While most fields in ecology and evolution have taken advantage of large-scale studies as a means to understand the role of spatially varying selection on the predictability of adaptations across species [152,153], comparative studies at such scales remain a ‘pending debt' in the field of host–brood parasite interactions (but see [44,154]). This approach could now be used to address the outstanding questions regarding when and with what strength hosts should evolve social defences against brood parasites.
A broad range of sources of selection that vary along geographical gradients are core candidates to shape predictable spatial patterns of adaptive variation in defences against brood parasites. Factors such as variation in resource availability [155], the effects of seasonality as a source of varying intensity of fecundity selection on clutch size [152,156], variation in predator intensity [155,157], and the intrinsic variation of species richness across space [158] could affect the balance between the costs to the donor of defending versus the benefits gained via indirect fitness. This offers a robust theoretical motivation to explore the adaptive expression of large-scale patterns of variation in defences, which can then be linked to the phylogenetic patterns of emergence (and reversals) of host–brood parasite interactions. This will ultimately draw a broader perspective on the factors and contexts that make the evolution of social immunity a viable strategy to counterbalance the costs of parasitism. Any insights gained from this approach could then be applied to social immune responses in general, and may inform how and under what circumstances these defences evolve [5,23,159]. The rapid accumulation of phylogenetic and environmental data reinforces the timely opportunity to expand the field in the context of macroecology.
Acknowledgements
We thank Ros Gloag and an anonymous reviewer whose suggestions greatly improved the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed equally to the manuscript.
Competing interests
We have no competing interests.
Funding
R.T. was supported by an Independent Research Fellowship from the Natural Environment Research Council (NE/K00929X/1).
References
- 1.Kilner RM. 2006. The evolution of egg colour and patterning in birds. Biol. Rev. 81, 383–406. ( 10.1017/S1464793106007044) [DOI] [PubMed] [Google Scholar]
- 2.Hamilton WD, Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387. ( 10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 3.Wilson K, Cotter SC. 2013. Host-parasite interactions and the evolution of immune defense. In Advances in the study of behavior, vol. 45 (eds Brockmann HJ, Roper TJ, Naguib M, Mitani JC, Simmons LW, Barrett L), pp. 81–174. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 4.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 5.Cotter SC, Kilner RM. 2010. Personal immunity versus social immunity. Behav. Ecol. 21, 663–668. ( 10.1093/beheco/arq070) [DOI] [Google Scholar]
- 6.Soler M. 2014. Long-term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704. ( 10.1111/brv.12075) [DOI] [PubMed] [Google Scholar]
- 7.Day T. 2003. Virulence evolution and the timing of disease life-history events. Trends Ecol. Evol. 18, 113–118. ( 10.1016/S0169-5347(02)00049-6) [DOI] [Google Scholar]
- 8.Cunningham-Rundles S, McNeeley DF, Moon A. 2005. Mechanisms of nutrient modulation of the immune response. J. Allergy Clin. Immunol. 115, 1119–1128. ( 10.1016/j.jaci.2005.04.036) [DOI] [PubMed] [Google Scholar]
- 9.Lafferty KD, Kuris AM. 2009. Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol. 25, 564–572. ( 10.1016/j.pt.2009.09.003) [DOI] [PubMed] [Google Scholar]
- 10.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Smiseth PT, Kölliker M, Royle NJ. 2012. What is parental care? In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 1–20. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Costa JT. 2018. The other insect societies: overview and new directions. Curr. Opin. Insect Sci. 28, 40–49. ( 10.1016/j.cois.2018.04.008) [DOI] [PubMed] [Google Scholar]
- 13.Groulx AF, Forrest JR. 2018. Nesting aggregation as a predictor of brood parasitism in mason bees (Osmia spp.). Ecol. Entomol. 43, 182–191. ( 10.1111/een.12484) [DOI] [Google Scholar]
- 14.Costa JT. 2006. The other insect societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 15.Davies N. 2015. Cuckoo: cheating by nature. London, UK: Bloomsbury Publishing. [Google Scholar]
- 16.Spottiswoode CN, Kilner RM, Davies NB. 2012. Brood parasitism. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 226–356. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Thorogood R, Spottiswoode CN, Portugal SJ, Gloag R. 2019. The coevolutionary biology of brood parasitism: a call for integration. Phil. Trans. R. Soc. B 374, 20180190 ( 10.1098/rstb.2018.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cini A, Sumner S, Cervo R. 2019. Inquiline social parasites as tools to unlock the secrets of insect sociality. Phil. Trans. R. Soc. B 374, 20180193 ( 10.1098/rstb.2018.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuura K, Yashiro T, Shimizu K, Tatsumi S, Tamura T. 2009. Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme β-glucosidase. Curr. Biol. 19, 30–36. ( 10.1016/j.cub.2008.11.030) [DOI] [PubMed] [Google Scholar]
- 20.Gilbert JDJ, Mound LA, Simpson SJ. 2012. Biology of a new species of socially parasitic thrips (Thysanoptera: Phlaeothripidae) inside Dunatothrips nests, with evolutionary implications for inquilinism in thrips. Biol. J. Linn. Soc. 107, 112–122. ( 10.1111/j.1095-8312.2012.01928.x) [DOI] [Google Scholar]
- 21.Kaur R, Stoldt M, Jongepier E, Feldmeyer B, Menzel F, Bornberg-Bauer E, Foitzik S. 2019. Ant behaviour and brain gene expression of defending hosts depend on the ecological success of the intruding social parasite. Phil. Trans. R. Soc. B 374, 20180192 ( 10.1098/rstb.2018.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartally A, et al. 2019. Patterns of host use by brood parasitic Maculinea butterflies across Europe. Phil. Trans. R. Soc. B 374, 20180202 ( 10.1098/rstb.2018.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meunier J. 2015. Social immunity and the evolution of group living in insects. Phil. Trans. R. Soc. B 370, 10 ( 10.1098/rstb.2014.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cremer S, Armitage SAO, Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702. ( 10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 25.Cremer S, Pull CD, Furst MA. 2018. Social immunity: emergence and evolution of colony-level disease protection. In A. Rev. Entomol. 63, 105–123. [DOI] [PubMed] [Google Scholar]
- 26.Evans SS, Repasky EA, Fisher DT. 2015. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 15, 335–349. ( 10.1038/nri3843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas MB, Blanford S. 2003. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 17, 344–350. ( 10.1016/S0169-5347(03)00069-7) [DOI] [Google Scholar]
- 28.Schieber AMP, Ayres JS. 2016. Thermoregulation as a disease tolerance defense strategy. Pathog. Dis. 74, 15 ( 10.1093/femspd/ftw106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starks PT, Blackie CA, Seeley TD. 2000. Fever in honeybee colonies. Naturwissenschaften 87, 229–231. ( 10.1007/s001140050709) [DOI] [PubMed] [Google Scholar]
- 30.Sadd BM, Schmid-Hempel P. 2007. Facultative but persistent transgenerational immunity via the mother's eggs in bumblebees. Curr. Biol. 17, R1046–R1047. ( 10.1016/j.cub.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 31.Grindstaff JL, Brodie ED, Ketterson ED. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319. ( 10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosengaus RB, Traniello JFA, Lefebvre ML, Maxmen AB. 2004. Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Sociaux 51, 259–264. ( 10.1007/s0040-004-0749-x) [DOI] [Google Scholar]
- 33.Trivers RL. 1974. Parent–offspring conflict. Integr. Comp. Biol. 14, 249–264. [Google Scholar]
- 34.Foster KR, Ratnieks FL. 2000. Social insects: facultative worker policing in a wasp. Nature 407, 692 ( 10.1038/35037665) [DOI] [PubMed] [Google Scholar]
- 35.Smiseth PT, Royle NJ. 2018. The resolution of conflict in families. Curr. Opin. Insect Sci. 28, 8–12. ( 10.1016/j.cois.2018.03.007) [DOI] [PubMed] [Google Scholar]
- 36.Davies NB, Butchart SHM, Burke TA, Chaline N, Stewart IRK. 2003. Reed warblers guard against cuckoos and cuckoldry. Anim. Behav. 65, 285–295. ( 10.1006/anbe.2003.2049) [DOI] [Google Scholar]
- 37.Fialho VS, Rodrigues VB, Elliot SL. 2018. Nesting strategies and disease risk in necrophagous beetles. Ecol. Evol. 8, 3296–3310. ( 10.1002/ece3.3919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinze J, Walter B. 2010. Moribund ants leave their nests to die in social isolation. Curr. Biol. 20, 249–252. ( 10.1016/j.cub.2009.12.031) [DOI] [PubMed] [Google Scholar]
- 39.Epsky ND, Capinera JL. 1988. Efficacy of the entomogenous nematode Steinernema feltiae against a subterranean termite, Reticulitermes tibialis (Isoptera: Rhinotermitidae). J. Econ. Entomol. 81, 1313–1317. ( 10.1093/jee/81.5.1313) [DOI] [Google Scholar]
- 40.Oien IJ, Honza M, Moksnes A, Roskaft E. 1996. The risk of parasitism in relation to the distance from reed warbler nests to cuckoo perches. J. Anim. Ecol. 65, 147–153. ( 10.2307/5717) [DOI] [Google Scholar]
- 41.Strohm E, Laurien-Kehnen C, Bordon S. 2001. Escape from parasitism: spatial and temporal strategies of a sphecid wasp against a specialised cuckoo wasp. Oecologia 129, 50–57. ( 10.1007/s004420100702) [DOI] [PubMed] [Google Scholar]
- 42.Tolvanen J, Forsman JT, Thomson RL. 2017. Reducing cuckoo parasitism risk via informed habitat choices. Auk 134, 553–563. ( 10.1642/auk-17-30.1) [DOI] [Google Scholar]
- 43.Anderson RM, May RM. 1981. The population dynamics of microparasites and their invertebrate hosts. Phil. Trans. R. Soc. Lond. B 291, 451–524. ( 10.1098/rstb.1981.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina I, Langmore NE. 2019. Host density predicts the probability of parasitism by avian brood parasites. Phil. Trans. R. Soc. B 374, 20180204 ( 10.1098/rstb.2018.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosengaus RB, Maxmen AB, Coates LE, Traniello JFA. 1998. Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav. Ecol. Sociobiol. 44, 125–134. ( 10.1007/s002650050523) [DOI] [Google Scholar]
- 46.Walker TN, Hughes WOH. 2009. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 5, 446–448. ( 10.1098/rsbl.2009.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feeney WE, Welbergen JA, Langmore NE. 2012. The frontline of avian brood parasite–host coevolution. Anim. Behav. 84, 3–12. ( 10.1016/j.anbehav.2012.04.011) [DOI] [Google Scholar]
- 48.Welbergen JA, Davies NB. 2009. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240. ( 10.1016/j.cub.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 49.Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. 2013. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim. Behav. 86, 1023–1029. ( 10.1016/j.anbehav.2013.09.007) [DOI] [Google Scholar]
- 50.Molnár B. 1944. The cuckoo in the Hungarian plain. Aquila 51, 100–112. [Google Scholar]
- 51.Ma L, Yang C, Liu J, Zhang J, Liang W, Møller AP. 2018. Costs of breeding far away from neighbors: isolated host nests are more vulnerable to cuckoo parasitism. Behav. Process 157 , 327–332. ( 10.1016/j.beproc.2018.07.017) [DOI] [PubMed] [Google Scholar]
- 52.Litman JR. 2019. Under the radar: detection avoidance in brood parasitic bees. Phil. Trans. R. Soc. B 374, 20180196 ( 10.1098/rstb.2018.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welbergen JA, Davies NB. 2008. Reed warblers discriminate cuckoos from sparrowhawks with graded alarm signals that attract mates and neighbours. Anim. Behav. 76, 811–822. ( 10.1016/j.anbehav.2008.03.020) [DOI] [Google Scholar]
- 54.Davies NB, Welbergen JA. 2009. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320. ( 10.1126/science.1172227) [DOI] [PubMed] [Google Scholar]
- 55.Thorogood R, Davies NB. 2012. Cuckoos combat socially transmitted defenses of reed warbler hosts with a plumage polymorphism. Science 337, 578–580. ( 10.1126/science.1220759) [DOI] [PubMed] [Google Scholar]
- 56.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 57.Christe P, Oppliger A, Bancala F, Castella G, Chapuisat M. 2003. Evidence for collective medication in ants. Ecol. Lett. 6, 19–22. ( 10.1046/j.1461-0248.2003.00395.x) [DOI] [Google Scholar]
- 58.Gwinner H, Berger S. 2005. European starlings: nestling condition, parasites and green nest material during the breeding season. J. Ornithol. 146, 365–371. ( 10.1007/s10336-005-0012-x) [DOI] [Google Scholar]
- 59.Fleming RI, Mackenzie CD, Cooper A, Kennedy MW. 2009. Foam nest components of the túngara frog: a cocktail of proteins conferring physical and biological resilience. Proc. R. Soc. B 276, 1787–1795. ( 10.1098/rspb.2008.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knouft JH, Page LM, Plewa MJ. 2003. Antimicrobial egg cleaning by the fringed darter (Perciformes: Percidae: Etheostoma crossopterum): implications of a novel component of parental care in fishes. Proc. R. Soc. Lond. B 270, 2405–2411. ( 10.1098/rspb.2003.2501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giacomello E, Marri L, Marchini D, Mazzoldi C, Rasotto MB. 2008. Sperm-duct gland secretion of the grass goby Zosterisessor ophiocephalus exhibits antimicrobial activity. J. Fish Biol. 73, 1823–1828. ( 10.1111/j.1095-8649.2008.02069.x) [DOI] [Google Scholar]
- 62.Little TJ, Perutz M, Palmer M, Crossan C, Braithwaite VA. 2008. Male three-spined sticklebacks Gasterosteus aculeatus make antibiotic nests: a novel form of parental protection? J. Fish Biol. 73, 2380–2389. ( 10.1111/j.1095-8649.2008.02086.x) [DOI] [Google Scholar]
- 63.Rosengaus RB, Guldin MR, Traniello JFA. 1998. Inhibitory effect of termite fecal pellets on fungal spore germination. J. Chem. Ecol. 24, 1697–1706. ( 10.1023/A:1020872729671) [DOI] [Google Scholar]
- 64.Cotter SC, Kilner RM. 2010. Sexual division of antibacterial resource defence in breeding burying beetles, Nicrophorus vespilloides. J. Anim. Ecol. 79, 35–43. ( 10.1111/j.1365-2656.2009.01593.x) [DOI] [PubMed] [Google Scholar]
- 65.Cardoza YJ, Klepzig KD, Raffa KF. 2006. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 31, 636–645. ( 10.1111/j.1365-2311.2006.00829.x) [DOI] [Google Scholar]
- 66.Shukla SP, Plata C, Reichelt M, Steiger S, Heckel DG, Kaltenpoth M, Vilcinskas A, Vogel H. 2018. Microbiome-assisted carrion preservation aids larval development in a burying beetle. Proc. Natl Acad. Sci. USA 115, 11 274–11 279. ( 10.1073/pnas.1812808115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duarte A, Cotter SC, De Gasperin O, Houslay TM, Boncoraglio G, Welch M, Kilner RM. 2017. No evidence of a cleaning mutualism between burying beetles and their phoretic mites. Sci. Rep. 7, 13838 ( 10.1038/s41598-017-14201-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duarte A, Welch M, Swannack C, Wagner J, Kilner RM. 2018. Strategies for managing rival bacterial communities: lessons from burying beetles. J. Anim. Ecol. 87, 414–427. ( 10.1111/1365-2656.12725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freeman S. 1988. Egg variability and conspecific nest parasitism in the Ploceus weaverbirds. Ostrich 59, 49–53. ( 10.1080/00306525.1988.9633694) [DOI] [Google Scholar]
- 70.Munster-Swendsen M, Calabuig I. 2000. Interaction between the solitary bee Chelostoma florisomne and its nest parasite Sapyga clavicornis–empty cells reduce the impact of parasites. Ecol. Entomol. 25, 63–70. ( 10.1046/j.1365-2311.2000.00225.x) [DOI] [Google Scholar]
- 71.Medzhitov R, Janeway C. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173, 89–97. ( 10.1034/j.1600-065X.2000.917309.x) [DOI] [PubMed] [Google Scholar]
- 72.Pull CD, Ugelvig LV, Wiesenhofer F, Grasse AV, Tragust S, Schmitt T, Brown MJF, Cremer S. 2018. Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. eLife 7, e32073 ( 10.7554/eLife.32073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rohlfs M, Obmann B, Petersen R. 2005. Competition with filamentous fungi and its implication for a gregarious lifestyle in insects living on ephemeral resources. Ecol. Entomol. 30, 556–563. ( 10.1111/j.0307-6946.2005.00722.x) [DOI] [Google Scholar]
- 74.Parker BJ, Elderd BD, Dwyer G. 2010. Host behaviour and exposure risk in an insect–pathogen interaction. J. Anim. Ecol. 79, 863–870. ( 10.1111/j.1365-2656.2010.01690.x) [DOI] [PubMed] [Google Scholar]
- 75.Davis HE, Meconcelli S, Radek R, McMahon DP. 2018. Termites shape their collective behavioural response based on stage of infection. Sci. Rep. 8, 14433 ( 10.1038/s41598-018-32721-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA. 1999. Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften 86, 544–548. ( 10.1007/s001140050672) [DOI] [PubMed] [Google Scholar]
- 77.Myles TG. 2002. Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Sociobiology 40, 243–255. [Google Scholar]
- 78.Akino T, Knapp J, Thomas J, Elmes G. 1999. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 266, 1419–1426. ( 10.1098/rspb.1999.0796) [DOI] [Google Scholar]
- 79.Stoddard MC, Marshall KLA, Kilner RM. 2011. Imperfectly camouflaged avian eggs: artefact or adaptation? Avian Biol. Res. 4, 196–213. ( 10.3184/175815511X13207484398647) [DOI] [Google Scholar]
- 80.Langmore NE, Stevens M, Maurer G, Heinsohn R, Hall ML, Peters A, Kilner RM. 2011. Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463. ( 10.1098/rspb.2010.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stoddard MC, Kilner RM, Town C. 2014. Pattern recognition algorithm reveals how birds evolve individual egg pattern signatures. Nat. Commun. 5, 4117 ( 10.1038/ncomms5117) [DOI] [PubMed] [Google Scholar]
- 82.Stoddard MC, Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B 277, 1387–1393. ( 10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoddard MC, Stevens M. 2011. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution 65, 2004–2013. ( 10.1111/j.1558-5646.2011.01262.x) [DOI] [PubMed] [Google Scholar]
- 84.Caves EM, Stevens M, Iversen ES, Spottiswoode CN. 2015. Hosts of avian brood parasites have evolved egg signatures with elevated information content. Proc. R. Soc. B 282, 20150598 ( 10.1098/rspb.2015.0598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spottiswoode CN, Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676. ( 10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Troscianko J, Stevens M. 2015. Image calibration and analysis toolbox—a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol. 6, 1320–1331. ( 10.1111/2041-210x.12439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blazek R, Polacik M, Smith C, Honza M, Meyer A, Reichard M. 2018. Success of cuckoo catfish brood parasitism reflects coevolutionary history and individual experience of their cichlid hosts. Sci. Adv. 4, eaar4380 ( 10.1126/sciadv.aar4380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanley D, López AV, Fiorini VD, Reboreda JC, Grim T, Hauber ME. 2019. Variation in multicomponent recognition cues alters egg rejection decisions: a test of the optimal acceptance threshold hypothesis. Phil. Trans. R. Soc. B 374, 20180195 ( 10.1098/rstb.2018.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang C, Liang W, Møller AP. 2019. Egg retrieval versus egg rejection in cuckoo hosts. Phil. Trans. R. Soc. B 374, 20180200 ( 10.1098/rstb.2018.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen MS, Hawkins MB, Stock DW, Cruz A. 2019. Early life-history features associated with brood parasitism in the cuckoo catfish, Synodontis multipunctatus (Siluriformes: Mochokidae). Phil. Trans. R. Soc. B 374, 20180205 ( 10.1098/rstb.2018.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polačik M, Reichard M, Smith C, Blažek R. 2019. Parasitic cuckoo catfish exploit parental responses to stray offspring. Phil. Trans. R. Soc. B 374, 20180412 ( 10.1098/rstb.2018.0412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lihoreau M, Rivault C. 2009. Kin recognition via cuticular hydrocarbons shapes cockroach social life. Behav. Ecol. 20, 46–53. ( 10.1093/beheco/arn113) [DOI] [Google Scholar]
- 93.Steiger S, Peschke K, Francke W, Muller JK. 2007. The smell of parents: breeding status influences cuticular hydrocarbon pattern in the burying beetle Nicrophorus vespilloides. Proc. R. Soc. B 274, 2211–2220. ( 10.1098/rspb.2007.0656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steiger S, Peschke K, Muller JK. 2008. Correlated changes in breeding status and polyunsaturated cuticular hydrocarbons: the chemical basis of nestmate recognition in the burying beetle Nicrophorus vespilloides? Behav. Ecol. Sociobiol. 62, 1053–1060. ( 10.1007/s00265-007-0532-x) [DOI] [Google Scholar]
- 95.Richard FJ, Hunt JH. 2013. Intracolony chemical communication in social insects. Insectes Sociaux 60, 275–291. ( 10.1007/s00040-013-0306-6) [DOI] [Google Scholar]
- 96.d'Ettorre P, Mondy N, Lenoir A, Errard C. 2002. Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. Lond. B 269, 1911–1918. ( 10.1098/rspb.2002.2110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kleeberg I, Menzel F, Foitzik S. 2017. The influence of slavemaking lifestyle, caste and sex on chemical profiles in Temnothorax ants: insights into the evolution of cuticular hydrocarbons. Proc. R. Soc. B 284, 20162249. ( 10.1098/rspb.2016.2249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. ( 10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hosoi SA, Rothstein SI. 2000. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim. Behav. 59, 823–840. ( 10.1006/anbe.1999.1370) [DOI] [PubMed] [Google Scholar]
- 100.Thomas JA, Wardlaw JC. 1992. The capacity of a Myrmica ant nest to support a predacious species of Maculinea butterfly. Oecologia 91, 101–109. ( 10.1007/bf00317247) [DOI] [PubMed] [Google Scholar]
- 101.Spottiswoode CN, Busch R. 2019. Vive la difference! Self/non-self recognition and the evolution of signatures of identity in arms races with parasites. Phil. Trans. R. Soc. B 374, 20180206 ( 10.1098/rstb.2018.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Roode JC, Lefèvre T, Hunter MD. 2013. Self-medication in animals. Science 340, 150–151. ( 10.1126/science.1235824) [DOI] [PubMed] [Google Scholar]
- 103.Lefevre T, Oliver L, Hunter MD, de Roode JC. 2010. Evidence for trans-generational medication in nature. Ecol. Lett. 13, 1485–1493. ( 10.1111/j.1461-0248.2010.01537.x) [DOI] [PubMed] [Google Scholar]
- 104.Stokke BG, Ratikainen II, Moksnes A, Roskaft E, Schulze-Hagen K, Leech DI, Moller AP, Fossoy F. 2018. Characteristics determining host suitability for a generalist parasite. Sci. Rep. 8, 1 ( 10.1038/s41598-018-24627-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang CC, et al. 2013. Host selection in parasitic birds: are open-cup nesting insectivorous passerines always suitable cuckoo hosts? J. Avian Biol. 44, 216–220. ( 10.1111/j.1600-048X.2013.00123.x) [DOI] [Google Scholar]
- 106.Svensson EI, Raberg L. 2010. Resistance and tolerance in animal enemy–victim coevolution. Trends Ecol. Evol. 25, 267–274. ( 10.1016/j.tree.2009.12.005) [DOI] [PubMed] [Google Scholar]
- 107.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936–941. ( 10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kutzer MAM, Armitage SAO. 2016. Maximising fitness in the face of parasites: a review of host tolerance. Zoology 119, 281–289. ( 10.1016/j.zool.2016.05.011) [DOI] [PubMed] [Google Scholar]
- 109.Graham AL, Allen JE, Read AF. 2005. Evolutionary causes and consequences of immunopathology. A. Rev. Ecol. Evol. System. 36, 373–397. ( 10.1146/annurev.ecolsys.36.102003.152622) [DOI] [Google Scholar]
- 110.Miller CVL, Cotter SC. 2018. Resistance and tolerance: the role of nutrients on pathogen dynamics and infection outcomes in an insect host. J. Anim. Ecol. 87, 500–510. ( 10.1111/1365-2656.12763) [DOI] [PubMed] [Google Scholar]
- 111.Vale PF, Little TJ. 2012. Fecundity compensation and tolerance to a sterilizing pathogen in Daphnia. J. Evol. Biol. 25, 1888–1896. ( 10.1111/j.1420-9101.2012.02579.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calleri DV, Rosengaus RB, Traniello JFA. 2006. Disease and colony establishment in the dampwood termite Zootermopsis angusticollis: survival and fitness consequences of infection in primary reproductives. Insectes Sociaux 53, 204–211. ( 10.1007/s00040-005-0859-0) [DOI] [Google Scholar]
- 113.Hauber ME. 2003. Interspecific brood parasitism and the evolution of host clutch sizes. Evol. Ecol. Res. 5, 559–570. [Google Scholar]
- 114.Aviles JM. 2018. Can hosts tolerate avian brood parasites? An appraisal of mechanisms. Behav. Ecol. 29, 509–519. ( 10.1093/beheco/arx150) [DOI] [Google Scholar]
- 115.Cotter S, Topham E, Price A, Kilner R. 2010. Fitness costs associated with mounting a social immune response. Ecol. Lett. 13, 1114–1123. ( 10.1111/j.1461-0248.2010.01500.x) [DOI] [PubMed] [Google Scholar]
- 116.Reavey CE, Beare L, Cotter SC. 2014. Parental care influences social immunity in burying beetle larvae. Ecol. Entomol. 39, 395–398. ( 10.1111/een.12099) [DOI] [Google Scholar]
- 117.Arce AN, Smiseth PT, Rozen DE. 2013. Antimicrobial secretions and social immunity in larval burying beetles, Nicrophorus vespilloides. Anim. Behav. 86, 741–745. ( 10.1016/j.anbehav.2013.07.008) [DOI] [Google Scholar]
- 118.Duarte A, Cotter SC, Reavey CE, Ward RJS, De Gasperin O, Kilner RM. 2016. Social immunity of the family: parental contributions to a public good modulated by brood size. Evol. Ecol. 30, 123–135. ( 10.1007/s10682-015-9806-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol. 18, 41–47. ( 10.1016/S0169-5347(02)00004-6) [DOI] [Google Scholar]
- 120.Beekman M, Komdeur J, Ratnieks FL. 2003. Reproductive conflicts in social animals: who has power? Trends Ecol. Evol. 18, 277–282. ( 10.1016/S0169-5347(03)00068-5) [DOI] [Google Scholar]
- 121.Samas P, Rutila J, Grim T. 2016. The common redstart as a suitable model to study cuckoo-host coevolution in a unique ecological context. BMC Evol. Biol. 16, 255 ( 10.1186/s12862-016-0835-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Medina I, Langmore NE. 2016. The evolution of acceptance and tolerance in hosts of avian brood parasites. Biol. Rev. 91, 569–577. ( 10.1111/brv.12181) [DOI] [PubMed] [Google Scholar]
- 123.Takasu F, Kawasaki K, Nakamura H, Cohen JE, Shigesada N. 1993. Modeling the population-dynamics of a cuckoo-host association and the evolution of host defenses. Am. Nat. 142, 819–839. ( 10.1086/285574) [DOI] [PubMed] [Google Scholar]
- 124.Medina I, Langmore NE. 2015. The costs of avian brood parasitism explain variation in egg rejection behaviour in hosts. Biol. Lett. 11, 20150296 ( 10.1098/rsbl.2015.0296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stokke BG, Moksnes A, Røskaft E. 2005. The enigma of imperfect adaptations in hosts of avian brood parasites. Ornithol. Sci. 4, 17–29. [Google Scholar]
- 126.Hamilton WD. 1963. Evolution of altruistic behaviour. Am. Nat. 97, 354. [Google Scholar]
- 127.Gloag R, Beekman M. 2019. The brood parasite's guide to inclusive fitness theory. Phil. Trans. R. Soc. B 374, 20180198 ( 10.1098/rstb.2018.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Canestrari D, Bolopo D, Turlings TC, Röder G, Marcos JM, Baglione V. 2014. From parasitism to mutualism: unexpected interactions between a cuckoo and its host. Science 343, 1350–1352. ( 10.1126/science.1249008) [DOI] [PubMed] [Google Scholar]
- 129.Soler M, de Neve L, Roldán M, Pérez-Contreras T, Soler JJ. 2017. Great spotted cuckoo nestlings have no antipredatory effect on magpie or carrion crow host nests in southern Spain. PLoS ONE 12, e0173080 ( 10.1371/journal.pone.0173080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boncoraglio G, Saino N, Garamszegi LZ. 2008. Begging and cowbirds: brood parasites make hosts scream louder. Behav. Ecol. 20, 215–221. ( 10.1093/beheco/arn137) [DOI] [Google Scholar]
- 131.Remeš V. 2006. Growth strategies of passerine birds are related to brood parasitism by the brown-headed cowbird (Molothrus ater). Evolution 60, 1692–1700. ( 10.1111/j.0014-3820.2006.tb00513.x) [DOI] [PubMed] [Google Scholar]
- 132.Payne RB, Payne LL. 1998. Brood parasitism by cowbirds: risks and effects on reproductive success and survival in indigo buntings. Behav. Ecol. 9, 64–73. ( 10.1093/beheco/9.1.64) [DOI] [Google Scholar]
- 133.Cohen MS, Hawkins MB, Knox-Hayes J, Vinton AC, Cruz A. 2018. A laboratory study of host use by the cuckoo catfish Synodontis multipunctatus. Environ. Biol. Fishes 101, 1417–1425. ( 10.1007/s10641-018-0788-1) [DOI] [Google Scholar]
- 134.Arce AN, Johnston PR, Smiseth PT, Rozen DE. 2012. Mechanisms and fitness effects of antibacterial defences in a carrion beetle. J. Evol. Biol. 25, 930–937. ( 10.1111/j.1420-9101.2012.02486.x) [DOI] [PubMed] [Google Scholar]
- 135.Wilson K, Thomas MB, Blanford S, Doggett M, Simpson SJ, Moore SL. 2002. Coping with crowds: density-dependent disease resistance in desert locusts. Proc. Natl Acad. Sci. USA 99, 5471–5475. ( 10.1073/pnas.082461999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cotter SC, Hails RS, Cory JS, Wilson K. 2004. Density-dependent prophylaxis and condition-dependent immune function in lepidopteran larvae: a multivariate approach. J. Anim. Ecol. 73, 283–293. ( 10.1111/j.0021-8790.2004.00806.x) [DOI] [Google Scholar]
- 137.Wilson K, Cotter S, Reeson A, Pell J. 2001. Melanism and disease resistance in insects. Ecol. Lett. 4, 637–649. ( 10.1046/j.1461-0248.2001.00279.x) [DOI] [Google Scholar]
- 138.Wilson K, Reeson AF. 1998. Density-dependent prophylaxis: evidence from Lepidoptera–baculovirus interactions? Ecol. Entomol. 23, 100–101. ( 10.1046/j.1365-2311.1998.00107.x) [DOI] [Google Scholar]
- 139.Wilson K, Cotter SC. 2009. Density-dependent prophylaxis in insects. In Insects and phenotypic plasticity: mechanisms and consequences (eds Ananthakrishnan TN, Whitman DW), pp. 137–176. Plymouth, UK: Science Publishers. [Google Scholar]
- 140.Ruiz-Gonzalez MX, Moret Y, Brown MJF. 2009. Rapid induction of immune density-dependent prophylaxis in adult social insects. Biol. Lett. 5, 781–783. ( 10.1098/rsbl.2009.0505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mills S. 2012. Density-dependent prophylaxis in the coral-eating crown-of-thorns sea star, Acanthaster planci. Coral Reefs 31, 603–612. ( 10.1007/s00338-012-0883-2) [DOI] [Google Scholar]
- 142.Brutsch T, Avril A, Chapuisat M. 2017. No evidence for social immunity in co-founding queen associations. Sci. Rep. 7, 16262 ( 10.1038/s41598-017-16368-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saino N, Canova L, Fasola M, Martinelli R. 2000. Reproduction and population density affect humoral immunity in bank voles under field experimental conditions. Oecologia 124, 358–366. ( 10.1007/s004420000395) [DOI] [PubMed] [Google Scholar]
- 144.Stillson LL, Platt TR. 2007. The crowding effect and morphometric variability in Echinostoma caproni (Digenea: Echinostomatidae) from ICR mice. J. Parasitol. 93, 242–246. ( 10.1645/ge-1015r.1) [DOI] [PubMed] [Google Scholar]
- 145.Young HS, Dirzo R, Helgen KM, McCauley DJ, Nunn CL, Snyder P, Veblen KE, Zhao S, Ezenwa VO. 2016. Large wildlife removal drives immune defence increases in rodents. Funct. Ecol. 30, 799–807. ( 10.1111/1365-2435.12542) [DOI] [Google Scholar]
- 146.Moller AP, Martin-Vivaldi M, Merino S, Soler JJ. 2006. Density-dependent and geographical variation in bird immune response. Oikos 115, 463–474. ( 10.1111/j.2006.0030-1299.15312.x) [DOI] [Google Scholar]
- 147.Feeney WE, Langmore NE. 2013. Social learning of a brood parasite by its host. Biol. Lett. 9, 20130443 ( 10.1098/rsbl.2013.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Simpson SJ, Despland E, Hagele BF, Dodgson T. 2001. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl Acad. Sci. USA 98, 3895–3897. ( 10.1073/pnas.071527998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Langmore NE, Feeney WE, Crowe-Riddell J, Luan H, Louwrens KM, Cockburn A. 2012. Learned recognition of brood parasitic cuckoos in the superb fairy-wren Malurus cyaneus. Behav. Ecol. 23, 798–805. ( 10.1093/beheco/ars033) [DOI] [Google Scholar]
- 150.Thorogood R, Davies NB. 2016. Combining personal with social information facilitates host defences and explains why cuckoos should be secretive. Sci. Rep. 6, 19872 ( 10.1038/srep19872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dall SRX, Giraldeau LA, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193. ( 10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 152.Jetz W, Sekercioglu CH, Böhning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, e303 ( 10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blackburn TM, Gaston KJ. 2003. Macroecology: concepts and consequences: 43rd Symp. British Ecol. Soc. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 154.Suhonen J, Ilvonen JJ, Nyman T, Sorvari J. 2019. Brood parasitism in eusocial insects (Hymenoptera): role of host geographical range size and phylogeny. Phil. Trans. R. Soc. B 374, 20180203 ( 10.1098/rstb.2018.0203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pincheira-Donoso D, Hunt J. 2017. Fecundity selection theory: concepts and evidence. Biol. Rev. 92, 341–356. ( 10.1111/brv.12232) [DOI] [PubMed] [Google Scholar]
- 156.Pincheira-Donoso D, Tregenza T. 2011. Fecundity selection and the evolution of reproductive output and sex-specific body size in the Liolaemus lizard adaptive radiation. Evol. Biol. 38, 197–207. ( 10.1007/s11692-011-9118-7) [DOI] [Google Scholar]
- 157.Griebeler E, Caprano T, Böhning-Gaese K. 2010. Evolution of avian clutch size along latitudinal gradients: do seasonality, nest predation or breeding season length matter? J. Evol. Biol. 23, 888–901. ( 10.1111/j.1420-9101.2010.01958.x) [DOI] [PubMed] [Google Scholar]
- 158.Roll U, et al. 2017. The global distribution of tetrapods reveals a need for targeted reptile conservation. Nat. Ecol. Evol. 1, 1677–1682. ( 10.1038/s41559-017-0332-2) [DOI] [PubMed] [Google Scholar]
- 159.Van Meyel S, Körner M, Meunier J. 2018. Social immunity: why we should study its nature, evolution and functions across all social systems. Curr. Opin. Insect Sci. 28, 1–7. ( 10.1016/j.cois.2018.03.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.